Abstract
Objective
To examine the changes in AMH levels longitudinally over time and their relationship with both body composition, particularly abdominal adiposity, and milestones of pubertal development in female children.
Design
Secondary analysis of a prospective, longitudinal study.
Setting
University affiliated research center and laboratories.
Patient(s)
Eighty-nine females were examined between 1990 and 2015 to study child growth and development.
Intervention(s)
Demographic, anthropometric, growth, and pubertal milestone data with serum samples stored and subsequently analyzed for AMH.
Main Outcome Measure(s)
Longitudinal change in AMH and predicted AMH levels based on body composition, age, and pubertal milestones including, pubarche, thelarche, and menarche.
Result(s)
Natural log-transformed AMH (AMHlog) levels appeared to have a nonlinear relationship with age, decreasing between 10 and 14 years of age, increasing until 16 years. A mixed effect linear model demonstrated that increased abdominal adiposity (waist/height ratio, WHtR) was significantly associated with the predicted increased AMHlog levels (β=1.37). As females progressed through the Tanner stages, the model predicted decreasing AMHlog values when adjusting for age and WHtR.
Conclusion(s)
Declining AMH levels during puberty may not be reflective of diminished ovarian reserve as observed in adults, but may suggest a permissive role of AMH in the activation of the hypothalamic-pituitary-ovarian axis.
Key Words: Antimüllerian hormone, menarche, puberty, pubarche, thelarche
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-20-00028
Antimüllerian hormone (AMH), a member of the transforming growth factor-β family, is produced by the granulosa cells of the ovary and prevents excessive recruitment of primordial follicles by pituitary gonadotropins (1). Most of the studies on AMH levels have been conducted in infertile adult women. AMH levels are believed to be a surrogate marker of the remaining follicular pool (2) and are regarded as a marker of ovarian reserve that may be useful in predicting the response to ovarian stimulation (3). These previous findings suggest that AMH levels both reflect the remaining follicular pool and provide some insights into ovarian function. However, despite these relationships observed in the infertile female population, AMH levels do not appear to be predictive of spontaneous conception, as measured by positive pregnancy testing (4). Thus, the relationship between AMH levels and reproductive potential in the general population remains controversial.
Intrigued by this potential marker of ovarian function, several investigators have evaluated the natural course of AMH production in childhood and adolescence to gain a better understanding of the reference values in the noninfertile population. In cross-sectional and longitudinal studies, AMH levels tend to rise in childhood and adolescence, peak in young adulthood and decline until ovarian senescence (5, 6, 7). Moreover, some studies have established that obese children have higher AMH values than normal-weight children and will reach puberty earlier (7, 8). Paradoxically, studies on factors that affect AMH levels in adults have found that obese adult females have lower AMH values compared with their normal-weight counterparts (9, 10). To our knowledge, no studies have previously examined AMH levels through the entire pubertal transition period into adulthood with respect to body composition (7). We postulate that AMH levels will decrease throughout puberty in female children, but the changes will be variable depending on their body composition, especially abdominal adiposity. Thus, we seek to examine longitudinal changes in AMH from childhood to adulthood, analyzing its relationship with both developmental pubertal milestones and body composition.
Materials and methods
Data Collection
This study was approved by the University of Southern California Institutional Review Board (IRB #HS-16-07659) and Wright State University (IRB #HSC 3187) and was a secondary analysis of the Fels Longitudinal Study, which is the oldest continuous study of growth, development, and aging in the world (11). The Fels Longitudinal Study was initiated in Yellow Springs, Ohio, in 1929 to follow child growth and development during the Great Depression to help protect the health of children. Throughout the years, more data have emerged on the risk factors for various chronic diseases such as cardiovascular disease (CVD) and obesity, as well as establishing growth charts to follow maturation (11). Since its inception, the Fels Longitudinal Study participants have not been selected based on health status or any other obesity, CVD, or diabetes-related traits. At enrollment, the participants lived in Southwestern Ohio within a 30-mile radius of Yellow Springs, Ohio. Enrollment in the study began with approximately 10 newborns per year, increasing since the 1930s to 15–20 per year, with the oldest participants with long-term serial data from birth now in their late 80s. Participants were not examined during menstruation, pregnancy, or when there were any transient conditions (e.g., infectious disease) that could affect data quality (11). The Fels Longitudinal Study population comprised of 98% non-Hispanic European Americans.
Informed consent for participants was obtained at all visits at Wright State University in Dayton, OH. At each study visit, demographics, physical growth parameters, pubertal milestones, body composition measurements, and fasting blood samples were collected annually. All female participants with available frozen, serum (at −80 °C) were included in our analysis. We included 89 participants, aged 8 to 18 years, who had between 1 and 7 visits, totaling 226 visits between the years of 1990 and 2015. Scheduled study visits were set for specific target ages, but this has not always been feasible (e.g., health conditions at the target age study visit). Due to this, study participants were examined at different ages, and subsequently time intervals between serial examinations varied by visits and by study participants (visit range, 1–7 visits). On average, the available data span an interval of approximately 4.7 years (SD, 2.3 years) between the first and last follow-up among participants with more than one study visit. A total of 36 participants had one study visit, 15 participants had 2 study visits, and 38 participants had ≥3 visits.
Demographic, anthropometric, growth, and pubertal milestone data were recorded and kept in a secure database at Wright State University. Parameters collected included: age; height; weight; body mass index (BMI); age and sex-adjusted BMI percentile; waist circumference; and estimated ages at reaching individual pubertal milestones. At each visit, weight (kg), stature (cm), and waist circumference (cm) were obtained, using techniques similar to corresponding measurements in the Anthropometric Standardization Reference Manual (12, 13) or to corresponding measurements in the National Health and Nutrition Examination Survey (NHANES) III and the current NHANES. BMI was calculated using the formula, BMI = weight (kg)/height2 (m2), and age-specific and sex-specific BMI percentiles were calculated. Each child also used a standardized method to self-assess her sexual maturity according to the stages of breast and pubic hair development. This standardized self-assessment was originally described by Reynolds and Wines using the Fels Longitudinal Study data and was later popularized by Tanner (14, 15). The purpose of the self-assessment was explained in private to each study participant, and the participants were shown sex-appropriate sets of standardized photographs along with verbal descriptions matching participants’ developmental stages (Tanner stages I to V)(14, 15). Self-asssessment has been validated against physical examination, correlates well with pubic hair stages, and has a moderate correlation with the breast and genital stages (16, 17, 18). Pubertal milestones were defined as Tanner stage II for both thelarche and pubarche, and the onset of the first menses for menarche. Reproductive history including gynecological health status was collected for age at menarche (in years) and for supplemental hormone use. Self-reported oral contraceptive use was included as either present or absent.
For laboratory collection, venipuncture was performed during study visits after at least eight hours of fasting. Serum samples were processed within 60 minutes after blood collection and were stored at −80 °C until analysis for AMH as described below.
Antimüllerian Hormone Measurement
Frozen, unthawed, and deidentified serum samples were shipped on dry ice to the Reproductive Endocrine Research Laboratory at the University of Southern California for biomarker assays. The Ultrasensitive AMH ELISA from Ansh Labs (Webster, TX) was used to determine AMH levels, with an assay sensitivity of 60 pg/mL. The interassay coefficient of variation was 9.7% at 1.6 ng/mL and 12.0% at 4.5 ng/mL, respectively.
Statistical Analysis
Pubertal milestones were dichotomized as present or absent for analysis at each study visit. BMI percentile and waist to height ratio (WHtR) were used for body composition. Due to the lack of normal distribution, AMH values were natural log-transformed (AMHlog) for analysis. A generalized linear mixed effect (LME) model with a random participant intercept and unstructured covariance matrix was used for analysis between AMHlog levels and milestones of pubertal development and body composition. The best model including significant body composition variables was based on the approach using the information criteria such as the Akaike information criterion and Bayesian information criterion (19). The final statistical LME model based on WHtR, rather than BMI due to model fit, was used to calculate the predicted AMH values based on a subject’s age and BMI as well as before and after reaching thelarche and pubarche to illustrate the associations using Figures. All data were analyzed using SAS version 9.4. P values <.05 were set as statistically significant.
Results
Descriptive characteristics of participants on their first visit are represented in Supplemental Table 1 (available online). At their first visit, of the participants included in the analysis, 12.4% were obese and 11.2% were overweight. A total of 83 participants (93.3%) were non-Hispanic Whites. Mean ± SD for the age of menarche was 12.6 ± 1.2 years. Mean BMI was 19.4 ± 4.3 kg/m2 and WHtR was 0.47 ± 0.06. Approximately 19.6% of study visits were examined at Tanner stage II; 27.3% at Tanner stage III; 25.9% at Tanner stage IV; and 7.3% at Tanner stage V. Overall, the mean AMH from all visits was 5.3 ± 3.4 ng/mL and the median was 4.2 ng/mL (interquartile range: ± 4.4 ng/mL). Mean AMH values for study subjects on reaching thelarche, pubarche, and menarche were 4.3 ± 2.2 ng/mL; 4.3 ± 1.8 ng/mL; and 3.9 ± 2.7 ng/mL, respectively. Overall, 7 participants reported using oral contraceptives at their last study visit, resulting in 8 observations out of 226 study visits.
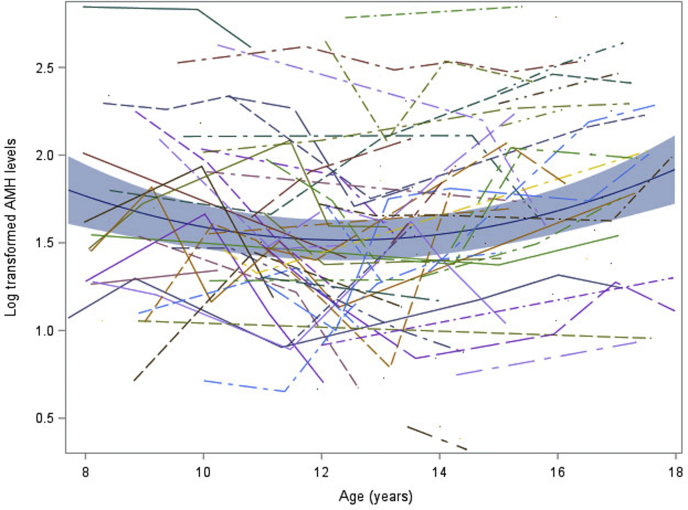
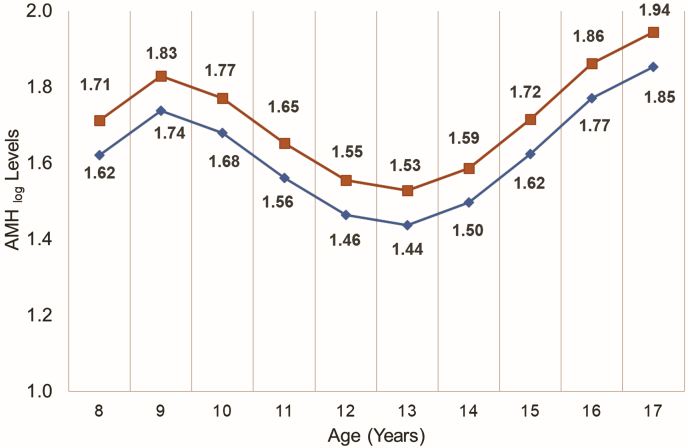
A significant, nonlinear association was observed with AMHlog levels over time (Fig. 1), decreasing between ages 10 and 14 years of age then increasing until 16 years. The relationship between oral contraceptive use and AMHlog was negative (β=-0.007), but it was not significantly associated with AMHlog levels (P=.966). Using values from our study subjects and a mixed effect linear model, we calculated the predicted AMH levels at each age for those of normal weight and those with a WHtR one SD above the mean. Increased abdominal adiposity significantly correlated with an increase in AMHlog levels (Fig. 2, β=1.37, P=.035). AMHlog levels for subjects with a WHtR one SD above the mean were consistently higher, while still following the overall nonlinear trend seen throughout childhood and adolescence in normal-weight counterparts (Fig. 2).
Figure 1.
Longitudinal change in AMHlog of study participants. The blue line is the predicted value from mixed effect modeling. The blue shade is 95%CI prediction interval. Log-transformed AMH values from each participant are also plotted (individual lines).
Figure 2.
Predicted AMHlog levels by waist to height ratio across different ages. The red line (squares) is the sample mean WHtR (0.40). The blue line (diamonds) is one SD above the mean WHtR (0.54).
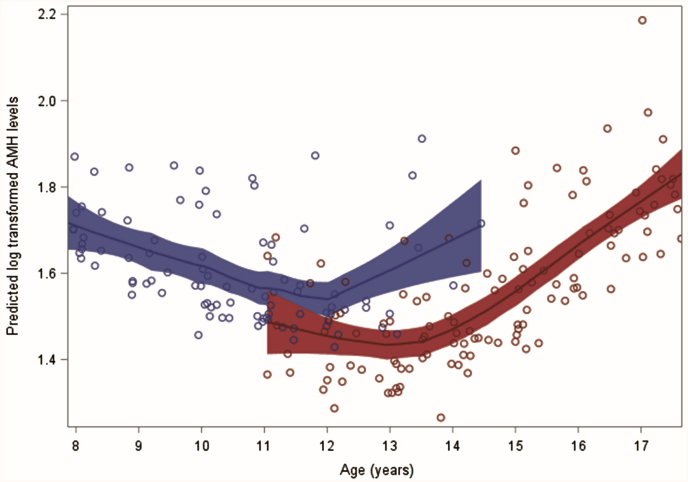
Our model also allowed us to examine predicted AMH levels on the basis of pubarche and thelarche status in those with a normal WHtR. At each age, the predicted AMHlog level of a girl who reached pubarche was lower than one who had not reached pubarche, provided she had a normal WHtR (Fig. 3). For example, in 12-year-old girls, the predicted, back-transformed AMH value for having reached pubarche was 4.15 ng/mL compared with 4.72 ng/mL for those who had not reached pubarche. The overall trajectory of predicted AMHlog levels throughout adolescence in our pubarche model matched those seen from the analysis of AMH values in our study subjects, demonstrating a nonlinear trend with an initial decrease in predicted AMH levels at age 10 and then a subsequent rise around age 14.
Figure 3.
Predicted AMHlog levels by pubarche status across different ages. Back-transformed AMH values as follows: 1.55 = 4.72 ng/mL; 1.42 = 4.15 (ng/mL). Red = pubarche; blue = no pubarche.
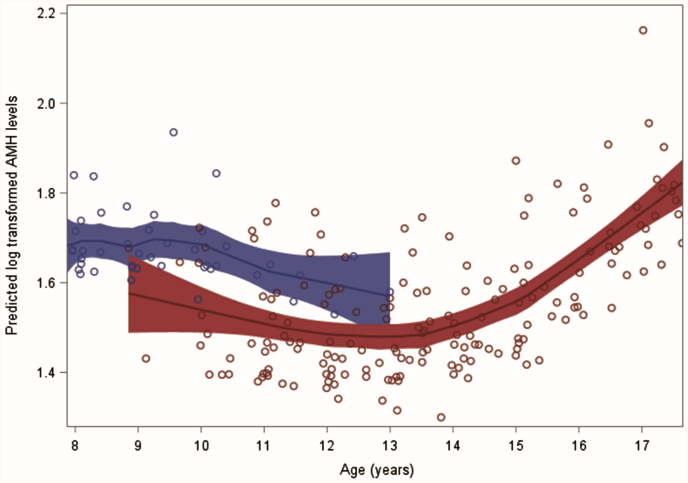
Similarly, after age 9, girls with a normal WHtR who had reached thelarche had lower predicted AMHlog levels than those who had not reached thelarche (Fig. 4). For 12-year-old girls, the predicted, back-transformed AMH value for those having reached thelarche was 4.05 ng/mL compared with 6.85 ng/mL for those who had not reached puberty. However, the overall trend of predicted AMH levels did not follow the same nonlinear curve, as seen in the other analysis. Rather, predicted AMH levels in those who had not yet reached thelarche start below those at ages 8 and 9, with a shift seen around 9-and-a-half years of age.
Figure 4.
Predicted AMH levels by hypothetical thelarche status across different ages. Back-transformed AMH values as follows: 1.92 = 6.85 ng/mL; 1.40= 4.05 (ng/mL). Red = thelarche; blue = no thelarche.
Discussion
Following the discovery of an association between AMH and ovarian reserve, several studies have analyzed how this relationship influences fertility outcomes, especially in relation to assisted reproduction. However, the function of AMH, the changes in its levels over time, and the consequences of those changes on fertility in the general population have remained elusive. Of particular interest is the function of AMH at the time of pubertal development. Given that prior studies showing obese girls undergo puberty at earlier ages, it is interesting that our study found that changes in AMH levels are not only correlated with pubertal milestones but also with body composition, specifically abdominal adiposity. Indeed, this secondary analysis of a prospective study was designed to investigate and understand this trend in relation to obesity and pubertal development. To our knowledge, this is the largest study to date specifically to analyze the changes in AMH levels throughout puberty with respect to body composition.
A previous longitudinal study also examined the changes in AMH over time. Hagen et al. studied Danish girls from birth through menopausal adults, and reported an elevation of AMH right before puberty, which remained stable until approximately 25 years of age, then slowly declined until menopause (20). Our study was more focused on the narrow age range across puberty and the possible effects of childhood body composition on AMH changes. Similar to Hagen et al. (20), we found a high level of AMH before puberty but we could also detect a marked decline in AMH, correlating with the transition through puberty (approximately 14 years) and then a return of AMH to the higher levels by the age of 17. Our study included multiple samples over time from individual girls, a data set that may have allowed more sensitivity to slight changes in AMH during the transition window. Importantly, from both studies, it can be inferred that decreasing values of AMH during puberty are not necessarily reflective of diminishing ovarian reserve, as occurs in adult females. This dip in AMH levels during puberty may indicate an oscillation in the number of growing follicles during the window of transition, although only ultrasound or histologic data may be used to directly corroborate this.
Our study also characterized the changes in predicted AMH levels with the development of pubertal milestones, observing that the onset of thelarche and pubarche were associated with lower predicted AMH values compared with those who have not yet reached these milestones. Moreover, we found that the predicted AMH levels in those who have not reached thelarche remained higher than in those who have. Given that thelarche signals the successful initiation of gonadotropin stimulation of the ovary with concomitant sex steroid production, we hypothesize that the decrease in AMH around the initiation of puberty is either necessary or a function of the pubertal transition.
Previous studies have examined differences in AMH levels in those with central precocious puberty (CPP), or premature thelarche (PT). Savas-Erdeve et al. (21) evaluated AMH levels in individuals with CPP (n = 21) and PT (n = 24) compared with age-appropriate controls (n = 22). Those with PT had higher AMH levels than those with CPP. Similarly, Sahin et al. (22) reported that AMH levels were significantly lower in those with CPP vs. PT, again helping delineate those who are undergoing the progressive changes of puberty versus those in whom estrogen-stimulated breast development has occurred, but are otherwise halted in further pubertal development. Given that PT is not necessarily puberty, which must be coupled with a progressive sequence of defined events, it does appear that AMH levels could be used to distinguish between female children in whom a progressive process is occurring and in whom it is not. Chen et al. (23) analyzed 83 individuals with CPP and observed lower levels of AMH in those that have more rapidly progressive CPP and ultimately required treatment to achieve full adult height, suggesting that the decrease in AMH around puberty signals a fully functional hypothalamic-pituitary-ovarian axis. Additional studies should explore the potential diagnostic value of AMH in CPP. However, this is not currently feasible because AMH cutoffs are yet to be established.
Our study spanned 25 years between 1990 and 2005. Therefore, it may have been influenced by changes in dietary trends and environmental exposures during that period. The calendar time of study visit may serve as an overall indicator of any specific secular trends. Thus, we examined the relationship between sample collection timing (study visit date) by decades (e.g., visit between 1990 and 2000 as first visit group) and AMH levels. However, we did not observe any significant visit time effect on AMH levels in LME model analyses.
With respect to the interplay between anthropomorphic attributes and initiation of puberty, our analysis revealed that girls with a WHtR or waist circumference adjusted for height one SD above the mean had higher AMH levels throughout puberty compared with those who had normal WHtRs. However, the overall trend in terms of AMH over time mirrored that of normal-weight counterparts, with an inflection point around the pubertal transition. Some previous studies have reported that women with polycystic ovarian syndrome (PCOS) often have high AMH levels, and, from our data, it appears that a transient decrease in AMH is associated with the start of normal folliculogenesis regardless of WHtR. At present, it is unclear whether being an obese adolescent with higher AMH levels confers an increased risk of abnormal folliculogenesis and development of PCOS, as our study was not able to evaluate PCOS versus non-PCOS. Although PCOS is very difficult to diagnose during adolescence, it is an area that requires further investigation For instance, the analysis of androgen values may provide useful insights into the relationship between obesity and AMH in this study population.
The strengths of this study include its longitudinal nature and the large number of females observed from early adolescence into their reproductive years. This enabled us to connect pubertal milestones with hormonal and anthropomorphic data. Some of the limitations of our study include its retrospective nature, the fact that the accuracy of the recorded information could not be verified, and the difficulty of generalizing our results generalized to populations other than non-Hispanic European Americans. Moreover, diagnostic criteria have changed over time as well as the coding of metabolic disease(s), particularly PCOS. As such, we could not include PCOS status in our analysis, which may have been illuminating in the context of the available anthropometric data. Lastly, given that the attainment of puberty was characterized as a dichotomous variable, we were unable to track AMH levels with all five of the specific Tanner stages.
Conclusion
Overall, our finding of a significant, nonlinear relationship between AMH levels and a noted decrease around the onset of puberty suggests that AMH levels may be a biochemical marker of ovarian activity. Moreover, this suggests that a decrease in AMH value in puberty is not necessarily reflective of diminished ovarian reserve, although subsequent fertility was not recorded. The elevated AMH levels seen with elevated abdominal adiposity, represented as WHtR, is an association that requires further investigation in terms of the cause-effect relationship. Whether this influences the development of PCOS remains unclear, but it is strongly suggestive of a larger number of activated and growing follicles in adolescents with high WHtR (24). Our data also suggest a permissive role of AMH in the activation of the hypothalamic-pituitary-ovarian axis, although this needs to be further investigated.
Acknowledgments
Supported by Goldhirsh-Yellin Foundation Research Grant, Dayton Area Graduate Medical Education Community Research Grant (T.L.G.), and National Institutes of Health (R01HD12252).
Footnotes
M.B.S. has nothing to disclose. J.H. has nothing to disclose. L.M. has nothing to disclose. M.L. has nothing to disclose. S.A.C. has nothing to disclose. T.L.G. has nothing to disclose. D.R.C. has nothing to disclose. P.G. has nothing to disclose. F.Z.S. has nothing to disclose. L.K.M. has nothing to disclose. S.R.L. has nothing to disclose.
M.B.S. and J.H. should be considered similar in author order.
Supplementary data
References
- 1.Visser J.A., Themmen A.P. Anti-Mullerian hormone and folliculogenesis. Mol Cell Endocrinol. 2005;234:81–86. doi: 10.1016/j.mce.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Hansen K.R., Hodnett G.M., Knowlton N., Craig L.B. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95:170–175. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Broer S.L., van Disseldorp J., Broeze K.A., Dolleman M., Opmeer B.C., Bossuyt P. Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: an individual patient data approach. Hum Reprod Update. 2013;19:26–36. doi: 10.1093/humupd/dms041. [DOI] [PubMed] [Google Scholar]
- 4.Steiner A.Z., Pritchard D., Stanczyk F.Z., Kesner J.S., Meadows J.W., Herring A.H. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. J Am Med Assoc. 2017;318:1367–1376. doi: 10.1001/jama.2017.14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lie Fong S., Visser J.A., Welt C.K., de Rijke Y.B., Eijkemans M.J., Broekmans F.J. Serum anti-mullerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97:4650–4655. doi: 10.1210/jc.2012-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui L., Qin Y., Gao X., Lu J., Geng L., Ding L. Antimüllerian hormone: correlation with age and androgenic and metabolic factors in women from birth to postmenopause. Fertil Steril. 2016;105:481–485.e481. doi: 10.1016/j.fertnstert.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Lashen H., Dunger D.B., Ness A., Ong K.K. Peripubertal changes in circulating antimüllerian hormone levels in girls. Fertil Steril. 2013;99:2071–2075. doi: 10.1016/j.fertnstert.2013.01.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biro F.M., Greenspan L.C., Galvez M.P., Pinney S.M., Teitelbaum S., Windham G.C. Onset of breast development in a longitudinal cohort. Pediatrics. 2013;132:1019–1027. doi: 10.1542/peds.2012-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Leonibus C., Marcovecchio M.L., Chiavaroli V., de Giorgis T., Chiarelli F., Mohn A. Timing of puberty and physical growth in obese children: a longitudinal study in boys and girls. Pediatr Obes. 2014;9:292–299. doi: 10.1111/j.2047-6310.2013.00176.x. [DOI] [PubMed] [Google Scholar]
- 10.Nardo L.G., Yates A.P., Roberts S.A., Pemberton P., Laing I. The relationships between AMH, androgens, insulin resistance and basal ovarian follicular status in non-obese subfertile women with and without polycystic ovary syndrome. Hum Reprod. 2009;24:2917–2923. doi: 10.1093/humrep/dep225. [DOI] [PubMed] [Google Scholar]
- 11.Roche A.F. Cambridge University Press; Cambridge: 1992. Growth, Maturation, and Body Composition: The Fels Longitudinal Study 1929–1991. [Google Scholar]
- 12.Lohman T.G. Applicability of body composition techniques and constants for children and youths. Exerc Sport Sci Rev. 1986;14:325–357. [PubMed] [Google Scholar]
- 13.Lohman T.G., Roche A.F., Martorell R. Human Kinetics Publishers, Inc; champaign, IL: 1988. Anthropometric standardization reference manual. [Google Scholar]
- 14.Reynolds E.L., Wines J.V. Individual differences in physical changes associated with adolescence in girls. Am J Dis Child. 1948;75:329–350. doi: 10.1001/archpedi.1948.02030020341006. [DOI] [PubMed] [Google Scholar]
- 15.Marshall W.A., Tanner J.M. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duke P.M., Litt I.F., Gross R.T. Adolescents' self-assessment of sexual maturation. Pediatrics. 1980;66:918–920. [PubMed] [Google Scholar]
- 17.Neinstein L.S. Adolescent self-assessment of sexual maturation: reassessment and evaluation in a mixed ethnic urban population. Clin Pediatr (Phila) 1982;21:482–484. doi: 10.1177/000992288202100806. [DOI] [PubMed] [Google Scholar]
- 18.Taylor S.J., Whincup P.H., Hindmarsh P.C., Lampe F., Odoki K., Cook D.G. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr Perinat Epidemiol. 2001;15:88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- 19.Regnault N., Gillman M.W., Kleinman K., Rifas-Shiman S., Botton J. Comparative study of four growth models applied to weight and height growth data in a cohort of US children from birth to 9 years. Ann Nutr Metab. 2014;65:167–174. doi: 10.1159/000365894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagen C.P., Aksglaede L., Sorensen K., Main K.M., Boas M., Cleemann L. Serum levels of anti-Mullerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 Turner syndrome patients. J Clin Endocrinol Metab. 2010;95:5003–5010. doi: 10.1210/jc.2010-0930. [DOI] [PubMed] [Google Scholar]
- 21.Savas-Erdeve S., Sagsak E., Keskin M., Cetinkaya S., Aycan Z. AMH levels in girls with various pubertal problems. J Pediatr Endocrinol Metab. 2017;30:333–335. doi: 10.1515/jpem-2016-0217. [DOI] [PubMed] [Google Scholar]
- 22.Sahin N.M., Kinik S.T., Tekindal M.A., Bayraktar N. AMH levels at central precocious puberty and premature thelarche: is it a parameter? J Pediatr Endocrinol Metab. 2015;28:1351–1356. doi: 10.1515/jpem-2014-0521. [DOI] [PubMed] [Google Scholar]
- 23.Chen T., Wu H., Xie R., Wang F., Chen X., Sun H. Serum anti-mullerian hormone and inhibin b as potential markers for progressive central precocious puberty in girls. J Pediatr Adolesc Gynecol. 2017;30:362–366. doi: 10.1016/j.jpag.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Ibanez L., Oberfield S.E., Witchel S., Auchus R.J., Chang R.J., Codner E. An international consortium update: pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence. Horm Res Paediatr. 2017;88:371–395. doi: 10.1159/000479371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.