Abstract
Background
The reporting quality of clinical practice guidelines (CPGs) for gliomas has not yet been thoroughly assessed. The International Reporting Items for Practice Guidelines in Healthcare (RIGHT) statement developed in 2016 provides a reporting framework to improve the quality of CPGs. We aimed to estimate the reporting quality of glioma guidelines using the RIGHT checklist and investigate how the reporting quality differs by selected characteristics.
Methods
We systematically searched electronic databases, guideline databases, and medical society websites to retrieve CPGs on glioma published between 2018 and 2020. We calculated the compliance of the CPGs to individual items, domains and the RIGHT checklist overall. We performed stratified analyses by publication year, country of development, reporting of funding, and impact factor (IF) of the journal.
Results
Our search revealed 20 eligible guidelines. Mean overall adherence to the RIGHT statement was 54.6%. Eight CPGs reported more than 60% of the items, and five reported less than 50%. All guidelines adhered to the items 1a, 3, 7a, 13a, while no guidelines reported the items 17 or 18b (see http://www.right-statement.org/right-statement/checklist for a description of the items). Two of the seven domains, “Basic information” and “Background”, had mean reporting rates above 60%. The “Review and quality assurance” domain had the lowest mean reporting rate, 12.5%. The reporting quality of guidelines published in 2020, guidelines developed in the United States, and guidelines that reported funding tended to be above average.
Conclusions
The reporting quality of CPGs on gliomas is low and needs improvement. Particular attention should be paid on reporting the external review and quality assurance process. The use of the RIGHT criteria should be encouraged to guide the development, reporting and evaluation of CPGs.
Keywords: Gliomas, clinical practice guidelines (CPGs), Reporting Items for Practice Guidelines in Healthcare checklist (RIGHT checklist), reporting quality
Introduction
Gliomas are the most common primary brain tumors in all age groups, with an average annual incidence of 6.0 per 100 000 population between 2010 and 2014 worldwide (1). Glioblastoma, the most aggressive type of glioma, account for the majority of all diagnosed gliomas (57.7%), having a median overall survival of 8 months (2). Current therapy possibilities for malignant gliomas are limited. Despite aggressive surgery, radiation and chemotherapies, the additional survival thanks to standard therapeutic regimens is usually only months. Due to the high mortality and its inherent disabling effects on patients, the global burden of malignant gliomas has increased (3).
Compared with other types of cancer, the development of treatment strategies for gliomas has been very complicated and slow. However, some important advances, motivated by basic and translational research, have been seen in recent years. Thorough understanding of gliomas at both genetic and molecular level can provide insights into tumor classification (4). Tumor classification has in turn been used to develop personalized rational therapy. New surgical techniques have emerged to improve the extent of tumor resection and yield better tumor control (5). Intensity-modulated and image-guided radiation therapy has advanced (6). The use of temozolomide concurrently and after radiotherapy has clearly improved overall survival (7). Results of immunotherapy have been promising in patients with newly diagnosed glioblastoma, although no immunotherapeutic agents are yet available (8). Given these numerous emerging new treatment opportunities, rational selection of the optimal therapeutic strategy for every patient is essential.
Clinical practice guidelines (CPGs) are systematically developed statements supported by a systematic review of evidence to optimize patient care. Recently, many guidelines regarding the neuropathology (9), surgery, radiation therapy, and chemotherapeutic and antiangiogenic treatment on gliomas (8), have emerged or updated due to the growing evidence. The guidelines also addressed the management of gliomas in children (10,11). However, the quality of the guidelines influences their potential benefits. Rigorous development strategies and explicit reporting are important factors in the acceptance and effective use of the CPG recommendations.
The International Reporting Items for Practice Guidelines in Healthcare (RIGHT) tool has become a widely accepted international resource for assessing the reporting quality of CPGs (12). It provides a reporting framework to inform the development, reporting, and evaluation of CPGs. The RIGHT tool targets all components of reporting: basic information, background, evidence, recommendations, review and quality assurance, funding and statements and management of conflicts of interest, and other information. The RIGHT compliance is particularly important for CPGs on glioma because of the heterogeneous biological background of gliomas and the high associated mortality. However, there is to our knowledge no assessment of reporting quality for glioma guidelines so far. In this study, we aimed to identify all relevant CPGs for gliomas and evaluate their reporting quality according to the RIGHT statement. We also investigated the reporting quality differed between selected subgroups of CPGs to inform the development of future glioma guidelines.
Methods
Study design
We conducted a systematic review and critical appraisal of the reporting quality of CPGs for glioma using the RIGHT checklist.
Literature search
A systematic search of the scientific literature was done using words and Medical Subject Headings related to “guidelines” and “gliomas”. We searched Medline (via PubMed), Chinese Biomedical Literature Database (CBM), Wan Fang Database and Chinese National Knowledge Infrastructure (CNKI) from their inception to November 27, 2020. The search was limited to studies published in Chinese and English. Additionally, we searched the websites of The National Institute for Health and Care Excellence (NICE, https://www.nice.org.uk/), The National Comprehensive Cancer Network (NCCN, https://www.nccn.org/), World Health Organization guidelines (WHO, https://www.who.int/publications/guidelines/year/en/), Scottish Intercollegiate Guidelines Network (SIGN, https://www.sign.ac.uk/our-guidelines/) and Guidelines International Network (GIN, https://guidelines.ebmportal.com/), as well as Google Scholar.
Inclusion criteria and exclusion criteria
All CPGs published between 2018 and 2020 focusing on screening, testing, diagnosis, treatment or management of gliomas were considered eligible for inclusion. Duplicate reports, summaries and interpretations of guidelines, and unpublished drafts of guidelines were excluded.
Screening
The titles and abstracts of all records were screened by two authors (JM Zhang and HY Meng) to identify reports for full text review. Then, full texts and any related supplementary materials were assessed to reach a final decision on inclusion or exclusion. Discrepancies or inconsistent findings were adjudicated by a third reviewer (Z Chen).
Data extraction of guidelines
Data for each included CPG were collected independently and in duplicate by QW Zhang and SZ Du with the help of a standardized electronic form. Any disagreements were settled by discussion or adjudication (XJ Zhang). Collected data items included authors, year of publication, publication language, region/country where the CPG was developed, developers (institution or working group), format of publication (peer-reviewed journal, or website only), impact factor (IF) of the journal according to Science Citation Index (SCI), and the scope/purpose and target population of CPGs.
Reporting quality assessment using RIGHT checklists
The reporting quality of CPGs was evaluated using the RIGHT tool. This instrument includes 35 items categorized into seven domains: basic information (6 items), background (8 items), evidence (5 items), recommendations (7 items), review and quality assurance (2 items), funding and conflicts of interest statements and management (4 items), and other information (3 items). Each item was rated as “Reported” (fully presented relevant information) or “Not reported” (lacked some relevant information). In certain conditions, when an item was not applicable to evaluate in the specific guideline, we rated the item as “Not applicable”. The assessment was completed by two authors (YJ Yang and YF Ma); Disagreements were resolved by a third investigator (JL Lu).
Statistical analysis
For each guideline, we calculated the domain reporting rate by dividing the number of reported items by the total number of items in the domain, and the overall reporting rate was estimated through dividing the number of reported items by the total number of items. We carried out subgroup analyses stratifying for the year of publication, region/country of origin, funding support, and format of publication (journal categorized by IF, or website only).
Results
Identification of guidelines
Of 486 potentially relevant records identified by the initial search, duplicates were first eliminated, leaving 467 records for title and abstract screening (Figure 1). After screening titles and abstracts, 437 CPGs were excluded due to not fulfilling the inclusion criteria, leaving 30 CPGs for extensive full-text review. Finally, 20 CPGs were eligible for inclusion in our analysis.
Figure 1.
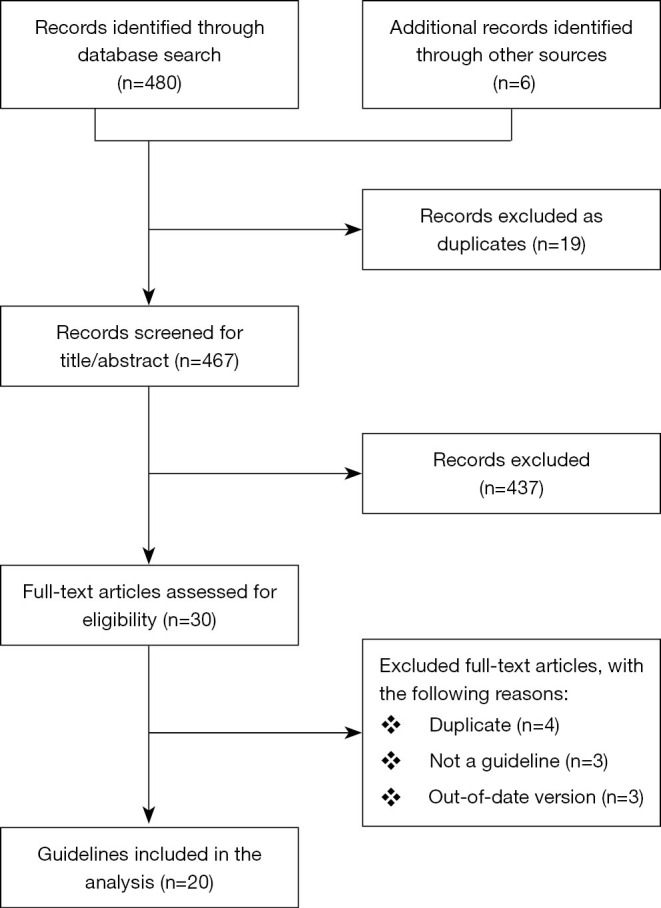
The flow chart of the selection process.
Basic characteristics of included studies
Table 1 shows the characteristics of all included CPGs. The number of CPGs for gliomas published annually increased over time, with four published in 2018, six in 2019, and ten in 2020. Two of the CPGs were published in Chinese, and the rest in English. Six CPGs were from the United States, three CPGs were developed by global consortiums, and the remaining were from South Korea (n=3), China (n=3), Europe (n=3), Italy (n=1) and India (n=1). Five CPGs exclusively dealt with pediatric gliomas, nine focused on high-grade gliomas, and two focused on low-grade gliomas. The majority of CPGs did not report the funding source (n=13), five were funded by government or specialty societies, and two stated explicitly not to have received funding.
Table 1. Characteristics of the included clinical practice guidelines.
| No | Year | Country/region | Language | Developer | Journal IF (2019) | Topic | Funding source |
|---|---|---|---|---|---|---|---|
| 1, (13) | 2020 | USA | English | AANS, CNS | 3.267 | Glioblastoma | Society |
| 2, (11) | 2020 | Global | English | RAPNO | 33.752 | Pediatric LGG | Unreported |
| 3, (10) | 2020 | Global | English | RAPNO | 33.752 | Pediatric HGG | Unreported |
| 4, (14) | 2020 | Global | English | RAPNO | 33.752 | Pediatric DIPG | Unreported |
| 5, (8) | 2020 | USA | English | AANS, CNS | 3.267 | Glioblastoma | Society |
| 6, (15) | 2020 | USA | English | AANS, CNS | 3.267 | Glioblastoma | Society |
| 7, (16) | 2020 | China | English | CGCG, SNO-China, CBCA | 7.36 | Adult diffuse gliomas | Government |
| 8, (9) | 2020 | USA | English | AANS, CNS | 3.267 | Glioblastoma | Unreported |
| 9, (17) | 2020 | Italy | English | SINch/AINO/SIN | 1.645 | LGG | Unreported |
| 10, (18) | 2020 | USA | English | NCCN | Website | Grade III Glioma | Unreported |
| 11, (19) | 2019 | South Korea | English | KSNO | None | Grade II Gliomas | Unreported |
| 12, (20) | 2019 | South Korea | English | KSNO | None | Pediatric LGG | Unreported |
| 13, (21) | 2019 | Europe | English | SIOP-E-BTG/GPOH | 0.882 | Pediatric LGG | Unreported |
| 14, (22) | 2019 | India | English | ISNO | 2.128 | Adult diffuse gliomas | No funding |
| 15, (23) | 2019 | Europe | English | EANO/EURACAN | 33.752 | Medulloblastoma | Unreported |
| 16, (24) | 2019 | South Korea | English | KSNO | None | Glioblastomas | Unreported |
| 17, (25) | 2018 | USA | English | VHA | None | Diffuse gliomas | Unreported |
| 18, (26) | 2018 | Europe | English | EANO | 10.247 | Ependymal tumors | No funding |
| 19, (27) | 2018 | China | Chinese | NHC | Chinese | Gliomas | Unreported |
| 20, (28) | 2018 | China | Chinese | CGCG | Chinese | Gliomas | Government |
AANS, American Association of Neurological Surgeons; AINO, Italian Association of Neuro-Oncology; BGC-CMDA, Brain Glioma Committee-Chinese Medical Doctor Association; CBCA, Chinese Brain Cancer Association; CGCG, committee of Chinese Glioma Cooperative Group; CNS, the Congress of Neurological Surgeons; DIPG, Diffuse intrinsic pontine glioma; EANO, European Association of Neuro-Oncology; EURACAN, EUropean RAre CANcer; GPOH, society of pediatric oncology and hematology; ISNO, Indian Society of Neuro-oncology; KSNO, Korean Society for Neuro-Oncology; LGG, low-grade glioma; HGG, high-grade glioma; NHC, National Health Commission of the People’s Republic of China; RAPNO, the Response Assessment in Pediatric Neuro-Oncology; SIN, Italian Association of Neurology; SINch, (Italian Society of Neurosurgery) Neuro-Oncology section; SIOP-E-BTG, International Society of Pediatric Oncology-Europe-brain tumor group; SNO-China, Society for Neuro- Oncology of China; VHA, Veterans Health Administration.
Overall analysis of reporting quality
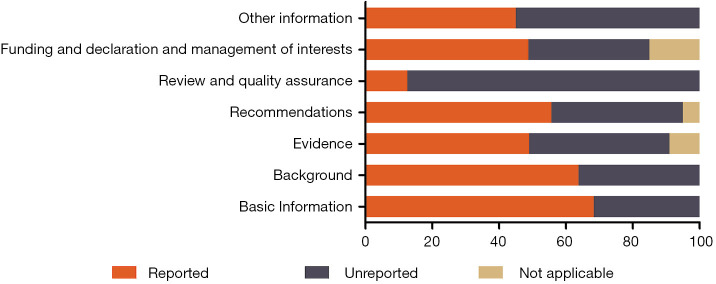
The mean reporting rate was greater than 60% in two domains, “Basic information” (68.3%) and “Background” (63.8%). The “Review and quality assurance” domain had the lowest reporting proportion, 12.5%. The remaining domains were also underreported, with rates of 49.0% for “Evidence”, 55.7% for “Recommendations”, 48.8% for “Funding and declaration and management of interests”, and 45.0% for “other information” (Figure 2). The mean overall reporting rate was 56.1%, ranging between 34.2% and 71.4% across the guidelines. Only eight of the 20 CPGs presented a reporting rate above 60% (Table 2).
Figure 2.
The mean reporting rates of the seven RIGHT checklist domains in the included clinical practice guidelines. RIGHT, Reporting Items for Practice Guidelines in Healthcare.
Table 2. The number of included clinical practice guidelines reporting each RIGHT checklist item (12).
| Section/topic | No. | Item | Reported, n (%) | Unreported, n (%) | Not applicable, n (%) |
|---|---|---|---|---|---|
| Basic information | |||||
| Title/subtitle | 1a | Identify the report as a guideline, that is, with ‘guideline(s)’ or ‘recommendation(s)’ in the title | 20 (100.0) | 0 (0.0) | 0 (0.0) |
| 1b | Describe the year of publication of the guideline | 6 (30.0) | 14 (70.0) | 0 (0.0) | |
| 1c | Describe the focus of the guideline, such as screening, diagnosis, treatment, management, prevention, or others | 14 (70.0) | 6 (30.0) | 0 (0.0) | |
| Executive summary | 2 | Provide a summary of the recommendations contained in the guideline | 4 (20.0) | 16 (80.0) | 0 (0.0) |
| Abbreviations and acronyms | 3 | Define new or key terms, and provide a list of abbreviations and acronyms if applicable | 20 (100.0) | 0 (0.0) | 0 (0.0) |
| Corresponding developer | 4 | Identify at least 1 corresponding developer or author who can be contacted about the guideline | 18 (90.0) | 2 (10.0) | 0 (0.0) |
| Background | |||||
| Brief description of the health problem(s) | 5 | Describe the basic epidemiology of the problem, such as the prevalence/incidence, morbidity, mortality, and burden (including financial) resulting from the problem | 15 (75.0) | 5 (25.0) | 0 (0.0) |
| Aim(s) of the guideline and specific objectives | 6 | Describe the aim(s) of the guideline and specific objectives, such as improvements in health indicators (e.g., mortality and disease prevalence), quality of life, or cost savings | 19 (95.0) | 1 (5.0) | 0 (0.0) |
| Target population(s) | 7a | Describe the primary population(s) that is affected by the recommendation(s) in the guideline | 20 (100.0) | 0 (0.0) | 0 (0.0) |
| 7b | Describe any subgroups that are given special consideration in the guideline | 17 (85.0) | 3 (15.0) | 0 (0.0) | |
| End users and settings | 8a | Describe the intended primary users of the guideline (such as primary care providers, clinical specialists, public health practitioners, program managers, and policymakers) and other potential users of the guideline | 12 (60.0) | 8 (40.0) | 0 (0.0) |
| 8b | Describe the setting(s) for which the guideline is intended, such as primary care, low- and middle-income countries, or inpatient facilities | 3 (15.0) | 17 (85.0) | 0 (0.0) | |
| Guideline development groups | 9a | Describe how all contributors to the guideline development were selected and their roles and responsibilities (e.g., steering group, guideline panel, external reviewers, systematic review team, and methodologists) | 8 (40.0) | 12 (60.0) | 0 (0.0) |
| 9b | List all individuals involved in developing the guideline, including their title, role(s), and institutional affiliation(s) | 8 (40.0) | 12 (60.0) | 0 (0.0) | |
| Evidence | |||||
| Health care questions | 10a | State the key questions that were the basis for the recommendations in PICO (population, intervention, comparator, and outcome) or other format as appropriate | 10 (50.0) | 10 (50.0) | 0 (0.0) |
| 10b | Indicate how the outcomes were selected and sorted | 10 (50.0) | 10 (50.0) | 0 (0.0) | |
| Systematic reviews | 11a | Indicate whether the guideline is based on new systematic reviews done specifically for this guideline or whether existing systematic reviews were used | 6 (30.0) | 14 (70.0) | 0 (0.0) |
| 11b | If the guideline developers used existing systematic reviews, reference these and describe how those reviews were identified and assessed (provide the search strategies and the selection criteria, and describe how the risk of bias was evaluated) and whether they were updated | 10 (50.0) | 1 (5.0) | 9 (45.0) | |
| Assessment of the certainty of the body of evidence | 12 | Describe the approach used to assess the certainty of the body of evidence | 13 (65.0) | 7 (35.0) | 0 (0.0) |
| Recommendations | |||||
| Recommendations | 13a | Provide clear, precise, and actionable recommendations | 20 (100.0) | 0 (0.0) | 0 (0.0) |
| 13b | Present separate recommendations for important subgroups if the evidence suggests that there are important differences in factors influencing recommendations, particularly the balance of benefits and harms across subgroups | 17 (85.0) | 3 (15.0) | 0 (0.0) | |
| 13c | Indicate the strength of recommendations and the certainty of the supporting evidence | 13 (65.0) | 0 (0.0) | 7 (35.0) | |
| Rationale/explanation for recommendations | 14a | Describe whether values and preferences of the target population(s) were considered in the formulation of each recommendation. If yes, describe the approaches and methods used to elicit or identify these values and preferences. If values and preferences were not considered, provide an explanation | 6 (30.0) | 14 (70.0) | 0 (0.0) |
| 14b | Describe whether cost and resource implications were considered in the formulation of recommendations. If yes, describe the specific approaches and methods used (such as cost-effectiveness analysis) and summarize the results. If resource issues were not considered, provide an explanation | 7 (35.0) | 13 (65.0) | 0 (0.0) | |
| 14c | Describe other factors taken into consideration when formulating the recommendations, such as equity, feasibility, and acceptability | 3 (15.0) | 17 (85.0) | 0 (0.0) | |
| Evidence to decision processes | 15 | Describe the processes and approaches used by the guideline development group to make decisions, particularly the formulation of recommendations (such as how consensus was defined and achieved and whether voting was used) | 12 (60.0) | 8 (40.0) | 0 (0.0) |
| Review and quality assurance | |||||
| External review | 16 | Indicate whether the draft guideline underwent independent review and, if so, how this was executed and the comments considered and addressed | 5 (25.0) | 15 (75.0) | 0 (0.0) |
| Quality assurance | 17 | Indicate whether the guideline was subjected to a quality assurance process. If yes, describe the process | 0 (0.0) | 20 (100.0) | 0 (0.0) |
| Funding and declaration and management of interests | |||||
| Funding source(s) and role(s) of the funder | 18a | Describe the specific sources of funding for all stages of guideline development | 8 (40.0) | 12 (60.0) | 0 (0.0) |
| 18b | Describe the role of funder(s) in the different stages of guideline development and in the dissemination and implementation of the recommendations | 0 (0.0) | 8 (40.0) | 12 (60.0) | |
| Declaration and management of interests | 19a | Describe what types of conflicts (financial and nonfinancial) were relevant to guideline development | 17 (85.0) | 3 (15.0) | 0 (0.0) |
| 19b | Describe how conflicts of interest were evaluated and managed and how users of the guideline can access the declarations | 14 (70.0) | 6 (30.0) | 0 (0.0) | |
| Other information | |||||
| Access | 20 | Describe where the guideline, its appendices, and other related documents can be accessed | 1 (5.0) | 19 (95.0) | 0 (0.0) |
| Suggestions for further research | 21 | Describe the gaps in the evidence and/or provide suggestions for future research | 13 (65.0) | 7 (35.0) | 0 (0.0) |
| Limitations of the guideline | 22 | Describe any limitations in the guideline development process (such as the development groups were not multidisciplinary or patients’ values and preferences were not sought), and indicate how these limitations might have affected the validity of the recommendations | 13 (65.0) | 7 (35.0) | 0 (0.0) |
RIGHT, Reporting Items for Practice Guidelines in Healthcare. (Details of the RIGHT checklist is available on: http://www.right-statement.org/right-statement/checklist).
Items 1b (the year of publication within the title) and 2 (a summary of the recommendations) from the “Basic information” domain were reported by 30.0% and 20.0% of the CPGs, respectively. Although most items in “Background” domain were well reported (>60%), items 8b, 9a and 9b were reported less frequently, by 15.0%, 40.0%, 40.0% of the CPGs, respectively. Most items of the “Evidence” domain were reported by about half of the CPGs, with the exception of item 11a (30.0%). Items 14a, 14b and 14c in the “Recommendations” domain were reported by 30.0%, 35.0%, and 15.0% of the CPGs, respectively. Among the items of the “Review and quality assurance” domain, external review (item 16) was reported by 25.0% of the CPGs, and quality assurance (item 17) by none of the CPGs. In the domain “Funding and declaration and management of interests”, funding source (item 18a) was reported by 40.0% of the CPGs, but no CPG reported the role of the funder (item 18b) (Table 2).
Subgroup analyses of reporting quality
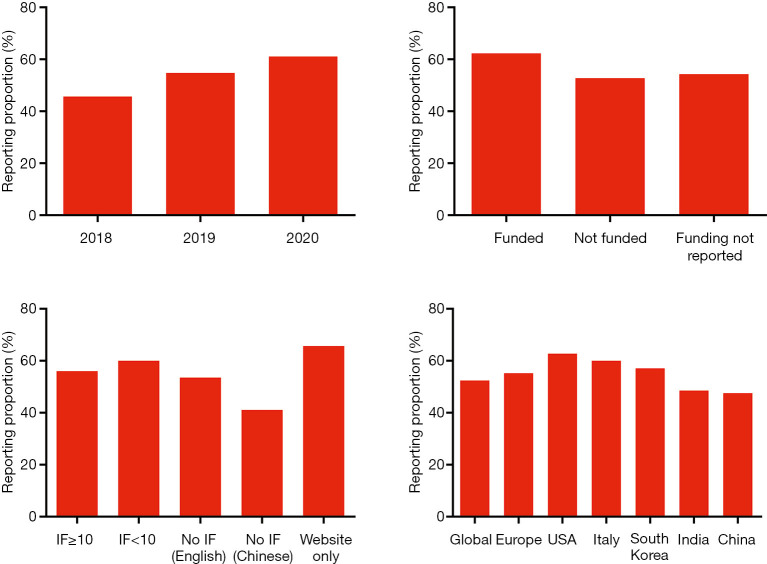
The overall reporting quality improved over years, the mean overall reporting rate being 45.7% for CPGs published in 2018, 54.8% in 2019, and 61.1% in 2020. The reporting quality of English-language CPGs did not differ between journals with different IFs, whereas CPGs published in Chinese-language journals had a lower reporting rate of 41.1%. The guidelines supported by funding had a high reporting proportion compared to those without funding or with unreported funding support. Guidelines from the USA had the highest reporting rate of 62.8% among the regions of origin (Figure 3).
Figure 3.
The reporting proportion of included clinical practice guidelines according to subgroup analyses. IF, impact factor.
Discussion
The present study investigated the reporting quality of CPGs for gliomas using the RIGHT instrument. The methodological quality of glioma guidelines has been assessed before using the Appraisal of guidelines for research & evaluation II (AGREE II) tool (29), but to our knowledge, the current analysis is the first to evaluate the reporting quality, one of the most important aspects of guidelines. We reviewed 20 guidelines that met our inclusion criteria and found that the overall adherence to the RIGHT statement was low. Only one of the 20 CPGs presented a reporting quality above 70%, and twelve had rates below 60%.
In the majority of CPGs included in this analysis, details about the review and quality assurance were underreported. External review and quality assurance are needed to ensure the balance, comprehensiveness and quality of the guidelines (30). It allows experts, potential end users and the industry to challenge the evidence characterization, recommendations and other aspects of CPGs. Unfortunately, only a limited number of CPGs described the details of external review, and none of the CPGs reported the quality assurance process. The finding is in line with previously published studies on other diseases, such as idiopathic pulmonary fibrosis (31). If the review process is poorly reported, it is not possible to determine if and how the review was used to improve the guideline and its recommendations. Attention needs to be paid on clear and explicit report the review and quality assurance process when developing CPG.
Very few CPGs reported the “Recommendations” domain completely. This is unfortunate, because recommendations are the core and most important output of a guideline. The recommendations should summarize the benefits and harms and consider patient preferences; cost and resource implications are also vital in clinical decision-making (32). Importantly, a recent study also emphasized the importance of equality, the evidentiary acceptability and implementation feasibility in the formulation of each recommendation (33). Seventy-five percent of the CPGs we analyzed failed to report whether the values and preferences of patients were considered, and 65 percent neglected to address the cost and resource implication. Eighty-five percent did not capture implementation feasibility and excluded details of equality and acceptability for clinical recommendations. Overall, these issues appear to be poorly considered in the development of CPGs.
It should be noted that some items that could be expected to be easy to report, had a poor reporting rate. For example, the year of publication was rarely reported within the title, even in high-quality guidelines (13). Three CPGs described the settings for which the guideline was intended (16,22,28); and only one CPG described where the guideline, its appendices, and other related documents could be accessed (18). Complete reporting of the above information accelerates the dissemination of guidelines and implementation of the recommendations. These findings reinforce the need for glioma CPGs to improve the reporting of this essential information.
The IF of the journal of publication did not seem to correlate with the reporting quality of English-language CPGs. This finding might be explained by the fact that many high-ranking specialty journals in the field of neuro-oncology have only a moderate-level IF; meanwhile, the reporting quality of the guidelines published in high-impact journals also fell short of expectations. When publishing CPGs which need to be up-to-date and are commonly endorsed by health authorities, the authors may give more value to aspects such as fast publication process or open access than the IF. The Chinese-language guidelines were poorly reported, which has also been found in previous studies have shown (34), but it is worth noting that the quality of the Chinese-originated CPGs published in English was better. This result may suggest that publication in English-language journals means an attempt to disseminate the guidelines globally, and the authors will pay more attention on the reporting quality.
The reporting proportion did not substantially vary across the subgroups we studied. For differences across regions and countries, it is difficult to draw conclusions due to the limited number of CPGs. Nevertheless, we did see a good adherence to RIGHT statement in the six CPGs developed in the United States, all having an overall reporting rate >60%. They should be regarded as an example to other CPG developers on adequate reporting. We chose to restrict our analysis to CPGs published in the past three years to cover only the currently valid guidelines. We saw that the reporting quality improved over time, but it is not clear whether this feature is related to the use of the RIGHT tool. We also observed a better reporting quality in CPGs that reported funding support. Given that the development, maintenance, and revision of CPG are a costly and time-consuming intellectual work, adequate funding support may allow a better reporting quality. However, the influence of funders on conclusions and recommendations needs to be considered and understood; none of the CPGs specified the role of funders in the different stages of guideline development, or in the dissemination and implementation of the recommendations. Thus, disclosure mechanisms, management strategies are needed to improve the transparency of the process.
Several limitations should be highlighted. We did not use methods such as a multivariate regression or linear correlation; the findings of the subgroup analyses should be interpreted cautiously as they do not necessarily reflect true associations. In particular, some subgroups only consisted of one or two CPGs. Although designed as a descriptive analysis of the reporting quality of CPGs, the poor reporting rates of many RIGHT items also demonstrate the potential of improving quality by the adhering to RIGHT statement for future guidelines. More studies are needed to better understand the relative benefits of RIGHT tool. The restriction to Chinese- and English-language guidelines was an additional limitation, as the results may not reflect the overall reporting quality of worldwide CPGs.
Questions to be further discussed and considered
Question 1: What impact do you think the low reporting quality of clinical practice guidelines on gliomas will have on clinicians and clinical practices?
Expert opinion: Dr. Wenyin Shi
The low reporting quality of CPGs on gliomas clearly reflects the slow adoption of SMART guideline in the guideline developing process. However, assessment of quality of CPGs is comprehensive. Multiple tools may be used to better determine the quality, for example, AGREE II tool targets the entire CPG development process and all components of CPG report, while AGREE-REX tool focuses on evaluating the quality of CPG recommendations. Despite the low reporting quality, the CPGs can help reduce variation in practice, lessen disparities, and promote shared the decision making between patients and clinicians. Due to the low reporting quality, extra rigor should be taken by the clinicians to fully understand the applicability of guidelines to individual patients. Lower quality CPGs may suit well for the average patients, but unlikely to meet the demand for more nuanced recommendations with comprehensive consideration of patients’ factors, molecular genetics, radiomics, psychosocial and economical considerations. This is highlighted the importance for clinician’s judgement to apply the appropriate CPGs to individual clinical decision making.
Expert opinion: Dr. Francesco Girolamo
Beyond meaningful use, practice guidelines on gliomas are the cornerstones to cure the diseases while maintaining the physical and psychosocial integrity of the affected patients. Low reporting quality of guidelines negatively affects important aspects of performance improvement of care delivery and patient outcome, for example reducing larger adhesion in specific clinical contexts or impeding the assessment of high impact quality measures responsible for reimbursement and incentive payments by governments and payers. High-quality patient care determined by high-quality guidelines directly aligns with the scope of clinical practice and patient expectations, including those of patients affected by rare types of gliomas, or frail.
Expert opinion: Dr. Santiago Cepeda
Low-quality clinical practice guidelines undoubtedly lead to erroneous decision-making in the management of glioma patients. Due to the exponential growth of scientific evidence related to this pathology, it is challenging to select beneficial information. If clinical practice guidelines start from a deficient information base, their quality could be seriously compromised. Furthermore, if these errors are transferred to the field of clinical practice, there is a risk that inappropriate decisions will be made to the detriment of patients’ health.
Question 2: What do you think the most important aspects needed for developing high-quality clinical practice guidelines on gliomas are?
Expert opinion: Dr. Wenyin Shi
High quality CPGs should follow good practice guidelines, such as SMART guideline. High quality of CPGs need to be built on high quality data, and comprehensive analysis of data. It should provide clearly worded recommendations with ratings of quality of evidence and level of recommendation. The target population should be precisely defined to ensure the appropriateness of the applications of the CPGs, such as patient subgroups based on clinical factors, molecular information, etc. Another important aspect of CPG development is timeliness. The understanding of the gliomas, new treatment development, and management options evolve constantly. New data from key clinical trials become available may significantly change the pattern of practice. As a result, CPGs often become obsolete in several years.
Expert opinion: Dr. Francesco Girolamo
As experienced for other neoplasms, important aspects to be kept in mind during guidelines drafting are: the clinical care objectives, the patient safety, the strategy to improve coordination of clinicians with patients and health information technology developers to work together in the care trajectory, the patient and caregiver experiences, the prevention and population health, and the maintenance of affordable costs. In the molecular era of studying glioma mutational burden, there is an increasing interest in specific targeting of the glioma cells, as well as of their blood vessels and inflammatory compartments. To develop high-quality clinical practice guidelines on gliomas, the most recent acquisition on patient molecular profiling should be promptly incorporated into tailored clinical practice for hopeful better patient outcome.
Expert opinion: Dr. Santiago Cepeda
One of the most important aspects when preparing high-quality clinical practice guidelines is having studies that include sufficiently broad information about the immunophenotypic properties of gliomas, responses to treatment, and adequate follow-up time.
Question 3: How do you think conflicts of interest in the guidelines should be handled?
Expert opinion: Dr. Wenyin Shi
Conflicts of interest have been shown to influence the opinions of experts, thus may compromise the CPG quality and validity. Full disclosure of possible conflicts of interest is routinely required for authors on CPGs. However, management on reducing/eliminating conflicts is non-consistent. It is critical that procedures of managing conflicts are implemented throughout the CPGs development. Many strategies have been established, such as reducing/eliminating conflicted experts, having non-conflicted experts serve as chair of expert group, balancing experts with non-overlapping conflicts, and tasking conflict experts on evaluating quality of evidence rather than formation of recommendations.
Expert opinion: Dr. Francesco Girolamo
Despite the role of funders in guideline development and implementation has been not perceived as a real risk in gliomas management so far, the potential future conflict of interest should be prevented integrating the concerns eventually raised by the third parties (e.g., associations of family caregivers) into the process of development of glioma guidelines.
Expert opinion: Dr. Santiago Cepeda
A possible source of conflict of interest in developing clinical practice guidelines is the relationship of some clinicians with the pharmaceutical industry. Furthermore, the lack of independent external evaluators may compromise the validity of these guidelines.
Conclusions
In summary, the adherence of CPGs on gliomas published in the last three years to the RIGHT checklist was low. It is important to improve the reporting quality of CPGs for gliomas, especially with respect to the external review and quality assurance. Structured and transparent reporting can enable clinicians to easily adhere to guidelines and effectively implement recommendations.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors appreciate the academic support from the AME Reporting Guideline Collaborative Group.
Funding: This work was supported by the National Science and Technology Major Project of China (grant number 2020ZX09201009).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-21-2604). WS received research funding from Regeneron, Novocure, consulting fees from Novocure, Zai lab, Brainlab, Varian, and honoraria for lectures from Zai lab; outside the submitted work. The other authors have no conflicts of interest to declare.
References
- 1.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol 2017;19:v1-v88. 10.1093/neuonc/nox158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrom QT, Patil N, Cioffi G, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013-2017. Neuro Oncol 2020;22:iv1-iv96. 10.1093/neuonc/noaa200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global, regional, and national burden of brain and other CNS cancer, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:376-93. 10.1016/S1474-4422(18)30468-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molinaro AM, Taylor JW, Wiencke JK, et al. Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol 2019;15:405-17. 10.1038/s41582-019-0220-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollon T, Hervey-Jumper SL, Sagher O, et al. Advances in the Surgical Management of Low-Grade Glioma. Semin Radiat Oncol 2015;25:181-8. 10.1016/j.semradonc.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grosshans DR, Mohan R, Gondi V, et al. The role of image-guided intensity modulated proton therapy in glioma. Neuro Oncol 2017;19:ii30-7. 10.1093/neuonc/nox002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schreck KC, Grossman SA. Role of Temozolomide in the Treatment of Cancers Involving the Central Nervous System. Oncology (Williston Park) 2018;32:555-60, 69. [PubMed]
- 8.Farrell C, Shi W, Bodman A, et al. Congress of neurological surgeons systematic review and evidence-based guidelines update on the role of emerging developments in the management of newly diagnosed glioblastoma. J Neurooncol 2020;150:269-359. 10.1007/s11060-020-03607-4 [DOI] [PubMed] [Google Scholar]
- 9.Velázquez Vega JE, Brat DJ, Ryken TC, et al. The role of neuropathology in the management of newly diagnosed glioblastoma: a systematic review and evidence-based clinical practice guideline. J Neurooncol 2020;150:143-64. 10.1007/s11060-020-03616-3 [DOI] [PubMed] [Google Scholar]
- 10.Erker C, Tamrazi B, Poussaint TY, et al. Response assessment in paediatric high-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol 2020;21:e317-29. 10.1016/S1470-2045(20)30173-X [DOI] [PubMed] [Google Scholar]
- 11.Fangusaro J, Witt O, Hernaiz Driever P, et al. Response assessment in paediatric low-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol 2020;21:e305-16. 10.1016/S1470-2045(20)30064-4 [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Yang K, Marusic A, et al. A Reporting Tool for Practice Guidelines in Health Care: The RIGHT Statement. Ann Intern Med 2017;166:128-32. 10.7326/M16-1565 [DOI] [PubMed] [Google Scholar]
- 13.Ziu M, Kim BYS, Jiang W, et al. The role of radiation therapy in treatment of adults with newly diagnosed glioblastoma multiforme: a systematic review and evidence-based clinical practice guideline update. J Neurooncol 2020;150:215-67. 10.1007/s11060-020-03612-7 [DOI] [PubMed] [Google Scholar]
- 14.Cooney TM, Cohen KJ, Guimaraes CV, et al. Response assessment in diffuse intrinsic pontine glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol 2020;21:e330-6. 10.1016/S1470-2045(20)30166-2 [DOI] [PubMed] [Google Scholar]
- 15.Redjal N, Nahed BV, Dietrich J, et al. Congress of neurological surgeons systematic review and evidence-based guidelines update on the role of chemotherapeutic management and antiangiogenic treatment of newly diagnosed glioblastoma in adults. J Neurooncol 2020;150:165-213. 10.1007/s11060-020-03601-w [DOI] [PubMed] [Google Scholar]
- 16.Jiang T, Nam DH, Ram Z, et al. Clinical practice guidelines for the management of adult diffuse gliomas. Cancer Lett 2021;499:60-72. 10.1016/j.canlet.2020.10.050 [DOI] [PubMed] [Google Scholar]
- 17.Rudà R, Angileri FF, Ius T, et al. Italian consensus and recommendations on diagnosis and treatment of low-grade gliomas. An intersociety (SINch/AINO/SIN) document. J Neurosurg Sci 2020;64:313-34. 10.23736/S0390-5616.20.04982-6 [DOI] [PubMed] [Google Scholar]
- 18.Nabors LB, Portnow J, Ahluwalia M, et al. Central Nervous System Cancers, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1425. [DOI] [PubMed]
- 19.Kim YZ, Kim CY, Lim J, et al. The Korean Society for Neuro-Oncology (KSNO) Guideline for WHO Grade III Cerebral Gliomas in Adults: Version 2019.01. Brain Tumor Res Treat 2019;7:63-73. 10.14791/btrt.2019.7.e42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YZ, Kim CY, Wee CW, et al. The Korean Society for Neuro-Oncology (KSNO) Guideline for WHO Grade II Cerebral Gliomas in Adults: Version 2019.01. Brain Tumor Res Treat 2019;7:74-84. 10.14791/btrt.2019.7.e43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gnekow AK, Kandels D, Tilburg CV, et al. SIOP-E-BTG and GPOH Guidelines for Diagnosis and Treatment of Children and Adolescents with Low Grade Glioma. Klin Padiatr 2019;231:107-35. 10.1055/a-0889-8256 [DOI] [PubMed] [Google Scholar]
- 22.Santosh V, Sravya P, Gupta T, et al. ISNO consensus guidelines for practical adaptation of the WHO 2016 classification of adult diffuse gliomas. Neurol India 2019;67:173-82. [DOI] [PubMed] [Google Scholar]
- 23.Franceschi E, Hofer S, Brandes AA, et al. EANO-EURACAN clinical practice guideline for diagnosis, treatment, and follow-up of post-pubertal and adult patients with medulloblastoma. Lancet Oncol 2019;20:e715-28. 10.1016/S1470-2045(19)30669-2 [DOI] [PubMed] [Google Scholar]
- 24.Kim YZ, Kim CY, Lim J, et al. The Korean Society for Neuro-Oncology (KSNO) Guideline for Glioblastomas: Version 2018.01. Brain Tumor Res Treat 2019;7:1-9. 10.14791/btrt.2019.7.e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulich S, Becker D, Dacic S, et al. VHA Practice Guideline Recommendations for Diffuse Gliomas. Fed Pract 2018;35:S28-S35. [PMC free article] [PubMed] [Google Scholar]
- 26.Rudà R, Reifenberger G, Frappaz D, et al. EANO guidelines for the diagnosis and treatment of ependymal tumors. Neuro Oncol 2018;20:445-56. 10.1093/neuonc/nox166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bureau of Medical Administration NHCotPsRoC . Guidelines for diagnosis and Treatment of Glioma (2018 version). Chinese Journal of Neurosurgery 2019;35:217-39. [Google Scholar]
- 28.Chinese Glioma Cooperative Group , China SfN-Oo. Guidelines for the removal of functional brain during awake glioma surgery (2018 version). Chinese Journal of Minimally Invasive Neurosurgery 2018;23:383-4. [Google Scholar]
- 29.Romeo V, Stanzione A, Ugga L, et al. A Critical Appraisal of the Quality of Glioma Imaging Guidelines Using the AGREE II Tool: A EuroAIM Initiative. Front Oncol 2019;9:472. 10.3389/fonc.2019.00472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cruz JE, Fahim G, Moore K. Practice Guideline Development, Grading, and Assessment. P T 2015;40:854-7. [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Yu X, Xie Y, et al. Critical appraisal of the quality of clinical practice guidelines for idiopathic pulmonary fibrosis. Ann Transl Med 2020;8:1405. 10.21037/atm-20-3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenfeld RM, Shiffman RN. Clinical practice guideline development manual: a quality-driven approach for translating evidence into action. Otolaryngol Head Neck Surg 2009;140:S1-43. 10.1016/j.otohns.2009.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glenton C, Lewin S, Gulmezoglu AM. Expanding the evidence base for global recommendations on health systems: strengths and challenges of the OptimizeMNH guidance process. Implement Sci 2016;11:98. 10.1186/s13012-016-0470-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, Duan Y, Liang J, et al. Reporting quality of 2014-2018 clinical practice guidelines on diabetes according to the RIGHT checklist. Endocrine 2019;65:531-41. 10.1007/s12020-019-02005-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as