Abstract
Objective
To evaluate racial differences in the anxiety and depression prevalence and scores in women with polycystic ovary syndrome (PCOS).
Design
Cross-sectional.
Setting
Academic institution.
Patient(s)
Reproductive-aged women with PCOS (n = 272) and controls (n = 295).
Intervention(s)
Hospital anxiety and depression scale and modified PCOS quality-of-life survey (MPCOS-Q).
Main Outcome Measure(s)
Differences in depression and anxiety scores and quality-of-life score measured using the hospital anxiety and depression scale and MPCOS-Q were determined between White and Black women with PCOS. Multivariable correlation regressions assessed the association of the Ferriman-Gallwey score, total testosterone, body mass index (BMI), and homeostatic model assessment of insulin resistance with anxiety, depression, and quality-of-life scores.
Result(s)
Multivariable regression controlling for age, BMI, and socioeconomic status showed that White women with PCOS had a significantly higher prevalence of anxiety than Black women with PCOS (75.9% vs. 61.3%) and significantly higher anxiety scores (mean ± SD, 10.3 ± 4.1 vs. 8.7 ± 4.6). The prevalence of depression (24.4% vs. 29%) and depression scores (4.8 ± 3.6 vs. 5.1 ± 4.0) was not significantly different. In multivariable correlation regressions, the interaction between BMI and race in its association with anxiety scores was significant. The association of race with Ferriman-Gallwey score, total testosterone, or homeostatic model assessment of insulin resistance was not significant. In multivariable models, although the total MPCOS-Q scores were similar, the infertility domain was significantly lower in Black women with PCOS (mean ± SD, 12.6 ± 7.8 vs. 17.5 ± 6.8) indicating a lower quality of life related to infertility.
Conclusion
Racial differences identified in the prevalence of anxiety and MPCOS-Q domains suggest the importance of routine screening and provide an opportunity for targeted interventions based on race.
Key Words: Anxiety, depression, PCOS, quality of life, racial differences
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-20-00221
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder affecting reproductive-aged women (1). Its prevalence ranges from 8% to 13% depending on the diagnostic criteria used and the race and ethnicity of the population studied (2). For example, although the prevalence of PCOS in the United States ranges from 5% to 7% (3), it is as low as 2.2% in China (4) and as high as 14.1% in Iran (5). The prevalence of different phenotypes of PCOS also varies depending upon race or ethnicity. In a cross-sectional retrospective study in the United Kingdom (n = 1,310), South Asian women were significantly more likely to have hyperandrogenic symptoms compared with White women (6). Similar differences in phenotypes have been described in other populations, including African Americans and Hispanics (7, 8), leading recent international guidelines to suggest ethnicity-based cutoffs for Ferriman-Gallwey (FG) scores (2).
In addition, metabolic risk in women with PCOS also varies with ethnicity. In an international study, Norwegian women with PCOS were more likely to have metabolic syndrome, Indian women were more likely to have impaired glucose tolerance, and Brazilian women were more likely to have low high-density lipoprotein (HDL) compared with White women residing in the United States (9). In the United States, Black adolescents and young adults have been reported to have a higher prevalence of metabolic syndrome and its components compared with their White counterparts (relative risk 2.65, 95% confidence interval [CI]: 1.29–5.4) (10, 11).
Although racial disparities in phenotype and metabolic risk have been studied, there is no data on racial differences in emotional wellness in the PCOS population. It is important to consider the effect of racial disparities and differences on mental health, including the emerging evidence that racial experiences in minority populations can affect multiple aspects of mental health care, including distress due to anxiety, access to care, and the use of treatment (12, 13, 14, 15, 16). In the US general population, the lifetime prevalence of generalized anxiety disorder (GAD) is higher in White women (8.6%) compared with that in Black women (5.1%) (17). A survey-based study reported higher 12-month odds of GAD diagnosis (odds ratio [OR] 2.9, 95% CI: 2.1–4.1) and social anxiety disorders diagnosis (OR 2.4, 95% CI: 1.8–3.2) in White adults compared with African Americans after controlling for sociodemographic variables (18). Another survey of 43,093 adults found that Black adults had significantly lower lifetime odds of social anxiety disorder compared with White adults (OR 0.6, 95% CI: 0.5–0.73) (19). The prevalence of depression has also been shown to be influenced by race, although the 2013–2016 National Health and Nutrition Examination Survey found no significant differences in the prevalence of major depression between White (10.5% ± 0.9%) and Black (11.0% ± 0.8%) women (20). Despite the evidence in the non-PCOS population, there are no studies directly comparing the racial differences in anxiety and depression within the PCOS population. Understanding these differences is important as it could influence screening guidelines, counseling tools, or allocation of resources when evaluating mental health in PCOS.
Therefore, the aim of our study was to evaluate the racial differences in the prevalence of anxiety and depression between Black and White women with PCOS and to determine if physical and biochemical attributes of PCOS correlate with anxiety, depression, and quality of life.
Materials and methods
Subjects
This was a retrospective, cross-sectional study conducted at the University of Pennsylvania from November 2015 to October 2018 that included nonpregnant women of 18–50 years of age. Women seen at the Penn PCOS center meeting the Rotterdam criteria (21) were approached for participation. Data on clinical and biochemical measures related to PCOS were obtained by chart review. The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated by multiplying fasting glucose levels (mg/dL) times fasting insulin levels (mIU/mL) divided by 405. Women without PCOS were recruited from the gynecology clinic as controls and were excluded if they reported both hirsutism and irregular menses on the demographic questionnaires. The University of Pennsylvania Institutional Review Board approved this study.
Surveys
Demographic questionnaires containing items on race, socioeconomic status (SES), and psychiatric, menstrual, and pregnancy history, the hospital anxiety and depression scale (HADS) survey, and the modified-PCOSQ (MPCOS-Q) survey were administered. White or Black race was self-reported. The HADS is a validated 14-item questionnaire with 7 questions dedicated to the assessment of anxiety and 7 to the assessment of depressive symptoms (22, 23). HADS is scored on a Likert scale, and scores ≥8 were considered clinical cutoffs as described in other studies (24). The MPCOS-Q is a 30-item questionnaire containing the following domains: emotions, body hair, weight, infertility, menstruation, and acne (25). Questions relate to the impact of each domain on the quality of life, with answers presented on Likert scales, for example, none of the time to all of the time. Higher values in each domain and in total scores indicate a higher quality of life. The study data were collected and managed using REDCap (Vanderbilt University, Nashville, TN) (26) electronic data capture tools.
Outcomes
The primary outcome was the differences in the prevalence of anxiety and depression in women with PCOS as well as differences in anxiety, depression, and quality-of-life scores between White and Black women with PCOS. The secondary outcomes included the same comparisons in White and Black controls and correlations between anxiety, depression, quality-of-life scores and FG scores, body mass index (BMI), testosterone levels, and HOMA-IR between races in women with PCOS.
Statistical Analyses
Continuous outcomes were compared using either rank-sum or student t-test as dictated by normality assessments. Normality assessments were based on graphical displays as well as quantitatively through the Shapiro–Wilk test. Categorical outcomes were compared using Pearson chi-square tests or Fisher’s exact test. For correlation analyses, FG score, BMI, total testosterone levels, and HOMA-IR were included.
Multivariable logistic regression modeling was used to examine differences in the prevalence of anxiety and depression between White and Black women with PCOS. Multivariable linear regression modeling was used to examine differences in continuous HADS scores and MPCOS-Q scores between White and Black women with PCOS. Covariates to include in the model were determined a priori as well as assessment of confounding using a backward elimination strategy and included patient age, BMI, and SES. Linear regression with interaction terms as well as Pearson’s correlation were employed to evaluate racial differences in association between FG scores, BMI, testosterone levels, and HOMA-IR, HADS, and MPCOS-Q scores. For comparative purposes, the preceding analyses were replicated for the controls, including examining for interactive effects for race between women with PCOS and controls.
Results
Demographic Characteristics
The demographic characteristics of the 272 women with PCOS and 295 controls are shown in Table 1. Black women with PCOS had a significantly higher BMI (P<.001), were less likely to have a college degree or higher (P = .006), had lower incomes (P<.001), and were less likely to be married or in a domestic partnership (P = .034) than White women with PCOS. FG score, total testosterone, and free testosterone did not differ significantly between Black and White women with PCOS (Table 2).
Table 1.
Demographic characteristics of women with PCOS and controls.
| PCOS (n = 272) |
Controls (n = 295) |
|||||
|---|---|---|---|---|---|---|
| White (n = 202) | Black (n = 70) | P value | White (n = 109) | Black (n = 186) | P value | |
| Median age in years (range) | 28.7 (24.5–32.2) | 29.2 (25.2–32.2) | .36 | 32.6 (27.9–40.7) | 31.6 (26.5–39.3) | .22 |
| Median BMI in kg/m2 (range) | 31.1 (25.5–38.4) | 36.5 (31.0–41.8) | <.001 | 25.2 (22.3–31.5) | 31.8 (26.1–39.3) | <.001 |
| Highest degree, n (%) | .006 | <.001 | ||||
| Some high school | 1 (0.5%) | 2 (2.9%) | 1 (0.9%) | 12 (6.6%) | ||
| High school | 6 (3.0%) | 7 (10.1%) | 8 (7.3%) | 53 (29.3%) | ||
| Some college | 45 (22.4%) | 25 (36.2%) | 14 (12.8%) | 53 (29.3%) | ||
| College | 78 (38.8%) | 20 (29.0%) | 36 (33.0%) | 38 (21.0%) | ||
| Some professional | 16 (8.0%) | 3 (4.4%) | 7 (6.4%) | 6 (3.3%) | ||
| Professional | 55 (27.4%) | 12 (17.4%) | 43 (39.5%) | 19 (10.3%) | ||
| Employment, n (%) | .228 | .004 | ||||
| Full time | 152 (75.3%) | 52 (75.4%) | 79 (73.2%) | 117 (64.6%) | ||
| Part time | 12 (5.9%) | 2 (2.9%) | 10 (9.3%) | 11 (6.1%) | ||
| Unemployed, not looking for job | 35 (17.3%) | 11 (15.9%) | 16 (14.8%) | 25 (13.8%) | ||
| Unemployed, looking for job | 3 (1.5%) | 4 (5.8%) | 3 (2.8%) | 28 (15.5%) | ||
| Income in dollars, n (%) | <.001 | <.001 | ||||
| <20,000 | 26 (13.2%) | 10 (14.5%) | 9 (8.3%) | 58 (33.0%) | ||
| 20,001-50,000 | 37 (18.8%) | 32 (46.4%) | 28 (25.9%) | 72 (40.9%) | ||
| 50,001-100,000 | 63 (32.0%) | 22 (31.9%) | 35 (32.4%) | 29 (16.5%) | ||
| 100,001-150,000 | 41 (20.8%) | 4 (5.8%) | 19 (17.6%) | 13 (7.4%) | ||
| >150,000 | 30 (15.2%) | 1 (1.5%) | 17 (15.7%) | 4 (2.3%) | ||
| Marital status, n (%) | .034 | <.001 | ||||
| Single, never married | 130 (64.4%) | 49 (70.0%) | 56 (51.4%) | 140 (76.1%) | ||
| Married or domestic partnership | 71 (35.2%) | 18 (25.7%) | 46 (42.2%) | 35 (19.0%) | ||
| Divorced or separated | 1 (0.5%) | 3 (4.3%) | 7 (6.4%) | 9 (4.9%) | ||
| Antidepressant use, n (%) | 22 (10.8%) | 5 (7.1%) | .366 | 16 (14.7%) | 18 (9.7%) | .194 |
Note: BMI = body mass index; PCOS = polycystic ovary syndrome.
Table 2.
Phenotypic characteristics and biochemical data in White versus Black women with PCOS.
| White women with PCOS (n = 202) | Black women with PCOS (n = 70) | P value | |
|---|---|---|---|
| Measures of androgen excess | |||
| FG score | 10 (4–15) | 12 (5–17) | .128 |
| Total testosterone (ng/dL) | 49 (32–60) | 48 (44–74) | .055 |
| Free testosterone (pg/mL) | 4.9 (2.5–8) | 6.3 (3.7–9.7) | .086 |
| DHEAS (μg/dL) | 222.5 (136.0–307.0) | 177.5 (118.0–264.8) | .036 |
| SHBG (nmol/L) | 53.5 (31.6–100.9) | 38 (24–67) | .031 |
| Measures of hyperlipidemia | |||
| Total cholesterol (mg/dL) | 179.5 (162–204) | 164 (149–177) | <.001 |
| Triglycerides (mg/dL) | 103.5 (75.5–163.5) | 68 (51–92) | <.001 |
| LDL (mg/dL) | 100 (83–118) | 97 (86–110) | .584 |
| HDL (mg/dL) | 54 (45–67) | 50 (41.5–59) | .043 |
| Measures of insulin resistance | |||
| HgbA1c | 5.3 ± 0.4 | 5.6 ± 0.4 | <.001 |
| HOMA-IR | 2.8 (1.5–5.6) | 3.7 (1.8–6.2) | .334 |
| Fasting glucose | 85 (78–91) | 85 (81–90) | .730 |
| Measures of ovarian reserve | |||
| AMH (ng/mL) | 6.0 (3.1–9.1) | 7.0 (4.4–10.4) | .251 |
| Total AFC | 37 (27–40) | 40 (31–55) | .044 |
Note: Data are presented as median (IQR) and mean ± SD. AFC = antral follicle count; AMH = antimüllerian hormone; DHEAS = dehydroepiandrosterone sulfate; FG = Ferriman-Gallwey score; HDL = high-density lipoprotein; HgbA1c = hemoglobin A1c; HOMA-IR = homeostatic model assessment of insulin resistance; IQR = interquartile range; LDL = low-density lipoprotein; PCOS = polycystic ovary syndrome; SHBG = sex hormone-binding globulin.
Racial Differences in Anxiety and Depression Prevalence
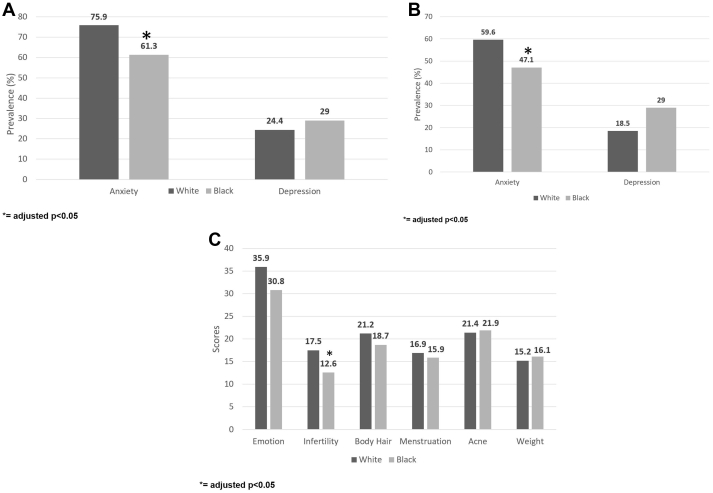
White women with PCOS had a significantly higher prevalence of anxiety (defined as HADS >8) than Black women (75.9% vs. 61.3%), and the odds of anxiety in multivariable logistic regression models controlling for age, BMI, and SES (adjusted odds ration [aOR] 2.21, 95% CI: 1.17–4.19) was also higher in White versus Black women with PCOS (P = .014). However, the prevalence of depression (defined as HADS >8) and the odds of depression were not significantly different (White women 24.4% vs. Black women 29.0%) in adjusted models (aOR 1.03, 95% CI: 0.50–2.14, P = .933) (Table 3, Fig. 1A).
Table 3.
Racial differences in anxiety and depression in White versus Black women with PCOS and controls.
| White vs. Black PCOS (n = 272) |
White vs. Black controls (n = 295) |
Interaction P value | |||
|---|---|---|---|---|---|
| aORb | P value | aORb | P value | ||
| HADS anxiety prevalencea | 2.21 (1.17, 4.19) | .014 | 2.04 (1.17, 3.53) | .011 | .839 |
| HADS depression prevalencea | 1.03 (0.50, 2.14) | .933 | 1.46 (0.65, 3.28) | .361 | .514 |
| Coefficient | P value | Coefficient | P value | ||
| Mean HADS anxiety score | 1.80 (0.51, 3.10) | .006 | 1.03 (−0.13, 2.19) | .083 | .363 |
| Mean HADS depression score | 0.23 (−0.80, 1.27) | .659 | 0.77 (−0.16, 1.70) | .105 | .425 |
Note: aOR = adjusted odds ratio; BMI = body mass index; HADS = hospital anxiety and depression scale; PCOS = polycystic ovary syndrome.
Defined as HADS score >8.
Adjusted for age, BMI, and socioeconomic status variables.
Figure 1.
(A) Racial differences in the prevalence of anxiety and depression symptoms in women with PCOS. (B) Racial differences in the prevalence of anxiety and depression symptoms in controls. (C) Racial differences in the quality-of-life domain scores in women with PCOS. PCOS = polycystic ovary syndrome.
In multivariable logistic regression models controlling for age, BMI, and SES, White controls had a significantly higher prevalence of anxiety and odds of anxiety than Black controls (59.6% vs. 47.1%; aOR 2.04, 95% CI: 1.17–3.53, P = .011). The prevalence of depression and odds of depression were not significantly different between White and Black controls in models adjusted for age, BMI, and SES (18.5% vs. 13.2%; aOR 1.46, 95% CI: 0.65–3.28, P = .361) (Table 3, Fig. 1B).
Thus, in both women with PCOS and controls, a higher prevalence of anxiety was seen among White women compared with Black women. To determine if the differences between White women and Black women varied significantly between women with PCOS and controls, the data were combined and the above models extended to include the interaction between groups. Both interaction models were nonsignificant (P = .839 for anxiety prevalence; P = .514 for depression prevalence) indicating that there were no racial differences in prevalence when comparing the PCOS cohort with the control cohort.
Racial Differences in Anxiety and Depression Scores
In multivariable linear regression models controlling for age, BMI, and SES, White women with PCOS had significantly higher anxiety scores (mean ± SD 10.3 ± 4.1 vs. 8.7 ± 4.6, β = 1.80, P = .006). Depression scores were not significantly different in adjusted models for comparisons of White and Black women with PCOS (mean 4.8 ± 3.6 vs. 5.1 ± 4.0, β = 0.23, P = .659) (Table 3).
In multivariable linear regression models adjusted for age, BMI, and SES, White controls did not have significantly different anxiety scores compared with those of Black controls (mean 8.1 ± 3.8 vs. 7.5 ± 4.8, β = 1.03, P = .083). In models adjusted for age, BMI, and SES, White controls did not have significantly different depression scores compared with those of Black controls (mean 3.6 ± 3.3 vs. 4.1 ± 3.5, β = 0.77, P = .105) (Table 3).
Combining the PCOS and controls data, interaction models yielded nonsignificant interactions for both anxiety (P = .363) and depression (P = .425) scores, indicating that there were no racial differences in scores when comparing the PCOS cohort with the controls.
Correlation Between Anxiety, Depression, and FG Scores, Total Testosterone, BMI, and HOMA-IR
In multivariable correlation regressions of anxiety and depression, within the PCOS sample, the interaction term between BMI and race in its association with anxiety scores was significant (P = .031), indicating that the relationship between BMI and anxiety varied differentially between White and Black women. Further evaluation of correlation estimates separately within White and Black women with PCOS revealed a direct relationship in White women in whom higher BMI was associated with higher anxiety scores (r = 0.11, P = .147) but an inverse relationship in Black women in whom higher BMI was associated with lower anxiety scores (r = −0.17, P = .184). The interaction term between race and FG score, total testosterone, and HOMA-IR was not significant.
Racial Differences in Quality-of-Life Scores
In unadjusted analyses, Black women with PCOS had significantly lower total MPCOS-Q scores compared with those of White women (98.6 vs. 110.7, P = .009). In multivariable linear regression models adjusted for age, BMI, and SES, there was no difference in the total MPCOS-Q score (β = 2.75, P = .572). When broken down by domain, in unadjusted analyses the emotions domain and infertility domain scores were significantly lower in Black women with PCOS compared with those in White women with PCOS (P = .002 and P<.001, respectively), meaning that Black women had lower quality of life related to emotional well-being and infertility concerns. In multivariable linear regression models adjusted for age, BMI, and SES, the infertility domain score remained significantly lower in Black women with PCOS (mean 12.6 vs. 17.5, β = 3.51, P = .001) (Fig. 1C). In correlation regressions of total quality-of-life scores in women with PCOS, the interaction terms between race and FG score, total testosterone, and HOMA-IR were not significant (P = .644, P = .276, and P = .300, respectively).
Discussion
Racial differences in PCOS phenotypes and long-term metabolic risk are well recognized. In our study, the prevalence of anxiety after adjusting for age, BMI, and SES and the anxiety levels were significantly higher in adult White women with PCOS compared with those in Black women with PCOS. There were no significant differences in the prevalence of depression or depressive symptoms between the 2 groups.
In keeping with the literature, we found that White controls had a significantly higher prevalence of anxiety compared with that of Black controls, with no difference in the prevalence of depression. In addition, although the finding that higher BMI was associated with higher anxiety in White women with PCOS but lower anxiety in Black women with PCOS did not reach statistical significance, this may relate in part to a lack of power and warrants further investigation. Examination of quality-of-life scores showed that infertility had the most significant impact on lowering quality of life, with Black women with PCOS having lower infertility domain scores compared with those of White women with PCOS.
Anxiety disorders contribute significantly to the financial burden related to health care (27) such that GAD is associated with 1.5–5.4 days of work impairment in 1 month and 5 times greater likelihood of moderate/severe occupational dysfunction and physical disability (28, 29). Women with PCOS are at an increased risk of anxiety compared with controls as seen in a large meta-analysis of >1,400 women (OR 5.62; 95% CI: 3.22–9.80) (30). Thus, understanding the risk factors that mediate the relationship between PCOS and anxiety is vital.
Although our study was not designed to determine causative factors, potential causes for the lower anxiety scores in Black women include greater resilience to stressors secondary to strong social support and religiosity (18). Also, preexisting substance-abuse disorders, known to be more common in White adults, may contribute to an increase in certain forms of anxiety (18). Further, higher body image dissatisfaction in White compared with Black adults (31) has also been linked to mood disorders (32, 33, 34). Of note, body image dissatisfaction is a known mediator for anxiety in women with PCOS. In contrast, other studies have emphasized that sociocultural differences in beliefs and attitudes may affect the expression and assessment of anxiety in Black adults, thus advising caution in the interpretation of observed differences (35). The impact of racial experiences on mental health cannot be understated. Unconscious bias and other race-related factors have been associated with differences in access to care, the use of treatment, and stress (12, 13, 14, 15, 16). It is possible that differences in mental health are also driven by cultural and systemic differences as well as structural racism (36). Although our study was not constructed to evaluate this, the contribution and interplay of these factors must not be ignored.
The underlying etiology for increased anxiety and depression in women with PCOS is unclear; in 1 study, women with PCOS and concurrent depression had higher BMI and hirsutism scores, whereas women with PCOS and concurrent anxiety had higher BMI, hirsutism scores, and free testosterone levels, although the effect sizes were small (30). The association between BMI and anxiety has been studied in the general population (37), with a recent meta-analysis of 25 studies reporting increased anxiety in obese individuals (pooled OR 1.30, 95% CI: 1.20–1.41) (38). Dysregulated biological pathways, including neurotransmitter imbalances, oxidative stress, and hypothalamus-pituitary-adrenal axis disturbances, have been implicated in the association between psychiatric disorders and obesity (39). In addition, anxiety has been associated with disordered eating, which can affect obesity, and obese women may be at a higher risk of facing stigma and discrimination leading to dissatisfaction (38). Another study demonstrated that insulin resistance was associated with increased odds of depression in women with PCOS after controlling for age and BMI (40). To understand the etiology for increased anxiety in White women with PCOS, we examined the association with the previously described risk factors and found a significant interaction between race and BMI as it associates with anxiety scores.
Racial differences in the health-related quality of life in women with PCOS have been described with varying results (41, 42). In our study, Black women with PCOS were noted to have significantly lower scores on the emotion and infertility domains of the quality-of-life survey. With regards to the relationship between infertility and quality of life, several studies have shown that infertile women suffer from poor quality of life scores. An Iranian matched case-control study of 180 infertile and 540 fertile women found that infertility affected the women’s physical health, mental health, and social health significantly (43). A cross-sectional study of US women veterans also found that of the 996 veterans studied, those reporting a history of infertility had worse perceived physical health and higher rates of depression in analyses adjusted for age (44). In a meta-analysis of studies comparing racial differences in in vitro fertilization treatment outcomes in the general infertile population, Black women had lower live birth rates compared with those of White women, although there was considerable heterogeneity (45). In addition to differences in access to infertility services, Black and Hispanic women use infertility services far less frequently compared with White women (46). Black women have a higher prevalence of uterine fibroids and tubal disease, which affect miscarriage and live birth rates even in equal access settings (47). These studies underscore the complex relationship between infertility and race and may provide insights into our findings of lower quality-of-life scores related to infertility. In our population, Black women were less likely to be married or in a domestic partnership, which could have also contributed to lower quality-of-life scores related to fertility. As the majority of women with anovulatory infertility have PCOS (48), practitioners should be sensitive to the impact that infertility has on overall quality of life, particularly in Black populations.
Cognitive behavioral therapy combined with nutritional counseling, lifestyle changes and the use of continuous hormonal contraceptives improve anxiety scores in women with PCOS (49, 50). Cognitive behavioral therapy is a form of psychotherapy that focuses on changing the dysfunctional thoughts that lead to negative mood states and is recommended by the American Psychological Association as a first-line treatment for several mental health disorders (51, 52). Although most of these studies were small, they suggested that targeting weight and hyperandrogenism improved anxiety symptoms. Our findings of an interaction between race and BMI also suggested that BMI may play a role in the differences observed. As it is estimated that 80% of women with PCOS in the United States are obese (53), future trials should examine if the impact of lifestyle modifications and hormonal contraceptives on anxiety and depression symptoms is modified by race.
Our study is the first to examine differences in anxiety and depressive symptoms and health-related quality-of-life scores between White and Black women with PCOS residing in the United States. Relatively large sample sizes and interaction assessment with clinical parameters in women with PCOS add to the strengths of this study. We were unable to examine racial differences in anxiety and depression based on PCOS phenotypes, and our findings may not be generalizable to adolescents. Although women with hirsutism and irregular menses were excluded from the control group, it is possible that some controls were incorrectly labeled; however, we would expect this to result in more conservative estimates. Hormonal contraceptive use data, which can impact mood, was not available, although antidepressant use was not significantly different between the populations. In addition, although the focus of this paper was on Black and White women, exploration of other races is also necessary. Although there were insufficient numbers and power to evaluate additional races in this analysis, future studies evaluating a larger cohort of races should be conducted. They should also examine the impact of factors such as substance abuse or body image distress on the relationship between race and anxiety in these populations.
Conclusion
White women with PCOS are more likely to have anxiety symptoms and higher anxiety scores compared with Black women. Black women with PCOS also had lower quality-of-life scores, particularly in the infertility domain, compared with those of White women with PCOS. The Androgen Excess-Polycystic Ovary Syndrome Society recommends screening all women with PCOS for anxiety and depression and having adequate resources for follow-up, referral, and ongoing care (54). Understanding the factors that contribute to differences in the prevalence of anxiety may provide an opportunity for improved individualized counseling. Currently, there appears to be a role for recommending lifestyle management as studies in the general population suggest that exercise, such as resistance training or Pilates, can lead to significant reductions in anxiety levels both in obese individuals and those with anxiety disorders (55, 56, 57). Further, routine management of women with PCOS must include assessment of quality-of-life symptoms, and research trials should continue to evaluate the contribution of race and race-related factors to these findings. Management of long-term comorbidities in this young, reproductive age population may be best addressed by multidisciplinary teams, including physicians, nutritionists, mental health therapists, and psychiatrists, as proposed in the international guidelines (2).
Footnotes
S.A.G. was a paid consultant for Bayer and reports an NIH T32 training grant outside the submitted work. I.L. has nothing to disclose. A.C. has nothing to disclose. C.L. has nothing to disclose. J.L. has nothing to disclose. K.A. reports personal fees from Weight Watchers International and grants from Novo Nordisk outside the submitted work. R.G. has nothing to disclose. A.D. has nothing to disclose.
Supported by NIH T32 training grant: HD007440 (to S.A.G.). This source did not play any role in study design; in the analysis or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
References
- 1.De Leo V., Musacchio M.C., Cappelli V., Massaro M.G., Morgante G., Petraglia F. Genetic, hormonal and metabolic aspects of PCOS: an update. Reprod Biol Endocrinol. 2016;14:1–7. doi: 10.1186/s12958-016-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teede H.J., Misso M.L., Costello M.F., Dokras A., Laven J., Moran L. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364–379. doi: 10.1016/j.fertnstert.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lizneva D., Suturina L., Walker W., Brakta S., Gavrilova-Jordan L., Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106:6–15. doi: 10.1016/j.fertnstert.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Chen X., Yang D., Mo Y., Li L., Chen Y., Huang Y. Prevalence of polycystic ovary syndrome in unselected women from southern China. Eur J Obstet Gynecol Reprod Biol. 2008;139:59–64. doi: 10.1016/j.ejogrb.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 5.Rashidi H., Tehrani F.R., Khomami M.B., Tohidi M., Azizi F. To what extent does the use of the Rotterdam criteria affect the prevalence of polycystic ovary syndrome? A community-based study from the southwest of Iran. Eur J Obstet Gynecol Reprod Biol. 2014;174:100–105. doi: 10.1016/j.ejogrb.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Mani H., Davies M.J., Bodicoat D.H., Levy M.J., Gray L.J., Howlett T.A. Clinical characteristics of polycystic ovary syndrome: investigating differences in white and South Asian women. Clin Endocrinol. 2015;83:542–549. doi: 10.1111/cen.12784. [DOI] [PubMed] [Google Scholar]
- 7.Legro R.S., Myers E.R., Barnhart H.X., Carson S.A., Diamond M.P., Carr B.R. The pregnancy in polycystic ovary syndrome study: baseline characteristics of the randomized cohort including racial effects. Fertil Steril. 2006;86:914–933. doi: 10.1016/j.fertnstert.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y., Qiao J. Ethnic differences in the phenotypic expression of polycystic ovary syndrome. Steroids. 2013;78:755–760. doi: 10.1016/j.steroids.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Chan J.L., Kar S., Vanky E., Morin-Papunen L., Piltonen T., Puurunen J. Racial and ethnic differences in the prevalence of metabolic syndrome and its components of metabolic syndrome in women with polycystic ovary syndrome: a regional cross-sectional study. Am J Obstet Gynecol. 2017;217:189.e1–189.e8. doi: 10.1016/j.ajog.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Single-race population estimates, United States, 2010–2017. July 1st resident population by state, county, age, sex, single-race, and Hispanic origin. http://wonder.cdc.gov/single-race-v2017.html Available at:
- 11.Hillman J.K., Johnson L.N., Limaye M., Feldman R.A., Sammel M., Dokras A. Black women with polycystic ovary syndrome (PCOS) have increased risk for metabolic syndrome and cardiovascular disease compared with white women with PCOS. Fertil Steril. 2014;101:530–535. doi: 10.1016/j.fertnstert.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 12.Manseau M., Case B.G. Racial-ethnic disparities in outpatient mental health visits to U.S. physicians, 1993–2008. Psychiatr Serv. 2014;65:59–67. doi: 10.1176/appi.ps.201200528. [DOI] [PubMed] [Google Scholar]
- 13.Williams D.R. Stress and the mental health of populations of color: advancing our understanding of race-related stressors. J Health Soc Behav. 2018;59:466–485. doi: 10.1177/0022146518814251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sosoo E.E., Bernard D.L., Neblett E.W. The influence of internalized racism on the relationship between discrimination and anxiety. Cultur Divers Ethnic Minor Psychol. 2020;26:570–580. doi: 10.1037/cdp0000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feagin J., Bennefield Z. Systemic racism and U.S. health care. Soc Sci Med. 2014;103:7–14. doi: 10.1016/j.socscimed.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Biener AI, Zuvekas SH. Do racial and ethnic disparities in mental health treatment vary with underlying mental health? Med Care Res Rev. In press. [DOI] [PubMed]
- 17.Breslau J., Aguilar-Gaxiola S., Kendler K.S., Su M., Williams D., Kessler R.C. Specifying race-ethnic differences in risk for psychiatric disorder in a USA national sample. Psychol Med. 2006;36:57–68. doi: 10.1017/S0033291705006161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Himle J.A., Baser R.E., Taylor R.J., Campbell R.D., Jackson J.S. Anxiety disorders among African Americans, blacks of Caribbean descent, and non-Hispanic whites in the United States. J Anxiety Disord. 2009;23:578–590. doi: 10.1016/j.janxdis.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant B.F., Hasin D.S., Blanco C., Stinson F.S., Chou S.P., Goldstein R.B. The epidemiology of social anxiety disorder in the United States: results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry. 2005;66:1351–1361. doi: 10.4088/jcp.v66n1102. [DOI] [PubMed] [Google Scholar]
- 20.Brody D.J., Pratt L.A., Hughes J.P. Prevalence of depression among adults aged 20 and over: United States, 2013–2016. NCHS data brief. 2018:1–8. Available at: https://www.cdc.gov/nchs/products/databriefs/db303.htm. Accessed January 1, 2019. [PubMed] [Google Scholar]
- 21.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Bjelland I., Dahl A.A., Haug T.T., Neckelmann D. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 23.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 24.Deeks A.A., Gibson-Helm M.E., Paul E., Teede H.J. Is having polycystic ovary syndrome a predictor of poor psychological function including anxiety and depression? Hum Reprod. 2011;26:1399–1407. doi: 10.1093/humrep/der071. [DOI] [PubMed] [Google Scholar]
- 25.Barnard L., Ferriday D., Guenther N., Strauss B., Balen A.H., Dye L. Quality of life and psychological well being in polycystic ovary syndrome. Hum Reprod. 2007;22:2279–2286. doi: 10.1093/humrep/dem108. [DOI] [PubMed] [Google Scholar]
- 26.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chisholm D., Sweeny K., Sheehan P., Rasmussen B., Smit F., Cuijpers P. Scaling-up treatment of depression and anxiety: a global return on investment analysis. Lancet Psychiatry. 2016;3:415–424. doi: 10.1016/S2215-0366(16)30024-4. [DOI] [PubMed] [Google Scholar]
- 28.Ormel J., VonKorff M., Ustun T.B., Pini S., Korten A., Oldehinkel T. Common mental disorders and disability across cultures. Results from the WHO collaborative study on psychological problems in general health care. J Am Med Assoc. 1994;272:1741–1748. doi: 10.1001/jama.272.22.1741. [DOI] [PubMed] [Google Scholar]
- 29.Kessler R.C., Berglund P.A., Dewit D.J., Ustun T.B., Wang P.S., Wittchen H.U. Distinguishing generalized anxiety disorder from major depression: prevalence and impairment from current pure and comorbid disorders in the US and Ontario. Int J Methods Psychiatr Res. 2002;11:99–111. doi: 10.1002/mpr.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooney L.G., Lee I., Sammel M.D., Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2017;32:1075–1091. doi: 10.1093/humrep/dex044. [DOI] [PubMed] [Google Scholar]
- 31.Javier S.J., Moore M.P., Belgrave F.Z. Racial comparisons in perceptions of maternal and peer attitudes, body dissatisfaction, and eating disorders among African American and white women. Women Health. 2016;56:615–633. doi: 10.1080/03630242.2015.1118721. [DOI] [PubMed] [Google Scholar]
- 32.Silveira M.L., Ertel K.A., Dole N., Chasan-Taber L. The role of body image in prenatal and postpartum depression: a critical review of the literature. Arch Womens Ment Health. 2015;18:409–421. doi: 10.1007/s00737-015-0525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benedict C., Rodriguez V.M., Carter J., Temple L., Nelson C., DuHamel K. Investigation of body image as a mediator of the effects of bowel and GI symptoms on psychological distress in female survivors of rectal and anal cancer. Support Care Cancer. 2016;24:1795–1802. doi: 10.1007/s00520-015-2976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alur-Gupta S., Chemerinski A., Liu C., Lipson J., Allison K., Sammel M.D. Body-image distress is increased in women with polycystic ovary syndrome and mediates depression and anxiety. Fertil Steril. 2019;112:930–938.e1. doi: 10.1016/j.fertnstert.2019.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunter L.R., Schmidt N.B. Anxiety psychopathology in African American adults: literature review and development of an empirically informed sociocultural model. Psychol Bull. 2010;136:211–235. doi: 10.1037/a0018133. [DOI] [PubMed] [Google Scholar]
- 36.Bailey Z.D., Krieger N., Agénor M., Graves J., Linos N., Bassett M.T. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453–1463. doi: 10.1016/S0140-6736(17)30569-X. [DOI] [PubMed] [Google Scholar]
- 37.Gariepy G., Nitka D., Schmitz N. The association between obesity and anxiety disorders in the population: a systematic review and meta-analysis. Int J Obes (Lond) 2010;34:407–419. doi: 10.1038/ijo.2009.252. [DOI] [PubMed] [Google Scholar]
- 38.Amiri S., Behnezhad S. Obesity and anxiety symptoms: a systematic review and meta-analysis. Neuropsychiatr. 2019;33:72–89. doi: 10.1007/s40211-019-0302-9. [DOI] [PubMed] [Google Scholar]
- 39.DeJesus R.S., Breitkopf C.R., Ebbert J.O., Rutten L.J., Jacobson R.M., Jacobson D.J. Associations between anxiety disorder diagnoses and body mass index differ by age, sex and race: a population based study. Clin Pract Epidemiol Ment Health. 2016;12:67–74. doi: 10.2174/1745017901612010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenwood E.A., Pasch L.A., Cedars M.I., Legro R.S., Eisenberg E., Huddleston H.G. Insulin resistance is associated with depression risk in polycystic ovary syndrome. Fertil Steril. 2018;110:27–34. doi: 10.1016/j.fertnstert.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones G.L., Palep-Singh M., Ledger W.L., Balen A.H., Jenkinson C., Campbell M.J. Do South Asian women with PCOS have poorer health-related quality of life than Caucasian women with PCOS? A comparative cross-sectional study. Health Qual Life Outcomes. 2010;8:1–8. doi: 10.1186/1477-7525-8-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmid J., Kirchengast S., Vytiska-Binstorfer E., Huber J. Infertility caused by PCOS—health-related quality of life among Austrian and Moslem immigrant women in Austria. Hum Reprod. 2004;19:2251–2257. doi: 10.1093/humrep/deh432. [DOI] [PubMed] [Google Scholar]
- 43.Bakhtiyar K., Beiranvand R., Ardalan A., Changaee F., Almasian M., Badrizadeh A. An investigation of the effects of infertility on women's quality of life: a case-control study. BMC Womens Health. 2019;19:1–9. doi: 10.1186/s12905-019-0805-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mancuso A.C., Summers K.M., Mengeling M.A., Torner J.C., Ryan G.L., Sadler A.G. Infertility and health-related quality of life in United States women veterans. J Womens Health (Larchmt) 2020;29:412–419. doi: 10.1089/jwh.2019.7798. [DOI] [PubMed] [Google Scholar]
- 45.Humphries L.A., Chang O., Humm K., Sakkas D., Hacker M.R. Influence of race and ethnicity on in vitro fertilization outcomes: systematic review. Am J Obstet Gynecol. 2016;214:212.e1–212.e17. doi: 10.1016/j.ajog.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Quinn M., Fujimoto V. Racial and ethnic disparities in assisted reproductive technology access and outcomes. Fertil Steril. 2016;105:1119–1123. doi: 10.1016/j.fertnstert.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Feinberg E.C., Larsen F.W., Catherino W.H., Zhang J., Armstrong A.Y. Comparison of assisted reproductive technology utilization and outcomes between Caucasian and African American patients in an equal-access-to-care setting. Fertil Steril. 2006;85:888–894. doi: 10.1016/j.fertnstert.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 48.Consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod. 2008;23:462–477. doi: 10.1093/humrep/dem426. [DOI] [PubMed] [Google Scholar]
- 49.Cooney L.G., Milman L.W., Hantsoo L., Kornfield S., Sammel M.D., Allison K.C. Cognitive-behavioral therapy improves weight loss and quality of life in women with polycystic ovary syndrome: a pilot randomized clinical trial. Fertil Steril. 2018;110:161–171.e1. doi: 10.1016/j.fertnstert.2018.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dokras A., Sarwer D.B., Allison K.C., Milman L., Kris-Etherton P.M., Kunselman A.R. Weight loss and lowering androgens predict improvements in health-related quality of life in women with PCOS. J Clin Endocrinol Metab. 2016;101:2966–2974. doi: 10.1210/jc.2016-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hofmann S.G., Smits J.A. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69:621–632. doi: 10.4088/jcp.v69n0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cuijpers P., Berking M., Andersson G., Quigley L., Kleiboer A., Dobson K.S. A meta-analysis of cognitive-behavioural therapy for adult depression, alone and in comparison with other treatments. Can J Psychiatry. 2013;58:376–385. doi: 10.1177/070674371305800702. [DOI] [PubMed] [Google Scholar]
- 53.Dumesic D.A., Oberfield S.E., Stener-Victorin E., Marshall J.C., Laven J.S., Legro R.S. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36:487–525. doi: 10.1210/er.2015-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dokras A., Stener-Victorin E., Yildiz B.O., Li R., Ottey S., Shah D. Androgen excess-polycystic ovary syndrome society: position statement on depression, anxiety, quality of life, and eating disorders in polycystic ovary syndrome. Fertil Steril. 2018;109:888–899. doi: 10.1016/j.fertnstert.2018.01.038. [DOI] [PubMed] [Google Scholar]
- 55.Vancini R.L., Rayes A.B., Lira C.A., Sarro K.J., Andrade M.S. Pilates and aerobic training improve levels of depression, anxiety and quality of life in overweight and obese individuals. Arq Neuropsiquiatr. 2017;75:850–857. doi: 10.1590/0004-282X20170149. [DOI] [PubMed] [Google Scholar]
- 56.Gordon B.R., McDowell C.P., Lyons M., Herring M.P. The effects of resistance exercise training on anxiety: a meta-analysis and meta-regression analysis of randomized controlled trials. Sports Med. 2017;47:2521–2532. doi: 10.1007/s40279-017-0769-0. [DOI] [PubMed] [Google Scholar]
- 57.Stubbs B., Vancampfort D., Rosenbaum S., Firth J., Cosco T., Veronese N. An examination of the anxiolytic effects of exercise for people with anxiety and stress-related disorders: a meta-analysis. Psychiatry Res. 2017;249:102–108. doi: 10.1016/j.psychres.2016.12.020. [DOI] [PubMed] [Google Scholar]