Abstract
Background & aims
Sleep-wake abnormalities [poor nighttime sleep and excessive daytime sleepiness (EDS)] are common in patients with cirrhosis. The aim of this study was to assess the prevalence of sleep-wake abnormalities and clinical factors associated with these abnormalities in a group of patients with cirrhosis.
Methods
1098 patients with cirrhosis [Child Turcotte Pugh (CTP) class A, 22.2%; CTP class B, 29.2% and CTP class C, 48.6%], with either no ascites or mild ascites controlled on diuretics, and no history of or current overt hepatic encephalopathy were included in the study.
Results
Poor nighttime sleep and EDS were found in 569 (51.8%) and 489 (44.5%) patients respectively. On multivariate analysis, factors associated with poor nighttime sleep were CTP class C (vs. class A), presence of minimal hepatic encephalopathy (MHE), intermediate or evening type of diurnal preference category (vs. morning type), high risk for obstructive sleep apnea (OSA), diuretic use, presence of major depression, and presence of generalized anxiety disorder (GAD). Factors associated with EDS on multivariate analysis were CTP class B and C (vs. class A), intermediate or evening type of diurnal preference category (vs. morning type), high risk for OSA, presence of major depression, and presence of GAD.
Conclusions
Sleep-wake abnormalities are common in patients with cirrhosis. CTP status, diurnal preference chronotype, risk of OSA, major depression and GAD are associated with both poor nighttime sleep and EDS. MHE and diuretic use are associated with poor nighttime sleep, but not with EDS.
Keywords: cirrhosis, insomnia, sleep disturbances, sleep-wake abnormalities
Abbreviations: ACLF, Acute on chronic liver failure; BQ, Berlin questionnaire; CFF, Critical flicker frequency; CLDQ, Chronic liver disease questionnaire; CSM, Composite scale of morningness; CTP, Child Turcotte Pugh; EDS, Excessive daytime sleepiness; ESS, Epworth sleepiness scale; GAD, generalized anxiety disorder; HE, Hepatic encephalopathy; HRQOL, Health related quality of life; MHE, Minimal hepatic encephalopathy; NASH, Non-alcoholic steatohepatitis; OSA, Obstructive sleep apnea; PHQ, Patient health questionnaire; PSQI, Pittsburgh sleep quality index
Sleep–wake disturbances are common in patients with cirrhosis, with poor nighttime sleep reported in 47–69%, and excessive daytime sleepiness (EDS) in 18.5–38% of patients with cirrhosis.1, 2, 3, 4, 5, 6 Sleep-wake disturbances are considered a classic sign of overt hepatic encephalopathy (HE),7 but such disturbances may also occur in patients without overt HE.1,3,4
There is conflicting/confusing evidence of a direct relationship between poor nighttime sleep and EDS in patients with cirrhosis4,5
Itching and tense ascites are obvious causes of sleep-wake disturbances in patients with cirrhosis, but these can also occur in the absence of such obvious causes.1,4 The associations of poor nighttime sleep and EDS with other clinical factors like Child Turcotte Pugh (CTP) status1,3,4 minimal hepatic encephalopathy (MHE)1,4,5 evening chronotype,1,4 obstructive sleep apnea (OSA),6,8 and depression or anxiety1,2 have been previously studied in small studies, with differing results. Patients with cirrhosis are often taking diuretics and beta-blockers which have the potential to cause sleep-wake disturbances.1,9 No previous study has assessed association of these drugs with poor nighttime sleep or EDS in patients with cirrhosis.
There are no large studies that have assessed the association of all these clinical parameters in a single cohort of patients with cirrhosis.
Aims
The aim of this study was to assess the prevalence of sleep-wake abnormalities (poor nighttime sleep and EDS) and clinical factors associated with these abnormalities in a group of patients with cirrhosis.
Methods
Study design: This was a single center prospective cross-sectional study.
Setting: The study was conducted at the Department of Hepatology and Liver transplantation, Institute of Liver and Biliary Sciences (ILBS), New Delhi from June 30, 2015 to May 20, 2017. The study was approved by the Institutional Review Board (IRB) of the Institute of Liver and Biliary Sciences (D1 Vasant Kunj, New Delhi, India) [Institutional ethical committee number: F. 25/5/75/ILBS/AC/2014/392, dated May 22, 2015].
Participants
Patients with cirrhosis who presented to the outpatient clinic of Hepatology department of the institute were assessed for inclusion in the study.
The inclusion criteria were the following: Patients with cirrhosis, >18 years age, either no ascites or mild ascites controlled on diuretics, and no history of overt hepatic encephalopathy.
Exclusion criteria were the following: Patients with acute-on-chronic liver failure (ACLF) as defined by Asia Pacific Association for study of Liver (APASL) 2014 criteria10; total serum bilirubin ≥5 mg/dL (but not fulfilling criteria for ACLF); hepatocellular carcinoma (HCC); patients with history of overt hepatic encephalopathy (HE) or currently having overt encephalopathy; prior history of transjugular intrahepatic portosystemic shunt (TIPS) placement; gastrointestinal bleed in the last 1 month; alcohol intake within 6 months of enrollment; active substance abuse or intake within 1 month of enrollment; patients using antidepressants and anticonvulsants; pregnancy or lactation; complaints of itching interfering with sleep; moderate-to-tense ascites; active infection; acute febrile illness; and no consent.
We excluded patients with currently in overt HE or had previous history of overt HE, as such patients are likely to be on treatment with ammonia-lowering therapies like lactulose or rifaximin, which could be a confounder for the prevalence of minimal hepatic encephalopathy.
Variables assessed
Patients underwent the following investigations at baseline: complete hemogram, renal function tests, serum electrolytes, liver function tests (LFTs) including International normalized ratio (INR), etiological workup for cirrhosis as needed, ultrasound abdomen with Doppler splenoportal axis and hepatic veins, blood sugar fasting, venous ammonia, chest X-ray, Pittsburgh sleep quality index (PSQI) questionnaire, Epworth sleepiness scale (ESS) questionnaire, critical flicker frequency (CFF), composite scale of morningness (CSM) questionnaire, Berlin questionnaire (BQ), patient health questionnaire (PHQ-9) questionnaire, generalized anxiety disorder 7 (GAD-7) questionnaire and chronic liver disease questionnaire (CLDQ) questionnaire.
Cirrhosis was diagnosed by clinical, radiological criteria or liver biopsy (if required). Twenty g/d of alcohol use was taken as significant for males and females.11 Non-alcoholic steatohepatitis (NASH) cirrhosis was diagnosed using histological (histological evidence of NASH with cirrhosis on liver biopsy within last 12 months) or clinical criteria [If biopsy was not available within 12 months, the diagnosis of NASH cirrhosis was made using any one of the following after excluding other causes of cirrhosis: (1) current or prior documentation of hepatic steatosis with non-invasive evidence of cirrhosis, or (2) two or more risk factors for NASH (overweight/obesity, type 2 diabetes mellitus, hypertension, or dyslipidemia) with evidence of cirrhosis].
Venous ammonia was measured by the ammonia checker II (Daiichi Kagaku Co. Ltd., Kyoto, Japan), within 3 min of blood sampling.
Methods of assessments (measurements)
Pittsburgh sleep quality index (PSQI) andEpworth sleepiness scale (ESS): The PSQI is a self-administered questionnaire; with a global PSQI score greater than 5 indicating a “poor” sleeper.12,13 ESS is used to assess daytime sleepiness, with values higher than 10 reflecting above normal daytime sleepiness.14
Methods for measuring nighttime sleep disturbances include physiologic measurement instruments (polysomnography and actigraphy) and self-report measures (sleep diary and sleep questionnaires like PSQI). While physiologic measurement instruments are preferable for studying nighttime sleep disturbances, many studies have used sleep diaries and self report measures (like PSQI) as well. In patients with primary insomnia, PSQI score has sensitivity of 98.7% and specificity of 84.4% in separating patients with poor nighttime sleep and good sleepers.13 Many previous studies on sleep disturbances in patients with cirrhosis have also used PSQI.4,5 Similarly methods for assessing EDS include physiologic measurement instruments (polysomnography, multiple sleep latency test and maintenance of wakefulness test) and self-report measures (like ESS). Many previous studies on excessive daytime sleepiness in patients with cirrhosis have also used ESS.3, 4, 5
Measurement of critical flicker frequency (CFF) threshold: CFF was analyzed by HEP Atonorm analyzer (Accelab GmbH, Kusterdingen, Germany).15 Patients were diagnosed as minimal hepatic encephalopathy (MHE) if mean of eight CFF readings was <39 Hz.15
Composite scale of morningness (CSM) questionnaire: The 13-item CSM is a self-reported questionnaire widely used to assess morningness/eveningness. Total CSM scores may range from 13 to 55. CSM score of 36 and below were considered as evening types, those with scores between 36 and 49 would represent intermediates and scores above 49 were considered as morning types. For any individual, morningness-eveningness refers to the diurnal preferences, and sleep-wake patterns and alertness for activity in the morning and evening. Habitual sleep patterns and sleep parameters can be influenced by the morningness/eveningness of any individual.16
Berlin questionnaire (BQ): The BQ, which consists of three categories of symptoms, classifies patients as having a high risk of obstructive sleep apnea (OSA) if two or more categories had positive score; and as low risk of OSA if only one or no category had positive score.17
Generalized anxiety disorder 7 (GAD-7) questionnaire: GAD 7 is a self-reported questionnaire for screening and severity measuring of generalized anxiety disorder, with GAD 7 score of ≥10 indicated probable diagnosis of GAD.18
Patient health questionnaire (PHQ-9): The PHQ-9 is the depression module, with PHQ-9 score ≥10 being taken as indication of major depression.19
Chronic liver disease questionnaire (CLDQ): CLDQ is a CLD specific health related quality of life (HRQOL) instrument.20
All of the above questionnaires used in this study were in both English and a Hindi translated version, and the patients were asked to read in the language they preferred. These questionnaires were administered within 2 days of enrollment.
Statistical methods
Assuming the prevalence of sleep-wake abnormalities to be 60%, with confidence limits of 5%, and design effect of 1, sample size of 1039 was required for a confidence level of 99.9%. Data were processed using the SPSS version 20.0 software. Chi-square and Fisher's exact tests were used for comparison of categorical variables. For comparison of continuous variables, t-test and Mann–Whitney test were used for normally distributed and non-normally distributed data respectively. A univariate logistic-regression analysis was performed for the assessment of variables associated with poor nighttime sleep and EDS Variables likely to be associated with poor nighttime sleep and EDS based on a priori theory were used in multivariate logistic regression analysis, and forward selection was applied at a 5% significance level.
Results
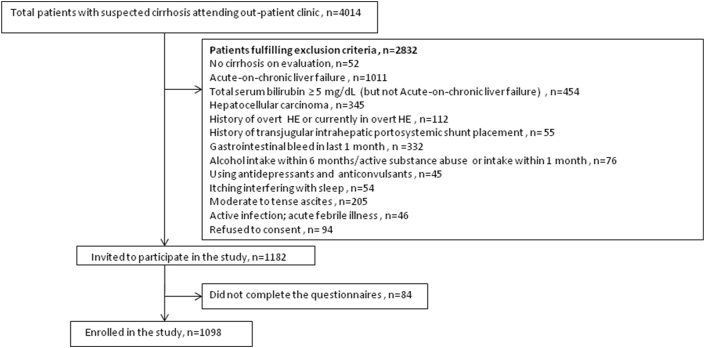
Participants: 1098 patients fulfilling the inclusion and exclusion criteria were enrolled for this study (Figure 1).
Figure 1.
Patient enrollment in the study.
Baseline patient characteristics
The etiology of the cirrhosis was non-alcoholic steatohepatitis (NASH) in 530 (48.3%) of the 1098 patients, alcohol in 457 (41.6%), hepatitis B in 50 (4.5%) and hepatitis C in 39 (3.6%). Overall, 243 patients (22.2%) were classified as Child Turcotte Pugh (CTP) class A, 321 (29.2%) as CTP class B, and 534 (48.6%) as CTP class C. On the day of study, 626 (57%) of patients had critical flicker frequency (CFF) < 39 Hz (i.e. classified as having minimal hepatic encephalopathy). None of the patients had overt hepatic encephalopathy.
Sleep–wake disturbances
Overall, 724 (65.9%) patients had sleep-wake disturbances. Disturbed nighttime sleep (poor sleepers, Pittsburgh sleep quality index (PSQI) score >5) was seen in 569 (51.8%) patients. Excessive daytime sleepiness (EDS) [Epworth sleepiness scale (ESS) score >10] was seen in 489 (44.5%) patients.
Nighttime sleep: There were no significant differences in age, gender and etiology of cirrhosis between ‘good’ and ‘poor’ sleepers. As compared to good sleepers, poor sleepers had higher CTP scores and more likely to have CTP class C; had more minimal hepatic encephalopathy (MHE) and higher venous ammonia levels; were more likely to be evening type; more likely to have higher body mass index (BMI), and high risk for obstructive sleep apnea (OSA); more often using diuretics and beta-blockers; more likely to have major depression and generalized anxiety disorder; significantly more impaired health-related quality of life (HRQOL); and had higher ESS score; and more likely to have EDS (Table 1).
Table 1.
Baseline Characteristics of Patients According to Night Time Sleep Status.
| Parameters | Total (n = 1098) | Poor Sleepers (PSQI score >5) (n = 569) | Good Sleepers (PSQI score ≤5) (n = 529) | P value |
|---|---|---|---|---|
| Demographic parameters | ||||
| Age (years), mean ± SD | 48.3 ± 10.5 | 48.4 ± 10.1 | 48.2 ± 10.9 | 0.820 |
| Male sex, n (%) | 1010 (92%) | 517 (90.9%) | 493 (93.2%) | 0.182 |
| Etiology of cirrhosis, n (%) | ||||
| NASH | 530 (48.3%) | 274 (48.2%) | 256 (48.4%) | 0.440 |
| Alcohol | 457 (41.6%) | 233 (40.9%) | 224 (42.3%) | |
| HBV | 50 (4.5%) | 29 (5.1%) | 21 (4.0%) | |
| HCV | 39 (3.6%) | 18 (3.2%) | 21 (4.0%) | |
| Others | 22 (2%) | 15 (2.6%) | 7 (1.3%) | |
| BMI (kg/m2), mean ± SD | 25.9 ± 3.7 | 26.4 ± 3.8 | 25.4 ± 3.6 | <0.001 |
| Laboratory parameters | ||||
| Bilirubin (mg/dL), mean ± SD | 2.6 ± 1.1 | 2.7 ± 1.1 | 2.4 ± 1.1 | <0.001 |
| Serum albumin (g/L), mean ± SD | 2.9 ± 0.6 | 2.9 ± 0.5 | 3.0 ± 0.6 | <0.001 |
| INR, mean ± SD | 1.9 ± 0.5 | 2.0 ± 0.5 | 1.8 ± 0.5 | <0.001 |
| CTP class A/B/C, n (%) | 243 (22.2%)/321 (29.2%)/534 (48.6%) | 96 (16.9%)/151 (26.5%)/322 (56.6%) | 147 (27.8%)/170 (32.1%)/212 (40.1%) | <0.001 |
| CTP score, mean ± SD | 8.5 ± 2.2 | 8.8 ± 2.1 | 8.1 ± 2.3 | <0.001 |
| Sleep parameters | ||||
| PSQI variables, mean ±SD | ||||
| Subjective sleep quality | 1.8 ± 1.1 | 2.7 ± 0.6 | 0.8 ± 0.4 | <0.001 |
| Sleep latency | 1.7 ± 1.1 | 2.7 ± 0.5 | 0.7 ± 0.5 | <0.001 |
| Sleep duration | 1.6 ± 1.1 | 2.5 ± 0.6 | 0.6 ± 0.5 | <0.001 |
| Habitual sleep efficiency | 1.4 ± 1.2 | 2.3 ± 0.8 | 0.3 ± 0.5 | <0.001 |
| Sleep disturbances | 1.2 ± 1.2 | 2.2 ± 0.9 | 0.2 ± 0.4 | <0.001 |
| Sleeping medication | 0.02 ± 0.1 | 0.1 ± 0.2 | 0.0 ± 0.0 | <0.001 |
| Daytime dysfunction | 0.5 ± 0.5 | 0.6 ± 0.6 | 0.4 ± 0.4 | <0.001 |
| Total PSQI score [0–21] | 8.3 ± 5.4 | 13.1 ± 2.9 | 3.1 ± 0.8 | <0.001 |
| Day-time sleepiness score | ||||
| ESS score (0–24), mean ± SD | 9.8 ± 4.7 | 11.9 ± 5.1 | 7.7 ± 2.9 | <0.001 |
| ESS >10, n (%) | 489 (44.5%) | 334 (58.7%) | 155 (29.3%) | <0.001 |
| MHE and ammonia levels | ||||
| CFF (Hz), mean ± SD | 36.3 ± 5.0 | 33.8 ± 5.5 | 38.9 ± 2.5 | <0.001 |
| CFF < 39 Hz, n (%) | 626 (57%) | 371 (65.2%) | 255 (48.2%) | <0.001 |
| Ammonia (μmol/L), mean ± SD | 94.7 ± 26.8 | 101.5 ± 27.3 | 87.5 ± 24.4 | <0.001 |
| Diurnal preference category, n(%) | ||||
| Morning type | 307 (28.0%) | 94 (16.5%) | 213 (40.3%) | <0.001 |
| Intermediate type | 427 (38.9%) | 216 (38.0%) | 211 (39.9%) | |
| Evening type | 364 (33.2%) | 259 (45.5%) | 105 (19.8%) | |
| Risk for OSA, n(%) | ||||
| Low risk | 826 (75.2%) | 399 (70.1%) | 468 (88.5%) | <0.001 |
| High risk | 272 (24.8%) | 170 (29.9%) | 102 (19.3%) | |
| Concomitant medications, n(%) | ||||
| Diuretics | 135 (12.3%) | 83 (14.6%) | 52 (9.8%) | 0.017 |
| Beta-blockers | 587 (53.5%) | 336 (59.1%) | 251 (47.4%) | <0.001 |
| Depression and anxiety scores | ||||
| PHQ 9 score, mean ± SD | 11.3 ± 6.5 | 14.3 ± 6.9 | 8.0 ± 4.0 | <0.001 |
| PHQ 9 score ≥10, n (%) | 420 (38.3%) | 358 (62.9%) | 62 (11.7%) | <0.001 |
| GAD 7 score, mean ± SD | 9.1 ± 5.4 | 11.2 ± 5.9 | 6.8 ± 3.9 | <0.001 |
| GAD 7 score ≥10, n (%) | 374 (34.1%) | 317 (55.7%) | 57 (10.8%) | <0.001 |
| Quality of life score | ||||
| CLDQ variables, mean±SD | ||||
| Abdominal symptoms | 4.7 ± 1.9 | 3.3 ± 1.6 | 6.3 ± 0.7 | <0.001 |
| Activity | 4.7 ± 1.8 | 3.3 ± 1.4 | 6.2 ± 0.8 | <0.001 |
| Fatigue | 4.5 ± 1.9 | 2.9 ± 1.5 | 6.1 ± 0.8 | <0.001 |
| Emotional function | 4.4 ± 1.7 | 3.1 ± 1.5 | 5.8 ± 0.6 | <0.001 |
| Systemic symptoms | 4.9 ± 1.7 | 3.5 ± 1.3 | 6.4 ± 0.6 | <0.001 |
| Worry | 4.7 ± 1.8 | 3.5 ± 1.7 | 6.0 ± 0.7 | <0.001 |
| Total CLDQ score | 4.7 ± 1.7 | 3.3 ± 1.3 | 6.1 ± 0.5 | <0.001 |
Abbreviations: NASH-Non-alcoholic steatohepatitis;HBV-Hepatitis B virus; HCV-Hepatitis C virus; BMI-Body mass index;INR-International normalized ratio;CTP-Child turcotte pugh;PSQI-, Pittsburgh sleep quality index;ESS-Excessive day-time sleepiness;MHE-Minimal hepatic encephalopathy;CFF-Critical flicker frequency;OSA-Obstructive sleep apnea;PHQ-Patient health questionnaire;GAD-Generalized anxiety disorder;CLDQ-Chronic liver disease questionnaire.
Daytime sleepiness: EDS (ESS score >10) was seen in 489/1098 (44.5%) patients. There were no significant differences in age, gender and etiology of cirrhosis between patients with excessive EDS and those without (Table 2).
Table 2.
Baseline Characteristics of Patients According to Daytime Sleepiness Status.
| Parameters | Excessive day time sleepiness present (ESS score >10) (n = 489) | Excessive day time sleepiness absent (ESS score ≤10) (n = 609) | P value |
|---|---|---|---|
| Demographic parameters | |||
| Age (years), mean ± SD | 48.2 ± 10.8 | 48.3 ± 10.3 | 0.838 |
| Male sex, n (%) | 442 (90.4%) | 568 (93.3%) | 0.093 |
| Etiology of cirrhosis, n (%) | |||
| NASH | 244 (49.9%) | 286 (47.0%) | 0.470 |
| Alcohol | 195 (39.9%) | 262 (43.0%) | |
| HBV | 23 (4.7%) | 27 (4.4%) | |
| HCV | 20 (4.1%) | 19 (3.1%) | |
| Others | 7 (1.4%) | 15 (2.5%) | |
| BMI (kg/m2), mean ± SD | 26.6 ± 3.8 | 25.4 ± 3.5 | <0.001 |
| Laboratory parameters | |||
| Bilirubin (mg/dL), mean ± SD | 2.7 ± 1.1 | 2.5 ± 1.1 | 0.019 |
| Serum albumin (g/L), mean ± SD | 2.9 ± 0.5 | 2.9 ± 0.6 | 0.024 |
| INR, mean ± SD | 1.9 ± 0.5 | 1.8 ± 0.5 | 0.005 |
| CTP class A/B/C, n (%) | 86 (17.6%)/145 (29.7%)/258 (52.8%) | 157 (25.8%)/176 (28.9%)/276 (45.3%) | 0.003 |
| CTP score, mean ± SD | 8.6 ± 2.1 | 8.3 ± 2.3 | 0.008 |
| Sleep parameters | |||
| PSQI variables, mean ±SD | |||
| Subjective sleep quality | 2.1 ± 0.9 | 1.5 ± 1.0 | <0.001 |
| Sleep latency | 2.0 ± 1.0 | 1.4 ± 1.1 | <0.001 |
| Sleep duration | 1.9 ± 1.0 | 1.4 ± 1.0 | <0.001 |
| Habitual sleep efficiency | 1.6 ± 1.2 | 1.2 ± 1.1 | <0.001 |
| Sleep disturbances | 1.4 ± 1.1 | 1.1 ± 1.2 | <0.001 |
| Sleeping medication | 0.02 ± 0.1 | 0.02 ± 0.1 | 0.196 |
| Daytime dysfunction | 0.8 ± 0.6 | 0.2 ± 0.4 | <0.001 |
| Total PSQI score [0–21] | 10.0 ± 5.3 | 6.8 ± 5.1 | <0.001 |
| PSQI score >5 | 334 (68.3%) | 235 (38.6%) | <0.001 |
| Day-time sleepiness score | |||
| ESS score (0–24), mean ± SD | 14.0 ± 3.8 | 6.6 ± 1.9 | <0.001 |
| MHE and ammonia levels | |||
| CFF (Hz), mean ± SD | 35.4 ± 5.4 | 37.0 ± 4.6 | <0.001 |
| CFF < 39 Hz, n (%) | 298 (60.9%) | 328 (53.9%) | 0.020 |
| Ammonia (μmol/L), mean ± SD | 99.7 ± 26.6 | 90.8 ± 26.4 | <0.001 |
| Diurnal preference category, n(%) | |||
| Morning type | 104 (21.3%) | 203 (33.3%) | 0 < 0.001 |
| Intermediate type | 192 (39.3%) | 235 (38.6%) | |
| Evening type | 193 (39.5%) | 171 (28.1%) | |
| Risk for OSA, n(%) | |||
| Low risk | 293 (59.9%) | 533 (87.5%) | <0.001 |
| High risk | 196 (40.1%) | 76 (12.5%) | |
| Concomitant medications, n(%) | |||
| Diuretics | 58 (11.9%) | 77 (12.6%) | 0.712 |
| Beta-blockers | 284 (58.1%) | 303 (49.8%) | 0.006 |
| Depression and anxiety scores | |||
| PHQ 9 score, mean ± SD | 14.2 ± 6.5 | 8.9 ± 5.5 | <0.001 |
| PHQ 9 score ≥10, n (%) | 324 (66.3%) | 96 (15.8%) | <0.001 |
| GAD 7 score, mean ± SD | 12.1 ± 5.6 | 6.5 ± 3.8 | <0.001 |
| GAD 7 score ≥10, n (%) | 311 (63.6%) | 63 (10.3%) | <0.001 |
| Quality of life score | |||
| CLDQ variables, mean±SD | |||
| Abdominal symptoms | 4.3 ± 1.9 | 5.1 ± 1.8 | <0.001 |
| Activity | 4.2 ± 1.8 | 5.1 ± 1.8 | <0.001 |
| Fatigue | 4.0 ± 1.9 | 4.8 ± 1.9 | <0.001 |
| Emotional function | 4.0 ± 1.8 | 4.7 ± 1.7 | <0.001 |
| Systemic symptoms | 4.4 ± 1.7 | 5.2 ± 1.7 | <0.001 |
| Worry | 4.2 ± 1.9 | 5.1 ± 1.7 | <0.001 |
| Total CLDQ score | 4.2 ± 1.7 | 5.0 ± 1.6 | <0.001 |
Abbreviations: NASH-Non-alcoholic steatohepatitis;HBV-Hepatitis B virus; HCV-Hepatitis C virus; BMI-Body mass index;INR-International normalized ratio; CTP-Child turcotte pugh;PSQI-, Pittsburgh sleep quality index;ESS- Excessive day-time sleepiness; MHE- Minimal hepatic encephalopathy;CFF- Critical flicker frequency;OSA- Obstructive sleep apnea;PHQ- Patient health questionnaire;GAD- Generalized anxiety disorder;CLDQ- Chronic liver disease questionnaire.
As compared to patients without EDS, patients with EDS had higher CTP scores and more likely to have CTP class C; had more MHE and higher venous ammonia levels; were more likely to be evening type; more likely to have higher BMI, and high risk for OSA; more often using beta-blockers; were more likely to have major depression, and generalized anxiety disorder; significantly more impaired HRQOL; and had higher PSQI score and were more likely to have poor nighttime sleep (PSQI >5).
There was no significant difference as regards to diuretic use between patients with and without EDS.
Correlation of venous ammonia levels, beta-blocker doses, and diuretics doses with PSQI score and ESS score
Correlation of venous ammonia levels, beta-blocker doses, and diuretics doses with PSQI score and ESS score was assessed in all the patients and patients with different etiologies (NASH and alcohol). The analysis was not done for etiologies other than NASH and alcohol, as the number of subjects with these etiologies (hepatitis B virus infection, hepatitis C virus infection, others) were small.
There was a significant correlation between venous ammonia levels and the PSQI score [Pearson correlation coefficient (P value), 0.244 (P < 0.001) for all patients; 0.278 (P < 0.001) for NASH patients; 0.306 (P < 0.001) for patients with alcohol as etiology of cirrhosis]. There was also a significant correlation between venous ammonia levels and the ESS score [Pearson correlation coefficient (P value), 0.173 (P < 0.001) for all patients; 0.247 (P < 0.001) for NASH patients; 0.172 (P < 0.001) for patients with alcohol as etiology of cirrhosis].
Of 1098 patients, 587 (53.5%) were on beta-blockers; 340 (31.01%) were on carvedilol (mean ± SD of dose, 13.4 ± 4.4 mg) and 247 (22.5%) were on propranolol (mean ± SD of dose, 115.3 ± 30.4 mg). There was no correlation between the PSQI score and dose of beta-blockers [Pearson correlation coefficient (P value) for carvedilol, 0.030 (P = 0.581) for all patients, −0.057 (P = 0.472 for NASH patients; 0.130 (P = 0.123) for patients with alcohol as etiology of cirrhosis; for propranolol, 0.019 (P = 0.770), for all patients, −0.023 (P = 0.804) for NASH patients; 0.048 (P = 0.643) for patients with alcohol as etiology of cirrhosis]. Similarly, there was no correlation between the ESS score and dose of beta-blockers [Pearson correlation coefficient (P value) for carvedilol, 0.093 (P = 0.086 for all patients, 0.067 (P = 0.394) for NASH patients; 0.118 (P = 0.161) for patients with alcohol as etiology of cirrhosis; for propranolol, −0.0333 (P = 0.610) for all patients, −0.006 (P = 0.951) for NASH patients; −0.009 (P = 0.928) for patients with alcohol as etiology of cirrhosis].
Of 1098 patients, 135 (12.3%) were on diuretics; 20 (1.8%) on aldosterone antagonists and 115 (10.5%) were additionally on loop diuretics also (mean ± SD of spironolactone equivalent dose, 168.4 ± 80.9 mg and furosemide equivalent dose, 72.6 ± 39.2 mg). Dose equivalencies for aldosterone antagonists used were amiloride–spironolactone 3.3 to 1, and for loop diuretics were torsemide-furosemide 4 to 1. There was significant correlation between the PSQI score and dose of diuretics [Pearson correlation coefficient (P value) for aldosterone antagonists, 0.664 (P < 0.001) for all patients, 0.953 (P < 0.001 for NASH patients; 0.708 (P < 0.001 for patients with alcohol as etiology of cirrhosis; for loop diuretics, 0.714 (P < 0.001), for all patients, 0.617 (P < 0.001) for NASH patients; 0.743 (P < 0.001) for patients with alcohol as etiology of cirrhosis]. However, there was no correlation between the ESS score and dose of diuretics [Pearson correlation coefficient (P value) for aldosterone antagonists, 0.165 (P = 0.078 for all patients, 0.119 (P = 0.378) for NASH patients; −0.033 (P = 0.606) for patients with alcohol as etiology of cirrhosis; for loop diuretics, 0.173 (P = 0.063) for all patients, −0.089 (P = 0.540) for NASH patients; 0.051 (P = 0.935) for patients with alcohol as etiology of cirrhosis].
Analysis of factors associated with poor nighttime sleep (Pittsburgh Sleep Quality Index score >5)
The factors assessed for association with poor nighttime sleep and excessive daytime sleepiness were Child Turcotte Pugh (CTP) class, presence of minimal hepatic encephalopathy (MHE), diurnal preference category, risk for obstructive sleep apnea (OSA), diuretics use, beta-blockers use, presence of major depression, presence of generalized anxiety disorder, presence of Excessive daytime sleepiness (EDS). Chronic liver disease questionnaire (CLDQ) was not included as a factor in analysis, as poor quality of life is a consequence of, rather than contributing to, the sleep-wake disturbances. Table 3 shows univariate analysis, adjusted univariate analysis (adjusted for Child-Turcotte-Pugh Class and MHE status) and multivariate analysis.
Table 3.
Analysis of Factors Associated With Poor Sleepers (PSQI Score >5).
| Parameters | Univariate analysis OR (95% CI), P value | Adjusted univariate analysisa OR (95% CI), P value | Multivariate analysis OR (95% CI), P value |
|---|---|---|---|
| Child-Turcotte-Pugh Class | |||
| A | 1 | 1 | |
| B | 1.360 (0.970–1.907), P = 0.074 | – | 1.040 (0.658–1.644), P = 0.865 |
| C | 2.326 (1.706–3.172), P < 0.001 | 2.158 (1.431–3.255), P < 0.001 | |
| Minimal hepatic encephalopathy | |||
| No | 1 | 1 | |
| Yes | 2.013 (1.580–2.566), P < 0.001 | – | 1.658 (1.211–2.271), P = 0002 |
| Diurnal preference category | |||
| Morning type | 1 | 1 | 1 |
| Intermediate type | 2.320 (1.705–3.156), P < 0.001 | 2.267 (1.654–3.105), P < 001 | 2.751 (1.855–4.078), P < 0.001 |
| Evening type | 5.589 (4.010–7.791), P < 0.001 | 5.383 (3.835–7.557), P < 0.001 | 5.851 (3.864–8.861), P < 0.001 |
| Risk for OSA | |||
| Low risk | 1 | 1 | 1 |
| High risk | 1.784 (1.347–2.362), P < 0.001 | 1.959 (1.463–2.622), P < 0.001 | 2.400 (1.489–3.866), P < 0.001 |
| Diuretics use | |||
| No | 1 | 1 | 1 |
| Yes | 1.567 (1.083–2.266), P = 0.017 | 1.304 (0.890–1.912), P = 0.174 | 2.129 (1.344–3.371), P = 0.001 |
| Beta blocker use | |||
| No | 1 | 1 | 1 |
| Yes | 1.597 (1.258–2.028), P < 0.001 | 1.114 (0.829–1.498), P = 0.474 | 1.204 (0.821–1.767), P = 0.341 |
| Major depression | |||
| No | 1 | 1 | 1 |
| Yes | 12.780 (9.328–17.509), P < 0.001 | 14.644 (10.487–20.448),P < 0.001 | 11.437 (7.039–18.584), P < 0.001 |
| Generalized anxiety disorder | |||
| No | 1 | 1 | 1 |
| Yes | 10.417 (7.558–14.356), P < 0.001 | 10.176 (7.740–15.002), P < 0.001 | 2.977 (1.823–4.861), P < 0.001 |
| Excessive daytime sleepiness | |||
| No | 1 | 1 | 1 |
| Yes | 3.429 (2.669–4.407),P < 0.001 | 3.314 (2.564–4.283), P < 0.001 | 0.752 (0.518–1.091), P = 0.133 |
Abbreviations: OSA- Obstructive sleep apnea.
Adjusted for Child-Turcotte-Pugh Class and Minimal hepatic encephalopathy status.
On multivariate analysis, factors associated with poor nighttime sleep (PSQI > 5) were CTP class C (vs. class A), presence of MHE, intermediate or evening type of diurnal preference category (vs. morning type), patients with high risk for OSA, diuretics use, presence of major depression, and presence of generalized anxiety disorder. Beta-blocker use and EDS were not associated with poor sleep on multivariate analysis (Table 3).
Supplementary table 1shows analysis of factors predictive of poor nighttime sleep for the two most common etiologies of cirrhosis (NASH and alcohol) and found results that were similar to the combined analysis. The analysis was not done for other etiologies as the number of subjects with these etiologies (hepatitis B virus infection, hepatitis C virus infection, others) was small.
Analysis of factors associated with excessive daytime sleepiness (ESS score >10)
Table 4 shows univariate analysis, adjusted univariate analysis (adjusted for Child-Turcotte-Pugh Class and MHE status) and multivariate analysis of factors associated with excessive daytime sleepiness.
Table 4.
Analysis of Factors Associated With Excessive day Time sleepiness (ESS Score >10).
| Parameters | Univariate analysis OR (95% CI) P value |
Adjusted univariate analysisa OR (95% CI) P value |
Multivariate analysis OR (95% CI) P value |
|---|---|---|---|
| Child-Turcotte-Pugh Class | |||
| A | 1 | – | 1 |
| B | 1.504 (1.068–2.119), P = 0.020 | 1.904 (1.224–2.960), P = 0.004 | |
| C | 1.707 (1.248–2.334), P = 0.001 | 2.126 (1.414–3.198), P < 0.001 | |
| Minimal hepatic encephalopathy | |||
| No | 1 | – | 1 |
| Yes | 1.337 (1.050–1.702), P = 0.019 | 0.987 (0.725–1.344), P = 0.932 | |
| Diurnal preference category | |||
| Morning type | 1 | 1 | 1 |
| Intermediate type | 1.595 (1.177–2.161), P = 0.003 | 1.552 (1.144–2.107), P = 0.005 | 1.563 (1.076–2.271), P = 0.019 |
| Evening type | 2.203 (1.610–3.014), P < 0.001 | 2.117 (1.544–2.904), P < 0.001 | 1.618 (1.014–2.393), P = 0.016 |
| Risk for OSA | |||
| Low risk | 1 | 1 | 1 |
| High risk | 4.691 (3.473–6.338), P < 0.001 | 5.205 (3.814–7.102),P < 0.001 | 2.110 (1.437–3.101), P < 0.001 |
| Diuretics use | |||
| No | 1 | 1 | 1 |
| Yes | 10.930 (0.646–1.338), P = 0.695 | 0.822 (0.566–1.192), P = 0.301 | 1.021 (0.675–1.213), P = 0.543 |
| Beta blocker use | |||
| No | 1 | 1 | 1 |
| Yes | 1.399 (1.101–1.778), P = 0.006 | 1.226 (0.915–1.644), P = 0.172 | 1.352 (0.941–1.945), P = 0.103 |
| Major depression | |||
| No | 1 | 1 | 1 |
| Yes | 10.493 (7.827–13.988). P < 0.001 | 10.799 (8.054–14.480), P < 0.001 | 2.749 (1.843–4.100), P < 0.001 |
| Generalized anxiety disorder | |||
| No | 1 | 1 | 1 |
| Yes | 15.142 (11.003–20.838), P < 0.001 | 15.979 (11.511–22.181), P < 0.001 | 6.055 (3.941–9.303), P < 0.001 |
| Sleep status | |||
| Good sleeper | 1 | 1 | 1 |
| Poor sleeper | 3.429 (2.669–4.407), P < 0.001 | 3.314 (2.565–4.284), P < 0.001 | 0.821 (0.564–1.193),P = 0.300 |
Abbreviations: OSA- Obstructive sleep apnea.
Adjusted for Child-Turcotte-Pugh Class and Minimal hepatic encephalopathy status.
On multivariate analysis, factors associated with EDS were CTP class B and C (vs. class A), intermediate or evening type of diurnal preference category (vs. morning type), patients with high risk for OSA, presence of major depression, and presence of generalized anxiety disorder. Presence of MHE, diuretics use, beta-blocker use, and poor nighttime sleep were not associated with excessive daytime sleepiness on multivariate analysis (Table 4).
Supplementary Table 2 shows analysis of factors predictive of EDS for the two most common etiologies of cirrhosis (NASH and alcohol) and found results that were similar to the combined analysis.
Discussion
In this study on patients with cirrhosis,65.9% had sleep-wake disturbances [51.8%, poor nighttime sleep and 44.5%, EDS]. Previous studies have found sleep-wake disturbances in 47%–69%1,2 of patients with cirrhosis, with 26%–67%3, 4, 5, 6 complaining of poor nighttime sleep and 18.5%–38%3, 4, 5, 6 having EDS. The likely reasons for the variations in the reported prevalence of sleep-wake disturbances reported in various studies include, heterogeneity of the assessment tools used, differences in the studies population characteristics (e.g differences in the severity of cirrhosis and proportion of patients with history of overt HE or MHE).
Prevalence of MHE varies between 22% and 74% in patients with cirrhosis, depending on the inclusion criteria and the diagnostic cut-offs used the study.21 In the previous study from our Institute in 91 patients with cirrhosis (patients with previous history of or currently in hepatic encephalopathy were excluded), MHE was seen in 53 (58.2%) patients (7/18, 39% in CTP-A; 32/54, 59% in CTP-B and 14/19, 74% in Child CTP-C patients).22 Similarly another Indian study from another tertiary care center in 100 patients with cirrhosis (patients with history of or the presence of overt HE were excluded) also found MHE in 46% of patients (18/53, 33.9% in CTP-A; 19/34, 55.8% in CTP-B and 9/13,69.2% in CTP-C patients).5 The high prevalence of MHE in our study (among patients with cirrhosis without prior history of HE) could be due to higher number of patients with CTP-C (48.6%) and CTP-B (29.2%) as compared to CTP-A (22.2%). This may be due to referral bias with more sick patients getting referred to our institute. The high prevalence of MHE in our cohort of patients with cirrhosis encephalopathy could have added to the observation of higher sleep disturbances.
The prevalence of poor nighttime sleep and excessive daytime sleepiness in patients with cirrhosis are much higher as compared to the general population in India. There have been a few studies on prevalence of poor nighttime sleep and EDS in the general population in India. Prevalence of poor nighttime sleep in general population has been reported from 6.2% to 15.4%23, 24, 25 One study reported EDS prevalence of 0.2% in general population.24
Sleep-wake disturbances amongst patients with cirrhosis are due to abnormalities in both circadian sleep-wake regulation and homeostatic sleep-wake regulation. Abnormalities in circadian sleep-wake regulation include delayed clearance of melatonin (resulting in high daytime melatonin levels,26 low urinary 6-sulfatoxymelatonin (aMT6s),27 reduced overnight melatonin clearance28) and delays the start of the nocturnal rise of melatonin and the time to peak melatonin levels at night.26 These abnormalities in circadian rhythm are due to central circadian dysfunction.28 The delayed sleep phase syndrome (DSPS), characterized by evening preference, delayed sleep habits, impaired sleep quality, and delayed circadian rhythms, is a common feature in patients with cirrhosis.28 Abnormalities in homeostatic sleep-wake regulation include HE/hyperammonemia related compromised homeostatic build-up of sleep pressure,29 and decreased density of the adenosine receptor A1AR in cortical and subcortical regions of the brain of patients with cirrhosis.30 EDS may be attributable to a dysfunction of the neural circuits responsible for the maintenance of wakefulness. Hyperammonemia reduces serotonin and noradrenaline levels in the central nervous system and leads to low alertness and attention-associated problems, including EDS.31,32
In this study, excessive daytime sleepiness was not associated with poor nighttime sleep on multivariate analysis. There is confusing evidence of a direct relationship between nighttime sleep disturbance and excessive daytime sleepiness in patients with cirrhosis, with study by Montagnese et al. finding no significant association between excessive daytime somnolence and disturbed nighttime sleep,4 while other studies finding significant correlation between excessive daytime somnolence and disturbed nighttime sleep.6,8 Poor nighttime sleep and EDS in patients with cirrhosis, may be different disease processes altogether.
We found that, on multivariate analysis, CTP class C (vs. class A) was associated with poor nighttime sleep, and CTP class B and C (vs. class A) was associated with EDS. Some previous studies have found significant association of CTP score with poor nighttime sleep,4 whereas others have not found any association of CTP with either poor nighttime1,3 sleep or EDS.3,4,6 Poor night time sleep has been related to melatonin metabolism disturbances. The delay in the timing of melatonin/aMT6s peaks are correlated to the degree of hepatic failure.28,33 Also, in decompensated cirrhosis, sympathetic/parasympathetic imbalance affects the autonomic transmission of the suprachiasmatic nuclei signal to the periphery; the vascular mechanisms involved in sleep onset are affected by hyperdynamic circulation, peripheral vasodilatation, and loss of temperature regulation; and reduced/blunted response to exercise cues occur due to sarcopenia. 31 Although poor sleep is related to melatonin metabolism, EDS seems to be related to degree of HE (and not to disturbances in melatonin metabolism).28 In a study of 106 patients with cirrhosis, it has been found that patients complaining of EDS had slower EEGs than those without EDS. Also, the absence of EDS had a 92% negative predictive value for HE-related hospitalizations during an 8-month follow-up period.34 Since the degree of HE/MHE correlates with the CTP scores, it might explain the correlation of EDS with CTP class B and C in our study.
In this study, on multivariate analysis, intermediate or evening type of diurnal preference category (vs. morning type) was associated with poor nighttime sleep, and EDS. patients with cirrhosis with sleep disturbance have significant delay of the nocturnal period of rest and significantly higher morningness/eveningness score than those with normal sleep.1 Another study found that patients with cirrhosis with total PSQI scores ≥11 were significantly more likely to be moderate/definite evening chronotypes (22 vs. 4%; P = 0.03).4 In patients with cirrhosis, the delay in hepatic metabolism of melatonin may exacerbate the DSPS. 33
This study (which did not include patients with history of or current overt HE) found that on multivariate analysis, presence of MHE was associated with poor sleep, but not with EDS. Some experimental animal studies support the association of sleep-wake abnormalities with HE.35 Regarding human studies, some studies have found no association between MHE and poor nighttime sleep1,2,4 and EDS,4 whereas others have found significant association between MHE and poor nighttime sleep3,5 and EDS.3,5 In a study, induced hyperammonemia resulted in increased subjective sleepiness in patients with cirrhosis as well as matched controls, which were correlated with blood ammonia levels. However, in healthy controls, the sleep was longer and deeper; where as in patients with cirrhosis, the sleep was more superficial (indicating difficulty in producing restorative sleep in patients with cirrhosis).29 This highlights ammonia to be an important contributor for sleep disturbance among patients with cirrhosis. Neuroinflammation can also contribute to sleep-wake abnormalities in cirrhosis. 35 Ammonia and proinflammatory cytokines may act synergistically to produce sleep-wake abnormalities as well as cognitive alterations in MHE. On the other hand, hyperammonemia is associated with both increasing Child-Pugh grade of liver cirrhosis and hepatic encephalopathy36 and proinflammatory cytokines are also related to severity of cirrhosis.37
In our study, on multivariate analysis, high risk for OSA (vs. low risk) was associated with poor nighttime sleep, and excessive daytime sleepiness. Another study amongst patients with cirrhosis without overt HE, found that high risk of OSA was equally frequent among patients with cirrhosis with and without insomnia. Patients having both EDS and high risk OSA were more frequent among patients with cirrhosis with insomnia vs those without insomnia. High risk of OSA was associated with EDS (EDS was related to neck size and neck size was related to high risk OSA].6 The contribution of OSA to sleep disturbances can be important in patients with cirrhosis, given the increasing prevalence of obesity. Besides obesity, OSA might also be considered as a complication in cirrhosis per se (edema of the pharyngeal and laryngeal soft tissues with fluid retention could increase the collapsibility of the upper airways).38
This study also found that on multivariate analysis, diuretics use was associated with poor sleep, but not with excessive daytime sleepiness. Nocturia associated with diuretic administration can lead to sleep fragmentation.
In this study, we found that on multivariate analysis, generalized anxiety disorder and major depression were associated with both poor nighttime sleep, and EDS. Anxiety and depression questionnaire scores are significantly higher among patients with cirrhosis with unsatisfactory sleep.1,2 Unrecognised depression or anxiety may lead to disturbed sleep–wake patterns in patients with cirrhosis; and disturbed sleep-wake patterns may themselves lead to these psychological problems.
Our study found that, poor night time sleepers (as compared to good sleepers) and patients with EDS (as compared to those without EDS) had significantly more impaired HRQOL. Multiple studies have found that impaired HRQOL is associated with poor night-time sleep4,5,39, 40, 41 and EDS.4,5,40,41 Poor HRQOL is associated with chronic insomnia in otherwise healthy individuals also, and patients with chronic sleep disturbances have occupational maladjustment, physical and social role malfunctioning, and poor mental health.42 Additionally, EDS also impairs HRQOL, even in the absence of significant night-time sleep disturbance.43
There are a few limitations to our study. This study did not dwell into mechanistic basis of sleep disturbances among patients with cirrhosis. Most of the subjects in this study were male (92%). This may be important as men and women differ somewhat in the phenotype of sleep problems. As compared to men, women have better sleep quality (i.e. shorter sleep-onset latency, longer total sleep time and higher sleep efficiency). But, women do have more sleep-related complaints as compared to men.44 This study may represent a select group of patients with cirrhosis with advanced liver disease as, only 243 (22.2%) cases in this study were CTP-A. Hence the results may not be representative of findings in patients with compensated cirrhosis. This may reflect the referral bias of our institute. Patients with HBV as the etiology of cirrhosis were small in number in this study (4.5% of all patients). One recent study has shown that the disturbed sleep in patients with hepatitis B liver cirrhosis causes symptoms of anxiety. Thus, the evaluation of sleep quality and anxiety in patients with hepatitis B liver cirrhosis are important.45 Recently, muscle cramps, a common problem in patients with cirrhosis, has been shown to independently and negatively impact the quality of life and sleep.46 However our study did not assess the impact of muscle cramps on sleep problems. Melatonin levels were not assessed in this study. Also the impact of social status and occupation on sleep pattern was not studied in the study.
In conclusion, sleep-wake abnormalities are common in patients with cirrhosis. CTP status, diurnal preference chronotype, risk of OSA, major depression and GAD are associated with both poor nighttime sleep and EDS. MHE and diuretic use are associated with poor nighttime sleep, but not with EDS.
CRediT authorship contribution statement
Manoj Kumar: Conceptualization, Methodology, Software, Writing - original draft. Sumeet Kainth: Data curation, Writing - original draft. Sachin Kumar: Conceptualization, Methodology. Ankit Bhardwaj: Methodology, Software. Hemant KumarAggarwal: Conceptualization, Methodology, Writing - review & editing. Rakhi Maiwall: Conceptualization, Methodology, Writing - review & editing. Kapil D. Jamwal: Conceptualization, Methodology, Writing - review & editing. Saggere M. Shasthry: Conceptualization, Methodology, Writing - review & editing. Ankur Jindal: Conceptualization, Methodology, Writing - review & editing. Ashok Choudhary: Conceptualization, Methodology, Writing - review & editing. Lovkesh Anand: Conceptualization, Methodology, Writing - review & editing. Ravinder M. Dhamija: Conceptualization, Methodology, Writing - review & editing. Guresh Chibbar: Formal analysis, Data curation, Software. Barjesh Chander Sharma: Conceptualization, Methodology, Supervision. Shiv K. Sarin: Conceptualization, Methodology, Supervision.
Conflicts of interest
All authors have none to declare.
Funding
The resources required for the completion of the study were provided by the Institute of Liver and Biliary Sciences. No funding was received from any source.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2020.10.006.
Contributor Information
Manoj Kumar, Email: manojkumardm@gmail.com.
Barjesh Chander Sharma, Email: mksharma@ilbs.in.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Cordoba J., Cabrera J., Lataif L., Penev P., Zee P., Blei A.T. High prevalence of sleep disturbance in cirrhosis. Hepatology. 1998;27:339–345. doi: 10.1002/hep.510270204. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi G., Marchesini G., Nicolino F. Psychological status and depression in patients with liver cirrhosis. Dig Liver Dis. 2005;37:593–600. doi: 10.1016/j.dld.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 3.Mostacci B., Ferlisi M., Baldi Antognini A. Sleep disturbance and daytime sleepiness in patients with cirrhosis: a case control study. Neurol Sci. 2008;29:237–240. doi: 10.1007/s10072-008-0973-7. [DOI] [PubMed] [Google Scholar]
- 4.Montagnese S., Middleton B., Skene D.J., Morgan M.Y. Nighttime sleep disturbance does not correlate with neuropsychiatric impairment in patients with cirrhosis. Liver Int. 2009;29:1372–1382. doi: 10.1111/j.1478-3231.2009.02089.x. [DOI] [PubMed] [Google Scholar]
- 5.Samanta J., Dhiman R.K., Khatri A. Correlation between degree and quality of sleep disturbance and the level of neuropsychiatric impairment in patients with liver cirrhosis. Metab Brain Dis. 2013;28:249–259. doi: 10.1007/s11011-013-9393-3. [DOI] [PubMed] [Google Scholar]
- 6.Enezi A.A., Al-Jahdali F., Ahmed A.E. Symptoms of daytime sleepiness and sleep apnea in liver cirrhosis patients. Ann Hepatol. 2017;16:591–598. doi: 10.5604/01.3001.0010.0304. [DOI] [PubMed] [Google Scholar]
- 7.Sherlock S., Summerskill W.H., White L.P., Phear E.A. Portal-systemic encephalopathy: neurological complications of liver disease. Lancet. 1954;267:454–457. [PubMed] [Google Scholar]
- 8.Bajaj J.S., Thacker L.R., Leszczyszyn D. Effects of obstructive sleep apnea on sleep quality, cognition, and driving performance in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:390–397. doi: 10.1016/j.cgh.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang C.H., Yang Y.H., Lin S.J., Su J.J., Cheng C.L., Lin L.J. Risk of insomnia attributable to β-blockers in elderly patients with newly diagnosed hypertension. Drug Metabol Pharmacokinet. 2013;28:53–58. doi: 10.2133/dmpk.dmpk-12-rg-004. [DOI] [PubMed] [Google Scholar]
- 10.Sarin S.K., Kedarisetty C.K., Abbas Z. Acute-on-chronic liver failure: consensus recommendations of the Asian pacific association for the study of the liver (APASL) 2014. Hepatol Int. 2014 Oct;8:453–471. doi: 10.1007/s12072-014-9580-2. [DOI] [PubMed] [Google Scholar]
- 11.Duseja A., Singh S.P., Saraswat V.A. Non-alcoholic fatty liver disease and metabolic syndrome-position paper of the Indian national association for the study of the liver, endocrine society of India, Indian College of Cardiology and Indian society of gastroenterology. J Clin Exp Hepatol. 2015;5:51–68. doi: 10.1016/j.jceh.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manzar M.D., Moiz J.A., Zannat W. Validity of the pittsburgh sleep quality index in Indian university students. Oman Med J. 2015;30:193–202. doi: 10.5001/omj.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Backhaus J., Junghanns K., Broocks A., Riemann D., Hohagen F. Test-retest reliability and validity of the pittsburgh sleep quality index in primary insomnia. J Psychosom Res. 2002;53:737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 14.Johns M.W. Reliability and factor analysis of the epworth sleepiness scale. Sleep. 1992;15:376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 15.Sharma P., Sharma B.C., Puri V., Sarin S.K. Critical flicker frequency: diagnostic tool for minimal hepatic encephalopathy. J Hepatol. 2007;47:67–73. doi: 10.1016/j.jhep.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Bhatia T., Agrawal A., Beniwal R.P. A Hindi version of the composite scale of morningness. Asian J Psychiatr. 2013;6:581–584. doi: 10.1016/j.ajp.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta R., Ali R., Dhyani M., Das S., Pundir A. Hindi translation of Berlin questionnaire and its validation as a screening instrument for obstructive sleep apnea. J Neurosci Rural Pract. 2016;7:244–249. doi: 10.4103/0976-3147.176187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spitzer R.L., Kroenke K., Williams J.B., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 19.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benjamin J., Mani K., Saraya A., Joshi Y.K. Translation and validation of the Hindi version of chronic liver disease questionnaire (CLDQ) for the assessment of health related quality of life in patient with chronic liver disease in India. Trop Gastroenterol. 2016;37:112–122. [PubMed] [Google Scholar]
- 21.Dhiman R.K., Saraswat V.A., Sharma B.K. Indian national association for study of the liver. Minimal hepatic encephalopathy: consensus statement of a working party of the Indian national association for study of the liver. J Gastroenterol Hepatol. 2010 Jun;25:1029–1041. doi: 10.1111/j.1440-1746.2010.06318.x. [DOI] [PubMed] [Google Scholar]
- 22.Sharma P., Kumar A. Minimal hepatic encephalopathy in patients with cirrhosis by measuring liver stiffness and hepatic venous pressure gradient. Saudi J Gastroenterol. 2012 Sep-Oct;18:316–321. doi: 10.4103/1319-3767.101126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan I.W., Juyal R., Shikha D., Gupta R. Generalized Anxiety disorder but not depression is associated with insomnia: a population based study. Sleep Sci. 2018;11:166–173. doi: 10.5935/1984-0063.20180031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panda S., Taly A.B., Sinha S., Gururaj G., Girish N., Nagaraja D. Sleep-related disorders among a healthy population in South India. Neurol India. 2012;60:68–74. doi: 10.4103/0028-3886.93601. [DOI] [PubMed] [Google Scholar]
- 25.Roy S.K., Bhattacharjee A.K., Chakraborti C., Singh R. Prevalence of insomnia in urban population of West Bengal: a community based cross sectional study. Int J Med Publ Health. 2015;5:293–296. [Google Scholar]
- 26.Steindl P.E., Finn B., Bendok B., Rothke S., Zee P.C., Blei A.T. Disruption of the diurnal rhythm of plasma melatonin in cirrhosis. Ann Intern Med. 1995;123:274–277. doi: 10.7326/0003-4819-123-4-199508150-00005. [DOI] [PubMed] [Google Scholar]
- 27.Steindl P.E., Ferenci P., Marktl W. Impaired hepatic catabolism of melatonin in cirrhosis. Ann Intern Med. 1997;127:494. doi: 10.7326/0003-4819-127-6-199709150-00025. [DOI] [PubMed] [Google Scholar]
- 28.Montagnese S., Middleton B., Mani A.R., Skene D.J., Morgan M.Y. On the origin and the consequences of circadian abnormalities in patients with cirrhosis. Am J Gastroenterol. 2010;105:1773–1781. doi: 10.1038/ajg.2010.86. [DOI] [PubMed] [Google Scholar]
- 29.Bersagliere A., Raduazzo I.D., Nardi M. Induced hyperammonemia may compromise the ability to generate restful sleep in patients with cirrhosis. Hepatology. 2012;55:869–878. doi: 10.1002/hep.24741. [DOI] [PubMed] [Google Scholar]
- 30.Boy C., Meyer P.T., Kircheis G. Cerebral A1 adenosine receptors (A1AR) in liver cirrhosis. Eur J Nucl Med Mol Imag. 2008;35:589–597. doi: 10.1007/s00259-007-0586-z. [DOI] [PubMed] [Google Scholar]
- 31.Montagnese S., De Pittà C., De Rui M. Sleep-wake abnormalities in patients with cirrhosis. Hepatology. 2014 Feb;59:705–712. doi: 10.1002/hep.26555. [DOI] [PubMed] [Google Scholar]
- 32.Ahabrach H., Piedrafita B., Ayad A. Chronic hyperammonemia alters the circadian rhythms of corticosteroid hormone levels and of motor activity in rats. J Neurosci Res. 2010;88:1605–1614. doi: 10.1002/jnr.22311. [DOI] [PubMed] [Google Scholar]
- 33.Montagnese S., Middleton B., Mani A.R., Skene D.J., Morgan M.Y. Sleep and circadian abnormalities in patients with cirrhosis: features of delayed sleep phase syndrome? Metab Brain Dis. 2009;24:427–439. doi: 10.1007/s11011-009-9146-5. [DOI] [PubMed] [Google Scholar]
- 34.De Rui M., Schiff S., Aprile D. Excessive daytime sleepiness and hepatic encephalopathy: it is worth asking. Metab Brain Dis. 2013;28:245–248. doi: 10.1007/s11011-012-9360-4. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigo R., Cauli O., Gomez-Pinedo U. Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology. 2010;139:675–684. doi: 10.1053/j.gastro.2010.03.040. [DOI] [PubMed] [Google Scholar]
- 36.Khan A., Ayub M., Khan W.M. Hyperammonemia is associated with increasing severity of both liver cirrhosis and hepatic encephalopathy. Int J Hepatol. 2016;2016:6741754. doi: 10.1155/2016/6741754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prystupa A., Kiciński P., Sak J., Boguszewska-Czubara A., Toruń-Jurkowska A., Załuska W. Proinflammatory cytokines (IL-1α, IL-6) and hepatocyte growth factor in patients with alcoholic liver cirrhosis. Gastroenterol Res Pract. 2015;2015:532615. doi: 10.1155/2015/532615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crespo J., Cifrián J., Pinto J.A., Jiménez-Gómez A., Pons-Romero F. Sleep apnea obstructive syndrome: a new complication previously undescribed in cirrhotic patients with ascites. Am J Gastroenterol. 2003;98:2815–2816. doi: 10.1111/j.1572-0241.2003.08754.x. [DOI] [PubMed] [Google Scholar]
- 39.Ghabril M., Jackson M., Gotur R. Most individuals with advanced cirrhosis have sleep disturbances, which are associated with poor quality of life. Clin Gastroenterol Hepatol. 2017;15:1271–1278. doi: 10.1016/j.cgh.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao X., Wong P. Managing sleep disturbances in cirrhosis. Scientifica (Cairo) 2016;2016:6576812. doi: 10.1155/2016/6576812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Formentin C., Garrido M., Montagnese S. Assessment and management of sleep disturbance in cirrhosis. Curr Hepat Rep. 2018;17:52–69. doi: 10.1007/s11901-018-0390-1. [published correction appears in Curr Hepatol Rep. 2018;17(3):300] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sateia M.J., Nowell P.D. Insomnia. Lancet. 2004;364:1959–1973. doi: 10.1016/S0140-6736(04)17480-1. [DOI] [PubMed] [Google Scholar]
- 43.van der Klaauw A.A., Dekkers O.M., Pereira A.M., van Kralingen K.W., Romijn J.A. Increased daytime somnolence despite normal sleep patterns in patients treated for non-functioning pituitary macroadenoma. J Clin Endocrinol Metab. 2007;92:3898–3903. doi: 10.1210/jc.2007-0944. [DOI] [PubMed] [Google Scholar]
- 44.Krishnan V., Collop N.A. Gender differences in sleep disorders. Curr Opin Pulm Med. 2006;12:383–389. doi: 10.1097/01.mcp.0000245705.69440.6a. [DOI] [PubMed] [Google Scholar]
- 45.Xiao G., Ye Q., Han T., Yan J., Sun L., Wang F. Study of the sleep quality and psychological state of patients with hepatitis B liver cirrhosis. Hepatol Res. 2018;48:E275–E282. doi: 10.1111/hepr.12981. [DOI] [PubMed] [Google Scholar]
- 46.Iwasa M., Karino Y., Kawaguchi T. Relationship of muscle cramps to quality of life and sleep disturbance in patients with chronic liver diseases: a nationwide study. Liver Int. 2018;38:2309–2316. doi: 10.1111/liv.13745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.