Abstract
The novel coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused a global pandemic in the last year. Along with major respiratory distress, a myriad of neurological manifestations was also reported to be associated with COVID-19 patients. These cases indicate that SARS-CoV-2 can be considered as an opportunistic pathogen of the brain. SARS-CoV-2 enters the brain through the olfactory bulb, retrograde axonal transport from peripheral nerve endings, or via hematogenous or lymphatic routes. Notably, COVID-19 infection can cause or even present with different neurological features including encephalopathy, impaired consciousness, confusion, agitation, seizure, ataxia, headache, anosmia, ageusia, neuropathies, and neurodegenerative diseases. In this paper, we provide a brief review of observed neurological manifestations associated with COVID-19.
Keywords: COVID-19, SARS-CoV-2, Neurological manifestations, Neurodegenerative diseases, Neuroinvasion, Sleep
1. Introduction
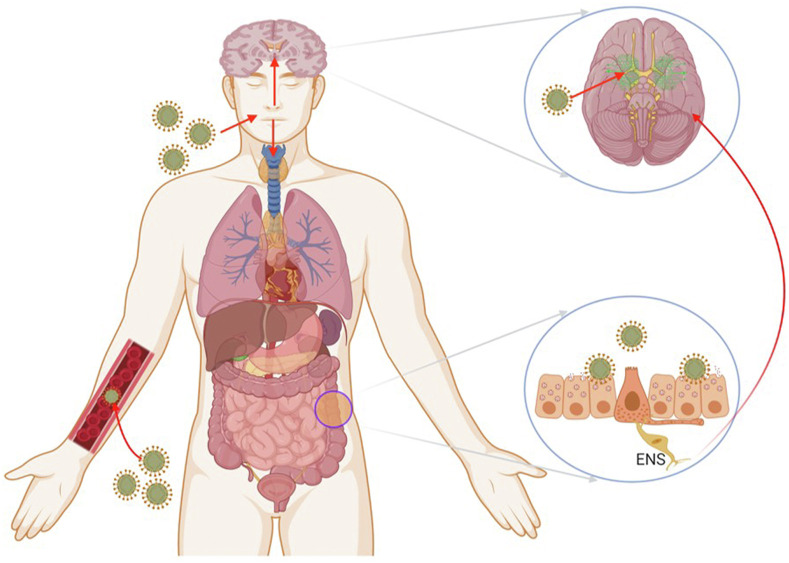
The novel coronavirus disease 2019 (COVID-19) has infected people throughout the globe in an uncontrolled manner showing no sign of disappearing on its own. Till the end of June 2021, this virus has infected almost 178 million people around the globe and caused the death of 38 million people (https://covid19.who.int/). The majority of the SARS-CoV-2 infected cases were asymptomatic (80%) or displayed moderate flu-like symptoms, including shortness of breath, fever, sore throat, cough, myalgia, loss of taste and smell, and fatigue. SARS-CoV-2 is a single-stranded positively sensed RNA virus, comprising a 26-32 kb-sized genome. The virus is spherical or oval-shaped with an average of 100 nm diameter, covered by a crown-like spike (S) protein that binds to the host cellular angiotensin-converting enzyme 2 (ACE-2) receptors and enters the host cell [1]. SARS-CoV-2 shares the common neuroinvasive nature with the SARS-CoV and MERS-CoV viruses [2,3] and enters the brain by several routes including direct and indirect pathways. It may enter the central nervous system (CNS) by infecting endothelial cells of the blood–brain-barrier (BBB) and epithelial cells of the blood-CSF barrier. The other routes to the CNS are through retrograde axonal transport, synapse-connected route after entering peripheral nerve terminals of the respiratory network [4], sympathetic afferent neurons of the enteric nervous system (ENS), and also through blood circulation [5,6] (Fig. 1 ).
Fig. 1.
Possible access of coronavirus to the nervous system. The SARS-CoV-2 can enter the nervous system directly through the olfactory nerve, blood circulation, ENS, and its sympathetic afferent neurons. Adapted from Ref. [6].
Several studies reported that over one-third of COVID-19 patients developed a broad spectrum of neurological symptoms affecting the CNS, peripheral nervous system (PNS), and skeletal muscles [7,8]. By direct activation of immune response and neurotoxicity, SARS-CoV-2 causes damage in a host's body. Moreover, SARS-CoV-2 infection leads to a cerebral vascular injury that increases the risk of chronic brain damage, because of the collective damaging effect of multifocal cerebral or haemorrhage, endothelial and BBB dysfunction, and upregulation of pro-inflammatory cytokines within the brain [9]. The neurological manifestations associated with COVID-19 can be classified as CNS and PNS manifestations (see also preceding brief overview by Chokroverty in this issue).
2. Central nervous system manifestations
2.1. Headache and dizziness
Recent studies reported that headaches and dizziness are the most common neurological symptoms in COVID-19 patients [7,10]. Mao et al. [7] reported a wide array of manifestations, including dizziness and headache in 16.8% and 13.1% of patients, respectively. A population-based study [10] was conducted across China which included 1099 patients, of which 13.6% of patients reported headaches. However, headaches, in general, are not considered as specific symptoms in any viral infections, but in most studies conducted on COVID-19 patients, it has been largely reported across the globe, from as low as 3% to as high as 15% in some studies [10,11]. Nevertheless, headache can often be considered as a precursor of viral meningitis or encephalitis and can often be the predictive symptom of cerebrovascular disease [12].
2.2. Anosmia and hypogeusia/ageusia
In adults, post-viral anosmia is one of the major reasons for olfactory dysfunction in COVID-19, identified in up to 40% of infected patients [13]. Reports from the United States of America, Italy, China, United Kingdom, France, and Korea bring forth the symptoms of hypogeusia/ageusia in the infected patients [11]. A study from Italy reports that around 33.9% of infected patients have either disturbance in taste or the olfactory system, whereas around 18.3% of infected patients experienced both disturbances [13]. Anosmia was also reported as the first symptom in about 83% of infected patients. In most cases, despite the absence of any significant inflammation in nasal linings or any coryzal symptoms, disturbances in olfactory systems still occurred [14]. Thus, it has been suggested that the odor-processing mechanism is directly targeted by the SARS-CoV-2 [15].
2.3. Cerebrovascular events
In the middle of this ongoing pandemic period, several studies have reported cerebrovascular events including ischemic stroke, intracerebral hemorrhage (ICH), and cerebral venous sinus thrombosis in the COVID-19 patients [16,17]. A recent study finds that out of 214 patients, 6 patients in the later course of infection experienced acute cerebrovascular disease, 5 out of 6 patients developed ischemic stroke and only a single patient showed an ICH and later succumbed to respiratory failure [7]. Studies conducted across China have reported cerebrovascular events such as ischemic stroke, ICH, and cerebral venous sinus thrombosis in the infected patients from as high as 17% to as low as 2% [11]. The larger proportion of the study showed acute ischemic stroke and about 38% of total deaths in this study occurred due to cerebrovascular events [17]. Cerebrovascular events consisted of acute ischemia in 62%, intracerebral haemorrhage in 74%, and CNS vasculitis reported in 12% of patients in a study of 153 total COVID-19 patients across Britain [18]. In most of the cases, strokes were reported in elderly patients with prior history of stroke along with other comorbidities (eg, hypertension and diabetes). Some of the studies also reported stroke in patients less than 50 years and may occur earlier in the course of infection than the average duration of 10–12 days [19,20]. COVID-19 patients with stroke have a significantly higher in-hospital mortality rate [21].
2.4. Encephalopathy
Encephalopathy in COVID-19 patients shows common symptoms such as headache, fatigue, fever, and neck rigidity. More severe cases may have a seizure, stroke, agitation, altered sensorium, coma, and focal neurological defects. The first case of COVID-19 viral meningo-encephalitis was reported from Japan in a young man of 24 years [22]. The man presented with unconsciousness and generalized convulsions. Since then, many other studies have reported encephalitis as a clinical manifestation of COVID-19. The study by Helms et al. [8] reported agitation in 40 infected patients out of 58. The majority of the patients showed confusion of varying degrees and almost 67% of cases demonstrated diffuse cortico-spinal tract signs. Another study described Acute Necrotising Encephalopathy (ANE) in COVID-19 patients, most likely because of cytokine storm occurring during infection [23].
2.5. Seizures and SARS-CoV-2
It has been reported that virus particles directly invade cerebral arteries and cause vasculopathy [24,25]. COVID-19 patients may also have swollen legs and purple rashes due to blood clots. A study conducted in Europe confirms blood clotting abnormalities in 20–30% of COVID-19 patients [26]. The clots were even seen in small capillaries. It is presumed that clotting in the small arteries lessens cerebral oxygen supply and leads to ischemic stroke. An infected patient with a history of diabetes, hypertension, and on kidney dialysis without known seizures developed multiple episodes of new-onset seizures during infection and died shortly after the onset of seizure [27]. Many other cases have subsequently been reported of new-onset seizures in COVID-19 patients [28,29]. Whether such seizures are secondary to multi-organ failure or intravascular coagulopathy-related brain injury is still not clear.
2.6. Hypoxia and brain damage
COVID-19 can also lead to CNS damage and neurological symptoms without directly invading the brain itself. In the lungs, the respiratory viruses can cause inflammation which leads to alveolar and lung tissue damage. This inflammation and edema affect the exchange of oxygen at the alveolar–capillary interface and finally leads to brain hypoxia [30]. Brain metabolic activity requires high amounts of energy and oxygen for proper functioning. Hypoxia is one of the most serious health problems and can cause brain-related injury, cardiorespiratory arrest, carbon monoxide poisoning, stroke, and hypotension. Symptoms of poor judgment, incoordination, and temporary loss of memory can also arise due to brain hypoxia. Severe hypoxia of the brain can lead to coma, seizure, and even brain death [31]. SARS-CoV-2 has also been shown to cause significant hypoxemia in COVID-19 patients and could represent one of the possible pathways of brain injury [32].
3. Peripheral nervous system (PNS) manifestations
3.1. Guillain-Barre syndrome
Guillain-Barre syndrome (GBS) is a condition in which the host immune system mistakenly starts attacking the PNS. GBS is also a common reason for acute flaccid paralysis. Around two-thirds of GBS cases can be associated with viral infections such as H1N1, Zika, and influenza [33]. The first case of an association between GBS and COVID-19 was reported from China. The patient showed symmetric weakness and areflexia in both legs and feet along with impaired sensation to light touch and pinprick [34]. Since this report, about 73 more cases of GBS have been reported across the globe show, a preponderance of male patients [26]. Another case from Italy reported a man of 71 years showing GBS in association with COVID-19 infection; the patient did not have any previous neurological history [35]. Fortunately, during immunoglobulin treatment, 70% of COVID-19 patients with GBS symptoms were resolved [36]. However, whether the SARS-CoV-2 itself causes GBS, or secondary to other infections in COVID-19 patients such as dengue remains to be determined [37].
3.2. Myopathy
The skeletal muscle damage of myopathy is observed in COVID-19 patients, ranging from an asymptomatic increase in creatine kinase and lactate dehydrogenase to rhabdomyolysis [38]. About 11–70% of COVID-19 patients shared common symptoms including myalgia, weakness, and fatigue as seen with other viral illnesses [12]. Even though very few cases of rhabdomyolysis have been reported in the pandemic period, there were significant elevations of levels of serum myoglobin, lactate dehydrogenase, and creatine kinase in COVID-19 patients [38,39]. Myopathy can be caused by multiple routes which include viral invasion via ACE2 receptors, damage of muscle cell membranes by circulating viral toxins, and by a strongly generated cytokine storm. Hepatic and renal disease may also be risk factors for myopathy.
3.3. Somatic Symptom Disorder
Somatic Symptom Disorder (SSD) was initially ignored despite being commonly observed in COVID-19 patients. SSD is associated with disturbing emotion, cognition, and behavior. Nausea, headache, chest discomfort, dizziness, and palpitation are some of the symptoms of SSD. One study reported a 16-year-old child with extreme and persisting health concerns responding rapidly to low doses of antidepressant and antipsychotic drugs [40]. Intensive care unit (ICU) nurses in China were found to be vulnerable to SSD with varying and overlapping symptoms which were mainly associated with the failure of personal protective equipment (PPE) [41]. Similarly, in Italy, health care workers were showing increased irritability, a change in food habits, insomnia, and muscle tension [42]. Similar cases have also been reported in India and Brazil [43,44].
4. Similarities between neurological manifestations of SARS-CoV infections
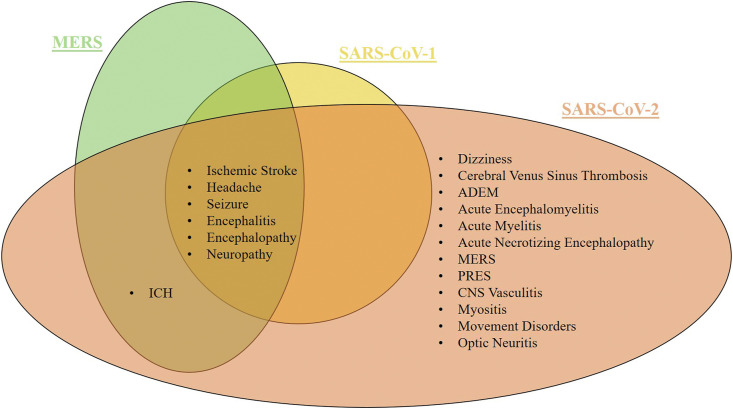
Current and prior coronavirus (CoV) infections share similarities in neurological manifestations. Encephalitis is the most common neurological manifestation in HCoV-OC43, SARS-CoV-1, MERS-CoV, and SARS-CoV-2 infections [[45], [46], [47], [48], [49]]. Whereas in post-infections of HCoV-OC43 and SARS-CoV-2, Accurate Disseminated Encephalomyelitis (ADEM) was commonly observed. The common neurological manifestations associated with SARS-CoV-1, MERS-CoV, and SARS-CoV-2 include headache, ischemic stroke, encephalitis, encephalopathy, seizure, and neuropathy. Intracerebral haemorrhage (ICH) was also observed in MERS-CoV and SARS-CoV-2, while myopathy and anosmia were observed in SARS-CoV-1 and SARS-CoV-2 [50] (Fig. 2 ).
Fig. 2.
Neurological manifestations are recurrent in different types of viral disorders Adapted from Ref. [50].
5. Neurodegenerative diseases and SARS-CoV-2
The long-term post-infectious complications of COVID-19 can be associated with neurodegenerative diseases [[51], [52], [53], [54]]. Infection from human immunodeficiency virus (HIV), West Nile virus, herpes simplex virus type 1 (HSV-1), H1N1 influenza A virus, and the respiratory syncytial virus can cause several neurological manifestations, including encephalitis, protein aggregation, neurodegeneration, and Parkinsonian- or dementia symptoms [55]. SARS-CoV-2 invasion into the CNS increases BBB permeability to both cytokines and peripheral leukocytes which in turn induces the activation of microglia and their maturation into the M1 neurotoxic phenotype, thereby contributing to the propagation of neurodegenerative processes [56,57]. Moreover, viral replication can also hijack protein synthesis machinery in neurons, promote unfolded protein response, and impair proteostasis, thus leading to the accumulation of misfolded protein and subsequent aggregation [52,54,58]. The common risk factors between COVID-19 and neurodegenerative diseases include genetic variations such as APOE e4 genotype and metabolic risk factors such as diabetes mellitus or hyperlipidaemia [54] (Table 1 ).
Table 1.
Neurological manifestations associated to COVID-19.
| Study | Clinical presentation | References |
|---|---|---|
| CNS Diseases | ||
| Moriguchi et al. | Headache, fever, fatigue, sore throat, seizures, decreased consciousness, and meningism. | [22] |
| Sohal et al. | Weakness, hypoglycaemia, difficulty in breathing, altered mental status, and seizures. | [27] |
| Wong et al. | Ataxia, ascillopsia, and bilateral facial weakness, shortness of breath on exertion, cough and diarrhoea, | [76] |
| Dugue et al. | Cough, fever, bilateral leg stiffening, and sustained upward gaze. | [77] |
| Helms st al | Agitation, confusion, corticospinal tract signs, momentary ischaemic attack, partial epilepsy, and mild cognitive impairment. | [8] |
| Mao et al. | Impaired consciousness, seizures, limb twitching, and loss of consciousness. | [7] |
| Poyiadji et al. | Cough, fever, and disturbed mental status, ANE | [23] |
| Paniz-Mondolfi et al. | History of Parkinson's disease with fever, confusion, and agitation. | [78] |
| Zhou et al. | COVID-19 pneumonia | [79] |
| PNS manifestations | ||
| Camdessanche et al. | Cough, fever, paraesthesia in hands and feet, weakness in limbs with areflexia and loss of vibration sense, dysphagia, and respiratory insufficiency. | [80] |
| Toscano et al. | Flaccid, areflexic limb weakness, facial weakness, dysphagia, respiratory failure, facial diplegia, arelexia, limb paraesthesia, and ataxia | [81] |
| Zhao et al. | Cough, fever, weakness of limbs, fatigue, areflexia, neurological symptoms. | [34] |
| Skeletal muscle disease | ||
| Jin et al. | Cough, fever, weakness, and tenderness in lower limbs. | [17] |
| Taste and smell dysfunction | ||
| Lechien et al. | Taste and Smell dysfunction | [82] |
| Ischemic stroke | ||
| Avula et al. | Hypertension, dyslipidaemia, diabetes and neuropathy, carotid stenosis, chronic kidney disease, focal neurological deficit, altered mental status, fever, respiratory distress, nausea, and vomiting | [83] |
| Beyrouti et al. | Hypertension, ischemia heart disease, atrial fibrillation, stroke, hemiparesis, decreased consciousness, and respiratory symptoms. | [84] |
| Li et al. | Hypertension, diabetes, cardiovascular disease, malignancy, cardioembolic, respiratory symptoms. | [17] |
| Morassi et al. | Hypertension, stroke, ischaemia attack, aortic valve disease, myocardial infarction, respiratory symptoms. | [16] |
| Oxly et al. | Hemiplegia, reduced consciousness, dysarthria, dysphasia, sensory deficit, respiratory symptoms. | [19] |
6. COVID-19 and sleep
Sleep dysfunction can be seen commonly in COVID-19 infected patients but also those staying home because of the lockdown (Bhat and Chokroverty in this Issue). People in quarantine reported acute stress disorder, anxiety, poor concentration, failing work performance, depressive symptoms, and insomnia along with other factors [59]. There is a two-way link between sleep and immune function. Activation of the immune system alters sleep and innate and adaptive immune system are affected by sleep as well. Adequate sleep strengthens the immune system whereas a lack of sleep weakens it. Depending upon the time and magnitude of the inflammatory responses generated by the immune system, sleep can be increased in duration and in intensity but can also be disrupted [60]. Recently, a study conducted in June 2020 on the effect of lockdown on mental health and sleep disturbance on 1515 Italian people reported that 42.2% of the patients reported sleep disturbances with 17% of them meeting the criteria for insomnia disorder. The study also reported that females and people already having any chronic condition were associated with a higher risk of sleep disturbances [61]. A study conducted on 7236 Chinese people showed disturbed sleep quality in 18.2% of patients [62]. An international COVID-19 sleep study has been initiated to assess disorders of insomnia, nightmares, sleep apnea, fatigue, exhaustion, and REM sleep through translated questionaries and has the relationship to COVID-19 disease and psychiatric or other physiological disorders [63].
The anxiety of getting infected by COVID-19 and not being able to go to work was also a contributor to shifting of sleep patterns If people are confined to home all day, circadian time cue of daily morning sunlight is lost, disrupting their internal clock and sleep pattern [64]. A study by Dempsey et al. [65] reported a link between obstructive sleep apnea (OSA) and cardiovascular disease such as hypertension, left ventricular dysfunction, stroke, coronary artery disease, cardiac arrhythmias, and pulmonary hypertension. Several studies have studied an association between OSA, obesity, and insulin resistance [66,67]. OSA is a central part of metabolic syndrome combining obesity, hypertension, hyperlipidaemia, and insulin resistance. People with diabetes will be more likely to have COVID-19 infection along with increased inflammation and increased insulin requirement to control diabetes [68,69].
A study by Philip et al. [70] was conducted on 2069 people to identify good versus poor sleepers during the pandemic period. The AI and smartphone technology were used to conduct the study. Out of the total test subjects, ∼37% of people had moderate to severe insomnia. Similarly, Schlarb et al. [71] conducted a study on children of age 5–10 years old along with their parents. The study found that 67% of children showed reduced sleep patterns during COVID-19. A study by Kim et al. [72] includes 2884 high-risk health workers from six countries. The study reported that an increase in 1 h in nocturnal sleep duration was associated with 12% lower odds of COVID-19, whereas having severe sleep disorder was associated with 88% greater odd of covid19. Also, work burnouts was associated with a 2.6-fold greater odds of COVID-19 including greater disease duration and severity.
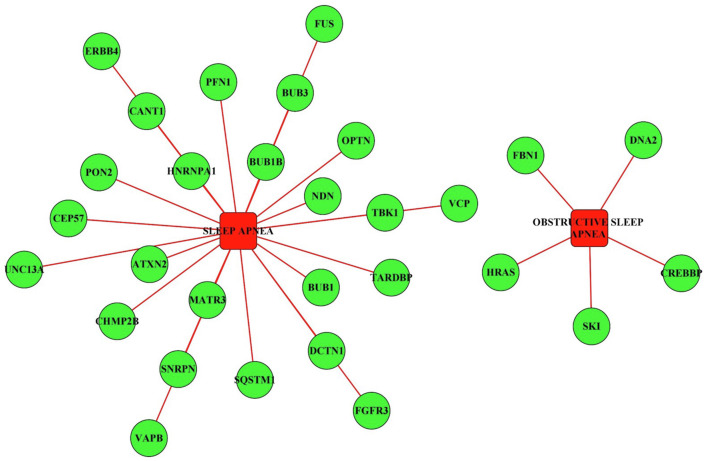
Recently, our group reported brain disease network analysis to elucidate neurological manifestations of COVID-19, identifying both direct and indirect links with COVID-19 infection [73]. This study also reported the genes which were influenced by the SARS-CoV-2 proteins directly or indirectly and were also known to be associated with OSA in COVID-19 patients (Fig. 3 ). This study reported a total of 29 genes of which 24 were known to be associated with sleep apnea and 5 being more specific for obstructive sleep apnea. The genes involved in sleep apnea were significantly enriched in pathways such as mitophagy, cell cycle, necroptosis, Rap1 signaling pathways, regulation of actin cytoskeleton, Human T-cell leukemia virus 1 infection, Ras signaling pathway, endocytosis, bladder cancer, and MAPK signaling pathways [74,75]. Genes involved in sleep apnea and obstructive sleep apnea have also been reported to be associated with other neurological diseases, suggesting the association of obstruction in sleep with neurologically related disorders and vice versa.
Fig. 3.
Network of SARS-CoV-2 virus targeted host genes involved in sleep apnea and chronic sleep apnea.
7. Conclusion
The global problem of COVID-19 affected millions of human lives with a high infectivity rate. The major proportion of SARS-CoV-2 infected patients showed more frequent respiratory disorders compared to neurological manifestations. Apart from neurological manifestations, sleep-related disorders are also prominent in infected patients. People having a history of neurological or sleep-related disorders are more prone to covid19 infection and vice-versa. The nervous system and the sleep cycle are connected in a two-way manner, any disturbance in one can cause a disturbance in the function of the other as well. As the number of cases increase globally, it is essential to focus on sleep and neurological complications of COVID-19. Clinical, diagnostic, and epidemiological studies during acute and recovery phases are required for better diagnosis and management of the patients with COVID-19.
CRediT author statement
Fatima Khatoon: writing-original draft preparation; Kartikay Prasad: Data curation and analysis, writing- original draft; Vijay Kumar: Conceptualization, Supervision, Review and Editing. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors sincerely thank the Amity University, Noida for providing facilities.
Footnotes
No potential conflict of interest was reported by the authors.
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: https://doi.org/10.1016/j.sleep.2021.07.005.
Conflict of interest
The following is the supplementary data related to this article:
Multimedia component 1
References
- 1.Wrapp D., Wang N., Corbett K.S., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Netland J., Meyerholz D.K., Moore S., et al. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82:7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li K., Wohlford-Lenane C., Perlman S., et al. Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis. 2016;213:712–722. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohmwald K., Galvez N., Ríos M., et al. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386. doi: 10.3389/fncel.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baig A.M., Khaleeq A., Ali U., et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 6.Khatoon F., Prasad K., Kumar V. Neurological manifestations of COVID-19: available evidences and a new paradigm. J Neurovirol. 2020;26:619–630. doi: 10.1007/s13365-020-00895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helms J., Kremer S., Merdji H., et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsivgoulis G., Palaiodimou L., Katsanos A.H., et al. Neurological manifestations and implications of COVID-19 pandemic. Ther Adv Neurol Dis. 2020;13 doi: 10.1177/1756286420932036. 1756286420932036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan W-j, Ni Z-y, Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy D., Ghosh R., Dubey S., et al. Neurological and neuropsychiatric impacts of COVID-19 pandemic. Can J Neurol Sci. 2020:1–16. doi: 10.1017/cjn.2020.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giacomelli A., Pezzati L., Conti F., et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020;71:889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heidari F., Karimi E., Firouzifar M., et al. Anosmia as a prominent symptom of COVID-19 infection. Rhinology. 2020;58:302–303. doi: 10.4193/Rhin20.140. [DOI] [PubMed] [Google Scholar]
- 15.Einstein A., Podolsky B., Rosen N., et al. Can quantum-mechanical description of physical reality be considered complete? Phys Rev. 1935;47:777–780. [Google Scholar]
- 16.Morassi M., Bagatto D., Cobelli M., et al. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. 2020;267:2185–2192. doi: 10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y., Li M., Wang M., et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vas Neurol. 2020;5 doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varatharaj A., Thomas N., Ellul M., et al. 2020. UK-wide surveillance of neurological and neuropsychiatric complications of COVID-19: the first 153 patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oxley T.J., Mocco J., Majidi S., et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grau A.J., Buggle F., Heindl S., et al. Recent infection as a risk factor for cerebrovascular ischemia. Stroke. 1995;26:373–379. doi: 10.1161/01.str.26.3.373. [DOI] [PubMed] [Google Scholar]
- 21.Benussi A., Pilotto A., Premi E., et al. Clinical characteristics and outcomes of inpatients with neurologic disease and COVID-19 in Brescia, Lombardy, Italy. Neurology. 2020;95:e910–e920. doi: 10.1212/WNL.0000000000009848. [DOI] [PubMed] [Google Scholar]
- 22.Moriguchi T., Harii N., Goto J., et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poyiadji N., Shahin G., Noujaim D., et al. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296:E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagel M., Mahalingam R., Cohrs R., et al. Virus vasculopathy and stroke: an under-recognized cause and treatment target. Infect Disord - Drug Targets. 2010;10:105–111. doi: 10.2174/187152610790963537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilden D., Kleinschmidt-DeMasters B., Wellish M., et al. Varicella zoster virus, a cause of waxing and waning vasculitis: the New England Journal of Medicine case 5-1995 revisited. Neurology. 1996;47:1441–1446. doi: 10.1212/wnl.47.6.1441. [DOI] [PubMed] [Google Scholar]
- 26.Karuppan M.K.M., Devadoss D., Nair M., et al. SARS-CoV-2 infection in the central and peripheral nervous system-associated morbidities and their potential mechanism. Mol Neurobiol. 2021:1–16. doi: 10.1007/s12035-020-02245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sohal S., Mansur M. COVID-19 presenting with seizures. IDCases. 2020;20 doi: 10.1016/j.idcr.2020.e00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asadi-Pooya A.A. Seizures associated with coronavirus infections. Seizure. 2020;79:49–52. doi: 10.1016/j.seizure.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang S.T., Ballout A.A., Mirza U., et al. Acute seizures occurring in association with SARS-CoV-2. Front Neurol. 2020;11:576329. doi: 10.3389/fneur.2020.576329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdennour L., Zeghal C., Deme M., et al. Annales francaises d'anesthesie et de reanimation; 2012. Interaction brain-lungs; pp. e101–e107. [DOI] [PubMed] [Google Scholar]
- 31.Fugate J.E. Anoxic-ischemic brain injury. Neurol Clin. 2017;35:601–611. doi: 10.1016/j.ncl.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Xu K., Cai H., Shen Y., et al. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. J Zhejiang Univ. 2020;49 [Google Scholar]
- 33.Vellozzi C., Iqbal S., Broder K. Guillain-Barre syndrome, influenza, and influenza vaccination: the epidemiologic evidence. Clin Infect Dis. 2014;58:1149–1155. doi: 10.1093/cid/ciu005. [DOI] [PubMed] [Google Scholar]
- 34.Zhao H., Shen D., Zhou H., et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alberti P., Beretta S., Piatti M., et al. Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflammation. 2020;7 doi: 10.1212/NXI.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abu-Rumeileh S., Abdelhak A., Foschi M., et al. Guillain–Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol. 2020:1–38. doi: 10.1007/s00415-020-10124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joob B., Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82:e177. doi: 10.1016/j.jaad.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin M., Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis. 2020;26:1618. doi: 10.3201/eid2607.200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suwanwongse K., Shabarek N. Rhabdomyolysis as a presentation of 2019 novel coronavirus disease. Cureus. 2020:12. doi: 10.7759/cureus.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colizzi M., Bortoletto R., Silvestri M., et al. Medically unexplained symptoms in the times of Covid-19 pandemic: a case-report. Brain, Behav Immun Health. 2020;5:100073. doi: 10.1016/j.bbih.2020.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yifan T., Ying L., Chunhong G., et al. Symptom Cluster of ICU nurses treating COVID-19 pneumonia patients in Wuhan, China. J Pain Symptom Manag. 2020;60:e48–e53. doi: 10.1016/j.jpainsymman.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barello S., Palamenghi L., Graffigna G. Burnout and somatic symptoms among frontline healthcare professionals at the peak of the Italian COVID-19 pandemic. Psychiatr Res. 2020;290:113129. doi: 10.1016/j.psychres.2020.113129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goularte J.F., Serafim S.D., Colombo R., et al. COVID-19 and mental health in Brazil: psychiatric symptoms in the general population. J Psychiatr Res. 2021;132:32–37. doi: 10.1016/j.jpsychires.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naskar C., Grover S., Sharma A. Telephonic survey and psychological aid for patients with somatic symptom disorders for the impact of lockdown and COVID-19 pandemic. Int J Soc Psychiatr. 2021 Mar;67(2):203–204. doi: 10.1177/0020764020954247. Epub 2020 Aug 29. PMID: 32865082. [DOI] [PubMed] [Google Scholar]
- 45.Nilsson A., Edner N., Albert J., et al. Fatal encephalitis associated with coronavirus OC43 in an immunocompromised child. Infect Dis. 2020;52:419–422. doi: 10.1080/23744235.2020.1729403. [DOI] [PubMed] [Google Scholar]
- 46.Morfopoulou S., Brown J.R., Davies E.G., et al. Human coronavirus OC43 associated with fatal encephalitis. N Engl J Med. 2016;375:497–498. doi: 10.1056/NEJMc1509458. [DOI] [PubMed] [Google Scholar]
- 47.Xu J., Zhong S., Liu J., et al. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis. 2005;41:1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arabi Y., Harthi A., Hussein J., et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43:495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95:e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dorche M.S., Huot P., Osherov M., et al. Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVID-19 pandemic. J Neurol Sci. 2020:117085. doi: 10.1016/j.jns.2020.117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solomon I.H., Normandin E., Bhattacharyya S., et al. Neuropathological features of covid-19. N Engl J Med. 2020;383:989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lippi A., Domingues R., Setz C., et al. SARS-CoV-2: at the crossroad between aging and neurodegeneration. Mov Disord. 2020;35:716–720. doi: 10.1002/mds.28084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tavassoly O., Safavi F., Tavassoly I. Seeding brain protein aggregation by SARS-CoV-2 as a possible long-term complication of COVID-19 infection. ACS Chem Neurosci. 2020;11:3704–3706. doi: 10.1021/acschemneuro.0c00676. [DOI] [PubMed] [Google Scholar]
- 54.Dolatshahi M., Sabahi M., Aarabi M.H. Pathophysiological clues to how the emergent SARS-CoV-2 can potentially increase the susceptibility to neurodegeneration. Mol Neurobiol. 2021;58:2379–2394. doi: 10.1007/s12035-020-02236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou L., Miranda-Saksena M., Saksena N.K. Viruses and neurodegeneration. Virol J. 2013;10:172. doi: 10.1186/1743-422X-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grozdanov V., Bliederhaeuser C., Ruf W.P., et al. Inflammatory dysregulation of blood monocytes in Parkinson's disease patients. Acta Neuropathol. 2014;128:651–663. doi: 10.1007/s00401-014-1345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Domingues C., da Cruz E.S.O.A.B., Henriques A.G. Impact of cytokines and chemokines on Alzheimer's disease neuropathological hallmarks. Curr Alzheimer Res. 2017;14:870–882. doi: 10.2174/1567205014666170317113606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steenblock C., Todorov V., Kanczkowski W., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the neuroendocrine stress axis. Mol Psychiatr. 2020;25:1611–1617. doi: 10.1038/s41380-020-0758-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brooks S.K., Webster R.K., Smith L.E., et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912–920. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Physiopedia. Covid-19 and sleep. 2020. [Google Scholar]
- 61.Gualano M.R., Lo Moro G., Voglino G., et al. Effects of Covid-19 lockdown on mental health and sleep disturbances in Italy. Int J Environ Res Publ Health. 2020;17:4779. doi: 10.3390/ijerph17134779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang Y., Zhao N. Generalized anxiety disorder, depressive symptoms and sleep quality during COVID-19 outbreak in China: a web-based cross-sectional survey. Psychiatr Res. 2020;288:112954. doi: 10.1016/j.psychres.2020.112954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Partinen M., Bjorvatn B., Holzinger B., et al. Sleep and circadian problems during the coronavirus disease 2019 (COVID-19) pandemic: the International COVID-19 Sleep Study (ICOSS) J Sleep Res. 2021;30 doi: 10.1111/jsr.13206. [DOI] [PubMed] [Google Scholar]
- 64.NeurologyToday . 2020, July 9. Sleep neurologists call it ‘COVID-somnia’—increased sleep disturbances linked to the pandemic. [Google Scholar]
- 65.Dempsey J.A., Veasey S.C., Morgan B.J., et al. Pathophysiology of sleep apnea. Physiol Rev. 2010 doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gharib S.A., Hayes A.L., Rosen M.J., et al. A pathway-based analysis on the effects of obstructive sleep apnea in modulating visceral fat transcriptome. Sleep. 2013;36:23–30. doi: 10.5665/sleep.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prasad K., AlOmar S.Y., Alqahtani S.A.M., et al. Brain disease network analysis to elucidate the neurological manifestations of COVID-19. Mol Neurobiol. 2021;58:1875–1893. doi: 10.1007/s12035-020-02266-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jornayvaz F.R., Assouline B., Pugin J., et al. Extremely high-dose insulin requirement in a diabetic patient with COVID-19: a case report. BMC Endocr Disord. 2020;20:1–4. doi: 10.1186/s12902-020-00632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seggelke S.A., Ingram C.C., Crawley S., et al. Insulin resistance in a hospitalized COVID-19 patient: a case review. Clin Diabetes. 2021;39:228–232. doi: 10.2337/cd20-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Philip P., Dupuy L., Morin C.M., et al. Smartphone-based virtual agents to help individuals with sleep concerns during COVID-19 confinement: feasibility study. J Med Internet Res. 2020;22 doi: 10.2196/24268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schlarb A.A., Schulte H., Selbmann A., et al. Online cognitive behavioral group therapy (iCBT-I) for insomnia for school children and their parents. Somnologie. 2020;24:259–266. doi: 10.1007/s11818-020-00280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim H., Hegde S., LaFiura C., et al. COVID-19 illness in relation to sleep and burnout. BMJ Nutr Prev Health. 2021 doi: 10.1136/bmjnph-2021-000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prasad K., AlOmar S.Y., Alqahtani S.A.M., et al. Brain disease network analysis to elucidate the neurological manifestations of COVID-19. Mol Neurobiol. 2021:1–19. doi: 10.1007/s12035-020-02266-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen E.Y., Tan C.M., Kou Y., et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinf. 2013;14:1–14. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kanehisa M., Furumichi M., Sato Y., et al. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 2021;49:D545–D551. doi: 10.1093/nar/gkaa970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wong P.F., Craik S., Newman P., et al. Lessons of the month 1: a case of rhombencephalitis as a rare complication of acute COVID-19 infection. Clin Med. 2020;20:293. doi: 10.7861/clinmed.2020-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dugue R., Cay-Martínez K.C., Thakur K.T., et al. Neurologic manifestations in an infant with COVID-19. Neurology. 2020;94:1100–1102. doi: 10.1212/WNL.0000000000009653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paniz-Mondolfi A., Bryce C., Grimes Z., et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J Med Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou L., Zhang M., Wang J., et al. Sars-Cov-2: underestimated damage to nervous system. Trav Med Infect Dis. 2020:101642. doi: 10.1016/j.tmaid.2020.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Camdessanche J.-P., Morel J., Pozzetto B., et al. COVID-19 may induce Guillain–Barré syndrome. Rev Neurol. 2020;176:516. doi: 10.1016/j.neurol.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Toscano G., Palmerini F., Ravaglia S., et al. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino-Laryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elkhider H., Ibrahim F., Sharma R., et al. COVID-19 and stroke, a case series and review of literature. Brain, Behav Immun Health. 2020;9:100172. doi: 10.1016/j.bbih.2020.100172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beyrouti R., Adams M.E., Benjamin L., et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatr. 2020;91:889–891. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1