Abstract.
Klinefelter syndrome (KS) is a sex chromosome disorder characterized by the presence of one or more extra X chromosomes. KS is well known by the common karyotype 47, XXY and presents as male infertility with hypogonadism in adults. Pediatric patients with KS commonly show neurodevelopmental disorders and cryptorchidism. We have reported a case of a 14-yr-old boy with KS and severe obesity (body mass index, 38.1 kg/m2), insulin (IRI) resistance (homeostatic model assessment 1 IRI resistance, 9.26), hyperlipidemia (serum low-density lipoprotein cholesterol level, 192 mg/dL; serum triglyceride level, 239 mg/dL), hypergonadotropic hypogonadism, and learning difficulties. The karyotype was 47, XXY, t(4;5) (q21.2;q32). Initially, he was unwilling to accept dietary restrictions and perform physical exercise against obesity. Testosterone replacement therapy was initiated at 16 years of age, which successfully improved the body composition, IRI resistance, and hyperlipidemia and increased the serum testosterone levels. Additionally, he adhered to recommendations for exercise and dietary restrictions. Patients with KS have risks of obesity and metabolic syndrome with sarcopenic conditions due to hypergonadotropic hypogonadism. Pediatricians should be aware of KS as a primary disease causing obesity. Testosterone replacement therapy could help ameliorate obesity and its comorbidities in patients with obesity and KS.
Keywords: Klinefelter syndrome, obesity, metabolic syndrome, hyperlipidemia, testosterone therapy
Introduction
Klinefelter syndrome (KS) is a sex chromosome disorder characterized by the presence of one or more extra X chromosomes. The classical KS karyotype is 47, XXY, and the incidence of KS is approximately 1 in 660 births (1). KS in adults is characterized by tall stature, gynecomastia, late-onset puberty, azoospermia, erectile dysfunction, reduced libido, and neurodevelopmental disorders (1). Stereotypically, patients with KS are tall and slender with narrow shoulders, long extremities, and sparse body hair. In recent studies, 44% and 42.6% patients with KS aged >18 yr had metabolic syndrome (2) and obesity (3), respectively. Some adult patients with KS have insulin (IRI) resistance and type 2 diabetes mellitus (2,3,4,5). In patients with KS, the risks of obesity, metabolic syndrome, and type 2 diabetes mellitus increase from adolescence to adulthood. Diabetes-related mortality and comorbidities are serious problems in adulthood in patients with this syndrome (6, 7).
In prepubertal patients, the common features of KS are cryptorchidism and neurodevelopmental disorders, whereas those in pubertal patients are small penises, small testes, cryptorchidism, gynecomastia, and neurodevelopmental disorders. In pediatric patients, obesity is seldom observed as a clinical symptom of KS (8).
Here, we have reported a case of a pubertal patient with KS who developed severe obesity and hypergonadotropic hypogonadism. Testosterone replacement therapy led to an improvement obesity and hyperlipidemia. We suggest that testosterone therapy not only improves sexual development but also has anti-obesity and metabolic effects in adolescent patients with KS.
Patient and Methods
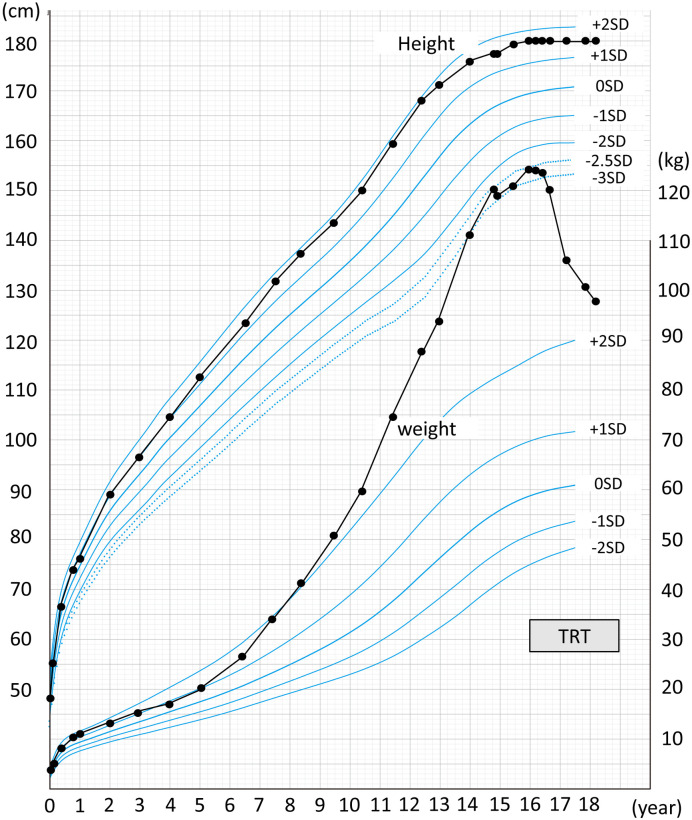
A 14-yr-old boy with severe obesity, developmental delay, microphallus, and small testes was referred to our outpatient clinic. He was born to non-consanguineous Japanese parents at 38 wk of gestation by urgent cesarean section with fetal distress. His birth weight was 2,576 g, and Apgar scores were 4 and 7 at 1 and 5 min after birth, respectively. However, he immediately recovered from neonatal asphyxia, and no other abnormalities were observed during the neonatal period. His progress was normal at the developmental milestones throughout the infancy and toddler periods. At 6 yr of age, he became significantly obese, although the growth in height was within the normal range (Fig. 1). At 10 yr of age, he required educational support due to learning difficulties. At 13 yr of age, his developmental quotient was 75.
Fig. 1.
Growth chart of the patient (height and weight); testosterone replacement therapy improved severe obesity.
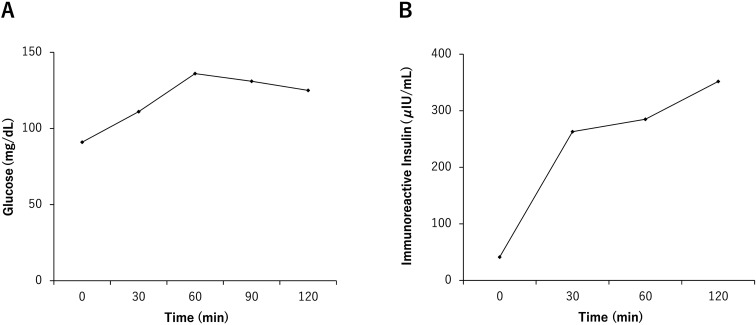
At the first referral at 14 yr of age, his height and weight were 177.6 cm (+ 1.84 standard deviation score [SDS]) and 120.2 kg (+ 6.19 SDS), respectively. His body mass index (BMI), percentage of overweight (POW; calculated using the method by Murata et al. (9, 10)), and body fat percentage (BF%) were 38.1 kg/m2, 87.3, and 50.9%, respectively. These values corresponded to “severe obesity,” defined by the 2017 update of the Japanese guideline for childhood obesity. His blood pressure and heart rate were 122/96 mmHg and 78/min, respectively. The Tanner stage (rating of sexual maturity) was 1 (pubic hair, absent; penile length, 3 cm; testis volume, 4 mL). Blood tests revealed the following results: aspartate transaminase (AST) level, 50 U/L; alanine transaminase (ALT) level, 84 U/L; fasting blood glucose (FBG) level, 91 mg/dL; serum IRI level, 41.2 μIU/mL; homeostatic model assessment 1 IRI resistance (HOMA1-IR), 9.26; glycated hemoglobin (HbA1c) level, 5.5%; total cholesterol level, 261 mg/dl; low-density lipoprotein cholesterol (LDL-C) level, 192 mg/dL; high-density lipoprotein cholesterol (HDL-C) level, 59 mg/dL; triglyceride (TG) level, 239 mg/dL; leptin level, 65.2 ng/mL; TSH level, 2.464 μIU/mL; free T3 level, 3.51 pg/mL; free T4 level, 1.28 ng/dL; LH level, 10.92 mIU/mL; FSH level, 21.66 mIU/mL; and testosterone level, 1.28 ng/mL. Although a 75-g oral glucose tolerance test showed IRI resistance, glucose tolerance was preserved (Figs. 2A and 2B). Indirect calorimetry showed a resting oxygen consumption and resting energy expenditure of 339 mL/min and 2,357 kcal/d, respectively. Abdominal ultrasonography revealed fatty liver, and FibroScan documented high lipid accumulation and high stiffness of the liver (controlled attenuation parameter index, 288 dB/m; liver stiffness measurement index, 5.4 kPa). He was diagnosed with KS with karyotype 47, XXY, t(4;5) (q21.2;q32). We found no structural abnormalities in chromosome 15 on fluorescence in situ hybridization analysis. A clinical nutritionist estimated that his daily calorie intake was 3000–4000 kcal/d.
Fig. 2.
(A) Plasma glucose levels during a 75-g oral glucose tolerance test (75 g OGTT); (B) Insulin reactivity during a 75-g OGTT.
After admission, dietary restriction (1800–1900 kcal/d) and routine exercise using an exercise bike were indicated. Two weeks after admission, his weight, BMI, and POW reduced to 118.3 kg (+ 6.01 SDS), 36.1 kg/m2, and 77.2%, respectively. After discharge, he exercised for 30 min per day using an exercise bike. Nevertheless, he had difficulty in adhering to a calorie-restricted diet.
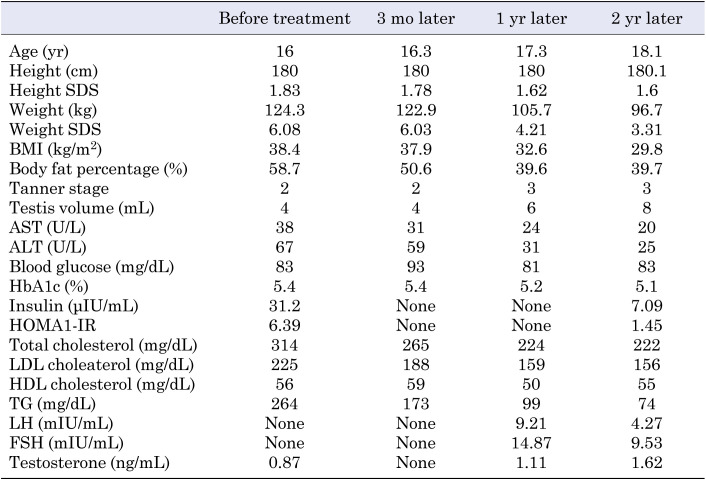
Fifteen months after admission (at 16 yr of age), we found no improvement in obesity. He continued the prescribed physical exercise; however, his dietary energy intake increased (2500–3000 kcal/d), leading to weight gain. His height and weight were 180 cm (+ 1.83 SDS) and 124.3 kg (+ 6.08 SDS), respectively, whereas his BMI, POW, and BF% were 38.4 kg/m2, 87.6%, and 58.7%, respectively. Blood test results revealed worsened hyperlipidemia and fatty liver, and IRI resistance persisted (Table 1). The testicular volume was 4 mL (Tanner stage 2). Testosterone therapy was initiated to induce normal sex development (50-mg injection of testosterone enanthate monthly). At the age of 16 yr and 6 mo, the dose of testosterone was increased to 125 mg monthly.
Table 1. Clinical findings after testosterone replacement therapy in an adolescent patient with Klinefelter syndrome.
Results
Twelve months after the initiation of testosterone therapy (at 17 yr of age), his weight, BMI, POW, and BF% reduced to 105.7 kg (+ 4.21 SDS), 32.6 kg/m2, 46.8%, and 39.6%, respectively; however, his height remained unchanged at 180 cm (+ 1.62 SDS).
Two years later, at the age of 18 yr, his height, weight, BMI, obesity index, and BF% were 180.1 cm (+ 1.60 SDS), 96.7 kg (+ 3.31 SDS), 29.8 kg/m2, 35.5%, and 39.7%, respectively. Testosterone treatment was continued at 125 mg monthly, and his testicular volume was 8 ml (Tanner stage 3). Blood test results showed the following findings: AST level, 20 U/L; ALT level, 25 U/L; FBG level, 83 mg/dL; HbA1c level, 5.1%; IRI level, 7.09 μIU/mL; HOMA1-IR, 1.45; total cholesterol level, 222 mg/dL; LDL-C level, 156 mg/dL; HDL-C, level 55 mg/dL; TG level, 74 mg/dL; LH level, 4.27 mIU/mL; FSH level, 9.53 mIU/mL; and testosterone level, 1.62 ng/mL. Abdominal ultrasonography showed improvement in fatty liver.
Discussion
We have reported a case of KS in an adolescent presenting with severe obesity, IRI resistance, hyperlipidemia, and primary hypogonadism. Bejesen et al. have described higher incidences of metabolic syndrome and IRI resistance in adult patients with KS than in an age-matched control population (2). In fact, 44% of Danish adult patients with KS aged > 18 yr had obesity and metabolic syndrome associated with hypoandrogenemia and low serum HDL values (2). Han et al. reported that the prevalence of obesity (BMI ≥ 25 kg/m2) in patients with KS was 42.6% (3). Moreover, a low testosterone level was an independent risk factor of obesity and hyperglycemia in patients with KS (3). In summary, these results establish that KS is a risk factor for obesity, metabolic syndrome, diabetes mellitus, and dyslipidemia.
According to Aksglaede et al., physicians should be aware of the comorbidities of KS such as obesity, metabolic syndrome, hyperlipidemia, diabetes mellitus, and IRI resistance, even if patients are young or prepubertal (9). A study in 89 prepubertal boys with KS revealed that 37% boys had elevated LDL-C levels, 24% boys had IRI resistance, and 7% boys had metabolic syndrome (10). In our case, obesity was assumed to have developed at the age of 6 yr. We speculate that hyperphagia must have begun in the background of difficult behavioral development in our patient. Subsequently, in prepuberty, obesity accelerated by hyperphagia resulted from developmental characteristics due to the duplicated X-chromosome abnormality rather than hypogonadism.
Obesity supposedly induces hypogonadism (9), and low levels of testosterone, free testosterone, and sex hormone-binding globulin have been found in both men with obesity (11) and diabetes (12). Additionally, increased amounts of body fat in prepubertal boys with KS indicate causes other than low testosterone levels (4). In summary, these findings suggest the presence of a vicious circle between obesity and hypogonadism that accelerates sarcopenic adiposity in patients with KS having metabolic morbidities; this might have occurred in our patient.
We concluded that testosterone replacement therapy was effective in improving obesity, IRI resistance, metabolic syndrome, and dyslipidemia in our patient. Moreover, testosterone therapy could improve body composition by decreasing fat accumulation. In a previous study, testosterone replacement therapy increased the prostate volume and testosterone levels and improved HOMA1-IR, total cholesterol levels, TG levels, and visceral obesity in adult patients with KS (13). Similarly, testosterone therapy improved BMI, waist circumference, and hypertension in hypogonadal men who were overweight or obese in another study (14). The anti-obesity effects of testosterone therapy may be explained by various mechanisms. Testosterone therapy improves sarcopenic obesity by increasing the muscle mass in hypogonadal men (15); thus, testosterone increases the lean body mass and basal metabolic rate (16). Androgens, including testosterone, play a critical role in the regulation of adipogenesis and body fat distribution. Testosterone inhibits the differentiation of human adipose stem cells into preadipocytes by activating bone morphogenetic protein 4 (17). Androgen receptor expression is greater in visceral adipocytes than that in subcutaneous adipocytes (18). Moreover, testosterone improves IRI sensitivity by upregulating the expressions of IRI receptors and glucose transporter 4 and suppressing inflammation. Similarly, it increases the expression of anti-inflammatory cytokine (interleukin 10) and decreases those of proinflammatory cytokines such as interleukin 6 and tumor necrosis factor alpha (19, 20). These mechanisms may explain the anti-obesity effect of testosterone on visceral fat obesity and metabolic syndrome in KS. The common assumption is that the effects of testosterone treatment in patients with KS are equivalent to those in hypogonadal men, and there is a consensus that testosterone treatment should be offered to most patients with KS. Treatment should be initiated in the peripubertal period to ensure optimal masculine development of sexual characteristics, whole muscle mass, and bone structure to prevent the long-term negative consequences of hypergonadotropic hypogonadism (21).
The timing of initiating testosterone therapy in children with KS is controversial. Aksglaede et al. reported no significant difference in BMI between the studied groups, but reported a significant increase in the BF% in patients with KS aged 4–18 yr compared to that in healthy controls (9). In contrast, Mehta et al. reported that testosterone replacement therapy in adolescents with KS aged 10–21 yr increased the serum testosterone levels to those within the normal range with no adverse effects (22). Testosterone therapy can have adverse side effects such as acne, oily skin, hypercythemia, exacerbation of gynecomastia, and dysregulation of spermatogenesis (23). In children requiring testosterone therapy, the recommended starting dose is 50–75 mg, which is followed by a 50-mg increase every 4–6 mo to a maximum dose of 150 mg (23). In our case, the starting dose was 50 mg monthly, and after 6 mo, the dose was increased to 125 mg monthly. This was followed by a marked decrease in the weight and body fat percentage. At 14 yr of age, he was admitted to the hospital for education, and he underwent a regimen of increased physical exercise and dietary restriction, which resulted in weight loss. After being discharged from the hospital, he continued to exercise for approximately 30 min/d; however, his dietary energy intake increased to approximately 2,500–3,000 kcal/d, and he had difficulty adhering to the strict diet; therefore, he gradually gained weight. Notably, our patient adhered to the prescribed physical exercise regimen during testosterone replacement therapy. However, the patient dramatically lost weight after testosterone therapy. The significant weight loss after testosterone replacement suggests that testosterone therapy may be an effective option for the treatment of obesity in patients with KS. The goals of testosterone therapy for patients with KS are promotion of linear growth, development of secondary sexual characteristics, normal distribution of body fat, and increased muscle mass and bone mineral content (23).
Although most boys and men with KS have various cognitive disabilities, the most common disability is verbal deficits (24). Similarly, patients with KS are at risks of depression, autistic disorder, anxiety, and attention-deficit/hyperactivity disorder (24). Moreover, testosterone is known to influence brain development (25). In our case, the patient was highly motivated and had a positive attitude toward daily exercise during the testosterone replacement therapy. Additionally, he seemed to be more fluent in verbal communication after testosterone replacement therapy was initiated. In KS, prenatal and postnatal androgen deficiency affects the neuropsychological phenotype and brain volume. However, evidence regarding the effects of testosterone replacement therapy on neuropsychological aspects is lacking. The development of the neuropsychological phenotype and changes in brain volume may be gene dosage-related effects due to the presence of a supernumerary X chromosome (24). Nielsen et al. reported that testosterone therapy elevated mood; reduced irritability, fatigue, and the need for sleep; increased energy, drive, endurance, strength, and concentration ability; and improved relations with others. They suggested that testosterone therapy could be beneficial in patients with KS and that therapy should be preferably initiated at 11–12 yr of age (26). We agree with this opinion, considering our experience with the current patient.
Conclusion
Testosterone therapy not only induced pubertal development but also exerted anti-obesity and metabolic effects in an adolescent case of KS, while also ameliorating hyperlipidemia and IRI resistance. In pediatric and adolescent patients with KS, testosterone replacement therapy can improve excessive adiposity, metabolic syndrome, and IRI sensitivity.
Conflicts of interest
None of the authors reports financial relationships relevant to this article.
Acknowledgements
We appreciate Dr. Atsushi Umemura and Dr. Kanji Yamaguchi (Department of Gastroenterology and Hepatology, Kyoto Prefectural University of Medicine) performing the FibroScan examinations on the current patient.
References
- 1.Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab 2003;88: 622–6. doi: 10.1210/jc.2002-021491 [DOI] [PubMed] [Google Scholar]
- 2.Bojesen A, Kristensen K, Birkebaek NH, Fedder J, Mosekilde L, Bennett P, et al. The metabolic syndrome is frequent in Klinefelter’s syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care 2006;29: 1591–8. doi: 10.2337/dc06-0145 [DOI] [PubMed] [Google Scholar]
- 3.Han SJ, Kim KS, Kim W, Kim JH, Lee YH, Nam JS, et al. Obesity and hyperglycemia in korean men with Klinefelter syndrome: The Korean Endocrine Society registry. Endocrinol Metab (Seoul) 2016;31: 598–603. doi: 10.3803/EnM.2016.31.4.598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bojesen A, Høst C, Gravholt CH. Klinefelter’s syndrome, type 2 diabetes and the metabolic syndrome: the impact of body composition. Mol Hum Reprod 2010;16: 396–401. doi: 10.1093/molehr/gaq016 [DOI] [PubMed] [Google Scholar]
- 5.O’Connor MJ, Snyder EA, Hayes FJ. Klinefelter syndrome and diabetes. Curr Diab Rep 2019;19: 71. doi: 10.1007/s11892-019-1197-3 [DOI] [PubMed] [Google Scholar]
- 6.Swerdlow AJ, Higgins CD, Schoemaker MJ, Wright AF, Jacobs PA, United Kingdom Clinical Cytogenetics Group. Mortality in patients with Klinefelter syndrome in Britain: a cohort study. J Clin Endocrinol Metab 2005;90: 6516–22. doi: 10.1210/jc.2005-1077 [DOI] [PubMed] [Google Scholar]
- 7.Bojesen A, Juul S, Birkebaek NH, Gravholt CH. Morbidity in Klinefelter syndrome: a Danish register study based on hospital discharge diagnoses. J Clin Endocrinol Metab 2006;91: 1254–60. doi: 10.1210/jc.2005-0697 [DOI] [PubMed] [Google Scholar]
- 8.Pacenza N, Pasqualini T, Gottlieb S, Knoblovits P, Costanzo PR, Stewart Usher J, et al. Clinical presentation of Klinefelter’s syndrome: Differences according to age. Int J Endocrinol 2012;2012: 324835. doi: 10.1155/2012/324835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aksglaede L, Molgaard C, Skakkebaek NE, Juul A. Normal bone mineral content but unfavourable muscle/fat ratio in Klinefelter syndrome. Arch Dis Child 2008;93: 30–4. doi: 10.1136/adc.2007.120675 [DOI] [PubMed] [Google Scholar]
- 10.Bardsley MZ, Falkner B, Kowal K, Ross JL. Insulin resistance and metabolic syndrome in prepubertal boys with Klinefelter syndrome. Acta Paediatr 2011;100: 866–70. doi: 10.1111/j.1651-2227.2011.02161.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasquali R, Casimirri F, Cantobelli S, Melchionda N, Morselli Labate AM, Fabbri R, et al. Effect of obesity and body fat distribution on sex hormones and insulin in men. Metabolism 1991;40: 101–4. doi: 10.1016/0026-0495(91)90199-7 [DOI] [PubMed] [Google Scholar]
- 12.Goodman-Gruen D, Barrett-Connor E. Sex differences in the association of endogenous sex hormone levels and glucose tolerance status in older men and women. Diabetes Care 2000;23: 912–8. doi: 10.2337/diacare.23.7.912 [DOI] [PubMed] [Google Scholar]
- 13.Selice R, Caretta N, Di Mambro A, Torino M, Palego P, Ferlin A, et al. Prostate volume and growth during testosterone replacement therapy is related to visceral obesity in Klinefelter syndrome. Eur J Endocrinol 2013;169: 743–9. doi: 10.1530/EJE-13-0488 [DOI] [PubMed] [Google Scholar]
- 14.Saad F, Doros G, Haider KS, Haider A. Differential effects of 11 years of long-term injectable testosterone undecanoate therapy on anthropometric and metabolic parameters in hypogonadal men with normal weight, overweight and obesity in comparison with untreated controls: real-world data from a controlled registry study. Int J Obes 2020;44: 1264–78. doi: 10.1038/s41366-019-0517-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giannoulis MG, Martin FC, Nair KS, Umpleby AM, Sonksen P. Hormone replacement therapy and physical function in healthy older men. Time to talk hormones? Endocr Rev 2012;33: 314–77. doi: 10.1210/er.2012-1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welle S, Jozefowicz R, Forbes G, Griggs RC. Effect of testosterone on metabolic rate and body composition in normal men and men with muscular dystrophy. J Clin Endocrinol Metab 1992;74: 332–5. doi: 10.1210/jcem.74.2.1730811. [DOI] [PubMed] [Google Scholar]
- 17.Chazenbalk G, Singh P, Irge D, Shah A, Abbott DH, Dumesic DA. Androgens inhibit adipogenesis during human adipose stem cell commitment to preadipocyte formation. Steroids 2013;78: 920–6. doi: 10.1016/j.steroids.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groti K, Žuran I, Antonič B, Foršnarič L, Pfeifer M. The impact of testosterone replacement therapy on glycemic control, vascular function, and components of the metabolic syndrome in obese hypogonadal men with type 2 diabetes. Aging Male 2018;21: 158–69. doi: 10.1080/13685538.2018.1468429 [DOI] [PubMed] [Google Scholar]
- 19.Zitzmann M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat Rev Endocrinol 2009;5: 673–81. doi: 10.1038/nrendo.2009.212 [DOI] [PubMed] [Google Scholar]
- 20.Li SY, Zhao YL, Yang YF, Wang X, Nie M, Wu XY, et al. Metabolic effects of testosterone replacement therapy in patients with type 2 diabetes mellitus or metabolic syndrome: A meta-analysis. Int J Endocrinol 2020;2020: 4732021. doi: 10.1155/2020/4732021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bojesen A, Gravholt CH. Klinefelter syndrome in clinical practice. Nat Clin Pract Urol 2007;4: 192–204. doi: 10.1038/ncpuro0775 [DOI] [PubMed] [Google Scholar]
- 22.Mehta A, Clearman T, Paduch DA. Safety and efficacy of testosterone replacement therapy in adolescents with Klinefelter syndrome. J Urol 2014;191(Suppl): 1527–31. doi: 10.1016/j.juro.2013.09.015 [DOI] [PubMed] [Google Scholar]
- 23.Rogol AD, Tartaglia N. Considerations for androgen therapy in children and adolescents with Klinefelter syndrome (47, XXY). Pediatr Endocrinol Rev 2010;8(Suppl 1): 145–50. [PubMed] [Google Scholar]
- 24.Høst C, Skakkebæk A, Groth KA, Bojesen A. The role of hypogonadism in Klinefelter syndrome. Asian J Androl 2014;16: 185–91. doi: 10.4103/1008-682X.122201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knickmeyer RC, Baron-Cohen S. Fetal testosterone and sex differences in typical social development and in autism. J Child Neurol 2006;21: 825–45. doi: 10.1177/08830738060210101601 [DOI] [PubMed] [Google Scholar]
- 26.Nielsen J, Pelsen B, Sørensen K. Follow-up of 30 Klinefelter males treated with testosterone. Clin Genet 1988;33: 262–9. doi: 10.1111/j.1399-0004.1988.tb03447.x [DOI] [PubMed] [Google Scholar]