Abstract
Simple Summary
In the last decades, many researchers produced promising data concerning genetics and tumor microenvironment of poorly differentiated thyroid cancer (PDTC) and anaplastic thyroid cancer (ATC). They are trying to tear the veil covering these orphan cancers, suggesting new therapeutic weapons as single or combined therapies.
Abstract
PDTC and ATC present median overall survival of 6 years and 6 months, respectively. In spite of their rarity, patients with PDTC and ATC represent a significant clinical problem, because of their poor survival and the substantial inefficacy of classical therapies. We reviewed the newest findings about genetic features of PDTC and ATC, from mutations occurring in DNA to alterations in RNA. Therefore, we describe their tumor microenvironments (both immune and not-immune) and the interactions between tumor and neighboring cells. Finally, we recapitulate how this upcoming evidence are changing the treatment of PDTC and ATC.
Keywords: anaplastic thyroid cancer, poorly thyroid cancer, genetic landscape, tumor microenvironment, genetically guided therapy
1. Introduction
Thyroid cancer is the most common endocrine tumor and its incidence has been raising up over the last 20 years, mostly due to the flowering diagnosis of micro thyroid carcinomas [1]. Thyroid cancer is subcategorized into follicular and non-follicular derived carcinoma (e.g., medullary thyroid carcinoma). Among the first, World Health Organization (WHO) identifies papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC), poorly differentiated thyroid carcinoma (PDTC), and anaplastic thyroid carcinoma (ATC) [2].
Thyroid cancer 5 year-survival is variable among the different hystotypes. According to a recent epidemiological study performed in Denmark by using a national cancer registry, the 5 year-survival rates were 91.1% and 79.9% in PTC and FTC, respectively, 63.6% in PDTC and 12.2% in ATC [3]. Unfortunately, PDTC and ATC median overall survival is 6 years and 6 months, respectively [4,5]. Although PDTC and ATC are rare, therapy for patients affected by PDTC and ATC represents an unmet clinical need that should be addressed, considering their poor survival. In addition, PDTC and ATC harbor diagnostic pitfalls that make difficult their clinical management. Although PDTC was added in WHO classification in 2004, its diagnostic criteria are not widely shared and many pathologists are following criteria of Turin consensus conference [6] and others Memorial Sloan Kettering Cancer Center ones [7]. Likewise, the wide spectrum of ATC hystotypes could challenge the differential diagnosis with other cancers (e.g., angiomatoid variant ATC with thyroid angiosarcoma) [8] or even with benign lesions (e.g., acute thyroiditis) [9] (Figure 1).
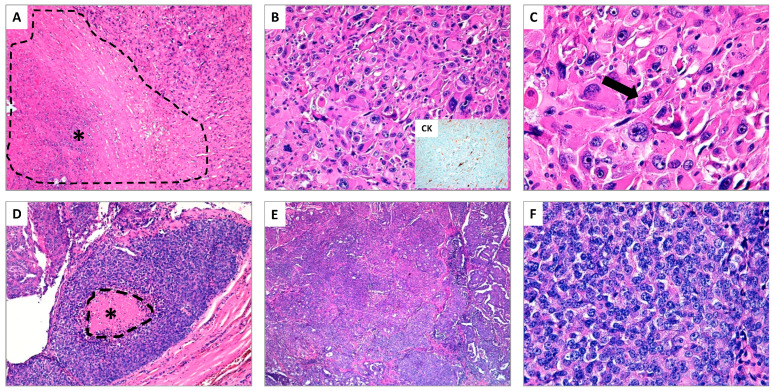
Figure 1.
(A–C) Anaplastic thyroid carcinoma (ATC) (hematoxylin and eosin stain); (A) The presence of extensive tumor necrosis is a typical aspect of ATC (see asterisk *) (original magnification ×10); (B) The neoplastic cells show marked nuclear atypia with spindled and pleomorphic morphology, associated to elevated mitotic rate, simulating high-grade pleomorphic sarcoma. In the insert, focal immunostaining for cytokeratins supports the epithelial nature of ATC (original magnification ×20); (C) At higher magnification, the pronounced nuclear atypia and an atypical mitosis (see arrow) are shown (original magnification ×40). (D–F) Poorly differentiated thyroid carcinoma (PDTC) (hematoxylin and eosin stain); (D) The typical example of PDTC is the so-called “insular carcinoma”. In this field, the tumor shows a small focus of tumor necrosis (see asterisk *) (original magnification ×10); (E) The neoplasm exhibits a prevalent solid growth pattern (original magnification ×20); (F) At higher magnification, the tumor cells appear small and uniform, the nuclei are generally rounded and hyperchromatic, in absence of the typical aspects of papillary thyroid carcinoma (original magnification ×40).
In the past, many treatments were proposed to answer this aforementioned need, but with disappointing results [10]. Nowadays many clinicians are proposing genetically guided treatments for PDTC and ATC, according to the new discoveries about their genetic landscape [11,12].
In the current review, as first, we summarize the upcoming findings about genetic features of PDTC and ATC, from mutations occurring in DNA to alterations in RNA; therefore, we describe their tumor microenvironments and the interactions between tumor and other neighboring cells; finally, we recapitulate how this upcoming evidence are changing the treatment of PDTC and ATC.
2. Genetics Features
Genomic instability is universally considered as a driver of carcinogenesis, supporting the generation of all hallmarks of cancer (i.e., resistance to cell death, promotion of proliferative signaling, escape from growth suppressors, invasion and metastasis capacity, activation of replicative immortality, evasion of immune destruction, deregulation cellular energetics and neo-angiogenesis) [13,14]. Across several neoplasia, thyroid cancer presents lower genomic instability, expressed as the number of mutations per tumor, compared to other adult neoplasia (e.g., endometrial and colorectal cancers) [15]; this evidence has also been confirmed in metastatic cases [16]. However, thyroid cancer shows a heterogenous mutational burden across its histotypes: ATC presents an increased number of genetic alterations per tumor (median 4, range 0–29) compared to PTC and FTC [17]; likewise, according to data from Tissue Cancer Genome Atlas (TCGA), PDTC mutational burden is higher than PTC, even if lower than ATC [18,19]. Genomic instability in PDTC and ATC embraces both somatic driver mutations and gene fusions.
2.1. Somatic Driver Mutations
Vogelstein et al. considered a driver mutation as a genomic variant that directly or indirectly induces a selective growth advantage [20]. As shown in Table 1, ATC and PDTC harbor many driver mutations, occurring mainly in both MAPK and PI3K-AKT pathways.
Table 1.
ATC and PDTC genetic landscape: somatic mutations.
| Cellular Function | Gene | Mutation Rate (%) | ||
|---|---|---|---|---|
| ATC [21,29] |
PDTC [18,22,23,24] |
|||
| Intracellular signaling | MAPK pathway | BRAF | 27.63 | 15.38–33.33 |
| NRAS | 19.25 | 4.35–30.77 | ||
| NF1 | 5.56 | 0 | ||
| KRAS | 4.92 | 0–5.31 | ||
| HRAS | 4.51 | 2.45–4.88 | ||
| PI3K-AKT pathway | PIK3CA | 11.24 | 2.38–19.51 | |
| PTEN | 9.27 | 0–4.35 | ||
| NF2 | 5.10 | 0 | ||
| IRS1 | 3.64 | - | ||
| AKT1 | - | 0-8.70 | ||
| WNT pathway | AXIN1 | 4.51 | - | |
| CTNNB1 | 3.88 | 0–2.44 | ||
| APC | 3.05 | 17.39 | ||
| Cell cycle regulation | TERT promoter | 75 | 21.95–40.48 | |
| TP53 | 45.67 | 8.33–43.48 | ||
| ATM | 4.91 | 7.14–13.04 | ||
| RB1 | 4.36 | 1.19–4.35 | ||
| CDKN2A | 4.01 | - | ||
| Chromatin remolding | KMT2D | 4.42 | - | |
| CREBBP | 4.17 | - | ||
| ARID2 | 3.93 | - | ||
| ARID1A | 3.69 | - | ||
| DNMT3A | 3.38 | - | ||
| KMT2A | 3.36 | - | ||
| DNA damage response | MDC1 | 3.18 | - | |
| MSH2 | 3.05 | - | ||
| Protein metabolism | EIF1AX | 9.24 | 4.88–10.71 | |
| CALR | 4.85 | - | ||
| RBM10 | 3.38 | - | ||
BRAF and RAS genes (HRAS, KRAS, and NRAS) are main members of MAPK pathway. Both of them occur in more than 25% of ATCs, according to catalogue of somatic mutations in cancer (COSMIC) database [21], while 15.38–33.33% and 6.8–41.2% of PDTCs harbor BRAF and RAS mutations, respectively (Table 1) [18,22,23,24]. Interestingly, although BRAF and RAS mutations are present in a relevant percentage of both ATC and PDTC cases, they seem to play different roles. In ATC, neither BRAF or RAS mutations seem to be sufficient to induce neoplastic cell anaplasia. McFadden et al. produced a thyroid-specific CreER transgenic mouse in order to specifically induce BRAFV600E mutation in thyroid cells; although this mutation induces PTC foci, it was capable to promote ATC tumorigenesis only in the presence of p53 mutation [25]. Likewise, KRASG12D mutation developed anaplastic foci with complete deregulation of normal thyroid follicular morphology in mice model only in association with a homozygous mutation of TSH receptor [26]. However, BRAF-RAS signaling retains a crucial role in ATC cells and its inhibition by siRNA anti-BRAF produces growth arrest in ATC cell lines [27], even stronger in combination with MEK inhibition [25]. Otherwise, the mechanisms seem to be different in PDTC: Vitagliano et al. were able to promote progression of FTC foci into PDTC in mouse model by NRASG61K mutation [28].
In addition to mutations of MAPK pathway, next generation analysis showed that ATC harbors higher prevalence of mutations in PI3K-AKT pathway compared to other histotypes [30]: according to COSMIC database, PI3KCA and PTEN were found mutated in 11.24% and 9.27%, respectively (Table 1) [21]. Likewise, also PDTC harbors frequently PIK3CA or AKT1 mutations (2.38–19.51% and 0–8.70%, respectively) (Table 1) [18,22,23,24].
Interestingly, in ATC series provided by Liu et al., the 81.3% of samples presented genetic alterations affecting both MAPK and PI3K-AKT pathways [31]. Accordingly, in mouse model, the presence of mutations occurring in both pathways induced ATC foci, confirming the synergistic interactions between these pathways [32]. On one hand, MAPK pathway has a crucial role in cell proliferation and survival, and, on the other hand, upregulated PI3K-AKT pathway has been related to tumor aggressiveness [33].
Beyond mutations occurring at members of MAPK and PI3K-AKT pathways, many variants have been reported in cell cycle regulators. Many reports showed that mutations occurring in p53 and TERT promoter (pTERT) are highly prevalent in ATC, occurring even simultaneously [17,18,21] (Table 1). Likewise, PDTC presents both mutations, even if less frequently than ATC [18,34]. Intriguingly, in the presence of an impaired cell-cycle checkpoint pathway (e.g., p53), the occurrence of a concomitant mutation in telomerase activity (e.g., pTERT) could induce an indefinite cell proliferation [35]. In addition, the interplays between the duet BRAF-pTERT have recently been described by Tan and colleagues [36]. In particular, in case of mutation of both of them, cancer cells suppress apoptosis mainly thank to pTERT activity, while in case of mutation occurring only on BRAF gene, apoptosis activity seems to be not significantly affected [36]. Accordingly, the inhibition of TERT activity could represent an Achilles heel, as recently shown in-vitro and in-vivo model by Bu et al. In these models, BIBR1532 (a TERT inhibitor) significantly inhibited tumor growth as well as cell invasion, migration and angiogenesis [37].
If regulation of cell cycle has a crucial role in oncogenesis, also protein metabolism control has been deeply involved in tumorigenesis [14,38]. Not surprisingly, both PDTC and ATC harbor EIF1AX mutations in about 10% of cases (Table 1) [18,22,23,24]. EIF1AX is a member of 43S preinitiation complexes, responsible of translation initiation, and its mutation has recently been involved in preinitiation complex stabilization and, further, in deregulating protein synthesis [39,40]. Interestingly, EIF1AX mutations are mutually exclusive with other drivers in PTC [19], while they co-occur with RAS mutations in ATC and PDTC [18]. Recently, Krishnamoorthy et al. showed a positive feedback relationship between RAS and EIF1AX proteins, which reinforces c-MYC gene expression [40].
2.2. Gene Fusions
Fusion genes are common driver mutations described in both hematopoietic and solid tumors [41]. They usually involve a driver gene, which expresses a receptor tyrosine kinase (e.g., RET) or its downstream kinase (e.g., BRAF), and a partner gene (e.g., NCOA4). If in physiologic state most of these kinases require the ligand to induce their dimerization, these rearrangements are capable to induce a ligand-independent dimerization and a deregulated kinase activity [42]. In the past, all the tumorigenic effects were considered as consequence of a non-controlled expression of the driver gene; however, new evidence suggests that also the partner gene may play a crucial oncogenic role [43].
Although fusion genes have been extensively described in thyroid cancer, their prevalence is lower compared to other solid tumors [41]. PDTC harbors gene fusions in 10–14% of cases while ATC in 3–5% [44] (Table 2). Interestingly, when present, fusions usually involve the same few oncogenes. RET fusions are the most common, mainly CCDC6-RET (RET/PTC1) and NCOA4-RET (RET/PTC3), while NTRK, ALK and BRAF fusions are quite rare (Table 2) [44]. Recently, Nikitski et al. developed a mouse model of STRN-ALK fusion gene that was capable of inducing PTC, PDTC and ATC foci [45]. This model revealed the presence of two clusters of PDTC with specific cell morphology, immunohistochemical characteristics and different levels of expression of thyroid differentiation markers [45].
Table 2.
ATC and PDTC genetic landscape: gene fusions.
| Gene Fusions | Mutation Rate [16,18,29,44,46] |
||
|---|---|---|---|
| ATC | PDTC | ||
| PAX8-PPARγ Fusions | 0 | 3/84 | |
| NTRK fusions | NTRK1-IRF2BP2 | 1/126 | 0 |
| NTRK2-CRNDE | 1/126 | 0 | |
| ETV6-NTRK3 | 0 | 1/60 | |
| RET fusions | CCDC6-RET | 2/126 | 3/84, 2/60 |
| NCOA4-RET | Case report | 2/84, 1/23 | |
| PDCD10-RET | 0 | 1/60 | |
| TFG-RET | 0 | 1/60 | |
| ALK fusions | STRN-ALK | Case report | 1/23 |
| EML4-ALK | 0 | 2/84 | |
| BRAF fusions | KIAA1549-BRAF | Case report | 0 |
| SCRIB-BRAF | 0 | 1/60 | |
| Other fusions | NUT-BRD4 | 1/33 | 0 |
Although rare, gene fusions could represent precious targets for targeted therapies. Moreover, any histotype of thyroid cancers with gene fusions has recently been proposed as a discrete group with specific histologic characteristics such as multinodular growth and extensive fibrotic features. For this reason, they have been named “kinase fusion-related thyroid carcinomas” [46].
2.3. Copy Number Variations
In oncology, copy number variations (CNVs) are well characterized as prognostic factors for recurrence and death [47]. This evidence has been confirmed also in advanced thyroid cancer [18]. If they are quite rare in differentiated thyroid cancer (less than 10%) [19], in PDTC and ATC they are widespread, especially in cancers without known driver mutation (losses of 1p, 8p, 13q, 15q, 17p, 22q, and gains of 1q and 20q) [18]. Interestingly, they seem to be hystotipes-specific: 8p and 17p losses and 20q gains are more frequent in ATC while loss of 1p was substantially more recurring in PDTCs [18]. Moreover, CNVs correlate with gene context where occur: 1p, 13q, and 15q losses were enriched in PDTCs without known driver mutation while loss of 22q was associated with RAS-mutated PDTCs [18]. In ATC, beyond large chromosomal variations, Pozdeyev et al. reported more restricted CNVs such as losses of CDKN2A and CDKN2B or amplification of KIT, PDGFRA and KDR, further confirmed by other authors [17,24].
Finally, since CNVs have recently been related to resistance to target therapies in thyroid cancer [48], it would be very interesting to ascertain if some of them (e.g., PDGFRA amplification) could induce resistance to target therapy (e.g., multikinase inhibitors, MKIs) in ATC and PDTC.
2.4. RNA Alterations
It is universally recognized that messenger RNA (mRNA) synthesis and translation are deeply modified in cancer; however, new evidence shows that all kinds of RNA are universally impaired [49]. In normal condition, cells produce different types of RNAs: mRNA, ribosomal RNA (rRNA), transfer RNA (tRNA), microRNA (miRNA), long non-coding (lncRNA) and circular RNA (circRNA). Accordingly, neoplastic cells could deregulate all kinds of RNAs that could promote the cells growth and invasiveness [49].
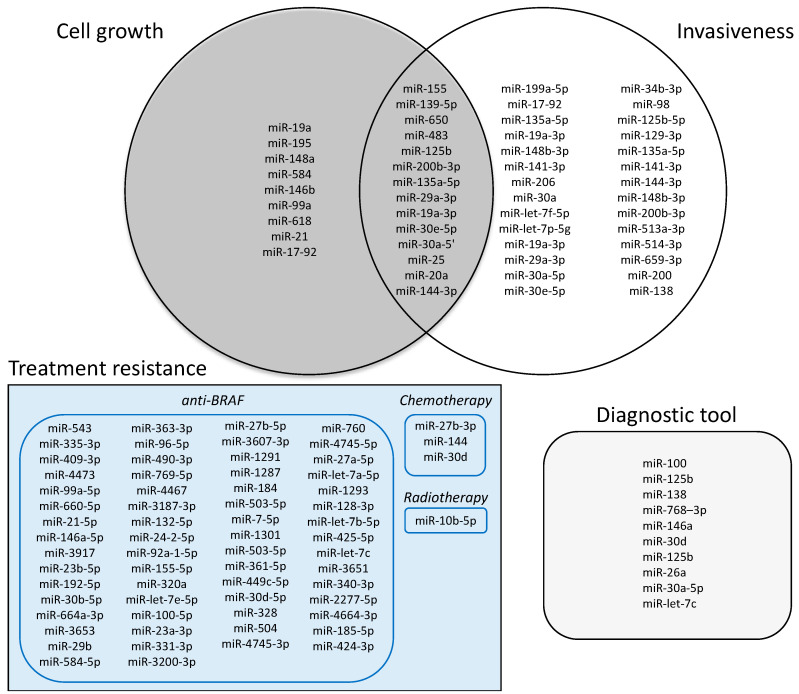
In particular, miRNA are usually 20–23 nucleotides in length that can bind multiple mRNA, regulating their catabolism and further their translation [50]. 127 and 18 different miRNAs have been characterized in ATC and PDTC, respectively. Among them, 69 miRNAs resulted decreased and 54 increased in ATC, while 10 resulted decreased and 8 increased in PDTC. If their role in PDTC is not fully elucidated and they might be used as an ancillary diagnostic tool and prognostic marker [51], they have been fully characterized in ATC. According to literature data, we grouped them into 3 main roles: regulation of growth tumor, invasiveness and resistance to therapy (Figure 2). We found that 9 miRNA were related to tumor growth [52,53,54,55,56,57,58,59,60,61], 14 to tumor growth and invasiveness [62,63,64,65,66,67,68,69], 28 to invasiveness [67,70,71,72,73,74,75,76], and 66 to therapies resistance (62 to anti-BRAF treatment, 3 to chemotherapy and 1 to radiotherapy) [77,78,79,80,81]. Additionally, 10 miRNAs were considered as an ancillary diagnostic tool [82,83] (Figure 2).
Figure 2.
miRNA that were discovered in ATC according to their function.
Beyond miRNA, growing evidence is showing the role of lncRNAs in cancer. lncRNA are RNAs longer than 200 nucleotides that do not encode proteins but regulate gene expression, splicing and nucleation of subnuclear domains [49]; moreover, lncRNAs may have cytoplasmic functions, such as miRNA sponging, interaction with signaling proteins, and further modulation of mRNA translation [49]. In ATC, lncRNAs may regulate tumor growth [84,85], invasiveness [86], and both tumor growth and invasiveness [87,88,89,90,91]; they can also regulate cancer sensitivity to treatments [92]. In particular, lncRNA PTCSC3 was described at low levels both in ATC tissue and cell lines and it was demonstrated that its upregulation inhibited the resistance to doxorubicin by suppressing stem cell proprieties [92].
Finally, circRNAs are usually consequence of back-splicing events, producing in a covalently closed circRNA molecule instead of linear ones. Although circRNAs have usually been detected at low levels in normal and in cancer cells, some of them are at higher concentration and have functional roles: miRNA sponging and proteins stabilization [49]. In ATC, a recent study showed that circRNA may produce resistance to chemotherapy. In particular, Liu et al. showed that circRNA EIF6 could sponge miR-144-3p to promote autophagy and cisplatin-resistance [93].
3. Tumor Microenvironment
Tumor microenvironment (TME) is the dynamic milieu that harbors tumor cells [94]. It comprises blood vessels, extracellular matrix (ECM), non-neoplastic cells, and signaling molecules [95]. Neoplastic cells interact with other TME members in order to regulate self-growth, invasiveness and resistance to therapy [94]. In thyroid, many reports showed that TME may promote tumor growth, metastatic power, and resistance to therapy, both in differentiated and anaplastic thyroid cancer [96,97,98].
In TME we should distinguish not immune and immune related cells. Among the former, cancer associated fibroblasts play a relevant role in both ATC and PDTC [99,100]. In ATC, tumor cells present paracrine communication with fibroblast: ATC cells activate fibroblasts by reprogramming their metabolism, phenotype and secretome, and then activated fibroblasts reinforce thyroid cancer progression, by enhancing tumor invasion and proliferation [100]. Likewise, interactions between PDTC cells and cancer associated fibroblasts may potentiate tumor progression, by collagen remodeling [99]. Analog interplays have been recently demonstrated between ATC and endothelial cells, partially rescued by sorafenib [94].
Giannini et al. provided significant evidence about immune TME in ATC and PDTC [101]. ATC TME was enriched of tumor infiltrating leukocytes (both macrophage and lymphocytes) and characterized by hot or altered–immunosuppressed phenotype, since a relevant part of CD8+ lymphocytes presented exhausted features. Accordingly, Caillou et al. showed that tumor-associated macrophages build up a dense network in whom cancer cells reside [102,103] and their presence is associated with a worse prognosis in ATC [104]. Cameselle-García and colleagues elucidated that ATC tumors are enriched of tumor infiltrating lymphocytes (mainly CD8+ cytotoxic T cells), which mainly reside in the interface between tumor ant thyroid tissue [105]. Otherwise, PDTC harbored less tumor infiltrating leukocytes compared to ATC, and presented a cold immune contexture in 65% of cases [101]. In these immune contexts, PD/PD-L1 pathway (programmed cell death protein-1/programmed cell death ligand-1) plays a crucial role in ATC and less frequently also in PDTC [97,105,106]. If in physiologic conditions, PD/PD-L1 pathway regulates T cell immune suppression, in neoplastic milieu it is exploited by cancer cells in order to avoid immune attack, by inducing T-cell exhaustion [107]. In ATC, PD/PD-L1 proteins expression was shown to be regulated by BRAF mutation and is was associated to a worst prognosis [106,108]. Accordingly, Brauner et al. demonstrated that dual inhibition of BRAF and PD/PD-L1 pathways induced a powerful shrinkage of ATC tumor in orthotopic immune-competent mouse model [108].
4. Contemporary Treatment in ATC and PDTC
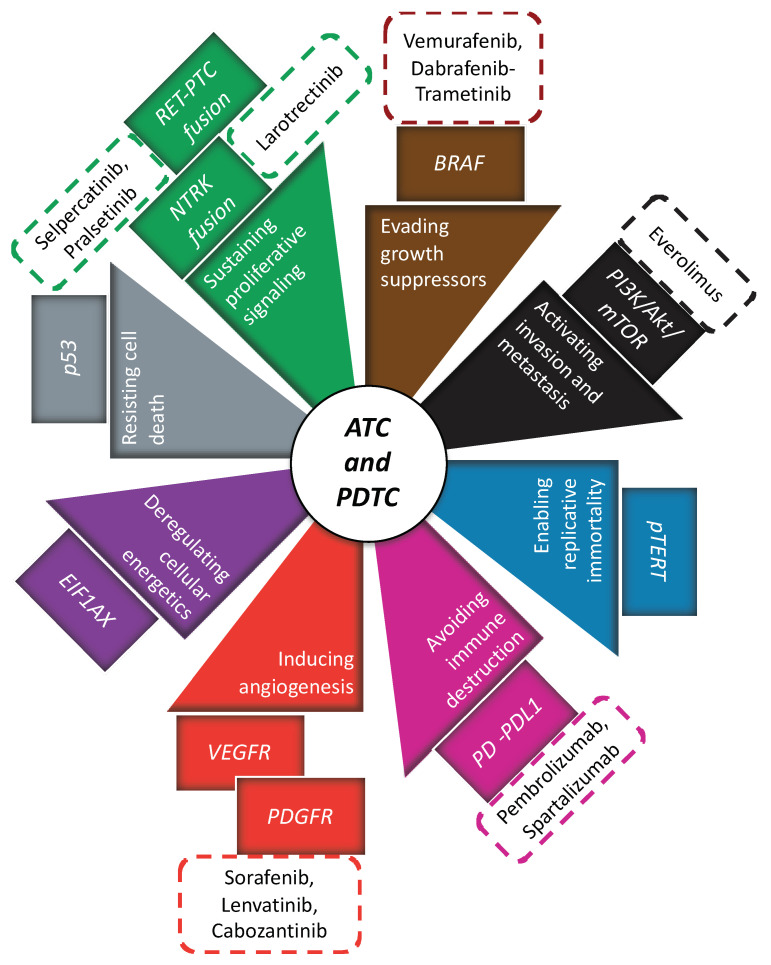
Traditionally, treatments against ATC and PDTC globally provided disappointing results [12,109]. ATC presents very low median overall survival [109,110]. Although PDTC presents higher 5-year survival (62%) compared to ATC [111], disease control in patients with metastatic PDTC is still poor (59%) and 85% of their disease specific deaths is related to the presence of distant metastasis [12]. In both of them, surgery represents a cornerstone of multimodal treatment; nonetheless, systemic treatment is necessary in case of diffuse disease. Systemic treatment comprises chemotherapy (in case of ATC, elsewhere reviewed [112]), anti-angiogenic therapy, immunotherapy and genetically guided therapy (Figure 3). Since their individual use provided encouraging but insufficient results, they have recently been proposed in multimodal approach.
Figure 3.
All innovative therapies that have been suggested in ATC and PDTC (rectangular box with dotted lines); each of them has been related to a specific inhibition of hallmark of cancer (triangle box) and cellular pathway (rectangular box).
4.1. Antiangiogenic Therapy
As previously shown, neo-angiogenesis is a main hallmark of cancer, sustaining its limitless growth [113]. Accordingly, neoplastic cells regulate neo-angiogenesis in order to guarantee their progression in ATC as well as in PDTC [114,115]. Many anti-angiogenic drugs have been employed to inhibit ATC and PDTC growth. Year by year, sorafenib, lenvatinib, cabozantinib, pazopanib, gefitinib and imatinib have been used with fluctuating results.
Sorafenib was the first anti-angiogenic drug proposed for ATC treatment. It was employed in two different phase-2 trials [116,117]; in both of them, disease control (partial response and stable disease) was reached in about 40% of patients but the median overall survival was still lower than 5 months [116,117]. Similarly, sorafenib seemed not to produce exciting results in PDTC [118]. From 2009 to 2011, 40 patients with PDTC were randomly allocated to sorafenib and placebo arms in a randomized, double-blind, multicentric, phase-3 trial (DECISION trial). Although not statistically significant, the sub analysis on PDTC showed a consistent improvement of PFS in patients treated with sorafenib as in all the other histotypes [119]. As reported in DECISION trial, sorafenib toxicity is mainly characterized by grade 1 and grade 2 adverse events such as hand–foot skin reaction, diarrhoea, alopecia, rash/desquamation, fatigue, weight loss, and hypertension [119].
Lenvatinib had very promising results in in-vitro and in-vivo models of ATC that were partially confirmed in clinical settings [120]. Takahashi et al. performed a phase II clinical trial, showing an overall survival of 10.6 months (95% CI: 3.8–19.8) and disease control in 16 out of 17 patients [121]. However, different results were given by a recent post-marketing observational study which reviewed 124 patients affected by ATC and treated with lenvatinib [122]. It showed a disease control in 76.2% (66.89–83.96%) of patients; however, this response seemed to be only transient, because the time-to-treatment failure was 74.5 (57.0–108.0) days, and the median overall survival was still poor (3.4, 95% CI 2.66–4.33 months) [122]. At the same time, baseline clinical conditions of enrolled patients were poor (ECOG > 1) in more than 70% and this could partially explain these disappointing findings. Further studies should be employed in order to verify lenvatinib efficacy in ATC patients with better clinical conditions. Otherwise, lenvatinib produced interesting results for PDTC patients. In SELECT trial, which explored lenvatinib efficacy in patients with radioiodine-refractory thyroid cancer, 28 patients harboring PDTC were enrolled. In this selected population, lenvatinib confirmed its efficacy compared to placebo (HR 0.21, 0.08–0.56) [123]. Accordingly, a retrospective multicentric analysis of real-world data confirmed these encouraging results [124]. In this analysis of clinical practice in Austria enrolling 43 patients, the overall survival seemed to be not modified by tumor subtype (differentiated vs. poorly differentiated/anaplastic TC), whereas a maintenance dosage higher than 14 mg was associated with better prognosis [124]. About toxicity, in spite of high proportion of adverse events with grade ≥ III in SELECT trial (75.9%), Austrian, Italian, and French real-world data reported lower rates of adverse events of grade ≥ III (44%, 22.3% and 48%, respectively) [123,124,125,126]. Fatigue, hypertension, diarrhea, decreased weight, stomatitis, and anorexia are the most common reported adverse events [125,126].
More recently, cabozantinib has been explored as a salvage therapy for patients with radioiodine-refractory thyroid cancer already treated with MKIs. In this trial, 7 patients with PDTC were enrolled to receive cabozantinib and all of them presented clinical benefit (3 PR and 4 SD) [127]. Other antiangiogenic agents (pazopanib, gefitinib and imatinib) were employed for ATC therapy but they did not produce encouraging results [128,129,130].
4.2. Genetically Guided Therapy
Many reports showed that ATC has a singular genomic and transcriptomic landscape (ATC-like) [131]. In this singular genomic landscape, BRAF-MEK pathway was proposed as a potential target.
After the exciting results of BRAF inhibition in BRAF-mutated melanoma [132], a multicenter prospective “basket” trial, encompassing tumors with BRAF mutation, enrolled 7 patients affected by ATC for treatment with vemurafenib: 2 of them experienced a durable partial response (more than 11 months) [133]. In order to produce a stronger inhibition of BRAF-MEK pathway, dual inhibition of BRAF and MEK with dabrafenib and trametinib was proposed. Accordingly, in in-vitro ATC model, combined therapy induced greater growth inhibition than single agents [134]. Likewise, dabrafenib-trametinib therapy produced about 80% of 12-months progression free survival and overall survival in phase II clinical trial enrolling 16 patients [135]. Moreover, 1 patient experienced a complete response, 10 partial response, 3 stable disease and only 1 disease progression. In this trial, fatigue (44%), pyrexia (31%), and nausea (31%), were the most common adverse events, although the 50% of enrolled patients reported an adverse event with grade ≥ III [135]. This trial permitted the approval of this combination by FDA for treatment of BRAFV600E mutated ATC.
As previously reported, PI3K/Akt/mTOR has a crucial role in ATC cells and also anti-mTOR inhibitors have been proposed with conflicting results. Everolimus was used to treat one patient with ATC harboring a mutation of Tuberous Sclerosis 2 protein (TSC2), member of PI3K/AKT/mTOR pathway, obtaining an extraordinary 18-month response [136]. However, these promising results were not confirmed in other studies [137,138].
New perspectives have recently been opened for patients with ATC or PDTC harboring RET fusion genes, since highly selective RET inhibitors, such as selpercatinib and pralsetinib, are currently under investigation [139,140]. In 2020, a phase 1–2 clinical trial enrolled patients with thyroid cancer harboring an activating RET alteration for treatment with selpercatinib (LIBRETTO-001). In this trial, one patient with ATC and 3 with PDTC were enrolled. Interestingly, the patient with ATC reached PR as best response as well as 2 out of 3 patients with PDTC, while the other one with PDTC obtained SD [139]. Furthermore, selpercatinib presents a more tolerable toxicity profile with a rate of adverse events ≥ III of only 30% compared to other targeted therapies and the most common reported adverse events were hypertension, increased alanine or aspartate aminotransferase level, hyponatremia and diarrhea. Likewise, Cabanillas et al. have recently presented data about the use of larotrectinib, a NTRK fusion gene inhibitor, in 7 patients with ATC. Intriguingly, 3 out of 7 reached PR and SD, while 3 patients experienced PD [141]. Grade ≥ III adverse events occurred in 46% of patients, although only 7% of patients presented ones that were considered related to larotrectinib [141]. Recently, an excellent response was documented with crizotinib in one patient with ATC, harboring ALK-RET fusion gene [142].
Other agents such as HDAC inhibitors have been used but with disappointing results (NCT03002623 trial).
4.3. Immunotherapy
Immunotherapy is inducing a deep change in anticancer therapy, regulating immune cells attack against neoplastic cells. Interestingly, many reports showed that ATC presents higher PD-L1+ cells compared to DTC, proposing PD/PD-L1 pathway as targetable [101,143], and, as shown above, preclinical data produced interesting result in mouse model [108]. Accordingly, PD-1 antibodies (e.g., pembrolizumab and spartalizumab), after promising data about their use in BRAF-mutated melanoma [144], have been used as single agents in patients affected by ATC [145].
Pembrolizumab induced a durable response (16 months) in one patient with unresectable ATC: after its second cycle the patient referred a significant improvement of dysphagia and after 3 cycle a complete response was almost reached [146]. However, after a severe toxicity related to pembrolizumab (grade 4 colitis), it was suspended and the patient died 8 months later, after the appearance of cerebral metastasis [146]. On the other hand, it seemed to do not produce the same result when co-administrated with chemoradiotherapy. Chintakuntlawar et al. treated 3 patients with pembrolizumab and chemoradiotherapy, but, in spite of a prompt an early tumor response, all patients passed away <6 months [147].
Spartalizumab toxicity and efficacy were evaluated in a phase I/II trial enrolling 42 patients with locally advanced and/or metastatic anaplastic thyroid carcinoma [148]. The overall response rate was 19% in the whole cohort, while it was higher in patients defined as PD-L1–positive (29%), and even better in the subset of patients with PD-L1 expression > 50% (35%). In this last subset of patients, the 1-year survival rate reached 52.1% [148]. About toxicity profile, the most frequent adverse events were diarrhea, pruritus, fatigue, and pyrexia and grade ≥ III adverse events related to treatment were observed in 10% of patients [148].
4.4. Multimodal Therapy
Considering the promising data about single regimens, many clinicians proposed a multimodal therapy against ATC in order to reduce therapy resistance. Moreover, driver mutations such as BRAF were proposed as master regulators of immune TME in thyroid cancer [114,149]. Multimodal therapy against immune TME and main mutated pathways could induce a deep inhibition of ATC growth and progression, evading resistance mechanisms.
In 2018, Cabanillas et al. showed one case of locally aggressive unresectable ATC treated with neo-adjuvant therapy composed by dabrafenib, trametinib and pembrolizumab (DTP) [150]. Interestingly, the patient had a relevant response, allowing a complete surgical resection followed by postoperative chemoradiation. Likewise, other 4 clinical cases have recently been reported about DTP use as neoadjuvant therapy in ATC, with unexpected high PFS (19.5, 95% CI: 13.75–24.5, months) [151].
Immunotherapy has been proposed also in adjuvant therapy with dabrafenib and trametinib or lenvatinib. Iyer et al. [152] used pembrolizumab as salvage therapy in 5 patients treated with dabrafenib and trametinib, 1 with trametinib, and 6 with lenvatinib. Although 2 patients experienced PD, 5 patients had PR and 5 had SD and, from the start of targeted therapies, the median OS was 10.4 months (95% CI = 6.02, 14.83, range 5.4–40 months) [152]. In this series, fatigue, anemia and hypertension were the most common AEs associated with this combination and drug-induced rash and altered mental status (likely related to PD) induced drug interruption [152]. Similarly, Dierks et al. showed interesting results about lenvatinib and pembrolizumab combined treatment both in ATC and PDTC: 5/6 patients with ATC reached CR/PR and 2/2 with PDTC obtained PR [153]. Similarly, nivolumab (anti-PD1 antibody) was added to vemurafenib in patients affected by metastatic ATC, obtaining a prolonged response (more than 20 months) [154]. According to these results, new clinical trials are ongoing (e.g., NCT03181100).
In 2017, 6 patients with PDTC and 2 with ATC were enrolled to receive sorafenib and temsirolimus (mTOR inhibitor) in a non-randomized clinical trial. In one hand, results in patients with PDTC were encouraging and 4 patients reached PR and 2 SD; on the other hand, one patient with ATC had PR and the other one had PD [155]. Furthermore, 14% of enrolled patients discontinued the treatment for toxicity and most common adverse events grade ≥ 3 were hyperglycemia, fatigue, anemia, and oral mucositis [155].
5. Conclusions
PDTC and ATC are rare but, unfortunately, they are lethal although a relevant different 5-year survival rate (5 years vs. 6 months). Nowadays, we know many elements of their genetic landscape and tumor microenvironment. This knowledge helped the scientific community to identify therapies which specifically target these cancers. Some of them (e.g., DTP) has recently reached the clinical practice and could be prescribed for BRAFV600E mutated ATC. However, their therapeutic benefit is still scarce and many other studies are necessary to answer these unmet clinical needs.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kitahara C.M., Sosa J.A. The Changing Incidence of Thyroid Cancer. Nat. Rev. Endocrinol. 2016;12:646–653. doi: 10.1038/nrendo.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borda A., Zahan A.-E., Piciu D., Barbuș E., Berger N., Nechifor-Boilă A. A 15 Year Institutional Experience of Well-Differentiated Follicular Cell-Derived Thyroid Carcinomas; Impact of the New 2017 TNM and WHO Classifications of Tumors of Endocrine Organs on the Epidemiological Trends and Pathological Characteristics. Endocrine. 2020;67:630–642. doi: 10.1007/s12020-019-02158-7. [DOI] [PubMed] [Google Scholar]
- 3.Mirian C., Grønhøj C., Jensen D.H., Jakobsen K.K., Karnov K., Jensen J.S., Hahn C.H., Klitmøller T.A., Bentzen J., von Buchwald C. Trends in Thyroid Cancer: Retrospective Analysis of Incidence and Survival in Denmark 1980–2014. Cancer Epidemiol. 2018;55:81–87. doi: 10.1016/j.canep.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 4.de la Fouchardière C., Decaussin-Petrucci M., Berthiller J., Descotes F., Lopez J., Lifante J.C., Peix J.L., Giraudet A.L., Delahaye A., Masson S., et al. Predictive Factors of Outcome in Poorly Differentiated Thyroid Carcinomas. Eur. J. Cancer. 2018 doi: 10.1016/j.ejca.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 5.Smallridge R.C., Copland J.A. Anaplastic Thyroid Carcinoma: Pathogenesis and Emerging Therapies. Clin. Oncol. 2010 doi: 10.1016/j.clon.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volante M., Collini P., Nikiforov Y.E., Sakamoto A., Kakudo K., Katoh R., Lloyd R.V., LiVolsi V.A., Papotti M., Sobrinho-Simoes M., et al. Poorly Differentiated Thyroid Carcinoma: The Turin Proposal for the Use of Uniform Diagnostic Criteria and an Algorithmic Diagnostic Approach. Am. J. Surg. Pathol. 2007;31:1256–1264. doi: 10.1097/PAS.0b013e3180309e6a. [DOI] [PubMed] [Google Scholar]
- 7.Hiltzik D., Carlson D.L., Tuttle R.M., Chuai S., Ishill N., Shaha A., Shah J.P., Singh B., Ghossein R.A. Poorly Differentiated Thyroid Carcinomas Defined on the Basis of Mitosis and Necrosis: A Clinicopathologic Study of 58 Patients. Cancer. 2006;106:1286–1295. doi: 10.1002/cncr.21739. [DOI] [PubMed] [Google Scholar]
- 8.Njim L., Moussa A., Hadhri R., Gassab I., Ben Yahia N., Mahmoudi H., Zakhama A. Angiomatoid tumor of the thyroid gland: Primitive angiosarcoma or variant of anaplastic carcinoma? Ann. Pathol. 2008;28:221–224. doi: 10.1016/j.annpat.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Prete A., Cosentino G., Manetti L., Ambrosini C.E., Papini P., Marinò M., Torregrossa L., Marcocci C., Elisei R., Lupi I. Firm Mass in Thyroid of an Elderly Patient: Not Always Cancer. Endocrinol. Diabetes Metab. Case Rep. 2020;2020 doi: 10.1530/EDM-20-0137. [DOI] [PubMed] [Google Scholar]
- 10.Ain K.B. Anaplastic Thyroid Carcinoma: Behavior, Biology, and Therapeutic Approaches. Thyroid. 1998;8:715–726. doi: 10.1089/thy.1998.8.715. [DOI] [PubMed] [Google Scholar]
- 11.Molinaro E., Romei C., Biagini A., Sabini E., Agate L., Mazzeo S., Materazzi G., Sellari-Franceschini S., Ribechini A., Torregrossa L., et al. Anaplastic Thyroid Carcinoma: From Clinicopathology to Genetics and Advanced Therapies. Nat. Rev. Endocrinol. 2017;13:644–660. doi: 10.1038/nrendo.2017.76. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahimpasic T., Ghossein R., Shah J.P., Ganly I. Poorly Differentiated Carcinoma of the Thyroid Gland: Current Status and Future Prospects. Thyroid. 2019;29:311–321. doi: 10.1089/thy.2018.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andor N., Maley C.C., Ji H.P. Genomic Instability in Cancer: Teetering on the Limit of Tolerance. Cancer Res. 2017;77:2179–2185. doi: 10.1158/0008-5472.CAN-16-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanahan D., Weinberg R.A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Martincorena I., Raine K.M., Gerstung M., Dawson K.J., Haase K., Van Loo P., Davies H., Stratton M.R., Campbell P.J. Universal Patterns of Selection in Cancer and Somatic Tissues. Cell. 2017;171:1029–1041.e21. doi: 10.1016/j.cell.2017.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zehir A., Benayed R., Shah R.H., Syed A., Middha S., Kim H.R., Srinivasan P., Gao J., Chakravarty D., Devlin S.M., et al. Mutational Landscape of Metastatic Cancer Revealed from Prospective Clinical Sequencing of 10,000 Patients. Nat. Med. 2017;23:15. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pozdeyev N., Gay L.M., Sokol E.S., Hartmaier R., Deaver K.E., Davis S., French J.D., Borre P.V., LaBarbera D.V., Tan A.-C., et al. Genetic Analysis of 779 Advanced Differentiated and Anaplastic Thyroid Cancers. Clin. Cancer Res. 2018;24:3059–3068. doi: 10.1158/1078-0432.CCR-18-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landa I., Ibrahimpasic T., Boucai L., Sinha R., Knauf J.A., Shah R.H., Dogan S., Ricarte-Filho J.C., Krishnamoorthy G.P., Xu B., et al. Genomic and Transcriptomic Hallmarks of Poorly Differentiated and Anaplastic Thyroid Cancers. J. Clin. Investig. 2016;126:1052–1066. doi: 10.1172/JCI85271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cancer Genome Atlas Research Network Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell. 2014;159:676–690. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogelstein B., Papadopoulos N., Velculescu V.E., Zhou S., Diaz L.A., Kinzler K.W. Cancer Genome Landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tate J.G., Bamford S., Jubb H.C., Sondka Z., Beare D.M., Bindal N., Boutselakis H., Cole C.G., Creatore C., Dawson E., et al. COSMIC: The Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res. 2019;47:D941–D947. doi: 10.1093/nar/gky1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerber T.S., Schad A., Hartmann N., Springer E., Zechner U., Musholt T.J. Targeted Next-Generation Sequencing of Cancer Genes in Poorly Differentiated Thyroid Cancer. Endocr. Connect. 2018;7:47–55. doi: 10.1530/EC-17-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan H., Li Y., Hu P., Gao J., Ying J., Xu W., Zhao D., Wang Z., Ye J., Lizaso A., et al. Mutational Profiling of Poorly Differentiated and Anaplastic Thyroid Carcinoma by the Use of Targeted Next-Generation Sequencing. Histopathology. 2019;75:890–899. doi: 10.1111/his.13942. [DOI] [PubMed] [Google Scholar]
- 24.Chen H., Luthra R., Routbort M.J., Patel K.P., Cabanillas M.E., Broaddus R.R., Williams M.D. Molecular Profile of Advanced Thyroid Carcinomas by Next-Generation Sequencing: Characterizing Tumors Beyond Diagnosis for Targeted Therapy. Mol. Cancer Ther. 2018;17:1575–1584. doi: 10.1158/1535-7163.MCT-17-0871. [DOI] [PubMed] [Google Scholar]
- 25.McFadden D.G., Vernon A., Santiago P.M., Martinez-McFaline R., Bhutkar A., Crowley D.M., McMahon M., Sadow P.M., Jacks T. P53 Constrains Progression to Anaplastic Thyroid Carcinoma in a Braf-Mutant Mouse Model of Papillary Thyroid Cancer. Proc. Natl. Acad. Sci. USA. 2014;111:E1600–E1609. doi: 10.1073/pnas.1404357111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu X., Zhao L., Park J.W., Willingham M.C., Cheng S.-Y. Synergistic Signaling of KRAS and Thyroid Hormone Receptor β Mutants Promotes Undifferentiated Thyroid Cancer through MYC Up-Regulation. Neoplasia. 2014;16:757–769. doi: 10.1016/j.neo.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salvatore G., De Falco V., Salerno P., Nappi T.C., Pepe S., Troncone G., Carlomagno F., Melillo R.M., Wilhelm S.M., Santoro M. BRAF Is a Therapeutic Target in Aggressive Thyroid Carcinoma. Clin. Cancer Res. 2006;12:1623–1629. doi: 10.1158/1078-0432.CCR-05-2378. [DOI] [PubMed] [Google Scholar]
- 28.Vitagliano D., Portella G., Troncone G., Francione A., Rossi C., Bruno A., Giorgini A., Coluzzi S., Nappi T.C., Rothstein J.L., et al. Thyroid Targeting of the N-Ras(Gln61Lys) Oncogene in Transgenic Mice Results in Follicular Tumors That Progress to Poorly Differentiated Carcinomas. Oncogene. 2006;25:5467–5474. doi: 10.1038/sj.onc.1209527. [DOI] [PubMed] [Google Scholar]
- 29.Xu B., Ghossein R. Genomic Landscape of Poorly Differentiated and Anaplastic Thyroid Carcinoma. Endocr. Pathol. 2016;27:205–212. doi: 10.1007/s12022-016-9445-4. [DOI] [PubMed] [Google Scholar]
- 30.Li Z., Zhang Y., Wang R., Zou K., Zou L. Genetic Alterations in Anaplastic Thyroid Carcinoma and Targeted Therapies. Exp. Ther. Med. 2019;18:2369–2377. doi: 10.3892/etm.2019.7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z., Hou P., Ji M., Guan H., Studeman K., Jensen K., Vasko V., El-Naggar A.K., Xing M. Highly Prevalent Genetic Alterations in Receptor Tyrosine Kinases and Phosphatidylinositol 3-Kinase/Akt and Mitogen-Activated Protein Kinase Pathways in Anaplastic and Follicular Thyroid Cancers. J. Clin. Endocrinol. Metab. 2008;93:3106–3116. doi: 10.1210/jc.2008-0273. [DOI] [PubMed] [Google Scholar]
- 32.Charles R.-P., Silva J., Iezza G., Phillips W.A., McMahon M. Activating BRAF and PIK3CA Mutations Cooperate to Promote Anaplastic Thyroid Carcinogenesis. Mol. Cancer Res. 2014;12:979–986. doi: 10.1158/1541-7786.MCR-14-0158-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xing M. Molecular Pathogenesis and Mechanisms of Thyroid Cancer. Nat. Rev. Cancer. 2013;13:184–199. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romei C., Tacito A., Molinaro E., Piaggi P., Cappagli V., Pieruzzi L., Matrone A., Viola D., Agate L., Torregrossa L., et al. Clinical, Pathological and Genetic Features of Anaplastic and Poorly Differentiated Thyroid Cancer: A Single Institute Experience. Oncol. Lett. 2018;15:9174–9182. doi: 10.3892/ol.2018.8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shay J.W., Wright W.E. Senescence and Immortalization: Role of Telomeres and Telomerase. Carcinogenesis. 2005;26:867–874. doi: 10.1093/carcin/bgh296. [DOI] [PubMed] [Google Scholar]
- 36.Tan J., Liu R., Zhu G., Umbricht C.B., Xing M. TERT Promoter Mutation Determines Apoptotic and Therapeutic Responses of BRAF-Mutant Cancers to BRAF and MEK Inhibitors: Achilles Heel. Proc. Natl. Acad. Sci USA. 2020;117:15846–15851. doi: 10.1073/pnas.2004707117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bu R., Siraj A.K., Divya S.P., Kong Y., Parvathareddy S.K., Al-Rasheed M., Al-Obaisi K.A.S., Victoria I.G., Al-Sobhi S.S., Al-Dawish M., et al. Telomerase Reverse Transcriptase Mutations Are Independent Predictor of Disease-Free Survival in Middle Eastern Papillary Thyroid Cancer. Int. J. Cancer. 2018;142:2028–2039. doi: 10.1002/ijc.31225. [DOI] [PubMed] [Google Scholar]
- 38.Vander Heiden M.G., DeBerardinis R.J. Understanding the Intersections between Metabolism and Cancer Biology. Cell. 2017;168:657–669. doi: 10.1016/j.cell.2016.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pestova T.V., Borukhov S.I., Hellen C.U. Eukaryotic Ribosomes Require Initiation Factors 1 and 1A to Locate Initiation Codons. Nature. 1998;394:854–859. doi: 10.1038/29703. [DOI] [PubMed] [Google Scholar]
- 40.Krishnamoorthy G.P., Davidson N.R., Leach S.D., Zhao Z., Lowe S.W., Lee G., Landa I., Nagarajah J., Saqcena M., Singh K., et al. EIF1AX and RAS Mutations Cooperate to Drive Thyroid Tumorigenesis through ATF4 and C-MYC. Cancer Discov. 2019;9:264–281. doi: 10.1158/2159-8290.CD-18-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stransky N., Cerami E., Schalm S., Kim J.L., Lengauer C. The Landscape of Kinase Fusions in Cancer. Nat. Commun. 2014;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao Q., Liang W.-W., Foltz S.M., Mutharasu G., Jayasinghe R.G., Cao S., Liao W.-W., Reynolds S.M., Wyczalkowski M.A., Yao L., et al. Driver Fusions and Their Implications in the Development and Treatment of Human Cancers. Cell Rep. 2018;23:227–238. doi: 10.1016/j.celrep.2018.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santoro M., Carlomagno F. Central Role of RET in Thyroid Cancer. Cold Spring Harb. Perspect. Biol. 2013;5:a009233. doi: 10.1101/cshperspect.a009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yakushina V.D., Lerner L.V., Lavrov A.V. Gene Fusions in Thyroid Cancer. Thyroid. 2018;28:158–167. doi: 10.1089/thy.2017.0318. [DOI] [PubMed] [Google Scholar]
- 45.Nikitski A.V., Rominski S.L., Condello V., Kaya C., Wankhede M., Panebianco F., Yang H., Altschuler D.L., Nikiforov Y.E. Mouse Model of Thyroid Cancer Progression and Dedifferentiation Driven by STRN-ALK Expression and Loss of P53: Evidence for the Existence of Two Types of Poorly Differentiated Carcinoma. Thyroid. 2019;29:1425–1437. doi: 10.1089/thy.2019.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chu Y.-H., Wirth L.J., Farahani A.A., Nosé V., Faquin W.C., Dias-Santagata D., Sadow P.M. Clinicopathologic Features of Kinase Fusion-Related Thyroid Carcinomas: An Integrative Analysis with Molecular Characterization. Mod. Pathol. 2020;33:2458–2472. doi: 10.1038/s41379-020-0638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hieronymus H., Murali R., Tin A., Yadav K., Abida W., Moller H., Berney D., Scher H., Carver B., Scardino P., et al. Tumor Copy Number Alteration Burden Is a Pan-Cancer Prognostic Factor Associated with Recurrence and Death. Elife. 2018;7 doi: 10.7554/eLife.37294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Antonello Z.A., Hsu N., Bhasin M., Roti G., Joshi M., Van Hummelen P., Ye E., Lo A.S., Karumanchi S.A., Bryke C.R., et al. Vemurafenib-Resistance via de Novo RBM Genes Mutations and Chromosome 5 Aberrations Is Overcome by Combined Therapy with Palbociclib in Thyroid Carcinoma with BRAFV600E. Oncotarget. 2017;8:84743–84760. doi: 10.18632/oncotarget.21262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goodall G.J., Wickramasinghe V.O. RNA in Cancer. Nat. Rev. Cancer. 2021;21:22–36. doi: 10.1038/s41568-020-00306-0. [DOI] [PubMed] [Google Scholar]
- 50.Lin S., Gregory R.I. MicroRNA Biogenesis Pathways in Cancer. Nat. Rev. Cancer. 2015;15:321–333. doi: 10.1038/nrc3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dettmer M.S., Perren A., Moch H., Komminoth P., Nikiforov Y.E., Nikiforova M.N. MicroRNA Profile of Poorly Differentiated Thyroid Carcinomas: New Diagnostic and Prognostic Insights. J. Mol. Endocrinol. 2014;52:181–189. doi: 10.1530/JME-13-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calabrese G., Dolcimascolo A., Caruso G., Forte S. MiR-19a Is Involved In Progression And Malignancy Of Anaplastic Thyroid Cancer Cells. Onco Targets Ther. 2019;12:9571–9583. doi: 10.2147/OTT.S221733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maroof H., Irani S., Arianna A., Vider J., Gopalan V., Lam A.K.-Y. Interactions of Vascular Endothelial Growth Factor and P53 with MiR-195 in Thyroid Carcinoma: Possible Therapeutic Targets in Aggressive Thyroid Cancers. Curr. Cancer Drug Targets. 2019;19:561–570. doi: 10.2174/1568009618666180628154727. [DOI] [PubMed] [Google Scholar]
- 54.Sheng W., Chen Y., Gong Y., Dong T., Zhang B., Gao W. MiR-148a Inhibits Self-Renewal of Thyroid Cancer Stem Cells via Repressing INO80 Expression. Oncol. Rep. 2016;36:3387–3396. doi: 10.3892/or.2016.5203. [DOI] [PubMed] [Google Scholar]
- 55.Orlandella F.M., Di Maro G., Ugolini C., Basolo F., Salvatore G. TWIST1/MiR-584/TUSC2 Pathway Induces Resistance to Apoptosis in Thyroid Cancer Cells. Oncotarget. 2016;7:70575–70588. doi: 10.18632/oncotarget.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang S., Chen Y., Bai Y. P21 Participates in the Regulation of Anaplastic Thyroid Cancer Cell Proliferation by MiR-146b. Oncol. Lett. 2016;12:2018–2022. doi: 10.3892/ol.2016.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haghpanah V., Fallah P., Tavakoli R., Naderi M., Samimi H., Soleimani M., Larijani B. Antisense-MiR-21 Enhances Differentiation/Apoptosis and Reduces Cancer Stemness State on Anaplastic Thyroid Cancer. Tumour Biol. 2016;37:1299–1308. doi: 10.1007/s13277-015-3923-z. [DOI] [PubMed] [Google Scholar]
- 58.Huang H.-G., Luo X., Wu S., Jian B. MiR-99a Inhibits Cell Proliferation and Tumorigenesis through Targeting MTOR in Human Anaplastic Thyroid Cancer. Asian Pac. J. Cancer Prev. 2015;16:4937–4944. doi: 10.7314/APJCP.2015.16.12.4937. [DOI] [PubMed] [Google Scholar]
- 59.Cheng Q., Zhang X., Xu X., Lu X. MiR-618 Inhibits Anaplastic Thyroid Cancer by Repressing XIAP in One ATC Cell Line. Ann. Endocrinol. (Paris) 2014;75:187–193. doi: 10.1016/j.ando.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 60.Frezzetti D., De Menna M., Zoppoli P., Guerra C., Ferraro A., Bello A.M., De Luca P., Calabrese C., Fusco A., Ceccarelli M., et al. Upregulation of MiR-21 by Ras in Vivo and Its Role in Tumor Growth. Oncogene. 2011;30:275–286. doi: 10.1038/onc.2010.416. [DOI] [PubMed] [Google Scholar]
- 61.Takakura S., Mitsutake N., Nakashima M., Namba H., Saenko V.A., Rogounovitch T.I., Nakazawa Y., Hayashi T., Ohtsuru A., Yamashita S. Oncogenic Role of MiR-17-92 Cluster in Anaplastic Thyroid Cancer Cells. Cancer Sci. 2008;99:1147–1154. doi: 10.1111/j.1349-7006.2008.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang W., Ji W., Zhao X. MiR-155 Promotes Anaplastic Thyroid Cancer Progression by Directly Targeting SOCS1. BMC Cancer. 2019;19:1093. doi: 10.1186/s12885-019-6319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Montero-Conde C., Graña-Castro O., Martín-Serrano G., Martínez-Montes Á.M., Zarzuela E., Muñoz J., Torres-Perez R., Pita G., Cordero-Barreal A., Leandro-García L.J., et al. Hsa-MiR-139-5p Is a Prognostic Thyroid Cancer Marker Involved in HNRNPF-Mediated Alternative Splicing. Int. J. Cancer. 2020;146:521–530. doi: 10.1002/ijc.32622. [DOI] [PubMed] [Google Scholar]
- 64.Orlandella F.M., Mariniello R.M., Iervolino P.L.C., Imperlini E., Mandola A., Verde A., De Stefano A.E., Pane K., Franzese M., Esposito S., et al. MiR-650 Promotes Motility of Anaplastic Thyroid Cancer Cells by Targeting PPP2CA. Endocrine. 2019;65:582–594. doi: 10.1007/s12020-019-01910-3. [DOI] [PubMed] [Google Scholar]
- 65.Zhang X., Liu L., Deng X., Li D., Cai H., Ma Y., Jia C., Wu B., Fan Y., Lv Z. MicroRNA 483-3p Targets Pard3 to Potentiate TGF-Β1-Induced Cell Migration, Invasion, and Epithelial-Mesenchymal Transition in Anaplastic Thyroid Cancer Cells. Oncogene. 2019;38:699–715. doi: 10.1038/s41388-018-0447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bu Q., You F., Pan G., Yuan Q., Cui T., Hao L., Zhang J. MiR-125b Inhibits Anaplastic Thyroid Cancer Cell Migration and Invasion by Targeting PIK3CD. Biomed. Pharmacother. 2017;88:443–448. doi: 10.1016/j.biopha.2016.11.090. [DOI] [PubMed] [Google Scholar]
- 67.Liu G., Wu K., Sheng Y. Elucidation of the Molecular Mechanisms of Anaplastic Thyroid Carcinoma by Integrated MiRNA and MRNA Analysis. Oncol. Rep. 2016;36:3005–3013. doi: 10.3892/or.2016.5064. [DOI] [PubMed] [Google Scholar]
- 68.Aherne S.T., Smyth P., Freeley M., Smith L., Spillane C., O’Leary J., Sheils O. Altered Expression of Mir-222 and Mir-25 Influences Diverse Gene Expression Changes in Transformed Normal and Anaplastic Thyroid Cells, and Impacts on MEK and TRAIL Protein Expression. Int. J. Mol. Med. 2016;38:433–445. doi: 10.3892/ijmm.2016.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiong Y., Zhang L., Kebebew E. MiR-20a Is Upregulated in Anaplastic Thyroid Cancer and Targets LIMK1. PLoS ONE. 2014;9:e96103. doi: 10.1371/journal.pone.0096103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hao F., Bi Y.-N., Wang L., Wang Y., Ma J., Cui P., Li X., Sun S., Ning L., Huang Y., et al. MiR-199a-5p Suppresses Epithelial- Mesenchymal-Transition in Anaplastic Thyroid Carcinoma Cells via Targeting Snail Signals. Cancer Biomark. 2020;29:317–326. doi: 10.3233/CBM-201518. [DOI] [PubMed] [Google Scholar]
- 71.Fuziwara C.S., Saito K.C., Kimura E.T. Thyroid Follicular Cell Loss of Differentiation Induced by MicroRNA MiR-17-92 Cluster Is Attenuated by CRISPR/Cas9n Gene Silencing in Anaplastic Thyroid Cancer. Thyroid. 2020;30:81–94. doi: 10.1089/thy.2018.0601. [DOI] [PubMed] [Google Scholar]
- 72.Zhang W.-L., Lv W., Sun S.-Z., Wu X.-Z., Zhang J.-H. MiR-206 Inhibits Metastasis-Relevant Traits by Degrading MRTF-A in Anaplastic Thyroid Cancer. Int. J. Oncol. 2015;47:133–142. doi: 10.3892/ijo.2015.2993. [DOI] [PubMed] [Google Scholar]
- 73.Boufraqech M., Nilubol N., Zhang L., Gara S.K., Sadowski S.M., Mehta A., He M., Davis S., Dreiling J., Copland J.A., et al. MiR30a Inhibits LOX Expression and Anaplastic Thyroid Cancer Progression. Cancer Res. 2015;75:367–377. doi: 10.1158/0008-5472.CAN-14-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hébrant A., Floor S., Saiselet M., Antoniou A., Desbuleux A., Snyers B., La C., de Saint Aubain N., Leteurtre E., Andry G., et al. MiRNA Expression in Anaplastic Thyroid Carcinomas. PLoS ONE. 2014;9:e103871. doi: 10.1371/journal.pone.0103871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Braun J., Hoang-Vu C., Dralle H., Hüttelmaier S. Downregulation of MicroRNAs Directs the EMT and Invasive Potential of Anaplastic Thyroid Carcinomas. Oncogene. 2010;29:4237–4244. doi: 10.1038/onc.2010.169. [DOI] [PubMed] [Google Scholar]
- 76.Mitomo S., Maesawa C., Ogasawara S., Iwaya T., Shibazaki M., Yashima-Abo A., Kotani K., Oikawa H., Sakurai E., Izutsu N., et al. Downregulation of MiR-138 Is Associated with Overexpression of Human Telomerase Reverse Transcriptase Protein in Human Anaplastic Thyroid Carcinoma Cell Lines. Cancer Sci. 2008;99:280–286. doi: 10.1111/j.1349-7006.2007.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu Y., Han Y.-F., Ye B., Zhang Y.-L., Dong J.-D., Zhu S.-J., Chen J. MiR-27b-3p Is Involved in Doxorubicin Resistance of Human Anaplastic Thyroid Cancer Cells via Targeting Peroxisome Proliferator-Activated Receptor Gamma. Basic Clin. Pharmacol. Toxicol. 2018;123:670–677. doi: 10.1111/bcpt.13076. [DOI] [PubMed] [Google Scholar]
- 78.Liu J., Feng L., Zhang H., Zhang J., Zhang Y., Li S., Qin L., Yang Z., Xiong J. Effects of MiR-144 on the Sensitivity of Human Anaplastic Thyroid Carcinoma Cells to Cisplatin by Autophagy Regulation. Cancer Biol. Ther. 2018;19:484–496. doi: 10.1080/15384047.2018.1433502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Penha R.C.C., Pellecchia S., Pacelli R., Pinto L.F.R., Fusco A. Ionizing Radiation Deregulates the MicroRNA Expression Profile in Differentiated Thyroid Cells. Thyroid. 2018;28:407–421. doi: 10.1089/thy.2017.0458. [DOI] [PubMed] [Google Scholar]
- 80.Varmeh S., Borre P.V., Gunda V., Brauner E., Holm T., Wang Y., Sadreyev R.I., Parangi S. Genome-Wide Analysis of Differentially Expressed MiRNA in PLX4720-Resistant and Parental Human Thyroid Cancer Cell Lines. Surgery. 2016;159:152–162. doi: 10.1016/j.surg.2015.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y., Yang W.Q., Zhu H., Qian Y.Y., Zhou L., Ren Y.J., Ren X.C., Zhang L., Liu X.P., Liu C.G., et al. Regulation of Autophagy by MiR-30d Impacts Sensitivity of Anaplastic Thyroid Carcinoma to Cisplatin. Biochem. Pharmacol. 2014;87:562–570. doi: 10.1016/j.bcp.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vriens M.R., Weng J., Suh I., Huynh N., Guerrero M.A., Shen W.T., Duh Q.-Y., Clark O.H., Kebebew E. MicroRNA Expression Profiling Is a Potential Diagnostic Tool for Thyroid Cancer. Cancer. 2012;118:3426–3432. doi: 10.1002/cncr.26587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schwertheim S., Sheu S.-Y., Worm K., Grabellus F., Schmid K.W. Analysis of Deregulated MiRNAs Is Helpful to Distinguish Poorly Differentiated Thyroid Carcinoma from Papillary Thyroid Carcinoma. Horm. Metab. Res. 2009;41:475–481. doi: 10.1055/s-0029-1215593. [DOI] [PubMed] [Google Scholar]
- 84.Xu B., Qin T., Yu J., Giordano T.J., Sartor M.A., Koenig R.J. Novel Role of ASH1L Histone Methyltransferase in Anaplastic Thyroid Carcinoma. J. Biol. Chem. 2020;295:8834–8845. doi: 10.1074/jbc.RA120.013530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou Q., Chen J., Feng J., Wang J. Long Noncoding RNA PVT1 Modulates Thyroid Cancer Cell Proliferation by Recruiting EZH2 and Regulating Thyroid-Stimulating Hormone Receptor (TSHR) Tumour Biol. 2016;37:3105–3113. doi: 10.1007/s13277-015-4149-9. [DOI] [PubMed] [Google Scholar]
- 86.Zhang R., Hardin H., Huang W., Chen J., Asioli S., Righi A., Maletta F., Sapino A., Lloyd R.V. MALAT1 Long Non-Coding RNA Expression in Thyroid Tissues: Analysis by In Situ Hybridization and Real-Time PCR. Endocr. Pathol. 2017;28:7–12. doi: 10.1007/s12022-016-9453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim D., Lee W.K., Jeong S., Seol M.-Y., Kim H., Kim K.-S., Lee E.J., Lee J., Jo Y.S. Upregulation of Long Noncoding RNA LOC100507661 Promotes Tumor Aggressiveness in Thyroid Cancer. Mol. Cell Endocrinol. 2016;431:36–45. doi: 10.1016/j.mce.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 88.Wang Y., Hou Z., Li D. Long Noncoding RNA UCA1 Promotes Anaplastic Thyroid Cancer Cell Proliferation via MiR-135a-mediated C-myc Activation. Mol. Med. Rep. 2018;18:3068–3076. doi: 10.3892/mmr.2018.9276. [DOI] [PubMed] [Google Scholar]
- 89.Pellecchia S., Sepe R., Decaussin-Petrucci M., Ivan C., Shimizu M., Coppola C., Testa D., Calin G.A., Fusco A., Pallante P. The Long Non-Coding RNA Prader Willi/Angelman Region RNA5 (PAR5) Is Downregulated in Anaplastic Thyroid Carcinomas Where It Acts as a Tumor Suppressor by Reducing EZH2 Activity. Cancers. 2020;12:235. doi: 10.3390/cancers12010235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tan X., Wang P., Lou J., Zhao J. Knockdown of LncRNA NEAT1 Suppresses Hypoxia-Induced Migration, Invasion and Glycolysis in Anaplastic Thyroid Carcinoma Cells through Regulation of MiR-206 and MiR-599. Cancer Cell Int. 2020;20:132. doi: 10.1186/s12935-020-01222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang L., Zhang J., Li S., Zhang Y., Liu Y., Dong J., Zhao W., Yu B., Wang H., Liu J. Genomic Amplification of Long Noncoding RNA HOTAIRM1 Drives Anaplastic Thyroid Cancer Progression via Repressing MiR-144 Biogenesis. RNA Biol. 2021;18:547–562. doi: 10.1080/15476286.2020.1819670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang X.-M., Liu Y., Fan Y.-X., Liu Z., Yuan Q.-L., Jia M., Geng Z.-S., Gu L., Lu X.-B. LncRNA PTCSC3 Affects Drug Resistance of Anaplastic Thyroid Cancer through STAT3/INO80 Pathway. Cancer Biol. Ther. 2018;19:590–597. doi: 10.1080/15384047.2018.1449610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu F., Zhang J., Qin L., Yang Z., Xiong J., Zhang Y., Li R., Li S., Wang H., Yu B., et al. Circular RNA EIF6 (Hsa_circ_0060060) Sponges MiR-144-3p to Promote the Cisplatin-Resistance of Human Thyroid Carcinoma Cells by Autophagy Regulation. Aging (Albany N.Y.) 2018;10:3806–3820. doi: 10.18632/aging.101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hui L., Chen Y. Tumor Microenvironment: Sanctuary of the Devil. Cancer Lett. 2015;368:7–13. doi: 10.1016/j.canlet.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 95.Junttila M.R., de Sauvage F.J. Influence of Tumour Micro-Environment Heterogeneity on Therapeutic Response. Nature. 2013;501:346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 96.Bergdorf K., Ferguson D.C., Mehrad M., Ely K., Stricker T., Weiss V.L. Papillary Thyroid Carcinoma Behavior: Clues in the Tumor Microenvironment. Endocr. Relat. Cancer. 2019;26:601–614. doi: 10.1530/ERC-19-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bastman J.J., Serracino H.S., Zhu Y., Koenig M.R., Mateescu V., Sams S.B., Davies K.D., Raeburn C.D., McIntyre R.C., Haugen B.R., et al. Tumor-Infiltrating T Cells and the PD-1 Checkpoint Pathway in Advanced Differentiated and Anaplastic Thyroid Cancer. J. Clin. Endocrinol. Metab. 2016;101:2863–2873. doi: 10.1210/jc.2015-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prete A., Lo A.S., Sadow P.M., Bhasin S.S., Antonello Z.A., Vodopivec D.M., Ullas S., Sims J.N., Clohessy J., Dvorak A.M., et al. Pericytes Elicit Resistance to Vemurafenib and Sorafenib Therapy in Thyroid Carcinoma via the TSP-1/TGFβ1 Axis. Clin. Cancer Res. 2018;24:6078–6097. doi: 10.1158/1078-0432.CCR-18-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jolly L.A., Novitskiy S., Owens P., Massoll N., Cheng N., Fang W., Moses H.L., Franco A.T. Fibroblast-Mediated Collagen Remodeling Within the Tumor Microenvironment Facilitates Progression of Thyroid Cancers Driven by BrafV600E and Pten Loss. Cancer Res. 2016;76:1804–1813. doi: 10.1158/0008-5472.CAN-15-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fozzatti L., Alamino V.A., Park S., Giusiano L., Volpini X., Zhao L., Stempin C.C., Donadio A.C., Cheng S.-Y., Pellizas C.G. Interplay of Fibroblasts with Anaplastic Tumor Cells Promotes Follicular Thyroid Cancer Progression. Sci. Rep. 2019;9:8028. doi: 10.1038/s41598-019-44361-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Giannini R., Moretti S., Ugolini C., Macerola E., Menicali E., Nucci N., Morelli S., Colella R., Mandarano M., Sidoni A., et al. Immune Profiling of Thyroid Carcinomas Suggests the Existence of Two Major Phenotypes: An ATC-Like and a PDTC-Like. J. Clin. Endocrinol. Metab. 2019;104:3557–3575. doi: 10.1210/jc.2018-01167. [DOI] [PubMed] [Google Scholar]
- 102.Varricchi G., Loffredo S., Marone G., Modestino L., Fallahi P., Ferrari S.M., de Paulis A., Antonelli A., Galdiero M.R. The Immune Landscape of Thyroid Cancer in the Context of Immune Checkpoint Inhibition. Int. J. Mol. Sci. 2019;20:3934. doi: 10.3390/ijms20163934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Caillou B., Talbot M., Weyemi U., Pioche-Durieu C., Al Ghuzlan A., Bidart J.M., Chouaib S., Schlumberger M., Dupuy C. Tumor-Associated Macrophages (TAMs) Form an Interconnected Cellular Supportive Network in Anaplastic Thyroid Carcinoma. PLoS ONE. 2011;6:e22567. doi: 10.1371/journal.pone.0022567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ryder M., Ghossein R.A., Ricarte-Filho J.C., Knauf J.A., Fagin J.A. Increased Density of Tumor Associated Macrophages Is Associated with Decreased Survival in Advanced Thyroid Cancer. Endocr. Relat. Cancer. 2008;15:1069–1074. doi: 10.1677/ERC-08-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cameselle-García S., Abdulkader-Sande S., Sánchez-Ares M., Rodríguez-Carnero G., Garcia-Gómez J., Gude-Sampedro F., Abdulkader-Nallib I., Cameselle-Teijeiro J.M. PD-L1 Expression and Immune Cells in Anaplastic Carcinoma and Poorly Differentiated Carcinoma of the Human Thyroid Gland: A Retrospective Study. Oncol. Lett. 2021;22:553. doi: 10.3892/ol.2021.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chintakuntlawar A.V., Rumilla K.M., Smith C.Y., Jenkins S.M., Foote R.L., Kasperbauer J.L., Morris J.C., Ryder M., Alsidawi S., Hilger C., et al. Expression of PD-1 and PD-L1 in Anaplastic Thyroid Cancer Patients Treated With Multimodal Therapy: Results From a Retrospective Study. J. Clin. Endocrinol. Metab. 2017;102:1943–1950. doi: 10.1210/jc.2016-3756. [DOI] [PubMed] [Google Scholar]
- 107.Hirsch L., Zitvogel L., Eggermont A., Marabelle A. PD-Loma: A Cancer Entity with a Shared Sensitivity to the PD-1/PD-L1 Pathway Blockade. Br. J. Cancer. 2019;120:3–5. doi: 10.1038/s41416-018-0294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brauner E., Gunda V., Vanden Borre P., Zurakowski D., Kim Y.S., Dennett K.V., Amin S., Freeman G.J., Parangi S. Combining BRAF Inhibitor and Anti PD-L1 Antibody Dramatically Improves Tumor Regression and Anti Tumor Immunity in an Immunocompetent Murine Model of Anaplastic Thyroid Cancer. Oncotarget. 2016;7:17194–17211. doi: 10.18632/oncotarget.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alobuia W., Gillis A., Kebebew E. Contemporary Management of Anaplastic Thyroid Cancer. Curr. Treat. Opt. Oncol. 2020;21:78. doi: 10.1007/s11864-020-00776-2. [DOI] [PubMed] [Google Scholar]
- 110.Xu B., Fuchs T., Dogan S., Landa I., Katabi N., Fagin J.A., Tuttle R.M., Sherman E., Gill A.J., Ghossein R. Dissecting Anaplastic Thyroid Carcinoma: A Comprehensive Clinical, Histologic, Immunophenotypic, and Molecular Study of 360 Cases. Thyroid. 2020;30:1505–1517. doi: 10.1089/thy.2020.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ibrahimpasic T., Ghossein R., Carlson D.L., Nixon I., Palmer F.L., Shaha A.R., Patel S.G., Tuttle R.M., Shah J.P., Ganly I. Outcomes in Patients with Poorly Differentiated Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2014;99:1245–1252. doi: 10.1210/jc.2013-3842. [DOI] [PubMed] [Google Scholar]
- 112.Tiedje V., Stuschke M., Weber F., Dralle H., Moss L., Führer D. Anaplastic Thyroid Carcinoma: Review of Treatment Protocols. Endocr. Relat. Cancer. 2018;25:R153–R161. doi: 10.1530/ERC-17-0435. [DOI] [PubMed] [Google Scholar]
- 113.Bergers G., Hanahan D. Modes of Resistance to Anti-Angiogenic Therapy. Nat. Rev. Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Husain A., Hu N., Sadow P.M., Nucera C. Expression of Angiogenic Switch, Cachexia and Inflammation Factors at the Crossroad in Undifferentiated Thyroid Carcinoma with BRAF(V600E) Cancer Lett. 2016;380:577–585. doi: 10.1016/j.canlet.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Song Y.S., Kim M.J., Sun H.J., Kim H.H., Shin H.S., Kim Y.A., Oh B.-C., Cho S.W., Park Y.J. Aberrant Thyroid-Stimulating Hormone Receptor Signaling Increases VEGF-A and CXCL8 Secretion of Thyroid Cancer Cells, Contributing to Angiogenesis and Tumor Growth. Clin. Cancer Res. 2019;25:414–425. doi: 10.1158/1078-0432.CCR-18-0663. [DOI] [PubMed] [Google Scholar]
- 116.Savvides P., Nagaiah G., Lavertu P., Fu P., Wright J.J., Chapman R., Wasman J., Dowlati A., Remick S.C. Phase II Trial of Sorafenib in Patients with Advanced Anaplastic Carcinoma of the Thyroid. Thyroid. 2013;23:600–604. doi: 10.1089/thy.2012.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ito Y., Onoda N., Ito K.-I., Sugitani I., Takahashi S., Yamaguchi I., Kabu K., Tsukada K. Sorafenib in Japanese Patients with Locally Advanced or Metastatic Medullary Thyroid Carcinoma and Anaplastic Thyroid Carcinoma. Thyroid. 2017;27:1142–1148. doi: 10.1089/thy.2016.0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gupta-Abramson V., Troxel A.B., Nellore A., Puttaswamy K., Redlinger M., Ransone K., Mandel S.J., Flaherty K.T., Loevner L.A., O’Dwyer P.J., et al. Phase II Trial of Sorafenib in Advanced Thyroid Cancer. J. Clin. Oncol. 2008;26:4714–4719. doi: 10.1200/JCO.2008.16.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brose M.S., Nutting C.M., Jarzab B., Elisei R., Siena S., Bastholt L., de la Fouchardiere C., Pacini F., Paschke R., Shong Y.K., et al. Sorafenib in Radioactive Iodine-Refractory, Locally Advanced or Metastatic Differentiated Thyroid Cancer: A Randomised, Double-Blind, Phase 3 Trial. Lancet. 2014;384:319–328. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tohyama O., Matsui J., Kodama K., Hata-Sugi N., Kimura T., Okamoto K., Minoshima Y., Iwata M., Funahashi Y. Antitumor Activity of Lenvatinib (E7080): An Angiogenesis Inhibitor That Targets Multiple Receptor Tyrosine Kinases in Preclinical Human Thyroid Cancer Models. J. Thyroid Res. 2014;2014:638747. doi: 10.1155/2014/638747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Takahashi S., Kiyota N., Yamazaki T., Chayahara N., Nakano K., Inagaki L., Toda K., Enokida T., Minami H., Imamura Y., et al. A Phase II Study of the Safety and Efficacy of Lenvatinib in Patients with Advanced Thyroid Cancer. Future Oncol. 2019;15:717–726. doi: 10.2217/fon-2018-0557. [DOI] [PubMed] [Google Scholar]
- 122.Takahashi S., Tahara M., Ito K., Tori M., Kiyota N., Yoshida K., Sakata Y., Yoshida A. Safety and Effectiveness of Lenvatinib in 594 Patients with Unresectable Thyroid Cancer in an All-Case Post-Marketing Observational Study in Japan. Adv. Ther. 2020;37:3850–3862. doi: 10.1007/s12325-020-01433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schlumberger M., Tahara M., Wirth L.J., Robinson B., Brose M.S., Elisei R., Habra M.A., Newbold K., Shah M.H., Hoff A.O., et al. Lenvatinib versus Placebo in Radioiodine-Refractory Thyroid Cancer. N. Engl. J. Med. 2015;372:621–630. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 124.Rendl G., Sipos B., Becherer A., Sorko S., Trummer C., Raderer M., Hitzl W., Ardelt M., Gallowitsch H.J., Pirich C. Real-World Data for Lenvatinib in Radioiodine-Refractory Differentiated Thyroid Cancer (RELEVANT): A Retrospective Multicentric Analysis of Clinical Practice in Austria. Int. J. Endocrinol. 2020;2020:1–8. doi: 10.1155/2020/8834148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Locati L.D., Piovesan A., Durante C., Bregni M., Castagna M.G., Zovato S., Giusti M., Ibrahim T., Puxeddu E., Fedele G., et al. Real-World Efficacy and Safety of Lenvatinib: Data from a Compassionate Use in the Treatment of Radioactive Iodine-Refractory Differentiated Thyroid Cancer Patients in Italy. Eur. J. Cancer. 2019;118:35–40. doi: 10.1016/j.ejca.2019.05.031. [DOI] [PubMed] [Google Scholar]
- 126.Berdelou A., Borget I., Godbert Y., Nguyen T., Garcia M.-E., Chougnet C.N., Ferru A., Buffet C., Chabre O., Huillard O., et al. Lenvatinib for the Treatment of Radioiodine-Refractory Thyroid Cancer in Real-Life Practice. Thyroid. 2018;28:72–78. doi: 10.1089/thy.2017.0205. [DOI] [PubMed] [Google Scholar]
- 127.Cabanillas M.E., de Souza J.A., Geyer S., Wirth L.J., Menefee M.E., Liu S.V., Shah K., Wright J., Shah M.H. Cabozantinib As Salvage Therapy for Patients With Tyrosine Kinase Inhibitor-Refractory Differentiated Thyroid Cancer: Results of a Multicenter Phase II International Thyroid Oncology Group Trial. J. Clin. Oncol. 2017;35:3315–3321. doi: 10.1200/JCO.2017.73.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ha H.T., Lee J.S., Urba S., Koenig R.J., Sisson J., Giordano T., Worden F.P. A Phase II Study of Imatinib in Patients with Advanced Anaplastic Thyroid Cancer. Thyroid. 2010;20:975–980. doi: 10.1089/thy.2010.0057. [DOI] [PubMed] [Google Scholar]
- 129.Pennell N.A., Daniels G.H., Haddad R.I., Ross D.S., Evans T., Wirth L.J., Fidias P.H., Temel J.S., Gurubhagavatula S., Heist R.S., et al. A Phase II Study of Gefitinib in Patients with Advanced Thyroid Cancer. Thyroid. 2008;18:317–323. doi: 10.1089/thy.2007.0120. [DOI] [PubMed] [Google Scholar]
- 130.Bible K.C., Suman V.J., Menefee M.E., Smallridge R.C., Molina J.R., Maples W.J., Karlin N.J., Traynor A.M., Kumar P., Goh B.C., et al. A Multiinstitutional Phase 2 Trial of Pazopanib Monotherapy in Advanced Anaplastic Thyroid Cancer. J. Clin. Endocrinol. Metab. 2012;97:3179–3184. doi: 10.1210/jc.2012-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yoo S.-K., Song Y.S., Lee E.K., Hwang J., Kim H.H., Jung G., Kim Y.A., Kim S., Cho S.W., Won J.-K., et al. Integrative Analysis of Genomic and Transcriptomic Characteristics Associated with Progression of Aggressive Thyroid Cancer. Nat. Commun. 2019;10:2764. doi: 10.1038/s41467-019-10680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Flaherty K.T., Puzanov I., Kim K.B., Ribas A., McArthur G.A., Sosman J.A., O’Dwyer P.J., Lee R.J., Grippo J.F., Nolop K., et al. Inhibition of Mutated, Activated BRAF in Metastatic Melanoma. N. Engl. J. Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hyman D.M., Puzanov I., Subbiah V., Faris J.E., Chau I., Blay J.-Y., Wolf J., Raje N.S., Diamond E.L., Hollebecque A., et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N. Engl. J. Med. 2015;373:726–736. doi: 10.1056/NEJMoa1502309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kurata K., Onoda N., Noda S., Kashiwagi S., Asano Y., Hirakawa K., Ohira M. Growth Arrest by Activated BRAF and MEK Inhibition in Human Anaplastic Thyroid Cancer Cells. Int. J. Oncol. 2016;49:2303–2308. doi: 10.3892/ijo.2016.3723. [DOI] [PubMed] [Google Scholar]
- 135.Subbiah V., Kreitman R.J., Wainberg Z.A., Cho J.Y., Schellens J.H.M., Soria J.C., Wen P.Y., Zielinski C., Cabanillas M.E., Urbanowitz G., et al. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J. Clin. Oncol. 2018;36:7–13. doi: 10.1200/JCO.2017.73.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wagle N., Grabiner B.C., Van Allen E.M., Amin-Mansour A., Taylor-Weiner A., Rosenberg M., Gray N., Barletta J.A., Guo Y., Swanson S.J., et al. Response and Acquired Resistance to Everolimus in Anaplastic Thyroid Cancer. N. Engl. J. Med. 2014;371:1426–1433. doi: 10.1056/NEJMoa1403352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Harris E.J., Hanna G.J., Chau N., Rabinowits G., Haddad R., Margalit D.N., Schoenfeld J., Tishler R.B., Barletta J.A., Nehs M., et al. Everolimus in Anaplastic Thyroid Cancer: A Case Series. Front. Oncol. 2019;9:106. doi: 10.3389/fonc.2019.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schneider T.C., de Wit D., Links T.P., van Erp N.P., van der Hoeven J.J.M., Gelderblom H., Roozen I.C.F.M., Bos M., Corver W.E., van Wezel T., et al. Everolimus in Patients With Advanced Follicular-Derived Thyroid Cancer: Results of a Phase II Clinical Trial. J. Clin. Endocrinol. Metab. 2017;102:698–707. doi: 10.1210/jc.2016-2525. [DOI] [PubMed] [Google Scholar]
- 139.Wirth L.J., Sherman E., Robinson B., Solomon B., Kang H., Lorch J., Worden F., Brose M., Patel J., Leboulleux S., et al. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N. Engl. J. Med. 2020;383:825–835. doi: 10.1056/NEJMoa2005651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Subbiah V., Yang D., Velcheti V., Drilon A., Meric-Bernstam F. State-of-the-Art Strategies for Targeting RET-Dependent Cancers. J. Clin. Oncol. 2020;38:1209–1221. doi: 10.1200/JCO.19.02551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cabanillas M.E., Drilon A., Farago A.F., Brose M.S., McDermott R., Sohal D., Oh D.-Y., Almubarak M., Bauman J., Chu E., et al. 1916P Larotrectinib Treatment of Advanced TRK Fusion Thyroid Cancer. Ann. Oncol. 2020;31:S1086. doi: 10.1016/j.annonc.2020.08.1404. [DOI] [Google Scholar]
- 142.Godbert Y., Henriques de Figueiredo B., Bonichon F., Chibon F., Hostein I., Pérot G., Dupin C., Daubech A., Belleannée G., Gros A., et al. Remarkable Response to Crizotinib in Woman With Anaplastic Lymphoma Kinase–Rearranged Anaplastic Thyroid Carcinoma. JCO. 2014;33:e84–e87. doi: 10.1200/JCO.2013.49.6596. [DOI] [PubMed] [Google Scholar]
- 143.Ahn J., Jin M., Song E., Ryu Y.-M., Song D.E., Kim S.-Y., Kim T.Y., Kim W.B., Shong Y.K., Jeon M.J., et al. Immune Profiling of Advanced Thyroid Cancers Using Fluorescent Multiplex Immunohistochemistry. Thyroid. 2021;31:61–67. doi: 10.1089/thy.2020.0312. [DOI] [PubMed] [Google Scholar]
- 144.Robert C., Schachter J., Long G.V., Arance A., Grob J.J., Mortier L., Daud A., Carlino M.S., McNeil C., Lotem M., et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 145.De Leo S., Trevisan M., Fugazzola L. Recent Advances in the Management of Anaplastic Thyroid Cancer. Thyroid Res. 2020;13:17. doi: 10.1186/s13044-020-00091-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Spalart V., Legius B., Segers K., Coolen J., Maes B., Decoster L. Dramatic Response to First Line Single Agent Pembrolizumab in Anaplastic Thyroid Carcinoma. Case Rep. Endocrinol. 2019;2019:9095753. doi: 10.1155/2019/9095753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chintakuntlawar A.V., Yin J., Foote R.L., Kasperbauer J.L., Rivera M., Asmus E., Garces N.I., Janus J.R., Liu M., Ma D.J., et al. A Phase 2 Study of Pembrolizumab Combined with Chemoradiotherapy as Initial Treatment for Anaplastic Thyroid Cancer. Thyroid. 2019;29:1615–1622. doi: 10.1089/thy.2019.0086. [DOI] [PubMed] [Google Scholar]
- 148.Capdevila J., Wirth L.J., Ernst T., Ponce Aix S., Lin C.-C., Ramlau R., Butler M.O., Delord J.-P., Gelderblom H., Ascierto P.A., et al. PD-1 Blockade in Anaplastic Thyroid Carcinoma. J. Clin. Oncol. 2020;38:2620–2627. doi: 10.1200/JCO.19.02727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vanden Borre P., McFadden D.G., Gunda V., Sadow P.M., Varmeh S., Bernasconi M., Jacks T., Parangi S. The next Generation of Orthotopic Thyroid Cancer Models: Immunocompetent Orthotopic Mouse Models of BRAF V600E-Positive Papillary and Anaplastic Thyroid Carcinoma. Thyroid. 2014;24:705–714. doi: 10.1089/thy.2013.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cabanillas M.E., Ferrarotto R., Garden A.S., Ahmed S., Busaidy N.L., Dadu R., Williams M.D., Skinner H., Gunn G.B., Grosu H., et al. Neoadjuvant BRAF- and Immune-Directed Therapy for Anaplastic Thyroid Carcinoma. Thyroid. 2018;28:945–951. doi: 10.1089/thy.2018.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang J.R., Zafereo M.E., Dadu R., Ferrarotto R., Busaidy N.L., Lu C., Ahmed S., Gule-Monroe M.K., Williams M.D., Sturgis E.M., et al. Complete Surgical Resection Following Neoadjuvant Dabrafenib Plus Trametinib in BRAFV600E-Mutated Anaplastic Thyroid Carcinoma. Thyroid. 2019;29:1036–1043. doi: 10.1089/thy.2019.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Iyer P.C., Dadu R., Gule-Monroe M., Busaidy N.L., Ferrarotto R., Habra M.A., Zafereo M., Williams M.D., Gunn G.B., Grosu H., et al. Salvage Pembrolizumab Added to Kinase Inhibitor Therapy for the Treatment of Anaplastic Thyroid Carcinoma. J. Immunother. Cancer. 2018;6:68. doi: 10.1186/s40425-018-0378-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dierks C., Seufert J., Aumann K., Ruf J., Klein C., Kiefer S., Rassner M., Boerries M., Zielke A., La Rosée P., et al. The Lenvatinib/Pembrolizumab Combination Is an Effective Treatment Option for Anaplastic and Poorly Differentiated Thyroid Carcinoma. Thyroid. 2021 doi: 10.1089/thy.2020.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kollipara R., Schneider B., Radovich M., Babu S., Kiel P.J. Exceptional Response with Immunotherapy in a Patient with Anaplastic Thyroid Cancer. Oncologist. 2017;22:1149–1151. doi: 10.1634/theoncologist.2017-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sherman E.J., Dunn L.A., Ho A.L., Baxi S.S., Ghossein R.A., Fury M.G., Haque S., Sima C.S., Cullen G., Fagin J.A., et al. Phase 2 Study Evaluating the Combination of Sorafenib and Temsirolimus in the Treatment of Radioactive Iodine-Refractory Thyroid Cancer. Cancer. 2017;123:4114–4121. doi: 10.1002/cncr.30861. [DOI] [PMC free article] [PubMed] [Google Scholar]