Abstract
Background/Objective
Obesity is a strong risk factor for adverse outcomes in patients hospitalized with COVID-19, however, the distribution of fat and the amount of muscle mass are more accurate risk factors than BMI. The objective of this study was to assess body composition measures obtained on opportunistic abdominal CTs as predictors of outcome in patients hospitalized with COVID-19. We hypothesized that elevated visceral and intermuscular adipose tissue would be associated with adverse outcome.
Subjects/Methods
Our retrospective study was IRB-approved and HIPAA-compliant. The study group comprised 124 patients (median age: 68 years, IQR: 56, 77; 59 weeks, 65 months) who were admitted with COVID-19 to a single hospital and who had undergone abdominal CT for clinical purposes. Visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT), intermuscular adipose tissue (IMAT), and paraspinal and abdominal muscle cross-sectional areas (CSA) were assessed. Clinical information including prognostic factors, time of admission to the intensive care unit (ICU) and time of death within 28 days were obtained. Multivariate time-to-event competing risk models were fitted to estimate the hazard ratio (HR) for a composite outcome of ICU admission/mortality associated with a one standard deviation increase in each body compositional measure. Each model was adjusted for age, sex, race, BMI, and cardiometabolic comorbidities.
Results
There were 50 patients who were admitted to the ICU or deceased over a median time of 1 day [IQR 1, 6] from hospital admission. Higher VAT/SAT ratio (HR of 1.30; 95% CI 1.04–1.62, p = 0.022) and higher IMAT CSA (HR of 1.44; 95% CI 1.10–1.89, p = 0.008) were associated with a reduced time to ICU admission or death in adjusted models.
Conclusion
VAT/SAT and IMAT are predictors of adverse outcome in patients hospitalized with COVID-19, independent of other established prognostic factors. This suggests that body composition measures may serve as novel biomarkers of outcome in patients with COVID-19.
Subject terms: Obesity, Obesity
Introduction
Several studies suggest that obesity is a strong risk factor for adverse outcome in patients hospitalized with coronavirus disease 2019 (COVID-19) [1–4]. Body mass index (BMI) is commonly used to assess obesity, but cardiometabolic risk and mortality vary considerably among patients with the same BMI, which is partially attributable to differences in body composition [5, 6]. The distribution of fat and the amount of muscle mass are more accurate risk factors than BMI for cardiometabolic risk. For example, visceral adipose tissue (VAT) is more strongly associated with fasting glucose and adverse serum lipid profile, and increased odds ratios for hypertension, diabetes, and the metabolic syndrome than subcutaneous adipose tissue (SAT) [7, 8], which may be relatively protective [9]. Low skeletal muscle mass and sarcopenia are known risk factors for cardiometabolic disease [6, 10–13], longer hospital stay, and increased mortality [14]; and fat between muscle cells, intermuscular adipose tissue (IMAT), has been identified as an independent risk factor of impaired glucose tolerance, impaired lipid profile, chronic inflammation, and lower muscle strength and quality [15].
Patients admitted to the hospital for COVID-19 often undergo computed tomography (CT) of the chest or abdomen for clinical care and these CTs could be used to assess body composition without additional costs or radiation exposure. Several smaller studies [16–18], three larger studies [19–21] and a recent meta-analysis [22] have identified visceral adiposity as a risk factor for adverse outcome (admission to the ICU, mechanical ventilation, death) in patients with COVID-19. However, no study has examined detailed measures of body composition, including IMAT, and their impact on patients hospitalized with COVID-19.
The purpose of our study was to determine the value of body composition measures obtained using opportunistic abdominal CTs to predict admission to the intensive care unit (ICU) and mortality in patients hospitalized with COVID-19. We hypothesized that elevated VAT and IMAT would be associated with a higher risk of ICU admission and mortality.
Methods
This retrospective study was Institutional Review Board approved and Health Insurance Portability and Accountability Act compliant with exemption status for individual informed consent.
Subjects
The Massachusetts General Hospital (MGH) COVID-19 Patient Registry, described previously [23], includes patients who tested positive for SARS-CoV-2 by PCR and were subsequently hospitalized at our institution between March 11 and May 31, 2020. Patients in the registry who underwent an abdominal CT exam within 2 months of hospital admission were included in our study. Clinical data were obtained by manual chart review and data extraction from electronic health records and included demographic information (age, sex, BMI, race/ethnicity), cardiometabolic comorbidities, and outcomes (admission to the ICU or death).
Computed tomography
CT of the abdomen was performed on a 64- or 128-slice multidetector CT scanner (Siemens, Erlangen, Germany or GE Healthcare, Chicago, IL, USA), slice thickness, 2–5 mm; table feed, 23–79.37 mm/s; pitch, 0.4–1; tube voltage, 90–140 kVP; tube current variable mAs (maximum 450 mAs); sagittal reconstruction thickness, 2 mm with 2-mm intervals. Intravenous contrast was administered in 97 studies (78.2%) and 27 studies (21.8%) were performed without contrast. The CT scanners used in this study were tested on an annual basis according to American Association of Physicists in Medicine and American College of Radiology guidelines, and standard clinical quality assurance measures were performed to assess for reproducibility of scans.
Image analysis
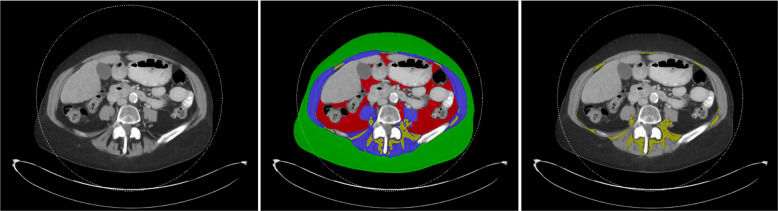
Measurements were performed at the mid portion of the L4 vertebral body using an in-house automated algorithm [24]. Cross-sectional areas (CSA) in cm2 of abdominal SAT, VAT, IMAT, and paraspinal and abdominal muscle were determined (Fig. 1). The relative distribution of abdominal adipose tissue was assessed by using the VAT/SAT ratio. In addition, body composition measurements were standardized by subtracting the mean of each measurement and dividing by the standard deviation (SD).
Fig. 1. Segmentation of body composition from abdominal CTs.
Subcutaneous adipose tissue in green, visceral adipose tissue in red, muscle in blue, and intermuscular adipose tissue in yellow.
A single trained observer blinded to the clinical information and patient outcome visually inspected and made corrections to the segmentation predictions using the Horos DICOM viewer (version 6.5.2, www.horosproject.com). The analyst was trained by two senior fellowship-trained musculoskeletal radiologists (MT and MAB).
Using our in-house automated algorithm, there is high concordance in CSA assessment using CTs performed with and without intravenous contrast. Interclass correlation coefficients (95% confidence intervals) are as follows: SAT 0.998 (0.993–0.999), VAT 0.999 (0.994–1.000), muscle 0.991 (0.963–0.998), IMAT 0.985 (0.940–0.996), and concordance correlation coefficients (95% confidence intervals) are as follows: SAT 0.997 (0.987–0.999), VAT 0.995 (0.981–0.999), muscle 0.964 (0.884–0.989), IMAT 0.955 (0.897–0.981).
Statistical analysis
The primary outcome measure was time to the composite endpoint of ICU admission or death. Patients were followed from hospital admission until they were deceased, discharged, or transferred to another facility within 28 days after presentation to care, whichever came first. Event times were indexed by the day before hospital admission in order to include patients who experienced the outcome on the day of hospital admission in the analysis.
Multivariate time-to-event competing risks models were used to estimate the hazard ratio (HR) associated with a one SD increase in each body compositional measure [25, 26]. Competing risk models were used in order to appropriately account for the fact that patients who were discharged or transferred were likely healthier than those who remained hospitalized [27]. Each model was adjusted for age, sex, race (White/Black/Hispanic/other), type 2 diabetes mellitus (T2DM), cardiovascular or metabolic disease not including T2DM (congenital heart disease, coronary artery disease, myocardial infarction, congestive heart failure, cardiomyopathy, hypertension, arrhythmia, type 1 diabetes, dyslipidemia; binary), and BMI at hospitalization, via natural cubic splines with three nodes [28].
In a small number of patients, SAT and paraspinal and abdominal muscle CSA were cut off due to body size and a sensitivity analysis was conducted that excluded these scans. A second sensitivity analysis was conducted excluding scans that occurred two or more days after hospital admission in order to avoid bias due to reverse causation from rapid body weight changes occurring as a result of COVID-19 hospitalization. Statistical significance was defined as a two-tailed p < 0.05. Analyses were performed with R version 3.6.1 and R package cmprsk version 2.2–10 (https://CRAN.R-project.org/package=cmprsk).
Results
Patient characteristics
Patient characteristics are shown in Table 1. We identified 124 patients (47.6% female; median age 68 years; median BMI 28.8 kg/m2) from the MGH COVID-19 patient registry who underwent an abdominal CT. Indications for the abdominal CT are summarized in Supplementary Table 1. The demographic characteristics and outcome rates of patients who did and did not undergo CT scans are summarized in Supplementary Table 2. Patients who underwent abdominal CTs appeared to be younger and were more likely to be White/Non-Hispanic and less likely to be Hispanic.
Table 1.
Patient characteristics.
| Number of patients | 124 |
|---|---|
| Age | 68 [56,77] |
| Body mass index (BMI) | 28.8 [24.3, 32.3] |
| Female | 59 (47.6%) |
| Race/Ethnicity | |
| Hispanic | 31 (25.0%) |
| Black/Non-Hispanic | 15 (12.1%) |
| White/Non-Hispanic | 66 (53.2%) |
| Other | 12 (9.7%) |
| History of type 2 diabetes | 43 (34.7%) |
| History of cardiac or metabolic diseasea, excluding type 2 diabetes | 97 (78.2%) |
Data presented as median and interquartile range [IQR] for continuous variables and n (%) for categorical variables.
aIncludes congenital heart disease, coronary artery disease, myocardial infarction, congestive heart failure, cardiomyopathy, hypertension, arrhythmia, type 1 diabetes, and dyslipidemia.
Among the 50 patients (40.3%) who experienced the composite outcome, admission to the ICU or death, the median time to event was 1 day [IQR 1, 6]. Of those, 39 patients (31.5%) were admitted to the ICU with a median time of 1 day [IQR 1, 2] and 23 patients (18%) died with a median follow-up period of 11 days [IQR 6, 18]. Of patients who died, 12 patients had also been admitted to the ICU.
Body composition
Median abdominal SAT CSA was 269.9 cm2 [IQR 198.1, 386.6] and median VAT CSA was 145.6 cm2 [IQR 86.2, 210.9], with a median VAT/SAT ratio of 0.51 [IQR 0.29, 0.83]. Median IMAT CSA was 12.1 cm2 [IQR 6.1, 20.7] and median paraspinal and abdominal muscle CSA was 134.5 cm2 [IQR 113.4, 167.3]. The SD of SAT CSA and VAT CSA were 110.2 and 148.0 cm2, respectively. The SD of the VAT/SAT ratio was 0.38. The SD of IMAT CSA was 13.7 cm2 and the SD of paraspinal and abdominal muscle CSA was 37.3 cm2. A significant association between increased VAT/SAT ratio and IMAT and the composite outcome, ICU or death, was found (Fig. 2 and Table 2).
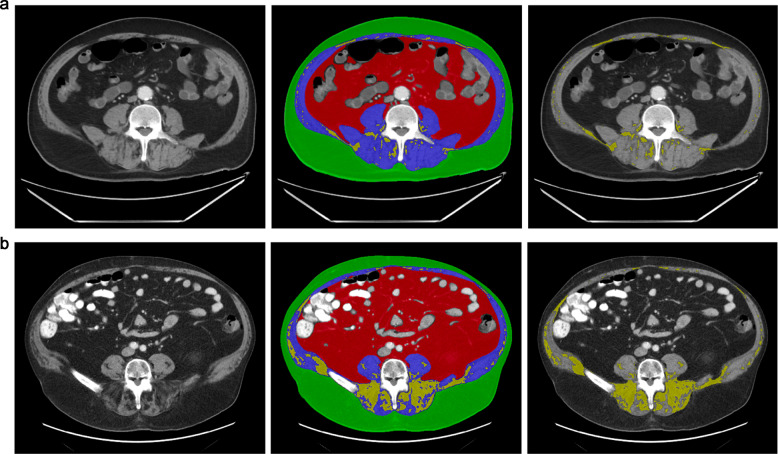
Fig. 2. Body composition assessment in patients with COVID-19.
a 73-year-old woman with obesity (BMI 33.4 kg/m2) admitted with COVID-19, who was alive after the follow-up period. Subcutaneous adipose tissue (SAT) cross-sectional area (CSA) 155.1 cm2, visceral adipose tissue (VAT) CSA 161.2 cm2, VAT/SAT ratio 1.04, muscle CSA 124.5 cm2, intermuscular adipose tissue (IMAT) CSA 15.3 cm2. b 76-year-old woman, BMI 35.4 kg/m2, admitted for COVID-19 who died 8 days after hospital admission: SAT CSA 217.9 cm2, VAT CSA 417.7 cm2, VAT/SAT ratio 1.92, muscle CSA 119.2 cm2, IMAT CSA 66.4 cm2. Despite similar BMI, the patient who died had higher visceral adiposity and higher IMAT.
Table 2.
Estimated hazard ratios for time to adverse outcome (ICU admission or death) from a model for a one standard deviation change in each body composition measure accounting for competing risks (N = 124).
| Body composition measure (CSA) | Standard deviation (cm2) | Hazard ratio (95% CI) | p value |
|---|---|---|---|
| VAT | 110 | 1.28 (0.93, 1.76) | 0.130 |
| VAT/SAT ratio | 0.38 | 1.30 (1.04, 1.62) | 0.022 |
| TAT | 213 | 1.58 (0.95, 2.63) | 0.078 |
| Muscle | 37 | 0.80 (0.48, 1.35) | 0.410 |
| IMAT | 13.7 | 1.44 (1.10, 1.89) | 0.008 |
Each model was additionally adjusted for age, sex, race (White/Black/Hispanic/other), type 2 diabetes, cardiovascular or metabolic disease not including type 2 diabetes, (binary), and body mass index (natural cubic splines with three nodes). Hazard ratios refer to subdistributional proportional hazard ratios for ICU admission and/or death, obtained from competing risks models.
CSA cross-sectional area, VAT visceral adipose tissue, SAT subcutaneous adipose tissue, IMAT intermuscular adipose tissue.
Higher VAT/SAT ratio was associated with adverse outcome in the model adjusted for age, sex, race, BMI, and cardiometabolic comorbidities (HR = 1.30, 95% CI 1.04–1.62, p = 0.022, SD of VAT/SAT ratio = 0.38). Higher IMAT was associated with adverse outcome in the model adjusted for age, sex, race, BMI, and cardiometabolic comorbidities (HR = 1.44, 95% CI 1.10–1.89, p = 0.008, SD of IMAT = 13.7 cm2). Paraspinal and abdominal muscle CSA was not associated with adverse outcome (Table 2).
The first sensitivity analysis excluded 19 measures of SAT CSA and 3 measures of paraspinal and abdominal muscle CSA that were “cut-off”. The second sensitivity analysis excluded 12 patients whose scans were performed between 3 and 14 days after admission. In both cases, the main results were robust to these exclusions (results not shown).
Discussion
Our study shows that higher VAT/SAT ratio and higher IMAT, assessed among patients admitted to MGH with COVID-19 who received opportunistic abdominal CTs, are positive predictors of ICU admission or death, independent of other established prognostic factors, such as age, sex, race, BMI, and cardiometabolic comorbidities. Our data suggest that these body composition measures could serve as novel biomarkers for adverse outcome in patients with COVID-19, who often undergo CT routinely as part of clinical care.
Several studies have shown that obesity presents an independent risk factor of disease severity, including the requirement for mechanical ventilation or death, in patients hospitalized with COVID-19 [1, 2, 4]. Cai et al. found patients with obesity who were hospitalized were at 3.40-fold odds of developing severe disease, after adjusting for age, sex, epidemiological characteristics, and comorbidities [1]. Palaiodimos et al. identified BMI ≥35 kg/m2, male sex, and age as independent predictors of mortality in patients hospitalized with COVID-19 [4]. However, BMI is an imperfect measure to characterize disease risk and several studies have shown that low muscle mass (sarcopenia) and high-fat mass, especially VAT, are stronger predictors of disease risk and mortality than BMI [29]. VAT is a significant risk factor for cardiometabolic disease [7, 8], while SAT may be relatively protective [9]. In addition, low skeletal muscle mass and sarcopenia are established indicators of cardiometabolic risk and mortality [6, 10–13]. Loss of skeletal muscle is accompanied by shifts in adipose tissue and accumulation of fat in ectopic depots. One of those ectopic fat depots is IMAT, adipose tissue located between muscle groups and separated from SAT by a well-defined fascia. Increased IMAT is associated with higher fasting glucose and insulin resistance, and greater prevalence of T2DM [15, 30].
Many patients hospitalized for COVID-19 undergo CTs of the chest or abdomen for clinical care and these CTs could be used to assess body composition without additional costs or radiation exposure. Recent studies have suggested that visceral adiposity is a risk factor for adverse outcome in patients with COVID-19 [19–21, 31]. Petersen et al. showed in 36 patients with COVID-19 that VAT is associated with increased risk of ICU admission and/or mechanical ventilation [18]. Of note, body composition was assessed using chest CTs at the level of the first lumbar vertebra. This anatomical location encompasses less VAT compared to the validated levels at L4 or L5 [32]. Chandarana et al. found higher VAT in patients with COVID-19 who required hospital admission compared to outpatients, despite similar BMI [16]. Three larger studies examining more than 100 patients hospitalized with COVID-19, showed that visceral adiposity was an independent risk factor for adverse outcome, including the need for ICU admission [19–21]. Yang et al. also identified fatty infiltration of muscle, assessed using CT attenuation, as an independent risk factor for adverse outcome [21]. However, no study has examined detailed measures of body composition, including IMAT, and their impact on outcome in patients admitted with COVID-19.
Our study in 124 patients hospitalized with COVID-19 who underwent abdominal CT demonstrated high visceral relative to subcutaneous adiposity (VAT/SAT ratio) and high IMAT as independent predictors of admission to the ICU or death, independent of established prognostic factors, such as age, sex, race, BMI, and cardiometabolic comorbidities. Our finding of an independent association between high VAT/SAT and adverse outcome is consistent with the aforementioned studies. Of note, VAT/SAT was an independent predictor of ICU admission and/or death after controlling for covariates, including BMI, suggesting that characteristics specific to VAT might serve as a mechanism for adverse outcome in COVID-19. A reason for the detrimental effects of VAT on cardiometabolic health is its pro-inflammatory characteristics. Individuals with visceral adiposity have higher concentrations of circulating inflammatory cytokines compared to lean controls [33–35]. In addition, visceral adiposity can contribute to adverse outcome in COVID-19 by diminishing diaphragmatic excursion and increasing airway resistance, and thereby impairing breathing and mechanical ventilation [36].
This is the first study showing high IMAT as an independent risk factor for adverse outcome in COVID-19. Higher IMAT is associated with dyslipidemia, impaired glucose tolerance and the metabolic syndrome and has been shown to be a predictor of subclinical atherosclerosis, independent of traditional risk factors including other fat depots, such as VAT [37]. Studies have suggested that excess IMAT may be pro-inflammatory and promotes and sustains an inflammatory microenvironment [38, 39]. Increased inflammatory markers have been linked to chronic inflammatory airway disease, and studies in patients with COVID-19 have shown higher levels of IL-6 in patients who died from COVID-19 [40, 41]. Therefore, our observed high VAT/SAT and IMAT content in patients admitted to the ICU or patients who died from COVID-19, may be due to their pro-inflammatory nature.
In our study, fat compartments and paraspinal and abdominal muscle areas on CT were easily quantified using a supervised automated algorithm. The impact of routine assessment of body composition using opportunistic CTs performed for clinical purposes should be evaluated to provide additional information on prognosis in patients with COVID-19 undergoing CT.
A limitation of our study is its retrospective design which limits our ability to infer causality. The study may also be subject to selection bias, since only patients who underwent abdominal CTs were included. It is possible that the observed relationships between the body composition measures considered and adverse outcomes differ between hospitalized patients who had CT scans and hospitalized patients who did not. Patients who underwent abdominal CT scans appeared to be younger and were more likely to be White/Non-Hispanic and less likely to be Hispanic. Further, given that our initial cohort consisted of subjects who were hospitalized at MGH with COVID, we could not evaluate whether body composition affected the rate at which patients were hospitalized following COVID-19 infection. Another limitation inherent to hospitalized cohorts of COVID-19 patients is that patients presented to the hospital care in different stages of disease progression. As such, our time-to-event analysis is indexed on a reference point that is not necessarily comparable for each patient. We also performed body composition measurements using a single slice at the level of L4 instead of the entire abdomen. However, abdominal fat and paraspinal and abdominal muscle CSA determined by single-slice measurements have been shown to be closely correlated with total body fat and muscle volumes [32, 42]. The use of our in-house algorithm limits generalizability of the study. Strengths of our study include the large number of patients admitted to a single hospital with COVID-19 and detailed measures of body composition by CT. Moreover, our statistical approach considered the time from hospitalization to severe disease while accounting for the competing risk of discharge, and adjusted for a wide variety of covariates; however, due to the limited sample size, we could not adjust for all known prognostic factors (e.g., medications patients were taking prior to admission, laboratory parameters, and other comorbidities).
In conclusion, VAT/SAT ratio and IMAT are associated with increased risk of ICU admission or death in patients hospitalized with COVID-19, independent of other established prognostic factors. With further investigation into the generalizability and predictive power of this finding, body composition measures could serve as novel biomarkers of outcome in patients with COVID-19.
Supplementary information
Acknowledgements
This work was funded in part by support from National Institutes of Health (NIH) grant K24DK109940 and P30DK040561.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Katherine M. Bunnell, Tanayott Thaweethai.
Supplementary information
The online version contains supplementary material available at 10.1038/s41366-021-00907-1.
References
- 1.Cai Q, Chen F, Wang T, Luo F, Liu X, Wu Q, et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care. 2020;43:1392–8. doi: 10.2337/dc20-0576. [DOI] [PubMed] [Google Scholar]
- 2.Gao F, Zheng KI, Wang XB, Sun QF, Pan KH, Wang TY, et al. Obesity is a risk factor for greater COVID-19 severity. Diabetes Care. 2020;43:e72–e4. doi: 10.2337/dc20-0682. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y, Lu Y, Huang YM, Wang M, Ling W, Sui Y, et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism. 2020;113:154378. doi: 10.1016/j.metabol.2020.154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haines MS, Dichtel LE, Santoso K, Torriani M, Miller KK, Bredella MA. Association between muscle mass and insulin sensitivity independent of detrimental adipose depots in young adults with overweight/obesity. Int J Obes. 2020;44:1851–8. doi: 10.1038/s41366-020-0590-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schorr M, Dichtel LE, Gerweck AV, Valera RD, Torriani M, Miller KK, et al. Sex differences in body composition and association with cardiometabolic risk. Biol Sex Differ. 2018;9:28. doi: 10.1186/s13293-018-0189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Fox CS, Hickson DA, May WD, Hairston KG, Carr JJ, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010;95:5419–26. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snijder MB, Visser M, Dekker JM, Goodpaster BH, Harris TB, Kritchevsky SB, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48:301–8. doi: 10.1007/s00125-004-1637-7. [DOI] [PubMed] [Google Scholar]
- 10.Batsis JA, Mackenzie TA, Jones JD, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and inflammation: results from the 1999-2004 National Health and Nutrition Examination Survey. Clin Nutr. 2016;35:1472–83. doi: 10.1016/j.clnu.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011;96:2898–903. doi: 10.1210/jc.2011-0435. [DOI] [PubMed] [Google Scholar]
- 12.Bower JK, Meadows RJ, Foster MC, Foraker RE, Shoben AB. The association of percent body fat and lean mass with HbA1c in US adults. J Endocr Soc. 2017;1:600–8. doi: 10.1210/js.2017-00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalyani RR, Metter EJ, Ramachandran R, Chia CW, Saudek CD, Ferrucci L. Glucose and insulin measurements from the oral glucose tolerance test and relationship to muscle mass. J Gerontol A Biol Sci Med Sci. 2012;67:74–81. doi: 10.1093/gerona/glr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boutin RD, Yao L, Canter RJ, Lenchik L. Sarcopenia: current concepts and imaging implications. AJR Am J Roentgenol. 2015;205:W255–66. doi: 10.2214/AJR.15.14635. [DOI] [PubMed] [Google Scholar]
- 15.Waters DL. Intermuscular adipose tissue: a brief review of etiology, association with physical function and weight loss in older adults. Ann Geriatr Med Res. 2019;23:3–8. doi: 10.4235/agmr.19.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandarana H, Dane B, Mikheev A, Taffel MT, Feng Y, Rusinek H. Visceral adipose tissue in patients with COVID-19: risk stratification for severity. Abdom Radiol. 2021;46:818–25. doi: 10.1007/s00261-020-02693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kottlors J, Zopfs D, Fervers P, Bremm J, Abdullayev N, Maintz D, et al. Body composition on low dose chest CT is a significant predictor of poor clinical outcome in COVID-19 disease - a multicenter feasibility study. Eur J Radiol. 2020;132:109274. doi: 10.1016/j.ejrad.2020.109274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen A, Bressem K, Albrecht J, Thiess HM, Vahldiek J, Hamm B, et al. The role of visceral adiposity in the severity of COVID-19: Highlights from a unicenter cross-sectional pilot study in Germany. Metabolism. 2020;110:154317. doi: 10.1016/j.metabol.2020.154317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Favre G, Legueult K, Pradier C, Raffaelli C, Ichai C, Iannelli A, et al. Visceral fat is associated to the severity of COVID-19. Metabolism. 2021;115:154440. doi: 10.1016/j.metabol.2020.154440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe M, Caruso D, Tuccinardi D, Risi R, Zerunian M, Polici M, et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism. 2020;111:154319. doi: 10.1016/j.metabol.2020.154319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Ding L, Zou X, Shen Y, Hu D, Hu X, et al. Visceral adiposity and high intramuscular fat deposition independently predict critical illness in patients with SARS-CoV-2. Obesity. 2020;28:2040–8. doi: 10.1002/oby.22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foldi M, Farkas N, Kiss S, Dembrovszky F, Szakacs Z, Balasko M, et al. Visceral adiposity elevates the risk of critical condition in COVID-19: a systematic review and meta-analysis. Obesity. 2021;29:521–8. doi: 10.1002/oby.23096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bassett IV, Triant VA, Bunda BA, Selvaggi CA, Shinnick DJ, He W, et al. Massachusetts general hospital Covid-19 registry reveals two distinct populations of hospitalized patients by race and ethnicity. PLoS One. 2020;15:e0244270. doi: 10.1371/journal.pone.0244270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemke R, Buckless CG, Tsao A, Wang B, Torriani M. Deep learning for automated segmentation of pelvic muscles, fat, and bone from CT studies for body composition assessment. Skeletal Radiol. 2020;49:387–95. doi: 10.1007/s00256-019-03289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017;36:4391–400. doi: 10.1002/sim.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Zhang MJ, Fine J. A proportional hazards regression model for the subdistribution with right-censored and left-truncated competing risks data. Stat Med. 2011;30:1933–51. doi: 10.1002/sim.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolkewitz M, Lambert J, von Cube M, Bugiera L, Grodd M, Hazard D, et al. Statistical analysis of clinical COVID-19 data: a concise overview of lessons learned, common errors and how to avoid them. Clin Epidemiol. 2020;12:925–8. doi: 10.2147/CLEP.S256735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perperoglou A, Sauerbrei W, Abrahamowicz M, Schmid M. A review of spline function procedures in R. BMC Med Res Methodol. 2019;19:46. doi: 10.1186/s12874-019-0666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller MJ, Braun W, Enderle J, Bosy-Westphal A. Beyond BMI: conceptual issues related to overweight and obese patients. Obes Facts. 2016;9:193–205. doi: 10.1159/000445380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Granados A, Gebremariam A, Gidding SS, Terry JG, Carr JJ, Steffen LM, et al. Association of abdominal muscle composition with prediabetes and diabetes: the CARDIA study. Diabetes Obes Metab. 2019;21:267–75. doi: 10.1111/dom.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pediconi F, Rizzo V, Schiaffino S, Cozzi A, Della Pepa G, Galati F, et al. Visceral adipose tissue area predicts intensive care unit admission in COVID-19 patients. Obes Res Clin Pract. 2021;15:89–92. doi: 10.1016/j.orcp.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr. 2004;80:271–8. doi: 10.1093/ajcn/80.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barchetta I, Cimini FA, Ciccarelli G, Baroni MG, Cavallo MG. Sick fat: the good and the bad of old and new circulating markers of adipose tissue inflammation. J Endocrinol Investig. 2019;42:1257–72. doi: 10.1007/s40618-019-01052-3. [DOI] [PubMed] [Google Scholar]
- 34.Stefan N, Haring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013;1:152–62. doi: 10.1016/S2213-8587(13)70062-7. [DOI] [PubMed] [Google Scholar]
- 35.Stienstra R, Stefan N. Tipping the inflammatory balance: inflammasome activation distinguishes metabolically unhealthy from healthy obesity. Diabetologia. 2013;56:2343–6. doi: 10.1007/s00125-013-3040-8. [DOI] [PubMed] [Google Scholar]
- 36.Dietz W, Santos-Burgoa C. Obesity and its implications for COVID-19 mortality. Obesity. 2020;28:1005. doi: 10.1002/oby.22818. [DOI] [PubMed] [Google Scholar]
- 37.Terry JG, Shay CM, Schreiner PJ, Jacobs DR, Jr, Sanchez OA, Reis JP, et al. Intermuscular adipose tissue and subclinical coronary artery calcification in midlife: The CARDIA Study (Coronary Artery Risk Development in Young Adults) Arterioscler Thromb Vasc Biol. 2017;37:2370–8. doi: 10.1161/ATVBAHA.117.309633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beasley LE, Koster A, Newman AB, Javaid MK, Ferrucci L, Kritchevsky SB, et al. Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity. 2009;17:1062–9. doi: 10.1038/oby.2008.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haam JH, Kim YS, Koo HS, Haam J, Seo NK, Kim HY, et al. Intermuscular adipose tissue is associated with monocyte chemoattractant protein-1, independent of visceral adipose tissue. Clin Biochem. 2016;49:439–43. doi: 10.1016/j.clinbiochem.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park YS, Kwon HT, Hwang SS, Choi SH, Cho YM, Lee J, et al. Impact of visceral adiposity measured by abdominal computed tomography on pulmonary function. J Korean Med Sci. 2011;26:771–7. doi: 10.3346/jkms.2011.26.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97:2333–8. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.