Abstract
Objectives
We aimed to assess the prevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and factors associated with seropositivity and asymptomatic coronavirus disease 2019 (COVID-19) among people with HIV (PWH).
Methods
This was a cross-sectional study carried out within the cohort of the Spanish HIV Research Network. Participants were consecutive PWH with plasma collected from 1st April to 30th September 2020. We determined SARS-CoV-2 antibodies (Abs) in plasma. Illness severity (NIH criteria) was assessed by a review of medical records and, if needed, participant interviews. Multivariable logistic regression analysis was used to identify predictors of seropositivity among the following variables: sex, age, country of birth, education level, comorbidities (hypertension, chronic heart disease, diabetes mellitus, non-AIDS-related cancer, chronic kidney disease, cirrhosis), route of HIV acquisition, prior AIDS, CD4+ cell count, HIV viral load, nucleoside/nucleotide reverse transcriptase inhibitor (N [t]RTI) backbone, type of third antiretroviral drug, and month of sample collection.
Results
Of 1076 PWH (88.0% males, median age 43 years, 97.7% on antiretroviral therapy, median CD4+ 688 cells/mm3, 91.4% undetectable HIV viral load), SARS-CoV-2 Abs were detected in 91 PWH, a seroprevalence of 8.5% (95%CI 6.9–10.3%). Forty-five infections (45.0%) were asymptomatic. Variables independently associated with SARS-CoV-2 seropositivity were birth in Latin American countries versus Spain (adjusted odds ratio (aOR) 2.30, 95%CI 1.41–3.76, p 0.001), and therapy with tenofovir disoproxil fumarate plus emtricitabine (TDF/FTC) versus tenofovir alafenamide (TAF)/FTC as the N(t)RTI backbone (aOR 0.49, 95%CI 0.26–0.94, p 0.031).
Conclusions
Many SARS-CoV-2 infections among PWH were asymptomatic, and birth in Latin American countries increased the risk of SARS-CoV-2 seropositivity. Our analysis, adjusted by comorbidities and other variables, suggests that TDF/FTC may prevent SARS-CoV-2 infection among PWH.
Keywords: COVID-19, HIV, SARS-CoV-2, Serology, Seroprevalence
Introduction
Human immunodeficiency virus (HIV) infection has been an uncommon underlying condition in large case series of coronavirus disease 2019 (COVID-19) associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [[1], [2], [3]]. Nevertheless, COVID-19 in people with HIV (PWH) has been the subject of substantial research and controversy [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]]. Notwithstanding, whether HIV increases the risk of acquiring or dying from COVID-19 remains uncertain.
Seroprevalence studies are essential to better characterize viral infections, including their true prevalence/incidence among populations, geographical distribution, and groups at risk. Besides, seroprevalence studies reflect both clinical and subclinical infection, giving better insight into the disease spectrum and allowing a more reliable estimation of the infection fatality risk [23,24]. SARS-CoV-2 seroprevalence studies have been carried out among the general population in different countries [[25], [26], [27], [28]] or specific populations at risk, such as healthcare workers [[29], [30], [31]] or patients with immune-mediated inflammatory diseases [32].
To the best of our knowledge, two SARS-CoV-2 seroprevalence studies in PWH have been reported so far. The first was a single-centre study carried out in May 2020 in the region of Perugia (Italy) with 270 asymptomatic participants, all of whom tested negative when 4% of the general population in the area was seropositive for SARS-CoV-2 [33]. In the second study, 500 PWH attending an HIV centre in Munich for routine laboratory controls between 29th May and 15th July 2020 were consecutively asked to participate [34]. Nine patients tested positive for SARS-CoV-2 with an IgG immunoassay, two of whom had been diagnosed with COVID-19. The estimated seroprevalence (accounting for the sensitivity and specificity of the test) was 1.5% (95%CI 0.69–3.13).
The objective of our work was to determine the prevalence of SARS-CoV-2 antibodies in a prospective cohort of PWH in Spain under the hypothesis that serological testing provides a suitable approach to assess the frequency of COVID-19 among PWH, to estimate the percentage of asymptomatic infections, and to identify potential risk factors for SARS-CoV-2 infection.
Patients and methods
Design and patient selection
In this cross-sectional study we determined antibodies against SARS-CoV-2 in plasma samples consecutively collected from 1st April 2020 to 30th September 2020 from PWH recruited in the Cohort of the Spanish HIV Research Network (CoRIS).
CoRIS is a prospective cohort of PWH older than 13 years, naive to antiretroviral therapy (ART) at study entry, seen for the first time from 1st January 2004, in 46 participating centres from 13 of 17 regions in Spain. The CoRIS database collects demographic and clinical data, HIV transmission category, ART history, previous opportunistic diseases, specific non-AIDS diseases, and serological and immunovirological data [35]. Blood and other biological samples from PWH in CoRIS are collected yearly and stored in the Spanish HIV BioBank [36]. The Ethics Committee of Hospital General Universitario Gregorio Marañón approved the study (Internal Ref# 162/20).
Data source
The data source for demographics, HIV-related characteristics, and comorbidities in this study was the CoRIS database. COVID-19-related clinical data were retrospectively collected from the electronic hospital and primary-care medical records and, if needed, participant phone interviews. The severity of illness was categorized following the National Institutes of Health (NIH) criteria: asymptomatic infection, and mild, moderate, severe, or critical illness [37].
Laboratory methods
Blood samples were collected at study sites by venepuncture in EDTA tubes, which were then sent the same day to the HIV HGM BioBank where they were processed, and plasma was immediately stored until use at –80°C. For this study, plasma samples were sent to the National Centre for Microbiology, Instituto de Salud Carlos III, where they were tested using the Platelia SARS-CoV-2 Total Ab assay (BioRad, Hercules, CA, USA), following the manufacturer's protocol. This is a qualitative combined enzyme-linked immunoassay (ELISA) for detecting IgG, IgA, and IgM antibodies against the nucleocapsid protein (N protein) of SARS-CoV-2.
Statistics
Descriptive analysis of individuals' characteristics was carried out using frequency tables with percentages for categorical variables and median and quartiles for continuous variables. We calculated the prevalence of SARS-CoV-2 antibodies according to potential risk factors and estimated the magnitude of the association between risk factors and seropositivity by computing odds ratios (ORs) and their 95% confidence intervals (95%CIs). Wald tests were used to derive pairwise p-values for differences in seropositivity between the different categories of each potential risk factor and the reference category. Multivariable models included all the following variables: sex, age, country of birth, education level, comorbidities (hypertension, chronic heart disease, diabetes mellitus, non-AIDS related cancer, chronic kidney disease, cirrhosis), route of HIV acquisition, prior AIDS, CD4+ cell count, HIV viral load, nucleoside/nucleotide reverse transcriptase inhibitors (N [t]RTI) backbone, type of third antiretroviral drug, and month of sample collection. The association of antiretroviral drugs and SARS-CoV-2 seropositivity was investigated because of the protective effect against COVID-19 of the tenofovir disoproxil fumarate plus emtricitabine (TDF/FTC) backbone in two extensive observational studies [14,18]. Given that the number of events (seropositivity) per covariate was ≤10 in some variables, we used penalization through data augmentation to perform multivariate logistic regression to avoid sparse data bias, setting the prior odds ratio as 1 (uncertain direction) [38]. Statistical analyses were performed by Stata 15.0 software (StataCorp, College Station, TX, USA).
Results
Of 16 178 PWH in active follow-up in CoRIS, plasma samples were collected and stored at the BioBank from 1076 (6.7%) during the study period. These samples are approximately half of those collected during the same period in the preceding year due to circumstances related to the COVID-19 pandemic.
The characteristics of the entire cohort and those PWH included or not in the serosurvey are shown in Table 1 . Of the 1076 PWH with plasma samples available for this study, 88.0% were males at birth, their median age was 43 years, 72.3% self-identified as men having sex with men (MSM), 70.0% were native-born Spaniards, 70.9% had high school or university education, 12.3% had had prior AIDS-defining conditions, 97.7% were on ART, their median CD4 cell count was 688 cells/mm3, and 91.4% had an undetectable HIV viral load. Arterial hypertension was the most common comorbidity (30.5%); less frequent comorbidities (<4.0%) were non-AIDS-defining cancers, diabetes mellitus, chronic heart disease, chronic kidney disease, and liver cirrhosis. Compared with PWH not included in the seroprevalence study, those included were more frequently males at birth, MSM, native Spaniards, and had a higher educational level. Furthermore, they were more frequently diagnosed with arterial hypertension and less frequently had had prior AIDS-defining conditions. Finally, they were more frequently receiving an ART regimen based on tenofovir alafenamide plus emtricitabine (TAF/FTC). The characteristic of PWH included in the serosurvey categorized according to the different N(t)TRI backbones are shown in the Supplementary Material (Table S1). The groups were well matched according to baseline characteristics except for arterial hypertension which was more frequent among PWH receiving TAF/FTC.
Table 1.
Characteristics of people with HIV (PWH) in active follow-up in CoRIS during the study period
| Variable | Total PWH n = 16 178 | PWH included in the serosurvey n = 1076 |
PWH not included in the serosurvey n = 15 102 |
p |
|---|---|---|---|---|
| Male sex at birth: n/with data (%) | 13 802/16 178 (85.3) | 947/1076 (88.0) | 12 855/15 102 (85.1) | 0.010 |
| Age | ||||
| Distribution: n/with data (%) | 0.279 | |||
| 18–34 | 3356/16 178 (20.7) | 235/1076 (21.8) | 3121/15 102 (20.7) | |
| 35–49 | 7939/16 178 (49.1) | 537/1076 (49.9) | 7402/15 102 (49.0) | |
| 50–64 | 4112/16 178 (25.4) | 264/1076 (24.5) | 3848/15 102 (25.5) | |
| ≥65 | 771/16 178 (4.8) | 40/1076 (3.7) | 731/15 102 (4.8) | |
| Median (Q1; Q3) yr | 44 (36; 52) | 43 (36; 51) | 44 (36:52) | 0.101 |
| Mechanism of HIV acquisition: n/with data (%) | < 0.001 | |||
| Men having sex with men | 10 013/15 717 (63.7) | 745/1030 (72.3) | 9268/14 687 (63.1) | |
| Heterosexual | 4430/15 717 (28.2) | 258/1030 (25.0) | 4172/14 687 (28.4) | |
| Injection drug use | 1118/15 717 (7.1) | 20/1030 (1.9) | 1098/14 687 (7.5) | |
| Other | 156/15 717 (1.0) | 7/1030 (0.7) | 149/14 687 (1.0) | |
| Country of birth: n/with data (%) | < 0.001 | |||
| Spain | 9480/16 112 (58.8) | 753/1075 (70.0) | 8727/15 037 (58.0) | |
| Latin American countries | 3457/16 112 (21.5) | 231/1075 (21.5) | 3226/15 037 (21.5) | |
| Other | 3175/16 112 (19.7) | 91/1075 (8.5) | 3084/15 037 (20.5) | |
| Level of education: n/with data (%) | < 0.001 | |||
| Compulsory education/no education | 4688/13 536 (34.6) | 257/951 (27.0) | 4431/12 585 (35.2) | |
| High school/university | 8581/13 536 (63.4) | 674/951 (70.9) | 7907/12 585 (62.8) | |
| Other | 267/13 536 (2.0) | 20/951 (2.1) | 247/12 585 (2.0) | |
| Comorbidities n/with data (%) | ||||
| Hypertension | 2402/16 178 (14.9) | 328/1076 (30.5) | 2074/15 102 (13.7) | < 0.001 |
| Chronic heart disease | 390/15 969 (2.4) | 29/1067 (2.7) | 361/14 902 (2.4) | 0.546 |
| Diabetes | 459/15 969 (2.9) | 25/1067 (2.3) | 434/14 902 (2.9) | 0.282 |
| History of non-AIDS-related cancer | 770/15 969 (4.8) | 42/1067 (3.9) | 728/14 902 (4.9) | 0.162 |
| Chronic kidney disease | 254/9298 (2.7) | 19/890 (2.1) | 235/8408 (2.8) | 0.251 |
| liver cirrhosis | 183/15 969 (1.2) | 6/1067 (0.6) | 177/14 902 (1.2) | 0.064 |
| Prior AIDS-defining conditions n/with data (%) | 2363/16 178 (14.6) | 132/1076 (12.3) | 2231/15 102 (14.8) | 0.025 |
| Last CD4+ count | ||||
| Distribution n/with data (%) | 0.606 | |||
| <350 | 1116/9836 (11.4) | 100/957 (10.4) | 1016/8879 (11.4) | |
| 350–499 | 1344/9836 (13.7) | 136/957 (14.2) | 1208/8879 (13.6) | |
| ≥500 | 7376/9836 (75.0) | 721/957 (75.3) | 6655/8879 (75.0) | |
| Median (Q1; Q3) cells/mm3 | 702 (499; 921) | 688 (500; 909) | 702 (499–922) | 0.477 |
| Last HIV-RNA load ≤50 copies/mm3n/with data (%) | 8996/10 005 (89.9) | 918/1004 (91.4) | 8078/9001 (89.7) | 0.092 |
| Antiretroviral therapy (N [t]RTI backbone) n/with data (%) | < 0.001 | |||
| TAF/FTC | 4289/14 587 (29.4) | 416/1062 (39.2) | 3873/13 525 (28.6) | |
| ABC/3TC | 3912/14 587 (26.8) | 279/1062 (26.3) | 3633/13 525 (26.9) | |
| TDF/FTC | 3036/14 587 (20.8) | 154/1062 (14.5) | 2882/13 525 (21.3) | |
| Other | 1927/14 587 (13.2) | 188/1062 (17.7) | 1739/13 525 (12.9) | |
| No antiretroviral therapy | 1423/14 587 (9.8) | 25/1062 (2.3) | 1398/13 525 (10.3) | |
| Antiretroviral therapy (third drug) | < 0.001 | |||
| NNRTI | 3434/14 587 (23.5) | 247/1062 (23.3) | 3187/13 525 (23.6) | |
| Protease inhibitor | 1451/14 587 (10.0) | 63/1062 (5.9) | 1388/13 525 (10.3) | |
| Integrase inhibitor | 6322/14 587 (43.3) | 530/1062 (49.9) | 5792/13 525 (42.8) | |
| Other | 1957/14 587 (13.4) | 197/1062 (18.6) | 1760/13 525 (13.0) | |
| No antiretroviral therapy | 1423/14 587 (9.8) | 25/1062 (2.3) | 1398/13 525 (10.3) | |
CoRIS, Spanish HIV Research Network Cohort; Q1, 1st quartile; Q3, 3rd quartile; N(t)RTI, nucleoside/nucleotide reverse transcriptase inhibitors; TAF, tenofovir alafenamide; FTC, emtricitabine; ABC, abacavir; 3TC, lamivudine; TDF; tenofovir disoproxil fumarate; NNRTI, non-nucleoside reverse transcriptase inhibitors.
SARS-CoV-2 antibodies were detected in 91 of 1076 PWH, a prevalence of 8.5% (95%CI 6.9–10.3%). The estimated seroprevalence for the full cohort of 16 178 PWH weighted by sex, age, transmission category, country of birth and level of education was 8.1% (95%CI 6.5–10.0%). The seroprevalence remained relatively stable during the study period, being lowest in samples collected in April (4 of 55, 7.3%) and highest in those collected in September (19 of 194, 9.8%).
Forty-one PWH with serologically confirmed COVID-19 had asymptomatic infections; the disease was mild in 43, moderate in four, severe in three, and none had critical disease. Seven PWH were admitted to the hospital, six required oxygen therapy, and three received non-invasive ventilation. Treatment with corticosteroids was administered to three PWH, and no one was treated with remdesivir or cytokine inhibitors. None was admitted to intensive care units or died. Laboratory confirmation of COVID-19 by RT-PCR or antigen testing had been performed in 22 PWH.
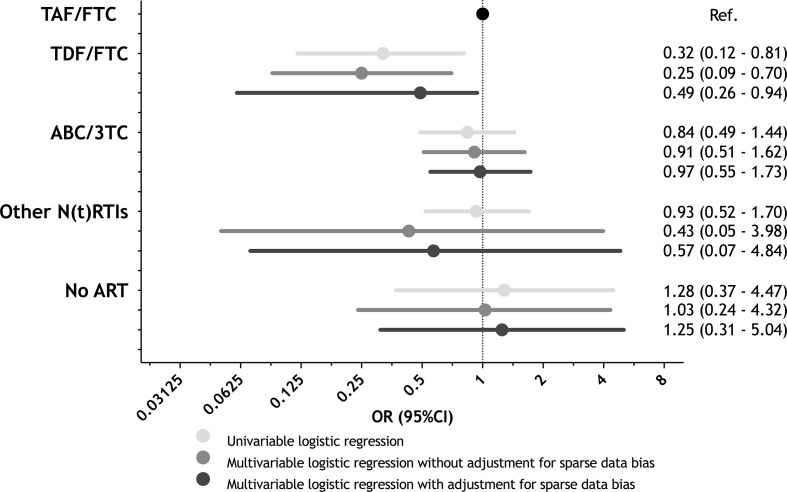
The prevalence of SARS-CoV-2 antibodies broken down by patients' characteristics and unadjusted and adjusted OR (95%CI) for the association between each potential risk factor and SARS-CoV-2 seropositivity are described in Table 2 . Multivariable analyses adjusted for sparse data bias showed that individuals born in Latin American countries had twice the odds of being SARS-CoV-2-seropositive than native-born Spaniards (adjusted OR (aOR) 2.30, 95%CI 1.41–3.76], p 0.001). In contrast, treatment with the TDF/FTC backbone was associated with half the odds of SARS-CoV-2 seropositivity compared to that based on TAF/FTC (aOR 0.49, 95%CI 0.26–0.94, p 0.031) (Fig. 1 ). No statistically significant differences in SARS-CoV-2 seropositivity were found concerning age, mechanism of HIV acquisition, level of education, comorbidities, prior AIDS-defining conditions, CD4+ cell counts, HIV detectability, type of third antiretroviral drug used, and month of sample collection.
Table 2.
Prevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies broken down by patient characteristics and factors associated with SARS-CoV-2 seropositivity by univariable and multivariable logistic regression analyses adjusted and non-adjusted for sparse data bias
| Variable | Antibody+/total (%) | Univariable logistic regression |
Multivariable logistic regression |
||||
|---|---|---|---|---|---|---|---|
| Non-adjusted for sparse data bias |
Adjusted for sparse data bias |
||||||
| OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | ||
| Sex | |||||||
| Male | 85/947 (9.0) | Ref | Ref | Ref | |||
| Female | 6/129 (4.6) | 0.49 (0.21–1.16) | 0.104 | 0.42 (0.15–1.16) | 0.095 | 0.64 (0.32–1.27) | 0.206 |
| Age, years | |||||||
| 18–34 | 21/235 (8.9) | Ref | Ref | Ref | |||
| 35–49 | 47/537 (8.7) | 0.98 (0.57–1.68) | 0.934 | 1.13 (0.63–2.04) | 0.685 | 1.13 (0.64–1.98) | 0.677 |
| 50–64 | 20/264 (7.6) | 0.84 (0.44–1.58) | 0.581 | 0.96 (0.46–2.01) | 0.923 | 0.99 (0.50 -1.99) | 0.984 |
| ≥65 | 3/40 (7.5) | 0.83 (0.23–2.91) | 0.766 | 0.81 (0.16–4.04) | 0.795 | 0.99 (0.44–2.23) | 0.984 |
| Mechanism of HIV acquisition | |||||||
| MSM | 68/745 (9.1) | Ref | Ref | Ref | |||
| Heterosexual | 20/258 (7.7) | 0.84 (0.50–1.41) | 0.501 | 1.36 (0.70–2.65) | 0.364 | 1.13 (0.61–2.10) | 0.702 |
| IDU | 1/20 (5.0) | 0.52 (0.07–3.97) | 0.532 | 0.78 (0.09–6.87) | 0.827 | 0.95 (0.40–2.91) | 0.912 |
| Other | 1/7 (14.3) | 1.66 (0.20–13.99) | 0.641 | 2.67 (0.23–30.84) | 0.431 | 1.13 (0.45–2.87) | 0.796 |
| Country of birth | |||||||
| Spain | 54/753 (7.2) | Ref | Ref | Ref | |||
| Latin American countries | 33/231 (14.3) | 2.16 (1.36–3.42) | 0.001 | 2.34 (1.41–3.89) | 0.001 | 2.30 (1.41–3.76) | 0.001 |
| Other | 4/91 (4.4) | 0.60 (0.21–1.68) | 0.328 | 0.64 (0.22–1.89) | 0.420 | 0.82 (0.41–1.64) | 0.572 |
| Level of education | |||||||
| Compulsory education/no education | 16/257 (6.2) | Ref | Ref | Ref | |||
| High school/University | 65/674 (9.6) | 1.61 (0.91–2.83) | 0.101 | 1.45 (0.79–2.68) | 0.234 | 1.43 (0.79–2.61) | 0.239 |
| Other | 0/20 (0) | — | — | — | — | — | — |
| Comorbidities | |||||||
| Hypertension | |||||||
| No | 56/748 (7.5) | Ref | Ref | Ref | |||
| Yes | 35/328 (10.7) | 1.48 (0.95–2.30) | 0.086 | 1.57 (0.95–2.58) | 0.078 | 1.61 (0.99–2.62) | 0.056 |
| Chronic heart disease | |||||||
| No | 88/1038 (8.5) | Ref | Ref | Ref | |||
| Yes | 3/29 (10.3) | 1.25 (0.37–4.20) | 0.723 | 1.54 (0.39–6.08) | 0.540 | 1.14 (0.51–2.55) | 0.750 |
| Diabetes | |||||||
| No | 89/1042 (8.5) | Ref | Ref | Ref | |||
| Yes | 2/25 (8.0) | 0.93 (0.22–4.01) | 0.924 | 0.77 (0.14–4.24) | 0.764 | 0.94 (0.41–2.16) | 0.889 |
| History of non-AIDS-related cancer | |||||||
| No | 88/1025 (8.6) | Ref | Ref | Ref | |||
| Yes | 3/42 (7.1) | 0.82 (0.25–2.70) | 0.743 | 0.86 (0.25–2.97) | 0.810 | 0.95 (0.44–2.01) | 0.884 |
| Chronic kidney disease | |||||||
| No | 70/871 (8.0) | Ref | Ref | Ref | |||
| Yes | 3/19 (15.8) | 2.15 (0.61–7.54) | 0.234 | 2.61 (0.62–10.95) | 0.188 | 1.37 (0.59–3.18) | 0.465 |
| Liver cirrhosis | |||||||
| No | 91/1061 (8.6) | Ref | Ref | Ref | |||
| Yes | 0/6 (0) | — | — | — | — | — | |
| Prior AIDS-defining conditions | |||||||
| No | 80/944 (8.5) | Ref | Ref | Ref | |||
| Yes | 11/132 (8.3) | 0.98 (0.51–1.90) | 0.956 | 1.01 (0.47–2.17) | 0.971 | 1.04 (0.50–2.17) | 0.909 |
| CD4+ count cells/mm3 | |||||||
| <350 | 9/100 (9.0) | Ref | Ref | Ref | |||
| 350–499 | 13/136 (9.6) | 1.07 (0.44–2.61) | 0.884 | 0.96 (0.37–2.48) | 0.933 | 0.98 (0.50–1.92) | 0.947 |
| ≥500 | 62/721 (8.6) | 0.95 (0.46–1.98) | 0.894 | 0.87 (0.38–2.00) | 0.740 | 9.90 (0.45–1.83) | 0.778 |
| Last HIV-RNA load copies/mm3 | |||||||
| ≤50 | 80/918 (8.7) | Ref | Ref | Ref | |||
| >50 | 5/86 (5.8) | 0.65 (0.25–1.64) | 0.359 | 0.48 (0.17–1.35) | 0.162 | 0.68 (0.35–1.34) | 0.266 |
| Unknown | 6/72 (8.3) | 0.95 (0.40–2.27) | 0.912 | 2.14 (0.59–7.70) | 0.245 | 2.11 (0.59–7.50) | 0.248 |
| Antiretroviral therapy (NRTI backbone) | |||||||
| TAF/FTC | 40/416 (9.6) | Ref | Ref | Ref | |||
| TDF/FTC | 5/154 (3.2) | 0.32 (0.12–0.81) | 0.017 | 0.25 (0.09–0.70) | 0.008 | 0.49 (0.26–0.94) | 0.031 |
| ABC/3TC | 23/279 (8.2) | 0.84 (0.49–1.44) | 0.537 | 0.91 (0.51–1.62) | 0.741 | 0.97 (0.55–1.73) | 0.926 |
| Other | 17/188 (9.0) | 0.93 (0.52–1.70) | 0.824 | 0.43 (0.05–3.98) | 0.458 | 0.57 (0.07–4.84) | 0.607 |
| No antiretroviral therapy | 3/25 (12.0) | 1.28 (0.37–4.47) | 0.697 | 1.03 (0.24–4.32) | 0.970 | 1.25 (0.31–5.04) | 0.756 |
| Antiretroviral therapy (third drug) | |||||||
| NNRTI | 21/247 (8.5) | Ref | Ref | Ref | |||
| Protease inhibitor | 4/63 (6.3) | 0.73 (0.24–2.21) | 0.577 | 0.53 (0.16–1.74) | 0.293 | 0.80 (0.38–1.66) | 0.547 |
| Integrase inhibitor | 42/530 (7.9) | 0.93 (0.54–1.60) | 0.784 | 0.67 (0.35–1.27) | 0.219 | 0.82 (0.44–1.50) | 0.511 |
| Other | 18/197 (9.1) | 1.08 (0.56–2.09) | 0.814 | 1.51 (0.17–13.56) | 0.711 | 1.48 (1.82–12.20) | 0.711 |
| No antiretroviral therapy | 3/25 (12.0) | 1.47 (0.41–5.31) | 0.559 | — | — | ||
| Month of sample collection | |||||||
| April | 4/55 (7.3) | Ref | Ref | Ref | |||
| May | 23/240 (9.6) | 1.35 (0.45–4.08) | 0.593 | 1.39 (0.44–4.40) | 0.580 | 1.36 (0.44–4.27) | 0.593 |
| June | 26/329 (7.9) | 1.09 (0.37–3.27) | 0.872 | 1.12 (0.36–3.50) | 0.849 | 1.14 (0.37–3.51) | 0.819 |
| July | 12/173 (6.9) | 0.95 (0.29–3.08) | 0.932 | 1.00 (0.29–3.40) | 0.998 | 0.96 (0.289–3.21) | 0.951 |
| August | 7/85 (8.2) | 1.14 (0.32–4.11) | 0.836 | 1.44 (0.37–5.55) | 0.596 | 1.32 (0.35–4.98) | 0.410 |
| September | 19/194 (9.8) | 1.38 (0.45–4.25) | 0.570 | 1.49 (0.46–4.81) | 0.501 | 1.42 (0.45–4.50) | 0.550 |
PWH, people with HIV; Q1, 1st quartile; Q3, 3rd quartile; N(t)RTI, nucleoside/nucleotide reverse transcriptase inhibitors; TAF, tenofovir alafenamide; FTC, emtricitabine; ABC, abacavir; 3TC, lamivudine; TDF; tenofovir disoproxil fumarate.
Fig. 1.
Association of the nucleoside/nucleotide reverse transcriptase inhibitors (N(t)RTI) backbone with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) seropositivity by logistic regression analysis. Multivariable models were adjusted by sex, age, country of birth, education level, comorbidities, route of HIV acquisition, prior AIDS, CD4+ cell count, HIV viral load, type of third antiretroviral drug used, and month of sample collection. To avoid sparse data bias, we used penalization through data augmentation to perform multivariate logistic regression. TAF, tenofovir alafenamide; FTC, emtricitabine; TDF, tenofovir disoproxil fumarate; ABC, abacavir; 3TC, lamivudine; ART, antiretroviral therapy; OR, odds ratio; CI, confidence interval.
Discussion
In this study, with plasma samples consecutively collected from 1076 PWH enrolled in a prospective cohort in Spain during the first wave of the pandemic in Spain, the prevalence of SARS-CoV-2 antibodies was 8.5%. Of 91 PWH with serologically confirmed COVID-19, almost half had asymptomatic infections. Birth in Latin American countries was associated with an increased risk of SARS-CoV-2 seropositivity, whereas therapy with TDF/FTC was associated with a lower risk of seropositivity.
The seropositivity against SARS-CoV-2 in our study is somewhat higher than that found in a population-based serosurvey carried out in Spain during the second trimester of 2020 [25]. In this previous study, with 61 075 participants, the seroprevalence was 5.0% by a point-of-care test and 4.6% by a qualitative immunoassay to detect IgG against SARS-CoV-2. Differences in study design must be taken into account when interpreting the results of both studies. First, the nationwide survey was based on a random sampling of households from the national municipal register representative of the Spanish population, whose sociodemographic characteristics differ from individuals in CoRIS who are younger, more frequently male, and less frequently Spanish born than patients in the nationwide survey. Second, the specimens for serological testing were collected in 2 weeks between April and May 2020 in the nationwide survey and over 6 months between April and September in the present study.
Various tests have been used to detect antibodies against SARS-CoV-2, with different diagnostic performances. ELISA tests detecting combined IgG and IgM have shown higher diagnostic sensitivity than ELISA tests for only IgG or IgM [39,40]. The total antibody serological test used in this study has shown excellent diagnostic accuracy for COVID-19, with an AUC of 0.933 and sensitivity and specificity >90% [41], and with a higher sensitivity than other ELISA tests 0–10 days after PCR for detecting SARS-CoV-2-infected patients [42].
The proportion of serologically proved infections in our study that were asymptomatic was 45.0%; this is a noteworthy finding because, to the best of our knowledge, a reliable estimate of the fraction of asymptomatic COVID-19 episodes among PWH has not previously been reported. In the Spanish serosurvey mentioned before, approximately one third of SARS-CoV-2 infections in the general population were asymptomatic [25], a proportion similar to that found in a recently published systematic review on the subject [43].
The association between birth in Latin American countries and a higher risk of SARS-CoV-2 seropositivity is concordant with observations that racial/ethnic minorities or economically disadvantaged people of any background are more susceptible to becoming infected by SARS-CoV-2, most likely due to living or working conditions that increase exposure to the infection, and because of a higher burden of comorbidities [44].
A relevant finding was that TDF/FTC use was associated with a significantly lower risk of serologically confirmed infection after adjustment by demographics, country of birth, education level, comorbidities, and HIV-related variables, including the third drug. Tenofovir diphosphate is a permanent terminator for the SARS-CoV-2 RNA-dependent RNA polymerase [45] with activity against SARS-CoV-2 in vitro [46] and in an animal model in ferrets [47]. Besides, the potential beneficial effect of TDF/FTC against COVID-19 has also been found in other large observational studies. In a multicentric cohort in Spain with 77 590 PWH receiving ART, the risk for COVID-19 hospitalization was lower in those receiving TDF/FTC versus those receiving other regimens, although residual confounding by comorbid conditions could not be ruled out [14]. In another study based on real-world data in the Western Cape, South Africa, with 54 0552 PWH of whom 3978 had COVID-19, TDF was associated with lower COVID-19 mortality compared to other antiretrovirals among those on ART, an association that remained when adjusting for kidney disease, viral suppression and ART duration [18]. The association of TDF/TFC but not TAF/FTC with a protective effect against SARS-CoV-2 infection could be related, at the doses used for HIV, to the fact that TAF yields lower plasma albeit higher intracellular levels of tenofovir than those achieved with TDF [48].
Our study is limited because it was not a random sampling survey among PWH included in CoRIS, and because clinical data related to COVID-19 were collected retrospectively. Another limitation relates to the large number of tests performed in the multivariable model because this increases the probability of finding associations below the significance level of p < 0.05. Our study is also limited because socioeconomic factors different from country of birth and education level, such as housing and work, were not available. Finally, some characteristics of the participants in CoRIS (relatively young age, good control of HIV infection, and low frequency of comorbidities) raise a question about the applicability of some of the results of this study to settings where PWH characteristics are less favourable.
Our study's strengths include the leverage within a prospective cohort of PWH, the large sample size, the adjustment for important covariables, including comorbidities, and the adjustment for sparse data bias in the multivariable regression analysis.
In conclusion, the seropositivity against SARS-CoV-2 among PWH in CoRIS was slightly higher than that found in Spain's general population during the first wave of the pandemic, and birth in Latin American countries increased the risk of SARS-CoV-2 seropositivity among PWH. We also found that many SARS-CoV-2 infections among PWH are asymptomatic, as in the general population. Our analysis, adjusted by comorbidities and other variables, further suggests that TDF/FTC may prevent SARS-CoV-2 infection among PWH. We believe that the accumulated evidence justifies randomized trials with TDF/FTC to treat and prevent COVID-19.
Author contributions
JB, CD, SM, JG-G, SR and IJ: study conception and design. MM-V and SR: laboratory procedures. IJ: statistical analyses. JB, IJ and SR: interpretation of data. JB: obtaining funding and drafting of the manuscript. All authors: acquisition of data, critical revision of the manuscript for important intellectual content, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Transparency declaration
Juan Berenguer reports honoraria for advice or public speaking from ABBVIE, GILEAD, MSD, JANSSEN, and ViiV Healthcare, and grants from ABBVIE, GILEAD, MSD, and ViiV Healthcare. María J. Pérez-Elías reports honoraria for advice or public speaking from GILEAD, MSD, JANSSEN, and ViiV Healthcare. Lucio J. García-Fraile. reports honoraria for advice or public speaking from MSD, and grants from GILEAD. Inés Suárez-García. reports honoraria for advice or public speaking from GILEAD, MSD, and ViiV Healthcare. Daniel Podzamczer reports honoraria for advice or public speaking from GILEAD, MSD, JANSSEN, and ViiV Healthcare, and grants from GILEAD, MSD, and ViiV Healthcare. Federico Pulido reports honoraria for advice or public speaking from ABBVIE, GILEAD, MSD, JANSSEN, and ViiV Healthcare. Félix Gutiérrez reports honoraria for advice or public speaking from JANSSEN and ViiV Healthcare. Víctor Asensi reports honoraria for advice or public speaking from ABBVIE, GILEAD, MSD, JANSSEN, and ViiV Healthcare, and grants from JANSSEN and ViiV Healthcare. Juan C. López reports honoraria for advice or public speaking from GILEAD, MSD, JANSSEN, and ViiV Healthcare. José R. Arribas reports honoraria for advice or public speaking from ALEXA, GILEAD, MSD, JANSSEN, SERONO, TEVA, and ViiV Healthcare, and grants from ALEXA, GILEAD, JANSSEN, MSD, SERONO, TEVA, and ViiV Healthcare. Santiago Moreno reports honoraria for advice or public speaking from GILEAD, MSD, JANSSEN, and ViiV Healthcare, and grants from GILEAD, MSD, and ViiV Healthcare. Juan González-García reports honoraria for advice or public speaking from GILEAD, MSD, JANSSEN, and ViiV Healthcare. Inmaculada Jarrín reports honoraria for advice or public speaking from GILEAD, and ViiV Healthcare, and grants from MSD. Cristina Díez, María Martín-Vicente, Rafael Micán, Francisco Vidal, Jorge Del Romero, José A. Iribarren, Eva Poveda, Carlos Galera, Rebeca Izquierdo, Joaquín Portilla, and Salvador Resino have nothing to disclose. This work was supported by the Instituto de Salud Carlos III (ISCII) (grant number COV20/00108) and the Spanish AIDS Research Network (RD16/0025), which is included in the Spanish I + D + I Plan and is co-funded by ISCIII-Subdirección General de Evaluacion and European Funding for Regional Development (FEDER).
Acknowledgements
We are indebted to all the patients who participated in this research.
Editor: C. Roy
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.06.023.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berenguer J., Ryan P., Rodriguez-Bano J., Jarrin I., Carratala J., Pachon J., et al. Characteristics and predictors of death among 4035 consecutively hospitalized patients with COVID-19 in Spain. Clin Microbiol Infect. 2020;26:1525–1536. doi: 10.1016/j.cmi.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco J.L., Ambrosioni J., García F., Martínez E., Soriano A., Mallolas J., et al. COVID-19 in HIV Investigators. COVID-19 in patients with HIV: clinical case series. Lancet HIV. 2020;7:E314–E316. doi: 10.1016/S2352-3018(20)30111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vizcarra P., Perez-Elias M.J., Quereda C., Moreno A., Vivancos M.J., Dronda F., et al. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV. 2020;7:e554–e564. doi: 10.1016/S2352-3018(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gervasoni C., Meraviglia P., Riva A., Giacomelli A., Oreni L., Minisci D., et al. Clinical features and outcomes of patients with human immunodeficiency virus with COVID-19. Clin Infect Dis. 2020;71:2276–2278. doi: 10.1093/cid/ciaa579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Childs K., Post F.A., Norcross C., Ottaway Z., Hamlyn E., Quinn K., et al. Hospitalized patients with COVID-19 and human immunodeficiency virus: a case series. Clin Infect Dis. 2020;71:2021–2022. doi: 10.1093/cid/ciaa657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karmen-Tuohy S., Carlucci P.M., Zervou F.N., Zacharioudakis I.M., Rebick G., Klein E., et al. Outcomes among HIV-positive patients hospitalized with COVID-19. J Acquir Immune Defic Syndr. 2020;85:6–10. doi: 10.1097/QAI.0000000000002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins L.F., Moran C.A., Oliver N.T., Moanna A., Lahiri C.D., Colasanti J.A., et al. Clinical characteristics, comorbidities and outcomes among persons with HIV hospitalized with coronavirus disease 2019 in Atlanta, Georgia. AIDS. 2020;34:1789–1794. doi: 10.1097/QAD.0000000000002632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrd K.M., Beckwith C.G., Garland J.M., Johnson J.E., Aung S., Cu-Uvin S., et al. SARS-CoV-2 and HIV coinfection: clinical experience from Rhode Island, United States. J Int AIDS Soc. 2020;23 doi: 10.1002/jia2.25573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyerowitz E.A., Kim A.Y., Ard K.L., Basgoz N., Chu J.T., Hurtado R.M., et al. Disproportionate burden of coronavirus disease 2019 among racial minorities and those in congregate settings among a large cohort of people with HIV. AIDS. 2020;34:1781–1787. doi: 10.1097/QAD.0000000000002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etienne N., Karmochkine M., Slama L., Pavie J., Batisse D., Usubillaga R., et al. HIV infection and COVID-19: risk factors for severe disease. AIDS. 2020;34:1771–1774. doi: 10.1097/QAD.0000000000002651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inciarte A., Gonzalez-Cordon A., Rojas J., Torres B., de Lazzari E., de la Mora L., et al. Clinical characteristics, risk factors, and incidence of symptomatic coronavirus disease 2019 in a large cohort of adults living with HIV: a single-center, prospective observational study. AIDS. 2020;34:1775–1780. doi: 10.1097/QAD.0000000000002643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Amo J., Polo R., Moreno S., Diaz A., Martinez E., Arribas J.R., et al. Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy : a cohort study. Ann Intern Med. 2020;173:536–541. doi: 10.7326/M20-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Biagio A., Ricci E., Calza L., Squillace N., Menzaghi B., Rusconi S., et al. Factors associated with hospital admission for COVID-19 in HIV patients. AIDS. 2020;34:1983–1985. doi: 10.1097/QAD.0000000000002663. [DOI] [PubMed] [Google Scholar]
- 16.Hadi Y.B., Naqvi S.F.Z., Kupec J.T., Sarwari A.R. Characteristics and outcomes of COVID-19 in patients with HIV: a multicentre research network study. AIDS. 2020;34:F3–F8. doi: 10.1097/QAD.0000000000002666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shalev N., Scherer M., LaSota E.D., Antoniou P., Yin M.T., Zucker J., et al. Clinical characteristics and outcomes in people living with human immunodeficiency virus hospitalized for coronavirus disease 2019. Clin Infect Dis. 2020;71:2294–2297. doi: 10.1093/cid/ciaa635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boulle A., Davies M.A., Hussey H., Ismail M., Morden E., Vundle Z., et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2020:ciaa1198. doi: 10.1093/cid/ciaa1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geretti A.M., Stockdale A.J., Kelly S.H., Cevik M., Collins S., Waters L., et al. Outcomes of COVID-19 related hospitalization among people with HIV in the ISARIC WHO Clinical Characterization Protocol (UK): a prospective observational study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sigel K., Swartz T., Golden E., Paranjpe I., Somani S., Richter F., et al. Coronavirus 2019 and people living with human immunodeficiency virus: outcomes for hospitalized patients in New York City. Clin Infect Dis. 2020;71:2933–2938. doi: 10.1093/cid/ciaa880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhaskaran K., Rentsch C.T., MacKenna B., Schultze A., Mehrkar A., Bates C.J., et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV. 2021;8:e24–e32. doi: 10.1016/S2352-3018(20)30305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park L.S., Rentsch C.T., Sigel K., Rodriguez-Barradas M., Brown S.T., Goetz M.B., et al. 2020. COVID-19 in the largest US HIV cohort 23rd International AIDS Conference. Abstract # LBPEC23; 6-10 July; Virtual meeting. [Google Scholar]
- 23.Abubakar I., Stagg H., Cohen T., Rodrigues L. Oxford University Press; Oxford: 2016. Oxford specialist handbook of infectious disease epidemiology. [Google Scholar]
- 24.Peeling R.W., Wedderburn C.J., Garcia P.J., Boeras D., Fongwen N., Nkengasong J., et al. Serology testing in the COVID-19 pandemic response. Lancet Infect Dis. 2020;20:e245–e249. doi: 10.1016/S1473-3099(20)30517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollan M., Perez-Gomez B., Pastor-Barriuso R., Oteo J., Hernan M.A., Perez-Olmeda M., et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gudbjartsson D.F., Norddahl G.L., Melsted P., Gunnarsdottir K., Holm H., Eythorsson E., et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383:1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basavaraju S.V., Patton M.E., Grimm K., Rasheed M.A.U., Lester S., Mills L., et al. Serologic testing of U.S. blood donations to identify SARS-CoV-2-reactive antibodies: december 2019–January 2020. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stringhini S., Wisniak A., Piumatti G., Azman A.S., Lauer S.A., Baysson H., et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396:313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korth J., Wilde B., Dolff S., Anastasiou O.E., Krawczyk A., Jahn M., et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128:104437. doi: 10.1016/j.jcv.2020.104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mansour M., Leven E., Muellers K., Stone K., Mendu D.R., Wajnberg A. Prevalence of SARS-CoV-2 antibodies among healthcare workers at a tertiary academic hospital in New York City. J Gen Intern Med. 2020;35:2485–2486. doi: 10.1007/s11606-020-05926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grant J.J., Wilmore S.M.S., McCann N.S., Donnelly O., Lai R.W.L., Kinsella M.J., et al. Seroprevalence of SARS-CoV-2 antibodies in healthcare workers at a London NHS Trust. Infect Control Hosp Epidemiol. 2020:1–3. doi: 10.1017/ice.2020.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon D., Tascilar K., Kronke G., Kleyer A., Zaiss M.M., Heppt F., et al. Patients with immune-mediated inflammatory diseases receiving cytokine inhibitors have low prevalence of SARS-CoV-2 seroconversion. Nat Commun. 2020;11:3774. doi: 10.1038/s41467-020-17703-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papalini C., Paciosi F., Schiaroli E., Pierucci S., Busti C., Bozza S., et al. Seroprevalence of anti-SARS-CoV2 antibodies in Umbrian persons living with HIV. Mediterr J Hematol Infect Dis. 2020;12 doi: 10.4084/MJHID.2020.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noe S., Schabaz F., Heldwein S., Mayer W., Ruecker K., Tiller F.W., et al. HIV and SARS-CoV-2 co-infection: cross-sectional findings from a German 'hotspot. Infection. 2021;49:313–320. doi: 10.1007/s15010-020-01564-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sobrino-Vegas P., Gutierrez F., Berenguer J., Labarga P., Garcia F., Alejos-Ferreras B., et al. [The Cohort of the Spanish HIV Research Network (CoRIS) and its associated biobank; organizational issues, main findings and losses to follow-up] Enferm Infecc Microbiol Clin. 2011;29:645–653. doi: 10.1016/j.eimc.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Merino I., de Las Cuevas N., Jimenez J.L., Gallego J., Gomez C., Prieto C., et al. The Spanish HIV BioBank: a model of cooperative HIV research. Retrovirology. 2009;6:27. doi: 10.1186/1742-4690-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Institutes of Health . 2020. COVID-19 treatment guidelines.https://www.covid19treatmentguidelines.nih.gov Available from: [PubMed] [Google Scholar]
- 38.Greenland S., Mansournia M.A., Altman D.G. Sparse data bias: a problem hiding in plain sight. BMJ. 2016;352:i1981. doi: 10.1136/bmj.i1981. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z.L., Hou Y.L., Li D.T., Li F.Z. Diagnostic efficacy of anti-SARS-CoV-2 IgG/IgM test for COVID-19: a meta-analysis. J Med Virol. 2021;93:366–374. doi: 10.1002/jmv.26211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H., Ai J., Loeffelholz M.J., Tang Y.W., Zhang W. Meta-analysis of diagnostic performance of serology tests for COVID-19: impact of assay design and post-symptom-onset intervals. Emerg Microbe. Infect. 2020;9:2200–2211. doi: 10.1080/22221751.2020.1826362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tre-Hardy M., Wilmet A., Beukinga I., Favresse J., Dogne J.M., Douxfils J., et al. Analytical and clinical validation of an ELISA for specific SARS-CoV-2 IgG, IgA, and IgM antibodies. J Med Virol. 2021;93:803–811. doi: 10.1002/jmv.26303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brochot E., Demey B., Handala L., Francois C., Duverlie G., Castelain S. Comparison of different serological assays for SARS-CoV-2 in real life. J Clin Virol. 2020;130:104569. doi: 10.1016/j.jcv.2020.104569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oran D.P., Topol E.J. The proportion of SARS-CoV-2 infections that are asymptomatic : a systematic review. Ann Intern Med. 2021:M20–M697. doi: 10.7326/M20-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Webb Hooper M., Napoles A.M., Perez-Stable E.J. COVID-19 and racial/ethnic disparities. JAMA. 2020;323:2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chien M., Anderson T.K., Jockusch S., Tao C., Li X., Kumar S., et al. Nucleotide analogues as inhibitors of SARS-CoV-2 polymerase, a key drug target for COVID-19. J Proteome Res. 2020;19:4690–4697. doi: 10.1021/acs.jproteome.0c00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clososki G., Soldi R., da Silva R., Guaratini T., Lopes J., Pereira P., et al. Tenofovir Disoproxil Fumarate: new chemical developments and encouraging in vitro biological results for SARS-CoV-2. J Braz Chem Soc. 2020;31 [Google Scholar]
- 47.Park S.J., Yu K.M., Kim Y.I., Kim S.M., Kim E.H., Kim S.G., et al. Antiviral efficacies of FDA-approved drugs against SARS-CoV-2 infection in ferrets. mBio. 2020;11 doi: 10.1128/mBio.01114-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Markowitz M., Zolopa A., Squires K., Ruane P., Coakley D., Kearney B., et al. Phase I/II study of the pharmacokinetics, safety and antiretroviral activity of tenofovir alafenamide, a new prodrug of the HIV reverse transcriptase inhibitor tenofovir, in HIV-infected adults. J Antimicrob Chemother. 2014;69:1362–1369. doi: 10.1093/jac/dkt532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.