Abstract
Polymeric biomaterials have been used in a variety of applications, like cargo delivery and tissue scaffolding, because they are easily synthesized and can be adapted to many systems. However, there is still a need to further enhance and improve their functions to progress their use in the biomedical field. A promising solution is to modify the polymer surfaces with peptides that can increase biocompatibility, cellular interactions, and receptor targeting. In recent years, peptide modifications have been used to overcome many challenges to polymer biomaterial development. This review discusses recent progress in developing peptide-modified polymers for therapeutic applications including cell-specific targeting and tissue engineering. Furthermore, we will explore some of the most frequently studied base components of these biomaterials.
Keywords: polymers, peptide modifications, polymeric therapeutics, targeting, tissue engineering
Graphical Abstract
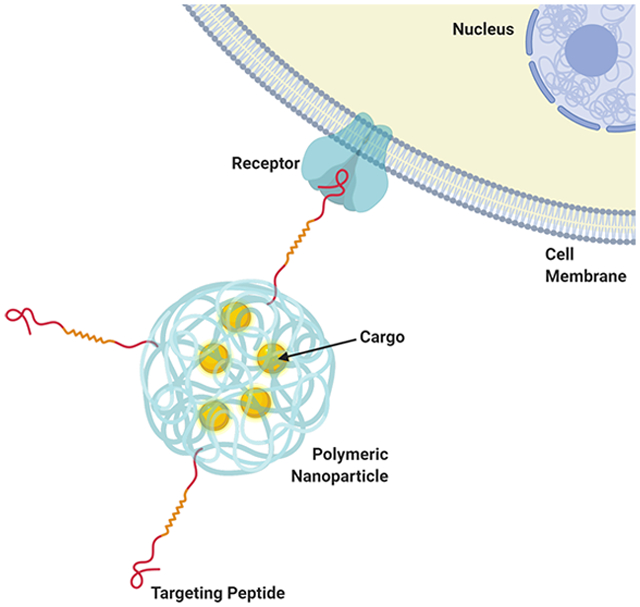
1. INTRODUCTION
Polymers are long-chain molecules formed with repeating units and are commonly used as plastics (such as polypropylene) and fibers (such as nylon).1 They are extremely versatile materials that also have a wide range of applications in the biomedical field. They are extremely adaptable, are easy to synthesize and functionalize, and can be biodegradable and biocompatible.1–5 Polymers have a long history in the biomedical field, and their applications have been constantly changing and evolving. The first polymer biomaterial used was poly(methyl methacrylate), which was used for tooth fillings in the 1930s.6 Specific applications of polymer biomaterials from the 30s through the mid-90s are thoroughly covered in the review by Moukwa.6 Polymer nanoparticles were first studied in 1969 by Dr. Speiser for biopharmaceutical applications.7 This represented a new era of polymer biomaterials, as they were now being studied for drug delivery and targeted therapeutic purposes. Ten years later, the first reports surfaced of polymer nanoparticles being used for cancer therapy, indicating further developments in this area.8 Taking polymers in another direction, the first biologically active polymer scaffold was created in 1974, utilizing collagen for tissue engineering.9
Despite all of these achievements, the use of polymers has been limited by difficulties with stability, aggregation, toxicity, and minimal cellular interaction.1–5 Peptide-modification of surfaces is one method that can alleviate many of the challenges associated with a given polymer by enhancing biocompatibility, targeting capabilities, cellular interactions, and more. Thanks to advances from researchers such as Robert Merrifield, who first demonstrated solid-phase peptide synthesis,10 and George Smith, who pioneered phage display technology,11 peptide-modified polymers became a significant research focus in the mid 1990s and have since blossomed into an extremely fruitful field. Advances made in this area have demonstrated how these peptide modifications can both overcome challenges with and add new capabilities to polymer biomaterials.
Peptide-modified polymers are extremely versatile tools in biomaterial design, making them of great interest to researchers in all areas of the field. Figure 1 depicts a timeline showing the evolution and merging of research into polymer and peptide biomedical applications. This area of research began just a few decades ago and has already produced fascinating and promising results.8–11 In this review, we first discuss the basic components to formulating peptide-modified polymer biomaterials. We then discuss recent applications of these biomaterials from the past decade as well as how peptides have been used to overcome challenges and enhance the properties of biopolymers in these applications.
Figure 1.
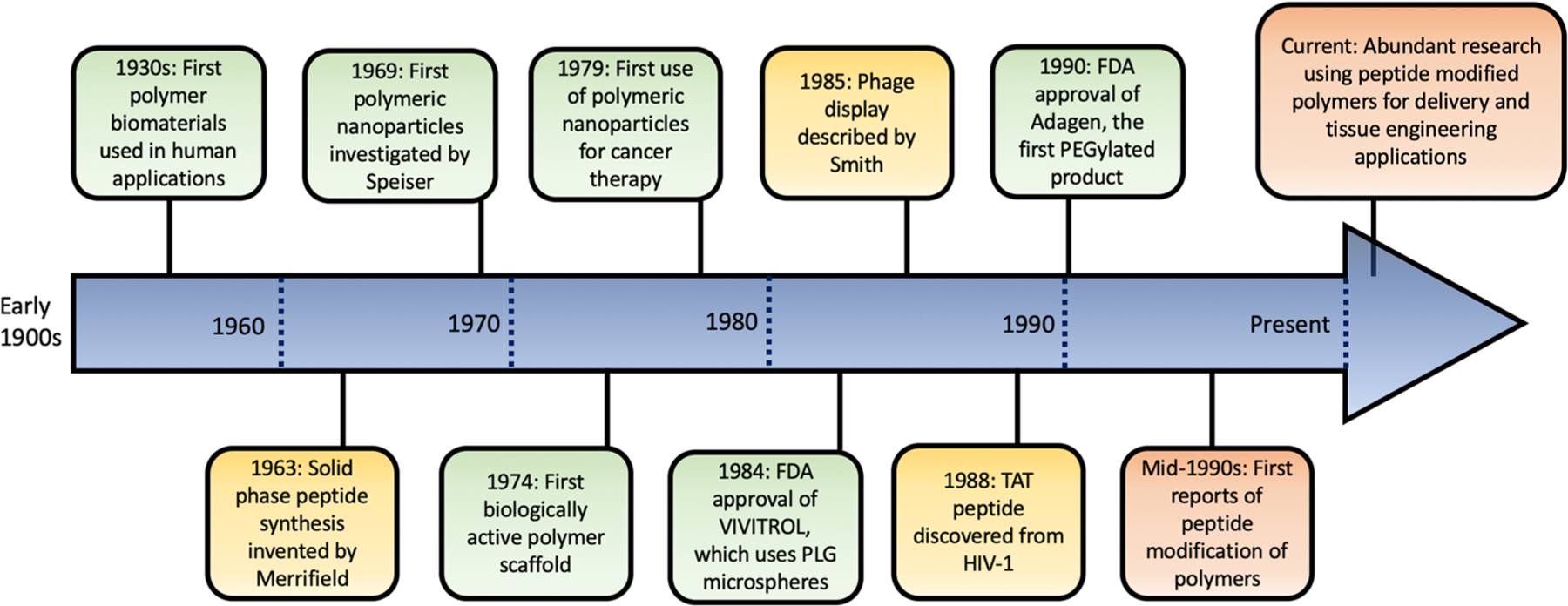
Timeline depicting some major discoveries made regarding polymer and peptide development for biomedical applications.
2. BASIC COMPONENTS
Polymers and peptides are the building blocks of the biomaterials discussed in this review. It is essential to understand the most important characteristics of frequently used polymers as well as which peptides are commonly used and how they are derived. In this section, an overview of the basic components will be given to prepare the reader for the applications which are described in section 3.
2.1. Polymers.
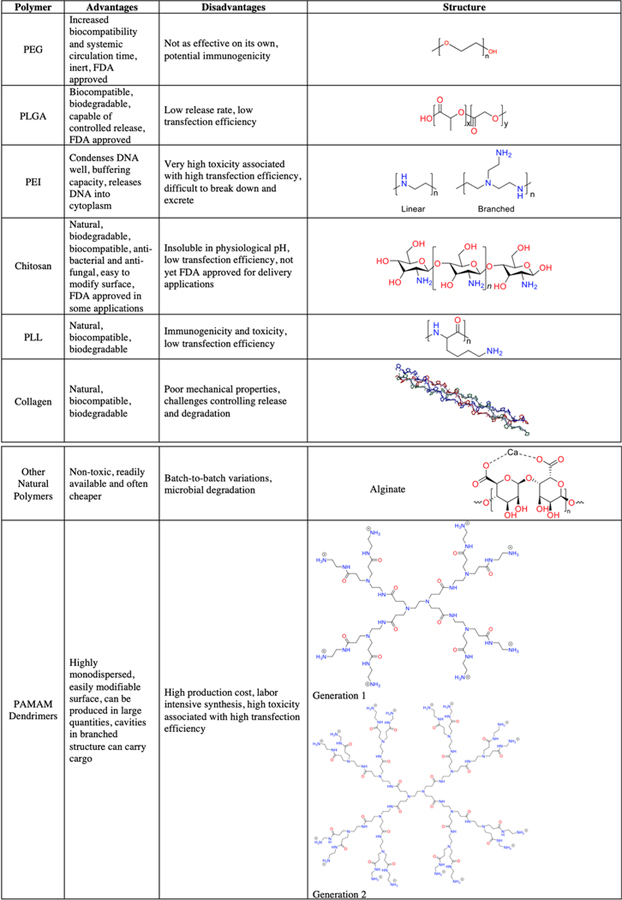
Polymers are extremely versatile and have a vast array of properties.1–5 Developments in medicine have provided us with a plethora of therapies which can be used to treat a variety of diseases, and polymer biomaterials have assumed an integral role in the construction of delivery vesicles for these therapies.1–5 Polymer scaffolding is also frequently used as implant material in tissue engineering to aid in cell growth and proliferation.33 Table 1 summarizes some advantages and disadvantages of various polymers and depicts their structures. Here, we briefly describe some of the most commonly used polymers and their important properties. Each polymer overview will also guide the reader to focused reviews on these materials as they have been covered thoroughly in the literature.
Table 1.
Summary of Different Types of Polymers Used, Written in Their Common Abbreviations, in Biomedical Applications4,12–32
|
2.1.2. Polyethylene Glycol (PEG).
PEG is one of the most frequently utilized polymers and is one of the few polymers that has been FDA approved in drug and pharmaceutical applications.34 PEG molecules are hydrophilic and will self-assemble into amphiphilic molecules when joined to hydrophobic polymers.12 The molecular weights used are generally 400–40 000 g/mol.35 While it can be used as the primary polymer to form a biomaterial, it is most frequently used as a modifying polymer. Polymeric and lipid nanoparticles can be coated with PEG in a process known as PEGylation, a process that results in materials of increased stability, reduced aggregation, and increased biocompatibility and is thus frequently used in designing delivery vesicles.12 PEG is also often combined with other polymers to form copolymers which take advantage of the properties of both PEG and the other polymer.36,37 Because of the hydroxyl groups at either end of the PEG molecule, it is easy to modify it so it can be attached to almost any other molecule. The PEGylation process has been reviewed extensively by Suk et al.12
2.1.3. Poly(lactic-co-glycolic acid) (PLGA).
PLGA polymers are negatively charged copolymers made of glycolic acid and lactic acid. PLGA is often used in biomaterials because it is biodegradable, biocompatible, and can protect cargo from degradation.30 PLGA molecular weights are typically between 10 000 and 20 000 g/mol, but higher molecular weights can be obtained.38 The ratio of lactic acid to glycolic acid can be varied to alter properties like circulation time and degradation rate.31 PLGA is also FDA approved and has been studied extensively, resulting in numerous applications,31 and has been adapted as a biomaterial for delivery32 and tissue engineering applications.39 PLGA and its applications are extensively reviewed by Xu et al., Danhier et al., and Mir et al.30–32
2.1.4. Polyethylenimine (PEI).
PEI polymers are positively charged branched or linear polymers.40,41 They are most frequently used for gene delivery applications because their charge enables them to encapsulate nucleic acids and they are able to achieve high transfection efficiencies.42 PEI has a wide range of molecular weights, from as low as 600 g/mol to as high as 800 000 g/mol and the effective range varies depending on the application.13 The amine groups enable it to be easily modified and functionalized. However, PEI is not very biocompatible, presenting a challenge for in vivo applications.41 PEI biomaterials can benefit greatly from methods like peptide-modification or PEGylation to increase biocompatibility while still maintaining high transfection efficiency. Properties of PEI and gene delivery applications were recently reviewed by Pandey et al. and Zou et al.13,14
2.1.5. Polyamidoamine (PAMAM) Dendrimers.
Polyamidoamine dendrimers are highly monodisperse, branched structures made up of repeating layers, abundant surface amine or carboxylate groups, and internal cavities.15 PAMAM dendrimers use generation numbers to reflect the number of concentric layers of branched monomers, and the number of surface groups doubles with each additional layer. Whole numbers, such as G2 or G5, have surface amine groups, while half numbers, such as G1.5 or G2.5, have surface carboxylate groups.43 Regardless of the generation, their surfaces have many functional groups allowing for easy modification. Because of their extremely controlled structure and advantageous surface properties, they are frequently studied for biomedical applications. However, they have challenges associated with high cytotoxicity and low biocompatibility which have limited their progress in clinical applications.4,16 These challenges can be overcome with peptide-modifications. Zhong et al. and Labieniec-Watala et al. have recently reviewed the applications and properties of PAMAM dendrimers.15,16
2.1.6. Chitosan.
Chitosan is a natural polymer that is derived from the deacetylation of chitin.27 Chitin is naturally produced by organisms such as mollusks and insects, making it very abundant and, therefore, economical to obtain. Chitosan is nontoxic, biodegradable, and has antibacterial and antifungal properties.17,27 Additionally, it has a positive surface charge that enables electrostatic interactions to bind anionic cargo like DNA. For gene therapy applications, chitosan can protect DNA from nuclease degradation.44 Finally, the surface is very easily modifiable due to the extensive hydroxyl and amine groups, allowing for the binding of ligands such as peptides.45 Chitosan is generally characterized by its molecular weight and degree of acetylation, both of which have a major impact on applications.46 Chitosan has been recently reviewed extensively by Ahsan et al., and Muxika et al., and Bellich et al. covered the physicochemical properties.17,18,46
2.1.7. Poly-l-lysine (PLL).
Poly-l-lysine is a natural, biodegradable, cationic polypeptide that is known to interact with and adhere to cells.47 Because of this, it has been studied in delivery applications for anionic cargo such as DNA. However, PLL does not release well from endosomes, resulting in low delivery and transfection efficiencies. PLL can also have adverse immunogenicity and toxicity, making modifications desirable.28 Francoia et al. reviewed properties and applications of PLL polymers.19
2.1.8. Collagen.
Collagen is a protein that is found in the extracellular matrix and is the most abundant protein in the animal kingdom.48 This protein cross-links to form scaffolding, making it a highly biocompatible, biodegradable, natural polymer.49 Collagen is frequently cross-linked or blended with other materials, including other types of polymers, to enhance its mechanical properties.20 Some limitations of collagen include difficulty in controlling release and degradation rate.49 Collagen has been used in drug delivery, tissue engineering, injectables, and sponges for wounds.29,49 Applications of collagen have been reviewed recently by Dong et al. and An et al.20,21
2.1.9. Other Natural Polymers.
There are many other natural polymers that could be adapted for targeted delivery and tissue engineering but have not yet been explored as thoroughly as the other polymers. Chitosan and collagen are the most heavily studied, and other natural polymers include gelatin, alginates, fibrin, and hyaluronic acid.50 Natural polymers have the benefits of being highly nontoxic, readily available, and cheaper than many synthetic polymers.22,50 However, unlike their synthetic counterparts, natural polymers can have batch to batch variations and are susceptible to microbial degradation.22,24 Kulkarni et al., Garg et al. and Samadian et al. recently reviewed biomedical applications of natural polymers.22,24,25
2.2. Peptides.
Peptides are short chains of two to 50 amino acids linked by peptide bonds. Peptides encode a primary sequence that can fold into secondary structures supporting a diverse and complex array of functions. A variety of hybrid materials could be prepared by combining biological molecules such as peptides and proteins with polymers.51 However, challenges in controlling sites of protein modification and in maintaining protein structure have fueled continued interest in making peptide functionalized hybrid materials with polymers. Additionally, function of natural proteins could be mimicked by designing peptides that borrow from the specific secondary and tertiary structure of the protein. Self-assembling peptides have also been used in the development of functional hydrogels with exquisite biological, mechanical and material properties.52 Peptides can be designed to maintain selectivity, specificity, and dynamic responses to external stimuli at the molecular level. The versatility of chemical functionality available in peptides can be taken advantage of in chemical modification of polymers with peptides. Although the physical limitations of biomolecules, such as their sensitivity to temperature, pH, organic solvents, and degradation, is a problem, this is much less of an issue when using peptides compared to proteins. Peptide sequences can be designed such that they have secondary structure and can also form a desired tertiary structure. These peptide–polymer hybrid materials can be designed to contain novel structures and functions for a wide range of biomedical and nonbiomedical applications.
Modification of polymers with peptides impart many useful properties to polymers. For example, peptide-modifications enhance the biocompatibility of polymers and provide them with new characteristics and properties needed for bio-recognition.53 Peptides that interact with specific receptors and ligands can be identified through phage display, making them tunable for a desired biomedical application.53,54 Peptide-modifications can enhance polymers for drug and gene delivery applications and increase cell attachment in tissue applications.53,55 Compared to proteins, they have the benefits of being smaller in size and easier to synthesize, which allows for making hybrid materials with polymers that are stable and compact. Additionally, they can increase biocompatibility by decreasing cytotoxicity and immunogenicity of the polymeric carrier molecule.53–56 Peptides can be used to make hierarchical structures with controlled geometry on polymers that result in the creation of novel material. Peptide functionalization can impart hydrophilic characteristics to hydrophobic polymers improving the solubility of the hybrid material. In the subsections below, we describe phage display method of selecting peptides as well as some common types of peptides employed in modification of polymers used in biomedical applications.
2.2.1. Identifying Peptides from Phage Display.
Phage display is a very common method of identifying peptides. Phages are bacterial viruses that can be engineered to carry foreign DNA inserts and display peptides on their surfaces.57 By developing a phage display library, peptides that have a high affinity for specific receptors can be selected and applied for the purpose of modifying a delivery system.57 Phages can display up to 10 billion peptides on their surface, making them extremely useful for identifying their binding targets.58 Phage libraries can be injected into mice to determine which peptides have targeting capabilities.59 Some researchers even use phage display libraries to determine which peptides will specifically bind to their polymer. For example, one group used phage display techniques to identify a specific binding peptide for the polymer PPyCl.60 This enabled the identification of ligands that can bind other modifiers, like antibodies, without altering the polymer properties. This method of identifying which peptides can bind to a desired target is invaluable to researchers working on creating delivery biomaterials.
2.2.2. Cell-Penetrating Peptides (CPP).
Cell-penetrating peptides are short, cationic peptides that are commonly used by researchers because they aid in fast transportation of biomaterials across cell membranes. They have been used to deliver various cargo including drugs and genes.61 Some CPPs can be activated and controlled by temperature. This feature allows an optimized targeted delivery of drugs into specified tissues, like tumors that need focal intervention.62 CPPs can even transport macromolecules across strong membrane barriers like the blood brain barrier, gastro-intestinal mucosa, and skin dermis.63
The Tat peptide, derived from the trans-activating transcriptional activator encoded by HIV-1, is one of the most frequently studied and well characterized CPPs.64 An 11-amino acid sequence from Tat (YGRKKRRQRRR) was identified to be the shortest peptide capable of stimulating cellular and nuclear uptake, making this specific sequence the most frequently used. Another example of a CPP is penetratin (RQIKIWFQNRRMKWKK), which was derived from the natural protein Antennapedia homeodomain.65,66 CPPs often contain large amounts of positively charged amino acids, which are thought to enhance internalization by interacting with the anionic cell surface.67 CPPs aid in increasing cellular uptake, a major barrier for most delivery systems, by translocating across the plasma membrane and facilitating endocytosis.56 In one study, modifying a PLGA nanoparticle with various CPPs resulted in enhanced cellular internalization via clathrin-mediated endocytosis.68 This and similar studies demonstrate how CPP modification can enhance cell internalization and therefore increase the efficacy of delivery biomaterials.
2.2.3. Arginine-Glycine-Aspartate (RGD)-Containing Peptides.
RGD-containing peptide sequences are capable of binding integrin and are frequently used in tissue engineering applications because of their ability to promote cell attachment and proliferation.69 RGD is known to be the minimal sequence capable of interacting with cells, resulting in the use of RGD-containing peptides when designing polymeric scaffolds.70 One example of an RGD-containing peptide is internalizing RGD (iRGD, CRGDKGPDC).71 Modification with RGD peptides allows biomaterials to imitate extracellular matrix proteins, thus enhancing interactions with cells and decreasing any immunogenic responses.72 RGD-containing peptides are used in many of the tissue engineering applications described later in this review.
2.2.4. Cell Targeting Peptides (CTP).
Cell targeting peptides are utilized for targeting of specific tissues mostly through the receptors. These peptides help facilitate specific targeting due to high affinity to a given cell and can enhance cellular uptake like CPPs.73 CTPs minimize off targeting effects, which can reduce adverse side effects, decrease the required dose, and enhance the therapeutic effect of cargo.55 While antibodies are used for specific targeting, CTPs are smaller and easier to synthesize.73 Antibodies also suffer from nonspecific uptake to the liver and reticuloendothelial system, making CTPs a more optimal targeting moiety.74
Tumor targeting peptides (TTPs) are a subset of CTPs. Researchers in nanomedicine have been exploring ways to reduce off targeting effects and increase efficacy of cancer treatments.75,76 Tumor targeting peptides function to increase specificity to cancer cells to minimize these adverse reactions.77 RGD peptides can also function as TTPs for cancer cells with elevated αvβ3 integrin receptors.75
CTPs and TTPs are often identified using phage display, as explained above, but can also be derived from known proteins and molecules. For example, some researchers will derive peptides by studying bacteria and how they interact with cells.78,79 R. Liu et al. extensively covered many ways to identify TTPs, and the methods they describe can be applied to all types of CTPs.77
2.3. Peptide–Polymer Conjugation Strategies.
There are many different approaches and methodologies for conjugating peptides to polymers and unfortunately there is no single optimal method that can be universally applied to all conjugations. One strategy that gives the best results can be completely inappropriate for another application. Each conjugation strategy needs to be considered carefully for the impact and the suitability of each technique and their impact on the production of the desired construct. Some factors need to be considered before the reaction is undertaken, such as the biorthogonality of the conjugation techniques to other conjugations schemes, reaction rates and the efficiencies of the selected reactions, cost of the chemicals as well as the surface orientation of the peptides on the polymeric nanomaterials all play a crucial role.80 Therefore, these type of conjugation strategies has been a great interest for the scientific community and many different conjugation strategies have been developed. These conjugation strategies have been described in several excellent reviews.51,54,80–83
Briefly, these conjugation strategies can be split into covalent and noncovalent conjugations. Noncovalent conjugations are a set of tools used to establish a conjugation between the peptide of interest and the biopolymers. These conjugations consist of electrostatic interactions, peptidic binding sequences, self-assembling peptides, host–guest chemistries, biotin–streptavidin interactions, nucleic acids, and their hybridization products. For simple electrostatic interactions, a positively charged peptide can be attached to the positively charged polymeric materials such as alginate gels84 or DNA molecules can be condensed on positively charged polymers.85
Another example of a noncovalent conjugation is the use of host–guest chemistries. For example, Boekhoven et al. utilized the interaction between the β-cyclodextrin (β-CD) modified alginate with naphtyl modified RGDS peptide to attach the RGDS peptide noncovalently to the alginate polymer and turn on or off the biological activity of the biopolymer by displacing the RGDS peptide with RGES peptide conjugated to an adamantyl group.86 Interactions between the adamantyl group and the β-CD moiety is about 30x stronger, therefore it can replace the naphtyl group thereby turning the biological activity of the RGDS peptide off. This is one of the main advantages of the noncovalent interactions where binding activity can be turned on or off depending of the binding partner used.
Another very commonly used noncovalent conjugation is the exploitation of the binding interactions between streptavidin and biotin. Biotin moiety is one of the most commonly used binding moiety because it is a small molecule and contains a carboxylic functional group which can be readily modified. Since the biotin–streptavidin interaction is one of the strongest interactions in nature, with dissociation constants in the range of 10–14–10–15 M, biotin–streptavidin interaction can be viewed as a covalent conjugation, a property which makes this interaction one of the most widely used one. Biotinylation of peptides can be achieved using the nonspecific lysine modifications,87 or the peptide modifying enzyme, biotin ligase, can be used to site-specifically biotinylate a specific peptide sequence. This peptide sequence, which is a short 15-amino acid sequence, commercially known as the AviTag enables efficient biotinylation of the peptide sequences.88
Many of the covalent conjugation strategies of peptides to the biopolymers are based on the more general chemical reactions that were developed for normal chemical synthesis. These covalent conjugations strategies can be divided into two broad categories: targeting a specific site for modification, and modifications that modify multiple groups promiscuously. This concept of selectivity is crucial for bioconjugation strategies since it has a profound effect on the biological activity of the final product. However, since the diversity of the chemical functionality is very limited in biomolecules, achieving desired selectivity can be extremely difficult.89 To achieve the desired selectivity, the following general strategies can be employed: targeting a single motif, among many others, by exploiting subtle differences in reactivities such as targeting the N-terminal amine groups, site-specific incorporation of unnatural functionalities, or incorporation of these unique reactivities through chemical synthesis.
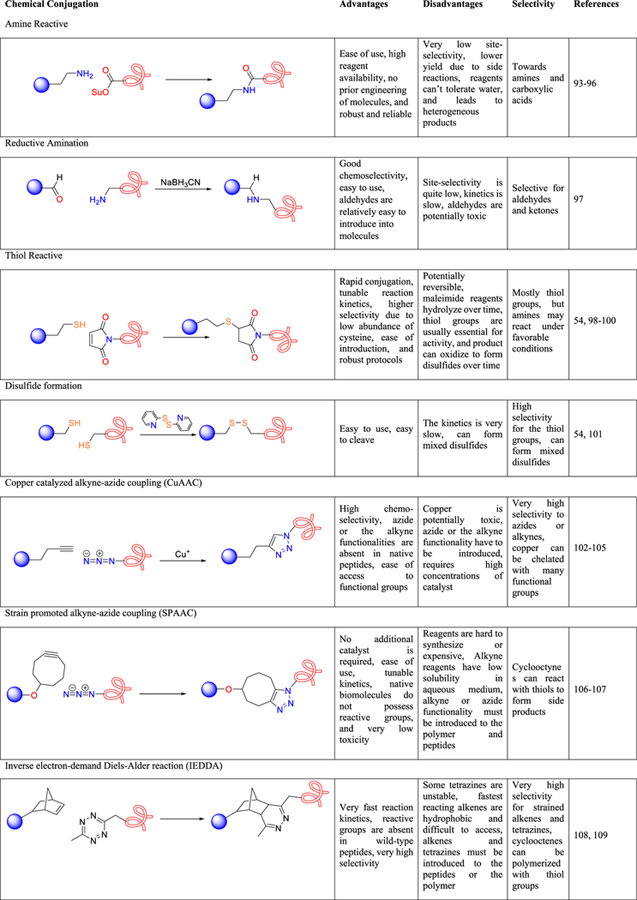
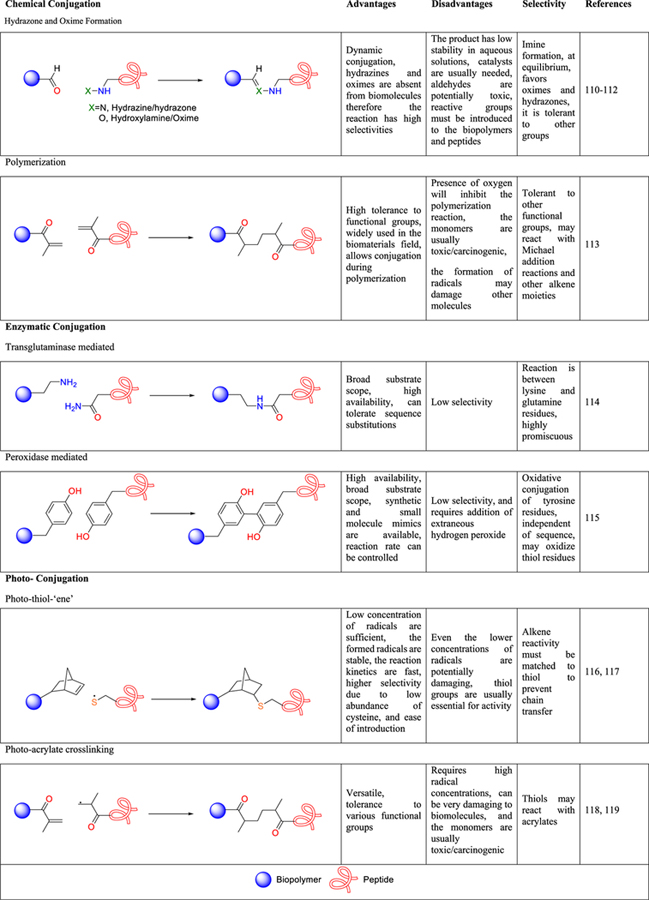
Chemical conjugation strategies are among the most widely applied methods for creating peptide–polymer conjugates, and there are many reactive handles that one can employ and due to space constraints going into the details of each reaction is not possible. Therefore, the readers are referred to many excellent reviews on these modification strategies that have been published.54,89–92 Some of the most commonly employed covalent modification strategies, their selectivities, advantages and disadvantages, and some representative literature examples are summarized in Table 2.
Table 2.
Key Covalent Conjugation Methods: Their Advantages, Disadvantages, Selectivities, and Some Representative References
|
3. APPLICATIONS OF TARGETED PEPTIDE-MODIFIED POLYMERS
3.1. Targeted Delivery.
There are a wide variety of therapies used in medicine including gene and drug therapies. These therapies can often have their intended effects enhanced by using targeted delivery methods, in which the therapy is delivered to a specific tissue or organ. These targeted therapies can dramatically reduce nonspecific uptake that causes adverse side effects in traditional drug therapies.120
In targeted delivery, a cargo such as nucleic acids or small-molecule therapeutics is bound to a carrier molecule for safe delivery to the target. This is beneficial because it brings the cargo to the area of interest and protects it from premature degradation. Gene therapies benefit greatly from targeted, vehicle-based delivery because this method provides a secure way to transport the genetic material. As DNA degradation is a common issue with gene therapies, delivery vessels can protect the DNA from being degraded before reaching the target.3,121 Additionally, targeted delivery significantly reduces off-target effects that commonly lead to collateral damage. For example, targeted delivery of chemotherapeutic agents can reduce adverse side effects while proving to be as effective and even potentially more effective at tumor reduction than traditional chemotherapeutic treatments.76
There are two main types of targeted delivery: passive targeting and active targeting (Figure 2). In passive targeting, the delivery molecules are injected directly into circulation or at the intended site and then accumulate by leveraging properties such as enhanced permeability and retention (EPR) effect. In this, a combination of high blood flow and poor lymphatic drainage sets up a choke point for macromolecular drugs and has been studied for applications like chemotherapeutic delivery to vascularized tumor tissues.122,123 Although the existence of such an effect is not universally accepted, passive targeting is still the most common method for delivery and persists in spite of issues such as bioavailability and off-target effects. Conversely, active targeting relies on the incorporation of a targeting ligand modification on the delivery molecules that can often be specifically tailored to a surface receptor on a desired cell or tissue.124,125 Although this method requires more complex synthetic chemistry and the identification of specific, high-affinity targeting ligands, the increased number of applications as well as the reduction in off-target effects warrants continued research.
Figure 2.
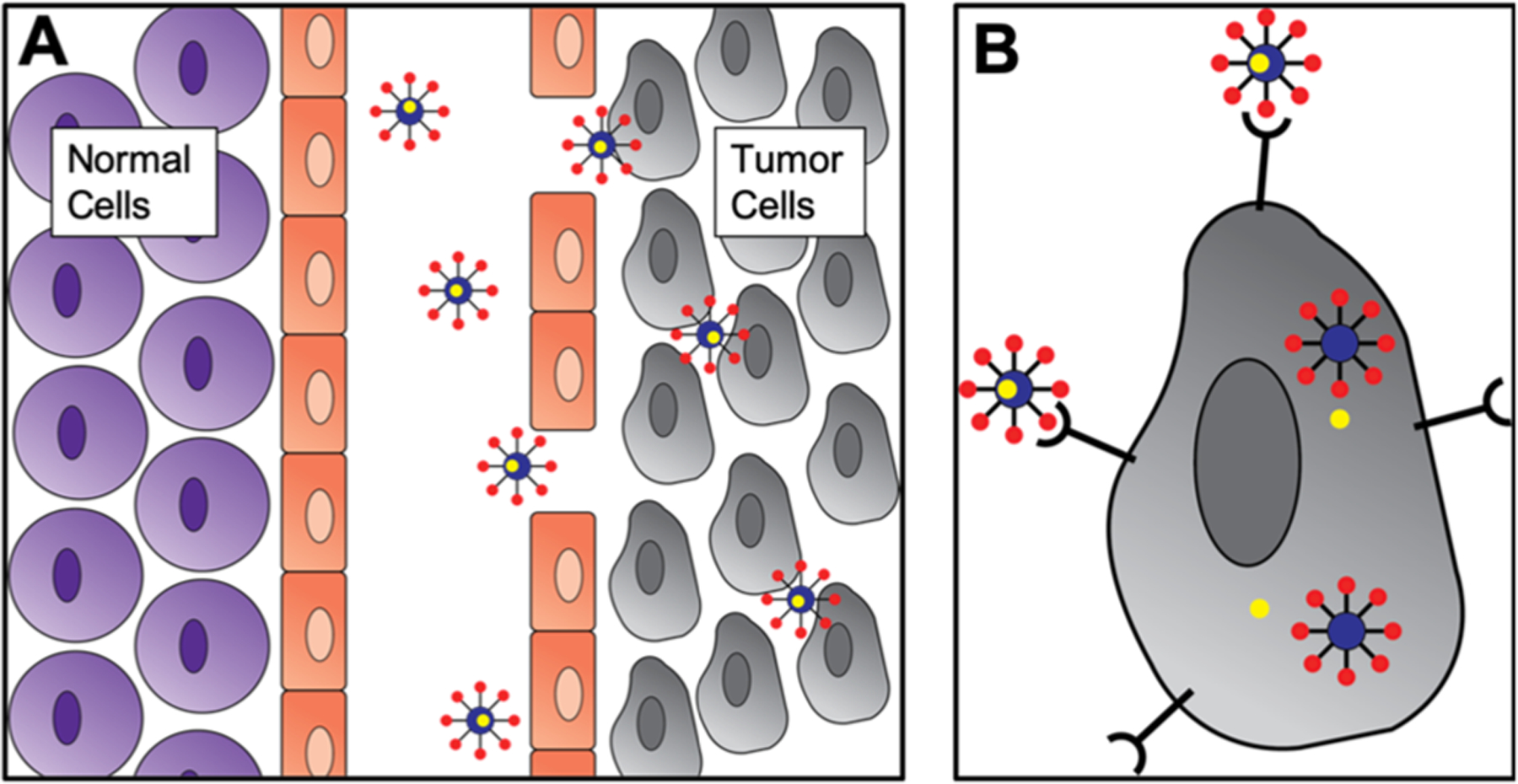
Passive and active targeting mechanisms. (a) Drug (yellow) loaded nanoparticles entering tumor cells through leaky vasculature. (b) Drug loaded nanoparticles entering tumor cells using receptor mediated targeting.
The process of targeting a specific cell or tissue begins with surface modification of a carrier with antibodies or recognition peptides, and this modification is frequently referred to as functionalization because it adds to or enhances a function of the carrier. This can be useful in delivery applications because it can also stimulate cellular uptake. Table 3 provides an overview of targeting/cell penetrating peptides and their targets, as discussed within this review.
Table 3.
Some Peptide Sequences and Their Targets
| peptide sequence | polymer used in study | target | cell type | ref |
|---|---|---|---|---|
| EPRNEEK | PLL | Laminin receptor | Brain cells | 78 |
| SYPGWSW | mPEG–PLA | Blood brain barrier | Brain cells | 79 |
| ASSLNIA | PAMAM | Skeletal muscle cells | Skeletal muscle cells | 85 |
| Tet1 (HLNILSTLWKYRC) | PEI, other polycations | Peripheral neurons | Brain cells | 133 |
| YQQVLTSLPSQNVLQI-ANDLENLRDLLHLL | PLL | Leptin receptor | Brain cells | 134 |
| CGHKAKGPRK | PEG–PLA | Transferrin receptor | Brain, cancer cells | 136 |
| RGDPAYQGRFL | PEO-b-PCL | Breast cancer cells | Breast cancer cells | l45 |
| WXEAAYQRFL | PEO-b-PCL | Breast cancer cells | Breast cancer cells | l45 |
| PGFAPLTSRGSQQYAA | P(LA-co-TMCC)-g-PEG | Taxane chemotherapeutic agents | Drug loading | 147 |
| YHWYGYTPQNVIGGGGC | PAMAM–PLA–OH | EGFR receptor | Triple negative breast cancer | 148 |
| IFLLWQR | Aldehyde–PEG-PLA and mPEG–PLA | Annexin 1 | Breast cancer | 149 |
| YLFFVFER | PEG–PAMAM | HER2 | Breast cancer cells | 150 |
| KTLLPTP | PEGylated PEI | Plectin-1 | Pancreatic cancer cells | 153 |
| d-Tyr-Arg-Arg-2-Nal-Gly | Chitosan | CXCR4 | Lung cancer, CXCR4 overexpressing cancer cells | 155 |
| RLWMRWYSPRTRAYGC | PEG-P(TMC-DTC)-PEI | Tumor cells | Lung, colon, breast, liver, leukemia cancers | 156 |
| YTIWMPENPRPGTPCDI-FTNSRGKRASNG | PAM-ABP | Nicotinic acetylcholine receptor | Stem cell | 157 |
| SGHQLLLNKMPNGGGSC | PAMAM | Mesenchymal stem cells | Stem cell | 158 |
| aKXVAAWTLKAAaZC | PAMAM | Major histocompatibility complex class II | Antigen presenting cells | 160 |
| HAIYPRH | PEI | Transferrin receptor | Breast, colon, liver, and lung cancer cells | 168 |
| CAGW | PLGA-g-PEI | Endothelial cells | Cardiovascular cells | 177 |
| REDV | Chitosan | Endothelial cells | Cardiovascular cells | 178 |
| WLSEAGPVVTVRALR-GTGSW | CBA-DAH | Primary cardiomyocytes | Cardiovascular cells | 180 |
| SDSSD | Polyurethane | Osteoblasts | Bone cells | 183 |
| QHREDGS | Chitosan, collagen | Angiopoietin-1 | Cardiomyocytes | 192 |
| LTVSPWY | Chitosan | HER2 | Breast cancer cells | 196 |
3.2. Therapeutic Delivery to Specific Cells and Tissues.
Peptide-modification of cargo delivery vehicles presents a powerful method for introducing molecular and nucleic acid effectors to certain tissues in the body. These functionalized vehicles are interesting because by changing the cargo, they can be adapted to treat a variety of diseases and disorders. The efficacy of a drug or gene therapy can be greatly enhanced by delivering it directly to the desired cell. Targeted polymer delivery vesicles have some major advantages over generalized delivery systems. Most importantly, they can greatly increase the efficacy of the delivered cargo. They can also minimize off targeting effects which can decrease adverse reactions and reduce the necessary dosage. For example, numerous studies have demonstrated that anticancer therapies can be made more effective with the use of specific targeting against tumor cells.126–129 Similarly, targeted delivery of antimalarial drugs was shown to improve the efficacy 10-fold when compared to generalized delivery.130 A different study demonstrated a 40-fold reduction in dose for colitis treatment when using a colon-targeting system.131 These are some of many examples in which targeted delivery has greatly improved an existing therapy. Here, we discuss the various methods in which researchers have targeted specific cells using peptide-modified polymer systems. Some of these systems have been designed around a specific cargo, while others can be adapted for various payloads by simply changing the targeting peptide.
3.2.1. Brain Cells.
One of the most frequently studied delivery targets is the brain. Delivery to the brain is more difficult than to other tissues due to the need to cross the blood-brain barrier (BBB).132 However, there are several treatments for central nervous system diseases which can benefit tremendously from gene and drug delivery. As such, researchers are utilizing peptides that can, in many cases, enhance targeting to the brain and facilitate transportation across the BBB. Fortunately, there are many known receptors which can aid in brain delivery, as discussed in the following examples.
One method for identifying peptides is to study the mechanisms of neuronal diseases and utilize them to formulate a delivery system. In one example, Kwon et al. identified the peptide Tet1, a 12-mer peptide that mirrors the binding characteristics of tetanus toxin, using phage display. Tetanus toxin binds to peripheral neurons, so a peptide with similar abilities would be useful for neuronal targeting. They also incorporated the HIV-1 gp41 (HGP) peptide to aid in endosomal escape. By observing luciferase activity, they determined that their dual peptide-modified polyplex increased transfection by almost 1000-fold when compared to the unmodified polymer, and a 9-fold and 2-fold increase compared to the HGP- and Tet1-modified polymers.133
Another approach is to focus on the mechanisms by which natural hormones and proteins enter the brain. One group derived the peptide leptin30 from the endogenic hormone leptin, which is known to be taken up into all regions of the brain with leptin receptors, making this an effective brain-targeting ligand. They utilized this 30-amino acid peptide to modify a poly-l-lysine dendrigraft carrier to facilitate delivery of pDNA to the brain. They used a luciferase assay to study their transfection efficiency and found that their polyplex performed as well as lipofectamine, a positive control for transfection.134 A similar strategy makes use of the transferrin receptor, a highly expressed ionic iron transporter found in blood brain barrier endothelial cells.135 To take advantage of this receptor, Z. Liu et al. used phage display to identify a peptide (B6) that has high affinity to the transferrin receptor. They created B6 peptide-modified poly(ethylene glycol)-poly(lactic acid) (PEG–PLA) block copolymers to deliver a secondary neuroprotective peptide in a mouse model of Alzheimer’s disease.136 Their delivery system exhibited high accumulation in the brain and had a longer circulation time than unmodified PEG–PLA. Figure 3 shows how their B6 peptide modification enhanced accumulation in the brain and greatly reduced accumulation in the spleen and lungs.
Figure 3.
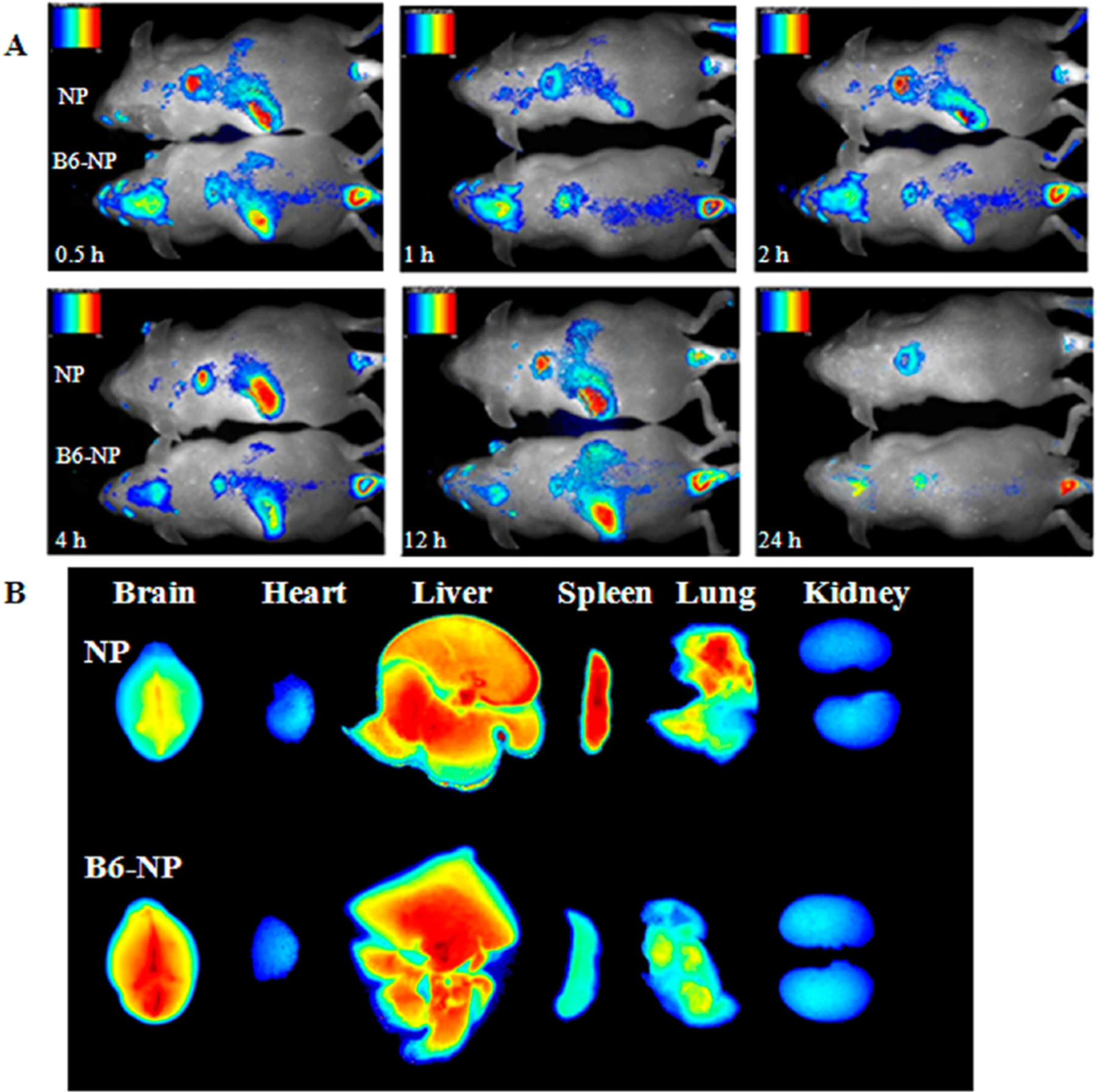
Distribution of nanoparticles with and without a B6 brain-targeting peptide modification (A) following tail vein IV administration in a mouse model and (B) in specific organs 1 h after administration. The bare nanoparticle does not localize as well to the brain and instead collects in the spleen and lungs. The modified nanoparticle accumulates only in the brain and the liver, where it is excreted. Reprinted with permission from reference.136 Copyright 2013 American Chemical Society.
An alternative method for brain delivery is to circumvent the BBB completely. This can be achieved by taking advantage of direct routes, like from the nose to the brain.137 To take advantage of this route, cell penetrating peptides are extremely useful. To leverage this, Yan et al. created poly(lactic-co-glycolic acid) (PLGA) nanoparticles modified with the cell-penetrating peptide Tat to deliver insulin as an Alzheimer’s therapy.138 Similarly, another group used Tat to modify poly(ethylene glycol)-poly(caprolactone) (PEG–PCL) copolymers to deliver siRNA as a treatment for various neural disorders.139 Both of these “direct” delivery system designs are of special significance because they allow for the design and transport of larger molecules. By modifying delivery vehicles with the cell-penetrating peptide Tat, conventional transport barriers such as cellular penetration and intracellular transport along the nose-to-brain pathway were easily defeated.
3.2.2. Brain Cancer Cells.
Targeting tumors in the brain has similar challenges to targeting any brain tissue; the delivery system not only needs to be able to cross the BBB but also requires specific functionality to target tumor cells. Brain tumors are most often gliomas, and certain gliomas, like glioblastoma, have very poor survival.140 Targeting brain cancer cells requires overcoming similar challenges to targeting brain cells. In one of the previously described studies, a peptide was identified by studying the neuronal disease tetanus toxin.133 In a similar study, a gene delivery system was created to treat glioma using a dendrigraft poly-l-lysine polymer modified with a peptide derived from Streptococcus pneumonia, the organism responsible for bacterial meningitis.78 This peptide contains a critical sequence that enables the bacteria to bind to the laminin receptor and undergo mediated transport across the blood brain barrier. They compared their delivery system to a positive control system modified with a pentapeptide derived from laminin and found that their system better targeted brain tumors than the control.
In other studies, peptides are used because they have been previously identified to penetrate cells or the blood brain barrier. For example, Ran et al. used a quorum-sensing peptide derived from Clostridium acetobutylicum for their system that had previously been reported to cross the BBB. Their system used mPEG–PLA copolymer micelles modified with the d-retroenantiomer of this bacteria-derived peptide to deliver the chemotherapeutic agent paclitaxel to glioma cells.79 They used the retro-inverso isomerization technique to develop their peptide because the d-retroenantiomer has enhanced stability and glioma-targeting properties, and their mouse model demonstrated successful targeting of brain tumors. In a similar approach that alternatively utilized a cell-penetrating peptide, Yao et al. used a dendrigraft poly-l-lysine and PEG copolymer and modified it with the nucleolar translocation signal sequence of the LIM Kinase 2 protein to deliver apoptosis-inducing therapeutic genes to glioma cells.141 This peptide enhanced BBB-crossing efficiency as well as transfection efficiency as a result of facilitated nuclear transport. These “dual functionality” peptides demonstrate that crossing the BBB and targeting a vehicle to glioma cells can be performed with a minimal system incorporating a single recognition element.
Brain cancer therapies can also take advantage of the nose to brain pathway, like in the following study. Kanazawa et al. expanded on their previous research involving Tat-modified mPEG–PCL block copolymers by using their system to codeliver siRNA and camptothecin, a topoisomerase inhibitor, to glioma cells.142 Their peptide-modified delivery system was able to induce cell death in glioma cells in a rat model, indicating the potential for high therapeutic effect. To illustrate this effect, Figure 4 shows brain samples of treated and untreated rats, demonstrating how the drug-loaded polymer micelles provide the greatest reduction in tumor size. This system provides an excellent example of rational vehicle design and modification for brain cancer treatment applications.
Figure 4.
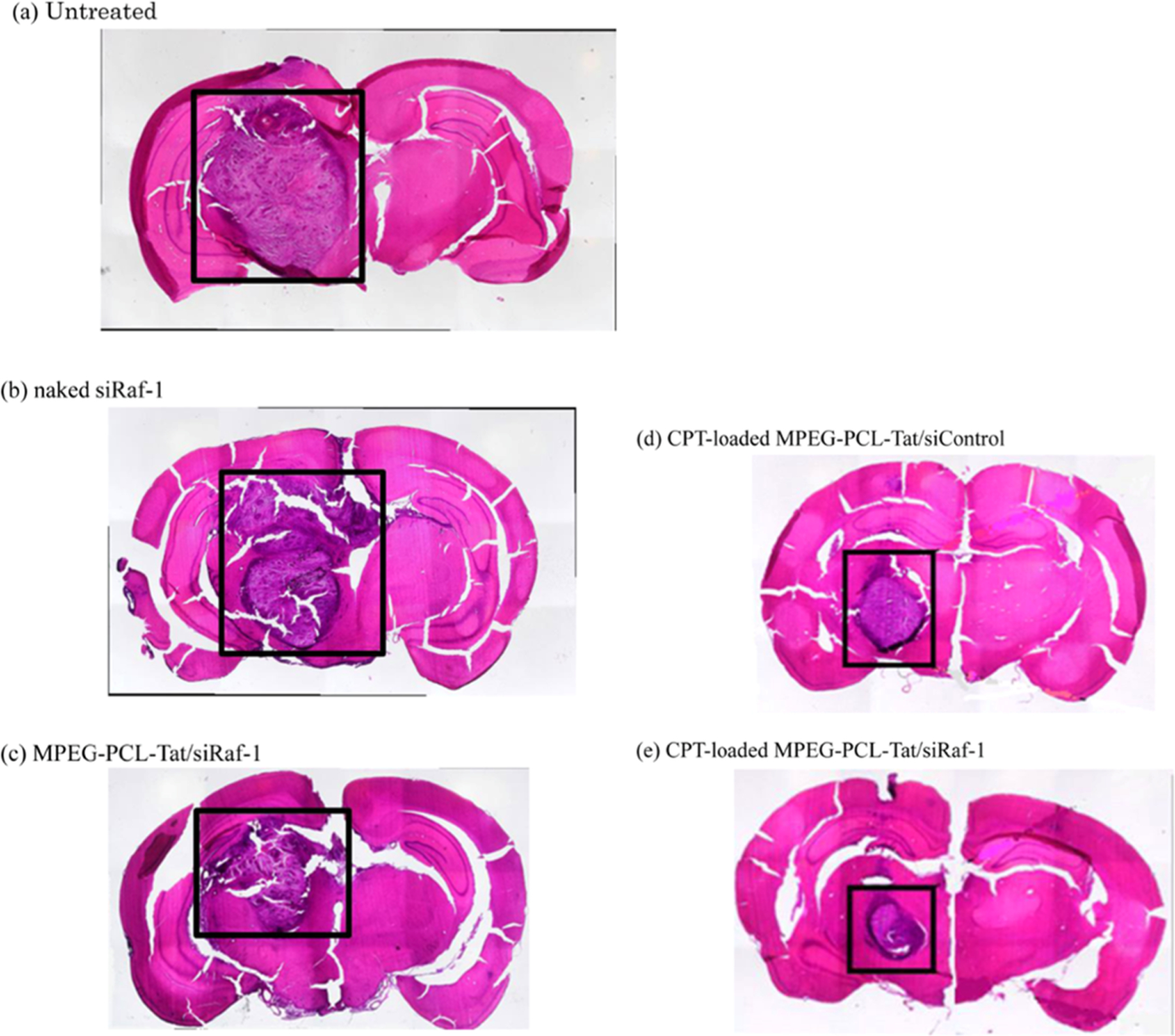
Brain tissue slices for (A) untreated rats and rats treated with (B) naked siRaf-1, (C) CPT-loaded MPEG-PCL-Tat/siControl, (D) MPEG-PCL-Tat/siRaf-1, and (E) CPT-loaded MPEG-PCL-Tat/siRaf-1. The greatest reduction in tumor size was seen with the peptide modified polymer micelle loaded with both drug and gene therapeutics. Reprinted with permission from reference.142 Copyright 2014 American Chemical Society.
3.2.3. Breast Cancer Cells.
Although not as complex as targeting the brain, targeting breast cells is of significant importance, as breast cancer is the most common type of cancer affecting women worldwide, with one in eight women in the United States being impacted.143,144 To target breast cancer cells, Mathews et al. engineered two breast cancer-targeting peptides and used them to modify poly(ethylene oxide)-b-poly(caprolactone) (PEO-b-PCL) diblock polymeric micelles.145 The polymeric micelles are biocompatible, biodegradable, and capable of encapsulating hydrophobic anticancer drugs. In comparing their engineered peptides to previously known cancer targeting peptides, they found that their engineered peptide delivery system demonstrated better targeting and higher binding affinity to breast cancer cells than the controls. Although their system demonstrated some selectivity issues, as MDA-MB-435 has been demonstrated to be a melanoma cell line,146 this system proved to effectively target cancer cells; by loading a chemotherapeutic agent into the micelle core, it could be used as an effective cancer therapy. Similarly, Logie et al. created a biodegradable system loaded with the chemotherapeutic drug docetaxel to target breast cancer cells. They used poly(d,l-lactide-co-2-methyl-2-carboxytrimeethylene carbonate)-graft-polyethylene glycol (P(LA-co-TMCC)-g-PEG) micelles modified with a novel taxane-binding peptide and conjugated with the antibody 73JFab.147 Their peptide increased the drug loading 2-fold, indicating that their novel peptide can greatly enhance the capacity and, therefore, the efficacy of the delivery system. Interestingly, they found that the 73JFab antibody did not improve targeting, indicating that their system could benefit from a breast cancer targeting peptide to further enhance delivery.
Some research into breast cancer targeting involves targeting a known biomarker. Many researchers have exploited knowledge of these receptors to design their targeting systems. In one study, researchers modified a PAMAM–PLA polymer with a peptide to target epidermal growth factor receptor to treat triple negative breast cancer, which has EGFR amplification.148 In another study, the annexin 1 biomarker was targeted for use in a therapy for multidrug resistant breast cancer.149 This system used a ligand isolated from phage display to bind annexin 1 and attached it to a block copolymer that included aldehyde PEG–PLA and mPEG–PLA and was loaded with paclitaxel. A final example involves targeting HER2, a very common biomarker associated with breast cancer, by using PEGylated PAMAM dendrimers.150 These studies all utilize known breast cancer cell receptors in their systems, and demonstrate an effective way to approach targeting challenges.
3.2.4. Ocular Cells.
Most eye treatments are in the form of eye drops or injections into the eye. The need for specific targeting to the eye is minimal, as these methods tend to localize extremely well.151 Therefore, modification is used to enhance the intended effect or targeting within the eye. For example, Chu et al. designed a PEG–PLGA polymer nanoparticle modified with RGD and Tat peptides to target drug delivery to choroidal neovascularization (CNV).71 CNV is new, invasive blood vessel growth from the choroid and frequently indicates ophthalmic diseases such as macular degeneration, making it an ideal target for drug delivery.152 Their system used an RGD peptide to target CNV because it is bound by integrin αvβ3, a receptor expressed on blood vessels. The Tat peptide was included to penetrate the ocular barrier. On the basis of the dual recognition of RGD and Tat, this system was able to target CNV and penetrate vascular endothelial cells, indicating a promising tool for ocular drug delivery.
3.2.5. Pancreas Cancer Cells.
A combination therapy for pancreatic cancer involving the delivery of both siRNA and an anticancer drug was developed by Li et al. using branched PEG with G2 dendrimers (PSPG). This dendrimer vehicle was modified with a plectin-1 targeting peptide and condensed with paclitaxel and siRNA as a targeted therapeutic against pancreatic cancer cells.153 Plectin-1, a known biomarker for pancreatic cancer, was targeted to localize the delivery system to the pancreatic tumor, while this siRNA sequence silenced TR3 genes responsible for the expression of a protein involved in growth and survival of pancreatic tumor cells. Figure 5 demonstrates that codelivery of the chemotherapeutic agent and anti-TR3 siRNA was more effective at minimizing tumor volume than any other tested method.
Figure 5.
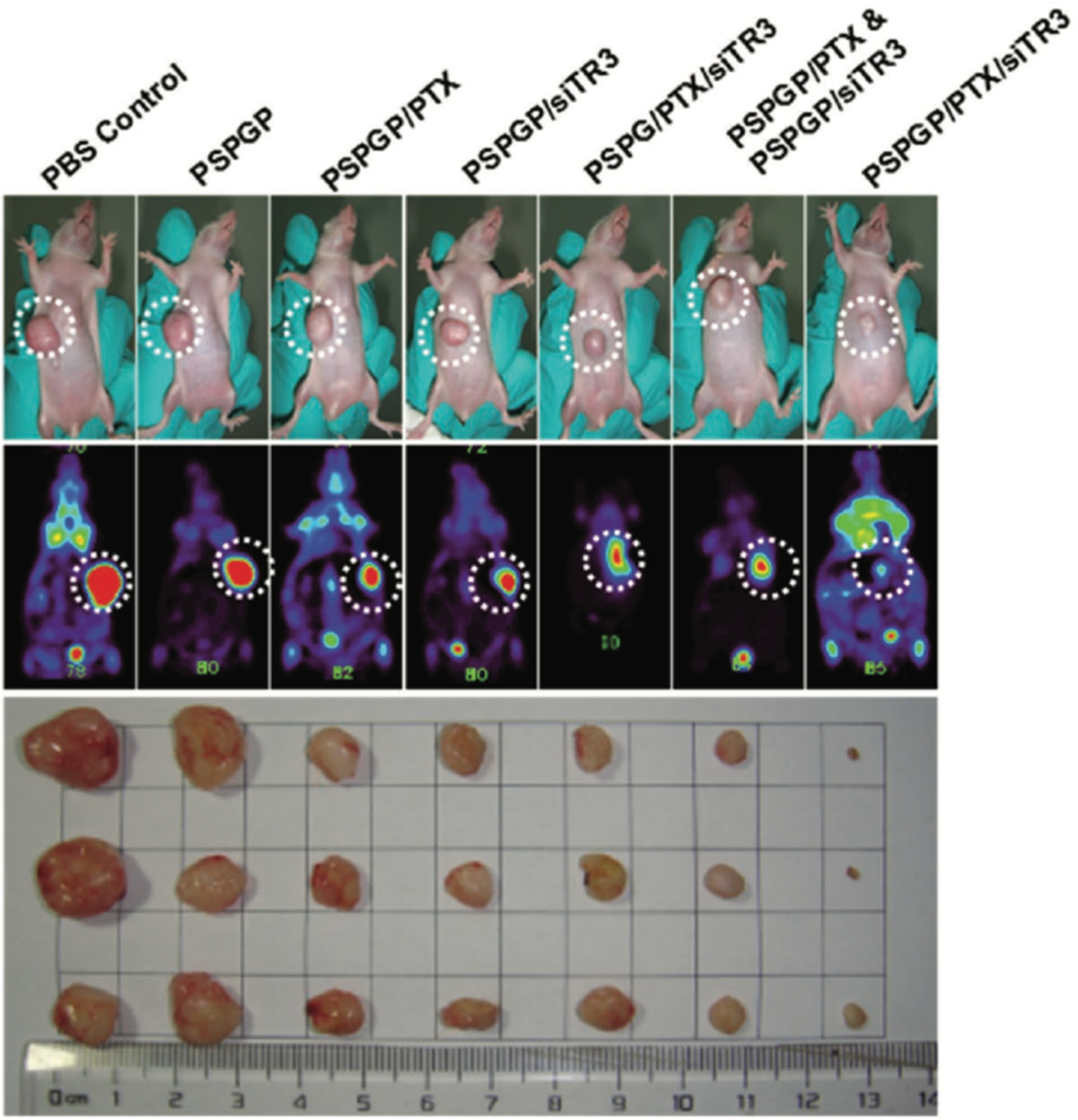
Tumor growth and dissected tumor tissues from mouse models 18 days after initial treatment with various combinations of the peptide-modified polymer (PSPGP), the chemotherapeutic drug paclitaxel (PTX), and small interfering RNA (siTR3). The first row of images shows the tumors on the mice, and the second row shows the PET scans of these tumors. The final image shows the dissected tumor tissues. The smallest tumor size was found with a peptide-modified polymer delivering both paclitaxel and siRNA. Reprinted with permission from ref 153. Copyright 2016 WILEY-VCH Verlag GmbH and Co. KGaA, Weinheim.
3.2.6. Lung Cancer Cells.
As lung cancer has significant mortality and a large affected population, it presents an important target for tissue-selective therapeutics. To address this, an RGD-modified lipid-polymer nanoparticle decorated with PEG was synthesized and loaded with two chemotherapeutic drugs, paclitaxel and cisplatin, to target lung cancer cells.154 The lipid-polymer nanoparticles consist of a PLGA polymer core surrounded by a lipid-PEG shell. This system was tested in a mouse model and was able to significantly inhibit tumor growth when compared to the free drugs, demonstrating an effective and readily generalizable anticancer therapeutic. Another group developed O-carboxymethyl chitosan nanoparticles and modified them with a peptide that targets CXCR4 receptors to deliver docetaxel to lung cancer cells.155 Their targeted system performed significantly better than a nontargeted control at inducing apoptosis and causing cancer cell death. In a different lung cancer study, PEG-P(TMC-DTC)-PEI was modified with a tumor targeting peptide that had been determined to be effective for lung, colon, breast, liver, and leukemia cancer cells.156 This system was used to deliver methotrexate to lung cancer cells, where they determined their delivery system was able to lower the IC50 and inhibit tumor progression when compared to the untargeted and free drug. Their system represents a method of enhancing anticancer drug delivery and minimizing the required dose for efficacy, which are both critical aspects of cancer therapy development.
3.2.7. Stem Cells.
Stem cells have traditionally been difficult to compromise as they are poorly permissive to nontargeted polymer delivery systems. However, recognition peptides have the potential to circumvent some of the shortcomings of passive delivery. Beloor et al. loaded pDNA onto a dendrimertype arginine-grafted polymer and modified it with a targeting peptide against the nicotinic acetylcholine receptor for targeted gene delivery to stem cells.157 This particular peptide was derived from Rabies virus glycoprotein and selectively binds human mesenchymal stem cells and human embryonic stem cells. The authors discovered that this functionality enabled the creation of a biocompatible gene delivery system that could transfect poorly permissive cells. They were able to transfect 60% of the mesenchymal stem cells and 50% of the embryonic stem cells, significantly more than the lipofectamine positive control. This study demonstrates an improvement to a slightly older study, in which PAMAM dendrimers were loaded with pDNA and modified with peptides having either a high affinity or low affinity toward mesenchymal stem cells.158 Although this older study found that their high binding affinity peptide had a better transfection than both the low affinity peptides, their binding efficiency, at around 45%, was lower than the new study. This is an example of how researchers are continuously able to identify new targeting peptides. An interesting comparison could be made by using the same polymer carrier and modifying with the peptides from different studies to directly compare their efficacy.
Alternatively, stem cells themselves can be delivered to a specific tissue for wound healing and tissue repair. One example used the sE-sel peptide to target E-selectin, a ligand that is expressed on endothelial cells during injury. For this, PAMAM dendrimer nanoparticles were modified with sE-sel peptide and vascular endothelial growth factor (VEGF) to deliver bone marrow stem cells.159 These nanocarriers were able to target endothelial cells and attach to the endothelium, a process that resulted in extravasation to the site of injury for delivery of the therapeutic stem cells. Figure 6 shows the nanocarrier biodistribution in a mouse model, demonstrating how well the peptide-modified dendrimer was able to localize to the wound tissue. Not only was this system very effective at targeting injured tissue, it could also be modified with other therapeutic cells to adapt it to different applications.
Figure 6.
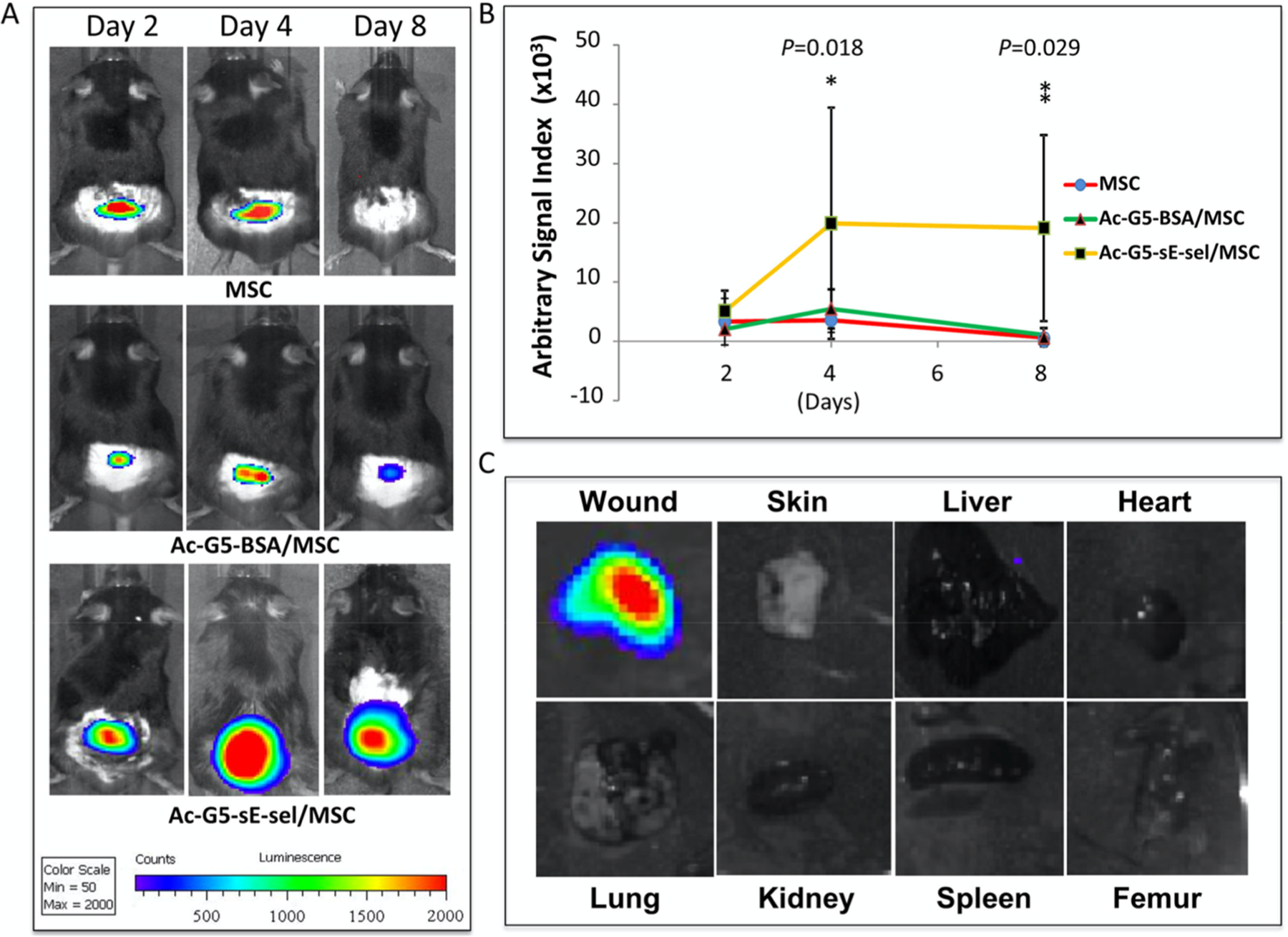
Localization of the modified nanocarriers to wound tissues. (A) Modified nanocarrier (Ac-G5-sE-sel/MSC) localized much better than the uncoated cells (MSC) or the unmodified nanocarrier (Ac-G5-BSA/MSC). (B) Quantitative plot of the bioluminescence from panel A. (C) Distribution of the modified nanocarrier (Ac-G5-sE-sel/MSC) in various organs, demonstrating how well it localized to the wound tissue. Reprinted with permission from ref 159. Copyright 2016 Liu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License.
3.2.8. Antigen Presenting Cells.
Systems are often designed with a specific disease in mind. For instance, Daftarian et al. wanted to focus on a therapy for leishmaniasis, a parasitic disease which affects macrophages. Leishmaniasis can be used as a model for studying diseases such as HIV and tuberculosis because they are all obligate intracellular diseases.160 By targeting antigen presenting cells (APCs), the diseased macrophages that are the source of the infection can be identified while stimulating other immune cells for enhanced immune response. For this, they developed a G5 PAMAM dendrimer modified with a Pan-DR-binding epitope (PADRE) peptide that targets the major histocompatibility complex (MHC) class II unique to professional APCs. This system was used to deliver liposomal amphotericin B (LAmB),160 which is an effective but highly toxic therapy against leishmaniasis. Their system was able to selectively target APCs and minimize both the effective dose and the toxicity of the drug, as shown in Figure 7. Additionally, they were able to induce T-cell responses and antiparasitic immunity. Although the mechanism responsible for the immune response enhancement was unclear, this study was able to leverage these two beneficial effects synergistically to provide a more effective treatment.
Figure 7.
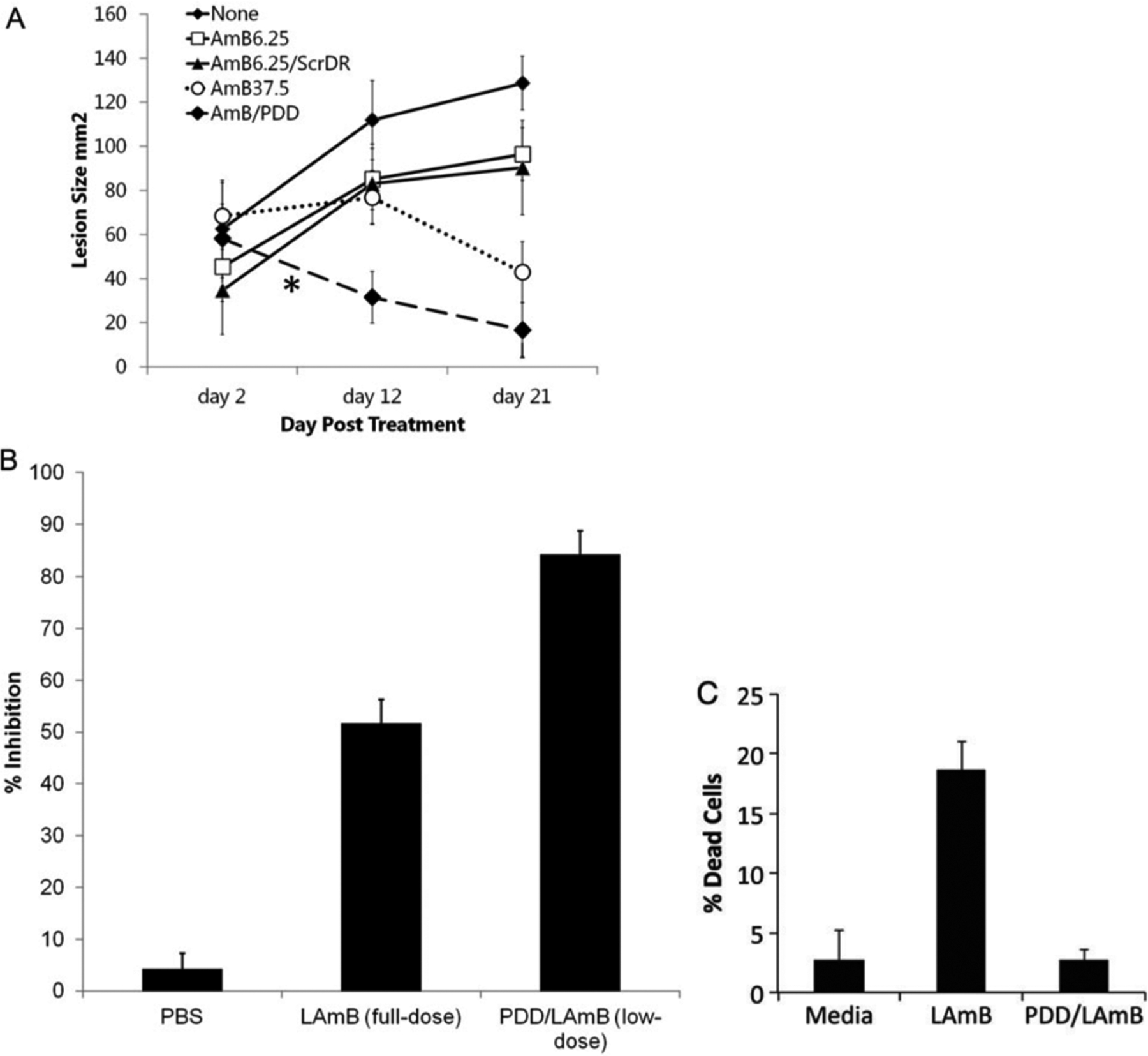
Peptide-modified nanocarriers were able to lower the effective dosage and the toxicity of the drug AmB. (A) Change in mouse lesion size over time for the following treatments: untreated (none), low dose of AmB drug (AmB6.25), low doses of AmB drug encapsulated in an unmodified nanoparticle (AmB6.25/ScrDR), full (high) dose AmB drug (AmB37.5), and low dose of AmB drug in the modified nanoparticle (AmB/PDD). From this graph, it can be seen that the low dose in the modified nanoparticle was the most effective at reducing lesion size. (B) Drug delivered in the modified nanoparticle has the highest percent inhibition, despite using a lower dose. (C) When encapsulated by the modified nanoparticle, the cytotoxicity of the drug is much lower. Reprinted with permission from ref 160. Copyright 2013 Oxford University Press.
3.2.9. Specific Receptors of Different Types of Cancer Cells.
There are plenty of studies designed around increasing the efficacy of anticancer treatments by targeting specific receptors on the surface of cancer cells. Although some previous studies attempted to find unique receptors to a specific cell, a newer focus is on receptors which are found on many cells but overexpressed on cancer cells. For instance, there are various types of tumor cells which overexpress the αvβ3 receptor given its crucial role in tumor angiogenesis, thus making this receptor a good target for solid tumor therapeutics.161,162 Some cancers that rely on rapid angiogenesis include lung cancer, glioblastoma, renal cell carcinoma, and thyroid cancer,163,164 and the fact that the RGD peptide binds to the αvβ3 receptor makes this an ideal candidate to target a broad range of cancer types. For instance, He et al. modified a G5 PAMAM dendrimer with RGD via a PEG spacer for delivery of doxorubicin.165 The authors were able to demonstrate specific targeting of αvβ3 overexpressing cells as well as successful delivery of the chemotherapeutic cargo. Similarly, Li et al. created PEG–PLA polymer micelles loaded with docetaxel and modified them with the anti-αvβ3 receptor peptide C(RGDfK).166 These peptide-modified polymer micelles were shown to selectively inhibit cancer cells over nonfunctionalized micelles, with a 20% higher cytotoxicity in a Hela cell in vitro model.
Another popular target for cancer cells is the transferrin receptor (TfR), which is overexpressed in various types of tumor cells including breast, colon, liver, and lung cancers.167 Xie et al. identified a transferrin receptor binding peptide via phage display and used it to modify branched PEI. The PEI vehicle was shown to transport siRNA and selectively target high TfR expressing cancer cells compared to low TfR expressing cancer cells.168 However, they discovered that they were only able to silence the reporter gene in the presence of transfection-enhancing chloroquine, indicating that TfR receptor binding may not be sufficient for uptake and a further modification for cell entry may be required.
An interesting target is CXCR4, which is overexpressed on some tumor cells including metastatic cancer cells and plays a critical role in tumor metastasis. Metastatic cancer cells travel away from their originating tissue and populate other tissue types; this represents the most severe stage of cancer.169 Targeting metastatic cancer can be more difficult because of the characteristic loss of surface marker expression that reduces the number of identifiable targets.170 X. Liu et al. modified chitosan micelles with a BKT-140 peptide to target metastatic cancer cells and deliver doxorubicin.171 The BKT-140 peptide inhibits CXCR4. The free doxorubicin drug has very high cytotoxicity, while the polymer system has lower cytotoxicity. The system they designed was able to selectively target CXCR4high cells and demonstrate a decrease in metastatic tumor sizes in the lungs of mice. Thus, the authors could deliver a chemotherapeutic drug to metastatic tumor cells without the high cytotoxicity found in the free drug.
3.2.10. Tumor-Associated Macrophages.
Tumor-associated macrophages (TAMs) are macrophages that create the immunosuppressive tumor microenvironment and promote tumor growth and metastasis.172 Pang et al. targeted TAMs by coating the surface of PLGA nanoparticles with a peptide which binds to M2-polarized macrophages and used it to deliver a tumor growth inhibitor.173 They found that their system was able to decrease the tumor size and tumor growth rate by 50% compared to the inhibitor alone. However, the addition of the peptide did not significantly lower the tumor growth when compared to the unmodified nanoparticle, implying that the benefit of the peptide was minimal. System optimization could improve the function of the peptide and thus further reduce the tumor size, perhaps by enhancing the peptide attachment or adding another modifying peptide.
3.2.11. Cardiovascular Cells.
Targeting cardiovascular cells is, in many ways, very similar to targeting metastatic cancer cells because cardiovascular tissue is found throughout the body, meaning that even specific targeting to these tissues could result in systemic circulation. The most abundant cardiovascular cell types are cardiomyocytes (CMs) and endothelial cells (ECs). In particular, ECs line the interior surface of blood vessels and are a target for various treatments and therapies.174 For instance, many patients require artificial blood vessels, which can fail after implantation. To help promote vascular growth and proliferation, gene therapy techniques can be used.175,176 Duo et al. synthesized a gene delivery system by fusing an EC-targeting peptide (CAGW) onto a poly(d,l-lactide-co-glycolide)-graft-PEI (PLGA-g-PEI) copolymer.177 This star-shaped polymer is biodegradable, with a low cytotoxicity and high transfection efficiency, while the CAGW peptide recognizes ECs with high selectivity and aids in rapid endothelialization. Another group also targeted ECs by using trimethyl chitosan modified with the REDV peptide for miRNA delivery.178 As this peptide is specific to ECs and mediates the adhesion and migration of these cells, it was applied to the delivery of miRNA for promotion of rapid endothelialization. Although the methods were different, both of these delivery systems were shown to provide the same benefit of preventing engraftment failure.
Cardiomyocytes are responsible for muscle contractions in the heart, and the death of these cells is associated with heart failure,179 making them an important target for delivery-based therapies. Nam et al. designed a bioreducible polymer, cystamine bis(acrylamide)-diaminohexane (CBA-DAH), that was modified with a novel, primary cardiomyocyte-specific peptide and the cell-penetrating peptide Tat to deliver siRNA to cardiomyocytes.180 The incorporation of Tat helped facilitate cellular uptake of their carrier system. The 20 amino acid cardiomyocyte targeting peptide, when used as part of a delivery system, specifically recognizes primary cardiomyocytes for improved transfection efficiency.181 This combination of peptide modifications could then enhance the recognition and uptake of a gene delivery system for targeted inhibition of cardiomyocyte cell death.
3.2.12. Bone Cells.
Similar to cardiovascular cells, osteoblasts are found throughout the body and serve, in the form of bone tissue, not only as a scaffold for muscle attachment but also as reservoirs for hematopoietic production and differentiation;182 therapeutic targeting to the bones could see utility in a vast array of diseases such as osteoporosis and cancer. Sun et al. used polyurethane nanomicelles modified with an osteoblast-targeting peptide, SDSSD, to deliver an antiosteoporosis gene for increased bone formation.183 Their peptide-modified polymer was identified via phage display and was able to target osteoblasts in vitro and in vivo in both mice and humans. Their system demonstrated an excellent proof-of-concept for future targeted therapies of various bone diseases.
3.2.13. Skeletal Muscle Cells.
Skeletal muscle cells are also found throughout the body and can be susceptible to a variety of diseases including the different types of muscular dystrophy. To target these cells for gene delivery therapies, Jativa et al. modified G5 PAMAM dendrimers with a skeletal muscle targeting peptide (SMTP). They also used a DLC8-binding peptide to utilize the dynein motor protein complex for intracellular trafficking.85 They found that SMTP was able to selectively target skeletal muscle cells both in vitro and in vivo in mouse models, causing a 3-fold increase in transfection efficiency when compared to unmodified dendrimer. Additionally, the DLC8-binding peptide was able to further enhance the transfection efficiency of their system, resulting in an 18-fold increase when compared to the unmodified dendrimer. This system demonstrated the concept of stacking functionality from multiple peptides to achieve a variety of enhancements in a delivery system.
4. TISSUE ENGINEERING APPLICATIONS
There are many applications involving the use of polymers for tissue engineering, especially as implant scaffolds and for promoting tissue growth.33 The design process can be quite different than previously described targeting studies, as there is no requirement that the peptides are specific for a certain cell type. Instead, the peptides used will often interact with components found in all cell types to increase or enhance interactions with cells, as seen in many of the studies described in the next subsection. These peptide modifications allow them to create polymer scaffolds which can be used both in vitro and in vivo.
4.1. Tissue Scaffolding Applications.
Earlier work in this area focused on hydrogel characterization under conditions of peptide addition. Drury et al. examined the mechanical integrity of alginate polymers that were embedded with C2C12 cells and modified with RGDSP-containing peptides.184 They discovered that a synergistic increase in mechanical integrity existed between the encapsulated cells and increasing densities of embedded peptides. This indicated the possibility of increased alginate polymer cross-linking due to cell–peptide interactions. These results can also be applied to other polymer systems for use in a diversity of tissue engineering applications.
A follow-up goal was the design of adaptive materials which could conform to a wide array of tissue types or could change properties in situ to enable adaptation to environmental changes. For example, Garty et al. designed a polyethylene-oxide and polypropylene-oxide block copolymer thermoresponsive gel modified with RGD peptides. Under ambient temperature conditions, the gel exhibited the low-viscosity properties of a liquid that allowed direct injection at the site of damage. Increasing temperature induced polymerization, enabling the formation of a rigid, supportive matrix for restoring function to damaged tissues or organs via previously embedded engineered stem cells.185 Similarly, Jones et al. developed a PEGylated polyamino acid hydrogel modified with lysine- and glutamine-containing peptides.186 This system functioned by directing the enzymatic coupling of the synthetic polymer molecules to cartilage and provided a generalizable method for structural enhancement of various other tissues such as those lining the oral cavity, gastro-intestinal tract, and those exposed during invasive surgery.
Furthermore, many studies have focused on trying to mimic the extracellular matrix to promote cell adhesion. These platforms focus on leveraging peptides known to influence cell adhesion, attachment, and spreading such as integrin-binding, ECM-derived RGD peptides and heparin-binding peptides.187 A significant research focus has been on the development of biodegradable ECM analogs for use as in vivo scaffolds. For example, a mixture of PLGA and PLGA–PEG block copolymer was used to make a nanofiber mesh, and this mesh was modified with a GRGDY peptide to create a biodegradable and biocompatible scaffold.188 The addition of the RGD-containing peptide was found to significantly increase cell attachment, spreading, and proliferation, making this nanofiber scaffold very effective for tissue generation. Cell adhesion and proliferation was enhanced on the RGD-modified nanofiber mesh relative to the bare mesh, indicating the potential for broad usage in a variety of tissue engineering applications.
Another method of mimicking the ECM is by creating a thermoresponsive hydrogel modified with peptide nanofibrils. Cao et al. achieved this by using the thermoresponsive polymer poly(N-isopropylacrylamide) and adding it to I3K fibrillar nanostructures.189 These peptides have drug carrying properties, so their incorporation not only helps with the three-dimensional structure through cross-linking but also enables the hydrogel to have therapeutic applications. They tested their design by releasing an antibacterial peptide and found the hydrogel displayed a controlled, linear release over time. Their results indicate their thermoresponsive hydrogel could be used as an injectable hydrogel for drug delivery applications.
Recently, Desseaux et al. used poly(2-hydroxyethyl methacrylate) polymer brushes modified with RGD and PHSRN peptides to study cell–substrate interactions with respect to adhesion, spreading, and focal contact formation.190 The PHSRN peptide is derived from fibronectin and acts synergistically with RGD. They found that these functionalized polymer brushes could be used to study the effects of copresentation of PHSRN and RGD and that copresentation enhances cell adhesion. This effect was most significant when the peptides had equal surface concentrations. These results, and similar experiments, can be used to adapt biomaterials for tissue regeneration and improve their function as scaffold materials. In another study, Wisse et al. used thermoplastic elastomers functionalized with one of 8 different peptides containing 2-ureido-4-pyrimidinone (Upy) groups to generate biocompatible and bioactive polymer nanofibers that represent the ECM.191 The Upy modification aids in lateral stacking interactions when urea or urethane are added and facilitates the formation of the nanofibers. By modifying biologically relevant peptides with the Upy group and incorporating them into polymers, the resulting nanofibers can be used in many in vivo applications because they have both morphological and functional characteristics of the natural ECM.
4.2. Promoting Growth for Specific Tissues.
Although many of the applications in this field are designed with peptides that can work with many cell types, there are projects which aim to promote the growth of a specific tissue to counter selective damage within an organ. This area of research is much more limited than research into nonspecific tissue growth and presents an exciting new direction for researchers to explore. For instance, Reis et al. used a chitosan-collagen hydrogel modified with an angiopoietin-1-derived peptide to aid in cardiomyocyte growth in patients with myocardial infarction.192 This angiopoietin-1-derived peptide promotes adhesion, survival, and maturation of remaining cardiomyocytes following smooth muscle destruction during myocardial infarction. They found that their hydrogel was able to localize at the infarction site and that increased peptide concentrations within the gel resulted in a higher success rate of in vitro cardiomyocyte activation. Cross-striated cardiomyocytes were more commonly found in the hydrogel with a high concentration of peptide, and even the low-concentration hydrogel outperformed the control.
In bone tissue, Barber et al. surrounded titanium implants with an interpenetrating polymer network modified with an RGD-containing peptide to promote cell growth and enhance bone formation around titanium implants.193 As this peptide was known to promote cell growth, they hypothesized they would see enhanced bone formation and interfacial bond strength. Interestingly, although their peptide-modified polymer coated implants had greater bone-implant contact than the positive control implants, only the positive control implants supported significant interfacial shear strength. The peptide-modification facilitated cell growth, as indicated by the increased bone/implant contact, but they discovered that this growth was not the primary mode of implant fixation. Studies such as this open up new avenues of basic research, as fundamental assumptions about the proper application of a technology can be challenged and redirected by observing its interaction with intact biological processes.
5. OTHER APPLICATIONS
There are many other technologies that have benefited from peptide-modified polymers. Although much of the work described here has been focused on therapeutic applications, this field can also aid the progress of basic research. In the following unique applications, peptide modifications aid researchers in sample analysis. These samples can be biological, like cells, enabling researchers to study cellular reactions to drugs and other molecules, or they can be environmental, providing researchers with a useful methodology for reliable field testing.
One example application involves a method for more easily studying cell cultures by designing a polymeric substrate for cell growth to determine how drugs and chemicals affect cell viability and morphology. Oyman et al. used RGD peptides to functionalize 3-(2,5-di(thiophen-2-yl)-1H-pyrrol-1-yl)aniline polymers for this purpose.194 They examined numerous cell lines (monkey kidney epithelial, human neuroblastoma, and human immortalized skin keratinocyte) on a control surface, an unmodified polymer-coated surface, and a peptide-modified polymer-coated surface. Their results (as shown in Figure 8) demonstrated that their RGD-modified polymer surface had the best cell adhesion and proliferation, with about 50% more cells than the control surface for all three cell lines. They were, therefore, able to create a novel, bifunctional substrate which could be used for cell studies or even adapted for cell-culture-on-a-chip applications.
Figure 8.
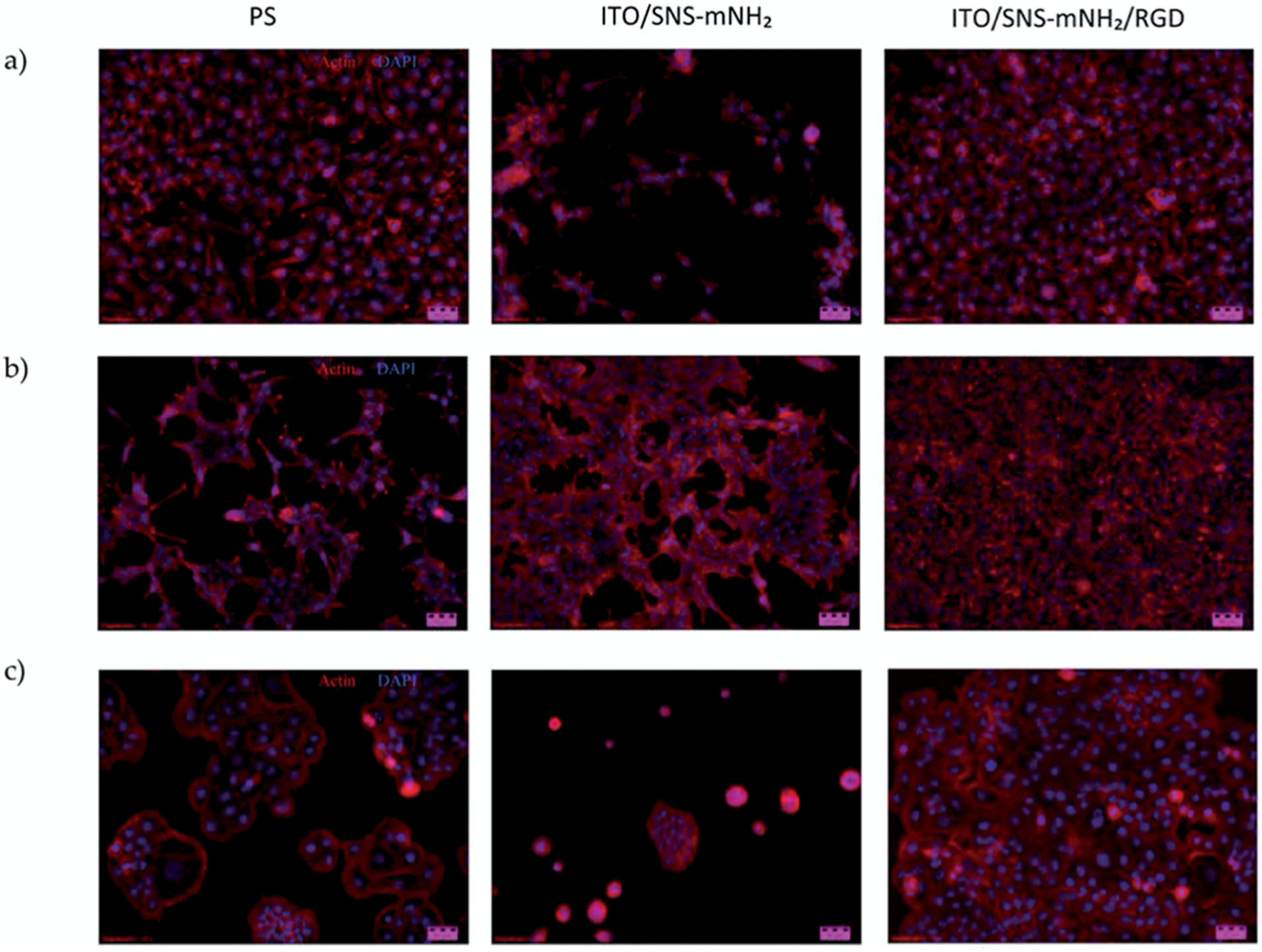
Proliferation of (a) Vero, (b) HaCaT, and (c) SH-SY5Y cells on a control polystyrene (PS) surface, unmodified glass (ITO/SNS-mNH2) surface, and peptide-modified (ITO/SNS-mNH2/RGD) surface. All three cell lines had the highest growth on the peptide-modified surface, indicating that the RGD peptide aided in cellular proliferation. Reprinted with permission from ref 194. Copyright 2014 The Royal Society of Chemistry.
Another application involved the deployment of peptide-modified polymers as chemical sensors for applications like soil and water analysis. Aguilar et al. used nanoelectrodes separated by peptide-modified polyanilines to form nano-junctions. The peptides specifically bound to copper and nickel; when bound, a conformational change was induced in the polymer that resulted in a change in conductance.195 This enabled real-time detection of these metal ions and achieved an impressive detection limit in the parts-per-trillion range. This nanojunction sensor could be easily adapted to test drinking water for heavy metal contamination.
Tumor imaging presents another interesting application, as this has become an important part of diagnosing and monitoring cancer. For example, mammograms, which use X-rays, are the most common tool for diagnosing breast cancer but are qualitative and prone to false negatives, leading to the need for an enhanced-sensitivity technique such as MRI. To aid in imaging breast cancer tumors, Jie et al. synthesized MRI contrast agents using PEGylated chitosan magnetic nanoparticles modified with a HER2 homing peptide to target HER2+ breast cancer.196 This modifying peptide was found to facilitate selective targeting to HER2 overexpressing tumor cells, providing a potential route for imaging and diagnosis of HER2 positive breast cancer.
A similar system was designed for gastric cancer diagnosis. Cheng et al. utilized a peptide which recognizes GRP78, a gastric cancer biomarker, in polymeric micelles that were coupled with a radioisotope to target and identify gastric cancers using computer tomography.197 They compared their peptide-modified system with an unmodified system in mice with gastric cancer xenografts and determined that the peptide modification significantly increased radioactive signal.197 Although their system presents a promising method of locating and diagnosing gastric cancer, they did note there was some accumulation in the liver and spleen and therefore identification of a higher affinity peptide may help to further enhance their system.
Peptide modifications can also serve to diagnosis other maladies such as food poisoning. One group used poly-d-lysine-based polymers and modified them with peptides that were identified using phage display to have high affinity for either Salmonella enteritidis or Salmonella typhimurium. They conjugated their system to a fluorescent dye to use it to detect Salmonella.198 They were able to obtain a detection of as low as 102 colony-forming units per mL, which is on par with other Salmonella detection methods.198 They have designed a system which has the potential to rapidly detect Salmonella and could outperform other methods with further enhancement of their design by identifying new, effective peptides.
6. CHALLENGES AND LIMITATIONS
While peptide modifications have enabled researchers to make great strides in polymer biomaterial applications, there are still some significant challenges left to overcome. Some of these challenges relate to the peptides themselves. While techniques like phage display have helped researchers to identify specific peptides, it is often still difficult to discover multirole peptides or isolate multiple interactors for a single peptide. Researchers can encounter difficulties related to peptide stability that may stem from aggregation, hydrophobicity/hydrophilicity, charge distributions, or the effects of ambient conditions.199 There are also challenges regarding the different conformations of the peptides and the possibility of secondary structures, which may interfere with conjugation and function.53,199 A related challenge is the modification of the polymer material, for which it can be difficult to create multifunctionalized structures that have the cargo and ligands in precise proportions and binding sites.200 This can cause a need for optimizing peptide conjugations to ensure both the peptide and the cargo maintain their original functionality.
Additional challenges address the applications of these polymer biomaterials. For tissue engineering applications, the peptides used often do not promote growth of a specific cell type, meaning any cell type could be stimulated. This is evident by the numerous studies demonstrating enhanced cell growth, but the lack of studies focusing on specific tissues. For delivery applications, some of the receptors targeted can be found on various cell types, a situation that can reduce the specific targeting capabilities. Additionally, specific receptors still need to be identified for certain therapeutic targets to enable targeted delivery. To address these limitations, further work must be done studying peptide–cell interactions.
7. CONCLUSIONS AND FUTURE PERSPECTIVES
Polymer-based biomaterials can be used in a wide variety of biomedical applications because they are easy to modify and can be biocompatible. Their applications are vast and include everything from delivery systems to tissue scaffolding. As research into polymer biomaterials has progressed, peptide modifications have been found to greatly enhance their intended effects and even add additional functionality. Researchers have utilized peptide modifications to target polymeric delivery systems to specific cell types and enhance cellular uptake, modifications that have vastly improved the selectivity and efficiency of therapeutic cargo delivery. For tissue applications, some groups have used peptide modifications to make their polymers more “life-like” for better compatibility and effectiveness in tissue engineering applications. Peptide modifications have thus expanded the field of polymer biomaterials and enabled researchers to utilize these in more biomedical applications.
Although peptide modifications have significantly enhanced the utility of polymers, there is still room for development. There are many barriers to overcome to take the greatest advantage of this tool, and the advancement of this field relies heavily on the identification of peptides relevant to a desired function. Plenty of future work can be devoted just to peptide identification technologies that provide for the creation of rapid and selective high throughput peptide libraries, as improving these methods will greatly improve the actual biomedical applications of the polymers. Some of the challenges directly associated with the applications, like improving the targeting efficiency of the peptides or enhancing growth of specific cell types, can be overcome by identifying the “best” peptide for the particular application. This draws a heavy focus on the development of peptide identification techniques. Related future work can involve utilizing these newly identified peptides for enhancing growth of specific cell types to make these biomaterials more useful in in vivo tissue engineering applications.
A related focus for future work is on the design of chemical methods for the facile and efficient attachment of the peptides to the polymers. Identification and utilization of biorthogonal chemical conjugation methods that maintain the binding ability of the peptides while creating reproducible and uniform peptide–polymer conjugate will also be highly useful. By overcoming all of the aforementioned challenges, these biomaterials could dramatically reshape the landscape of precision medicine, making them a very promising tool for future work.
ACKNOWLEDGMENTS
The authors would like to thank NIGMS (R01GM114321, R01GM127706), NHLBI (R01 HL149452), and the National Science Foundation (CBET-1841419) for funding support. S.D. thanks the Miller School of Medicine of the University of Miami for the Lucille P. Markey Chair in Biochemistry and Molecular Biology. J.H. thanks the University of Miami Dean’s Fellowship for funding.
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acsabm.0c01145
The authors declare no competing financial interest.
Contributor Information
Jessica Hersh, Department of Biochemistry and Molecular Biology, University of Miami Leonard M. Miller School of Medicine, Miami, Florida 33136, United States;; The Dr. John T. McDonald Foundation Bionanotechnology Institute of University of Miami, Miami, Florida 33136, United States;
David Broyles, Department of Biochemistry and Molecular Biology, University of Miami Leonard M. Miller School of Medicine, Miami, Florida 33136, United States;; The Dr. John T. McDonald Foundation Bionanotechnology Institute of University of Miami, Miami, Florida 33136, United States
José Manuel Condor Capcha, Interdisciplinary Stem Cell Institute and Division of Cardiology, Department of Medicine, University of Miami Leonard M. Miller School of Medicine, Miami, Florida 33136, United States.
Emre Dikici, Department of Biochemistry and Molecular Biology, University of Miami Leonard M. Miller School of Medicine, Miami, Florida 33136, United States;; The Dr. John T. McDonald Foundation Bionanotechnology Institute of University of Miami, Miami, Florida 33136, United States
Lina A. Shehadeh, Interdisciplinary Stem Cell Institute and Division of Cardiology, Department of Medicine, University of Miami Leonard M. Miller School of Medicine, Miami, Florida 33136, United States
Sylvia Daunert, Department of Biochemistry and Molecular Biology, University of Miami Leonard M. Miller School of Medicine, Miami, Florida 33136, United States;; The Dr. John T. McDonald Foundation Bionanotechnology Institute of University of Miami, Miami, Florida 33136, United States; Clinical and Translational Science Institute of University of Miami, Miami, Florida 33136, United States;
Sapna Deo, Department of Biochemistry and Molecular Biology, University of Miami Leonard M. Miller School of Medicine, Miami, Florida 33136, United States;; The Dr. John T. McDonald Foundation Bionanotechnology Institute of University of Miami, Miami, Florida 33136, United States;
REFERENCES
- (1).Rai R; Alwani S; Badea I Polymeric Nanoparticles in Gene Therapy: New Avenues of Design and Optimization for Delivery Applications. Polymers (Basel, Switz.) 2019, 11 (4), 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Sun Y; Zhao Y; Zhao X; Lee RJ; Teng L; Zhou C Enhancing the Therapeutic Delivery of Oligonucleotides by Chemical Modification and Nanoparticle Encapsulation. Molecules 2017, 22 (10), 1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Amreddy N; Babu A; Muralidharan R; Munshi A; Ramesh R Polymeric Nanoparticle-Mediated Gene Delivery for Lung Cancer Treatment. Top. Curr. Chem 2017, 375 (2), 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Jin L; Zeng X; Liu M; Deng Y; He N Current Progress in Gene Delivery Technology Based on Chemical Methods and NanoCarriers. Theranostics 2014, 4 (3), 240–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).He D; Wagner E Defined Polymeric Materials for Gene Delivery. Macromol. Biosci 2015, 15 (5), 600–612. [DOI] [PubMed] [Google Scholar]
- (6).Moukwa M The Development of Polymer-Based Biomaterials since the 1920s. JOM 1997, 49 (2), 46–50. [Google Scholar]
- (7).Kreuter J Nanoparticles-a Historical Perspective. Int. J. Pharm 2007, 331 (1), 1–10. [DOI] [PubMed] [Google Scholar]
- (8).Bolhassani A; Javanzad S; Saleh T; Hashemi M; Aghasadeghi MR; Sadat SM Polymeric Nanoparticles Potent Vectors for Vaccine Delivery Targeting Cancer and Infectious Diseases. Hum. Vaccines Immunother 2014, 10 (2), 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Dhandayuthapani B; Yoshida Y; Maekawa T; Kumar DS Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci 2011, 2011 (ii), 19. [Google Scholar]
- (10).Merrifield RB Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc 1963, 85 (14), 2149–2154. [Google Scholar]
- (11).Molek P; Strukelj B; Bratkovic T Peptide Phage Display as a Tool for Drug Discovery: Targeting Membrane Receptors. Molecules 2011, 16 (1), 857–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Suk JS; Xu Q; Kim N; Hanes J; Ensign LM PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Delivery Rev 2016, 99, 28–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Pandey AP; Sawant KK Polyethylenimine: A Versatile, Multifunctional Non-Viral Vector for Nucleic Acid Delivery. Mater. Sci. Eng., C 2016, 68, 904–918. [DOI] [PubMed] [Google Scholar]
- (14).Zou Y; Li D; Shen M; Shi X Polyethylenimine-Based Nanogels for Biomedical Applications. Macromol. Biosci 2019, 19 (11), 1–11. [DOI] [PubMed] [Google Scholar]
- (15).Zhong T; Ai P; Zhou J Structures and Properties of PAMAM Dendrimer: A Multi-Scale Simulation Study. Fluid Phase Equilib. 2011, 302 (1–2), 43–47. [Google Scholar]
- (16).Labieniec-Watala M; Watala C PAMAM Dendrimers: Destined for Success or Doomed to Fail? Plain and Modified PAMAM Dendrimers in the Context of Biomedical Applications. J. Pharm. Sci 2015, 104 (1), 2–14. [DOI] [PubMed] [Google Scholar]
- (17).Muxika A; Etxabide A; Uranga J; Guerrero P; de la Caba K Chitosan as a Bioactive Polymer: Processing, Properties and Applications. Int. J. Biol. Macromol 2017, 105, 1358–1368. [DOI] [PubMed] [Google Scholar]
- (18).Ahsan SM; Thomas M; Reddy KK; Sooraparaju SG; Asthana A; Bhatnagar I Chitosan as Biomaterial in Drug Delivery and Tissue Engineering. Int. J. Biol. Macromol 2018, 110, 97–109. [DOI] [PubMed] [Google Scholar]
- (19).Francoia JP; Vial L Everything You Always Wanted to Know about Poly-l-Lysine Dendrigrafts (But Were Afraid to Ask). Chem. - Eur. J 2018, 24 (12), 2806–2814. [DOI] [PubMed] [Google Scholar]
- (20).Dong C; Lv Y Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers (Basel, Switz.) 2016, 8 (2), 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).An B; Lin YS; Brodsky B Collagen Interactions: Drug Design and Delivery. Adv. Drug Delivery Rev 2016, 97, 69–84. [DOI] [PubMed] [Google Scholar]
- (22).Kulkarni V; Butte K; Rathod S Natural Polymers – A Comprehensive Review. Int. J. Res. Pharm. Biomed. Sci 2012, 3 (4), 1597–1613. [Google Scholar]
- (23).Liu K; Jiang X; Hunziker P Carbohydrate-Based Amphiphilic Nano Delivery Systems for Cancer Therapy. Nanoscale 2016, 8 (36), 16091–16156. [DOI] [PubMed] [Google Scholar]
- (24).Garg A; Garg S; Kumar M; Kumar S; Shukla AK; Kaushik SPC Applications of Natural Polymers in Mucoadhesive Drug Delivery: An Overview. Adv. Pharm. J 2018, 3 (2), 38–42. [Google Scholar]
- (25).Samadian H; Maleki H; Allahyari Z; Jaymand M Natural Polymers-Based Light-Induced Hydrogels: Promising Biomaterials for Biomedical Applications. Coord. Chem. Rev 2020, 420, 213432. [Google Scholar]
- (26).Matoba T; Koga J-I; Nakano K; Egashira K; Tsutsui H Nanoparticle-Mediated Drug Delivery System for Atherosclerotic Cardiovascular Disease. J. Cardiol 2017, 70 (3), 206–211. [DOI] [PubMed] [Google Scholar]
- (27).Kean T; Thanou M Biodegradation, Biodistribution and Toxicity of Chitosan. Adv. Drug Delivery Rev 2010, 62 (1), 3–11. [DOI] [PubMed] [Google Scholar]
- (28).Zhang X; Oulad-Abdelghani M; Zelkin AN; Wang Y; Haîkel Y; Mainard D; Voegel JC; Caruso F; Benkirane-Jessel N Poly(l-Lysine) Nanostructured Particles for Gene Delivery and Hormone Stimulation. Biomaterials 2010, 31 (7), 1699–1706. [DOI] [PubMed] [Google Scholar]
- (29).Friess W Collagen - Biomaterial for Drug Delivery. Eur. J. Pharm. Biopharm 1998, 45 (2), 113–136. [DOI] [PubMed] [Google Scholar]
- (30).Xu Y; Kim CS; Saylor DM; Koo D Polymer Degradation and Drug Delivery in PLGA-Based Drug–Polymer Applications: A Review of Experiments and Theories. J. Biomed. Mater. Res., Part B 2017, 105 (6), 1692–1716. [DOI] [PubMed] [Google Scholar]
- (31).Danhier F; Ansorena E; Silva JM; Coco R; Le Breton A; Préat V PLGA-Based Nanoparticles: An Overview of Biomedical Applications. J. Controlled Release 2012, 161 (2), 505–522. [DOI] [PubMed] [Google Scholar]
- (32).Mir M; Ahmed N; Rehman A. ur. Recent Applications of PLGA Based Nanostructures in Drug Delivery. Colloids Surf., B 2017, 159, 217–231. [DOI] [PubMed] [Google Scholar]
- (33).Guo B; Ma PX Synthetic Biodegradable Functional Polymers for Tissue Engineering: A Brief Review. Sci. China: Chem 2014, 57 (4), 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Alconcel SNS; Baas AS; Maynard HD FDA-Approved Poly(Ethylene Glycol)-Protein Conjugate Drugs. Polym. Chem 2011, 2 (7), 1442–1448. [Google Scholar]
- (35).Mishra J; Tiwari SK; Abolhasani MM; Azimi S; Nayak GC Fundamental of Polymer Blends and Its Thermodynamics. In Micro and Nano Fibrillar Composites (MFCs and NFCs) from Polymer Blends; Woodhead Publishing Series in Composites Science and Engineering, 2017; pp 27–55. [Google Scholar]
- (36).Bley H; Fussnegger B; Bodmeier R Characterization and Stability of Solid Dispersions Based on PEG/Polymer Blends. Int. J. Pharm 2010, 390 (2), 165–173. [DOI] [PubMed] [Google Scholar]
- (37).Tessmar JK; Göpferich AM Customized PEG-Derived Copolymers for Tissue-Engineering Applications. Macromol. Biosci 2007, 7 (1), 23–39. [DOI] [PubMed] [Google Scholar]
- (38).Gentile P; Chiono V; Carmagnola I; Hatton PV An Overview of Poly(Lactic-Co-Glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci 2014, 15 (3), 3640–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Lee JY; Bashur CA; Goldstein AS; Schmidt CE Polypyrrole-Coated Electrospun PLGA Nanofibers for Neural Tissue Applications. Biomaterials 2009, 30 (26), 4325–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Ulasov AV; Khramtsov YV; Trusov GA; Rosenkranz AA; Sverdlov ED; Sobolev AS Properties of PEI-Based Polyplex Nanoparticles That Correlate with Their Transfection Efficacy. Mol. Ther 2011, 19 (1), 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Brownlie A; Uchegbu IF; Schätzlein AG PEI-Based Vesicle-Polymer Hybrid Gene Delivery System with Improved Biocompatibility. Int. J. Pharm 2004, 274 (1–2), 41–52. [DOI] [PubMed] [Google Scholar]
- (42).Taranejoo S; Liu J; Verma P; Hourigan K A Review of the Developments of Characteristics of PEI Derivatives for Gene Delivery Applications. J. Appl. Polym. Sci 2015, 132 (25), 2–9. [Google Scholar]
- (43).Klajnert B; Bryszewska M Dendrimers: Properties and Applications. Acta Biochim. Polym 2001, 48 (1), 199–208. [PubMed] [Google Scholar]
- (44).Bravo-Anaya LM; Soltero JFA; Rinaudo M DNA/Chitosan Electrostatic Complex. Int. J. Biol. Macromol 2016, 88, 345–353. [DOI] [PubMed] [Google Scholar]
- (45).Hozumi K; Nomizu M Mixed Peptide-Conjugated Chitosan Matrices as Multi-Receptor Targeted Cell-Adhesive Scaffolds. Int. J. Mol. Sci 2018, 19 (9), 2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Bellich B; D’Agostino I; Semeraro S; Gamini A; Cesàro A The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99 DOI: 10.3390/md14050099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Krikorian V; Kurian M; Galvin ME; Nowak AP; Deming TJ; Pochan DJ Polypeptide-Based Nanocomposite: Structure and Properties of Poly(L-Lysine)/Na+-Montmorillonite. J. Polym. Sci., Part B: Polym. Phys 2002, 40 (22), 2579–2586. [Google Scholar]
- (48).Lodish H; Berk A; Zipursky S Section 22.3, Collagen: The Fibrous Proteins of the Matrix. In Mol. Cell. Biol, 4th ed.; W. H. Freeman: New York, 2000. [Google Scholar]
- (49).Lee CH; Singla A; Lee Y Biomedical Applications of Collagen. Int. J. Pharm 2001, 221, 1–22. [DOI] [PubMed] [Google Scholar]
- (50).Dang JM; Leong KW Natural Polymers for Gene Delivery and Tissue Engineering. Adv. Drug Delivery Rev 2006, 58 (4), 487–499. [DOI] [PubMed] [Google Scholar]
- (51).Shu JY; Panganiban B; Xu T Peptide-Polymer Conjugates: From Fundamental Science to Application. Annu. Rev. Phys. Chem 2013, 64 (1), 631–657. [DOI] [PubMed] [Google Scholar]
- (52).Raza F; Zafar H; Zhu Y; Ren Y; Ullah A; Khan AU; He X; Han H; Aquib M; Boakye-Yiadom KO; Ge L A Review on Recent Advances in Stabilizing Peptides/Proteins upon Fabrication in Hydrogels from Biodegradable Polymers. Pharmaceutics 2018, 10 (1), 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Krishna OD; Kiick KL Protein- and Peptide-Modified Synthetic Polymeric Biomaterials. Biopolymers 2010, 94 (1), 32–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Gauthier MA; Klok HA Peptide/Protein-Polymer Conjugates: Synthetic Strategies and Design Concepts. Chem. Commun 2008, No. 23, 2591–2611. [DOI] [PubMed] [Google Scholar]
- (55).Sun Y; Yang Z; Wang C; Yang T; Cai C; Zhao X; Yang L; Ding P Exploring the Role of Peptides in Polymer-Based Gene Delivery. Acta Biomater. 2017, 60, 23–37. [DOI] [PubMed] [Google Scholar]
- (56).Torchilin VP Cell Penetrating Peptide-Modified Pharmaceutical Nanocarriers for Intracellular Drug and Gene Delivery. Biopolymers 2008, 90 (5), 604–610. [DOI] [PubMed] [Google Scholar]
- (57).Smith GP; Petrenko VA Phage Display. Chem. Rev 1997, 97 (2), 391–410. [DOI] [PubMed] [Google Scholar]
- (58).Koivunen E; Arap W; Rajotte D; Lahdenranta J; Pasqualini R Identification of Receptor Ligands with Phage Display Peptide Libraries. J. Nucl. Med 1999, 40 (5), 883–888. [PubMed] [Google Scholar]
- (59).Pasqualini R; Ruoslahti E Organ Targeting in Vivo Using Phage Display Peptide Libraries. Nature 1996, 380 (6572), 364–366. [DOI] [PubMed] [Google Scholar]
- (60).Sanghvi AB; Miller KPH; Belcher AM; Schmidt CE Biomaterials Functionalization Using a Novel Peptide That Selectively Binds to a Conducting Polymer. Nat. Mater 2005, 4 (6), 496–502. [DOI] [PubMed] [Google Scholar]
- (61).Bolhassani A Potential Efficacy of Cell-Penetrating Peptides for Nucleic Acid and Drug Delivery in Cancer. Biochim. Biophys. Acta, Rev. Cancer 2011, 1816 (2), 232–246. [DOI] [PubMed] [Google Scholar]
- (62).Raucher D; Ryu JS Cell-Penetrating Peptides: Strategies for Anticancer Treatment. Trends Mol. Med 2015, 21 (9), 560–570. [DOI] [PubMed] [Google Scholar]
- (63).Zhang D; Wang J; Xu D Cell-Penetrating Peptides as Noninvasive Transmembrane Vectors for the Development of Novel Multifunctional Drug-Delivery Systems. J. Controlled Release 2016, 229, 130–139. [DOI] [PubMed] [Google Scholar]
- (64).Torchilin VP Tat Peptide-Mediated Intracellular Delivery of Pharmaceutical Nanocarriers. Adv. Drug Delivery Rev 2008, 60 (4–5), 548–558. [DOI] [PubMed] [Google Scholar]
- (65).Nielsen EJB; Kamei N; Takeda-Morishita M Safety of the Cell-Penetrating Peptide Penetratin as an Oral Absorption Enhancer. Biol. Pharm. Bull 2015, 38 (1), 144–146. [DOI] [PubMed] [Google Scholar]
- (66).Derakhshankhah H; Jafari S Cell Penetrating Peptides: A Concise Review with Emphasis on Biomedical Applications. Biomed. Pharmacother 2018, 108 (June), 1090–1096. [DOI] [PubMed] [Google Scholar]
- (67).Endoh T; Ohtsuki T Cellular SiRNA Delivery Using Cell-Penetrating Peptides Modified for Endosomal Escape. Adv. Drug Delivery Rev 2009, 61 (9), 704–709. [DOI] [PubMed] [Google Scholar]
- (68).Steinbach JM; Seo YE; Saltzman WM Cell Penetrating Peptide-Modified Poly(Lactic-Co-Glycolic Acid) Nanoparticles with Enhanced Cell Internalization. Acta Biomater. 2016, 30, 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Shu XZ; Ghosh K; Liu Y; Palumbo FS; Luo Y; Clark RA; Prestwich GD Attachment and Spreading of Fibroblasts on an RGD Peptide-Modified Injectable Hyaluronan Hydrogel. J. Biomed. Mater. Res 2004, 68 (2), 365–375. [DOI] [PubMed] [Google Scholar]
- (70).Jo S; Shin H; Mikos AG Modification of Oligo(Poly-(Ethylene Glycol) Fumarate) Macromer with a GRGD Peptide for the Preparation of Functionalized Polymer Networks. Biomacromolecules 2001, 2 (1), 255–261. [DOI] [PubMed] [Google Scholar]
- (71).Chu Y; Chen N; Yu H; Mu H; He B; Hua H; Wang A; Sun K Topical Ocular Delivery to Laser-Induced Choroidal Neovascularization by Dual Internalizing RGD and TAT Peptide-Modified Nanoparticles. Int. J. Nanomed 2017, 12, 1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Cook AD; Hrkach JS; Gao NN; Johnson IM; Pajvani UB; Cannizzaro SM; Langer R Characterization and Development of RGD-Peptide-Modified Poly(Lactic Acid-Co-Lysine) as an Interactive, Resorbable Biomaterial. J. Biomed. Mater. Res 1997, 35 (4), 513–523. [DOI] [PubMed] [Google Scholar]
- (73).Mousavizadeh A; Jabbari A; Akrami M; Bardania H Cell Targeting Peptides as Smart Ligands for Targeting of Therapeutic or Diagnostic Agents: A Systematic Review. Colloids Surf., B 2017, 158, 507–517. [DOI] [PubMed] [Google Scholar]
- (74).Aina OH; Sroka TC; Chen ML; Lam KS Therapeutic Cancer Targeting Peptides. Biopolymers 2002, 66 (3), 184–199. [DOI] [PubMed] [Google Scholar]
- (75).Dissanayake S; Denny WA; Gamage S; Sarojini V Recent Developments in Anticancer Drug Delivery Using Cell Penetrating and Tumor Targeting Peptides. J. Controlled Release 2017, 250, 62–76. [DOI] [PubMed] [Google Scholar]
- (76).Steichen SD; Caldorera-Moore M; Peppas NA A Review of Current Nanoparticle and Targeting Moieties for the Delivery of Cancer Therapeutics. Eur. J. Pharm. Sci 2013, 48 (3), 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Liu R; Li X; Xiao W; Lam KS Tumor-Targeting Peptides from Combinatorial Libraries. Adv. Drug Delivery Rev 2017, 110–111, 13–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Liu Y; He X; Kuang Y; An S; Wang C; Guo Y; Ma H; Lou J; Jiang C A Bacteria Deriving Peptide Modified Dendrigraft Poly- l -Lysines (DGL) Self-Assembling Nanoplatform for Targeted Gene Delivery. Mol. Pharmaceutics 2014, 11 (10), 3330–3341. [DOI] [PubMed] [Google Scholar]
- (79).Ran D; Mao J; Zhan C; Xie C; Ruan H; Ying M; Zhou J; Lu WL; Lu W D -Retroenantiomer of Quorum-Sensing Peptide-Modified Polymeric Micelles for Brain Tumor-Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9 (31), 25672–25682. [DOI] [PubMed] [Google Scholar]
- (80).Spicer CD; Pashuck ET; Stevens MM Achieving Controlled Biomolecule–Biomaterial Conjugation. Chem. Rev 2018, 118 (16), 7702–7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Devaraj NK The Future of Bioorthogonal Chemistry. ACS Cent. Sci 2018, 4 (8), 952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Jeong W; Bu J; Kubiatowicz LJ; Chen SS; Kim Y; Hong S Peptide–Nanoparticle Conjugates: A next Generation of Diagnostic and Therapeutic Platforms? Nano Converg. 2018, 5 (1), 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Xu D; Heck AJ; Kuan SL; Weil T; Wegner SV Precise Tetrafunctional Streptavidin Bioconjugates towards Multi-faceted Drug Delivery Systems. Chem. Commun 2020, 56 (68), 9858–9861. [DOI] [PubMed] [Google Scholar]
- (84).Borges J; Sousa MP; Cinar G; Caridade SG; Guler MO; Mano JF Nanoengineering Hybrid Supramolecular Multi-layered Biomaterials Using Polysaccharides and Self-Assembling Peptide Amphiphiles. Adv. Funct. Mater 2017, 27 (17), 1605122. [Google Scholar]
- (85).Jativa SD; Thapar N; Broyles D; Dikici E; Daftarian P; Jiménez JJ; Daunert S; Deo SK Enhanced Delivery of Plasmid DNA to Skeletal Muscle Cells Using a DLC8-Binding Peptide and ASSLNIA-Modified PAMAM Dendrimer. Mol. Pharmaceutics 2019, 16 (6), 2376–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Boekhoven J; Rubert Pérez CM; Sur S; Worthy A; Stupp SI Dynamic Display of Bioactivity through Host-Guest Chemistry. Angew. Chem., Int. Ed 2013, 52 (46), 12077–12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Hynd MR; Frampton JP; Dowell-Mesfin N; Turner JN; Shain W Directed Cell Growth on Protein-Functionalized Hydrogel Surfaces. J. Neurosci. Methods 2007, 162 (1–2), 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Tirat A; Freuler F; Stettler T; Mayr LM; Leder L Evaluation of Two Novel Tag-Based Labelling Technologies for Site-Specific Modification of Proteins. Int. J. Biol. Macromol 2006, 39 (1–3), 66–76. [DOI] [PubMed] [Google Scholar]
- (89).Boutureira O; Bernardes GJL Advances in Chemical Protein Modification. Chem. Rev 2015, 115 (5), 2174–2195. [DOI] [PubMed] [Google Scholar]
- (90).Spicer CD; Davis BG Selective Chemical Protein Modification. Nat. Commun 2014, 5 (1), 4740. [DOI] [PubMed] [Google Scholar]
- (91).Krall N; da Cruz FP; Boutureira O; Bernardes GJL Site-Selective Protein-Modification Chemistry for Basic Biology and Drug Development. Nat. Chem 2016, 8 (2), 103–113. [DOI] [PubMed] [Google Scholar]
- (92).Gunnoo SB; Madder A Bioconjugation – Using Selective Chemistry to Enhance the Properties of Proteins and Peptides as Therapeutics and Carriers. Org. Biomol. Chem 2016, 14 (34), 8002–8013. [DOI] [PubMed] [Google Scholar]
- (93).Hermanson G Bioconjugate Techniques, 3rd ed.; Elsevier/AP: Waltham, MA, 2013. [Google Scholar]
- (94).Baslé E; Joubert N; Pucheault M Protein Chemical Modification on Endogenous Amino Acids. Chem. Biol 2010, 17 (3), 213–227. [DOI] [PubMed] [Google Scholar]
- (95).Matos MJ; Oliveira BL; Martínez-Sáez N; Guerreiro A; Cal PMSD; Bertoldo J; Maneiro M; Perkins E; Howard J; Deery MJ; Chalker JM; Corzana F; Jiménez-Osés G; Bernardes GJL Chemo- and Regioselective Lysine Modification on Native Proteins. J. Am. Chem. Soc 2018, 140 (11), 4004–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Valeur E; Bradley M Amide Bond Formation: Beyond the Myth of Coupling Reagents. Chem. Soc. Rev 2009, 38 (2), 606–631. [DOI] [PubMed] [Google Scholar]
- (97).Lane C Sodium Cyanoborohydride - Highly Selective Reducing Agent for Organic Functional Groups. Synthesis 1975, 3, 135–146. [Google Scholar]
- (98).Chalker JM; Bernardes GJL; Lin YA; Davis BG Chemical Modification of Proteins at Cysteine: Opportunities in Chemistry and Biology. Chem. - Asian J 2009, 4 (5), 630–640. [DOI] [PubMed] [Google Scholar]
- (99).Thiol-X Chemistries in Polymer and Materials Science; Lowe A, Bowman C, Eds.; Polymer Chemistry Series; Royal Society of Chemistry: Cambridge, 2013. DOI: 10.1039/9781849736961. [DOI] [Google Scholar]
- (100).Nair DP; Podgórski M; Chatani S; Gong T; Xi W; Fenoli CR; Bowman CN The Thiol-Michael Addition Click Reaction: A Powerful and Widely Used Tool in Materials Chemistry. Chem. Mater 2014, 26 (1), 724–744. [Google Scholar]
- (101).Xu Q; Zhang Z; Xiao C; He C; Chen X Injectable Polypeptide Hydrogel as Biomimetic Scaffolds with Tunable Bioactivity and Controllable Cell Adhesion. Biomacromolecules 2017, 18 (4), 1411–1418. [DOI] [PubMed] [Google Scholar]
- (102).Rostovtsev VV; Green LG; Fokin VV; Sharpless KB A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem., Int. Ed 2002, 41 (14), 2596–2599. [DOI] [PubMed] [Google Scholar]
- (103).Tornøe CW; Christensen C; Meldal M Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem 2002, 67 (9), 3057–3064. [DOI] [PubMed] [Google Scholar]
- (104).McKay CS; Finn MG Click Chemistry in Complex Mixtures: Bioorthogonal Bioconjugation. Chem. Biol 2014, 21 (9), 1075–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Pickens CJ; Johnson SN; Pressnall MM; Leon MA; Berkland CJ Practical Considerations, Challenges, and Limitations of Bioconjugation via Azide–Alkyne Cycloaddition. Bioconjugate Chem. 2018, 29 (3), 686–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Baskin JM; Prescher JA; Laughlin ST; Agard NJ; Chang PV; Miller IA; Lo A; Codelli JA; Bertozzi CR Copper-Free Click Chemistry for Dynamic in Vivo Imaging. Proc. Natl. Acad. Sci. U. S. A 2007, 104 (43), 16793–16797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Guo C; Kim H; Ovadia EM; Mourafetis CM; Yang M; Chen W; Kloxin AM Bio-Orthogonal Conjugation and Enzymatically Triggered Release of Proteins within Multi-Layered Hydrogels. Acta Biomater. 2017, 56, 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Oliveira BL; Guo Z; Bernardes GJL Inverse Electron Demand Diels–Alder Reactions in Chemical Biology. Chem. Soc. Rev 2017, 46 (16), 4895–4950. [DOI] [PubMed] [Google Scholar]
- (109).Mayer S; Lang K Tetrazines in Inverse-Electron-Demand Diels–Alder Cycloadditions and Their Use in Biology. Synthesis 2017, 49 (04), 830–848. [Google Scholar]
- (110).Kölmel DK; Kool ET Oximes and Hydrazones in Bioconjugation: Mechanism and Catalysis. Chem. Rev 2017, 117 (15), 10358–10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Deng G; Tang C; Li F; Jiang H; Chen Y Covalent Cross-Linked Polymer Gels with Reversible Sol–Gel Transition and Self-Healing Properties. Macromolecules 2010, 43 (3), 1191–1194. [Google Scholar]
- (112).Kalia J; Raines RT Hydrolytic Stability of Hydrazones and Oximes. Angew. Chem., Int. Ed 2008, 47 (39), 7523–7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).O’Brien-Simpson NM; Ede NJ; Brown LE; Swan J; Jackson DC Polymerization of Unprotected Synthetic Peptides: A View toward Synthetic Peptide Vaccines. J. Am. Chem. Soc 1997, 119 (6), 1183–1188. [DOI] [PubMed] [Google Scholar]
- (114).Schense JC; Bloch J; Aebischer P; Hubbell JA Enzymatic Incorporation of Bioactive Peptides into Fibrin Matrices Enhances Neurite Extension. Nat. Biotechnol 2000, 18 (4), 415–419. [DOI] [PubMed] [Google Scholar]
- (115).Wang L-S; Boulaire J; Chan PPY; Chung JE; Kurisawa M The Role of Stiffness of Gelatin–Hydroxyphenylpro-pionic Acid Hydrogels Formed by Enzyme-Mediated Crosslinking on the Differentiation of Human Mesenchymal Stem Cell. Biomaterials 2010, 31 (33), 8608–8616. [DOI] [PubMed] [Google Scholar]
- (116).Hoyle CE; Bowman CN Thiol-Ene Click Chemistry. Angew. Chem., Int. Ed 2010, 49 (9), 1540–1573. [DOI] [PubMed] [Google Scholar]
- (117).Lowe AB Thiol-Ene “Click” Reactions and Recent Applications in Polymer and Materials Synthesis. Polym. Chem 2010, 1 (1), 17–36. [Google Scholar]
- (118).Nguyen KT; West JL Photopolymerizable Hydrogels for Tissue Engineering Applications. Biomaterials 2002, 23 (22), 4307–4314. [DOI] [PubMed] [Google Scholar]
- (119).Ligon SC; Husár B; Wutzel H; Holman R; Liska R Strategies to Reduce Oxygen Inhibition in Photoinduced Polymerization. Chem. Rev 2014, 114 (1), 557–589. [DOI] [PubMed] [Google Scholar]
- (120).Pinto-Alphandary H; Andremont A; Couvreur P Targeted Delivery of Antibiotics Using Liposomes and Nanoparticles: Research and Applications. Int. J. Antimicrob. Agents 2000, 13 (3), 155–168. [DOI] [PubMed] [Google Scholar]
- (121).Dinari A; Moghadam TT; Abdollahi M; Sadeghizadeh M Synthesis and Characterization of a Nano-Polyplex System of GNRs-PDMAEA-PDNA: An Inert Self-Catalyzed Degradable Carrier for Facile Gene Delivery. Sci. Rep 2018, 8 (1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Fang J; Nakamura H; Maeda H The EPR Effect: Unique Features of Tumor Blood Vessels for Drug Delivery, Factors Involved, and Limitations and Augmentation of the Effect. Adv. Drug Delivery Rev 2011, 63 (3), 136–151. [DOI] [PubMed] [Google Scholar]
- (123).Maeda H; Wu J; Sawa T; Matsumura Y; Hori K Tumor Vascular Permeability and the EPR Effect in Macromolecular Therapeutics: A Review. J. Controlled Release 2000, 65 (65), 271–284. [DOI] [PubMed] [Google Scholar]
- (124).Béduneau A; Saulnier P; Benoit JP Active Targeting of Brain Tumors Using Nanocarriers. Biomaterials 2007, 28 (33), 4947–4967. [DOI] [PubMed] [Google Scholar]
- (125).Byrne JD; Betancourt T; Brannon-Peppas L Active Targeting Schemes for Nanoparticle Systems in Cancer Therapeutics. Adv. Drug Delivery Rev 2008, 60 (15), 1615–1626. [DOI] [PubMed] [Google Scholar]
- (126).Pérez-Herrero E; Fernández-Medarde A Advanced Targeted Therapies in Cancer: Drug Nanocarriers, the Future of Chemotherapy. Eur. J. Pharm. Biopharm 2015, 93, 52–79. [DOI] [PubMed] [Google Scholar]
- (127).Park JW; Hong K; Kirpotin DB; Colbern G; Shalaby R; Baselga J; Shao Y; Nielsen UB; Marks JD; Moore D; Papahadjopoulos D; Benz CC Anti-HER2 Immunoliposomes: Enhanced Efficacy Attributable to Targeted Delivery. Clin. Cancer Res 2002, 8 (4), 1172–1181. [PubMed] [Google Scholar]
- (128).Kumari S; Kondapi AK Lactoferrin Nanoparticle Mediated Targeted Delivery of 5-Fluorouracil for Enhanced Therapeutic Efficacy. Int. J. Biol. Macromol 2017, 95, 232–237. [DOI] [PubMed] [Google Scholar]
- (129).Dhar S; Kolishetti N; Lippard SJ; Farokhzad OC Targeted Delivery of a Cisplatin Prodrug for Safer and More Effective Prostate Cancer Therapy in Vivo. Proc. Natl. Acad. Sci. U. S. A 2011, 108 (5), 1850–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Urbán P; Estelrich J; Adeva A; Cortés A; Fernàndez-Busquets X Study of the Efficacy of Antimalarial Drugs Delivered inside Targeted Immunoliposomal Nanovectors. Nanoscale Res. Lett 2011, 6, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Tambuwala MM; Manresa MC; Cummins EP; Aversa V; Coulter IS; Taylor CT Targeted Delivery of the Hydroxylase Inhibitor DMOG Provides Enhanced Efficacy with Reduced Systemic Exposure in a Murine Model of Colitis. J. Controlled Release 2015, 217, 221–227. [DOI] [PubMed] [Google Scholar]
- (132).Pardridge WM The Blood-Brain Barrier: Bottleneck in Brain Drug Development. NeuroRx 2005, 2 (1), 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Kwon EJ; Bergen JM; Park IK; Pun SH Peptide-Modified Vectors for Nucleic Acid Delivery to Neurons. J. Controlled Release 2008, 132 (3), 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Liu Y; Li J; Shao K; Huang R; Ye L; Lou J; Jiang C A Leptin Derived 30-Amino-Acid Peptide Modified Pegylated Poly-l-Lysine Dendrigraft for Brain Targeted Gene Delivery. Biomaterials 2010, 31 (19), 5246–5257. [DOI] [PubMed] [Google Scholar]
- (135).Pardridge WM; Eisenberg J; Yang J Human Blood-Brain Barrier Transferrin Receptor. Metab., Clin. Exp 1987, 36 (9), 892–895. [DOI] [PubMed] [Google Scholar]
- (136).Liu Z; Gao X; Kang T; Jiang M; Miao D; Gu G; Hu Q; Song Q; Yao L; Tu Y; Chen H; Jiang X; Chen J B6 Peptide-Modified PEG-PLA Nanoparticles for Enhanced Brain Delivery of Neuroprotective Peptide. Bioconjugate Chem. 2013, 24 (6), 997–1007. [DOI] [PubMed] [Google Scholar]
- (137).Mittal D; Ali A; Md S; Baboota S; Sahni JK; Ali J Insights into Direct Nose to Brain Delivery: Current Status and Future Perspective. Drug Delivery 2014, 21 (2), 75–86. [DOI] [PubMed] [Google Scholar]
- (138).Yan L; Wang H; Jiang Y; Liu J; Wang Z; Yang Y; Huang S; Huang Y Cell-Penetrating Peptide-Modified PLGA Nanoparticles for Enhanced Nose-to-Brain Macromolecular Delivery. Macromol. Res 2013, 21 (4), 435–441. [Google Scholar]
- (139).Kanazawa T; Akiyama F; Kakizaki S; Takashima Y; Seta Y Delivery of SiRNA to the Brain Using a Combination of Nose-to-Brain Delivery and Cell-Penetrating Peptide-Modified Nano-Micelles. Biomaterials 2013, 34 (36), 9220–9226. [DOI] [PubMed] [Google Scholar]
- (140).Schwartzbaum JA; Fisher JL; Aldape KD; Wrensch M Epidemiology and Molecular Pathology of Glioma. Nat. Clin. Pract. Neurol 2006, 2 (9), 494–503. [DOI] [PubMed] [Google Scholar]
- (141).Yao H; Wang K; Wang Y; Wang S; Li J; Lou J; Ye L; Yan X; Lu W; Huang R Enhanced Blood-Brain Barrier Penetration and Glioma Therapy Mediated by a New Peptide Modified Gene Delivery System. Biomaterials 2015, 37, 345–352. [DOI] [PubMed] [Google Scholar]
- (142).Kanazawa T; Morisaki K; Suzuki S; Takashima Y Prolongation of Life in Rats with Malignant Glioma by Intranasal SiRNA/Drug Codelivery to the Brain with Cell-Penetrating Peptide-Modified Micelles. Mol. Pharmaceutics 2014, 11 (5), 1471–1478. [DOI] [PubMed] [Google Scholar]
- (143).DeSantis C; Ma J; Bryan L; Jemal A Breast Cancer Statistics, 2013. Ca-Cancer J. Clin 2014, 64 (1), 52–62. [DOI] [PubMed] [Google Scholar]
- (144).Tao ZQ; Shi A; Lu C; Song T; Zhang Z; Zhao J Breast Cancer: Epidemiology and Etiology. Cell Biochem. Biophys 2015, 72 (2), 333–338. [DOI] [PubMed] [Google Scholar]
- (145).Mathews AS; Ahmed S; Shahin M; Lavasanifar A; Kaur K Peptide Modified Polymeric Micelles Specific for Breast Cancer Cells. Bioconjugate Chem. 2013, 24 (4), 560–570. [DOI] [PubMed] [Google Scholar]
- (146).Korch C; Hall EM; Dirks WG; Ewing M; Faries M; Varella-Garcia M; Robinson S; Storts D; Turner JA; Wang Y; Burnett EC; Healy L; Kniss D; Neve RM; Nims RW; Reid YA; Robinson WA; Capes-Davis A Authentication of M14 Melanoma Cell Line Proves Misidentification of MDA-MB-435 Breast Cancer Cell Line. Int. J. Cancer 2018, 142 (3), 561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Logie J; Ganesh AN; Aman AM; Al-awar RS; Shoichet MS Preclinical Evaluation of Taxane-Binding Peptide-Modified Polymeric Micelles Loaded with Docetaxel in an Orthotopic Breast Cancer Mouse Model. Biomaterials 2017, 123, 39–47. [DOI] [PubMed] [Google Scholar]
- (148).Brinkman AM; Chen G; Wang Y; Hedman CJ; Sherer NM; Havighurst TC; Gong S; Xu W Aminoflavone-Loaded EGFR-Targeted Unimolecular Micelle Nanoparticles Exhibit Anti-Cancer Effects in Triple Negative Breast Cancer. Biomaterials 2016, 101, 20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Yu DH; Liu YR; Luan X; Liu HJ; Gao YG; Wu H; Fang C; Chen HZ IF7-Conjugated Nanoparticles Target Annexin 1 of Tumor Vasculature against P-Gp Mediated Multidrug Resistance. Bioconjugate Chem. 2015, 26 (8), 1702–1712. [DOI] [PubMed] [Google Scholar]
- (150).Rostami I; Zhao ZJ; Wang ZH; Zhang WK; Zhong Y; Zeng Q; Jia XR; Hu ZY Peptide-Conjugated PEGylated PAMAM as a Highly Affinitive Nanocarrier towards HER2-Overexpressing Cancer Cells. RSC Adv. 2016, 6 (109), 107337–107343. [Google Scholar]
- (151).Johnson L; Cashman S; Kumar-Singh R Cell-Penetrating Peptide for Enhanced Delivery of Nucleic Acids and Drugs to Ocular Tissues Including Retina and Cornea. Mol. Ther 2008, 1 (16), 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Grossniklaus HE; Green WR Choroidal Neovascularization. Am. J. Ophthalmol 2004, 137 (3), 496–503. [DOI] [PubMed] [Google Scholar]
- (153).Li Y; Wang H; Wang K; Hu Q; Yao Q; Shen Y; Yu G; Tang G Targeted Co-Delivery of PTX and TR3 SiRNA by PTP Peptide Modified Dendrimer for the Treatment of Pancreatic Cancer. Small 2017, 13 (2), 1–9. [DOI] [PubMed] [Google Scholar]
- (154).Wang G; Wang Z; Li C; Duan G; Wang K; Li Q; Tao T RGD Peptide-Modified, Paclitaxel Prodrug-Based, Dual-Drugs Loaded, and Redox-Sensitive Lipid-Polymer Nanoparticles for the Enhanced Lung Cancer Therapy. Biomed. Pharmacother 2018, 106 (287), 275–284. [DOI] [PubMed] [Google Scholar]
- (155).Wang RT; Zhi XY; Yao SY; Zhang Y LFC131 Peptide-Conjugated Polymeric Nanoparticles for the Effective Delivery of Docetaxel in CXCR4 Overexpressed Lung Cancer Cells. Colloids Surf., B 2015, 133, 43–50. [DOI] [PubMed] [Google Scholar]
- (156).Yang W; Xia Y; Fang Y; Meng F; Zhang J; Cheng R; Deng C; Zhong Z Selective Cell Penetrating Peptide-Functionalized Polymersomes Mediate Efficient and Targeted Delivery of Methotrexate Disodium to Human Lung Cancer In Vivo. Adv. Healthcare Mater 2018, 7 (7), 1–8. [DOI] [PubMed] [Google Scholar]
- (157).Beloor J; Ramakrishna S; Nam K; Seon Choi C; Kim J; Kim SH; Cho HJ; Shin HS; Kim H; Kim SW; Lee SK; Kumar P Effective Gene Delivery into Human Stem Cells with a Cell-Targeting Peptide-Modified Bioreducible Polymer. Small 2015, 11 (17), 2069–2079. [DOI] [PubMed] [Google Scholar]
- (158).Santos JL; Pandita D; Rodrigues J; Pêgo AP; Granja PL; Balian G; Tomás H Receptor-Mediated Gene Delivery Using PAMAM Dendrimers Conjugated with Peptides Recognized by Mesenchymal Stem Cells. Mol. Pharmaceutics 2010, 7 (3), 763–774. [DOI] [PubMed] [Google Scholar]
- (159).Liu ZJ; Daftarian P; Kovalski L; Wang B; Tian R; Castilla DM; Dikici E; Perez VL; Deo S; Daunert S; Velazquez OC Directing and Potentiating Stem Cell-Mediated Angiogenesis and Tissue Repair by Cell Surface e-Selectin Coating. PLoS One 2016, 11 (4), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Daftarian PM; Stone GW; Kovalski L; Kumar M; Vosoughi A; Urbieta M; Blackwelder P; Dikici E; Serafini P; Duffort S; Boodoo R; Rodríguez-Cortés A; Lemmon V; Deo S; Alberola J; Perez VL; Daunert S; Ager AL A Targeted and Adjuvanted Nanocarrier Lowers the Effective Dose of Liposomal Amphotericin B and Enhances Adaptive Immunity in Murine Cutaneous Leishmaniasis. J. Infect. Dis 2013, 208 (11), 1914–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).Danhier F; Le Breton A; Préat V RGD-Based Strategies to Target Alpha(v) Beta(3) Integrin in Cancer Therapy and Diagnosis. Mol. Pharmaceutics 2012, 9 (11), 2961–2973. [DOI] [PubMed] [Google Scholar]
- (162).Liu Z; Wang F; Chen X Integrin AlphaV-Beta3-Targeted Cancer Therapy. Drug Dev. Res 2008, 69 (6), 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (163).Rajabi M; Mousa SA The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5 (2), 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (164).Nishida N; Yano H; Nishida T; Kamura T; Kojiro M Angiogenesis in Cancer. Vasc. Health Risk Manag 2006, 2 (3), 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (165).He X; Alves CS; Oliveira N; Rodrigues J; Zhu J; Bányai I; Tomás H; Shi X RGD Peptide-Modified Multifunctional Dendrimer Platform for Drug Encapsulation and Targeted Inhibition of Cancer Cells. Colloids Surf., B 2015, 125, 82–89. [DOI] [PubMed] [Google Scholar]
- (166).Li C; Wang W; Xi Y; Wang J; Chen JF; Yun J; Le Y Design, Preparation and Characterization of Cyclic RGDfK Peptide Modified Poly(Ethylene Glycol)-Block-Poly(Lactic Acid) Micelle for Targeted Delivery. Mater. Sci. Eng., C 2016, 64, 303–309. [DOI] [PubMed] [Google Scholar]
- (167).Shen Y; Li X; Dong D; Zhang B; Xue Y; Shang P Transferrin Receptor 1 in Cancer: A New Sight for Cancer Therapy. Am. J. Cancer Res 2018, 8 (6), 916–931. [PMC free article] [PubMed] [Google Scholar]
- (168).Xie Y; Killinger B; Moszczynska A; Merkel OM Targeted Delivery of SiRNA to Transferrin Receptor Overexpressing Tumor Cells via Peptide Modified Polyethylenimine. Molecules 2016, 21 (10), 1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (169).Zeeshan R; Mutahir Z Cancer Metastasis - Tricks of the Trade. Bosnian J. Basic Med. Sci 2015, 17 (3), 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (170).Steeg PS Targeting Metastasis. Nat. Rev. Cancer 2016, 16 (4), 201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (171).Liu X; Cheng B; Meng T; You J; Zhu Y; Lu B; Yuan H; Huang X; Hu F Synthesis and Biological Application of BKT-140 Peptide Modified Polymer Micelles for Treating Tumor Metastasis with an Enhanced Cell Internalization. Polym. Chem 2016, 7 (7), 1375–1386. [Google Scholar]
- (172).Lin Y; Xu J; Lan H Tumor-Associated Macrophages in Tumor Metastasis: Biological Roles and Clinical Therapeutic Applications. J. Hematol. Oncol 2019, 12 (1), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (173).Pang L; Pei Y; Uzunalli G; Hyun H; Lyle LT; Yeo Y Surface Modification of Polymeric Nanoparticles with M2pep Peptide for Drug Delivery to Tumor-Associated Macrophages. Pharm. Res 2019, 36, 65 DOI: 10.1007/s11095-019-2596-5.Surface. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Talman V; Kivelä R Cardiomyocyte—Endothelial Cell Interactions in Cardiac Remodeling and Regeneration. Front. Cardiovasc. Med 2018, 5 (July), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (175).Kakisis JD; Liapis CD; Breuer C; Sumpio BE Artificial Blood Vessel: The Holy Grail of Peripheral Vascular Surgery. J. Vasc. Surg 2005, 41 (2), 349–354. [DOI] [PubMed] [Google Scholar]
- (176).Fields RC; Solan A; McDonagh KT; Niklason LE; Lawson JH Gene Therapy in Tissue-Engineered Blood Vessels. Tissue Eng. 2003, 9 (6), 1281–1287. [DOI] [PubMed] [Google Scholar]
- (177).Duo X; Wang J; Li Q; Neve AL; Akpanyung M; Nejjari A; Ali ZSS; Feng Y; Zhang W; Shi C CAGW Peptide Modified Biodegradable Cationic Copolymer for Effective Gene Delivery. Polymers (Basel, Switz.) 2017, 9 (5), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (178).Zhou F; Jia X; Yang Q; Yang Y; Zhao Y; Fan Y; Yuan X Targeted Delivery of MicroRNA-126 to Vascular Endothelial Cells: Via REDV Peptide Modified PEG-Trimethyl Chitosan. Biomater. Sci 2016, 4 (5), 849–856. [DOI] [PubMed] [Google Scholar]
- (179).Takemura G; Kanoh M; Minatoguchi S; Fujiwara H Cardiomyocyte Apoptosis in the Failing Heart - A Critical Review from Definition and Classification of Cell Death. Int. J. Cardiol 2013, 167 (6), 2373–2386. [DOI] [PubMed] [Google Scholar]
- (180).Nam HY; Kim J; Kim S; Yockman JW; Kim SW; Bull DA Cell Penetrating Peptide Conjugated Bioreducible Polymer for SiRNA Delivery. Biomaterials 2011, 32 (22), 5213–5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (181).Nam HY; McGinn A; Kim PH; Kim SW; Bull DA Primary Cardiomyocyte-Targeted Bioreducible Polymer for Efficient Gene Delivery to the Myocardium. Biomaterials 2010, 31 (31), 8081–8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (182).Mohamed AMFS An Overview of Bone Cells and Their Regulating Factors of Differentiation. Malaysian J. Med. Sci 2008, 15 (1), 4–12. [PMC free article] [PubMed] [Google Scholar]
- (183).Sun Y; Ye X; Cai M; Liu X; Xiao J; Zhang C; Wang Y; Yang L; Liu J; Li S; Kang C; Zhang B; Zhang Q; Wang Z; Hong A; Wang X Osteoblast-Targeting-Peptide Modified Nanoparticle for SiRNA/MicroRNA Delivery. ACS Nano 2016, 10 (6), 5759–5768. [DOI] [PubMed] [Google Scholar]
- (184).Drury JL; Boontheekul T; Mooney DJ Cellular Cross-Linking of Peptide Modified Hydrogels. J. Biomech. Eng 2005, 127 (2), 220–228. [DOI] [PubMed] [Google Scholar]
- (185).Garty S; Kimelman-Bleich N; Hayouka Z; Cohn D; Friedler A; Pelled G; Gazit D Peptide-Modified “Smart” Hydrogels and Genetically Engineered Stem Cells for Skeletal Tissue Engineering. Biomacromolecules 2010, 11 (6), 1516–1526. [DOI] [PubMed] [Google Scholar]
- (186).Jones MER; Messersmith PB Facile Coupling of Synthetic Peptides and Peptide-Polymer Conjugates to Cartilage via Transglutaminase Enzyme. Biomaterials 2007, 28 (35), 5215–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (187).Lebaron RG; Athanasiou KA Extracellular Matrix Cell Adhesion Peptides: Functional Applications in Orthopedic Materials. Tissue Eng. 2000, 6 (2), 85–103. [DOI] [PubMed] [Google Scholar]
- (188).Kim TG; Park TAEG; D P Biomimicking Extracellular Matrix: Cell Adhesive RGD Peptide Modified Electrospun Poly(D,L-Lactic-Co-Glycolic Acid) Nanofiber Mesh. Tissue Eng. 2006, 12 (2), 221–233. [DOI] [PubMed] [Google Scholar]
- (189).Cao M; Wang Y; Hu X; Gong H; Li R; Cox H; Zhang J; Waigh TA; Xu H; Lu JR Reversible Thermoresponsive Peptide-PNIPAM Hydrogels for Controlled Drug Delivery. Biomacromolecules 2019, 20 (9), 3601–3610. [DOI] [PubMed] [Google Scholar]
- (190).Desseaux S; Klok HA Fibroblast Adhesion on ECM-Derived Peptide Modified Poly(2-Hydroxyethyl Methacrylate) Brushes: Ligand Co-Presentation and 3D-Localization. Biomaterials 2015, 44, 24–35. [DOI] [PubMed] [Google Scholar]
- (191).Wisse E; Spiering AJH; Dankers PYW; Mezari B; Magusin PCMM; Meijer EW Multicomponent Supramolecular Thermoplastic Elastomer with Peptide-Modified Nanofibers. J. Polym. Sci., Part A: Polym. Chem 2011, 49 (8), 1764–1771. [Google Scholar]
- (192).Reis LA; Chiu LLY; Liang Y; Hyunh K; Momen A; Radisic M A Peptide-Modified Chitosan-Collagen Hydrogel for Cardiac Cell Culture and Delivery. Acta Biomater. 2012, 8 (3), 1022–1036. [DOI] [PubMed] [Google Scholar]
- (193).Barber TA; Ho JE; De Ranieri A; Virdi AS; Sumner DR; Healy KE Peri-Implant Bone Formation and Implant Integration Strength of Peptide-Modified p(AAm-Co-EG/AAc) Interpenetrating Polymer Network-Coated Titanium Implants. J. Biomed. Mater. Res., Part A 2007, 80A, 306–320. [DOI] [PubMed] [Google Scholar]
- (194).Oyman G; Geyik C; Ayranci R; Ak M; Odaci Demirkol D; Timur S; Coskunol H Peptide-Modified Conducting Polymer as a Biofunctional Surface: Monitoring of Cell Adhesion and Proliferation. RSC Adv. 2014, 4 (96), 53411–53418. [Google Scholar]
- (195).Aguilar AD; Forzani ES; Li X; Tao N; Nagahara LA; Amlani I; Tsui R Chemical Sensors Using Peptide-Functionalized Conducting Polymer Nanojunction Arrays. Appl. Phys. Lett 2005, 87 (19), 1–3. [Google Scholar]
- (196).Jie LY; Cai LL; Wang LJ; Ying XY; Yu RS; Zhang MM; Du YZ Actively-Targeted LTVSPWY Peptide-Modified Magnetic Nanoparticles for Tumor Imaging. Int. J. Nanomed 2012, 7, 3981–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).Cheng CC; Huang CF; Ho AS; Peng CL; Chang CC; Der Mai F; Chen LY; Luo TY; Chang J Novel Targeted Nuclear Imaging Agent for Gastric Cancer Diagnosis: Glucose-Regulated Protein 78 Binding Peptide-Guided 111In-Labeled Polymeric Micelles. Int. J. Nanomed 2013, 8, 1385–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (198).Lee SC; Kim MS; Yoo KC; Ha NR; Moon JY; Lee SJ; Yoon MY Sensitive Fluorescent Imaging of Salmonella Enteritidis and Salmonella Typhimurium Using a Polyvalent Directed Peptide Polymer. Microchim. Acta 2017, 184 (8), 2611–2620. [Google Scholar]
- (199).Bysell H; Månsson R; Hansson P; Malmsten M Microgels and Microcapsules in Peptide and Protein Drug Delivery. Adv. Drug Delivery Rev 2011, 63 (13), 1172–1185. [DOI] [PubMed] [Google Scholar]
- (200).Lallana E; Fernandez-Trillo F; Sousa-Herves A; Riguera R; Fernandez-Megia E Click Chemistry with Polymers, Dendrimers, and Hydrogels for Drug Delivery. Pharm. Res 2012, 29 (4), 1134–1142. [DOI] [PubMed] [Google Scholar]


