Abstract
Background:
Humans are primary drivers of environmental contamination worldwide, including in drinking-water resources. In the United States (US), federal and state agencies regulate and monitor public-supply drinking water while private-supply monitoring is rare; the current lack of directly comparable information on contaminant-mixture exposures and risks between private- and public-supplies undermines tapwater (TW) consumer decision-making.
Methods:
We compared private- and public-supply residential point-of-use TW at Cape Cod, Massachusetts, where both supplies share the same groundwater source. TW from 10 private- and 10 public-supply homes was analyzed for 487 organic, 38 inorganic, 8 microbial indicators, and 3 in vitro bioactivities. Concentrations were compared to existing protective health-based benchmarks, and aggregated Hazard Indices (HI) of regulated and unregulated TW contaminants were calculated along with ratios of in vitro exposure-activity cutoffs.
Results:
Seventy organic and 28 inorganic constituents were detected in TW. Median detections were comparable, but median cumulative concentrations were substantially higher in public supply due to 6 chlorine-disinfected samples characterized by disinfection byproducts and corresponding lower heterotrophic plate counts. Public-supply applicable maximum contaminant (nitrate) and treatment action (lead and copper) levels were exceeded in private-supply TW samples only. Exceedances of health-based HI screening levels of concern were common to both TW supplies.
Discussion:
These Cape Cod results indicate comparable cumulative human-health concerns from contaminant exposures in private- and public-supply TW in a shared source-water setting. Importantly, although this study’s analytical coverage exceeds that currently feasible for water purveyors or homeowners, it nevertheless is a substantial underestimation of the full breadth of contaminant mixtures documented in the environment and potentially present in drinking water.
Conclusion:
Regardless of the supply, increased public engagement in source-water protection and drinking-water treatment, including consumer point-of-use treatment, is warranted to reduce risks associated with long-term TW contaminant exposures, especially in vulnerable populations.
Keywords: tapwater, organics, inorganics, disinfection byproducts, sole-source aquifer, human health, public supply, private supply
1. Introduction
Current understanding of the role of drinking-water contaminant-mixture exposures in human-health outcomes remains limited, in part, because routine compliance monitoring and more comprehensive multi-contaminant chemical and biological investigations in the United States (US) and worldwide are, with notable exceptions (e.g., Olson et al. 1999), not commonly conducted at the point and time of consumption and, thus, do not encompass biochemical changes that may occur during distribution and delivery, whether via public-supply infrastructure, private-well/premise plumbing, or commercial packaging (“bottled” water). In the extant Anthropocene, humans are acknowledged as primary drivers of many contaminants of potential human-health concern in water resources, including in drinking source waters, (Crutzen and Stoermer 2000; Crutzen 2006; Steffen et al. 2011). Many of the same anthropogenic (human generated or driven) contaminant concerns affect public, private, and bottled drinking-water supplies (Bradley et al. 2017; Moschet et al. 2014; Olson et al. 1999; Wang et al. 2020). Notable differences in management and monitoring, however, engender a fundamental information imbalance that influences the perceived quality and safety of different drinking-water supplies and, consequently, directly impacts consumer decision making. In the US and elsewhere, the general lack of comparable water-quality information on private supplies versus public supplies contributes to public concern for the safety and socioeconomic equity of tapwater (TW) (Collier et al. 2019; Hobson et al. 2007; Huerta-Saenz et al. 2012; Javidi and Pierce 2018; Pierce and Gonzalez 2017; Rosinger et al. 2018; Wescoat et al. 2007) and undermines consumer decisions (Flanagan et al. 2016b; MacDonald Gibson et al. 2014; MacDonald Gibson and Pieper 2017; Pieper et al. 2018; Ranganathan and Balazs 2015; Seltenrich 2017; Zheng and Flanagan 2017).
In the US, approximately 87% of the population receives its drinking water from public water systems (i.e., ≥15 service connections or ≥25 people served for ≥60 days per year (U.S. Environmental Protection Agency 2020g); referred to herein as public supply) with the remainder from private supply (Dieter and Maupin 2017; Dieter et al. 2018; Maupin 2018). In development of public-supply drinking-water contaminant regulations under the Safe Drinking Water Act (SDWA), the US Environmental Protection Agency (EPA) first sets non-enforceable, margin-of-safety Maximum Contaminant Level Goal(s) (MCLG), based solely on public health and specifically in consideration of sensitive subpopulations (infants, children, elderly, immune and disease compromised), and then sets and promulgates enforceable primary standards (National Primary Drinking Water Regulations, NPDWR), including Maximum Contaminant Level(s) (MCL) or treatment techniques and corresponding action level(s) (AL), as close to the MCLG as economically and technically feasible (U.S. Environmental Protection Agency 2017, 2018, 2020k). In general, regulated chemical contaminants are monitored for compliance and, as needed, treated at drinking-water facilities prior to distribution, with notably limited monitoring in the distribution system (e.g., disinfection byproduct(s) (DBP) and residual disinfectant (U.S. Environmental Protection Agency 2010, 2020i)) and at select tapwater (TW) consumer endpoints (lead and copper (U.S. Environmental Protection Agency 2008, 2020f)). Acknowledged challenges to the perceived quality of public-supply TW (Doria 2010; Doria et al. 2009; Doria 2006; Pierce and Gonzalez 2017) include 1) the disparity between the number of regulated contaminants and the breadth of anthropogenic chemicals in commercial use (Wang et al. 2020) and in ambient (surface water, groundwater) source waters (Altenburger et al. 2015; Bradley et al. 2017; de Jesus Gaffney et al. 2015; DeSimone et al. 2015; Moschet et al. 2014; Toccalino and Hopple 2010; Toccalino et al. 2012), 2) persistence of generally low-level unregulated organic contaminants in existing treatment processes and occurrence in pre-distribution, treated drinking water (Conley et al. 2017b; Furlong et al. 2017; Glassmeyer et al. 2017; Guelfo and Adamson 2018; Hu et al. 2016; Klarich et al. 2017; Klarich Wong et al. 2019; Stackelberg et al. 2004; Stackelberg et al. 2007), 3) distribution-system water-quality changes (Makris et al. 2014; Triantafyllidou and Edwards 2012; U.S. Environmental Protection Agency 2020f, i) and inadequate water-quality characterization at the tap (Bondy and Campbell 2018; Bradley et al. 2018; Bradley et al. 2020; Braun and Gray 2017) and 4) chlorine-based disinfection to control water-borne diseases (Reynolds et al. 2008; Richardson et al. 2007; Schoenen 2002) also impacts taste and odor (organoleptic quality) (Doria 2010; Doria et al. 2009; Doria 2006; Pierce and Gonzalez 2017) and produces genotoxic/cytotoxic DBP (Jeong et al. 2015; Krasner et al. 2016; Muellner et al. 2007; Pressman et al. 2010; Richardson et al. 2007; Stalter et al. 2020; Villanueva et al. 2018; Wang et al. 2015). The latter is a particularly challenging public-health tradeoff and cogent argument for continued water-disinfection innovation (Hrudey et al. 2006; Hrudey 2009; Richardson and Plewa 2020; Stalter et al. 2020).
On the other hand, EPA is not authorized to regulate or monitor private supplies (i.e., <25 people or <15 service connections (U.S. Environmental Protection Agency 2020g)), and more than 40 million people in the US depend on private wells for drinking water (Dieter and Maupin 2017; Dieter et al. 2018; Maupin 2018). Although prior studies have documented multiple concerns and the need for expanded monitoring in private-well systems (de Jesus Gaffney et al. 2015; DeSimone et al. 2015; Focazio et al. 2006; Knobeloch et al. 2013; MacDonald Gibson and Pieper 2017; Rogan and Brady 2009; Zheng and Flanagan 2017; Zogorski et al. 2006), corresponding water quality data remain rare in part because high analytical costs and conflation of organoleptic quality with safety undermine owner self-monitoring (Doria 2010; Doria et al. 2009; Flanagan et al. 2016a; Flanagan et al. 2016b; Focazio et al. 2006; MacDonald Gibson and Pieper 2017; Seltenrich 2017; Swistock and Clemens 2013; Zheng and Flanagan 2017).
The resultant lack of drinking-water contaminant characterization in private-supply, relative to public-supply, and misplaced confidence in organoleptic quality as an indicator of safety contribute to 1) public-supply TW mistrust and its notable socioeconomic underpinnings (Collier et al. 2019; Hobson et al. 2007; Huerta-Saenz et al. 2012; Javidi and Pierce 2018; Pierce and Gonzalez 2017; Rosinger et al. 2018; Wescoat et al. 2007), 2) reluctance or inability to switch to monitored public supply in high risk (e.g., urbanizing or geogenic contaminant) locations (Flanagan et al. 2016b; MacDonald Gibson et al. 2014; MacDonald Gibson and Pieper 2017; Pieper et al. 2018; Ranganathan and Balazs 2015; Seltenrich 2017; Thomas et al. 2019; Zheng and Flanagan 2017), 3) delayed adoption of appropriate point-of-use treatment (MacDonald Gibson and Pieper 2017; Pieper et al. 2018), and 4) consumption of sugary drink or bottled water alternatives (Hawkins 2017; Saylor et al. 2011) with documented human-health (e.g., for the former, obesity, diabetes, dental caries, Auerbach et al. 2016; Legler et al. 2015; Onufrak et al. 2014; Scherzer et al. 2010; World Health Organization 2016) as well as economic and environmental consequences (e.g., plastic pollution, cost, sustainability, Collier et al. 2019; Knox and McDermott 2019; Olson et al. 1999; Onufrak et al. 2014; Pierce and Gonzalez 2017; Saylor et al. 2011).
The U.S. Geological Survey (USGS) is collaborating with the EPA, National Institute of Environmental Health Science (NIEHS), Colorado School of Mines (Mines), utilities, and others to inform drinking-water exposure data gaps and associated water-supply information imbalances by assessing TW mixture exposures and associated distal (e.g., ambient source water) and proximal (e.g., premise plumbing, point-of-use treatment) drivers in a range of source-water vulnerability settings (Bradley et al. 2018; Bradley et al. 2020). Demographic and hydrologic settings are critical determinants of source-water vulnerability, and populated locations that depend on surface water or shallow unconfined aquifers are particular concerns for elevated drinking-water contaminant risk (Gallagher et al. 2010; Schaider et al. 2014; Schaider et al. 2016; Swartz et al. 2003; Swartz et al. 2006). The Cape Cod aquifer is a single continuous unconfined aquifer that currently serves as the “sole source” of drinking water for more than 140,000 permanent and 400,000 seasonal residents of Cape Cod, Massachusetts (Cape Cod Commission 2020b; Cape Cod Groundwater Guardians 2020; Masterson and Walter 2009). More broadly, the vulnerable Cape Cod sole-source aquifer system provides insight into other east coast Coastal Plain packages and vulnerable, shallow, unconfined groundwater drinking-water sources across the US.
USGS, EPA, NIEHS, and Mines assessed TW exposures in 20 homes at Cape Cod in 2018, in order to compare estimates of cumulative risk (exposure, toxicity/hazard; Moretto et al. 2017; National Research Council 1983; Norton et al. 1992) to human health from contaminants in private- and public-supply drinking water in an example populated and unconfined aquifer source-water setting. For this study, TW exposure is operationally defined as concentrations of 487 unique organics, 38 inorganics, and select microbiological contaminant and bioactivity indicators detected in residential point-of-consumption TW samples. Cape Cod private and public supplies rely on the same unconsolidated, surficial-aquifer source (Cape Cod Groundwater Guardians 2020; Masterson and Walter 2009), which has well-documented legacy and emerging anthropogenic contaminant concerns (for summary and data viewer see, Cape Cod Commission 2020a; Cape Cod Commission 2020b). Potential human-health risks of individual and aggregate TW exposures were explored based on multiple lines of evidence, comprising: 1) cumulative detections and concentrations of designed-bioactive (e.g., pesticides, pharmaceuticals) chemicals (Bradley et al. 2017; Bradley et al. 2018; Bradley et al. 2020), 2) individual contaminant exposure exceedances of health-only benchmarks (i.e., hazard quotient (HQ) approach, Goumenou and Tsatsakis 2019; U.S. Environmental Protection Agency 2011, 2012), including MCLG (U.S. Environmental Protection Agency 2017, 2020k) and other public-health advisories, and 3) exposure metrics based on cumulative Exposure-Activity Ratio(s) (∑EAR) (Blackwell et al. 2017) and hazard indices (HI, cumulative hazard quotients for mixtures, Goumenou and Tsatsakis 2019; U.S. Environmental Protection Agency 2011, 2012) of cumulative benchmark-based Toxicity Quotients (∑TQ) (Corsi et al. 2019). The same general sampling protocol and analytical toolbox employed previously (Bradley et al. 2018; Bradley et al. 2020) were preserved herein to inform TW chemical and biological exposures at Cape Cod, while supporting inter-study comparisons.
Multiple TW-exposure hypotheses relevant specifically to Cape Cod and, more generally, to shared source waters from unconfined, highly permeable aquifers in populated areas were assessed, as follows. Because of the shared aquifer source and unregulated status of most of the organic analytes in this study, exposures and estimates of aggregate effects were expected to be comparable in private- and public-supply TW (Hypothesis I), with the following exceptions. Exceedances of NPDWR, National Secondary Drinking Water Regulations (NSDWR), and other water-quality outliers were expected to be more common in private supply, due to a lack of SDWA regulation, monitoring, and treatment (Hypothesis II). DBP were expected to dominate organic detections and concentrations in TW samples from public supplies that employed chlorine-based disinfection (Hypothesis III) and microbial indicators were expected to be generally lower (Hypothesis IV) in these chlorine-disinfected public-supply TW samples (public-health tradeoff).
2. Methods
2.1. Site Selection and Sample Collection
In line with national water-use statistics (Dieter and Maupin 2017; Dieter et al. 2018; Maupin 2018), drinking water for approximately 85% of the Cape Cod populace is from public supply and 15% from private wells (Cape Cod Commission 2020a, b; Cape Cod Groundwater Guardians 2020). The unconfined surficial Cape Cod aquifer provides 100% of Cape Cod drinking water (Cape Cod Commission 2020b; Cape Cod Groundwater Guardians 2020; Masterson and Walter 2009) and, consequently, is designated a sole source aquifer (U.S. Environmental Protection Agency 2020b, e) by the EPA. For this synoptic pilot study, TW sample locations were selected based on community volunteers and to ensure broad spatial coverage of Cape Cod (Figures 1 and S1) and equal coverage of public/private supply (10 public, 10 private). All TW samples were sourced from the interconnected Sagamore and Monomoy lenses of the Cape Cod aquifer system (Barbaro et al. 2014; Cape Cod Commission 2020a; Masterson and Walter 2009). Public-supply TW samples were from 8 drinking-water suppliers (DWS), of which 5 (DWS: 1, 3, 4, 7, 8; total TW samples: 6) employed chlorine-based disinfection and the remainder did not (Table S1). Complete sampling details are provided elsewhere (Romanok et al. 2018; Romanok et al. 2020). Taps (cold water) were sampled once each in August 2018, with sample times varying throughout the day and without pre-cleaning, screen removal, or flushing (Romanok et al. 2018); relevant to the interpretation of observed lead (Pb) and copper (Cu) concentrations, 6h-stagnant first draw Lead and Copper Rule (LCR) monitoring protocols (first-draw, 6h-stagnant sampling, U.S. Environmental Protection Agency 2008; U.S. Environmental Protection Agency 2020f) were not employed.
Figure 1.
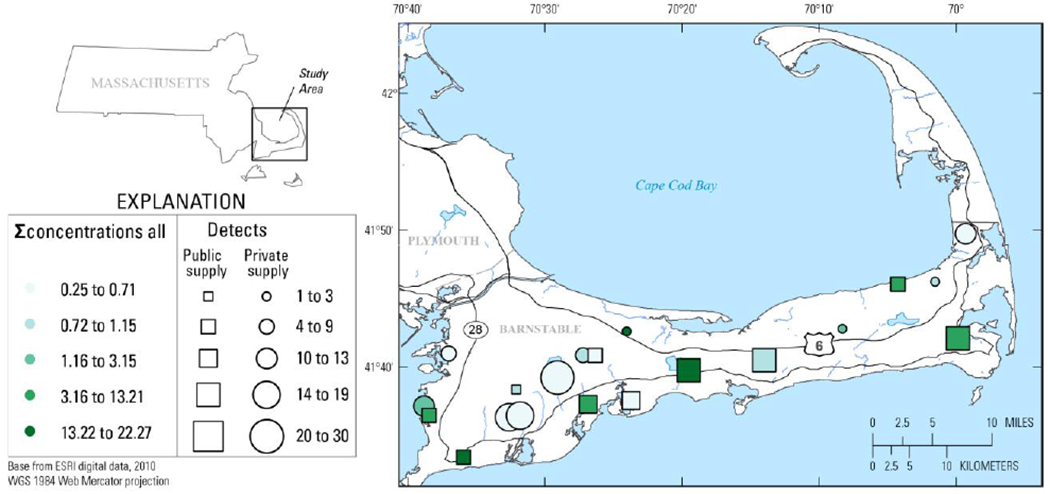
Cumulative (sum of all detected) concentrations (μg L−1) and numbers of organic compounds in samples of treated public-supply (squares, ∎) and untreated private-supply (circles, ●) home tapwater collected during 2018 at Cape Cod, Massachusetts. Sample locations are anonymized.
2.2. Analytical Methods
TW samples were analyzed by USGS using 7 target-organic (487 unique analytes), 3 inorganic/field (38 analytes), and 4 microbial (8 analytes) methods (Table S2), as discussed (Bradley et al. 2018; Bradley et al. 2020; Romanok et al. 2018) and presented in detail previously (American Public Health Association et al. 2017; Ball and McCleskey 2003; Fishman and Friedman 1989; Furlong et al. 2014; Graham et al. 2010; Hergenreder 2011; Hladik et al. 2008; Hoffman et al. 1996; Loftin et al. 2016; Pfaff 1993; Rose et al. 2016; U.S. Environmental Protection Agency 2014). Per/polyfluoroalkyl substance(s) (PFAS) were analyzed (Murray et al. 2019) at Mines, as described (Bradley et al. 2020; Murray et al. 2019; Romanok et al. 2018). In vitro estrogen (ER), androgen (AR), and glucocorticoid (GR) bioactivities were assessed by EPA using the T47D-KBluc cell line (American Type Cell Culture, Manassas, Virginia; ATCC CRL-2865) human estrogen receptor (Wilson et al. 2002; Wilson et al. 2004) and the CV1 cell line (ATCC CCL-70) adenovirally transduced with the chimpanzee androgen receptor (Hartig et al. 2002; Hartig et al. 2007) or the human glucocorticoid receptor (Conley et al. 2017a), as described previously (Conley et al. 2017a; Conley et al. 2017b; Medlock Kakaley et al. 2020). Bioassay standards, controls, and samples were run in quadruplicate, and each sample screen was at least duplicated. Endocrine-active samples were identified using a tiered screening process (Medlock Kakaley et al. 2020), except for ER bioactivity, which was statistically compared to field blanks. Biological equivalency values (BioEq) were calculated using an enrichment factor (EF) of 10,000 (Escher and Leusch 2011) and BioEq above the respective assay method detection limit were considered positive for endocrine activity (Figure S2), as described (Medlock Kakaley et al. 2020). All results are in Tables S3-S8 and in Romanok et al (2020).
2.3. Data Handling, Quality Assurance, Statistics, and Risk Assessments
Quantitative (≥limit of quantitation, ≥LOQ) and semi-quantitative (between LOQ and long-term method detection limit, MDL, Childress et al. 1999; U.S. Environmental Protection Agency 2020c) results were treated as detections (Childress et al. 1999; Mueller et al. 2015). Quality-assurance/quality-control included analyses of field blanks from 2 sites, laboratory blanks and spikes, and stable-isotope surrogates. Only calcium, silica, sodium, zinc, and alkalinity were detected in inorganic-field blanks (Table S5); their reporting limits were blank-corrected to the maximum value detected in any blank. Among those organic compounds detected at least once in residential-TW samples, 1,1-difluoroethane (0.015-0.018 μg L−1) was detected twice and tert-butylalcohol (0.33 μg L−1) was detected once at concentrations comparable to those observed in residential TW samples; these analytes were removed from the interpretive dataset. Of the nine PFAS compounds detected at least once in residential-TW samples, 5 were detected 1-2 times each (maximum 0.0033 μg L−1) in organic-field blanks (Table S5); the reporting limits for these compounds were blank-corrected to twice the highest field blank concentration as footnoted in Tables S4 and S5. The median stable-isotope surrogate recovery (Table S6) for all samples and relevant methods (pesticides, pharmaceuticals) was 99.5% (interquartile range: 90-110%). Differences (data centroids and dispersions) between TW-sample groups were assessed by nonparametric One-way PERMANOVA (n = 9999 permutations) on Euclidean distance (Paleontological Statistics, PAST, vers. 4) (Hammer et al. 2001).
A screening-level assessment (Goumenou and Tsatsakis 2019; U.S. Environmental Protection Agency 2011) of potential cumulative biological activity of mixed-organic contaminants in each TW sample was conducted as described (Blackwell et al. 2017; Bradley et al. 2018; Bradley et al. 2019), using the toxEval version 1.1.0 package (De Cicco et al. 2018) of the open source statistical software R (R Development Core Team 2019) to sum (non-interactive concentration addition model (e.g., Altenburger et al. 2018; Cedergreen et al. 2008; Stalter et al. 2020) individual EAR (ratio of the detected concentration to the activity concentration at cutoff (ACC) from the Toxicity ForeCaster (ToxCast, U.S. Environmental Protection Agency 2020d) high-throughput screening data (U.S. Environmental Protection Agency 2019, 2020a)) to estimate sample-specific cumulative EAR (∑EAR) (Blackwell et al. 2017; Bradley et al. 2018; Bradley et al. 2020). ACC estimates the point of departure concentration at which a defined threshold of response (cutoff) is achieved for a given biological activity and is less prone to violations of relative potency assumptions (for discussion see, Blackwell et al. 2017). ACC data in the toxEval v1.1.0 employed in the present study were from the May 2019 invitroDBv3.0 release of the ToxCast database (publicly available at, U.S. Environmental Protection Agency 2020a). Non-specific-endpoint, baseline, and unreliable response-curve assays were excluded (Blackwell et al. 2017; Bradley et al. 2018; Bradley et al. 2020). ∑EAR results and exclusions are summarized in Tables S9–S11.
Because the ∑EAR approach was constrained to organic chemicals in ToxCast, an analogous human-health benchmark-based HI assessment (Goumenou and Tsatsakis 2019; U.S. Environmental Protection Agency 2011, 2012) of aggregate inorganic and organic contaminant risk also was conducted using toxEval v1.1.0 (De Cicco et al. 2018) to sum the TQ (ratio of detected concentration to corresponding health-based benchmark) of individual detections to estimate sample-specific cumulative TQ (∑TQ) (Corsi et al. 2019). A precautionary screening-level approach was employed based on the most protective human-health benchmark (i.e., lowest benchmark concentration) among the following: MCLG (U.S. Environmental Protection Agency 2017, 2020k), WHO guidelines (World Health Organization (WHO) 2011), USGS Health-Based Screening Level (HBSL; (Norman et al. 2018)), and state drinking-water MCL or health advisories (DWHA). Drinking-water contaminants (e.g., bromodichloromethane, Pb) with no safe-exposure threshold, “below which there is no known or expected risk to the health” of sensitive sub-populations, including infants, children, the elderly, and those with compromised immune systems and chronic diseases (U.S. Environmental Protection Agency 2020j, k), are assigned MCLG of zero (Lanphear 2017; U.S. Environmental Protection Agency 2017, 2020j, k). For the purpose of the ∑TQ assessment employed herein, MCLG values of zero were set to the method reporting limit, except for Pb, which was set to 1 μg L−1 as suggested by the American Academy of Pediatrics (Lanphear et al. 2016). ∑TQ results and respective health-based benchmarks are summarized in Tables S12–S13.
3. Results
3.1. Private/Public-Supply TW Exposures
Multiple detections of regulated and unregulated chemical (inorganic, organic) analytes were typical in both private- and public-supply TW samples at Cape Cod (Tables S3–S4; Figures 1–4). Importantly, 86% of the organic analytes assessed in this study were not detected in any TW sample. Conversely, 70 (14%) of the 487 organic analytes and 28 (74%) of the 38 inorganic analytes and field parameters were detected. No differences in cumulative detections of inorganic (PERMANOVA: 9999 permutations; probability of being the same p = 0.5227) and organic (p = 0.7911) constituents were observed between private- and public-supply TW groups. Of note, pesticides and pharmaceuticals, which are designed to be biologically active, were detected in private- and public-supply TW samples with similar (p = 0.1946 and p = 0.2034 for pesticides and pharmaceuticals, respectively) detection frequencies. Notable exceptions to this general pattern of detections included the higher (p = 0.0009) median and range in the number of detected DBP in public-supply TW and the order of magnitude greater number of pharmaceuticals detected in a single private-well sample (22 versus median = 1 in all other samples; Figures 3–4).
Figure 4.
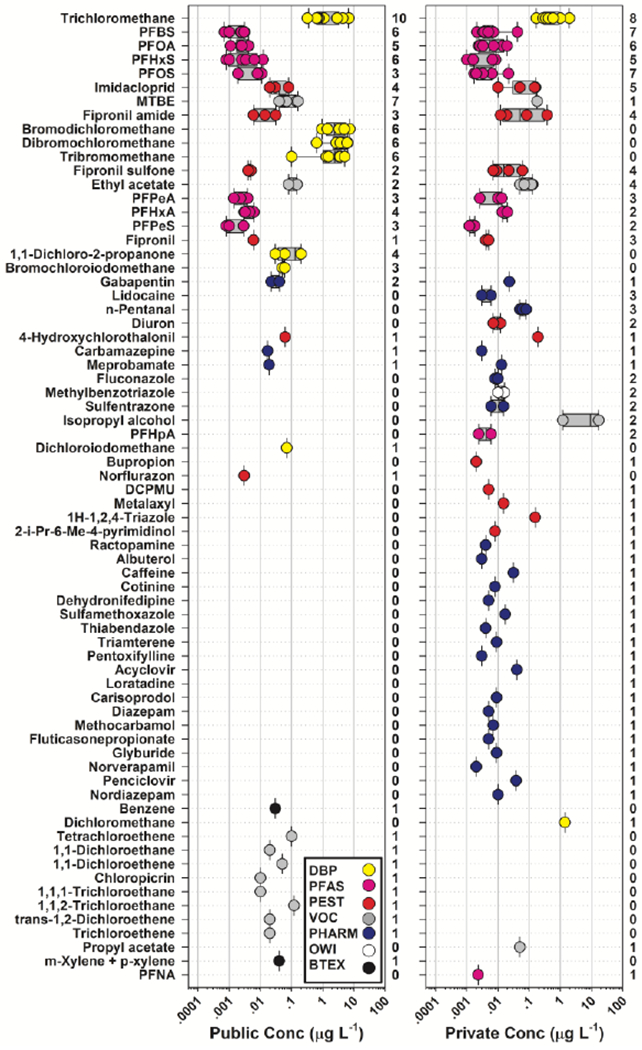
Detected concentrations (circles, μg L−1) and number of sites (right axes) for 70 organic analytes (left axis, in order of decreasing total detections) detected in samples of treated public-supply (left plot, 10 total samples) and untreated private-supply (right plot, 10 total samples) home tapwater collected during 2018 at Cape Cod, Massachusetts. Circles (●) are data for individual samples. Boxes, centerlines, and whiskers indicate interquartile range, median, and 5th and 95th percentiles, respectively.
Figure 3.
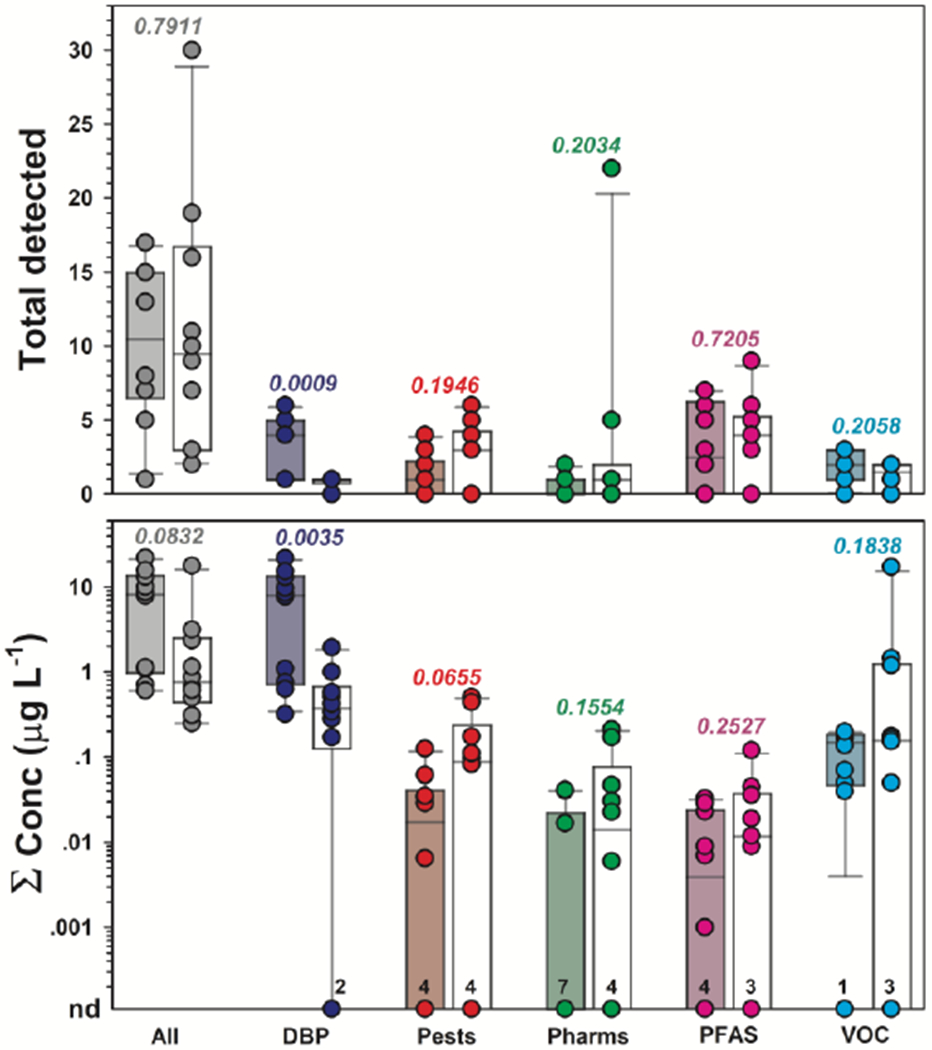
Cumulative numbers (top) and concentrations (bottom) of all organic analytes (All) and disinfection byproduct (DBP), pesticide (Pests), pharmaceutical (Pharms), per/poly-fluorinated alkyl substances (PFAS), and volatile organic chemical (VOC) group analytes detected in samples of treated public-supply (shaded boxplots) and untreated private-supply (white boxplots) home tapwater collected during 2018 at Cape Cod, Massachusetts. Circles (●) are data for individual samples. Boxes, centerlines, and whiskers indicate interquartile range, median, and 5th and 95th percentiles, respectively. Numbers above the x-axis indicate number of samples in which respective analytes were not detected (nd). Numbers above each boxplot pair indicate the permuted probability that the centroids and dispersions are the same (PERMANOVA; 9999 permutations).
Several differences in individual and aggregate concentrations of detected constituents also were observed between private- and public-supply TW samples in this study. Although, no differences in the centroids and dispersions of specific conductance (p = 0.5553) or cumulative concentrations of major ions (p = 0.4705) were observed between private- and public-supply TW samples (Table S3), the pH was substantially lower (p = 0.0001; medians 5.7 and 6.7, respectively; Figure 2) and cumulative concentrations of trace metals were substantially higher (p = 0.0008; medians 1145 μg L−1 and 299 μg L−1, respectively) in private-supply TW samples than in public supply (Figure 2). The latter observations support Hypothesis II, because pH and several metals are monitored in public supply under the SDWA and treated prior to distribution. Concentrations of Cu and Pb were higher (p = 0.0023 and p = 0.0211, respectively) in private-supply TW than in public supply. Elevated Cu and Pb concentrations in some private-supply samples generally corresponded to lower TW pH. Low pH is associated with increased corrosion (U.S. Environmental Protection Agency 2020h) and with mobilization of Cu, Pb, and other metals in aquifers, distribution systems, and premise plumbing (Boulay and Edwards 2001; Edwards and Triantafyllidou 2007; Makris et al. 2014).
Figure 2.
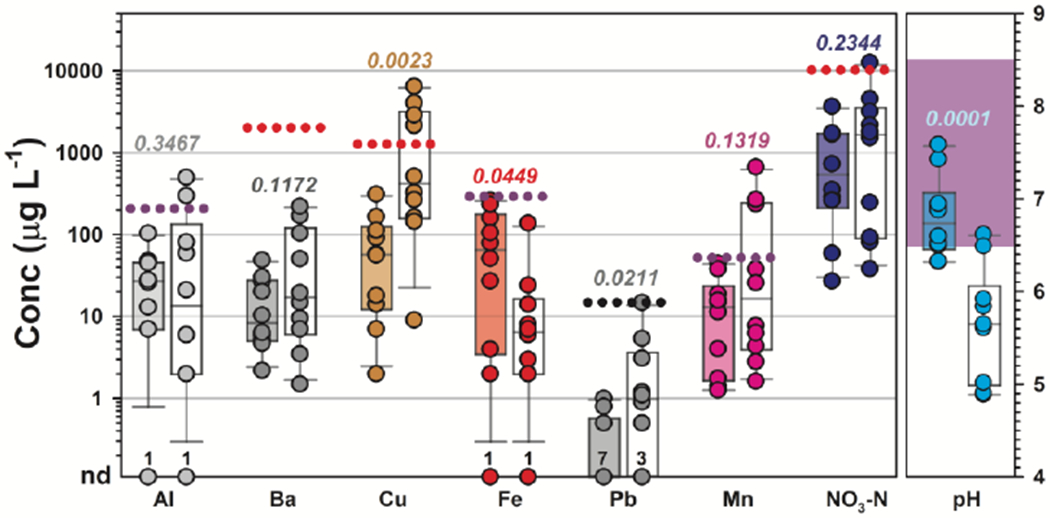
Concentrations (left, μg L−1) of aluminum (Al), barium (Ba), copper (Cu), iron (Fe), lead (Pb), manganese (Mn), and nitrate nitrogen (NO3-N) and pH (right) in samples of treated public-supply (shaded boxplots) and untreated private-supply (white boxplots) home tapwater collected during 2018 at Cape Cod, Massachusetts. Circles (●) are data for individual samples. Boxes, centerlines, and whiskers indicate interquartile range, median, and 5th and 95th percentiles, respectively. For each element (left), dotted lines indicate health-based National Primary Drinking Water Regulation Maximum Contaminant Level (MCL: Ba, Cu, NO3-N) and non-health-based National Primary Drinking Water Regulation Technology Treatment Action Level (Pb) or National Secondary Drinking Water Regulation standards (Al, Fe) for public supply. For pH (right), shaded area indicates National Secondary Drinking Water Regulation range for public supply. The MCL Goal (MCLG) for Pb is zero. Numbers above the x-axis indicate number of samples in which respective analytes were not detected (nd). Numbers above each boxplot pair indicate the permuted probability that the centroids and dispersions are the same (PERMANOVA; 9999 permutations).
No difference in the centroids and dispersions of cumulative organic detections (p = 0.7910) was apparent between TW groups (Figure 3), but the median cumulative concentration of detected organics was an order-of-magnitude higher in public-supply TW samples than in private-supply TW (medians: 8.27 μg L−1 and 0.77 μg L−1, respectively), albeit with substantial overlap in the respective data distributions (p = 0.0832). Consistent with previous TW findings (Bradley et al. 2018; Bradley et al. 2020), with chlorine-based disinfection of TW for 6 of the 10 Cape Cod public-supply locations (Cape Cod Groundwater Guardians 2020), and with Hypothesis III, the elevated cumulative concentration of organics in public supply was attributable to order-of-magnitude higher (medians: 8.10 μg L−1 and 0.47 μg L−1, respectively; p = 0.0035) concentrations of chlorine-disinfection DBP, which comprised 98% of the mass concentration of organics detected in public-supply samples on average (Table S4). Of note, while trichloromethane (chloroform) is a ubiquitous byproduct of chlorine-based drinking-water disinfection and was substantially higher in chlorine-disinfected TW (p = 0.0006), it also has natural sources (Laturnus et al. 2002; McCulloch 2003) and is reported in groundwater (e.g., up to >4.1 μg L−1 Hunkeler et al. 2012), including in untreated drinking water at Cape Cod (Centerville-Osterville-Marstons Mills Water Department 2019; Mashpee Water District 2020; Yarmouth Water 2019). Common occurrences of trichloromethane in Cape Cod TW samples from private supply and from public supplies that were not treated with chlorine-based disinfection (14 samples; median: 0.47 μg L−1; range: not detected (nd) up to 1.95 μg L−1) are consistent with a naturally occurring background (Hunkeler et al. 2012; Laturnus et al. 2002; McCulloch 2003). For microbial indicators, only heterotrophic bacteria were consistently detected; heterotrophic plate counts (HPC per 100 mL) did not differ significantly between private- and public-supply samples (p = 0.8544) but were substantially (p = 0.0763) lower in chlorine-disinfected (median: 41; range: 2-127) than in undisinfected (median: 155; range: 1-959) TW (Figure 5).
Figure 5.
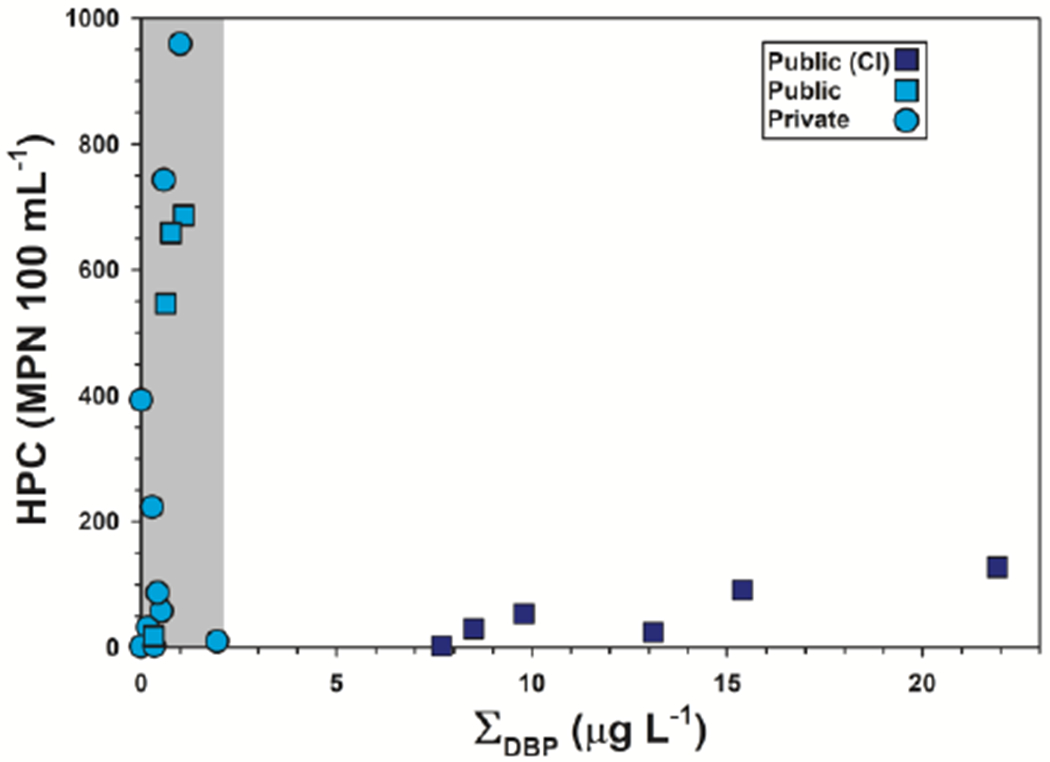
Plot of Heterotrophic Plate Count (HPC; most probable number per 100 mL) vs cumulative concentrations of DBP (μg L−1) detected in TW samples from treated public-supply (chlorine-based disinfection, blue squares, ■; no disinfection, cyan squares, ■) and untreated private-supply (cyan circles, ●) home tapwater collected during 2018 at Cape Cod, Massachusetts. Samples without chlorine-based disinfection (cyan symbols, gray shaded area) only contained trichloromethane (chloroform), which is naturally-occurring in Cape Cod groundwater.
3.2. TW In Vitro ER, AR, and GR Bioactivity
Biological activities, indicative of androgenic or glucocorticoid-like compounds, were not detected in any TW samples. However, estrogenic activity was detected above the MDL and elevated compared to field blanks in three private-supply samples (Figure S2, Table S8), ranging 0.047-2.97 ng estradiol equivalents (E2Eq) L−1 (median: 0.078 ng E2Eq L−1). All three TW samples were below a human-health Effects-Based Trigger (EBT) value of 3.8 ng E2Eq L−1 for drinking water reported previously for the ER CALUX assay (Brand et al. 2013).
3.3. TW Exposures and Health-Based Benchmarks
EPA sets non-enforceable, margin-of-safety MCLG for public-supply drinking water, which are based solely on public health and in consideration of sensitive sub-populations, including infants, children, the elderly, and those with compromised immune systems and chronic diseases (U.S. Environmental Protection Agency 2020j, k). Further, EPA promulgates non-enforceable NSDWR guidelines for contaminants that may cause cosmetic or aesthetic effects (U.S. Environmental Protection Agency 2017, 2020h). Consistent with Hypothesis II and with the lack of SDWA regulatory authority over private supplies, concentrations above health-based benchmarks for inorganics were more common in private-supply samples (Figure 2, Tables S3). MCLG are established for 7 of the detected inorganics in this study and 3 were exceeded at least once. The MCLG for nitrate-nitrogen (NO3-N; 10 mg L−1) and Cu (1300 μg L−1) were exceeded in 1 and 4 TW samples, respectively, from 4 (40%) private-supply homes. In contrast, the Pb MCLG is zero and was exceeded (i.e., detected; MDL: 0.02 μg L−1) in half (3 public, 7 private) of the samples in this study, with concentrations up to 14.7 μg L−1 (median: 0.3 μg L−1; IQR: nd-1.1 μg L−1). Pb also exceeded the 10 μg L−1 WHO drinking-water guideline (World Health Organization (WHO) 2011) in a private-supply sample that also exceeded the Cu MCLG.
MCLG are established for 13 of the 70 organics detected in TW samples and 6, including 2 DBP and 4 volatile organic compounds (VOC), were exceeded at least once (Table S4). The 4 VOC have well-established carcinogenic concerns and concomitant MCLG of zero (U.S. Environmental Protection Agency 2018, 2020k). Each was detected once in TW samples from 4 different homes (public supply: benzene, tetrachloroethene, trichloroethene; private supply: dichloromethane). The remaining, bromodichloromethane and tribromomethane, exceeded their MCLG of zero (U.S. Environmental Protection Agency 2017, 2020k) in all of the samples from chlorine-disinfected public-supply sources, but not in any sample from private supply or from public supplies without chlorine-based disinfection. PFAS were detected in 65% (13/20) of TW samples in this study, with 9 detected at least once at individual concentrations up to 0.041 μg L−1 for perfluorobutanesulfonate (PFBS). Although no samples exceeded the EPA advisory of 0.070 μg L−1 for PFOS/PFOA combined (U.S. Environmental Protection Agency 2016a), one sample exceeded the draft Agency for Toxic Substances and Disease Registry (2018) minimum risk level (MRL) of 0.014 μg L−1 for perfluorooctanesulfonate (PFOS). In October 2020, the Massachusetts Department of Environmental Protection (MassDEP) set a revised state drinking-water MCL of 0.020 μg L−1 for the sum of PFOS, PFOA, perflurodecanoic acid (PFDA), perflouroheptanoic acid (PFHpA), perfluorohexanesulfonate (PFHxS), and perfluorononanoic acid (PFNA) (Massachusetts Department of Environmental Protection 2019, 2020); 3 private-supply and a single public-supply TW sample exceeded the benchmark. No exceedances of WHO drinking-water guidance values (World Health Organization (WHO) 2011) were observed for any organic analytes in this study.
Non-enforceable, non-health-based, public-supply NSDWR also are promulgated (U.S. Environmental Protection Agency 2017, 2020h) for 9 of the inorganic analytes and parameters detected in this study; 4 were not met in at least one residential TW sample (Figure 2; Table S3). Notably, the NSDWR for pH is 6.5-8.5, due in part to metallic taste and corrosion concerns at lower pH values. Eight (80%) of the private-supply TW samples in this study fell below this standard, with the remainder at the NSDWR lower limit; only 2 public-supply TW samples (pH = 6.3) fell below the NSDWR range. NSDWR for aluminum (Al), Manganese (Mn), and Cu, established primarily for color, staining, and metallic taste, were also exceeded, but only in private-supply TW samples from 2, 3, and 4 homes, respectively.
3.4. TW Aggregated Screening Assessment: ∑EAR and ∑TQ
Of the 70 compounds detected in residential TW in this study, 43 (61%) had exact Chemical Abstract Services (CAS) number matches in the ToxCast™ invitroDBv3.0 database. Site-specific ∑EAR were substantially higher (p = 0.0304) in public-supply TW than in private supply, ranging 0.0004-1.0762 (median: 0.2929; IQR: 0.0273-0.6919) in public-supply TW and 0.0016-0.3955 (median: 0.0466; interquartile range (IQR): 0.0040-0.1133) in private-supply TW (Figure 6; Table S10). Individual chemical EAR or sample-specific ∑EAR in excess of 1 (solid red line, Figure 6) indicate exposure at concentrations shown to modulate molecular targets in vitro. Two public-supply TW samples had ∑EAR = 1 indicating high probability of molecular effects, which were attributable to chlorodibromomethane. The remaining 4 chlorine-disinfected public-supply samples exhibited elevated probabilities of effects (∑EAR ≥ 0.1), likewise due to chlorodibromomethane. Similarly, ∑EAR ≥ 0.1 in 3 private-supply samples indicated elevated probability of effects driven primarily by exposures to perfluorohexanoic acid (PFHxA) in two samples and to the corticosteroid pharmaceutical, fluticasone propionate, in the third. Exceedance of ∑EAR = 0.001 (precautionary screening-level threshold of concern) in all but 2 samples indicated that further investigation of the cumulative biological activity from private- and public-supply Cape Cod TW exposures is warranted, even when considering only detected organic contaminant mixtures.
Figure 6.
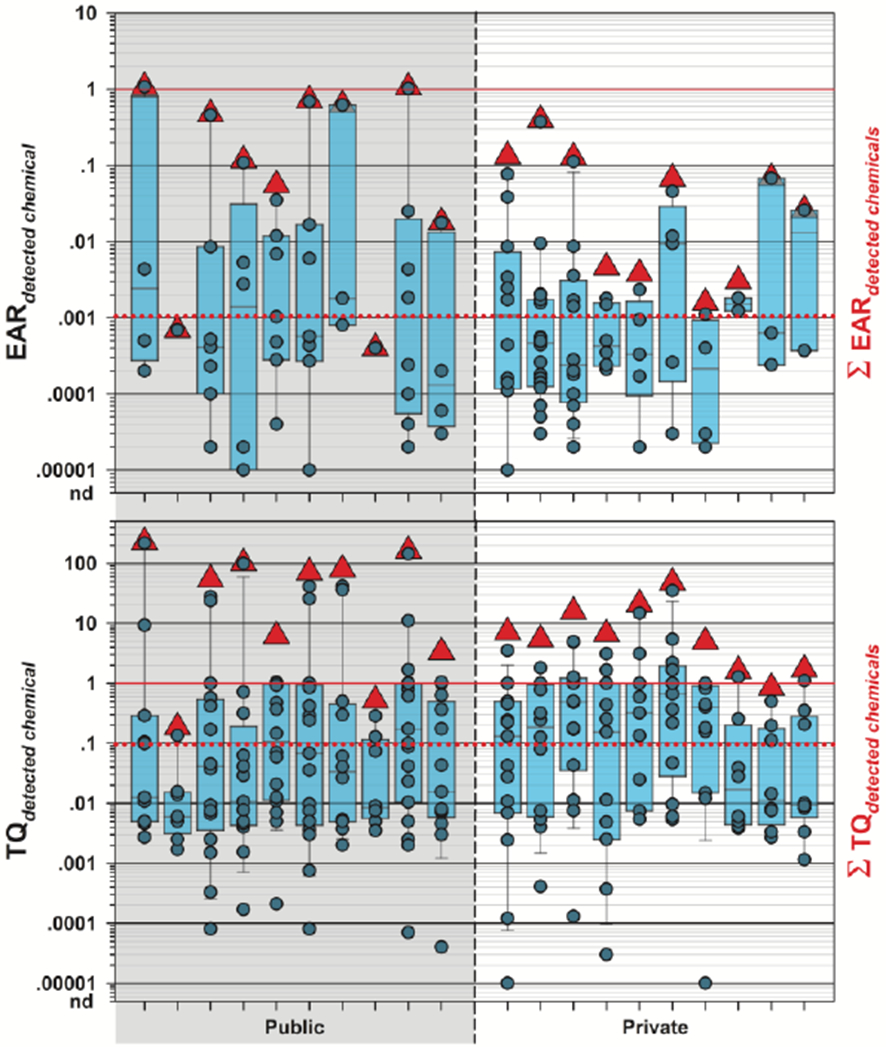
Top. Individual EAR values (circles) and cumulative EAR (ΣEAR, sum of all detected; red triangles, ▲) across all assays for 43 organic analytes listed in ToxCast and detected in samples of treated public-supply (left shaded, 10 total samples) and untreated private-supply (right unshaded, 10 total samples) home tapwater collected during 2018 at Cape Cod, Massachusetts. Solid and dashed red lines indicate concentrations shown to modulate effects in vitro and effects-screening-level thresholds (EAR = 0.001), respectively. Bottom. Human health benchmark-based individual TQ values (circles) and cumulative TQ (ΣTQ, sum of all detected; red triangles, ▲) for inorganic and organic analytes listed in Table S11 and detected in samples of treated public-supply (left shaded) and untreated private-supply (right unshaded) home tapwater. Solid and dashed red lines indicate benchmark equivalent concentrations and effects-screening-level threshold of concern (TQ = 0.1), respectively. Boxes, centerlines, and whiskers indicate interquartile range, median, and 5th and 95th percentiles, respectively, for both plots.
Benchmark-based ∑TQ HI were employed to estimate the cumulative risks from simultaneous exposures to mixtures of organic and inorganic contaminants. Individual benchmark comparisons suggested HI were dominated by public-supply DBP and private-supply exceedances for SDWA-regulated metals. A precautionary approach comprising the lowest available benchmark for each detected chemical, typically a state or federal human-health drinking-water advisory, was employed (Table S12). For chemicals with MCLG of zero, the benchmark value was set equal to the analytical detection limit. These ∑TQ results indicate comparable and high probabilities of aggregated risks in Cape Cod private- and public-supply TW samples when considering exposures to both organic and inorganic chemicals (Figure 6; Table S13). Every sample exceeded the ∑TQ = 0.1 screening threshold of concern and all but 3 samples (1 private, 2 public) exceeded ∑TQ = 1.
4. Discussion
In the extant Anthropocene (Crutzen and Stoermer 2000; Crutzen 2006; Steffen et al. 2011), humans are primary drivers of the Earth systems environmental contaminants subsystem (Steffen et al. 2015) and of human exposures. Water is a biological necessity and, consequently, an intrinsic route of human exposure to environmental chemical/biological hazards (human-health risk vector). Thus, drinking water is a critical nexus of anthropogenic-contaminant cause and human effects and a high leverage point for individual and community-level engagement in risk mitigation at various points along the anthropogenic contaminant cause-effect continuum, including chemical design, production, use, and disposal; source-water protection; drinking-water supply selection; and community and point-of-use treatment. Because perceptions of relative safety and acceptable risk (risk tolerance) vary widely individually and across communities and cultures (Hrudey et al. 2006; Hrudey 2009), directly comparable contaminant exposure and human-health risk assessment datasets, which realistically reflect extant environmental contaminant complexity (Bradley et al. 2017; Glassmeyer et al. 2017; Moschet et al. 2014) and encompass available drinking-water supplies (e.g., public, private, bottled/packaged) and multiple assessment endpoints (e.g., pre-distribution, tap), are required to encourage public engagement and inform decision-making by regulatory agencies, water suppliers, and, critically, end-users. The current Cape Cod study and others across the US (Bradley et al. 2018; Bradley et al. 2020) provide a standardized, broad characterization of inorganics and organics in water supplies to help address data gaps on mixed contaminant exposures in US residential TW and to inform public-health research assessments of the importance of drinking-water contaminant exposures as potential drivers of human-health outcomes.
Detections of regulated and unregulated chemical (inorganic and organic) analytes, which are not systematically monitored together at the drinking-water point of consumption, were common in both private- and public-supply TW samples at Cape Cod (Tables S3–S4; Figures 1–4). This is consistent with the fact that most of the analytes in this study are organic contaminants not currently regulated under the SDWA and, thus, not public-supply treatment targets (Hypothesis I), and with the lack of SDWA regulation and monitoring of private supplies (Hypothesis II). Frequent detections of co-occurring contaminants, which can originate in (e.g., Cu, Pb) or are known to undergo significant changes within (e.g., DBP) public-supply distribution systems and premise plumbing, further emphasize the importance of at-the-tap monitoring of wide-ranging contaminant indicators that more realistically reflect the documented complexity of source-water contaminant profiles (e.g., Bradley et al. 2017; Moschet et al. 2014). Broad agreement between the number of detections in private- and public-supply TW is consistent with well-documented contaminant concerns in the Cape Cod sole-source drinking-water aquifer (Cape Cod Commission 2020a, b) and emphasizes the mutual challenge that anthropogenic contaminants pose to unregulated and regulated TW supplies.
Differences between Cape Cod private- and public-supply TW exposures also were observed and attributable to respective differences in drinking water regulation and compliance monitoring under the SDWA. Consistent with previous TW studies, DBP dominated public-supply concentrations due to chlorine-based disinfection of 6 of 10 Cape Cod public-supply TW samples. The disease prevention benefits of chemical disinfection prior to distribution and of residual disinfectant at the tap are well documented (Reynolds et al. 2008; Richardson et al. 2007; Schoenen 2002) and the low number of microorganisms observed in chlorine-treated Cape Cod public-supply TW samples in this study are consistent with protective disinfection (Figure 5, Table S7). Water-quality outliers, including substantially lower pH, elevated concentrations of inorganics that are regulated in public supply, and the order-of-magnitude higher detections in one sample of pharmaceuticals most readily reconciled with upgradient septic systems (Schaider et al. 2016; Swartz et al. 2006), illustrated the high variability and elevated risk of unrecognized exposures inherent in unmonitored private-supply TW. The results align with the findings of previous TW exposure studies by this group and others in the US (Bradley et al. 2018; Bradley et al. 2020; Evans et al. 2019; Stoiber et al. 2019) and elsewhere (de Jesus Gaffney et al. 2015; Gonzalez et al. 2013; Leusch et al. 2018; Stalter et al. 2020; Tröger et al. 2018). Because the analytes detected in this study included chemicals with documented hazard concerns, the potential human-health risks of these TW exposures were assessed individually and cumulatively (National Research Council 1983).
Concentrations above human-health benchmarks for several inorganic and organic TW constituents (e.g., Pb, Cu, NO3-N, PFAS, DBP) in private- and public-supply samples illustrate the shared TW quality concerns associated with unconfined source waters in populated areas and emphasize the importance of balanced private-/public-supply contaminant exposure information to support drinking-water supply decisions and inform point-of-use treatment selection. TW Pb is a well-established public health concern (Lanphear et al. 2016; Pfadenhauer et al. 2016; Renner 2010; Rosen et al. 2017; Triantafyllidou and Edwards 2012), primarily associated with neurocognitive impairment in infants and children (Lanphear et al. 2016; Levallois et al. 2018) and has no known safe level of exposure (i.e., MCLG of zero). Importantly, the Cape Cod Pb results cannot be interpreted as indicative of worst-case TW Pb exposures, because TW flushing can effectively reduce the concentration of plumbing-derived TW contaminants like Pb (Triantafyllidou and Edwards 2012; U.S. Environmental Protection Agency 2016b) and same-day prior use was typical in this study. The MCLG for NO3-N in public-supply drinking water (10 mg L−1) and the effectively equivalent WHO guideline value (11 mg L−1) were established to protect against bottle-fed infant (< 6 months) methemoglobinemia (U.S. Environmental Protection Agency 2020k). Recent reviews report evidence of epidemiological relationships between other adverse health outcomes (e.g., cancers (Jones et al. 2016; Jones et al. 2017; Quist et al. 2018), thyroid disease (Aschebrook-Kilfoy et al. 2012), neural tube defects (Brender et al. 2013)) and ingestion of drinking-water NO3 at levels below those associated with methemoglobinemia (Ward et al. 2005; Ward et al. 2018). The MCLG and MCL for Cu in public-supply (1.3 mg L−1), which is below the WHO guideline value (2.0 mg L−1), was established to protect against gastrointestinal distress from short-term exposure and liver and kidney damage from long-term exposure (Georgopoulos et al. 2001; Stern et al. 2007; Stern 2010; U.S. Environmental Protection Agency 2020k).
PFAS are targets of an increasing number of US public-health advisories and regulations at the state and federal level (see updating list at Interstate Technology Regulatory Council 2020), due to global occurrence and persistence in the environment, biota, and humans (Post et al. 2012; Suja et al. 2009), human-health concerns (Sunderland et al. 2019), and widespread drinking-water exposures (Kaboré et al. 2018). State and federal health-based benchmark concentrations have consistently decreased as exposure and toxicity data for more PFAS compounds become available (Post 2020). Importantly, the updated MassDEP MCL (Massachusetts Department of Environmental Protection 2019, 2020) for combined exposure to 6 long-chain PFAS (20 ng L−1 combined PFOA, PFOS, PFHxS, PFDA, PFNA, PFHpA) was exceeded in 3 of 7 private-supply and 1 of 6 public-supply homes where PFAS were detected. None of the homes with PFAS detection exceeded the EPA advisory level of 70 ng L−1 for combined exposure to PFOS and PFOA. For perspective, the 45 target PFAS assessed in this study, of which 9 were detected, are only a fraction of the hundreds of PFAS in direct production and thousands of PFAS precursors and byproducts that have been detected in environmental matrices (Wang et al. 2017). These compounds share similar structures, environmental persistence, and poorly understood health consequences (Conley et al. 2019; Gomis et al. 2015; Gomis et al. 2018; Strynar et al. 2015; Wang et al. 2017). Similarly, while the disease prevention benefits of chemical disinfection prior to distribution and of residual disinfectant at the tap are well documented (Reynolds et al. 2008; Richardson et al. 2007; Schoenen 2002), the carcinogenic/genotoxic potentials of currently regulated DBP (U.S. Environmental Protection Agency 2017, 2020k), the linkages between TW DBP exposure and DBP in blood (Rivera-Núñez et al. 2012), the reported in vitro cyto/genotoxicity of 103 unregulated DBP (Jeong et al. 2015; Krasner et al. 2016; Muellner et al. 2007; Pressman et al. 2010; Richardson et al. 2007; Villanueva et al. 2018; Wang et al. 2015), the association between drinking-water DBP and bladder cancer (Hrudey et al. 2015; Hrudey and Fawell 2015), and the remaining majority of largely unidentified DBP in drinking water (Krasner et al. 2006; Weinberg et al. 2002) are public-health concerns and a clear public-health tradeoff (Hrudey 2009; Richardson and Plewa 2020). Thus, the frequent detections of DBP in chlorine-disinfected public-supply TW and PFAS in private- and public-supply TW, some of which were greater than EPA MCLG or MassDEP MCL levels, are both consistent with previous TW findings (Bradley et al. 2018; Bradley et al. 2020) and emphasize the need for improved understanding of cumulative adverse health risks of DBP and PFAS exposures in TW.
The estrogenic activity detected in three private-supply samples fell below an EBT derived previously for the ER CALUX assay (Brand et al. 2013). To our knowledge, however, no EBT currently exists for the T47D-KBluc method and relative potency factors affecting measured cumulative biological activity can vary between different platforms with the same nominal endpoint (Brand et al. 2013; Conley et al. 2016; Houtman et al. 2009; Sonneveld et al. 2005). Notably, the 2.97 ng E2Eq L−1 activity in one private-supply TW sample is consistent with that reported in untreated surface-water sources (Conley et al. 2017a; Medlock Kakaley et al. 2020), suggesting that follow-up, precautionary monitoring for recycled water (e.g., California State Water Resources Control Board 2018) or prophylactic use of suitable point-of-use treatment (Brown et al. 2017) is warranted.
Detections of multiple unregulated organics as well as inorganics combined with exceedances of multiple health-based benchmarks in individual samples also demonstrate the need for aggregated risk assessment of simultaneous exposures to organic and to organic/inorganic TW mixtures at Cape Cod. The results of the precautionary ∑EAR and ∑TQ assessments for Cape Cod and across all studies by this group (Bradley et al. 2018; Bradley et al. 2020) indicate that biological activity and human-health risks from drinking-water contaminant exposures are common to and comparable in private- and public-supply TW. Common DBP-driven exceedance of HI screening-levels of concern, primarily based on MCLG, in public-supply TW reemphasize the public-health tradeoff of chlorine disinfection (Cortés and Marcos 2018; Hrudey 2009; Hrudey et al. 2015; Hrudey and Fawell 2015; Nieuwenhuijsen et al. 2009) and the need for better understanding of the exposure-effects relations and cumulative adverse health risks of DBP, including unregulated DBP and unidentified compounds (Figure 5; Cortés and Marcos 2018; Hrudey and Fawell 2015; Krasner et al. 2006; Nieuwenhuijsen et al. 2009; Richardson and Plewa 2020; Weinberg et al. 2002) and for improved pre-disinfection treatment to remove DBP precursors like natural organic matter (Bond et al. 2011; Bond et al. 2012; Bond et al. 2014; Chu et al. 2011). Common HI exceedances of screening-levels of concern in unregulated and generally unmonitored private-supply TW reiterate the inherent human-health risks of unmonitored TW (DeSimone et al. 2015; Focazio et al. 2006; MacDonald Gibson and Pieper 2017; Rogan and Brady 2009; Zheng and Flanagan 2017) and argue for systematic monitoring of private-supply TW (Zheng and Flanagan 2017) with an analytical scope that realistically reflects the documented complexity of environmental organic contamination (Bradley et al. 2017; Glassmeyer et al. 2017; Moschet et al. 2014; Schaider et al. 2014; Schaider et al. 2016).
5. Conclusions
The results emphasize the importance of directly comparable private-/public-supply contaminant exposure information to support consumer decisions, such as the choice of private- versus public-supply source and selection of point-of-use treatment options. Importantly, the results indicate that incorporation of well-maintained, point-of-use treatment as an integral additional line of consumer protection for public-supply TW during normal operation and outbreak conditions and as a prudent proactive treatment in private-supply homes merits consideration (Brown et al. 2017; Verhougstraete et al. 2019). Improved characterization of TW contaminant profiles at the point-of-exposure (tap) and increased availability of monitoring data including results below current enforceable standards are essential to support point-of-use treatment decisions by the consumer.
Analytically extensive datasets to inform scientific and public health understanding of the role of drinking water as a vector for human contaminant exposures and associated human-health outcomes remain limited because the TW point-of-exposure is not commonly monitored or tested in the US and worldwide. This study addresses a critical gap in the comprehension of TW exposures nationwide by informing TW contaminant mixtures in a source-water vulnerability archetype wherein the sole source of public- and private-supply drinking water to a densely populated area is a shallow unconfined permeable aquifer, a setting with numerous hydrologic and drinking-water source analogues along the extensive, densely populated US east coast (DeSimone et al. 2015). As noted previously (Bradley et al. 2018; Bradley et al. 2020), these results highlight the potential cumulative risk of low-level, mixed inorganic/organic TW exposures. These results argue for continued systematic, quantitatively robust TW exposure assessments and a range of methods, including whole-water high throughput bioassay platforms with broad molecular-endpoint coverage (Baken et al. 2018; Braun and Gray 2017; Gross and Birnbaum 2017; Lanphear 2017; Schriks et al. 2010), to support models of contaminant exposures and related risks at the drinking-water point of consumption.
Supplementary Material
Acknowledgements
We thank Alana B. Spaetzel, Riley L. Blais, and Shannon M. Meppelink, of the U.S. Geological Survey for logistical and field support and the Cape Cod homeowner study participants. This research was conducted and funded by the USGS Environmental Health Mission Area. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. The findings and conclusions in this article do not necessarily represent the views or policies of the US Environmental Protection Agency or the NIH/National Institute of Environmental Health Sciences. This report contains CAS Registry Numbers®, which is a registered trademark of the American Chemical Society. CAS recommends the verification of the CASRNs through CAS Client ServicesSM.
Footnotes
The authors declare they have no actual or potential competing financial interests.
Appendix A. Supplementary data
Data discussed in this paper are summarized in Supporting Information (Figures S1–S3, Tables S1–S14) and in the USGS data release (Romanok et al. 2020). Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.envint.2021.106487. NOTE TO REVIEWERS: LINKS NOT LIVE; SEE SUPPLEMENTAL DATA FILE FOR ALL DATA.
References
- Agency for Toxic Substances and Disease Registry. 2018. Toxicological profile for Perfluoroalkyls. (Draft for Public Comment).US Department of Health and Human Services, Public Health Service; Atlanta, GA, 10.15620/cdc:59198. [DOI] [Google Scholar]
- Altenburger R, Ait-Aissa S, Antczak P, Backhaus T, Barceló D, Seiler T-B, et al. 2015. Future water quality monitoring—adapting tools to deal with mixtures of pollutants in water resource management. Sci Total Environ 512-513:540–551, 10.1016/j.scitotenv.2014.12.057. [DOI] [PubMed] [Google Scholar]
- Altenburger R, Scholze M, Busch W, Escher BI, Jakobs G, Krauss M, et al. 2018. Mixture effects in samples of multiple contaminants—An inter-laboratory study with manifold bioassays. Environ Int 114:95–106, 10.1016/j.envint.2018.02.013. [DOI] [PubMed] [Google Scholar]
- American Public Health Association et al. 2017. Standard methods for the examination of water and wastewater: American Public Health Association. [Google Scholar]
- Aschebrook-Kilfoy B, Heltshe SL, Nuckols JR, Sabra MM, Shuldiner AR, Mitchell BD, et al. 2012. Modeled nitrate levels in well water supplies and prevalence of abnormal thyroid conditions among the Old Order Amish in Pennsylvania. Environmental Health 11:6, 10.1186/1476-069X-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach S, Filer D, Reif D, Walker V, Holloway AC, Schlezinger J, et al. 2016. Prioritizing Environmental Chemicals for Obesity and Diabetes Outcomes Research: A Screening Approach Using ToxCast High Throughput Data. Environ Health Perspect 124:1141–1154, 10.1289/ehp.1510456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baken KA, Sjerps RMA, Schriks M, van Wezel AP. 2018. Toxicological risk assessment and prioritization of drinking water relevant contaminants of emerging concern. Environ Int 118:293–303, 10.1016/j.envint.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Ball JW, McCleskey RB. 2003. A new cation-exchange method for accurate field speciation of hexavalent chromium. Talanta 61:305–313, 10.1016/S0039-9140(03)00282-0. [DOI] [PubMed] [Google Scholar]
- Barbaro JR, Masterson J, LeBlanc DR. 2014. Science for the stewardship of the groundwater resources of Cape Cod, Massachusetts. U.S. Geological Survey Fact Sheet; 2014–3067. 10.3133/fs20143067. [DOI] [Google Scholar]
- Blackwell BR, Ankley GT, Corsi SR, De Cicco LA, Houck KA, Judson RS, et al. 2017. An″ EAR″ on environmental surveillance and monitoring: A case study on the use of exposure-activity ratios (EARs) to prioritize sites, chemicals, and bioactivities of concern in Great Lakes waters. Environ Sci Technol 51:8713–8724, 10.1021/acs.est.7b01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond T, Huang J, Templeton MR, Graham N. 2011. Occurrence and control of nitrogenous disinfection by-products in drinking water — A review. Water Res 45:4341–4354, 10.1016/j.watres.2011.05.034. [DOI] [PubMed] [Google Scholar]
- Bond T, Templeton MR, Graham N. 2012. Precursors of nitrogenous disinfection by-products in drinking water—A critical review and analysis. J Hazard Mater 235-236:1–16, 10.1016/j.jhazmat.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Bond T, Mokhtar Kamal NH, Bonnisseau T, Templeton MR. 2014. Disinfection by-product formation from the chlorination and chloramination of amines. J Hazard Mater 278:288–296, 10.1016/j.jhazmat.2014.05.100. [DOI] [PubMed] [Google Scholar]
- Bondy SC, Campbell A. 2018. Water quality and brain function. International Journal of Environmental Research and Public Health 15, 10.3390/ijerph15010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay N, Edwards M. 2001. Role of temperature, chlorine, and organic matter in copper corrosion by-product release in soft water. Water Res 35:683–690, 10.1016/S0043-1354(00)00320-1. [DOI] [PubMed] [Google Scholar]
- Bradley P, Journey C, Romanok K, Barber L, Buxton HT, Foreman WT, et al. 2017. Expanded Target-Chemical Analysis Reveals Extensive Mixed-Organic-Contaminant Exposure in USA Streams. Environ Sci Technol 51:4792–4802, 10.1021/acs.est.7b00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PM, Kolpin DW, Romanok KM, Smalling KL, Focazio MJ, Brown JB, et al. 2018. Reconnaissance of Mixed Organic and Inorganic Chemicals in Private and Public Supply Tapwaters at Selected Residential and Workplace Sites in the United States. Environ Sci Technol, 10.1021/acs.est.8b04622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PM, Journey CA, Berninger JP, Button DT, Clark JM, Corsi SR, et al. 2019. Mixed-chemical exposure and predicted effects potential in wadeable southeastern USA streams. Sci Total Environ 655:70–83, 10.1016/j.scitotenv.2018.11.186. [DOI] [PubMed] [Google Scholar]
- Bradley PM, Argos M, Kolpin DW, Meppelink SM, Romanok KM, Smalling KL, et al. 2020. Mixed organic and inorganic tapwater exposures and potential effects in greater Chicago area, USA. Sci Total Environ:137236, 10.1016/j.scitotenv.2020.137236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand W, de Jongh CM, van der Linden SC, Mennes W, Puijker LM, van Leeuwen CJ, et al. 2013. Trigger values for investigation of hormonal activity in drinking water and its sources using CALUX bioassays. Environ Int 55:109–118, 10.1016/j.envint.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Braun JM, Gray K. 2017. Challenges to studying the health effects of early life environmental chemical exposures on children’s health. PLOS Biology 15:e2002800, 10.1371/journal.pbio.2002800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brender JD, Weyer PJ, Romitti PA, Mohanty BP, Shinde MU, Vuong AM, et al. 2013. Prenatal Nitrate Intake from Drinking Water and Selected Birth Defects in Offspring of Participants in the National Birth Defects Prevention Study. Environ Health Perspect 121:1083–1089, 10.1289/ehp.1206249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, Gessesse B, Butler LJ, MacIntosh DL. 2017. Potential Effectiveness of Point-of-Use Filtration to Address Risks to Drinking Water in the United States. Environmental health insights 11:1178630217746997–1178630217746997, 10.1177/1178630217746997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- California State Water Resources Control Board. 2018. Water Quality Control Policy for Recycled Water. https://www.waterboards.ca.gov/board_decisions//adopted_orders/resolutions/2018/121118_7_final_amendment_oal.pdf.
- Cape Cod Commission. 2020a. 2018 Regional Policy Plan (RPP) Data Viewer. https://capecodcommission.org/our-work/2018-rpp-data-viewer/ [accessed June 24, 2020].
- Cape Cod Commission. 2020b. Drinking Water and Groundwater. https://capecodcommission.org/our-work/drinkingwater/ [accessed June 24, 2020].
- Cape Cod Groundwater Guardians. 2020. Drinking water on Cape Cod. https://www.capecodgroundwater.org/learn-more/drinking-water-supplies/ [accessed March 14, 2020].
- Cedergreen N, Christensen AM, Kamper A, Kudsk P, Mathiassen SK, Streibig JC, et al. 2008. A review of independent action compared to concentration addition as reference models for mixtures of compounds with different molecular target sites. Environ Toxicol Chem 27:1621–1632, 10.1897/07-474.1. [DOI] [PubMed] [Google Scholar]
- Centerville-Osterville-Marstons Mills Water Department. 2019. Centerville-Osterville-Marstons Mills 2018 Water Quality Report. http://www.commwater.com/wp-content/uploads/2019/01/Final-WQR-2018-1.pdf.
- Childress C, Foreman W, Conner B, Maloney T. 1999. New reporting procedures based on long-term method detection levels and some considerations for interpretations of water-quality data provided by the U.S. Geological Survey National Water Quality Laboratory. U.S. Geological Survey Open-File Report; 99–193. 10.3133/ofr99193. [DOI] [Google Scholar]
- Chu W, Gao N, Deng Y, Templeton MR, Yin D. 2011. Impacts of drinking water pretreatments on the formation of nitrogenous disinfection by-products. Bioresour Technol 102:11161–11166, 10.1016/j.biortech.2011.09.109. [DOI] [PubMed] [Google Scholar]
- Collier DN, Robinson A, Mitra S, Taft N, Raad A, Hudson S, et al. 2019. Tapping Out: Influence of Organoleptic and Perceived Health Risks on Bottled Versus Municipal Tap Water Consumption Among Obese, Low Socioeconomic Status Pediatric Patients. Exposure and Health, 10.1007/s12403-019-00302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley J, Evans N, Cardon M, Rosenblum L, Iwanowicz L, Hartig P, et al. 2017a. Occurrence and in vitro bioactivity of estrogen, androgen, and glucocorticoid compounds in a nationwide screen of United States stream waters. Environ Sci Technol 51:4781–4791, 10.1021/acs.est.6b06515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley J, Evans N, Mash H, Rosenblum L, Schenck K, Glassmeyer S, et al. 2017b. Comparison of in vitro estrogenic activity and estrogen concentrations in source and treated waters from 25 US drinking water treatment plants. Sci Total Environ 579:1610–1617, 10.1016/j.scitotenv.2016.02.093. [DOI] [PubMed] [Google Scholar]
- Conley JM, Hannas BR, Furr JR, Wilson VS, Gray LE Jr. 2016. A Demonstration of the Uncertainty in Predicting the Estrogenic Activity of Individual Chemicals and Mixtures From an In Vitro Estrogen Receptor Transcriptional Activation Assay (T47D-KBluc) to the In Vivo Uterotrophic Assay Using Oral Exposure. Toxicol Sci 153:382–395, 10.1093/toxsci/kfw134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley JM, Lambright CS, Evans N, Strynar MJ, McCord J, McIntyre BS, et al. 2019. Adverse Maternal, Fetal, and Postnatal Effects of Hexafluoropropylene Oxide Dimer Acid (GenX) from Oral Gestational Exposure in Sprague-Dawley Rats. Environ Health Perspect 127:037008, 10.1289/EHP4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi SR, De Cicco LA, Villeneuve DL, Blackwell BR, Fay KA, Ankley GT, et al. 2019. Prioritizing chemicals of ecological concern in Great Lakes tributaries using high-throughput screening data and adverse outcome pathways. Sci Total Environ 686:995–1009, 10.1016/j.scitotenv.2019.05.457. [DOI] [PubMed] [Google Scholar]
- Cortés C, Marcos R. 2018. Genotoxicity of disinfection byproducts and disinfected waters: A review of recent literature. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 831:1–12, 10.1016/j.mrgentox.2018.04.005. [DOI] [PubMed] [Google Scholar]
- Crutzen PJ, Stoermer EF. 2000. The Anthropocene. International Geosphere-Biosphere Programme (IGBP): Global Change Newsletter 41:17–18, http://www.igbp.net/download/18.316f18321323470177580001401/1376383088452/NL41.pdf. [Google Scholar]
- Crutzen PJ. 2006. The “Anthropocene”. In: Earth System Science in the Anthropocene. Berlin, Heidelberg:Springer Berlin Heidelberg, 13–18, 10.1007/3-540-26590-2_3. [DOI] [Google Scholar]
- De Cicco L, Corsi SR, Villeneuve D, Blackwell BR, Ankley GT. 2018. toxEval: Evaluation of measured concentration data using the ToxCast high-throughput screening database or a user-defined set of concentration benchmarks. R package version 1.0.0. https://owi.usgs.gov/R/gran.html [accessed May 1, 2018].
- de Jesus Gaffney V, Almeida CM, Rodrigues A, Ferreira E, Benoliel MJ, Cardoso VV. 2015. Occurrence of pharmaceuticals in a water supply system and related human health risk assessment. Water Res 72:199–208, 10.1016/j.watres.2014.10.027. [DOI] [PubMed] [Google Scholar]
- DeSimone LA, McMahon PB, Rosen MR. 2015. The quality of our Nation’s waters: water quality in Principal Aquifers of the United States, 1991-2010. (Circular). 1360. Reston, VA, https://doi.org/10.3133/cir1360http://pubs.er.usgs.gov/publication/cir1360http://pubs.er.usgs.gov/publication/cir1360 . [Google Scholar]
- Dieter CA, Maupin MA. 2017. Public supply and domestic water use in the United States, 2015. (Open-File Report). 2017–1131. Reston, VA, https://doi.org/10.3133/ofr20171131http://pubs.er.usgs.gov/publication/ofr20171131http://pubs.er.usgs.gov/publication/ofr20171131 . [Google Scholar]
- Dieter CA, Maupin MA, Caldwell RR, Harris MA, Ivahnenko TI, Lovelace JK, et al. 2018. Estimated use of water in the United States in 2015. (Circular). 1441. Reston, VA, 10.3133/cir1441. [DOI] [Google Scholar]
- Doria dFM. 2010. Factors influencing public perception of drinking water quality. Water policy 12:1–19, 10.2166/wp.2009.051. [DOI] [Google Scholar]
- Doria MdF, Pidgeon N, Hunter PR. 2009. Perceptions of drinking water quality and risk and its effect on behaviour: A cross-national study. Sci Total Environ 407:5455–5464, 10.1016/j.scitotenv.2009.06.031. [DOI] [PubMed] [Google Scholar]
- Doria MF. 2006. Bottled water versus tap water: understanding consumers’ preferences. Journal of Water and Health 4:271–276, 10.2166/wh.2006.0023. [DOI] [PubMed] [Google Scholar]
- Edwards M, Triantafyllidou S. 2007. Chloride-to-sulfate mass ratio and lead leaching to water. Journal - AWWA 99:96–109, 10.1002/j.1551-8833.2007.tb07984.x. [DOI] [Google Scholar]
- Escher B, Leusch F. 2011. Bioanalytical tools in water quality assessment:IWA Publishing.
- Evans S, Campbell C, Naidenko OV. 2019. Cumulative risk analysis of carcinogenic contaminants in United States drinking water. Heliyon 5:e02314, 10.1016/j.heliyon.2019.e02314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman MJ, Friedman LC. 1989. Methods for determination of inorganic substances in water and fluvial sediments. U.S. Geological Survey Techniques of Water-Resources Investigations 05-A1. 10.3133/twri05A1. [DOI]
- Flanagan SV, Spayd SE, Procopio NA, Chillrud SN, Braman S, Zheng Y. 2016a. Arsenic in private well water part 1 of 3: Impact of the New Jersey Private Well Testing Act on household testing and mitigation behavior. Sci Total Environ 562:999–1009, 10.1016/j.scitotenv.2016.03.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan SV, Spayd SE, Procopio NA, Marvinney RG, Smith AE, Chillrud SN, et al. 2016b. Arsenic in private well water part 3 of 3: Socioeconomic vulnerability to exposure in Maine and New Jersey. Sci Total Environ 562:1019–1030, 10.1016/j.scitotenv.2016.03.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focazio MJ, Tipton D, Dunkle SS, Geiger LH. 2006. The chemical quality of self-supplied domestic well water in the United States. Ground Water Monit Rem 26:92–104, 10.1111/j.1745-6592.2006.00089.x. [DOI] [Google Scholar]
- Furlong E, Noriega M, Kanagy C, Kanagy L, Coffey L, Burkhardt M. 2014. Methods of the National Water Quality Laboratory. Chapter B10. Determination of human-use pharmaceuticals in filtered water by direct aqueous injection—high-performance liquid chromatography/tandem mass spectrometry. U.S. Geological Survey Techniques and Methods. Book 5. Laboratory Analysis. Chap. B10. 10.3133/tm5B10. [DOI]
- Furlong ET, Batt AL, Glassmeyer ST, Noriega MC, Kolpin DW, Mash H, et al. 2017. Nationwide reconnaissance of contaminants of emerging concern in source and treated drinking waters of the United States: Pharmaceuticals. Sci Total Environ 579:1629–1642, 10.1016/j.scitotenv.2016.03.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher LG, Webster TF, Aschengrau A, Vieira VM. 2010. Using Residential History and Groundwater Modeling to Examine Drinking Water Exposure and Breast Cancer. Environ Health Perspect 118:749–755, 10.1289/ehp.0901547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos PG, Roy A, Yonone-Lioy MJ, Opiekun RE, Lioy PJ. 2001. Environmental copper: its dynamics and human exposure issues. Journal of Toxicology and Environmental Health, Part B 4:341–394, 10.1080/109374001753146207. [DOI] [PubMed] [Google Scholar]
- Glassmeyer ST, Furlong ET, Kolpin DW, Batt AL, Benson R, Boone JS, et al. 2017. Nationwide reconnaissance of contaminants of emerging concern in source and treated drinking waters of the United States. Sci Total Environ 581-582:909–922, 10.1016/j.scitotenv.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis MI, Wang Z, Scheringer M, Cousins IT. 2015. A modeling assessment of the physicochemical properties and environmental fate of emerging and novel per- and polyfluoroalkyl substances. Sci Total Environ 505:981–991, 10.1016/j.scitotenv.2014.10.062. [DOI] [PubMed] [Google Scholar]
- Gomis MI, Vestergren R, Borg D, Cousins IT. 2018. Comparing the toxic potency in vivo of long-chain perfluoroalkyl acids and fluorinated alternatives. Environ Int 113:1–9, 10.1016/j.envint.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Lopez-Roldan R, Cortina J-L. 2013. Presence of metals in drinking water distribution networks due to pipe material leaching: a review. Toxicol Environ Chem 95:870–889, 10.1080/02772248.2013.840372. [DOI] [Google Scholar]
- Goumenou M, Tsatsakis A. 2019. Proposing new approaches for the risk characterisation of single chemicals and chemical mixtures: The source related Hazard Quotient (HQS) and Hazard Index (HIS) and the adversity specific Hazard Index (HIA). Toxicology Reports 6:632–636, 10.1016/j.toxrep.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JL, Loftin KA, Meyer MT, Ziegler AC. 2010. Cyanotoxin mixtures and taste-and-odor compounds in cyanobacterial blooms from the Midwestern United States. Environ Sci Technol 44:7361–7368, 10.1021/es1008938. [DOI] [PubMed] [Google Scholar]
- Gross L, Birnbaum LS. 2017. Regulating toxic chemicals for public and environmental health. PLOS Biology 15:e2004814, 10.1371/journal.pbio.2004814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelfo JL, Adamson DT. 2018. Evaluation of a national data set for insights into sources, composition, and concentrations of per- and polyfluoroalkyl substances (PFASs) in U.S. drinking water. Environ Pollut 236:505–513, 10.1016/j.envpol.2018.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer Ø, Harper DA, Ryan PD. 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontologia electronica 4:9, https://palaeo-electronica.org/2001_1/past/issue1_01.htm. [Google Scholar]
- Hartig PC, Bobseine KL, Britt BH, Cardon MC, Lambright CR, Wilson VS, et al. 2002. Development of Two Androgen Receptor Assays Using Adenoviral Transduction of MMTV-Luc Reporter and/or hAR for Endocrine Screening. Toxicol Sci 66:82–90, 10.1093/toxsci/66.1.82. [DOI] [PubMed] [Google Scholar]
- Hartig PC, Cardon MC, Lambright CR, Bobseine KL, Gray LE, Wilson VS. 2007. Substitution of synthetic chimpanzee androgen receptor for human androgen receptor in competitive binding and transcriptional activation assays for EDC screening. Toxicol Lett 174:89–97, 10.1016/j.toxlet.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Hawkins G 2017. The impacts of bottled water: an analysis of bottled water markets and their interactions with tap water provision. WIREs Water 4:e1203, 10.1002/wat2.1203. [DOI] [Google Scholar]
- Hergenreder RL. 2011. Trace Metals in Waters by GFAAS, in Accordance with U.S. EPA and Health Canada Requirements. Waltham, MA:Perkin Elmer, Inc., https://www.perkinelmer.com/lab-solutions/resources/docs/PinAAcleTraceMetalsinWaterbyGFAAAppNote.pdf. [Google Scholar]
- Hladik ML, Smalling KL, Kuivila KM. 2008. A Multi-residue Method for the Analysis of Pesticides and Pesticide Degradates in Water Using HLB Solid-phase Extraction and Gas Chromatography—Ion Trap Mass Spectrometry. Bull Environ Contam Toxicol 80:139–144, 10.1007/s00128-007-9332-2. [DOI] [PubMed] [Google Scholar]
- Hobson WL, Knochel ML, Byington CL, Young PC, Hoff CJ, Buchi KF. 2007. Bottled, filtered, and tap water use in latino and non-latino children. Archives of Pediatrics & Adolescent Medicine 161:457–461, 10.1001/archpedi.161.5.457. [DOI] [PubMed] [Google Scholar]
- Hoffman GL, Fishman MJ, Garbarino JR. 1996. Methods of Analysis by the US Geological Survey National Water Quality Laboratory: In-bottle Acid Digestion of Whole-water Samples. U.S. Geological Survey Open-File Report 96-225.US Department of the Interior, US Geological Survey, 10.3133/ofr96225. [DOI] [Google Scholar]
- Houtman CJ, Sterk SS, van de Heijning MPM, Brouwer A, Stephany RW, van der Burg B, et al. 2009. Detection of anabolic androgenic steroid abuse in doping control using mammalian reporter gene bioassays. Anal Chim Acta 637:247–258, 10.1016/j.aca.2008.09.037. [DOI] [PubMed] [Google Scholar]
- Hrudey SE, Hrudey EJ, Pollard SJT. 2006. Risk management for assuring safe drinking water. Environ Int 32:948–957, 10.1016/j.envint.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Hrudey SE. 2009. Chlorination disinfection by-products, public health risk tradeoffs and me. Water Res 43:2057–2092, 10.1016/j.watres.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Hrudey SE, Backer LC, Humpage AR, Krasner SW, Michaud DS, Moore LE, et al. 2015. Evaluating Evidence for Association of Human Bladder Cancer with Drinking-Water Chlorination Disinfection By-Products. Journal of Toxicology and Environmental Health, Part B 18:213–241, 10.1080/10937404.2015.1067661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrudey SE, Fawell J. 2015. 40 years on: what do we know about drinking water disinfection by-products (DBPs) and human health? Water Supply 15:667–674, 10.2166/ws.2015.036. [DOI] [Google Scholar]
- Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, et al. 2016. Detection of poly-and perfluoroalkyl substances (PFASs) in US drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ Sci Technol Lett 3:344–350, 10.1021/acs.estlett.6b00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta-Saenz L, Irigoyen M, Benavides J, Mendoza M. 2012. Tap or Bottled Water: Drinking Preferences Among Urban Minority Children and Adolescents. Journal of Community Health 37:54–58, 10.1007/s10900-011-9415-1. [DOI] [PubMed] [Google Scholar]
- Hunkeler D, Laier T, Breider F, Jacobsen OS. 2012. Demonstrating a Natural Origin of Chloroform in Groundwater Using Stable Carbon Isotopes. Environ Sci Technol 46:6096–6101, 10.1021/es204585d. [DOI] [PubMed] [Google Scholar]
- Interstate Technology Regulatory Council. 2020. Regulations, Guidance, and Advisories for Per- and Polyfluoroalkyl Substances (PFAS): Water and Soil Values. https://pfas-1.itrcweb.org/wp-content/uploads/2020/08/ITRCPFASWaterandSoilValuesTables_JULY_2020-final-1.xlsx.
- Javidi A, Pierce G. 2018. US Households’ Perception of Drinking Water as Unsafe and its Consequences: Examining Alternative Choices to the Tap. Water Resour Res 54:6100–6113, 10.1029/2017WR022186. [DOI] [Google Scholar]
- Jeong CH, Postigo C, Richardson SD, Simmons JE, Kimura SY, Mariñas BJ, et al. 2015. Occurrence and Comparative Toxicity of Haloacetaldehyde Disinfection Byproducts in Drinking Water. Environ Sci Technol 49:13749–13759, 10.1021/es506358x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RR, Weyer PJ, DellaValle CT, Inoue-Choi M, Anderson KE, Cantor KP, et al. 2016. Nitrate from Drinking Water and Diet and Bladder Cancer Among Postmenopausal Women in Iowa. Environ Health Perspect 124:1751–1758, 10.1289/EHP191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RR, Weyer PJ, DellaValle CT, Robien K, Cantor KP, Krasner S, et al. 2017. Ingested Nitrate, Disinfection By-products, and Kidney Cancer Risk in Older Women. Epidemiology 28:703–711, 10.1097/EDE.0000000000000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaboré HA, Vo Duy S, Munoz G, Méité L, Desrosiers M, Liu J, et al. 2018. Worldwide drinking water occurrence and levels of newly-identified perfluoroalkyl and polyfluoroalkyl substances. Sci Total Environ 616-617:1089–1100, 10.1016/j.scitotenv.2017.10.210. [DOI] [PubMed] [Google Scholar]
- Klarich KL, Pflug NC, DeWald EM, Hladik ML, Kolpin DW, Cwiertny DM, et al. 2017. Occurrence of Neonicotinoid Insecticides in Finished Drinking Water and Fate during Drinking Water Treatment. Environ Sci Technol Lett 4:168–173, 10.1021/acs.estlett.7b00081. [DOI] [Google Scholar]
- Klarich Wong KL, Webb DT, Nagorzanski MR, Kolpin DW, Hladik ML, Cwiertny DM, et al. 2019. Chlorinated Byproducts of Neonicotinoids and Their Metabolites: An Unrecognized Human Exposure Potential? Environ Sci Technol Lett 6:98–105, 10.1021/acs.estlett.8b00706. [DOI] [Google Scholar]
- Knobeloch L, Gorski P, Christenson M, Anderson H. 2013. Private Drinking Water Quality in Rural Wisconsin. Journal of Environmental Health 75:16–21, www.jstor.org/stable/26329567. [PubMed] [Google Scholar]
- Knox R, McDermott R. 2019. Tap Water versus Bottled Water: A Pilot Study. Journal of Water Resource and Protection Journal of Water Resource and Protection:1398–1407, 10.4236/jwarp.2019.1111081. [DOI] [Google Scholar]
- Krasner SW, Weinberg HS, Richardson SD, Pastor SJ, Chinn R, Sclimenti MJ, et al. 2006. Occurrence of a New Generation of Disinfection Byproducts. Environ Sci Technol 40:7175–7185, 10.1021/es060353j. [DOI] [PubMed] [Google Scholar]
- Krasner SW, Lee TCF, Westerhoff P, Fischer N, Hanigan D, Karanfil T, et al. 2016. Granular Activated Carbon Treatment May Result in Higher Predicted Genotoxicity in the Presence of Bromide. Environ Sci Technol 50:9583–9591, 10.1021/acs.est.6b02508. [DOI] [PubMed] [Google Scholar]
- Lanphear B, Lowry J, Ahdoot S, Baum C, Bernstein A, Bole A, et al. 2016. Prevention of Childhood Lead Toxicity: Policy Statement of the American Academy of Pediatrics Council on Environmental Health. Pediatrics 138:e20161493, 10.1542/peds.2016-1493. [DOI] [PubMed] [Google Scholar]
- Lanphear BP. 2017. Low-level toxicity of chemicals: No acceptable levels? PLoS Biology 15:e2003066, 10.1371/journal.pbio.2003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laturnus F, Haselmann KF, Borch T, Grøn C. 2002. Terrestrial natural sources of trichloromethane (chloroform, CHCl3) — An overview. Biogeochemistry 60:121–139, 10.1023/A:1019887505651. [DOI] [Google Scholar]
- Legler J, Fletcher T, Govarts E, Porta M, Blumberg B, Heindel JJ, et al. 2015. Obesity, Diabetes, and Associated Costs of Exposure to Endocrine-Disrupting Chemicals in the European Union. The Journal of Clinical Endocrinology & Metabolism 100:1278–1288, 10.1210/jc.2014-4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leusch FDL, Neale PA, Arnal C, Aneck-Hahn NH, Balaguer P, Bruchet A, et al. 2018. Analysis of endocrine activity in drinking water, surface water and treated wastewater from six countries. Water Res 139:10–18, 10.1016/j.watres.2018.03.056. [DOI] [PubMed] [Google Scholar]
- Levallois P, Barn P, Valcke M, Gauvin D, Kosatsky T. 2018. Public Health Consequences of Lead in Drinking Water. Current Environmental Health Reports 5:255–262, 10.1007/s40572-018-0193-0. [DOI] [PubMed] [Google Scholar]
- Loftin KA, Graham JL, Hilborn ED, Lehmann SC, Meyer MT, Dietze JE, et al. 2016. Cyanotoxins in inland lakes of the United States: Occurrence and potential recreational health risks in the EPA National Lakes Assessment 2007. Harmful Algae 56:77–90, 10.1016/j.hal.2016.04.001. [DOI] [PubMed] [Google Scholar]
- MacDonald Gibson J, DeFelice N, Sebastian D, Leker H. 2014. Racial disparities in access to community water supply service in Wake County, North Carolina. Frontiers in Public Health Services and Systems Research 3:7, 10.13023/FPHSSR.0303.06. [DOI] [Google Scholar]
- MacDonald Gibson J, Pieper K. 2017. Strategies to Improve Private-Well Water Quality: A North Carolina Perspective. Environ Health Perspect 125:076001–076001, 10.1289/EHP890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris KC, Andra SS, Botsaris G. 2014. Pipe Scales and Biofilms in Drinking-Water Distribution Systems: Undermining Finished Water Quality. Critical Reviews in Environmental Science and Technology 44:1477–1523, 10.1080/10643389.2013.790746. [DOI] [Google Scholar]
- Mashpee Water District. 2020. Mashpee Water District 2020 Water Quality Report. http://www.mashpeewaterdistrict.com/waterquality/Mashpee%20Water%20Qual%20Rpt%201-16%20REV.pdf.
- Massachusetts Department of Environmental Protection. 2019. Technical Support Document Per- and Polyfluoroalkyl Substances (PFAS): An Updated Subgroup Approach to Groundwater and Drinking Water Values. https://www.mass.gov/doc/per-and-polyfluoroalkyl-substances-pfas-an-updated-subgroup-approach-to-groundwater-and/download.
- Massachusetts Department of Environmental Protection. 2020. Documentation for Updated Office of Research and Standards Guidelines (ORSG) for Per- and Polyfluoroalkyl Substances (PFAS) in Drinking Water. https://www.mass.gov/doc/massdep-ors-guideline-for-pfas/download.
- Masterson J, Walter D. 2009. Hydrogeology and Groundwater Resources of the Coastal Aquifers of Southeastern Massachusetts. U.S. Geological Survey Circular 1338. http://pubs.usgs.gov/circ/circ1338/. [Google Scholar]
- Maupin MA. 2018. Summary of estimated water use in the United States in 2015. (Fact Sheet). 2018-3035. Reston, VA, 10.3133/fs20183035. [DOI] [Google Scholar]
- McCulloch A 2003. Chloroform in the environment: occurrence, sources, sinks and effects. Chemosphere 50:1291–1308, 10.1016/S0045-6535(02)00697-5. [DOI] [PubMed] [Google Scholar]
- Medlock Kakaley EK, Blackwell BR, Cardon MC, Conley JM, Evans N, Feifarek DJ, et al. 2020. De Facto Water Reuse: Bioassay suite approach delivers depth and breadth in endocrine active compound detection. Sci Total Environ 699:134297, 10.1016/j.scitotenv.2019.134297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretto A, Bachman A, Boobis A, Solomon KR, Pastoor TP, Wilks MF, et al. 2017. A framework for cumulative risk assessment in the 21st century. Crit Rev Toxicol 47:85–97, 10.1080/10408444.2016.1211618. [DOI] [PubMed] [Google Scholar]
- Moschet C, Wittmer I, Simovic J, Junghans M, Piazzoli A, Singer H, et al. 2014. How a complete pesticide screening changes the assessment of surface water quality. Environ Sci Technol 48:5423–5432, 10.1021/es500371t. [DOI] [PubMed] [Google Scholar]
- Mueller DK, Schertz TL, Martin JD, Sandstrom MW. 2015. Design, analysis, and interpretation of field quality-control data for water-sampling projects. U.S. Geological Survey Techniques and Methods Book 4 Chapter C4.US Geological Survey, 10.3133/tm4C4. [DOI] [Google Scholar]
- Muellner MG, Wagner ED, McCalla K, Richardson SD, Woo Y-T, Plewa MJ. 2007. Haloacetonitriles vs. Regulated Haloacetic Acids: Are Nitrogen-Containing DBPs More Toxic? Environ Sci Technol 41:645–651, 10.1021/es0617441. [DOI] [PubMed] [Google Scholar]
- Murray CC, Vatankhah H, McDonough CA, Nickerson A, Hedtke TT, Cath TY, et al. 2019. Removal of per- and polyfluoroalkyl substances using super-fine powder activated carbon and ceramic membrane filtration. J Hazard Mater 366:160–168, 10.1016/j.jhazmat.2018.11.050. [DOI] [PubMed] [Google Scholar]
- National Research Council. 1983. Risk Assessment in the Federal Government: Managing the Process. Washington, DC:The National Academies Press, doi:10.17226/36610.17226/366https://www.nap.edu/catalog/366/risk-assessment-in-the-federal-government-managing-the-processhttps://www.nap.edu/catalog/366/risk-assessment-in-the-federal-government-managing-the-process [PubMed] [Google Scholar]
- Nieuwenhuijsen MJ, Martinez D, Grellier J, Bennett J, Best N, Iszatt N, et al. 2009. Chlorination Disinfection By-Products in Drinking Water and Congenital Anomalies: Review and Meta-Analyses. Environ Health Perspect 117:1486–1493, 10.1289/ehp.0900677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JE, Toccalino PL, Morman SA. 2018. Health-Based Screening Levels for evaluating water-quality data (2nd ed.). Available: 10.5066/F71C1TWP [accessed February 10, 2020]. [DOI]
- Norton SB, Rodier DJ, van der Schalie WH, Wood WP, Slimak MW, Gentile JH. 1992. A framework for ecological risk assessment at the EPA. Environ Toxicol Chem 11:1663–1672, 10.1002/etc.5620111202. [DOI] [Google Scholar]
- Olson ED, Poling D, Solomon G. 1999. Bottled water: pure drink or pure hype?:National Resources Defense Council, https://www.nrdc.org/sites/default/files/bottled-water-pure-drink-or-pure-hype-report.pdf. [Google Scholar]
- Onufrak SJ, Park S, Sharkey JR, Sherry B. 2014. The relationship of perceptions of tap water safety with intake of sugar-sweetened beverages and plain water among US adults. Public Health Nutrition 17:179–185, 10.1017/S1368980012004600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfadenhauer LM, Burns J, Rohwer A, Rehfuess EA. 2016. Effectiveness of interventions to reduce exposure to lead through consumer products and drinking water: a systematic review. Environ Res 147:525–536, 10.1016/j.envres.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Pfaff JD. 1993. Method 300.0, Determination of inorganic anions by ion chromatography. Revision 2.1. USEPA Method 300.0.U.S. Environmental Protection Agency, https://www.epa.gov/sites/production/files/2015-08/documents/method_300-0_rev_2-1_1993.pdf.
- Pieper KJ, Nystrom VE, Parks J, Jennings K, Faircloth H, Morgan JB, et al. 2018. Elevated Lead in Water of Private Wells Poses Health Risks: Case Study in Macon County, North Carolina. Environ Sci Technol 52:4350–4357, 10.1021/acs.est.7b05812. [DOI] [PubMed] [Google Scholar]
- Pierce G, Gonzalez S. 2017. Mistrust at the tap? Factors contributing to public drinking water (mis) perception across US households. Water Policy 19:1–12, 10.2166/wp.2016.143. [DOI] [Google Scholar]
- Post GB, Cohn PD, Cooper KR. 2012. Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: a critical review of recent literature. Environ Res 116:93–117, 10.1016/j.envres.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Post GB. 2020. Recent US State and Federal Drinking Water Guidelines for Per- And Polyfluoroalkyl Substances (PFAS). Environ Toxicol Chem n/a, 10.1002/etc.4863. [DOI] [PubMed] [Google Scholar]
- Pressman JG, Richardson SD, Speth TF, Miltner RJ, Narotsky MG, Hunter I, Sidney E, et al. 2010. Concentration, chlorination, and chemical analysis of drinking water for disinfection byproduct mixtures health effects research: US EPA’s four lab study. Environ Sci Technol 44:7184–7192, 10.1021/es9039314. [DOI] [PubMed] [Google Scholar]
- Quist AJL, Inoue-Choi M, Weyer PJ, Anderson KE, Cantor KP, Krasner S, et al. 2018. Ingested nitrate and nitrite, disinfection by-products, and pancreatic cancer risk in postmenopausal women. Int J Cancer 142:251–261, 10.1002/ijc.31055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. 2019. R: A Language and Environment for Statistical Computing. Version 3.5.2. Vienna Austria:R Foundation for Statistical Computing, https://www.R-project.org. [Google Scholar]
- Ranganathan M, Balazs C. 2015. Water marginalization at the urban fringe: environmental justice and urban political ecology across the North—South divide. Urban Geography 36:403–423, 10.1080/02723638.2015.1005414. [DOI] [Google Scholar]
- Renner R 2010. Exposure on tap: drinking water as an overlooked source of lead. Environ Health Perspect 118:A68, 10.1289/ehp.118-a68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds KA, Mena KD, Gerba CP. 2008. Risk of waterborne illness via drinking water in the United States. In: Reviews of environmental contamination and toxicology:Springer, 117–158, 10.1007/978-0-387-71724-1_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson SD, Plewa MJ, Wagner ED, Schoeny R, DeMarini DM. 2007. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutation Research/Reviews in Mutation Research 636:178–242, 10.1016/j.mrrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Richardson SD, Plewa MJ. 2020. To regulate or not to regulate? What to do with more toxic disinfection by-products? Journal of Environmental Chemical Engineering 8:103939, 10.1016/j.jece.2020.103939. [DOI] [Google Scholar]
- Rivera-Núñez Z, Wright JM, Blount BC, Silva LK, Jones E, Chan RL, et al. 2012. Comparison of trihalomethanes in tap water and blood: a case study in the United States. Environ Health Perspect 120:661, 10.1289/ehp.1104347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan WJ, Brady MT. 2009. Drinking water from private wells and risks to children. Pediatrics 123:e1123–e1137, 10.1542/peds.2009-0752. [DOI] [PubMed] [Google Scholar]
- Romanok KM, Kolpin DW, Meppelink SM, Argos M, Brown J, DeVito M, et al. 2018. Methods used for the collection and analysis of chemical and biological data for the Tapwater Exposure Study, United States, 2016–17. U.S. Geological Survey Open-File Report 2018-1098. Reston, VA, 10.3133/ofr20181098. [DOI] [Google Scholar]
- Romanok KM, Bradley PM, LeBlanc DR. 2020. Target-Chemical Concentration Results of Mixed-Organic/Inorganic Chemical Exposures in Cape Cod, Massachusetts Tapwater, 2018. U.S. Geological Survey data release. 10.5066/P9RKZK1E. [DOI] [Google Scholar]
- Rose D, Sandstrom M, Murtagh L. 2016. Methods of the National Water Quality Laboratory. Chapter B12. Determination of heat purgeable and ambient purgeable volatile organic compounds in water by gas chromatography/mass spectrometry. U.S. Geological Survey Techniques and Methods. Book 5. Laboratory Analysis. 10.3133/tm5B12. [DOI] [Google Scholar]
- Rosen MB, Pokhrel LR, Weir MH. 2017. A discussion about public health, lead and Legionella pneumophila in drinking water supplies in the United States. Sci Total Environ 590:843–852, 10.1016/j.scitotenv.2017.02.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosinger AY, Herrick KA, Wutich AY, Yoder JS, Ogden CL. 2018. Disparities in plain, tap and bottled water consumption among US adults: National Health and Nutrition Examination Survey (NHANES) 2007–2014. Public Health Nutrition 21:1455–1464, 10.1017/S1368980017004050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor A, Prokopy LS, Amberg S. 2011. What’s wrong with the tap? Examining perceptions of tap water and bottled water at Purdue University. Environmental management 48:588–601, 10.1007/s00267-011-9692-6. [DOI] [PubMed] [Google Scholar]
- Schaider LA, Rudel RA, Ackerman JM, Dunagan SC, Brody JG. 2014. Pharmaceuticals, perfluorosurfactants, and other organic wastewater compounds in public drinking water wells in a shallow sand and gravel aquifer. Sci Total Environ 468:384–393, 10.1016/j.scitotenv.2013.08.067. [DOI] [PubMed] [Google Scholar]
- Schaider LA, Ackerman JM, Rudel RA. 2016. Septic systems as sources of organic wastewater compounds in domestic drinking water wells in a shallow sand and gravel aquifer. Sci Total Environ 547:470–481, 10.1016/j.scitotenv.2015.12.081. [DOI] [PubMed] [Google Scholar]
- Scherzer T, Barker JC, Pollick H, Weintraub JA. 2010. Water consumption beliefs and practices in a rural Latino community: implications for fluoridation. Journal of Public Health Dentistry 70:337–343, 10.1111/j.1752-7325.2010.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenen D 2002. Role of disinfection in suppressing the spread of pathogens with drinking water: possibilities and limitations. Water Res 36:3874–3888, 10.1016/S0043-1354(02)00076-3. [DOI] [PubMed] [Google Scholar]
- Schriks M, Heringa MB, van der Kooi MME, de Voogt P, van Wezel AP. 2010. Toxicological relevance of emerging contaminants for drinking water quality. Water Res 44:461–476, 10.1016/j.watres.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Seltenrich N 2017. Unwell: The Public Health Implications of Unregulated Drinking Water. Environ Health Perspect 125:114001–114001, 10.1289/EHP2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneveld E, Riteco JAC, Jansen HJ, Pieterse B, Brouwer A, Schoonen WG, et al. 2005. Comparison of In Vitro and In Vivo Screening Models for Androgenic and Estrogenic Activities. Toxicol Sci 89:173–187, 10.1093/toxsci/kfj009. [DOI] [PubMed] [Google Scholar]
- Stackelberg PE, Furlong ET, Meyer MT, Zaugg SD, Henderson AK, Reissman DB. 2004. Persistence of pharmaceutical compounds and other organic wastewater contaminants in a conventional drinking-water-treatment plant. Sci Total Environ 329:99–113, 10.1016/j.scitotenv.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Stackelberg PE, Gibs J, Furlong ET, Meyer MT, Zaugg SD, Lippincott RL. 2007. Efficiency of conventional drinking-water-treatment processes in removal of pharmaceuticals and other organic compounds. Sci Total Environ 377:255–272, 10.1016/j.scitotenv.2007.01.095. [DOI] [PubMed] [Google Scholar]
- Stalter D, O’Malley E, von Gunten U, Escher BI. 2020. Mixture effects of drinking water disinfection by-products: implications for risk assessment. Environmental Science: Water Research & Technology, 10.1039/C9EW00988D. [DOI] [Google Scholar]
- Steffen W, Grinevald J, Crutzen P, McNeill J. 2011. The Anthropocene: conceptual and historical perspectives. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 369:842–867, 10.1098/rsta.2010.0327. [DOI] [PubMed] [Google Scholar]
- Steffen W, Richardson K, Rockström J, Cornell SE, Fetzer I, Bennett EM, et al. 2015. Planetary boundaries: Guiding human development on a changing planet. Science 347:1259855, 10.1126/science.1259855. [DOI] [PubMed] [Google Scholar]
- Stern BR, Solioz M, Krewski D, Aggett P, Aw T-C, Baker S, et al. 2007. Copper and Human Health: Biochemistry, Genetics, and Strategies for Modeling Dose-response Relationships. Journal of Toxicology and Environmental Health, Part B 10:157–222, 10.1080/10937400600755911. [DOI] [PubMed] [Google Scholar]
- Stern BR. 2010. Essentiality and Toxicity in Copper Health Risk Assessment: Overview, Update and Regulatory Considerations. Journal of Toxicology and Environmental Health, Part A 73:114–127, 10.1080/15287390903337100. [DOI] [PubMed] [Google Scholar]
- Stoiber T, Temkin A, Andrews D, Campbell C, Naidenko OV. 2019. Applying a cumulative risk framework to drinking water assessment: a commentary. Environmental Health 18:37, 10.1186/s12940-019-0475-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strynar M, Dagnino S, McMahen R, Liang S, Lindstrom A, Andersen E, et al. 2015. Identification of novel perfluoroalkyl ether carboxylic acids (PFECAs) and sulfonic acids (PFESAs) in natural waters using accurate mass time-of-flight mass spectrometry (TOFMS). Environ Sci Technol 49:11622–11630, 10.1021/acs.est.5b01215. [DOI] [PubMed] [Google Scholar]
- Suja F, Pramanik BK, Zain SM. 2009. Contamination, bioaccumulation and toxic effects of perfluorinated chemicals (PFCs) in the water environment: a review paper. Water Sci Technol 60:1533–1544, 10.2166/wst.2009.504. [DOI] [PubMed] [Google Scholar]
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. 2019. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. Journal of Exposure Science & Environmental Epidemiology 29:131–147, 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz CH, Rudel RA, Kachajian JR, Brody JG. 2003. Historical reconstruction of wastewater and land use impacts to groundwater used for public drinking water:Exposure assessment using chemical data and GIS. Journal of Exposure Science & Environmental Epidemiology 13:403–416, 10.1038/sj.jea.7500291. [DOI] [PubMed] [Google Scholar]
- Swartz CH, Reddy S, Benotti MJ, Yin H, Barber LB, Brownawell BJ, et al. 2006. Steroid Estrogens, Nonylphenol Ethoxylate Metabolites, and Other Wastewater Contaminants in Groundwater Affected by a Residential Septic System on Cape Cod, MA. Environ Sci Technol 40:4894–4902, 10.1021/es052595+. [DOI] [PubMed] [Google Scholar]
- Swistock BR, Clemens S. 2013. Water quality and management of private drinking water wells in Pennsylvania. Journal of environmental health 75:60, https://www.jstor.org/stable/26329557. [PubMed] [Google Scholar]
- Thomas ED, Gittelsohn J, Yracheta J, Powers M, O’Leary M, Harvey DE, et al. 2019. The Strong Heart Water Study: Informing and designing a multi-level intervention to reduce arsenic exposure among private well users in Great Plains Indian Nations. Sci Total Environ 650:3120–3133, 10.1016/j.scitotenv.2018.09.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toccalino PL, Hopple JA. 2010. Quality of Water from Public-Supply Wells in the United States, 1993-2007: Overview of Major Findings. (Circular). 1346. 10.3133/cir1346. [DOI]
- Toccalino PL, Norman JE, Scott JC. 2012. Chemical mixtures in untreated water from public-supply wells in the US—occurrence, composition, and potential toxicity. Sci Total Environ 431:262–270, 10.1016/j.scitotenv.2012.05.044. [DOI] [PubMed] [Google Scholar]
- Triantafyllidou S, Edwards M. 2012. Lead (Pb) in tap water and in blood: implications for lead exposure in the United States. Critical Reviews in Environmental Science & Technology 42:1297–1352, 10.1080/10643389.2011.556556. [DOI] [Google Scholar]
- Tröger R, Klöckner P, Ahrens L, Wiberg K. 2018. Micropollutants in drinking water from source to tap - Method development and application of a multiresidue screening method. Sci Total Environ 627:1404–1432, 10.1016/j.scitotenv.2018.01.277. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. 2008. Lead and Copper Rule: A Quick Reference Guide. https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=60001N8P.txt [accessed January 25, 2018].
- U.S. Environmental Protection Agency. 2010. Comprehensive Disinfectants and Disinfection Byproducts Rules (Stage 1 and Stage 2): Quick Reference Guide. https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=P100C8XW.txt [accessed January 25, 2018].
- U.S. Environmental Protection Agency. 2011. EPA’s National-scale Air Toxics Assessment, An Overview of Methods for EPA’s National-Scale Air Toxics Assessment. https://www.epa.gov/sites/production/files/2015-10/documents/2005-nata-tmd.pdf.
- U.S. Environmental Protection Agency. 2012. Sustainable Futures / Pollution Prevention (P2) Framework Manual. EPA-748-B12-001. Washington, D.C.:U.S. Environmental Protection Agency, https://www.epa.gov/sustainable-futures/sustainable-futures-p2-framework-manual. [Google Scholar]
- U.S. Environmental Protection Agency. 2014. Inductively coupled plasma-optical emission spectrometry, Method 6010D. EPA SW-846 Update V, accessed November 2, 2017. https://www.epa.gov/sites/production/files/2015-12/documents/6010d.pdf.
- U.S. Environmental Protection Agency. 2016a. PFOA & PFOS Drinking Water Health Advisories. EPA 800-F-16-003. https://www.epa.gov/sites/production/files/2016-06/documents/drinkingwaterhealthadvisories_pfoa_pfos_updated_5.31.16.pdf.
- U.S. Environmental Protection Agency. 2016b. Quick Guide to Drinking Water Sample Collection. https://www.epa.gov/sites/production/files/2017-04/documents/quick-guide-drinking-water-sample-collection-2ed-update-508.pdf [accessed February 26, 2018].
- U.S. Environmental Protection Agency. 2017. 40 C.F.R. § 131: Water Quality Standards. https://www.ecfr.gov/cgi-bin/text-idx?SID=454a7b51118b27f20cef29ff071c1440&node=40:22.0.1.1.18&rgn=div5.
- U.S. Environmental Protection Agency. 2018. 2018 Edition of the Drinking Water Standards and Health Advisories. EPA 822-F-18-001. https://www.epa.gov/sites/production/files/2018-03/documents/dwtable2018.pdf.
- U.S. Environmental Protection Agency. 2019. ToxCast & Tox21 Summary Files from invitrodb_v3. https://www.epa.gov/chemical-research/toxicity-forecaster-toxcasttm-data [accessed July 5, 2019].
- U.S. Environmental Protection Agency. 2020a. EPA’s National Center for Computational Toxicology: Previously published ToxCast data. v3. Available: 10.23645/epacomptox.6062551.v3. [DOI]
- U.S. Environmental Protection Agency. 2020b. EPA Sole Source Aquifers. Available: https://epa.maps.arcgis.com/apps/webappviewer/index.html?id=9ebb047ba3ec41ada1877155fe31356b [accessed March 14, 2020].
- U.S. Environmental Protection Agency. 2020c. 40 C.F.R. § 136: Guidelines establishing test procedures for the analysis of pollutants. http://www.ecfr.gov/cgi-bin/text-idx?SID=3c78b6c8952e5e79268e429ed98bad84&mc=true&node=pt40.23.136&rgn=div5
- U.S. Environmental Protection Agency. 2020d. CompTox Chemicals Dashboard. https://comptox.epa.gov/dashboard [accessed January 22, 2020].
- U.S. Environmental Protection Agency. 2020e. Sole Source Aquifers for Drinking Water. https://www.epa.gov/dwssa [accessed March 14 2020].
- U.S. Environmental Protection Agency. 2020f. Drinking Water Requirements for States and Public Water Systems: Lead and Copper Rule. https://www.epa.gov/dwreginfo/lead-and-copper-rule#additional-resources [accessed January 24, 2018].
- U.S. Environmental Protection Agency. 2020g. Information about public water systems. Available: https://www.epa.gov/dwreginfo/information-about-public-water-systems [accessed September 30].
- U.S. Environmental Protection Agency. 2020h. National Secondary Drinking Water Regulations. https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations [accessed March 16, 2020].
- U.S. Environmental Protection Agency. 2020i. Drinking Water Requirements for States and Public Water Systems: Stage 1 and Stage 2 Disinfectants and Disinfection Byproducts Rules. https://www.epa.gov/dwreginfo/stage-1-and-stage-2-disinfectants-and-disinfection-byproducts-rules [accessed January 24, 2020].
- U.S. Environmental Protection Agency. 2020j. How EPA Regulates Drinking Water Contaminants. https://www.epa.gov/dwregdev/how-epa-regulates-drinking-water-contaminants [accessed July 26, 2018].
- U.S. Environmental Protection Agency. 2020k. National Primary Drinking Water Regulations. https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations [accessed March 16, 2020].
- Verhougstraete MP, Gerald JK, Gerba CP, Reynolds KA. 2019. Cost-benefit of point-of-use devices for lead reduction. Environ Res 171:260–265, 10.1016/j.envres.2019.01.016. [DOI] [PubMed] [Google Scholar]
- Villanueva CM, Gracia-Lavedan E, Julvez J, Santa-Marina L, Lertxundi N, Ibarluzea J, et al. 2018. Drinking water disinfection by-products during pregnancy and child neuropsychological development in the INMA Spanish cohort study. Environ Int 110:113–122, 10.1016/j.envint.2017.10.017. [DOI] [PubMed] [Google Scholar]
- Wang X, Mao Y, Tang S, Yang H, Xie YF. 2015. Disinfection byproducts in drinking water and regulatory compliance: A critical review. Frontiers of Environmental Science & Engineering 9:3–15, 10.1007/s11783-014-0734-1. [DOI] [Google Scholar]
- Wang Z, DeWitt JC, Higgins CP, Cousins IT. 2017. A Never-Ending Story of Per-and Polyfluoroalkyl Substances (PFASs)? Environ Sci Technol 51:2508–2518, 10.1021/acs.est.6b04806. [DOI] [PubMed] [Google Scholar]
- Wang Z, Walker GW, Muir DCG, Nagatani-Yoshida K. 2020. Toward a Global Understanding of Chemical Pollution: A First Comprehensive Analysis of National and Regional Chemical Inventories. Environ Sci Technol, 10.1021/acs.est.9b06379. [DOI] [PubMed] [Google Scholar]
- Ward MH, deKok TM, Levallois P, Brender J, Gulis G, Nolan BT, et al. 2005. Workgroup Report: Drinking-Water Nitrate and Health - Recent Findings and Research Needs. Environ Health Perspect 113:1607–1614, 10.1289/ehp.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MH, Jones RR, Brender JD, De Kok TM, Weyer PJ, Nolan BT, et al. 2018. Drinking water nitrate and human health: an updated review. International journal of environmental research and public health 15:1557, 10.3390/ijerph15071557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg H, Krasner S, Richardson S, Thruston A. 2002. The Occurrence of Disinfection By-Products (DBPs) of Health Concern in Drinking Water: Results of a Nationwide DBP Occurrence Study, EPA/600/R-02/068. (Athens, GA: ). EPA/600/R-02/068. https://cfpub.epa.gov/si/si_public_file_download.cfm?p_download_id=525040&Lab=NERL. [Google Scholar]
- Wescoat JL, Headington L, Theobald R. 2007. Water and poverty in the United States. Geoforum 38:801–814, 10.1016/j.geoforum.2006.08.007. [DOI] [Google Scholar]
- Wilson VS, Bobseine K, Lambright CR, Gray LE Jr. 2002. A Novel Cell Line, MDA-kb2, That Stably Expresses an Androgen- and Glucocorticoid-Responsive Reporter for the Detection of Hormone Receptor Agonists and Antagonists. Toxicol Sci 66:69–81, 10.1093/toxsci/66.1.69. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Bobseine K, Gray LE. 2004. Development and characterization of a cell line that stably expresses an estrogen-responsive luciferase reporter for the detection of estrogen receptor agonist and antagonists. Toxicol Sci 81:69–77, 10.1093/toxsci/kfh180. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2016. Global report on diabetes. France, http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf.
- World Health Organization (WHO). 2011. Guidelines for drinking-water quality, Fourth edition incorporating first addendum, https://apps.who.int/iris/bitstream/handle/10665/254637/9789241549950-eng.pdf;jsessionid=4A5CF097C24BF0987FE379E9B140CFCA?sequence=1. [PubMed]
- Yarmouth Water. 2019. Yarmouth Water 2018 Annual Drinking Water Quality Report. http://www.yarmouth.ma.us/DocumentCenter/View/12180/Water-Quality-Report-2018?bidId=.
- Zheng Y, Flanagan SV. 2017. The Case for Universal Screening of Private Well Water Quality in the U.S. and Testing Requirements to Achieve It: Evidence from Arsenic. Environ Health Perspect 125:085002, https://doi.org/0.1289/EHP629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zogorski JS, Carter JM, Ivahnenko T, Lapham WW, Moran MJ, Rowe BL, et al. 2006. Volatile organic compounds in the nation’s ground water and drinking-water supply wells. (Circular). 1292. http://pubs.er.usgs.gov/publication/cir1292. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


