Abstract
目的
探讨猪诱导多能干细胞(iPSCs)体外定向分化为GABA能神经元前体的方法体系。
方法
猪iPSCs诱导分化为GABA能神经元前体遵循两个阶段,第1阶段,猪iPSCs悬浮培养,第3天时形成类胚体,采用神经诱导培养基NIM(SB431542、DMH1、FGF2)继续诱导,第12天分化为原始神经上皮细胞。第2阶段,使用含Pur、B27的NIM培养基悬浮培养形成神经球,至第21天时形成GABA能神经元前体。CM-DiI标记后,定向移植帕金森(PD)模型大鼠黑质纹状体,检测其在宿主脑内存活、迁移及分化状况。
结果
猪iPSCs在饲养层细胞上稳定传代,表达多能性标记OCT4、Nanog、SSEA1和TRA-160,并且核型分析显示没有其他物种来源细胞污染。第12天经诱导分化获得原始神经上皮细胞能够形成玫瑰花环结构,并表达其表面标记物(PAX6、SOX2和Nestin)与神经微管蛋白标志物Tuj1。第21天诱导细胞高表达GABA能神经元前体的表面特异性抗原NKX2.1和前脑标志物FOXG1。移植8周后,体内可分化为GABA能神经元与多巴胺能神经元,明显改善PD大鼠运动行为。
结论
结合无血清培养基筛选法逐步定向诱导猪iPSCs高效分化为前脑GABA能神经元前体,移植后能够显著改善PD大鼠的运动功能障碍,为诱导GABA能神经元前体移植治疗神经损伤疾病奠定基础。
Keywords: 诱导多能干细胞,猪, 细胞分化, 原始神经上皮细胞, GABA能神经元前体, 帕金森模型大鼠
Abstract
Objective
To establish an efficient protocol for directed differentiation of miniature-swine induced pluripotent stem cells (iPSCs) into GABAergic progenitors in a chemically defined system.
Methods
We adopted a two-stage protocol for inducing the differentiation of porcine iPSCs. In the first stage, embryoid bodies (EBs) derived from porcine iPSCs after 3 days of suspension culture were induced in neural induction medium (containing SB431542, DMH1 and FGF2) till day 12 to differentiate into primitive neuroepithelia cells (NECs). In the second stage, the primitive NECs were induced in neural induction medium (containing Pur and B27) to obtain neural rosettes, which further differentiated into GABAergic neuron progenitors on day 21. After labeling with CM-DiI, the progenitor cells were stereotactically transplanted into the substantia nigra (SN) of 6-OHDA-lesioned PD model rats, and the cell survival, migration and differentiation in vivo were observed.
Results
Porcine iPSCs could be passaged stably on the feeder cell layer and expressed the pluripotent stem cell markers OCT4, Nanog, SSEA1and TRA-160. Karyotype analysis demonstrated the absence of contamination by cells from other species. On day 12 of induced differentiation, the cells formed adherent colonies containing NECs in the form of neural rosettes, which expressed the neuroepithelial markers PAX6, SOX2 and Nestin and the neurite marker beta Ⅲ Tubulin (Tuj1). After induction for 21 days, the NECs differentiated into GABAergic neural progenitors highly expressing NKX2.1 and FOXG1. Eight weeks after transplantation, the iPSCs-iGABA progeniters survived in the striatum of the PD rats, where they differentiate into GABAergic neurons and TH+ neurons and significantly improved dyskinesia of the rats.
Conclusion
The miniature-swine iPSCsderived GABA progenitors may serve as promising donor cells for neural grafting for treatment of neurodegenerative diseases.
Keywords: miniature-swine induced pluripotent stem cells, cell differentiation, primitive neuroepithelia, GABAergic progenitors, rat models Parkinson's disease
2006年,有研究将4种转录因子Oct4、Sox2、c-Myc和Klf4转入成年小鼠皮肤成纤维细胞,获得与胚胎干细胞特征类似的细胞,并具有自我更新能力和多向分化潜能,命名为“诱导多能干细胞”(iPSCs)[1]。该技术是近年来干细胞领域中一个里程碑式的突破,它解决了干细胞应用由来已久的伦理学和免疫排斥等问题,具有无穷的开发潜力。
帕金森综合症(PD)的生化和病理改变不仅仅是由于黑质纹状体多巴胺能神经元缺失,可能与脑内重要的抑制性神经递质GABA的缺失也存在很大关系[2-3]。Martinez-Cerdeno实验组将胚胎来源的GABA能神经元前体移植到帕金森大鼠纹状体,75%移植细胞能分化为GABA能神经元,整合到宿主纹状体回路中,发挥功效改善帕金森大鼠运动行为[4],但有限的胚胎组织来源限制了其应用。iPSCs可以分化为各种组织细胞,使无限制备各种供体细胞成为可能[5-8]。因此iPSCs来源的GABA能神经元前体用于帕金森病等神经退行性疾病的治疗,具有极大的应用前景[9-10]。
近年来,多能干细胞定向分化研究取得了显著进展。Goulburn等[11]将人胚胎干细胞(hESCs)分化为GABA能神经元前体,并提出维甲酸和成纤维细胞生长因子2(FGF2)在干细胞诱导GABA能神经元前体中发挥重要作用,但该方法诱导的前体细胞效率低,仅占总细胞数的10%。2012年,研究发现SHH信号在诱导的前脑腹侧NKX2.1+表达中起关键作用,他们采用改良的单层神经分化方案,辅以SHH处理,提高诱导效率,证明了腹侧前体细胞的产生数量随SHH剂量的增加而增加[12]。2013年,学者相继报道了从人诱导多能干细胞(hiPSCs)产生前脑GABA能神经元前体的方法,但这些方法需要多种小分子和/或生长因子的组合(SB431542+LSB+XAV939+SHH+Purmorphamine)或(SB431542 + BMPRIA- Fc + Dkk1 + Purmorphamine + Y27632),增加了实验成本和可变性[13-14]。同年,有研究仅使用高浓度的SHH或Pur激活hedgehog途径,就能将hiPSCs分步诱导分化为高纯度的GABA能神经元前体(大于90%)[15]。随后相继出现许多可以获得成熟GABA能神经元前体的分化方案[16-17],但是依然存在分化过程繁琐、耗时长等问题。2016年,有研究利用慢病毒转导的方法,仅需7 d就能高效地将hiPSCs诱导成GABA能神经元[18],此法体系为之后GABA能神经元的研究提供了很好的参考。
五指山小型猪来源的神经元在生理和生物学特性等方面与人类神经元具有高度相似性[19]。因此,猪来源细胞替代人类细胞,可能是一种有吸引力的异种神经移植供体材料[20]。目前五指山小型猪模型已被广泛应用于人类神经系统疾病的研究,但建立成熟猪GABA能神经元前体的报道却十分有限,因此亟须建立一个成熟的猪GABA能神经元前体的体外分化体系。本研究首次成功建立猪iPSCs到GABA能神经元前体的分化体系,并通过定向移植PD大鼠黑质纹状体区,观察移植细胞存活和分化情况,为研究PD的发病机制和探索新的治疗方法提供实验依据。
1. 材料和方法
1.1. 细胞系
猪iPS细胞系由中国农业科学院北京畜牧兽医研究所关伟军教授惠赠;饲养层细胞(赛业生物科技公司)。
1.2. 实验动物
30只健康雄性Sprague-Dawley(SD)大鼠,约6周龄,体质量180~220 g,由安徽医科大学提供。清洁级动物房中饲养,环境温度控制在26 ℃,饲养密度≤4只/盒,定期更换垫料,自由活动。高温消毒饮水垫料,喂食商品化的颗粒饲料。
1.3. 主要试剂
DMEM高糖培养基购自Hyclone,DMEM/F12培养基、非必需氨基酸(NEAA)、L-谷氨酰胺、β-巯基乙醇、Knockout血清替代物、B27(Without VitaminA)、Laminin(Gibco),胎牛血清(Lonsa science srl),碱性成纤维细胞生长因子(FGF2, R & D),DMH1(TocrisBioscience),N2(Gemini),Poly-l-ornithine hydrobromide(PLO)、Ⅳ型胶原酶、6-羟基多巴胺(6-OHDA)、阿扑吗啡(APO) (Sigma),肝素(MCE),SB431542(StemGent),Purmorphamine(Millipore),兔抗Oct4、鼠抗SSEA1、鼠抗TRA-160、鼠抗Nestin、兔抗Sox2、兔抗PAX6、鼠抗Tuj1、兔抗FOXG1购自Abcam,鼠抗NKX2.1 (Chemicon, Millipore),兔抗Nanog(CST),驴抗兔Cy3 (Jackson Lab),驴抗鼠Alexa Fluor 488、CM-DiI、抗荧光淬灭封片剂(Invitrogen),维生素C(BioShap),DAPI染液(碧云天),Albumin Bovine(BSA, Solarbio。
1.4. 五指山小型猪iPS细胞的传代培养
复苏五指山小型猪iPS细胞系(iPSCs),接种于饲养层细胞(γ射线辐照小鼠胚胎成纤维细胞)上,采用ES培养基培养,2 d/次更换培养液。
1.5. 猪iPSCs表型鉴定及遗传学特征检测
猪iPSCs的多能性检测利用免疫荧光技术对猪iPS细胞克隆多潜能性标记分子SSEA1、OCT4、Nanog和TRA-160的表达进行特异性检测。猪iPSCs的染色体G显带核型分析猪iPSCs处于增殖期时,按照染色体核型制备标准方法进行低渗、固定并滴片,Giemsa染色后,在油浸物镜下观察到100个扩散良好的中期分裂相,并进行核型分析[21]。
1.6. 猪iPSCs向GABA能神经元前体分化
1.6.1. 猪iPSCs诱导为原始神经上皮细胞(iNECs)
猪iPSCs克隆长满饲养层后,用Ⅳ型胶原酶消化收集细胞,加1 mL iPS培养基(不含FGF2)重悬后,将细胞转入六孔板中,置37 ℃,5% CO2的培养箱培养3 d,2 d/1次换液。当细胞聚集形成类胚体(EBs)时,更换为神经诱导培养基NIM(SB431542、DMH1、FGF2)继续培养3 d。第7天,使用NIM培养基(含10% FBS)将EBs接种至PLO-laminin包被的六孔板,过夜培养后更换NIM培养基。在分化的第12天左右观察EBs形态,用免疫荧光技术检测表面特异性抗原SOX2、PAX6、Nestin与Tuj1表达。
1.6.2. iNECs诱导为GABA能神经元前体
吸弃培养基,向诱导原始神经上皮细胞(iNECs)中加入Ⅳ型胶原酶1 mL,置37 ℃,5% CO2培养箱中消化5 min,拍打至细胞团飘起,加1 mL培养基(含10% FBS)终止消化,移至15 mL离心管,静置5 min,去上清。加1 mL的NIM (Pur、B27)培养基重悬细胞并移至新六孔板,悬浮培养9 d,2 d/次更换培养基。取神经球至15 mL离心管,静置5 min后去上清,加1 mLTrypLE置于37 ℃,5% CO2的培养箱消化5 min,加入1 mL培养基(含10% FBS)终止消化,移至15 mL离心管,1000 r/min,离心5 min,去上清。加NDM培养基重悬成单细胞,接种于PLOLaminin包被的玻片(Cover Slip),每个玻片约10 000个细胞。置于37 ℃,5% CO2的培养箱过夜,次日用免疫荧光法检测特征性蛋白NKX2.1和FOXG1。
1.7. 猪iPSCs、iNECs和iGABA能神经元前体表面特征性抗原的免疫荧光检测
用4%多聚甲醛固定玻片上的细胞18 min,用0.2% Triton X-100渗透8 min,之后用2%胎牛血清白蛋白(BSA)和10%驴或羊血清在室温下封闭50 min[12]。通过以下一抗与标本孵育4 ℃过夜,细胞多潜能标志物:SSEA1(1∶200)、Oct4(1∶200)、Nanog(1∶200)、TRA- 160(1∶200);神经元标志物:鼠抗Nestin(1∶200)、兔抗Sox2(1∶300)、兔抗PAX6(1∶50)、MTUJ1(1∶300);GABA神经元前体标志物:鼠抗NKX2.1(1∶500)、兔抗FOXG1(1∶500)。次日,在避光、室温条件下与二抗Alexa Fluor 488(1∶500)或Cy3(1∶800)孵育1 h后,用碘化丙啶(PI)或4',6-二脒基-2-苯基吲哚(DAPI)复染细胞核10 min,共聚焦显微镜观察(Olympus,FV-1200MPE SHARE)发光情况。
1.8. 猪iPSCs-iGABA神经元前体定向移植帕金森大鼠
将大鼠深度麻醉后,头部用脑立体定位仪固定,采用6-OHDA单侧毁损法制备稳定的PD模型大鼠(图 6A),以冠状缝和矢状缝相交点即为前囟点,参考大鼠脑立体定位图谱,确定SD大鼠右侧前脑内侧束损伤位点:(1)AP-4.4 mm,ML-1.2 mm,DV-7.8 mm;(2)AP- 4.0 mm,ML-0.8 mm,DV-8.0 mm,分别向这两个预定靶点注入6-OHDA 4 μL(图 6B)。术后2周,腹腔注射APO(0.5 mg/kg)诱发大鼠的旋转行为,以大鼠旋转数≥210 r/30 min为建模成功的标准。将生长状态良好的猪iGABA神经元前体经CM-DiI标记(红色荧光,图 6A),细胞浓度约为1.5×107/mL,采用PD模型相同的坐标,将细胞立体定向移植入PD大鼠脑内黑质-纹状体束。
6.
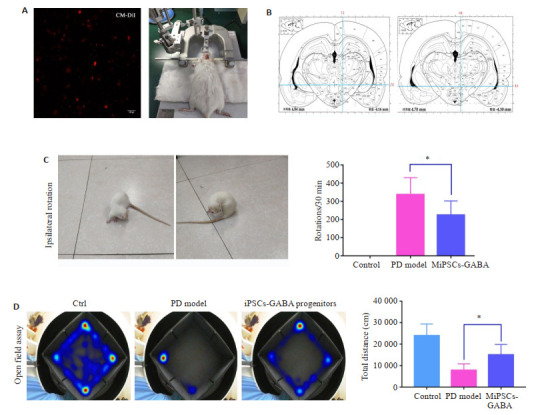
细胞移植帕金森大鼠行为学测试及量化
Behavioral test of Parkinson's disease rat models before and after cell transplantation. A: iPSCs-iGABA progenitors were labeled with Cell Tracker CM-DiI before transplantation (× 100). B: Two coordinates were identified in the ipsilateral right striatum during cell transplantation and establishment of PD model. C: Ipsilateral rotation in APO test; D: Heat maps of open field assay. *P < 0.01.
1.9. 行为学与免疫荧光检测
APO诱导旋转实验:移植细胞8周后,APO诱导大鼠30 min内旋转的圈数与实验组相比较,观察治疗组大鼠的运动功能改善情况。旷场实验:将大鼠放入旷场箱中央格内,迅速拉上周围布帘,系统自动捕捉大鼠5 min内的活动情况并分析其在中央格的逗留时间、运动总距离以及直立次数。选取移植细胞8周后大鼠,心脏灌注取脑后冰冻切片,用2% BSA、10%驴或羊血清和0.2% Triton X-100封闭1 h。加入稀释过的一抗TH(1∶300)与GABA(1∶100),4 ℃孵育过夜。避光、室温与Alexa Fluor 488(1∶500)或Cy3(1∶800)标记的二抗孵育1 h。DAPI复染后,滴加适量抗荧光淬灭剂,封片,激光共聚焦显微镜观察(Olympus,FV-1200MPE SHARE)。
2. 结果
2.1. 猪iPSCs形态特点及多能性鉴定
猪iPSCs呈典型的团状克隆生长,呈圆形或椭圆形,细胞核大浆小,边缘有较为清晰的界限,中央逐渐突起,倒置显微镜下观察立体感更强,细胞排列更紧密(图 3A)。免疫荧光结果显示,猪iPSCs高表达多能性标记Nanog、OCT4、SSEA1和TRA-160(图 1A)。染色体G显带结果验证了猪的染色体数目2n=38条,且形态正常(图 1B)。
3.

猪iPSCs-GABA能神经元前体各阶段细胞的形态变化
Morphological changes of miniature-swine iPSCs-GABA progenitors at different stages. A: Phase contrast images of iPSCs. B: iPSCs form embryoid bodies (EBs) in suspension culture for the first 6 days. C: On day 12, each EB develops into a colony containing neuroepithelial cells in the form of rosettes; D: On day 14, the rosette-containing colonies are detached and grown in suspension to form neuroepithelial spheres (NS). E: iPSCs-GABAprogenitors are induced.
1.

猪iPSCs生物学特征鉴定
Biological characteristics of miniature-swine iPSCs. A: Immunofluorescence detection of pluripotent markers of miniature-swine iPSCs (Original magnification: ×100). B: G-band staining of miniature-swine iPSCs.
2.2. 猪iPSCs-NECs诱导与特征检测
猪iPSCs诱导分化为GABA能神经元前体遵循两个阶段(图 2),第1阶段,将猪iPSCs诱导分化为原始神经上皮细胞(iNECs)。第2阶段,继续将iPSCs-NECs诱导分化形成GABA能神经元前体细胞。
2.

猪iPSCs-GABA神经元前体的时间轴
Timeline of generation of forebrain GABAneural progenitors.
猪iPSCs形成的类胚体呈球形,胚体饱满,细胞间紧密粘附(图 3B)。第12天镜下观察到每个EB发育成一个集落,集落中包含典型玫瑰花结式的神经管样“rosette”结构,即为诱导原始神经上皮细胞(iNECs) (图 3C)。
免疫荧光染色结果显示,分化得到的“rosette”样结构能够表达神经上皮细胞标记物PAX6、SOX2、Nestin以及神经微管蛋白标记Tuj1,具有神经上皮细胞特征(图 4)。
4.

猪iPSCs-原始神经上皮细胞表面标志物的表达及量化
Expression of surface markers of miniature-swine iPSCs-iNECs. A: NECs induced from miniature-swine iPSCs express SOX2, Nestin, PAX6 and TUJ1. B: Expression of specific markers of iPSCs-iNECs analyzed by flow cytometry.
2.3. 猪iPSCs-NECs-iGABA能神经元前体的诱导及鉴定
将猪iPSCs-NECs更换含有高浓度Pur的NIM培养基悬浮培养,形成神经上皮球(NS)(图 3D),第21天诱导成GABA能神经元前体(图 3E)。经免疫荧光染色结果显示,90%以上的细胞诱导成为NKX2.1+和FOXG1+ 的iGABA神经元前体细胞(图 5)。
5.

猪iPSCs-前脑GABA能神经元前体表面标志物的表达及量化
Characteristics of miniature-swine iPSCs-iGABA progenitors. A: The induced iPSCs-GABA progenitors are positive for the forebrain marker FOXG1 (red) and GABA progenitor marker NKX2.1 (green). B: More than 90% of the cells are positive for NKX2.1 and FOXG1. C: Expression of FOXG1 and NKX2.1 analyzed by flow cytometry.
2.4. iPSCs-GABA能神经元前体对PD模型大鼠的修复效应
6-OHDA单侧毁损法建模成功的SD大鼠(图 6A、B)在APO诱导旋转实验中表现出压尾、弓背、嗅探等行为,并出现以左后肢为支点,不断地向损伤对侧旋转的行为,转圈数≥210 r/30 min。与模型组相比(n=10),iPSCs-GABA能神经元前体移植8周后,APO诱导的旋转行为显著减少(P < 0.01,n=12,图 6C),此外,GABA前体细胞移植后的大鼠比PD模型组更兴奋、更活跃,旷场运动总距离显著增加(P < 0.01,图 6D)。
大鼠脑组织冰冻切片免疫荧光检测结果显示,移植后8周,70%以上的GABA能神经元前体细胞表达GABA(+ 图 7)。此外,CM-DiI标记的细胞形成了明显的移植物区域,而且许多CM-DiI标记的细胞,TH染色也呈阳性,TH+细胞团散布于移植物各处,并整合于宿主纹状体(图 7)。
7.

猪iPSCs-iGABA能神经元前体在大鼠脑内的存活、分化
Survival and differentiation of MiPSCs-iGABAprogenitors in vivo (×200).
3. 讨论
目前,iPSCs的体内试验多局限于大小鼠,但是大小鼠无论在生理生化特征还是免疫特性上和人类都有很大的区别,尤其是对脊髓损伤与神经退行性病变,啮齿类模型往往不能有效地模拟疾病。并且能够实现长时间实验追踪的医学模型,大小鼠并不是最佳选择。为了实现大小鼠和人之间的过渡,我们注意到另外一种模式生物-猪,五指山小型猪的器官和人类器官大小相近,解剖生理、血液指标、免疫功能及50多项生理生化指标近似人类,被用作皮肤科或心脏科的供体材料,更适合作为人类疾病研究的动物模型[20, 22]。同时研究发现,猪和人的诱导多能干细胞系在维持多能性方面的信号通路具有相似性[23-24]。
GABA能神经元属于抑制性神经元,它的丢失会导致一些神经系统疾病[25]。以细胞为基础治疗,旨在替代这些功能失调或受损的抑制细胞。本试验在Liu等[15]的研究基础上,首次成功建立猪iPSCs到GABA能神经元前体的分化体系。猪iPSCs经历21 d包括两个不同的分化阶段后分化形成GABA能神经元前体。本实验还发现经过PLO和Laminin双重包被的培养皿、适当的细胞接种密度将极大的提高存活率。这种无血清的分化体系,与之前的分化体系相比较,过程更简单,耗时更短。与Sun等[18]利用慢病毒转导将iPSCs诱导成GABA能神经元的方法相比,本实验采用在培养基中添加小分子化合物组合诱导法,既避免了使用病毒载体可能产生的外源基因插入,又提高了诱导效率,具有更高的生物安全性和应用潜力。
基于猪iPSCs的生长特性,本实验在Liu等[15]的研究基础上作了适当的调整:第1阶段,猪iPSCs形成EBs后,使用NIM培养基(含SB431542、DMH1、FGF2)成功诱导分化获得NECs,发现NIM(10% FBS)培养基更容易使EBs贴壁,并且培养时间由5~8 h延长至过夜,而适当密度接种细胞以及PLO-Laminin双包被培养皿,可增强NECs的黏附性;第2阶段,形成神经球后,使用NIM培养基(含Pur、B27)诱导得到GABA能神经元前体,并表达其特异性标志物,说明猪iPSCs成功分化为前脑GABA能神经元前体。第2阶段是本诱导方法最关键的部分,通过高浓度的Pur,激活hedgehog通路[15, 26],前脑内NKX2.1+细胞的数量和Pur的浓度呈剂量依赖性[12]。同时,为了维持特定神经前体的前脑同一性,要避免常用的神经诱导剂如维甲酸或有丝分裂原如(FGF2),因为它们在不同程度上尾化神经上皮[27]。
将猪iPSCs-iGABA能神经元前体移植到6-OHDA损毁的大鼠黑质纹状体8周后,可以明显恢复PD大鼠的运动功能障碍与神经功能。而且,移植8周后,70%的iGABA能神经元前体细胞表达GABA,这些GABA能神经元可能负责调控纹状体兴奋性和抑制性信号的平衡,从而产生行为改变。此外,部分移植细胞分化为TH+神经元,TH在多巴胺的生物合成中起着重要的调节作用。供体源性TH+细胞(DA神经元)的成熟和增加以及与宿主脑之间突触的形成可能有助于功能恢复。因此,PD大鼠的行为改善可能与纹状体多巴胺能神经元、GABA能神经元的联合作用有关。
综上所述,本研究通过分步诱导法,成功将猪iPSCs诱导形成前脑GABA能神经元前体细胞,将其移植至PD大鼠脑黑质纹状体区,能够分化为功能性神经元,改善PD大鼠的运动功能障碍。猪iPSCs-iGABA能神经元前体的成功获取为帕金森病的治疗提供重要的细胞来源。
Biography
朱缓,硕士,E-mail: 921268155@qq.com
Funding Statement
国家自然科学基金(81771381);安徽省自然科学基金(1908085MH277);安徽省高校自然科学基金重点项目(KJ2017A215,KJ2019A0322);国家级大学生创新创业训练项目资助(201910367005,201910367039,201910367046,202010367015)
Supported by National Natural Science Foundation of China (81771381)
Contributor Information
朱 缓 (Huan ZHU), Email: 921268155@qq.com.
郭 俣 (Yu GUO), Email: guoyu@bbmc.edu.cn.
References
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors[J]. Cell, 2006, 126(4): 663-76.] [DOI] [PubMed] [Google Scholar]
- 2.During MJ, Kaplitt MG, Stern MB, et al. Subthalamic GAD gene transfer in Parkinson disease patients who are candidates for deep brain stimulation. http://www.ncbi.nlm.nih.gov/pubmed/11529246. Hum Gene Ther. 2001;12(12):1589–91. [During MJ, Kaplitt MG, Stern MB, et al. Subthalamic GAD gene transfer in Parkinson disease patients who are candidates for deep brain stimulation[J]. Hum Gene Ther, 2001, 12(12): 1589-91.] [PubMed] [Google Scholar]
- 3.Huang LX, Ren YD, Zeng ZS, et al. Comparative study of striatum GABA concentrations and magnetic resonance spectroscopic imaging in Parkinson's disease monkeys. BMC Neurosci. 2019;20(1):42. doi: 10.1186/s12868-019-0522-8. [Huang LX, Ren YD, Zeng ZS, et al. Comparative study of striatum GABA concentrations and magnetic resonance spectroscopic imaging in Parkinson's disease monkeys[J]. BMC Neurosci, 2019, 20(1): 42.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martínez-Cerdeño V, Noctor SC, Espinosa A, et al. Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6-OHDA-lesioned rats. Cell Stem Cell. 2010;6(3):238–50. doi: 10.1016/j.stem.2010.01.004. [Martínez-Cerdeño V, Noctor SC, Espinosa A, et al. Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6-OHDA-lesioned rats[J]. Cell Stem Cell, 2010, 6(3): 238-50.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen H, Zarriello S, Coats A, et al. Stem cell therapy for neurological disorders: a focus on aging. Neurobiol Dis. 2019;126 doi: 10.1016/j.nbd.2018.09.011. [Nguyen H, Zarriello S, Coats A, et al. Stem cell therapy for neurological disorders: a focus on aging[J]. Neurobiol Dis, 2019, 126.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu GL, David BT, Trawczynski M, et al. Advances in pluripotent stem cells: history, mechanisms, technologies, and applications. Stem Cell Rev Rep. 2020;16(1):3–32. doi: 10.1007/s12015-019-09935-x. [Liu GL, David BT, Trawczynski M, et al. Advances in pluripotent stem cells: history, mechanisms, technologies, and applications[J]. Stem Cell Rev Rep, 2020, 16(1): 3-32.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang MY, Zhang L, Gage FH. Modeling neuropsychiatric disorders using human induced pluripotent stem cells. Protein Cell. 2020;11(1):45–59. doi: 10.1007/s13238-019-0638-8. [Wang MY, Zhang L, Gage FH. Modeling neuropsychiatric disorders using human induced pluripotent stem cells[J]. Protein Cell, 2020, 11 (1): 45-59.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakayama N, Pothiawala A, Lee JY, et al. Human pluripotent stem cell-derived chondroprogenitors for cartilage tissue engineering. Cell Mol Life Sci. 2020;77(13):2543–63. doi: 10.1007/s00018-019-03445-2. [Nakayama N, Pothiawala A, Lee JY, et al. Human pluripotent stem cell-derived chondroprogenitors for cartilage tissue engineering[J]. Cell Mol Life Sci, 2020, 77(13): 2543-63.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murueta-Goyena A, Andikoetxea A, Gómez-Esteban JC, et al. Contribution of the GABAergic system to non-motor manifestations in premotor and early stages of Parkinson's disease. Front Pharmacol. 2019;10:1294. doi: 10.3389/fphar.2019.01294. [Murueta-Goyena A, Andikoetxea A, Gómez-Esteban JC, et al. Contribution of the GABAergic system to non-motor manifestations in premotor and early stages of Parkinson's disease[J]. Front Pharmacol, 2019, 10: 1294.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolagar TA, Farzaneh M, Nikkar N, et al. Human pluripotent stem cells in neurodegenerative diseases: potentials, advances and limitations. Curr Stem Cell Res Ther. 2020;15(2):102–10. doi: 10.2174/1574888X14666190823142911. [Kolagar TA, Farzaneh M, Nikkar N, et al. Human pluripotent stem cells in neurodegenerative diseases: potentials, advances and limitations[J]. Curr Stem Cell Res Ther, 2020, 15(2): 102-10.] [DOI] [PubMed] [Google Scholar]
- 11.Goulburn AL, Alden D, Davis RP, et al. A targeted NKX2.1 human embryonic stem cell reporter line enables identification of human basal forebrain derivatives. Stem Cells. 2011;29(3):462–73. doi: 10.1002/stem.587. [Goulburn AL, Alden D, Davis RP, et al. A targeted NKX2.1 human embryonic stem cell reporter line enables identification of human basal forebrain derivatives[J]. Stem Cells, 2011, 29(3): 462-73.] [DOI] [PubMed] [Google Scholar]
- 12.Germain ND, Banda EC, Becker S, et al. Derivation and isolation of NKX2.1-positive basal forebrain progenitors from human embryonic stem cells. Stem Cells Dev. 2013;22(10):1477–89. doi: 10.1089/scd.2012.0264. [Germain ND, Banda EC, Becker S, et al. Derivation and isolation of NKX2.1-positive basal forebrain progenitors from human embryonic stem cells[J]. Stem Cells Dev, 2013, 22(10): 1477-89.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maroof AM, Keros S, Tyson JA, et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell. 2013;12(5):559–72. doi: 10.1016/j.stem.2013.04.008. [Maroof AM, Keros S, Tyson JA, et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells[J]. Cell Stem Cell, 2013, 12(5): 559-72.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholas CR, Chen J, Tang Y, et al. Functional maturation of hPSCderived forebrain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell. 2013;12(5):573–86. doi: 10.1016/j.stem.2013.04.005. [Nicholas CR, Chen J, Tang Y, et al. Functional maturation of hPSCderived forebrain interneurons requires an extended timeline and mimics human neural development[J]. Cell Stem Cell, 2013, 12(5): 573-86.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Liu H, Sauvey C, et al. Directed differentiation of forebrain GABA interneurons from human pluripotent stem cells. Nat Protoc. 2013;8(9):1670–9. doi: 10.1038/nprot.2013.106. [Liu Y, Liu H, Sauvey C, et al. Directed differentiation of forebrain GABA interneurons from human pluripotent stem cells[J]. Nat Protoc, 2013, 8(9): 1670-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim TG, Yao RQ, Monnell T, et al. Efficient specification of interneurons from human pluripotent stem cells by dorsoventral and rostrocaudal modulation. Stem Cells. 2014;32(7):1789–804. doi: 10.1002/stem.1704. [Kim TG, Yao RQ, Monnell T, et al. Efficient specification of interneurons from human pluripotent stem cells by dorsoventral and rostrocaudal modulation[J]. Stem Cells, 2014, 32(7): 1789-804.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colasante G, Lignani G, Rubio A, et al. Rapid conversion of fibroblasts into functional forebrain GABAergic interneurons by direct genetic reprogramming. Cell Stem Cell. 2015;17(6):719–34. doi: 10.1016/j.stem.2015.09.002. [Colasante G, Lignani G, Rubio A, et al. Rapid conversion of fibroblasts into functional forebrain GABAergic interneurons by direct genetic reprogramming[J]. Cell Stem Cell, 2015, 17(6): 719- 34.] [DOI] [PubMed] [Google Scholar]
- 18.Sun AX, Yuan Q, Tan S, et al. Direct induction and functional maturation of forebrain GABAergic neurons from human pluripotent stem cells. Cell Rep. 2016;16(7):1942–53. doi: 10.1016/j.celrep.2016.07.035. [Sun AX, Yuan Q, Tan S, et al. Direct induction and functional maturation of forebrain GABAergic neurons from human pluripotent stem cells[J]. Cell Rep, 2016, 16(7): 1942-53.] [DOI] [PubMed] [Google Scholar]
- 19.Larsson LC, Frielingsdorf H, Mirza B, et al. Porcine neural xenografts in rats and mice: donor tissue development and characteristics of rejection. Exp Neurol. 2001;172(1):100–14. doi: 10.1006/exnr.2001.7738. [Larsson LC, Frielingsdorf H, Mirza B, et al. Porcine neural xenografts in rats and mice: donor tissue development and characteristics of rejection[J]. Exp Neurol, 2001, 172(1): 100-14.] [DOI] [PubMed] [Google Scholar]
- 20.Mine Y, Momiyama T, Hayashi T, et al. Grafted miniature-swine neural stem cells of early embryonic mesencephalic neuroepithelial origin can repair the damaged neural circuitry of Parkinson's disease model rats. Neuroscience. 2018;386:51–67. doi: 10.1016/j.neuroscience.2018.06.007. [Mine Y, Momiyama T, Hayashi T, et al. Grafted miniature-swine neural stem cells of early embryonic mesencephalic neuroepithelial origin can repair the damaged neural circuitry of Parkinson's disease model rats[J]. Neuroscience, 2018, 386: 51-67.] [DOI] [PubMed] [Google Scholar]
- 21.Li XC, Guo Y, Yao YX, et al. Reversine increases the plasticity of long-term cryopreserved fibroblasts to multipotent progenitor cells through activation of Oct4. Int J Biol Sci. 2016;12(1):53–62. doi: 10.7150/ijbs.12199. [Li XC, Guo Y, Yao YX, et al. Reversine increases the plasticity of long-term cryopreserved fibroblasts to multipotent progenitor cells through activation of Oct4[J]. Int J Biol Sci, 2016, 12(1): 53-62.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma CY, Guo Y, Liu H, et al. Isolation and biological characterization of a novel type of pulmonary mesenchymal stem cells derived from Wuzhishan miniature pig embryo. Cell Biol Int. 2016;40(10):1041–9. doi: 10.1002/cbin.10643. [Ma CY, Guo Y, Liu H, et al. Isolation and biological characterization of a novel type of pulmonary mesenchymal stem cells derived from Wuzhishan miniature pig embryo[J]. Cell Biol Int, 2016, 40(10): 1041-9.] [DOI] [PubMed] [Google Scholar]
- 23.Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. http://onlinelibrary.wiley.com/resolve/reference/PMED?id=16179608. J Cell Sci. 2005;118(Pt 19):4495–509. doi: 10.1242/jcs.02553. [Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells[J]. J Cell Sci, 2005, 118(Pt 19): 4495-509.] [DOI] [PubMed] [Google Scholar]
- 24.Alberio R, Croxall N, Allegrucci C. Pig epiblast stem cells depend on activin/nodal signaling for pluripotency and self-renewal. Stem Cells Dev. 2010;19(10):1627–36. doi: 10.1089/scd.2010.0012. [Alberio R, Croxall N, Allegrucci C. Pig epiblast stem cells depend on activin/nodal signaling for pluripotency and self-renewal[J]. Stem Cells Dev, 2010, 19(10): 1627-36.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lozovaya N, Eftekhari S, Cloarec R, et al. GABAergic inhibition in dual-transmission cholinergic and GABAergic striatal interneurons is abolished in Parkinson disease. Nat Commun. 2018;9(1):1422. doi: 10.1038/s41467-018-03802-y. [Lozovaya N, Eftekhari S, Cloarec R, et al. GABAergic inhibition in dual-transmission cholinergic and GABAergic striatal interneurons is abolished in Parkinson disease[J]. Nat Commun, 2018, 9(1): 1422.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ericson J, Muhr J, Placzek M, et al. Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell. 1995;81(5):747–56. doi: 10.1016/0092-8674(95)90536-7. [Ericson J, Muhr J, Placzek M, et al. Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube[J]. Cell, 1995, 81(5): 747- 56.] [DOI] [PubMed] [Google Scholar]
- 27.LaVaute TM, Yoo YD, Pankratz MT, et al. Regulation of neural specification from human embryonic stem cells by BMP and FGF. Stem Cells. 2009;27(8):1741–9. doi: 10.1002/stem.99. [LaVaute TM, Yoo YD, Pankratz MT, et al. Regulation of neural specification from human embryonic stem cells by BMP and FGF [J]. Stem Cells, 2009, 27(8): 1741-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]


