Abstract
Introduction
IDH1/2 mutations are prevalent in cartilaginous tumors including chondrosarcoma. This meta‐analysis using individual patient data (IPD) aimed to investigate the clinical and prognostic association of these mutations in chondrosarcoma patients.
Methods
Two electronic databases including PubMed and Web of Science were searched for relevant data. We included studies providing IPD of chondrosarcoma with available IDH1/2 mutational status for meta‐analysis. Chi‐square and t‐test were performed to compare the groups with and without IDH1/2 mutations. For survival analysis, log‐rank test, and Cox proportional hazards model were used to investigate the association of IDH mutations with patient outcomes.
Results
Fourteen studies with 488 patients were analyzed. IDH1 and IDH2 mutations were detected in 38.7% and 12.1% of cases, respectively. IDH1/2 mutations were significantly associated with an older age (p = 0.003), tumor origins (p < 0.001), tumor grades (p < 0.001), larger diameter (p = 0.003), relapse (p = 0.014), and patient mortality (p = 0.04). Multivariate Cox regression analysis adjusted for age, gender, tumor grade, and tumor sites confirmed the negative impact of IDH1/2 mutations on patient overall survival (HR = 1.90; 95% CI = 1.06–3.42; p = 0.03).
Conclusion
Our meta‐analysis demonstrated the distinct characteristics of IDH1/2‐mutated chondrosarcomas in comparison to those without mutations. These mutations could serve as an independent prognostic biomarker to better prognosticate patient outcomes and design appropriate treatment plans.
Keywords: chondrosarcoma, IDH, isocitrate dehydrogenase, meta‐analysis, overall survival, recurrence‐free survival
1. INTRODUCTION
Chondrosarcoma is a common primary malignant bone tumor, and is the second most common malignancy following osteosarcoma. 1 They are classified into different grades based on tumor cellularity and nuclear changes in chondrocytes. Low‐grade chondrosarcomas correspond to grade I tumors. These tumors are usually treated with curettage and wide excision and likely have a good prognosis 2 , 3 whereas, high‐grade (grade II–III) chondrosarcomas have a high risk of relapse and even metastasis which requires more aggressive treatment. 3 , 4 The most aggressive form of chondrosarcoma is dedifferentiated chondrosarcoma, which is associated with a dismal prognosis and rapid development of widespread metastases. 5
Besides tumor grades, several clinicopathological features have been shown to be of prognostic importance in chondrosarcoma patients including gender, tumor location, diameter, and extent of resection. 6 , 7 , 8 Recently, emerging data have clarified the genomic landscape of chondrosarcomas. 9 , 10 , 11 , 12 Isocitrate dehydrogenase (IDH) mutation is a common genetic alteration in gliomas 13 and is also found in about 50% of central chondrosarcomas. 10 In gliomas, this mutation is associated with a favorable prognosis. 14 , 15 However, published data are equivocal regarding the association of IDH1/2 mutations and outcomes of chondrosarcoma patients. 12 , 16 , 17 This meta‐analysis aims to explore the clinicopathological and prognostic characteristics of IDH1/2 mutations in chondrosarcoma.
2. METHODS
2.1. Ethical approval
An ethical approval is not needed for this study because this is a meta‐analysis and systematic review based on published studies.
2.2. Literature search
PubMed and Web of Science databases were searched for relevant articles from inception to November 2020. We used the following term: chondrosarcoma AND (IDH1 OR IDH2 OR IDH1/2 OR IDH OR isocitrate dehydrogenase). This study generally followed the recommendations of the Preferred Reporting Items for Systematic Review and Meta‐Analysis (PRISMA) statement. 18
2.3. Selection criteria and abstract screening
All search results from two electronic databases were imported into EndNote (Clarivate, PA, US) and duplicates were removed. Two reviewers independently screened the title and abstract of these search results. Studies were included if they provided individual patient data (IPD) of chondrosarcoma patients and IDH1/2 mutation data. We excluded studies without IPD; reviews; case reports, proceeding papers, conference abstracts, or books. If there are any discrepancies among the two reviewers, discussion and consensus were reached.
2.4. Full‐text screening and data extraction
The full‐text of suspected studies were independently read by two reviewers and IPD were extracted into a standardized worksheet. The following IPD were extracted: authors, institution, country, year of publication, demographic information, tumor location, tumor diameter, histopathological subtypes, tumor grades, patient outcomes (recurrence, recurrence‐free survival [RFS] time, metastasis, metastasis‐free survival [MFS] time, overall survival [OS] status, OS time), and status of IDH1/2 mutations.
2.5. Statistical analyses
Categorical data were presented as frequency (percentage), and comparisons between groups were performed using the chi‐square test. Continuous variables are expressed as mean ± standard deviation (SD) for normal distributions and median + interquartile range (IQR) for non‐normal distributions. The distribution of continuous variables was assessed using skewness, kurtosis, and visual inspection of the histogram. Continuous variables were compared between two groups by t‐test or Mann–Whitney U‐test, as appropriate. Univariate and multivariable Cox proportional hazards models were conducted to determine the association of IDH1/2 mutations with clinical outcomes (recurrence, metastasis, and overall survival). Proportionality assumptions of the Cox regression models were assessed by log‐log survival curves and with the use of Schoenfeld residuals. The deviance residuals and the dfbeta values were used to examine influential observations. Hazard ratios (HR) are presented as mean and 95% confidence interval (CI). A two‐sided p‐value of <0.05 was considered statistically significant. The statistical analyses were performed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, N.Y., USA) and R software, version 3.6.1 (The R Foundation, Vienna, Austria).
2.6. Quality assessment and risk of bias analysis
We evaluated the quality of included studies in our meta‐analysis using the Newcastle–Ottawa Scale (NOS). 19 Two reviewers independently awarded the number of stars using a standardized checklist. We considered studies moderate to high quality if they have six stars or more.
3. RESULTS
We identified 136 articles for the title and abstract screening; 34 of these were selected for full‐text reading. Following this step, we included 14 studies comprising 488 chondrosarcoma patients with available IDH1/2 mutational status for data analyses (Figure 1). 10 , 11 , 12 , 17 , 29 All studies but three had moderate to good quality using the NOS tool assessment. Table 1 and Table S1 present the characteristics of all included studies.
FIGURE 1.
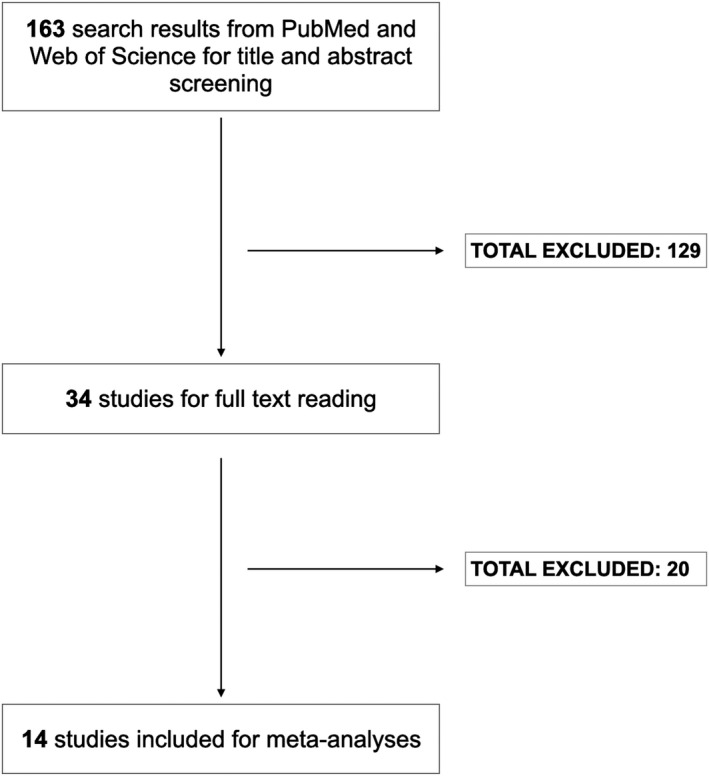
Study flowchart
TABLE 1.
Characteristics of included studies
| Study | Institution | Country | Study period | Detection methods | No. of cases | NOS stars | ||
|---|---|---|---|---|---|---|---|---|
| S | C | O | ||||||
| Amary (2011) 10 | Multicenter | UK | NA | Sequenom, Sanger, IHC | 69 | 4 | 0 | 3 |
| Arai (2012) 20 | Gunma University School of Medicine | Japan | NA | Sanger | 13 | 4 | 0 | 1 |
| Asioli (2020) 17 | Multicenter | Italy | 1998–2019 | Sanger, IHC | 46 | 4 | 0 | 3 |
| Chen (2017) 21 | Multicenter | USA | NA | IDH1/2 Rotor‐Gene Q PCR, IHC | 43 | 4 | 0 | 1 |
| Gambarotti (2020) 22 | IRCCS Instituto Ortopedico Rizzoli | Italy | 1990–2020 | Sanger | 6 | 4 | 0 | 3 |
| Kanamori (2015) 23 | Keio University Hospital | Japan | 1997–2010 | Sanger | 7 | 4 | 0 | 3 |
| Kerr (2013) 11 | Massachusetts General Hospital | USA | 1990–2012 | SNaPshot, Sanger | 23 | 4 | 0 | 3 |
| Lam (2019) 24 | Leiden University Medical Center | Netherlands | NA | NGS, Sanger | 5 | 4 | 0 | 3 |
| Lucas (2020) 25 | University of California at San Francisco | USA | NA | NGS, IHC | 11 | 4 | 0 | 2 |
| Mohammad (2020) 26 | University of British Columbia | Canada | 1996–2016 | Quantitative PCR, Sanger | 21 | 4 | 0 | 3 |
| Nicolle (2019) 27 | Multicenter | France | 1997–2013 | NGS | 76 | 4 | 0 | 3 |
| Tallegas (2019) 28 | French Bone Pathology Group Network RESOS | France | 2000–2018 | Pyrosequencing, Sanger | 71 | 4 | 0 | 1 |
| Yang (2020) 29 | Shanghai Jiaotong University Affiliated Sixth People's Hospital | China | 2011–2017 | Sanger | 18 | 4 | 0 | 3 |
| Zhu (2020) 12 | Memorial Sloan Kettering Cancer Center | USA | 1984–2017 | Sequenom, NGS, Sanger | 79 | 4 | 0 | 3 |
Abbreviations: C, comparability; IHC, immunohistochemistry; NA, not available; NGS, next‐generation sequencing; NOS, Newcastle‐Ottawa Scale; O, outcome; PCR, polymerase chain reaction; S, selection.
3.1. Prevalence of IDH mutations in chondrosarcomas
IDH1/2 mutations were detected in 250 patients (51.2%). The prevalence of IDH1 and IDH2 were 38.7% and 12.1%, respectively. IDH1 and IDH2 mutations were mutually exclusive except for one case. Among 488 analyzed cases, IDH1 and IDH2 mutation genotypes were reported in 237 cases. The detailed genotypes of IDH1 and IDH2 mutations are shown in Table 2. The prevalence of IDH mutations was statistically different between tumor locations with the highest prevalence seen in phalanges (100%) and femur (83.0%) and the lowest prevalence found in vertebrae and sternum (0%) (Table 3).
TABLE 2.
Distribution of IDH genotypes in chondrosarcomas
| IDH gene | IDH genotypes | No. of positive cases (%) |
|---|---|---|
| IDH1 | R132, unspecified | 13 (5.5) |
| R132C | 81(34.2) | |
| R132F | 1(0.4) | |
| R132G | 30 (12.7) | |
| R132H | 17 (7.2) | |
| R132I | 1 (0.4) | |
| R132L | 24 (10.1) | |
| R132S | 13 (5.5) | |
| IDH2 | R172, unspecified | 11 (4.6) |
| R172G | 4 (1.7) | |
| R172 M | 6 (2.5) | |
| R172S | 31 (13.1) | |
| R172T | 2 (0.8) | |
| T172W | 1 (0.4) | |
| Others | 2 (0.8) | |
| Total | 237 |
TABLE 3.
The associations of IDH mutations with clinicopathological factors and prognostic outcome
| Variables | Groups | IDH1/2‐mut | IDH1/2‐wt | p‐value |
|---|---|---|---|---|
| Age (year) | Mean ±SD | 57.2 ± 15.3 | 52.0 ± 16.2 | 0.003 |
| Gender | Female | 66 (37.3) | 72 (43.9) | 0.21 |
| Male | 111 (62.7) | 92 (56.1) | ||
| Tumor origins | Extraosseous | 5 (2.4) | 53 (25.6) | <0.001 |
| Flat bone | 95 (46.3) | 95 (43.3) | ||
| Irregular bone | 0 (0) | 14 (9.0) | ||
| Long bone | 104 (50.7) | 39 (18.8) | ||
| Multiple sites | 1 (0.5) | 6 (2.9) | ||
| Tumor sites | Cranium | 44 (21.5) | 52 (25.1) | <0.001 |
| Tracheolarynx | 4 (2.0) | 41 (19.8) | ||
| Scapula | 8 (3.9) | 5 (2.4) | ||
| Humerus | 20 (9.8) | 18 (8.7) | ||
| Chest wall (rib & sternum) | 4 (2.0) | 19 (9.2) | ||
| Vertebra | 0 (0.0) | 14 (6.8) | ||
| Pelvis | 40 (19.5) | 29 (14.0) | ||
| Femur | 73 (35.6) | 15 (7.2) | ||
| Fibula/Tibia | 7 (3.4) | 6 (2.9) | ||
| Phalanges | 4 (2.0) | 0 (0.0) | ||
| Others | 1 (0.5) | 8 (3.8) | ||
| Tumor grades | I | 24 (10.0) | 54 (23.8) | <0.001 |
| II | 103 (43.1) | 124 (54.6) | ||
| III | 24 (10.0) | 13 (5.7) | ||
| Dedifferentiated | 88 (36.8) | 36 (15.9) | ||
| Largest diameter (cm) | Mean ±SD | 12.1 ± 7.2 | 9.5 ± 6.7 | 0.003 |
| Recurrence | Yes | 12 (31.6) | 19 (61.3) | 0.014 |
| No | 26 (68.4) | 12 (38.7) | ||
| Metastasis | Yes | 52 (54.7) | 42 (55.3) | 0.95 |
| No | 43 (45.3) | 34 (44.7) | ||
| Death | Yes | 64 (47.8) | 26 (33.3) | 0.04 |
| No | 70 (52.2) | 52 (66.7) |
Abbreviations: HG‐CCS, high‐grade chondrosarcoma; IQR, interquartile range; LG‐CCS, low‐grade chondrosarcoma; SD, standard deviation.
Bold values indicate a statistically significant result.
3.2. Associations of IDH mutations with clinicopathological factors and patient outcomes
Table 3 shows the correlations of IDH mutations with clinicopathological features. Compared to IDH‐wild type (IDH‐wt) tumors, IDH‐mut chondrosarcomas were associated with older age and larger tumor diameter (p = 0.003). In addition, IDH mutations were more likely to occur in long bones chondrosarcomas (e.g., femur, humerus, tibia) and flat bones (e.g., pelvis, cranium) whereas, these mutations were completely absent in irregular bones (e.g., vertebrae, sternum). We also found an association of IDH mutations with chondrosarcoma grades; the prevalence of IDH mutations was significantly increased with grades with the highest prevalence being identified in dedifferentiated tumors.
The median follow‐up duration of chondrosarcomas was 20.5 months. The presence of IDH1/2 mutations were associated with a decreased recurrence rate but a significantly higher risk of patient death (Table 3). For time‐to‐event analysis, IDH1/2 mutations were not associated with tumor RFS (HR = 0.63; 95% CI = 0.30–1.31; p = 0.21) and MFS (HR = 1.75; 95% CI = 0.91–3.36; p = 0.09), but they were significantly correlated with patient OS (HR = 1.81; 95% CI = 1.15–2.87; p = 0.01) (Figure 2). The significant result was retained in the multivariate model adjusted for age, gender, tumor grades, and tumor locations (HR = 1.90; 95% CI = 1.06–3.42; p = 0.03).
FIGURE 2.
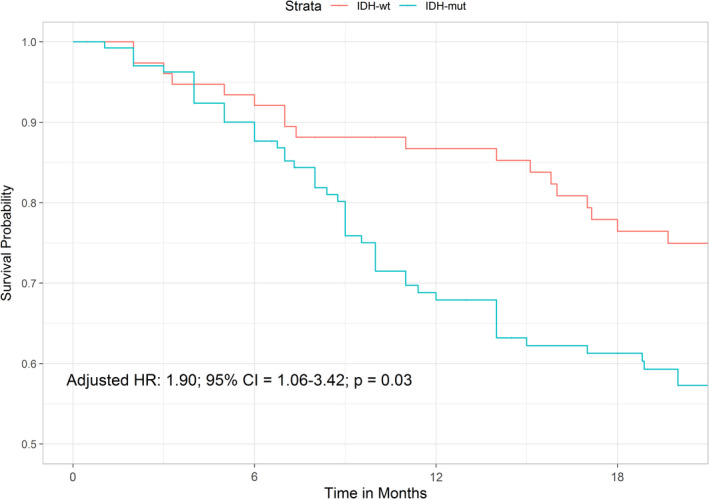
Kaplan–Meier curve illustrating the impact of IDH mutations on OS of chondrosarcoma
Stratified by tumor grades, we found an association of IDH mutation with longer RFS in the high‐grade group (Figure 3) using the log‐rank test (p = 0.049). Because of the small sample size with available survival data when dividing into different subgroups, we did not found any associations of IDH1/2 mutations with patient RFS, MFS, and OS using the Cox proportional hazards model in other chondrosarcoma grades (Table 4).
FIGURE 3.
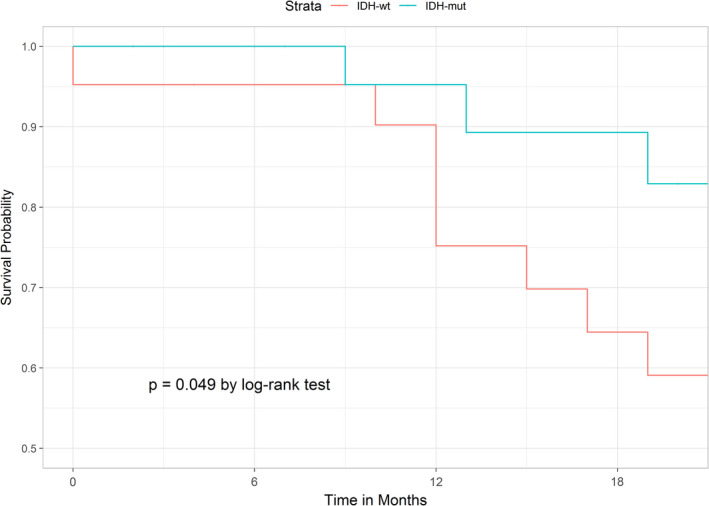
Kaplan–Meier curve illustrating the impact of IDH mutations on RFS of high‐grade chondrosarcoma
TABLE 4.
Subgroup analyses of impacts of IDH mutations on patient outcomes in different chondrosarcoma grades
| Tumor grades | Hazard Ratio (95% CI) | p‐value |
|---|---|---|
| Low‐grade | ||
| RFS | NA | NA |
| MFS | NA | NA |
| OS | NA | NA |
| High‐grade | ||
| RFS | 0.41 (95% CI = 0.17–1.03) | 0.06 |
| MFS | 1.55 (95% CI = 0.57–4.24) | 0.39 |
| OS | 1.21 (95% CI = 0.53–2.79) | 0.65 |
| Dedifferentiated | ||
| RFS | 1.54 (95% CI = 0.25–9.27) | 0.64 |
| MFS | 1.35 (95% CI = 0.54–3.34) | 0.52 |
| OS | 1.63 (95% CI = 0.89–3.01) | 0.12 |
Abbreviations: CI, confidence interval; MFS, metastasis‐free survival; NA, not available; OS, overall survival; RFS, recurrence‐free survival.
3.3. The characteristics of cranial chondrosarcomas
There were 96 cases of cranial chondrosarcoma included in our meta‐analysis. The characteristics of these neoplasms are summarized in Table 5. IDH mutations were only detected in skull base chondrosarcoma and were not present in craniofacial tumors (p < 0.001). Patient outcomes were missing in the majority of cranial chondrosarcomas so we excluded them from the analyses.
TABLE 5.
Characteristics of cranial chondrosarcomas
| Variables | Groups |
Craniofacial (%) (n = 23) |
Skull base (%) (n = 73) |
p‐value |
|---|---|---|---|---|
| Gender | Male | 6 (42.9) | 17 (37.8) | 0.734 |
| Female | 8 (57.1) | 28 (62.2) | ||
| Age | Mean ±SD | 57.5 ± 19.7 | 49.9 ± 15.4 | 0.135 |
| Grade | I | 8 (36.4) | 20 (30.8) | 0.160 |
| II | 13 (59.1) | 44 (67.7) | ||
| III | 0 (0.0) | 1 (1.5) | ||
| Dedifferentiated | 1 (4.5) | 0 (0.0) | ||
| IDH1/2 status | Mutated | 0 (0.0) | 44 (60.3) | <0.001 |
| Wild‐type | 23 (100) | 29 (39.7) |
Abbreviations: SD, standard deviation.
Bold values indicate a statistically significant result.
4. DISCUSSION
IDH mutation is a highly recurrent genetic event in glial and hematopoietic tumors. 13 , 30 In mice, there is established evidence that mutant IDH can trigger enchondromatosis, which could subsequently undergo additional tumorigenesis to become chondrosarcoma. 31 Our study confirmed the presence of this mutation in over 50% of chondrosarcoma patients and its prevalence is elevated with tumor grade with the highest prevalence seen in dedifferentiated tumors. IDH mutations are very specific to cartilaginous tumors but do not exist in other mesenchymal neoplasms such as osteosarcoma or undifferentiated pleomorphic sarcoma. 11 , 21 , 29 Of note, it is crucial to distinguish chondrosarcoma from osteosarcoma because the former is not chemosensitive while the primary treatment of osteosarcoma involves neoadjuvant chemotherapy. 32 Additionally, about a quarter of conventional osteosarcoma can produce cartilaginous areas, making it challenging to distinguish these two tumor entities in small biopsies. Unlike gliomas where IDH1 R132H mutation is predominant and can be diagnosed by immunohistochemistry, 33 the distribution of IDH1 mutations is more heterogeneous in chondrosarcoma with R132C being the most frequent followed by R132G and R132L. As there are no antibodies specific for R132C currently available, the best approach to detect this hotspot mutation is to use traditional Sanger sequencing or pyrosequencing. IDH2 mutations are prevalent in patients with acute myeloid leukemia and occur most frequently at codon 140. 13 We did not find any IDH2 R140 mutations among the included studies. IDH2 mutation solely occurred at codon 172 in chondrosarcomas with R172S being the most frequent IDH2 genotype.
Our results established a strikingly heterogeneous distribution of IDH1/2 mutations in specific anatomical locations. The distribution of IDH‐positive tumors in the bones of extremities was significantly higher than other types, suggesting different pathways of tumorigenesis among these neoplasms. Besides IDH mutation, other common genetic alterations in chondrosarcoma include COL2A1, CDKN2A/B, TP53 mutations with a prevalence of 20–30%. 12 , 27 TERT promoter mutation/amplification was found in a subset of high‐grade chondrosarcomas and likely to concurrently occur with IDH mutations, CDKN2A/B deletions, or TP53 mutations. 12 That the effect of the TERT promoter mutation on patient survival can be modulated by IDH mutation and other genetic events in glioma patients has been suggested in prior studies. 34 , 35 , 36
In cranial chondrosarcomas, we demonstrated different patterns of IDH1/2 mutations by the anatomical site of neoplasms. These mutations were found in 60% of skull base tumors but did not exist in craniofacial chondrosarcomas. These discrepancies might stem from the distinct modes of ossification where skull base bones are structured by endochondral ossification and facial bones originate from intramembranous ossification. The latter mode involves direct differentiation of progenitor cells into osteoblasts and the cartilaginous phase does not take place. The high frequency of IDH1/2 mutations in skull base chondrosarcoma makes it a useful diagnostic tool to differentiate from chordoma, particularly in small biopsy specimens. The effect of IDH mutations on patient outcome in this group is limited and most of the included studies did not provide survival data. Kanamori et al. 23 reported an insignificant correlation of IDH1 mutation with tumor relapse but only seven patients were enrolled in their study. Additional studies are necessary to elucidate the prognostication of IDH1/2 mutations in chondrosarcomas of the head and neck.
The impact of IDH1/2 mutations on chondrosarcoma patient survival is unclear. 12 , 16 , 17 Lugowska et al. indicated an association of IDH mutation with a shorter OS while other studies failed to establish this relationship. 12 , 26 This study concluded a negative impact of IDH mutation on patient OS, emphasizing the independent role of IDH mutation as a prognostic marker in chondrosarcomas. In this study, we found that IDH mutation is more commonly seen in dedifferentiated tumors which could explain the link between IDH mutation and mortality rate. It can help clinicians better predict the clinical course and tailor appropriate treatment decisions. The association of IDH mutation and tumor relapse remains investigational. IDH mutation was found to be associated with longer RFS in grade II‐III chondrosarcomas. 12 We also observed the same trend of correlation of IDH mutation and patient RFS in chondrosarcomas and a decreased risk of tumor relapse in patients harboring IDH1/2 mutations. Although the Cox regression model does not reach statistical significance, it should be noted that the length of follow‐up in the Zhu et al. study 12 was quite long compared to other studies (over 21 years), increasing the power of their statistical adjustment. Raw patient survival data was not provided in this study so we could not include them in our survival analyses.
4.1. Strengths and limitations
Our study is the first meta‐analysis investigating the clinical and prognostic significance of IDH mutations in chondrosarcomas. Our meta‐analyses were solely based on individual participant data which significantly increases the statistical power compared to meta‐analyses using aggregate data. Given the rarity of chondrosarcomas, 8 it was difficult to reach statistical significance when examining the prognosis of this tumor. Most of the published data failed to demonstrate the prognostic impact of IDH mutations in chondrosarcomas. 17 , 23 , 26 We incorporated nearly 500 cases of chondrosarcoma and highlighted that IDH mutation is an independent prognostic marker regardless of age, gender, tumor grades, and locations. However, there are a few limitations that need to be addressed. First, all included studies were designed retrospectively, resulting in unavoidable selection bias. Next, few studies only selected a specific grade or type of chondrosarcoma (e.g., dedifferentiated) which can affect the true frequency of IDH mutations. We were unable to calculate the survival of IDH‐mut and IDH‐wt low‐grade chondrosarcomas and cranial chondrosarcomas because of the small sample size and lack of follow‐up data among the included studies. Future meta‐analyses are needed to investigate the prognostic value of IDH1/2 mutations in these neoplasms. Finally, the median follow‐up time of chondrosarcomas in our series was only 20.5 months which could affect the long‐term survival analysis.
In summary, we demonstrated the distinct characteristics of chondrosarcoma patients harboring IDH1/2 mutations as compared with patients without these mutations. IDH1/2 mutations were prevalent in some specific types of chondrosarcoma and assessment of these mutations in challenging cases could help distinguish from chordoma or osteosarcoma. These mutations could be used as an independent prognostic marker to better predict patient outcomes.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVAL
Not applicable.
Supporting information
Table S1
ACKNOWLEDGMENTS
None.
Vuong HG, Ngo TNM, Dunn IF. Prognostic importance of IDH mutations in chondrosarcoma: An individual patient data meta‐analysis. Cancer Med. 2021;10:4415–4423. 10.1002/cam4.4019
Funding information
This study receives no funding support.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing's sarcoma: national cancer data base report. Clin Orthop Relat Res. 2007;459:40‐47. [DOI] [PubMed] [Google Scholar]
- 2. Bauer HC, Brosjö O, Kreicbergs A, Lindholm J. Low risk of recurrence of enchondroma and low‐grade chondrosarcoma in extremities. 80 patients followed for 2–25 years. Acta Orthop Scand. 1995;66:283‐288. [DOI] [PubMed] [Google Scholar]
- 3. Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer. 1977;40:818‐831. [DOI] [PubMed] [Google Scholar]
- 4. Fiorenza F, Abudu A, Grimer RJ, et al. Risk factors for survival and local control in chondrosarcoma of bone. J Bone Joint Surg Br. 2002;84:93‐99. [DOI] [PubMed] [Google Scholar]
- 5. Grimer RJ, Gosheger G, Taminiau A, et al. Dedifferentiated chondrosarcoma: prognostic factors and outcome from a European group. Eur J Cancer. 2007;43:2060‐2065. [DOI] [PubMed] [Google Scholar]
- 6. Kreicbergs A, Boquist L, Borssén B, Larsson SE. Prognostic factors in chondrosarcoma: a comparative study of cellular DNA content and clinicopathologic features. Cancer. 1982;50:577‐583. [DOI] [PubMed] [Google Scholar]
- 7. Sheth DS, Yasko AW, Johnson ME, Ayala AG, Murray JA, Romsdahl MM. Chondrosarcoma of the pelvis. Prognostic factors for 67 patients treated with definitive surgery. Cancer. 1996;78:745‐750. [DOI] [PubMed] [Google Scholar]
- 8. van Praag Veroniek VM, Rueten‐Budde AJ, Ho V, et al. Incidence, outcomes and prognostic factors during 25 years of treatment of chondrosarcomas. Surg Oncol. 2018;27:402‐408. [DOI] [PubMed] [Google Scholar]
- 9. Tarpey PS, Behjati S, Cooke SL, et al. Frequent mutation of the major cartilage collagen gene COL2A1 in chondrosarcoma. Nat Genet. 2013;45:923‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Amary MF, Bacsi K, Maggiani F, et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol. 2011;224:334‐343. [DOI] [PubMed] [Google Scholar]
- 11. Kerr DA, Lopez HU, Deshpande V, et al. Molecular distinction of chondrosarcoma from chondroblastic osteosarcoma through IDH1/2 mutations. Am J Surg Pathol. 2013;37:787‐795. [DOI] [PubMed] [Google Scholar]
- 12. Zhu GG, Nafa K, Agaram N, et al. Genomic profiling identifies association of IDH1/IDH2 mutation with longer relapse‐free and metastasis‐free survival in high‐grade chondrosarcoma. Clin Cancer Res. 2020;26:419‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dang L, Jin S, Su SM. IDH mutations in glioma and acute myeloid leukemia. Trends Mol Med. 2010;16:387‐397. [DOI] [PubMed] [Google Scholar]
- 14. Houillier C, Wang X, Kaloshi G, et al. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low‐grade gliomas. Neurology. 2010;75:1560‐1566. [DOI] [PubMed] [Google Scholar]
- 15. Vuong HG, Altibi AMA, Duong UNP, et al. TERT promoter mutation and its interaction with IDH mutations in glioma: combined TERT promoter and IDH mutations stratifies lower‐grade glioma into distinct survival subgroups‐A meta‐analysis of aggregate data. Crit Rev Oncol Hematol. 2017;120:1‐9. [DOI] [PubMed] [Google Scholar]
- 16. Lugowska I, Teterycz P, Mikula M, et al. IDH1/2 mutations predict shorter survival in chondrosarcoma. J Cancer. 2018;9:998‐1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Asioli S, Ruengwanichayakun P, Zoli M, et al. Association of clinicopathological features with outcome in chondrosarcomas of the head and neck. Otolaryngol Head Neck Surg. 2020;164:807–814. [DOI] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wells G, Shea B, O’connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. 2000.
- 20. Arai M, Nobusawa S, Ikota H, Takemura S, Nakazato Y. Frequent IDH1/2 mutations in intracranial chondrosarcoma: a possible diagnostic clue for its differentiation from chordoma. Brain Tumor Pathol. 2012;29:201‐206. [DOI] [PubMed] [Google Scholar]
- 21. Chen S, Fritchie K, Wei S, et al. Diagnostic utility of IDH1/2 mutations to distinguish dedifferentiated chondrosarcoma from undifferentiated pleomorphic sarcoma of bone. Hum Pathol. 2017;65:239‐246. [DOI] [PubMed] [Google Scholar]
- 22. Gambarotti M, Pacheco M, Ruengwanichayakun P, et al. Synovial chondrosarcoma: a single‐institution experience with molecular investigations and review of the literature. Histopathology. 2020;77:391‐401. [DOI] [PubMed] [Google Scholar]
- 23. Kanamori H, Kitamura Y, Kimura T, Yoshida K, Sasaki H. Genetic characterization of skull base chondrosarcomas. J Neurosurg. 2015;123:1036‐1041. [DOI] [PubMed] [Google Scholar]
- 24. Lam SW, van Langevelde K, Suurmeijer AJH, Cleven AHG, Bovée J. Conventional chondrosarcoma with focal clear cell change: a clinicopathological and molecular analysis. Histopathology. 2019;75:843‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lucas CG, Grenert JP, Horvai A. Targeted next‐generation sequencing identifies molecular and genetic events in dedifferentiated chondrosarcoma. Arch Pathol Lab Med. 2020; 10.5858/arpa.2020-0379-OA. [DOI] [PubMed] [Google Scholar]
- 26. Mohammad N, Wong D, Lum A, et al. Characterisation of isocitrate dehydrogenase 1/isocitrate dehydrogenase 2 gene mutation and the d‐2‐hydroxyglutarate oncometabolite level in dedifferentiated chondrosarcoma. Histopathology. 2020;76:722‐730. [DOI] [PubMed] [Google Scholar]
- 27. Nicolle R, Ayadi M, Gomez‐Brouchet A, et al. Integrated molecular characterization of chondrosarcoma reveals critical determinants of disease progression. Nat Commun. 2019;10:4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tallegas M, Miquelestorena‐Standley É, Labit‐Bouvier C, et al. IDH mutation status in a series of 88 head and neck chondrosarcomas: different profile between tumors of the skull base and tumors involving the facial skeleton and the laryngotracheal tract. Hum Pathol. 2019;84:183‐191. [DOI] [PubMed] [Google Scholar]
- 29. Yang T, Bai Y, Chen J, et al. Clonality analysis and IDH1 and IDH2 mutation detection in both components of dedifferentiated chondrosarcoma, implicated its monoclonal origin. J Bone Oncol. 2020;22:100293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clark O, Yen K, Mellinghoff IK. Molecular pathways: isocitrate dehydrogenase mutations in cancer. Clin Cancer Res. 2016;22:1837‐1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hirata M, Sasaki M, Cairns RA, et al. Mutant IDH is sufficient to initiate enchondromatosis in mice. Proc Natl Acad Sci USA. 2015;112:2829‐2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carrle D, Bielack SS. Current strategies of chemotherapy in osteosarcoma. Int Orthop. 2006;30:445‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reuss DE, Sahm F, Schrimpf D, et al. ATRX and IDH1‐R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an "integrated" diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol. 2015;129:133‐146. [DOI] [PubMed] [Google Scholar]
- 34. Eckel‐Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372:2499‐2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Labussiere M, Boisselier B, Mokhtari K, et al. Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology. 2014;83:1200‐1206. [DOI] [PubMed] [Google Scholar]
- 36. Vuong HG, Nguyen TQ, Ngo TNM, Nguyen HC, Fung KM, Dunn IF. The interaction between TERT promoter mutation and MGMT promoter methylation on overall survival of glioma patients: a meta‐analysis. BMC Cancer. 2020;20:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
Not applicable.


