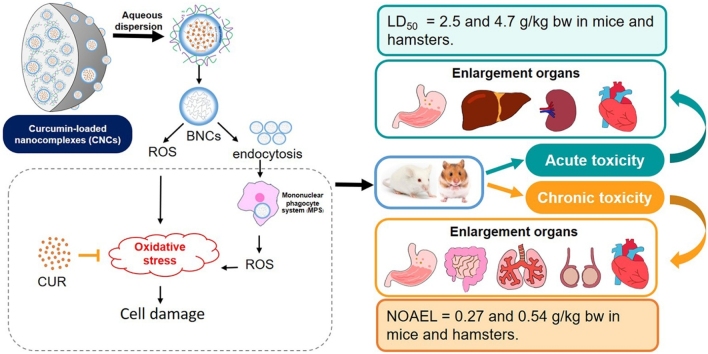
Graphical abstract
Keywords: Curcumin, LD50, NOAEL, Safety assessment, ER stress
Highlights
-
•
CNCs have potential to stabilize curcuminoid content for at least 12-months.
-
•
CNCs are safe in appropriate dose as low and medium doses of toxicity testing.
-
•
High dose of CNCs could produce toxicity in blood parameters and histopathology.
-
•
Most altered parameters in chronic toxicity test could recover within 28 days.
-
•
CNCs have very low toxicity in non-clinical trials and are ready for clinical study.
Abstract
We recently developed a modified solid dispersion of curcumin-loaded nanocomplexes (CNCs) in gums which promoted the prolonged and sustained release of curcumin. However, its safety assessment has not yet been investigated. Here, acute and chronic toxicities of CNCs were assayed using mice and hamsters. CNCs were orally administered to the animals. Doses of CNCs used for acute toxicity testing were 0.1, 1.1, 11.0 g/kg body weight for mice and 0.2, 2.1 and 21.4 g/kg body weight for hamsters. Doses of CNCs for chronic toxicity testing were 0.09, 0.27, 0.8 g/kg body weight/day for mice and 0.18, 0.54 and 1.61 g/kg body weight/day for hamsters. This regimen was followed daily for 6 months. Low and medium doses of CNCs did not induce any side effects in acute and chronic toxicity tests in either animal species. However, in acute toxicity testing, the organ-weight to body-weight ratio of spleen was significantly increased in mice treated with 11 g/kg body weight along with elevated levels of some biochemical parameters. There was a significant increase in organ-weight to body-weight ratios of stomach, liver and heart in hamsters treated with 21.4 g/kg body weight, but no elevated levels of biochemical parameters. Oral LD50 of CNCs in mice and hamsters were 8.9 and 16.8 g/kg body weight (equivalent to 2.5 and 4.7 g curcumin/kg body weight), respectively. Daily CNCs high-dose treatment for 6 months significantly increased organ-weight to body-weight ratios of stomach and intestine in mice and of lung and heart in hamsters. Elevated levels of glucose, total protein, ALT, AST and globulin in mice, and increased levels of AST, but decrease in cholesterol, in hamsters were concurrently observed with inflammation in liver and lung. These abnormalities were resolved within 28 days after cessation of treatment. The no-observed-adverse-effect level of CNCs was determined at 0.27 and 0.54 g/kg body weight/day in mice and hamsters. In conclusion, toxicity of high-dose CNCs treatment was graded as very low, possibly due to the components of the nanocomplex.
1. Introduction
Curcumin, the principal bioactive constituent of turmeric (Curcuma longa), has been investigated for its antioxidant, anti-inflammatory, anti-parasitic infections, anti-fibrosis and anti-cancer activities [[1], [2], [3]]. It is also beneficial for alleviating various chronic diseases [[4], [5], [6]]. Its water-insolubility, instability, and poor bioavailability [7] account for uncertain therapeutic efficacies [8]. To obtain therapeutic effects, high doses of oral curcumin, up to 1 g/kg/day [[9], [10], [11], [12]] are required, leading to concerns about its safety and efficacy in long-term use [13]. It dose-dependently acts as anti-oxidant, inhibiting reactive oxygen species (ROS), as well as a pro-oxidant and producing ROS [14]. At high doses, curcumin can react with thiol groups of cysteine residues [15], eventually damaging DNA [16] or inactivating p53 [17]. To improve its bioavailability without using high doses, a nanoparticle delivery system for curcumin has been developed [[18], [19], [20], [21]].
Nanoparticles with a particle size range of (10−600 nm) enhance therapeutic efficacy of curcumin [22]. Delivery of nanoparticles of curcumin can improve oral bioavailability due to enhanced solubility [24,25] or increased mucosal permeation and cell-selective uptake [26,27]. Although several formulations of nanoparticle-based curcumin have been developed, only few of them such as curcumin-loaded polymeric nanoparticles of Eudragit S100 [28] and curcuminoid-essential oil complex [29] have evaluated the safety of nanoparticle delivery systems in animal models. These formulations were non-toxic in acute toxicity studies at doses equivalent to 2 g curcumin /kg of body weight [28]. Daily administration of a curcuminoid-essential oil complex (CEC) at a dose of 1 g CEC/kg body weight [29] or curcumin-loaded polymeric nanoparticles of Eudragit S100 at 0.1 g curcumin/kg of body weight [28] also revealed no toxicity after 90 and 28 days, respectively. However, further evaluation of safety, particularly in the event of long-term consumption of nanoparticles is required. It is widely accepted that the toxicity of nanodelivery systems vary according to nanoparticle size [30], nanomaterials used and their exact formulation [23], dosage [31] and cell types targeted [32]. Therefore, although curcumin has been shown to be safe in several studies [33,34], development of novel nanoparticle-based delivery system for curcumin still requires safety assessment.
Nanoparticles taken orally are generally absorbed through the intestinal mucosa or lymphatics whereby distributed and excreted through clearance systems, i.e. renal clearance, hepatobiliary clearance, the reticuloendothelial system or the mononuclear phagocyte system, depending on several factors such as particle sizes [35], surface morphology, charges, and properties of nanomaterials [36]. Renal clearance, through glomerular filtration and tubular secretion, deals with small particles (about 5 nm) [36]. The epithelial lining of hepatic sinusoids deals with nano-sizes up to 200 nm, however, other hepatic mechanisms, i.e. endocytosis, metabolism and enzymatic cleavage, also play roles in hepatic clearance of foreign particles and eliminate them via the bile duct. Nanoparticles with sizes of >200 nm tend to remain in the body until degradation [35,37].
Previously, mucoadhesive polymers were used to form nanoparticles of curcumin and shown to promote its release and absorption [38] with chemoprevention potential for opisthorchiasis-associated cholangiocarcinoma (CCA) [39]. To solve the indispensability in water of the curcumin nanoparticles, hydrophilic solid dispersions using arabic and xanthan gums have been used to develop curcumin-loaded nanocomplexes (CNCs) with particle sizes in a range of 400−1,000 nm. These showed improved oral delivery of curcumin by enhancing gastrointestinal mucoadhesion and potentially extending curcumin retention [40]. Because CNCs tend to be retained in the body for long periods, it is essential to determine their safety for human use as a novel drug. Non-clinical risk assessment in an animal model is a necessary step on the path for translation of CNCs to use in human patients. The objective of this study was to investigate acute and chronic toxicities of oral CNCs in two different animal species, mice and hamsters. Different doses of CNCs were administered to evaluate their toxicity as indicated by physiological, biochemical parameters, ultrastructural effects and histopathological changes in various organs. Safety assessment of CNCs have very low toxicity in non-clinical trials and are ready for clinical study.
2. Materials and methods
2.1. CNCs preparation
Arabic gum, xanthan gum and isoflurane were purchased from Sigma-Aldrich (St. Louis, MO, USA). Curcumin (>98 % purity w/w) was purchased from ACROS Organics (Geel, Belgium). Powdered CNCs (WellCap® Kaminn, with encapsulation efficiency of 80 % and loading capacity of 28 % [40] and blank nanocomplexes (BNCs, WellCap® Capsule) kindly gifted by Welltech Biotechnology Co. Ltd. Bangkok, Thailand. These were stored following the manufacturer’s instructions. In brief, the curcumin-encapsulated nanoparticles, using ethylcellulose and methylcellulose formula dispersed in deionized water, were mixed with a solution containing 1% each of arabic gum and xanthan gum and then subjected to a spray-drying process as previously described [40].
2.2. Physicochemical characterization of CNCs
2.2.1. Morphology
Focused ion beam-field emission scanning electron microscopy (FIB-SEM, FEI Helios Nanolab G3CX, USA) was used to observed dry CNCs powder at 10 kV, after the powder was spread on adhesive tape and then vacuum-coated with a thin layer of gold at 15 kV for 90 s. CNCs were also dispersed in deionized water, dropped onto analytic glass plate, desiccated and then gold-coated before visualization using a JSM-IT100, SEM (JEOL, Japan).
CNCs in deionized water were dropped onto a 200-mesh grid and visualized by transmission electron microscopy (TEM, JEM-1010, JEOL, Japan) at 100 kV.
2.2.2. Stability
Ten light-proof foil/polyamide-sealed packets containing CNCs (5 g each) were randomly sampled from three batches of thirty packs (ten packs / batches) to be stored at 25 ± 2 °C/60 ± 5 % RH for stability monitoring. After 6 or 12 months, samples were diluted with ethyl acetate for UV spectroscopic determination of curcuminoid concentration at 416 nm (Spectroquant UV2400PC, China) in comparison to standard solutions of curcuminoid (1−5 μg/mL). Data were presented as the average of % w/w of curcumin in CNCs and % of initial.
2.3. Ethics statement and animals used
This study has been reviewed and approved by the Animal Ethics Committee of Khon Kaen University based on the Ethics of Animal Experimentation of National Research Council of Thailand (ACUC-KKU-59/2559). Two species of animals were used in this study. Swiss albino mice of both sexes (JcL:ICR) (4–5 weeks old, 25−40 g, total 197 mice) were purchased from Nomura Siam International Co. Ltd., Bangkok, Thailand and reared at Northeast Laboratory Animal Center, Khon Kaen University. This strain of mice has been used in tests for sensitivity to chemicals, susceptibility to toxic substances and for tumor induction and is commonly used for preclinical toxicity testing. Syrian golden hamsters (Mesocricetus auratus), a susceptible animal model for opisthorchiasis-associated cholangiocarcinoma [41], of both sexes (4–6 weeks old, 80−100 g, total 207 hamsters) were reared at the Animal Unit, the Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand. All female rodents were nulliparous and non-pregnant, as recommended by Organization for Economic Co-operation and Development (OECD) Guidelines for Testing of Chemicals (Sections 423 and 452). This study was performed under good laboratory practice according to OECD principles on good laboratory practice guideline [42]. All animals were maintained under clean conventional conditions at 23 °C (± 2 °C) with relative humidity 30–60 % and 12 h light/ dark cycles, and fed ad libitum a commercial pellet diet (CP-SWT, Thailand) with unlimited access to food and drinking water. All animals were randomly assigned to cages for at least 5 days before experimentation. All cages were monitored every day and bedding material was changed thrice a week.
2.4. Acute toxicity study
Female animals (total 29 mice and 23 hamsters) were randomly assigned to non-treated (normal control group) or intervention groups. Acute toxicity testing was carried out following OECD Guideline 423 for Testing of Chemicals with slight modification, including dose level, rodent species and number of animals used. The acute oral toxicity of CNCs was classified based on the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) 2003 in which the most severe toxicity is classified in category 1 (LD50 ≤ 0.005 g/kg bw) and relatively low toxicity in category 5 (LD50 > 2−5 g/kg bw). Agents that have very low toxicity (LD50 > 5 g/kg bw) [43] are placed into the unclassified hazards category as indicated by the ∞ symbol based on OECD guidelines [44]. Most previous articles have reported LD50 of curcumin to be approximately 2 g/kg bw (in GHS category 5 of OECD guidelines for oral toxicity studies) in rats and mice [45,46]. Therefore, in this study, we used the higher dose levels from OECD Guideline 423 to determine the actual dose for translation from animal to human. For rodent species, mice and hamsters were selected as described above. CNCs were given on a single occasion at low, medium and high doses, which were 0.1, 1.1 or 11 g/kg bw (equivalent to 0.03, 0.3 or 3 g/kg bw of curcumin, respectively) for mice, and 0.2, 2.1 and 21.4 g/kg bw (equivalent to 0.06, 0.6, 6 g/kg bw of curcumin, respectively) for hamsters. Mice and hamsters in the blank nanocomplexes (BNCs) group received a single oral dose of 7.9 g/kg bw BNCs and 15.4 g/kg bw BNCs, respectively. Administration of the single dose was performed by oral gavage in a volume of 10 mL/kg bw. The BNCs or CNCs powder to be administered was diluted with distilled water at a ratio of 10:1 w/v. The diluted samples were pushed through gavage tubes within 45 min. Animals in the negative control group received no intervention. Clinical signs of toxidromes (depression, rising fur, tremors, excitability, twitching, salivation, morbidity) and mortality were observed and recorded twice daily for 14 days post-treatment.
2.5. Chronic toxicity study
The protocol was performed based on the OECD Guidelines for Testing of Chemicals (Section 452) with slight modification including rodent species, dose levels and number of animals used. Swiss albino mice (12/sex/group, total 168, bodyweight 25−40 g) and hamsters (13/sex/group, total 182, bodyweight 80−100 g) were randomly divided into seven groups as follows: Group 1 (control) normal diet without any treatment, Group 2 daily oral gavage with BNCs (0.58 g/kg bw/day in mice or 1.16 g/kg bw/day in hamsters), Groups 3–5 daily oral gavage of CNCs at low, medium and high doses in mice (0.09, 0.27 and 0.8 g/kg bw, equivalent to 0.025, 0.075, 0.225 g/kg bw of curcumin, respectively) and CNCs at low, medium and high doses in hamsters 0.18, 0.54, 1.61 g/kg bw (equivalent to 0.05, 0.15, 0.45 g/kg bw of curcumin, respectively), for 6 months. Groups 6 and 7 of both species (n = 24 mice and 26 hamsters), termed the recovery groups, were respectively given 0.58 g/kg bw/day of BNCs or the high-dose CNCs regimen daily for 6 months. Following cessation of the treatments at 6 months, animals in groups 6 and 7 were held for a further 28 days. The dose volume in all animals used was 10 mL/kg bw. The dose levels to be used for chronic toxicity testing were based on the results from the acute toxicity testing. The high-dose level in the chronic toxicity test was approximately equivalent to the medium dose level used in the acute toxicity tests: the medium and low doses used in the chronic toxicity tests were successive approximately three-fold reductions of the high dose. During the experiment, all animals were daily checked for overall health condition, body weight, morbidity and mortality. All animals were starved for 1 day before euthanasia.
2.6. Sample collection and histopathological study
Animals were anesthetized using isoflurane inhalation and euthanized by cardiac puncture. Blood samples obtained were immediately divided into 3 portions, one for hematological analysis (stored in an EDTA tube), one for coagulation analysis (in a citrate tube) and one for serum biochemistry. Internal organs including liver, lung, kidney, heart, spleen, pancreas, stomach, intestine and ovaries or testes were collected and immediately fixed in 10 % buffered formalin for histopathological study. The tissues were processed using an Automatic Tissue Processor (Hestion, England) and embedded with paraffin using a tissue processor (Bio-Optica, Italy). The paraffin-embedded tissues were cut using a Microm HM 315 microtome (Thermo Fisher Scientific, USA) and stained with hematoxylin and eosin (H&E). The slides were observed under a light microscope.
2.7. Hematological and biochemical parameters
All analyses of blood samples, with results reported as means ± SD, were conducted at the Laboratory Unit, Srinagarind Hospital, Faculty of Medicine, Khon Kaen University, Thailand. A Sysmex Xs-800i1000i Automated Hematology Analyzer (Sysmex Corporation, Kobe, Japan) provided hematological parameters. A Cobas 8000 Chemistry Autoanalyzer (Roche Diagnostics International Ltd., Scotland) was used to determine serum biochemistry parameters, among which were glucose levels, activity of liver function enzymes and lipid profile. An automated blood coagulation analyzer ACLTOP550 (AWerfen Company, Germany) provided coagulation parameters.
2.8. Scanning electron microscopy (SEM)
To achieve our ultimate goal of CNCs use in opisthorchiasis-associated CCA patients, we focused on hamsters, a susceptible animal model for O. viverrini infection [41]. Tissue samples of hamster stomach and intestine (1 mm3 each) of Groups 5and 7 in the chronic toxicity study were excised, fixed with Karnovsky's fixative, washed twice with buffered solution for 10 min and post-fixed using osmium tetroxide (OsO4) for 2 h and then, re-washed twice in buffered solution for 10 min. The samples were dehydrated using ethyl alcohol for 10 min at each of the following concentrations: 50 %, 70 %, 80 %, 90 % and 95 %. Final dehydration was achieved using 3 changes of absolute alcohol (10 min each) followed by amyl acetate for 15 min. All tissues were dried using a critical-point drier (CPD) K850 (ASHFORD, Kent, UK) liquid carbon dioxide, coated with a thin layer of gold under vacuum at 15 kV for 90 s (EMITECH K550X, England) and the mucosal side of each sample was observed and imaged using SEM (JSM-IT200, JEOL, Japan).
2.9. Transmission electron microscopy (TEM)
Samples of liver (1 mm3, each) of hamsters from Groups 1, 2 and 5 in the chronic toxicity study were fixed in Karnovsky's fixative and dehydrated using an ethanol concentration gradient. The samples were embedded in propylene at 60 °C for 48 h. The sections were cut using an Ultracut N, Reichert-Nissei microtome. The TEM grids holding samples were viewed using a JEM-1010 TEM, (JEOL, Japan). TEM images of CNCs were obtained with an accelerating voltage of 100 kV.
2.10. Statistical analysis
Survival rates were statistically analyzed using Kaplan-Meier analysis and Cox regression. All parameters were compared statistically between the normal (control) group and any treatment group. Blood parameters were analyzed using analysis of variance (one-way ANOVA) with a post-hoc Tukey’s HSD (Honestly Significant Difference) test, implemented in the IBM SPSS Statistics 19 program (SPSS, Inc., Chicago, IL, USA), and reported as means ± SD. Comparisons yielding p values of 0.05 or lower were regarded as statistically significant differences.
3. Results
3.1. Characteristics of CNCs
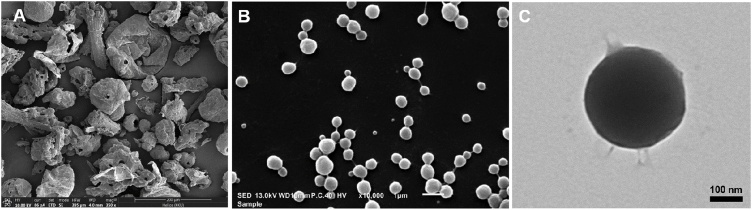
Fig. 1(A) illustrates FIB-SEM images of CNCs powder in aggregates of nanoparticles which were segregated after being dispersed in water (Fig. 1(B) and (C)). The segregated nanoparticles of CNCs forming nano-encapsulated curcumin were similar to those in our previous report [40]. The segregated nanoparticles of CNCs were within a size range of 400−1,000 nm. The TEM image, Fig. 1(C), reveals a loose surface detaching from the nanoparticle. After storage at 25 ± 2 °C/60 ± 5 % RH in light-protected and sealed conditions for 6 and 12 months, curcuminoid contents of CNCs remained higher than 97 % of the initial amount with little or no changes in pH and bulk densities (Table 1).
Fig. 1.
Images of the CNCs-based nanodelivery system. CNCs powder was visualized using FIB-SEM (A). CNCs after dispersal in water and visualized using SEM (B) and TEM (C).
Table 1.
Physicochemical characteristics of curcumin-loaded nanocomplexes (CNCs) and their stability following storage for 6 or 12 months.
| Characteristics | |||
|---|---|---|---|
| Appearance: Yellowish-orange, mild turmeric odor and tasteless powder | |||
| Condition: Stored CNCs in foil-sealed 5-g packs at 25 ± 2 °C/60 ± 5 %RH (n = 3 lots) | |||
| Tests | Initial | 6 months | 12 months |
| 1. pH (0.1 % in water, 25 °C) | 6.4 ± 0.8 | 5.8 ± 0.2 | 5.9 ± 0.4 |
| 2. Bulk density (g/cm3) | 0.07 ± 0.02 | 0.07 ± 0.02 | 0.08 ± 0.01 |
| 3. Curcuminoids (%) | 29.7 ± 1.1 | 28.9 ± 1.2 | 29.1 ± 1.1 |
| 4. % curcuminoids remaining | 100 ± 0 | 97.2 ± 1.0 | 98.1 ± 0.8 |
3.2. Acute toxicity
In generally, mice engage in high-energy activities, such as climbing around in their cage [47]. Similarly, hamsters spend much time running around their habitat and do a lot of chewing [48]. In acute toxicity testing, all animals treated with high doses (11 g/kg bw in mice and 21.4 g/kg bw in hamsters) exhibited different behaviors from the normal group: they moved more slowly and squeezed or curled themselves against the walls or into corners of their cages a few minutes after the dosing. Half of the animals died within 24 h after administration of a single high dose of CNCs (3 of the 5 hamsters and 3 of the 6 mice), enabling the determination of LD50. The surviving animals recovered after some hours and showed no further signs of toxicity or died during the remaining 14 days of observation. Based on Lorke’s method [49], the estimated oral LD50 values of CNCs were 8.9 and 16.8 g/kg bw (equivalent to 2.5 and 4.7 g/kg bw of curcumin) for mice and hamsters, respectively.
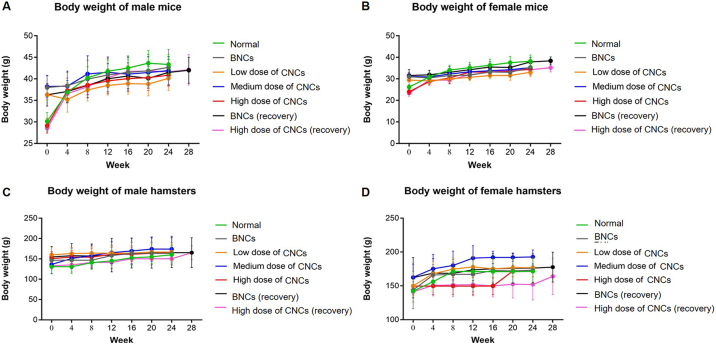
There was no significant change in body weights of mice or hamsters throughout the period of study relative to initial (p > 0.05 both) (Fig. S1). Gross pathology and histopathology did not find necrosis or other severe abnormal changes, but inflammation was found in liver after high doses (11 g/kg bw in mice and 21.4 g/kg bw in hamsters) (Fig. S2). In the animals treated with a high dose of CNCs, as shown in Table 2, there were significant increases in organ-weight to body-weight ratios of spleen in mice and of heart and stomach in hamsters (p < 0.05, all). Liver weight was significantly increased in hamsters compared to the normal group, but not in mice. Mice treated with a high dose of CNCs exhibited significantly elevated total protein, globulin and BUN with an increasing trend of ALT and alkaline phosphatase but decreasing trend of AST. In hamsters receiving the high dose, there was a non-significant increase of ALT, AST and alkaline phosphatase compared to the normal group (Table 3). Notably, BNCs-treated mice showed significant elevation of total protein, globulin, ALT, AST, alkaline phosphatase and BUN compared to the normal control group. Significantly elevated levels of total protein, globulin, ALT and alkaline phosphatase were also observed in BNCs-treated hamsters.
Table 2.
Relative organ weights of animals in the acute toxicity study at 14 days after oral administration.
| Organs | Normal | Intervention |
||||
|---|---|---|---|---|---|---|
| BNCs | CNCs |
|||||
| Low dose | Medium dose | High dose | ||||
| 7.9 g/kg bw | 0.1 g/kg bw | 1.1 g/kg bw | 11 g/kg bw | |||
| n = 5 | n = 8 | n = 2 | n = 2 | n = 6 | ||
| Mice | Stomach | 1.80 ± 0.32 | 1.53 ± 0.50 | 1.54 ± 0.01 | 1.51 ± 0.59 | 1.70 ± 0.65 |
| Intestine | 8.18 ± 0.67 | 8.66 ± 0.99 | 8.30 ± 1.02 | 8.00 ± 0.64 | 8.97 ± 0.79 | |
| Liver | 4.56 ± 0.05 | 4.36 ± 0.89 | 4.66 ± 0.47 | 4.78 ± 0.69 | 5.25 ± 0.77 | |
| Pancreas | 0.84 ± 0.12 | 0.68 ± 0.27 | 0.61 ± 0.05 | 0.75 ± 0.04 | 0.74 ± 0.34 | |
| Kidneys | 1.52 ± 0.16 | 1.33 ± 0.15 | 1.35 ± 0.07 | 1.41 ± 0.08 | 1.45 ± 0.20 | |
| Spleen | 0.28 ± 0.05 | 0.42 ± 0.09 | 0.46 ± 0.16 | 0.43 ± 0.26 | 0.46 ± 0.09* | |
| Lung | 0.74 ± 0.11 | 0.73 ± 0.09 | 0.73 ± 0.15 | 0.83 ± 0.16 | 0.71 ± 0.17 | |
| Heart | 0.51 ± 0.05 | 0.48 ± 0.13 | 0.49 ± 0.07 | 0.55 ± 0.01 | 0.51 ± 0.05 | |
| Ovaries | 1.16 ± 0.48 | 1.65 ± 0.44 | 1.49 ± 0.20 | 1.27 ± 0.32 | 1.10 ± 0.50 | |
| 15.4 g/kg bw | 0.2 g/kg bw | 2.1 g/kg bw | 21.4 g/kg bw | |||
|---|---|---|---|---|---|---|
| n = 7 | n = 5 | n = 2 | n = 2 | n = 5 | ||
| Hamsters | Stomach | 1.18 ± 0.41 | 1.30 ± 0.29 | 1.39 ± 0.17 | 1.97 ± 0.06 | 2.02 ± 0.68* |
| Intestine | 8.76 ± 1.75 | 8.16 ± 0.34 | 9.49 ± 1.47 | 11.54 ± 0.71 | 9.86 ± 3.62 | |
| Liver | 3.95 ± 0.73 | 4.55 ± 0.15 | 4.18 ± 1.32 | 3.99 ± 0.91 | 5.09 ± 0.54* | |
| Pancreas | 0.26 ± 0.04 | 0.31 ± 0.14 | 0.47 ± 0.13 | 0.32 ± 0.10 | 0.34 ± 0.19 | |
| Kidneys | 0.98 ± 0.08 | 1.01 ± 0.04 | 0.97 ± 0.08 | 0.97 ± 0.02 | 1.01 ± 0.09 | |
| Spleen | 0.17 ± 0.03 | 0.15 ± 0.04 | 0.13 ± 0.02 | 0.17 ± 0.01 | 0.15 ± 0.02 | |
| Lung | 0.69 ± 0.08 | 0.79 ± 0.17 | 0.70 ± 0.08 | 0.82 ± 0.22 | 0.75 ± 0.08 | |
| Heart | 0.38 ± 0.03 | 0.41 ± 0.03 | 0.44 ± 0.03 | 0.50 ± 0.02* | 0.45 ± 0.07* | |
| Ovaries | 1.48 ± 0.37 | 1.20 ± 0.37 | 1.15 ± 0.03 | 1.32 ± 0.06 | 0.93 ± 0.40 |
Note: BNCs - blank nanocomplexes (7.9 g/kg bw in mice or 15.4 g in hamsters), CNCs – Curcumin-loaded nanocomplexes at low doses (0.1 g/kg bw in mice or 0.2 g/kg bw in hamsters), medium doses (1.1 g/kg bw in mice or 2.1 g/kg bw in hamsters) or high doses (11.0 g/kg bw in mice or 21.4 g/kg bw in hamsters); Data are mean ± SD, *P value < 0.05 one-way ANOVA, n = number of animals.
Table 3.
Average (±standard deviation) of serum chemistry parameters of animals that consumed a single dose of blank nanocomplexes (BNCs) or of curcumin-loaded nanocomplexes (CNCs) in the acute toxicity study.
| Parameters | Normal range | Normal | BNCs | CNCs |
|||
|---|---|---|---|---|---|---|---|
| Low dose | Medium dose | High dose | |||||
| 7.9 g/kg bw | 0.1 g/kg bw | 1.1 g/kg bw | 11 g/kg bw | ||||
| n = 5 | n = 8 | n = 2 | n = 2 | n = 6 | |||
| Mice | Blood glucoseU | 60−150 | 175.00 ± 35.36 | 136.33 ± 67.57 | 209.00 | 127.00 | 175.75 ± 37.25 |
| Blood Urea NitrogenU | 15−33 | 16.06 ± 2.64 | 21.90 ± 4.62 | 20.80 | 18.40 | 23.87 ± 5.99* | |
| CreatinineU | 0.2−1.0 | 0.15 ± 0.02 | 0.10 ± 0.05 | 0.11 | 0.09 | 0.13 ± 0.06 | |
| CholesterolU | 55−181 | 83.80 ± 22.39 | 109.00 ± 33.47 | 104.00 | 98.00 | 105.50 ± 10.41 | |
| TriglycerideU | 72−227 | 120.60 ± 10.26 | 151.00 ± 82.02 | NA | NA | 142.00 ± 79.20 | |
| Total protein (g/dL) | 4.5−7.5 | 4.72 ± 0.50 | 5.80 ± 0.64* | 6.40 | 5.50 | 5.85 ± 0.64* | |
| Albumin (g/dL) | 2.3−4.3 | 3.42 ± 0.04 | 3.55 ± 0.21 | 3.70 | 3.50 | 3.67 ± 0.35 | |
| Globulin (g/dL) | 2.4−4.2 | 1.28 ± 0.13 | 2.25 ± 0.69* | 2.70 | 2.00 | 2.20 ± 0.31* | |
| Bilirubin TotalU | 0−1.0 | 0.03 ± 0.05 | 0.08 ± 0.10 | 0.20 | NA | 0.10 ± 0.00 | |
| ALT (U/L) | 22−128 | 59.60 ± 33.22 | 82.00 ± 40.26 | 200.00 | 220.00 | 115.00 ± 175.14 | |
| AST (U/L) | 20−150 | 223.67 ± 96.72 | 256.33 ± 160.50 | NA | NA | 128.00 ± 34.51 | |
| Alkaline phosphatase (U/L) | 50−186 | 47.60 ± 16.09 | 70.60 ± 20.54 | 93.00 | 84.00 | 78.83 ± 29.92 | |
| 15.4 g/kg bw | 0.2 g/kg bw | 2.1 g/kg bw | 21.4 g/kg bw | ||||
|---|---|---|---|---|---|---|---|
| n = 7 | n = 5 | n = 2 | n = 2 | n = 5 | |||
| Hamsters | Blood glucoseU | 60−150 | 147.00 ± 47.48 | 166.50 ± 19.09 | 107.00 ± 0.00 | 146.00 ± 25.46 | 216.50 ± 47.38 |
| Blood Urea NitrogenU | 15−33 | 26.34 ± 4.01 | 24.60 ± 2.67 | 27.75 ± 6.15 | 30.40 ± 5.09 | 26.98 ± 4.72 | |
| CreatinineU | 0.2−1.0 | 0.21 ± 0.05 | 0.18 ± 0.01 | 0.20 ± 0.03 | 0.18 ± 0.03 | 0.22 ± 0.03 | |
| CholesterolU | 55−181 | 123.17 ± 26.72 | 136.50 ± 24.12 | 119.00 ± 46.67 | 139.50 ± 41.72 | 139.60 ± 30.39 | |
| TriglycerideU | 72−227 | 181.86 ± 81.93 | 187.60 ± 25.40 | 186.50 ± 152.03 | 176.00 ± 113.14 | 219.20 ± 60.61 | |
| Total protein (g/dL) | 4.5−7.5 | 6.14 ± 0.49 | 6.26 ± 0.44 | 5.90 ± 0.42 | 6.00 ± 0.00 | 5.96 ± 0.40 | |
| Albumin (g/dL) | 2.3−4.3 | 3.27 ± 0.13 | 3.26 ± 0.15 | 3.20 ± 0.14 | 3.30 ± 0.00 | 3.06 ± 0.25 | |
| Globulin (g/dL) | 2.4−4.2 | 2.87 ± 0.37 | 3.00 ± 0.34 | 2.70 ± 0.28 | 2.70 ± 0.00 | 2.90 ± 0.24 | |
| Bilirubin TotalU | 0−1.0 | 0.07 ± 0.05 | 0.02 ± 0.04 | 0.05 ± 0.07 | 0.05 ± 0.07 | 0.24 ± 0.43 | |
| ALT (U/L) | 22−128 | 120.40 ± 41.73 | 126.20 ± 67.34 | 141.00 ± 38.18 | 180.00 ± 115.97 | 236.50 ± 208.37 | |
| AST (U/L) | 20−150 | 145.50 ± 31.20 | 139.60 ± 66.08 | 287.00 ± 59.40 | 318.00 ± 199.40 | 264.25 ± 312.05 | |
| Alkaline phosphatase (U/L) | 50−186 | 148.00 ± 15.56 | 153.50 ± 20.51 | 183.00 ± 55.15 | 177.50 ± 43.13 | 198.20 ± 43.48 |
Note: BNCs - blank nanocomplexes (7.9 g/kg bw in mice or 15.4 g in hamsters), CNCs – Curcumin-loaded nanocomplexes at low doses (0.1 g/kg bw in mice or 0.2 g/kg bw in hamsters), medium doses (1.1 g/kg bw in mice or 2.1 g/kg bw in hamsters) or high doses (11.0 g/kg bw in mice or 21.4 g/kg bw in hamsters); superscripted U – units are mg/dL, Data are mean ±SD, NA = not available; *P value < 0.05 one-way ANOVA, n = number of animals.
3.3. Chronic toxicity study
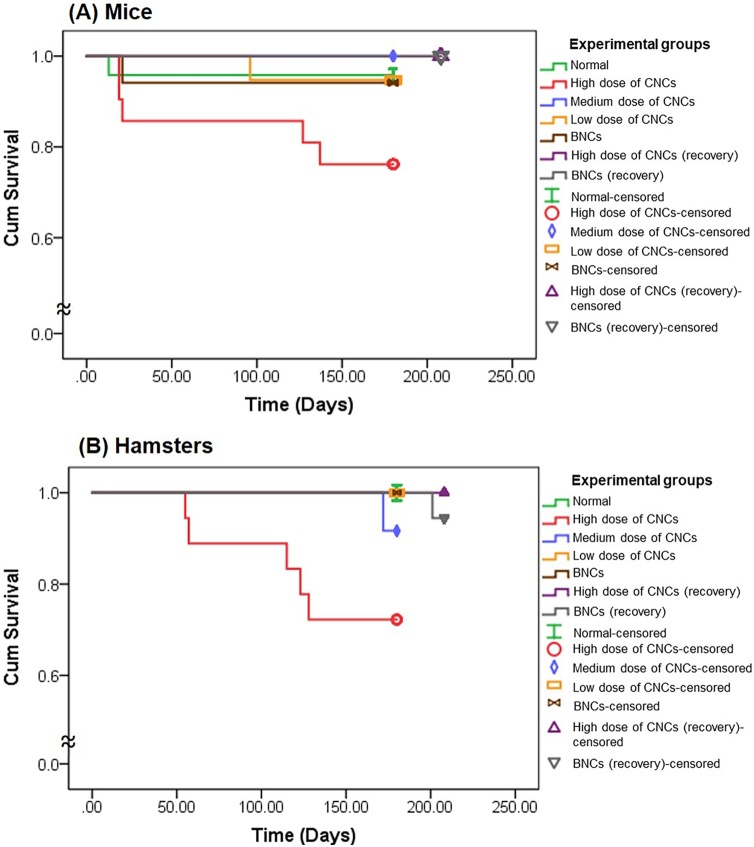
3.3.1. Survival, clinical observations and body weights
Kaplan-Meier plots, Fig. 2 (A) and (B), illustrate significant decreases in survival rates of both species of animals treated for 6 months with high doses of CNCs (0.8 g/kg bw in mice and 1.61 g/kg bw in hamsters). The number of animals in each group surviving until euthanasia (251 of 350) is shown in the Table 4. Interestingly, BNCs (0.58 g/kg bw in mice and 1.16 g/kg bw in hamsters) as well as low- and medium-dose CNCs regimens, did not affect survival of the animals. No abnormal clinical signs were evident nor was food and water intake affected in any treated animals. There was no significant difference in either species in body weights of experimental groups relative to controls at any time point throughout the period of study (p > 0.05 both) (Fig. S3).
Fig. 2.
Kaplan-Meier plots of survival rates of (A) mice and (B) hamsters randomly assigned to receive blank nanocomplexes (BNCs, brown), low-dose of curcumin-loaded nanocomplexes (CNCs, yellow), medium-dose of CNCs (blue), high-dose of CNCs (red), recovery BNCs (gray) and recovery high-dose of CNCs (purple), relative to the normal group (green) in the chronic toxicity study (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Table 4.
Relative organ weights of animals in the chronic toxicity study.
| Organs | Sex | Normal | BNCs | BNCs (recovery) | CNCs |
||||
|---|---|---|---|---|---|---|---|---|---|
| Low | Medium | High | High (recovery) | ||||||
| 0.58 g/kg bw | 0.58 g/kg bw | 0.09 g/kg bw | 0.27 g/kg bw | 0.8 g/kg bw | 0.8 g/kg bw | ||||
| n = 23 | n = 16 | n = 17 | n = 18 | n = 22 | n = 16 | n = 17 | |||
| Mice | Stomach | M | 1.33 ± 0.31 | 1.09 ± 0.23 | 1.15 ± 0.15 | 1.04 ± 0.23 | 1.22 ± 0.27 | 1.77 ± 0.39* | 1.29 ± 0.39 |
| F | 1.68 ± 0.56 | 1.65 ± 0.30 | 1.63 ± 0.15 | 1.74 ± 0.20 | 2.24 ± 0.69* | 2.07 ± 0.33 | 2.23 ± 0.61 | ||
| Intestine | M | 6.26 ± 0.76 | 5.47 ± 0.34 | 5.54 ± 0.39 | 5.84 ± 0.51 | 5.23 ± 1.30* | 7.03 ± 0.53 | 6.13 ± 0.86 | |
| F | 6.70 ± 0.58 | 6.66 ± 0.75 | 6.30 ± 0.46 | 6.74 ± 0.42 | 6.99 ± 0.62 | 7.38 ± 0.58* | 6.55 ± 0.44 | ||
| Liver | M | 4.02 ± 0.34 | 4.30 ± 0.46 | 4.33 ± 0.17 | 4.10 ± 0.25 | 4.17 ± 0.29 | 3.85 ± 0.21 | 3.94 ± 0.33 | |
| F | 4.15 ± 0.34 | 4.07 ± 0.22 | 3.92 ± 1.45 | 4.15 ± 0.31 | 4.22 ± 0.57 | 4.08 ± 0.45 | 4.20 ± 0.28 | ||
| Pancreas | M | 0.55 ± 0.11 | 0.63 ± 0.08 | 0.68 ± 0.10* | 0.69 ± 0.08* | 0.66 ± 0.08* | 0.63 ± 0.03 | 0.65 ± 0.09 | |
| F | 0.66 ± 0.18 | 0.74 ± 0.05 | 0.75 ± 0.09 | 0.75 ± 0.09 | 0.70 ± 0.10 | 0.75 ± 0.16 | 0.73 ± 0.13 | ||
| Kidneys | M | 1.85 ± 0.29 | 2.19 ± 0.25* | 2.26 ± 0.21* | 1.98 ± 0.15 | 2.07 ± 0.24 | 1.96 ± 0.17 | 2.06 ± 0.22 | |
| F | 1.46 ± 0.21 | 1.57 ± 0.08 | 1.57 ± 0.16 | 1.52 ± 0.13 | 1.57 ± 0.11 | 1.52 ± 0.07 | 1.52 ± 0.14 | ||
| Spleen | M | 0.24 ± 0.07 | 0.23 ± 0.08 | 0.26 ± 0.09 | 0.24 ± 0.08 | 0.24 ± 0.10 | 0.26 ± 0.07 | 0.28 ± 0.19 | |
| F | 0.39 ± 0.10 | 0.37 ± 0.07 | 0.51 ± 0.23 | 0.36 ± 0.22 | 0.51 ± 0.50 | 0.36 ± 0.19 | 0.32 ± 0.06 | ||
| Lung | M | 0.77 ± 0.13 | 0.73 ± 0.05 | 0.89 ± 0.28 | 0.81 ± 0.32 | 0.70 ± 0.08 | 0.75 ± 0.09 | 0.97 ± 0.60 | |
| F | 0.80 ± 0.10 | 0.91 ± 0.17 | 1.00 ± 0.37 | 0.84 ± 0.10 | 1.00 ± 0.33 | 0.82 ± 0.15 | 0.87 ± 0.10 | ||
| Heart | M | 0.59 ± 0.10 | 0.61 ± 0.05 | 0.66 ± 0.07 | 0.60 ± 0.04 | 0.58 ± 0.06 | 0.55 ± 0.04 | 0.67 ± 0.09 | |
| F | 0.59 ± 0.11 | 0.62 ± 0.07 | 0.59 ± 0.07 | 0.55 ± 0.04 | 0.58 ± 0.07 | 0.56 ± 0.05 | 0.59 ± 0.06 | ||
| Testes | M | 1.08 ± 0.31 | 0.85 ± 0.16 | 0.90 ± 0.13 | 1.17 ± 0.23 | 0.98 ± 0.17 | 1.12 ± 0.15 | 0.84 ± 0.12* | |
| Ovaries | F | 1.61 ± 0.56 | 1.42 ± 0.45 | 1.47 ± 0.20 | 1.47 ± 0.39 | 1.45 ± 0.81 | 1.65 ± 0.74 | 1.82 ± 0.70 | |
| 1.16 g/kg bw | 1.16 g/kg bw | 0.18 g/kg bw | 0.54 g/kg bw | 1.61 g/kg bw | 1.61 g/kg bw | ||||
|---|---|---|---|---|---|---|---|---|---|
| n = 20 | n = 18 | n = 18 | n = 24 | n = 14 | n = 14 | n = 14 | |||
| Hamsters | Stomach | M | 0.85 ± 0.25 | 1.24 ± 0.34* | 1.30 ± 0.28* | 1.23 ± 0.25* | 0.90 ± 0.14 | 1.10 ± 0.17 | 1.61 ± 0.21* |
| F | 1.55 ± 0.28 | 1.62 ± 0.37 | 1.53 ± 0.34 | 1.22 ± 0.26 | 1.35 ± 0.09 | 1.30 ± 0.24 | 1.96 ± 0.42* | ||
| Intestine | M | 5.98 ± 0.58 | 7.22 ± 1.75 | 7.45 ± 1.59* | 6.80 ± 0.78 | 7.14 ± 0.88 | 6.60 ± 0.86 | 7.58 ± 1.16* | |
| F | 7.88 ± 2.63 | 10.67 ± 2.34 | 8.68 ± 3.70 | 8.56 ± 1.44 | 9.46 ± 2.40 | 7.59 ± 1.08 | 11.05 ± 2.29* | ||
| Liver | M | 2.71 ± 0.27 | 2.41 ± 0.24 | 2.62 ± 0.38 | 2.89 ± 0.19 | 2.90 ± 0.19 | 2.98 ± 0.32 | 2.43 ± 0.23 | |
| F | 4.07 ± 0.91 | 3.39 ± 0.56 | 3.22 ± 0.28 | 3.38 ± 0.21 | 4.13 ± 2.07 | 3.14 ± 0.22 | 3.10 ± 0.24 | ||
| Pancreas | M | 0.42 ± 0.18 | 0.44 ± 0.11 | 0.65 ± 0.28* | 0.48 ± 0.20 | 0.36 ± 0.17 | 0.50 ± 0.17 | 0.56 ± 0.08 | |
| F | 0.42 ± 0.11 | 0.47 ± 0.11 | 0.59 ± 0.09* | 0.57 ± 0.12* | 0.50 ± 0.13 | 0.41 ± 0.15 | 0.46 ± 0.09 | ||
| Kidneys | M | 0.85 ± 0.05 | 0.78 ± 0.13 | 0.81 ± 0.06 | 0.86 ± 0.05 | 0.81 ± 0.09 | 0.91 ± 0.12 | 0.77 ± 0.09 | |
| F | 1.10 ± 0.16 | 0.97 ± 0.17 | 0.87 ± 0.07* | 0.90 ± 0.08 | 1.03 ± 0.21 | 0.95 ± 0.06 | 0.93 ± 0.13 | ||
| Spleen | M | 0.13 ± 0.02 | 0.13 ± 0.02 | 0.14 ± 0.03 | 0.14 ± 0.05 | 0.16 ± 0.07 | 0.14 ± 0.03 | 0.12 ± 0.02 | |
| F | 0.23 ± 0.13 | 0.25 ± 0.19 | 0.21 ± 0.05 | 0.24 ± 0.06 | 0.27 ± 0.10 | 0.22 ± 0.07 | 0.21 ± 0.04 | ||
| Lung | M | 0.59 ± 0.04 | 0.64 ± 0.06 | 0.66 ± 0.07 | 0.64 ± 0.05 | 0.70 ± 0.05* | 0.74 ± 0.09* | 0.60 ± 0.03 | |
| F | 0.78 ± 0.13 | 0.75 ± 0.04 | 0.69 ± 0.05 | 0.69 ± 0.04 | 0.78 ± 0.13 | 0.73 ± 0.08 | 0.76 ± 0.10 | ||
| Heart | M | 0.45 ± 0.06 | 0.40 ± 0.03 | 0.40 ± 0.04 | 0.43 ± 0.04 | 0.44 ± 0.04 | 0.53 ± 0.06* | 0.40 ± 0.02 | |
| F | 0.47 ± 0.03 | 0.44 ± 0.03 | 0.42 ± 0.03 | 0.44 ± 0.04 | 0.40 ± 0.02 | 0.49 ± 0.05 | 0.47 ± 0.07 | ||
| Testes | M | 2.68 ± 0.22 | 2.60 ± 0.29 | 2.35 ± 0.61 | 2.35 ± 0.35 | 2.53 ± 0.38 | 2.71 ± 0.15 | 2.47 ± 0.30 | |
| Ovaries | F | 0.68 ± 0.31 | 0.87 ± 0.18 | 0.85 ± 0.08 | 0.77 ± 0.13 | 0.73 ± 0.20 | 0.64 ± 0.20 | 0.89 ± 0.19 |
Note: BNCs - blank nanocomplexes (0.58 g/kg bw in mice or 1.16 g in hamsters), CNCs – Curcumin-loaded nanocomplexes at low doses (0.09 g/kg bw in mice or 0.18 g/kg bw in hamsters), medium doses (0.27 g/kg bw in mice or 0.54 g/kg bw in hamsters) or high doses (0.8 g/kg bw in mice or 1.61 g/kg bw in hamsters); Data are mean ± SD, *P value < 0.05 one-way ANOVA, n = number of animals.
3.3.2. Organ-weight to body-weight ratios
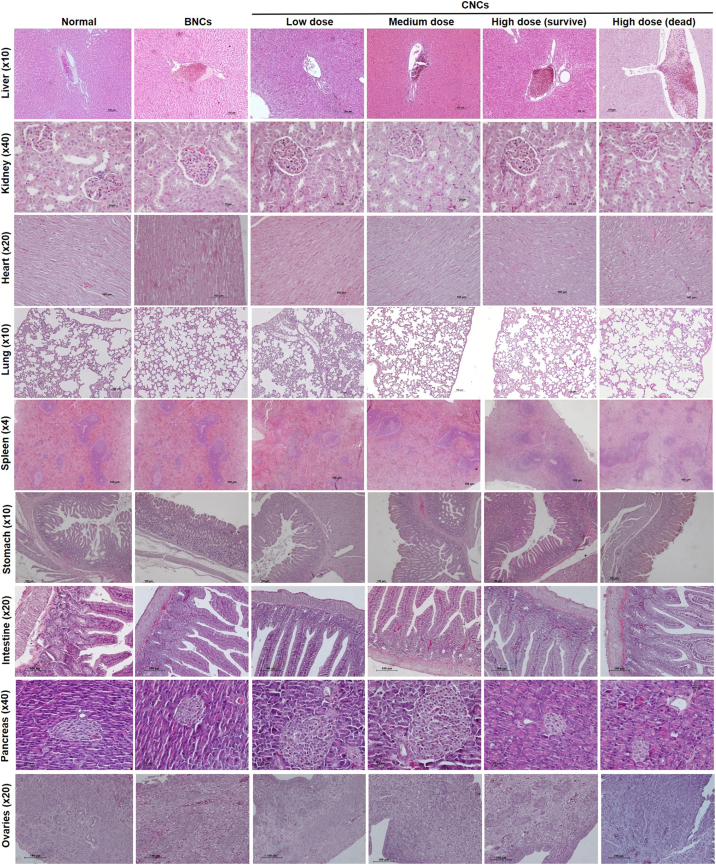
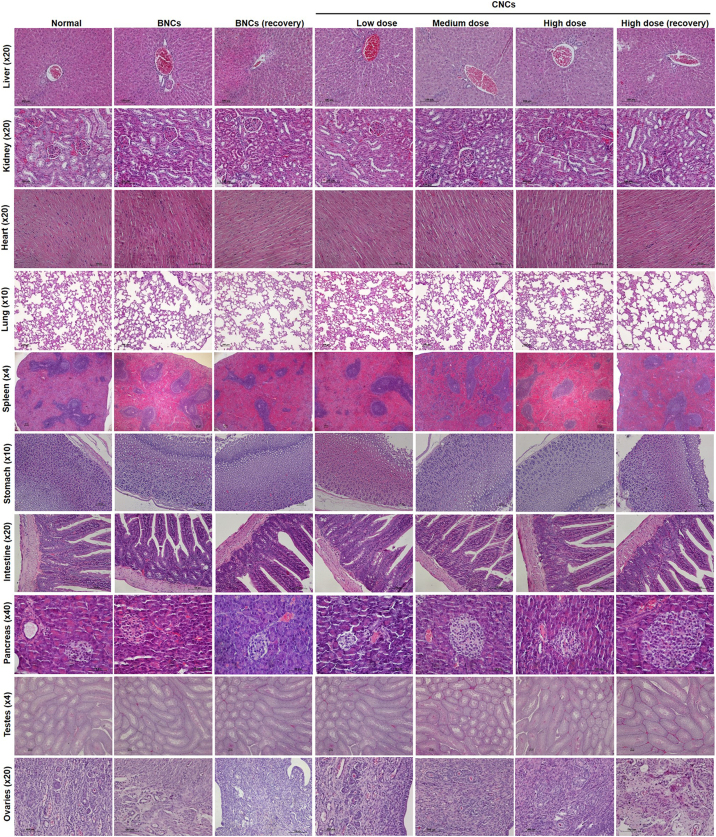
Daily consumption of both BNCs and CNCs for 6 months had some effects on the organ weights and organ-weight to body-weight ratios of mice and hamsters (Table 4). Compared to the normal control group, organ-weight to body-weight ratios of stomach, intestine, pancreas, lung, heart and testes were increased in both species of animal according to doses and the conditions of treatment. These changes were markedly observed in animals receiving the high CNCs doses. Although high dose CNCs treatment induced weights of some organs significant changes, histological study did not show any severe abnormalities. Moreover, most of organ-weight to body-weight ratios reverted to normal after 28 days in the high-CNCs recovery groups.
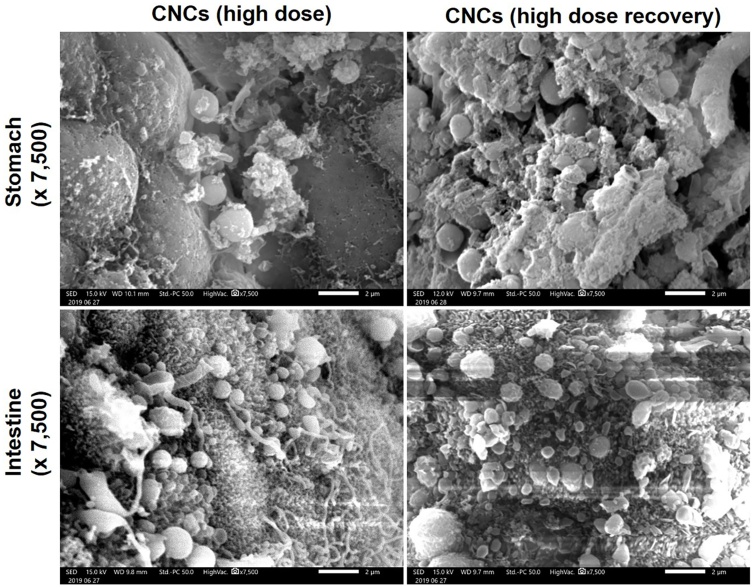
3.3.3. Particle adherence
SEM photographs of stomach and small intestine of hamsters in the group treated with high doses of CNCs and the high-dose CNCs recovery group are shown in Fig. 3. The deposition of CNCs on the stomach and small intestine walls 24 h after oral administration, due to mucoadhesive effects of the gum polymers, was still apparent 28 days after the last high-dose CNCs treatment.
Fig. 3.
Scanning electron microscopy (SEM) images illustrating curcumin-loaded nanocomplexes (CNCs) deposited on the walls of the stomach and small intestine of hamsters 24 h after oral administration of a high dose of CNCs (1.61 g/kg bw, equivalent to 0.45 g/kg bw of curcumin) and of the recovery group 28 days after the final high-dose CNCs treatment. The SEM images were obtained at 15 kV and recorded using JSM-IT200, JEOL, Japan. The scale bar is 2 μm. The original magnifications were x 7,500.
3.3.4. Biochemical analysis, hematological, and coagulation parameters
Treatment with blank nanocomplexes and high doses of CNCs yielded elevated levels of blood glucose and creatinine in mice and blood urea nitrogen in hamsters, as shown in Table 5. The liver function markers ALT and AST, but not alkaline phosphatase, were significantly increased both animal species in high-dose CNCs treatment compared to the normal control group. In contrast, triglycerides significantly decreased in the BNCs and BNCs recovery groups in mice and low-dose CNCs group in hamsters compared with the normal group. Likewise, cholesterol significantly decreased in the high-dose CNCs recovery group relative to the high-dose and normal groups of hamsters. BNCs-recovered hamsters exhibited significant decrease of cholesterol compared with the BNCs and normal groups.
Table 5.
Average (±standard deviation) of serum chemistry parameters of animals that consumed blank nanocomplexes (BNCs) daily and curcumin-loaded nanocomplexes (CNCs) at various doses daily for 6 months in the chronic toxicity study.
| Parameters | Sex | Normal range | Normal | BNCs | BNCs (recovery) | CNCs |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low | Medium | High | High (recovery) | |||||||
| 0.58 g/kg bw | 0.58 g/kg bw | 0.09 g/kg bw | 0.27 g/kg bw | 0.8 g/kg bw | 0.8 g/kg bw | |||||
| n = 23 | n = 16 | n = 17 | n = 18 | n = 22 | n = 16 | n = 17 | ||||
| Mice | Blood glucoseU | M | 60−150 | 82.44 ± 14.59 | 93.44 ± 16.17 | 108.83 ± 16.83 | 101.00 ± 27.86 | 96.00 ± 28.30 | 120.38 ± 25.44* | 111.33 ± 27.73* |
| F | 60−150 | 75.50 ± 11.86 | 103.83 ± 15.04* | 98.00 ± 12.39 | 86.11 ± 15.42 | 92.91 ± 23.77 | 116.29 ± 29.80* | 97.88 ± 23.94 | ||
| Blood Urea NitrogenU | M | 15−28 | 27.03 ± 8.31 | 25.59 ± 5.96 | 24.40 ± 6.05 | 28.57 ± 10.47 | 26.60 ± 19.16 | 24.73 ± 3.39 | 22.51 ± 4.65 | |
| F | 15−33 | 17.43 ± 4.44 | 16.14 ± 2.36 | 16.63 ± 2.34 | 17.89 ± 2.67 | 22.38 ± 12.09 | 19.64 ± 2.61 | 15.53 ± 1.95 | ||
| CreatinineU | M | 0.2−1.0 | 0.14 ± 0.04 | 0.18 ± 0.02 | 0.11 ± 0.02 | 0.08 ± 0.03* | 0.10 ± 0.03* | 0.14 ± 0.03 | 0.15 ± 0.03 | |
| F | 0.2−1.0 | 0.15 ± 0.04 | 0.18 ± 0.03 | 0.11 ± 0.03 | 0.09 ± 0.04* | 0.11 ± 0.05* | 0.14 ± 0.03 | 0.17 ± 0.02 | ||
| CholesterolU | M | 55−181 | 95.60 ± 21.21 | 93.63 ± 17.69 | 78.11 ± 13.27 | 88.00 ± 16.56 | 83.73 ± 14.58 | 90.50 ± 15.76 | 90.25 ± 20.40 | |
| F | 55−181 | 71.00 ± 17.97 | 71.29 ± 18.09 | 77.25 ± 19.40 | 67.49 ± 10.94 | 65.14 ± 12.65 | 77.00 ± 12.18 | 68.13 ± 11.46 | ||
| TriglycerideU | M | 72−227 | 84.55 ± 28.85 | 65.44 ± 27.19 | 53.00 ± 17.69* | 59.00 ± 20.70 | 69.80 ± 19.99 | 97.50 ± 12.56 | 64.25 ± 19.07 | |
| F | 72−227 | 104.25 ± 31.82 | 70.86 ± 13.47* | 79.63 ± 13.79 | 79.44 ± 14.93 | 78.22 ± 28.49 | 104.86 ± 26.02 | 92.88 ± 20.52 | ||
| Total protein (g/dL) | M | 4.5−7.5 | 4.95 ± 0.23 | 5.06 ± 0.32 | 4.84 ± 0.61 | 5.04 ± 0.27 | 5.05 ± 0.29 | 5.26 ± 0.30* | 4.96 ± 0.16 | |
| F | 4.5−7.5 | 4.94 ± 0.37 | 5.31 ± 0.23 | 5.20 ± 0.41 | 5.12 ± 0.19 | 5.13 ± 0.72 | 5.27 ± 0.42 | 4.91 ± 0.39 | ||
| Albumin (g/dL) | M | 2.3−4.3 | 3.43 ± 0.18 | 3.47 ± 0.24 | 3.26 ± 0.42 | 3.29 ± 0.33 | 3.30 ± 0.19 | 3.53 ± 0.21 | 3.30 ± 0.14 | |
| F | 2.3−4.3 | 3.56 ± 0.30 | 3.89 ± 0.16 | 3.63 ± 0.20 | 3.67 ± 0.28 | 3.52 ± 0.58 | 3.74 ± 0.19 | 3.46 ± 0.18 | ||
| Globulin (g/dL) | M | 2.4−4.2 | 1.52 ± 0.21 | 1.62 ± 0.18 | 1.60 ± 0.26 | 1.76 ± 0.35 | 1.75 ± 0.30 | 1.78 ± 0.17* | 1.68 ± 0.09 | |
| F | 2.4−4.2 | 1.39 ± 0.27 | 1.40 ± 0.23 | 1.58 ± 0.35 | 1.46 ± 0.19 | 1.71 ± 0.70 | 1.51 ± 0.40 | 1.44 ± 0.27 | ||
| Bilirubin TotalU | M | 0−1.0 | 0.09 ± 0.03 | 0.12 ± 0.07 | 0.09 ± 0.03 | 0.08 ± 0.11 | 0.08 ± 0.09 | 0.08 ± 0.05 | 0.15 ± 0.11 | |
| F | 0−1.0 | 0.08 ± 0.06 | 0.08 ± 0.04 | 0.05 ± 0.05 | 0.00 ± 0.00* | 0.02 ± 0.04* | 0.06 ± 0.05 | 0.1 ± 0.00 | ||
| Bilirubin DirectU | M | NA | 0.07 ± 0.05 | 0.10 ± 0.05 | 0.06 ± 0.05 | 0.03 ± 0.05 | 0.04 ± 0.05 | 0.06 ± 0.05 | 0.06 ± 0.05 | |
| F | NA | 0.03 ± 0.05 | 0.00 ± 0.00 | 0.01 ± 0.04 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.04 | ||
| ALT (U/L) | M | 22−128 | 33.08 ± 7.13 | 56.44 ± 18.84 | 54.00 ± 18.13 | 35.56 ± 10.93 | 36.36 ± 17.56 | 61.63 ± 51.57* | 29.88 ± 8.43 | |
| F | 22−128 | 33.79 ± 8.47 | 30.14 ± 4.18 | 43.29 ± 12.50 | 32.56 ± 7.58 | 31.25 ± 7.78 | 37.14 ± 13.51 | 29.63 ± 6.52 | ||
| AST (U/L) | M | 20−150 | 167.00 ± 26.58 | 214.17 ± 41.95 | 227.50 ± 43.90 | 221.38 ± 78.51 | 210.60 ± 57.17 | 245.50 ± 69.64* | 142.63 ± 38.49 | |
| F | 20−150 | 187.69 ± 51.43 | 174.14 ± 42.70 | 184.57 ± 43.60 | 188.00 ± 38.85 | 159.89 ± 79.79 | 243.43 ± 72.32 | 173.75 ± 69.39 | ||
| Alkaline phosphatase (U/L) | M | 50−186 | 43.30 ± 10.70 | 45.43 ± 7.83 | 42.44 ± 15.61 | 36.38 ± 17.39 | 46.55 ± 15.76 | 40.50 ± 11.71 | 31.11 ± 11.69 | |
| F | 50−186 | 65.46 ± 21.47 | 62.43 ± 9.18 | 67.38 ± 17.71 | 49.71 ± 8.77 | 58.43 ± 27.60 | 62.00 ± 24.26 | 54.50 ± 17.63 | ||
| 1.16 g/kg bw | 1.16 g/kg bw | 0.18 g/kg bw | 0.54 g/kg bw | 1.61 g/kg bw | 1.61 g/kg bw | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 20 | n = 18 | n = 18 | n = 24 | n = 14 | n = 14 | n = 14 | ||||
| Hamsters | Blood glucoseU | M | 60−150 | 100.75 ± 25.46 | 82.40 ± 10.02 | 84.73 ± 10.29 | 85.42 ± 14.21 | 100.67 ± 18.03 | 74.40 ± 14.71* | 92.83 ± 13.00 |
| F | 60−150 | 105.00 ± 26.38 | 81.13 ± 19.75 | 83.71 ± 8.24 | 91.58 ± 15.71 | 98.00 ± 46.41 | 82.56 ± 13.94 | 92.88 ± 8.81 | ||
| Blood Urea NitrogenU | M | 15−28 | 20.00 ± 3.95 | 21.65 ± 3.75 | 24.99 ± 6.44 | 27.62 ± 6.54* | 23.57 ± 4.11 | 21.16 ± 3.59 | 23.13 ± 1.91 | |
| F | 15−33 | 28.23 ± 10.32 | 24.55 ± 7.02 | 27.84 ± 4.07 | 29.16 ± 5.20 | 28.64 ± 7.60 | 31.27 ± 8.92 | 28.21 ± 4.36 | ||
| CreatinineU | M | 0.2−1.0 | 0.28 ± 0.05 | 0.29 ± 0.03 | 0.24 ± 0.04* | 0.22 ± 0.03* | 0.22 ± 0.02* | 0.24 ± 0.03 | 0.31 ± 0.04 | |
| F | 0.2−1.0 | 0.28 ± 0.11 | 0.25 ± 0.02 | 0.24 ± 0.03 | 0.17 ± 0.02* | 0.20 ± 0.03* | 0.25 ± 0.03 | 0.25 ± 0.03 | ||
| CholesterolU | M | 55−181 | 92.33 ± 14.04 | 74.30 ± 15.27* | 77.73 ± 17.84 | 76.75 ± 17.98 | 101.50 ± 7.26 | 95.40 ± 16.73 | 60.00 ± 5.93* | |
| F | 55−181 | 143.00 ± 18.22 | 127.25 ± 29.30 | 106.29 ± 19.64 | 125.00 ± 15.47 | 140.88 ± 62.20 | 108.56 ± 10.16* | 84.88 ± 27.68* | ||
| TriglycerideU | M | 72−227 | 115.08 ± 28.82 | 138.20 ± 19.94 | 125.00 ± 36.11 | 78.78 ± 24.94* | 135.67 ± 65.33 | 103.60 ± 22.83 | 127.83 ± 8.42 | |
| F | 72−227 | 151.43 ± 51.01 | 156.00 ± 16.22 | 151.00 ± 44.31 | 102.42 ± 13.13 | 144.00 ± 57.50 | 123.56 ± 36.29 | 139.38 ± 49.59 | ||
| Total protein (g/dL) | M | 4.5−7.5 | 6.11 ± 0.49 | 6.13 ± 0.24 | 5.82 ± 0.36 | 6.10 ± 0.27 | 5.80 ± 0.68 | 6.10 ± 0.33 | 6.45 ± 0.21 | |
| F | 4.5−7.5 | 6.61 ± 0.85 | 6.40 ± 0.38 | 6.07 ± 0.59 | 6.21 ± 0.23 | 6.39 ± 0.70 | 6.21 ± 0.25 | 6.04 ± 0.37 | ||
| Albumin (g/dL) | M | 2.3−4.3 | 3.59 ± 0.16 | 3.54 ± 0.16 | 3.37 ± 0.22 | 3.45 ± 0.13 | 3.47 ± 0.64 | 3.54 ± 0.11 | 3.62 ± 0.13 | |
| F | 2.3−4.3 | 3.31 ± 0.39 | 3.39 ± 0.36 | 3.29 ± 0.15 | 3.34 ± 0.14 | 3.20 ± 0.74 | 3.47 ± 0.14 | 3.33 ± 0.29 | ||
| Globulin (g/dL) | M | 2.4−4.2 | 2.50 ± 0.39 | 2.60 ± 0.13 | 2.44 ± 0.29 | 2.65 ± 0.17 | 2.35 ± 0.24 | 2.56 ± 0.24 | 2.82 ± 0.18 | |
| F | 2.4−4.2 | 3.09 ± 0.29 | 3.03 ± 0.14 | 2.79 ± 0.47 | 2.87 ± 0.13 | 2.83 ± 0.64 | 2.74 ± 0.18* | 2.76 ± 0.16* | ||
| Bilirubin TotalU | M | 0−1.0 | 0.03 ± 0.05 | 0.09 ± 0.03* | 0.03 ± 0.05 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.02 ± 0.04 | 0.08 ± 0.04* | |
| F | 0−1.0 | 0.05 ± 0.05 | 0.19 ± 0.33 | 0.06 ± 0.05 | 0.00 ± 0.00 | 0.05 ± 0.05 | 0.02 ± 0.04 | 0.10 ± 0.00 | ||
| Bilirubin DirectU | M | NA | 0.01 ± 0.03 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.03 ± 0.05 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| F | NA | 0.00 ± 0.00 | 0.03 ± 0.05 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.04 | 0.01 ± 0.03 | 0.03 ± 0.05 | ||
| ALT (U/L) | M | 22−128 | 78.33 ± 17.42 | 71.30 ± 10.61 | 70.27 ± 11.65 | 68.42 ± 17.50 | 67.27 ± 29.27 | 93.20 ± 15.55 | 59.78 ± 25.35 | |
| F | 22−128 | 90.75 ± 41.51 | 88.13 ± 46.30 | 123.57 ± 56.30 | 87.83 ± 38.03 | 109.38 ± 43.74 | 128.33 ± 54.59 | 122.00 ± 60.33 | ||
| AST (U/L) | M | 20−150 | 111.33 ± 38.86 | 142.29 ± 18.93 | 100.27 ± 33.65 | 129.58 ± 21.59 | 134.80 ± 28.11 | 158.40 ± 38.26* | 146.20 ± 28.66 | |
| F | 20−150 | 116.88 ± 34.98 | 124.88 ± 51.18 | 126.57 ± 50.30 | 91.50 ± 46.52 | 117.20 ± 26.90 | 152.89 ± 49.04 | 164.29 ± 50.61 | ||
| Alkaline phosphatase (U/L) | M | 50−186 | 77.75 ± 6.37 | 84.50 ± 19.90 | 90.06 ± 26.29 | 73.33 ± 8.33 | 71.00 ± 12.75 | 70.60 ± 10.71 | 89.50 ± 12.72 | |
| F | 50−186 | 96.80 ± 25.19 | 109.71 ± 20.91 | 92.86 ± 18.58 | 79.83 ± 17.72 | 111.23 ± 38.62 | 89.00 ± 15.05 | 117.50 ± 32.09 |
Note: BNCs - blank nanocomplexes (0.58 g/kg bw in mice or 1.16 g in hamsters), CNCs – Curcumin-loaded nanocomplexes at low doses (0.09 g/kg bw in mice or 0.18 g/kg bw in hamsters), medium doses (0.27 g/kg bw in mice or 0.54 g/kg bw in hamsters) or high doses (0.8 g/kg bw in mice or 1.61 g/kg bw in hamsters); superscripted U – units are mg/dL, Data are mean ±SD, NA = not available; *P value < 0.05 one-way ANOVA, n = number of animals.
The hematological analysis of male and female mice (Table S1) revealed that RBC and HCT in female mice of the high-dose CNCs group were significantly increased (p < 0.05). The WBC values for female mice were significantly higher in the low-dose and medium-dose CNCs groups (p < 0.05). Similarly, RDW was significantly higher in female medium-dose, high-dose and high-dose (recovery) CNCs groups (p < 0.05). In female mice of the medium-dose group, MCH value was significantly lower (p < 0.05). Percentage of monocytes was significantly higher in mice treated with BNCs and low-dose CNCs for 6 months (but returned to normal 28 days after the last dose) while there were significant increases in eosinophils in male mice treated with low-dose CNCs. In addition, eosinophils of hamsters were significantly elevated after recovery from BNCs and high-dose CNCs treatment. Basophils were significantly higher in medium-dose treated males. Blood coagulation parameters, including PT and aPTT of male and female hamsters, were within normal ranges for all experimental groups. However, mice yielded insufficient whole blood for us to do this analysis (Table S1).
3.3.5. Gross and histopathology
There was no significant necrosis and no severe abnormal gross pathology of the liver, kidney, lung, heart, spleen, stomach, intestine, pancreas, testes and ovaries in any of the treatment groups. Histopathological examination did not identify any dose-related abnormalities or lesions, such as severe damage or necrosis, in any animal group, but animals given the high-dose CNCs treatment exhibited slight changes in tissues with some precipitates, indicating mild inflammation (Fig. S4).
3.3.6. Ultrastructural changes of liver tissue
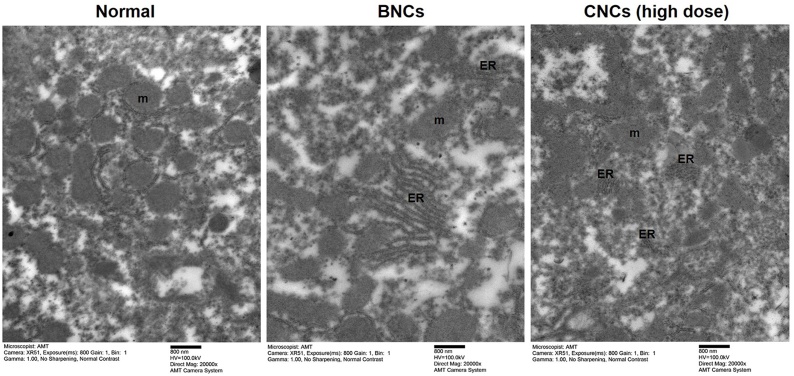
The liver, as one of the main target organs for nanoparticle clearance, was shown to gain weight in hamsters after a single high-dose CNCs treatment (Table 1). Also, increases in biochemical parameters indicate alterations in liver function in hamsters with ALT, AST, globulins, total protein (Table 4). Thorough observations by TEM occasionally found intense endoplasmic-reticulum stress and destruction of mitochondria in liver samples in the BNCs-treated group and high-CNCs group compared to the normal group (Fig. 4). Organelle structure, including endoplasmic reticulum, nucleus and mitochondria were clearly seen in normal liver samples.
Fig. 4.
Ultrastructural changes in hamster livers were revealed by transmission electron microscopy (TEM). Hamsters were evaluated in the control group and 24 h after oral administration of blank nanocomplexes (BNCs) or a high dose of curcumin-loaded nanocomplexes (CNCs) (1.61 g/kg bw, equivalent to 0.45 g/kg bw of curcumin). The TEM images were obtained at 100 kV and recorded using JEM-1010 TEM, (JEOL, Japan). The scale bar is 800 nm. The original magnification for liver tissues of hamsters was x 20,000. Representative data are shown (n = 2 animals/group) in the normal, BNCs-treated and CNCs high-dose groups. Abbreviations are for mitochondria (m) and endoplasmic reticulum (ER).
4. Discussion
CNCs, formed as a complex of nanoparticles of curcumin in a solid dispersion form, could be well dispersed in water to give segregated nanoparticles and mucoadhesive gums, providing sustained release of curcumin in the GI tract. Curcuminoid contents were stable in the CNCs for at least 12 months under the specified storage conditions. This demonstrates the advantage of CNCs, as curcumin is prone to rapid degradation [40].
It has been reported that curcumin can induce DNA damage both in vitro and in vivo [13] potentially due to pro-oxidant effects [50]. Nanoparticles are speculated to enhance absorption [25] and could potentially promote the toxicity of the encapsulated curcumin. The toxicity of nanoparticles might differ according to the encapsulated substances [51]. Here, we assessed the actual LD50 for curcumin in CNCs form in vivo in mice and hamsters. The oral LD50 values of CNCs estimated using Lorke’s method were 8.9 and 16.8 g/kg bw (equivalent to 2.5 and 4.5 g/kg bw of curcumin) for mice and hamsters, respectively. Our data could provide the actual LD50 of nanoparticles of curcumin in vivo, which was 4.5-fold higher than the LD50 level of curcumin in mice (approximately 2 g/kg bw) [46].
A single dose of CNCs followed by an observation period of 14 days to assess acute toxicity and daily oral CNCs treatment for 6 months, to test chronic toxicity, did not produce obvious toxicologically significant changes in clinical observations or blood chemistry in groups given the low-dose and medium-dose treatment. However, a high dose could produce toxicity in tissues via inflammation-mediated injury in both animal species. A possible mechanism of CNCs-induced toxicity is shown in Fig. 5 .
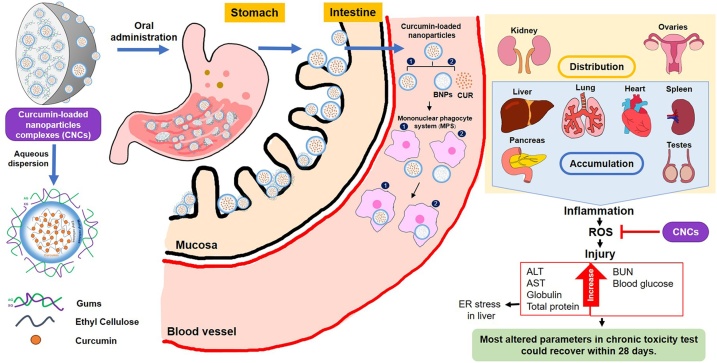
Fig. 5.
Possible mechanism by which a high dose of CNCs induces toxicity in mice and hamsters. Curcumin-loaded nanocomplexes (CNCs) with arabic gum and xanthan gum adhere to the stomach wall after oral administration. After dispersal in water, CNCs forms an amorphous nanoprecipitate partially covered by gums. In this form, it is moved onwards to the small intestine, and then absorbed into the bloodstream via many pathways. Curcumin-loaded nanoparticles and curcumin released from nanocomplexes are engulfed by the mononuclear phagocyte system and then distributed to various organs. Accumulation of phagocytes containing nanoparticles is primarily resided in the liver, kidney, lung, spleen, pancreas and testes, leading to injuries in those organs via inflammation-mediated ROS production. These were resolved within 28 days after cessation of treatment.
Increases in weights of some organs, both in acute (Table 2) and chronic (Table 4) toxicity tests may be due to mucoadhesive characteristics of CNCs with additional effects of arabic and xanthan gums [40]. Interestingly, hamsters in the high-CNCs chronic recovery group showed significant increases in stomach weight (1.89-fold) and intestine (1.27-fold) (Table 4) (p < 0.05), which was supported by SEM results (Fig. 3). Notably, this effect persisted even at 28 days after the last dose as a result of the extended release of CNCs [40,52] and their mucoadhesive properties [38]. In contrast, in mice the increase in weight of stomach and intestine of high-dose CNCs-treated group (0.8 g/kg bw) was reversed by 28 days after the last dose (Table 4). The reasons for these species-specific differences are unknown. Moreover, CNCs were apparent in the stomach and intestinal mucosa of hamsters 24 h after oral administration were still present in both organs in reduced quantities 28 days after the last dose, suggesting extended release of CNCs (Fig. 2). Increased organ-weight to body-weight ratios, particularly of the liver and spleen after acute CNCs treatments, and pancreas, lung, heart, kidney and testes after 6 months of daily CNCs intake (Table 2, Table 4), might be related to the pharmacokinetics of nanoparticles, their biodistribution and clearance-related side effects [36]. Those processes occurred after absorption of nanoparticles into the bloodstream and distribution into other organs [36].
The clearance of nanoparticles from the body depends on many factors including particle size, shape, materials and surface modifications [35]. Perhaps, CNCs product with particle size of 400−1000 nm after dispersal in water, as previously described in [40], may be eliminated by the reticuloendothelial system or the mononuclear phagocyte system (MPS) [35]. Macrophages might phagocytose CNCs or BNCs and excrete them into the blood circulation with smaller sizes. Kidney and liver are main clearance organs to eliminate them from body. An overdose of nanoparticles might extend the elimination process in some organs [36], leading to tissue damage via production of enhanced reactive oxygen species (ROS) by inflammatory cells [53,54], leading to increased organ weight. The distribution of curcumin in various organs after uptake such as in the liver, kidney, stomach, small intestine and also blood was supported by a previously reported [55]. In contrast, some MPS phagocytes that carry CNCs may exert an inhibitory effect on BNCs-induced inflammation by curcumin [56] as proposed in Fig. 5.
Daily oral administration of CNCs at low, medium and high doses (0.09, 0.27 and 0.8 g/kg bw, which is equivalent to 0.025, 0.075, 0.225 g/kg bw of curcumin, respectively) was defined as the appropriate dose for testing in mice for 6 months. Those dose levels are close to levels used in a previous study of solid-lipid curcumin particles (SLCP) containing approximately 30 % curcumin (0.18, 0.36, 0.72 g SLCP/kg bw in low, medium and high doses, respectively, in rats [46]. High-dose administration of CNCs for 6 months led to 1.86- and 1.47-fold increases of of ALT and AST levels in mice, respectively. In hamsters, the same treatment led to 1.19-fold increase in ALT and a significant increase of 1.42-fold in AST, indicating that high-dose and long-term CNCs treatment induced inflammation-mediated liver injury. Notably, these changes were restored to normal levels in the recovery groups by 28 days after the final dose.
In addition, although high dose CNCs-treated mice and hamsters exhibited slight changes in blood glucose (an increase 1.46-fold in mice and decrease of 0.74-fold in hamsters), these levels were still within the normal range. Increases in blood glucose observed in high-dose CNCs groups might be an effect of gum arabic, which has been reported to adversely interfere with electrolyte balance and vitamin D in mice, and to induce hypersensitivity in humans [52]. This, however, requires further studies to identify the exact mechanism. BNCs at a high dose had the same result as high-dose CNCs treatment that produced significant changes in total protein and globulin in the acute toxicity test (Table 3) and had same trend in the chronic toxicity test (Table 5), suggesting that high BNCs dose might induce toxicity. BNCs are composed of cellulose-based materials, mainly ethylcellulose and methylcellulose. These are popular in pharmaceutical technology and side effects seem to be limited [57], although an overdose of cellulose-based materials can induce cellular damage [58]. Alternatively, an overdose of curcumin could enhance glycolysis by upregulation of metabolism-related genes [59], leading to increased glucose levels.
We found that liver-injury markers such as ALT and AST were increased by high-dose and long-term CNCs treatment, but not by low- and medium-dose treatments. Oral consumption of high-dose CNCs for 6 months decreased survival rates in animals (Fig. 2). Adverse effects of CNCs treatment are likely to be dose dependent. In agreement, overdose and long-term administration (100 mg/kg/90 days) of curcumin could induce imbalance in rats including overproduction of ROS, increased production of pro-oxidant cytokine IL6 and decreased antioxidant enzymes i.e. SOD and GST, leading to oxidative stress-mediated liver injury and inflammatory disorders which, however, could recover to normal 1 month after cessation of treatment [59]. Likewise, a higher dose of curcumin (400 mg/kg/15 days) mediated ROS induction, leading to myocardial damage in rats. On the other hand, curcumin can enhance endogenous antioxidant systems at lower doses [60]. Taken together, based on our results and previous study, it seems likely that high doses CNCs treatment might be involved in induction toxicity in both animal species due to excessive of curcumin deposition, and nanomaterials of gums and methy- and ethycelluloase.
Several studies have recommended doses of curcumin for various disease conditions ranging from 0.02 to up to 2.5 g/person/day (around up to 42 mg/kg bw/day for an individual weighing 60 kg) [[61], [62], [63]]. Our chronic toxicity trial revealed that the NOAEL of CNCs at doses within a range of up to 0.27 and 0.54 g/kg bw/day does not cause obvious toxicity in mice and hamsters, respectively. Based on the results of our study, NOAEL of curcumin from CNCs might be converted to a maximum recommended starting dose for clinical trials at 13.13 and 43.7 mg/person/day (an individual weighing 60 kg) of CNCs. Also, toxicokinetics/exposure data to correlate with the findings of the study should be obtained in future study. Safety of CNCs will thus be confirmed.
5. Conclusion
CNCs segregated to provide nanoparticles after dispersion in water and showed potential to stabilize curcuminoid contents for at least 12 months in the storage conditions used. Acute and chronic toxicity studies were conducted to confirm the safety of CNCs. A single low or medium dose of CNCs is safe in both mice and hamsters. Likewise, low and medium daily CNCs doses are safe for long-term administration. We observed that CNCs treatment have the potential to produce toxicity in high-dose treatments, but most abnormal parameters returned to normal levels by 28 days after the final dose. Therefore, CNCs exhibit relatively low toxicity and are ready for use in clinical study in human beings, but requiring more study of their toxicity in high doses.
Authors statement
Somchai Pinlaor: Project administration; Supervision; Data curation; Writing - review & editing. Chanakan Jantawong: Methodology; Data curation; Writing - original draft. Aroonsri Priprem: Supervision; Data curation; Writing - review & editing. Kitti Intuyod: Methodology; Data curation; Writing - review & editing. Chawalit Pairojkul: Resources; Supervision; Writing - review & editing. Porntip Pinlaor, Sakda Waraasawapati, Itnarin Mongkon and Yaovalux Chamgramol: Methodology; Data curation; Writing - original draft.
Declaration of Competing Interest
Welltech Biotechnology Co., Ltd, Thailand, the producer and supplier of CNCs and BNCs, had no part in the planning and conducting the acute and chronic toxicity testing in this project. All authors declare no conflict of interest.
Acknowledgments
We would like to acknowledge the financial support from Center of Excellence on Medical Biotechnology (CEMB), S&T Postgraduate Education and Research Development Office (PERDO), Office of Higher Education Commission (OHEC), Thailand (No. SN-59-002-05). We would like to thank Welltech Biotechnology Co., Ltd, Thailand for producing CNCs and BNCs. Chanakan Jantawong is a graduate student supported by Cholangiocarcinoma Research Institute (CARI), Khon Kaen University, Khon Kaen, Thailand (No. CARI 02/2561). We would like to thank Prof. David Blair for invaluable suggestions and editing the manuscript via publication clinic, Khon Kaen University, Thailand.
Handling Editor Dr. Aristidis Tsatsakis
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2021.06.021.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Haddad M., Sauvain M., Deharo E. Curcuma as a parasiticidal agent: a review. Planta Med. 2011;77(6):672–678. doi: 10.1055/s-0030-1250549. [DOI] [PubMed] [Google Scholar]
- 2.Kunnumakkara A.B., Bordoloi D., Padmavathi G., Monisha J., Roy N.K., Prasad S., Aggarwal B.B. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017;174(11):1325–1348. doi: 10.1111/bph.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prakobwong S., Gupta S.C., Kim J.H., Sung B., Pinlaor P., Hiraku Y., Wongkham S., Sripa B., Pinlaor S., Aggarwal B.B. Curcumin suppresses proliferation and induces apoptosis in human biliary cancer cells through modulation of multiple cell signaling pathways. Carcinogenesis. 2011;32(9):1372–1380. doi: 10.1093/carcin/bgr032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He Y., Yue Y., Zheng X., Zhang K., Chen S., Du Z. Curcumin, inflammation, and chronic diseases: how are they linked? Molecules. 2015;20(5):9183–9213. doi: 10.3390/molecules20059183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal B.B., Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol. Sci. 2009;30(2):85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Giordano A., Tommonaro G. Curcumin and cancer. Nutrients. 2019;11(10):1–20. doi: 10.3390/nu11102376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jager R., Lowery R.P., Calvanese A.V., Joy J.M., Purpura M., Wilson J.M. Comparative absorption of curcumin formulations. Nutr. J. 2014;13:1–8. doi: 10.1186/1475-2891-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahran R.I., Hagras M.M., Sun D., Brenner D.E. Bringing curcumin to the clinic in cancer prevention: a review of strategies to enhance bioavailability and efficacy. AAPS J. 2017;19(1):54–81. doi: 10.1208/s12248-016-0003-2. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S.K., Kumar B., Nag T.C., Agrawal S.S., Agrawal R., Agrawal P., Saxena R., Srivastava S. Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J. Ocul. Pharmacol. Ther. 2011;27(2):123–130. doi: 10.1089/jop.2010.0123. [DOI] [PubMed] [Google Scholar]
- 10.Hanai H., Iida T., Takeuchi K., Watanabe F., Maruyama Y., Andoh A., Tsujikawa T., Fujiyama Y., Mitsuyama K., Sata M., Yamada M., Iwaoka Y., Kanke K., Hiraishi H., Hirayama K., Arai H., Yoshii S., Uchijima M., Nagata T., Koide Y. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin. Gastroenterol. Hepatol. 2006;4(12):1502–1506. doi: 10.1016/j.cgh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Golombick T., Diamond T.H., Badmaev V., Manoharan A., Ramakrishna R. The potential role of curcumin in patients with monoclonal gammopathy of undefined significance--its effect on paraproteinemia and the urinary N-telopeptide of type I collagen bone turnover marker. Clin. Cancer Res. 2009;15(18):5917–5922. doi: 10.1158/1078-0432.CCR-08-2217. [DOI] [PubMed] [Google Scholar]
- 12.Dhillon N., Aggarwal B.B., Newman R.A., Wolff R.A., Kunnumakkara A.B., Abbruzzese J.L., Ng C.S., Badmaev V., Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008;14(14):4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 13.Burgos-Moron E., Calderon-Montano J.M., Salvador J., Robles A., Lopez-Lazaro M. The dark side of curcumin. Int. J. Cancer. 2010;126(7):1771–1775. doi: 10.1002/ijc.24967. [DOI] [PubMed] [Google Scholar]
- 14.Aggeli I.K., Koustas E., Gaitanaki C., Beis I. Curcumin acts as a pro-oxidant inducing apoptosis via JNKs in the isolated perfused Rana ridibunda heart. J. Exp. Zool. A. Ecol. Genet. Physiol. 2013;319(6):328–339. doi: 10.1002/jez.1797. [DOI] [PubMed] [Google Scholar]
- 15.Ahsan H., Parveen N., Khan N.U., Hadi S.M. Pro-oxidant, anti-oxidant and cleavage activities on DNA of curcumin and its derivatives demethoxycurcumin and bisdemethoxycurcumin. Chem. Biol. Interact. 1999;121(2):161–175. doi: 10.1016/s0009-2797(99)00096-4. [DOI] [PubMed] [Google Scholar]
- 16.Banerjee A., Kunwar A., Mishra B., Priyadarsini K.I. Concentration dependent antioxidant/pro-oxidant activity of curcumin studies from AAPH induced hemolysis of RBCs. Chem. Biol. Interact. 2008;174(2):134–139. doi: 10.1016/j.cbi.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Moos P.J., Edes K., Mullally J.E., Fitzpatrick F.A. Curcumin impairs tumor suppressor p53 function in colon cancer cells. Carcinogenesis. 2004;25(9):1611–1617. doi: 10.1093/carcin/bgh163. [DOI] [PubMed] [Google Scholar]
- 18.Yallapu M.M., Jaggi M., Chauhan S.C. Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov. Today. 2012;17(1-2):71–80. doi: 10.1016/j.drudis.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rai M., Ingle A.P., Pandit R., Paralikar P., Anasane N., Santos C.A.D. Curcumin and curcumin-loaded nanoparticles: antipathogenic and antiparasitic activities. Expert Rev. Anti. Infect Ther. 2020;18(4):367–379. doi: 10.1080/14787210.2020.1730815. [DOI] [PubMed] [Google Scholar]
- 20.Salehi B., Del Prado-Audelo M.L., Cortes H., Leyva-Gomez G., Stojanovic-Radic Z., Singh Y.D., Patra J.K., Das G., Martins N., Martorell M., Sharifi-Rad M., Cho W.C., Sharifi-Rad J. Therapeutic applications of curcumin nanomedicine formulations in cardiovascular diseases. J. Clin. Med. 2020;9(3):1–25. doi: 10.3390/jcm9030746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delavarian Z., Pakfetrat A., Ghazi A., Jaafari M.R., Homaei Shandiz F., Dalirsani Z., Mohammadpour A.H., Rahimi H.R. Oral administration of nanomicelle curcumin in the prevention of radiotherapy-induced mucositis in head and neck cancers. Spec. Care Dentist. 2019;39(2):166–172. doi: 10.1111/scd.12358. [DOI] [PubMed] [Google Scholar]
- 22.Naksuriya O., Okonogi S., Schiffelers R.M., Hennink W.E. Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35(10):3365–3383. doi: 10.1016/j.biomaterials.2013.12.090. [DOI] [PubMed] [Google Scholar]
- 23.Jeevanandam J., Barhoum A., Chan Y.S., Dufresne A., Danquah M.K. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018;9:1050–1074. doi: 10.3762/bjnano.9.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hani U., Shivakumar H.G. Solubility enhancement and delivery systems of curcumin a herbal medicine: a review. Curr. Drug Deliv. 2014;11(6):792–804. doi: 10.2174/1567201811666140825130003. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y., Rauf Khan A., Fu M., Zhai Y., Ji J., Bobrovskaya L., Zhai G. Advances in curcumin-loaded nanopreparations: improving bioavailability and overcoming inherent drawbacks. J. Drug Target. 2019:1–32. doi: 10.1080/1061186X.2019.1572158. [DOI] [PubMed] [Google Scholar]
- 26.Ipar V.S., Dsouza A., Devarajan P.V. Enhancing curcumin oral bioavailability through nanoformulations. Eur. J. Drug Metab. Pharmacokinet. 2019;44(4):459–480. doi: 10.1007/s13318-019-00545-z. [DOI] [PubMed] [Google Scholar]
- 27.Jamwal R. Bioavailable curcumin formulations: a review of pharmacokinetic studies in healthy volunteers. J. Integr. Med. 2018;16(6):367–374. doi: 10.1016/j.joim.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Dandekar P., Dhumal R., Jain R., Tiwari D., Vanage G., Patravale V. Toxicological evaluation of pH-sensitive nanoparticles of curcumin: acute, sub-acute and genotoxicity studies. Food Chem. Toxicol. 2010;48(8-9):2073–2089. doi: 10.1016/j.fct.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Aggarwal M.L., Chacko K.M., Kuruvilla B.T. Systematic and comprehensive investigation of the toxicity of curcuminoidessential oil complex: a bioavailable turmeric formulation. Mol. Med. Rep. 2016;13(1):592–604. doi: 10.3892/mmr.2015.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gliga A.R., Skoglund S., Wallinder I.O., Fadeel B., Karlsson H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014;11:1–17. doi: 10.1186/1743-8977-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong T., Zhang S., Kang Z., Zhang T., Li S. Dose-dependent physiological and transcriptomic responses of lettuce (Lactuca sativa L.) to copper oxide nanoparticles-insights into the phytotoxicity mechanisms. Int. J. Mol. Sci. 2021;22(7):1–26. doi: 10.3390/ijms22073688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim I.Y., Joachim E., Choi H., Kim K. Toxicity of silica nanoparticles depends on size, dose, and cell type. Nanomedicine. 2015;11(6):1407–1416. doi: 10.1016/j.nano.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa) J. Altern. Complement. Med. 2003;9(1):161–168. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 34.Soleimani V., Sahebkar A., Hosseinzadeh H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: review. Phytother. Res. 2018;32(6):985–995. doi: 10.1002/ptr.6054. [DOI] [PubMed] [Google Scholar]
- 35.Poon W., Zhang Y.N., Ouyang B., Kingston B.R., Wu J.L.Y., Wilhelm S., Chan W.C.W. Elimination pathways of nanoparticles. ACS Nano. 2019;13(5):5785–5798. doi: 10.1021/acsnano.9b01383. [DOI] [PubMed] [Google Scholar]
- 36.Wei Y., Quan L., Zhou C., Zhan Q. Factors relating to the biodistribution & clearance of nanoparticles & their effects on in vivo application. Nanomed. (Lond). 2018;13(12):1495–1512. doi: 10.2217/nnm-2018-0040. [DOI] [PubMed] [Google Scholar]
- 37.Longmire M., Choyke P.L., Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomed. (Lond). 2008;3(5):703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suwannateep N., Banlunara W., Wanichwecharungruang S.P., Chiablaem K., Lirdprapamongkol K., Svasti J. Mucoadhesive curcumin nanospheres: biological activity, adhesion to stomach mucosa and release of curcumin into the circulation. J. Control. Release. 2011;151(2):176–182. doi: 10.1016/j.jconrel.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Charoensuk L., Pinlaor P., Wanichwecharungruang S., Intuyod K., Vaeteewoottacharn K., Chaidee A., Yongvanit P., Pairojkul C., Suwannateep N., Pinlaor S. Nanoencapsulated curcumin and praziquantel treatment reduces periductal fibrosis and attenuates bile canalicular abnormalities in Opisthorchis viverrini-infected hamsters. Nanomedicine. 2016;12(1):21–32. doi: 10.1016/j.nano.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Pinlaor S., Jantawong C., Intuyod K., Sirijindalert T., Pinlaor P., Pairojkul C., Charoensuk L., Sitthirach C., Vaeteewoottacharn K., Puthongking P., Priprem A. Solid dispersion improves release of curcumin from nanoparticles: potential benefit for intestinal absorption. Mater Today Commun. 2021;26:1–9. [Google Scholar]
- 41.Thamavit W., Bhamarapravati N., Sahaphong S., Vajrasthira S., Angsubhakorn S. Effects of dimethylnitrosamine on induction of cholangiocarcinoma in Opisthorchis viverrini-infected Syrian golden hamsters. Cancer Res. 1978;38(12):4634–4639. [PubMed] [Google Scholar]
- 42.OECD [OECD series on principles of good laboratory practice and compliance monitoring] Ann. Ist. Super. Sanita. 1997;33(1):1–172. [PubMed] [Google Scholar]
- 43.Erhirhie E.O., Ihekwereme C.P., Ilodigwe E.E. Advances in acute toxicity testing: strengths, weaknesses and regulatory acceptance. Interdiscip. Toxicol. 2018;11(1):5–12. doi: 10.2478/intox-2018-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Creton S., Dewhurst I.C., Earl L.K., Gehen S.C., Guest R.L., Hotchkiss J.A., Indans I., Woolhiser M.R., Billington R. Acute toxicity testing of chemicals-Opportunities to avoid redundant testing and use alternative approaches. Crit. Rev. Toxicol. 2010;40(1):50–83. doi: 10.3109/10408440903401511. [DOI] [PubMed] [Google Scholar]
- 45.Krishnaraju A.V., Sundararaju D., Sengupta K., Venkateswarlu S., Trimurtulu G. Safety and toxicological evaluation of demethylatedcurcuminoids; a novel standardized curcumin product. Toxicol. Mech. Methods. 2009;19(6-7):447–460. doi: 10.1080/15376510903200766. [DOI] [PubMed] [Google Scholar]
- 46.Dadhaniya P., Patel C., Muchhara J., Bhadja N., Mathuria N., Vachhani K., Soni M.G. Safety assessment of a solid lipid curcumin particle preparation: acute and subchronic toxicity studies. Food Chem. Toxicol. 2011;49(8):1834–1842. doi: 10.1016/j.fct.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Speakman J.R. Measuring energy metabolism in the mouse - theoretical, practical, and analytical considerations. Front. Physiol. 2013;4:1–23. doi: 10.3389/fphys.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flamand A., Rebout N., Bordes C., Guinnefollau L., Berges M., Ajak F., Siutz C., Millesi E., Weber C., Petit O. Hamsters in the city: a study on the behaviour of a population of common hamsters (Cricetus cricetus) in urban environment. PLoS One. 2019;14(11):1–15. doi: 10.1371/journal.pone.0225347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chinedu E., Arome D., Ameh F.S. A new method for determining acute toxicity in animal models. Toxicol. Int. 2013;20(3):224–226. doi: 10.4103/0971-6580.121674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshino M., Haneda M., Naruse M., Htay H.H., Tsubouchi R., Qiao S.L., Li W.H., Murakami K., Yokochi T. Prooxidant activity of curcumin: copper-dependent formation of 8-hydroxy-2’-deoxyguanosine in DNA and induction of apoptotic cell death. Toxicol. In Vitro. 2004;18(6):783–789. doi: 10.1016/j.tiv.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 51.Khan I., Saeed K., Khan I. Nanoparticles: properties, applications and toxicities. Arab. J. Chem. 2019;12(7):908–931. [Google Scholar]
- 52.Ali B.H., Ziada A., Blunden G. Biological effects of gum arabic: a review of some recent research. Food Chem. Toxicol. 2009;47(1):1–8. doi: 10.1016/j.fct.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 53.Gustafson H.H., Holt-Casper D., Grainger D.W., Ghandehari H. Nanoparticle uptake: the phagocyte problem. Nano Today. 2015;10(4):487–510. doi: 10.1016/j.nantod.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song G., Petschauer J.S., Madden A.J., Zamboni W.C. Nanoparticles and the mononuclear phagocyte system: pharmacokinetics and applications for inflammatory diseases. Curr. Rheumatol. Rev. 2014;10(1):22–34. doi: 10.2174/1573403x10666140914160554. [DOI] [PubMed] [Google Scholar]
- 55.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 56.Abdollahi E., Momtazi A.A., Johnston T.P., Sahebkar A. Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: a nature-made jack-of-all-trades? J. Cell. Physiol. 2018;233(2):830–848. doi: 10.1002/jcp.25778. [DOI] [PubMed] [Google Scholar]
- 57.Wasilewska K., Winnicka K. Ethylcellulose-a pharmaceutical excipient with multidirectional application in drug dosage forms development. Mater. (Basel). 2019;12(20):1–21. doi: 10.3390/ma12203386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guidi P., Bernardeschi M., Palumbo M., Genovese M., Scarcelli V., Fiorati A., Riva L., Punta C., Corsi I., Frenzilli G. Suitability of a cellulose-based nanomaterial for the remediation of heavy metal contaminated freshwaters: a case-study showing the recovery of cadmium induced DNA integrity loss, cell proliferation increase, nuclear morphology and chromosomal alterations on Dreissena polymorpha. Nanomater. (Basel). 2020;10(9):1–16. doi: 10.3390/nano10091837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiu P., Man S., Li J., Liu J., Zhang L., Yu P., Gao W. Overdose intake of curcumin initiates the unbalanced state of bodies. J. Agric. Food Chem. 2016;64(13):2765–2771. doi: 10.1021/acs.jafc.6b00053. [DOI] [PubMed] [Google Scholar]
- 60.Tanwar V., Sachdeva J., Kishore K., Mittal R., Nag T.C., Ray R., Kumari S., Arya D.S. Dose-dependent actions of curcumin in experimentally induced myocardial necrosis: a biochemical, histopathological, and electron microscopic evidence. Cell Biochem. Funct. 2010;28(1):74–82. doi: 10.1002/cbf.1623. [DOI] [PubMed] [Google Scholar]
- 61.Goel A., Kunnumakkara A.B., Aggarwal B.B. Curcumin as "Curecumin": from kitchen to clinic. Biochem. Pharmacol. 2008;75(4):787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 62.Ramirez Bosca A., Soler A., Carrion-Gutierrez M.A., Pamies Mira D., Pardo Zapata J., Diaz-Alperi J., Bernd A., Quintanilla Almagro E., Miquel J. An hydroalcoholic extract of Curcuma longa lowers the abnormally high values of human-plasma fibrinogen. Mech. Ageing Dev. 2000;114(3):207–210. doi: 10.1016/s0047-6374(00)00089-0. [DOI] [PubMed] [Google Scholar]
- 63.Basnet P., Skalko-Basnet N. Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16(6):4567–4598. doi: 10.3390/molecules16064567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.