Abstract
Objective
To assess if the newer Kruger strict morphology (WHO5; normal ≥4%) adds any clinical value beyond the criteria of the World Health Organization fourth edition (WHO4; normal ≥14%).
Design
Retrospective study.
Setting
Tertiary hospital.
Patient(s)
Men without known azoospermia who had semen analysis (SA) collected over a 10-year period of time.
Intervention(s)
Morphology classification under Kruger WHO5 strict criteria and WHO4 criteria.
Main Outcome Measure(s)
Correlation between the WHO5 and WHO4 morphological classifications.
Result(s)
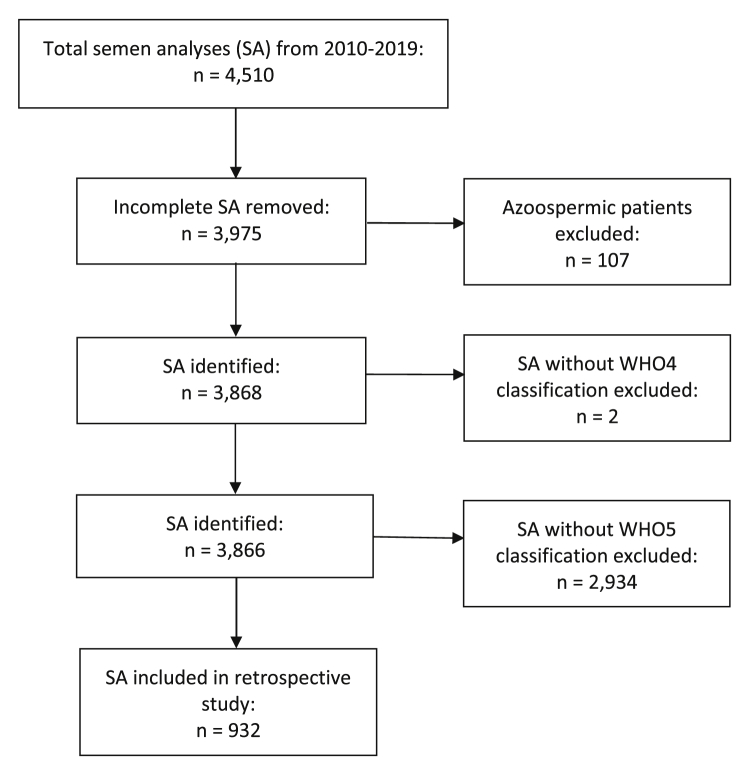
A total of 4,510 SAs were identified during the study period. Of these, both Kruger WHO5 and WHO4 morphologies were included in 932 SAs (20.7%) from a total of 691 men. The median age of the men was 37 years (interquartile range, 32.0–43.8 years). The mean (±SD) semen volume, sperm concentration, and motility were 2.6 ± 1.4 mL, 50.0 ± 35.6 × 106/mL, and 53.1% ± 18.6%, respectively. The correlation between the WHO4 and WHO5 morphology assessments was high (Spearman correlation coefficient = 0.94). Only 545 (58.5%) of 932 SAs had abnormal Kruger WHO5 morphology, of which 543 (99.6%) of 545 also had abnormal morphology by the WHO4 criteria.
Conclusion(s)
The Kruger WHO5 and WHO4 morphologic criteria correlate closely. Only two men (0.4%) with an abnormal Kruger morphology had normal WHO4 morphology. Given the limited predictive value of sperm morphology, the additional cost and effort of Kruger criteria may not be warranted in lieu of, or in addition to, the WHO4 classification.
Key Words: Infertility, semen analysis, sperm morphology
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-20-00256
Male factor infertility is responsible for up to 50% of cases of infertility (1, 2). Semen analysis (SA) is the minimum and gold standard assessment tool for the workup of male infertility (3). According to the American Urologic Association, two samples should be obtained at least 4 weeks apart and after a 2–7 day period of abstinence (3, 4). The World Health Organization (WHO) creates standard cutoffs for semen parameter values based on large subsets of fertile individuals, with the most recent version published in 2010 (WHO5) and the previous version (WHO4) published in 1999 (4, 5). However, the predictive value of semen parameters for identifying patients who are subfertile remains low (6, 7). Specifically, for morphology, the cutoff was reduced from 14% in the 1999 edition to 4% in the 2010 edition. In addition to the cutoff change, the actual criteria for assessing sperm morphology were also modified by Kruger (4).
The observed decline in morphologically normal sperm over the last several decades has been attributed, at least partially, to a stricter evaluation approach (8). In a 15-year series of sperm smears, the proportion of spermatozoa with normal form was originally reported to decline from 42.8% to 36.7%; however, reevaluation of the sampled smears showed minimal change from 13.7% to 13.3% (9). More recently, the over-criticality of the Kruger criteria was debated because of its controversial clinical utility (8). In the fifth edition of the WHO manual, the Kruger strict criteria require apparently normal spermatozoa to be measured for head size and determine that if any one structural feature (head, appearance, width, length, neck, or tail) has a defect, then the spermatozoa are considered morphologically abnormal. In contrast, the WHO4 methodology embraces a more liberal approach in which spermatozoa have a wider definition of normal morphology (5).
Although studies have shown that Kruger normal sperm have a better prognosis for in vitro fertilization (10), the advent of intracytoplasmic sperm injection may render this tedious analysis obsolete. Further, limited data exist describing the time and cost of classifying the Kruger morphology. In this article, we evaluate if the WHO5 strict Kruger assessment for morphology captures additional patients beyond the WHO4 edition given the additional financial and labor implications. We hypothesize that the evaluation of sperm morphology under the WHO4 manual and WHO5 Kruger assessment will be closely correlated.
Materials and methods
Study Population
Study approval was obtained from the Weill Cornell Institutional Review Board. A retrospective chart review from a prospectively collected database at the Andrology Laboratory at Weill Cornell Medicine was completed. Samples were reviewed over a 10-year period between January 2010 and December 2019. All semen samples were reviewed and only included if the SA contained morphology readings under both the WHO4 and WHO5 methods. Men with azoospermia and/or incomplete data were excluded.
Sample Collection
All individuals were provided instructions on sample collection, including collection after self-stimulation into a clean container. Samples were immediately provided to the laboratory, for processing by the andrologist.
Samples were prepared according to the WHO laboratory manual using CELL-VU Pre-Stained Morphology slides (Millennium Sciences, Inc; New York, NY) (4, 5). A total of 100 cells were systematically evaluated in four different areas of each slide under ×400 magnification by a trained andrologist. Sperm morphology was characterized with two sets of criteria based on the WHO manual. First, the samples were assessed using the WHO4 edition, which included an assessment of sperm morphology based on normal-appearing heads, midpieces, and tails for which a cutoff of ≥14% was employed. Second, the samples were then assessed using the WHO5 edition, which required a cutoff of ≥4% and a strict morphometric assessment of the sperm characteristics (4, 11).
Statistical Analysis
Patient age was computed electronically using the date of the sample collection. The period of abstinence was recorded in days. The correlation between the normal sperm morphology under WHO4 and WHO5 was assessed using the Spearman correlation coefficient. For analyzing the sperm substructures, a multivariable logistic regression model was used to predict the morphology classification according to the percentage of head and tail defects. All statistical analyses were completed with GraphPad Prism (v 8.4.2) (GraphPad Software; San Diego, CA).
Results
A total of 4,510 SAs were identified between January 2010 and December 2019 (Fig. 1). A total of 932 SAs from 691 men included morphology assessments using both the WHO4 and WHO5 criteria. Demographic data are summarized in Table 1. The median age was 37 years (interquartile range [IQR], 32–43.8 years) and the median abstinence period was 3 days (IQR, 2.0–3.5 days). The mean ± SD semen volume, sperm concentration, and motility were 2.6 ± 1.4 mL, 50.0 ± 35.6 ×106/mL, and 53.1% ± 18.6%, respectively. The mean normal sperm morphology under WHO4 and WHO5 were 6.4% ± 4.8% and 3.3% ± 3.2%, respectively.
Figure 1.
Flow diagram of the study exclusion criteria. SAs = semen analyses; WHO4 = World Health Organization laboratory manual, fourth edition criteria; WHO5 = Kruger criteria.
Table 1.
Summary of demographic data.
| Summary data | Mean ± SD |
|---|---|
| Age (y) | 37 (32.0–43.8)a |
| Abstinence period (d) | 3 (2.0–3.5)a |
| Total SA analyzed | 932 |
| Semen volume (mL) | 2.6 ± 1.4 |
| Sperm concentration (millions/mL) | 50.0 ± 35.6 |
| Sperm motility (%) | 53.1 ± 18.6 |
| WHO4 normal sperm Morphology (%) | 6.4 ± 4.8 |
| Kruger WHO5 normal sperm morphology (%) | 3.3 ± 3.2 |
Note: SAs = semen analyses; WHO4 = criteria of the World Health Organization fourth edition; WHO5 = Kruger criteria.
Values reported as median (interquartile range).
According to the WHO4 morphologic criteria, 847 SAs (90.9%) were read as abnormal. Of those, 304 SAs (35.9%) were normal based on the Kruger strict criteria. In patients with abnormal WHO4 yet normal WHO5 sperm morphology, the mean semen volume, sperm concentration, and motility were 2.6 ± 1.3 mL, 68.6 ± 31.1 ×106/mL, and 60.5% ± 8.5%, respectively.
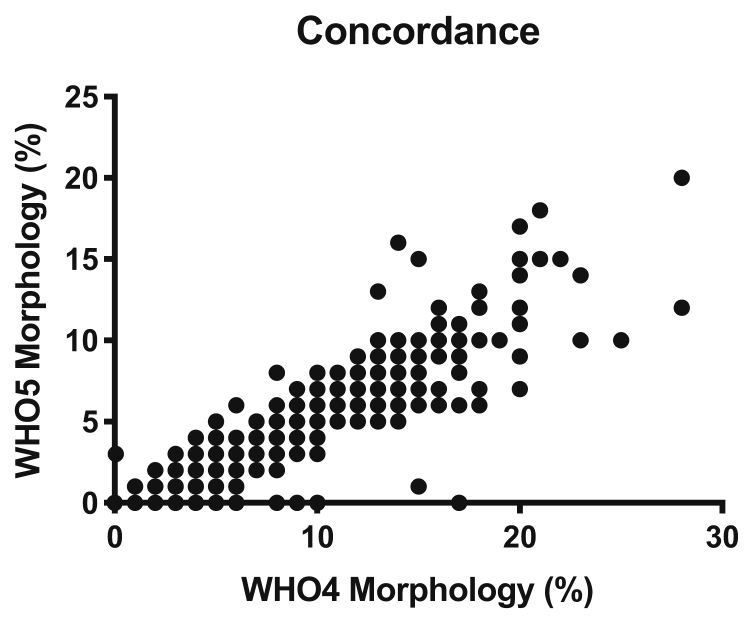
The Spearman correlation coefficient of Kruger WHO5 and WHO4 morphology was r = 0.94 (P<.0001) (Fig. 2). Only 545 SAs (58.5%) had abnormal Kruger WHO5 morphology, of which 543 (99.6%) of 545 also had abnormal morphology by WHO4. The remaining two patients (0.4%) had abnormal sperm morphology according to only the Kruger classification.
Figure 2.
Relationship of the World Health Organization laboratory manual, fourth edition (WHO4) and Kruger WHO5 morphology assessments. Concordance: r = 0.9352; P<.0001.
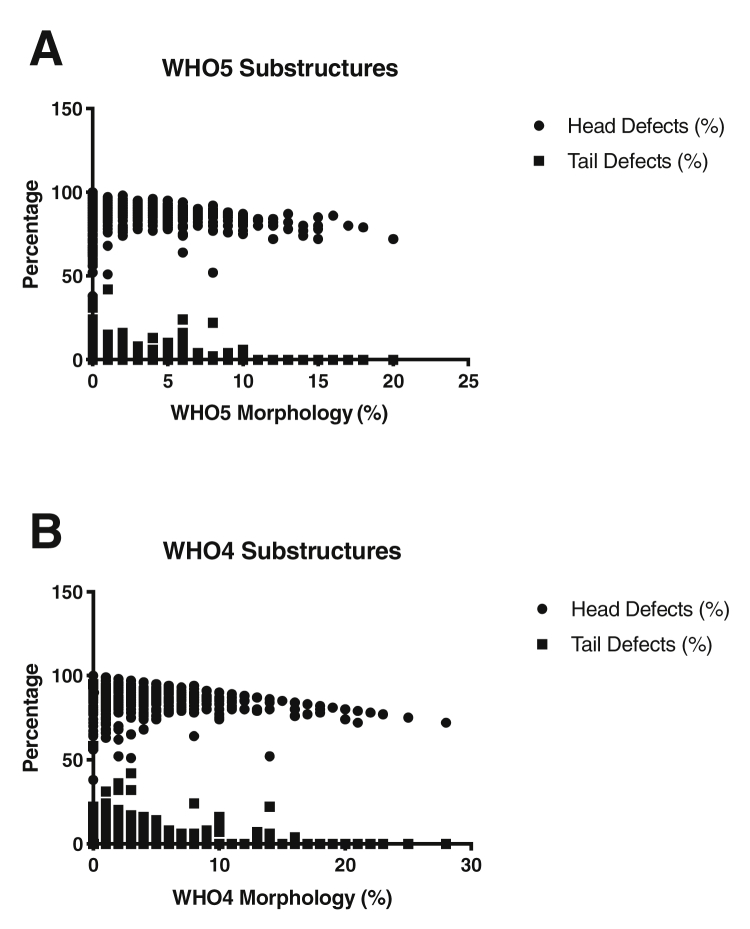
Semen analyses with a low percentage of normal sperm morphology were associated with a high percentage of substructure defects (Fig. 3). In predicting the morphology under WHO4, the odds ratios for head and tail defects were 1.30 (95% confidence interval [CI], 1.24–1.38) and 1.63 (95% CI, 1.41–1.98), respectively. In predicting the morphology under the Kruger strict criteria, the odds ratios for head and tail defects were 1.14 (95% CI, 1.11–1.78) and 1.43 (95% CI, 1.33–1.55), respectively.
Figure 3.
Relationship of sperm morphology and substructure defects. (A) Association of Kruger WHO5 morphology with head and tail defects. (B) Association of World Health Organization laboratory manual, fourth edition (WHO4) morphology assessment with head and tail defects.
Discussion
Semen analysis remains the gold standard in assessing male infertility and includes numerous parameters. Sperm morphology has had significant changes in the evolution of the WHO guidelines from 1999 and 2010. Our study illustrates a significant correlation (r = 0.94) between the Kruger WHO5 and WHO4 morphologic criteria. Only 2 (0.4%) of 545 men with an abnormal Kruger WHO5 morphology had normal WHO4 morphology. With our logistic regression model, head and tail defects predicted sperm morphology classification under both sets of criteria.
Sperm morphological defects are usually mixed and not limited to external structural abnormalities. Sperm with morphological defects generally have lower fertilizing potential, possibly because of other intrinsic issues such as increased DNA fragmentation, increased incidence of structural chromosomal aberrations, immature chromatin, and aneuploidy (12, 13, 14, 15). Yet in most cases, fertilization and pregnancy with assisted reproductive technology are feasible with a low normal form percentage and a variety of morphological appearances (16). One rare exception, however, is the round-headed spermatozoa (globozoospermia) that lack acrosomal structures and its contents. With an incidence in the infertile population of <0.05%, globozoospermic patients have lower fertilization rates in intracytoplasmic sperm injection, although some pregnancies have been reported (11). In addition to having a reduced capacity to bind to the zona pellucida and penetrate the oocyte, these spermatozoa were shown to have chromatin structural abnormalities and be deficient in oocyte activation factor (17, 18). Similarly, abnormal sperm functions, as well as low fertilization and pregnancy rates, were seen in patients with short tail syndrome and small or large-headed spermatozoa (19, 20, 21). Unfortunately, the strict criteria fail to stratify spermatozoa based on the success of fertilization. Kruger groups genetic defects, such as globozoospermia and short tail syndrome, with morphologically abnormal spermatozoa of potentially fertile patients into the subthreshold (<4%) category. This reinforces the lack of predictive utility in the Kruger morphological criteria and its inability to distinguish the patients most at risk for infertility.
Numerous studies investigated the effects of Kruger sperm morphology on intrauterine insemination and in vitro fertilization success (22, 23). A systematic review and meta-analysis of 20 observational trials demonstrated no clinical or statistical difference in the rate of pregnancy per intrauterine insemination when comparing men at >4% and <4% thresholds (24). In addition, the study looked at a 1% threshold and similarly found no apparent differences. Kovac et al. (25) further reported that 29.2% of men with zero normal forms did not require assisted reproductive technology for their first pregnancy and 75% of the cohort did not require in vitro fertilization. As suggested by the American Urologic Association Best Practice statement, sperm morphology by the Kruger strict criteria was not shown to be a predictive indicator of fertility, and its use as a tool in isolation to make clinical management decisions was not recommended (3). In effect, the process of analyzing semen samples according to Kruger strict criteria may not add any clinical value.
Although the clinical implications of abnormal sperm morphology remain controversial, it is important to acknowledge that an abnormal label of SAs may cause additional anxiety to patients. A diagnosis of infertility may be a large burden to patients and may contribute to additional psychological problems, such as an increased level of stress and depression (26, 27). Given the higher cutoff of normal sperm morphology, additional patients may be considered abnormal than otherwise in the WHO4 edition. In our study, we demonstrate 35.9% of those with abnormal sperm morphology under WHO4 were considered to have normal morphology under WHO5. Notably, the mean semen volume, concentration, and motility in these patients were similar to those of the entire cohort. Given their otherwise normal semen parameters, their sperm morphology should not be used in isolation to guide prognostic or therapeutic decisions.
To our knowledge, no published study has examined the labor and financial differences between the Kruger morphology classification and that of prior WHO editions. However, our institution has internally collected data illustrating the discrepancy. Depending on the health insurance plan, complete SA (CSA) with WHO4 criteria ranges in price in New York City from $125–$415. In contrast, complete SA with Kruger criteria ranges in price from $175–$465. Anecdotally, an experienced andrologist, such as the one in our study, takes approximately 10 minutes to classify according to WHO4 per sample, but those without experience take at least 20 minutes. For Kruger morphology, an experienced andrologist similarly takes 10 minutes, but novice technicians take 20–30 minutes a sample because of the complexity of using an ocular ruler to accurately measure sperm substructures. For practical purposes, according to our anecdotal experience, Kruger morphology is at least $50 more expensive and is a more laborious process for the inexperienced andrologist. Therefore, the added cost and effort to analyze strict morphology further reinforces not using a controversial classification system that provides limited clinical utility.
Our study has some important limitations. It is a retrospective chart review from a prospectively collected database of SAs, which limited our ability to measure key parameters such as fertilization or pregnancy outcomes. Although all the semen samples in this study were examined by a single andrologist with >30 years of experience in our institution, the possibility of measurement bias cannot be ignored because no independent review of the samples was done by another andrologist. Lastly, some patients provided multiple semen samples. Although our article aimed to examine the correlation of the two classification systems, it can be argued that these repeated samples were not independent of each other and may have affected our results.
Conclusion
Given the limited predictive value of sperm morphology, the additional cost and effort of performing strict morphometric assessments using the Kruger strict criteria may not be warranted in comparison with the simpler WHO4 morphologic assessment. Future work is needed to establish the correlation with pregnancy outcomes in order to determine the optimal assessment strategy. Although widely accepted as a tedious evaluation, further studies should conduct a cost analysis delineating the magnitude of work necessary to carry out the Kruger criteria.
Acknowledgments
The authors thank the staff of the Center for Reproductive Medicine and Surgery at Weill Cornell Medicine, Department of Urology.
Footnotes
G.W. has nothing to disclose. N.P. is supported by the Frederick J. and Theresa Dow Wallace Fund of the New York Community Trust. R.H. has nothing to disclose. M.F. has nothing to disclose. V.D. has nothing to disclose. M.G. has nothing to disclose.
References
- 1.Kumar N., Singh A.K. Trends of male factor infertility, an important cause of infertility: a review of literature. J Hum Reprod Sci. 2015;8:191–196. doi: 10.4103/0974-1208.170370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thonneau P., Marchand S., Tallec A., Ferial M.L., Ducot B., Lansac J. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989) Hum Reprod. 1991;6:811–816. doi: 10.1093/oxfordjournals.humrep.a137433. [DOI] [PubMed] [Google Scholar]
- 3.Jarow J., Sigman M., Kolettis P., Lipshultz L., McClure R., Nangia A. American Urological Association Education and Research. Inc. Available at: https://www.auanet.org/guidelines/archived-documents/male-infertility-optimal-evaluation-best-practice-statement. Accessed June 20, 2020; 2010. The optimal evaluation of the infertile male: AUA Best Practice statement. [Google Scholar]
- 4.World Health Organization . 5th ed. World Health Organzation; Switzerland: 2010. Laboratory manual for the examination and processing of human semen. [Google Scholar]
- 5.World Health Organisation . 4th ed. The Press Syndicate of the University of Cambridge; Cambridge: 1999. Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. [Google Scholar]
- 6.Patel A.S., Leong J.Y., Ramasamy R. Prediction of male infertility by the World Health Organization laboratory manual for assessment of semen analysis: a systematic review. Arab J Urol. 2018;16:96–102. doi: 10.1016/j.aju.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X., Zhang W., Luo Y., Long X., Sun X. Predictive value of semen parameters in in vitro fertilisation pregnancy outcome. Andrologia. 2009;41:111–117. doi: 10.1111/j.1439-0272.2008.00898.x. [DOI] [PubMed] [Google Scholar]
- 8.Menkveld R. Clinical significance of the low normal sperm morphology value as proposed in the fifth edition of the WHO laboratory manual for the examination and processing of human semen. Asian J Androl. 2010;12:47–58. doi: 10.1038/aja.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horte A., Vierula M., Toppari J., Suominen J. Reassessment of sperm morphology of archival semen smears from the period 1980--1994. Int J Androl. 2001;24:120–124. doi: 10.1046/j.1365-2605.2001.00285.x. [DOI] [PubMed] [Google Scholar]
- 10.Kruger T.F., Acosta A.A., Simmons K.F., Swanson R.J., Matta J.F., Oehninger S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988;49:112–117. doi: 10.1016/s0015-0282(16)59660-5. [DOI] [PubMed] [Google Scholar]
- 11.Kruger T.F., Franken D.R., editors. Atlas of human sperm morphology evaluation. Taylor & Francis; London: 2004. [Google Scholar]
- 12.Gandini L., Lombardo F., Paoli D., Caponecchia L., Familiari G., Verlengia C. Study of apoptotic DNA fragmentation in human spermatozoa. Hum Reprod. 2000;15:830–839. doi: 10.1093/humrep/15.4.830. [DOI] [PubMed] [Google Scholar]
- 13.Lee J.D., Kamiguchi Y., Yanagimachi R. Analysis of chromosome constitution of human spermatozoa with normal and aberrant head morphologies after injection into mouse oocytes. Hum Reprod. 1996;11:1942–1946. doi: 10.1093/oxfordjournals.humrep.a019521. [DOI] [PubMed] [Google Scholar]
- 14.Dadoune J.P., Mayaux M.J., Guihard-Moscato M.L. Correlation between defects in chromatin condensation of human spermatozoa stained by aniline blue and semen characteristics. Andrologia. 1988;20:211–217. [PubMed] [Google Scholar]
- 15.Martin R.H., Rademaker A.W., Greene C., Ko E., Hoang T., Barclay L. A comparison of the frequency of sperm chromosome abnormalities in men with mild, moderate, and severe oligozoospermia. Biol Reprod. 2003;69:535–539. doi: 10.1095/biolreprod.102.015149. [DOI] [PubMed] [Google Scholar]
- 16.Hotaling J.M., Smith J.F., Rosen M., Muller C.H., Walsh T.J. The relationship between isolated teratozoospermia and clinical pregnancy after in vitro fertilization with or without intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril. 2011;95:1141–1145. doi: 10.1016/j.fertnstert.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 17.Rybouchkin A., Dozortsev D., Pelinck M.J., De Sutter P., Dhont M. Analysis of the oocyte activating capacity and chromosomal complement of round-headed human spermatozoa by their injection into mouse oocytes. Hum Reprod. 1996;11:2170–2175. doi: 10.1093/oxfordjournals.humrep.a019071. [DOI] [PubMed] [Google Scholar]
- 18.Vicari E., Perdichizzi A., De Palma A., Burrello N., D'Agata R., Calogero A.E. Globozoospermia is associated with chromatin structure abnormalities: case report. Hum Reprod. 2002;17:2128–2133. doi: 10.1093/humrep/17.8.2128. [DOI] [PubMed] [Google Scholar]
- 19.Chemes H.E. Phenotypes of sperm pathology: genetic and acquired forms in infertile men. J Androl. 2000;21:799–808. [PubMed] [Google Scholar]
- 20.Kihaile P., Hirotsuru K., Kumasako Y., Misumi J., Utsunomiya T. Fertilization rates of small-head sperm in conventional IVF and ICSI. Arch Androl. 2003;49:327–329. doi: 10.1080/01485010390219692. [DOI] [PubMed] [Google Scholar]
- 21.Chelli M.H., Albert M., Ray P.F., Guthauser B., Izard V., Hammoud I. Can intracytoplasmic morphologically selected sperm injection be used to select normal-sized sperm heads in infertile patients with macrocephalic sperm head syndrome? Fertil Steril. 2010;93:1347.e1–1347.e5. doi: 10.1016/j.fertnstert.2008.10.059. [DOI] [PubMed] [Google Scholar]
- 22.Keegan B.R., Barton S., Sanchez X., Berkeley A.S., Krey L.C., Grifo J. Isolated teratozoospermia does not affect in vitro fertilization outcome and is not an indication for intracytoplasmic sperm injection. Fertil Steril. 2007;88:1583–1588. doi: 10.1016/j.fertnstert.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 23.Lockwood G.M., Deveneau N.E., Shridharani A.N., Strawn E.Y., Sandlow J.I. Isolated abnormal strict morphology is not a contraindication for intrauterine insemination. Andrology. 2015;3:1088–1093. doi: 10.1111/andr.12098. [DOI] [PubMed] [Google Scholar]
- 24.Kohn T.P., Kohn J.R., Ramasamy R. Effect of sperm morphology on pregnancy success via intrauterine insemination: a systematic review and meta-analysis. J Urol. 2018;199:812–822. doi: 10.1016/j.juro.2017.11.045. [DOI] [PubMed] [Google Scholar]
- 25.Kovac J.R., Smith R.P., Cajipe M., Lamb D.J., Lipshultz L.I. Men with a complete absence of normal sperm morphology exhibit high rates of success without assisted reproduction. Asian J Androl. 2017;19:39–42. doi: 10.4103/1008-682X.189211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagórska M., Obrzut B., Ulman D., Darmochwał-Kolarz D. The need of personalized medicine in coping with stress during infertility treatment. J Pers Med. 2021;11:56. doi: 10.3390/jpm11010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masoumi S.Z., Garousian M., Khani S., Oliaei S.R., Shayan A. Comparison of quality of life, sexual satisfaction and marital satisfaction between fertile and infertile couples. Int J Fertil Steril. 2016;10:290–296. doi: 10.22074/ijfs.2016.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]