Abstract
Coronavirus disease 2019 causes respiratory and systemic disease and has led to a sudden epidemic affecting people of all ages. Patients with congenital heart disease represent a high-risk population. In this article, we present a newborn who required extracorporeal membrane oxygenation support for acute respiratory failure in the early postoperative period due to exposure to severe acute respiratory syndrome coronavirus 2 after aortic arch repair and ventricular septal defect closure. To the best of our knowledge, this patient represents the first neonatal case of severe acute respiratory syndrome coronavirus 2 infection after congenital heart surgery and is the youngest patient to need extracorporeal membrane oxygenation support.
Keywords: SARS-CoV-2, neonate, congenital heart surgery, ECMO
Coronavirus disease 2019 – the illness caused by severe acute respiratory syndrome coronavirus 2 – was first announced in an adult in China on 17 November, 2019, and the World Health Organization classified the epidemic of severe acute respiratory syndrome coronavirus 2 as an emergency of international concern on 30 January, 2020.1,2
Babies with congenital heart disease continue to be born at the rate of 1 in 100 live births during the pandemic, and approximately 25% of these are considered to have critical congenital heart disease requiring surgery or other interventions in the first year of life.3 A history of cardiac surgery may be associated with the risk of hospitalisation in an intensive care unit (ICU), intubation and mechanical ventilation, particularly more severe form of the disease in newborns and children.4
In this report, we present the management of a severe acute respiratory syndrome coronavirus 2 infection in newborns after successful repair of the aortic arch and ventricular septal defect closure surgery. We performed this operation approximately 6 months after the epidemic was declared. Although extracorporeal membrane oxygenation support was applied twice due to respiratory failure, the patient died due to multiorgan failure. To our knowledge, this patient was the first newborn to receive extracorporeal membrane oxygenation support for severe acute respiratory syndrome coronavirus 2 infection after congenital heart surgery. Consent was obtained from the family for the publication of this study.
Case report
A full-term 16-day-old newborn boy weighing 3.5 kg was admitted to our department with an absence of femoral pulses and suspected coarctation of the aorta. Echocardiography revealed aortic arch hypoplasia with important aortic coarctation (35–40 mmHg gradient with diastolic extension), the diameter of the proximal transverse arch was 4,9 m (z score −3,27), the diameter of the distal transverse arch was 4 mm (z score −2,62), and the diameter of the isthmus was 2,8 mm (z score −4,29). She also had a restrictive muscular outlet ventricular septal defect with no evidence of left ventricular volume overload, a bicuspid aortic valve with mild stenosis, left aortic arch, and systemic pulmonary hypertension. There were no abnormalities in the patient’s preoperative laboratory values, and she did not have a fever or cough preoperatively.
The patient underwent surgery with a median sternotomy, and cardiopulmonary bypass was performed. During arch repair, selective antegrade cerebral perfusion was initiated, and del Nido cardioplegia was given. The aortic arch incision was started from the descending aorta and extended to the midportion of the ascending aorta, and the aortic arch was reconstructed with the anterior patch augmentation technique using an autologous pericardial patch. The ventricular septal defect was closed with the pericardial patch via right atriotomy. After the cessation of cardiopulmonary bypass, modified ultrafiltration was performed, and the skin was closed with a patch, leaving the sternum open. The patient was transferred to the ICU with moderate doses of inotropic support and was in stable haemodynamic condition. The cardiopulmonary bypass, aortic cross-clamping, and antegrade cerebral perfusion times were 152 min, 75 min, and 35 min, respectively. In the control echocardiography examination, no significant gradient was observed on the aortic arch, and no residual ventricular septal defect was detected. Peritoneal dialysis was started postoperatively. The sternum was closed uneventfully on postoperative day 2. Laboratory test results included a normal white blood cell count (6.2 kU/ul, ref range ≤ 4 kU/ul) with elevated polymorphonuclear neutrophils of 77,9% and a low lymphocyte count of 750 cells/mm3 (ref range ≤ 800 cells/mm3) on postoperative day 2. The lymphocyte count (570 cells/mm3) was lower than that on the previous day, and the C-reactive protein level (7.7 mg/dL, ref interval ≤ 0.5 mg/dL) was higher on postoperative day 3.
Acute respiratory deterioration occurred with refractory hypoxemia and worsening hypercapnia despite lung protective ventilation and high positive end-expiratory pressure therapy on postoperative day 3 (Fig 1). Extracorporeal membrane oxygenation support was used because of acute respiratory dysfunction on postoperative day 4. Venoarterial extracorporeal membrane oxygenation support was initiated via a 14 Fr venous cannula inserted into the right atrium and an 8 Fr arterial cannula inserted into the ascending aorta. The support was started at a flow rate of 100 ml/kg/min (80–140 ml/kg/min), and the arterial waveform demonstrated normal pulsatility. The white blood cell count decreased to 4 kU/ul with an elevated polymorphonuclear neutrophil percentage of 72% and a low lymphocyte count of 690 cells/mm3. The patient’s reverse transcription-polymerase chain reaction test for severe acute respiratory syndrome coronavirus 2 was positive, with the sample taken from the endotracheal tube on postoperative day 4. Hydroxychloroquine and favipiravir therapy was started immediately and given for 7 days. A heparin infusion was started, and the activated clotting time was maintained in the target range of 180–220. We did not see major complications such as thrombosis or bleeding during the extracorporeal membrane oxygenation support. The initial echocardiogram on extracorporeal membrane oxygenation showed normal left ventricle systolic function. The patient was weaned from extracorporeal membrane oxygenation after 6 days of support. Because oxygen saturation decreased and respiratory functions deteriorated 7 days after weaning from extracorporeal membrane oxygenation, the support was applied again. After 18 days of care, including hydroxychloroquine and favipiravir therapy, the reverse transcription-polymerase chain reaction test for severe acute respiratory syndrome coronavirus 2 was negative; however, there was no significant improvement in the patient’s clinical condition. During this period, the patient’s sternum being open also made the patient vulnerable to infections in addition to coronavirus disease 2019. Candida parapsilosis mediastinitis occurred, and Sterotrophomonas maltophilia was found to have reproduced in blood culture obtained during the second extracorporeal membrane oxygenation support. Unfortunately, the patient was unable to survive and died on extracorporeal membrane oxygenation due to sepsis and multiorgan failure despite extended antibiotic therapy on postoperative day 28.
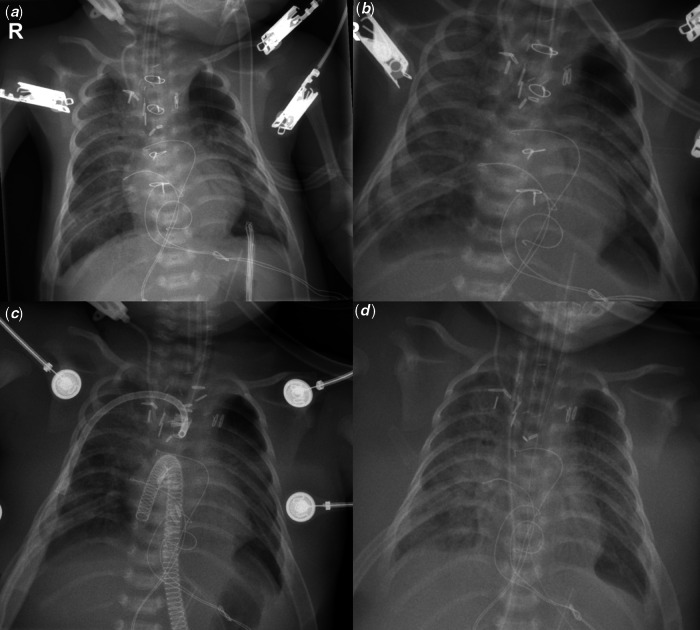
Figure 1.
Chest radiography during hospitalisation. (a) Chest radiography on postoperative day 2 after sternum closure. (b) Chest radiography on the postoperative day 3. (c) Chest radiography after central cannulation for Venoarterial (VA) extracorporeal membrane oxygenation. Venous cannula in the right atrium and arterial cannula in the ascending aorta. (d) Chest radiography after weaning from the first extracorporeal membrane oxygenation support.
Discussion
It is necessary to understand that although the majority of children are not seriously affected, there may be a subset of group children for whom coronavirus disease 2019 can progress rapidly, leading to respiratory function deterioration.5 Our patient is the youngest patient ever reported with a progression of severe acute respiratory syndrome coronavirus 2 to severe disease requiring extracorporeal membrane oxygenation support after cardiac surgery. The respiratory deterioration in this case is important for understanding severe acute respiratory syndrome coronavirus 2 disease in newborns undergoing cardiac surgery. Our patient – who developed a rapid respiratory disorder in the early postoperative period – required extracorporeal membrane oxygenation support within the next 24 hours despite lung protective ventilation and high positive end-expiratory pressure therapy.
Current evidence shows that excessive inflammation, oxidation and an extreme immune reaction play significant roles in the pathogenesis of coronavirus disease 2019.6 Direct myocardial damage via angiotensin-converting enzyme 2, viral pneumonia, acute respiratory distress syndrome, acute lung injury, hypoxia-induced myocardial damage, cardiopulmonary bypass -related systemic inflammatory response syndrome and pulmonary hypertension related to congenital heart disease may contribute to the inflammation course.6 Moreover, these events cause a cytokine storm and result in acute respiratory distress syndrome.6 The immunity of the patient has a major influence on coronavirus disease 2019 severity, and those with low immune function, such as neonates, may be more susceptible, especially after cardiopulmonary bypass.6
According to the Extracorporeal Life Support Organization registry, as of 5 December, 2020, extracorporeal membrane oxygenation support has been used in 3543 confirmed severe acute respiratory syndrome coronavirus 2 patients, and the in-hospital mortality is 45%.7 Thrombosis and bleeding complications are well-known risks of extracorporeal membrane oxygenation support. Some studies have indicated a 70% incidence of bleeding and a 37% incidence of thrombosis complications in children supported by extracorporeal membrane oxygenation.8 However, the experience with extracorporeal membrane oxygenation and the corresponding literature are limited with regard to severe acute respiratory syndrome coronavirus 2.9 Kaushik et al reported a 5-year-old male patient who underwent extracorporeal membrane oxygenation support with carotid artery cannulation due to severe acute respiratory syndrome coronavirus 2 multisystem inflammatory syndrome.9 Unfortunately, right anterior and middle cerebral artery infarction occurred on extracorporeal membrane oxygenation day 6, and finally, the patient succumbed to the disease. The cause of stroke in the present case may be multifactorial and could include severe acute respiratory syndrome coronavirus 2-associated thromboembolic complications or the carotid artery cannulation strategy. A single centre reported a 5% incidence of acute ischaemic stroke in 221 patients with severe acute respiratory syndrome coronavirus 2, and in addition, the incidence of extracorporeal membrane oxygenation -associated stroke in children is known to be between 5.6 and 7.8%.10,11
In our case, the acute respiratory distress syndrome findings were first observed on the 3rd postoperative day, and the respiratory functions of the patient deteriorated rapidly. Given that the thromboembolic complications associated with severe acute respiratory syndrome coronavirus 2 could increase, venoarterial extracorporeal membrane oxygenation support was applied with central cannulation. Perhaps for this reason, we did not observe thromboembolic neurological complications, which may produce symptoms such as anisocoria or a decrease in near infrared spectroscopy (NIRS) values over a total of 17 days of extracorporeal membrane oxygenation support.
Cardiopulmonary bypass circuit complications during cardiac surgery are another critical issue in coronavirus disease 2019. A multicentre study showed that the rate of coagulation complications (4.1% versus 0.2%) and oxygenator gas exchange complications (2% versus 0.2%) during adult cardiac surgery were higher in patients who tested positive for coronavirus disease 2019 than negative ones.12
Extracorporeal membrane oxygenation support in acute respiratory distress syndrome due to coronavirus disease 2019 is associated with high mortality. Henry et al reviewed 234 coronavirus disease 2019-related acute respiratory distress syndrome patients in China, 17 of whom (7.25%) received extracorporeal membrane oxygenation, with a mortality rate of 94.1% versus 70.9% in conventional patients.13 In our opinion, in addition to having undergone cardiac surgery in the neonatal period, the high mortality rate of extracorporeal membrane oxygenation in acute respiratory distress syndrome due to coronavirus disease 2019 negatively affected survival in our patient.
On the other hand, several multicentre studies have demonstrated that extracorporeal membrane oxygenation can play a helpful role in rescuing selected seriously ill adult patients with coronavirus disease 2019.14,15 Jacobs et al conducted a prospective study on 100 adult patients with coronavirus disease 2019 who needed extracorporeal membrane oxygenation support. Of these, 50 patients weaned from extracorporeal membrane oxygenation and 49 were discharged from hospital. Although the rate of survival with veno-arterial extracorporeal membrane oxygenation was 25% (1 of 4 patients), that with veno-venous was 51% (49 of 96 patients). These studies show that extracorporeal membrane oxygenation may facilitate salvage, and provides a sensible rescue strategy.
Conclusion
Because little is known about the clinical course and progression of severe acute respiratory syndrome coronavirus 2 infection in newborns after cardiac surgery, the duration of extracorporeal membrane oxygenation support, cannulation and anticoagulation strategy, and the recovery timetable for guiding treatment are unclear. Our case emphasises the clinical course of this disease leading to mortality, as well as the difficulties in managing acute respiratory distress syndrome and extracorporeal membrane oxygenation in the context of severe acute respiratory syndrome coronavirus 2 syndrome, especially in newborns who undergo cardiac surgery.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees.
References
- 1.The first COVID-19 case originated on November 17, according to Chinese officials searching for ‘Patient Zero’, 2020, from www. msn.com
- 2. World Health Organization: WHO, from https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 3. Dolk H, Loane M, Garne E. Congenital heart defects in Europe: prevalence and perinatal mortality, 2000 to 2005. Circulation 2011; 123: 841–849. [DOI] [PubMed] [Google Scholar]
- 4. Sanna G, Serrau G, Bassareo PP, Neroni P, Fanos V, Marcialis MA. Children’s heart and COVID-19: up-to-date evidence in the form of a systematic review. Eur J Pediatr 2020; 1–9. doi: 10.1007/s00431-020-03699-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qiu H, Wu J, Hong L, et al. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis 2020; 20: 689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dilli D, Tasoglu I. Perioperative care of the newborns with congenital heart diseases in the time of COVID-19. Cardiol Young 2020; 30: 946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Extracorporeal Life Support Organization (ELSO) Registry, from https://www.elso.org/Registry/Statistics/ InternationalSummary.aspx (accessed 5 December 2020)
- 8. Dalton HJ, Garcia-Filion P, Holubkov R, et al. Association of bleeding and thrombosis with outcome in extracorporeal life support. Pediatr Crit Care Med 2015; 16: 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaushik S, Ahluwalia N, Gangadharan S, et al. ECMO support in SARS-CoV2 multisystem inflammatory syndrome in children in a child. Perfusion 2021; 36: 524–528. [DOI] [PubMed] [Google Scholar]
- 10. AHA/ASA Stroke Council Leadership. Temporary emergency guidance to US stroke centers during the coronavirus disease 2019 (COVID-19) pandemic: on behalf of the American Heart Association/American Stroke Association Stroke Council Leadership. Stroke 2020; 51: 1910–1912. [DOI] [PubMed]
- 11. Werho DK, Pasquali SK, Yu S, et al. Epidemiology of stroke in pediatric cardiac surgical patients supported with extracorporeal membrane oxygenation. Ann Thorac Surg 2015; 100: 1751–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stammers AH, Mongero LB, Tesdahl EA, et al. The assessment of patients undergoing cardiac surgery for Covid-19: Complications occuring during cardiopulmonary bypass. Perfusion 2021; 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Henry BM, Lippi G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to cor- onavirus disease 2019 (COVID-19): pooled analysis of early reports. J Crit Care 2020; 58: 27–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jacobs JP, Stammers AH, St Louis J, et al. Extracorporeal membrane oxygenation in the treatment of severe pulmonary and cardiac compromise in coronavirus disease 2019: Experience with 32 patients. ASAIO J 2020; 66: 722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jacobs JP, Stammers AH, St Louis J, et al. Multi-institutional analysis of 100 consecutive patients with COVID-19 and severe pulmonary compromise treated with extracorporeal membrane oxygenation: outcomes and trends over time. ASAIO J 2021; 67: 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]