ABSTRACT:
Background:
In January 2020, the first case of Guillain Barre syndrome (GBS) due to COVID-19 was documented in China. GBS is known to be postinfectious following several types of infections. Although causality can only be proven through large epidemiological studies, we intended to study this association by a thorough review of the literature.
Methods:
We searched PubMed, EMBASE, and Google scholar and included all papers with English or Spanish full text and original data of patients with GBS and recent COVID infection. Variables of interest were demographics, diagnostic investigations, and the latency between arboviral and neurological symptoms. Further variables were pooled to identify GBS clinical and electrophysiological variants, used treatments, and outcomes. The certainty of GBS diagnosis was verified using Brighton criteria.
Results:
We identified a total of 109 GBS cases. Ninety-nine cases had confirmed COVID-19 infection with an average age of 56.07 years. The average latency period between the arboviral symptoms and neurologic manifestations for confirmed COVID-19 cases was 12.2 d. The predominant GBS clinical and electromyography variants were the classical sensorimotor GBS and acute demyelinating polyneuropathy respectively. Forty cases required intensive care, 33 cases required mechanical ventilation, and 6 cases were complicated by death.
Conclusions:
Studies on COVID-19-related GBS commonly reported sensorimotor demyelinating GBS with frequent facial palsy. The time between the onset of infectious and neurological symptoms suggests a postinfectious mechanism. Early diagnosis of GBS in COVID-19 patients is important as it might be associated with a severe disease course requiring intensive care and mechanical ventilation.
Keywords: Guillain Barre syndrome, GBS, Miller Fisher syndrome, MFS, SARS-CoV2, COVID-19
RÉSUMÉ :
Apparition du syndrome de Guillain-Barré à la suite d’une infection à la COVID-19 : une étude systématique.
Contexte :
C’est en janvier 2020 qu’on a documenté en Chine le premier cas de syndrome de Guillain-Barré (SGB) attribuable à une infection à la COVID-19. Le SGB est connu pour être post-infectieux et pour apparaître à la suite de plusieurs types d'infections. Bien qu’une réelle causalité puisse seulement être établie par l’entremise de vastes études épidémiologiques, nous nous sommes penchés sur cette association au moyen d’un examen approfondi de la littérature sur le sujet.
Méthodes :
Pour ce faire, nous avons interrogé les bases de données suivantes : PubMed, EMBASE et Google Scholar. À cet égard, nous avons inclus dans notre étude tous les articles complets rédigés en anglais ou en espagnol contenant des données originales à propos de patients atteints du SGB et ayant été infectés récemment à la COVID-19. Les variables qui nous ont le plus intéressés portaient sur leurs caractéristiques démographiques, sur les examens diagnostics qui avaient été effectués et sur la période de latence entre les symptômes dits « arboviraux » et ceux de nature neurologique. Davantage de variables ont été par la suite regroupées pour identifier les variantes cliniques et électro-physiologiques du SGB, les traitements utilisés et l’évolution de l’état de santé de ces patients. On a aussi pu valider la certitude d’un diagnostic de SGB à l’aide des critères de Brighton.
Résultats :
Au total, ce sont 109 cas de SGB que nous avons identifiés. De ce nombre, 99 étaient liés à des cas confirmés d’infection à la COVID-19, l’âge moyen des patients étant de 56,07 ans. La période moyenne de latence entre les premiers symptômes dits « arboviraux » et des manifestations neurologiques pour des cas confirmés d’infection à la COVID-19 a été de 12,2 jours. À noter que les variantes cliniques et électromyographiques prédominantes de la SGB ont relevé respectivement de la forme classique sensorimotrice et de la polyradiculonévrite inflammatoire démyélinisante associées à ce syndrome. Enfin, soulignons que 40 cas ont nécessité le recours aux soins intensifs, que 33 d’entre eux ont entraîné l’utilisation de la ventilation artificielle tandis que 6 autres se sont soldés par un décès.
Conclusion :
Il n’est pas rare que des études portant sur les liens entre le SGB et l’infection à la COVID-19 aient signalé un syndrome de type sensorimoteur démyélinisant avec de fréquentes manifestations de paralysie faciale. La période qui sépare une infection à la COVID-19 de l’apparition de symptômes neurologiques suggère ainsi un mécanisme post-infectieux. Un diagnostic précoce de SGB chez des patients infectés à la COVID-19 est donc important car un tel syndrome peut être associé à une évolution préoccupante de leur état de santé nécessitant des soins intensifs et une ventilation artificielle.
Introduction
In December 2019, the COVID-19 epidemic emerged in Wuhan, China, causing global alterations not only in the field of healthcare, but also in all walks of life. The viral agent responsible for this clinical illness is described as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It was documented that SARS-CoV-2 is associated with neurologic manifestations, including headache, dizziness, hypogeusia, and hyposmia.1 Beside hypogeusia and hyposmia, there has been increased reporting of distinct peripheral nervous system (PNS) diseases in COVID-19 patients.
Guillain Barre syndrome (GBS) is an inflammatory disease of the PNS, characterized by rapidly progressive, symmetrical, and typically ascending weakness of the limbs with reduced or absent deep tendon reflexes, and upper and lower extremities non-length-dependent paresthesia and sensory symptoms at onset. Cranial nerves involvement can also be present in GBS patients, with facial and bulbar muscles often being affected.2 GBS can be classified into different distinct clinical variants including classical sensorimotor, paraparetic, pure motor, pure sensory, Miller Fisher syndrome (MFS), pharyngeal-cervical-brachial variant (PCB), bilateral facial palsy with paranesthesia, and Bickerstaff brainstem encephalitis.3 Another classification of GBS based on the electromyography (EMG) findings has also been described, with acute inflammatory demyelinating polyneuropathy (AIDP) being the most common variant. Other EMG variants of GBS according to this classification include acute motor axonal neuropathy (AMAN) and acute motor and sensory axonal neuropathy (AMSAN).4
GBS has been linked to a variety of causative pathogens; campylobacter jejuni (C. jejuni), cytomegalovirus (CMV), hepatitis E virus, mycoplasma pneumoniae, Epstein–Barr virus (EBV), and Zika virus.5–8 The emergence of Zika virus epidemic in 2016 was noticeably linked to increased incidence of GBS.9 GBS has also been linked to Middle East respiratory syndrome coronavirus (MERS-CoV) which is genetically similar to SARS-CoV-2 and was responsible for the outbreak of Middle East Respiratory Syndrome in 2013.10 In January 2020, the first case of GBS due to SARS-CoV-2 infection was documented in China.11 In this article, we are reviewing all the published cases of GBS that have been linked to SARS-CoV-2, to study their clinical presentations, the average latency period till the onset of GBS symptoms, the global distribution of these cases, and the findings of the ancillary GBS investigations.
Methods
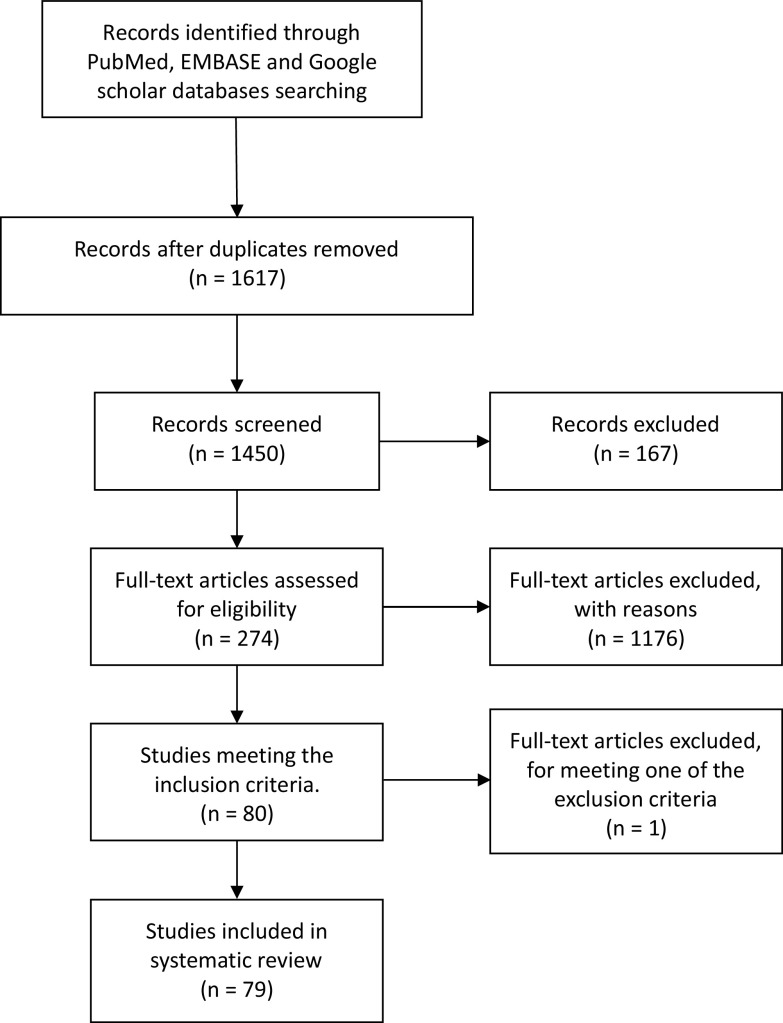
We searched PubMed, EMBASE, and Google scholar and included all papers with full text available in English or Spanish and reporting original data of patients with GBS and recent COVID infection. This systematic literature review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Figure 1).12 We used the following keywords on our search: GBS, MFS, COVID-19, SARS-CoV2, and neurological manifestations, and these databases were searched from August 26, 2020 and to February 7, 2021. Titles and abstracts were screened by two researchers (M. Aladawi and M. Elfil). The full texts of the selected papers were read in full by five researchers (M. Aladawi, B. Abu-Esheh, D. Abu Jazar, A. Armouti, and A. Bayoumi), and their extracted data were then revised by M. Aladawi.
Figure 1:
PRISMA figure showing the steps of literature search and paper selection for the systematic review.
We included all papers, reports, or bulletins with the full text available in English or Spanish, reporting data of patients with GBS and a probable or confirmed recent COVID-19 diagnosis. Preidentified exclusion criteria were: (1) GBS with proven triggering infection other than SARS-CoV2 (e.g., C. jejuni), (2) presence of alternative diagnosis for weakness (e.g., critical illness neuropathy), and (3) latency period between COVID-19 infection and the onset of GBS symptoms of more than 6 weeks. Variables of interest were demographics, COVID-19 diagnostic investigations, latency between constitutional viral symptoms and neurological symptoms, presence of a negative SARS-Cov2 polymerase chain reaction (PCR) at the time of neurological manifestations (Table 1). Studied variables of cases with confirmed COVID-19 infection were pooled into another table to identify clinical characteristics (viral symptoms and neurological symptoms), GBS ancillary diagnostic investigations (cerebrospinal fluid [CSF] findings and testing for antiganglioside antibodies), the predominant clinical and electrophysiological variants of COVID-19-related GBS, received immunomodulatory therapy, disease progression, and clinical outcome (Table 2).
Table 1:
Demographics, diagnostic confirmation of COVID-19, latency duration of neurologic symptoms, and PCR testing at the time of neurological manifestations of both suspected and confirmed cases of COVID-19
| COVID diagnostics at time of arboviral symptoms | Duration between arboviral and neurological symptoms | Negative repeat PCR at time of neurological symptoms | |||||
|---|---|---|---|---|---|---|---|
| Author | Country | PCR | Serology | Chest radiographic features | Nasopharyngeal swab | Cerebrospinal fluid | |
| Confirmed cases | |||||||
| Diez-Porras14 | Spain | Yes | No | No | 5 d | No | NA |
| Granger15 | Italy | Yes | No | No | 22 d | No | NA |
| Hirayama16 | Japan | Yes | No | Yes | 20 d | Yes | NA |
| Liberatore17 | Italy | Yes | No | Yes | 23 d | No | NA |
| Nanda18 | India | Yes | No | No | 10 d | No | NA |
| Nanda18 | India | Yes | No | No | 6 d | No | NA |
| Nanda18 | India | Yes | No | No | 7 d | No | NA |
| Nanda18 | India | Yes | No | Yes | 10 d | No | NA |
| Atakla19 | Guinea | Yes | No | Yes | 14 d | No | NA |
| Rajdev20 | USA | Yes | No | Yes | 18 d | No | NA |
| Senel21 | Germany | Yes | Yes | No | NA | Yes | Yes |
| Tard22 | France | Yes | No | Yes | 10 d | No | NA |
| Chan23 | USA | Yes | No | No | 18 d | No | Yes |
| Chan23 | USA | Yes | No | No | 23 d | Yes | Yes |
| Sedaghat24 | Iran | Yes | No | Yes | 11 d | No | NA |
| Ebrahimzadeh25 | Iran | Yes | No | Yes | 18 d | No | NA |
| Ebrahimzadeh25 | Iran | Yes | No | No | 10 d | No | NA |
| Arnaud26 | France | Yes | No | Yes | 22 d | No | Yes |
| Paybast27 | Iran | Yes | No | No | 16 d | No | NA |
| Paybast27 | Iran | Yes | No | No | 19 d | No | NA |
| Coen28 | Switzerland | Yes | Yes | No | 6 d | No | Yes |
| Dinkin29 | USA | Yes | No | No | 4 d | No | NA |
| Dinkin29 | USA | Yes | No | Yes | NA | No | NA |
| Manganotti30 | Italy | Yes | No | No | 18 d | No | Yes |
| Manganotti30 | Italy | Yes | No | No | 30 d | No | Yes |
| Manganotti30 | Italy | Yes | No | No | 14 d | No | Yes |
| Manganotti30 | Italy | Yes | No | No | 33 d | No | NA |
| Manganotti30 | Italy | Yes | No | No | 22 d | No | Yes |
| Fernández-Domínguez31 | Spain | Yes | No | No | 15 d | Yes | Yes |
| Hutchins32 | USA | Yes | No | Yes | 16 d | No | NA |
| Kilinc33 | Netherlands | No | Yes | No | 28 d | No | Yes |
| Naddaf34 | USA | No | Yes | Yes | 17 d | Yes | Yes |
| Abrams35 | USA | Yes | No | Yes | 10 d | No | Yes |
| Gigli36 | Italy | No | Yes | Yes | 17 d | Yes | NA |
| Bracaglia37 | Italy | Yes | No | No | 0 d | No | NA |
| Sidig38 | Sudan | Yes | No | Yes | 5 d | No | NA |
| Lascano39 | Switzerland | Yes | Yes | No | 15 d | No | Yes |
| Lascano39 | Switzerland | Yes | No | No | 7 d | No | NA |
| Lascano39 | Switzerland | Yes | No | No | 22 d | No | Yes |
| Camdessanche40 | France | Yes | No | Yes | 11 d | No | NA |
| Abolmaali41 | Iran | Yes | No | Yes | 0 d | No | NA |
| Abolmaali41 | Iran | Yes | No | Yes | 10 d | No | NA |
| Abolmaali41 | Iran | Yes | No | Yes | 9 d | No | NA |
| L.Chan42 | Canada | Yes | No | Yes | 0 d | No | Yes |
| Sancho-Saldaña43 | Spain | Yes | No | No | 15 d | No | Yes |
| Assini44 | Italy | Yes | No | No | 20 d | No | Yes |
| Assini44 | Italy | Yes | No | Yes | 23 d | No | Yes |
| Frank45 | Brazil | Yes | Yes | No | 5 d | No | Yes |
| Caamaño46 | Spain | Yes | No | Yes | 10 d | No | Yes |
| Oguz-Akarsu47 | Turkey | Yes | No | Yes | 0 d | No | Yes |
| Toscano48 | Italy | Yes | No | Yes | 7 d | No | Yes |
| Toscano48 | Italy | Yes | No | No | 10 d | No | Yes |
| Toscano48 | Italy | Yes | No | Yes | 10 d | No | Yes |
| Toscano48 | Italy | Yes | No | No | 5 d | No | Yes |
| Toscano48 | Italy | No | Yes | Yes | 7 d | Yes | Yes |
| Reyes-Bueno49 | Spain | No | Yes | No | 15 d | Yes | NA |
| Bigaut50 | France | Yes | No | Yes | 21 d | No | Yes |
| Bigaut50 | France | Yes | No | Yes | 10 d | No | Yes |
| Padroni51 | Italy | Yes | No | No | 24 d | Yes | NA |
| Tiet52 | UK | Yes | No | No | 14 d | No | Yes |
| Ameer53 | UK | Yes | No | No | 4 d before arboviral symptoms | No | Yes |
| Wada54 | China | Yes | No | Yes | 17 d | No | NA |
| Ray55 | UK | Yes | No | No | 0 d | No | NA |
| Guijarro-Castro56 | Spain | Yes | No | Yes | 21 d | No | NA |
| Gutiéerrez-Ortiz57 | Spain | Yes | No | No | 5 d | No | Yes |
| Gutiéerrez-Ortiz57 | Spain | Yes | No | No | 3 d | No | Yes |
| Agosti58 | Italy | Yes | No | Yes | 5 d | No | NA |
| Zhao11 | China | Yes | No | Yes | 8 d before arboviral symptoms | No | NA |
| Khalifa59 | KSA | Yes | No | Yes | 20 d | No | NA |
| Farzi60 | Iran | Yes | No | Yes | 10 d | No | NA |
| Alberti61 | Italy | Yes | No | Yes | 7 d | No | Yes |
| Sandeep62 | US | Yes | No | Yes | 14 d | Yes | NA |
| Korem63 | USA | Yes | No | No | 14 d | No | NA |
| Civardi64 | Italy | Yes | No | No | 10 d | No | Yes |
| Virani65 | USA | Yes | No | No | 10 d | No | NA |
| Khaja66 | USA | Yes | No | No | 0 d | No | Yes |
| Lampe67 | Germany | Yes | No | No | 2 d | No | NA |
| Ottaviani68 | Italy | Yes | No | Yes | 10 d | No | Yes |
| Scheidl69 | Germany | Yes | No | No | 3 weeks | Yes | NA |
| El Otmani70 | France | Yes | No | Yes | 13 d | No | Yes |
| Lantos71 | USA | Yes | No | No | 4 d | No | NA |
| Riva72 | Italy | No | Yes | Yes | 20 d | Yes | Yes |
| Helbok73 | Austria | No | Yes | Yes | 14 d | Yes | Yes |
| Webb74 | UK | Yes | No | No | 7 d | No | Yes |
| Pfefferkorn75 | Germany | Yes | No | Yes | 14 d | No | Yes |
| Dufour76 | USA | Yes | No | No | 21 d | Yes | NA |
| Jones77 | UK | Yes | No | No | 22 d | No | NA |
| Ghosh78 | India | Yes | No | No | 0 d | No | NA |
| Mackenzie79 | Columbia | Yes | No | No | 0 d | No | NA |
| Mansour80 | Morroco | Yes | No | Yes | 12 d | No | Yes |
| Petrelli81 | Italy | Yes | No | No | 15 d | No | NA |
| Yaqoob82 | NA | Yes | No | Yes | 12 d | No | NA |
| Bueso83 | USA | Yes | No | Yes | 22 d | No | NA |
| Manji84 | Tanzania | Yes | No | No | 7 d | No | NA |
| Su85 | USA | Yes | No | Yes | 6 d | No | Yes |
| Galán86 | Spain | Yes | No | Yes | 10 d | No | NA |
| Barrachina-Esteve87 | Spain | Yes | No | Yes | 0 d | No | Yes |
| Marta-Enguita88 | Spain | Yes | No | Yes | 8 d | No | NA |
| Gigli89 | Italy | No | Yes | Yes | NA | Yes | Yes |
| Suspected cases | |||||||
| Gigli89 | Italy | No | No | Yes | NA | Yes | NA |
| Gigli89 | Italy | No | No | No | NA | Yes | NA |
| Gigli89 | Italy | No | No | No | NA | Yes | Yes |
| Gigli89 | Italy | No | No | No | NA | Yes | Yes |
| Gigli89 | Italy | No | No | No | NA | Yes | NA |
| Gigli89 | Italy | No | No | No | NA | Yes | Yes |
| Gigli89 | Italy | No | No | Yes | NA | Yes | NA |
| Manganotti90 | Italy | No | No | No | 16 d | No | NA |
| Gale91 | UK | No | No | Yes | NA | Yes | NA |
| García-Manzanedo92 | Spain | No | No | Yes | 21 d | No | NA |
Table 2:
Demographics, clinical features, and GBS classification in patients with confirmed cases of COVID-19
| Demographics | |
|---|---|
| Mean age (years) | 56.07 |
| Males | 71 |
| Females | 28 |
| Average latency of neurological symptoms (days) | 12.2 (±7.5) |
| Arboviral symptoms | |
| Fever | 67/95 |
| Sore throat | 12/95 |
| Anosmia/dysgeusia | 25/95 |
| Dry cough | 60/95 |
| Rash | 2/95 |
| Arthralgia/myalgia | 18/95 |
| Chest pain | 1/95 |
| Shortness of breath | 27/95 |
| Headache | 10/95 |
| Gastrointestinal symptoms | 17/95 |
| Neurological signs and symptoms | |
| Dysphagia | 18/99 |
| Dysarthria | 11/99 |
| Sensory symptoms | 65/99 |
| Diplopia | 11/99 |
| Facial palsy | 42/99 |
| Bulbar palsy | 12/99 |
| Ocular palsy | 11/99 |
| Tetraparesis | 64/99 |
| Paraparesis | 81/99 |
| Sensory deficits | 41/99 |
| Areflexia or hyporeflexia | 93/99 |
| Ataxia | 18/99 |
| Respiratory dysfunction | 30/99 |
| Dysautonomia | 20/99 |
| GBS clinical variant | |
| Classical sensorimotor GBS | 64/99 |
| Paraparetic GBS | 16/99 |
| Miller Fisher syndrome | 9/99 |
| Pharyngeal-cervical-brachial GBS | 2/99 |
| Bilateral facial palsy with paranesthesia | 3/99 |
| Bickerstaff brainstem encephalitis | 0/99 |
| Pure motor GBS | 0/99 |
| Pure sensory GBS | 1/99 |
| Unclassified | 4/99 |
| CSF analysis | |
| Albuminocytologic dissociation | 74/86 |
| Oligoclonal bands | 2/86 |
| Normal | 10/86 |
| Neuroimaging findings | |
| Cranial nerve enhancement | 9/61 |
| Spinal nerve root enhancement | 10/61 |
| Unremarkable | 44/61 |
| Antiganglioside antibodies | |
| Anti-GM1 | 3/50 |
| Anti-GM2 | 2/50 |
| Anti-GD1a | 3/50 |
| Anti-GD1b | 3/50 |
| Anti-GD3 | 1/50 |
| Anti-GQ1b | 1/50 |
| Anti-GT1b | 1/50 |
| Anti-Gal-C | 1/50 |
| Negative antiganglioside Ab | 43/50 |
| GBS EMG variant | |
| AIDP | 59/77 |
| AMAN | 8/77 |
| AMSAN | 10/77 |
| Immunomodulatory treatment | |
| IVIG | 72/98 |
| PLEX | 10/98 |
| IVIG and PLEX | 7/98 |
| No treatment | 8/98 |
| Clinical outcome | |
| ICU admission | 40/99 |
| Mechanical ventilation | 33/99 |
| Death | 6/99 |
| Brighton criteria | |
| Level 1–3 | 84/99 |
| Level 4 | 9/99 |
| Other variants | 6/99 |
AIDP= acute inflammatory demyelinating polyneuropathy; AMAN=acute motor axonal neuropathy; AMSAN=acute motor and sensory axonal neuropathy; CSF=cerebrospinal fluid; GBS=Guillain Barre syndrome; ICU=intensive care unit; IVIG=intravenous immunoglobulin; PLEX=plasmapheresis.
Cases were classified according to the reported diagnostic certainty levels for GBS and COVID-19 infection. To classify the diagnosis of GBS, we employed the Brighton Collaboration Criteria.13 The diagnostic certainty of COVID-19 infection was classified as confirmed and suspected. As confirmed cases were identified by the presence of positive PCR at the time of arboviral symptoms or the presence of positive SARS-CoV2 antibodies whether during arboviral or neurological presentation as in some cases GBS was the presenting manifestation.
Results
We identified 1450 articles in the databases researched, of which 79 papers were included in our systematic review (66 case reports and 13 cases series). The selected studies reported on a total of 109 GBS cases with a confirmed or a suspected COVID-19 infection. One case was excluded as it met one of the exclusion criteria; the latency between the onset of COVID-19 infection and the GBS onset of symptoms was 53 d (>6 weeks).93
The applied investigations in confirming COVID-19 infection at the time of arboviral symptoms were COVID-19 PCR testing, detection of SARS-CoV2 antibodies, and suggestive features on chest radiography. Cases with either positive PCR or SARS-CoV2 antibodies were categorized as confirmed cases, whereas patients diagnosed based on abnormal chest radiographs or clinical suspicion only were categorized as suspected cases. We have identified 99 cases of COVID-19 complicated by GBS that has been confirmed with either PCR testing or serology (Table 1). Table 1 also includes the latency period between arboviral symptoms and neurologic manifestations, the country of reported cases, and repeat COVID-19 PCR at the time of neurological symptoms either from nasopharyngeal, swabs, or in the CSF.
The global distribution of cases was as follows: 32 cases in Italy, 16 cases in the United States, 12 cases in Spain, 9 cases in Iran, 6 cases in France, 6 cases in the United Kingdom, 5 cases in India, 4 cases in Germany, 4 cases in Switzerland, 2 cases in China, 1 case in Guinea, 1 case in Austria, 1 case in Brazil, 1 case in Canada, 1 case in Columbia, 1 case in Japan, 1 case in Morocco, 1 case in Netherlands, 1 case in Sudan, 1 case in Tanzania, 1 case in Turkey, and 1 case in Saudi Arabia.
At the time of the patient’s demonstrated neurologic signs and symptoms, repeat SARS-CoV2 PCR swab was negative in 23 cases. Reverse transcription PCR (RT-PCR) for SARS-CoV-2 in the CSF was performed in 50 cases in which it was negative. The average latency period between the arboviral symptoms and neurologic manifestations for confirmed COVID-19 cases was 12.2 d (Table 2). There were two cases where neurological manifestations have preceded arboviral symptoms, and nine cases where patients only presented with neurologic deficits with no symptoms of COVID-19, but they had positive COVID-19 testing.
Table 2 shows the pooled data of GBS cases that have been preceded by a confirmed COVID-19 infection. There was a total of 99 cases (71 males and 28 females), the average age was 56.07 years. The most common arboviral symptoms prior to GBS were fever, dry cough, dyspnea, and gastrointestinal symptoms. There were four cases which did not report patient’s arboviral symptoms prior to GBS manifestations. The most commonly reported neurological signs and symptoms were ascending motor weakness (tetraparesis and paraparesis), diminished deep tendon reflexes, sensory disturbances (paresthesia), sensory loss, and facial palsy. GBS was complicated by respiratory failure in 30 cases and dysautonomia in 20 cases.
Clinical GBS variants have been identified in these cases. The most commonly reported GBS variants were classical sensorimotor GBS (64 cases), followed by paraparetic GBS (16 cases), MFS (9 cases), facial diplegia with paresthesia (3 cases), pharyngeal-cervical-brachial GBS (2 cases), and pure sensory GBS (1 case). There were four cases that could not be classified into any of the GBS clinical variants. CSF analysis was performed in 86 cases. Seventy-four cases have shown albuminocytologic dissociation (normal CSF protein <45 mg/dl94), 2 cases have shown oligoclonal band, and 10 cases had no abnormalities in the CSF analysis. Antiganglioside antibodies were investigated in 50 cases. The majority of cases had negative antiganglioside antibodies (43 cases). Each of anti-GM1, anti-GD1a, and anti-GD1b were positive in three cases; anti-GM2 was positive in two cases; and each of anti-GD3, anti-GQ1b, anti-GT1b, and anti-Gal-C were positive in one case.
Electromyography (EMG) was performed in 77 cases. The predominant EMG variant of GBS was AIDP (59 cases), followed by AMSAN (10 cases), and AMAN (8 cases). Eighty-nine reports confirmed the use of immunomodulatory treatment for GBS. Seventy-two cases received intravenous immunoglobulin (IVIG) therapy, 10 cases were treated with plasmapheresis (PLEX), and 7 cases were treated with both IVIG and PLEX. In terms of disease progression and the clinical outcomes, 40 cases required admission to the intensive care unit (ICU), 33 cases required mechanical ventilation, and 6 cases were complicated by death.
Brighton criteria were applied to improve the diagnostic certainty for the cases; valid symptomatology included bilateral and flaccid weakness of limbs at the time of presentation, decreased deep tendon reflexes in affected limbs, the presence of a monophasic course of neurologic symptoms, CSF cell count <50/μl, elevated CSF protein, EMG findings consistent with one of the subtypes of GBS, and the absence of alternative diagnosis. Accordingly, cases were classified from level 1–4 of diagnostic certainty.13 Cases with MFS where the complete triad of ophthalmoplegia, ataxia, and areflexia was not present were classified as level 4.95 Cases with other variants such as facial diplegia with paresthesia, PCB variant, and pure sensory GBS has been excluded. Accordingly, 51 cases have fulfilled level 1 of diagnostic certainty, 26 cases have fulfilled level 2, 7 cases have fulfilled level 3, and 9 cases fulfilled level 4. We have concluded that the reported cases have a high-diagnostic certainty of GBS as most of the cases have been classified into level 1–3 of Brighton criteria.
Discussion
Our systematic review shows that the published literature on COVID-19-related GBS commonly report a classic sensorimotor variant of GBS with often facial palsy and a demyelinating electrophysiological subtype. The disease course is frequently severe with high rates of respiratory dysfunction and ICU admission.96 The time elapsed between infection and neurologic manifestations, and a negative PCR in spinal fluid might suggest that there is a postinfectious mechanism implicated in the etiology of COVID-19-related GBS. However, these results should be interpreted with caution as the cases included in this systematic review varied widely in diagnostic ascertainment and reporting of different variables. Moreover, the reported cases were limited to certain geographical areas, which might provide a source of bias.
The constellation of sensorimotor signs with facial palsy, respiratory insufficiency, and a demyelinating electrophysiological subtype has been described in GBS patients with other viral infections such as CMV and Zika virus, which might indicate that this clinical and electrophysiological variant of GBS is related to viral infections in general.8,97 On the other hand, C. jejuni is typically associated with pure motor and axonal type of GBS.98 Although GBS is generally more common in men as compared with women,99 in our systematic review, we have found that the male to female ratio was 2.5:1 which is significantly higher than what is usually reported.100 This suggests that men might be more prone to COVID-19-related GBS.
In our review, the most common arboviral symptoms were fever and dry cough, which is typical in COVID-19 infection.101 We could not identify a specific arboviral symptom that could be typically preceding the development of GBS. However, we have identified two cases in which GBS manifestations preceded COVID-19 arboviral symptoms, and nine cases that did not present with arboviral symptoms initially. This chronology of GBS preceding the arboviral symptoms has not been previously reported with GBS related to other viral agents. In addition, the asymptomatic infection of COVID-19 might limit the ability to accurately determine the latency period between viral symptoms and the GBS presentation.
The mean duration between the onset of COVID-19 infectious symptoms and GBS presentation was 2 weeks, which is similar to other infections preceding GBS.102 The latency between COVID-19 infection and GBS was more than a week for most cases, but it should be taken into consideration that COVID-19 can initially be asymptomatic which makes the latency duration arguably longer than reported. This suggests a postinfectious immunopathogenesis rather than direct neuronal damage or a parainfectious mechanism. The fact that COVID-19 PCR of the CSF was not positive in a single report, the negativity of repeat nasopharyngeal PCR at the time of symptoms in almost one-third of the cases, and the absence of elevated white blood cell count in the CSF in majority of cases, further argues against the assumption of COVID-19 infection being directly responsible for the GBS development in this proportion of patients.
Despite the fact that previous epidemiological studies have suggested that COVID-19 might not be associated with GBS,103 the chronology of publication of the COVID-19-related GBS cases followed the same pattern of the global spread of COVID-19, as the first cases report was from China followed by Italy, Iran, and USA indicates a positive association.11,24,48,65 GBS has been historically related to various pathogens including C. jejuni, M. pneumoniae, EBV, CMV, Hepatitis E virus, and Zika virus.5–9 However, in certain pathogens such as Hepatitis E virus, this association has not been established globally, as it was only reported in Netherlands and Bangladesh.104 Therefore, immunogenicity of COVID-19 in the development of GBS should consider the variations between different populations,105–108 as epidemiologic studies involving certain populations might introduce bias in reporting results.
Interestingly, almost half of the cases were tested for the presence of antiganglioside antibodies in serum. There were only seven cases have tested positive for different antiganglioside antibodies. Historically, different antigangliosides have been linked to different variants of GBS, such as anti-GQ1b in MFS and anti-GD1a in PCB variant.109,110 Antiganglioside antibodies are considered to be biomarkers of axonal injury rather demyelination, as they directly target the neuronal membrane gangliosides.111 Because most of the COVID-19-related GBS cases reported a demyelinating variant of GBS, it can be anticipated that the presence of antiganglioside antibodies would be low. Thus, the spectrum of immune cascade in COVID-19-related GBS should be expanded by studying other different antibodies affecting the myelin sheath, Schwann cell components, and the neuronal axolemma.112,113 One case was reported with positive NF-155 and NF-186 antibodies, which are structural proteins in the node of Ranvier.22
The possible role of host immunogenetic background in the development of GBS and its variants has been related to human leukocyte antigen (HLA) polymorphism in different populations, this observation might explain the increased reporting of COVID-19 related GBS in the Italy, as one-third of the cases identified in our review were Italian.114,115 The role of HLA polymorphism in COVID-19 related GBS has been emphasized in one of the cases reported by Gigli et al.,36 in which SARS-CoV2 antibodies were detected in the CSF. Interestingly, HLA analysis of the reported case showed several HLA alleles that are known to be associated with GBS, such as: HLA-A33,116 DRB1 * 03:01,117 and DQB1 * 05:01.118
With the emergence of COVID-19 pandemic, there have been increasing reports of various neurological complications in infected patients, which was well documented and studied in other coronaviruses.1 Genomic analysis shows that SARS-CoV-2 is in the same beta-coronavirus (βCoV) clade as MERS-CoV and SARS-CoV, and shares a highly homological sequence with SARS-CoV.119 There has been clinical evidence of neuromuscular sequela in SARS CoV and MERS infection and the most documented neuromuscular syndromes related to these viruses are critical illness polyneuropathy and myopathy, which are hypothesized to occur in the context of severe inflammatory response syndrome (SIRS).120 Cases of MERS-related GBS have been reported, yet GBS in these cases has been linked to the treatment received for MERS infection, such as interferon alpha2 and Lopinavir/ritonavir.10 In contrast to MERS, SARS-CoV2 is likely associated with GBS.
Conclusion
Based on this systematic review, most cases of COVID-19-related GBS are of the sensorimotor demyelinating subtype with frequent facial palsy. The latency between infection and onset of neurologic symptoms as well as the absence of viral genome detected by PCR suggest a postinfectious, rather than a direct infectious or para-infectious mechanism. Global reporting of COVID-19-related GBS cases, in addition to testing for different antibodies to different structural proteins and glycolipids in the peripheral nerves, would improve the understanding of the immunological cascade of COVID-19-related GBS. Finally, early diagnosis and identification of GBS in COVID-19 patients is important as COVID-19-related GBS might be associated with a severe disease course that frequently requires ICU admission and mechanical ventilation.
Disclosures
The authors declare no conflicts of interest.
Statement of Authorship
MA: contributed with the conception and design of the study, acquisition, analysis, and interpretation of data, drafting, revising, and final approval of the article.
ME: contributed with the conception and design of the study, acquisition, analysis and interpretation of data, drafting, revising, and final approval of the article.
BA: contributed with acquisition and extraction of data and drafting the article.
DA: contributed with extraction of data and final approval of the article.
AA: contributed with extraction of data and final approval of the article.
AB: contributed with extraction of data and final approval of the article.
EP: contributed with conception and design of the study, drafting, revising and final approval of the article.
References
- 1. Mao L, Wang M, Chen S, et al. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. SSRN Electron J. 2020. doi: 10.2139/ssrn.3544840 [DOI] [Google Scholar]
- 2. Leonhard SE, Mandarakas MR, Gondim FAA, et al. Diagnosis and management of Guillain–Barré syndrome in ten steps. Nat Rev Neurol. 2019;15:671–83. doi: 10.1038/s41582-019-0250-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hiew FL, Ramlan R, Viswanathan S, Puvanarajah S. Guillain-Barré syndrome, variants & forms fruste: reclassification with new criteria. Clin Neurol Neurosurg. 2017;158:114–18. doi: 10.1016/j.clineuro.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 4. Dimachkie MM, Barohn RJ. Guillain-Barré syndrome and variants. Neurol Clin. 2013;31:491–510. doi: 10.1016/j.ncl.2013.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nachamkin I, Allos BM, Ho T. Campylobacter species and Guillain-Barre syndrome. Clin Microbiol Rev. 1998;11:555–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meyer Sauteur PM, Huizinga R, Tio-Gillen AP, et al. Mycoplasma pneumoniae triggering the Guillain-Barré syndrome: a case-control study. Ann Neurol. 2016;80:566–80. [DOI] [PubMed] [Google Scholar]
- 7. Masajtis-Zagajewska A, Muras K, Mochecka-Thoelke A, Kurnatowska I, Nowicki M. Guillain-Barre syndrome in the course of EBV infection after kidney transplantation—a case report. Ann Transplant. 2012;17:133–37. [DOI] [PubMed] [Google Scholar]
- 8. Orlikowski D, Porcher R, Sivadon-Tardy V, et al. Guillain-barré syndrome following primary cytomegalovirus infection: a prospective cohort study. Clin Infect Dis. 2011;52:837–44. doi: 10.1093/cid/cir074 [DOI] [PubMed] [Google Scholar]
- 9. Counotte MJ, Meili KW, Taghavi K, Calvet G, Sejvar J, Low N. Zika virus infection as a cause of congenital brain abnormalities and Guillain-Barré syndrome: a living systematic review [version 1; peer review: 2 approved]. F1000Research. 2019;8. doi: 10.12688/f1000research.19918.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim JE, Heo JH, Kim HO, et al. Neurological complications during treatment of middle east respiratory syndrome. J Clin Neurol. 2017;13:227–33. doi: 10.3988/jcn.2017.13.3.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–84. doi: 10.1016/S1474-4422(20)30109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis of Guillain-Barré syndrome and validation of Brighton criteria. Brain. 2014;137:33–43. doi: 10.1093/brain/awt285 [DOI] [PubMed] [Google Scholar]
- 14. Diez-Porras L, Vergés E, Gil F, Vidal MJ, Massons J, Arboix A. Guillain-Barré-Strohl syndrome and COVID-19: case report and literature review. Neuromuscul Disord. 2020;30:859–861. doi: 10.1016/j.nmd.2020.08.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Granger A, Omari M, Jakubowska-Sadowska K, Boffa M, Zakin E. SARS-CoV-2–Associated Guillain–Barre syndrome with good response to plasmapheresis. J Clin Neuromuscular Dis. 2020;22:58–9. [DOI] [PubMed] [Google Scholar]
- 16. Hirayama T, Hongo Y, Kaida K, Kano O. Guillain-Barré syndrome after COVID-19 in Japan. BMJ Case Rep. 2020;13:1–4. doi: 10.1136/bcr-2020-239218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liberatore G, De Santis T, Doneddu PE, Gentile F, Albanese A, Nobile-Orazio E. Clinical reasoning: a case of COVID-19-associated pharyngeal-cervical-brachial variant of Guillain-Barré syndrome. Neurology. 2020;95:978–83. doi: 10.1212/WNL.0000000000010817 [DOI] [PubMed] [Google Scholar]
- 18. Nanda S, Handa R, Prasad A, et al. Covid-19 associated Guillain-Barre syndrome: contrasting tale of four patients from a tertiary care centre in India. Am J Emerg Med. 2021;39:125–28. doi: 10.1016/j.ajem.2020.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Atakla HG, Noudohounsi MMUD, Sacca H, Tassiou NRA, Noudohounsi WC, Houinato DS. Acute Guillain-Barré polyradiculoneuritis indicative of covid-19 infection: a case report. Pan Afr Med J. 2020;35:1–6. doi: 10.11604/pamj.supp.2020.35.150.25745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rajdev K, Victor N, Buckholtz ES, et al. A case of Guillain-Barré syndrome associated with COVID-19. J Investig Med High Impact Case Rep. 2020;8. doi: 10.1177/2324709620961198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Senel M, Abu-Rumeileh S, Michel D, et al. Miller-Fisher syndrome after COVID-19: neurochemical markers as an early sign of nervous system involvement. Eur J Neurol. 2020;27:2378–80. doi: 10.1111/ene.14473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tard C, Maurage CA, de Paula AM, et al. Anti-pan-neurofascin IgM in COVID-19-related Guillain-Barré syndrome: Evidence for a nodo-paranodopathy. Neurophysiol Clin. 2020;50:397–99. doi: 10.1016/j.neucli.2020.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan M, Han SC, Kelly S, Tamimi M, Giglio B, Lewis A. A case series of Guillain-Barré Syndrome following Covid-19 infection in New York. Neurol Clin Pract. Published online 2020. doi: 10.1212/cpj.0000000000000880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sedaghat Z, Karimi N. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19. The COVID-19 resource centre is hosted on Elsevier Connect, the company’s public news and information. 2020;(January). [Google Scholar]
- 25. Ebrahimzadeh SA, Ghoreishi A, Rahimian N. Guillain-Barré Syndrome associated with the coronavirus disease 2019 (COVID-19). Neurol Clin Pract. 2020;2019. doi: 10.1212/cpj.0000000000000879 [DOI] [PMC free article] [PubMed]
- 26. Arnaud S, Budowski C, Ng Wing Tin S, Degos B. Post SARS-CoV-2 Guillain-Barré syndrome. Clin Neurophysiol. 2020;131:1652–54. doi: 10.1016/j.clinph.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paybast S, Gorji R, Mavandadi S. Guillain-Barré syndrome as a neurological complication of novel COVID-19 infection: a case report and review of the literature. Neurologist. 2020;25:101–103. doi: 10.1097/NRL.0000000000000291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coen M, Jeanson G, Culebras Almeida LA, et al. Guillain-Barré syndrome as a complication of SARS-CoV-2 infection. Brain Behav Immun. 2020;87:111–12. doi: 10.1016/j.bbi.2020.04.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dinkin M, Gao V, Kahan J, et al. COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology. 2020;95:221–23. doi: 10.1212/WNL.0000000000009700 [DOI] [PubMed] [Google Scholar]
- 30. Manganotti P, Bellavita G, D’Acunto L, et al. Clinical neurophysiology and cerebrospinal liquor analysis to detect Guillain-Barré syndrome and polyneuritis cranialis in COVID-19 patients: a case series. J Med Virol. 2020:1–9. doi: 10.1002/jmv.26289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fernández-Domínguez J, Ameijide-Sanluis E, García-Cabo C, García-Rodríguez R, Mateos V. Miller–Fisher-like syndrome related to SARS-CoV-2 infection (COVID 19). J Neurol. 2020;267:2495–96. doi: 10.1007/s00415-020-09912-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hutchins KL, Jansen JH, Comer AD, et al. COVID-19-associated bifacial weakness with paresthesia subtype of Guillain-Barré syndrome. Am J Neuroradiol. 2020;41:1707–11. doi: 10.3174/ajnr.A6654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kilinc D, van de Pasch S, Doets AY, Jacobs BC, van Vliet J, Garssen MPJ. Guillain–Barré syndrome after SARS-CoV-2 infection. Eur J Neurol. 2020;27:1757–58. doi: 10.1111/ene.14398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Naddaf E, Laughlin RS, Klein CJ, et al. Guillain-Barré syndrome in a patient with evidence of recent SARS-CoV-2 infection. Mayo Clin Proc. 2020;95:1799–801. doi: 10.1016/j.mayocp.2020.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abrams RMC, Kim BD, Markantone DM, et al. Severe rapidly progressive Guillain-Barré syndrome in the setting of acute COVID-19 disease. J Neurovirol. 2020;26:797–99. doi: 10.1007/s13365-020-00884-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gigli GL, Vogrig A, Nilo A, et al. HLA and immunological features of SARS-CoV-2-induced Guillain-Barré syndrome. Neurol Sci. 2020;41:3391–94. doi: 10.1007/s10072-020-04787-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bracaglia M, Naldi I, Govoni A, Brillanti Ventura D, De Massis P. Acute inflammatory demyelinating polyneuritis in association with an asymptomatic infection by SARS-CoV-2. J Neurol. 2020;267:3166–168. doi: 10.1007/s00415-020-10014-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sidig A, Abbasher K, Digna MF, et al. COVID-19 and Guillain-Barre Syndrome – a case report. Res Sq Prepr. Published online 2020:1–8. [Google Scholar]
- 39. Lascano AM, Epiney JB, Coen M, et al. SARS-CoV-2 and Guillain–Barré syndrome: AIDP variant with a favourable outcome. Eur J Neurol. 2020;27:1751–53. doi: 10.1111/ene.14368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Camdessanche JP, Morel J, Pozzetto B, Paul S, Tholance Y, Botelho-Nevers E. COVID-19 may induce Guillain–Barré syndrome. Rev Neurol (Paris). 2020;176:516–18. doi: 10.1016/j.neurol.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Abolmaali M, Heidari M, Zeinali M, et al. Guillain–Barré syndrome as a parainfectious manifestation of SARS-CoV-2 infection: a case series. J Clin Neurosci. 2021;83:119–22. doi: 10.1016/j.jocn.2020.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chan JL, Ebadi H, Sarna JR. Guillain-Barré syndrome with facial Diplegia related to SARS-CoV-2 infection. Can J Neurol Sci/J Can des Sci Neurol. Published online 2020:1–3. doi: 10.1017/cjn.2020.106 [DOI] [PMC free article] [PubMed]
- 43. Sancho-Saldaña A, Lambea-Gil Á, Capablo Liesa JL, et al. Guillain-Barré syndrome associated with leptomeningeal enhancement following SARS-CoV-2 infection. Clin Med J R Coll Physicians London. 2020;20:E93–94. doi: 10.7861/CLINMED.2020-0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Assini A, Benedetti L, Di Maio S, Schirinzi E, Del Sette M. Correction to: New clinical manifestation of COVID-19 related Guillain-Barrè syndrome highly responsive to intravenous immunoglobulins: two Italian cases (Neurological Sciences, (2020), 41, 7, (1657–1658), 10.1007/s10072-020-04484-5). Neurol Sci. 2020;41:2307. doi: 10.1007/s10072-020-04517-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Frank CHM, Almeida TVR, Marques EA, et al. Guillain–Barré syndrome associated with SARS-CoV-2 infection in a pediatric patient. J Trop Pediatr. Published online 2020:1–6. doi: 10.1093/tropej/fmaa044 [DOI] [PMC free article] [PubMed]
- 46. Juliao Caamaño DS, Alonso Beato R. Facial diplegia, a possible atypical variant of Guillain-Barré syndrome as a rare neurological complication of SARS-CoV-2. J Clin Neurosci. 2020;77:230–32. doi: 10.1016/j.jocn.2020.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oguz-Akarsu E, Ozpar R, Mirzayev H, et al. Guillain-Barré syndrome in a patient with minimal symptoms of COVID-19 infection. Muscle and Nerve. 2020;2:54–57. doi: 10.1002/mus.26992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Toscano G, Palmerini F, Ravaglia S, et al. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574–76. doi: 10.1056/NEJMc2009191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reyes-Bueno JA, García-Trujillo L, Urbaneja P, et al. Miller-Fisher syndrome after SARS-CoV-2 infection. Eur J Neurol. 2020;27:1759–61. doi: 10.1111/ene.14383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bigaut K, Mallaret M, Baloglu S, et al. Guillain-Barré syndrome related to SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflammation. 2020;7:4–6. doi: 10.1212/NXI.0000000000000785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Padroni M, Mastrangelo V, Asioli GM, et al. Guillain-Barré syndrome following COVID-19: new infection, old complication? J Neurol. 2020;267:1877–79. doi: 10.1007/s00415-020-09849-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tiet MY, Alshaikh N. Guillain-Barré syndrome associated with COVID-19 infection: a case from the UK. BMJ Case Rep. 2020;13:1–4. doi: 10.1136/bcr-2020-236536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ameer N, Shekhda KM, Cheesman A. Guillain-Barré syndrome presenting with COVID-19 infection. BMJ Case Rep. 2020;13:3–5. doi: 10.1136/bcr-2020-236978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wada S, Nagasaki Y, Arimizu Y, et al. Neurological disorders Identified during treatment of a SARS-CoV-2 infection. Intern Med. 2020;59:2187–89. doi: 10.2169/internalmedicine.5447-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ray A. Miller Fisher syndrome and COVID-19: Is there a link. BMJ Case Rep. 2020;13:19–22. doi: 10.1136/bcr-2020-236419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guijarro-Castro C, Rosón-González M, Abreu A, García-Arratibel A, Ochoa-Mulas M. Guillain-Barré syndrome associated with SARS-CoV-2 infection. Comments after 16 published cases. Neurologia. 2020;35:412–15. doi: 10.1016/j.nrl.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95:e601–605. doi: 10.1212/WNL.0000000000009619 [DOI] [PubMed] [Google Scholar]
- 58. Agosti E, Giorgianni A, D’Amore F, Vinacci G, Balbi S, Locatelli D. Is Guillain-Barrè syndrome triggered by SARS-CoV-2? Case report and literature review. Neurol Sci. Published online 2020. doi: 10.1007/s10072-020-04553-9 [DOI] [PMC free article] [PubMed]
- 59. Khalifa M, Zakaria F, Ragab Y, et al. Guillain-Barré syndrome associated with severe acute respiratory syndrome coronavirus 2 detection and coronavirus disease 2019 in a child. J Pediatric Infect Dis Soc. 2020;9:510–13. doi: 10.1093/jpids/piaa086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Farzi MA, Ayromlou H, Jahanbakhsh N, Bavil PH, Janzadeh A, Shayan FK. Guillain-Barré syndrome in a patient infected with SARS-CoV-2, a case report. J Neuroimmunol. 2020;346:577294. doi: 10.1016/j.jneuroim.2020.577294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Alberti P, Beretta S, Piatti M, et al. Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol NeuroInflammation. 2020;7:1–3. doi: 10.1212/NXI.0000000000000741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rana S, Lima AA, Chandra R, et al. Novel coronavirus (Covid-19)-associated Guillain-Barré syndrome: Case report. J Clin Neuromuscul Dis. 2020;21:240–42. doi: 10.1097/cnd.0000000000000309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Korem S, Gandhi H, Dayag DB. Guillain-Barré syndrome associated with COVID-19 disease. BMJ Case Rep. 2020;13. doi: 10.1136/bcr-2020-237215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Civardi C, Collini A, Geda DJ, Geda C. Antiganglioside antibodies in Guillain-Barré syndrome associated with SARS-CoV-2 infection. J Neurol Neurosurg Psychiatry. 2020;91:1361–62. doi: 10.1136/jnnp-2020-324279 [DOI] [PubMed] [Google Scholar]
- 65. Virani A, Rabold E, Hanson T, et al. Guillain-Barré Syndrome associated with SARS-CoV-2 infection. IDCases. 2020;20:e00771. doi: 10.1016/j.idcr.2020.e00771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Khaja M, Roa Gomez GP, Santana Y, et al. A 44-year-old hispanic man with loss of taste and bilateral facial weakness diagnosed with Guillain-Barré syndrome and Bell’s Palsy associated with SARS-CoV-2 infection treated with intravenous immunoglobulin. Am J Case Rep. 2020;21:1–6. doi: 10.12659/AJCR.927956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lampe A, Winschel A, Lang C, Steiner T. Guillain-Barré syndrome and SARS-CoV-2. Neurol Res Pract 2020;2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ottaviani D, Boso F, Tranquillini E, et al. Early Guillain-Barré syndrome in coronavirus disease 2019 (COVID-19): a case report from an Italian COVID-hospital. Neurol Sci. 2020;41:1351–54. doi: 10.1007/s10072-020-04449-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Scheidl E, Canseco DD, Hadji-Naumov A, Bereznai B. Guillain-Barré syndrome during SARS-CoV-2 pandemic: a case report and review of recent literature. J Peripher Nerv Syst. 2020;25(2):204–207. doi: 10.1111/jns.12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. El Otmani H, El Moutawakil B, Rafai MA, et al. Covid-19 and Guillain-Barré syndrome: more than a coincidence! Rev Neurol (Paris). 2020;176:518–19. doi: 10.1016/j.neurol.2020.04.007 [DOI] [PMC free article] [PubMed]
- 71. Lantos JE, Strauss SB, Lin E. COVID-19–associated Miller Fisher syndrome: MRI findings. Am J Neuroradiol. 2020;41:1184–86. doi: 10.3174/ajnr.A6609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Riva N, Russo T, Falzone YM, et al. Post-infectious Guillain–Barré syndrome related to SARS-CoV-2 infection: a case report. J Neurol. 2020;267:2492–94. doi: 10.1007/s00415-020-09907-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Helbok R, Beer R, Löscher W, et al. Guillain-Barré syndrome in a patient with antibodies against SARS-COV-2. Eur J Neurol. 2020;27:1754–56. doi: 10.1111/ene.14388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Webb S, Wallace VCJ, Martin-Lopez D, Yogarajah M. Guillain-Barré syndrome following COVID-19: a newly emerging post-infectious complication. BMJ Case Rep. 2020;13:1–4. doi: 10.1136/bcr-2020-236182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pfefferkorn T, Dabitz R, von Wernitz-Keibel T, Aufenanger J, Nowak-Machen M, Janssen H. Acute polyradiculoneuritis with locked-in syndrome in a patient with Covid-19. J Neurol. 2020;267:1883–84. doi: 10.1007/s00415-020-09897-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dufour C, Co T-K, Liu A. GM1 ganglioside antibody and COVID-19 related Guillain Barre Syndrome – a case report, systemic review and implication for vaccine development. Brain, Behav Immun – Health. 2021;12:100203. doi: 10.1016/j.bbih.2021.100203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jones R, Kolbe H, Barton A. Post Covid-19 Guillain-Barre syndrome: case report. Morecambe Bay Med J. 2020;8:229–31. [Google Scholar]
- 78. Ghosh R, Roy D, Sengupta S, Benito-León J. Autonomic dysfunction heralding acute motor axonal neuropathy in COVID-19. J Neurovirol. 2020;26:964–66. doi: 10.1007/s13365-020-00908-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mackenzie N, Lopez-Coronel E, Alberto Dau C, et al. A concomitant Guillain-Barre syndrome with COVID-19: a first case-report in Colombia. 1–9. 10.21203/rs.3.rs-61279/v1 [DOI] [PMC free article] [PubMed]
- 80. Charra B. Guillain Barre syndrome & Covid-19: a case report. Ann Clin Med Case Rep. Published online 2021:V5.
- 81. Petrelli C, Scendoni R, Paglioriti M, Logullo FO. Acute motor axonal neuropathy related to COVID-19 infection: a new diagnostic overview. J Clin Neuromuscul Dis. 2020;22:120–21. doi: 10.1097/cnd.0000000000000322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yaqoob H, Jain A, Epelbaum O. A unique case of Guillain-Barré syndrome related to COVID-19 infection. Chest. 2020;158:A771. doi: 10.1016/j.chest.2020.08.718 [DOI] [Google Scholar]
- 83. Bueso T, Montalvan V, Lee J, et al. Guillain-Barre syndrome and COVID-19: a case report. Clin Neurol Neurosurg. 2021;200:2020–22. doi: 10.1016/j.clineuro.2020.106413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Manji HK, George U, Mkopi NP, Manji KP. Guillain-Barré syndrome associated with COVID-19 infection. Pan Afr Med J. 2020;35:118. doi: 10.11604/pamj.supp.2020.35.2.25003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Su XW, Palka S V., Rao RR, Chen FS, Brackney CR, Cambi F. SARS-CoV-2–associated Guillain-Barré syndrome with dysautonomia. Muscle and Nerve. 2020;62:E48–49. doi: 10.1002/mus.26988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Velayos Galán A, del Saz Saucedo P, Peinado Postigo F, Botia Paniagua E. Guillain-Barré syndrome associated with SARS-CoV-2 infection. Neurologia. 2020;35:268–69. doi: 10.1016/j.nrl.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Barrachina-Esteve O, Palau Domínguez A, Hidalgo-Torrico I, Viguera Martínez ML. Síndrome de Guillain-Barré como forma de presentación de la infección por SARS-CoV-2. Neurología. 2020;35:710–12. doi: 10.1016/j.nrl.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Marta-Enguita J, Rubio-Baines I, Gastón-Zubimendi I. Síndrome de Guillain-Barré fatal tras infección por el virus SARS-CoV-2. Neurología. 2020;35:265–67. doi: 10.1016/j.nrl.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gigli GL, Bax F, Marini A, et al. Guillain-Barré syndrome in the COVID-19 era: just an occasional cluster? J Neurol. 2020;0123456789:1–3. doi: 10.1007/s00415-020-09911-3 [DOI] [PMC free article] [PubMed]
- 90. Manganotti P, Pesavento V, Buoite Stella A, et al. Miller Fisher syndrome diagnosis and treatment in a patient with SARS-CoV-2. J Neurovirol. 2020;26:605–606. doi: 10.1007/s13365-020-00858-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gale A, Sabaretnam S, Lewinsohn A. Guillain-Barré syndrome and COVID-19: association or coincidence. BMJ Case Rep. 2020;13:10–14. doi: 10.1136/bcr-2020-239241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. García-Manzanedo S, López de la Oliva Calvo L, Ruiz Álvarez L. Síndrome de Guillain-Barré tras infección por COVID-19. Med Clin (Barc). 2020;155:366. doi: 10.1016/j.medcli.2020.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Raahimi MM, Kane A, Moore CE, Alareed AW. Late onset of Guillain-Barré syndrome following SARS-CoV-2 infection: part of “long COVID-19 syndrome”? BMJ Case Rep. 2021;14:1–4. doi: 10.1136/bcr-2020-240178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bourque PR, Breiner A, Moher D, et al. Adult CSF total protein: higher upper reference limits should be considered worldwide. a web-based survey. J Neurol Sci. 2019;396:48–51. doi: 10.1016/j.jns.2018.10.033 [DOI] [PubMed] [Google Scholar]
- 95. Tan CY, Razali SNO, Goh KJ, Shahrizaila N. Diagnosis of Guillain-Barré syndrome and validation of the Brighton criteria in Malaysia. J Peripher Nerv Syst. 2020;25:256–64. doi: 10.1111/jns.12398 [DOI] [PubMed] [Google Scholar]
- 96. Shang P, Zhu M, Baker M, Feng J, Zhou C, Zhang H-L. Mechanical ventilation in Guillain-Barré syndrome. Expert Rev Clin Immunol. 2020;16:1053–64. doi: 10.1080/1744666X.2021.1840355 [DOI] [PubMed] [Google Scholar]
- 97. Leonhard SE, Bresani-Salvi CC, Lyra Batista JD. et al. Guillain-barré syndrome related to Zika virus infection: a systematic review and meta-analysis of the clinical and electrophysiological phenotype. PLoS Negl Trop Dis. 2020;14:1–24. doi: 10.1371/journal.pntd.0008264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rees JH, Hughes RAC. Campylobacter jejuni infection and Guillain-Barré syndrome. N Engl J Med. 1995;333:1374–9. [DOI] [PubMed] [Google Scholar]
- 99. Katirji B, Ruff RL, Kaminski HJ. Neuromuscular disorders in clinical practice. Vol. 9781461465; 2014. doi: 10.1007/978-1-4614-6567-6 [DOI]
- 100. Piccione EA, Salame K, Katirji B. Guillain-Barré syndrome and related disorders BT – neuromuscular disorders in clinical practice. In: Katirji B, Kaminski HJ, Ruff RL, editors. Neuromuscular Disorders in Clinical Practice. New York: Springer; 2014, pp. 573–603. doi: 10.1007/978-1-4614-6567-6_28 [DOI]
- 101. Eastin C, Eastin T. Clinical characteristics of Coronavirus Disease 2019 in China: Guan W, Ni Z, Hu Y, et al. N Engl J Med. 2020 Feb 28 [Online ahead of print] DOI: 10.1056/NEJMoa2002032. J Emerg Med. 2020;58:711–12. doi: 10.1016/j.jemermed.2020.04.004 [DOI] [Google Scholar]
- 102. Rees JH, Soudain SE, Gregson NA, Hughes RAC. Campylobacter jejuni infection and Guillain–Barré syndrome. N Engl J Med. 1995;333:1374–79. doi: 10.1056/NEJM199511233332102 [DOI] [PubMed] [Google Scholar]
- 103. Keddie S, Pakpoor J, Mousele C, et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barré syndrome. Brain. 2021;144:682–93. doi: 10.1093/brain/awaa433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Liu H, Ma Y. Hepatitis E virus-associated Guillain-Barre syndrome: revision of the literature. Brain Behav. 2020;10:e01496. doi: 10.1002/brb3.1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hardy TA, Blum S, McCombe PA, Reddel SW. Guillain-Barré syndrome: modern theories of etiology. Curr Allergy Asthma Rep. 2011;11:197–204. doi: 10.1007/s11882-011-0190-y [DOI] [PubMed] [Google Scholar]
- 106. Jahan I, Ahammad RU, Khalid MM, et al. Toll-like receptor-4 299Gly allele is associated with Guillain-Barré syndrome in Bangladesh. Ann Clin Transl Neurol. 2019;6:708–15. doi: 10.1002/acn3.744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Jaramillo-Valverde L, Levano KS, Villanueva I, et al. Guillain–Barre syndrome outbreak in Peru: association with polymorphisms in IL-17, ICAM1, and CD1. Mol Genet Genomic Med. 2019;7:e00960. doi: 10.1002/mgg3.960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Nyati KK, Prasad KN, Verma A, et al. Association of TLR4 Asp299Gly and Thr399Ile polymorphisms with Guillain-Barré syndrome in Northern Indian population. J Neuroimmunol. 2010;218:116–19. doi: 10.1016/j.jneuroim.2009.10.018 [DOI] [PubMed] [Google Scholar]
- 109. Willison HJ, O’Hanlon GM. The immunopathogenesis of Miller Fisher syndrome. J Neuroimmunol. 1999;100:3–12. doi: 10.1016/S0165-5728(99)00213-1 [DOI] [PubMed] [Google Scholar]
- 110. Nagashima T, Koga M, Odaka M, Hirata K, Yuki N. Continuous spectrum of pharyngeal-cervical-brachial variant of Guillain-Barré syndrome. Arch Neurol. 2007;64:1519–23. doi: 10.1001/archneur.64.10.1519 [DOI] [PubMed] [Google Scholar]
- 111. Willison HJ, Yuki N. Peripheral neuropathies and anti-glycolipid antibodies. Brain. 2002;125:2591–625. doi: 10.1093/brain/awf272 [DOI] [PubMed] [Google Scholar]
- 112. Soliven B. Animal models of autoimmune neuropathy. ILAR J. 2014;54:282–90. doi: 10.1093/ilar/ilt054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Stathopoulos P, Alexopoulos H, Dalakas MC. Autoimmune antigenic targets at the node of Ranvier in demyelinating disorders. Nat Rev Neurol. 2015;11:143–56. doi: 10.1038/nrneurol.2014.260 [DOI] [PubMed] [Google Scholar]
- 114. Rodríguez Y, Rojas M, Pacheco Y, et al. Guillain–Barré syndrome, transverse myelitis and infectious diseases. Cell Mol Immunol. 2018;15:547–62. doi: 10.1038/cmi.2017.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Safa A, Azimi T, Sayad A, Taheri M, Ghafouri-Fard S. A review of the role of genetic factors in Guillain–Barré syndrome. J Mol Neurosci. Published online 2020:1–19. [DOI] [PubMed]
- 116. Guo L, Wang W, Li C, Liu R, Wang G. The association between HLA typing and different subtypes of Guillain Barré syndrome [in Chinese]. Zhonghua Nei Ke Za Zhi. 2002;41:381–3. Available at http://europepmc.org/abstract/MED/12137599 [PubMed] [Google Scholar]
- 117. Hasan ZN, Zalzala HH, Mohammedsalih HR, et al. Association between human leukocyte antigen-DR and demylinating Guillain-Barre syndrome. Neurosciences (Riyadh). 2014;19:301–305. [PMC free article] [PubMed] [Google Scholar]
- 118. Schirmer L, Worthington V, Solloch U, et al. Higher frequencies of HLA DQB1*05:01 and anti-glycosphingolipid antibodies in a cluster of severe Guillain–Barré syndrome. J Neurol. 2016;263:2105–13. doi: 10.1007/s00415-016-8237-6 [DOI] [PubMed] [Google Scholar]
- 119. Hu T, Liu Y, Zhao M, Zhuang Q, Xu L, He Q. A comparison of COVID-19, SARS and MERS. PeerJ. 2020;8:e9725–25. doi: 10.7717/peerj.9725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Tsai L-K, Hsieh S-T, Chang Y-C. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol Taiwan. 2005;14:113–19. [PubMed] [Google Scholar]