Highlights
-
•
Dose escalation with IMRT to 60 Gy for esophageal cancer is feasible.
-
•
Dose escalation with cisplatin/5-FU still results in significant toxicity.
-
•
Improved local control, but comparable survival compared to historical controls.
-
•
Pretreatment weight loss was found to be an independent predictor of poor survival.
-
•
Our dose escalation study is one of few with predominant adenocarcinoma histology.
Keywords: Radiation therapy, Esophageal cancer, Dose escalation, Chemoradiation, Phase I
Abstract
Background and purpose
Radiation dose escalation to improve poor outcomes with chemoradiation in locally advanced esophageal carcinoma is limited in part by increased toxicity. This Phase I study investigates the use of IMRT to improve tolerability of dose escalation.
Materials and methods
A single-institution, prospective study was conducted between 2007 and 2013 for individuals with inoperable esophageal carcinoma. Gross disease received 60 Gy in 30 fractions and at-risk sites received 54 Gy with simultaneous integrated boost. Concurrent chemotherapy primarily consisted of cisplatin/5-FU. The primary objective was to assess feasibility (<15% rate of grade 4–5 toxicity). Secondary objectives included assessment of overall survival (OS), progression free survival (PFS), and locoregional (LRR) and distant recurrence.
Results
Twenty-six patients were enrolled with median follow up of 17.6 months (range 0.1 to 152.0). The majority were AJCC 7th edition Stage III (54%), distal esophagus primary (81%), and adenocarcinoma histology (85%). Twenty-one patients (81%) completed their course of radiation therapy, while only 55% received 2 cycles of concurrent cisplatin/5-FU. One grade 5 and one grade 4 cardiac event occurred, both during chemoradiation and before receiving 50 Gy. The 3-year OS was 48.6% (95% CI: 32.5 to 72.2%) and PFS was 28.5% (95% CI: 14.6 to 55.5%). Half developed distant failure with LRR occurring in 10 patients (38%), isolated in 5 patients.
Conclusion
While feasibility was demonstrated, toxicity and compliance remained limiting factors with outcomes similar to historical controls. There remains an uncertain role for dose escalation in definitive management of locally advanced esophageal cancer.
Introduction
Despite recent advances in treatment, esophageal cancer remains a significant therapeutic challenge. Of the estimated 18,440 new cases in the United States in 2020, nearly an equal number of individuals are expected to die from this disease [1]. For locally advanced patients, optimal outcomes result from trimodality therapy with neoadjuvant chemoradiation followed by surgery [2], however nearly half of patients are not candidates for surgery. Concurrent chemoradiation is the alternative definitive approach with improved outcomes over radiation alone [3]. Few prospective studies have directly compared chemoradiation to trimodality therapy, though available data suggest improved locoregional control but comparable overall survival with the addition of surgery, particularly for squamous cell carcinoma [4], [5]. For inoperable patients, chemoradiation is the only potentially curative approach despite continued poor outcomes.
Radiation dose escalation has been one approach to help improve disease control and survival with chemoradiation. Historically, outcomes with dose escalation have been discouraging. Intergroup 0123 evaluated dose escalation from 50.4 Gy to 64.8 Gy with 3D conformal radiation therapy and concurrent cisplatin/5-FU, but the study was terminated at interim analysis when significant toxicity and treatment-related death was observed in the high dose arm [6]. The majority of treatment-related deaths occurred at a delivered dose below 50.4 Gy, however analysis of patients who received 64.8 Gy still failed to show a benefit and dose escalation was generally abandoned based on these results [6]. With subsequent improvements in radiotherapy delivery including intensity modulated radiation therapy (IMRT), there has been renewed interest in dose escalation. Various single- or multi-institution experiences have been reported [7], [8], [9], [10], though a consensus on the benefit and ideal dose/fractionation has not been determined.
At our institution, we evaluated the feasibility of radiation dose escalation with IMRT in a prospective Phase I feasibility study in patients with inoperable, locally advanced esophageal cancer. Patients were treated to 60 Gy in 2 Gy per fraction with concurrent chemotherapy. The primary objective was to determine the feasibility of this treatment as defined as < 15% grade 4 toxicity and no grade 5 toxicity related specifically to external beam treatment. Secondary objectives included evaluation of overall and disease-free survival as well as local, regional, and distant recurrence rates.
Materials and methods
Patients
Eligibility criteria included pathologically confirmed esophageal cancer, T1-4, N0-3, M0 (AJCC 7th edition), age ≥ 18, Karnofsky performance status ≥ 60, determined to be unfit for resection by a thoracic surgeon, but medically fit for concurrent chemotherapy. Patients were excluded if they had radiographic or pathologic evidence of metastatic disease or prior radiation therapy to the thorax or upper abdomen. The study protocol was approved by the Institutional Review Board at Washington University in St Louis and all patients gave written informed consent. Clinicaltrials.gov Identifier: NCT00593723.
Chemotherapy
Concurrent and consolidation chemotherapy consisted of cisplatin (75 mg/m2) on day 1 and 5-FU (1,000 mg/m2) on days 1–4 on weeks 1, 5, 10, and 14 of therapy. However, drug regimens and doses could be varied at the discretion of the treating medical oncologist.
Radiotherapy
CT simulation was required for all patients with body-mold immobilization. Oral esophageal contrast was utilized if tolerated. Radiation therapy was delivered as IMRT with 6 MV photons in 30 fractions via Tomotherapy or fixed angle IMRT. The clinical target volume (CTV) was defined as a 4 cm expansion on the primary gross tumor volume (GTV) along the esophagus and adjacent lymph node regions in N0 patients. For GE junction tumors, a full 4 cm expansion into the stomach was not required and the final extent of CTV expansion inferiorly was at the discretion of the treating radiation oncologist. CTV for nodal disease was defined as a 0.5 cm expansion on nodal GTV(s). Elective nodal coverage consisted of celiac axis lymph nodes for distal/GE junction primaries and supraclavicular lymph nodes for upper esophageal primaries. Two planning target volumes (PTVs) were generated. PTV1 was defined as a 7–10 mm expansion on the CTV. PTV2 was defined as a 7–10 mm expansion on the GTV (primary and nodal, if applicable). Radiation was delivered with simultaneous integrated boost (SIB). PTV1 received 5400 cGy in 180 cGy/fraction while PTV2 received 6000 cGy in 200 cGy/fraction. PTV coverage goal was 98% receiving ≥ 95% prescription dose and maximum dose of 107% of a 2 cm3 volume and entirely within the PTV. Normal tissue constraints were as follows: Total lung minus PTV: V20 ≤ 25%, Heart: V60 ≤ 33%, V40 ≤ 66%, V20 ≤ 100%, Liver: V30 ≤ 30%, Spinal cord: max ≤ 47 Gy.
Assessment and follow up
Feasibility was defined as achieving all of the following: (1) ≤ 15% of patients experiencing any grade 4 acute toxicity judged to be related to radiation therapy, (2) ≤ 15% of patients experiencing any grade 4 late toxicity judged to be related to radiation therapy within 1 year of registration, (3) no deaths judged to be related to radiation therapy within 1 year of registration. Toxicity was assessed using the Common Toxicity Criteria for Adverse Events, version 3.0. After completion of radiation therapy, patients were followed at 6 weeks, then every 3 months in the first 2 years, every 6 months years 2–5, and annually thereafter. Routine use of endoscopy for surveillance was not mandated per protocol. Half of patients underwent endoscopy at some point during follow up (median time from completion of chemoradiation was 6.9 months) with 31% strictly for surveillance in the absence of symptoms. Imaging studies were performed 2–4 months after completion of therapy and then at the discretion of the treating physician(s).
Statistical analysis
The primary aim was to assess feasibility of a single radiation dose level as detailed above. Secondary objectives included overall survival (OS), disease free survival (DFS), local recurrence, regional recurrence, and distant recurrence. OS was defined as the time from diagnosis to death from any cause. DFS was defined as the time from radiation completion to first recurrence (any type) or death from any cause. Local or regional recurrence (LRR) was defined as recurrence at the primary site (per endoscopy and/or imaging, all but one recurrence confirmed with endoscopy) or nodal sites respectively. When combined for analysis, if an individual experienced metachronous local and regional recurrences, only the date of first recurrence was used for analysis. Distant recurrence was defined as recurrence outside of the esophagus or mediastinal or upper abdominal lymph nodes. Time-to-event outcomes were summarized using Kaplan-Meier analysis. Locoregional control and distant control specifically were determined from treatment start with the time to event noted regardless of whether this was the first site of failure or not. Due to the small number of events, 25 variables including age, sex, race, ethnicity, marital status, performance status, pretreatment weight loss, smoking status, treatment lymphocyte nadir, age-adjusted Charlson comorbidity score, histology, clinical stage, absence or presence of consolidation chemotherapy, were considered for the univariate analysis. Cox proportional hazards model was generated to identify how the clinical variables associated with the increased risk of overall mortality. Fast backward variable selection method was applied to further eliminate the redundant variables in the Cox model. Wilcoxon test was performed to identify cardiac dosimetric variable associated with high grade acute and late cardiac toxicity. P values < 0.05 were considered statistically significant. Statistical analyses were performed in R (Version 3.6.2).
Results
Patient characteristics
A total of 26 patients were enrolled and initiated treatment between February 2007 and January 2013 (Table 1). The median age was 67.5 with median follow up of 17.6 months, 31.1 months for living patients. Patients were predominantly Caucasian (81%), male (69%), and married (58%). The majority were clinical stage II or III (89%), however Stage I patients were included as well. The vast majority had a performance status of 0 or 1 (92%) and the median Age-adjusted Charlson comorbidity index score was 4. Most tumors were located in the lower third of the esophagus and/or gastroesophageal junction (88%) and were of adenocarcinoma histology (85%). The latter is a notable difference from prior prospective dose-escalation studies where squamous cell carcinoma predominated within each cohort and our cohort is a more accurate reflection of the current adenocarcinoma-predominant presentation in Western countries.
Table 1.
Patient Characteristics.
| Age (median, range) | 67.5 (46-88) |
| Sex | |
| Male | 18 (69%) |
| Female | 8 (31%) |
| Race | |
| White | 21 (81%) |
| Black | 5 (19%) |
| Marital Status | |
| Married | 15 (58%) |
| Single | 2 (8%) |
| Divorced | 3 (11%) |
| Widowed | 6 (23%) |
| Performance Status | |
| 0 | 4 (15%) |
| 1 | 20 (77%) |
| 2 | 2 (8%) |
| Smoking Status | |
| Current | 7 (27%) |
| Former | 12 (46%) |
| Never | 7 (27%) |
| Charlson Comorbidity Index | |
| 1-3 | 8 (31%) |
| 4-6 | 16 (61%) |
| 7 | 2 (8%) |
| Pretreatment Enteral Nutrition | |
| Yes | 22 (85%) |
| No | 22 (85%) |
| Tumor Location | |
| Upper Thoracic | 3 (11%) |
| Lower Thoracic | 23 (89%) |
| GE Junction | 18 (69%) |
| Histology | |
| Adenocarcinoma | 22 (85%) |
| Squamous Cell Carcinoma | 3 (11%) |
| Other | 1 (4%) |
| Grade | |
| Moderately Differentiated | 12 (46%) |
| Poorly Differentiated | 13 (50%) |
| Unknown/Not Specified | 1 (4%) |
| Clinical Stage (AJCC 7th Ed) | |
| I | 3 (11%) |
| II | 9 (35%) |
| III | 14 (54%) |
| T Stage | |
| T1 | 2 (8%) |
| T2 | 4 (15%) |
| T3 | 19 (73%) |
| T4 | 1 (4%) |
| N Stage | |
| N0 | 9 (35%) |
| N1 | 13 (50%) |
| N2 | 4 (15%) |
| Concurrent Chemotherapy | |
| Cisplatin/5-FU | 18 (70%) |
| Carboplatin/5-FU | 4 (15%) |
| Other/None | 4 (15%) |
| Consolidation Chemotherapy | |
| Yes | 9 (35%) |
| No | 17 (65%) |
Treatment
All 26 patients initiated radiation therapy, though 81% received the full prescription dose of 60 Gy. Total radiation dose received for the remaining 5 patients was as follows: 56 Gy, 48 Gy, 46 Gy, 30 Gy, and 18 Gy. Reasons for not completing radiation therapy as prescribed included treatment-related toxicity, proceeding to surgery, and poor patient compliance. One patient had a prolonged treatment break (195 days) due to toxicity and non-compliance. The vast majority received platinum/5-FU based concurrent chemotherapy (85%), with other regimens consisting of FOLFOX and carboplatin/paclitaxel (Supplemental Table S1). Of the patients who received concurrent cisplatin/5-FU (18 patients), 6 patients (33%) received only one cycle due to toxicity or patient refusal of additional chemotherapy. One patient was ultimately not given concurrent chemotherapy due to large tumor volume and concern for toxicity with concurrent therapy. Consolidation chemotherapy was given to 9 patients (36%) with 6 of these patients receiving cisplatin/5-FU recommended per protocol (Supplemental Table S2). In those not receiving consolidation chemotherapy, the most common reason was toxicity from chemoradiation or patient refusal.
Toxicity and feasibility
Feasibility criteria as defined in the protocol was met. There was no grade 4 acute toxicity, 12% grade 4 late toxicity, and no treatment-related deaths attributed to the radiation therapy. Acute grade 2 esophagitis was seen in 11 patients (42%) with grade 3 esophagitis in 8 patients (31%) (Table 2). There was one grade 5 toxicity during chemoradiation from hypoxemic respiratory failure. This was secondary to pneumonia precipitated by neutropenia primarily attributed to chemotherapy. One patient experienced acute grade 4 cardiac toxicity (acute myocardial infarction) due to chemotherapy and discontinued treatment on clinical trial and was lost to follow up for any further outcome or toxicity evaluation. Late grade 3 esophagitis was seen in 3 patients (11%) and this was primarily persistence of acute esophagitis requiring either prolonged PEG use or post-treatment hospitalization for altered swallowing. Of the 2 patients with sufficient post-treatment survival, the esophagitis did ultimately resolve. Grade 3 esophageal stricture developed in 2 patients (8%), and stricture was treated with balloon dilation in both these patients and in 5 of 8 patients with grade 2 stricture. Late grade 4 cardiac toxicity developed in 3 patients (11%) for arrhythmia, hypotension, and cardiac troponin respectively (Table 2). No grade 2 or higher pneumonitis was observed.
Table 2.
Toxicity.
| Acute Toxicity (n=26) | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| Esophagitis | 0 | 11 (42%) | 8 (31%) | 0 | 0 |
| Cardiac | 0 | 0 | 0 | 1 (4%) | 0 |
| Hypoxemia | 0 | 0 | 0 | 0 | 1 (4%) |
| Fatigue | 4 (15%) | 15 (58%) | 3 (12%) | 0 | 0 |
| Nausea | 4 (15%) | 9 (35%) | 3 (12%) | 0 | 0 |
| Vomiting | 5 (19%) | 5 (19%) | 2 (8%) | 0 | 0 |
| Diarrhea | 6 (23%) | 8 (31%) | 1 (4%) | 0 | 0 |
| Gastritis | 1 (4%) | 2 (8%) | 0 | 0 | 0 |
| Late Toxicity (n=24) | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
| Esophagitis | 1 (4%) | 3 (12%) | 3 (12%) | 0 | 0 |
| Esophageal Stricture | 1 (4%) | 8 (33%) | 2 (8%) | 0 | 0 |
| Pneumonitis | 2 (8%) | 0 | 0 | 0 | 0 |
| Cardiac | 3 (12%) | 1 (4%) | 2 (8%) | 3 (12%) | 0 |
Given the rate of high-grade cardiac toxicity in our cohort, we then evaluated cardiac dosimetric parameters to determine if there was any correlation with these events. The average maximum heart dose was 6270 cGy (range: 5836 cGy to 6483 cGy) and average mean dose was 2719 cGy (range: 1456 cGy to 3998 cGy). We also examined V40 and V5 with average values being 20.5% (range: 5% to 48%) and 94.3% (range: 73% to 100%) respectively. When comparing patients who developed acute grade 4 (no grade 3 toxicity seen) and late grade 3/4 cardiac toxicity to those who did not, there was no significant difference in any dosimetric parameter that might account for this difference (p value = 0.7 to 1).
Outcomes
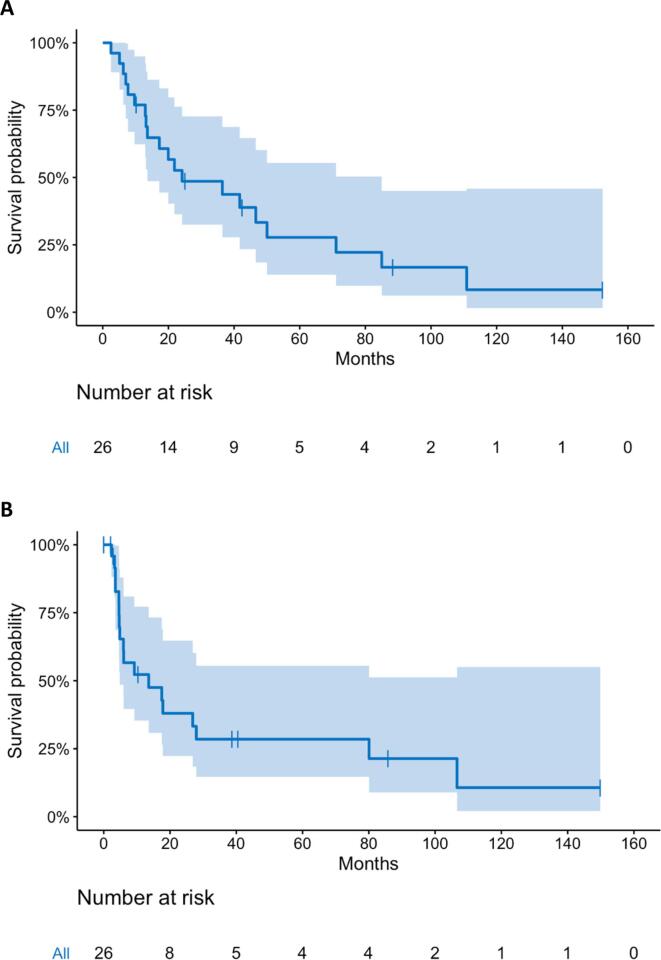
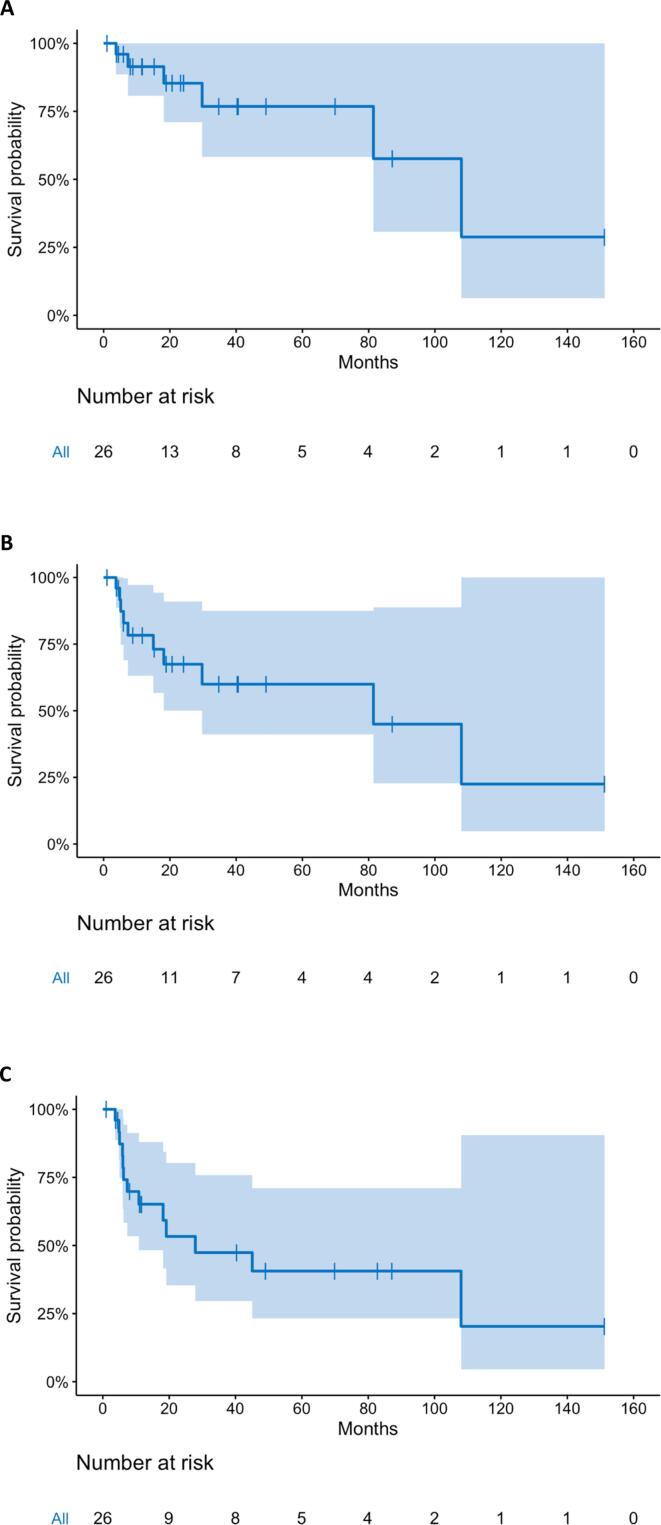
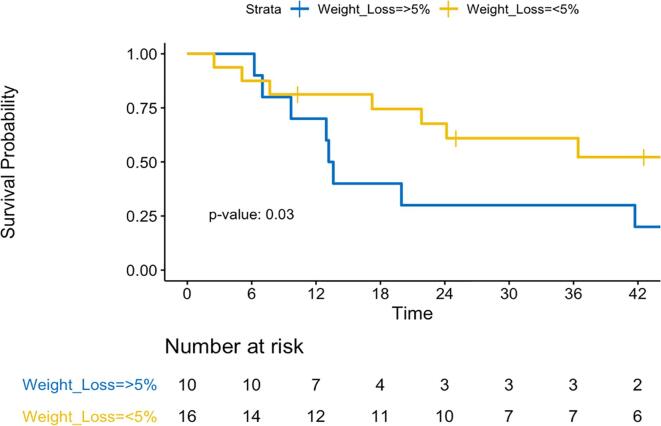
Median follow up was 17.6 months (range 0.1 to 152.0) with a median overall and progression free survival of 24.2 months and 13.6 months respectively. At 3 years, overall survival was 48.6% (95% CI: 32.5 to 72.2%) and progression free survival was 28.5% (95% CI: 14.6 to 55.5%) (Fig. 1). Local control at 3 years was 78.6% (95% CI: 52.8 to 100%) and locoregional control was 60% (95% CI: 41.1 to 87.5%), while distant control was only 47.4% (95% CI: 29.6 to 75.8%) (Fig. 2). The site(s) and frequency of first recurrence are shown in Supplemental Table S3. Of the 6 regional failures, only 2 (33%) were within the radiation field. One patient underwent esophagectomy after chemoradiation in this cohort and had an R0 resection with a pathologic partial response. Cox regression analysis found that only the extent of pre-treatment weight loss independently correlated with survival. Specifically, weight loss of > 5% prior to starting therapy was independently associated with worse overall survival (HR 1.03). Patients with < 5% pretreatment weight loss demonstrated improved survival with 2-year OS of 67.7%, compared to 30% with > 5% pretreatment weight loss (p = 0.03) (Fig. 3). Receiving consolidation chemotherapy demonstrated borderline significance for improved overall survival (p = 0.058).
Fig. 1.
Kaplan-Meier estimates of (A) Overall Survival and (B) Disease-Free Survival with 95% confidence interval for dose-escalated chemoradiation for inoperable esophageal cancer.
Fig. 2.
Kaplan-Meier estimates of (A) Local Control, (B) Locoregional Control and (C) Distant Control with 95% confidence interval with dose-escalated chemoradiation for inoperable esophageal cancer.
Fig. 3.
Overall survival as a function of pretreatment weight loss. Kaplan-Meier estimates of overall survival comparing individuals with < 5% pretreatment weight loss from baseline (yellow) to individuals with > 5% pretreatment weight loss (blue). P = 0.03. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Discussion
Dose-escalated IMRT using a simultaneous integrated boost technique for locally advanced, inoperable esophageal cancer to 60 Gy in conventional fractionation with concurrent chemotherapy was feasible in this Phase I study. While the threshold for feasibility was met based on our pre-determined criteria, our regimen still exhibited significant toxicity. In the acute setting, 31% developed a grade 3 esophagitis with grade 4/5 toxicity developing in 2 patients, both felt to be related specifically to systemic therapy. Late toxicity was also significant with 42% developing grade 2 or 3 esophageal stricture and 3 patients developing grade 4 cardiac toxicity. While this rate of toxicity appears improved over historical controls [3], [6], this remains relatively high compared to more modern cohorts [2], [8], [10].
A likely contributing factor is the use of cisplatin/5-FU for concurrent therapy and consolidation. The RTOG 85–01 Phase III study showing a benefit of chemoradiation over radiation alone utilized this regimen, and this was considered standard of care for subsequent studies and clinical practice [11]. More recently, carboplatin/paclitaxel use has increased after favorable results from the CROSS trial, and this regimen with a relatively favorable toxicity profile is currently being investigated with dose escalation in the ARTDECO and SCOPE2 trials [2], [12]. Though there was some heterogeneity in systemic therapy for our cohort, the majority (85%) did receive cisplatin/5-FU and so this may explain the degree of toxicity seen independent of radiation dose escalation. Indeed, preliminary reports from the CONCORDE study using FOLFOX with radiation dose escalation showed comparable toxicity between standard and escalated dose arms, suggesting that the type of concurrent chemotherapy may play a more pivotal role in toxicity over an incremental increase in radiation dose [13].
While this phase I study was designed to test the feasibility of dose escalation, the ultimate goal is to improve disease outcome with this regimen. In that regard, we did not observe any significant difference in survival in our preliminary cohort. Overall survival was comparable to RTOG 85–01 (5 yr OS 26% vs 28% in our cohort) [3]. Locoregional control, however, appears to be improved as one might expect with dose escalation, but distant recurrence occurred early and was significant in this cohort. A potential shift in failure from dose escalation may explain how LRR is improved but OS remains unchanged from historical controls.
Though the study was not powered for survival analysis, Cox proportional hazards model identified the extent of pretreatment weight loss as an independent predictor of survival. Specifically, individuals who lost > 5% of the pretreatment baseline weight before chemoradiation had significantly worse survival compared to those with minimal or no pretreatment weight loss. The impact of pretreatment weight loss on survival was also observed in a large cohort study of patients undergoing surgery for their esophageal cancer. Individuals who lost > 10% of their pretreatment baseline weight over 3 months before diagnosis had worse survival, and similar to our cohort, this survival difference became apparent relatively early and continued out to 5 years [14]. This is the first description, to our knowledge, of a similar phenomenon for individuals who underwent definitive chemoradiation. The underlying reason for this difference in survival is not entirely clear, but as with the underlying cancer cachexia, altered immune function is thought to play a significant role [15].
Ultimately, despite decades of investigation, the role of dose escalation in the definitive treatment of locally advanced esophageal cancer remains controversial. Prospective studies in this field have been varied and surprisingly scarce (Table 3). An early approach used a brachytherapy boost (RTOG 9207), but grade 5 toxicity was high (10%) with esophageal fistula developing in 12% of patients and this approach was ultimately felt to be too toxic [16]. INT-0123 then attempted to dose escalate using external beam radiation to 64.8 Gy with concurrent cisplatin/5-FU, but as mentioned previously, no improvement in locoregional control or overall survival was observed with an uncertain cause of increased toxicity in the high-dose arm [6]. With the advent and more broad utilization of IMRT and other components of treatment delivery (e.g. image guidance), re-attempting dose escalation became more feasible from a toxicity standpoint. A more recent institutional comparison between 3D conformal and IMRT treatment with standard dose suggested that IMRT not only decreases toxicity, but improves survival [17]. A follow-up Phase I/II study using intensity modulated therapy for dose escalation with SIB found dose escalation was reasonably well tolerated with no grade 4 or 5 toxicity and showed improved overall survival and local control compared to standard-dose institutional controls [10]. Nevertheless, the lack of prospective comparison to standard dose confirming these relative benefits continues to limit the widespread use of dose escalation in standard clinical practice.
Table 3.
Prospective Studies on Dose Escalation/Elevated Dose in Non-Operative Management of Esophageal Cancer.
|
Completed |
||||
|---|---|---|---|---|
| Study | Patient Population | Treatment | Results | Comments |
| INT 0123 (Minsky et al., 2002) | Phase III 218 eligible patients T1-T4 N0-1 M0 Adeno: 14% Squamous CC: 86% |
RT: 50.4 Gy in 1.8 Gy/fx vs. 64.8 Gy in 1.8 Gy/fx 2D Treatment Chemo: Cisplatin/5-FU |
High dose vs standard: MS: 13 mo vs 18.1 mo (NS) 2 yr OS: 31% vs 40% (NS) 2 yr LRF: 56% vs 52% (NS) Distant failure: 9% vs 16% Grade 5 toxicity: 11 pts vs 2 pts |
• 7 deaths in high dose arm occurred at or before 50.4 Gy • When early deaths factored out, survival still comparable between high and standard dose arms |
| RTOG 9207 (Gaspar et al., 2000) |
Phase I/II 49 eligible patients T1-2 NX-1 M0 No cervical tumors or within 1 cm GE junction Adeno: 8% Squamous CC: 92% |
RT: EBRT: 50 Gy in 2 Gy/fx Brachytherapy boost: LDR: 20 Gy HDR: Initially 15 Gy in 3 fx, later changed 10 Gy in 2 fx Chemo: Cisplatin/5-FU |
MS: 11 months “Life threatening” toxicity: 12 pts (24%) Grade 5 toxicity: 5 pts (10%) Esophageal fistula developed in 6 pts (12%) |
• Regimen felt not to be feasible due to high rate of toxicity |
| MD Anderson (Chen et al., 2019) |
Phase I/II 46 eligible patients All stages including IV with up to 3 metastatic sites Adeno: 48% Squamous CC: 52% |
RT: Simultaneous integrated boost (SIB) 50.4 Gy in 1.8 Gy/fx to PTV 63.0 Gy in 2.25 Gy/fx to IGTV IMRT: 85% IMPT: 15% Chemo: Docetaxel + 5-FU or Capecitabine. Induction allowed but not mandated (37% received) 11% ultimately received surgery |
MS: 21.5 months 2 yr OS: 41.3% No difference between histologies 2 yr LR: 33% Grade 3 acute toxicity in 10 pts No Grade 4 or 5 toxicity 17 pts (37%) developed esophageal strictures |
• Study compared SIB dose escalated patients to similar institutional patients receiving standard dose RT • SIB showed improved OS (HR 0.66, CI: 0.47 to 0.94) •SIB showed reduced LR (HR 0.49, CI: 0.26 to 0.92) |
| ESO-Shanghai 1 (Chen et al., 2019) |
Phase III 436 eligible patients Stage IIA to IVA (AJCC, 6th) Adeno: 0% Squamous CC: 100% |
RT: 61.2 Gy in 1.8 Gy/fx Chemo: Cisplatin/5-FU vs Paclitaxel/5-FU |
Cisplatin/5-FU vs Paclitaxel/5-FU 2 yr OS: 61.5% vs 60.6% (NS) Median PFS: 24.3 mo vs 21.0 ms (NS) LR free survival and Metastasis free survival comparable Grade 3+ toxicity: 51.6% vs 48.8% (NS) |
• Total doses of 60-64 Gy in 1.8-2 Gy/fx considered standard of care in China per national guidelines [18] |
| Ongoing | ||||
|---|---|---|---|---|
| Study | Eligible Patient Population | Treatment | Preliminary Results | Status |
| CONCORD-PRODIGE 26 Clinicaltrials.gov: NCT01348217 |
Age ≥ 18 and <75 T1-3 N1-3 M0 Inoperable |
Arm A: RT: 40 Gy in 2 Gy/fx + boost 10 Gy in 5 fx (50 Gy total) Chemo: FOLFOX Arm B: RT: 40 Gy in 2 Gy/fx + boost 26 Gy in 13 fx(66 Gy total) Chemo: FOLFOX |
196 pts enrolled Preliminary Abstract [13]: 160 pts evaluated IMRT (vs 3DC) in 70% in both arms Standard vs High Dose: Grade 3+ toxicity: Non-heme: 77% vs 86% (NS) Heme: 83% vs 89% (NS) |
Completed, Final Results Pending |
| ARTDECO Netherlands Trial Register ID: 3532 |
Age > 18 T1-4 N0-3 M0 Inoperable |
Arm A: RT: 50.4 Gy in 1.8 Gy/fx Chemo: Carboplatin/paclitaxel Arm B: RT: 50.4 Gy in 1.8 Gy/fx with SIB to 61.6 Gy in 2.2 Gy/fx Chemo: Carboplatin/paclitaxel |
Preliminary Abstract [12]: 260 pts eligible 61% with Squamous CC and 39% adenocarcinoma Standard vs High Dose: 3 yr OS: 41% vs 40% (NS) 3 yr LPFS: 70% vs 76% (NS) 3 yr LRPFS: 53% vs 63% (p = 0.08) Grade 4 toxicity: 12% vs 14% Grade 5 toxicity: 4% vs 10% |
Completed, Final Results Pending |
| SCOPE 2 Clinicaltrials.gov: NCT02741856 |
Age ≥ 17 T1-4 N0 or N+ M0 |
Arm 1: RT: 50 Gy in 2 Gy/fx Chemo: Carboplatin/paclitaxel Arm 2: RT: 50 Gy in 2 Gy/fx Chemo: Cisplatin/capecitabine Arm 3: RT: 60 Gy in 2.4 Gy/fx Chemo: Carboplatin/paclitaxel Arm 4: RT: 60 Gy in 2.4 Gy/fx Chemo: Cisplatin/capecitabine |
None | Recruiting |
| Washington University Clinicaltrials.gov: NCT04046575 |
Age ≥ 18 Inoperable Amenable to definitive chemoradiation |
Phase I RT: 40.05 Gy in 2.67 Gy/fx to PTV Starting dose boost SIB: 50 Gy in 3.33 Gy/fx Chemo: carboplatin/paclitaxel |
None | Recruiting |
Our Phase I study further contributes to the limited prospective data on IMRT in dose escalation and demonstrates that this approach is generally feasible with cisplatin/5-FU, though significant toxicity remains. As the Phase I/II study with similar dose escalation but with concurrent docetaxel/5-FU demonstrated less toxicity [10], concurrent chemotherapy regimen clearly plays an important part in the feasibility of dose escalation. In China, where standard-of-care dose for esophageal cancer is 60–64 Gy [18], the recent Phase III ESO-Shanghai 1 study demonstrated high rates of Grade 3 acute toxicity in the cisplatin/5-FU arm with Grade 5 toxicity in 12 patients [19]. With this same concurrent regimen, we also observed relatively high rates of grade 3 toxicity with limited compliance, again suggesting that identifying both an appropriate radiation dose and concurrent chemotherapy regimen is critical to optimize tolerability.
With regard to treatment outcomes, we found some improvement in locoregional control compared to historical controls, but similar overall survival. A more recent National Cancer Data Base analysis also demonstrated no improvement in overall survival with dose escalation>50.4 Gy [20]. Additionally, preliminary results from the ARTDECO study showed no local control or overall survival benefit to 61.6 Gy with concurrent carboplatin/paclitaxel [12]. While the final results and detailed analysis of this study are still pending, these findings are nevertheless discouraging.
Overall, the role of dose escalation in the treatment of locally advanced, inoperable esophageal cancer remains uncertain, and our Phase I study further suggests potential benefit in some disease outcomes but ultimately no significant benefit over standard therapy. Whether or not alternative approaches to radiation therapy and dose escalation such as hypofractionation and/or proton beam therapy may better achieve disease control and improve survival is a topic of current investigation (NCT04046575 and NCT03801878). However, despite improved feasibility with current technology, more conventional dose escalation appears to have a limited role in definitive management for esophageal cancer.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
There was no source of funding to disclose for this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2021.06.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.van Hagen P., Hulshof M.C.C.M., van Lanschot J.J.B., Steyerberg E.W., Henegouwen M.I.V.B., Wijnhoven B.P.L. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 3.Cooper J., Guo M., Herskovic A., Macdonald J., Martenson J.J., Al-Sarraf M. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85–01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623–1627. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 4.Stahl M., Stuschke M., Lehmann N., Meyer H.-J., Walz M.K., Seeber S. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23(10):2310–2317. doi: 10.1200/JCO.2005.00.034. [DOI] [PubMed] [Google Scholar]
- 5.Bedenne L., Michel P., Bouché O., Milan C., Mariette C., Conroy T. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25(10):1160–1168. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 6.Minsky B.D., Pajak T.F., Ginsberg R.J., Pisansky T.M., Martenson J., Komaki R. INT 0123 (Radiation Therapy Oncology Group 94–05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20(5):1167–1174. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 7.Li C., Ni W., Wang X., Zhou Z., Deng W., Chang X. A phase I/II radiation dose escalation trial using simultaneous integrated boost technique with elective nodal irradiation and concurrent chemotherapy for unresectable esophageal Cancer. Radiat Oncol. 2019;14(1) doi: 10.1186/s13014-019-1249-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welsh J.W., Seyedin S.N., Allen P.K., Hofstetter W.L., Ajani J.A., Chang J.Y. Local Control and Toxicity of a Simultaneous Integrated Boost for Dose Escalation in Locally Advanced Esophageal Cancer: Interim Results from a Prospective Phase I/II Trial. J Thorac Oncol. 2017;12(2):375–382. doi: 10.1016/j.jtho.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Chen J., Guo H., Zhai T., Chang D., Chen Z., Huang R. Radiation dose escalation by simultaneous modulated accelerated radiotherapy combined with chemotherapy for esophageal cancer: a phase II study. Oncotarget. 2016;7(16):22711–22719. doi: 10.18632/oncotarget.8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen D., Menon H., Verma V., Seyedin S.N., Ajani J.A., Hofstetter W.L. Results of a Phase 1/2 trial of chemoradiotherapy with simultaneous integrated boost of radiotherapy dose in unresectable locally advanced esophageal cancer. JAMA Oncol. 2019;5(11):1597. doi: 10.1001/jamaoncol.2019.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herskovic A., Martz K., Al-Sarraf M., Leichman L., Brindle J., Vaitkevicius V. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326(24):1593–1598. doi: 10.1056/NEJM199206113262403. [DOI] [PubMed] [Google Scholar]
- 12.Hulshof M.C.C.M., Geijsen D., Rozema T., Oppedijk V., Buijsen J., Neelis K.J. A randomized controlled phase III multicenter study on dose escalation in definitive chemoradiation for patients with locally advanced esophageal cancer: ARTDECO study. J Clin Oncol. 2020;38(4_suppl):281. doi: 10.1200/JCO.20.03697. [DOI] [PubMed] [Google Scholar]
- 13.Crehange G., Bertaut A., Peiffert D., Le Prise E., Etienne P.-L., Rio E. Exclusive chemoradiotherapy with or without dose escalation in locally advanced esophageal carcinoma: The CONCORDE study (PRODIGE 26) J Clin Oncol. 2017;35(15_suppl):4037. [Google Scholar]
- 14.van der Schaaf M.K., Tilanus H.W., van Lanschot J.J.B., Johar A.M., Lagergren P., Lagergren J. The influence of preoperative weight loss on the postoperative course after esophageal cancer resection. J Thorac Cardiovasc Surg. 2014;147(1):490–495. doi: 10.1016/j.jtcvs.2013.07.072. [DOI] [PubMed] [Google Scholar]
- 15.Anandavadivelan P., Lagergren P. Cachexia in patients with oesophageal cancer. Nat Rev Clin Oncol. 2016;13(3):185–198. doi: 10.1038/nrclinonc.2015.200. [DOI] [PubMed] [Google Scholar]
- 16.Gaspar L.E., Winter K., Kocha W.I., Coia L.R., Herskovic A., Graham M. A phase I/II study of external beam radiation, brachytherapy, and concurrent chemotherapy for patients with localized carcinoma of the esophagus (Radiation Therapy Oncology Group Study 9207) Cancer. 2000;88(5):988–995. [PubMed] [Google Scholar]
- 17.Lin S., Wang L., Myles B., Thall P., Hofstetter W., Swisher S. Propensity score based comparison of long term outcomes with 3D conformal radiotherapy (3DCRT) versus Intensity Modulated Radiation Therapy (IMRT) in the treatment of esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;84:1078–1085. doi: 10.1016/j.ijrobp.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Health Commission of The People’s Republic of China. Chinese guidelines for diagnosis and treatment of esophageal carcinoma 2018 (English version). Chin J Cancer Res. 2019;31:223-58. [DOI] [PMC free article] [PubMed]
- 19.Chen Y., Ye J., Zhu Z., Zhao W., Zhou J., Wu C. Comparing paclitaxel plus fluorouracil versus cisplatin plus fluorouracil in chemoradiotherapy for locally advanced esophagea squamous cell cancer: a randomized, multicenter, Phase III clinical trial. J Clin Oncol. 2019;37:1695–1703. doi: 10.1200/JCO.18.02122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brower J.V., Chen S., Bassetti M.F., Yu M., Harari P.M., Ritter M.A. Radiation Dose Escalation in Esophageal Cancer Revisited: A Contemporary Analysis of the National Cancer Data Base, 2004 to 2012. Int J Radiat Oncol Biol Phys. 2016;96(5):985–993. doi: 10.1016/j.ijrobp.2016.08.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.