Abstract
Objective
To determine the impact of endometrial thickness on live birth outcomes and obstetric complication rate after hormone-replaced frozen embryo transfer.
Design
Retrospective cohort study.
Setting
Large, urban, academic fertility center.
Patient(s)
All patients with a singleton live birth after single euploid embryo transfer (by array comparative genomic hybridization or next-generation sequencing) in a hormone-replaced frozen embryo transfer cycle between January 2017 and December 2018 were reviewed.
Intervention(s)
None.
Main Outcome measure(s)
The primary outcomes were birth weight and obstetric complication rate.
Result(s)
A total of 492 patients were included. The median endometrial thickness was 8.60 mm (range, 6.0–20.0). The median gestational age at live birth was 39.4 weeks with a median birth weight of 3,345.2 g. Endometrial thickness was significantly correlated with birth weight. When patients were dichotomized into groups (those with an endometrial thickness of <7 mm and those with an endometrial thickness of >7 mm), neonates born from endometria with a thickness of <7 mm were born earlier (37.3 vs. 39.4 weeks and born with lower birth weights (2,749.9 vs. 3,345.2 g). It should be noted that only seven patients had an endometrium measuring <7 mm. Moreover, 7.1% (n = 35) of patients had an obstetric complication. Endometrial thickness was not significantly associated with obstetric complications, even with adjustments for age and medical history.
Conclusion(s)
Endometrial thickness may be a valuable predictor of placental health and birth weight. Further study is required to examine the relationship with individual obstetric complications, as our study may not have been powered to observe differences in obstetric complication rate, as well as the relationship between endometrial thickness and outcomes in natural frozen embryo transfer cycles.
Key Words: Birth weight, endometrial thickness, obstetric outcomes
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-21-00012
The use of assisted reproductive technology (ART) is increasing in the United States and worldwide (1), with more than 260,000 ART procedures reported to the US Centers for Disease Control in 2016 (2). Research has shown that women who undergo ART are at increased risk for a number of obstetric complications, even as multiple gestation rates among those who conceive via ART have declined (3). Possible complications include placenta accreta (4, 5), placenta previa (6, 7, 8), placental abruption (6, 7), preeclampsia (6, 9, 10) and other hypertensive disorders of pregnancy (6, 7, 8), premature rupture of membranes (8), preterm labor (8), preterm birth (7, 8, 10, 11), and low-birth-weight and small-for-gestational-age infants (10).
A healthy and receptive endometrium is crucial for healthy placentation. It is hypothesized that the endometrium plays a critical role in placentation via signaling molecules released from the uterine decidua that regulate both the timing and extent of trophoblastic invasion (12). Additionally, studies have shown that epigenetic changes to genes that control endometrial remodeling among women undergoing fresh embryo transfer may underlie altered trophoblastic invasion (13). Thus, evaluating for the health of endometrium has become routine before embryo transfer (14).
Abnormal placentation may underlie the pathophysiologic mechanisms leading to obstetric complications and disease among women undergoing ART (15). It is hypothesized that failure of the blastocyst trophoblastic cells to properly invade the uterine decidua and myometrium is key to the pathogenesis of preeclampsia, intrauterine growth restriction, placental abruption, and preterm birth. It is thought that improper trophoblastic invasion leads to abnormal spiral artery remodeling and alterations in blood flow and oxygen delivery to the fetus (15, 16). Given the increased incidence of ART use among women in the United States and worldwide, it is increasingly essential to understand the mechanisms behind the increased risk of placental-mediated complications and predictors of poor outcome among women undergoing ART.
Endometrial thickness is a key metric signifying the health of the endometrium and has been shown to be associated with clinical pregnancy and live birth rates among women undergoing both fresh and frozen embryo transfers (17, 18, 19, 20, 21, 22, 23, 24). However, few studies have evaluated the impact of endometrial thickness on neonatal and obstetric outcomes after embryo transfers that lead to an ongoing pregnancy and live birth. Additionally, most of these studies have investigated only either obstetric or neonatal outcomes, not both.
Therefore, we performed a retrospective cohort study among women who underwent hormone-replaced frozen embryo transfer (HR-FET) to address whether endometrial thickness is associated with both neonatal outcomes, such as neonatal birth weight and gestational age at birth, and placental-mediated adverse obstetric outcomes.
Methods
Study Design
We conducted a retrospective cohort study of all patients at a large, single, urban, academic fertility center in the United States with a singleton live birth from a single euploid HR-FET cycle between January 2017 and December 2018. The study was performed with New York University IRB approval (#s13-00389).
Subjects
All patients with a singleton live birth after single euploid embryo transfer (by array comparative genomic hybridization or next-generation sequencing) in an HR-FET cycle were included. We excluded patients with miscarriage after euploid embryo transfer, multiple gestation (monozygotic twins), and missing data.
Variables
All included patients were individually reviewed via the electronic medical record (EMR) by one researcher. Variables collected from the EMR included endometrial thickness at time of HR-FET, patient age at HR-FET, patient medical comorbidities, patient obstetric complications, gestational age of neonate at time of live birth, and neonatal birth weight. Patient medical comorbidities were reviewed by a separate researcher and grouped into three categories: mild, moderate, and severe. Classification was made on the basis of the severity of the condition and its likelihood of negatively impacting a pregnancy.
The primary independent variable, endometrial thickness, was measured by the physicians and physician assistants in the fertility center via ultrasound. Endometrial thickness referred to the peak thickness during the estradiol (E2) phase. Patients were characterized into two groups: one for patients with an endometrial thickness of <7 mm (“thin”); and the other for patients with an endometrial thickness of ≥7 mm (“thick”). This cutoff was chosen on the basis of our HR-FET protocol, in which the goal endometrial thickness is 7 mm and above, and a review of the literature.
The primary outcomes for this study were neonatal birth weight and a composite obstetric complication rate. The composite obstetric complication outcome was defined by the presence of any one of the following complications: preeclampsia or gestational hypertension, hemolysis, elevated liver enzymes, low platelet count (HELLP) syndrome, abnormal placentation and umbilical cord anomalies, premature rupture of membranes, oligohydramnios, or intrauterine growth restriction. These complications were included on the basis of their association with placental-mediated pathologies. The presence of any one of the aforementioned obstetric complications constituted the presence of the outcome.
The secondary outcomes included gestational age at delivery (calculated from the day of embryo transfer), rate of preterm delivery (defined as delivery at <37 weeks), and rate of small-for-gestational-age infants who were born at term (defined as infants born at >37 weeks at a birth weight of <2,500 g).
Ovarian Stimulation, Embryo Biopsy, Embryo Cryopreservation, and Preimplantation Genetic Testing for Aneuploidy Platforms
Controlled ovarian hyperstimulation utilizing a gonadotropin-releasing hormone antagonist protocol with administration of gonadotropins was prescribed for each patient on the basis of their antral follicle count, age, and follicle-stimulating hormone and anti-mullerian hormone levels as determined by their physician. Follicular growth and maturation were monitored by transvaginal ultrasound and serum E2 level. The gonadotropin- releasing hormone antagonist was added when a lead follicle was identified as ≥13 mm or the E2 level was >1,000 pg/mL. Either human chorionic gonadotropin alone or human chorionic gonadotropin with leuprolide acetate was used for trigger of final follicular maturation with planned oocyte aspiration scheduled for 35 h after administration. Oocyte retrieval was performed via ultrasound-guided transvaginal aspiration. Both insemination and intracytoplasmic sperm injection (ICSI) were used for fertilization of oocytes; ICSI was utilized if indicated by semen parameters, but in our center, preimplantation genetic testing for aneuploidy alone is not an indication for the use of ICSI.
Standard laboratory techniques were employed, and embryos were cultured to the blastocyst stage. Trophectoderm biopsy was performed at the blastocyst stage on day 5 or 6 before embryo cryopreservation via vitrification. Biopsy analysis was performed by array comparative genomic hybridization or next-generation sequencing. Blastocysts of patients who returned for frozen embryo transfer underwent standard embryo warming in our laboratory.
HR-FET Protocol
Hormone-replaced frozen embryo transfer cycles were defined by the treatment of oral E2 daily until the E2 level was >150 pg/mL and the endometrium measured a goal of ≥7 mm, followed by progesterone, 50–75 mg either intramuscularly in oil or as a vaginal suppository (most of the patients received intramuscular progesterone, with less than five patients receiving vaginal suppository). If the endometrium did not reach 7 mm, vaginal Estrace was added from 2 to 7 days or until the lining reached 7 mm. A direct embryo transfer under ultrasound guidance was performed on the sixth day of progesterone administration. Decision to proceed with HR-FET if the endometrium did not reach the goal of 7 mm was made on a case-by-case basis by the physician.
Statistical Analysis
All continuous variables were assessed for normality using the Kolmogorov–Smirnov test and determined to be nonparametric. Descriptive data are presented as median (range). Analysis was performed using the Mann–Whitney U test and Spearman’s rho correlation for nonparametric continuous variables and the Fisher exact and Pearson chi-square tests for categorical variables, where appropriate. A logistic regression was used to determine whether there was an association between endometrial thickness and obstetric complication rate, incidence of preterm birth, and incidence of small-for-gestational-age infants, where appropriate. Data analysis was conducted using SPSS (v.25), with P<.05 considered significant for all analyses.
Results
Demographics
In total, 492 patients were included in our analyses. The median age for all participants was 37.0 (range, 21–59) years. The median endometrial thickness for all patients was 8.6 (range, 6.0–20.0) mm. In this study cohort, seven unique patients (1.4%) had an endometrial thickness of <7 mm, and these patients had a median endometrial thickness of 6.6 mm (range, 6.0–6.9). There was no significant difference in age between those with an endometrial thickness of <7 mm and those with an endometrial thickness of ≥7 mm (35.00 vs. 37.00, P=.5). Moreover, 54.1% (n = 266) of patients had a medical comorbidity: 12.8%, mild; 28.5%, moderate; and 4.9%, major. There was no difference in rates of medical comorbidities between those with an endometrial thickness below and above 7 mm (57.1% vs. 54.0%, P=.76) (Table 1).
Table 1.
Baseline characteristics of the study participants by endometrial thickness.
| Endometrial thickness |
||
|---|---|---|
| <7 mm | ≥7 mm | |
| No. | 7 | 485 |
| Age (years) | 38.4 | 37.1 |
| Comorbidities (%) | 57.1 | 54 |
| Mild (%) | 14.3 | 12.8 |
| Moderate (%) | 28.6 | 27.5 |
| Severe (%) | 14.3 | 4.7 |
| Unknown (%) | 0 | 41 |
| Median number of total embryo transfers per patient | 1 (range, 1–9) | 1 (range, 1–15) |
Neonatal Outcomes
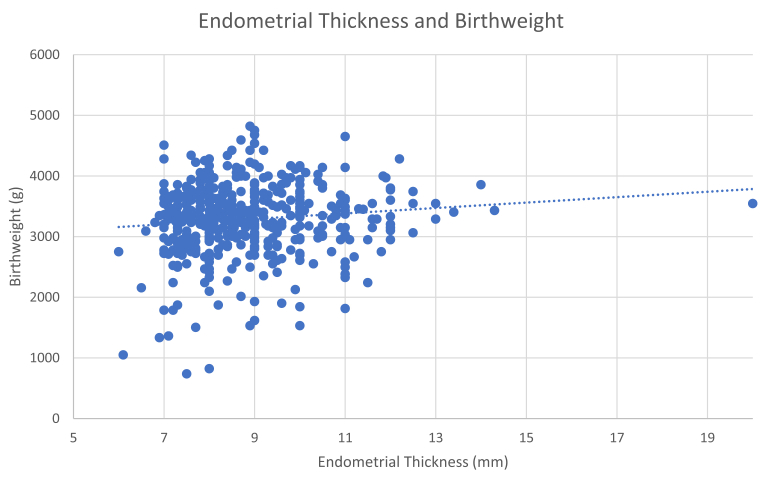
In the study cohort, the median birth weight was 3,345.2 g, and the median gestational age at live birth was 39.4 weeks. Overall, endometrial thickness was significantly positively correlated with birth weight (P<.03) (Fig. 1) but not with gestational age at live birth (P=.15). However, when patients were dichotomized into the two study subgroups (thin or thick), neonates born from a thin endometrium measuring <7 mm were more likely to be born at an earlier gestational age (37.3 vs. 39.4 weeks, P=.01) and had lower birth weights (2,749.9 vs. 3,345.2 g, P<.01) (Table 2).
Figure 1.
Correlation between endometrial thickness and neonatal birth weight.
Table 2.
Comparison of neonatal outcomes between infants by endometrial thickness.
| Endometrial thickness | ||
|---|---|---|
| <7 mm | ≥7 mm | |
| No. of neonates born | 7 | 485 |
| Mean birth weight (g)∗ | 2,421.9 | 3,300.4 |
| Mean gestational age (weeks)∗ | 36.2 | 38.9 |
| % Born at term (>37 weeks) | 71.4 (n = 5) | 91.1 (n = 442) |
| % Small for gestational age | 14.3 (n = 1) | 2.7 (n = 13) |
Difference reached statistical significance.
Overall, 8.7% (n = 43) of infants were born preterm, and 2.8% (n = 14) of term infants were born small for gestational age (Table 2). Endometrial thickness was not associated with the incidence of preterm birth (P=.67), and there was no significant difference in rates of preterm birth between those with a thin endometrium and those with a thick endometrium (28.6% vs. 8.5%, P=.12). Additionally, endometrial thickness was not associated with the incidence of small-for-gestational-age infants among those who were born at term (>37 weeks) (P=.32), and there was no significant difference in rates of small-for-gestational-age infants between those with an endometrial thickness of <7 mm and those with an endometrial thickness of ≥7 mm (20.0% vs. 3.0%, P=.15) (Table 2).
Adverse Obstetric Outcomes
In our cohort, 35 patients (7.1%) had an obstetric complication (Table 3). There was no significant association between endometrial thickness and a composite obstetric complication outcome (P=.19), even after controlling for age and medical comorbidities (P=.212). Additionally, there was no difference in obstetric complication rate between those with an endometrial thickness of <7 mm and those with an endometrial thickness of ≥7 mm (10% vs. 8.3%, P=.41) (Table 3).
Table 3.
Prevalence of delivery complications among participants by endometrial thickness.
| Delivery complications | Endometrial thickness |
|
|---|---|---|
| <7 mm | >7 mm | |
| Gestational hypertension | 0 | 10 |
| Preeclampsia | 1 | 8 |
| HELLP syndrome | 0 | 1 |
| Placenta previa | 0 | 3 |
| Placenta accreta | 0 | 1 |
| Bilobed placenta | 0 | 1 |
| Placental abruption | 0 | 2 |
| Abnormal cord insertion | 0 | 5 |
| Intrauterine growth restriction | 0 | 2 |
| Oligohydramnios | 0 | 1 |
| Premature rupture of membranes | 0 | 1 |
HELLP, hemolysis, elevated liver enzymes, low platelet count.
Discussion
Our study suggests that endometrial thickness is not only significant as a metric to predict probability of clinical pregnancy and live birth within ART but additionally helpful as an indicator of maternal and neonatal outcomes after pregnancy. Our results showed that endometrial thickness was significantly associated with neonatal birth weight and gestational age at live birth among women undergoing hormone-replaced frozen euploid embryo transfer. Specifically, our results demonstrate a positive correlation between endometrial thickness and neonatal birth weight and indicate that infants born from a “thin” endometrium of <7 mm are significantly more likely to have a lower birth weight and be born earlier than those born from a thick endometrium of ≥7 mm.
It must be noted that the number of patients in our study with an endometrial thickness of <7 mm was small at only seven patients. However, our results support prior studies showing that endometrial thickness is associated with live birth outcomes and, in particular, neonatal birth weight. Ribeiro et al. (25) similarly found that neonates born from an endometrium with a thickness of <7 mm were significantly more likely to have lower birth weight z-scores (25). In addition, other studies have demonstrated differences in neonatal birth weight by endometrial thickness, albeit with differing cutoffs. In an analysis of over 6,000 singleton newborns, Zhang et al. (26) found that among women undergoing day 3 frozen embryo transfer, an endometrial thickness of <8 mm was significantly associated with lower birth weight compared with an endometrial thickness of ≥10 mm after adjusting for gestational age. In addition, Guo et al. (27) found that endometrial thickness was significantly associated with birth weight and those with an endometrial thickness of ≤7.5 mm were significantly more likely to have a small-for-gestational-age neonate compared with to those with an endometrial thickness of >12 mm. Taken together, these studies indicate that endometrial thickness is a significant factor in healthy pregnancy and fetal development and support using a goal endometrial thickness of ≥7 mm as a conservative cutoff for progressing with embryo transfer.
The mechanism underlying the association between endometrial thickness and neonatal birth weight is likely multifaceted and has not been clearly elucidated. However, it has been hypothesized that a thin endometrium leads to higher oxygen tension and results in increased fetal exposure to reactive oxygen species (28) that are unhealthy for the developing fetus (29). There is discrepant vascularity between the functional and basal layers of the endometrium, with the large spiral arteries confined to the basal layer and a preponderance of capillaries in the functional layer (30). As thin endometria lack a sufficient functional layer, fetuses born from thin endometria suffer from increased proximity and exposure to the large spiral arteries and the increased oxygen tension that follows (28). Experiments demonstrating that the spiral arteries constrict after ovulation highlight the significance of limiting oxygen tension to the fetus in healthy pregnancies (31). In addition, it has been hypothesized that a thin endometrium leads to deficiencies in deep placentation and remodeling of the spiral arteries, leading to disruptions in blood flow to the placenta and within the uteroplacental circulation that adversely affect the growing fetus (26).
While our results did not demonstrate an association between endometrial thickness and prevalence of preterm and small-for-gestational-age neonates, those of other studies have shown such association (32, 33). Similarly, our results did not show an association between endometrial thickness and obstetric complication rate, while prior studies have shown significant correlations between endometrial thickness and a variety of individual obstetric complications. Oron et al. (33) found that an endometrial thickness of <7.5 mm is associated with a 1.5-fold increased risk of adverse obstetric outcome, as defined by preeclampsia, placenta previa, placental abruption, manual removal of an adherent placenta, small-for-gestational-age neonate, and preterm delivery. He et al. (34) demonstrated than an endometrial thickness of <8 mm is significantly associated with an increased risk of premature rupture of membranes and postpartum hemorrhage. Jing et al. (35) found that women with a thicker endometrium were at lower risk for placenta previa and cesarean section delivery. Rombauts et al. (36) similarly found that in those undergoing single embryo transfer, there is a fourfold increased risk for placenta previa among women with an endometrial thickness of <9 mm compared with those with an endometrial thickness of >12 mm, and Kaser et al. (4) described a significant association between placenta accreta and thinner endometria. Additionally, both Liu et al. (37) and Rombauts et al. (38) described an association between thinner endometria and risk of ectopic pregnancy among women undergoing frozen embryo transfer and in vitro fertilization, respectively.
The discrepancy between the results of this study and those seen in the literature may be explained by the fact that there were few women with obstetric complications (7.1% of participants) included in the study, and we thus may not have been powered to observe a statistically significant correlation. Additionally, the use of a composite score may have hidden significant associations within the total. Similarly, there were few small-for-gestational-age (2.8% of infants) and preterm infants (8.7% of infants).
Strengths and Limitations
The strengths of this study include its large sample size and comprehensive sampling method. Additionally, bias was limited by ensuring that the individual who completed chart review was not responsible for coding the severity of medical comorbidities.
This study has several limitations, chief among them the retrospective design and small number of women with an endometrial thickness of <7 mm. However, given that our results regarding birth weight are concordant with several recently published studies, it is likely that these results are valid. As stated previously, there were additionally few women with obstetric complications and with preterm and small-for-gestational-age infants. Combined with the fact that there were additionally few participants with an endometrial thickness of <7 mm, this study may not have had sufficient power to evaluate differences in these metrics between the two populations, explaining the discrepant results between our study and those previously published. Additionally, multiple providers were responsible for taking measurements of the endometrium, and there was likely slight variability between different providers. In addition, this study is limited by the information that could be extracted from the EMR, meaning that several baseline characteristics of the study participants, including smoking status and infertility duration, could not be assessed.
Future Directions
Prospectively designed studies are needed to further evaluate the relationship between endometrial thickness and birth weight and gestational age. Further study is required to examine the relationship between endometrial thickness and individual obstetric complications, such as placenta previa and accreta, preeclampsia and gestational hypertension, and placental abruption, and the relationship between endometrial thickness and miscarriage rate. Additionally, further study is needed to compare the risks of a thin endometrium in naturally prepared and hormone-controlled embryo transfer cycles. It is worth noting that, overall, infants born from ART appear to have no increased risk of neurodevelopmental (39), cognitive, behavioral, or growth delay (40) compared with those conceived without ART. In addition, future studies are needed to evaluate the long-term sequelae, if any, among infants born from mothers using ART with thin endometria.
In conclusion, among women undergoing HR-FET, an endometrial thickness of <7 mm was associated with lower neonatal birth weight and decreased gestational age at live birth. This indicates that endometrial thickness is a valuable metric when considering the health of the endometrium and is useful to assess routinely before embryo transfer. Further study is needed to elucidate individual obstetric complications that may be related to a thin endometrium, as prior studies have demonstrated an association between endometrial thickness and various obstetric complications, but our study may not have been powered to observe differences in obstetric complication rate. Moreover, further study is required to compare how endometrial thickness impacts outcomes in naturally prepared and hormone-controlled embryo transfer cycles.
Acknowledgments
The investigators would like to thank all of the participants who were included in this study.
Footnotes
R.A.M. has nothing to disclose. J.K.B. has nothing to disclose. J.A.G. has nothing to disclose.
References
- 1.De Geyter C. Assisted reproductive technology: impact on society and need for surveillance. Best Pract Res Clin Endocrinol Metab. 2019;33:3–8. doi: 10.1016/j.beem.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Sunderam S., Kissin D.M., Zhang Y., Folger S.G., Boulet S.L., Warner L. Assisted reproductive technology surveillance - United States, 2016. MMWR Surveill Summ. 2019;68:1–23. doi: 10.15585/mmwr.ss6804a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen S.H., Bergh C., Gissler M., Åsvold B.O., Romundstad L.B., Tiitinen A. Time trends in placenta-mediated pregnancy complications after assisted reproductive technology in the Nordic countries. Am J Obstet Gynecol. 2020;223:226.e1–226.e19. doi: 10.1016/j.ajog.2020.02.030. [DOI] [PubMed] [Google Scholar]
- 4.Kaser D.J., Melamed A., Bormann C.L., Myers D.E., Missmer S.A., Walsh B.W. Cryopreserved embryo transfer is an independent risk factor for placenta accreta. Fertil Steril. 2015;103:1176–1184.e2. doi: 10.1016/j.fertnstert.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Esh-Broder E., Ariel I., Abas-Bashir N., Bdolah Y., Celnikier D.H. Placenta accreta is associated with IVF pregnancies: a retrospective chart review. BJOG. 2011;118:1084–1089. doi: 10.1111/j.1471-0528.2011.02976.x. [DOI] [PubMed] [Google Scholar]
- 6.Shevell T., Malone F.D., Vidaver J., Porter T.F., Luthy D.A., Comstock C.H. Assisted reproductive technology and pregnancy outcome. Obstet Gynecol. 2005;106:1039–1045. doi: 10.1097/01.AOG.0000183593.24583.7c. [DOI] [PubMed] [Google Scholar]
- 7.Nagata C., Yang L., Yamamoto-Hanada K., Mezawa H., Ayabe T., Ishizuka K. Complications and adverse outcomes in pregnancy and childbirth among women who conceived by assisted reproductive technologies: a nationwide birth cohort study of Japan environment and children’s study. BMC Pregn Childbirth. 2019;19:77. doi: 10.1186/s12884-019-2213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santi E., Nencini G., Cerni A., Greco P., Spelzini F., Tormettino B. The PLART study: incidence of preterm labor and adverse pregnancy outcomes after assisted reproductive techniques-a retrospective cohort study. Arch Gynecol Obstet. 2019;300:911–916. doi: 10.1007/s00404-019-05261-2. [DOI] [PubMed] [Google Scholar]
- 9.Almasi-Hashiani A., Omani-Samani R., Mohammadi M., Amini P., Navid B., Alizadeh A. Assisted reproductive technology and the risk of preeclampsia: an updated systematic review and meta-analysis. BMC Pregnancy Childbirth. 2019;19:149. doi: 10.1186/s12884-019-2291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berntsen S., Söderström-Anttila V., Wennerholm U.B., Laivuori H., Loft A., Oldereid N.B. The health of children conceived by ART: ‘the chicken or the egg?’. Hum Reprod Update. 2019;25:137–158. doi: 10.1093/humupd/dmz001. [DOI] [PubMed] [Google Scholar]
- 11.Grady R., Alavi N., Vale R., Khandwala M., McDonald S.D. Elective single embryo transfer and perinatal outcomes: a systematic review and meta-analysis. Fertil Steril. 2012;97:324–331. doi: 10.1016/j.fertnstert.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 12.Knöfler M., Pollheimer J. IFPA Award in Placentology lecture: molecular regulation of human trophoblast invasion. Placenta. 2012;33:S55–S62. doi: 10.1016/j.placenta.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senapati S., Wang F., Ord T., Coutifaris C., Feng R., Mainigi M. Superovulation alters the expression of endometrial genes critical to tissue remodeling and placentation. J Assist Reprod Genet. 2018;35:1799–1808. doi: 10.1007/s10815-018-1244-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasius A., Smit J.G., Torrance H.L., Eijkemans M.J., Mol B.W., Opmeer B.C. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:530–541. doi: 10.1093/humupd/dmu011. [DOI] [PubMed] [Google Scholar]
- 15.Kroener L., Wang E.T., Pisarska M.D. Predisposing factors to abnormal first trimester placentation and the impact on fetal outcomes. Semin Reprod Med. 2016;34:27–35. doi: 10.1055/s-0035-1570029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim Y.M., Bujold E., Chaiworapongsa T., Gomez R., Yoon B.H., Thaler H.T. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189:1063–1069. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 17.Yuan X., Saravelos S.H., Wang Q., Xu Y., Li T.C., Zhou C. Endometrial thickness as a predictor of pregnancy outcomes in 10787 fresh IVF-ICSI cycles. Reprod Biomed Online. 2016;33:197–205. doi: 10.1016/j.rbmo.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Zhang T., Li Z., Ren X., Huang B., Zhu G., Yang W. Endometrial thickness as a predictor of the reproductive outcomes in fresh and frozen embryo transfer cycles: a retrospective cohort study of 1512 IVF cycles with morphologically good-quality blastocyst. Medicine (Baltimore) 2018;97:e9689. doi: 10.1097/MD.0000000000009689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bu Z., Wang K., Dai W., Sun Y. Endometrial thickness significantly affects clinical pregnancy and live birth rates in frozen-thawed embryo transfer cycles. Gynecol Endocrinol. 2016;32:524–528. doi: 10.3109/09513590.2015.1136616. [DOI] [PubMed] [Google Scholar]
- 20.Ma N.Z., Chen L., Dai W., Bu Z.Q., Hu L.L., Sun Y.P. Influence of endometrial thickness on treatment outcomes following in vitro fertilization/intracytoplasmic sperm injection. Reprod Biol Endocrinol. 2017;15:5. doi: 10.1186/s12958-016-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan Y., Hao G., Wang Q., Liu H., Wang Z., Jiang Q. Major factors affecting the live birth rate after frozen embryo transfer among young women. Front Med (Lausanne) 2020;7:94. doi: 10.3389/fmed.2020.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang R., Cai L., Xiong F., Chen J., Yang W., Zhao X. The effect of endometrial thickness on the day of hCG administration on pregnancy outcome in the first fresh IVF/ICSI cycle. Gynecol Endocrinol. 2016;32:473–476. doi: 10.3109/09513590.2015.1132304. [DOI] [PubMed] [Google Scholar]
- 23.Liu K.E., Hartman M., Hartman A., Luo Z.C., Mahutte N. The impact of a thin endometrial lining on fresh and frozen-thaw IVF outcomes: an analysis of over 40 000 embryo transfers. Hum Reprod. 2018;33:1883–1888. doi: 10.1093/humrep/dey281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Toukhy T., Coomarasamy A., Khairy M., Sunkara K., Seed P., Khalaf Y. The relationship between endometrial thickness and outcome of medicated frozen embryo replacement cycles. Fertil Steril. 2008;89:832–839. doi: 10.1016/j.fertnstert.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro V.C., Santos-Ribeiro S., De Munck N., Drakopoulos P., Polyzos N.P., Schutyser V. Should we continue to measure endometrial thickness in modern-day medicine? The effect on live birth rates and birth weight. Reprod Biomed Online. 2018;36:416–426. doi: 10.1016/j.rbmo.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J., Liu H., Mao X., Chen Q., Si J., Fan Y. Effect of endometrial thickness on birthweight in frozen embryo transfer cycles: an analysis including 6181 singleton newborns. Hum Reprod. 2019;34:1707–1715. doi: 10.1093/humrep/dez103. [DOI] [PubMed] [Google Scholar]
- 27.Guo Z., Xu X., Zhang L., Zhang L., Yan L., Ma J. Endometrial thickness is associated with incidence of small-for-gestational-age infants in fresh in vitro fertilization-intracytoplasmic sperm injection and embryo transfer cycles. Fertil Steril. 2020;113:745–752. doi: 10.1016/j.fertnstert.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Casper R.F. It’s time to pay attention to the endometrium. Fertil Steril. 2011;96:519–521. doi: 10.1016/j.fertnstert.2011.07.1096. [DOI] [PubMed] [Google Scholar]
- 29.Catt J.W., Henman M. Toxic effects of oxygen on human embryo development. Hum Reprod. 2000;15:199–206. doi: 10.1093/humrep/15.suppl_2.199. [DOI] [PubMed] [Google Scholar]
- 30.Bartelmez G.W. The form and the functions of the uterine blood vessels in the rhesus monkey. Contrib Embryol. 1957;36:153–182. [Google Scholar]
- 31.Rossman I., Bartelmez G.W. The injection of the blood vascular system of the uterus. Anat Rec. 1957;128:223–231. doi: 10.1002/ar.1091280207. [DOI] [PubMed] [Google Scholar]
- 32.Mouhayar Y., Franasiak J.M., Sharara F.I. Obstetrical complications of thin endometrium in assisted reproductive technologies: a systematic review. J Assist Reprod Genet. 2019;36:607–611. doi: 10.1007/s10815-019-01407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oron G., Hiersch L., Rona S., Prag-Rosenberg R., Sapir O., Tuttnauer-Hamburger M. Endometrial thickness of less than 7.5 mm is associated with obstetric complications in fresh IVF cycles: a retrospective cohort study. Reprod Biomed Online. 2018;37:341–348. doi: 10.1016/j.rbmo.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 34.He L., Zhang Z., Li H., Li Y., Long L., He W. Correlation between endometrial thickness and perinatal outcome for pregnancies achieved through assisted reproduction technology. J Perinat Med. 2019;48:16–20. doi: 10.1515/jpm-2019-0159. [DOI] [PubMed] [Google Scholar]
- 35.Jing S., Li X., Zhang S., Gong F., Lu G., Lin G. The risk of placenta previa and cesarean section associated with a thin endometrial thickness: a retrospective study of 5251 singleton births during frozen embryo transfer in China. Arch Gynecol Obstet. 2019;300:1227–1237. doi: 10.1007/s00404-019-05295-6. [DOI] [PubMed] [Google Scholar]
- 36.Rombauts L., Motteram C., Berkowitz E., Fernando S. Risk of placenta praevia is linked to endometrial thickness in a retrospective cohort study of 4537 singleton assisted reproduction technology births. Hum Reprod. 2014;29:2787–2793. doi: 10.1093/humrep/deu240. [DOI] [PubMed] [Google Scholar]
- 37.Liu H., Zhang J., Wang B., Kuang Y. Effect of endometrial thickness on ectopic pregnancy in frozen embryo transfer cycles: an analysis including 17,244 pregnancy cycles. Fertil Steril. 2020;113:131–139. doi: 10.1016/j.fertnstert.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Rombauts L., McMaster R., Motteram C., Fernando S. Risk of ectopic pregnancy is linked to endometrial thickness in a retrospective cohort study of 8120 assisted reproduction technology cycles. Hum Reprod. 2015;30:2846–2852. doi: 10.1093/humrep/dev249. [DOI] [PubMed] [Google Scholar]
- 39.Basatemur E., Sutcliffe A. Follow-up of children born after ART. Placenta. 2008;29:135–140. doi: 10.1016/j.placenta.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 40.Shankaran S. Outcomes from infancy to adulthood after assisted reproductive technology. Fertil Steril. 2014;101:1217–1221. doi: 10.1016/j.fertnstert.2014.03.049. [DOI] [PubMed] [Google Scholar]