Abstract
This study was conducted to investigate the efficacy of incomplete degradation products of galactomannan (IDPG) on the production performance, egg quality, plasma parameters, and lipid metabolites of laying hens. A total of 288 laying hens were allocated into 4 treatments and fed diets supplemented with 0%, 0.01%, 0.025%, and 0.05% IDPG. Results showed that IDPG supplementation significantly increases egg production and decreases feed conversion ratio (P < 0.05). Eggs laid by hens receiving IDPG exhibited higher eggshell strength (P < 0.05). Moreover, IDPG supplementation significantly increased the serum albumin content, and decreased the blood ammonia content as well as triglyceride levels in serum and liver (P < 0.05). Overall, IDPG can be considered as an effective feed additive due to its capacity of improving egg production, increasing plasma protein, and changing lipid metabolism of laying hens.
Key words: Incomplete degradation products of galactomannan, Laying hen, Production performance, Egg quality, Lipid metabolism
INTRODUCTION
Sesbania species, a leguminous crop, are locally known in many tropical and subtropical countries of Asia and Africa (Hossain et al., 2002). They grow quickly and are often used for saline land improvement due to their outstanding tolerance to salt, barren, and flooding stress (Cowan et al., 1982). Their major agricultural use has been as green manure to improve soil fertility (Olvera et al., 1988). Meanwhile, Sesbania seeds have been reported as plant-based protein sources for fish because of their high protein content of 30 to 36% (Hossain et al., 2003). Moreover, the galactomannan, main components of endosperm in Sesbania seed, may also provide a cheap source of dietary energy for fish. However, galactomannan is poorly utilized and in contrast identified as an antinutritive factor. Supplementation of the galactomannan-rich endosperm of Sesbania aculeate seeds in the diet of common carp (Cyprinus carpio) resulted in increased viscosity in the intestinal content, thereby affecting the nutrient absorption and utilization (Hossain et al., 2001). With the deepening understanding of polysaccharides, the reason of this phenomenon may be related to the solubility and molecular weight of galactomannan.
Generally, the molecular weight of native galactomannan isolated from legume seeds is as high as hundreds of thousands Dalton (Da, 1 Da=1 g/mol). For example, the molecular weight of galactomannans from seeds of Cassia obtusifolia (Feng et al., 2018) and Cyamopsis tetragonalobus (Shobha and Tharanathan, 2008) were 4.51 × 105 and 2.4 × 105 Da, respectively. Besides, galactomannan is chemically composed of β-(1,4) linked mannose backbone with abundant galactopyranosyl side chains attached through α-1,6-glycosidic linkages (Gu et al., 2020). A large number of galactose side-chains endow the galactomannan high solubility. As a consequence, the high molecular weight and high solubility of galactomannan lead to its high viscosity, which in turn results in a relatively high viscosity in the intestinal content followed by reduced nutrient digestibility when the galactomannan precursor was supplemented to the diet of fish (Sinha et al., 2020). Therefore, it has been recognized that the use of galactomannan after its molecular weight is reduced might eliminate the adverse effects caused by its high viscosity. One reported practice is partially hydrolyzed guar gum (PHGG), which was obtained by the treatment of guar gum with mannanase (Hidehisa et al., 1994). The molecular weight of PHGG was approximately one-tenth of the original guar gum and the viscosity was much lower (approximately one-thousandth). Interestingly, the PHGG exhibited many overall animal health enhanced properties such as, significant improvement of diarrhea in patients with gastrointestinal intolerance, reduction of serum cholesterol in the rat, and improvement of lipid metabolism without reduction of protein utilization (Idea et al., 1991; Yamatoya et al., 1997). However, there is currently not sufficient work available that the degradation products of galactomannan as feed additives for poultry especially for laying hens.
The incomplete degradation products of galactomannan (IDPG) refers to the degradation products of natural galactomannan with polymerization degree more than 2, which does not contain monosaccharides such as mannose and galactose (Tao et al., 2020). Herein, the IDPG was prepared by enzymatic hydrolysis of galactomannan from Sesbania canabina seeds. Noticeably, the average molecular weight of IDPG dropped to one-tenth of the original galactomannan after enzymatic hydrolysis. Once a viable additive was procured, evaluations were conducted in an effort to better understand the effects of IDPG as feed additives upon the production performance, egg qualities of laying hens. In addition, critical metabolites were recorded in an effort to pinpoint specific metabolic benefits of IDPG. The effect of IDPG on lipid metabolism in aged laying hens was also investigated. We hope that this work paves the way for IDPG to be considered a viable feed additive based upon its bioavailability and pertinent production benefits.
MATERIALS AND METHODS
Isolation and Preparation of the IDPG
IDPG was prepared by enzymatic hydrolysis of galactomannan from Sesbania canabina seeds. S. cannabina seeds were collected from a local farm in Yancheng city, Jiangsu province of China. After grinding (Mini plant shredder F2102, Taisite instrument Co., Ltd., Tianjin, China), the size-reduced seeds (the average size of 100 mesh) were added to a 50 mM citric acid buffer (pH 4.8) at a galactomannan concentration of 40 g/L, and then the obtained suspension was treated by β-mannanase, which was produced by Trichoderma reesei Rut C-30 with avicel as carbon source (Xie et al., 2017), with an enzyme dosage of 20 U/g-galactomannan at 50°C for 72 h. After enzymatic hydrolysis, the enzymatic hydrolysate was boiled at 100°C for 10 min to inactivate the enzymes followed by centrifugation (16,465 × g, 10 min) (Ledend Mach 1.6R centrifuge, Thermo Fisher, Franklin, MA). The supernatant was nanofiltrated (200 Da, ST-Recovery Tech Co., Ltd., Nanjing, Jiangsu Province, China) to remove galactose and mannose, and the solution of incomplete degradation products of galactomannan was obtained. Finally, the solution of IDPG was spray dried (B-191, BUCHI, Flawil, Switzerland) at 160°C to obtain solid.
Composition Analysis of the IDPG
The content of IDPG in the solid was determined by a sulfuric acid hydrolysis method and high performance anion exchange chromatography with pulsed amperometric detection as described by Tao et al. (2020). The weight-average molecular weight of IDPG was finally determined by high-performance size exclusion chromatography (HPSEC) as described by Tao et al. (2020).
Animal Care and Experimental Design
The experimental protocols used in this experiment, including animal care and use, were reviewed and approved by the Animal Care and Use Ethics Committee of Nanjing Forestry University (Nanjing, China). A total of two hundred and twenty-eight, 68-week-old laying hens (Hy-Line variety brown) were randomly distributed into 4 dietary treatments consisting of 6 replicates (cages) with 12 birds per replicate. The 4 groups were fed a basal diet supplemented with 0 (control group), 0.01%, 0.025% and 0.05% IDPG over the course of 8 wk (wk 68–76 of the laying hens age). The ingredients composition and nutrients content of the basal diet are shown in Table 1. Kept in an environmentally controlled house, the birds were allowed free access to water and mash feed in 3-level cages (120 cm × 60 cm × 50 cm; 0.09 m2 per chick) with controlled ventilation and lighting (16L:8D). The troughs are filled up twice a day (6 AM and 3 PM), and the diets were added in the trough. The feeding amount each day was appropriately adjusted according to the amount of feed remaining from the previous day. This was done to ensure no feed was leftover in the trough each night. Eggs were collected and weighed at the same time every afternoon. Furthermore, a 2-week pre-experiment was carried out to ensure the eggs production rate is the same for each replicate when the test officially starts.
Table 1.
Composition and nutrient levels of basal diets (as-fed basis).
| Ingredients | Content (%) | Nutrient levelsb | |
|---|---|---|---|
| Corn | 63.50 | Apparent metabolizable energy (kcal/kg) | 2667.24 |
| Soybean meal | 18.80 | Crude protein (%) | 15.37 |
| Fish meal | 1.50 | Calcium, Ca (%) | 3.79 |
| Rapeseed meal | 2.00 | Total phosphorus, P (%) | 0.64 |
| Corn gluten meal | 1.20 | ||
| Soybean phospholipid | 1.00 | ||
| Limestone | 9.17 | ||
| Dicalcium phosphate | 1.40 | ||
| DL-Methionine | 0.10 | ||
| Sodium chloride | 0.33 | ||
| 1% Premixa | 1.00 | ||
| Total | 100 | ||
1% Premix was provided by Huamu Institute of Animal Science and Technology, and provided per kilogram of diet: vitamin A (transretinyl acetate), 1.08 × 104 IU; vitamin D3 (cholecalciferol), 2.7 × 103 IU; vitamin E (all-rac-α-tocopherol), 27 mg; menadione, 0.84 mg; thiamin, 0.72 mg; riboflavin, 5.4 mg; nicotinamide, 9 mg; calcium pantothenate, 36 mg; pyridoxine•HCl, 2.7 mg; biotin, 0.09 mg; folic acid, 0.24 mg; vitamin B12 (cobalamin), 0.009 mg; Fe (from ferrous sulfate), 100 mg; Cu (from copper sulphate), 8.0 mg; Mn (from manganese sulphate), 100 mg; Zn (from zinc oxide), 100 mg; I (from calcium iodate), 0.9 mg; Se (from sodium selenite), 0.3 mg.
Nutrient levels were the calculated values.
Sample Collection
At 76 wk of age, 6 birds from each treatment group were randomly selected (one bird per replicate). Blood samples were collected from wing veins using sterilized needles and syringes. These samples were then centrifuged at 4,940 × g for 15 min at 4°C to separate serum. Serum was collected in new tubes and stored at -20°C for further analysis. The randomly selected laying hens were then euthanized by cervical dislocation and immediately necropsied. The liver was quickly excised, rapidly frozen in liquid nitrogen and then preserved at -80°C before further analysis.
Production Performance
The number of eggs, egg weight, feed consumption, and the mortality were recorded every day. Egg production rate and feed intake were expressed based on a hen-day during the intervals of 68-72 and 72-76 wk. Egg mass and feed conversion ratio (FCR) were calculated based on a hen-day during the intervals of 68-72 and 72-76 wk. Egg mass was calculated by multiplying egg weight by egg production rate, and FCR was determined by dividing feed intake by egg mass.
Egg Quality Analysis
To determine egg quality for wk 68-72 and 72-76, 18 eggs were randomly collected per treatment group (3 eggs per replicate). Each egg was individually weighed and broken using an egg force reader (Orka Food Technology, Bountiful, UT) to measure eggshell breaking strength. Eggshell thickness is reported as an average value based on the thickness of eggshell measured at 3 different places (upper end, lower end, and the middle) using a micrometer screw gauge (Suce measuring instrument Co., Ltd., Nanjing, Jiangsu, China). Albumen height, Haugh units, and yolk color were measured by an automatic egg quality analysis instrument (EMT-5200, Robotmation Co., Ltd., Tokyo, Japan). Yolk and eggshell were separated manually, and both were weighed to calculate yolk relative weight.
Determination of Serum and Liver Parameters
Total protein (TP), albumin (ALB), globulin (GLB), alkaline phosphatase (ALP), blood ammonia (BA), blood urea nitrogen (BUN), cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) in the serum and cholesterol and triglycerides in the liver of the samples in 4 groups (control group and IDPG group [0.01%, 0.025%, and 0.05%]) were measured by the automatic biochemical analyzer (Au264, Olympus, Tokyo, Japan) using commercially available kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, P. R. China) according to manufacturer's instructions.
Statistical Analysis
Data were analyzed by one-way analysis of variance (ANOVA) using SPSS (2008) statistical software (Ver.16.0 for windows, SPSS Inc., Chicago, IL). Differences in means among treatment groups were separated using least significant difference (LSD) test. Data were assumed to be statistically significant when P < 0.05.
RESULTS
Composition Analysis of the IDPG
The purity of IDPG was 45.96%. Moreover, the obtained IDPG powder contained monosaccharide of 9.28%. Through HPSEC, the weight-average molecular weight of IDPG was finally determined to 1.74–14.12 kDa.
Production Performance
Production performance of the laying hens fed with and without IDPG is shown in Table 2. It can be seen that the addition of IDPG in the diet of laying hens significantly increased egg production (P < 0.05). Specifically, compared with the control group, dietary 0.05% IDPG supplementation was shown to increase egg production by 4.24% throughout the trial period. It can also be seen that IDPG had no significant effect on egg mass and egg weight of laying hens during the 8-wk trial period. Two other indicators reflecting nutrient utilization, feed intake and feed conversion ratio were also calculated. The laying hens fed with diets containing IDPG showed a lower feed intake and FCR than those fed without IDPG, but the difference was only significant at a IDPG supplementation of 0.05% (P < 0.05).
Table 2.
Production performance of laying hens fed diets supplemented with different doses of incomplete degradation products of galactomannan (IDPG).
| IDPG level (%) |
||||||
|---|---|---|---|---|---|---|
| Items | 0 | 0.01 | 0.025 | 0.05 | SEM | P values |
| Values at 68 to 72 wk of age | ||||||
| Egg production (%) | 78.67b | 76.88b | 81.06ab | 82.09a | 0.68 | 0.015 |
| Egg weight (g) | 61.86 | 62.97 | 62.35 | 62.14 | 0.25 | 0.474 |
| Feed intake (g/hen/d) | 129.92 | 125.51 | 125.44 | 128.10 | 0.76 | 0.093 |
| FCR (g feed/g egg) | 2.68 | 2.60 | 2.48 | 2.45 | 0.03 | 0.129 |
| Egg mass (g/hen/d) | 48.65 | 48.35 | 50.55 | 51.01 | 0.44 | 0.062 |
| Values at 72 to 76 wk of age | ||||||
| Egg production (%) | 74.71b | 74.66b | 76.21ab | 77.96a | 0.47 | 0.037 |
| Egg weight (g) | 62.84 | 63.41 | 63.02 | 63.30 | 0.27 | 0.894 |
| Feed intake (g/hen/d) | 124.99 | 119.50 | 119.14 | 120.13 | 0.98 | 0.115 |
| FCR (g feed/g egg) | 2.66a | 2.55ab | 2.49ab | 2.42b | 0.03 | 0.036 |
| Egg mass (g/hen/d) | 47.30 | 47.36 | 48.40 | 49.39 | 0.38 | 0.162 |
| Values at 68 to 76 wk of age | ||||||
| Egg production (%) | 76.69b | 75.76b | 78.72ab | 79.94a | 0.57 | 0.028 |
| Egg weight (g) | 62.35 | 63.22 | 62.72 | 62.75 | 0.25 | 0.700 |
| Feed intake (g/hen/d) | 127.48 | 122.48 | 122.29 | 124.11 | 1.04 | 0.330 |
| FCR (g feed/g egg) | 2.67a | 2.56ab | 2.48ab | 2.44b | 0.03 | 0.048 |
| Egg mass (g/hen/d) | 47.98 | 47.85 | 49.48 | 50.21 | 0.37 | 0.053 |
a–cIndicates different superscript letters in the same row differ significantly (P < 0.05).
Egg Quality
In the fourth wk of the experiment, the addition of 0.05% IDPG significantly increased the eggshell strength (P < 0.05) when laying hens were at 72 wk of age (Table 3). However, in the eighth wk of the experiment, the supplement of IDPG exhibited no significant influence on eggshell strength. Moreover, in the fourth and eighth wk of the experiment, dietary supplementation with IDPG had no significant effect on other egg quality characteristics such as eggshell thickness, albumen height, Haugh unit, yolk relative weight and yolk color of eggs.
Table 3.
Egg quality of laying hens supplemented with different doses of incomplete degradation products of galactomannan (IDPG) at 72 and 76 wk of age.
| IDPG level (%) |
||||||
|---|---|---|---|---|---|---|
| Items | 0 | 0.01 | 0.025 | 0.05 | SEM | P values |
| Values at 72 wk of age | ||||||
| Eggshell strength (kg/cm2) | 2.93b | 3.44ab | 3.28ab | 3.78a | 0.12 | 0.041 |
| Eggshell thickness (mm) | 0.34 | 0.33 | 0.32 | 0.32 | 0.01 | 0.072 |
| Albumen height (mm) | 6.57 | 5.62 | 6.64 | 6.76 | 0.16 | 0.060 |
| Haugh unit | 75.79 | 70.26 | 77.72 | 78.69 | 1.43 | 0.156 |
| Yolk color | 8.21 | 7.98 | 7.62 | 7.83 | 0.13 | 0.437 |
| Yolk relative weight (%) | 25.06 | 25.13 | 25.99 | 24.60 | 0.29 | 0.414 |
| Values at 76 wk of age | ||||||
| Eggshell strength (kg/cm2) | 3.43 | 3.54 | 3.42 | 3.59 | 0.11 | 0.937 |
| Eggshell thickness (mm) | 0.35 | 0.33 | 0.32 | 0.32 | 0.01 | 0.102 |
| Albumen height (mm) | 6.80 | 6.54 | 6.31 | 6.43 | 0.15 | 0.710 |
| Haugh unit | 78.88 | 76.34 | 77.06 | 76.22 | 1.07 | 0.824 |
| Yolk color | 7.42 | 7.45 | 6.91 | 6.96 | 0.14 | 0.377 |
| Yolk relative weight (%) | 24.59 | 25.41 | 25.95 | 25.18 | 0.33 | 0.554 |
a–cMeans with different superscript letters in the same row differ significantly (P < 0.05).
Serum Biochemical Parameters
As shown in Table 4, the addition of IDPG resulted in an increase (P < 0.05) in ALB content but had no significant effect on the TP and GLB contents. In addition, there was no significant increase in the ALP and BUN content between the IDPG-supplemented group and the control group. More importantly, the content of BA in the serum was significantly decreased by the introduction of IDPG additives in the laying hens’ diet (P < 0.05).
Table 4.
Serum biochemical parameters of laying hens supplemented with different doses of incomplete degradation products of galactomannan (IDPG) at 76 wk of age.
| IDPG level (%) |
||||||
|---|---|---|---|---|---|---|
| Items | 0 | 0.01 | 0.025 | 0.05 | SEM | P values |
| Serum | ||||||
| TP (g/L) | 49.29 | 47.49 | 53.03 | 52.82 | 1.05 | 0.165 |
| ALB (g/L) | 18.78b | 19.96ab | 21.28a | 21.49a | 0.37 | 0.02 |
| GLB (g/L) | 30.51 | 27.53 | 31.75 | 31.33 | 0.91 | 0.37 |
| ALP (g/L) | 10.18 | 10.15 | 8.95 | 7.91 | 0.81 | 0.745 |
| BA (g/L) | 253.58a | 210.96b | 225.08ab | 200.00b | 7.35 | 0.045 |
| BUN (g/L) | 0.77 | 0.92 | 1.19 | 0.91 | 0.06 | 0.099 |
a–cMeans with different superscript letters in the same row differ significantly (P < 0.05).
Lipid Metabolites of Serum and Liver
As seen in Table 5, serum triglyceride content in the group supplemented with 0.01% or 0.05% IDPG was significantly lower than that in the control group (P < 0.05). Concerning the liver, the triglyceride content of the group fed with diet containing 0.025% IDPG significantly decreased compared with the control group (P < 0.05). However, no significant effects of IDPG on the cholesterol content in the liver and serum, and the content of HDL-C and LDL-C in the serum were observed.
Table 5.
Lipid parameters in serum and liver of laying hens supplemented with different doses of incomplete degradation products of galactomannan (IDPG) at 76 wk of age.
| IDPG level (%) |
||||||
|---|---|---|---|---|---|---|
| Items | 0 | 0.01 | 0.025 | 0.05 | SEM | P values |
| Serum | ||||||
| Triglyceride (mmol/L) | 15.86a | 8.29b | 12.06ab | 10.14b | 0.84 | 0.004 |
| Cholesterol (mmol/L) | 6.97 | 5.10 | 6.58 | 6.16 | 0.32 | 0.195 |
| HDL-C (mmol/L) | 0.62 | 0.68 | 0.72 | 0.73 | 0.03 | 0.658 |
| LDL-C (mmol/L) | 2.91 | 1.99 | 2.36 | 2.37 | 0.18 | 0.381 |
| Liver | ||||||
| Triglyceride (mmol/g prot) | 0.46a | 0.44a | 0.33b | 0.40a | 0.01 | 0.006 |
| Cholesterol (mmol/g prot) | 63.39 | 62.43 | 63.81 | 64.82 | 0.84 | 0.811 |
a–cMeans with different superscript letters in the same row differ significantly (P < 0.05).
DISCUSSION
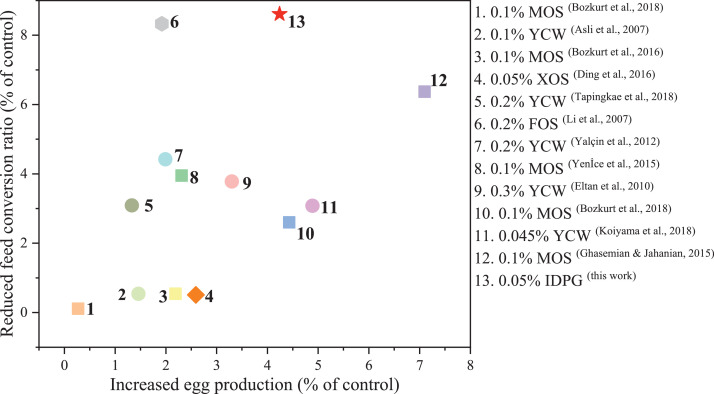
Recently, some studies have confirmed that supplemental mannan degradation products could improve the production performance of laying hens. Jahanian et al. (2016) carried out a 10-wk test on 55-week-old Hy-Line variety brown laying hens and revealed that dietary 0.2% manno-oligosaccharide (MOS) from yeast cell wall supplementation could significantly increase egg production and decrease FCR. Consistent with the results of Jahanian et al. (2016), this work showed a significant effect of IDPG on the production performance of laying hens in the later laying period. The usage of IDPG as additives increased the egg production of aged laying hens, and the greatest egg production was allotted to the hens fed diets supplemented with 0.05% IDPG. Furthermore, FCR, a parameter of concern to farmers who seek to maximize profit, was decreased due to the inclusion of IDPG in the diet of laying hens. This indicated that the addition of IDPG in the diet of laying hens could improve feed utilization. Moreover, the decrease of FCR may be partly attributed to the reduction of pathogenic colonies, eventually improving the digestive capacity of the intestine. Hassanein and Soliman (2010) suggested that the establishment of useful bacterial colonies could improve the nutrient digestibility in the intestine, thereby decreasing the value of FCR. Then we compared the effects of other oligosaccharides reported previously on the production performance of laying hens with IDPG, the results are shown in Figure 1. Compared with MOS (Bozkurt et al., 2018; Bozkurt et al., 2018; Bozkurt et al., 2016; Ghasemian and Jahanian, 2015; Yenİce et al., 2015) and yeast cell wall (containing mannan degradation products) (Asli et al., 2007; Eltan et al., 2010; Koiyama et al., 2018; Tapingkae et al., 2018; Yalçin et al., 2012), IDPG has a positive effect in improving the production performance. Furthermore, the supplement of IDPG is half of the MOS reported in other literature. Compared with the xylo-oligosaccharides (0.05%) (Ding et al., 2016) and fructo-oligosaccharides (0.2%) (Li et al., 2007), IDPG (0.05%) had a more pronounced effect on increasing egg production and decreasing FCR. Another issue that cannot be ignored is that supplemental IDPG had no marked effect on feed intake, egg mass and egg weight of laying hens, which is consistent with the previous report (Gao et al., 2008).
Figure 1.
Comparison of the effects of various oligosaccharides on the production performance of laying hens. Abbreviations: FOS, fructo-oligosaccharide; IDPG, incomplete degradation products of galactomannan; MOS, manno-oligosaccharide; XOS, xylo-oligosaccharide; YCW, yeast cell walls.
Egg quality is one of the factors that directly influence economic outcomes for livestock farmers in the intensive farm of laying hen (Ding et al., 2016). Eggshell strength and eggshell thickness are the 2 primary indicators of eggshell quality, as they influence the storage and transportation stability of eggs. Our results showed that the addition of IDPG into laying hen's diet significantly increased eggshell strength in the fourth wk of this trial, which is consistent with the findings of Bozkurt et al. (2016). However, in the eighth wk of this trial, this beneficial effect was found to lose its significance. This may be attributed to advanced age of hen, meaning the positive gains attributable early to IDPG inclusion eventually become masked by age-related performance decay. Furthermore, the eggshell thickness has no significant difference between each treatment. In agreement with our findings, other research has similarly found no significant effect of MOS on eggshell thickness (Jahanian and Ashnagar, 2018). In the further evaluation of eggs, their protein quality is another important judgment data of egg quality. Egg protein quality is mainly evaluated by albumen height and Haugh units (Leng et al., 2014). However, the introduction of IDPG to the basal diet failed to influence either albumen height or Haugh units. In the further analysis of egg, both yolk color and yolk relative weight are also used to examine yolk quality, while the yolk relative weight directly reflects yolk quality. Results from the current study did not show significant effect on yolk color and yolk relative weight when our laying hen diets included with IDPG. In summary regarding egg quality, supplemental IDPG to the basal diet of laying hens can improve eggshell strength in the age of 68 to 72 wk, but no significant influence was seen to the quality of either protein or yolk.
Generally, the nutrients digested and absorbed by animals and the hormones that regulate various functions of the body are mainly transported to various organs of the body through blood circulation. Therefore, the content of certain components in serum reflects the digestive metabolism of body. Serum protein levels reflect the condition of an organism and the changes happening to it under the influence of internal and external factors. The experimental results showed that serum ALB content was increased significantly by the inclusion of IDPG (except the level of 0.01%), while the content of GLB and TP did not statistically vary among treatments. The significant change in serum ALB indicates that the usage of IDPG may promote nutrient metabolism in laying hens, which is consistent with previous findings regarding production performance (Toghyani et al., 2010).
Apart from the protein content in the serum, we also measured certain catabolic products of protein: BA and BUN. Urea nitrogen is the main product of nitrogen metabolism, and the BUN level is an indicator reflecting ongoing protein metabolism within the animal body (Stanley et al., 2010). Increased BUN levels and decreased BA concentrations reflect improvement of protein catabolism. Our results showed that dietary inclusion of IDPG significantly decreased the content of BA (P < 0.05), but had an insignificant effect on the increase of BUN content. This indicates some enhancement of protein catabolism could be taken place in the laying hens being fed IDPG, which is congruent with the findings of Matur et al. (2018).
Recent industrial and research interest has been spent investigating different polysaccharide degradation products for their capabilities of lowering cholesterol levels. Idea et al. have observed that serum cholesterol level and triglyceride level were significantly lowered in the rat fed diet with PHGG (Idea et al., 1991). Consistent with the previous results, our supplementation of IDPG into laying hen's basal diet markedly reduced triglyceride contents in both the serum and liver. It has been reported that inhibition of hepatic lipogenesis is the major mode of action for the triglyceride-lowering effects of fructo-oligosaccharide (Delzenne and Kok, 1999). Based on this, it is possible that the consumption of IDPG can provide the decreases to serum triglyceride contents through this pathway. However, no significant effect of IDPG was observed on the cholesterol content in serum and liver.
CONCLUSION
Results from the current study indicated that dietary supplementation of IDPG at 0.025% or 0.05% increased egg production and eggshell strength, and decreased FCR, each at statistically significant levels (P < 0.05). Meanwhile, the IDPG supplementation exhibited lower blood ammonia content, and greater protein for the liver than the control treatment. Furthermore, IDPG supplementation in the basal diet can also beneficially modulate lipid metabolic profiles in laying hens. These results cumulatively indicate the value of including IDPG in basal feed for laying hens. More importantly, the precursor material of Sesbania cannabina for preparing IDPG is an annual herb and is widely cultivated, thereby IDPG is sustainable and renewable. However, further research is needed to elucidate the mechanism by which IDPG improves production performance in laying hens.
ACKNOWLEDGMENTS
This work was supported by the National Key R&D Program of China (2016YFD0600803) and the Priority Academic Program Development of Jiangsu Higher Education Institution (PAPD).
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Asli M.M., Hosseini S.A., Lotfollahian H., Shariatmadari F. Effect of probiotics, yeast, vitamin E and vitamin C supplements on performance and immune response of laying hen during high environmental temperature. Int. J. Poult. Sci. 2007;6:895–900. [Google Scholar]
- Bozkurt M., Binta E., Seyrek K. Comparative evaluation of dietary supplementation with mannan oligosaccharide and oregano essential oil in forced molted and fully fed laying hens between 82 and 106 weeks of age. Poult. Sci. 2018;95:2576–2591. doi: 10.3382/ps/pew140. [DOI] [PubMed] [Google Scholar]
- Bozkurt M., Küçükyilmaz K., Çatli A.U., Çınar M., Binta E., Çöven F. Performance, egg quality, and immune response of laying hens fed diets supplemented with mannan-oligosaccharide or an essential oil mixture under moderate and hot environmental conditions. Poult. Sci. 2018;91:1379–1386. doi: 10.3382/ps.2011-02023. [DOI] [PubMed] [Google Scholar]
- Bozkurt M., Tokuşoğlu Ö., Küçükyilmaz K., Akşit H., Çatli A.U., Seyrek K., Çinar M. Effects of dietary mannan oligosaccharide and herbal essential oil blend supplementation on performance and oxidative stability of eggs and liver in laying hens. Ital. J. Anim. Sci. 2016;11:e41. [Google Scholar]
- Cowan R.S., Allen O.N., Allen E.K. The Leguminosae: a source book of characteristics, uses, and nodulation. Taxon. 1982;31 [Google Scholar]
- Delzenne N.M., Kok N.N. Biochemical basis of oligofructose-induced hypolipidemia in animal models. J. Nutr. 1999;29:1467S–1470S. doi: 10.1093/jn/129.7.1467S. [DOI] [PubMed] [Google Scholar]
- Ding L.X.M., Bai K.Z.S.P., Zeng J.P.W.Q.F., Science A.F. Effects of dietary xylooligosaccharides on the performance, egg quality, nutrient digestibility and plasma parameters of laying hens. Anim. Feed Sci. Tech. 2016;225:20–26. [Google Scholar]
- Eltan Ö., Çakın K., Yalçın S., Dağaşan L., Yalçın S. Effects of dietary yeast autolysate (Saccharomyces cerevisiae) on performance, egg traits, egg cholesterol content, egg yolk fatty acid composition and humoral immune response of laying hens. J. Sci. Food Agric. 2010;90:1695–1701. doi: 10.1002/jsfa.4004. [DOI] [PubMed] [Google Scholar]
- Feng L., Yin J., Nie S., Wan Y., Xie M. Structure and conformation characterization of galactomannan from seeds of Cassia obtusifolia. Food Hydrocoll. 2018;76:67–77. [Google Scholar]
- Gao J., Zhang H.J., Yu S.H., Wu S.G., Yoon I., Quigley J., Gao Y.P., Qi G.H. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult. Sci. 2008;87:1377–1384. doi: 10.3382/ps.2007-00418. [DOI] [PubMed] [Google Scholar]
- Ghasemian M., Jahanian R. Dietary mannan-oligosaccharides supplementation could affect performance, immunocompetence, serum lipid metabolites, intestinal bacterial populations, and ileal nutrient digestibility in aged laying hens. Anim. Feed Sci. Tech. 2015;213:81–89. [Google Scholar]
- Gu J., Pei W., Tang S., Yan F., Peng Z., Huang C., Yang J., Yong Q. Procuring biologically active galactomannans from spent coffee ground (SCG) by autohydrolysis and enzymatic hydrolysis. Int. J. Biol. Macromol. 2020;149:572–580. doi: 10.1016/j.ijbiomac.2020.01.281. [DOI] [PubMed] [Google Scholar]
- Hassanein S.M., Soliman N.K. Effect of probiotic (Saccharomyces cerevisae) adding to diets on intestinal microflora and performance of Hy-line layer hens. J. Am. Sci. 2010;6:159–169. [Google Scholar]
- Hidehisa T., Yang S.I., Mujo K., Takehiko Y. Protein and energy utilization of growing rats fed on the diets containing intact or partially hydrolyzed guar gum. Comp. Biochem. Physiol. – Part A Physiol. 1994;107:255–260. [Google Scholar]
- Hossain M.A., Focken U., Becker K. Galactomannan-rich endosperm of Sesbania ž Sesbania aculeata /seeds responsible for retardation of growth and feed utilisation in common carp, Cyprinus carpio L. Aquaculture. 2001;203:121–132. [Google Scholar]
- Hossain M.A., Focken U., Becker K. Nutritional evaluation of dhaincha (Sesbania aculeata) seeds as dietary protein source for tilapia Oreochromis niloticus. Aquac. Res. 2002;33:653–662. [Google Scholar]
- Hossain M.A., Focken U., Becker K. Antinutritive effects of galactomannan-rich endosperm of Sesbania (Sesbania aculeata) seeds on growth and feed utilization in tilapia, Oreochromis niloticus. Aquac. Res. 2003;34:1171–1179. [Google Scholar]
- Idea T., Moriuchib H., Nihimotoh K. Hypolipidemic effects of guar gum and its enzyme hydrolysate in rats fed highly saturated fat diets. Ann. Nutr. Metab. 1991;35:34–44. doi: 10.1159/000177619. [DOI] [PubMed] [Google Scholar]
- Jahanian R., Ashnagar M. Effect of dietary supplementation of mannan-oligosaccharides on performance, blood metabolites, ileal nutrient digestibility, and gut microflora in Escherichia coli-challenged laying hens. Poult. Sci. 2018;94:2165. doi: 10.3382/ps/pev180. [DOI] [PubMed] [Google Scholar]
- Jahanian E., Mahdavi A.H., Asgary S., Jahanian R. Effect of dietary supplementation of mannanoligosaccharides on growth performance, ileal microbial counts, and jejunal morphology in broiler chicks exposed to aflatoxins. Livest. Sci. 2016;190:123–130. [Google Scholar]
- Koiyama N.T.G., Utimi N.B.P., Santos B.R.L., Bonato M.A., Barbalho R., Gameiro A.H., Araújo C.S.S., Araújo L.F. Effect of yeast cell wall supplementation in laying hen feed on economic viability, egg production, and egg quality. J. Appl. Poult. Res. 2018;27:116–123. [Google Scholar]
- Leng X., Hsu K.N., Austic R.E., Lei X.G. Effect of dietary defatted diatom biomass on egg production and quality of laying hens. J. Anim. Sci. Biotechnol. 2014;5:1–7. doi: 10.1186/2049-1891-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Liu L., Li K., Hao K., Xu C. Effect of fructooligosaccharides and antibiotics on laying performance of chickens and cholesterol content of egg yolk. Br. Poult. Sci. 2007;48:185–189. doi: 10.1080/00071660701261310. [DOI] [PubMed] [Google Scholar]
- Matur E., Ergul E., Akyazi I., Eraslan E., Cirakli Z.T. The effects of Saccharomyces cerevisiae extract on the weight of some organs, liver, and pancreatic digestive enzyme activity in breeder hens fed diets contaminated with aflatoxins. Poult. Sci. 2018;89:2213–2220. doi: 10.3382/ps.2010-00821. [DOI] [PubMed] [Google Scholar]
- Olvera M.A., Martinez C.A., Galvan R., Chavez C. The use of seed of the leguminous plant Sesbania grandiflora as a partial replacement for fish meal in diets for tilapia (Oreochromis mossambicus) Aquaculture. 1988;71:51–60. [Google Scholar]
- Shobha M.S., Tharanathan R.N. Nonspecific activity of Bacillus acidopullulyticus pullulanase on debranching of guar galactomannan. J. Agric. Food Chem. 2008;56:10858–10864. doi: 10.1021/jf801483j. [DOI] [PubMed] [Google Scholar]
- Sinha A.K., Kumar V., Makkar H.P.S., De Boeck G., Becker K. Non-starch polysaccharides and their role in fish nutrition – a review. Food Chem. 2020;127:1409–1426. [Google Scholar]
- Stanley C.C., Williams C.C., Jenny B.F., Fernandez J.M., Bateman H.G., Nipper W.A., Lovejoy J.C., Gantt D.T., Goodier G.E. Effects of feeding milk replacer once versus twice daily on glucose metabolism in Holstein and Jersey calves. J. Dairy Sci. 2010;85:2335–2343. doi: 10.3168/jds.S0022-0302(02)74313-0. [DOI] [PubMed] [Google Scholar]
- Tao Y., Yang L., Lai C., Huang C., Li X., Yong Q. A facile quantitative characterization method of incomplete degradation products of galactomannan by ethanol fractional precipitation. Carbohydr. Polym. 2020;250 doi: 10.1016/j.carbpol.2020.116951. [DOI] [PubMed] [Google Scholar]
- Tapingkae W., Panyachai K., Yachai M., Doan H.V. Effects of dietary red yeast (Sporidiobolus pararoseus) on production performance and egg quality of laying hens. J. Anim. Physiol. Anim. Nutr. (Berl). 2018;102:e337–e344. doi: 10.1111/jpn.12751. [DOI] [PubMed] [Google Scholar]
- Toghyani M., Toghyani M., Gheisari A., Ghalamkari G., Mohammadrezaei M. Growth performance, serum biochemistry and blood hematology of broiler chicks fed different levels of black seed (Nigella sativa) and peppermint (Mentha piperita) Livest. Sci. 2010;129:173–178. [Google Scholar]
- Xie Y., Huang C., Li X., Lai C., Yu S., Yong Q. Effect of agitation speed and aeration rate on fermentation synthesis of β-mannanase with Trichoderma reesei. J. For. Eng. 2017;2:92–96. [Google Scholar]
- Yalçin S., Yalçin S., Uzunoǧlu K., Duyum H.M., Eltan Ö. Effects of dietary yeast autolysate (Saccharomyces cerevisiae) and black cumin seed (Nigella sativa L.) on performance, egg traits, some blood characteristics and antibody production of laying hens. Livest. Sci. 2012;145:13–20. [Google Scholar]
- Yamatoya K., Kuwano K., Suzuki J. Effects of hydrolyzed guar gum on cholesterol and glucose in humans. Food Hydrocoll. 1997;11:239–242. [Google Scholar]
- Yenİce E., Mizrak C., Ceylan N. Effects of dietary sodium bentonite and mannan oligosaccharide supplementation on performance, egg quality, blood and digestion characteristics of laying hens fed aflatoxin contaminated diet. Kafkas Universitesi. Veteriner Fakultesi Dergisi. 2015;21:211–218. [Google Scholar]