Acute hepatic dysfunction is categorized as acute liver injury (ALI) i.e. presence of coagulopathy, with no encephalopathy and acute liver failure (ALF) i.e., presence of encephalopathy and coagulopathy.1 Higher degree of coagulopathy is loosely termed as severe ALI; however, this is not well defined. Patients with severe ALI or ALF need early recognition as seriously ill patients, requiring urgent specialized care.
The causes of ALF are strikingly different across the world, with major differences in the East and the West. Although viral hepatitis (mainly hepatitis E) is a common cause of ALF in northern India, paracetamol overdose is the commonest cause in the Western world.2 More recently, hepatotoxicity due to yellow phosphorus poison (YPP) is being increasingly recognized as a cause of ALF in some parts of the Indian subcontinent largely from southern3, 4, 5, 6 and western parts7,8 of the country.
A recent statewide multicenter survey across 6 districts in Tamil Nadu (TN), between January and June 2019, on causes of toxic hepatitis (under the aegis of the Tamil Nadu Chapter of Indian Society of Gastroenterology), found the ratio of rodenticide (yellow phosphorus) ingestion to paracetamol overdose to be 75: 1.4 Of the 450 YPP-induced hepatotoxicity, 131 patients (31%) died and 28 patients (6%) were discharged in a moribund state. Majority of the patients were managed in hospitals where high end modalities such as plasma exchange (PLEX) or liver transplantation (LT) options were not available. Extrapolating the study results to all 35 districts of TN state, the study investigators estimated that during the six-month study period the state had a case burden of 1584 rodenticide induced hepatotoxicity patients (95% CI: 265–6119) and among these, it was projected that 554 patients were likely to have a poor outcome. Clearly, in the statewide study, YPP-induced hepatotoxicity can be considered the equivalent to paracetamol overdose causing toxic liver necrosis and death in the West.
Factors that determine YPP-induced hepatotoxicity and mortality include
-
a.
Concentrations of metal phosphides such as aluminum phosphide, zinc phosphide, and warfarin-like derivatives that compound the multisystem injury, mortality being highest in those pastes containing high concentrations of aluminum phosphide.9,10
-
b.
High levels of plasma von Willebrand factor (vWF)11: There is impaired VWF clearance in hepatic sinusoids.12 The high levels are shown to correlate with disease severity and also serve as predictors for poor outcomes. In 24 consecutive patients with YPP-induced hepatotoxicity (ALI: 20 patients, ALF: 3 patients, uncomplicated acute hepatitis: 1 patient), the area under the receiver operating curve of plasma vWF level to predict survival was 0.92 and of Model for End-Stage Liver Disease (MELD) score was 0.66 indicating vWF levels to be a better indicator than MELD score in predicting survival. Similar observations on vWF levels have now been replicated in other causes of ALF.13
In the aforementioned context, an article in this issue by Mohanka et al7 discussing their experience on managing patients with YPP is timely. LT Society of India has also recently published guidelines on management of patients with YPP including indications for LT.14 Mohanka et al7 analyzed outcome in 19 YPP – patients with ALF managed in a center with a dedicated liver intensive care unit (ICU) and facilities for LT. Extrapolating this information to centers with massive case load as those reported from southern states of India, very few patients have an access for an urgent LT. For example, only one patient of 450 YPP-induced hepatotoxicity patients i.e. 0.2% underwent urgent LT in the multicenter study.4 There are other similar reports from India wherein very few patients underwent supra urgent LT for YPP, even in those centers which had a provision for LT.3,7,11
Management options for YPP induced hepatotoxicity can therefore be considered under 2 broad headings: Non-LT and LT options
-
I.
Non-LT options
Patients with YPP should be recognized as seriously ill with a need for admission to High Dependency Unit (HDU)/ICU, with focused care on reducing cerebral edema,15 and use of PLEX to circumvent the critical period i.e., intervention before the onset of severe ALI or ALF. PLEX can be provided to all patients in any hospital that has facilities for dialysis and blood banking. The American Society of Apheresis, 2019 guidelines, consider high volume PLEX as first line and as a sole treatment or as a supplement to other treatment modalities (category 1 indication) to treat ALF.16 Emerging evidence also suggests that standard volume PLEX17 and low volume PLEX18 are effective modality to treat ALF.
In a recent study, Varghese et al3 categorized their patients with YPP as those fulfilling Kings College Hospital (KCH) Criteria19 and those who did not and managed all their 43 patients with PLEX. Although all the patients in the latter group recovered, 4 of 20 in the former group had a LT, and 7 of 20 survived (without a LT) with PLEX; 9 patients died. In a study by Sharma et al,20 50 per cent of patients who opted out of LT, survived with PLEX and this served as a stand-alone treatment in these patients. In Mohanka's series,7 of the 19 patients, PLEX was included as a standard of care and served as a bridge to LT and not considered as a curative modality of treatment. The number of cases reported in their study is too few to reach any conclusion on efficacy of PLEX.
The efficacy of PLEX in improving survival, serving as a curative treatment and as a bridge to LT in patients who meet listing criteria for urgent LT (KCH criteria for ALF,19 Kochi criteria21 for YPP ALF) needs to be studied in large number of patients.
Other nontransplant options that deserve special consideration are treatment modalities to lower the raised vWF levels in liver diseases.11,12,22 Of the three easily available vWF lowering interventions, N-acetyl cysteine, ADAMTS13 supplementation by fresh frozen plasma infusions and PLEX, latter appears to be the most efficacious in patients with severe ALI/ALF.11
Preliminary reports suggest N-acetyl cysteine may also be beneficial in patients with YPP.6,23,24
Can avoiding/minimizing sedation in YPP-induced hepatotoxicity improve patient survival, while awaiting an urgent LT?1,7,25,26 This has been a subject of debate. With reference to this, one must recall that most of the sedative/hypnotic drugs are metabolized in the liver, and this function is affected in ALF, thereby enhancing the risk of respiratory depression. Caution therefore must be exerted in the use of sedatives in patients with compromised liver function.27 Ideal would be to use sedatives when absolutely indicated, in the smallest dose and for the shortest period.
Few highlights of the publication by Mohanka et al7 in this issue on nontransplant options deserve special mention. The authors had a protocol-driven management for specific complications in YPP-induced patients with ALF. These included mechanical ventilation for patients with high grades of hepatic encephalopathy, continuous veno-venous hemodiafiltration for those with renal failure, severe lactic acidosis (despite adequate resuscitation) and hyperammonemia; PLEX being reserved for systemic inflammatory response syndrome.
All this proactive non-LT lifesaving procedures hitherto mentioned are likely to help a significant proportion of patients without the need for a LT. This is possible and practical if HDU and ICU facilities are increased in hospitals managing patients with YPP-induced hepatotoxicity.
-
II.
Liver transplant as an option
Liver transplant is an obvious option in ALF, but one question remains unanswered. In which patient and when, in the course of YPP-induced hepatotoxicity, is an urgent listing for LT warranted? Although currently KCH criteria19 for patients with ALF are used for listing for LT, no patient in the original dataset from whom the KCH listing criteria was derived, had YPP-induced hepatotoxicity. Investigators from Kochi, India proposed listing criteria for urgent LT in patients with YPP-induced hepatotoxicity i.e. MELD ≥36 or a combination of international normalized ratio >6 and hepatic encephalopathy.21 In their series, all eight patients who fulfilled these listing criteria but did not undergo LT died. Sharma et al20 observed 50 per cent survival after PLEX, amongst a select group of patients who fulfilled the Kochi listing criteria for LT but opted out of LT due to economic constraints. In Mohanka's series, 5 of 15 patients underwent LT; however the criteria for listing for LT are not clear. PLEX in their series served as a bridge while listing for LT. Currently, there are no definite set guidelines for YPP-induced ALF especially in centers which have facilities for both non-LT and LT options.
In summary, we construe that in resource-constrained settings, patients with YPP-induced hepatotoxicity encounter markedly reduced access to urgent appropriate treatment owing to the double whammy effects of greatly increased need and scarce resources. Urgent multipronged steps are needed to tackle this. Primary prevention steps include enacting legislation to make rodenticide less easily accessible to public (this can be purchased over the counter at present) and setting up round-the-clock counseling services to help persons in mental distress.
The general principles of managing any poison should be applied immediately for YPP with an easily accessible dedicated facility within the same locality for monitored HDU/ICU care and for specialized treatment such as PLEX. There is a need for a dedicated trained team comprising of internal medicine, pediatrics, emergency medicine, and intensive care specialists for such cases.
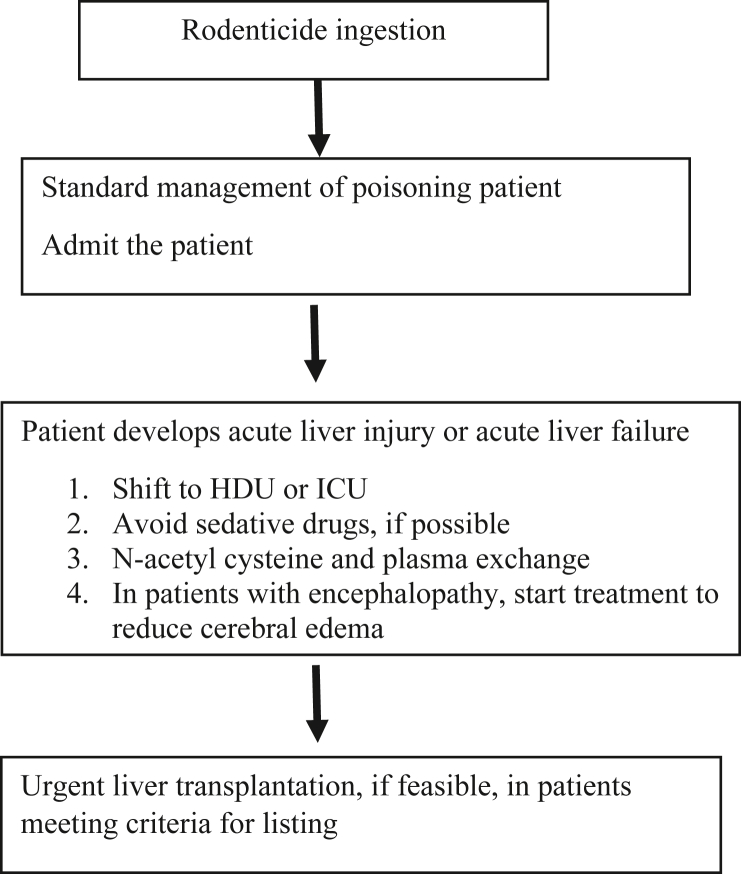
Figure 1 summarizes the approach to management of YPP-induced hepatotoxicity. Briefly, patients with YPP-induced ALI need close monitoring in HDU and those with ALF require ICU care. In patients with no realistic option of urgent LT, it appears prudent to try and avoid use of sedative/hypnotic drugs. Patients with worsening coagulopathy need to be started on nontransplant options that includes N-acetyl cysteine and PLEX. In patients with ALF i.e., with onset of encephalopathy and deteriorating clinical parameters, urgent LT is indicated.
Figure 1.
Algorithmic approach to management of patients with YPP.
One can hope that in coming years, ALF from all causes will be a ‘medically treatable disease, without the need for LT’.28
References
- 1.Anand A.C., Nandi B., Acharya S.K. Indian National Association for the study of the liver consensus statement on acute liver failure (Part 1): epidemiology, pathogenesis, presentation and prognosis. J Clin Exp Hepatol. 2020;10:339–376. doi: 10.1016/j.jceh.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acharya S.K., Batra Y., Hazari S., Choudhury V., Panda S.K., Dattagupta S. Etiopathogenesis of acute hepatic failure: Eastern versus Western countries. J Gastroenterol Hepatol. 2002;17(suppl 3):S268–S273. doi: 10.1046/j.1440-1746.17.s3.12.x. [DOI] [PubMed] [Google Scholar]
- 3.Varghese J., Joshi V., Bollipalli M.K. Role of therapeutic plasma exchange in acute liver failure due to yellow phosphorus poisoning. Indian J Gastroenterol. 2020;39:544–549. doi: 10.1007/s12664-020-01095-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Govindarajan R., Ramamoorthy G., Marimuthu R. Rodenticide ingestion is an important cause of acute hepatotoxicity in Tamil Nadu, Southern India. Indian J Gastroenterol. 2021 doi: 10.1007/s12664-021-01178-4. (in press) [DOI] [PubMed] [Google Scholar]
- 5.Suneetha D.K., Inbanathan J., Kannoth S., Reshma P.K., Shashank M.S. Profile of rat killer poisoning cases in a tertiary care hospital at Mysore. Int J Sci Stud. 2016;3:264–267. [Google Scholar]
- 6.Shashidhara K.C., Marijayanth M., Reddy P.K., Kaluvakuri S. Clinical profile and outcome of rodenticide poisoning in patients admitted to a tertiary care teaching hospital in Mysore, Karnataka, India. Int J Res Med Sci. 2016;4:5023–5027. [Google Scholar]
- 7.Mohanka R., Rao P., Shah M. Acute liver failure secondary to yellow phosphorus rodenticide poisoning: outcomes at a centre with dedicated liver intensive care and transplant unit. JCEH. 2021;11:425–435. doi: 10.1016/j.jceh.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patil Shilpa, Khatri Mohit, Avhad Aniket, Jaju Gaurav. Study of clinical profile and outcome of Ratol poisoning in tertiary care hospital. MedPulse Int J Med. 2019;9:200–204. [Google Scholar]
- 9.Nalabothu M., Monigari N., Acharya R. Clinical profile and outcomes of rodenticide poisoning in tertiary care hospital. IJSRP. 2015;5:1–12. [Google Scholar]
- 10.Mathews M., Sreedhar S., Valliyot B., Balakrishnan Rodenticidal; poisoning in a tertiary centre in North Kerala. Indian J Sci Res. 2016;5:464–466. [Google Scholar]
- 11.Sardar D., Mathews N., Mammen J. Rodenticidal hepatotoxicity: raised plasma Von Willebrand factor levels predict in-hospital survival and preliminary report of the outcome of Von Willebrand factor reducing management protocol. Indian J Gastroenterol. 2019;38:527–533. doi: 10.1007/s12664-019-00989-w. [DOI] [PubMed] [Google Scholar]
- 12.Goel A., Nair S.C., Balasubramanian K.A. Targeting raised von Willebrand factor levels in liver diseases: opening up newer therapeutic avenues. EMJ Hepatol. 2020;8:16–25. [Google Scholar]
- 13.Driever E.G., Stravitz R.T., Zhang J. vWF/ADAMTS13 imbalance, but not global coagulation or fibrinolysis, is associated with outcome and bleeding in acute liver failure. Hepatology. 2020 Aug 7 doi: 10.1002/hep.31507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy M.S., Rajakumar A., Mathew J.S. Liver transplantation society of India guidelines for the management of acute liver injury secondary to yellow phosphorus–containing rodenticide poisoning using the modified delphi technique of consensus development. JCEH. 2021;11:476–484. doi: 10.1016/j.jceh.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernal W., Hyyrylainen A., Gera A. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J Hepatol. 2013;59:74–80. doi: 10.1016/j.jhep.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Padmanabhan A., Connelly-Smith L., Aqui N. Guidelines on the use of therapeutic apheresis in clinical practice – evidence-based approach from the writing committee of the American Society for Apheresis: the eighth special issue. J Clin Apher. 2019;34:171–354. doi: 10.1002/jca.21705. [DOI] [PubMed] [Google Scholar]
- 17.Maiwall R., Bajpai M., Singh A. Standard-Volume plasma exchange improves outcomes in patients with acute liver failure: a Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2021 Jan 29 doi: 10.1016/j.cgh.2021.01.036. Epub ahead of print. PMID: 33524593. [DOI] [PubMed] [Google Scholar]
- 18.Zachariah U., Kumar S.E., Alexander V., Patel L., Goel A., Eapen C.E. Low-volume plasma exchange and low-dose steroid to treat severe liver injury. Gastroenterol Hepatol Endosc Pract. 2021;1:47–54. [Google Scholar]
- 19.O'Grady J.G., Alexander G.J., Hayllar K.M., Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439–445. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 20.Sharma A., Kumar S.E., Thomas L. Plasma exchange to treat yellow phosphorus induced liver injury. J Gastroenterol Hepatol. 2019;34:757. PE 0348. [Google Scholar]
- 21.Saraf V., Pande S., Gopalakrishnan U. Acute liver failure due to zinc phosphide containing rodenticide poisoning: clinical features and prognostic indicators of need for liver transplantation. Indian J Gastroenterol. 2015;34:325–329. doi: 10.1007/s12664-015-0583-2. [DOI] [PubMed] [Google Scholar]
- 22.Groeneveld D., Poole L.G., Luyendyk J.P. Targeting von Willebrand factor in liver diseases: a novel therapeutic strategy? J Thromb Haemost. 2021 Mar 28 doi: 10.1111/jth.15312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra A.K., Devakiruba N.S., Jasmine S., Sathyendra S., Anand Zachariah A., Iyadurai R. Clinical spectrum of yellow phosphorous poisoning in a tertiary care centre in South India: a case series. Trop Doct. 2017;47:245–249. doi: 10.1177/0049475516668986. [DOI] [PubMed] [Google Scholar]
- 24.Saravanan S., Karthik B. Efficacy of early N-acetylcysteine in rat killer paste poisoning. Int J Sci Stud. 2019;6:73–75. [Google Scholar]
- 25.Wendon J., Cordoba J., Dhawan A. EASL Clinical Practice Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047–1081. doi: 10.1016/j.jhep.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Anand A.C., Nandi B., Acharya S.K. INASL task-force on acute liver failure. Indian National Association for the study of liver consensus statement on acute liver failure (Part-2): management of acute liver failure. J Clin Exp Hepatol. 2020 Sep-Oct;10:477–517. doi: 10.1016/j.jceh.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vadivukkarasi T.J., Kandasamy S., Abhilash K.P.P., Zachariah U., Goel A., Eapen C.E. Safe sedation practices in acute liver failure in resource-constrained settings: a viewpoint. Gastroenterol Hepatol Endosc Pract. 2021;1:17–21. [Google Scholar]
- 28.Bernal W., Lee W.M., Wendon J., Larsen F.S., Williams R. Acute liver failure: a curable disease by 2024? J Hepatol. 2015;62:S112–S120. doi: 10.1016/j.jhep.2014.12.016. [DOI] [PubMed] [Google Scholar]