Abstract
Pregnancy is a complicated and insidious state with various aspects to consider, including the well-being of the mother and child. Developing better non-invasive tests that cover a broader range of disorders with lower false-positive rates is a fundamental necessity in the prenatal medicine field, and, in this sense, the application of metabolomics could be extremely useful. Metabolomics measures and analyses the products of cellular biochemistry. As a biomarker discovery tool, the integrated holistic approach of metabolomics can yield new diagnostic or therapeutic approaches. In this review, we identify and summarize prenatal metabolomics studies and identify themes and controversies. We conducted a comprehensive search of PubMed and Google Scholar for all publications through January 2020 using combinations of the following keywords: nuclear magnetic resonance, mass spectrometry, metabolic profiling, prenatal diagnosis, pregnancy, chromosomal or aneuploidy, pre-eclampsia, fetal growth restriction, pre-term labor, and congenital defect. Metabolite detection with high throughput systems aided by advanced bioinformatics and network analysis allowed for the identification of new potential prenatal biomarkers and therapeutic targets. We took into consideration the scientific papers issued between the years 2000–2020, thus observing that the larger number of them were mainly published in the last 10 years. Initial small metabolomics studies in perinatology suggest that previously unidentified biochemical pathways and predictive biomarkers may be clinically useful. Although the scientific community is considering metabolomics with increasing attention for the study of prenatal medicine as well, more in-depth studies would be useful in order to advance toward the clinic world as the obtained results appear to be still preliminary. Employing metabolomics approaches to understand fetal and perinatal pathophysiology requires further research with larger sample sizes and rigorous testing of pilot studies using various omics and traditional hypothesis-driven experimental approaches.
Keywords: congenital anatomic defects, fetal growth restriction, metabolomics, normal pregnancy, pre-eclampsia, prenatal diagnosis, prenatal medicine, pre-term labor and delivery
Introduction
The “-omics revolution” has brought promising options for high-throughput network-based analysis of DNA, RNA, proteins, and metabolites. Integrated analyses of the genome, transcriptome, proteome, and metabolome can reveal normal and pathophysiologic mechanisms that may not be detected by traditional hypothesis-driven experiments or focussed diagnostics (1). The analysis of end products of cellular or organismal biochemical processes using metabolomics approaches could revolutionize our approach to medical diagnosis and therapeutics. Metabolomics employs cutting-edge technologies to assess the presence of low molecular weight compounds, such as carbohydrates, amino acids, peptides, nucleic acids, organic acids, vitamins, and lipids, produced by cells, organs, or whole organisms (2, 3). The interaction and relationships among metabolites can be qualitatively and quantitatively characterized using powerful contemporary computational algorithms and increasingly effective informatics software (4). As a holistic characterization of physiology, metabolomics reflects genetics, environment, and the response to environmental stressors, and can reveal specific metabolic signatures due to genetics, disease, drugs, infection, nutrition, or exposures. Identifying metabolites or metabolic patterns that reflect specific disease states is a primary goal for metabolomics (5) research. In this review, we describe the techniques used in metabolomics, provide examples of metabolomics studies in prenatal care and maternal–fetal medicine, and highlight the tremendous opportunities for metabolomics applications in prenatal diagnosis.
Metabolomics Overview
The compounds produced by cellular metabolism are extremely diverse, involving wide variation in physicochemical properties and concentration. Their detection must be addressed by a suitable and adequate quantitative approach, and in fact, no single experimental assay can capture the full range of metabolic output (2). Therefore, investigators use multiple instruments and several analytic approaches. Two of the most common methods are metabolic profiling and metabolic fingerprinting (6). Metabolic profiling is the quantitative measurement of specific metabolites in a selected biochemical pathway or a particular class of compounds. Similarly, metabolic fingerprinting is a global screening approach that identifies metabolite patterns or “fingerprints” that are associated with known biochemical pathways, specific responses to external stimuli or endogenous signals, or disease processes. With metabolic fingerprinting, we attempt to identify discriminating metabolites that reflect specific alterations, responses, or dysfunction. Depending on the specific scientific question and the analytical approach, metabolomic analyses can be informative, discriminative, or predictive (7) (Figure 1).
Figure 1.
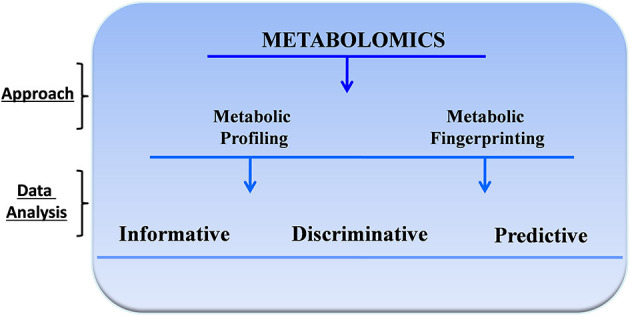
General approach for metabolomics studies.
Informative metabolomic analyses identify and quantify targeted or untargeted metabolites. This approach has been used to develop metabolic databases and to identify critical pathophysiologic pathways, bioactive molecules, and function or disease biomarkers. Discriminative analyses, in contrast, identify metabolic endpoint differences between samples from control and disease populations or before and after specific treatments or perturbation. This approach is typically performed using regression and other multivariate analyses to define clear diagnostic thresholds. Finally, in predictive studies, investigators use metabolic fingerprinting models based on a statistical analysis of metabolite profiles to generate predictive global algorithms that are difficult or impossible to achieve with other more focussed approaches.
Recent advances in robust high-throughput techniques such as 1H-Nuclear Magnetic Resonance spectroscopy (NMR) and Mass Spectrometry (MS) permit simultaneous measurement of many metabolites from a single biological sample (8–12) and more efficient metabolomic studies. Table 1 summarizes the key advantages and limitations of NMR and MS approaches in metabolomics research.
Table 1.
The advantages and limitations of NMR spectroscopy and MS spectrometry as an analytical tool for metabolomics research.
| Nuclear magnetic resonance | Mass spectrometry | |
|---|---|---|
| Analysis | Generally non-selective/untargeted | Both selective/targeted and non-selective/screening |
| Sensitivity | Lower | High using nanomolar detection limit |
| Reproducibility | Very high | Moderate; can depend on sample preparation or storage |
| Detection limits | Low micromolar to nanomolar (with specialized hardware) | Picomolar or lower (with specialized equipment) |
| Sample preparation | Minimal | Often requires specialized extraction, precipitation, or derivatization |
| Sample measurement | All metabolites detected in one measurement | Typically use different separation/preparation for different metabolite classes |
| Sample recovery | Non-destructive—specimen can be recovered | Destructive—but requires tiny amount of specimen |
| Amount of sample used | Usually 200–400 μL | 2–100 μL |
| Number of metabolites detected in biofluids | 40–200 depending on spectral resolution | >500 using various preparations |
| Molecular identification | Easy | Difficult |
| Robustness of the instruments | High | Low |
Although NMR and MS are powerful techniques, the voluminous raw metabolomics data produced is only valuable after careful organization and interpretation with sophisticated contemporary bioinformatics and biostatistical tools (13, 14). Multivariate or principal component analysis transforms an assortment of metabolites into informative profiles by comparing critical elements (or components) that define health, disease, degree of disease, or other conditions and exposures. Supervised learning (e.g., machine learning) is used to transform multivariate data from metabolite profiles into patterns that are biologically relevant (15–18). The general idea behind multivariate methods is to find, if possible, distinct metabolite profiles most strongly associated with the studied phenomenon (19). Once key metabolites have been identified, metabolic network analysis can generate hypotheses for a particular condition(s) (16). Other metabolomics analysis approaches include standard methods such as parametric/non-parametric univariate tests and ANOVA and more sophisticated methodologies. The use of multiple complementary statistical methods allows the investigator to extract the most important information from a single experiment (19). Figure 2 shows the typical workflow for metabolomics experiments and analysis.
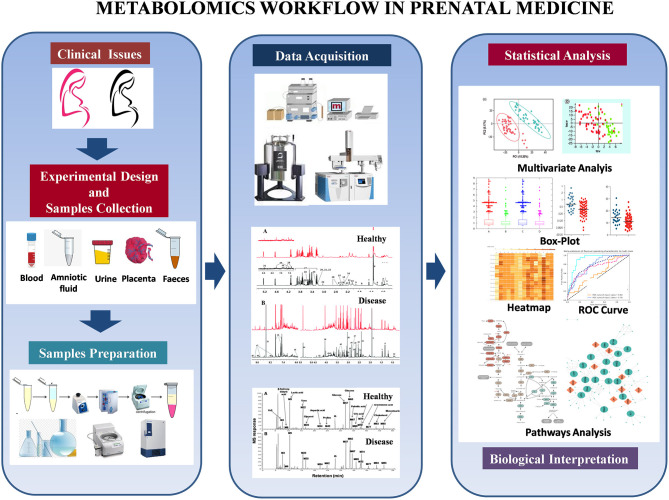
Figure 2.
Metabolomics workflow in prenatal medicine.
Methods
For this review of perinatal metabolomics, we conducted a comprehensive search of PubMed and Google Scholar throughout January 2020 using the following key terms: metabolomics, nuclear magnetic resonance, mass spectrometry, and metabolic profiling. We combined these with the following clinical terms: normal pregnancy, prenatal diagnosis, gestational disorders, chromosomal disorders, pre-eclampsia, fetal growth restriction, pre-term labor, and congenital anatomic defects. We identified additional articles by searching the reference lists of the identified studies. We considered peer-reviewed journal articles that appropriately described their methods and included only unique subjects, data, or analyses. We took into consideration the scientific papers issued between the years 2000–2020, thus observing that the larger number of them were mainly published in the last 10 years. Only papers dealing with the above-mentioned topics were included, those on other pathological conditions were omitted. Systematic reviews and meta-analyses were also excluded from the review together with studies focused on non-human subjects or studies published only in conference proceedings. Of 124 studies identified by the initial search, about 60 studies met the inclusion criteria. Table 2 reports the summary of each study. In particular, the data points taken from each study included the first author, year of publication, analytical technique, sample type and size, type of biomaterial examined, and results. Despite the fact that there have been a good number of pregnancy-related metabolite and metabolomics studies, the use of metabolomics specifically in prenatal diagnosis and pregnancy prediction has been more limited (63–65) although the number of pregnancy metabolomics studies is increasing (Figure 3). Next, we will review recent metabolomics publications for prenatal diagnosis and specific pregnancy pathologies.
Table 2.
Key data points extracted from the cited studies.
| Authors | Year | Pregnancy period | Analytical technique | Sample size | Biomaterial | Result | |
|---|---|---|---|---|---|---|---|
| Normal pregnancy physiology | Jauniaux et al. (20) | 2004 | 1st trimester | HPLC GC-MS |
24 HC | CF AF MP |
CF vs. plasma: ↓ GSH, DHA, ↑α-tocopherol, ↓γ-tocopherol, ≈ Ascorbic acid, Uric acid CF vs. AF: ↑α-tocopherol, Ascorbic acid, Uric acid, ≈ DHA |
| Heazell et al. (21) | 2008 | 1st trimester | GC-TOF-MS | 11 HC | P | With 1% O2: ↑ 2-deoxyribose, erythritol, hexadecanoic acid | |
| Jauniaux et al. (22) | 2005 | 1st trimester | HPLC | 16 CF 12 AF 7 IF 17 MS |
CF AF IF MS |
CF and AF vs. MS: ↑ Inositol, sorbitol, erythritol, ribitol, fructose; ↓ glucose and glycerol CF vs. AF: ↑ inositol, sorbitol, erythritol, ribitol, mannitol, galactose, galactosamine, and glucosamine IF vs. MS: ↑ inositol, sorbitol, mannitol | |
| Murgia et al. (23) | 2019 | 1st trimester |
1H-NMR GC-MS HPLC |
13 HC 8 CD |
CV | Positive correlation with the CRL: Myo-inositol, glutamine, citrate. inositol, glycerol, dehydroascorbic acid, and ribitol Negative correlation with the CRL: 1,5-anydro-D-Sorbitol, D-fructose, and D-mannose |
|
| Aneuploidy screening |
Troisi et al. (24) | 2017 | GC-MS | 220 HC 108 CD |
MS | CA vs HC:↓ Elaidic, mannose, stearic, myristic, ↑ benzoic, linolenic, citric and glyceric acid, 2-hydroxy butyrate, phenylalanine, proline, alanine and 3-methyl histidine | |
| Bahado-Singh et al. (25) | 2013 | 1st trimester | 1H-NMR | 60 HC 30 T21 |
MS | Trisomy 21 vs HC: ↑ 3-hydroxybutyrate, 3-hydroxyisovalerate, and 2-hydroxybutyrate | |
| Bahado-Singh et al. (26) | 2013 | 1st trimester | 1H-NMR | 114 HC 30 T18 30 T21 |
MS | Trisomy 18 vs. HC: ↑ 2-hydroxybutyrate, glycerol Trisomy 18 vs. Trsisomy 21: ↑ TMA, ↓ threonine,creatine, and formate |
|
| Pinto et al. (27) | 2015 | 1st/2nd trimester | 1H-NMR | 74 HC 45 CD |
MP | CD vs. HC, 1st trimester: ↑ketone bodies; ↓ glucose, pyruvate, citrate, HDL, proline, methanol CD vs. HC, 2nd trimester: urea, creatinine, acetate, LDL, VLDL |
|
| Diaz et al. (28) | 2013 | 2nd trimester | 1H-NMR | 34 HC 33 CD 13 T21 |
U | Trisomy 21 vs. others CD: ↓ Glucose, N-methyl-2-pyridone-5-carboxamide | |
| Trivedi and Iles (29) | 2015 | 1st/2nd trimester | ZIC-HILIC-IT-TOF RPLC-IT-TOF | 93 HC 23 T21 |
U | Trisomy 21 vs. HC: ↑ Dihydrouracil, ↓ Progesterone | |
| Murgia et al. (23) | 2019 | 1st trimester |
1H-NMR GC-MS HPLC |
13 HC 8 CD |
CV | CD vs HC: ↑ Lactate, asparagine, branched-chain aminoacids, D-sorbitol, 1,5-anydro-D-sorbitol, D-fructose, dehydroascorbic acid, and glucose, ↓ myo-inositol, glycerol, fumarate, betaine, and acetate, cholesterol, pyruvic acid, palmitic acid, inositol, homoserine, stearic acid, GSH and GSSG | |
| Pre-eclampsia | Dunn et al. (30) | 2009 | 1st trimester | UPLC–MS | 6 HC 6 PE |
Explanted CV 1% O2 or 6% O2 |
PE 1% O2 vs. HC: ↑ Progesterone, Glycerol, Valinol or choline, Diglyceride. Alteration in glutamate and glutamine, tryptophan metabolism and leukotriene or prostaglandin metabolism |
| Austdal et al. (31) | 2014 | 2nd trimester | 1H-NMR | 10 HC preg. 10 HC not preg. 10 PE |
U MS |
Urine PE vs. HC pregnant: ↑ choline, ↓ glycine, p-cresol sulfate and hippurate Serum PE vs. HC pregnant: ↑ lipids, VLDL/LDL, histidine, glycerol |
|
| Zhou et al. (32) | 2017 | Delivery | GC-MS | 11 HC 11 PE |
Placental mitochondria | PE vs. HC: ↓ ATP, citraconate and caprylate; ↑ arachidonate, bihomo-γ-linoleate, and γ-linoleate, docosapentaenoate, myristate in PE | |
| Bahado-Singh et al. (33) | 2017 | 1st trimester | 1H-NMR | 55 HC 29 PE |
MS | PE vs. HC: alteration in Branch chain amino acids | |
| Bahado-Singh et al. (34) | 2015 | 1st trimester | 1H-NMR | 108 HC 50 PE |
MS | PE vs. HC: ↑ 2-hydroxybutyrate, 3-hydroxyisovalerate, citrate, ↓ arginine, acetone, glycerol | |
| Bahado-Singh et al. (35) | 2017 | 1st trimester 3rd trimester |
1H-NMR LC-MS MALDI-TOF |
35 PE 63 HC |
MS | 1st trimester: putrescine, urea and carnitine, TNF-α, RPL41, ATP5E, TBP 3rd trimester: methylhistidine, serotonin, citrate, hexose and propylene glycol, HLA-DR B1, GTP binding protein-3 |
|
| Koster et al. (36) | 2015 | 1st trimester | UPLC-MS/MS | 500 HC 68 early PE 99 late PE |
MS | Early PE: combination of MC, MAP, PAPPA, PLGF, taurine, stearoylcarnitine Late PE: combination of MC, MAP, PAPPA, PLGF, stearoylcarnitine |
|
| Kuc et al. (37) | 2014 | 1st trimester | UPLC-MS/MS | 500 HC 68 Early PE 99 Late PE |
MS | Early PE vs. HC: ↓ taurine and asparagine Late PE vs. HC: ↓ glycylglycine |
|
| Fetal | Bernard et al. (38) | 2017 | 2nd trimester 3rd trimester Post-natal |
GC | 1,171 Preg | MS | Linoleic acid positively associated with birthweight, BMI, head circumference, neonatal abdominal adipose tissue volume High DHA levels were associated with greater length/height |
| Visentin et al. (39) | 2017 | 3rd trimester | GC-MS | 12 AGA 12 IUGR 10 SGA |
MP FUVP |
SGA vs. IUGR: ↑ C6:0 (in maternal plasma) SGA vs. AGA: ↑ C8:0, C10:0, and C12:0 (in maternal plasma) No statistical differences between AGA and IUGR MCFAs fetal to maternal ratio is >1 for IUGR group MCFAs fetal to maternal ratio is <1 for SGA and AGA |
|
| Clinton et al. (40) | 2020 | 1st trimester 2nd trimester |
GC-MS | 30 HC 30 FGR |
U | 1st trimester FGR vs HC: ↑ acetoacetate, 2-methylglutaric acid, benzoic acid 2nd trimester FGR vs HC: ↑ 1,2-propanediol, benzoic acid Increased level of cholesterol from 1st trimester FGR to 2nd trimester FGR |
|
| Dessì et al. (41) | 2014 | Post-natal | 1H-NMR | 17 AGA 12 IUGR 9 LGA |
U | IUGR vs. AGA: ↑ Myo-inositol, creatinine, creatine, citrate, betaine/TMAO glycine; ↓ urea, aromatic coumpounds, branched chain amicoacids LGA vs. AGA: ↑Myo-inositol, creatinine, aminoacids; ↓ urea, formate, citrate IUGR vs. LGA: ↑ Myo-inositol, creatinine, creatine, citrate, betaine/TMAO, glycine, acetate; ↓ urea, aromatic coumpounds |
|
| Dessì et al. (42) | 2011 | Post-natal | 1H-NMR | 30 HC 26 IUGR |
U | IUGR vs. HC: ↑ Myo-inositol, creatinine | |
| Favretto et al. (43) | 2012 | Post-natal | LC-MS | 22 IUGR 21 AGA |
FUVP | IUGR vs. AGA: ↑ Phenylalanine, tryptophan, and glutamate | |
| Sanz-Cortés et al. (44) | 2013 | Post-natal | 1H-NMR | 23 Early IUGR 23 AGA 56 Late IUGR 56 AGAs |
FUVP | Early and late IUGR vs. HC: ↑ Unsaturated lipids and VLDL levels; ↓ phenylalanine, tyrosine, choline Early IUGR vs. HC: ↓ glucose; ↑ acetone, glutamine and creatine Late IUGR vs. HC: ↓ Valine, leucine |
|
| Liu et al. (45) | 2016 | Post-natal | LC-MS | 60 IUGR 60 AGA |
Heel-stick blood | Newborns of different weight percentages: alteration in alanine, homocysteine, methionine, ornithine, serine, tyrosine, isovaleryl carnitine, and eicosenoyl carnitine IUGR vs. AGA: ↓ Alanine, homocysteine, methionine, ornithine, serine, and tyrosine Pre-term vs. full-term IUGR: ↑ homocysteine, heptanoyl carnitine decanoyl carnitine, methylmalonyl carnitine, glutaryl carnitine, sebacoyl carnitine, hydroxyacetyl carnitine, and hydroxyhexadecenyl carnitine |
|
| Porter et al. (46) | 2020 | 3rd trimester | LC-MS GC-MS |
14 Low EFW 9 Normal UmA 5 Abnormal UmA 10 Normal UtA 3 Abnormal UtA |
MP | Abnormal UmA vs. normal UmA: ↑ ornithine Abnormal UtA vs. normal UtA: ↓ dimethylglycine, isoleucine, methionine, phenylalanine, 1-methylhistidine |
|
| Bahado-Singh et al. (47) | 2020 | Post-natal |
1H-NMR DI-LC-MS/MS |
30 HC 19 FGR |
P | Combination of 3-hydroxybutyrate, glycine and PCaa C42:0 for FGR detection | |
| Sulek et al. (48) | 2014 | 2nd trimester | GC-MS | 30 Mother of SGA 42 Mother of HC |
Hair | Combination of lactate, levulinate, 2-methyloctadecanate, tyrosine, and margarate | |
| Pre-term labor and delivery | Caboni et al. (49) | 2014 | Term of gestation | GC-MS1H-NMR | 59 Preg | U | Alanine, glycine, acetone, 3-hydroxybutiyric acid, 2,3,4-trihydroxybutyric acid and succinic acid characterize the late phase of labor |
| Baraldi et al. (50) | 2016 | 3rd trimester | UPLC-MS | 13 Pre-term 11 Term |
AF | PTD vs. TD: ↑ 3-methoxybenzenepropanoic acid, 4-hydroxy nonenal alkyne, muconic dialdehyde. ↓ phosphatidylcholine | |
| Graça et al. (51) | 2010 | 2nd trimester | 1H-NMR | 27 Pre-term 82 Term |
AF | Alanine, allantoin, citrate, and myoinositol | |
| Menon et al. (52) | 2014 | 3rd trimester | GC-MS LC-MS |
25 Pre-term 25 Term |
AF | PTD vs. TD: Changes in Histidine metabolites (cis-urocanate, trans-urocanate, 1-methylimidazoleacetate) ↑ 4-acetamidophenol, 2-methoxyacetaminophen sulfate, 3-(cysteine-S-yl) acetaminophen, 3-(N-acetyl-L-cystein-S-yl) acetaminophen, p-acetamidophenylglucuronide, progesterone, bile acids; ↓ squalene, lathosterol, cortisolo, cortisone, metabolites related to caffeine, LCFAs, EFA, arachidonate, mead acid |
|
| Romero et al. (53) | 2010 | 2nd trimester | GC-MS LC-MS |
52 Pre-term without IAI 60 Pre-term with IAI 56 Term |
AF | Pre-term without IAI: ↓ carbohydrates and amino acids Pre-term with IAI:↓ carbohydrates; ↑ amino acids, Term:↑ mannose, galactose, fructose; ↓ alanine, glutamine, glutamic acid |
|
| Virgiliou et al. (54) | 2017 | 2nd trimester | UHPLC–MS | 35 Pre-term 35 Term |
AF MS |
Pre-term (amniotic fluid): ↓ pyruvic acid, inositol, glutamine; ↓ glutamate Pre-term (serum): ↑ unsaturated lipids, pyroglutamic acid; ↓ hypoxanthine, tryptophan |
|
| Lizewska et al. (55) | 2018 | Post-natal | LC-MS | 57 Pre-term 49 Threatened pre-term labor 25 Term |
MP | Threatened pre-term vs Pre-term and Term: ↓antiinflammatory omega 3, proinflammatory omega 6 fatty acids Pre-term vs Threatened pre-term: ↑ DHA |
|
| Tea et al. (56) | 2012 | Post-natal | 1H-NMR | 35 VLBW 35 Term |
FUVP MP |
Fetal umbilical vein plasma vs Maternal plasma: ↑ amino acids, glucose, and albumin-lysyl; ↓ LDL, VLDL, lipid VLBW vs term: ↓ acetate; ↑ lipids, pyruvate, glutamine, valine, threonine |
|
| Congenital anatomic defects | Groenen et al. (57) | 2004 | 2nd trimester 3rd trimester |
1H-NMR | 14 Spina bifida 18 HC |
AF | Spina bifida vs HC: ↑ succinate, glutamine; ↓ creatine, creatinine |
| Bock (58) | 1994 | 2nd trimester 3rd trimester |
1H-NMR | 70 Preg | AF | PE: ↑ Choline, succinate, acetate Spina bifida: ↑ Lactate, glutamate, acetate |
|
| Clifton et al. (59) | 2006 | 2nd trimester 3rd trimester |
1H-NMR | 3 Preg | AF | 3rd trimester vs. 2nd trimester: ↑ choline | |
| Pearce et al. (60) | 1993 | 2nd trimester 3rd trimester |
31P NMR | 16 Preg | AF | Disaturated phosphatidylcholines positively correlates with the gestational age and fetal maturation | |
| Graça et al. (61) | 2007 | 2nd trimester | 1H-NMR | 16 HC Preg | AF | Methodological article | |
| Graça et al. (62) | 2009 | 2nd trimester | 1H-NMR | 51 HC 12 FM |
AF | Fetal malformation vs HC: ↓ leucine, valine, ethanol, alanine, proline, glutamate, glucose; ↑ methionine, succinate, glutamine, citrate, glycine | |
| Bahado-Singh et al. (63) | 2014 | 1st trimester |
1H-NMR LC-MS |
27 CD 59 HC |
MS | CD vs. HC: ↓ C3-OH, C5-OH(C3-DC-M), C14:1, and SM C22:3, alteration in acetone, ethanol, acetate, and pyruvate levels | |
| Single gene disorders | Monni et al. (64) | 2019 | 1st trimester | GC-MS | 27 HC 7 β-thal het 7 β-thal hom |
CV | Homozygous vs HC and heterozygous: ↑ Glutamic acid, glycerol-1-phosphate, malic acid, arachidonic acid, glucose, and ribose; ↓ docosatetranoic acid, palmitoleic acid |
HC, healthy controls; CF, coelomic fluid; AF, amniotic fluid; IF, intervillous fluid; MS, maternal serum; MP, maternal plasma; U, urine; CV, chorionic villi; FUVP, Fetal umbilical vein plasma; P, placenta; CD, chromosomal disorders; T21, trisomy 21; T18, trisomy 18; T13, trisomy 13; PE, pre-eclampsia; FM, fetal malformation; Preg, pregnancies; MAP, Mean arterial pressure; TNF-α, Tumor necrosis factor-alpha; RPL41, 60S ribosomal protein L41; ATP5E: ATP synthase subunit epsilon; TBP, TATA box binding protein - associated factor; EFW, estimated fetal weight; UmA, umbilical artery; UtA, uterine artery; IUGR, intrauterine growth restriction; AGA, adequate-for-gestational-age; SGA, small-for-gestational-age; MCFAs, Medium chain fatty acids; PTD, pre-term delivery; TD, term delivery; LCFAs, long-chain fatty acids; EFA, essential fatty acids. IAI, intra-amniotic infection/inflammation; DHA, docosahexaenoic acid; HDL, High density lipoprotein; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein.
Figure 3.
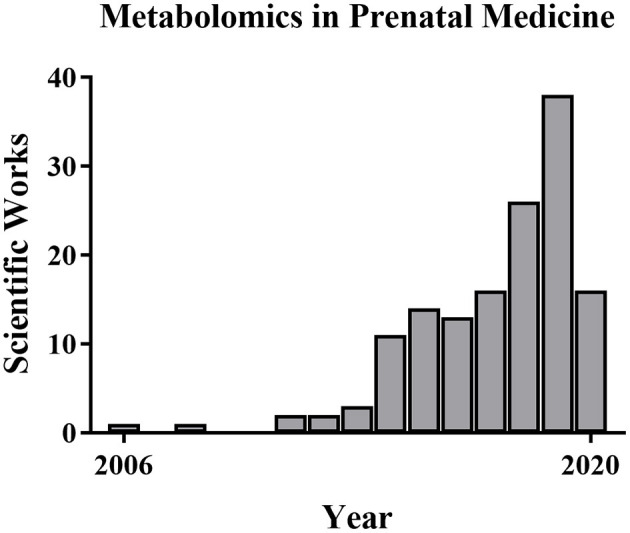
Metabolomics publications in prenatal medicine. The number of metabolomics publications in prenatal medicine is low but increased from 2006 to 2019 based on PubMed searches.
Prenatal Diagnosis
Contemporary fetal assessment uses both non-invasive and invasive methods. Non-invasive methods include maternal factors and history, fetal ultrasound imaging, and maternal serum analyte or cell-free fetal DNA (cfDNA) screening. Maternal blood analyte tests, such as the first trimester screen (66), sequential screen, and quadruple test (67), and maternal serum cfDNA screening carry no procedural risk for the pregnancy, but these only assess risk and cannot diagnose. In contrast, invasive diagnostic tests require fetal samples of the placenta (chorionic villus sampling) or skin cells (amniocentesis) but provide definitive results. Invasive testing incurs a small chance of procedural complications, including a 0.1–0.2% risk of fetal loss (68). The earliest possible diagnosis of fetal aneuploidy and other congenital defects is highly desired because it affords more time for decision making and reduces risks of pregnancy interruption if that is what the couple chooses.
In first trimester non-invasive screening, we estimate risk from a combination of maternal factors (e.g., age, weight, and medical considerations, such as diabetes), fetal factors (e.g., nuchal translucency [NT], nasal bone, and fetal heart rate), and feto-placental factors in maternal blood (ß-hCG and PAPP-A). The combined first trimester screen detects trisomies 21, 18, and 13 with 90, 97, and 92% sensitivity but with a set positive rate of 5% (69). The second trimester quadruple test assesses α-fetoprotein, ß-hCG, estriol, and inhibin-A and detects trisomy 21 with ~80% sensitivity and a false positive rate of 5% (67). The newest non-invasive screening test is maternal serum cfDNA which exhibits very high sensitivity and specificity for trisomies 21, 18, and 13 by examining free chromosomal DNA fragments in maternal blood that are released by normal placental apoptosis (70). Although screening tests are all helpful for alerting us about fetuses at higher risk for aneuploidy, their positive predictive value varies dramatically depending on maternal age-related risk. Many women decline invasive testing despite the minimal risk and high diagnostic accuracy of CVS or amniocentesis. Therefore, developing better non-invasive tests that cover a broader range of disorders and have lower false-positive rates is a critical need in the field (71). Omics approaches could be a reasonable solution.
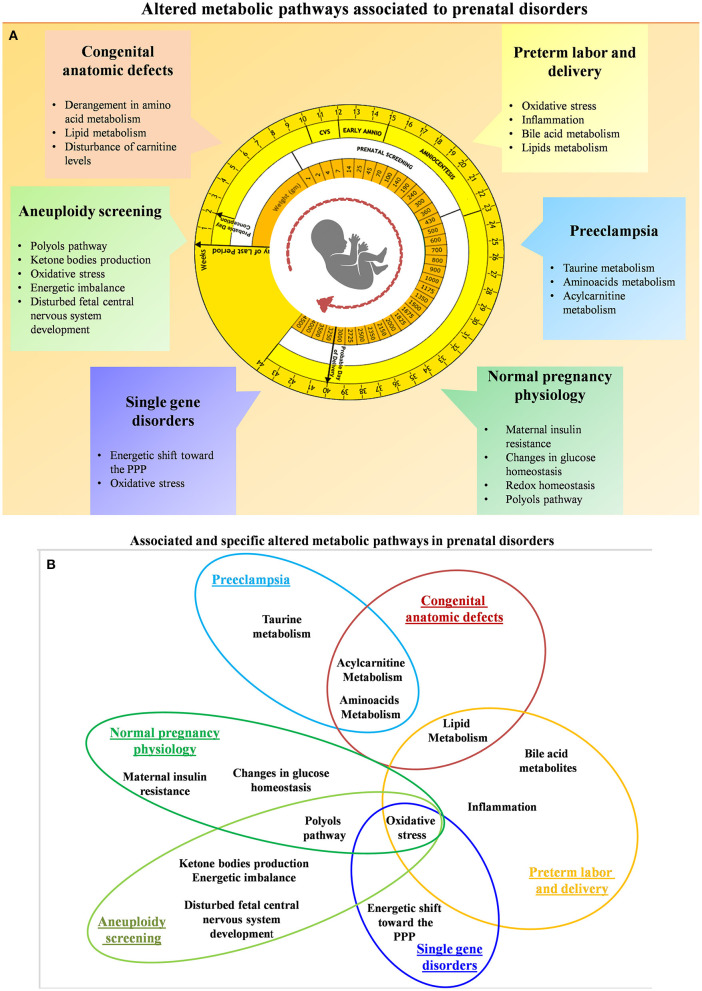
High-dimensional biology techniques (i.e., multi-omics) that combine genomic, transcriptomic, proteomic, and metabolomic approaches have been applied in both normal and complicated pregnancies (72–86). Because metabolomics provides a final readout of cellular physiology, it may be the best single omics approach to characterize normal vs. pathologic pregnancies. Examining different maternal-fetal compartments at different stages of pregnancy and clearly characterizing changes throughout gestation is required in order to determine the best tests and timing for particular clinical samples. Ideally, materia for non-invasive prenatal metabolomics would be obtained from maternal blood (plasma or serum) (87), urine (88), or cervico-vaginal secretions (89). Invasive sampling can also be used to obtain amniotic fluid (90), placental biopsy (23), and cord blood (43). Samples from invasive approaches might be useful to characterize the relationship between fetal and maternal metabolites or eventually might be used for confirmatory testing as it is today. Thorough metabolomic characterization of pregnancy across gestation might suggest new diagnostic or treatment options. So far, gestational metabolomics has been used to characterize normal physiologic changes of pregnancy as well as common complications including fetal aneuploidy, pre-eclampsia, pre-term birth, fetal growth restriction (FGR), pre-term parturition, congenital fetal anomalies, and single-gene disorders. A summary of the main investigated perinatal issues and the specific identified metabolic pattern is represented in Figures 4A,B. Below, we review recent work in each of these areas.
Figure 4.
(A) Summary of the altered metabolic pathways associated with prenatal disorders. (B) Associated and specific altered metabolic pathways in prenatal disorders.
Normal Pregnancy Physiology
Pregnancy requires a wide range of adaptive physiologic changes throughout gestation in maternal, fetal, and placental function. For example, circulating maternal metabolic products such as triglycerides, cholesterol, free fatty acids, and phospholipids change dramatically during pregnancy (91) to satisfy fetal energy and catabolic needs in utero and the production of an adequate maternal milk supply post-partum (92). Maternal insulin resistance also increases significantly with gestational age in normal pregnancy to ensure adequate transfer of glucose to the fetus (93). Maternal inositols increase with gestational age, positively correlating with fetal crown-rump length. They are correlated with insulin sensitivity and could be mechanistically linked to glucose homeostasis (91).
The placenta plays a key role in regulating the metabolic milieu of pregnancy. Several studies have described an altered placental metabolic profile, due to environmental factors. For example, the oxygen tension during placental explant culture in vitro can dramatically alter metabolic signatures. The human placenta is adapted for an initial hypoxic environment (20) then switches metabolism to accommodate increased oxygenation in the second trimester when extensive spiral artery remodeling occurs (94). The intervillous oxygen tension increases from 2–3% at 8 weeks to 8.5% by 12 weeks (95) of gestation with concurrent increased placental oxidative stress. Upregulation of placental antioxidant factors maintains redox homeostasis (96), and the metabolic profile of normal placental villus explants varies with oxygen culture conditions (21). Changes in hexadecanoic acid, erythritol, and 2-deoxyribose are particularly prominent. Moreover, placental cholesterol levels were found to be increased in correlation with the CRL. The higher concentration of cholesterol may be the result of increased levels of progestational hormones. In fact, maternal blood cholesterol represents the precursor of both progesterone and estrogen (23). Other gestational age-related metabolic changes (22, 23) include placental polyol pathways which are very active in the first trimester. One hypothesis regarding elevated polyols in early pregnancy is that they are an early carbohydrate source for the placenta and embryo. Additionally, the polyol pathway may facilitate the re-oxidation of pyridine nucleotides under low oxygen conditions, helping regulate intracellular pH during periods of robust glycolysis (23). These are just a few examples of the way in which metabolomics has been used to characterize the normal physiologic changes of pregnancy.
Aneuploidy Screening
Chromosomal abnormalities are the most frequent fetal problem diagnosed in the first trimester. Trisomy 21 (T21; Down syndrome), trisomy 18 (T18; Edwards syndrome), trisomy 13 (T13; Patau syndrome), and abnormal sex chromosomes (e.g., XO, Turner syndrome, or Monosomy X) are the most commonly screened chromosome errors. Standard routine prenatal care includes screening for these common aneuploidies (97). Although current screening tests (see above) successfully identify fetal aneuploidy in early pregnancy, the search for additional genetic biomarkers for a wider range of aneuploidies and improved sensitivity and specificity is an important goal in the science of prenatal diagnosis (24).
Several studies have examined the utility of metabolic screening for aneuploidy. For example, Bahado-Singh et al. used NMR to analyze first trimester maternal serum from T21 and control pregnancies (25). They found 11 metabolites differed between groups, including the novel biomarkers 3-hydroxybutyrate and 2-hydroxybutyrate. The first is an indispensable energy source for extrahepatic tissues such as the brain, involved in growth and myelination; the second is involved in oxidative stress defense (25). Bahado-Singh similarly studied first trimester maternal serum from normal and T18 pregnancies (26) and found that glycerol and 2-hydroxybutyrate best identified T18 fetuses. Combining discriminatory metabolites with clinical and demographic parameters, they detected T18 with 90% sensitivity and 100% specificity using the delta nuchal translucency (98) and 2-hydroxybutryate levels together. Several other metabolites appear to distinguish between T18 and T21 fetuses including threonine, trimethylamine, creatine, and formate (26). Troisi et al. published similar findings for pregnancies affected by any trisomy (combined group of T21, T18, and T13) vs. normal controls (24). A specific pattern of metabolites was altered in trisomic compared to normal fetuses, suggesting a metabolic environment of elevated oxidative stress and disturbed fetal central nervous system development. Yet another group found associations between T21 and abnormal high-density lipoprotein (HDL), methanol, and proline in the first trimester and abnormal creatinine, acetate, HDL, and low-density lipoprotein (LDL) in the second trimester (27). Overall, these studies suggest increased oxidative stress in aneuploid pregnancies and switched fuel metabolism resulting in β-oxidation and ketone body production. Aneuploid fetuses appear to utilize glucose, pyruvate, and citrate less as energy sources compared with normal pregnancies.
In addition to maternal plasma and serum, some metabolomics studies of aneuploidy have evaluated maternal urine specimens. Maternal urine metabolic signatures for chromosomal disorders generally showed altered 3-hydroxybutyrate, 2-ketoglutarate, and 1-methylhistidine. Urine metabolites specifically associated with T21 included N-methyl-2-pyridone-5-carboxamide (28). Trivedi and Iles clarified the altered cellular metabolism with T21, suggesting that metabolic profiles may improve detection of both aneuploidy and inborn errors of metabolism (29). Comparing the mass spectrometry urine metabolome of women with aneuploid or normal fetuses revealed altered levels of progesterone and dihydrouracil (29).
Our own laboratory has characterized metabolic networks from the first trimester placenta obtained via transabdominal chorionic villus sampling (TA-CVS) (23). CVS placental biopsies are ideal for metabolic analyses as they are obtained from the undisturbed placenta in situ rather than after delivery, miscarriage, or termination. We compared normal and aneuploid fetuses (T21, T18, T13) using NMR, GC-MS, and HPLC and found critical differences in energy metabolism and polyol pathways. The aneuploid placenta demonstrates excessive polyol pathway activation, decreased glutathione levels, and increased dehydroascorbate. Additionally, thorough characterization of the placental metabolome may significantly improve our ability to interpret changes in the maternal metabolic profile caused by fetal or placental dysfunction.
Pre-eclampsia
Pre-eclampsia (PE) is a gestational hypertensive syndrome that complicates 2–8% of pregnancies worldwide (99) and is a major cause of maternal/fetal morbidity and mortality. The current definition of PE, according to the International Society for the Study of Hypertension in Pregnancy (ISSHP) (100) and the American College of Obstetricians and Gynecologists (ACOG) (101), is a new onset of hypertension (blood pressure ≥140 mmHg systolic or ≥90 mmHg diastolic) at ≥20 weeks of gestation and proteinuria (≥300 mg/24 h or protein-to-creatinine ratio >30 mg/mmol or ≥2+ on dipstick testing) or cases without proteinuria but with severe range blood pressure (>160/110) or evidence of organ dysfunction (e.g., hematologic, renal, hepatic, and neurologic). This new definition of PE resulted in an increase in pregnancies diagnosed with PE but generally milder disease (102).
Although the exact cause has not been identified, PE is thought to be due to the interaction of maladaptive trophoblast-derived signaling factors [e.g., soluble FMS-like tyrosine kinase-1 (sFlt-1) and placental growth factor (PlGF)] (103, 104) with susceptible maternal physiology. One theory is that PE is triggered by reduced uterine perfusion leading to placental oxidative stress and apoptotic release of pro-pre-eclampsia signals. Despite decades of PE research, the only available treatment remains delivery (i.e., removal of the placenta), and the only marginally helpful prevention comes from maternal risk reduction and possibly low-dose aspirin prophylaxis (105). Combined screening using maternal factors, mean arterial pressure, uterine artery, Pulsatility Index (PI), and maternal serum PlGF has been proposed to predict about 90% of early PE (Early-PE; <34 weeks), 75% of late pre-term PE (Late Pre-term-PE; 34 to <37 weeks) and 45% of full-term PE (Term-PE; ≥37 weeks) (106). Identifying and integrating new biomarkers to predict PE prior to symptoms could facilitate diagnosis and prevention. Given the heterogeneous presentation of gestational hypertensive disorders, it is unlikely that only one or several biomarkers will predict all types of PE (e.g., gestational hypertension, PE, PE with severe features, HELLP, and eclampsia). Therefore, broad network analysis using metabolomics may be the most appropriate approach to solving this important recalcitrant clinical problem.
Several groups have examined differences in maternal metabolomics in normal and PE pregnancies. In vitro studies of villous trophoblast explants using LC-MS identified differences in culture media metabolites for normal and PE patients (30). Furthermore, the metabolic profile changes in response to different oxygen conditions varied between normal and PE placenta, suggesting not only baseline differences in PE metabolism but persistent functional differences in the response environmental stressors. Studies have not determined whether the changes in hypoxia responses in vitro preceded PE or were caused by it (30). In vivo changes due to PE have been studied in maternal urine and serum (31). Women with PE have increased urine markers of oxidative stress and renal or liver dysfunction as well as altered serum metabolites. In particular, higher total lipid content and lipoprotein levels were observed with PE (31). One study used metabolomics and Western blotting to examine placental mitochondrial funding during severe PE. Isolated placental mitochondria from severe preeclamptics showed reduced ATP levels, higher fatty acid levels, and decreased fatty acid catabolism (32).
Some of the most intriguing prenatal diagnostic studies in PE research have retrospectively assessed early metabolic changes from normal control pregnancies vs. PE cases. For instance, Bahado-Singh et al. investigated serum metabolic profiles in patients with early and late-onset PE compared with healthy controls using specific metabolic panels (33, 34). The metabolites 3-hydroxyisovalerate, arginine, and glycerol were particularly increased in Early-PE, especially when combined with uterine artery PI. An approach using metabolic, proteomic, and ultrasound parameters reached over 90% sensitivity and nearly 90% specificity for predicting PE. An important milestone in the PE study has been filed when metabolomic was combined with proteomic approaches (35) and clinical maternal features to enhance omics diagnosis from early pregnancy maternal blood samples. Significant changes in G-protein-coupled receptors, signal transduction serotonin, and glycosaminoglycan metabolisms emerged as the final result.
Similarly, Koster et al. constructed a prediction model using maternal clinical factors (e.g., maternal age, history of pre-eclampsia, other risk factors) for baseline risk and then adding protein and metabolite measures (36). More in detail, acylcarnitines of first-trimester maternal serum from women with and without PE were analyzed and correlated with the clinical factors. Interestingly, the correlation between markers selected for prediction modeling (hexanoylcarnitine, octanoylcarnitine, decenoylcarnitine, and decanoylcarnitine) showed an R > 0.8, suggesting a potential role of this class of compounds. That approach reached ~70% sensitivity and 90% specificity for Early-PE. For Late Pre-term-PE, the sensitivity and specificity were only ~30 and 90%. Moreover, these findings suggested the stearoylcarnitine as a biomarker for both EO- and LO-PE. The concentration of this biomolecule improved the prediction power of the clinical factors normally used to evaluate the baseline risk.
An important critique of PE metabolomics studies (and metabolomics diagnostic studies in general) is the wide variability in reported significant metabolites by different groups. Indeed, some studies evaluating metabolites for PE prediction have found that clinical risk factors are better than metabolite levels and that metabolite assessment does not add predictive power (37). Nonetheless, further studies may identify consistent factors we might use to create accurate, sensitive, specific prediction algorithms for PE (35).
Fetal Growth Restriction
Fetal growth restriction (FGR) is defined by a fetus that fails to meet expected growth for gestational age using estimated fetal weight below the 10th percentile. FGR occurs in 5–10% of all pregnancies (107) due to multiple causes and increases the risk of adverse perinatal outcomes. The primary challenge for FGR is that fetuses measuring below the 10th percentile for gestational age may be either constitutionally small for gestational age (SGA) or have pathologic growth restriction. Thus, there is no agreed-upon single gold standard for FGR diagnosis, and the possibility of developing metabolomics approaches to diagnose FGR pathology has generated significant interest.
Several metabolomics studies have highlighted abnormal lipid metabolism during pregnancies resulting in SGA newborns (38, 108). Interestingly, near delivery, the mother-to-newborn ratio of medium-chain fatty acids was decreased with FGR, suggesting increased energetic and structural metabolic demands of the infant (39). Differences in cholesterol synthesis were observed in FGR fetuses compared to normal fetuses (40). In particular cholesterol levels during pregnancy only increased slightly in FGR cases (2.48-fold change) while increasing substantially in normally grown controls (6.54-fold change). In neonatal urine, newborns from FGR pregnancies had increased myo-inositol, which correlated with downregulation of adipose free fatty acid release (41, 42).
Amino acid metabolism also appears to play a role in FGR, with several amino acids differing between the average for gestational age and FGR newborns (43, 44) especially ornithine (45). A role for ornithine in fetal growth was also identified in maternal serum from pregnancies affected by extreme FGR (<5th %ile) and abnormal umbilical artery Dopplers (46). Correlations between ornithine and umbilical blood flow may be related to angiogenesis via polyamine pathways. Placentae from FGR pregnancies show almost universally decreased metabolite levels (47), and generally reduced metabolite concentrations have also been detected in the maternal hair from FGR pregnancies (48). Recent mechanistic research suggests that there is a general disruption in fetal energy substrates and metabolism in FGR, but the unique metabolic adaptations of FGR vs. SGA have not been well-studied.
Pre-term Labor and Delivery
About 10% of all deliveries occur pre-term at <37 weeks. Pre-term birth (PTB) and the consequences of prematurity are the greatest contributors to neonatal morbidity and mortality (109, 110). Prematurity also has important long-term consequences for lifelong health and disease, and survivors of prematurity have increased risk for chronic medical conditions such as cardiovascular disease, metabolic syndrome, stroke, dyslipidemia, and neurocognitive dysfunction (111–113). Idiopathic pre-term labor (PTL) and pre-term prelabor rupture of membranes (PPROM) account for about two-thirds of pre-term deliveries while indicated deliveries (e.g., pre-eclampsia, placental abruption) account for the other third (114). PTB is the common pathophysiologic endpoint of a wide range of causes, and decades of research have failed to develop effective treatments or even provide adequate screening algorithms (115). Omics-sciences could play a role in defining normal pre-term/term physiology and the pathophysiologic pre-term processes leading to early labor and delivery (116, 117). Some have suggested that all of the great obstetrical syndromes, including PTB, represent different manifestations of various placental dysfunction or maladaptation which might be characterized effectively using metabolic assessment.
Many PTB investigators have focussed on specific markers of inflammation. Inflammation signaling is frequently associated with pre-term labor/PTB (118, 119), but a single universal marker or even several markers may not be sufficient to characterize the diverse etiologies leading to the common final pathway of labor. Analysis of urine from pregnant women before and after labor onset with GC/MS and NMR found 18 unique metabolic changes associated with labor status (49). Analysis of amniotic fluid metabolites has been of particular interest, as the fetus and placenta may be a critical cog in the pregnancy clock (50–54). One study compared amniotic fluid and maternal serum, identifying energy metabolism factors-associated PTB in the fetal compartment (pyruvate, glutamate, and glutamine) that were distinct from maternal serum metabolites that discriminated PTB (54). Another study used amniotic fluid metabolic profiling to compare women with pre-term contractions at risk for spontaneous PTB with or without intra-amniotic infection. Altered amniotic fluid carbohydrates were associated with PTB regardless of infection, while increased amino acids were present only with PTB and intrauterine infection (53). Graca et al. found decreased amino acids, citrate, and myo-inositol and increased allantoin and hexose in amniotic fluid from second trimester PTB cases (51).
Metabolic fingerprinting of pre-term labor that goes on to PTB has been attempted (55). This is a critical challenge in the field, as the majority of PTL does not proceed to PTB. Several studies found increased fatty acids associated with PTB (53). PTB was also associated with decreased levels of acetate and increased lipids (56) and a few amino acids. Interestingly, there may be important differences in PTB metabolic signatures by race and ethnicity. Menon et al. evaluated amniotic fluid from African American women with early spontaneous PTB vs. term birth. Bile acids, steroids, and xanthines were altered and the authors found 8-fold increased pantothenol levels in women who delivered early (52). To achieve the full potential of metabolomics in PTB research, careful selection of comparison groups and rigorous deep phenotyping of both maternal and fetal characteristics is needed.
Congenital Anatomic Defects
It is well-known that some congenital/structural defects lead to changes in perfusion, organ function, or other factors, which could change the fetal metabolic signature and perhaps be reflected in maternal serum. Thus, even though congenital structural defects are not metabolic issues per se, we may detect some anomalies through metabolic approaches. We might also exploit metabolomics to elucidate causative metabolic mechanisms. Although NMR of amniotic fluid has been used for decades to characterize neural tube defects (57) and fetal lung or kidney maturity (58–60), more recently NMR has been paired with MS to identify other malformations. Graça et al. evaluated second-trimester amniotic fluid from normal pregnancies and from fetuses affected by congenital anatomic anomalies (61, 62). Surprisingly, maternal and fetal characteristics (e.g., maternal age and fetal sex) had no effect on the metabolic profile of normal fetuses. However, in multivariate supervised analysis, specific changes in glucose, succinate, and some amino acids and proteins were clear (62). Overall, these changes suggested a shift to glycolysis, perhaps due to hypoxic stress. Thus, it is possible that metabolic assessment could be used to screen for anatomic anomalies in combination with detailed fetal ultrasound imaging, similar to maternal serum alpha-fetoprotein for neural tube and abdominal wall defects.
Metabolomics has also been used to specifically evaluate fetal congenital heart disease (CHD) (120, 121). A few studies report metabolic screening for both structural and functional fetal heart disorders. For example, one report described metabolic changes in first trimester maternal serum for chromosomally normal fetuses with structural heart disease, showing altered phosphatidyl-choline and sphingolipids (122). Whether these metabolic changes were related to cardiac energetic processes or other end-organ compensation for altered heart function (e.g., liver dysfunction) was not determined (122). This sort of finding suggests that metabolic characterization could be used as an additional tool for CHD screening.
Single-Gene Disorders
Common single-gene disorders are routinely evaluated with parental carrier testing and increasingly with cfDNA sequencing techniques. It is possible that some single-gene disorders, even those not affecting biochemical pathways, could be detected or at least flagged for increased scrutiny using metabolomics. That would be particularly helpful for de novo mutations. In some cases, phenotypic or genetic heterogeneity can make prenatal diagnosis difficult (e.g., β-thalassemia, Noonan's syndrome). Nonetheless, Monni et al. found that pregnancies affected by β-thalassemia exhibit significant metabolic changes (123). Comparing the metabolic profiles of placental samples obtained by TA-CVS from normal fetuses and fetuses with homozygous or heterozygous β-thalassemia identified consistent alterations in all β-thalassemia cases. We have proposed a specific metabolic fingerprint for β-thalassemia that is associated with high fetal demand for ribose 5-phosphate (for nucleotide synthesis) and nicotinamide adenine dinucleotide phosphate (for redox maintenance). It appears that fetal oxidative stress can be an important and frequent marker for a wide range of abnormal conditions, and metabolic markers of oxidative stress are readily detected with metabolomics techniques. Other heterogenous single-gene disorders may have common metabolic phenotypes as well that might be best detected by the end products of biochemical production through the analysis and quantification of metabolomics pathways.
Conclusions
Metabolomics is a novel and promising area of research in reproductive medicine. It can be placed in the field of precision medicine, aiming, in general, at developing personalized strategies to manage disease states by considering, at the same time, the patient's genetics, environment, lifestyle, and individual treatment responses. Considering the current prenatal screening methods, it is clear that genetics plays the most relevant role, but metabolomics can generate new insights into the biological and physio/pathological processes. In this perspective, metabolomics can offer the opportunity to find new therapeutic targets and a better understanding of pathological mechanisms.
Table 3 summarizes the advantages and limitations of metabolomics in prenatal medicine. Metabolite detection with high throughput systems coupled with advanced bioinformatics and network analysis holds promise for new prenatal biomarkers and therapeutic discoveries. This metabolomics approach can identify complex physiologic pathways that would not be detected by measuring single metabolites. Further metabolomic investigation of both normal and pathologic prenatal specimens may enhance our knowledge of pregnancy disorders and improve our ability to diagnose and treat fetal disease. Toward this goal, the implementation of large-scale metabolomics studies and secondary cohort validation will be needed. Indeed, despite the general success and the increasing number of publications in prenatal medicine as well, the impact of the metabolomics in the current clinical practice is still dim. Several aspects contribute to this statement: one of the main problems is the enormous variability (external stimuli not closely related to the disease conditions, such as diet, lifestyle in general, analytical and experimental conditions, and data analysis methods), which could influence the final result. Thus, the experimental design covers a fundamental significance and should be planned with extremely controlled conditions. In addition, based on our literature investigation, it has emerged that several clinical metabolomics research studies are affected by strong limitations (e.g., small size of the patient cohorts and incomplete patient's clinical data), and often studies with the same topics produce results that are poorly comparable. Standardization of the methods is mandatory to promote the translation of the metabolomics toward clinical practice. It is important to consider that unfortunately, validated findings have not been yet evidenced in the literature, probably by being metabolomics in prenatal medicine a pioneer new research field. For this reason, new works are still expected in this context. Clinicians who are interested in learning these new approaches and participating in such studies have much to offer the field. Hopefully, soon we will be able to offer our patients clinical metabolomics tools to more effectively characterize, diagnose, and develop treatments for a vast range of pregnancy conditions and fetal disorders.
Table 3.
Advantage, limitations, and the future of metabolomics in prenatal medicine.
| Metabolomics in prenatal medicine | |
|---|---|
| Advantages | Limitations and future directions |
| Evaluates several biomarkers in a single experiment | Possible over-interpretation of data |
| Rapid experimental turnaround and relatively low cost | High false discovery rates requiring expert analysis |
| Does not require a-priori hypotheses of specific metabolites | Proof of initial findings in cell line and animal models often lags initial reports |
| Can identify altered metabolic pathways from multiple metabolite analysis | Hypothesis generating approach, but cutoff values and normal ranges must be established for clinical studies |
| May permit earlier identification of fetal or pregnancy disorders | Collaboration is weak among clinicians, analytical chemists, and biotechnologists |
| Simultaneously analyze metabolome of several compartments (e.g., maternal, placental, fetal) | Simple, specific tests that do not use sophisticated equipment may need to be developed |
Author Contributions
FM and VC: conceptualization. FM, VC, and FD: methodology. LA and GM: resources. FM and AI: data curation. FM and KH: writing–original draft preparation. FM, KH, LA, and GM: writing–review & editing. GM: supervision. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Olivier M, Asmis R, Hawkins GA, Howard TD, Cox LA. The need for multi-omics biomarker signatures in precision medicine. Int J Mol Sci. (2019) 20:4781. 10.3390/ijms20194781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dettmer K, Aronov PA, Hammock BD. Mass spectrometry-based metabolomics. Mass Spectrom Rev. (2007) 26:51–78. 10.1002/mas.20108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang A, Sun H, Wang P, Han Y, Wang X. Modern analytical techniques in metabolomics analysis. Analyst. (2011) 137:293–300. 10.1039/C1AN15605E [DOI] [PubMed] [Google Scholar]
- 4.Madsen R, Lundstedt T, Trygg J. Chemometrics in metabolomics–a review in human disease diagnosis. Anal Chim Acta. (2010) 659:23–33. 10.1016/j.aca.2009.11.042 [DOI] [PubMed] [Google Scholar]
- 5.Nicholson JK, Lindon JC. Metabonomics. Nature. (2008) 455:1054–6. 10.1038/4551054a [DOI] [PubMed] [Google Scholar]
- 6.Dettmer K, Hammock BD. Metabolomics–a new exciting field within the “omics” sciences. Environ Health Perspect. (2004) 112:A396–7. 10.1289/ehp.112-1241997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cevallos-Cevallos JM, Reyes-De-Corcuera JI, Etxeberria E, Danyluk MD, Rodrick GE. Metabolomic analysis in food science: a review. Trends Food Sci Technol. (2009) 20:557–66. 10.1016/j.tifs.2009.07.002 [DOI] [Google Scholar]
- 8.Dunn WB, Ellis DavidI. Metabolomics: current analytical platforms and methodologies. TrAC Trends Analyt Chem. (2005) 24:285–94. 10.1016/j.trac.2004.11.02130830346 [DOI] [Google Scholar]
- 9.Issaq HJ, Van QN, Waybright TJ, Muschik GM, Veenstra TD. Analytical and statistical approaches to metabolomics research. J Separat Sci. (2009) 32:2183–99. 10.1002/jssc.200900152 [DOI] [PubMed] [Google Scholar]
- 10.Wishart DS. Quantitative metabolomics using NMR. TrAC Trends Analyt Chem. (2008) 27:228–37. 10.1016/j.trac.2007.12.001 [DOI] [Google Scholar]
- 11.Lei Z, Huhman DV, Sumner LW. Mass spectrometry strategies in metabolomics. J Biol Chem. (2011) 286:25435–42. 10.1074/jbc.R111.238691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moco S, Vervoort J, Moco S, Bino RJ, De Vos RCH, Bino R. Metabolomics technologies and metabolite identification. TrAC Trends Analyt Chem. (2007) 26:855–66. 10.1016/j.trac.2007.08.003 [DOI] [Google Scholar]
- 13.Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd ed. New York, NY: Springer Science & Business Media; (2009). [Google Scholar]
- 14.Oresic M, Clish CB, Davidov EJ, Verheij E, Vogels J, Havekes LM, Neumann E, et al. Phenotype characterisation using integrated gene transcript, protein and metabolite profiling. Appl Bioinformatics. (2004) 3:205–17. 10.2165/00822942-200403040-00002 [DOI] [PubMed] [Google Scholar]
- 15.Worley B, Powers R. Multivariate analysis in metabolomics. Curr Metabolomics. (2013) 1:92–107. 10.2174/2213235X130108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollywood K, Brison DR, Goodacre R. Metabolomics: current technologies and future trends. Proteomics. (2006) 6:4716–23. 10.1002/pmic.200600106 [DOI] [PubMed] [Google Scholar]
- 17.Gromski PS, Muhamadali H, Ellis DI, Xu Y, Correa E, Turner ML, et al. A tutorial review: metabolomics and partial least squares-discriminant analysis – a marriage of convenience or a shotgun wedding. Anal Chim Acta. (2015) 879:10–23. 10.1016/j.aca.2015.02.012 [DOI] [PubMed] [Google Scholar]
- 18.Martin M, Govaerts B. Feature selection in metabolomics with PLS-derived methods. Methods. (2019) 5:1046–2023. [Google Scholar]
- 19.Bartel J, Krumsiek J, Theis FJ. Statistical methods for the analysis of high-throughput metabolomics data. Comput Struct Biotechnol J. (2013) 4:e201301009. 10.5936/csbj.201301009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jauniaux E, Cindrova-Davies T, Johns J, Dunster C, Hempstock J, Kelly FJ, et al. Distribution and transfer pathways of antioxidant molecules inside the first trimester human gestational sac. J Clin Endocrinol Metab. (2004) 89:1452–8. 10.1210/jc.2003-031332 [DOI] [PubMed] [Google Scholar]
- 21.Heazell AEP, Brown M, Dunn WB, Worton SA, Crocker IP, Baker PN, et al. Analysis of the metabolic footprint and tissue metabolome of placental villous explants cultured at different oxygen tensions reveals novel redox biomarkers. Placenta. (2008) 29:691–8. 10.1016/j.placenta.2008.05.002 [DOI] [PubMed] [Google Scholar]
- 22.Jauniaux E, Hempstock J, Teng C, Battaglia FC, Burton GJ. Polyol concentrations in the fluid compartments of the human conceptus during the first trimester of pregnancy: maintenance of redox potential in a low oxygen environment. J Clin Endocrinol Metab. (2005) 90:1171–5. 10.1210/jc.2004-1513 [DOI] [PubMed] [Google Scholar]
- 23.Murgia F, Iuculano A, Peddes C, Santoru ML, Tronci L, Deiana M, et al. Metabolic fingerprinting of chorionic villous samples in normal pregnancy and chromosomal disorders. Prenat Diagnosis. (2019) 39:848–58. 10.1002/pd.5461 [DOI] [PubMed] [Google Scholar]
- 24.Troisi J, Sarno L, Martinelli P, Di Carlo C, Landolfi A, Scala G, et al. A metabolomics-based approach for non-invasive diagnosis of chromosomal anomalies. Metabolomics. (2017) 13:140. 10.1007/s11306-017-1274-z [DOI] [Google Scholar]
- 25.Bahado-Singh RO, Akolekar R, Mandal R, Dong E, Xia J, Kruger M, et al. Metabolomic analysis for first-trimester down syndrome prediction. Am J Obstet Gynecol. (2013) 208:371.e1–8. 10.1016/j.ajog.2012.12.035 [DOI] [PubMed] [Google Scholar]
- 26.Bahado-Singh RO, Akolekar R, Chelliah A, Mandal R, Dong E, Kruger M, et al. Metabolomic analysis for first-trimester trisomy 18 detection. Am J Obstet Gynecol. (2013) 209:65.e1–9. 10.1016/j.ajog.2013.03.028 [DOI] [PubMed] [Google Scholar]
- 27.Pinto J, Almeida LM, Martins AS, Duarte D, Marques Domingues MR, Barros AS, et al. Impact of fetal chromosomal disorders on maternal blood metabolome: toward new biomarkers? Am J Obstet Gynecol. (2015) 213:841.e1–15. 10.1016/j.ajog.2015.07.032 [DOI] [PubMed] [Google Scholar]
- 28.Diaz SO, Barros AS, Goodfellow BJ, Duarte IF, Galhano E, Pita C, et al. Second trimester maternal urine for the diagnosis of trisomy 21 and prediction of poor pregnancy outcomes. Journal of Proteome Research. (2013) 12:2946–57. 10.1021/pr4002355 [DOI] [PubMed] [Google Scholar]
- 29.Trivedi DK, Iles RK. Shotgun metabolomic profiles in maternal urine identify potential mass spectral markers of abnormal fetal biochemistry – dihydrouracil and progesterone in the metabolism of down syndrome. Biomed Chromatogr. (2015) 29:1173–83. 10.1002/bmc.3404 [DOI] [PubMed] [Google Scholar]
- 30.Dunn WB, Brown M, Worton SA, Crocker IP, Broadhurst D, Horgan R, et al. Changes in the metabolic footprint of placental explant-conditioned culture medium identifies metabolic disturbances related to hypoxia and pre-eclampsia. Placenta. (2009) 30:974–80. 10.1016/j.placenta.2009.08.008 [DOI] [PubMed] [Google Scholar]
- 31.Austdal M, Skråstad RB, Gundersen AS, Austgulen R, Iversen A-C, Bathen TF. Metabolomic biomarkers in serum and urine in women with preeclampsia. PLoS ONE. (2014) 9:e91923. 10.1371/journal.pone.0091923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou X, Han T-L, Chen H, Baker PN, Qi H, Zhang H. Impaired mitochondrial fusion, autophagy, biogenesis and dysregulated lipid metabolism is associated with preeclampsia. Exp Cell Res. (2017) 359:195–204. 10.1016/j.yexcr.2017.07.029 [DOI] [PubMed] [Google Scholar]
- 33.Bahado-Singh RO, Syngelaki A, Mandal R, Graham SF, Akolekar R, Han B, et al. Metabolomic determination of pathogenesis of late-onset preeclampsia. J Mater Fetal Neonat Med. (2017) 30:658–64. 10.1080/14767058.2016.1185411 [DOI] [PubMed] [Google Scholar]
- 34.Bahado-Singh RO, Syngelaki A, Akolekar R, Mandal R, Bjondahl CT, Beomsoo Han, et al. Validation of metabolomic models for prediction of early-onset preeclampsia. Am J Obstet Gynecol. (2015) 213:530.e1–10. 10.1016/j.ajog.2015.06.044 [DOI] [PubMed] [Google Scholar]
- 35.Bahado-Singh R, Poon LC, Yilmaz A, Syngelaki A, Turkoglu O, Kumar P, et al. Integrated proteomic and metabolomic prediction of term preeclampsia. Sci Rep. (2017) 7:16189. 10.1038/s41598-017-15882-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koster MPH, Vreeken RJ, Harms AC, Dane AD, Kuc S, Schielen PCJI, et al. First-Trimester serum acylcarnitine levels to predict preeclampsia: a metabolomics approach. Dis Mark. (2015) 2015:1–8. 10.1155/2015/857108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuc S, Koster MPH, Pennings JLA, Hankemeier T, Berger R, Harms AC, et al. Metabolomics profiling for identification of novel potential markers in early prediction of preeclampsia. PLoS ONE. (2014) 9:e98540. 10.1371/journal.pone.0098540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernard JY, Tint M-T, Aris IM, Chenc LW, Quaha PL, Tand KH, et al. Maternal plasma phosphatidylcholine polyunsaturated fatty acids during pregnancy and offspring growth and adiposity. Prostaglandins, Leukot Essent Fatty Acids. (2017) 121:21–9. 10.1016/j.plefa.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Visentin S, Crotti S, Donazzolo E, D'Aronco S, Nitti D, Cosmi E, et al. Medium chain fatty acids in intrauterine growth restricted and small for gestational age pregnancies. Metabolomics. (2017) 13:54. 10.1007/s11306-017-1197-8 [DOI] [Google Scholar]
- 40.Clinton CM, Bain JR, Muehlbauer MJ, Li YY, Li L, O'Neal SK, et al. Non-targeted urinary metabolomics in pregnancy and associations with fetal growth restriction. Sci Rep. (2020) 10:5307. 10.1038/s41598-020-62131-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dessì A, Marincola FC, Pattumelli MG, Ciccarelli S, Corbu S, Ossicini C, et al. Investigation of the 1H-NMR based urine metabolomic profiles of IUGR, LGA and AGA newborns on the first day of life. J Matern Fetal Neonat Med. (2014) 27:13–9. 10.3109/14767058.2014.955674 [DOI] [PubMed] [Google Scholar]
- 42.Dessì A, Atzori L, Noto A, Visser GHA, Gazzolo D, Zanardo V, et al. Metabolomics in newborns with intrauterine growth retardation (IUGR): urine reveals markers of metabolic syndrome. J Mater Fetal Neonat Med. (2011) 24:35–9. 10.3109/14767058.2011.605868 [DOI] [PubMed] [Google Scholar]
- 43.Favretto D, Cosmi E, Ragazzi E, Visentin S, Tucci M, Fais P, et al. Cord blood metabolomic profiling in intrauterine growth restriction. Anal Bioanal Chem. (2012) 402:1109–21. 10.1007/s00216-011-5540-z [DOI] [PubMed] [Google Scholar]
- 44.Sanz-Cortés M, Carbajo RJ, Crispi F, Figueras F, Pineda-Lucena A, Gratacós E. Metabolomic profile of umbilical cord blood plasma from early and late intrauterine growth restricted (IUGR) neonates with and without signs of brain vasodilation. PLoS ONE. (2013) 8:e80121. 10.1371/journal.pone.0080121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Chen X-X, Li X-W, Fu W, Zhang W-Q. Metabolomic research on newborn infants with intrauterine growth restriction. Medicine. (2016) 95:3564. 10.1097/MD.0000000000003564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Porter AC, Gumina DL, Armstrong M, Maclean KN, Reisdorph N, Galan HL, et al. Maternal amino acid profiles to distinguish constitutionally small versus growth-restricted fetuses defined by doppler ultrasound: a pilot study. Am J Perinatol. (2020) 37:1084–93. 10.1055/s-0040-1701504 [DOI] [PubMed] [Google Scholar]
- 47.Bahado-Singh RO, Turkoglu O, Yilmaz A, Kumar P, Zeb A, Konda S, et al. Metabolomic identification of placental alterations in fetal growth restriction. J Mater Fetal Neonatal Med. (2020) 11, 1–10. 10.1080/14767058.2020.1722632 [DOI] [PubMed] [Google Scholar]
- 48.Sulek K, Han T-L, Villas-Boas SG, Wishart DS, Soh SE, Kwek K, et al. Hair metabolomics: identification of fetal compromise provides proof of concept for biomarker discovery. Theranostics. (2014) 4:953–9. 10.7150/thno.9265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caboni P, Meloni A, Lussu M, Carta E, Barberini L, Noto A, et al. Urinary metabolomics of pregnant women at term: a combined GC/MS and NMR approach. J Matern Fetal Neonatal Med. (2014) 27:4–12. 10.3109/14767058.2014.956403 [DOI] [PubMed] [Google Scholar]
- 50.Baraldi E, Giordano G, Stocchero M, Moschino L, Patrizia Zaramella P, Tran MR, et al. Untargeted metabolomic analysis of amniotic fluid in the prediction of preterm delivery and bronchopulmonary dysplasia. PLoS ONE. (2016) 11:e0164211. 10.1371/journal.pone.0164211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Graça G, Duarte IF, Barros AS, Goodfellow BJ, Diaz SO, Pinto J, et al. Impact of prenatal disorders on the metabolic profile of second trimester amniotic fluid: a nuclear magnetic resonance metabonomic study. J Proteome Res. (2010) 9:6016–24. 10.1021/pr100815q [DOI] [PubMed] [Google Scholar]
- 52.Menon R, Jones J, Gunst PR, Kacerovsky M, Fortunato SJ, Saade GR, et al. Amniotic fluid metabolomic analysis in spontaneous preterm birth. Reprod Sci. (2014) 21:791–803. 10.1177/1933719113518987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Romero R, Mazaki-Tovi S, Vaisbuch E, Kusanovic JP, Chaiworapongsa T, Gomez R, et al. Metabolomics in premature labor: a novel approach to identify patients at risk for preterm delivery. J Matern Fetal Neonatal Med. (2010) 23:1344–59. 10.3109/14767058.2010.482618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Virgiliou C, Gika HG, Witting M, Bletsou AA, Athanasiadis A, Zafrakas M, et al. Amniotic fluid and maternal serum metabolic signatures in the second trimester associated with preterm delivery. J Proteome Res. (2017) 16:898–910. 10.1021/acs.jproteome.6b00845 [DOI] [PubMed] [Google Scholar]
- 55.Lizewska B, Teul J, Kuc P, Lemancewicz A, Charkiewicz K, Goscik J, et al. Maternal plasma metabolomic profiles in spontaneous preterm birth: preliminary results. Mediat Inflamm. (2018) 2018:9362820. 10.1155/2018/9362820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tea I, Le Gall G, Kuester A, Guignard N, Alexandre-Gouabau MC, Darmaun D, et al. H-1-NMR-Based metabolic profiling of maternal and umbilical cord blood indicates altered materno-foetal nutrient exchange in preterm infants. PLoS ONE. (2012) 7:e029947. 10.1371/journal.pone.0029947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Groenen PMW, Engelke UF, Wevers RA, Hendriks JCM, Eskes TKAB, Merkus HMWM, et al. High-resolution 1H NMR spectroscopy of amniotic fluids from spina bifida fetuses and controls. Euro J Obstet Gynecol Reproduct Biol. (2004) 112:16–23. 10.1016/S0301-2115(03)00279-3 [DOI] [PubMed] [Google Scholar]
- 58.Bock JL. Metabolic profiling of amniotic fluid by proton nuclear magnetic resonance spectroscopy: correlation with fetal maturation and other clinical variables. Clin Chem. (1994) 40:56–61. 10.1093/clinchem/40.1.56 [DOI] [PubMed] [Google Scholar]
- 59.Clifton MS, Joe BN, Zektzer AS, Kurhanewiczb J, Vigneronb DB, Coakley FV, et al. Feasibility of magnetic resonance spectroscopy for evaluating fetal lung maturity. J Pediatr Surg. (2006) 41:768–73. 10.1016/j.jpedsurg.2006.02.020 [DOI] [PubMed] [Google Scholar]
- 60.Pearce JM, Krone JT, Pappas AA, Komoroski RA. Analysis of saturated phosphatidylcholine in amniotic fluid by 31P NMR. Magnet Reson Med. (1993) 30:476–84. 10.1002/mrm.1910300410 [DOI] [PubMed] [Google Scholar]
- 61.Graça G, Duarte IF, Goodfellow BJ, Barros AS, Carreira IM, Couceiro AB, et al. Potential of NMR spectroscopy for the study of human amniotic fluid. Anal Chem. (2007) 79:8367–75. 10.1021/ac071278d [DOI] [PubMed] [Google Scholar]
- 62.Graça G, Duarte IF, Barros AS, Goodfellow BJ, Diaz S, Carreira IM, et al. 1H NMR based metabonomics of human amniotic fluid for the metabolic characterization of fetus malformations. J Proteome Res. (2009) 8:4144–50. 10.1021/pr900386f [DOI] [PubMed] [Google Scholar]
- 63.Bleicher SJ, O'Sullivan JB, Freinkel N. Carbohydrate metabolism in pregnancy. N Engl J Med. (1964) 271:866–72. 10.1056/NEJM196410222711702 [DOI] [PubMed] [Google Scholar]
- 64.Heaney RP, Skillman TG. Calcium metabolism in normal human pregnancy. J Clin Endocrinol Metab. (1971) 33:661–70. 10.1210/jcem-33-4-661 [DOI] [PubMed] [Google Scholar]
- 65.Knopp RH, Warth MR, Charles D, Childs M, Li JR, Mabuchi H, et al. Lipoprotein metabolism in pregnancy, fat transport to the fetus, and the effects of diabetes. Neonatology. (1986) 50:297–317. 10.1159/000242614 [DOI] [PubMed] [Google Scholar]
- 66.Nicolaides KH. First-Trimester screening for chromosomal abnormalities. Semi Perinatol. (2005) 29:190–4. 10.1053/j.semperi.2005.06.001 [DOI] [PubMed] [Google Scholar]
- 67.Nicolaides KH. Screening for fetal aneuploidies at 11 to 13 weeks. Prenat Diagn. (2011) 31:7–15. 10.1002/pd.2637 [DOI] [PubMed] [Google Scholar]
- 68.Akolekar R, Beta J, Picciarelli G, Ogilvie C, D'Antonio F. Procedure-related risk of miscarriage following amniocentesis and chorionic villus sampling: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. (2015) 45:16–26. 10.1002/uog.14636 [DOI] [PubMed] [Google Scholar]
- 69.Santorum M, Wright D, Syngelaki A, Karagioti N, Nicolaides KH. Accuracy of first-trimester combined test in screening for trisomies 21, 18 and 13. Ultrasound Obstet Gynecol. (2017) 49:714–20. 10.1002/uog.17283 [DOI] [PubMed] [Google Scholar]
- 70.Gil MM, Accurti V, Santacruz B, Plana MN, Nicolaides KH. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol. (2017) 50:302–14. 10.1002/uog.17484 [DOI] [PubMed] [Google Scholar]
- 71.Cheng W-L, Hsiao C-H, Tseng H-W, Lee T-P. Noninvasive prenatal diagnosis. Taiwan J Obstet Gynecol. (2015) 54:343–9. 10.1016/j.tjog.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 72.Buffat C, Mondon F, Rigourd V, Boubred F, Bessìeres B, Fayol L, et al. A hierarchical analysis of transcriptome alterations in intrauterine growth restriction (IUGR) reveals common pathophysiological pathways in mammals. J Pathol. (2007) 213:337–46. 10.1002/path.2233 [DOI] [PubMed] [Google Scholar]
- 73.Hansson SR, Chen Y, Brodszki J, Chen M, Hernandez-Andrade E, Inman JM, et al. Gene expression profiling of human placentas from preeclamptic and normotensive pregnancies. Mol Hum Reprod. (2006) 12:169–79. 10.1093/molehr/gal011 [DOI] [PubMed] [Google Scholar]
- 74.Hassan SS, Romero R, Tarca AL, Draghici S, Pineles B Bugrim A, et al. Signature pathways identified from gene expression profiles in the human uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol. (2007) 197:250.e1–7. 10.1016/j.ajog.2007.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Slonim DK, Koide K, Johnson KL, Tantravahid U, Cowanc JM, Jarrahc Z, et al. Functional genomic analysis of amniotic fluid cell-free mRNA suggests that oxidative stress is significant in down syndrome fetuses. Proc Natl Acad Sci USA. (2009) 106:9425–9. 10.1073/pnas.0903909106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Timmons BC, Mahendroo M. Processes regulating cervical ripening differ from cervical dilation and postpartum repair: insights from gene expression studies. Reprod Sci. (2007) 14:53–62. 10.1177/1933719107309587 [DOI] [PubMed] [Google Scholar]
- 77.Tsai M-S, Hwang S-M, Chen K-D, Lee YS, Hsu LW, Chang CJ, et al. Functional network analysis of the transcriptomes of mesenchymal stem cells derived from amniotic fluid, amniotic membrane, cord blood, and bone marrow. Stem Cells. (2007) 25:2511–23. 10.1634/stemcells.2007-0023 [DOI] [PubMed] [Google Scholar]
- 78.Breuiller-Fouche M, Charpigny G, Germain G. Functional genomics of the pregnant uterus: from expectations to reality, a compilation of studies in the myometrium. BMC Pregnancy Childbirth. (2007) 7:S4. 10.1186/1471-2393-7-S1-S4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chiu RWK, Chan KCA, Gao Y, Laua VYM, Zhenga W, Leunge TY, et al. Noninvasive prenatal diagnosis of fetal chromosomal aneuploidy by massively parallel genomic sequencing of DNA in maternal plasma. PNAS. (2008) 105:20458–20463. 10.1073/pnas.0810641105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dolan S, Biermann J, Damus K. Genomics for Health in Preconception and Prenatal Periods. Journal of Nursing Scholarship. (2007) 39:4–9. 10.1111/j.1547-5069.2007.00136.x [DOI] [PubMed] [Google Scholar]
- 81.Tromp G, Kuivaniemi H, Romero R, Chaiworapongsa T, Kim YM, Kim MR, et al. Genome-wide expression profiling of fetal membranes reveals a deficient expression of proteinase inhibitor 3 in premature rupture of membranes. Am J Obstet Gynecol. (2004) 191:1331–8. 10.1016/j.ajog.2004.07.010 [DOI] [PubMed] [Google Scholar]
- 82.Wilson RD. Genomics: new technology for obstetrics and gynaecology. J Obstet Gynaecol Can. (2005) 27:63–8. 10.1016/S1701-2163(16)30175-X [DOI] [PubMed] [Google Scholar]
- 83.Cho CKJ, Shan SJ, Winsor EJ, Diamandis EP. Proteomics analysis of human amniotic fluid. Mol Cell Proteomics. (2007) 6:1406–15 10.1074/mcp.M700090-MCP200 [DOI] [PubMed] [Google Scholar]
- 84.Park JS, Oh KJ, Norwitz ER, Han JS, Choi HJ, Seong HS, et al. Identification of proteomic biomarkers of preeclampsia in amniotic fluid using SELDI-TOF mass spectrometry. Reprod Sci. (2008) 15:457–68. 10.1177/1933719108316909 [DOI] [PubMed] [Google Scholar]
- 85.Gravett MG, Thomas A, Schneider KA, Reddy AP, Dasari S, Jacob T, et al. Proteomic analysis of cervical–vaginal fluid: identification of novel biomarkers for detection of intra-amniotic infection. J Proteome Res. (2007) 6:89–96. 10.1021/pr060149v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim Y-S, Kim M-S, Lee S-H, Choi BC, Lim JM, Cha KY, et al. Proteomic analysis of recurrent spontaneous abortion: Identification of an inadequately expressed set of proteins in human follicular fluid. Proteomics. (2006) 6:3445–54. 10.1002/pmic.200500775 [DOI] [PubMed] [Google Scholar]
- 87.Luan H, Meng N, Liu P, Feng Q, Lin S, Fu J, et al. Pregnancy-Induced metabolic phenotype variations in maternal plasma. J Proteome Res. (2014) 13:1527–36. 10.1021/pr401068k [DOI] [PubMed] [Google Scholar]
- 88.Diaz SO, Pinto J, Graça G, Duarte IF, Barros AS, Galhano E, et al. Metabolic biomarkers of prenatal disorders: an exploratory NMR metabonomics study of second trimester maternal urine and blood plasma. J Proteome Res. (2011) 10:3732–42. 10.1021/pr200352m [DOI] [PubMed] [Google Scholar]
- 89.Ghartey J, Brown A, Anglim L, Elovitz M. 11: women with preterm birth have a distinct cervico-vaginal metabolome. Am J Obstetr Gynecol. (2015) 212:S9. 10.1016/j.ajog.2014.10.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iuculano A, Murgia F, Peddes C, Santoru ML Tronci L, Deiana M, et al. Metabolic characterization of amniotic fluids of fetuses with enlarged nuchal translucency. J Perinat Med. (2019) 47:311–8. 10.1515/jpm-2018-0314 [DOI] [PubMed] [Google Scholar]
- 91.Catalano PM, Roman-Drago NM, Amini SB, Sims EAH. Longitudinal changes in body composition and energy balance in lean women with normal and abnormal glucose tolerance during pregnancy. Am J Obstetr Gynecol. (1998) 179:156–65. 10.1016/S0002-9378(98)70267-4 [DOI] [PubMed] [Google Scholar]
- 92.Di Cianni G, Ghio A, Resi V, Volpe L. Gestational diabetes mellitus: an opportunity to prevent type 2 diabetes and cardiovascular disease in young women. Womens Health. (2010) 6:97–105. 10.2217/WHE.09.76 [DOI] [PubMed] [Google Scholar]
- 93.McLachlan KA, O'Neal D, Jenkins A, Alford FP. Do adiponectin, TNFα, leptin and CRP relate to insulin resistance in pregnancy? Studies in women with and without gestational diabetes, during and after pregnancy. Diabet Metab Res Rev. (2006) 22:131–8. 10.1002/dmrr.591 [DOI] [PubMed] [Google Scholar]
- 94.Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab. (2002) 87:2954–9. 10.1210/jcem.87.6.8563 [DOI] [PubMed] [Google Scholar]
- 95.Jauniaux E, Watson AL, Hempstock J, Bao Y-P, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress: a possible factor in human early pregnancy failure. Am J Pathol. (2000) 157:2111–22. 10.1016/S0002-9440(10)64849-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. Reprod Sci. (2004) 11:342–52. 10.1016/j.jsgi.2004.03.003 [DOI] [PubMed] [Google Scholar]
- 97.National Collaborating Centre for Women's and Children's Health . Antenatal care: routine care for the healthy pregnant woman. In: Commissioned by the National Institute for Clinical Excellence. London: Royal College of Obstetrics and Gynaecology Press; (2008). p. 218–27. [Google Scholar]
- 98.Santiago JC, Ramos-Corpas D. Delta-NT and center-specific ultrasound nuchal translucency medians. Ultrasound Obstet Gynecol. (2007) 30:934–40. 10.1002/uog.5171 [DOI] [PubMed] [Google Scholar]
- 99.Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semi Perinatol. (2012) 36:56–9. 10.1053/j.semperi.2011.09.011 [DOI] [PubMed] [Google Scholar]
- 100.Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy F, Saito S, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. (2018) 72:24–43. 10.1161/HYPERTENSIONAHA.117.10803 [DOI] [PubMed] [Google Scholar]
- 101.ACOG . ACOG committee opinion No. 770: uterine morcellation for presumed leiomyomas. Obstet. Gynecol. (2019) 133:1. 10.1097/AOG.0000000000003126 [DOI] [PubMed] [Google Scholar]
- 102.Khan N, Andrade W, Castro HD, Wright A, Wright D, Nicolaides KH. Impact of new definitions of pre-eclampsia on incidence and performance of first-trimester screening. Ultrasound Obstet Gynecol. (2020) 55:50–7. 10.1002/uog.21867 [DOI] [PubMed] [Google Scholar]
- 103.Cnossen JS, Riet GT, Mol BW, Van der post JA, Leeflang MM, Meads CA, et al. Are tests for predicting pre-eclampsia good enough to make screening viable? A review of reviews and critical appraisal. Acta Obstet Gynecol Scand. (2009) 88:758–65. 10.1080/00016340903008953 [DOI] [PubMed] [Google Scholar]
- 104.Staff AC. Circulating predictive biomarkers in preeclampsia. Pregnancy Hypertens. (2011) 1:28–42. 10.1016/j.preghy.2010.10.012 [DOI] [PubMed] [Google Scholar]
- 105.Roberge S, Demers S, Bujold E. Low-Dose aspirin for prevention of morbidity and mortality from preeclampsia. Ann Inter Med. (2014). Available online at: https://www.acpjournals.org/doi/abs/10.7326/L14/5020/4 (accessed September 9, 2020). [DOI] [PubMed]
- 106.O'Gorman N, Wright D, Syngelaki A, Akolekar R, Wright A, Poon LC, et al. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11-13 weeks gestation. Am J Obstet Gynecol. (2016) 214:103.e1–2. 10.1016/j.ajog.2015.08.034 [DOI] [PubMed] [Google Scholar]
- 107.Vedmedovska N, Rezeberga D, Teibe U, Melderis I, Donders GGG. Placental pathology in fetal growth restriction. Euro J Obstet Gynecol Reproduct Biol. (2011) 155:36–40. 10.1016/j.ejogrb.2010.11.017 [DOI] [PubMed] [Google Scholar]
- 108.Leite DFB, Morillon A-C, Júnior EFM, Souza RT, Khashan AS, Baker PN, et al. Metabolomics for predicting fetal growth restriction: protocol for a systematic review and meta-analysis. BMJ Open. (2018) 8:e022743. 10.1136/bmjopen-2018-022743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Auray-Blais C, Raiche E, Gagnon R, Berthiaume M, Pasquier JC. Metabolomics and preterm birth: what biomarkers in cervicovaginal secretions are predictive of high-risk pregnant women? Int J Mass Spectrometry. (2011) 307:33–8. 10.1016/j.ijms.2011.02.009 [DOI] [Google Scholar]
- 110.Conde-Agudelo A, Romero R, Da Fonseca E, O'Brien JM, Cetingoz E, Creasy GW, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol. (2018) 219:10–25. 10.1016/j.ajog.2018.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Crump C. Preterm birth and mortality in adulthood: a systematic review. J Perinatol. (2020) 40:833–43. 10.1038/s41372-019-0563-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Luu TM, Katz SL, Leeson P, Thébaud B, Nuyt AM. Preterm birth: risk factor for early-onset chronic diseases. CMAJ. (2016) 188:736–40. 10.1503/cmaj.150450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes; Behrman RE Butler AS . Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academies Press; (2007). [PubMed] [Google Scholar]
- 114.Gotsch F, Gotsch F, Romero R, Erez O, Vaisbuch E, Kusanovic JP, et al. The preterm parturition syndrome and its implications for understanding the biology, risk assessment, diagnosis, treatment and prevention of preterm birth. J Matern Fetal Neonatal Med. (2009) 22:5–23. 10.1080/14767050902860690 [DOI] [PubMed] [Google Scholar]
- 115.Georgiou HM, Di Quinzio MKW, Permezel M, Brennecke SP. Predicting preterm labour: current status and future prospects. Dis Mark. (2015) 2015:1–9. 10.1155/2015/435014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kuć P, Laudański P, Kowalczuk O, Chyczewski L, Laudański T. Expression of selected genes in preterm premature rupture of fetal membranes. Acta Obstet Gynecol Scand. (2012) 91:936–43. 10.1111/j.1600-0412.2012.01445.x [DOI] [PubMed] [Google Scholar]
- 117.Tsiartas P, Holst RM, Wennerholm UB, Hagberg H, DM Hougaard DM, Skogstrand K, et al. Prediction of spontaneous preterm delivery in women with threatened preterm labour: a prospective cohort study of multiple proteins in maternal serum. BJOG. (2012) 119:866–73. 10.1111/j.1471-0528.2012.03328.x [DOI] [PubMed] [Google Scholar]
- 118.Chan RL. Biochemical markers of spontaneous preterm birth in asymptomatic women. Bio Med Res Int. (2014) 2014:1–8. 10.1155/2014/164081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Laudanski P, Raba G, Kuc P, Lemancewicz A, Kisielewski R, Laudanski T. Assessment of the selected biochemical markers in predicting preterm labour. J Matern Fetal Neonatal Med. (2012) 25:2696–9. 10.3109/14767058.2012.699116 [DOI] [PubMed] [Google Scholar]
- 120.Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. (2002) 39:1890–900. 10.1016/S0735-1097(02)01886-7 [DOI] [PubMed] [Google Scholar]
- 121.Mitchell SC, Korones SB, Berendes HW. Congenital heart disease in 56,109 births incidence and natural history. Circulation. (1971) 43:323–32. 10.1161/01.CIR.43.3.323 [DOI] [PubMed] [Google Scholar]
- 122.Bahado-Singh RO, Ertl R, Mandal R, Bjorndahl TC, Syngelaki A, Han B, et al. Metabolomic prediction of fetal congenital heart defect in the first trimester. Am J Obstet Gynecol. (2014) 211:240.e1–14. 10.1016/j.ajog.2014.03.056 [DOI] [PubMed] [Google Scholar]
- 123.Monni G, Murgia F, Corda V, Peddes C, Iuculano A, Tronci L, et al. Metabolomic investigation of β-thalassemia in chorionic villi samples. J Clin Med. (2019) 8:798. 10.3390/jcm8060798 [DOI] [PMC free article] [PubMed] [Google Scholar]