Abstract
Ischemic stroke is a fatal disease that has long-term disability. It induces excessive oxidative stress generation and cellular metabolic disorders, result in tissue damage. Epigallocatechin gallate (EGCG) is a naturally derived flavonoid with strong antioxidant property. We previously reported the neuroprotective effect of EGCG in ischemic stroke. The defensive mechanisms of stroke are very diverse and complex. This study investigated specific proteins that are regulated by EGCG treatment in the ischemic brain damage. Middle cerebral artery occlusion (MCAO) was performed to induce focal cerebral ischemia. EGCG (50 mg/kg) or vehicle was intraperitoneally administered just prior to MCAO. MCAO induced severe neurological deficits and disorders. EGCG treatment alleviated these neurological disorder and damage. Cerebral cortex was used for this study. Two-dimensional gel electrophoresis and mass spectrometry were performed to detect the proteins altered by EGCG. We identified various proteins that were changed between vehicle- and EGCG-treated animals. Among these proteins, isocitrate dehydrogenase, dynamin-like protein 1, and γ-enolase were decreased in vehicle-treated animals, while EGCG treatment prevented these decreases. However, pyridoxal-5’-phosphate phosphatase and 60 kDa heat shock protein were increased in vehicle-treated animals with MCAO injury. EGCG treatment attenuated these increases. The changes in these proteins were confirmed by Western blot and reverse transcription-PCR analyses. These proteins were associated with cellular metabolism and neuronal regeneration. Thus, these findings can suggest that EGCG performs a defensive mechanism in ischemic damage by regulating specific proteins related to energy metabolism and neuronal protection.
Keywords: epigallocatechin gallate, proteomics, stroke
Stroke is a serious disease that leads to permanent disabilities and disorders [46]. It has three different types: ischemic stroke, hemorrhagic stroke, and transient ischemic attack. Among these strokes, ischemic stroke is the most common type that accounts for 90 percent of all strokes. Ischemic stroke is caused by a blood clot that blocks a blood vessel in the brain. Vascular occlusion in the brain leads to reactive oxygen species generation and Ca2+ dependent protease activation [1, 7]. Moreover, it is accompanied by bioenergetics failure, excitotoxicity, oxidative stress, and inflammation [5, 38]. These pathological processes caused by ischemic conditions are very complex and difficult to reverse. Thus, potent neuroprotective drugs are needed to alleviate ischemic brain damage.
Epigallocatechin gallate (EGCG) is known as a representative catechin that abundantly exists in green tea [59]. EGCG exerts a powerful antioxidative effect and anti-tumorigenic effect [35]. It also reduces pathological events including inflammation, apoptosis, and aging [52, 54, 55]. It improves learning and memory deficits due to brain ischemic damage and reduces excitatory nerve damage [47]. In addition, EGCG exerts neuroprotective effects and alleviates cognitive impairment and nerve loss in hippocampal neurodegeneration [36]. EGCG plays beneficial functions on neurodegenerative diseases including Parkinson’s disease and Alzheimer’s disease [51, 62]. We previously showed the neuroprotective effect of EGCG in a focal cerebral ischemic animal model [49]. EGCG attenuates neurological disorders and infarction caused by ischemic damage. It exerts a neuroprotective effect by inhibiting the apoptosis process. Although the neuroprotective effect of EGCG on ischemic stroke has been reported, further studies are needed to elucidate the various neuroprotective mechanisms of EGCG. Thus, the aim of this study was to identify specific proteins and mechanisms that are regulated by EGCG in ischemic brain injury.
MATERIALS AND METHODS
Experimental animal preparation
Male Sprague-Dawley rats (n=60, 210 ± 10 g) were obtained from the Samtako Animal Breeding Center (Osan, Korea). Animals were maintained at constant temperatures and light conditions (18–22°C, 12/12 hr light/dark cycle), and kept free to access water and food. The experimental animal groups were randomly divided into four groups as follows; vehicle + sham, EGCG + sham, vehicle + middle cerebral artery occlusion (MCAO), and EGCG + MCAO. All experimental procedures were strictly performed according to the Animal Care Committee of Gyeongsang National University. EGCG (Sigma, St. Louis, MO, USA) was dissolved in phosphate buffered saline (PBS). EGCG (50 mg/kg) or vehicle was injected into the abdominal cavity before MCAO surgery [13]. The vehicle group was injected with PBS only.
Middle cerebral artery occlusion surgical operation
MCAO method was performed to induce focal cerebral ischemia according to the previously described method [41]. Experimental animals were treated with Zoletil (50 mg/kg, ie Virbac, Carros, France) for anesthesia. Animals were laid down on the operating table and central neck skin was incised. Right common carotid artery (CCA) was exposed by dividing the mandibular gland and omohyoid muscles, and carefully separated from the vagus nerve. The external carotid artery (ECA) and the internal carotid artery (ICA) were clearly exposed, and the CCA was temporarily blocked using a microclip. The ECA branch was ligated and cut. A 4/0 monofilament nylon suture was prepared with a heated blunt end. Nylon was placed in cut ECA and subsequently inserted into the ICA until the origin of the middle cerebral artery to block the blood flow. The length of inserted nylon was approximately 20–22 mm. Nylon sutures were ligated with ECA to fix the suture location and block blood flow. The same surgical procedure was carried out in the sham-operated group except nylon filament insertion.
Neurological functional test
Neurological functional tests were performed 24 hr after MCAO and scored on a 5-point scale [34, 41]. The criteria for the neurological deficit score were as follows: no neurological deficit (0), involuntary flexion of the contralateral forelimb (1, mild neurological deficit), contralateral circulation (2, moderate neurological deficit), gait and neuropathic pain loss (3, severe neurological deficit), loss of consciousness (4).
Corner test
Corner test was carried out as a previously described method [45]. The test device was prepared by connecting two identical cardboard rectangles with a size of 30 × 20 × 1 cm3. The two boards were at a 30° angle and the joint between the two boards was opened to motivate the animal to enter the corner. Animals were placed against the corners between the two boards. When the animals walked towards the corner, two vibrissae were stimulated at the same time, and the animals turned left or right. Motions were recorded 10 times per test and non-rotating backsteps were excluded from the recording. Animals were trained for 7 days before MCAO surgery, and only animals with similar bidirectional rotations were used for the corner test. The results expressed as the number of right turns per 10 trials.
Two-dimensional gel electrophoresis
The right cerebral cortex is considered to be an ipsilateral side or infarct region. Thus, the right cerebral cortex tissues were immediately isolated after neurological functional test and stored at −70°C until further experiments were performed. The frozen tissue samples were homogenized with lysis buffer solution [8 M urea, 4% 3-[(3-Cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS), ampholyte and 40 mM Tris-hydrochloric acid (Tris-HCl)] and sonicated for 3 min. The homogenates were centrifuged at 20,000 g for 20 min at 4°C and the supernatant was separated. The supernatant was mixed with 10% trichloroacetic acid for 30 min and centrifuged at 20,000 g for 20 min at 4°C. After centrifugation, the supernatant was discarded and the protein pellet was dissolved in sample buffer [8 M urea, 4% CHAPS, 0.2% ampholyte, 40 mM Tris-HCl, 2 µg/ml dithiothreitol (DTT)]. Protein concentration was measured by Bradford assay kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instruction. Total proteins (50 µg) were mixed with rehydration buffer (8 M urea, 2% CHAPS, 20 mM DTT, 0.5% immobilized pH gradient (IPG) buffer, bromophenol blue) and reacted with IPG gel strips (range pH 4–7, 17 cm, Bio-Rad) at room temperature for 13 hr. Rehydrated IPG gel strips were electrophoresed for first-dimensional isoelectric focusing (IEF) using an Ettan IPGphor 3 System (GE Healthcare, Little Chalfont, Buckinghamshire, UK) with the following protocol: 250 V for 15 min, 10,000 V for 3 hr, and then 10,000 V to 50,000 V. IPG gel strips were incubated with a equilibration buffer (6 M urea, 30% glycerol, 2% sodium dodecyl sulfate, 50 mM Tris-HCl, bromophenol blue) containing 1% DTT for 10 min and incubated with a equilibration buffer containing 2.5% iodoacetamide for 10 min. IPG gel strips were loaded into 7.5–17.5% gradient gel and second dimensional electrophoresis was performed at 10 mA using Protein-II XI electrophoresis equipment (Bio-Rad) at 10°C. Electrophoresis was performed until the bromophenol blue dye reached to the bottom of gel. The gels were isolated from electrophoresis device and fixed in a fixation solution (12% acetic acid in 50% methanol) for 2 hr, washed with 50% ethyl alcohol for 20 min, and dipped in 0.2% sodium thiosulfate for 1 min. They were stained with a silver solution (0.2% silver nitrate, 0.75 ml/l formaldehyde) for 20 min and washed with distilled water. They were reacted with a developer (0.2% sodium carbonate, 0.5 ml/l formaldehyde) and incubated with a stop solution (1% acetic acid) to stop the color reaction. The stained gel images were obtained using Agfar ARCUS 1200™ scanner (Agfar-Gevaert) and analyzed using PDQuest 2D analysis software (Bio-Rad) to identify differences in protein spot intensities of experimental groups. Matrix-assisted laser desorption ionization-time (MALDI-TOF) was performed to identify proteins. The protein spot was cut from the gel, reacted with a desalting solution (30 mM potassium hexacyanoferate, 100 mM sodium thiosulfate) and washed with a washing solution (10% acetic acid in 50% methanol). The gel particles were treated with 50 mM ammonium bicarbonate and acetonitrile, then lyophilized in a vacuum centrifuge (Biotron, Seoul, Korea) for 20 min. The dried gel particle was dissolved in reduction solution (10 mM DTT in 0.1 M ammonium bicarbonate) for 45 min at 56°C, treated with 0.1 M ammonium bicarbonate and acetonitrile, and lyophilized in vacuum centrifuge for 20 min. They were dissolved in a digestion solution (12.5 ng/ml trypsin, 0.1% octyl beta-D glycopyranside in 50 mM ammonium bicarbonate) and incubated for 12 hr at 37°C. The digested proteins were extracted with extraction buffer (1% trifluoroacetic acid in 66% acetonitrile). The extracted protein was lyophilized in a vacuum centrifuge. The dried protein was treated with an extraction buffer and a matrix solution (16 mg/ml alpha-cyano-4-hydroxinic acid, 4 mg/ml nitro cellulose acetone). The protein sample was placed on a MALDI-TOF metal plate and MALDI-TOF was performed with Voyager-DE STR (Applied Biosystem, Foster City, CA, USA). Results were analyzed using the National Center for Biotechnology Information and Mass Spectrometry-Fit protein sequence databases.
Western blot analysis
The right cerebral cortex was homogenized in a lysis buffer solution (1 M Tris-HCl, 5 M sodium chloride, 0.5% sodium deoxycholate, 10% sodium dodecyl sulfate, 1% sodium azide, 10% NP-40) containing phenylmethanesulfonylfluoride as a protease inhibitor. The homogenate was sonicated for 3 min and centrifuged at 15,000 g for 20 min at 4°C. The supernatant was isolated from the centrifuged homogenate and the protein concentration was measured by a bicinchoninic acid kit according to the manufacturer’s instructions (Pierce, Rockford, IL, USA). Total proteins (30 µg) were loaded on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and electrophoresis under the following condition: 10 mA for 30 min, 20 mA for 1 hr 30 min. The electrophoresed protein was transferred to a polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA) and the membrane was reacted with skim milk at room temperature for 1 hr to block non-specific reactions. The membrane was washed with Tris-buffered saline containing 0.1% Tween-20 (TBST) and treated with primary antibody at 4°C for 12 hr. The list of primary antibodies is as follows: anti-isocitrate dehydrogenase (ICDH, #8137S; Cell Signaling Technology, Beverly, MA, USA), anti-dynamin-like protein 1 (DLP-1, 611112; BD Bioscience, Franklin Lakes, NJ, USA), anti-γ-enolase (sc-31859; Santa Cruz Biotechnology, Dallas, TX, USA), anti-chronophin/PDXP (Cell Signaling Technology, #4686), anti-60 kDa heat shock protein (HSP60, Cell Signaling Technology, #4870S), (diluted 1:1,000 with TBST), and anti-β-actin (Santa Cruz Biotechnology, sc-4778). Chronophin/PDXP antibody was used to detect pyridoxal-5′-phosphate phosphatase (PLPP) protein expression. Chronophin is known as PLPP, and PDXP is an abbreviation of pyridoxal 5-phosphate phosphatase, a synonym for PLPP [20]. The membranes were washed in TBST and incubated with horseradish peroxidase-linked anti-mouse (Cell Signaling Technology, #7076), anti-goat (Santa Cruz Biotechnology, sc-2354), or anti-rabbit secondary antibody (Cell Signaling Technology, #7074, diluted 1:5,000 with TBST) at room temperature for 2 hr. Membranes were treated with enhanced chemiluminescence detection reagent (GE Healthcare) according to the manufacturer’s instructions to detect protein band signal. The relative densities of each protein expressions were normalized by β-actin expression, and the results were expressed as a ratio of β-actin intensity of each group.
Reverse transcription-polymerase chain reaction amplification
The right cerebral cortex was homogenized with Trizol Reagent (Life Technologies, Rockville, MD, USA). Homogenates were mixed with chloroform, stored on ice for 10 min, and centrifuged at 13,000 g for 15 min at 4°C. The supernatant was collected from the centrifuged sample, incubated with isopropanol for 5 min at 4°C, and centrifuged at 13,000 g for 5 min at 4°C. The pellet was washed with 70% ethyl alcohol and dissolved in RNase-free water. Single stranded complementary DNA (cDNA) was synthesized from extracted RNA samples (1 µg) using a Superscript III first strand system (Invitrogen, Carlsbad, CA, USA). Specific primers were used and target genes were amplified by polymerase chain reaction (PCR). DNA sequences of primers are described in a Table 1. cDNA was synthesized from RNA samples (1 µg) extracted using the Superscript III first strand system (Invitrogen). PCR reaction was performed under the following conditions: initial denaturation of cDNA for 5 min at 94°C; denaturation step for 30 sec at 94°C, primer annealing step for 30 sec at 54°C, and elongation step for 1 min at 72°C; and a final extension at 72°C for 10 min. It was carried out 30 cycles. The synthesized PCR product was mixed with a Loading star dye (Dyne Bio, Seongnam, Korea) and loaded on a 1% agarose gel for electrophoresis. The PCR product band was observed under an ultraviolet light. The relative densities of each mRNA expressions were normalized by β-actin expression. Results were expressed in the ratio of each mRNA to β-actin intensity.
Table 1. Sequence of the primers used for PCR amplification.
| Gene | Primer sequences (F, Forward; R, Reverse) | Product (bp) | |
|---|---|---|---|
| Isocitrate dehydrogenase [NAD+] | F: | 5′-AAAAATCCATGGCGGTTCTGTG-3′ | 404 |
| R: | 5′-GGTCCCCATAGGCGTGTCG-3′ | ||
| Dynamin-like protein 1 | F: | 5′-AAACACCCCGACTTTGCTGA-3′ | 196 |
| R: | 5′-GCATTCCTCTCCAGTTGCCT-3′ | ||
| γ-enolase | F: | 5′-GCACTCTACCAGGACTTTG-3′ | 284 |
| R: | 5′-CGATGACTCACCATAACCC-3′ | ||
| Pyridoxal-5′-phosphate phosphatase | F: | 5′-CCAATCCAGCGTCTTTTGAT-3′ | 159 |
| R: | 5′-AAACAATCCGCGTGTACCTC-3′ | ||
| 60 kDa heat shock protein | F: | 5′-AGGCATGAAGTTTGATAGAGG-3′ | 150 |
| R: | 5′-TTGGCAATTTCAAGAGCAGG- 3′ | ||
| β-actin | F: | 5′-GGGTCAGAAGGACTCCTACG-3′ | 238 |
| R: | 5′-GGTCTCAAACATGATCTGGG-3′ | ||
Statistical analysis
The densitometric analyses for western blot and RT-PCR were carried out using SigmaGel 1.0 (Jandel Scientific, San Rafael, CA, USA) and SigmaPlot 4.0 (SPSS Inc., Point Richmond, CA, USA). Data were expressed as the mean ± standard error of means (S.E.M.). Data from each group were compared by two-way analysis of variance (ANOVA) followed by post-hoc Scheffe’s test. P<0.05 was considered as statistically significant.
RESULTS
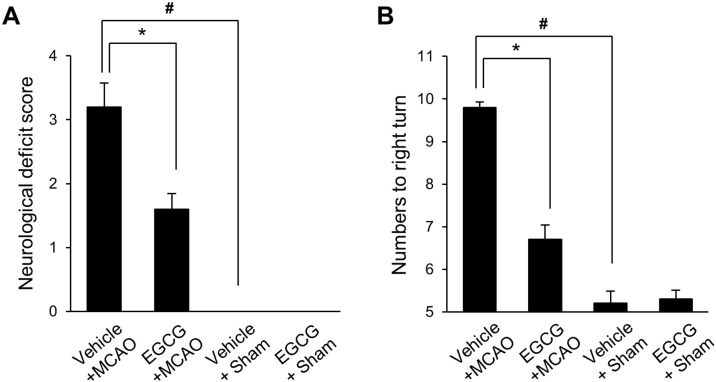
MCAO-induced injury caused neurological deficits and behavioral disorders. Behavioral disorders were confirmed by neurological deficit scoring and corner test. Results of the scoring test showed involuntary circling and loss of walking in vehicle + MCAO animals and contralateral forelimb flexion in EGCG + MCAO animals. Behavioral deficits were not observed in sham animals. The neurological deficit scores were 3.20 ± 0.41 and 1.60 ± 0.23 in vehicle + MCAO and EGCG + MCAO animals, respectively (Fig. 1A). Moreover, the corner test showed a significant increase in right-biased rotation in vehicle + MCAO animals compared to sham animals. EGCG treatment attenuated this increase. The frequency of rotation in sham animals was similar in both directions. The numbers of right turns were 9.80 ± 0.13 and 6.70 ± 0.33 in vehicle + MCAO and EGCG + MCAO animals, respectively (Fig. 1B).
Fig. 1.
Neurological deficit scores (A) and corner test (B) in vehicle + sham, epigallocatechin gallate (EGCG) + sham, vehicle + middle cerebral artery occlusion (MCAO), and EGCG + MCAO animals. EGCG improved the neurological deficit score and right-biased turn by ischemic stroke (A and B). Data (n=15 per group) are represented as the mean ± S.E.M. #P<0.05 vs. vehicle + sham animals, *P<0.05 vs. vehicle + MCAO animals.
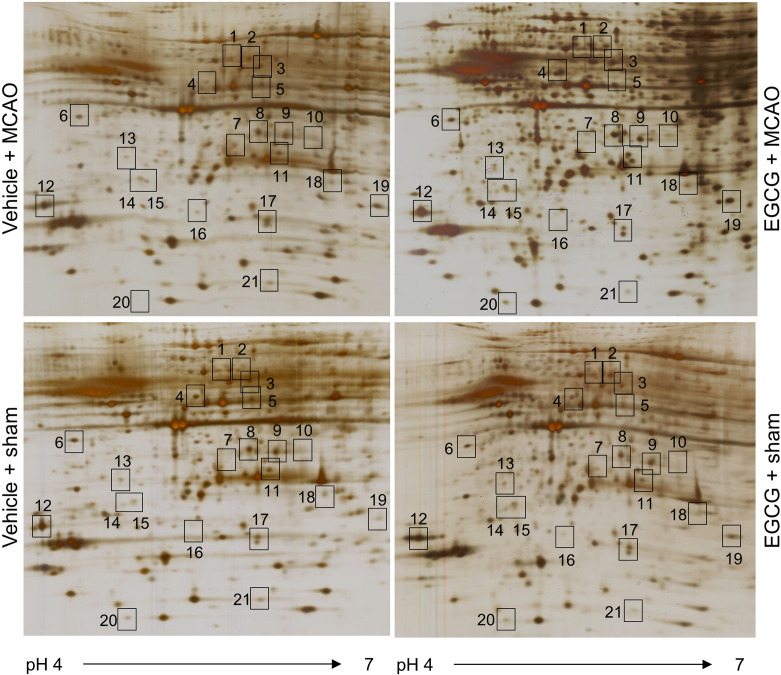
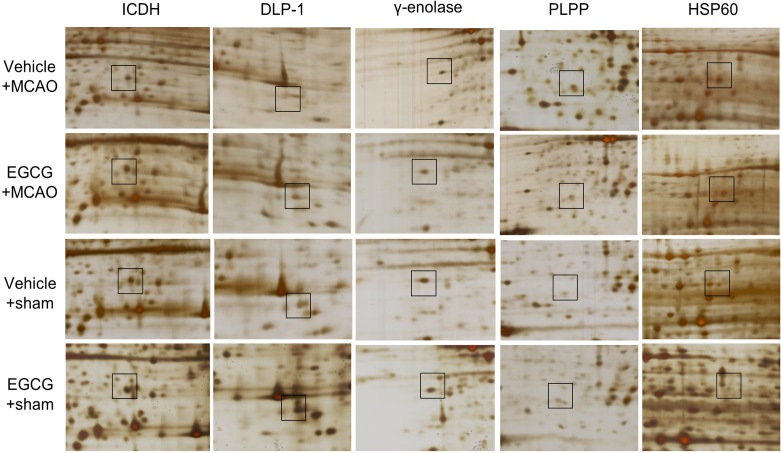
A proteomics approach showed the changed proteins between vehicle- and EGCG-treated animals with MCAO injury. Figure 2 shows images of two-dimensional gel electrophoresis. We detected almost 974 protein spots in each image and selected 43 protein spots that had more than two-fold changes in spot intensity between vehicle- and EGCG-groups with MCAO. Among these protein spots, 21 proteins were identified by MALDI-TOF analysis, and the matched sequence of these proteins was 21% to 53%. However, 5 protein spots were not identified and considered unknown proteins (Table 2). We focused on changes in ICDH, DLP-1, γ-enolase, PLPP, and HSP60 that are related to energy metabolism, neuronal regeneration, and neuroprotection (Fig. 3). The expressions of ICDH, DLP-1, and γ-enolase in the ischemic cerebral cortex were decreased, and these expressions were alleviated by EGCG treatment. However, PLPP and HSP60 expressions were increased in ischemic cortical damage, and EGCG treatment prevented these increases. These protein expressions did not change in sham-operated animals regardless of vehicle or EGCG treatment.
Fig. 2.
Silver stained gel image of two-dimensional sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis in the cerebral cortex from vehicle + sham, epigallocatechin gallate (EGCG) + sham, vehicle + middle cerebral artery occlusion (MCAO), and EGCG + MCAO animals (n=5 per group). Isoelectric focusing was performed at pH 4-7 IPG strips and electrophoresed on 7.5-17.5% gradient SDS gels. Each square indicates the significantly changed protein spots between vehicle + MCAO and EGCG + MCAO animals.
Table 2. List of identified proteins that were significantly differentially expressed in vehicle- and epigallocatechin gallate (EGCG)-treated animals with middle cerebral artery occlusion.
| Spot no. | Protein name | Accession no. | MW (kDa) | pI | Mass matched | Sequence coverage (%) |
|---|---|---|---|---|---|---|
| 1 | 60 kDa heat shock protein | P63039 | 60.91 | 5.91 | 11/133 | 32 |
| 2 | Dihydropyrimidinase-related protein 2 | P47942 | 83.85 | 6.64 | 7/109 | 29 |
| 3 | Unknown | |||||
| 4 | Rab, GTPase-GDP dissociation stimulation stimulator 1 | P52306 | 66.40 | 5.20 | 20/156 | 53 |
| 5 | Unknown | |||||
| 6 | γ-enolase | P07323 | 47.14 | 5.00 | 14/70 | 35 |
| 7 | Mu-crystallin | Q9QYU4 | 33.55 | 5.30 | 6/114 | 21 |
| 8 | Unknown | |||||
| 9 | Unknown | |||||
| 10 | Protein phosphatase 2A, subunit B | P58389 | 36.59 | 5.88 | 9/56 | 29 |
| 11 | Isocitrate dehydrogenase (NAD+) subunit alpha | Q99NA5 | 39.58 | 6.47 | 8/93 | 31 |
| 12 | 14-3-3 gamma | P61983 | 28.28 | 4.80 | 8/77 | 35 |
| 13 | Thioredoxin | Q920J4 | 32.23 | 4.84 | 8/87 | 42 |
| 14 | Ubiquitin carboxy-terminal hydrolase L1 | Q7TQI3 | 31.25 | 4.85 | 11/66 | 50 |
| 15 | Pyridoxal phosphate phosphatase | P60487 | 33.09 | 5.44 | 10/34 | 37 |
| 16 | Unknown | |||||
| 17 | Proteasome subunit alpha type3 | P18422 | 28.40 | 5.30 | 7/112 | 27 |
| 18 | Dynamin-like protein 1 | Q8K1M6 | 80.25 | 6.90 | 14/90 | 21 |
| 19 | Proteasome subunit α 1 | P18420 | 29.52 | 6.15 | 7/117 | 35 |
| 20 | Peroxiredoxin 2 | Q61171 | 21.64 | 5.34 | 6/51 | 46 |
| 21 | UMP-CMP kinase | Q4KM73 | 22.16 | 5.66 | 10/114 | 50 |
Protein names and accession numbers are listed according to the SWISS-PROT database. MW, molecular weight; pI, isoelectric point.
Fig. 3.
Magnified protein spots of isocitrate dehydrogenase (ICDH), dynamin-like protein 1 (DLP-1), γ-enolase, pyridoxal-5′-phosphate phosphatase (PLPP), and 60 kDa heat shock protein (HSP60) in the cerebral cortex from vehicle + sham, epigallocatechin gallate (EGCG) + sham, vehicle + middle cerebral artery occlusion (MCAO), and EGCG + MCAO animals. Each square indicates the protein spots.
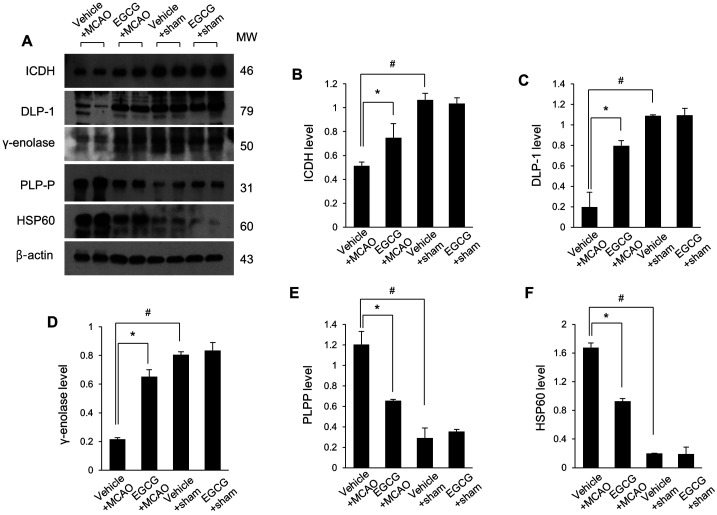
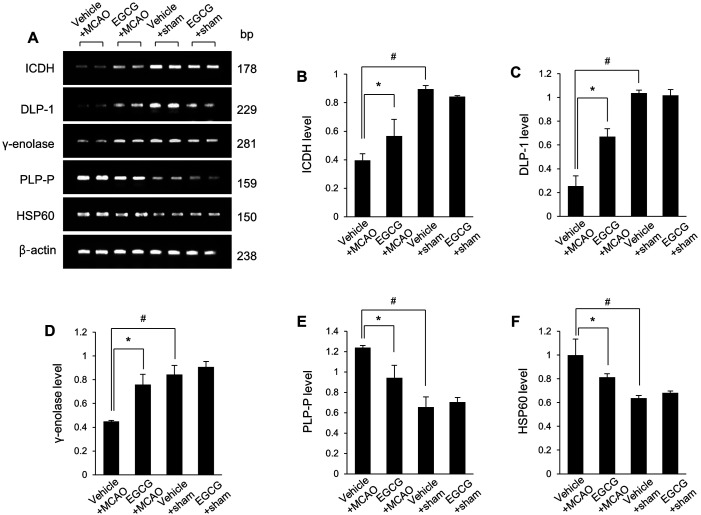
The changes in these protein expressions were confirmed by Western blot and reverse transcription-PCR analyses (Fig. 4A). ICDH protein levels were 0.51 ± 0.03 and 0.75 ± 0.12 in vehicle + MCAO and EGCG + MCAO animals, respectively (Fig. 4B). DLP-1 protein levels were 0.20 ± 0.14 in vehicle + MCAO and 0.80 ± 0.05 in EGCG + MCAO animals (Fig. 4C). γ-enolase protein levels were 0.22 ± 0.01 and 0.65 ± 0.05 in vehicle + MCAO and EGCG + MCAO animals, respectively (Fig. 4D). These proteins were decreased in MCAO-operated animals with vehicle and these decreases were alleviated by EGCG treatment. PLPP and HSP60 levels were increased in vehicle + MCAO animals and these increases were attenuated in the presence of EGCG. PLPP levels were 1.21 ± 0.13 in vehicle + MCAO and 0.65 ± 0.01 in EGCG + MCAO animals (Fig. 4E). HSP60 levels were 1.63 ± 0.06 and 0.93 ± 0.04 in vehicle + MCAO and EGCG + MCAO animals, respectively (Fig. 4F). The results of reverse transcription-PCR were similar to that of Western blot. EGCG treatment attenuated MCAO-induced ICDH, DLP-1, and γ-enolase mRNA levels decreases (Fig. 5A). ICDH mRNA levels were 0.40 ± 0.05 in vehicle + MCAO and 0.57 ± 0.11 in EGCG + MCAO animals (Fig. 5B). DLP-1 mRNA levels were 0.25 ± 0.09 in vehicle + MCAO and 0.67 ± 0.07 in EGCG + MCAO animals (Fig. 5C). γ-enolase mRNA levels were 0.45 ± 0.01 and 0.76 ± 0.09 in vehicle + MCAO and EGCG + MCAO animals, respectively (Fig. 5D). PLPP and HSP60 mRNA levels were significantly increased in vehicle + MCAO animals, and EGCG alleviated these increases. PLPP levels were 1.24 ± 0.02 and 0.94 ± 0.12 in vehicle + MCAO and EGCG + MCAO animals, respectively (Fig. 5E). HSP60 levels were 1.00 ± 0.14 in vehicle + MCAO and 0.81 ± 0.03 in EGCG + MCAO animals (Fig. 5F).
Fig. 4.
Western blot analysis of isocitrate dehydrogenase (ICDH), dynamin-like protein 1 (DLP-1), γ-enolase, pyridoxal-5′-phosphate phosphatase (PLPP), and 60 kDa heat shock protein (HSP60) in the cerebral cortex from vehicle + sham, epigallocatechin gallate (EGCG) + sham, vehicle + middle cerebral artery occlusion (MCAO), and EGCG + MCAO animals. Densitometric analysis is represented as a ratio of β-actin intensity. Data (n=5 per group) are represented as mean ± S.E.M. #P<0.05 vs. vehicle + sham animals, *P<0.05 vs. vehicle + MCAO animals.
Fig. 5.
Reverse transcription-PCR (RT-PCR) of isocitrate dehydrogenase (ICDH), dynamin-like protein 1 (DLP-1), γ-enolase, pyridoxal-5′-phosphate phosphatase (PLPP), and 60 kDa heat shock protein (HSP60) in the cerebral cortex from vehicle + sham, epigallocatechin gallate (EGCG) + sham, vehicle + middle cerebral artery occlusion (MCAO), and EGCG + MCAO animals. The band intensity of the RT-PCR product is expressed as a ratio of β-actin product intensity. Data (n=5 per group) are shown as mean ± S.E.M. #P<0.05 vs. vehicle + sham animals, *P<0.05 vs. vehicle + MCAO animals.
DISCUSSION
Ischemic brain injury causes severe neurological deficits that include sensorimotor impairment, memory loss, and cognitive impairment [57]. We previously demonstrated that EGCG has a neuroprotective effect against ischemic brain injury [49]. EGCG improves neurological deficits and disorders caused by ischemic brain damage and alleviates the increase in infarct volume [49]. To be precise, we focused on the preventive effects of EGCG in ischemic stroke and EGCG was administered before MCAO surgery. Thus, we showed that the presence of EGCG before brain damage affects the protection of neurons from ischemic brain damage. The neuroprotective effects of EGCG were confirmed through the neurological functional test and corner test. We also clearly identified the specific proteins that regulated by EGCG in MCAO damage. Among identified proteins, we further explain the changes of proteins and corresponding mRNA expressions including isocitrate dehydrogenase, dynamin-like protein 1, γ-enolase, pyridoxal-5′-phosphate phosphatase and 60 kDa heat shock protein. We discussed the relationship between these proteins and EGCG in ischemic brain damage.
Energy metabolic disorder is well known as a major cause of various diseases [17, 39]. Neurons are sensitive to energy metabolic disorders because they are very high-metabolizing cells [56]. ICDH is a Krebs cycle enzyme that catalyzes isocitrate to α-ketoglutarate and CO2 [27]. It widely presents in living organisms and contributes to glucose metabolism [18]. Glucose metabolism is closely related to glutamate activity and apoptotic process in neurons [58]. ICDH is particularly distributed in the cerebral cortex, hippocampus, and thalamus. These regions are accepted as vulnerable to ischemic stroke [48]. A decrease in ICDH expression leads to energy metabolic changes, while an increase in ICDH improves neuronal energy metabolism in ischemic brain injury [22]. A previous study reported reductions of mitochondrial enzyme including ICDH, α‐ketoglutarate dehydrogenase, and succinate dehydrogenase in ischemic-injuried brain tissue [25]. The preservation of mitochondria function is considered to be an important factor in neuroplasticity and axonal regeneration during brain repair process [12, 43]. Alzheimer’s disease and ischemic stroke patients showed a decrease in ICDH [6, 53]. In this study, ICDH mRNA and protein expressions were decreased in ischemic cerebral cortex damage and EGCG treatment alleviated these decrease. We suggest that regulating mitochondrial enzyme activity can contribute to neuroprotective mechanism of EGCG against focal cerebral ischemia. Moreover, EGCG regulates ICDH expression and alleviates nerve damage in focal cerebral ischemia. The regulation of ICDH expression is an important factor in the preservation of neurons. Based on this study, we described that the alleviation of reduction of ICDH activity by EGCG treatment contributes to neuroprotective effect of EGCG and reduces brain damage in ischemic brain injury.
Mitochondria fission and fusion are important cellular processes that associated with energy metabolism, cell signaling, and cell death [61]. Mitochondrial dysfunction in neurons inhibits energy generation and causes nerve damage. DLP-1 is a cytoplasmic GTPase responsible for mitochondrial fission and fusion [16]. DLP-1 is essential for synapse formation and regulates synaptic vesicle formation [30]. DLP-1 knockout mice have synaptic deformation, mitochondrial aggregation, and forebrain developmental malformation [30]. However DLP-1 overexpression mice reduce nitric oxide-induced neuronal cell death by regulation of mitochondrial fission [2]. We have shown the down-regulation of DLP-1 expression in the ischemic brain [33]. In addition, this study demonstrated that EGCG attenuates the reduction of DLP-1 caused by MCAO. DLP-1 also exerts a protective effect on ischemic hippocampus by controlling autophagy pathways and removing damaged mitochondria [64]. DLP-1 is considered a therapeutic target protein in ischemic stroke due to these beneficial functions. This study showed that EGCG regulates DLP-1 mRNA and protein expressions in focal cerebral ischemia and protects neurons from ischemic damage. Thus, our findings demonstrate that regulation of DLP-1 expression by EGCG in ischemia is involved in the neuroprotective mechanism of EGCG.
Enolase is a metalloenzyme that acts as a catalyst for converting 2-phosphoglycerate (2-PG) into phosphoenol pyruvate. It is also known as phosphopyruvate hydratase. Enolase exists in all tissues that perform glycolysis or fermentation [9]. Enolase is composed of three subunits, α, β, and γ. Among these subunits, γ-enolase is a neurospecific enolase that expressed in neurons and neuroendocrine cells [42]. γ-enolase is considered a neuronal marker due to its specific distribution [15]. It regulates neuronal survival, differentiation, and neuronal regeneration, also exerts a neuroprotective effect against Alzheimer’s disease [23, 24]. This study demonstrated that ischemic brain injury leads to a decrease in γ-enolase mRNA and protein expressions. EGCG treatment attenuated this decline from MCAO surgery. The decrease of γ-enolase inhibits neuronal regeneration and reduces cell viability in the ischemic cerebral cortex. Thus, maintenance of γ-enolase expression is important for neuroprotective function in ischemic brain damage. We clearly showed the recovery of γ-enolase by EGCG treatment in MCAO animal model. We clearly showed that pretreatment of EGCG in MCAO animal models alleviates the decrease in γ-enolase. Our results demonstrate that the preservation of γ-enolase expression by EGCG alleviates the brain damage caused by MCAO.
Neurotransmitters are essential chemical messengers that perform complex neuronal functions. Norepinephrine, γ-aminobutyric acid, dopamine, and serotonin are well known neurotransmitters. These neurotransmitters are synthesized by pyridoxal-5′-phosphate (PLP)-dependent enzymes [14]. PLP is an active form of vitamin B6 that maintains the physiological homeostasis of cells [44]. It exerts a neuroprotective effect in glucose deprivation-induced neuronal injury, and also protects neurons from nerve damage caused by ischemia [19, 63]. PLPP controls PLP levels by catabolism [3]. PLPP is also known as chronophin, which is an Mg2+-dependent haloacid dehydrogenase (HAD)-type phospho-aspartate transferase [20]. It is related to vitamin B6 metabolism in neurological disorders such as convulsive seizure and epilepsy [21]. PLPP expression was increased in the pyramidal neurons and glial cells of hippocampal CA1 region during ischemic brain injury [28]. The increase of PLPP in neurons induces the delayed neuronal cell death in ischemic-injured hippocampal CA1 region [28]. In particular, the increase in PLPP expression in astrocytes affects to the expression of glutamic acid decarboxylase that involved in neuronal cell death [29, 37]. Our proteomic approach elucidated an increase in PLPP expression in MCAO-induced cerebral cortex damage. EGCG alleviated an increase of PLPP by MCAO, and these results were confirmed by Western blot and RT-PCR analyses. An increase in PLPP expression results in the degradation of PLP. PLP synthesizes neurotransmitters and maintains neurophysiological homeostasis. EGCG attenuates an increase in PLPP protein expression and mRNA transcript level in focal cerebral ischemia and consequently maintains PLP level. Thus, these results suggest that EGCG modulates PLPP expression and protects neurons from ischemic brain damage.
Heat-shock proteins (HSPs) act as chaperone proteins for protein refolding, transport, and translocation [31]. They are immediately expressed in response to detrimental stress including high temperature, hypoxia, and ischemia [4, 60]. Moreover, they stimulate immature immune cells and activate the inflammatory response [10]. HSPs are the family proteins that present in most cell types. They are classified by molecular weight and distribution within cells [50]. Among these HSPs, HSP60 is specifically expressed in the mitochondrial matrix and regulates mitochondrial protein function. It interacts with the 10 kDa heat shock protein and contributes to folding of the newly imported protein [11]. When neurons are damaged, HSP60 is highly expressed and released into the extracellular space. The released HSP60 provides a signal to microglial cells and activates the innate immune system [40]. HSP60 is upregulated in various regions of the ischemic-damaged brain, including the cerebral cortex, striatum, and hippocampus [32]. This study showed that HSP60 protein expression and mRNA transcript level were significantly increased in MCAO-induced damaged cerebral cortex, while EGCG treatment alleviated this increase. The increase in HSP60 can be an indicator of harmful events including inflammation, apoptosis, and ischemic damage [8, 26]. We showed the neuroprotective effect and the alleviation of HSP60 increase by EGCG treatment in ischemic brain injury. We confirmed that EGCG modulates the stress-inducing protein HSP60 in MCAO injury using various experimental techniques. The results of this study demonstrate that EGCG can be used as a therapeutic agent for ischemic stroke.
This study showed that EGCG regulates various protein expression and mRNA transcription levels, and protects brain tissue from damage caused by MCAO. MCAO induces reduction of ICDH, DLP-1, and γ-enolase protein and mRNA expressions that associated with energy metabolism, neuronal regeneration, and neuroprotection. The presence of EGCG before brain damage alleviates the reduction of these proteins induced by MCAO. In contrast to these proteins, EGCG alleviated upregulation of PLPP and HSP60 proteins caused by MCAO. EGCG also acts as a powerful neuroprotector in focal cerebral ischemia. Therefore, our findings demonstrate that EGCG has neuroprotective effects on ischemic brain damage by controlling various functional proteins. Based on the results of this study, we can suggest that EGCG is effective as a therapeutic drug for ischemic brain damage.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (NRF-2018R1D1A1B07044074).
REFERENCES
- 1.Aronowski J., Grotta J. C., Waxham M. N.1992. Ischemia-induced translocation of Ca2+/calmodulin-dependent protein kinase II: potential role in neuronal damage. J. Neurochem. 58: 1743–1753. doi: 10.1111/j.1471-4159.1992.tb10049.x [DOI] [PubMed] [Google Scholar]
- 2.Barsoum M. J., Yuan H., Gerencser A. A., Liot G., Kushnareva Y., Gräber S., Kovacs I., Lee W. D., Waggoner J., Cui J., White A. D., Bossy B., Martinou J. C., Youle R. J., Lipton S. A., Ellisman M. H., Perkins G. A., Bossy-Wetzel E.2006. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 25: 3900–3911. doi: 10.1038/sj.emboj.7601253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell R. R., Haskell B. E.1971. Metabolism of vitamin B 6 in the I-strain mouse. I. Absorption, excretion, and conversion of vitamin to enzyme co-factor. Arch. Biochem. Biophys. 147: 588–601. doi: 10.1016/0003-9861(71)90417-6 [DOI] [PubMed] [Google Scholar]
- 4.Benjamin I. J., McMillan D. R.1998. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ. Res. 83: 117–132. doi: 10.1161/01.RES.83.2.117 [DOI] [PubMed] [Google Scholar]
- 5.Brouns R., De Deyn P. P.2009. The complexity of neurobiological processes in acute ischemic stroke. Clin. Neurol. Neurosurg. 111: 483–495. doi: 10.1016/j.clineuro.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 6.Bubber P., Haroutunian V., Fisch G., Blass J. P., Gibson G. E.2005. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann. Neurol. 57: 695–703. doi: 10.1002/ana.20474 [DOI] [PubMed] [Google Scholar]
- 7.Chan P. H.1996. Role of oxidants in ischemic brain damage. Stroke 27: 1124–1129. doi: 10.1161/01.STR.27.6.1124 [DOI] [PubMed] [Google Scholar]
- 8.Chandra D., Choy G., Tang D. G.2007. Cytosolic accumulation of HSP60 during apoptosis with or without apparent mitochondrial release: evidence that its pro-apoptotic or pro-survival functions involve differential interactions with caspase-3. J. Biol. Chem. 282: 31289–31301. doi: 10.1074/jbc.M702777200 [DOI] [PubMed] [Google Scholar]
- 9.Chen S. H., Giblett E. R.1976. Enolase: human tissue distribution and evidence for three different loci. Ann. Hum. Genet. 39: 277–280. doi: 10.1111/j.1469-1809.1976.tb00131.x [DOI] [PubMed] [Google Scholar]
- 10.Chen W., Syldath U., Bellmann K., Burkart V., Kolb H.1999. Human 60-kDa heat-shock protein: a danger signal to the innate immune system. J. Immunol. 162: 3212–3219. [PubMed] [Google Scholar]
- 11.Cheng M. Y., Hartl F. U., Martin J., Pollock R. A., Kalousek F., Neupert W., Hallberg E. M., Hallberg R. L., Horwich A. L.1989. Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature 337: 620–625. doi: 10.1038/337620a0 [DOI] [PubMed] [Google Scholar]
- 12.Cheng A., Hou Y., Mattson M. P.2010. Mitochondria and neuroplasticity. ASN Neuro 2: e00045. doi: 10.1042/AN20100019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi Y. B., Kim Y. I., Lee K. S., Kim B. S., Kim D. J.2004. Protective effect of epigallocatechin gallate on brain damage after transient middle cerebral artery occlusion in rats. Brain Res. 1019: 47–54. doi: 10.1016/j.brainres.2004.05.079 [DOI] [PubMed] [Google Scholar]
- 14.Dakshinamurti K., Paulose C. S., Viswanathan M.1990. Vitamin B6 and hypertension. Ann. N. Y. Acad. Sci. 585: 241–249. doi: 10.1111/j.1749-6632.1990.tb28057.x [DOI] [PubMed] [Google Scholar]
- 15.DeGiorgio C. M., Gott P. S., Rabinowicz A. L., Heck C. N., Smith T. D., Correale J. D.1996. Neuron-specific enolase, a marker of acute neuronal injury, is increased in complex partial status epilepticus. Epilepsia 37: 606–609. doi: 10.1111/j.1528-1157.1996.tb00623.x [DOI] [PubMed] [Google Scholar]
- 16.De Vos K. J., Allan V. J., Grierson A. J., Sheetz M. P.2005. Mitochondrial function and actin regulate dynamin-related protein 1-dependent mitochondrial fission. Curr. Biol. 15: 678–683. doi: 10.1016/j.cub.2005.02.064 [DOI] [PubMed] [Google Scholar]
- 17.Ferreira I. L., Resende R., Ferreiro E., Rego A. C., Pereira C. F.2010. Multiple defects in energy metabolism in Alzheimer’s disease. Curr. Drug Targets 11: 1193–1206. doi: 10.2174/1389450111007011193 [DOI] [PubMed] [Google Scholar]
- 18.Gálvez S., Gadal P.1995. On the function of the NADP-dependent isocitrate dehydrogenase isoenzymes in living organisms. Plant Sci. 105: 1–14. doi: 10.1016/0168-9452(94)04041-E [DOI] [Google Scholar]
- 19.Geng M. Y., Saito H., Katsuki H.1995. Effects of vitamin B6 and its related compounds on survival of cultured brain neurons. Neurosci. Res. 24: 61–65. doi: 10.1016/0168-0102(96)81279-X [DOI] [PubMed] [Google Scholar]
- 20.Gohla A., Birkenfeld J., Bokoch G. M.2005. Chronophin, a novel HAD-type serine protein phosphatase, regulates cofilin-dependent actin dynamics. Nat. Cell Biol. 7: 21–29. doi: 10.1038/ncb1201 [DOI] [PubMed] [Google Scholar]
- 21.Gospe S. M., Jr., Olin K. L., Keen C. L.1994. Reduced GABA synthesis in pyridoxine-dependent seizures. Lancet 343: 1133–1134. doi: 10.1016/S0140-6736(94)90236-4 [DOI] [PubMed] [Google Scholar]
- 22.Grelli K. N., Palubinsky A. M., Kale A. C., Lizama-Manibusan B. N., Stankowski J. N., Milne G. L., Singer R., McLaughlin B.2013. Alteration of isocitrate dehydrogenase following acute ischemic injury as a means to improve cellular energetic status in neuroadaptation. CNS Neurol. Disord. Drug Targets 12: 849–860. doi: 10.2174/18715273113129990062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hafner A., Glavan G., Obermajer N., Živin M., Schliebs R., Kos J.2013. Neuroprotective role of γ-enolase in microglia in a mouse model of Alzheimer’s disease is regulated by cathepsin X. Aging Cell 12: 604–614. doi: 10.1111/acel.12093 [DOI] [PubMed] [Google Scholar]
- 24.Hafner A., Obermajer N., Kos J.2012. γ-Enolase C-terminal peptide promotes cell survival and neurite outgrowth by activation of the PI3K/Akt and MAPK/ERK signalling pathways. Biochem. J. 443: 439–450. doi: 10.1042/BJ20111351 [DOI] [PubMed] [Google Scholar]
- 25.He W., Wang H., Zhao C., Tian X., Li L., Wang H.2020. Role of liraglutide in brain repair promotion through Sirt1-mediated mitochondrial improvement in stroke. J. Cell. Physiol. 235: 2986–3001. doi: 10.1002/jcp.29204 [DOI] [PubMed] [Google Scholar]
- 26.Hollander J. M., Lin K. M., Scott B. T., Dillmann W. H.2003. Overexpression of PHGPx and HSP60/10 protects against ischemia/reoxygenation injury. Free Radic. Biol. Med. 35: 742–751. doi: 10.1016/S0891-5849(03)00400-3 [DOI] [PubMed] [Google Scholar]
- 27.Huh T. L., Kim Y. O., Oh I. U., Song B. J., Inazawa J.1996. Assignment of the human mitochondrial NAD+ -specific isocitrate dehydrogenase alpha subunit (IDH3A) gene to 15q25.1-->q25.2by in situ hybridization. Genomics 32: 295–296. doi: 10.1006/geno.1996.0120 [DOI] [PubMed] [Google Scholar]
- 28.Hwang I. K., Yoo K. Y., Kim D. H., Lee B. H., Kwon Y. G., Won M. H.2007. Time course of changes in pyridoxal 5′-phosphate (vitamin B6 active form) and its neuroprotection in experimental ischemic damage. Exp. Neurol. 206: 114–125. doi: 10.1016/j.expneurol.2007.04.005 [DOI] [PubMed] [Google Scholar]
- 29.Hwang I. K., Yoo K. Y., Kim D. S., Eum W. S., Park J. K., Park J., Kwon O. S., Kang T. C., Choi S. Y., Won M. H.2004. Changes of pyridoxal kinase expression and activity in the gerbil hippocampus following transient forebrain ischemia. Neuroscience 128: 511–518. doi: 10.1016/j.neuroscience.2004.06.061 [DOI] [PubMed] [Google Scholar]
- 30.Ishihara N., Nomura M., Jofuku A., Kato H., Suzuki S. O., Masuda K., Otera H., Nakanishi Y., Nonaka I., Goto Y., Taguchi N., Morinaga H., Maeda M., Takayanagi R., Yokota S., Mihara K.2009. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat. Cell Biol. 11: 958–966. doi: 10.1038/ncb1907 [DOI] [PubMed] [Google Scholar]
- 31.Itoh H., Kobayashi R., Wakui H., Komatsuda A., Ohtani H., Miura A. B., Otaka M., Masamune O., Andoh H., Koyama K., Sato Y., Tashima Y.1995. Mammalian 60-kDa stress protein (chaperonin homolog). Identification, biochemical properties, and localization. J. Biol. Chem. 270: 13429–13435. doi: 10.1074/jbc.270.22.13429 [DOI] [PubMed] [Google Scholar]
- 32.Izaki K., Kinouchi H., Watanabe K., Owada Y., Okubo A., Itoh H., Kondo H., Tashima Y., Tamura S., Yoshimoto T., Mizoi K.2001. Induction of mitochondrial heat shock protein 60 and 10 mRNAs following transient focal cerebral ischemia in the rat. Brain Res. Mol. Brain Res. 88: 14–25. doi: 10.1016/S0169-328X(01)00012-2 [DOI] [PubMed] [Google Scholar]
- 33.Jang A. R., Koh P. O.2016. Ischemic brain injury decreases dynamin-like protein 1 expression in a middle cerebral artery occlusion animal model and glutamate-exposed HT22 cells. Lab. Anim. Res. 32: 194–199. doi: 10.5625/lar.2016.32.4.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin Z., Liang J., Wang J., Kolattukudy P. E.2015. MCP-induced protein 1 mediates the minocycline-induced neuroprotection against cerebral ischemia/reperfusion injury in vitro and in vivo. J. Neuroinflammation 12: 39. doi: 10.1186/s12974-015-0264-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung Y. D., Ellis L. M.2001. Inhibition of tumour invasion and angiogenesis by epigallocatechin gallate (EGCG), a major component of green tea. Int. J. Exp. Pathol. 82: 309–316. doi: 10.1046/j.1365-2613.2001.00205.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang K. S., Wen Y., Yamabe N., Fukui M., Bishop S. C., Zhu B. T.2010. Dual beneficial effects of (-)-epigallocatechin-3-gallate on levodopa methylation and hippocampal neurodegeneration: in vitro and in vivo studies. PLoS One 5: e11951. doi: 10.1371/journal.pone.0011951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang T. C., Park S. K., Hwang I. K., An S. J., Choi S. Y., Cho S. W., Won M. H.2002. Spatial and temporal alterations in the GABA shunt in the gerbil hippocampus following transient ischemia. Brain Res. 944: 10–18. doi: 10.1016/S0006-8993(02)02596-9 [DOI] [PubMed] [Google Scholar]
- 38.Khoshnam S. E., Winlow W., Farzaneh M., Farbood Y., Moghaddam H. F.2017. Pathogenic mechanisms following ischemic stroke. Neurol. Sci. 38: 1167–1186. doi: 10.1007/s10072-017-2938-1 [DOI] [PubMed] [Google Scholar]
- 39.Koroshetz W. J., Jenkins B. G., Rosen B. R., Beal M. F.1997. Energy metabolism defects in Huntington’s disease and effects of coenzyme Q10. Ann. Neurol. 41: 160–165. doi: 10.1002/ana.410410206 [DOI] [PubMed] [Google Scholar]
- 40.Lehnardt S., Schott E., Trimbuch T., Laubisch D., Krueger C., Wulczyn G., Nitsch R., Weber J. R.2008. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. J. Neurosci. 28: 2320–2331. doi: 10.1523/JNEUROSCI.4760-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Longa E. Z., Weinstein P. R., Carlson S., Cummins R.1989. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20: 84–91. doi: 10.1161/01.STR.20.1.84 [DOI] [PubMed] [Google Scholar]
- 42.Marangos P. J., Schmechel D. E.1987. Neuron specific enolase, a clinically useful marker for neurons and neuroendocrine cells. Annu. Rev. Neurosci. 10: 269–295. doi: 10.1146/annurev.ne.10.030187.001413 [DOI] [PubMed] [Google Scholar]
- 43.Mattson M. P.2007. Mitochondrial regulation of neuronal plasticity. Neurochem. Res. 32: 707–715. doi: 10.1007/s11064-006-9170-3 [DOI] [PubMed] [Google Scholar]
- 44.Meister A.1990. On the transamination of enzymes. Ann. N. Y. Acad. Sci. 585: 13–31. doi: 10.1111/j.1749-6632.1990.tb28038.x [DOI] [PubMed] [Google Scholar]
- 45.Michalski D., Küppers-Tiedt L., Weise C., Laignel F., Härtig W., Raviolo M., Schneider D., Hobohm C.2009. Long-term functional and neurological outcome after simultaneous treatment with tissue-plasminogen activator and hyperbaric oxygen in early phase of embolic stroke in rats. Brain Res. 1303: 161–168. doi: 10.1016/j.brainres.2009.09.038 [DOI] [PubMed] [Google Scholar]
- 46.Murray C. J., Vos T., Lozano R., Naghavi M., Flaxman A. D., Michaud C., Ezzati M., Shibuya K., Salomon J. A., Abdalla S., Aboyans V., Abraham J., Ackerman I., Aggarwal R., Ahn S. Y., Ali M. K., Alvarado M., Anderson H. R., Anderson L. M., Andrews K. G., Atkinson C., Baddour L. M., Bahalim A. N., Barker-Collo S., Barrero L. H., Bartels D. H., Basáñez M. G., Baxter A., Bell M. L., Benjamin E. J., Bennett D., Bernabé E., Bhalla K., Bhandari B., Bikbov B., Bin Abdulhak A., Birbeck G., Black J. A., Blencowe H., Blore J. D., Blyth F., Bolliger I., Bonaventure A., Boufous S., Bourne R., Boussinesq M., Braithwaite T., Brayne C., Bridgett L., Brooker S., Brooks P., Brugha T. S., et al. 2012. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2197–2223. doi: 10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- 47.Nagai K., Jiang M. H., Hada J., Nagata T., Yajima Y., Yamamoto S., Nishizaki T.2002. (-)-Epigallocatechin gallate protects against NO stress-induced neuronal damage after ischemia by acting as an anti-oxidant. Brain Res. 956: 319–322. doi: 10.1016/S0006-8993(02)03564-3 [DOI] [PubMed] [Google Scholar]
- 48.Nekrutenko A., Hillis D. M., Patton J. C., Bradley R. D., Baker R. J.1998. Cytosolic isocitrate dehydrogenase in humans, mice, and voles and phylogenetic analysis of the enzyme family. Mol. Biol. Evol. 15: 1674–1684. doi: 10.1093/oxfordjournals.molbev.a025894 [DOI] [PubMed] [Google Scholar]
- 49.Park D. J., Kang J. B., Koh P. O.2020. Epigallocatechin gallate alleviates neuronal cell damage against focal cerebral ischemia in rats. J. Vet. Med. Sci. 82: 639–645. doi: 10.1292/jvms.19-0703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pelham H. R.1986. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell 46: 959–961. doi: 10.1016/0092-8674(86)90693-8 [DOI] [PubMed] [Google Scholar]
- 51.Rezai-Zadeh K., Shytle D., Sun N., Mori T., Hou H., Jeanniton D., Ehrhart J., Townsend K., Zeng J., Morgan D., Hardy J., Town T., Tan J.2005. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J. Neurosci. 25: 8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riegsecker S., Wiczynski D., Kaplan M. J., Ahmed S.2013. Potential benefits of green tea polyphenol EGCG in the prevention and treatment of vascular inflammation in rheumatoid arthritis. Life Sci. 93: 307–312. doi: 10.1016/j.lfs.2013.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Safonova O. A., Popova T. N., Panchenko L. F.2011. Effects of 2,4-dimethoxyphenyl biguanide on glutathione system activity in rat tissues in brain ischemia-reperfusion. Bull. Exp. Biol. Med. 151: 556–559. doi: 10.1007/s10517-011-1381-1 [DOI] [PubMed] [Google Scholar]
- 54.Senthil Kumaran V., Arulmathi K., Srividhya R., Kalaiselvi P.2008. Repletion of antioxidant status by EGCG and retardation of oxidative damage induced macromolecular anomalies in aged rats. Exp. Gerontol. 43: 176–183. doi: 10.1016/j.exger.2007.10.017 [DOI] [PubMed] [Google Scholar]
- 55.Sheng R., Gu Z. L., Xie M. L., Zhou W. X., Guo C. Y.2007. EGCG inhibits cardiomyocyte apoptosis in pressure overload-induced cardiac hypertrophy and protects cardiomyocytes from oxidative stress in rats. Acta Pharmacol. Sin. 28: 191–201. doi: 10.1111/j.1745-7254.2007.00495.x [DOI] [PubMed] [Google Scholar]
- 56.Silver I., Erecińska M.1998. Oxygen and ion concentrations in normoxic and hypoxic brain cells. Adv. Exp. Med. Biol. 454: 7–16. doi: 10.1007/978-1-4615-4863-8_2 [DOI] [PubMed] [Google Scholar]
- 57.Toni D., Fiorelli M., Gentile M., Bastianello S., Sacchetti M. L., Argentino C., Pozzilli C., Fieschi C.1995. Progressing neurological deficit secondary to acute ischemic stroke. A study on predictability, pathogenesis, and prognosis. Arch. Neurol. 52: 670–675. doi: 10.1001/archneur.1995.00540310040014 [DOI] [PubMed] [Google Scholar]
- 58.Vaughn A. E., Deshmukh M.2008. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat. Cell Biol. 10: 1477–1483. doi: 10.1038/ncb1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vogiatzoglou A., Mulligan A. A., Lentjes M. A., Luben R. N., Spencer J. P., Schroeter H., Khaw K. T., Kuhnle G. G.2015. Flavonoid intake in European adults (18 to 64 years). PLoS One 10: e0128132. doi: 10.1371/journal.pone.0128132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagstaff M. J., Collaço-Moraes Y., Aspey B. S., Coffin R. S., Harrison M. J., Latchman D. S., de Belleroche J. S.1996. Focal cerebral ischaemia increases the levels of several classes of heat shock proteins and their corresponding mRNAs. Brain Res. Mol. Brain Res. 42: 236–244. doi: 10.1016/S0169-328X(96)00127-1 [DOI] [PubMed] [Google Scholar]
- 61.Westermann B.2010. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 11: 872–884. doi: 10.1038/nrm3013 [DOI] [PubMed] [Google Scholar]
- 62.Xu Y., Zhang Y., Quan Z., Wong W., Guo J., Zhang R., Yang Q., Dai R., McGeer P. L., Qing H.2016. Epigallocatechin Gallate (EGCG) Inhibits Alpha-Synuclein Aggregation: A Potential Agent for Parkinson’s Disease. Neurochem. Res. 41: 2788–2796. doi: 10.1007/s11064-016-1995-9 [DOI] [PubMed] [Google Scholar]
- 63.Yamashima T., Zhao L., Wang X. D., Tsukada T., Tonchev A. B.2001. Neuroprotective effects of pyridoxal phosphate and pyridoxal against ischemia in monkeys. Nutr. Neurosci. 4: 389–397. doi: 10.1080/1028415X.2001.11747375 [DOI] [PubMed] [Google Scholar]
- 64.Zuo W., Yang P. F., Chen J., Zhang Z., Chen N. H.2016. Drp-1, a potential therapeutic target for brain ischaemic stroke. Br. J. Pharmacol. 173: 1665–1677. doi: 10.1111/bph.13468 [DOI] [PMC free article] [PubMed] [Google Scholar]