Abstract
Simple Summary
The BCL2 family comprises pro- and anti-apoptotic proteins whose cellular abundance, modifications, and interactions determine the cell fate. Its contribution to pathogenesis of acute myeloid leukemia (AML) was not widely studied and none of the studies published before presented a complex picture of BCL2 family gene expression. Therefore, we decided to analyze the AML transcriptome sequencing data to outline this picture and look for relations between the expression of particular BCL2 family members and the genes encoding interacting proteins, presence of mutations, and clinical features. Our findings should not only shed light on apoptosis- and oncogenesis-related processes, but may also be implemented in clinical practice. Prognostic significance, association with response to therapy and potential application in the selection of therapeutic targets are of particular importance.
Abstract
The expression of apoptosis-related BCL2 family genes, fine-tuned in normal cells, is dysregulated in many neoplasms. In acute myeloid leukemia (AML), this problem has not been studied comprehensively. To address this issue, RNA-seq data were used to analyze the expression of 26 BCL2 family members in 27 AML FAB M1 and M2 patients, divided into subgroups differently responding to chemotherapy. A correlation analysis, analysis of variance, and Kaplan-Meier analysis were applied to associate the expression of particular genes with other gene expression, clinical features, and the presence of mutations detected by exome sequencing. The expression of BCL2 family genes was dysregulated in AML, as compared to healthy controls. An upregulation of anti-apoptotic and downregulation of pro-apoptotic genes was observed, though only a decrease in BMF, BNIP1, and HRK was statistically significant. In a group of patients resistant to chemotherapy, overexpression of BCL2L1 was manifested. In agreement with the literature data, our results reveal that BCL2L1 is one of the key players in apoptosis regulation in different types of tumors. An exome sequencing data analysis indicates that BCL2 family genes are not mutated in AML, but their expression is correlated with the mutational status of other genes, including those recurrently mutated in AML and splicing-related. High levels of some BCL2 family members, in particular BIK and BCL2L13, were associated with poor outcome.
Keywords: AML, BCL2 family, apoptosis, gene expression, RNA-seq, exome sequencing, response to therapy, mutation, splicing, correlation
1. Introduction
Acute myeloid leukemia (AML) is a heterogeneous hematological malignancy which develops as a result of hematopoietic differentiation impairment and excessive proliferation of myeloid progenitor cell clones [1,2]. Their accumulation in the bone marrow, peripheral blood, and organs leads to the manifestation of systemic symptoms and death within weeks to months, if the disease is left untreated [3]. Chromosomal rearrangements, small somatic mutations and dysregulation of the gene expression have been described as AML hallmarks [4,5,6,7]. Similarly to solid tumor cells, leukemic cells present enhanced survival rate and successfully evade apoptosis [8]. Apoptosis or programmed cell death is involved in a natural cell turnover, embryonic development, and the elimination of impaired or aging cells [9]. Therefore, dysregulation of this process usually has severe consequences [8,10].
Proteins belonging to the BCL2 (B-Cell lymphoma 2) family regulate all major types of cell death, including apoptosis, necrosis, and autophagy; although they are the best known for contribution to the intrinsic (mitochondrial) apoptotic pathway [11,12,13,14]. Their cellular content, post-translational modifications, and interactions determine cell fate, deciding between survival and death [14,15,16]. Up to date, 25 different BCL2 family members have been identified and most of them have two or more isoforms [15,17,18]. The family founder, the BCL2 gene, is involved in t(14;18) chromosomal translocation in B-cell lymphomas and frequently overexpressed in cancer [12,19,20]. The pro-apoptotic BCL2 family members include two subgroups: a “multi-domain” containing up to 4 BCL2 homology (BH1-BH4) domains and a “BH3-only” [12,20,21]. The multi-domain subgroup includes the BCL2 homologous antagonist/killer (BCL2-like protein 4 alias apoptosis regulator BAK, encoded by the BAK1 gene); the apoptosis regulator BAX (BCL2-like protein 4, encoded by BAX gene); and the BCL2-related ovarian killer (BCL2-like protein 9, encoded by the BOK gene). These three proteins trigger apoptosis by forming pores within the mitochondrial outer membrane. Mitochondrial outer membrane permeabilization (MOMP) is necessary to release pro-apoptogenic factors (e.g., cytochrome c) from the mitochondrial intermembrane space (IMS) into the cytosol, which activates a caspase cascade [14]. The pro-apoptotic BH3-only protein subgroup consists of inter alia BCL2-associated death promoter (BAD, encoded by the BAD gene); BH3-interacting domain death agonist (BID, encoded by the BID gene); BCL2-like protein 11 (BIM, encoded by the BCL2L11 gene); p53 upregulated modulator of apoptosis (PUMA, encoded by the BBC3 gene); BCL2 modifying factor (encoded by the BMF gene), activator of apoptosis harakiri (encoded by the HRK gene); and NADPH oxidase activator 1 (NOXA, alias Phorbol-12-myristate-13-acetate-induced protein 1, encoded by the PMAIP1 gene) [12,13,14,15]. The BH3-only proteins interact with anti-apoptotic proteins forming homo- or heterodimers, and the type of dimer partners determine the cell fate [13,14]. The anti-apoptotic BCL2 family members include apoptosis regulator BCL2 (encoded by the BCL2 gene); B-cell lymphoma-extra-large (BCL-XL, alias BCL2-like protein 1, encoded by the BCL2L1 gene); apoptosis regulator BCL-W (BCL2-like protein 2, encoded by the BCL2L2 gene); induced myeloid leukemia cell differentiation protein Mcl-1 (encoded by the MCL1 gene); and BCL2-related protein A1 (alias BFL-1, encoded by the BCL2A1 gene). These proteins sequester pro-apoptotic BH3-only proteins, thereby preventing the activation of multi-domain pro-apoptotic proteins [22,23].
The expression of genes encoding the BCL2 family proteins is frequently dysregulated in cancer [12]. The up-regulation of pro-survival BCL2 genes has also been associated with the pathogenesis of AML [24,25,26,27]. Thus, BCL2-targeted therapy has recently been introduced to cancer treatment with promising results [28,29]. In the AML induction treatment, the administration of anthracycline and cytosine arabinoside (3 + 7 regimen) has been used for more than 40 years [30,31,32]. Approximately 40–45% of younger and only 10–20% of older adults with AML may be cured with the current standard chemotherapy [33]. Despite the progress in the understanding of the disease biology, the three-year overall survival (OS) of AML patients is unacceptably low (~10%) in the case of relapsed or refractory (R/R) disease [1,34]. The allogenic stem cell transplantation remains the sole curative option, but is not always available [33]. Seventy percent of patients aged >65 die within 1 year from the moment of diagnosis [2]. Therefore, a lot of efforts have been made to individualize AML therapy according to the molecular profile of AML blast cells [35,36]. The application of next generation sequencing (NGS) has opened up new possibilities to precisely diagnose the disease, look for molecular bases of AML refractoriness, and try to overcome it. The introduction of targeted therapy with small molecule inhibitors of fms-like thyrosine kinase 3 (midostaurin, gilteritinib), dehydrogenase isocitrate 1 (AG-221), and dehydrogenase isocitrate 2 (AG-120) to the treatment of R/R AML are excellent examples [37,38,39,40]. Upon the absence of actionable mutations, the primary focus would be on non-chemotherapeutic strategies for the treatment of R/R AML [33]. One of them are drugs targeting the anti-apoptotic BCL2 family proteins [41,42].
In our previous studies, we demonstrated a number of genes which were overexpressed in AML, including STMN1, NPM1, CAT, and WT1 [43,44]. We also showed that at the time of AML diagnosis, high NPM1 expression correlated with poor outcome [44] and the WT1 expression increased with treatment resistance [43]. Due to an increasing interest in the role of pro-apoptotic drugs in overcoming leukemic cell resistance, the main aims of this study were to analyze the expression pattern of BCL2 family genes in AML patients and associate it with the retrospectively monitored response to therapy, as well as disease-free and overall survival. Looking for factors influencing the BCL2 family expression in AML, we also analyzed the expression of genes encoding proteins interacting with BCL2 and checked their mutational status. To do so, we took advantage of the RNA-seq and exome sequencing data collected in our laboratory.
2. Materials and Methods
Samples. Peripheral blood (PB) or bone marrow (BM) samples were collected in 2007–2010 from 27 adult patients with AML, M1 or M2 FAB, at the time of the first diagnosis (Table 1, Table S1). Control samples were collected from healthy volunteers (one BM and 12 PB samples) and from an AML patient after treatment (in complete remission stage, one BM sample). Patients were diagnosed and treated at the Department of Hematology and Bone Marrow Transplantation in the University Hospital of the Lord’s Transfiguration of Poznan University of Medical Sciences in Poznan, Poland. In the case of all AML patients, induction therapy was applied according to the following scheme: daunorubicin 60 mg/m2 per day given as a 1–3 h-long intravenous infusion on days 1–3 and cytosine arabinoside 200 mg/m2 per day as a continuous intravenous infusion on days 1–7 (3 + 7 regimen). Complete remission (CR) in AML was defined using the criteria developed by an International Working Group [45,46,47], including: (1) normal values for absolute neutrophil count (>1000/µL) and platelet count (>100,000/µL), and independence from red cell transfusion; (2) no clusters or collections of blast cells in bone marrow biopsy and no symptoms of extramedullary leukemia (e.g., the central nervous system or soft tissue involvement); (3) normal maturation of all cellular components (i.e., erythrocytic, granulocytic, and megakaryocytic series) in the bone marrow aspiration biopsy; (4) less than 5% percent blast cells in the bone marrow, and no blast cells expressing leukemic phenotype (e.g., Auer rods, aberrant immunophenotype blasts). The “primary refractory disease” (RES, resistant to treatment) was diagnosed when failure of only one induction cycle was confirmed [48].
Table 1.
The summarized characteristics of AML patients included in the study.
| Group | Number of Patients (F/M) | FAB Type (Number) | Age Median (Range) | WBC Count [×109/L] Mean (Range) |
NPM1 mut. | FLT3 mut. | RUNX1/RUNX1T1 | Karyotype |
|---|---|---|---|---|---|---|---|---|
| CR | 11 (6/5) | M1 (3) M2 (8) |
52 (19–64) |
36.3 (3.6–111) |
2 | 2 | 2 | normal (1) aberrant (4) NA (6) |
| RES | 8 (3/5) | M1 (2) M2 (6) |
53.5 (21–64) |
62.17 (1.3–146) |
2 | 1 | 1 | normal (1) aberrant (4) NA (3) |
| X | 8 (4/4) | M1 (4) M2 (4) |
51 (25–65) |
90.43 (19.9–233) |
3 | 1 | - | normal (1) aberrant (2) NA (5) |
| All patients | 27 (13/14) | M1 (9) M2 (18) |
52 (19–65) |
60.0 (1.3–233) |
7 | 4 | 3 | normal (3) aberrant (10) NA (14) |
AML patients were divided into three groups according to their response to the treatment (CR—complete remission; RES—resistance to therapy; X—death during therapy). FLT3 mut. = FLT3-ITD (internal tandem duplication in the FLT3 gene); RUNX1/RUNX1T—fusion gene, result of t(8;21) translocation; NA—data not available.
RNA-seq. PB or BM mononuclear cells were separated using density gradient centrifugation (Gradisol L, Aqua-Medica, Poland). Total RNA was extracted with a mirVana miRNA Isolation Kit (Ambion/Thermo Fisher Scientific, Waltham, MA, USA) and DNase-treated (TURBO DNA-free kit, Ambion/Thermo Fisher Scientific). RNA-seq was performed with a Genome Analyzer IIx (Illumina, San Diego, CA, USA). Up to 4 µg of total RNA was used to prepare sequencing libraries with the TruSeq RNA Sample Prep Kit (Illumina). The indexed libraries were sequenced on a single-read flow cell (TruSeq SR Cluster Kit v2 cBot, Illumina), two libraries per lane, with 72-nt long reads. The data were processed by STAR-aligner v. 2.5.3a (https://github.com/alexdobin/STAR, accessed on 15 November 2018, Alexander Dobin, Cold Spring Harbor Laboratory, New York, NY, USA) [49] for mapping, and RSEM ver. 1.3.0 (https://github.com/deweylab/RSEM, accessed on 14 February 2018, Dewey Lab, University of Wisconsin-Madison, Madison, WI, USA) [50] for gene quantification. The levels of expression were normalized first for the gene length and, subsequently, for the sequencing depth in order to obtain TPM (transcripts per million) values.
Statistical analysis. Statistical analyses were made in R ver. 4.0.4 (https://cran.r-project.org, accessed on 27 March 2021, R Foundation for Statistical Computing, Vienna, Austria) and R Studio ver. 1.4.1106 (https://www.rstudio.com, accessed on 27 March 2021, RStudio, Boston, MA, USA). The following R packages were used: base, DESeq2 [51], ggplot2, ggcorrplot, plyr, reshape2, ggsignif, ggpubr, Hmisc, and survival. Plots were generated with R or Microsoft Excel. To compare the levels of gene expression between AML and additional control samples from Human Protein Atlas, Mann-Whitney test with Bonferroni correction for multiple comparisons was used. To estimate the patient outcome and correlate it with particular gene expression levels, Kaplan-Meier analysis was applied. The differences between survival curves were tested with a log-rank test. For the association of gene expression with mutation status and clinical features, ANOVA and MANOVA tests were applied, for univariate and multivariate analysis of variance, respectively.
Mutation analysis. Whole exome sequencing (WES) data collected for the analyzed AML samples in our laboratory were used to extract information about mutational status of the genes of interest (26 BCL2 family members, 16 genes encoding BCL2-interacting proteins and 12 genes recurrently mutated in AML). Haplotype calling was made with GATK v. 4.0.12.0, (https://gatk.broadinstitute.org/, accessed on 2 February 2019) [52]. GATK-originated vcf files, generated for each sample separately, were analyzed with the use of eVai v. 0.6 (enGenome, www.engenome.com/evai/, accessed on 24 November 2019), with the application of the following filters: effect (all splice and coding variants, except for synonymous, were selected); sample (coverage ≥ 6; quality ≥ 40; allele frequency ≥ 0.1), and classification (only pathogenic, likely pathogenic and uncertain variants were maintained). Alternative allele coverage was used as an additional threshold (≥3 for SNPs and ≥4 for indels).
3. Results
3.1. Expression of Genes Encoding Proteins from the BCL2 Family
The list of 25 genes encoding proteins from the BCL2 family (Table S2) was downloaded from the HUGO Gene Nomenclature Committee (HGNC) repository (https://www.genenames.org/data/genegroup/#!/group/1057 accessed on 4 March 2021). Based on the differences in the protein structure described above, 14 genes are classified as the “BCL2 family” and the remaining 11 as the “BCL2 homology region 3 (BH3) only”. We decided to add also one fusion transcript (BCL2L2-PABPN1) to the list, which naturally occurs as a product of read-through transcription of BCL2L2 and its neighboring gene, PABPN1. This chimeric RNA was shown to be overexpressed in bladder cancer [53].
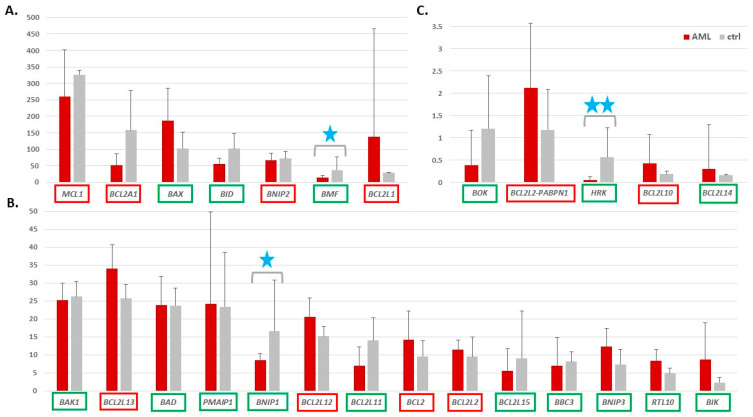
The normalized expression values (TPM, transcripts per million) for the 26 BCL2 family genes mentioned above were extracted from RNA-seq data generated in our laboratory for 27 AML M1 and M2 patients (Table 1, Table S1), from BM or PB samples collected at the time of AML diagnosis. Control samples (ctrl) were represented by three specimens: (i) a BM sample from a healthy volunteer; (ii) a pool of 12 PB samples delivered by healthy volunteers; (iii) a BM sample from an AML patient after treatment (in complete remission stage). Mean TPMs of all 26 genes in AML and control samples are presented in Figure 1, whereas the source data (TPM values for individual samples) are available in Table S3. The statistical data was extracted from the results of differential expression analysis, generated for the whole RNA-seq data set with the use of DESeq2 (Table S3).
Figure 1.
Expression levels of 26 genes from the BCL2 family in AML versus control (ctrl) samples. The expression levels were extracted from the RNA-seq data and presented as mean TPM (transcripts per million) values with standard deviation bars (y axis). For clarity, the genes were ranked according to the TPM in control samples and divided into three separate graphs, from (A) the highest to (B) middle and (C) the lowest gene expression. The genes encoding the pro-apoptotic proteins are indicated in green, the anti-apoptotic are in red frames. Blue stars indicate genes with statistically significant expression change between AML and ctrl (adj. p value threshold 0.05; 1 star < 0.01; 2 stars < 0.001). The statistical data were calculated with DESeq2, which was applied for differential analysis of the whole RNA-seq data set, with a default method for multiple testing correction.
Only five genes, namely MCL1, BAX, BCL2A1, BCL2L1, and BID, revealed high expression levels (TPM > 100) in the AML or ctrl samples (Figure 1A). The expression of five other genes was barely detectable (TPM about 2 and below, Figure 1C) whereas the majority of the BCL2 family members showed low to middle expression level (TPM range 5–35), including the BCL2 itself with mean TPM 14 in AML and 10 in ctrl (Figure 1B). About half of pro-apoptotic genes (Figure 1, names in the green frames) revealed decreased expression trend in AML, as compared to ctrl, and for three of them, BMF, BNIP1, and HRK, the expression difference between AML and ctrl was statistically significant (adj. p value < 0.01) (Figure 1, blue stars).
The majority of the anti-apoptotic genes showed an upregulation trend in AML and only one (BCL2A1) was decreased in AML vs. ctrl but no difference was statistically significant. The reason for it might be high variability between both, AML patients and control samples, reflected by high standard deviation values.
Because the number of control samples was not high in our dataset, we extracted control RNA-level data (mean TPM for 6 PB mononuclear cell samples and mean TPM for 4 BM samples) from the Human Protein Atlas (HPA, www.proteinatlas.org accessed 5 June 2021, Table S4A) and repeated the above analysis including additional controls. Each gene expression was compared between AML and control with the use of Mann-Whitney test. The results were comparable with the results of previous analysis but only the increase of BCL2L13 in AML was statistically significant when Bonferroni correction for multiple comparisons was applied (Figure S1, Table S4B). The levels of BCL2 family transcripts in our controls were highly correlated with the levels of HPA transcripts (Spearman correlation coefficient 0.85 for PB and 0.93 for BM, Table S4C).
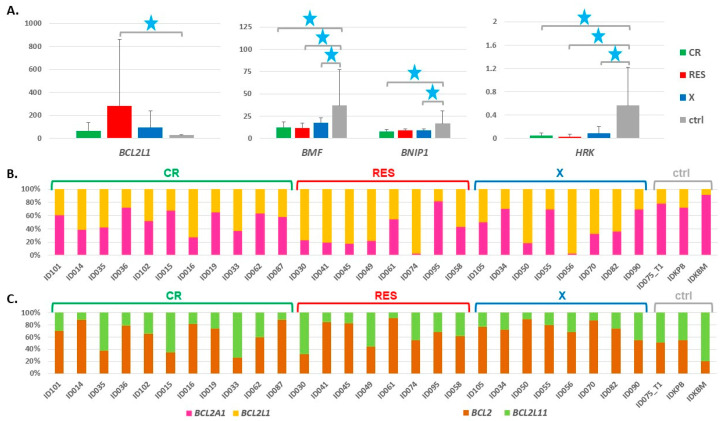
To retrospectively analyze the association between the BCL2 gene family expression at the time of AML diagnosis and the patient response to therapy monitored at a later stage, we divided AML into three groups: CR—patients who reached complete remission after the first induction therapy (n = 11); RES—patients resistant to the first induction therapy (n = 8); X—patients who succumbed to the disease during therapy (n = 8) (Table 1). The results are summarized in Table S3 (all 26 genes) and Figure 2A. Three genes (BMF, BNIP1, and HRK) were significantly decreased in two or three AML groups, as compared to ctrl. BCL2L1 was the only gene increased in AML patients resistant to therapy. The difference was statistically significant in a comparison between RES and ctrl (adj. p value 0.03). In the RES group, BCL2L1 revealed the highest level of expression among all the BCL2 family members (mean TPM 283). The variability of this anti-apoptotic gene expression within the RES group was also extremely high (TPM range 18–1702, Table S3).
Figure 2.
(A) Expression levels of four genes from the BCL2 family in AML and ctrl samples, with AML divided into three groups according to patient response to the first induction therapy (CR—complete remission; RES—resistance to therapy; X—death during therapy). The expression levels were extracted from the RNA-seq data collected at the time of diagnosis (before treatment) and presented as mean TPM (transcripts per million) values with standard deviation bars (y axis). Blue stars indicate statistically significant expression changes (adj. p value threshold 0.05; 1 star < 0.05). The statistical data was calculated with DESeq2, which was applied for differential analysis of the whole RNA-seq data set, with a default method for multiple testing correction. (B,C) Ratio of the expression of two pairs of genes: BCL2A1 and BCL2L1 (B); BCL2 and BCL2L11 (C) in individual AML and ctrl samples, presented as a percentage contribution of each gene into the sum of TPMs of both compared genes.
Comparing the BCL2 family members, we noticed similar expression levels but inverse relation between AML and ctrl for BCL2A1 and BCL2L1 genes. Looking at individual samples, we found that BCL2L1 remarkably predominated over BCL2A1 in AML, in particular in RES (Figure 2B). The difference in BCL2A1/BCL2L1 ratio was statistically significant not only between AML (as a whole group or divided into CR, RES and X) and ctrl, but also between RES and CR (Table 2). Another interesting relation was observed between BCL2 and BCL2L11 gene, which encodes one of the BCL2 antagonists, BIM [54]. The levels of these two genes were balanced in ctrl or shifted towards the pro-apoptotic BCL2L11. On the contrary, in the majority of the AML samples, including all from the X subgroup, the pro-apoptotic BCL2 evidently predominated (Figure 2C). The difference in BCL2/BCL2L11 ratio was statistically significant between AML and ctrl (p = 0.033) and between X and ctrl (p = 0.012) (Table 2).
Table 2.
Comparison of gene expression ratio for two pairs of genes: BCL2A1 and BCL2L1; BCL2 and BCL2L11 in AML and control samples.
| Group | BCL2A1/BCL2L1 Ratio | BCL2/BCL2L11 Ratio |
|---|---|---|
| AML—mean ratio (st. dev.) CR—mean ratio (st. dev.) RES—mean ratio (st. dev.) |
1.15 (1.02) | 3.26 (2.53) |
| 1.31 (0.7) | 2.93 (2.43) | |
| 0.92 (1.44) | 3.16 (3.05) | |
| X—mean ratio (st. dev.) ctrl—mean ratio (st. dev.) |
1.15 (0.99) | 3.81 (2.3) |
| 5.67 (4.56) | 0.83 (0.50) | |
| AML vs. ctrl * CR vs. ctrl* |
0.0034 | 0.033 |
| 0.0110 | 0.1264 | |
| RES vs. ctrl * X vs. ctrl * |
0.0485 | 0.1333 |
| 0.0121 | 0.0121 | |
| RES vs. X * | 0.5054 | 0.3823 |
| RES vs. CR * | 0.0506 | 0.9678 |
| CR vs. X * | 0.5448 | 0.3950 |
* p value (Wilcoxon rank sum exact test; statistically significant values are in bold); st. dev.—standard deviation.
A correlation analysis of the BCL2 family gene expression showed differences in the correlation pattern not only between AML and ctrl, but also between the CR, RES, and X AML subgroups (Figures S2 and S3), which suggests that the balance between the expression levels of particular family members is very delicate and dynamic, and can be easily disturbed. Only some correlations were statistically significant, e.g., in AML we observed a positive correlation between BCL2L1, BOK, and BCL2L11, a negative correlation between BCL2L1 and BID, a positive correlation between BAX, BBC3, PMAIP, positive correlation between BCL2, BMF, and BNIP3, a negative correlation between BCL2A1 and RTL10, or a negative correlation between BCL2A13 and BCL2A14 (Figure S2D). Strikingly, positive as well as negative correlations were observed between genes encoding both the pro- and anti-apoptotic proteins.
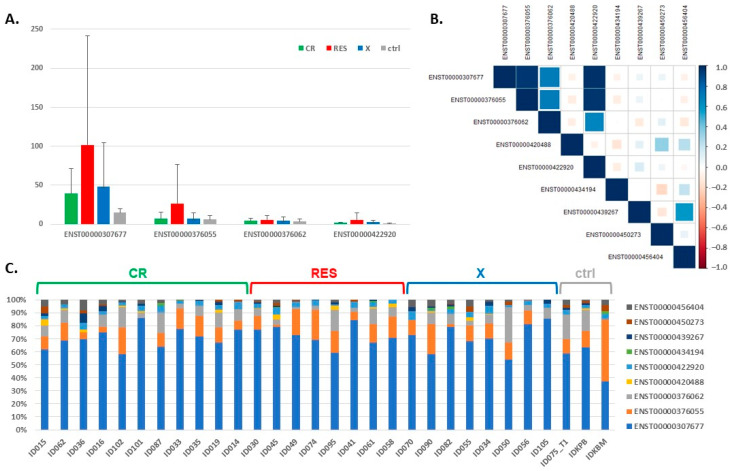
3.2. Expression of the BCL2L1 Gene Isoforms
Because BCL2L1 was the only gene specifically increased in RES and its function depends on the type of isoform generated as a result of alternative splicing [55], we compared the levels of expression of the BCL2L1 gene isoforms extracted from the RNA-seq data. Among nine isoforms identified, the expression of five was barely detectable (TPM ≤ 1, Table 3). Four isoforms with the highest expression level are presented in Figure 3A. Two of them, ENST00000307677 and ENST00000376062, encode a longer protein (BCL-XL, 233 aa), which acts as an apoptotic inhibitor, while the remaining two—a shorter one (BCL-XS, 170 aa)—acts as an apoptotic activator [55]. The expression of these four isoforms was highly correlated in individual patients (Spearman correlation coefficients 0.7–0.98) (Figure 3B). Isoform ENST00000307677, encoding an apoptosis inhibitor, contributed the most to the total expression of the BCL2L1 gene (Figure 3C). In AML, it was ~60–80%, with a mean of 71.3% (70.5% in CR, 72.4% in RES and 71.2% in the X group) (Table S5). In the control samples, this isoform was found in lower proportions (~40–60%, mean 53.2%), and the difference in the ENST00000307677 contribution to the total BCL2L1 expression was statistically significant between RES and ctrl, as well as between CR and ctrl (Wilcoxon rank sum exact test, p = 0.018 and p = 0.049, respectively).
Table 3.
Mean values of the expression levels of the BCL2L1 gene isoforms, extracted from the RNA-seq data.
| BCL2L1 Gene Isoform | Length of the Gene (Protein) | CR—Mean TPM (st. dev.) | RES—Mean TPM (st. dev.) | X—Mean TPM (st. dev.) | Ctrl—Mean TPM (st. dev.) |
|---|---|---|---|---|---|
| ENST00000307677 | 2574 bp (233 aa) | 39.14 (32.37) | 101.71 (140.27) | 48.01 (56.36) | 14.90 (4.68) |
| ENST00000376055 | 2496 bp (170 aa) | 7.05 (8.13) | 26.29 (50.13) | 6.89 (7.52) | 6.32 (5.18) |
| ENST00000376062 | 2578 bp (233 aa) | 4.49 (3.26) | 5.55 (5.80) | 4.67 (4.93) | 3.39 (2.95) |
| ENST00000420488 | 2444 bp (233 aa) | 0.41 (0.45) | 0.75 (0.88) | 0.22 (0.26) | 0.11 (0.19) |
| ENST00000422920 | 2227 bp (170 aa) | 1.82 (0.91) | 5.89 (8.84) | 2.44 (2.62) | 0.97 (0.15) |
| ENST00000434194 | 2441 bp (233 aa) | 0.10 (0.25) | 0.11 (0.19) | 0.23 (0.45) | 0.16 (0.28) |
| ENST00000439267 | 2426 bp (233 aa) | 0.48 (0.53) | 0.48 (0.51) | 0.63 (0.67) | 0.25 (0.22) |
| ENST00000450273 | 1292 bp (307 aa) | 0.35 (0.50) | 0.51 (0.52) | 0.48 (0.59) | 0.76 (0.40) |
| ENST00000456404 | 2562 bp (233 aa) | 0.97 (0.63) | 0.72 (0.83) | 1.20 (0.95) | 0.87 (0.47) |
Legend: CR—complete remission; RES—resistance to therapy; X—death during therapy; SD—standard deviation; TPM—transcripts per million; st. dev.—standard deviation. Dominant gene isoforms presented in Figure 3 are in bold.
Figure 3.
The expression of the BCL2L1 gene isoforms, extracted from the RNA-seq data. (A) The expression levels of four dominant isoforms in AML and control samples, with AML divided into three groups with different response to therapy; (B) the graphical presentation of Spearman correlation of the expression calculated for each pair of the BCL2L1 gene isoforms detected by the RNA-seq. The color intensity and size of the squares are proportional to the correlation coefficients. (C) The contribution of particular BCL2L1 isoforms to the total expression of the BCL2L1 gene in AML and control samples.
3.3. Expression of Genes Encoding Proteins Interacting with BCL2L1
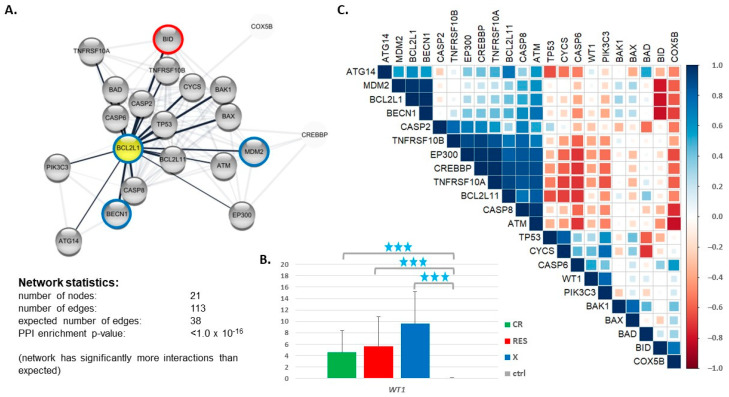
Looking for other factors which, in association with BCL2L1, may potentially contribute to AML therapy failure, we applied the STRING protein interaction database (https://string-db.org www.proteinatlas.org accessed on 7 March 2021) to generate a network of 20 proteins interacting with BCL2L1 (Figure 4A). The network included the BCL2 family members (BCL2L11, BAD, BAK1, BAX, BID) and other proteins engaged in the regulation of apoptosis and tumor development: caspases (CASP2, CASP6, CASP8); Tumor Protein P53 (TP53); tumor necrosis factor (TNF) receptor superfamily members (TNFRSF10A, TNFRSF10B); cytochromes (CYCS, COX5B); kinases (PIK3C3—Phosphatidylinositol 3-Kinase Catalytic Subunit Type 3 and ATM Serine/Threonine Kinase); autophagy related proteins (ATG14 and BECN1 (beclin 1)); transcription regulators with histone acetyltransferase activity (EP300 (E1A Binding Protein P300) and CREBBP (CREB Binding Protein)); and MDM2 Proto-Oncogene. Based on the literature data, we decided to also include the Wilms Tumor Protein (WT1), which was described as a mediator of TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) resistance in AML by regulating BCL2L1 [56]. However, according to STRING, WT1 does not interact directly with BCL2L1, but through TP53. The RNA-seq data revealed that the WT1 gene was highly upregulated in AML vs. ctrl, and the level of the WT1 expression presented an increasing trend from CR to X (Figure 4B), which confirm our earlier microarray-based results [43]. Any other gene from the BCL2L1-interacting network described above was differentially expressed in our dataset (Table S2).
Figure 4.
The expression of genes encoding proteins interacting with BCL2L1. (A) The network of 20 proteins interacting with BCL2L1 generated by STRING (https://string-db.org accessed on 7 March 2021); (B) WT1 gene expression in AML (divided into CR, RES and X groups) and ctrl, extracted from the RNA-seq data; (C) a graphical presentation of Spearman correlation of expression calculated for each pair of the genes encoding proteins interacting with BCL2L1, based on the RNA-seq data collected for RES AML patients. The color intensity and size of the squares are proportional to the correlation coefficients. The colored circles on the plot (A) indicate proteins encoded by the genes whose expression was significantly (p > 0.05) correlated with the expression of BCL2L1, blue circles mean a positive correlation and red—a negative one.
Correlation analysis of gene expression, performed exclusively for the RES group (Figure 4C, Table S6), revealed a cluster of strongly positively correlated genes, including BCL2L11, EP300, CREBBP, CASP8, ATM, TNFRSF10A, and TNFRSF10B. These genes were strongly negatively correlated with CASP6. BCL2L1 was highly positively correlated with two genes, namely BECN1 and proto-oncogene MDM2, and negatively correlated with BID. WT1 revealed a significant positive correlation only with PIK3C3. Other statistically significant positive correlations were noted for the following gene pairs: CASP2 & TNFRSF10B; TP53 & CYCS; CYCS & PIK3C3; BECN1 & ATM; BCL2L11 & ATG14; BAK1 & BAX, BID & COX5B. A statistically significant negative correlation was also found for CYCS & BAD.
3.4. Impact of the BCL2 Gene Family Expression Levels on the Patient Outcome
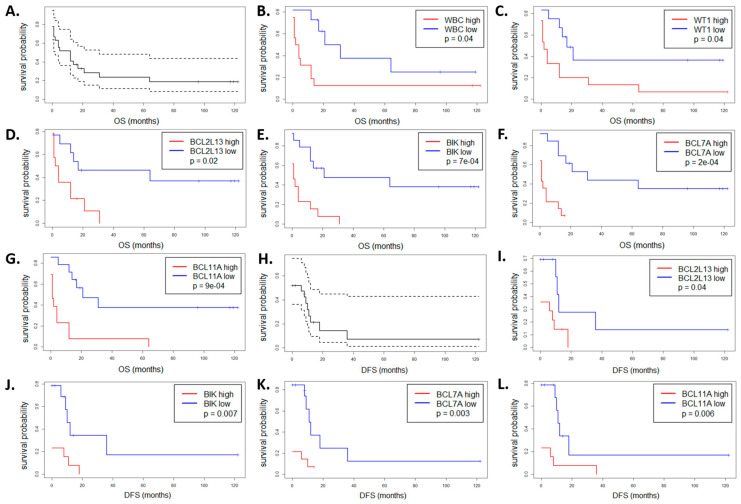
To associate the expression of genes from the BCL2 family, measured at the time of AML diagnosis, with disease prognosis, we performed a survival analysis with the use of Kaplan-Meier estimator. This type of retrospective analysis did not take into account patient response to the first induction therapy, but the time of disease-free and overall survival, which was monitored for up to 10 years after the first AML diagnosis. In the analyzed group of 27 AML patients, the median disease-free survival (DFS) and median overall survival (OS) were equal to 0 and 12 months, respectively (Figure 5A,H). As expected, high WBC count was associated with shorter OS and DFS (p = 0.04, Figure 5B and Figure S4F). Similarly, high level of the WT1 expression was associated with shorter OS (p = 0.04, Figure 5C).
Figure 5.
Overall survival (OS, A–G) and disease-free survival (DFS, H–L) of 27 AML patients as a whole group (A,H) and divided according to the WBC count (B) or the expression level of the following genes: WT1 (C); BCL2L13 (D,I); BIK (E,J); BCL7A (F,K); BCL11A (G,L). In each case, the blue curve means low level and the red curve high level of expression. Only the genes with statistically significant differences between the two curves are shown (the log-rank test p value threshold 0.05).
To analyze the impact of the BCL2 family gene expression levels on DFS and OS, we dichotomized samples based on the median level of a particular gene. The results indicated that the expression of seven genes may be associated with patient outcome, as presented in Figure 5 and Figure S4. In all cases except one (BCL2L1), high gene expression at the time of AML diagnosis was associated with worse prognosis. However, this effect was statistically significant for only two genes, namely BCL2L13 (p = 0.02 for OS and 0.04 for DFS, Figure 5D,I) and BIK (p = 7× 10−4 for OS and 0.007 for DFS, Figure 5E,J).
In addition, we performed a similar analysis for the members of other BCL families and found two genes whose expression levels were significantly associated with the patient outcome: BCL7A (p = 2× 10−4 for OS and 0.003 for DFS, Figure 5F,K) and BCL11A (p = 9 × 10−4 for OS and 0.006 for DFS, Figure 5G,L). They encode BAF chromatin remodeling complex subunits and are involved in lymphoma pathogenesis. Similarly as in the case of the BCL2 family members, high expression of these genes in AML at the time of diagnosis was associated with poor outcome.
3.5. Mutation Analysis of the BCL2 Family Genes and Genes Encoding Proteins Interacting with BCL2L1
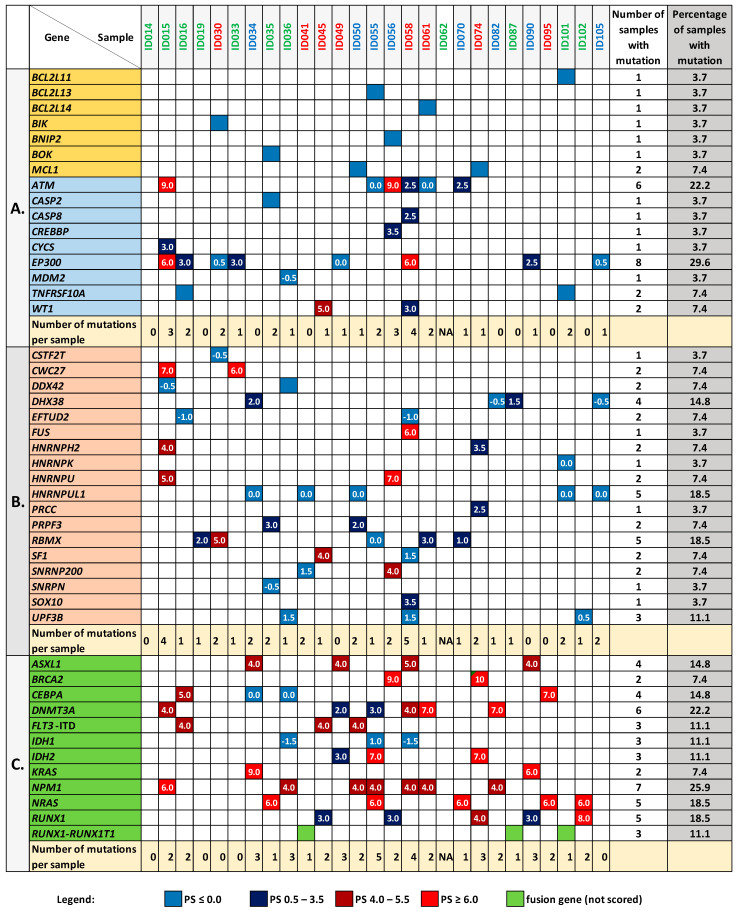
Taking advantage of exome sequencing, we explored the data collected in our laboratory for the analyzed sample set and checked whether any noteworthy mutation occurred in the BCL2 family genes and genes encoding proteins interacting with BCL2L1. The analysis was made with the use of eVai software (enGenome) and included only coding (except for synonymous) and splice variants that met the quality criteria described in the Methods section. Among the 26 BCL2 family members tested, we found single mutations in 7 genes: BCL2L11, BCL2L13, BCL2L14, BIK, BNIP2, BOK, and MCL1 (Figure 6A). MCL1 was the only gene mutated in two AML samples (ID050 and ID074), and in both cases, it was the same missense mutation (MCL1:c.680C>T; p.Ala227Val). This mutation, reported in the dbSNP database (rs11580946) with AF < 0.84% (GnomAD_exome), was classified by eVai software as a variant with uncertain significance (PaPI score: 0.8 (DAMAGING); PolyPhen-2: 0.303 (TOLERATED); DANN score: 0.99; SIFT: 0.02 (DAMAGING)). None of the mutations detected in the BCL2 family genes was classified as pathogenic, and none was assigned with any pathogenicity score (PS), which is a measure calculated by eVai based on a set of implemented variant classification criteria (Figure 6A, Table S7).
Figure 6.
The mutation patterns in the studied genes in the samples collected from patients at the time of AML diagnosis. (A) mutations in the BCL2 family members (yellow background) and genes encoding proteins interacting with BCL2L1 (blue background); (B) mutations in the genes encoding splicing-related proteins; (C) mutations in other genes, which are recurrently mutated in AML. The presence of a mutation is indicated by a colored square. The numbers inside squares reflect the pathogenicity scores (PS) estimated by eVai software (the higher PS, the more significant the mutation). If more than one mutation was detected in a sample, the highest PS value is presented. The lack of a number means no PS score assigned to a variant (PS NA). In panel (A), all genes with a mutation in at least one sample are shown, whereas in panels (B,C), only genes with PS-assigned mutations are presented. Tables S7 and S8 contain full lists of variants detected in the studied genes and analyzed samples.
With respect to the genes encoding proteins interacting with BCL2L1, 9 out of the 16 analyzed genes were mutated in at least one AML sample. In total, 35 different mutations were detected in these genes, including three which occurred in two samples. In contrast to the BCL2 family analysis, PS was not determined for only three variants here (Figure 6A, Table S7). The mutations in EP300 and ATM genes were the most significant (PS up to 6–9) and overrepresented, being detected in 8 (29.6%) and 6 (22.2%) samples, respectively. Mutations in WT1 and TNFRSF10A were present in two samples and the remaining genes (CASP2, CASP8, CREBBP, CYCS, MDM2) were mutated in only one sample. We identified no noteworthy mutation neither in ATG14, BECN1, CASP6, COX5B, PIK3C3, TNFRSF10B nor in TP53.
Notably, in one-third of AML samples (9 out of the 27 analyzed), we found no significant mutations neither in the BCL2 family members, nor in the genes encoding proteins interacting with them, and in another set of nine samples, only one mutation was noted (Figure 6A). The list of all the identified mutations is available in Table S7.
3.6. Mutation Analysis in the Genes Encoding Splicing-Associated Proteins
Dysregulation of proteins involved in apoptosis and oncogenesis may also be a consequence of splicing disruption, triggered by an uncontrolled overexpression of splicing factors or mutation of the genes which encode them. Therefore, we analyzed the mutational status of 188 genes implicated in the splicing. The list of genes was downloaded from the mRNA Splicing—Major Pathway (https://pathcards.genecards.org/Pathway/2004 accessed on 24 March 2021). Approximately half of the genes (98) were mutated in at least one AML sample and there was no sample without a mutation in the splicing-related genes (Figure S5). In total, 213 unique mutations were identified (Table S8); however, only 2 were pathogenic (frameshift variants in HNRNPU and CWC27 genes) and 3 were likely pathogenic (in CWC27, FUS and RBMX genes). 179 mutations had no PS at all. Figure 6B presents all the PS-attributed mutations which were identified in the 18 splicing-related genes. The most frequently mutated genes were RBMX and HNRNPUL1 (mutations detected in 5 samples), DHX38 (mutations detected in 4 samples), and UPF3B (mutations detected in 3 samples).
3.7. Relations between the BCL2 Family Gene Expression, Mutation Status, and Other Clinical Features
To associate the BCL2 family gene expression with the presence of particular mutations and other clinical features, we applied two types of analysis of variance. Only the mutations which occurred in at least two samples were taken into account. Apart from the mutations described above, we included also the mutations detected in the 12 genes recurrently mutated in AML (Figure 6C) in the analysis. A univariate analysis with two-way ANOVA revealed a total of 81 statistically significant (p value < 0.05) pair-wise relations between the expression levels of 25 BCL2 family members, the mutational status of 26 genes, and the following clinical features: AML FAB subtype, karyotype, tissue type, WBC count, the percentage of blasts in PB and BM, response to treatment (Table S9A).
MANOVA which included expression of all the BCL2 family members in one test, found a statistically significant relation to age (p = 0.02372) and mutational status of two genes, EP300 (p = 0.03293) and DDX42 (p = 0.006992) (Table S9B). Other statistically significant associations are presented in Table 4. The most significant relation was found between the expression of four genes (BCL2L1, BID, BOK, HRK) and the mutation status of two genes: BRCA2 (p = 4.145 × 10−10) and RUNX1 (p = 0.04287) (Figures S6 and S7). The overexpression of BOK, BCL2L1, BCL2L11, and MCL1 was also associated with the presence of the IDH2 mutation (p = 0.01203). It is not surprising, as the expression of three genes overrepresented in the above-mentioned tests, i.e., BCL2L1, BCL2L11 and BOK, was highly correlated in AML (Figure S2B). Notably, one sample (ID074) with an extremely high level of BCL2L1, was collected from a PB of a patient presenting mutations in BRCA2, IDH2, and RUNX1, and the lowest number of WBC in the whole group of the AML patients. Regarding single gene impact, the BCL2L1 expression was significantly associated only with the presence of the BRCA2 and IDH2 mutation (p = 1.179× 10−7 and 0.01512, respectively), the BCL2L11 expression with the IDH2 mutational status (p = 0.02728), the WBC count (0.00866) and the tissue type (0.01831), whereas the BOK expression—with the BRCA2 status (p = 6.211 × 10−5), the tissue type (0.0004571) and the IDH2 status (p = 0.0012835).
Table 4.
The associations between the expression levels of the BCL2 family genes and other factors, detected by MANOVA, in the studied AML patients. * p < 0.05, ** p < 0.01, *** p < 0.001.
| Expression of Genes | Associated Factor | p Value |
|---|---|---|
| BCL2L1, BID, BOK, HRK | BRCA2_status, RUNX1_status | 4.145 × 10−10 *** 0.04287 * |
| BCL2L1, BID, BOK, HRK | BRCA2 status | 2.106 × 10−9 *** |
| BCL2L1, BID, HRK | RUNX1 status | 0.0002187 *** |
| BCL2L10, BCL2L2, HRK | HNRNPU status | 8.038 × 10−7 *** |
| BCL2L1, BBC3, BOK, BCL2L10, BCL2L11 | HNRNPH2 status | 1.129 × 10−5 *** |
| BCL2L11, BCL2L13, BID, BIK, BNIP1 | WBC count | 6.459 × 10−5 *** |
| HRK, BAK1, BCL2A1, BID, BNIP1 | SNRNP200 status | 0.002168 ** |
| BCL2L10, BNIP2 | DDX42 status | 0.000545 *** |
| BCL2, BCL2A1, BCL2L10, BID, BNIP1, MCL1 | CWC27 status | 0.007744 ** |
| BMF, RTL10, BAX, BCL2L11, BCL2L2 | CEBPA status | 0.00383 ** |
| BCL2L13, BIK, PMAIP1 | RBMX status | 0.0004931 *** |
| MCL1, BCL2L11 | HNRNPUL1 status | 0.05703 |
| BCL2L2.PABPN1, BNIP2 | DHX38 status | 0.009772 ** |
| BCL2, BCL2L11, BCL2L12, BCL2L15 | EP300 status | 0.04793 * |
| BCL2L13, BCL2L2, BIK | ATM status | 0.003997 ** |
| BAX, BCL2A1, BNIP2 | KRAS status | 0.01254 * |
| BCL2L13, BCL2L2 | DNMT3A status | 0.02401 * |
| BNIP1, BCL2L2.PABPN1 | IDH1 status | 0.01978 * |
| BBC3, BCL2L1, BCL2L11, BOK, MCL1 | IDH2 status | 0.02641 * |
| BCL2L1, BOK |
IDH2 status, MCL1 status |
0.001286 ** 0.007325 ** |
| BCL2L1, BOK |
IDH2 status, MCL1 status, BRCA2 status |
1.755 × 10−5 *** 4.508 × 10−5 *** 4.624 × 10−6 *** |
| BCL2L1, BOK | MCL1 status | 0.001242 ** |
| BAD, BAK1, BCL2L11, BID, BNIP2, BNIP3 | RUNX1-RUNX1T1 status | 0.002101 ** |
| BCL2L13, BID, BIK, BNIP1 | NPM1 status | 0.02555 * |
| BAD, BAX, BCL2L1, BCL2L11, BCL2L13, BCL2L2.PABPN1, BID, BIK, BNIP1, BOK | Blast percentage in PB | 0.003099 ** |
| BCL2L1, BCL2L11, BCL2L15, BCL2L2.PABPN1, BID, BIK, BNIP1, BOK, HRK | Blast percentage in BM | 0.000261 *** |
| BAK1, BBC3, BCL2L1, BCL2L11, BCL2L12, BOK | Tissue type (PB, BM) | 0.0004833 *** |
| BCL2L15, BCL2L2, BNIP1, BNIP3 | FAB type (M1, M2) | 0.03326 * |
Other statistically significant associations were found between the expression of gene groups and FAB type or the presence of mutations in genes recurrently mutated in AML (NPM1, RUNX1, CEBPA, DNMT3A, KRAS), genes encoding proteins interacting with the BCL2 family (ATM, EP300) and genes encoding splicing-related proteins (RBMX, HNRNPU, HNRNPH2, HNRNPUL1, SNRNP200, DDX42, DHX38, CWC27) (Table 4). The results of all the tests performed are presented in Table S9. The selected examples are graphically presented in Figure 7.
Figure 7.
The expression patterns of selected BCL2 family genes in relation to (A) tissue type (PB or BM), (B) AML FAB type (M1 or M2), and mutation status of the following genes: (C) RUNX1-RUNX1T1 (fusion gene resulting from t(8;21)); (D) RBMX; (E) ATM; (F) EP300; (G) BRCA2; (H) IDH2; (I) RUNX1. On panels (A,D,G), the first sample from to the left is ID074.
4. Discussion
Apoptosis is a physiological process involved in the development of a multicellular organism and tissue renewal, e.g., hematopoiesis [10,57]. Relative levels of the BCL2 family members are critical in fine-tuning a cell’s fate [15,16,58] and change depending on the cell type and the stage of differentiation [59,60]. Although enhancing cell survival by BCL2 proteins usually does not affect cell proliferation and differentiation [61,62,63,64], a disturbed balance between pro-apoptotic and anti-apoptotic proteins often leads to tumorigenesis [8]. While excessive proliferation and blockade of differentiation are essential in the development of AML, inhibition of apoptosis is not negligible, particularly in refractory disease. Indeed, high BCL2 expression in the AML cells was detected at presentation, disease relapse, and treatment resistance [65,66]. To date, the BCL2 family research in AML has focused mainly on BCL2, and, though to a lesser extent, on MCL1 and BCL2L1. Nagy et al. [67] found that BCL2, BCL2A1, MCL1, and BAX were highly expressed in leukemia and lymphoma patients. Compared to healthy controls, BCL2L1 was underexpressed in chronic leukemias, but upregulated in mantle cell lymphoma and in some acute leukemia patients [67]. Here, we show that a dysregulation of the expression in AML concerns many other, although not all, BCL2 family members. Despite the general trend of upregulation of anti-apoptotic and downregulation of pro-apoptotic genes in AML, as compared to healthy controls, only a decrease in the genes encoding three pro-apoptotic proteins, i.e., BMF, BNIP1 and HRK, was statistically significant. This indicates the dominant role of the BH3-only-domain proteins in the apoptosis inhibition in AML. The main function of these proteins is activating BAX and BAX-like pro-apoptotic family members, and sequestering anti-apoptotic antagonists by forming heterodimers [68]. Therefore, insufficiency of BH3-only-domain proteins makes anti-apoptotic factors free and ready to act.
Comparing relative expression levels of particular family members, we found that in AML, the level of only three genes, MCL1, BAX, and BCL2L1, was high. MCL1 encodes one of the most essential proteins of the BCL2 family and its overexpression was associated with improved survival of hematopoietic stem cells in transgenic mice and immortalization of myeloid cell lineages [64]. Because BCL-XL and MCL-1 proteins appear to have complementary functions, acting by sequestering BAK [69], it is possible that the increased BCL2L1 observed here replaced the insufficiently expressed MCL1 to maintain anti-apoptotic activity in leukemic cells. Both genes revealed much higher expression than the expression of the BCL2 gene, the best known family member with an anti-apoptotic activity, commonly associated with drug resistance and considered a therapeutic target. The importance of BCL-XL in AML was perceived by Pallis et al. [70] who detected high levels of this protein in AML blasts with autonomous growth in vitro and associated it with poor response to therapy. Similarly, in our study, the BCL2L1 expression was increased in patients resistant to induction therapy, though it was highly variable between patients. This is consistent with the results of Kaufmann et al. [71] who found that protein levels of BCL2, MCL-1, BCL-XL, and BAX, measured at the time of AML or ALL diagnosis, varied over as much as a 40-fold range between individual patients.
BCL2L1 upregulation was found in different tumors [72,73,74,75,76,77]. In the study of a large cohort of colorectal cancers, BCL-XL, but not BCL2 or MCL-1, emerged as a highly active protein, and its transcript level was the highest among the anti-apoptotic BCL2 genes [77].
Functional studies of BCL2 family are impeded by the fact that the loss of Bcl-XL or Mcl-1 causes embryonic lethality in mice and the loss of Bcl2 reduces mice lifespan [78,79]. Bcl2a1 locus in mice is quadruplicated, which does not make it a perfect model in gene targeting studies, and the physiological role of this protein is still not completely understood. BCL2A1, regulated by the NF-kB transcription factor, is mainly expressed in the hematopoietic system, facilitating survival of leukocytes during their maturation and differentiation [80]. Here, BCL2A1 was the only anti-apoptotic family member, which was decreased in the majority of the AML samples. The lack of statistical significance was probably due to the high difference in BCL2A1 level between control PB and BM samples, reflected also in HPA data. In AML, BCL2A1 expression was much lower than in healthy BM but similar to that detected in healthy PB. The BCL2A1 decrease was accompanied by a decrease of the BID gene, encoding a BH3-only protein described as the BCL2A1 partner [80]. In accordance with the literature data, the expression of BCL2A1 was also highly correlated with the expression of two transcription factors, namely NFKB1 and NFKBIA (Figure S8).
Dysregulation of gene expression may also have a genetic background. Beroukhim et al. [81], studying genome-wide somatic copy-number alterations in a large collection of cancers, found frequent amplifications of the regions surrounding two anti-apoptotic genes, MCL1 and BCL2L1, and deletions containing two pro-apoptotic BCL2 family members, BOK and BBC3. Unfortunately, whole genome sequences of the AML patients studied here are not available to compare it. From the analysis of exome sequencing data, we concluded that the BCL2 family genes were rather not mutated in AML. Similarly, in the literature, small somatic mutations in the BCL2 family are noted occasionally [82,83,84,85,86,87]. Moreover, we did not detect mutations in the TP53 gene, encoding the tumor suppressor p53 protein, a regulator of cell cycle arrest or apoptosis upon DNA damage and cellular stress. In the studied samples, the mutations in genes recurrently mutated in AML, e.g., NPM1, DNMT3A, NRAS, RUNX1, ASXL1, ATM, CEBPA seemed to play a pivotal role in leukemogenesis, whereas changes in the BCL2 family gene expression probably played a secondary role. Recently, Bilbao-Sieyro et al. [88] reported a positive association between the absence of the NPM1 mutation and a higher BCL2 level. In our data, four other genes (pro-apoptotic BID, BIK, BNIP1, and BCL2L13 encoding a protein of controversial function) presented significantly higher expression in the NPM1-mutated samples. In consistence with our results, Gaidzik et al. [89] reported dysregulation of the apoptotic pathway, supported in particular by an increase in BCL2L1 expression, as a key feature of RUNX1-mutated AML. The presence of mutation in RUNX1 gene, encoding one of a crucial regulators of myelopoiesis, was associated by the authors with the presence of IDH1/IDH2 mutations and resistance to induction chemotherapy. In our data, the expression of three BCL2 family members (BCL2L1, BID and HRK) was correlated with RUNX1 mutation. BCL2L1 and HRK were upregulated whereas BID, encoding a pro-apoptotic BH3-only protein, was downregulated in RUNX1-mutated samples. BID inversely correlated with the level of antiapoptotic BCL2L1 in AML, in particular in RES. This suggests that RUNX1-mutation-driven apoptosis dysregulation is mainly based on increase of BCL2L1 and decrease of BID and both events contribute to treatment resistance. Other authors demonstrated that IDH1- and IDH2-mutated AML cells were highly dependent on the expression of BCL2 for their survival [90].
Furthermore, in line with our findings, Salmon et al. [91] depicted dysregulation of apoptosis-related genes as a possible consequence of the BRCA1- and BRCA2- mutations in breast cancer. Although solid tumors cells are believed to be more dependent on BCL-XL than hematologic malignancies, our results suggest that some AML cases, for example, BRCA2- or IDH2-mutated, may need this particular protein more than the other BCL2 family members for their survival. However, further research is advisable due to a small set of mutated samples studied here.
Another factor which may influence the BCL2 family gene expression is splicing, often impaired in malignancies [92,93]. Adamia et al. [94] proved that aberrant splicing was a common characteristic of AML and approximately 29% genes, encoding oncogenes, tumor suppressors, proteins implicated in splicing, apoptosis, cell cycle regulation, cell proliferation and differentiation, were aberrantly spliced in AML, as compared to normal CD34+ BM cells. Alternative splicing of BCL2 genes may result in the formation of protein isoforms exhibiting pro- or anti-apoptotic activity [18,55]. Based on the example of BCL2L1, we observed not only high variability of this gene expression in AML patients, but also different proportions of the BCL2L1 isoforms in AML and control samples. It was reported that the splicing events that control the expression of isoforms of the BCL2 family proteins were regulated by the Splicing Factor 3b Subunit 1 (SF3B1) [95]. The presence of mutations in the SF3B1 gene was confirmed in 3.8% and 48.9% of patients from the AMLCG cohort and AML patients with normal karyotype, respectively [96,97,98]. We did not detect any significant mutation in this gene in the group of AML patients studied here, but only three patients had a normal karyotype and in the case of half of the patients, the karyotype was unknown. Instead, we detected mutations in other genes encoding splicing-related proteins, e.g., RBMX, CWC27, HNRNPH2 or HNRPNHU, and correlated their presence with the expression of the selected BCL2 family members.
Regardless of the causes of the altered gene expression, the protein is the executive factor. Unfortunately, the BCL2 family protein-level data are limited and usually based on immunodetection in cells or tissues. According to the Human Protein Atlas (www.proteinatlas.org accessed 5 June 2021), only two family members (BID and BAX) were detected in blood by mass spectrometry. In BM samples, high protein level was noted only for BCL2 and BNIP2 and this does not correlate with the RNA levels (Table S4). The BM level of 13 proteins from BCL2 family was described in HPA as medium, 5 as low and six other proteins were not detected or have no data. In one of our earlier studies [99], we analyzed PB and BM proteomes of AML patients studied here, and no member of the BCL2 family was detected. Another problem is the BCL2 family proteins do not act alone, but are implicated in interactions with the family members and other partners, influenced also by their cellular localizations [100].
In the context of therapy, studies on the BCL2 family are of particular importance. In the light of numerous reports cited above, it seems that AML resistance to induction/consolidation therapy is a result of a complex network of protein interactions, in particular between the pro-survival and pro-death BCL2 family members. It has been shown that BCL-XL is about 10 times more efficient than BCL2 in preventing doxorubicin-induced apoptosis [101]. Inhibition of BCL-XL induced apoptosis and enhanced the effectiveness of chemotherapeutic agents in colorectal cancer cell lines [77]. Recently, a number of therapeutics targeting the BCL2 family has already been introduced into treatment or is now under investigation. One of the first evaluated drugs was ABT-737 (Venetoclax, BCL2-specific BH3 mimetic), a small molecule with high binding affinity to BCL-XL, BCL2, and BCL-W [102,103]. However, some inhibitors may elicit undesirable side-effects, as reported for the BCL-XL inhibition on platelets [60]. This is why future targeted therapies should be directed at specific targets and particular cell types, for example, leukemic cells and leukemic stem cells. In our opinion, a complex analysis of the impact of the BCL2 family proteins, also on transcript levels, on the AML pathogenesis should precede the introduction of a specific therapy.
Another aspect of the BCL2 family expression studies is connected with prognosis. Initial reports confirmed worse progression-free and overall survival of AML patients with high BCL2 expression in BM leukemic cells [104,105]. Del Poeta et al. [106] showed that a high BAX/BCL2 ratio level in the blast cells of de novo AML patients treated with standard induction and consolidation therapy was associated with a longer overall survival and disease-free survival. In addition, recently published data indicate that a high BCL2 mRNA level after induction therapy or at complete hematologic remission negatively affects the AML outcome [88]. On the contrary, low BCL2 level in the leukemic cells may help to identify patients with a favorable prognosis. It was shown that the absence of the BCL2 expression characterized a subgroup of AML patients with distinct molecular and clinical characteristics, including low BM blast percentage, low WT1 expression, underrepresentation of FLT3 mutations, positive response to chemotherapy, and better OS [107]. However, we did not observe a correlation between the BCL2 expression and OS. A similar conclusion was drawn by Zhou et al. [27] who noted an upregulation of BCL2 in AML, but without any impact on the patient outcome. Bilbao-Sieyro et al. [88] documented a marginal association between a high BCL2 level at the time of AML diagnosis and worse progression-free survival, but not with OS. The association with the negative outcome was stronger when a high BCL2 level was detected directly after induction therapy or at CR. Our results indicate a higher impact of other genes of the BCL2 family, in particular BIK and BCL2L13, on the AML patient outcome. Unexpectedly, BCL2L1 was the only gene which presented inverse correlation (low level associated with worse prognosis). Similar observation was made by Andreeff et al. [108] who associated low BCL2 content with poor survival in a group of patients with poor prognosis cytogenetics.
Summarizing, the following conclusion may be drawn from the literature data and results presented here: overexpression of anti-apoptotic BCL2 family genes and underexpression of pro-apoptotic genes may facilitate and accelerate tumor development, and support tumor cell viability, but it is rather not the main driver of oncogenesis [109]. Although the gene expression results cannot be directly transposed to protein-level, they may be particularly useful in designing further research as well as diagnostic and therapeutic strategies.
5. Conclusions
In conclusion, the comprehensive analysis of gene expression data presented here revealed a complex dysregulation of the BCL2 family in AML. Correlations with different factors, including other gene expressions, and the presence of mutations or patient outcome, was found for a number of the BCL2 family members, both, pro- and anti-apoptotic. Definitely, factors other than gene expression and mutational status contribute to the BCL2 family function—posttranslational modifications, intracellular localization, stabilization, affinities, and interactions with other proteins. Despite this, gene expression studies undoubtedly contribute to the research on basic cellular processes in which the BCL2 family is engaged. Moreover, gene expression studies have a high potential of translation to clinical practice. First of all, they may support the selection of treatment targets. Secondly, post-treatment monitoring of the BCL2 family gene expression should allow the estimation of treatment efficiency. In addition, high differences in the gene expression which we observed between individual patients suggested that not all of them might benefit from a BCL2-targeted therapy. In the end, the level of expression of particular genes from the BCL2 family may predict the patient outcome at the time of AML diagnosis.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13133175/s1, Figure S1: Expression levels of 26 genes from the BCL2 family in AML (27 patient samples from this study (TS)) versus control samples (ctrl) which included controls from this study and the additional controls from Human Protein Atlas (HPA), Figure S2: Graphical presentation of Spearman correlation of gene expression calculated for each pair of the genes encoding BCL2 family proteins, based on RNA-seq data collected for control samples (A,C) and AML patients as a whole group (B,D), Figure S3: Graphical presentation of Spearman correlation of gene expression calculated for each pair of the genes encoding BCL2 family proteins, based on RNA-seq data collected for AML divided into three subgroups according to the response to therapy: CR (A,D), RES (B,E) and X (C,F), Figure S4: Overall survival (OS, A–E) and disease free survival (DFS, F-L) of 27 AML patients divided according to the WBC count (F) or the expression level of the following genes: BCL2L2 (A,H); BCL2L12 (B,I); BAX (C,J); BID (D,K); BCL2L1 (E,L); WT1 (G), Figure S5: The mutation pattern in genes encoding proteins from mRNA Splicing-Major Pathway, Figure S6: The graphical presentation of BCL2 family genes whose expression was associated with RUNX1 mutation status by MANOVA, Figure S7: The graphical presentation of BCL2 family genes whose expression was associated with BRCA2 mutation status by MANOVA, Figure S8: The levels of expression of BCL2A1 gene, NFKB1 and NFKBIA genes encoding two transcription factors which are described to regulate BCL2A1 expression, and BID gene, encoding BH3-only partner of BCL2A1 protein, Table S1: The list of samples included into analysis, Table S2: Results of differential expression analysis of genes from BCL2 family (yellow background) and genes encoding proteins interacting with BCL2 family (blue background), Table S3: Expression levels (in TPM) of genes from BCL2 family (yellow background) and genes encoding proteins interacting with BCL2 family (blue background) in particular samples, Table S4: Data extracted from the Human Protein Atlas, Table S5: Isoforms of BCL2L1 gene, Table S6: Correlation of expression levels (in TPM) of BCL2L1 gene and the genes encoding proteins interacting with BLC2L1, indicated by STRING (https://string-db.org, accessed on 2 February 2019), in AML samples resistant to therapy (RES), Table S7: Mutations identified in BCL2 family genes and the genes encoding proteins interacting with BCL2L1, annotated with the use of eVai software (www.engenome.com/evai/, accessed on 2 February 2019), Table S8: Mutations identified in BCL2 family genes and the genes encoding proteins interacting with BCL2L1, annotated with the use of eVai software (www.engenome.com/evai/, accessed on 2 February 2019), Table S9: Results of uni- and multivariate analysis of relations between the levels of BCL2 family gene expression and other features.
Author Contributions
Conceptualization, L.H. and K.L.; Data curation, L.H. and P.W.; Formal analysis, L.H.; Funding acquisition, L.H.; Investigation, L.H. and P.W.; Methodology, L.H. and P.W.; Project administration, L.H.; Resources, M.K. and K.L.; Supervision, L.H. and K.L.; Visualization, L.H.; Writing—original draft, L.H. and K.L.; Writing—review & editing, L.H., P.W. and K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Science Centre (grant no. 2017/01/X/NZ2/01906) and the Polish Ministry of Science and Higher Education (grant no. PBZ-MNiI-2/1/2005 and the SPUB (5536/E-63/SPUB/2017/1) program). This research was also supported by PLGrid Infrastructure.
Institutional Review Board Statement
The study conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki. Appropriate approval was also obtained from the Bioethical Commission of Poznań University of Medical Sciences on day 5 January 2006 (ethic code 34/06).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Döhner H., Weisdorf D.J., Bloomfield C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015;373:1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 2.De Kouchkovsky I., Abdul-Hay M. Acute myeloid leukemia: A comprehensive review and 2016 update. Blood Cancer J. 2016;6:e441. doi: 10.1038/bcj.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deschler B., Lübbert M. Acute myeloid leukemia: Epidemiology and etiology. Cancer. 2006;107:2099–2107. doi: 10.1002/cncr.22233. [DOI] [PubMed] [Google Scholar]
- 4.Estey E., Döhner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrara F., Schiffer C.A. Acute myeloid leukaemia in adults. Lancet. 2013;381:484–495. doi: 10.1016/S0140-6736(12)61727-9. [DOI] [PubMed] [Google Scholar]
- 7.Handschuh L. Not Only Mutations Matter: Molecular Picture of Acute Myeloid Leukemia Emerging from Transcriptome Studies. J. Oncol. 2019;2019:1–36. doi: 10.1155/2019/7239206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerr J.F.R., Wyllie A.H., Currie A.R. Apoptosis: A Basic Biological Phenomenon with Wideranging Implications in Tissue Kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKenna S.L., Cotter T.G. Functional Aspects of Apoptosis in Hematopoiesis and Consequences of Failure. Adv. Cancer Res. 1997;71:121–164. doi: 10.1016/s0065-230x(08)60098-6. [DOI] [PubMed] [Google Scholar]
- 11.Reed J.C., Pellecchia M. Apoptosis-based therapies for hematologic malignancies. Blood. 2005;106:408–418. doi: 10.1182/blood-2004-07-2761. [DOI] [PubMed] [Google Scholar]
- 12.Yip K.W., Reed J.C. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–6406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- 13.Hatok J., Racay P. Bcl-2 family proteins: Master regulators of cell survival. Biomol. Concepts. 2016;7:259–270. doi: 10.1515/bmc-2016-0015. [DOI] [PubMed] [Google Scholar]
- 14.Kale J., Osterlund E.J., Andrews D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018;25:65–80. doi: 10.1038/cdd.2017.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardwick J.M., Soane L. Multiple Functions of BCL-2 Family Proteins. Cold Spring Harb. Perspect. Biol. 2013;5:a008722. doi: 10.1101/cshperspect.a008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Risberg K., Redalen K.R., Sønstevold L., Bjørnetrø T., Sølvernes J., Ree A.H. Pro-survival responses to the dual inhibition of anti-apoptotic Bcl-2 family proteins and mTOR-mediated signaling in hypoxic colorectal carcinoma cells. BMC Cancer. 2016;16:531. doi: 10.1186/s12885-016-2600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czabotar P.E., Lessene G., Strasser A., Adams J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 18.Akgul C., Moulding D.A., Edwards S.W. Alternative splicing of Bcl-2-related genes: Functional consequences and potential therapeutic applications. Cell. Mol. Life Sci. 2004;61:2189–2199. doi: 10.1007/s00018-004-4001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed J.C. Bcl-2 family proteins. Oncogene. 1998;17:3225–3236. doi: 10.1038/sj.onc.1202591. [DOI] [PubMed] [Google Scholar]
- 20.Delbridge A.R.D., Grabow S., Strasser A., Vaux D.L. Thirty years of BCL-2: Translating cell death discoveries into novel cancer therapies. Nat. Rev. Cancer. 2016;16:99–109. doi: 10.1038/nrc.2015.17. [DOI] [PubMed] [Google Scholar]
- 21.Popgeorgiev N., Jabbour L., Gillet G. Subcellular Localization and Dynamics of the Bcl-2 Family of Proteins. Front. Cell Dev. Biol. 2018;6:13. doi: 10.3389/fcell.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juin P., Geneste O., Gautier F., Depil S., Campone M. Decoding and unlocking the BCL-2 dependency of cancer cells. Nat. Rev. Cancer. 2013;13:455–465. doi: 10.1038/nrc3538. [DOI] [PubMed] [Google Scholar]
- 23.Niu X., Zhao J., Ma J., Xie C., Edwards H., Wang G., Caldwell J.T., Xiang S., Zhang X., Chu R., et al. Binding of Released Bim to Mcl-1 is a Mechanism of Intrinsic Resistance to ABT-199 which can be Overcome by Combination with Daunorubicin or Cytarabine in AML Cells. Clin. Cancer Res. 2016;22:4440–4451. doi: 10.1158/1078-0432.CCR-15-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campos L., Rouault J.P., Sabido O., Oriol P., Roubi N., Vasselon C., Archimbaud E., Magaud J.P., Guyotat D. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 1993;81:3091–3096. doi: 10.1182/blood.V81.11.3091.3091. [DOI] [PubMed] [Google Scholar]
- 25.Bensi L., Longo R., Vecchi A., Messora C., Garagnani L., Bernardi S., Tamassia M.G., Sacchi S. Bcl-2 oncoprotein expression in acute myeloid leukemia. Haematologica. 1995;80:98–102. [PubMed] [Google Scholar]
- 26.Lauria F., Raspadori D., Rondelli D., Ventura M., Fiacchini M., Visani G., Forconi F., Tura S. High bcl-2 expression in acute myeloid leukemia cells correlates with CD34 positivity and complete remission rate. Leukemia. 1997;11:2075–2078. doi: 10.1038/sj.leu.2400854. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J., Zhang T., Xu Z., Gu Y., Ma J., Li X., Guo H., Wen X., Zhang W., Yang L., et al. BCL2 overexpression: Clinical implication and biological insights in acute myeloid leukemia. Diagn. Pathol. 2019;14:68. doi: 10.1186/s13000-019-0841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell K.J., Tait S.W.G. Targeting BCL-2 regulated apoptosis in cancer. Open Biol. 2018;8:180002. doi: 10.1098/rsob.180002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lessene G., Czabotar P.E., Colman P.M. BCL-2 family antagonists for cancer therapy. Nat. Rev. Drug Discov. 2008;7:989–1000. doi: 10.1038/nrd2658. [DOI] [PubMed] [Google Scholar]
- 30.Malpas J., Mathé G., Hayat M. Comparison of the effects of cytosine arabinoside, cytosine arabinoside and thioguanine, and cytosine arabinoside and daunorubicin in acute myeloid leukemia. Bull. Cancer. 1974;61:411–418. [PubMed] [Google Scholar]
- 31.Lichtman M.A. A historical perspective on the development of the cytarabine (7days) and daunorubicin (3days) treatment regimen for acute myelogenous leukemia: 2013 the 40th anniversary of 7+3. Blood Cells. Mol. Dis. 2013;50:119–130. doi: 10.1016/j.bcmd.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Preliminary results of a trial on acute myeloid leukemia comparing the effect of cytosine arabinoside, the combination of cytosine arabinoside and thioguanine and the combination of cytosine arabinoside and daunorubicine. Recent Results Cancer Res. Fortschritte Krebsforsch. Progres Dans Rech. Sur Cancer. 1973;43:165–168. [PubMed] [Google Scholar]
- 33.Bose P., Vachhani P., Cortes J.E. Treatment of Relapsed/Refractory Acute Myeloid Leukemia. Curr. Treat. Options Oncol. 2017;18:17. doi: 10.1007/s11864-017-0456-2. [DOI] [PubMed] [Google Scholar]
- 34.Rowe J.M., Tallman M.S. How I treat acute myeloid leukemia. Blood. 2010;116:3147–3156. doi: 10.1182/blood-2010-05-260117. [DOI] [PubMed] [Google Scholar]
- 35.Rowe J.M. Will new agents impact survival in AML? Best Pract. Res. Clin. Haematol. 2019;32:101094. doi: 10.1016/j.beha.2019.101094. [DOI] [PubMed] [Google Scholar]
- 36.Dlamini Z., Shoba B., Hull R. Splicing machinery genomics events in acute myeloid leukaemia (AML): In search for therapeutic targets, diagnostic and prognostic biomarkers. Am. J. Cancer Res. 2020;10:2690–2704. [PMC free article] [PubMed] [Google Scholar]
- 37.Stone R.M., Mandrekar S.J., Sanford B.L., Laumann K., Geyer S., Bloomfield C.D., Thiede C., Prior T.W., Döhner K., Marcucci G., et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017;377:454–464. doi: 10.1056/NEJMoa1614359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perl A.E., Martinelli G., Cortes J.E., Neubauer A., Berman E., Paolini S., Montesinos P., Baer M.R., Larson R.A., Ustun C., et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3 -Mutated AML. N. Engl. J. Med. 2019;381:1728–1740. doi: 10.1056/NEJMoa1902688. [DOI] [PubMed] [Google Scholar]
- 39.DiNardo C.D., Stein E.M., de Botton S., Roboz G.J., Altman J.K., Mims A.S., Swords R., Collins R.H., Mannis G.N., Pollyea D.A., et al. Durable Remissions with Ivosidenib in IDH1 -Mutated Relapsed or Refractory AML. N. Engl. J. Med. 2018;378:2386–2398. doi: 10.1056/NEJMoa1716984. [DOI] [PubMed] [Google Scholar]
- 40.Stein E.M., DiNardo C.D., Pollyea D.A., Fathi A.T., Roboz G.J., Altman J.K., Stone R.M., DeAngelo D.J., Levine R.L., Flinn I.W., et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130:722–731. doi: 10.1182/blood-2017-04-779405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei A.H., Tiong I.S. Midostaurin, enasidenib, CPX-351, gemtuzumab ozogamicin, and venetoclax bring new hope to AML. Blood. 2017;130:2469–2474. doi: 10.1182/blood-2017-08-784066. [DOI] [PubMed] [Google Scholar]
- 42.Choi J.H., Bogenberger J.M., Tibes R. Targeting Apoptosis in Acute Myeloid Leukemia: Current Status and Future Directions of BCL-2 Inhibition with Venetoclax and Beyond. Target. Oncol. 2020;15:147–162. doi: 10.1007/s11523-020-00711-3. [DOI] [PubMed] [Google Scholar]
- 43.Handschuh L., Kaźmierczak M., Milewski M., Goralski M., Łuczak M., Wojtaszewska M., Uszczyńska-Ratajczak B., Lewandowski K., Komarnicki M., Figlerowicz M. Gene expression profiling of acute myeloid leukemia samples from adult patients with AML-M1 and -M2 through boutique microarrays, real-time PCR and droplet digital PCR. Int. J. Oncol. 2018;52:656–678. doi: 10.3892/ijo.2017.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Handschuh L., Wojciechowski P., Kazmierczak M., Marcinkowska-Swojak M., Luczak M., Lewandowski K., Komarnicki M., Blazewicz J., Figlerowicz M., Kozlowski P. NPM1 alternative transcripts are upregulated in acute myeloid and lymphoblastic leukemia and their expression level affects patient outcome. J. Transl. Med. 2018;16:232. doi: 10.1186/s12967-018-1608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Döhner H., Estey E.H., Amadori S., Appelbaum F.R., Büchner T., Burnett A.K., Dombret H., Fenaux P., Grimwade D., Larson R.A., et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 46.Cheson B.D., Bennett J.M., Kopecky K.J., Büchner T., Willman C.L., Estey E.H., Schiffer C.A., Doehner H., Tallman M.S., Lister T.A., et al. Revised Recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J. Clin. Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 47.de Greef G.E., van Putten W.L.J., Boogaerts M., Huijgens P.C., Verdonck L.F., Vellenga E., Theobald M., Jacky E., Lowenberg B., The Dutch-Belgian Hemato-Oncology Co-operative Group HOVON et al. Criteria for defining a complete remission in acute myeloid leukaemia revisited. An analysis of patients treated in HOVON-SAKK co-operative group studies. Br. J. Haematol. 2005;128:184–191. doi: 10.1111/j.1365-2141.2004.05285.x. [DOI] [PubMed] [Google Scholar]
- 48.Bloomfield C.D., Estey E., Pleyer L., Schuh A.C., Stein E.M., Tallman M.S., Wei A. Time to repeal and replace response criteria for acute myeloid leukemia? Blood Rev. 2018;32:416–425. doi: 10.1016/j.blre.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li B., Dewey C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., Garimella K., Altshuler D., Gabriel S., Daly M., et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu D., Singh S., Chen X., Zheng Z., Huang J., Lin T., Li H. The landscape of chimeric RNAs in bladder urothelial carcinoma. Int. J. Biochem. Cell Biol. 2019;110:50–58. doi: 10.1016/j.biocel.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 54.Del Gaizo Moore V., Brown J.R., Certo M., Love T.M., Novina C.D., Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J. Clin. Invest. 2007;117:112–121. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boise L.H., González-García M., Postema C.E., Ding L., Lindsten T., Turka L.A., Mao X., Nuñez G., Thompson C.B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-N. [DOI] [PubMed] [Google Scholar]
- 56.Bansal H., Seifert T., Bachier C., Rao M., Tomlinson G., Iyer S.P., Bansal S. The Transcription Factor Wilms Tumor 1 Confers Resistance in Myeloid Leukemia Cells against the Proapoptotic Therapeutic Agent TRAIL (Tumor Necrosis Factor α-related Apoptosis-inducing Ligand) by Regulating the Antiapoptotic Protein Bcl-xL. J. Biol. Chem. 2012;287:32875–32880. doi: 10.1074/jbc.C112.366559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Domen J. The Role of Apoptosis in Regulating Hematopoiesis and Hematopoietic Stem Cells. Immunol. Res. 2000;22:83–94. doi: 10.1385/IR:22:2-3:83. [DOI] [PubMed] [Google Scholar]
- 58.Lin J.-C., Tsao M.-F., Lin Y.-J. Differential Impacts of Alternative Splicing Networks on Apoptosis. Int. J. Mol. Sci. 2016;17:2097. doi: 10.3390/ijms17122097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cory S. Regulation of Lymphocyte Survival by the BCL-2 Gene Family. Annu. Rev. Immunol. 1995;13:513–543. doi: 10.1146/annurev.iy.13.040195.002501. [DOI] [PubMed] [Google Scholar]
- 60.Vikström I.B., Slomp A., Carrington E.M., Moesbergen L.M., Chang C., Kelly G.L., Glaser S.P., Jansen J.H.M., Leusen J.H.W., Strasser A., et al. MCL-1 is required throughout B-cell development and its loss sensitizes specific B-cell subsets to inhibition of BCL-2 or BCL-XL. Cell Death Dis. 2016;7:e2345. doi: 10.1038/cddis.2016.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaux D.L., Cory S., Adams J.M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 62.Tsujimoto Y. Overexpression of the human BCL-2 gene product results in growth enhancement of Epstein-Barr virus-immortalized B cells. Proc. Natl. Acad. Sci. USA. 1989;86:1958–1962. doi: 10.1073/pnas.86.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fairbairn L.J., Cowling G.J., Reipert B.M., Dexter T.M. Suppression of apoptosis allows differentiation and development of a multipotent hemopoietic cell line in the absence of added growth factors. Cell. 1993;74:823–832. doi: 10.1016/0092-8674(93)90462-Y. [DOI] [PubMed] [Google Scholar]
- 64.Zhou P., Qian L., Bieszczad C.K., Noelle R., Binder M., Levy N.B., Craig R.W. Mcl-1 in transgenic mice promotes survival in a spectrum of hematopoietic cell types and immortalization in the myeloid lineage. Blood. 1998;92:3226–3239. doi: 10.1182/blood.V92.9.3226. [DOI] [PubMed] [Google Scholar]
- 65.Maung Z.T., MacLean F.R., Reid M.M., Pearson A.D.J., Proctor S.J., Hamilton P.J., Hall A.G. The relationship between bcl-2 expression and response to chemotherapy in acute leukaemia. Br. J. Haematol. 1994;88:105–109. doi: 10.1111/j.1365-2141.1994.tb04984.x. [DOI] [PubMed] [Google Scholar]
- 66.Bincolettu C., Saad S.T.O., Silva E.S., Queiroz M.L.S. Haematopoietic response and bcl-2 expression in patients with acute myeloid leukaemia. Eur. J. Haematol. 2009;62:38–42. doi: 10.1111/j.1600-0609.1999.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 67.Nagy B., Lundán T., Larramendy M.L., Aalto Y., Zhu Y., Niini T., Edgren H., Ferrer A., Vilpo J., Elonen E., et al. Abnormal expression of apoptosis-related genes in haematological malignancies: Overexpression of MYC is poor prognostic sign in mantle cell lymphoma. Br. J. Haematol. 2003;120:434–441. doi: 10.1046/j.1365-2141.2003.04121.x. [DOI] [PubMed] [Google Scholar]
- 68.Bouillet P., Strasser A. BH3-only proteins—Evolutionarily conserved proapoptotic Bcl-2 family members essential for initiating programmed cell death. J. Cell Sci. 2002;115:1567–1574. doi: 10.1242/jcs.115.8.1567. [DOI] [PubMed] [Google Scholar]
- 69.Willis S.N. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pallis M., Zhu Y.-M., Russell N. Bcl-xL is heterogeneously expressed by acute myeloblastic leukaemia cells and is associated with autonomous growth in vitro and with P-glycoprotein expression. Leukemia. 1997;11:945–949. doi: 10.1038/sj.leu.2400705. [DOI] [PubMed] [Google Scholar]
- 71.Kaufmann S.H., Karp J.E., Svingen P.A., Krajewski S., Burke P.J., Gore S.D., Reed J.C. Elevated expression of the apoptotic regulator Mcl-1 at the time of leukemic relapse. Blood. 1998;91:991–1000. doi: 10.1182/blood.V91.3.991.991_991_1000. [DOI] [PubMed] [Google Scholar]
- 72.Adams C.M., Mitra R., Vogel A.N., Liu J., Gong J.Z., Eischen C.M. Targeting BCL-W and BCL-XL as a therapeutic strategy for Hodgkin lymphoma. Leukemia. 2020;34:947–952. doi: 10.1038/s41375-019-0611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leiter U., Schmid R.M., Kaskel P., Peter R.U., Krähn G. Antiapoptotic bcl-2 and bcl-xL in advanced malignant melanoma. Arch. Dermatol. Res. 2000;292:225–232. doi: 10.1007/s004030050479. [DOI] [PubMed] [Google Scholar]
- 74.Liu J.R., Fletcher B., Page C., Hu C., Nunez G., Baker V. Bcl-xLIs Expressed in Ovarian Carcinoma and Modulates Chemotherapy-Induced Apoptosis. Gynecol. Oncol. 1998;70:398–403. doi: 10.1006/gyno.1998.5125. [DOI] [PubMed] [Google Scholar]
- 75.España L., Fernández Y., Rubio N., Torregrosa A., Blanco J., Sierra A. Overexpression of Bcl-xL in Human Breast Cancer Cells Enhances Organ-Selective Lymph Node Metastasis. Breast Cancer Res. Treat. 2004;87:33–44. doi: 10.1023/B:BREA.0000041579.51902.89. [DOI] [PubMed] [Google Scholar]
- 76.Castilla C., Congregado B., Chinchón D., Torrubia F.J., Japón M.A., Sáez C. Bcl-xL Is Overexpressed in Hormone-Resistant Prostate Cancer and Promotes Survival of LNCaP Cells via Interaction with Proapoptotic Bak. Endocrinology. 2006;147:4960–4967. doi: 10.1210/en.2006-0502. [DOI] [PubMed] [Google Scholar]
- 77.Scherr A.-L., Mock A., Gdynia G., Schmitt N., Heilig C.E., Korell F., Rhadakrishnan P., Hoffmeister P., Metzeler K.H., Schulze-Osthoff K., et al. Identification of BCL-XL as highly active survival factor and promising therapeutic target in colorectal cancer. Cell Death Dis. 2020;11:875. doi: 10.1038/s41419-020-03092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sochalska M., Tuzlak S., Egle A., Villunger A. Lessons from gain- and loss-of-function models of pro-survival Bcl2 family proteins: Implications for targeted therapy. FEBS J. 2015;282:834–849. doi: 10.1111/febs.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rinkenberger J.L., Horning S., Klocke B., Roth K., Korsmeyer S.J. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 2000;14:23–27. [PMC free article] [PubMed] [Google Scholar]
- 80.Vogler M. BCL2A1: The underdog in the BCL2 family. Cell Death Differ. 2012;19:67–74. doi: 10.1038/cdd.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beroukhim R., Mermel C.H., Porter D., Wei G., Raychaudhuri S., Donovan J., Barretina J., Boehm J.S., Dobson J., Urashima M., et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Correia C., Schneider P.A., Dai H., Dogan A., Maurer M.J., Church A.K., Novak A.J., Feldman A.L., Wu X., Ding H., et al. BCL2 mutations are associated with increased risk of transformation and shortened survival in follicular lymphoma. Blood. 2015;125:658–667. doi: 10.1182/blood-2014-04-571786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamaguchi H., Inokuchi K., Tarusawa M., Dan K. Mutation of bcl-x gene in non-Hodgkin’s lymphoma. Am. J. Hematol. 2002;69:74–76. doi: 10.1002/ajh.10030. [DOI] [PubMed] [Google Scholar]
- 84.Park H., Cho S.-Y., Kim H., Na D., Han J.Y., Chae J., Park C., Park O.-K., Min S., Kang J., et al. Genomic alterations in BCL2L1 and DLC1 contribute to drug sensitivity in gastric cancer. Proc. Natl. Acad. Sci. USA. 2015;112:12492–12497. doi: 10.1073/pnas.1507491112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee J.W., Soung Y.H., Kim S.Y., Nam S.W., Kim C.J., Cho Y.G., Lee J.H., Kim H.S., Park W.S., Kim S.H., et al. Inactivating mutations of proapoptotic Bad gene in human colon cancers. Carcinogenesis. 2004;25:1371–1376. doi: 10.1093/carcin/bgh145. [DOI] [PubMed] [Google Scholar]
- 86.Singh K., Briggs J.M. Functional Implications of the spectrum of BCL2 mutations in Lymphoma. Mutat. Res. Mutat. Res. 2016;769:1–18. doi: 10.1016/j.mrrev.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 87.Fresquet V., Rieger M., Carolis C., García-Barchino M.J., Martinez-Climent J.A. Acquired mutations in BCL2 family proteins conferring resistance to the BH3 mimetic ABT-199 in lymphoma. Blood. 2014;123:4111–4119. doi: 10.1182/blood-2014-03-560284. [DOI] [PubMed] [Google Scholar]
- 88.Bilbao-Sieyro C., Rodríguez-Medina C., Florido Y., Stuckey R., Sáez M.N., Sánchez-Sosa S., González Martín J.M., Santana G., González-Pérez E., Cruz-Cruz N., et al. BCL2 Expression at Post-Induction and Complete Remission Impact Outcome in Acute Myeloid Leukemia. Diagnostics. 2020;10:1048. doi: 10.3390/diagnostics10121048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gaidzik V.I., Bullinger L., Schlenk R.F., Zimmermann A.S., Röck J., Paschka P., Corbacioglu A., Krauter J., Schlegelberger B., Ganser A., et al. RUNX1 mutations in acute myeloid leukemia: Results from a comprehensive genetic and clinical analysis from the AML study group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011;29:1364–1372. doi: 10.1200/JCO.2010.30.7926. [DOI] [PubMed] [Google Scholar]
- 90.Chan S.M., Thomas D., Corces-Zimmerman M.R., Xavy S., Rastogi S., Hong W.-J., Zhao F., Medeiros B.C., Tyvoll D.A., Majeti R. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat. Med. 2015;21:178–184. doi: 10.1038/nm.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Salmon A.Y., Salmon-Divon M., Zahavi T., Barash Y., Levy-Drummer R.S., Jacob-Hirsch J., Peretz T. Determination of Molecular Markers for BRCA1 and BRCA2 Heterozygosity Using Gene Expression Profiling. Cancer Prev. Res. 2013;6:82–90. doi: 10.1158/1940-6207.CAPR-12-0105. [DOI] [PubMed] [Google Scholar]
- 92.Thorsen K., Sørensen K.D., Brems-Eskildsen A.S., Modin C., Gaustadnes M., Hein A.-M.K., Kruhøffer M., Laurberg S., Borre M., Wang K., et al. Alternative Splicing in Colon, Bladder, and Prostate Cancer Identified by Exon Array Analysis. Mol. Cell. Proteom. 2008;7:1214–1224. doi: 10.1074/mcp.M700590-MCP200. [DOI] [PubMed] [Google Scholar]
- 93.David C.J., Manley J.L. Alternative pre-mRNA splicing regulation in cancer: Pathways and programs unhinged. Genes Dev. 2010;24:2343–2364. doi: 10.1101/gad.1973010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Adamia S., Haibe-Kains B., Pilarski P.M., Bar-Natan M., Pevzner S., Avet-Loiseau H., Lode L., Verselis S., Fox E.A., Burke J., et al. A Genome-Wide Aberrant RNA Splicing in Patients with Acute Myeloid Leukemia Identifies Novel Potential Disease Markers and Therapeutic Targets. Clin. Cancer Res. 2014;20:1135–1145. doi: 10.1158/1078-0432.CCR-13-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moore M.J., Wang Q., Kennedy C.J., Silver P.A. An Alternative Splicing Network Links Cell-Cycle Control to Apoptosis. Cell. 2010;142:625–636. doi: 10.1016/j.cell.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jeromin S., Bacher U., Bayer K., Dicker F., Eder C., Fasan A., Grossmann V., Kohlmann A., Kern W., Haferlach C., et al. SF3B1 Mutations Are Detectable in 48.9% of Acute Myeloid Leukemia with Normal Karyotype (AML-NK) and ≥15% Ring Sideroblasts and Are Closely Related to FLT3-ITD and RUNX1 Mutations. Blood. 2012;120:406. doi: 10.1182/blood.V120.21.406.406. [DOI] [Google Scholar]
- 97.Meggendorfer M., Jeromin S., Perglerová K., Haferlach C., Kern W., Haferlach T. SF3B1 Mutations in AML, MDS and MDS/MPN-RS-T Are Accompanied By Different Other Gene Mutations: Impact for Targeted Treatment Studies. Blood. 2016;128:1976. doi: 10.1182/blood.V128.22.1976.1976. [DOI] [Google Scholar]
- 98.Bamopoulos S.A., Batcha A.M.N., Jurinovic V., Rothenberg-Thurley M., Janke H., Ksienzyk B., Philippou-Massier J., Graf A., Krebs S., Blum H., et al. Clinical presentation and differential splicing of SRSF2, U2AF1 and SF3B1 mutations in patients with acute myeloid leukemia. Leukemia. 2020;34:2621–2634. doi: 10.1038/s41375-020-0839-4. [DOI] [PubMed] [Google Scholar]
- 99.Luczak M., Kaźmierczak M., Handschuh L., Lewandowski K., Komarnicki M., Figlerowicz M. Comparative proteome analysis of acute myeloid leukemia with and without maturation. J. Proteom. 2012;75:5734–5748. doi: 10.1016/j.jprot.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 100.Edlich F., Banerjee S., Suzuki M., Cleland M.M., Arnoult D., Wang C., Neutzner A., Tjandra N., Youle R.J. Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell. 2011;145:104–116. doi: 10.1016/j.cell.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fiebig A.A., Zhu W., Hollerbach C., Leber B., Andrews D.W. Bcl-XL is qualitatively different from and ten times more effective than Bcl-2 when expressed in a breast cancer cell line. BMC Cancer. 2006;6:213. doi: 10.1186/1471-2407-6-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oltersdorf T., Elmore S.W., Shoemaker A.R., Armstrong R.C., Augeri D.J., Belli B.A., Bruncko M., Deckwerth T.L., Dinges J., Hajduk P.J., et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 103.Kline M.P., Rajkumar S.V., Timm M.M., Kimlinger T.K., Haug J.L., Lust J.A., Greipp P.R., Kumar S. ABT-737, an inhibitor of Bcl-2 family proteins, is a potent inducer of apoptosis in multiple myeloma cells. Leukemia. 2007;21:1549–1560. doi: 10.1038/sj.leu.2404719. [DOI] [PubMed] [Google Scholar]
- 104.Karakas T., Maurer U., Weidmann E., Miething C.C., Hoelzer D., Bergmann L. High expression of bcl-2 mRNA as a determinant of poor prognosis in acute myeloid leukemia. Ann. Oncol. 1998;9:159–165. doi: 10.1023/A:1008255511404. [DOI] [PubMed] [Google Scholar]
- 105.Karakas T., Miething C., Maurer U., Weidmann E., Ackermann H., Hoelzer D., Bergmann L. The coexpression of the apoptosis-related genes bcl-2 and wt1 in predicting survival in adult acute myeloid leukemia. Leukemia. 2002;16:846–854. doi: 10.1038/sj.leu.2402434. [DOI] [PubMed] [Google Scholar]
- 106.Del Poeta G., Venditti A., Del Principe M.I., Maurillo L., Buccisano F., Tamburini A., Cox M.C., Franchi A., Bruno A., Mazzone C., et al. Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML) Blood. 2003;101:2125–2131. doi: 10.1182/blood-2002-06-1714. [DOI] [PubMed] [Google Scholar]
- 107.De haes I., Dendooven A., Mercier M.L., Puylaert P., Vermeulen K., Kockx M., Deiteren K., Maes M.-B., Berneman Z., Anguille S. Absence of BCL-2 Expression Identifies a Subgroup of AML with Distinct Phenotypic, Molecular, and Clinical Characteristics. J. Clin. Med. 2020;9:3090. doi: 10.3390/jcm9103090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Andreeff M., Jiang S., Zhang X., Konopleva M., Estrov Z., Snell V., Xie Z., Okcu M., Sanchez-Williams G., Dong J., et al. Expression of Bcl-2-related genes in normal and AML progenitors: Changes induced by chemotherapy and retinoic acid. Leukemia. 1999;13:1881–1892. doi: 10.1038/sj.leu.2401573. [DOI] [PubMed] [Google Scholar]
- 109.Hata A.N., Engelman J.A., Faber A.C. The BCL2 Family: Key Mediators of the Apoptotic Response to Targeted Anticancer Therapeutics. Cancer Discov. 2015;5:475–487. doi: 10.1158/2159-8290.CD-15-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.