Abstract
Background & Aims
Helicobacter pylori has been reported to modulate local immune responses to colonize persistently in gastric mucosa. Although the induced expression of programmed cell death ligand 1 (PD-L1) has been suggested as an immune modulatory mechanism for persistent infection of H pylori, the main immune cells expressing PD-L1 and their functions in Helicobacter-induced gastritis still remain to be elucidated.
Methods
The blockades of PD-L1 with antibody or PD-L1–deficient bone marrow transplantation were performed in Helicobacter-infected mice. The main immune cells expressing PD-L1 in Helicobacter-infected stomach were determined by flow cytometry and immunofluorescence staining. Helicobacter felis or H pylori–infected dendritic cell (DC)-deficient mouse models including Flt3–/–, Zbtb46–diphtheria toxin receptor, and BDCA2–diphtheria toxin receptor mice were analyzed for pathologic changes and colonization levels. Finally, the location of PD-L1–expressing DCs and the correlation with H pylori infection were analyzed in human gastric tissues using multiplexed immunohistochemistry.
Results
Genetic or antibody-mediated blockade of PD-L1 aggravated Helicobacter-induced gastritis with mucosal metaplasia. Gastric classical DCs expressed considerably higher levels of PD-L1 than other immune cells and co-localized with T cells in gastritis lesions from Helicobacter-infected mice and human beings. H felis– or H pylori–infected Flt3–/– or classical DC-depleted mice showed aggravated gastritis with severe T-cell and neutrophil accumulation with low bacterial loads compared with that in control mice. Finally, PD-L1–expressing DCs were co-localized with T cells and showed a positive correlation with H pylori infection in human subjects.
Conclusions
The PD-1/PD-L1 pathway may be responsible for the immune modulatory function of gastric DCs that protects the gastric mucosa from Helicobacter-induced inflammation, but allows persistent Helicobacter colonization.
Keywords: Gastric Inflammation, T Cell, Immune Regulation, Mucosal Metaplasia
Abbreviations used in this paper: Ab, antibody; ATPase, adenosine triphosphatase; BM, bone marrow; BMT, bone marrow transplanted; cDC, classical dendritic cell; CLU, clusterin; DC, dendritic cell; DN, double-negative; DT, diphtheria toxin; DTR, diphtheria toxin receptor; FBS, fetal bovine serum; Flt3, fms-like tyrosine kinase 3; IFN-γ, interferon-γ; IHC, immunohistochemistry; IL, interleukin; IRAE, immune-related adverse event; IRF, interferon regulatory factor; MHC, major histocompatibility complex; mRNA, messenger RNA; PCR, polymerase chain reaction; PD-1, programmed death 1; pDC, plasmacytoid DC; PD-L1, programmed death-ligand 1; SIRPα, signal regulatory protein alpha; SP-D, surfactant protein-D; SPEM, spasmolytic polypeptide-expressing metaplasia; TFF2, trefoil factor 2; Treg, regulatory T cell; WT, wild type; XCR1, X-C motif chemokine receptor 1; ZBTB46, zinc finger and BTB domain containing 46
Graphical abstract
Summary.
The induced programmed cell death ligand 1 expression in gastric epithelium has been suggested as an immunomodulatory mechanism for persistent gastric Helicobacter colonization. In this study, we found that classical dendritic cells mainly express programmed cell death ligand 1 in the gastric mucosal area and play a pivotal role in Helicobacter-induced gastritis.
Programmed death-ligand 1 (PD-L1; CD274) is a co-inhibitory molecule of the B7 family ligands and indispensable for the maintenance of T-cell tolerance. PD-L1 suppresses T-cell activity by transferring inhibitory signals to T cells and interfering with T-cell stimulatory signals via programmed death 1 (PD-1; CD279) receptor.1,2 Therefore, PD-1/PD-L1 signaling modulates T-cell–mediated immunity by regulating the number of T cells and their activity in multiple ways, for instance, by inducing T-cell exhaustion,3 anergy,4 apoptosis,5 or inhibiting cell proliferation.6 PD-1/PD-L1 interaction has been known to play a crucial role in tumor immune escape. Because PD-L1 on tumor cells suppresses antitumor T-cell response, PD-1/PD-L1 blockade has been considered a highly promising antitumor therapy. Meanwhile, PD-1/PD-L1 interaction also play important roles in various infectious diseases induced by virus, bacteria, or parasite.7 The T cells that infiltrate into the site of inflammation with infectious agents are suppressed by PD-1/PD-L1 engagement, allowing persistent infection but preventing immune-mediated tissue damage.8 Thus, the proper regulation of the PD-1/PD-L1 pathway appears to be very important to control inflammation and subsequent tissue damage during chronic infection.
Chronic infection of Helicobacter pylori causes persistent gastric inflammation. Approximately 50% of the human population is colonized by H pylori, 15% of whom develop severe lesions such as severe chronic gastritis, peptic ulcer, gastric adenocarcinoma, and lymphoma.9 Interestingly, the majority of gastric cancers are associated with H pylori.10 H pylori–induced gastropathy is affected mostly by the host’s immune response against H pylori.11 Various components of H pylori and signals from mucosal epithelial cells in contact with H pylori cause infiltration of innate immune cells including dendritic cells (DCs), macrophages, monocytes, and neutrophils. Although the responses of CD4+ or CD8+ T cells also are generated against H pylori,9,12, 13, 14 these T-cell–mediated immune responses were not effective in eradicating colonized H pylori in the gastric mucosa.15, 16, 17 T-cell–derived interferon-γ (IFN-γ) contributes to the development of severe gastritis and precancerous mucosal metaplasia.18,19
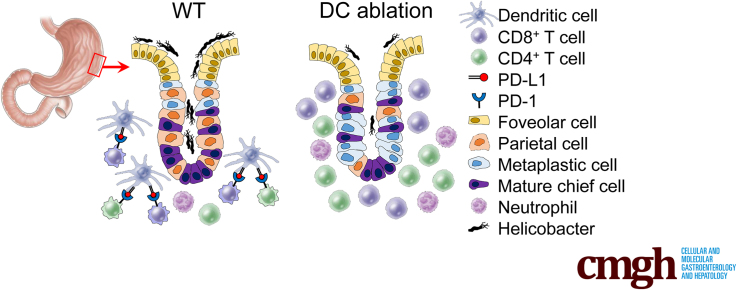
Previously, PD-1/PD-L1 has been reported to have a role in persistent gastric infection of H pylori. H pylori infection increases the PD-L1 expression on gastric epithelial cells and suppresses T-cell responses in gastric mucosa.20,21 However, antigen-presenting cells such as DCs and macrophages can express a higher level of PD-L1,8 and may play important roles in T-cell response to H pylori. Indeed, the PD-L1 expression of DCs was increased by H pylori, but its role still is unknown.22 In this study, we attempted to analyze the effect of PD-L1 blockade on bacterial colonization, inflammation, and precancerous mucosal changes in Helicobacter felis–infected gastric mucosa. Interestingly, gastric DCs were the main PD-L1–expressing cells and co-localized with T cells in the gastric mucosa of Helicobacter-infected mice and human beings. The ablation of classical DCs (cDCs) also induced severe lymphocytic gastritis with suppression of Helicobacter colonization. Collectively, PD-L1–expressing cDCs attenuate mucosal inflammation and precancerous mucosal metaplasia, but support the persistent Helicobacter colonization by modulating T-cell response.
Results
Blockade of PD-L1 With Antibody or PD-L1 Deficiency in Bone Marrow–Derived Cells Enhance Gastric Inflammation With Mucosal Metaplasia Upon Helicobacter Infection
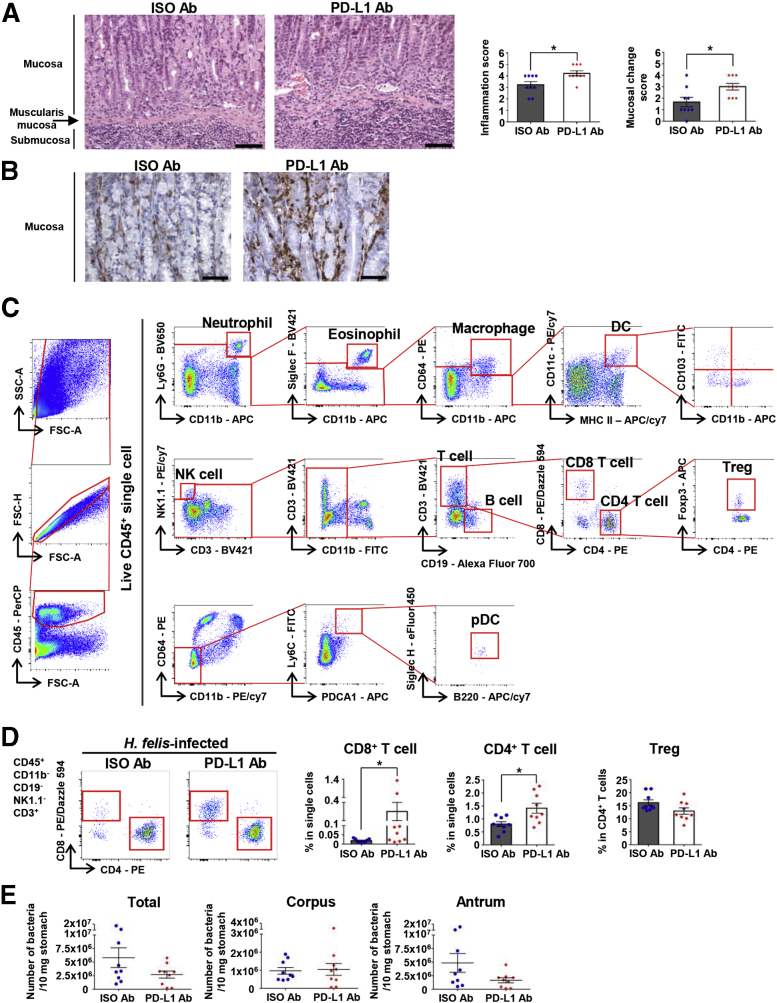
To confirm the role of PD-L1 in the host immune response to Helicobacter infection, we injected PD-L1 antibody (Ab) into H felis–infected wild-type (WT) mice to block PD-L1 signals. We analyzed gastric lesions at 4 weeks after infection. Histologically, lymphocyte infiltration into the gastric submucosa and mucosal lamina propria, as well as pathologic mucosal changes, were markedly more severe in the PD-L1 Ab–injected group than in the control Ab-injected group (Figure 1A). Furthermore, CD3+ T-cell infiltration into the mucosal lamina propria was more prominent in the PD-L1 Ab–injected group (Figure 1B). We performed flow cytometry analysis to observe the changes of various leukocyte populations in the H felis–infected stomach (Figure 1C). Among the leukocyte populations, T-cell infiltration was increased markedly by PD-L1 Ab injection. Infiltration of CD8+ T cells and CD4+ T cells was more prominent in the PD-L1 Ab–injected group, whereas the percentage of regulatory T cells (Tregs) in CD4+ T cells tended to be lower in the PD-L1 Ab–injected group (Figure 1D). The bacterial loads in the stomach were decreased in the PD-L1 Ab–injected group than in the control Ab-injected group (Figure 1E).
Figure 1.
Blockade of PD-L1 enhances gastric inflammation upon H felis infection for 4 weeks. (A) Histopathologic analysis of H felis–induced gastritis, oxyntic atrophy, and mucosal metaplasia according to grading criteria on H&E stain. Representative H&E images of the gastric corpus regions of isotype control mice and mice with PD-L1 blockade (left). Inflammation score and mucosal change score (right) (isotype control mice, n = 9; PD-L1 blockade mice, n = 9). Scale bar: 100 μm. (B) More severe mucosal infiltration of T cells in the gastric corpus regions of mice with PD-L1 blockade. Representative images of CD3 IHC (3,3'-diaminobenzidine substrate). Scale bar: 50 μm. (C) Gating strategy for the analysis of gastric immune cells. (D) Percentage of CD8+ T cells and CD4+ T cells in live single cells and of Tregs in live CD4+ T cells from the stomach of mice with PD-L1 blockade (n = 9) and isotype control mice (n = 9). Representative flow cytometry plots (left). (E) The bacterial loads were evaluated using quantitative real-time PCR for H felis flagellar filament B DNA extracted from the corpus and antrum, respectively (isotype control mice, n = 9; PD-L1 blockade mice, n = 9). Data are presented as means ± SEM. ∗P < .05. APC, allophycocyanin; FITC, fluorescein isothiocyanate; FSC, forward scatter; ISO, isotype control; NK, natural killer; PE, phycoerythrin; SSC, side scatter.
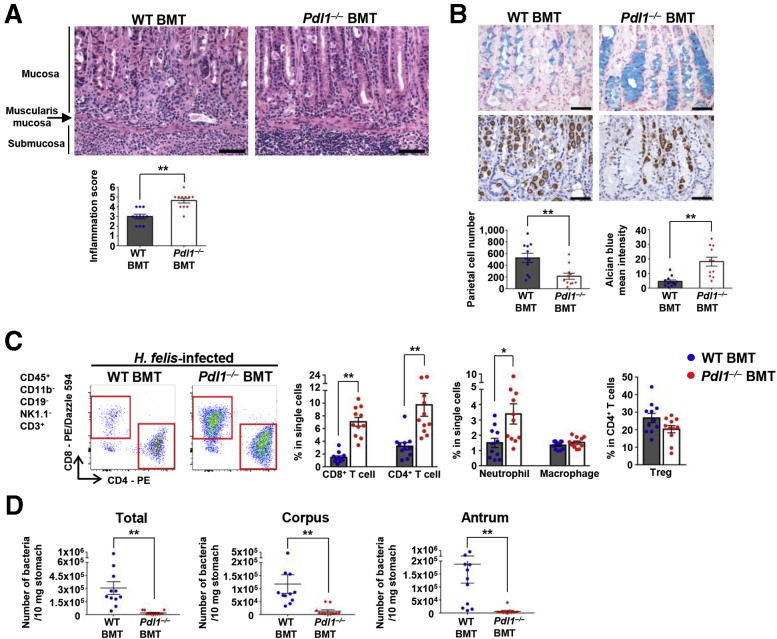
Several studies have reported PD-L1 expression on the gastric mucosal epithelium during Helicobacter infection.20,21,23 However, various hematopoietic lineage cells also express PD-L1.8 To determine the function of PD-L1 on hematopoietic lineage cells in Helicobacter infection, we created bone marrow (BM) chimeras by transplanting Cd274–/– (Pdl1–/–) or WT BM into irradiated WT mice. Histologically, inflammatory lesions were more severe in the Pdl1–/– BM transplanted (BMT) mice compared with control mice (Figure 2A). The inflammatory processes induced by Helicobacter infection can damage the mucosa, causing oxyntic atrophy and precancerous mucosal metaplasia. In the corpus mucosa of H felis–infected mice, varying numbers of parietal cells and mature chief cells were lost and replaced by mucous cells showing spasmolytic polypeptide-expressing metaplasia (SPEM). In addition, foveolar cell hyperplasia was observed. We confirmed the loss of parietal cells using immunohistochemistry (IHC) for H+/K+-adenosine triphosphatase (ATPase) and the degree of mucous neck cell hyperplasia and SPEM using Alcian blue staining. In the Pdl1–/– BMT mice, parietal cell loss worsened, and Alcian blue positive cells were more prominent (Figure 2B). Accumulation of T cells and neutrophils, but not macrophages, was more severe in the Pdl1–/– BMT mice than in control mice. However, the percentage of Tregs in CD4+ T cells tended to be lower in the Pdl1–/– BMT mice than those in control mice (Figure 2C). Bacterial load in the stomach was decreased markedly in Pdl1–/– BMT mice than in control mice (Figure 2D).
Figure 2.
PD-L1 deficiency in BM-derived cells enhances gastric inflammation with mucosal metaplasia upon H felis infection for 4 weeks. (A) Histopathologic analysis of H felis–induced gastritis. Representative H&E images of the gastric corpus regions of WT BMT and Pdl1–/– BMT mice (top). Inflammation score (bottom) (WT BMT, n = 11; Pdl1–/– BMT, n = 11). Scale bar: 100 μm. (B) Histopathologic analysis of H felis–induced oxyntic atrophy and mucosal metaplasia. Representative serial images of the gastric corpus regions of WT BMT (left) and Pdl1–/– BMT (right) mice after Alcian blue staining (top) and H+/K+-ATPase IHC (bottom). Quantification of H+/K+-ATPase+ cells (parietal cells) and Alcian blue–positive region (WT BMT, n = 11; Pdl1–/– BMT, n = 11). Scale bar: 50 μm. (C) Percentage of CD8+ T cells, CD4+ T cells, neutrophils, and macrophages in live single cells and of Tregs in live CD4+ T cells from the stomach of WT BMT (n = 11) and Pdl1–/– BMT (n = 11) mice. Representative flow cytometry plots of T cells (left). (D) H felis loads were evaluated using quantitative real-time PCR for H felis flagellar filament B DNA extracted from the corpus and antrum, respectively (WT BMT, n = 11; Pdl1–/– BMT, n = 11). Data are presented as means ± SEM. ∗P < .05 and ∗∗P < .01. PE, phycoerythrin.
CD11c+ Cells Mainly Express PD-L1 and Co-localize With PD-1–Expressing T Cells in Helicobacter-Infected Gastric Mucosa and Submucosa
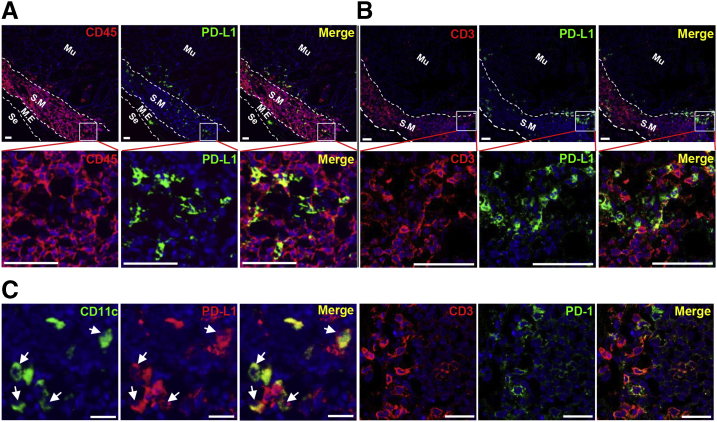
To analyze the pattern of PD-L1 expression in Helicobacter-infected gastric tissue, we performed immunofluorescence staining for PD-L1, CD45, CD3, CD11c, and PD-1 in H felis–infected gastric tissue. PD-L1+ cells were located mainly in the submucosa and mucosal laminar propria immediately above the muscularis mucosa. However, mucosal epithelial cells showed scant PD-L1 expression (Figure 3A and B). The majority of PD-L1+ cells co-stained with CD45 (Figure 3A), and these CD45+PD-L1+ cells were in contact with CD3+ cells (Figure 3B). In addition, CD11c+ cells expressed PD-L1, and the majority of CD3+ cells expressed PD-1 (Figure 3C). These results indicate that PD-L1–expressing CD11c+ cells interact with PD-1+CD3+ T cells in the H felis–infected gastric mucosa and submucosa.
Figure 3.
Location of PD-L1–expressing immune cells in the stomach during Helicobacter infection. (A–C) Immunofluorescence for PD-L1, CD45, CD3, CD11c, and PD-1 in the gastric corpus regions of H felis–infected WT mice. (A and B) Note the localization of PD-L1+ cells in the submucosa and mucosal laminar propria immediately above the muscularis mucosa. PD-L1+ cells are co-stained with CD45 and co-localize with CD3+ T cells. Scale bar: 50 μm. (C) PD-L1+CD11c+ cells (arrows) and PD-1+CD3+ T cells in the submucosa. Scale bar: 20 μm. M.E, muscularis externa; Mu, mucosa; Se, serosa; S.M, submucosa.
Gastric cDCs Express a High Level of PD-L1
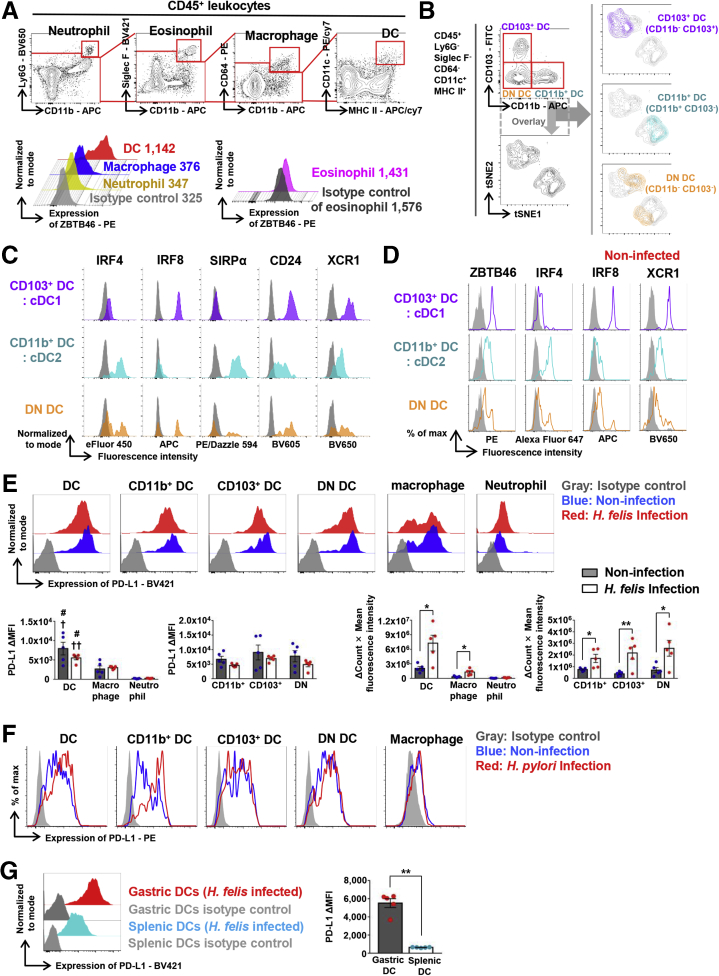
To further characterize PD-L1 expression in gastric CD11c-positive cells, we performed flow cytometry analysis on gastric immune cells. First, we gated out CD45+ leukocytes from the live cell population and then sequentially excluded neutrophils (Ly6G+CD11b+), eosinophils (Siglec F+CD11b+), and macrophages (CD64+CD11b+) from the CD45+ cells. Next, we defined CD11c+Major histocompatibility complex class II (MHC II)+ cells and further analyzed their CD103 and CD11b expression (Figure 4A and B). To confirm that these gastric CD11c+MHC II+ subsets were bona fide DCs, we analyzed the expression level of Zinc finger and BTB domain containing 46 (ZBTB46), a marker of human and murine DCs,24,25 in H felis–infected WT mice. As expected, gastric macrophages, neutrophils, and eosinophils did not express ZBTB46, while gastric CD11c+MHC II+ cells expressed high levels (Figure 4A). Furthermore, a t-stochastic neighborhood embedding algorithm was used to perform dimensionality reduction and visualization of DC subpopulations stained with CD45, CD11c, MHC II, Siglec F, Ly6G, CD64, CD11b, CD103, Interferon regulatory factor 4 (IRF4), Interferon regulatory factor 8 (IRF8), Signal regulatory protein alpha (SIRPα), CD24, and X-C motif chemokine receptor 1 (XCR1) antibodies. We found that gastric DCs can be divided into 2 populations containing CD103+CD11b- DCs—a part of double-negative (DN) DCs and CD103-CD11b+ DCs—the other half of DN DCs (Figure 4B). From the flow cytometry analysis, we defined CD103+CD11b- DCs as cDC1: IRF4-IRF8+SIRPα-CD24highXCR1high, and CD103-CD11b+ DCs as cDC2: IRF4+IRF8-SIRPα+CD24lowXCR1low. The DN subset, CD103-CD11b- DCs contained 2 populations: cDC1 and cDC2-like DCs (Figure 4B and C). These cDC populations also were well-conserved in normal gastric tissue (Figure 4D). Similar CD103-CD11b- DN DCs also were identified in the heart, lung, and liver, and even in solid tumors, and were assumed to represent an intermediate stage of cDC maturation.26,27
Figure 4.
Gastric cDCs express a high level of PD-L1. (A) Gating strategy of DCs, defined as CD45+Ly6G-Siglec F-CD64-CD11c+MHC II+ cells and expression of ZBTB46 in gastric DCs, macrophages, neutrophils, and eosinophils. Numbers in the histogram represent the median fluorescence intensity of ZBTB46 in each population. Note that DCs express considerably higher levels of ZBTB46 than other cell types. (B) A t-stochastic neighborhood embedding (tSNE) plot of total gastric cDCs and overlay of each cDC subset. (C) Expression of IRF4, IRF8, SIRPα, CD24, and XCR1 in 3 gastric cDC subsets under H felis infection. (D) Expression of ZBTB46, IRF4, IRF8, and XCR1 in 3 gastric cDC subsets under normal conditions. Gray indicates isotype control. (A–D) Concatenated data from 3 independent experiments. (E) PD-L1 expression on innate immune cells 8 weeks after H felis infection. Δ mean fluorescence intensity (MFI) = (sample MFI) – (isotype control MFI) Δ(count × MFI) = ([count × mean fluorescence intensity] of sample) – ([count × mean fluorescence intensity] of isotype control). Note that DCs and DC subsets express considerably higher levels of PD-L1 than other cell types. Each figure represents 5 independent experiments. Three mice per group were pooled for each experiment. Data are presented as means ± SEM. †P < .05, and ††P < .01, compared with macrophages; #P < .01, compared with neutrophils; ∗P < .05 and ∗∗P < .01, compared between the noninfected and H felis–infected groups. (F) As in panel E, but with H pylori infection. Note that DCs and DC subsets express considerably higher levels of PD-L1 than macrophages. (G) Comparison of PD-L1 expression levels in gastric DCs and splenic DCs 8 weeks after H felis infection. The left figure is representative of the 5 experiments. ΔMFI = (sample MFI) – (isotype control MFI). Three mice per group were pooled for each experiment. Stomach and spleen were collected from the same individuals. Data are presented as means ± SEM. ∗∗P < .01. APC, allophycocyanin; FITC, fluorescein isothiocyanate.
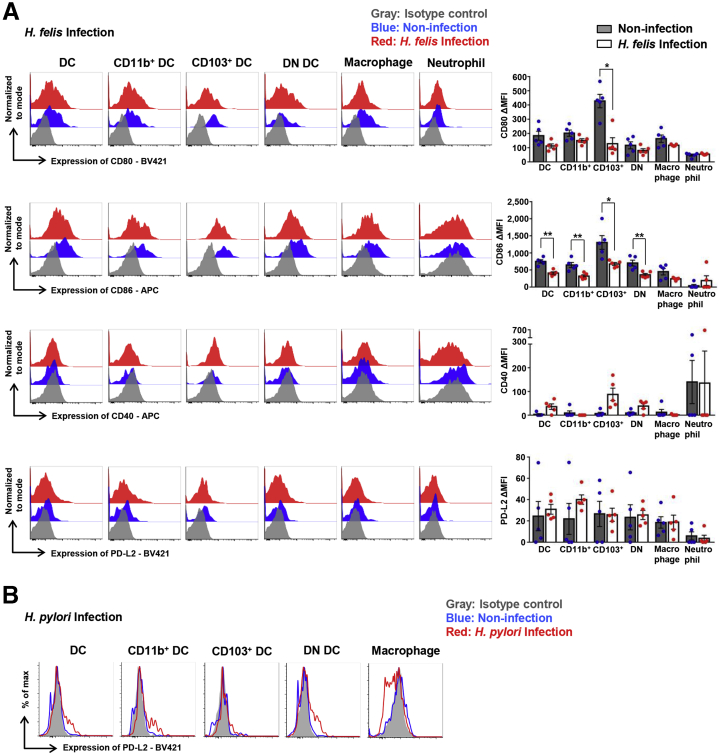
Next, we examined the expression level of PD-L1, PD-L2, and T-cell co-stimulatory molecules, including CD80, CD86, and CD40, on innate immune cells. Regardless of H felis infection, all gastric DC subsets expressed higher levels of PD-L1 than those of other gastric immune cells, including macrophages and neutrophils. Although H felis infection did not affect the PD-L1 expression level on individual gastric DCs regardless of the DC subsets, H felis infection did increase the number of PD-L1–expressing gastric DCs, resulting in an increase in the total level of PD-L1 expression (Figure 4E). To confirm whether similar results were observed in H pylori infection as in H felis infection, we infected mice with H pylori Sydney strain 1 containing virulence factors Cytotoxin-associated gene A (CagA) and Vacuolating cytotoxin A (VacA), and analyzed the results 18 months later. H pylori infection increased PD-L1 expression on gastric DCs, which also was significantly higher than on macrophages. In particular, PD-L1 expression on the CD11b+ DC subset was increased markedly by H pylori infection (Figure 4F). Gastric DCs also expressed higher levels of PD-L1 than splenic DCs upon H felis infection (Figure 4G). The CD80 expression level of gastric DCs, excluding CD103+ DCs, was not affected by H felis infection, whereas that of CD103+ DCs was decreased during H felis infection. In addition, the CD86 expression level of all gastric DC subsets was decreased during H felis infection. However, compared with the median fluorescence intensity of isotype control, CD40 or PD-L2 in gastric DCs and DC subsets showed scant expression, regardless of H felis infection (Figure 5A). A similar PD-L2 expression pattern of gastric DCs was observed in H pylori infection (Figure 5B).
Figure 5.
Expression level of co-stimulatory molecules and PD-L2 in gastric DCs. (A) Expression levels of CD80, CD86, CD40, and PD-L2 on innate immune cells 8 weeks after H felis infection. Data are presented as means ± SEM. ∗P < .05 and ∗∗P < .01. Each figure is representative of 5 independent experiments. Three mice per group were pooled for each experiment. (B) Expression level of PD-L2 on innate immune cells 18 months after H pylori infection. Three mice per group were pooled for the experiment. APC, allophycocyanin; max., maximum.
Helicobacter Infection Increases DCs in Gastric Mucosa and Submucosa
To confirm the location of DCs in the normal mouse stomach, we performed immunostaining for CD11c and observed the stomach of normal CD11c–Enhanced Yellow Fluorescent Protein (EYFP) mice using confocal microscopy. CD11c+ cells were very few and were located mainly in the superficial mucosal lamina propria and around the limiting ridge (Figure 6A). In contrast, during H felis infection, CD11c+ cells were located mainly in the inflamed submucosa and mucosal laminar propria immediately above the muscularis mucosa. A few cells also were distributed throughout the mucosal lamina propria. The number of CD11c+ cells infiltrating the stomach gradually increased at 2, 4, and 8 weeks of H felis infection (Figure 6B). Under normal conditions, gastric CD11c+MHC II+ cells comprised approximately 0.24% of the total live cells and were composed of 3 subsets, CD103+CD11b- (17.9%), CD103-CD11b+ (45.3%), and CD103-CD11b- (DN; 32.1%) populations (Figure 6C). During H felis infection, infiltration of leukocytes and DCs into the stomach was increased markedly. All subsets of CD11c+MHC II+ cells gradually increased at 2, 4, and 8 weeks of H felis infection (Figure 6C).
Figure 6.
Helicobacter infection increases DCs in gastric mucosa and submucosa. (A) Localization of CD11c+ DCs (arrows) under steady state. Left: Representative images of CD11c IHC (3,3'-diaminobenzidine substrate). Right: Representative images of normal CD11c-Enhanced Yellow Fluorescent Protein (EYFP) mouse stomach. White arrowhead indicates a limiting ridge at the boundary of the glandular and nonglandular stomach. Scale bar: 50 μm. (B) Localization and number of gastric CD11c+ DCs (arrows) in the gastric corpus regions of H felis–infected WT mice. Left: Representative images of CD11c IHC (3,3'-diaminobenzidine substrate). Right: Manual cell count of CD11c+ DCs in 2 paraffin sections (n = 5 for each group). Scale bar: 50 μm. (C) Numeric increase in gastric DCs during H felis infection. Percentage of leukocytes, DCs, and DC subsets in live single cells from the stomach of WT mice with H felis infection. Left: Representative flow cytometry plots. Right: Asterisks at 2, 4, and 8 weeks after H felis infection (n = 13–14 for each group) signify a significant increase compared with that in normal WT mice (n = 11). Data are presented as means ± SEM. ∗P < .01. Week, duration of infection. APC, allophycocyanin; FITC, fluorescein isothiocyanate; FSC, forward scatter; Lu, lumen; M.E, muscularis externa; Mu, mucosa; M.S, mucosal surface; PerCP, peridinin-chlorophyll-protein complex; Se, serosa.
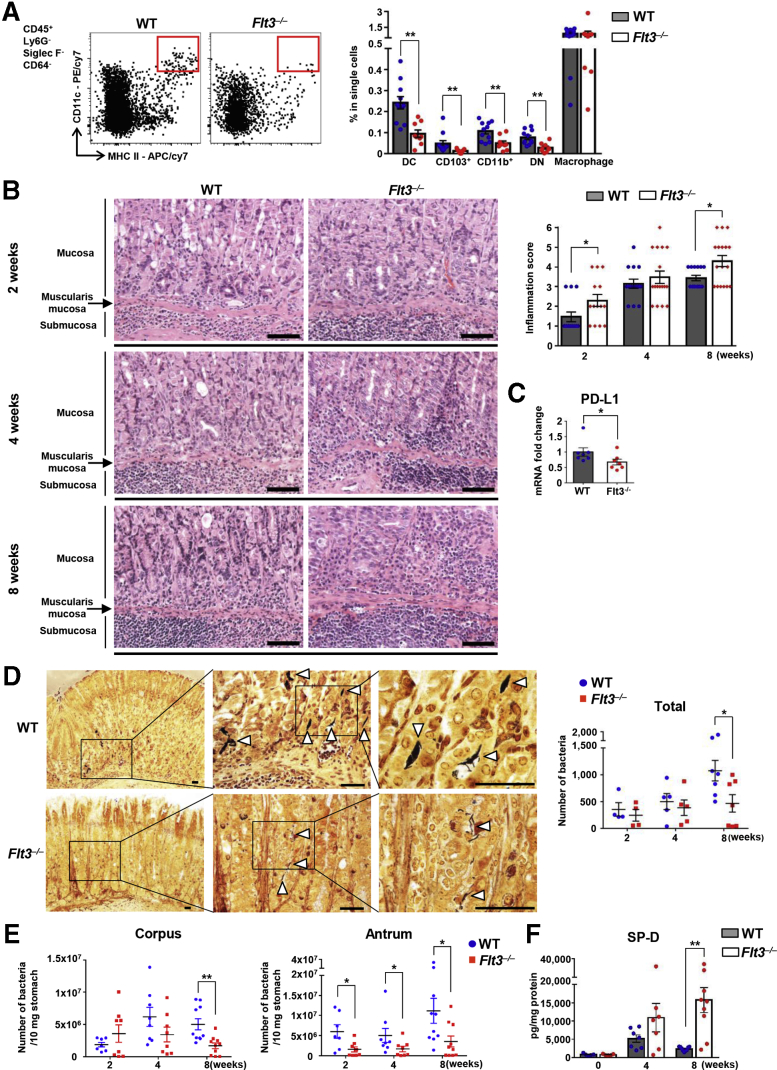
Flt3–/– Mice Showed Decreased Number of Gastric DCs and Developed Severe Gastric Inflammation With Less Helicobacter Colonization
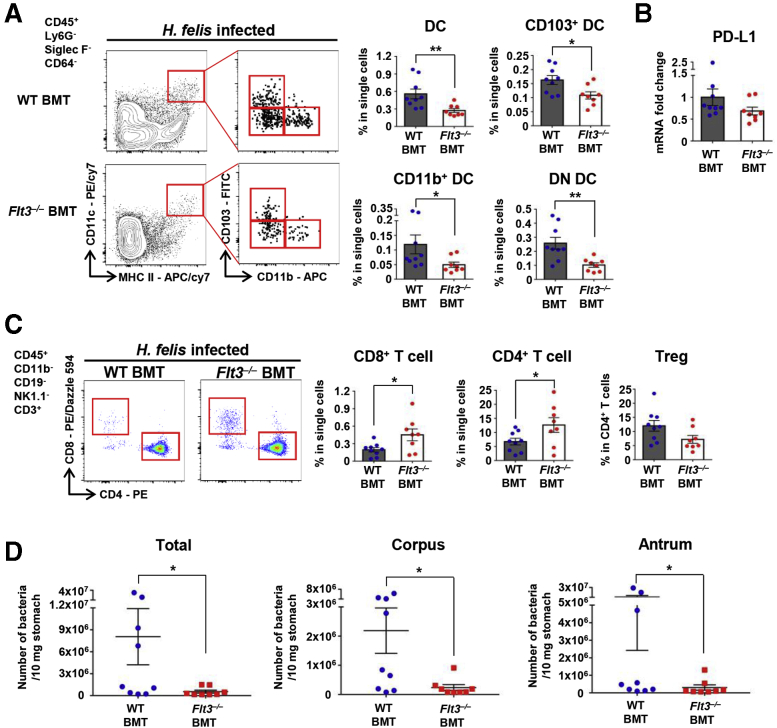
To determine the role of DCs in Helicobacter-induced gastritis, we investigated whether the immune response differed between H felis–infected fms-like tyrosine kinase 3 (Flt3) knockout (Flt3–/–) and WT mice. The receptor tyrosine kinase Flt3 is an important regulator of DC homeostasis at the periphery, and cDCs and plasmacytoid DCs (pDCs) are depleted in the peripheral lymphoid and nonlymphoid tissues by Flt3 deficiency.28, 29, 30 We confirmed that the number of total DCs and DC subsets, including CD103+, CD11b+, and DN DCs, but not macrophages, was markedly lower in the stomach of Flt3–/– mice than in WT mice (Figure 7A). Next, we compared the severity of gastritis between the 2 groups at 2, 4, and 8 weeks after H felis infection. Histologically, inflammation occurred mainly in the corpus. In WT mice, neutrophil infiltration into the submucosa was observed mainly after 2 weeks of infection, and lymphocyte and plasma cell infiltration was noted at 4 weeks, and most prominently at 8 weeks after infection. These inflammatory cells mostly infiltrated the submucosa, and as the severity of gastritis increased, infiltrated the mucosal lamina propria. In addition, lymphoid follicles occasionally were present in the submucosa or mucosa. In contrast, in Flt3–/– mice, infiltration of not only neutrophils but also many lymphocytes and plasma cells was noted at 2 weeks and continued to increase gradually until 8 weeks. Infiltration of inflammatory cells was observed mainly in the submucosa, but often diffusely expanded into the entire mucosal lamina propria, showing that Flt3–/– mice had more severe gastric inflammation compared with WT controls (Figure 7B). PD-L1 messenger RNA (mRNA) expression in gastric tissue was decreased significantly in Flt3–/– mice than in WT mice during H felis infection (Figure 7C).
Figure 7.
Flt3–/–mice with a decreased number of gastric DCs developed severe gastric inflammation with less Helicobacter colonization. (A) Percentage of DCs, DC subsets, and macrophages in live single cells from the stomach of normal Flt3–/– and WT mice. Representative flow cytometry plots (left). Gastric DCs and all DC subsets, but not macrophages, are deficient in Flt3–/– mice (n = 9) compared with WT mice (n = 11). (B) Histopathologic analysis of H felis–induced gastritis. Representative H&E images of the gastric corpus regions of WT and Flt3–/– mice were aligned (left). Inflammation score (right). Each mouse was scored (n = 13–17 for each group). Scale bar: 100 μm. (C) Relative PD-L1 mRNA expression levels in gastric corpus tissues from Flt3–/– and WT mice after H felis infection for 8 weeks. Results are shown as fold change compared with that in the H felis–infected WT group (n = 6–7 for each group). (D) Localization of H felis (white arrowheads) in the gastric corpus regions of WT and Flt3–/– mice (Warthin–Starry stain) and a manual count of H felis in 2 paraffin sections (n = 4–7 for each group). Scale bar: 50 μm. (E) H felis loads were evaluated using quantitative real-time PCR for H felis flagellar filament B DNA extracted from the corpus and antrum, respectively (n = 7–10 for each group). (F) Level of SP-D in gastric corpus tissues from Flt3–/– and WT mice using a magnetic luminex immunoassay (R&D Systems, Minneapolis, MN). Measured protein concentrations (pg/mL) were corrected for total protein content (mg/mL) (n = 7 and 9 for groups with 4 and 8 weeks of H felis infection, respectively; n = 5 for noninfection group). Data are presented as means ± SEM. ∗P < .05 and ∗∗P < .01. Weeks, duration of infection. APC, allophycocyanin.
H felis was mainly present in the lumen of the gastric pits and mucus layer throughout the glandular region (Figure 7D). The extent of H felis colonization in WT mice tended to increase gradually during 8 weeks of infection, although not in Flt3–/– mice. In particular, at 8 weeks after H felis infection, the extent of H felis colonization was lowest in the mucosa of Flt3–/– mice. Overall, the bacterial load was lower in Flt3–/– mice than in WT mice, and this difference was most noticeable after 8 weeks of infection (Figure 7D and E). Helicobacter colonization can be affected by various microenvironmental factors in gastric tissue. Previous studies have shown a positive correlation between the degree of gastritis and clearance of Helicobacter.19,31 In addition, surfactant protein-D (SP-D) has been reported to play a crucial role in the elimination of Helicobacter from the luminal mucus layer.32, 33, 34 In this study, the level of SP-D was increased during H felis infection and was much higher in H felis–infected Flt3–/– mice than in H felis–infected WT mice (Figure 7F). Therefore, the increased gastric inflammation and SP-D accumulation may be associated with high clearance of H felis in Flt3–/– mice.
Flt3–/– Mice Have Enhanced Mucosal Metaplasia Upon Helicobacter Infection
Because H felis–infected Flt3–/– mice showed profound inflammation in gastric mucosa, we further characterized precancerous changes including loss of parietal cells, mucous neck cell hyperplasia, and SPEM (Figure 8). Trefoil factor 2 (TFF2) is a marker of mucous neck cells and SPEM,35 and Clusterin (CLU) expression increases as SPEM progresses.36,37 In our results, as the duration of H felis infection progressed, parietal cell loss worsened, and Alcian blue–positive cells were seen to replace the lost parietal cells. The pattern of TFF2 and CLU expression was relatively similar to that of Alcian blue staining. TFF2 and CLU expression was observed in a few cells in the isthmus regions of the gastric mucosa in normal WT mice, whereas in H felis–infected mice, it was observed mainly at the base regions of the mucosa or throughout the mucosa. In particular, the areas of Alcian blue–, TFF2-, and CLU-positive mucosa in H felis–infected Flt3–/– mice were more prominent than in H felis–infected WT mice (Figure 8). Some of these metaplastic mucosal cells showed proliferative behavior according to Ki67 staining, and the number of Ki67-positive cells in the base regions of the mucosa was higher in H felis–infected Flt3–/– mice than in H felis–infected WT mice (Figure 8B and C).
Figure 8.
Flt3–/–mice have enhanced mucosal metaplasia upon Helicobacter infection. (A–C) Histopathologic analysis of H felis–induced metaplasia of the corpus mucosa. (A) Analysis of H felis–induced oxyntic atrophy and mucosal metaplasia. Representative serial images of H felis–infected WT (left) and H felis–infected Flt3–/– (right) mice for Alcian blue staining (top) and H+/K+-ATPase IHC (bottom) were aligned (2, 4, and 8 weeks after H felis infection). Scale bar: 50 μm. (B) Analysis of H felis–induced mucosal metaplasia and proliferation. Representative serial images of normal WT (top), H felis WT (middle) and H felis–infected Flt3–/– (bottom) mice after immunostaining for TFF2 (green), CLU (red), and Ki67 (3,3'-diaminobenzidine substrate) were aligned (8 weeks after H felis infection). Scale bar: 100 μm. (C) Quantification of H+/K+-ATPase+ cells (parietal cells) (n = 10 for each group), Alcian blue–positive region (n = 10 for each group), TFF2 and CLU double-positive region (WT, n = 9; Flt3–/–, n = 9), and Ki67-positive cells (WT, n = 9; Flt3–/–, n = 8) is shown. Data are presented as means ± SEM. ∗P < .05 and ∗∗P < .01. Weeks, duration of infection.
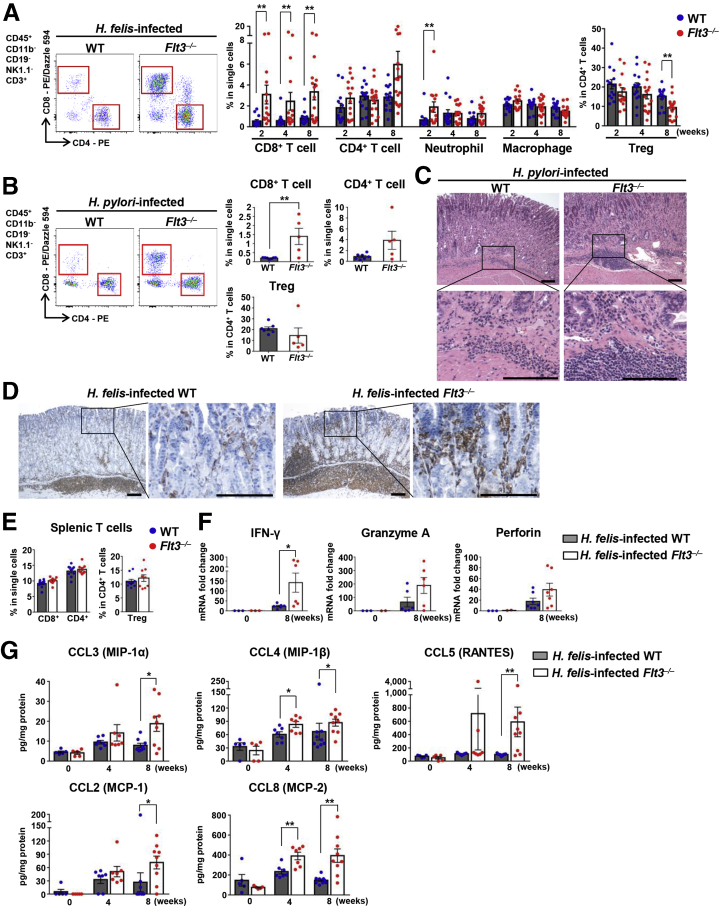
Flt3–/– Mice Showed More Prominent CD8+ T-Cell Accumulation Upon Helicobacter Infection
Among the various leukocyte populations that infiltrated the H felis–infected stomach, T-cell infiltration was the most prominent difference between WT and Flt3–/– mice. In Flt3–/– mice, infiltration of CD8+ T cells was dramatically higher than in WT mice, and infiltration of CD4+ T cells also tended to be greater in Flt3–/– mice than in WT mice when the H felis infection persisted for 8 weeks. In addition, in Flt3–/– mice compared with WT mice, neutrophil infiltration was more pronounced 2 weeks after H felis infection, and also tended to be more pronounced 8 weeks after H felis infection. However, no difference in the extent of macrophage infiltration was noted between the 2 groups. On the other hand, the percentage of Tregs in CD4+ T cells was reduced significantly in Flt3–/– mice compared with that in WT mice 8 weeks after H felis infection (Figure 9A). These phenotypes were recapitulated in the H pylori–infected Flt3–/– mice (Figure 9B). Furthermore, in H pylori infection, gastritis tended to be more severe in Flt3–/– mice than in WT mice. According to the histopathologic grading criteria, inflammation scores were 2 ± 1 in Flt3–/– mice and 1 or 2 in WT mice (Figure 9C). T-cell infiltration was more prominent not only in the submucosa but also in the mucosal lamina propria in H felis–infected Flt3–/– mice than in H felis–infected WT mice (Figure 9D). However, the number of splenic T cells was not different between the 2 groups (Figure 9E). H felis infection increased the mRNA expression of various factors related to inflammation. Expression of IFN-γ was approximately 6-fold higher in H felis–infected Flt3–/– mice than in H felis–infected WT mice. In addition, granzyme A and perforin were approximately 2.9- and 2.2-fold higher in Flt3–/– mice than in WT mice, respectively (Figure 9F). Levels of various chemokines, including CCL2 (monocyte chemoattractant protein-1), CCL3 (macrophage inflammatory protein-1α), CCL4 (macrophage inflammatory protein-1β), CCL5 (regulated on activation, normal T cell expressed and secreted), and CCL8 (monocyte chemoattractant protein-2), were higher in the gastric tissue of H felis–infected Flt3–/– mice than in H felis–infected WT mice. The difference between the 2 groups was clearly observed after 8 weeks of H felis infection (Figure 9G). These chemokines can contribute to T-cell migration into the peripheral site of infection,38,39 supporting enhanced CD8+ T-cell accumulation in gastric tissues in DC ablated mice. To confirm that the enhanced gastritis in Flt3–/– mice was driven by hematopoietic FLT3 deficiency, lethally irradiated WT mice were transplanted with Flt3–/– BM or WT BM as control. The H felis–infected recipient mice were analyzed 8 weeks after infection. Flow cytometry analysis of the gastric tissues showed that all subsets of gastric DCs were decreased significantly in Flt3–/– BMT mice compared with those in WT BMT mice (Figure 10A). Although the difference was not statistically significant, PD-L1 mRNA expression in gastric tissue tended to decrease in Flt3–/– BMT mice compared with that in WT BMT mice during H felis infection (Figure 10B) (P = .0927). CD8+ and CD4+ T-cell accumulation was more prominent in Flt3–/– BMT mice than that in WT BMT mice. The percentage of Tregs in CD4+ T cells tended to be lower in Flt3–/– BMT mice than that in WT BMT mice (Figure 10C). The bacterial load in the stomach was lower in Flt3–/– BMT mice than that in WT BMT mice (Figure 10D).
Figure 9.
Flt3–/–mice show more prominent CD8+T-cell accumulation upon Helicobacter infection. (A) Percentage of CD8+ T cells, CD4+ T cells, neutrophils, and macrophages in live single cells and of Tregs in live CD4+ T cells from the stomach of Flt3–/– and WT mice with H felis infection (n = 13–17 for each H felis infection group, n = 9–11 for the noninfection group). Representative flow cytometry plots of T cells (left). (B) As in panel A, but with H pylori infection (WT, n = 7; Flt3–/–, n = 5). Note that the number of CD8+ T cells is higher in Flt3–/– mice. (C) Representative H&E images of the gastric corpus regions of H pylori–infected WT (left) and H pylori–infected Flt3–/– (right) mice were aligned. Scale bar: 200 μm. (D) More severe mucosal infiltration of T cells in the stomach of H felis–infected Flt3–/– mice. Representative images of CD3 IHC (3,3'-diaminobenzidine substrate). Scale bar: 200 μm. (E) The T-cell fraction in the spleens of WT and Flt3–/– mice 8 weeks after H felis infection. Note that DC depletion did not affect CD8+ T cells, CD4+ T cells, or the Treg population in the spleen (WT, n = 10; Flt3–/–, n = 9). (F) Relative IFN-γ, Granzyme A, and Perforin mRNA expression levels in gastric corpus tissues from Flt3–/– and WT mice after H felis infection for 8 weeks. Results are shown as fold change compared with the noninfected WT group (n = 6–7 for the H felis infection group, n = 2–3 for the noninfection group). (G) Chemokine levels in gastric corpus tissues from Flt3–/– and WT mice using a magnetic Luminex immunoassay. Measured protein concentrations (pg/mL) were corrected for total protein content (mg/mL) (n = 7 and 9 for groups with 4 and 8 weeks of H felis infection, respectively; n = 5 for noninfection group). Data are presented as means ± SEM. ∗P < .05 and ∗∗P < .01. Weeks, duration of infection.
Figure 10.
Flt3–/–BMT mice with a decreased number of gastric DCs developed prominent T-cell responses with less Helicobacter colonization after H felis infection for 8 weeks. (A) The percentage of DCs and DC subsets in live single cells from the stomach of Flt3–/– BMT (n = 8) and WT BMT (n = 9) mice. Representative flow cytometry plots (left panel). (B) Relative PD-L1 mRNA expression levels in gastric corpus tissues from Flt3–/– BMT (n = 8) and WT BMT (n = 9) mice 8 weeks after H felis infection. Results are shown as fold change compared with that in the H felis–infected WT BMT group. (C) Percentage of CD8+ T cells and CD4+ T cells in live single cells and of Tregs in live CD4+ T cells from the stomach of Flt3–/– BMT (n = 8) and WT BMT (n = 9) mice. Representative flow cytometry plots (left panel). (D) H felis loads were evaluated using quantitative real-time PCR for H felis flagellar filament B DNA extracted from the corpus and antrum, respectively (WT BMT, n = 9; Flt3–/– BMT, n = 8). Data are presented as means ± SEM. ∗P < .05 and ∗∗P < .01. APC, allophycocyanin; PE, phycoerythrin.
Depletion of cDCs Enhanced Helicobacter-Induced Gastric Inflammation
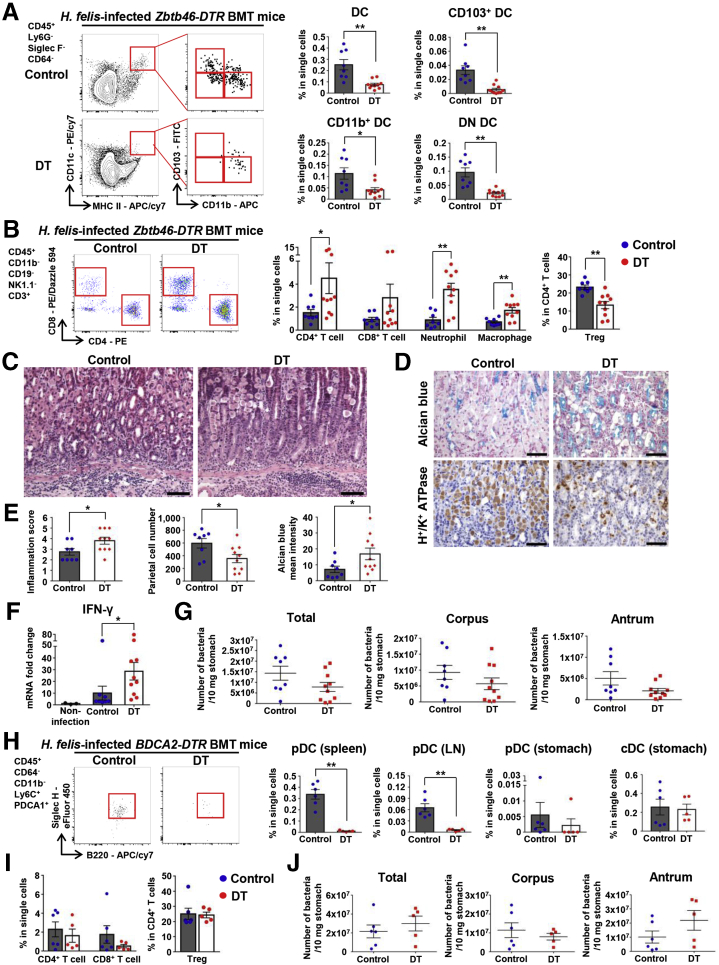
Because Flt3–/– mice lack pDCs as well as cDCs, we could not rule out the involvement of pDCs in the suppression of T-cell response to H felis. Therefore, we attempted to dissect the function of Flt3-dependent cDCs and pDCs in Helicobacter infection using Zbtb46-diphtheria toxin receptor (DTR) and BDCA2-DTR mice. Lethally irradiated mice were transplanted with BM from Zbtb46-DTR or BDCA2-DTR mice. We analyzed gastric lesions 4 weeks after H felis infection. Gastric DCs were effectively ablated by diphtheria toxin (DT) injection in Zbtb46-DTR BMT mice (Figure 11A). Both CD4+ T-cell and CD8+ T-cell accumulation was increased markedly in cDC-ablated mice. In addition, infiltration of neutrophils and macrophages also was increased significantly in cDC-ablated mice. However, the percentage of Tregs in CD4+ T cells was reduced significantly by cDC depletion (Figure 11B). Histologically, cDC ablation resulted in more severe inflammation and pathologic mucosal changes (Figure 11C–E). Expression of IFN-γ mRNA in gastric tissues was increased markedly in cDC-ablated mice (Figure 11F). Although there was no statistical significance, the bacterial loads in the stomach tended to decrease in cDC-ablated mice compared with those in control mice (Figure 11G).
Figure 11.
Depletion of cDCs enhances Helicobacter-induced gastric inflammation. (A) Percentage of DCs and DC subsets in live single cells from the stomach of DT-injected Zbtb46-DTR BMT mice (cDC-ablated mice; n = 10) and PBS-injected Zbtb46-DTR BMT mice (control mice; n = 8) 4 weeks after H felis infection. Representative flow cytometry plots (left). Note that gastric DCs and DC subsets are Zbtb46-dependent cDCs. (B) Percentage of CD8+ T cells, CD4+ T cells, neutrophils, and macrophages in live single cells and of Tregs in live CD4+ T cells from the stomach of cDC-ablated (n = 10)and control (n = 8) mice. Representative flow cytometry plots of T cells (left). (C) Histopathologic analysis of H felis–induced gastritis. Representative H&E images of the gastric corpus regions of control (left) and cDC-ablated (right) mice. Scale bar: 100 μm. (D) Histopathologic analysis of H felis–induced oxyntic atrophy and mucosal metaplasia. Representative serial images of the gastric corpus regions of control (left) and cDC-ablated (right) mice after Alcian blue staining (top) and H+/K+-ATPase IHC (bottom). Scale bar: 50 μm. (E) Inflammation score (left) and quantification of H+/K+-ATPase+ cells (parietal cells) (middle), and Alcian blue–positive region (right) (control mice, n = 8; cDC-ablated mice, n = 10). (F) Relative gastric mRNA expression levels of IFN-γ. Results are shown as fold change compared with the noninfection group (noninfection group, n = 3; control group, n = 8; cDC-ablated group, n = 10). (G) bacterial loads were evaluated using quantitative real-time PCR for H felis flagellar filament B DNA extracted from the corpus and antrum, respectively (control mice, n = 8; cDC-ablated mice, n = 10). (H) Percentage of pDCs (spleen, gastric lymph node [LN], and stomach) and cDCs (stomach) in live single cells of DT-injected BDCA2-DTR BMT mice (pDC-ablated mice; n = 5) and PBS-injected BDCA2-DTR BMT mice (control; n = 6). Representative pDCs flow cytometry plot of 4 pooled stomachs per group (left). (I) Percentage of CD8+ T cells and CD4+ T cells in live single cells and of Tregs in live CD4+ T cells from the stomach of pDC-ablated mice (n = 5) and control mice (n = 6). (J) Bacterial loads were evaluated using quantitative real-time PCR for H felis flagellar filament B DNA extracted from the corpus and antrum, respectively (control mice, n = 6; pDC-ablated mice, n = 5). Data are presented as means ± SEM. ∗P < .05 and ∗∗P < .01. Weeks, duration of infection. PE, phycoerythrin.
Gastric pDCs were identified as a CD64-CD11b-Ly6ChiPDCA1+B220+Siglec H+ population (Figure 1C). DT injection into BDCA2-DTR BMT mice effectively eliminated pDCs, but did not affect cDCs (Figure 11H). However, no differences in T-cell accumulation in the stomach were noted between pDC-ablated mice and control mice during H felis infection (Figure 11I). The bacterial loads in the stomach were not changed in pDC-ablated mice (Figure 11J). Taken together, these results indicate that gastric cDCs, but not pDCs, control gastric T-cell populations during Helicobacter infection.
PD-L1–Expressing DCs Co-localized With T Cells in Human Helicobacter-Positive Gastritis
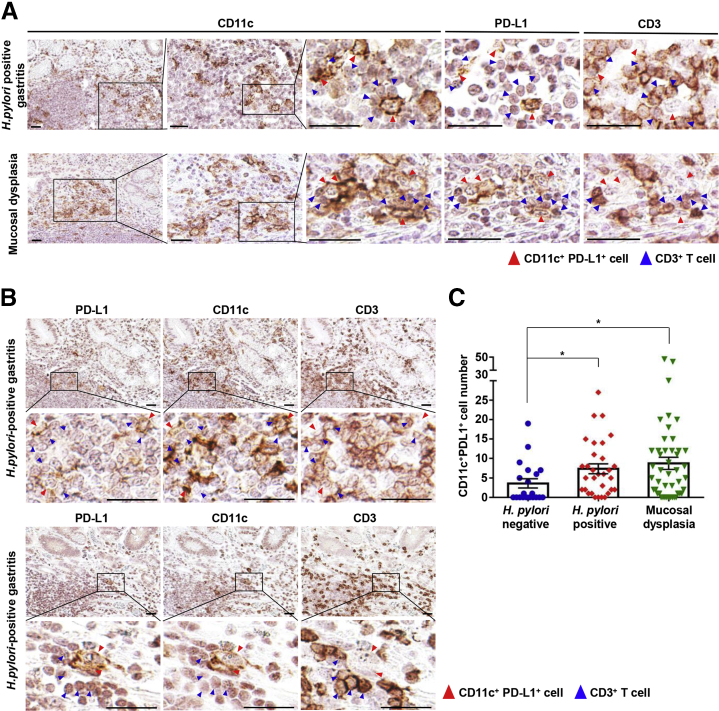
To determine whether PD-L1–expressing DCs co-localize with T cells in the gastric tissue of H pylori–infected patients, we performed multiplexed IHC for CD11c, PD-L1, and CD3. CD11c+ cells were distributed in the mucosal lamina propria and submucosa, and their numbers were significantly higher in the stomach of H pylori–positive patients and patients with mucosal dysplasia than in H pylori–negative patients. PD-L1 expression was found mostly in infiltrated cells, and not in epithelial cells. Importantly, the CD11c+PD-L1+ cells were in contact with CD3+ cells in the gastric mucosa (Figure 12A and B). There were more CD11c+PD-L1+ cells in the gastric mucosa of H pylori–positive patients than in H pylori–negative patients, and the gastric tissue showing mucosal dysplasia had more CD11c+PD-L1+ cells (Figure 12C).
Figure 12.
PD-L1–expressing DCs co-localize with T cells in human Helicobacter-positive gastritis. (A) Multiplexed IHC-stained representative images of the stomach of H pylori–positive patients (top) and patients with mucosal dysplasia (bottom). Multiplexed IHC was performed sequentially on each single section in the order of PD-L1, CD11c, and CD3. After each immunostained section was scanned, the section was placed in antibody stripping buffer to erase the staining and subjected to another immunostaining. Note that PD-L1+CD11c+ cells (red arrowheads) co-localize with CD3+ cells (blue arrowheads). Scale bar: 30 μm. (B) Multiplexed IHC stained representative images of the stomach of other H pylori–positive patients. Note that the positions of PD-L1+ and CD11c+ cells are similar (low-power fields) and PD-L1+CD11c+ cells (red arrowheads) co-localize with CD3+ cells (blue arrowheads) (high-power fields). Scale bar: 30 μm. (C) Quantification of PD-L1+CD11c+ cells in a section of a biopsy sample (H pylori–negative sample, n = 21; H pylori–positive sample, n = 31; mucosal dysplasia sample, n = 46). Data are presented as means ± SEM. ∗P < .05.
Discussion
In previous in vitro studies, DCs were shown to recognize H pylori via pattern recognition receptors such as Toll-like receptors,40 and H pylori–stimulated DCs secrete proinflammatory cytokines such as interleukin (IL)6, IL12, and IL23, and activate co-cultured autologous CD4+ T cells to secrete IFN-γ or IL17.41,42 In contrast to these proinflammatory roles, H pylori–stimulated DCs are involved in immune tolerance by inducing Tregs or producing anti-inflammatory cytokines such as IL10 and transforming growth factor β.14,43 CD11c-DTR mice have been used to understand the in vivo function of gastric DCs in H pylori infection.44,45 However, because DT injection also ablates tissue macrophages in CD11c-DTR mice,46 DC-specific cell ablation models are required to understand gastric DCs. Meanwhile, 1 study showed that gastric CD11b+ DCs engulf H pylori in the gastric mucosa.47 However, the exact immunologic role of gastric DCs in Helicobacter-induced gastritis remains largely unknown. In the present study, we showed that the PD-1/PD-L1 pathway plays a critical role in maintaining peripheral T-cell tolerance during Helicobacter infection, which appears to be mediated by PD-L1–expressing gastric DCs.
Compared with DCs in other organs, relatively little is known about gastric DCs; thus, we focused on characterizing DCs in the stomach. We defined gastric DCs as a Ly6G-Siglec F-CD64-CD11c+MHC IIhi population and determined that these DCs are composed of CD103+, CD11b+, and DN subsets. Because macrophages and eosinophils also can express CD11c and MHC II to varying levels and might be contained in the CD11b+ subset, we excluded macrophages and eosinophils in advance using CD64, Siglec F, and CD11b markers.48 The DCs and their subset populations adequately expressed the transcription factors ZBTB46, IRF8, and IRF4, as well as the DC subset surface markers SIRPα, CD24, and XCR1,25,49, 50, 51, 52, 53 and were dependent on the Flt3–Flt3-ligand pathway. Moreover, these populations were ablated by DT injection in Zbtb46-DTR BMT mice. From these results, we could confirm that the gastric Ly6G-Siglec F-CD64-CD11c+MHC IIhi population and its subsets are cDCs.
Although expression of the mature DC markers CD103 and CD11b was deficient in the DN subsets, they still can be regarded as bona fide DCs considering their similarity to mature DCs. Recent studies have shown that DN subsets are DC populations in an intermediate maturation stage, and reside in various organs, including the heart, lung, and liver.26 In the present study, gastric DN subsets showed a 2-peak pattern for the expression of IRF4, IRF8, SIRPα, CD24, and XCR1. These results seem to be consistent with those from another study showing that DN subsets consist of 2 subtypes with the ability to mature into CD103+ and CD11b+ DCs.26 Thus, our study suggests that bona fide DN DCs are present in the stomach. Conversely, a few CD103+CD11b+ cells were found in the gastric DC population. One study considered CD103+CD11b+ cells as one of the gastric DC subsets.47 However, in our study, CD103+CD11b+ cells were not significantly depleted in Flt3–/– and DT-treated Zbtb46-DTR BMT mice. Although the CD103+CD11b+ DC subset has been identified in the intestine, further studies are needed to determine whether this population is present in the stomach.
Interestingly, our study highlighted the fact that gastric cDCs are committed to the role of peripheral T-cell tolerance in the stomach during Helicobacter infection. One of the immune modulatory mechanisms of DCs is to activate the immune checkpoint pathway of T cells. In our study, during Helicobacter infection, gastric DCs were tolerogenic by expressing high levels of PD-L1 and low levels of CD80, CD86, and CD40. It should be noted here that not only the high level of PD-L1 was expressed on DCs, but also the low level of CD80 was expressed on DCs. CD80 of DCs binds cytotoxic T-lymphocyte–associated antigen 4, another co-inhibitory receptor expressed on T cells, or CD28, a costimulatory receptor expressed on T cells, but also binds PD-L1 expressed on DCs (cis-PD-L1/CD80 interaction) to disrupt PD-1/PD-L1 binding.54 Therefore, it is believed that the relative difference in the expression levels of PD-L1 and CD80 on gastric DCs triggered activation of the immune checkpoint pathway.
Furthermore, in Helicobacter infection, when cDCs were ablated, a phenotype similar to that noted in the depletion of PD-L1 on leukocytes was observed that included worsened gastritis and pathologic mucosal changes as well as an increase in the number of T cells with low bacterial loads. These findings indicate that impairment of cDCs is related to reduced PD-L1 levels in the stomach, which in turn causes a loss of T-cell regulation.
Gastric DCs may inhibit T-cell–mediated immunity through PD-L1 expression by directly regulating the number or activity of T cells or by promoting the differentiation of induced Tregs from conventional CD4+ T cells in the periphery.55,56 In particular, the in vivo experimental results of our study, such as changes in the Treg population and suboptimal costimulatory state of gastric DCs, support the role of gastric cDCs in promoting Treg development.
Some studies showed that the gastric epithelium expresses PD-L1. These studies used gastric epithelial cell lines20 or gastric organoid cultures differentiated from human-induced pluripotent stem cells,23 and thus may not have addressed whether PD-L1 is expressed by leukocytes. In the current study, we observed PD-L1 expression on DCs with flow cytometry of isolated single cells from the whole stomach and IHC on tissue sections of the stomach. With these methods, we identified differences in PD-L1 expression between leukocytes and nonleukocytes, including gastric epithelial cells, and concluded that gastric DCs express considerably higher levels of PD-L1 than gastric epithelial cells.
As mentioned previously, PD-L1+ gastric DCs were located primarily in the submucosa and mucosal laminar propria immediately above the muscularis mucosa with T cells. These DCs are considered a cellular barrier, protecting the gastric mucosa from T-cell infiltration. Without these barrier-functioning DCs, T cells indiscriminately could infiltrate the gastric mucosa and increase the immune response against Helicobacter infection by promoting the mobilization of other immune cells such as neutrophils,57 thereby decreasing bacterial loads but also inadvertently causing damage to the mucosa. During Helicobacter infection, mucosal damage is accompanied by the loss of parietal cells and mature chief cells (atrophy), SPEM, and further intestinal metaplasia as the lesion becomes more severe.58 Mucosal metaplasia has been regarded as a precancerous lesion because it is closely related to the incidence of gastric cancer in human beings.59 Interestingly, when DCs are absent, worsened oxyntic atrophy and mucosal metaplasia are accompanied by an increase in T-cell accumulation in the gastric mucosa. Gastric DCs may be involved in defense mechanisms against carcinogenesis by slowing the progression of gastritis into gastric cancer. However, considering that a recent study reported an association between immune stromal PD-L1 expression and poor prognosis in patients with gastric adenocarcinoma,10 and the fact that our results showed co-localization of PD-L1+ gastric DCs with T cells in some patients with mucosal dysplasia, suppression of T cells by gastric DCs via PD-L1 could have either a deleterious or a beneficial effect on the host, depending on the pathologic progression of the gastric lesions.
Along with other immune checkpoint inhibitors, PD-1 and PD-L1 inhibitors have been highlighted as a novel anticancer therapy. Some of them have received Food and Drug Administration approval and have proven effective, thereby increasing expectations. However, these inhibitors are reported to have inflammatory side effects called immune-related adverse events (IRAEs), and the stomach is one of the most IRAE-prone organs.60, 61, 62 Thus, the possibility of IRAE occurrence in the stomach should be an important consideration when treating cancer patients with checkpoint inhibitors. The inflammatory events shown in the current study might provide the basis to elucidate mechanisms underlying IRAE occurrence in the stomach.
In conclusion, we finely characterized gastric DCs in gastritis caused by Helicobacter infection. Moreover, the immune modulatory function of gastric cDCs possibly by the PD-1/PD-L1 pathway importantly protects the gastric mucosa against lymphocytic inflammation and precancerous mucosal changes, but allows persistent bacterial colonization. Our findings show that gastric cDCs are key immune cells that fine-tune inflammatory processes and bacterial colonization in Helicobacter-infected gastric mucosa.
Materials and Methods
Human Gastric Tissue Samples
Formalin-fixed paraffin-embedded tissue samples (20 H pylori–negative patients, 31 H pylori–positive patients, and 46 patients with mucosal dysplasia) from surgically resected human gastric tissue were retrieved from the archives of the Department of Pathology, Seoul National University Bundang Hospital (Seongnam, Korea). The collected tissue samples were the samples remaining after proper histologic diagnosis and relevant molecular study. Representative tissue areas were reviewed and selected for the construction of a tissue microarray. The study was approved by the Institutional Review Board of the Seoul National University Bundang Hospital (Institutional Review Board number: B-1606/349-308) and the Seoul National University Hospital (Institutional Review Board number: 1706-105-860) and performed in accordance with the principles of the Declaration of Helsinki. Informed consent was waived.
Mice
Flt3–/– mice63 were a generous gift from Professor Ihor R. Lemischka (Developmental and Stem Cell Biology, Mount Sinai School of Medicine, New York, NY). Flt3–/–, B6(Cg)-Zbtb46tm1(HBEGF)Mnz/J (Zbtb46-DTR, stock 019506; The Jackson Laboratory, Bar Harbor, ME), C57BL/6-Tg(CLEC4C-HBEGF)956Cln/J (BDCA2-DTR, stock 014176; The Jackson Laboratory), and B6.Cg-Tg(Itgax-Venus)1Mnz/J (CD11c-Enhanced Yellow Fluorescent Protein (EYFP), stock 008829; The Jackson Laboratory) mice on a C57BL/6J background were housed individually in the animal facility of Seoul National University or Hanyang University and provided a normal mouse diet and water ad libitum. Male C57BL/6J mice were purchased from Japan SLC, Inc (Hamamatsu, Shizuoka, Japan) through the Central Lab Animal, Inc (Seoul, Korea). All mice experiments were approved by the Animal Care and Use Committees of Seoul National University or Hanyang University (certification numbers: SNU-140320-2-9 and HY-IACUC-18-0048).
BM Transplantation
BM cells from Flt3–/–, Zbtb46-DTR, BDCA2-DTR, or Pdl1–/– mice64 (housed in the animal facility of Yonsei University) were injected intravenously into lethally irradiated (500 rad twice, 3-hour interval) WT C57BL/6J mice. BMT mice were fed water containing 7.6% Baytril (Bayer Korea, Seoul, Korea) for 2 weeks and monitored for approximately 6 weeks, until the transplanted BM was effectively reconstructed.
DT Injection
cDCs and pDCs were depleted via intraperitoneal administration of DT (322326; Calbiochem, San Diego, CA) to Zbtb46-DTR BMT mice and BDCA2-DTR BMT mice, respectively. A total of 500 ng DT was injected 2 days before H felis infection. During the infection period, Zbtb46-DTR BMT mice were treated with 100 ng DT every 2 days, and BDCA2-DTR BMT mice were treated with 200 ng DT every 3 days. The control group was treated with phosphate-buffered saline instead of DT, following the same schedule.
In Vivo PD-L1 Blockade
Anti-mouse PD-L1 (B7-H1) antibody (BE0101; Bio X Cell, West Lebanon, NH) was administered intraperitoneally to H felis–infected mice to block the PD-1/PD-L1 pathway during H felis infection. A total of 300 μg PD-L1 antibody diluted in InVivoPure pH 6.5 Dilution Buffer (IP0065; Bio X Cell) was administered 6 times, once every 3 days, before necropsy. A rat IgG2b isotype antibody (BE0090; Bio X Cell) diluted in InVivoPure pH 7.0 Dilution Buffer (IP0070; Bio X Cell) was used as a control.
Helicobacter Culture and Infection
H felis (ATCC 49179) was cultured in sterile-filtered brucella broth (211088; Becton Dickinson, Sparks, MD) containing 10% fetal bovine serum (FBS). Mouse-adapted H pylori Sydney strain 165 was cultured on the Brain Heart Infusion Agar plates (241830; Becton Dickinson) containing 10% sheep blood and H pylori Selective Supplement (SR0147E; Thermo Fisher Scientific, Rockford, IL). The brucella broth and brain heart infusion agar plates were maintained under microaerobic conditions produced using a GasPak EZ Campy Container System (260680; Becton Dickinson) at 37°C. The brucella broth was maintained at 150 rpm shaking. After 24-hour fasting, 8-week-old mice were orally administered 0.2 mL suspension containing 2 × 108 H felis or 1 × 109 colony-forming unit/mL H pylori 4 times every other day.
Necropsy and Tissue Preparation
At 2, 4, and 8 weeks after H felis infection, each group of mice was killed together with the noninfected control group, and the stomach, gastric lymph node, and spleen were removed. The stomach was incised along the greater curvature and spread onto filter paper. After cutting the lesser curvature, half of the stomach was used for flow cytometry and H felis quantification. The lesser curvature portion of the other half was fixed in 10% neutral buffered formalin for histopathologic examination, and the remaining tissue was stored at -70°C for subsequent analysis. At 18 months after H pylori infection, the H pylori–infected groups were treated as described previously.
Histopathologic Examinations and Quantitation
Gastric tissues fixed in 10% neutral buffered formalin were cut into 2 strips. After standard tissue processing, gastric tissues were embedded in paraffin wax. Paraffin sections were stained with H&E or an Alcian blue (pH 2.5) stain kit (H-3501; Vector Laboratories, Burlingame, CA). IHC for H+/K+-ATPase, TFF2, CLU, and Ki67 was performed to assess histopathologic changes. The degree of gastritis and pathologic mucosal changes were assessed based on methods described previously.19,66,67 Briefly, we graded the lesion with a scale of 0–6. The definition of inflammation scores are as follows: 0, none; 1, some infiltrates; 2, mild (few aggregates in submucosa and mucosa); 3, moderate (several aggregates in submucosa and mucosa); 4, marked (many aggregates in submucosa and mucosa); 5, nearly the entire mucosa contains a dense infiltrate; and 6, entire mucosa contains a dense infiltrate. Scores for pathologic mucosal changes were as follows: 0, none; 1, foci in which a few gastric glands are lost or replaced; 2, small areas in which gastric glands are lost or replaced; 3, less than 25% of gastric glands are lost or replaced; 4, 25%–50% of gastric glands are lost or replaced; 5, more than 50% of gastric glands are lost or replaced; and 6, only a few small areas of gastric glands remaining. Quantitation of Alcian blue–positive mucin and H+/K+-ATPase–, Ki67-, TFF2-, and CLU-positive cells was performed at the representative same region within the corpus mucosa for each sample. The number of H+/K+-ATPase–positive cells was counted manually. The amount of Alcian blue–positive mucin was measured using mean intensity with ImageJ software (National Institutes of Health, Bethesda, MD). The number of Ki67-positive cells was counted using ImageJ software. TFF2 and CLU expression was quantified by measuring the area of each positive signal region using CaseViewer software (3d Histech, Budapest, Hungary). Slides were imaged digitally using a Pannoramic scanner (3d Histech).
Single-Cell Preparations and Flow Cytometric Analysis
Gastric tissues were incubated in Hank’s balanced salt solution containing 2 mmol/L EDTA (CMS005; Cosmo Genetech, Seoul, Korea), 2% FBS, and 1 mmol/L DL-dithiothreitol (D0632-1G; Sigma-Aldrich, St. Louis, MO) for 15 minutes at 37°C with shaking. Next, the gastric tissue was finely trimmed and ground using gentleMACS Dissociator (130-093-235; Miltenyi Biotec, Bergisch Gladbach, Germany) and incubated in Hank’s balanced salt solution containing 100 U/mL collagenase D (11088866001; Roche, Mannheim, Germany), 10% FBS, 10 mmol/L HEPES (SH30237.01; GE Healthcare Life Sciences, Logan, UT), and 90 U/mL DNase 1 (4716728001; Roche) for 30 minutes at 37°C with shaking. After incubation, the gastric tissue was ground once more using the gentleMACS Dissociator, filtered through a 70-μm cell strainer, and centrifuged to collect the single cells. The final single cells were prepared by combining the cells collected from the suspension and from the gastric tissue. Gastric lymph node and spleen were incubated with 400 U/mL collagenase D at 37°C for 20 minutes to obtain dissociated single cells. The prepared single cells were stained primarily with Zombie Aqua (423102; BioLegend, San Diego, CA) to discriminate dead cells, according to the manufacturer’s protocol. Zombie Aqua–stained cells then were processed for Fragment crystallizable (Fc) receptor block by using a TruStain FcX antibody (anti-CD16/32, clone 93, 101320; BioLegend), to avoid nonspecific binding. After blocking, cells were incubated with a mixture of fluorochrome-labeled antibodies at 4°C for 30 minutes. To stain for transcription factors (Forkhead box P3 [FOXP3], IRF4, IRF8, and ZBTB46), cells were fixed and permeabilized with a FOXP3/Transcription Factor Staining Buffer Set (00-5523-00; Thermo Fisher Scientific), according to the manufacturer’s protocol, and stained with an antibody against each transcription factor. Antibodies are listed in Table 1. After washing briefly with RPMI 1640 medium containing 10% FBS, cells were analyzed using BD LSRFortessa (BD Biosciences, San Jose, CA) and FlowJo software (version 9.9 and 10.4; developed by FlowJo, LLC, Ashland, OR).
Table 1.
List of Antibodies for Flow Cytometry
| Antibody | Clone | Catalog number | Source |
|---|---|---|---|
| CD103 | 2E7 | 11-1031-81 | eBioscience (San Diego, CA) |
| Foxp3 | FJK-16s | 17-5773-82 | eBioscience |
| IRF8 | V3GYWCH | 17-9852 | eBioscience |
| IRF4 | 3E4 | 48-9858-80 | eBioscience |
| CD40 | 1C10 | 17-0401-81 | eBioscience |
| Siglec-H | eBio440c | 48-0333-80 | eBioscience |
| Rat IgG1κ isotype | eBRG1 | 48-4301-80 | eBioscience |
| CD4 | GK1.5 | 553730 | BD Bioscience |
| Zbtb46 | U4-1374 | 565832 | BD Bioscience |
| PD-L2 | TY25 | 564245 | BD Bioscience |
| Siglec-F | E50-2440 | 562681 | BD Bioscience |
| CD24 | M1/69 | 563060 | BD Bioscience |
| CD11b | M1/70 | 101206, 101216, 101212, 101226 | BioLegend |
| CD64 | X54-5/7.1 | 139304, 139320 | BioLegend |
| MHC II | M5/114.15.2 | 107621, 107628 | BioLegend |
| Ly6C | HK1.4 | 128006 | BioLegend |
| CD45 | 30-F11 | 103130 | BioLegend |
| CD11c | N418 | 117318 | BioLegend |
| NK1.1 | PK136 | 108714 | BioLegend |
| CD8α | 53-6.7 | 100762 | BioLegend |
| F4/80 | BM8 | 123146, 123149 | BioLegend |
| PDCA-1 | 927 | 127015 | BioLegend |
| CD86 | GL-1 | 105012 | BioLegend |
| CD19 | 6D5 | 115527 | BioLegend |
| B220 | RA3-6B2 | 103223 | BioLegend |
| PD-L1 | 10F.9G2 | 124308, 124315 | BioLegend |
| CD3 | 17A2 | 100228 | BioLegend |
| CD80 | 16-10A1 | 104725 | BioLegend |
| Ly6G | 1A8 | 127607, 127639, 127641 | BioLegend |
| XCR1 | ZET | 148220 | BioLegend |
| Siglec-F | S17007L | 155505 | BioLegend |
| SIRPα | P84 | 144015 | BioLegend |
| Armenian hamster IgG Isotype | HTK888 | 400905, 400921 | BioLegend |
| Rat IgG2α, κ Isotype | RTK2758 | 400505, 400507, 400511, 400526, 400540, 400542, 400549, 400557 | BioLegend |
Immunohistochemistry and Immunofluorescence
Paraffin sections were dewaxed and hydrated, and then antigen retrieval was performed using pH 6.0 citrate buffer (C9999; Sigma-Aldrich). Next, when visualizing the signal with 3,3'-diaminobenzidine substrate, sections were incubated with 0.3% hydrogen peroxide solution for 30 minutes to block endogenous peroxidase activity. An ImmPRESS Peroxidase Polymer kit (Vector Laboratories) was used according to the manufacturer’s protocol. Briefly, paraffin sections were blocked with 2.5% normal horse or goat serum for 1 hour and then incubated with the primary antibodies at 4°C overnight or at 20°C for 2 hours. The primary antibodies are listed in Table 2. For chromogenic detection, sections were incubated with an appropriate horseradish-peroxidase–conjugated secondary antibody (Vector Laboratories) for 1 hour followed by ImmPACT 3,3'-diaminobenzidine substrate (SK-4105; Vector Laboratories) and counterstained with hematoxylin. For fluorescence detection, Alexa488-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA) and Cy3-conjugated donkey anti-goat IgG (Jackson ImmunoResearch) were used at a 1:400 dilution. Sections were counterstained with 4′,6-diamidino-2-phenylindole (62248; Thermo Fisher Scientific) and mounted using mounting medium (Vector Laboratories). For anti-H+/K+-ATPase IHC, a Mouse on Mouse ImmPRESS Peroxidase Polymer Kit (MP-2400; Vector Laboratories) was used to block endogenous mouse immunoglobulin.
Table 2.
List of Primary Antibodies for Immunohistochemistry and Immunofluorescence
| Antibody | Clone | Catalog no. | Source | Dilution |
|---|---|---|---|---|
| CD3 | Polyclonal | A0452 | Dako | 1:200 (paraffin) |
| CD3 | 17A2 | 100201 | BioLegend | 1:50 (frozen) |
| CD45 | 30-F11 | 103101 | BioLegend | 1:100 (frozen) |
| CD11c | D1V9Y | 97585 | Cell Signaling Technology | 1:400 (paraffin) |
| CD11c | HL3 | 550283 | BD Biosciences | 1:100 (frozen) |
| PD-1 | D7D5W | 84651 | Cell Signaling Technology | 1:50 (frozen) |
| PD-L1 | Polyclonal | PA5-20343 | Invitrogen | 1:100 (frozen) |
| H+/K+-ATPase | 2B6 | D032-3H | MBL (Tokyo, Japan) | 1:4000 (paraffin) |
| TFF2 | Polyclonal | 13681-1-AP | Proteintech (Rosemont, IL) | 1:400 (paraffin) |
| Clusterin | Polyclonal | AF2747 | R&D Systems | 1:400 (paraffin) |
| Ki67 | Polyclonal | ab15580 | Abcam | 1:200 (paraffin) |
Frozen sections were blocked with 2.5% normal goat serum (Vector Laboratories) for 1 hour and incubated with primary antibodies (Table 2) overnight at 4°C. For fluorescence detection, Alexa594-conjugated goat anti-rat IgG (Jackson ImmunoResearch), Alexa488-conjugated goat anti-Armenian hamster IgG (Jackson ImmunoResearch), Alexa488-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch), and Alexa488- or Alexa594-conjugated goat anti-rabbit IgG (Invitrogen, Carlsbad, CA) were used at a 1:400 dilution. Sections were counterstained with 4′,6-diamidino-2-phenylindole and mounted using mounting medium (Vector Laboratories). Images were obtained using a confocal fluorescence microscope (Eclipse TE2000; Nikon, Tokyo, Japan; and LSM 800; Zeiss, Oberkochen, Germany).
H felis Quantification
Total DNA was extracted from gastric tissues (corpus and antrum) using a QIAamp DNA mini kit (51304; Qiagen, Hilden, Germany) and used to identify H felis DNA. Quantitative real-time polymerase chain reaction (PCR) was performed using specific primers for H felis flagellar filament B, Rotor-Gene SYBR Green PCR kit (204074; Qiagen), and Rotor-Gene Q (Qiagen). Amplified target DNA was analyzed using Rotor-Gene Q series software. The H felis flagellar filament B primers: forward: 5′-TTCGATTGGTCCTACAGGCTCAGA-3′ and reverse: 5′-TTCTTGTTGATGACATTGACCAACGCA-3′ were used.68 A Warthin–Starry staining kit (ab150688; Abcam, Cambridge, UK) was used to identify the location of H felis and to assess the extent of colonization in the stomach. Colonized H felis were counted manually on 2 sections from each sample.
Quantitative Real-Time Reverse-Transcription PCR
Total RNA from gastric corpus tissues was extracted using a Hybrid-R RNA purification kit (305-101; GeneAll, Seoul, Korea). Complementary DNA was synthesized from 0.1 μg RNA using a QuantiTect Reverse Transcription kit (205313; Qiagen). Quantitative real-time PCR was performed using specific primers, Rotor-Gene SYBR Green PCR kit (204074; Qiagen), and Rotor-Gene Q (Qiagen). The amplified target complementary DNA was analyzed using Rotor-Gene Q series software against the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase. Specific primer sequences are listed in Table 3. Primers for mouse Granzyme A (QT01551690) and perforin (QT00282002) were purchased from Qiagen.
Table 3.
List of Primers for Quantitative Real-Time Reverse-Transcription PCR
| Primer name | Sequence |
|---|---|
| Mouse GAPDH forward | 5′-TGCACCACCAACTGCTTAG-3′ |
| Mouse GAPDH reverse | 5′-GGATGCAGGGATGATGTTC-3′ |
| Mouse IFN-γ forward | 5′-AGCGGCTGACTGAACTCAGATTGTAG-3′ |
| Mouse IFN-γ reverse | 5′-GTCACAGTTTTCAGCTGTATAGGG-3′ |
| Mouse PD-L1 forward | 5′-TGGACAAACAGTGACCACCAA-3′ |
| Mouse PD-L1 reverse | 5′-CCCCTCTGTCCGGGAAGT-3′ |
| Mouse IL10 forward | 5′-GCTCCAAGACCAAGGTGTCT-3′ |
| Mouse IL10 reverse | 5′-CTAGGTCCTGGAGTCCAGCA-3′ |
| Mouse TGF-β forward | 5′-CTTCAATACGTCAGACATTCGGG-3′ |
| Mouse TGF-β reverse | 5′-GTAACGCCAGGAATTGTTGCTA-3′ |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; TGF-β, transforming growth factor β.
Magnetic Luminex Immunoassay
For protein extraction, gastric corpus tissues of H felis–infected and noninfected WT and Flt3–/– mice were lysed using Cell Lysis Buffer 2 (895347; R&D Systems, Minneapolis, MN). Chemokine and cytokine protein concentrations were measured using the Mouse Premixed Multi-Analyte kit (LXSAMSM; R&D Systems) according to the manufacturer’s protocol.
Multiplexed Immunohistochemistry
Multiplexed IHC was performed sequentially on each single paraffin section in the order of PD-L1, CD11c, and CD3 using Autostainer Link48 (Dako, Glostrup, Denmark) as previously described.69 Primary antibodies were as follows: rabbit anti–PD-L1 (1:30, 13684; Cell Signaling Technology, Danvers, MA), rabbit anti-CD11c (1:100, ab52632; Abcam), and rabbit anti-CD3 (1:300, A0452; Dako). After the immunostained sections were scanned using the Aperio AT2 (Leica Biosystems, Newcastle, UK), the slides were incubated in premixed stripping buffer (20% sodium dodecyl sulfate, 0.5 mol/L Tris-HCl pH 6.8, β-mercaptoethanol, and distilled water) for antibody stripping. For more effective stripping, slides were microwaved on low power. Sections then were incubated with the next primary antibody. For quantitation of PD-L1–expressing gastric DCs, CD11c+PD-L1+ cells were counted manually using QuPath software (available: https://qupath.github.io) on digitally scanned sections.
Statistical Analysis
All data are shown as means ± SE. Statistical analysis was performed using GraphPad Prism 7.00 (GraphPad Software, San Diego, CA). Two groups were compared using the Mann–Whitney U test or an unpaired t test with 2-tailed distributions after a Shapiro–Wilk normality test. When 3 or more groups were included, results were analyzed using the Kruskal–Wallis test or ordinary 1-way analysis of variance followed by the Mann–Whitney U test or an unpaired t test with 2-tailed distributions. A P value less than .05 was considered significant. All authors had access to the study data and reviewed and approved the final manuscript.
Acknowledgments
CRediT Authorship Contributions
Du-Min Go (Conceptualization: Equal; Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Methodology: Lead; Validation: Lead; Visualization: Lead; Writing – original draft: Lead; Writing – review & editing: Equal)
Seung Hyun Lee (Conceptualization: Equal; Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Methodology: Lead; Validation: Lead; Visualization: Lead; Writing – original draft: Lead; Writing – review & editing: Equal)
Su-Hyung Lee (Methodology: Equal)
Sang-Ho Woo (Methodology: Equal)
Kibyeong Kim (Methodology: Equal)
Kyeongdae Kim (Methodology: Equal)
Kyu Seong Park (Methodology: Equal)
Jong-Hwan Park (Resources: Equal)
Sang-Jun Ha (Resources: Equal)
Woo Ho Kim (Data curation: Equal; Formal analysis: Equal; Methodology: Equal)
Jae-Hoon Choi (Conceptualization: Lead; Data curation: Equal; Formal analysis: Equal; Funding acquisition: Lead; Investigation: Equal; Methodology: Equal; Project administration: Lead; Resources: Lead; Software: Equal; Supervision: Lead; Validation: Equal; Visualization: Equal; Writing – original draft: Lead; Writing – review & editing: Lead)
Dae-Yong Kim (Conceptualization: Lead; Data curation: Equal; Formal analysis: Equal; Funding acquisition: Lead; Investigation: Equal; Methodology: Equal; Project administration: Lead; Resources: Equal; Software: Equal; Supervision: Lead; Validation: Equal; Visualization: Equal; Writing – original draft: Equal; Writing – review & editing: Equal)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This research was supported by NRF-2016M3A9D5A01952416, 2016M3A9D5A01952413, 2018R1A2B6003393, and 2015M3A9B6029138, and by the National Research Foundation of the Korean government.
Contributor Information
Jae-Hoon Choi, Email: jchoi75@hanyang.ac.kr.
Dae-Yong Kim, Email: daeyong@snu.ac.kr.
References
- 1.Arasanz H., Gato-Cañas M., Zuazo M., Ibañez-Vea M., Breckpot K., Kochan G., Escors D. PD1 signal transduction pathways in T cells. Oncotarget. 2017;8:51936–51945. doi: 10.18632/oncotarget.17232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fife B.T., Pauken K.E., Eagar T.N., Obu T., Wu J., Tang Q., Azuma M., Krummel M.F., Bluestone J.A. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wherry E.J. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 4.Chikuma S., Terawaki S., Hayashi T., Nabeshima R., Yoshida T., Shibayama S., Okazaki T., Honjo T. PD-1-mediated suppression of IL-2 production induces CD8+ T cell anergy in vivo. J Immunol. 2009;182:6682–6689. doi: 10.4049/jimmunol.0900080. [DOI] [PubMed] [Google Scholar]
- 5.Dong H., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B., Roche P.C., Lu J., Zhu G., Tamada K., Lennon V.A., Celis E., Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 6.Patsoukis N., Sari D., Boussiotis V.A. PD-1 inhibits T cell proliferation by upregulating p27 and p15 and suppressing Cdc25A. Cell Cycle. 2012;11:4305–4309. doi: 10.4161/cc.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jubel J.M., Barbati Z.R., Burger C., Wirtz D.C., Schildberg F.A. The role of PD-1 in acute and chronic Infection. Front Immunol. 2020;11:487. doi: 10.3389/fimmu.2020.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharpe A.H., Wherry E.J., Ahmed R., Freeman G.J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 9.Kronsteiner B., Bassaganya-Riera J., Philipson N., Hontecillas R. Novel insights on the role of CD8+ T cells and cytotoxic responses during Helicobacter pylori infection. Gut Microbes. 2014;5:357–362. doi: 10.4161/gmic.28899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson E.D., Zahurak M., Murphy A., Cornish T., Cuka N., Abdelfatah E., Yang S., Duncan M., Ahuja N., Taube J.M., Anders R.A., Kelly R.J. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut. 2017;66:794–801. doi: 10.1136/gutjnl-2015-310839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kusters J.G., van Vliet A.H., Kuipers E.J. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;9:449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bimczok D., Clements R.H., Waites K.B., Novak L., Eckhoff D.E., Mannon P.J., Smith P.D., Smythies L.E. Human primary gastric dendritic cells induce a Th1 response to H. pylori. Mucosal Immunol. 2010;3:260–269. doi: 10.1038/mi.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quiding-Järbrink M., Lundin B.S., Lönroth H., Svennerholm A.M. CD4+ and CD8+ T cell responses in Helicobacter pylori-infected individuals. Clin Exp Immunol. 2001;123:81–87. doi: 10.1046/j.1365-2249.2001.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang M., Liu M., Luther J., Kao J.Y. Helicobacter pylori directs tolerogenic programming of dendritic cells. Gut Microbes. 2010;1:325–329. doi: 10.4161/gmic.1.5.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morey P., Pfannkuch L., Pang E., Boccellato F., Sigal M., Imai-Matsushima A., Dyer V., Koch M., Mollenkopf H.J., Schlaermann P., Meyer T.F. Helicobacter pylori depletes cholesterol in gastric glands to prevent interferon gamma signaling and escape the inflammatory response. Gastroenterology. 2018;154:1391–1404. doi: 10.1053/j.gastro.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Peek R.M.J., Fiske C., Wilson K.T. Role of innate immunity in Helicobacter pylori-induced gastric malignancy. Physiol Rev. 2010;90:831–858. doi: 10.1152/physrev.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Y.Y., Chen J.H., Kao J.T., Liu K.C., Lai C.H., Wang Y.M., Hsieh C.T., Tzen J.T., Hsu P.N. Expression of CD25(high) regulatory T cells and PD-1 in gastric infiltrating CD4(+) T lymphocytes in patients with Helicobacter pylori infection. Clin Vaccine Immunol. 2011;18:1198–1201. doi: 10.1128/CVI.00422-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osaki L.H., Bockerstett K.A., Wong C.F., Ford E.L., Madison B.B., DiPaolo R.J., Mills J.C. Interferon-γ directly induces gastric epithelial cell death and is required for progression to metaplasia. J Pathol. 2019;247:513–523. doi: 10.1002/path.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sayi A., Kohler E., Hitzler I., Arnold I., Schwendener R., Rehrauer H., Müller A. The CD4+ T cell-mediated IFN-gamma response to Helicobacter infection is essential for clearance and determines gastric cancer risk. J Immunol. 2009;182:7085–7101. doi: 10.4049/jimmunol.0803293. [DOI] [PubMed] [Google Scholar]
- 20.Das S., Suarez G., Beswick E.J., Sierra J.C., Graham D.Y., Reyes V.E. Expression of B7-H1 on gastric epithelial cells: its potential role in regulating T cells during Helicobacter pylori infection. J Immunol. 2006;176:3000–3009. doi: 10.4049/jimmunol.176.5.3000. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y.Y., Lin C.W., Cheng K.S., Lin C., Wang Y.M., Lin I.T., Chou Y.H., Hsu P.N. Increased programmed death-ligand-1 expression in human gastric epithelial cells in Helicobacter pylori infection. Clin Exp Immunol. 2010;161:551–559. doi: 10.1111/j.1365-2249.2010.04217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell P., Germain C., Fiori P.L., Khamri W., Foster G.R., Ghosh S., Lechler R.I., Bamford K.B., Lombardi G. Chronic exposure to Helicobacter pylori impairs dendritic cell function and inhibits Th1 development. Infect Immun. 2007;75:810–819. doi: 10.1128/IAI.00228-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holokai L., Chakrabarti J., Broda T., Chang J., Hawkins J.A., Sundaram N., Wroblewski L.E., Peek R.M.J., Wang J., Helmrath M., Wells J.M., Zavros Y. Increased programmed death-ligand 1 is an early epithelial cell response to Helicobacter pylori infection. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reizis B. Classical dendritic cells as a unique immune cell lineage. J Exp Med. 2012;209:1053–1056. doi: 10.1084/jem.20121038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satpathy A.T., KC W., Albring J.C., Edelson B.T., Kretzer N.M., Bhattacharya D., Murphy T.L., Murphy K.M. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med. 2012;209:1135–1152. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clemente-Casares X., Hosseinzadeh S., Barbu I., Dick S.A., Macklin J.A., Wang Y., Momen A., Kantores C., Aronoff L., Farno M., Lucas T.M., Avery J., Zarrin-Khat D., Elsaesser H.J., Razani B., Lavine K.J., Husain M., Brooks D.G., Robbins C.S., Cybulsky M., Epelman S. A CD103+ conventional dendritic cell surveillance system prevents development of overt heart failure during subclinical viral myocarditis. Immunity. 2017;47:974–989. doi: 10.1016/j.immuni.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Salmon H., Idoyaga J., Rahman A., Leboeuf M., Remark R., Jordan S., Casanova-Acebes M., Khudoynazarova M., Agudo J., Tung N., Chakarov S., Rivera C., Hogstad B., Bosenberg M., Hashimoto D., Gnjatic S., Bhardwaj N., Palucka A.K., Brown B.D., Brody J., Ginhoux F., Merad M. Expansion and activation of CD103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity. 2016;44:924–938. doi: 10.1016/j.immuni.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi J.H., Cheong C., Dandamudi D.B., Park C.G., Rodriguez A., Mehandru S., Velinzon K., Jung I.H., Yoo J.Y., Oh G.T., Steinman R.M. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity. 2011;35:819–831. doi: 10.1016/j.immuni.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Waskow C., Liu K., Darrasse-Jèze G., Guermonprez P., Ginhoux F., Merad M., Shengelia T., Yao K., Nussenzweig M. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008;9:676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yun T.J., Lee J.S., Machmach K., Shim D., Choi J., Wi Y.J., Jang H.S., Jung I.H., Kim K., Yoon W.K., Miah M.A., Li B., Chang J., Bego M.G., Pham T.N.Q., Loschko J., Fritz J.H., Krug A.B., Lee S.P., Keler T., Guimond J.V., Haddad E., Cohen E.A., Sirois M.G., El-Hamamsy I., Colonna M., Oh G.H., Choi J.H., Cheong C. Indoleamine 2,3-dioxygenase-expressing aortic plasmacytoid dendritic cells protect against atherosclerosis by induction of regulatory T cells. Cell Metab. 2016;23:852–866. doi: 10.1016/j.cmet.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Wunder C., Churin Y., Winau F., Warnecke D., Vieth M., Lindner B., Zähringer U., Mollenkopf H.J., Heinz E., Meyer T.F. Cholesterol glucosylation promotes immune evasion by Helicobacter pylori. Nat Med. 2006;12:1030–1038. doi: 10.1038/nm1480. [DOI] [PubMed] [Google Scholar]
- 32.Khamri W., Moran A.P., Worku M.L., Karim Q.N., Walker M.M., Annuk H., Ferris J.A., Appelmelk B.J., Eggleton P., Reid K.B., Thursz M.R. Variations in Helicobacter pylori lipopolysaccharide to evade the innate immune component surfactant protein D. Infect Immun. 2005;73:7677–7686. doi: 10.1128/IAI.73.11.7677-7686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khamri W., Worku M.L., Anderson A.E., Walker M.M., Hawgood S., Reid K.B., Clark H.W., Thursz M.R. Helicobacter infection in the surfactant protein D-deficient mouse. Helicobacter. 2007;12:112–123. doi: 10.1111/j.1523-5378.2007.00480.x. [DOI] [PubMed] [Google Scholar]
- 34.Murray E., Khamri W., Walker M.M., Eggleton P., Moran A.P., Ferris J.A., Knapp S., Karim Q.N., Worku M., Strong P., Reid K.B.M., Thursz M.R. Expression of surfactant protein D in the human gastric mucosa and during Helicobacter pylori infection. Infect Immun. 2002;70:1481–1487. doi: 10.1128/IAI.70.3.1481-1487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vange P., Bruland T., Doseth B., Fossmark R., Sousa M.M.L., Beisvag V., Sørdal Ø., Qvigstad G., Waldum H.L., Sandvik A.K., Bakke I. The cytoprotective protein clusterin is overexpressed in hypergastrinemic rodent models of oxyntic preneoplasia and promotes gastric cancer cell survival. PLoS One. 2017;12 doi: 10.1371/journal.pone.0184514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimizu T., Choi E., Petersen C.P., Noto J.M., Romero-Gallo J., Piazuelo M.B., Washington M.K., Peek R.M.J., Goldenring J.R. Characterization of progressive metaplasia in the gastric corpus mucosa of Mongolian gerbils infected with Helicobacter pylori. J Pathol. 2016;239:399–410. doi: 10.1002/path.4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weis V.G., Sousa J.F., LaFleur B.J., Nam K.T., Weis J.A., Finke P.E., Ameen N.A., Fox J.G., Goldenring J.R. Heterogeneity in mouse spasmolytic polypeptide-expressing metaplasia lineages identifies markers of metaplastic progression. Gut. 2013;62:1270–1279. doi: 10.1136/gutjnl-2012-302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J.J., Nottingham L.K., Sin J.I., Tsai A., Morrison L., Oh J., Dang K., Hu Y., Kazahaya K., Bennett M., Dentchev T., Wilson D.M., Chalian A.A., Boyer J.D., Agadjanyan M.G., Weiner D.B. CD8 positive T cells influence antigen-specific immune responses through the expression of chemokines. J Clin Invest. 1998;102:1112–1124. doi: 10.1172/JCI3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohtani N., Ohtani H., Nakayama T., Naganuma H., Sato E., Imai T., Nagura H., Yoshie O. Infiltration of CD8+ T cells containing RANTES/CCL5+ cytoplasmic granules in actively inflammatory lesions of human chronic gastritis. Lab Invest. 2004;84:368–375. doi: 10.1038/labinvest.3700039. [DOI] [PubMed] [Google Scholar]
- 40.Rad R., Ballhorn W., Voland P., Eisenächer K., Mages J., Rad L., Ferstl R., Lang R., Wagner H., Schmid R.M., Bauer S., Prinz C., Kirschning C.J., Krug A. Extracellular and intracellular pattern recognition receptors cooperate in the recognition of Helicobacter pylori. Gastroenterology. 2009;136:2247–2257. doi: 10.1053/j.gastro.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 41.Hafsi N., Voland P., Schwendy S., Rad R., Reindl W., Gerhard M., Prinz C. Human dendritic cells respond to Helicobacter pylori, promoting NK cell and Th1-effector responses in vitro. J Immunol. 2004;173:1249–1257. doi: 10.4049/jimmunol.173.2.1249. [DOI] [PubMed] [Google Scholar]
- 42.Khamri W., Walker M.M., Clark P., Atherton J.C., Thursz M.R., Bamford K.B., Lechler R.I., Lombardi G. Helicobacter pylori stimulates dendritic cells to induce interleukin-17 expression from CD4+ T lymphocytes. Infect Immun. 2010;78:845–853. doi: 10.1128/IAI.00524-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rizzuti D., Ang M., Sokollik C., Wu T., Abdullah M., Greenfield L., Fattouh R., Reardon C., Tang M., Diao J., Schindler C., Cattral M., Jones N.L. Helicobacter pylori inhibits dendritic cell maturation via interleukin-10-mediated activation of the signal transducer and activator of transcription 3 pathway. J Innate Immun. 2015;7:199–211. doi: 10.1159/000368232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hitzler I., Oertli M., Becher B., Agger E.M., Müller A. Dendritic cells prevent rather than promote immunity conferred by a helicobacter vaccine using a mycobacterial adjuvant. Gastroenterology. 2011;141:186–196. doi: 10.1053/j.gastro.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Oertli M., Sundquist M., Hitzler I., Engler D.B., Arnold I.C., Reuter S., Maxeiner J., Hansson M., Taube C., Quiding-Järbrink M., Muller A. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection. J Clin Invest. 2012;122:1082–1096. doi: 10.1172/JCI61029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bennett C.L., Clausen B.E. DC ablation in mice: promises, pitfalls, and challenges. Trends Immunol. 2007;28:525–531. doi: 10.1016/j.it.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 47.Arnold I.C., Zhang X., Urban S., Artola-Borán M., Manz M.G., Ottemann K.M., Müller A. NLRP3 controls the development of gastrointestinal CD11b+ dendritic cells in the steady state and during chronic bacterial infection. Cell Rep. 2017;21:3860–3872. doi: 10.1016/j.celrep.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 48.Bain C.C., Scott C.L., Uronen-Hansson H., Gudjonsson S., Jansson O., Grip O., Guilliams M., Malissen B., Agace W.W., Mowat A.M. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6:498–510. doi: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bachem A., Güttler S., Hartung E., Ebstein F., Schaefer M., Tannert A., Salama A., Movassaghi K., Opitz C., Mages H.W., Henn V., Kloetzel P.M., Gurka S., Kroczek R.A. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:273–1281. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guilliams M., Dutertre C.A., Scott C.L., McGovern N., Sichien D., Chakarov S., Van Gassen S., Chen J., Poidinger M., De Prijck S., Tavernier S.J., Low I., Irac S.E., Mattar C.N., Sumatoh H.R., Low G.H.L., Chung T.J.K., Chan D.K.H., Tan K.K., Hon T.L.K., Fossum E., Bogen B., Choolani M., Chan J.K.Y., Larbi A., Luche H., Henri S., Saeys Y., Newell E.W., Lambrecht B.N., Malissen B., Ginhoux F. Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species. Immunity. 2016;45:669–684. doi: 10.1016/j.immuni.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gurka S., Hartung E., Becker M., Kroczek R.A. Mouse conventional dendritic cells can be universally classified based on the mutually exclusive expression of XCR1 and SIRPα. Front Immunol. 2015;6:35. doi: 10.3389/fimmu.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mildner A., Jung S. Development and function of dendritic cell subsets. Immunity. 2014;40:642–656. doi: 10.1016/j.immuni.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 53.Seillet C., Jackson J.T., Markey K.A., Brady H.J., Hill G.R., Macdonald K.P., Nutt S.L., Belz G.T. CD8α+ DCs can be induced in the absence of transcription factors Id2, Nfil3, and Batf3. Blood. 2013;121:1574–1583. doi: 10.1182/blood-2012-07-445650. [DOI] [PubMed] [Google Scholar]
- 54.Sugiura D. Restriction of PD-1 function by cis-PD-L1/CD80 interactions is required for optimal T cell responses. Science. 2019;364:558–566. doi: 10.1126/science.aav7062. [DOI] [PubMed] [Google Scholar]
- 55.Francisco L.M., Salinas V.H., Brown K.E., Vanguri V.K., Freeman G.J., Kuchroo V.K., Sharpe A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmitt E.G., Williams C.B. Generation and function of induced regulatory T cells. Front Immunol. 2013;4:152. doi: 10.3389/fimmu.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silva M.T. Neutrophils and macrophages work in concert as inducers and effectors of adaptive immunity against extracellular and intracellular microbial pathogens. J Leukoc Biol. 2010;87:805–813. doi: 10.1189/jlb.1109767. [DOI] [PubMed] [Google Scholar]
- 58.Petersen C.P., Mills J.C., Goldenring J.R. Murine models of gastric corpus preneoplasia. Cell Mol Gastroenterol Hepatol. 2016;3:11–26. doi: 10.1016/j.jcmgh.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mera R.M., Bravo L.E., Camargo M.C., Bravo J.C., Delgado A.G., Romero-Gallo J., Yepez M.C., Realpe J.L., Schneider B.G., Morgan D.R., Peek R.M., Jr., Correa P., Wilson K.T., Blanca P. Dynamics of Helicobacter pylori infection as a determinant of progression of gastric precancerous lesions: 16-year follow-up of an eradication trial. Gut. 2018;67:1239–1246. doi: 10.1136/gutjnl-2016-311685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu J., Firpi-Morell R.J., Dang L.H., Lai J., Liu X. An unusual case of gastritis in one patient receiving PD-1 blocking therapy: coexisting immune-related gastritis and cytomegaloviral infection. Gastroenterology Res. 2018;11:383–387. doi: 10.14740/gr1068w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Postow M.A., Sidlow R., Hellmann M.D. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 62.Samaan M.A., Pavlidis P., Papa S., Powell N., Irving P.M. Gastrointestinal toxicity of immune checkpoint inhibitors: from mechanisms to management. Nat Rev Gastroenterol Hepatol. 2018;15:222–234. doi: 10.1038/nrgastro.2018.14. [DOI] [PubMed] [Google Scholar]
- 63.Mackarehtschian K., Hardin J.D., Moore K.A., Boast S., Goff S.P., Lemischka I.R. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity. 1995;3:147–161. doi: 10.1016/1074-7613(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 64.Latchman Y.E., Liang S.C., Wu Y., Chernova T., Sobel R.A., Klemm M., Kuchroo V.K., Freeman G.J., Sharpe A.H. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee A., O'Rourke J., De Ungria M.C., Robertson B., Daskalopoulos G., Dixon M.F. A standardized mouse model of Helicobacter pylori Infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 66.Chen X.Y., van der Hulst R.W., Bruno M.J., van der Ende A., Xiao S.D., Tytgat G.N., Ten Kate F.J. Interobserver variation in the histopathological scoring of Helicobacter pylori related gastritis. J Clin Pathol. 1999;52:612–615. doi: 10.1136/jcp.52.8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dixon M.F., Genta R.M., Yardley J.H., Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 68.El-Zaatari M., Kao J.Y., Tessier A., Bai L., Hayes M.M., Fontaine C., Eaton K.A., Merchant J.L. Gli1 deletion prevents Helicobacter-induced gastric metaplasia and expansion of myeloid cell subsets. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koh J., Kwak Y., Kim J., Kim W.H. High-throughput multiplex immunohistochemical imaging of the tumor and its microenvironment. Cancer Res Treat. 2020;52:98–108. doi: 10.4143/crt.2019.195. [DOI] [PMC free article] [PubMed] [Google Scholar]