Abstract
Liquid biopsy in cancer has gained momentum in clinical research and is experiencing a boom for a variety of applications. There are significant efforts to utilize liquid biopsies in cancer for early detection and treatment stratification, as well as residual disease and recurrence monitoring. Although most efforts have used circulating tumor cells and circulating tumor DNA for this purpose, exosomes and other extracellular vesicles have emerged as a platform with potentially broader and complementary applications. Exosomes/extracellular vesicles are small vesicles released by cells, including cancer cells, into the surrounding biofluids. These exosomes contain tumor-derived materials such as DNA, RNA, protein, lipid, sugar structures, and metabolites. In addition, exosomes carry molecules on their surface that provides clues regarding their origin, making it possible to sort vesicle types and enrich signatures from tissue-specific origins. Exosomes are part of the intercellular communication system and cancer cells frequently use them as biological messengers to benefit their growth. Since exosomes are part of the disease process, they have become of tremendous interest in biomarker research. Exosomes are remarkably stable in biofluids, such as plasma and urine, and can be isolated for clinical evaluation even in the early stages of the disease. Exosome-based biomarkers have quickly become adopted in the clinical arena and the first exosome RNA-based prostate cancer test has already helped >50 000 patients in their decision process and is now included in the National Comprehensive Cancer Network guidelines for early prostate cancer detection. This review will discuss the advantages and challenges of exosome-based liquid biopsies for tumor biomarkers and clinical implementation in the context of circulating tumor DNA and circulating tumor cells.
Keywords: liquid biopsy, exosome, extracellular vesicle, circulating tumor cell (CTC), cell-free DNA (cfDNA), circulating tumor DNA (ctDNA)
INTRODUCTION
Surgical tissue biopsies are considered the gold standard for solid tumor diagnosis. Historically, this has been a morphology-based approach, but more and more cancers are also being molecularly profiled to aid in treatment decisions and outcome-based predictions. However, tissue biopsies are not always available and clinicians continue to face the hurdle of tumor heterogeneity as they deliberate therapeutic options based on a patient’s tumor genomic profile, since it is known that various parts within a tumor can present a related but different genetic and epigenetic profile.1,2 Moreover, the molecular makeup of the tumor can dynamically evolve over time, driven by microenvironmental stimuli and clonal selection under treatment pressure,3,4 rendering future therapeutic decisions based on historical tissue biopsy suboptimal.5,6 Furthermore, the surgical biopsy procedure is constrained by accessibility, repeatability, patient age, cost, and time, some even causing harmful clinical complications. For instance, it has been reported that a lung biopsy costs on average US$14 587, which does not include the additional expense of complications from the surgery, reported in 19.3% of patients, where the cost averages US$37 745.7
These inadequacies of tissue biopsy, concomitant with the paradigm shift to molecular analysis, have begun a transition to molecular profiling of biofluids, also known as ‘liquid biopsy’. This approach offers a significant step forward because of its less invasive nature, lower cost, real-time insights into tumor status and, in some cases, the ability to overcome the issue of tumor heterogeneity (or multiple metastatic lesions). Another important consideration is that molecular tumor profiling by tissue biopsies cannot happen at optimal frequency for practical and patient welfare reasons, whereas the ease of use and increased availability of liquid biopsies can benefit more patients over time, even if the sensitivity is lower than the gold standard tissue test in some cases.8
In general, liquid biopsies for cancer fall into three major categories based on the circulating source of tumor-derived materials in biofluids: namely (i) circulating tumor DNA (ctDNA); (ii) circulating tumor cells (CTCs); and (iii) tumor-derived exosomes and other extracellular vesicles (EVs) (Figure 1). These topics are reviewed with an emphasis on exosomes as well as synergies that can result from combining multiple components in each category. There is a range of different terminologies/nomenclatures for these EVs, including exosomes, but for simplicity here, we will use the terminology exosomes and EVs interchangeably for all EVs smaller than 800 nm in diameter.
Figure 1. ctDNA, exosomes, and CTCs in circulation.
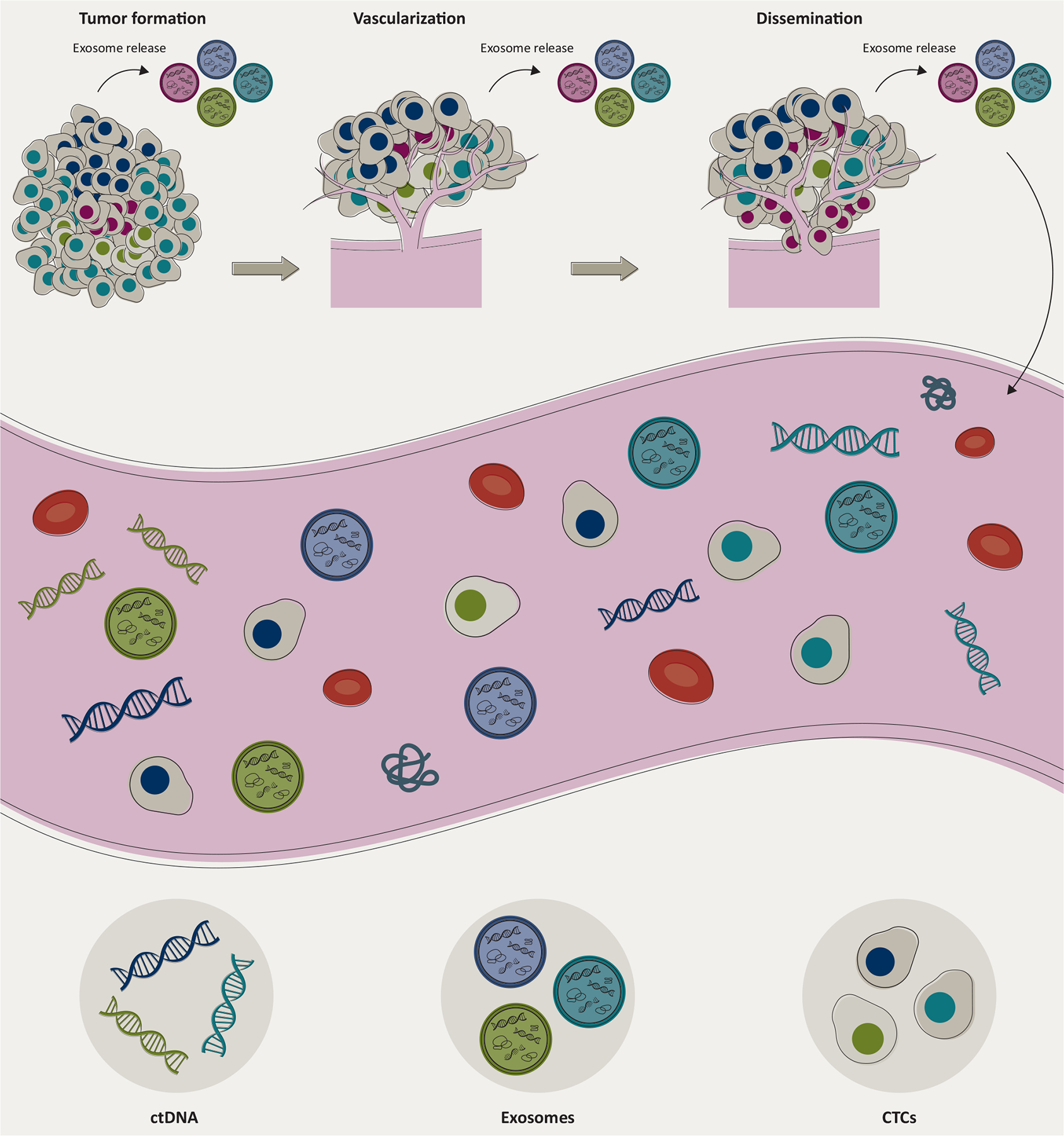
The molecular constituents from the tumor are released into biofluids at various stages of the tumor development through very different mechanisms and represent different biological entities. ctDNA is released from the dying tumor cells through apoptosis and/or necrosis, exosomes are actively released from cells at each stage of tumor formation, whereas CTCs are intact cancer cells disseminated from the tumor at later stages. Exosomes are involved in promoting the growth, vascularization, and even the dissemination and metastatic process of cancer. Elements are not drawn to scale.
CTCs, circulating tumor cells; ctDNA, circulating tumor DNA.
ctDNA approach
This nascent field has gained substantial momentum in clinical applications since the Food and Drug Administration (FDA) premarket approval of the cancer liquid biopsy test (cobas® epidermal growth factor receptor (EGFR) mutation test v2, 2016) as a companion diagnostic (CDx) to EGFR-targeted therapies in non-small-cell lung cancer (NSCLC)9 (Table 1). The ctDNA-based test detects EGFR mutations present in ~10%–30% of NSCLC cases. The ctDNA approach now encompasses single-gene mutation assays as well as large panels for broad molecular profiling strategies, including next-generation sequencing (NGS). Recently, Guardant360® CDx (Guardant Health, Redwood City, CA, 2020) became the first FDA-approved liquid biopsy NGS ctDNA test for treatment decisions on the use of osimertinib, a second-generation tyrosine kinase inhibitor, in NSCLC patients harboring EGFR mutations.10 This was followed by another FDA approval in the same space, the FoundationOne®Liquid CDx (Foundation Medicine, Cambridge, MA, 2020)11 indicated for treatment decisions for various targeted therapies in NSCLC, prostate, ovarian, and breast cancers (Table 1). These NGS panels include a large number of genes. Not all genes on the panels are clinically actionable today but can be useful for clinical trial enrollment or may prove clinically informative in the future.
Table 1.
FDA clearance and approvals over the years in the cancer liquid biopsy field for solid tumors
| FDA-approved assays | Indications |
|---|---|
| CellSearch™ | 2007: Circulating tumor cell blood test prognostic for breast, prostate, and colon cancer |
| Epi proColon® | 2016: cfDNA plasma epigenetic test for colorectal cancer screening |
| cobas® EGFR Mutation Test v2 | 2016: cfDNA qPCR plasma test indicated as a companion diagnostic to aid in selecting NSCLC patients for treatment with targeted therapies |
| therascreen PIK3CA RGQ PCR Kit | 2019: cfDNA qPCR plasma test indicated as a companion diagnostic to aid clinicians in identifying breast cancer patients who may be eligible for treatment with a targeted therapy |
| Guardant360® CDx | 2020: cfDNA NGS plasma test currently indicated as a companion diagnostic to identify patients who may benefit from targeted therapies in NSCLC |
| FoundationOne Liquid®CDx | 2020: cfDNA NGS plasma test currently indicated as a companion diagnostic to identify patients who may benefit from treatment with targeted therapies in NSCLC, prostate, ovarian and breast cancers |
CDx, companion diagnostic; cfDNA, cell-free DNA; EGFR, epidermal growth factor receptor; FDA, Food and Drug Administration; NGS, next-generation sequencing; NSCLC, non-small-cell lung cancer; qPCR, quantitative polymerase chain reaction.
Despite this progress, one limitation of this approach is that it relies on a single type of analyte, ctDNA, to be in sufficient quantities for analysis. This has proven to be particularly challenging for early-stage cancers. Some studies indicate that detection of ctDNA mutations alone requires impractically large input volumes of plasma to allow sufficient copy numbers of ctDNA-derived mutations in early stage cancer.12 Also, detecting low allelic frequency mutations in a broader gene panel increases the risk of false-positives due to clonal hematopoiesis of indeterminate potential (CHIP)13 or detection of the large numbers of indolent cancers that are better left undetected.14 Early cancer screening using ctDNA has recently gathered momentum and provides some insight into actionable mutations.15 Attention has also been drawn to methylation analysis of ctDNA for multi-cancer screening16 and even the detection of intracranial tumors,17 combination with other analytes, such as proteins.18,19 Moreover, the ctDNA liquid biopsy approach has been shown to outperform tissue in the case of detecting acquired resistance and tumor heterogeneity in gastrointestinal cancers.20 Challenges with multi-cancer screening assays, however, are even greater, and implementations of machine learning that utilize input from millions of parameters will require extensive studies to validate their performance and clinical utility. In this process, there are risks of overfitting to hidden variables in the initial training and validation sets when applied to the general population. Even large screening studies pose the risk of not having sufficient patient numbers in each category when broken down to tumor type, age, race, ethnicity, and other known risk factors.12
CTC approach
Tumor cells extravasate from the primary site into the circulation through the vasculature and can disseminate to distant sites. CTCs are a minimally invasive source of tumor material and have long been a focus of liquid biopsy.21–24 Characterization of the CTCs is appealing since they are assumed to drive the disease process by nucleating metastatic events.25 However, CTCs are a heterogenous group of cells, and only a tiny fraction of CTCs appears capable of colonization and metastasis.26
Since CTCs are cellular analytes, they confer the advantage of containing RNA, DNA, and protein from the tumor cells, with a spatial resolution of biomarkers (e.g. nuclear versus cytoplasmic), which is unattainable from ctDNA or exosomes. CTC analysis has yielded important information on the metastatic process. It was shown, for example, that CD44-dependent aggregation of CTCs provides cancer stem-cell-like properties, and that these cell clusters have enhanced metastatic capabilities.27 A challenge of CTCs is that some cancers only release a limited number of these cells into the circulation28,29 and that they appear to be more suitable for the evaluation of late-stage diseases.25 Nevertheless, several detection technologies based on unique surface markers have been developed early on to harness their clinical potential. The CellSearch® System (Veridex, Huntington Valley, PA, 2004) was the first and remains the only FDA-cleared CTC analysis device30 to quantify CTCs in whole blood and serves as the benchmark for CTC detection technologies. Clinical studies have validated the prognostic value of CTC counts in patients with early and metastatic breast,24,31,32 metastatic castration-resistant prostate,33 and colon cancers.23 Molecular characterization of CTC content can be valuable for therapy selection. For example, an androgen receptor variant ARv7 confers resistance to androgenic treatment, and the detection of both ARv7 mRNA and protein in CTCs is linked with resistance to treatment in metastatic prostate cancer.34,35 However, despite these promising developments, CTCs as a platform continue to be limited by two main factors: their rarity and heterogeneity in the circulation.36 The definition of a CTC also varies across different studies, since not all of the cells isolated by a CTC platform are of tumor origin and carry the genetic aberrations associated with the tumor.30,37
OPPORTUNITIES IN EXOSOME-BASED LIQUID BIOPSY
Here, we review the state-of-the-art in exosome-based liquid biopsies, with a particular focus on the exciting opportunities as to how this field is contributing to a new generation of tools for cancer diagnosis, patient stratification, monitoring, and treatment decision-making, with the potential to enrich for tumor-derived exosomes based on surface markers (Figure 2). We will also review the data wherein a combination of two or more types of liquid biopsy analysis can be synergistic to improve clinical performance, especially as the field moves toward early-stage cancer detection where tumor-derived material is more limited in liquid biopsies.
Figure 2. Exosomes enable cancer specific enrichment.
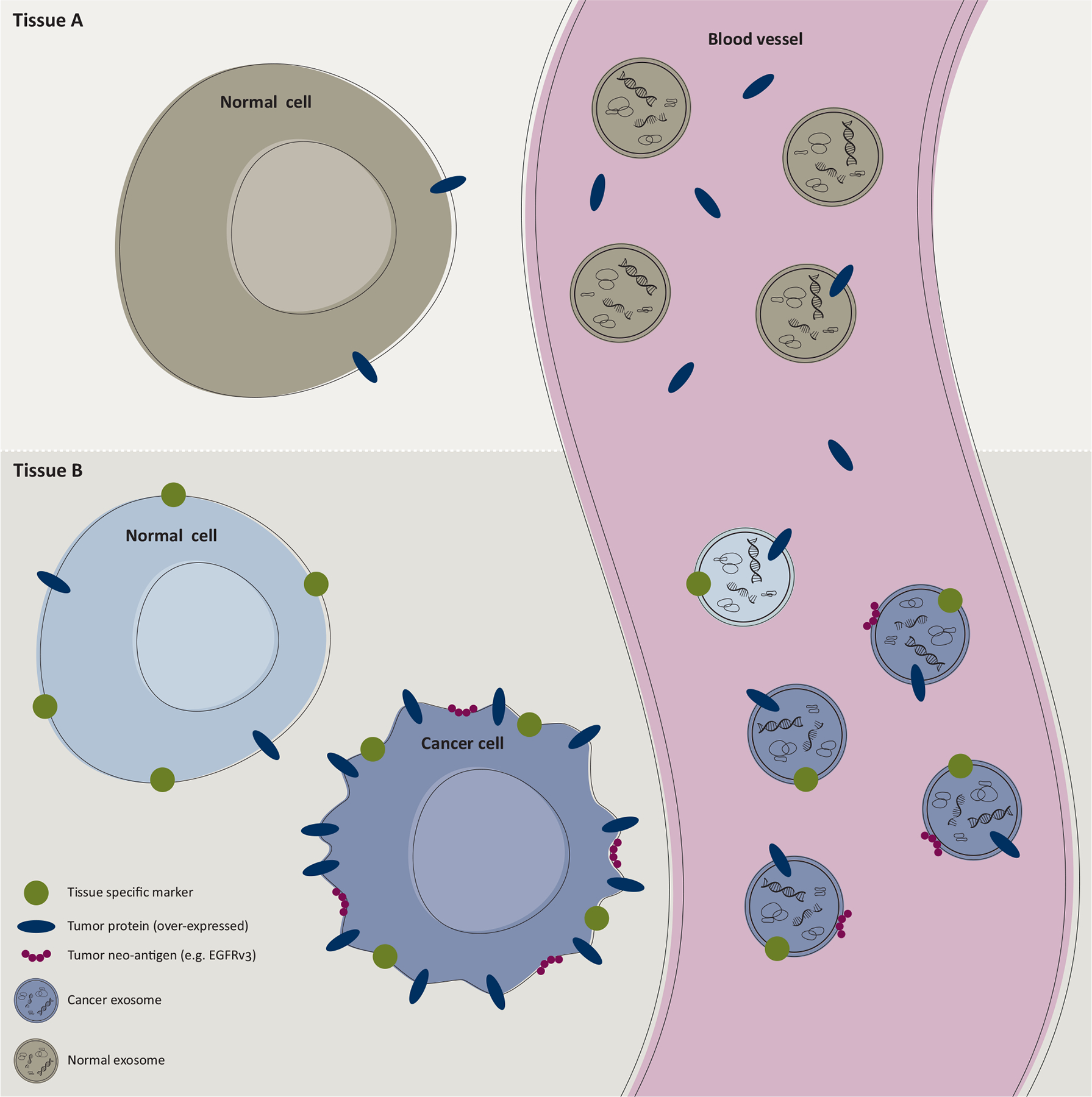
Proteins overexpressed in a tumor are not necessarily cancer-specific when isolated from the blood or other biofluid. Non-cancerous tissues can produce the same marker and generate a variable amount of the marker in normal individuals. By enriching exosomes with a tissue-specific (or cancer-specific) marker, higher sensitivity and/or specificity can be achieved.38 There are also known neoantigens (for example EGFRv3) that can be used to isolate cancer-specific exosomes.39,40
Exosomes and other EVs present an exciting alternative, and in some cases a complement, to other liquid biopsy forms for better overall diagnostic performances. Exosomes are released continuously by all living cells41 and provide broad opportunities for clinically relevant diagnostic content given that they contain DNA, RNA, and protein. Dying cells shed small pieces of ‘cell-free’ DNA (cfDNA) into circulation through apoptosis and necrosis, with a subfraction being DNA fragments from tumor cells as ctDNA. The stark contrast between how ctDNA and exosomes are released suggests that exosomes reveal information about living tumor cells and raises intriguing prospects for early lesion detection. For example, mutations in RNA revealed by exosomes can be added to the signal from the cfDNA to increase the sensitivity of mutation detection. More evidence is gathering that a multi-analyte approach will prove advantageous.18,42 Several studies have shown that combining cfDNA with exosomal RNA is advantageous over cfDNA analysis alone.43–45 In addition, the exosome component enables a combination of exosomal RNA, cfDNA, and disease-specific proteins as exemplified in Figure 3, but can also involve more complex analyses. The unique composition of the exosome compartment makes these vesicles particularly amenable for multi-analyte testing, since they carry cancer informative DNA, RNA, proteins, lipids, oligosaccharides, and metabolites.
Figure 3. Multi-analyte liquid biopsy approach utilizing exosomes for increased performance.
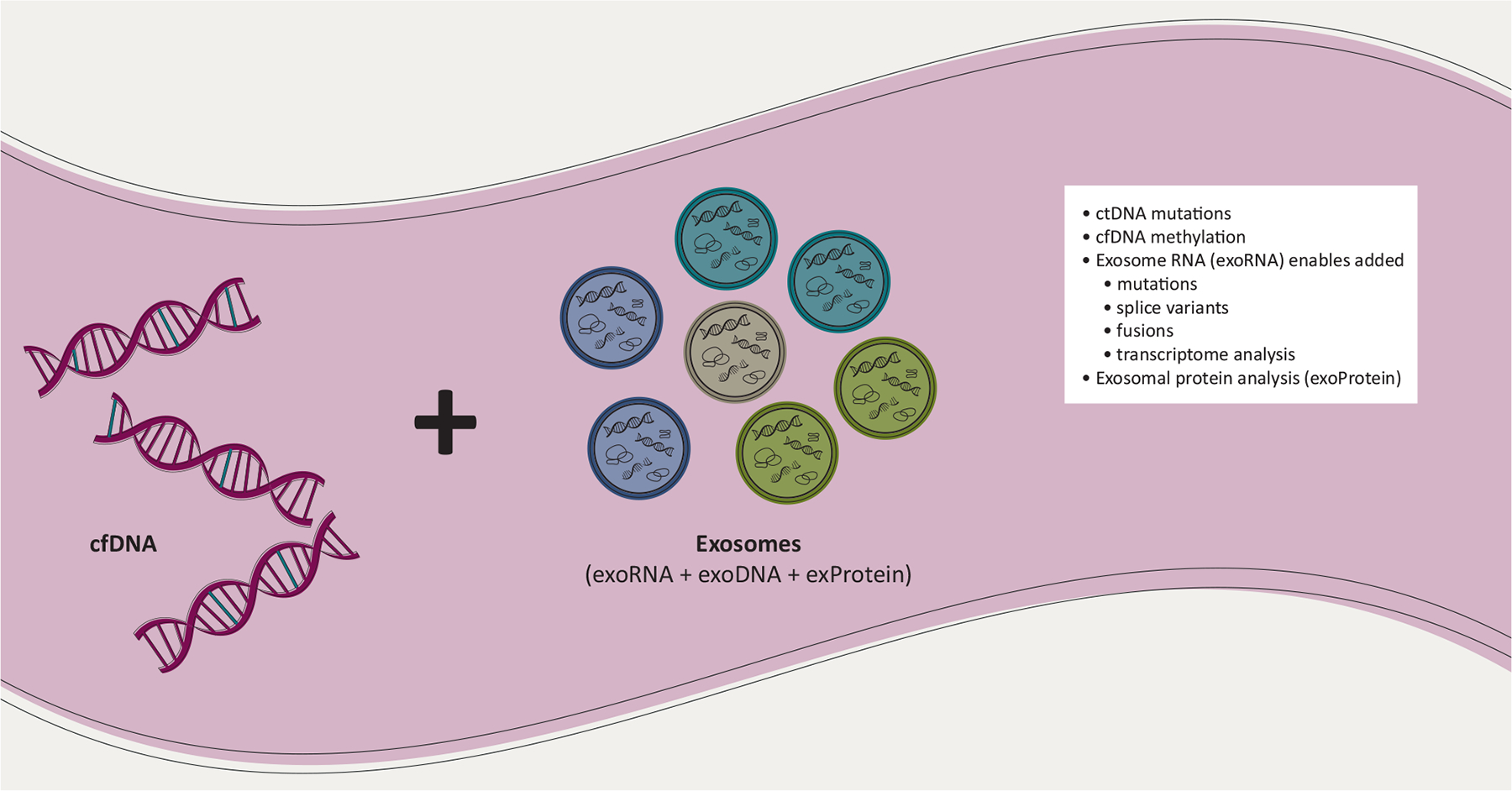
Combining features from cfDNA and exosomes have clear synergies to achieve increased liquid biopsy performance. The exosome fraction contains RNA and DNA (exoDNA) that give increased copy numbers of mutations43 and enable splice variant analysis of RNA,46–49 fusions,50,51 and transcriptome analysis.52,53 Combining the ctDNA mutations with cfDNA methylation and/or exosome analysis can enable a more complete picture of the tumor-derived biomarkers and potentially confer a better diagnostic performance. Exosomes from various sources (exemplified by the colors) can also be enriched to increase tissue specificity of the biomarker.
cfDNA, cell-free DNA; ctDNA, circulating tumor DNA.
Exosomes are nanoscale in size, with a majority being 30–200 nm in diameter. They are part of a broad class of EVs actively released into biofluids through either fusion with the plasma membrane and then exocytosis of multivesicular bodies, or direct budding of small cytoplasmic protrusion from the cell surface.54,55 The active shedding of vesicles from tumor cells is thought to involve the mitogen-activated protein kinase pathway which is up-regulated in most tumor cells.48,56–58 It has been reported that a cancer cell can release more than 20 000 of these vesicles over 48 h.59 Notably, there are also larger vesicles, large oncosomes, that can be up to 10 μm in diameter and also carry RNA, DNA, and protein from the donor cells.60–62 All these vesicles carry biological cargo, such as a variety of biomarkers from the tumor, including those involved in the tumorigenic process. Specifically, exosomes have been implicated in driving key attributes of malignant cell behavior, including stimulation of tumor cell growth, suppression of the immune response, induction of angiogenesis, promotion of tumor cell migration, and establishment of metastases,63 making them particularly attractive as cancer biomarkers. Indeed, the exosome field has experienced an explosion in research as summarized in literature numbers for the past 40 years (Figure 4A). Moreover, a snapshot of the current clinical trials database, ClinicalTrials. gov, reveals a torrent of clinical diagnostic and translational research studies of exosomes in cancers (72 studies, Figure 4B), remarkably outpacing areas such as exosomes in cardiovascular (seven studies), neurodegenerative/neuronal diseases (five studies), and diabetes (four studies). These are likely an underestimation since not all clinical studies are reported in this database.
Figure 4.
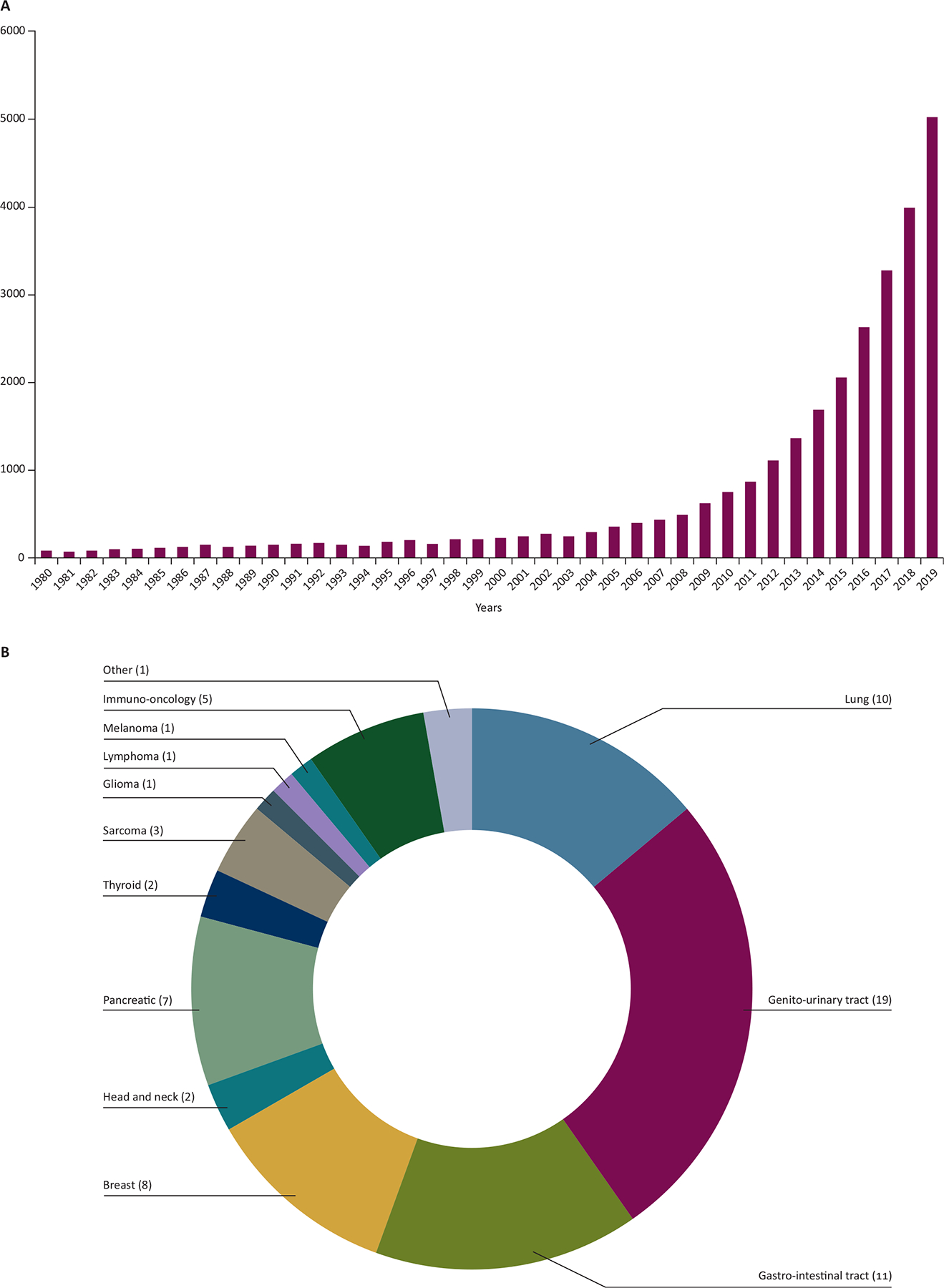
(A) Exosome-based research publications. The interest in exosomes for diagnostics, therapeutics and as an intricate part of intercellular communication has significantly increased in recent years. (B) Overview of a total of 71 exosome-based clinical trials registered worldwide in ClinicalTrials.gov (as of 22 October 2020) in diagnostics and translational biomarkers for various cancers, manually curated through searches with keywords (exosomes, extracellular vesicle, EVs, and microvesicles) with the elimination of both duplicate hits and those for therapeutic applications.
EXOSOMES PROVIDE CIRCULATING RNA BIOMARKERS
One development in the liquid biopsy field has been the characterization and analysis of extracellular RNA, propelled by various major funding mechanisms, including the Extracellular RNA Communication Consortium funded by the National Institutes of Health Common Fund. Extracellular RNA molecules are protected from degradation in the extracellular environment by forming ribonucleoprotein complexes, such as with Argonaute II (Ago2),64 associating with high-density lipoprotein/low-density lipoprotein particles,65 or being contained within exosomes and other EVs.41,66
An initial report in 2006 found RNA in exosomes from cell cultures,67 and a subsequent study by Skog et al.41 showed that tumor-derived mutations could be detected in exosomes isolated from plasma of patients, opening the opportunity to use these exosomes diagnostically. Exosomes mediate intercellular delivery of RNA and this was proposed as a novel medium of communication between cells.66 These and other studies showed that EVs are rich in small RNA (<200 nucleotides) molecules, with the majority of RNA <700 nucleotides, causing early investigations on circulating RNA to focus primarily on microRNAs (miRNAs),66,68–72 a fraction protected by ribonucleoprotein complexes as well as contained within EVs.
However, subsequent NGS-based studies on EV RNA reported that all RNA biotypes are contained within EVs, including messenger RNAs (mRNAs),41,67,73,74 mRNA fragments,75 long noncoding RNA (lncRNA),76,77 piwi-interacting RNA, and fragments of various noncoding RNAs, such as ribosomal RNA,76,78 transfer RNA, vault RNA, and Y RNAs in EVs.79–83 Whole transcriptome approaches, such as RNA sequencing (RNA-seq) are now being applied to interrogate the EV cargo of cerebrospinal fluid (CSF), plasma,84–86 and urine78 samples.
While the research space on clinical biomarkers seems currently dominated by miRNA studies, the biomarkers that have made it through validation and are used clinically are more typically mRNAs. The research interest in the miRNAs is mainly due to their abundance and known regulatory potential. However, from a liquid biopsy standpoint, the ‘low hanging fruit’ has been the longer RNA biotypes, especially the mRNA targets with known mutations and actionable alterations. In addition to the measurement of gene expression levels and detection of tumor-specific somatic alterations, long RNAs provide additional opportunities to study other processes that may indicate disease state/progression. For example, disease-specific detection of alternatively spliced isoforms of RNA (e.g. ARv7,48 MET exon 14 skipping49), fusion transcripts such as Echinoderm Microtubule-associated protein-Like 4 - Anaplastic Lymphoma Kinase (EML4-ALK)51 and BCR-ABL, RNA editing,87 and circular RNAs88 represent alternative approaches which may not be observable using DNA, small RNA, or protein assays. Previous studies employing RNA-seq to profile long RNAs have reported a relatively small proportion and poor transcript coverage of these long RNA transcripts which has led many to conclude that exosomes only carry short fragments of mRNA and lncRNA75,76,78 until recent improvements in exosomal long RNA-seq (sequencing of long RNA rather than long read sequencing).48,52,53
EXOSOMES IMPROVE MUTATION DETECTION SENSITIVITY FOR LIQUID BIOPSY
Circulating nucleic acids, including the RNA contained within exosomes, has been proven to increase the total number of mutant copies available for sampling compared with ctDNA alone.43 In one study, a direct, blind, comparative analysis of ctDNA alone versus exosomal RNA plus ctDNA found up to 10 times more copies of the mutation when combining exosomal RNA and ctDNA, compared with ctDNA alone (median of 24 copies/ml versus 234 copies/ml for EGFR activating mutations on ctDNA versus exoRNA + ctDNA, respectively).43 This substantially improves the odds of detecting the mutation in a blood sample and would be particularly beneficial in the earlier stages of disease where circulating copy numbers of ctDNA are very low. Another study showed a very high sensitivity (92%) of EGFR mutations utilizing combined exoRNA + ctDNA for detection of EGFR mutations, and the sensitivity remained high (88%) also in the subpopulation of intrathoracic lung cancer (M0/M1a) that have been challenging for ctDNA assays to detect.44 Moreover, a recent longitudinal study investigating levels of BRAF, KRAS, and EGFR mutations in exosomes and ctDNA over time demonstrated that the combined analysis significantly improved the correlation of biomarkers with treatment outcome as compared with ctDNA alone,45 highlighting that the interrogation of combined tumor materials from exosomes of living cells and cfDNA sourced from mostly dying cells could improve the likelihood of success for a liquid biopsy test.
EXOSOMES ENABLE ENRICHMENT OF DISEASE SIGNALS
As with other liquid biopsy approaches, differentiating the tumor signal from the ‘noise’ of normal cells remains a challenge when performing transcriptome analysis of RNA. This is particularly challenging when the RNA is directly extracted from plasma because of the sheer abundance of platelet-derived RNA that can give an overwhelming megakaryocyte RNA background that interferes with the analysis.89 However, the unique feature of exosomes is that they can be enriched from the biofluids using surface markers, and subpopulations of exosomes can be isolated with tumor-specific or tumor-enriched surface marker proteins. Indeed, multiple proof-of-concept studies have demonstrated the feasibility of this approach,90–92 including the enrichment of epithelial cell adhesion molecule (EpCAM)-positive tumor-derived exosomes from several types of cancer patients’ plasma.68,69 Enrichment of exosomal subpopulations is technically challenging and requires well-optimized protocols for its application, as antibodies that work well with tissues or purified proteins may not work efficiently with exosomes due to the orientation and/or folding of the surface protein in the membrane or the availability of the epitope on the exosome surface.93 Also, surface properties of exosomes render them capable of adhering to many surfaces including other exosomes. Nonspecific binding to the tube or bead surfaces used for immunological capture might result in loss of important biological material and reduction in specificity. Another emerging technology for disease/tissue-specific analysis of exosomes and other EVs is nano-flow cytometry. Although currently more suitable for lower throughput assays, this technique can sort/characterize individual EVs based on surface markers and has an immense potential to improve the performance of exosome-based liquid biopsies.94–96
Proteins located on the surface and within exosomes may also be used as cancer biomarkers. Several different protein detection or exosome capture methodologies may allow for discrimination of cancer types using various biofluids including plasma, serum, urine, saliva, CSF, and ascites. For detection of brain cancer using exosomes in plasma, biomarkers such as EGFRvIII have been successfully explored in brain cancer.39,97 Specific detection of HER2 as a breast cancer marker has also been widely reported by a few distinct methods including surface plasmon resonance,98 surface-enhanced Raman spectroscopy,99 and electrohydrodynamic micro shearing.100 Yoshioka et al.101 determined that surface markers CD147, carbohydrate antigen 19-9 (CA19-9), and carcinoembryonic antigen were specifically detected in the serum of patients with colorectal cancer using ExoScreen, a bead-based fluorescence detection system with signal enrichment. Another fluorescence-based microchip method was used to detect exosomes specifically in lung cancer patients using antibodies directed against α-insulin-like growth factor 1 receptor and EpCAM, and in ovarian cancer using EpCAM and CA125, respectively.102 Specific enrichment of exosomes containing ovarian cancer biomarkers has also been achieved using several different enrichment/detection methods for CA125, CA19-9, CD24, CLDN3, EpCAM, HER2, and MUC18.103 Lastly, prostate-specific antigen (PSA) in plasma exosomes can distinguish prostate cancer from benign hyperplasia.104 Protein detection technology has only recently improved in sensitivity to the single molecule level,105,106 and the proteomic landscape of EVs in cancers, such as prostate cancer is just beginning to be unraveled.107 These advances together with clinical understanding of cancer biology, could allow non-invasive protein biomarkers to fulfill their clinical promise.
ADVANCES AND CHALLENGES IN EXOSOME-BASED LIQUID BIOPSY
The first commercial exosome-based ExoDx™ Prostate (IntelliScore) (EPI) test became available for prostate cancer in 2016.108,109 This non-invasive urine test utilizes a novel expression signature of three exosomal RNA transcripts (ERG, PCA3, and SPDEF) for the risk management of men over 50 years of age with a PSA level in the ‘grey-zone’ of 2–10 ng/ml. The test gives a score from 0 to 100 where the risk of high-grade (Gleason score 7 and higher) increases with a higher score. The test was validated at a cut point of 15.6 to rule out high-grade prostate cancer (91% negative predictive value), such that it would avoid 27% of biopsies with potentially harmful procedural consequences. The test performance at this cut point has been validated in two prospective multicenter trials. A recent prospective utility study with a blinded control arm further found that doctors that used the EPI test in a real-world setting found 30% more high-grade prostate cancer than the randomized control arm that used the current best standard of care for the biopsy decision. This further highlights the utility of exosome-derived RNA biomarkers for early cancer detection. To date, this exosome-based test has been used by >50 000 patients in their decision process and is included in the National Comprehensive Cancer Network guidelines for early prostate cancer detection.110
Exosomes are known to also carry DNA, and the circulating DNA associated with exosomes has been shown to represent tumor DNA in a variety of cancer types.62,111–113 Cancer biomarkers of potential clinical utilities using exosome-derived DNA have been on the rise, encompassing genomic rearrangements,114 mutations,113 and copy number changes.115
In addition to nucleic acids and proteins, lipids and glycans are emerging as potential biomarkers with studies of lipid biomarkers related to prostate cancer.116–118 New technologies for exosome analysis are also being developed to move exosome-based assays into more portable point-of-care devices. Microfluidic devices and other small portable tools are now being employed for molecular analysis of exosomes,119,120 and technologies such as transmission surface plasmon resonance have already shown promise to analyze ovarian cancer exosome biomarkers.103
While there has been much effort in developing liquid biopsy in oncology, which is the focus of this article, studies in transplant rejection,121,122 cardiovascular diseases,123 neurodegenerative disorders,124,125 fetal or prenatal diagnostics,126 and immunological diseases127,128 have expanded the areas of research for circulating biomarkers that could be used in liquid biopsies. The application of liquid biopsy to neurodegenerative disease is intrinsically linked with oncology with respect to brain tumors, where the obstacles of sample collection are clear. EVs have been observed to cross an intact blood-brain barrier129 and thus can be used for developing tests for neurodegenerative disorders, which is a less invasive procedure than CSF sampling that can have problematic side-effects, especially for patients with a high intracranial pressure.
Clinical studies conducted using liquid biopsy platforms, similar to other clinical endeavors, are often costly and time-consuming. One advantage of cfDNA and exosomes is that they are stable enough to be analyzed retrospectively from frozen bio-banked samples. Exosome nucleic acid analysis does not require preservatives for collection and plasma can, for example, be stored for 2 days at 25°C or processed through many freeze-thaw cycles with no significant degradation of DNA or RNA when the sample is subsequently processed.130 Evidence exists that exosome RNA transcriptome profiling can be successfully carried out from plasma samples stored for more than 25 years at −80°C (unpublished data). As diagnostic analytes, RNA, DNA, or protein cargo in exosomes hold this informative profile stable during sample shipping and storage, which is critical for biomarker development using retrospective samples. Together with recent revelations that clinical studies based on exosomal RNA combined with cfDNA, in fact, made tests more clinically effective in sensitivity and specificity relative to either exosome RNA or cfDNA alone, the exosome-based liquid biopsy approach possesses some clinically fundamental advantages. Not all cancer characteristics are reflected in DNA mutations. There are currently no cfDNA mutation assays that have been able to discriminate clinically significant prostate cancer (Gleason 7 and higher) from benign and low-grade prostate cancer. However, a three-gene RNA signature from urine exosomes has shown good performance to do just that.108,109 Another proposed liquid biopsy platform utilizes RNA profiling of platelets from plasma,131 and its validity and clinical utility remain to be seen.
Despite the plethora of research in oncology biomarkers, only a few of them make it through validation to a commercial test. From a test developer’s viewpoint, it is necessary that not only the proper controls be conducted, but also the study population must be designed to ensure the biomarkers can be generalized to the intended use population. This is not trivial because many factors can affect biomarker performance, including age, ethnicity, environmental factors, and pre-existing conditions, as well as all the pre-analytical variables associated with sample collections.
CONCLUSION
Considerable progress has been made in the field of oncology liquid biopsy, and the number of tests available to clinicians is growing. While the obstacles to general adoption are not trivial, the promise of liquid biopsies is undeniable since they offer a number of advantages in addressing issues related to conventional biopsies. One example of challenges that rely solely on mutation-based assays is the issue of CHIP, as well as the potential detection of the large numbers of indolent cancers that are better left undiagnosed.14
The non-invasive nature and real-time assessment of the tumor’s molecular status make this type of diagnostic more readily available and can also be used to track patients over time to better monitor potential disease progression and therapeutic intervention. With these prospects, exosomes offer a unique biomarker content that can be used alone or in combination with other types of liquid biopsies. Increasingly sophisticated technologies for the analysis of tumor-derived exosomes should bring to the forefront transformational, robust, next-generation content-rich cancer diagnostics.
ACKNOWLEDGEMENTS
The authors thank Jason Alter for insightful edits and Eirini Tsilioni for the assistance in references.
FUNDING
This work was supported partly by the National Institutes of Health/National Cancer Institute [CA069246, CA232103 and CA229777] to XOB.
Footnotes
DISCLOSURE
WY, JH, DR, SC, DE, MN, and JS are employees of Exosome Diagnostics, a Bio-Techne brand.
REFERENCES
- 1.Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502: 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481:506–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driessens G, Beck B, Caauwe A, et al. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell PJ, Yachida S, Mudie LJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467: 1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navin N, Kendall J, Troge J, et al. Tumour evolution inferred by single-cell sequencing. Nature. 2011;472:90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lokhandwala T, Bittoni MA, Dann RA, et al. Costs of diagnostic assessment for lung cancer: a medicare claims analysis. Clin Lung Cancer. 2017;18:e27–e34. [DOI] [PubMed] [Google Scholar]
- 8.Leighl NB, Page RD, Raymond VM, et al. Clinical utility of comprehensive cell-free DNA analysis to identify genomic biomarkers in patients with newly diagnosed metastatic non-small cell lung cancer. Clin Cancer Res. 2019;25:4691–4700. [DOI] [PubMed] [Google Scholar]
- 9.FDA. Summary of Safety and Effectiveness Data (SSED) P150047. cobas® EGFR Mutation Test v2. 2016.
- 10.FDA. Summary of Safety and Effectiveness Data (SSED) P200010. Guardant360® CDx. 2020.
- 11.FDA. Summary of Safety and Effectiveness Data (SSED) P190032. FoundationOne® Liquid CDx. 2020.
- 12.Haque IS, Elemento O. Challenges in using ctDNA to achieve early detection of cancer. bioRxiv. 2017;237578. [Google Scholar]
- 13.Bauml J, Levy B. Clonal hematopoiesis: a new layer in the liquid biopsy story in lung cancer. Clin Cancer Res. 2018;24:4352–4354. [DOI] [PubMed] [Google Scholar]
- 14.Jahn JL, Giovannucci EL, Stampfer MJ. The high prevalence of undiagnosed prostate cancer at autopsy: implications for epidemiology and treatment of prostate cancer in the prostate-specific antigen-era. Int J Cancer. 2015;137:1749–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phallen J, Sausen M, Adleff V, et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci Transl Med. 2017;9: eaan2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu MC, Oxnard GR, Klein EA, et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol. 2020;31:745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nassiri F, Chakravarthy A, Feng S, et al. Detection and discrimination of intracranial tumors using plasma cell-free DNA methylomes. Nat Med. 2020;26:1044–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen JD, Li L, Wang Y, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. 2018;359: 926–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chalasani NP, Ramasubramanian TS, Bhattacharya A, et al. A novel blood-based panel of methylated DNA and protein markers for detection of early-stage hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2020;S1542–3565:31224–31226. [DOI] [PubMed] [Google Scholar]
- 20.Parikh AR, Leshchiner I, Elagina L, et al. Liquid versus tissue biopsy for detecting acquired resistance and tumor heterogeneity in gastrointestinal cancers. Nat Med. 2019;25:1415–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bidard FC, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15:406–414. [DOI] [PubMed] [Google Scholar]
- 22.Clifton GT, Sears AK, Patil R, et al. Monitoring of circulating tumor cell trends in a prospective, randomized, placebo-controlled HER2/neu peptide vaccine trial. J Clin Oncol. 2011;29:e11126. [Google Scholar]
- 23.Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26: 3213–3221. [DOI] [PubMed] [Google Scholar]
- 24.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. [DOI] [PubMed] [Google Scholar]
- 25.Castro-Giner F, Aceto N. Tracking cancer progression: from circulating tumor cells to metastasis. Genome Med. 2020;12:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodrigues P, Vanharanta S. Circulating tumor cells: come together, right now, over metastasis. Cancer Discov. 2018;9:22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Taftaf R, Kawaguchi M, et al. Homophilic CD44 interactions mediate tumor cell aggregation and polyclonal metastasis in patient-derived breast cancer models. Cancer Discov. 2019;9:96–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dawson S-J, Tsui DW, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368(13):1199–1209. [DOI] [PubMed] [Google Scholar]
- 29.Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.FDA. 510(k) Summary. CellSearch™ Circulating tumor cell kit. 2007.
- 31.Zhang L, Riethdorf S, Wu G, et al. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res. 2012;18:5701–5710. [DOI] [PubMed] [Google Scholar]
- 32.Riethdorf S, Fritsche H, Müller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13: 920–928. [DOI] [PubMed] [Google Scholar]
- 33.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. [DOI] [PubMed] [Google Scholar]
- 34.Antonarakis ES, Lu C, Luber B, et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015;1:582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scher HI, Jia X, de Bono JS, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10:233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossi E, Fabbri F. CTCs 2020: great expectations or unreasonable dreams. Cells. 2019;8:989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marchetti A, Del Grammastro M, Felicioni L, et al. Assessment of EGFR mutations in circulating tumor cell preparations from NSCLC patients by next generation sequencing: toward a real-time liquid biopsy for treatment. PLoS One. 2014;9:e103883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foroni C, Zarovni N, Bianciardi L, et al. When less is more: specific capture and analysis of tumor exosomes in plasma increases the sensitivity of liquid biopsy for comprehensive detection of multiple androgen receptor phenotypes in advanced prostate cancer patients. Biomedicines. 2020;8:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Nedawi K, Meehan B, Micallef B, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–624. [DOI] [PubMed] [Google Scholar]
- 40.Manda SV, Kataria Y, Tatireddy BR, et al. Exosomes as a biomarker platform for detecting epidermal growth factor receptor-positive high-grade gliomas. J Neurosurg. 2018;128:1091–1101. [DOI] [PubMed] [Google Scholar]
- 41.Skog J, Würdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Limaye S, Patil D, Akolkar DB, et al. Multi-analyte liquid biopsies based treatment in advanced refractory cancers. J Clin Oncol. 2020;38:e15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krug AK, Enderle D, Karlovich C, et al. Improved EGFR mutation detection using combined exosomal RNA and circulating tumor DNA in NSCLC patient plasma. Ann Oncol. 2018;29:2143. [DOI] [PubMed] [Google Scholar]
- 44.Castellanos-Rizaldos E, Grimm DG, Tadigotla V, et al. Exosome-based detection of EGFR T790M in plasma from non-small cell lung cancer patients. Clin Cancer Res. 2018;24:2944–2950. [DOI] [PubMed] [Google Scholar]
- 45.Möhrmann L, Huang HJ, Hong DS, et al. Liquid biopsies using plasma exosomal nucleic acids and plasma cell-free DNA compared with clinical outcomes of patients with advanced cancers. Clin Cancer Res. 2018;24:181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Figueroa JM, Skog J, Akers J, et al. Detection of wild-type EGFR amplification and EGFRvIII mutation in CSF-derived extracellular vesicles of glioblastoma patients. Neuro Oncol. 2017;19:1494–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nimir M, Ma Y, Jeffreys SA, et al. Detection of AR-V7 in liquid biopsies of castrate resistant prostate cancer patients: a comparison of AR-V7 analysis in circulating tumor cells, circulating tumor RNA and exosomes. Cells. 2019;8:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Del Re M, Biasco E, Crucitta S, et al. The detection of androgen receptor splice variant 7 in plasma-derived exosomal RNA strongly predicts resistance to hormonal therapy in metastatic prostate cancer patients. Eur Urol. 2017;71:680–687. [DOI] [PubMed] [Google Scholar]
- 49.Cortot AB, Kherrouche Z, Descarpentries C, et al. Exon 14 deleted MET receptor as a new biomarker and target in cancers. J Natl Cancer Inst. 2017;109:djw262. [DOI] [PubMed] [Google Scholar]
- 50.Nilsson J, Skog J, Nordstrand A, et al. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer. 2009;100:1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reclusa P, Laes J-F, Malapelle U, et al. EML4-ALK translocation identification in RNA exosomal cargo (ExoALK) in NSCLC patients: a novel role for liquid biopsy. Transl Cancer Res. 2019;8:S76–S78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodosthenous RS, Hutchins E, Reiman R, et al. Profiling extracellular long RNA transcriptome in human plasma and extracellular vesicles for biomarker discovery. iScience. 2020;23:101182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Everaert C, Helsmoortel H, Decock A, et al. Performance assessment of total RNA sequencing of human biofluids and extracellular vesicles. Sci Rep. 2019;9:17574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. [DOI] [PubMed] [Google Scholar]
- 55.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agarwal K, Saji M, Lazaroff SM, et al. Analysis of exosome release as a cellular response to MAPK pathway inhibition. Langmuir. 2015;31: 5440–5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cha DJ, Franklin JL, Dou Y, et al. KRAS-dependent sorting of miRNA to exosomes. Elife. 2015;4:e07197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dou Y, Cha DJ, Franklin JL, et al. Circular RNAs are down-regulated in KRAS mutant colon cancer cells and can be transferred to exosomes. Sci Rep. 2016;6:37982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balaj L, Lessard R, Dai L, et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011;2:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Vizio D, Kim J, Hager MH, et al. Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res. 2009;69:5601–5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Minciacchi VR, You S, Spinelli C, et al. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget. 2015;6:11327–11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vagner T, Spinelli C, Minciacchi VR, et al. Large extracellular vesicles carry most of the tumour DNA circulating in prostate cancer patient plasma. J Extracell Vesicles. 2018;7:1505403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu R, Rai A, Chen M, et al. Extracellular vesicles in cancer – implications for future improvements in cancer care. Nat Rev Clin Oncol. 2018;15:617–638. [DOI] [PubMed] [Google Scholar]
- 64.Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vickers KC, Palmisano BT, Shoucri BM, et al. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. [DOI] [PubMed] [Google Scholar]
- 67.Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, et al. Tumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol Immunother. 2006;55:808–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. [DOI] [PubMed] [Google Scholar]
- 69.Rabinowits G, Gerçel-Taylor C, Day JM, et al. Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer. 2009;10: 42–46. [DOI] [PubMed] [Google Scholar]
- 70.Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles. 2014;3:22743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pigati L, Yaddanapudi SC, Iyengar R, et al. Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS One. 2010;5:e13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bruno S, Grange C, Collino F, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7:e33115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deregibus MC, Cantaluppi V, Calogero R, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–2448. [DOI] [PubMed] [Google Scholar]
- 75.Batagov AO, Kurochkin IV. Exosomes secreted by human cells transport largely mRNA fragments that are enriched in the 3′-untranslated regions. Biol Direct. 2013;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang X, Yuan T, Tschannen M, et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013;14:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kogure T, Yan IK, Lin WL, Patel T. Extracellular vesicle-mediated transfer of a novel long noncoding RNA TUC339: a mechanism of intercellular signaling in human hepatocellular cancer. Genes Cancer. 2013;4:261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miranda KC, Bond DT, Levin JZ, et al. Massively parallel sequencing of human urinary exosome/microvesicle RNA reveals a predominance of non-coding RNA. PLoS One. 2014;9:e96094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bellingham SA, Coleman BM, Hill AF. Small RNA deep sequencing reveals a distinct miRNA signature released in exosomes from prion-infected neuronal cells. Nucleic Acids Res. 2012;40:10937–10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dhahbi JM, Spindler SR, Atamna H, et al. Deep sequencing of serum small RNAs identifies patterns of 5′ tRNA half and yRNA fragment expression associated with breast cancer. Biomark Cancer. 2014;6:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dhahbi JM, Spindler SR, Atamna H, et al. 5′-YRNA fragments derived by processing of transcripts from specific YRNA genes and pseudogenes are abundant in human serum and plasma. Physiol Genomics. 2013;45:990–998. [DOI] [PubMed] [Google Scholar]
- 82.Freedman JE, Gerstein M, Mick E, et al. Diverse human extracellular RNAs are widely detected in human plasma. Nat Commun. 2016;7: 11106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nolte-’t Hoen EN, Buermans HP, Waasdorp M, et al. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012;40:9272–9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saugstad JA, Lusardi TA, Van Keuren-Jensen KR, et al. Analysis of extracellular RNA in cerebrospinal fluid. J Extracell Vesicles. 2017;6: 1317577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Conley A, Minciacchi VR, Lee DH, et al. High-throughput sequencing of two populations of extracellular vesicles provides an mRNA signature that can be detected in the circulation of breast cancer patients. RNA Biol. 2017;14:305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei Z, Batagov AO, Schinelli S, et al. Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat Commun. 2017;8:1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Picardi E, D’Erchia AM, Gallo A, et al. Uncovering RNA editing sites in long non-coding RNAs. Front Bioeng Biotechnol. 2014;2:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barrett SP, Salzman J. Circular RNAs: analysis, expression and potential functions. Development. 2016;143:1838–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brinkman K, Meyer L, Bickel A, et al. Extracellular vesicles from plasma have higher tumour RNA fraction than platelets. J Extracell Vesicles. 2020;9:1741176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oksvold MP, Neurauter A, Pedersen KW. Magnetic bead-based isolation of exosomes. Methods Mol Biol. 2015;1218:465–481. [DOI] [PubMed] [Google Scholar]
- 91.Tauro BJ, Greening DW, Mathias RA, et al. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol Cell Proteomics. 2013;12:587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Laulagnier K, Motta C, Hamdi S, et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J. 2004;380:161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zaborowski MP, Cheah PS, Zhang X, et al. Membrane-bound Gaussia luciferase as a tool to track shedding of membrane proteins from the surface of extracellular vesicles. Sci Rep. 2019;9:17387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tiana Y, Gonga M, Hua Y, et al. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J Extracell Vesicles. 2019;9:1697028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Choi D, Montermini L, Jeong H, et al. Mapping subpopulations of cancer cell-derived extracellular vesicles and particles by nano-flow cytometry. ACS Nano. 2019;13:10499–10511. [DOI] [PubMed] [Google Scholar]
- 96.Jones PS,Yekula A, Lansbury E, et al. Characterization of plasma-derived protoporphyrin-IX-positive extracellular vesicles following 5-ALA use in patients with malignant glioma. EBioMedicine. 2019;48:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Choi D, Montermini L, Kim DK, et al. The impact of oncogenic EGFRvIII on the proteome of extracellular vesicles released from glioblastoma cells. Mol Cell Prot. 2018;17:1948–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grasso L, Wyss R, Weidenauer L, et al. Molecular screening of cancer-derived exosomes by surface plasmon resonance spectroscopy. Anal Bioanal Chem. 2015;407:5425–5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kwizera EA, O’Connor R, Vinduska V, et al. Molecular detection and analysis of exosomes using surface-enhanced raman scattering gold nanorods and a miniaturized device. Theranostics. 2018;8:2722–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vaidyanathan R, Naghibosadat M, Rauf S, et al. Detecting exosomes specifically: a multiplexed device based on alternating current electrohydrodynamic induced nanoshearing. Anal Chem. 2014;86:11125–11132. [DOI] [PubMed] [Google Scholar]
- 101.Yoshioka Y, Kosaka N, Konishi Y, et al. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat Commun. 2014;5:3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao Z, Yang Y, Zeng Y, He M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip. 2016;16:489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Im H, Shao H, Park YI, et al. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat Biotechnol. 2014;32:490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Logozzi M, Angelini DF, Giuliani A, et al. Increased plasmatic levels of PSA-expressing exosomes distinguish prostate cancer patients from benign prostatic hyperplasia: a prospective study. Cancers (Basel). 2019;11:1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li W, Bell NA, Hernández-Ainsa S, et al. Single protein molecule detection by glass nanopores. ACS Nano. 2013;7:4129–4134. [DOI] [PubMed] [Google Scholar]
- 106.Schubert SM, Arendt LM, Zhou W, et al. Ultra-sensitive protein detection via single molecule arrays towards early stage cancer monitoring. Sci Rep. 2015;5:11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dhondt B, Geeurickx E, Tulkens J, et al. Unravelling the proteomic landscape of extracellular vesicles in prostate cancer by density-based fractionation of urine. J Extracell Vesicles. 2020;9:1736935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McKiernan J, Donovan MJ, O’Neill V, et al. A novel urine exosome gene expression assay to predict high-grade prostate cancer at initial biopsy. JAMA Oncol. 2016;2:882–889. [DOI] [PubMed] [Google Scholar]
- 109.McKiernan J, Donovan MJ, Margolis E, et al. A prospective adaptive utility trial to validate performance of a novel urine exosome gene expression assay to predict high-grade prostate cancer in patients with prostate-specific antigen 2–10 ng/ml at initial biopsy. Eur Urol. 2018;74:731–738. [DOI] [PubMed] [Google Scholar]
- 110.National Comprehensive Cancer Network. Prostate Cancer EarlyDetection, (Version 2.2020). Available at: https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/PDF/prostate_detection.pdf. Accessed December 4, 2020.
- 111.Kahlert C, Melo SA, Protopopov A, et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem. 2014;289:3869–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thakur BK, Zhang H, Becker A, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24:766–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.San Lucas FA, Allenson K, Bernard V, et al. Minimally invasive genomic and transcriptomic profiling of visceral cancers by next-generation sequencing of circulating exosomes. Ann Oncol. 2015;27:635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Oldrini B, Vaquero-Siguero N, Mu Q, et al. MGMT genomic rearrangements contribute to chemotherapy resistance in gliomas. Nat Commun. 2020;11:3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee DH, Yoon H, Park S, et al. Urinary exosomal and cell-free DNA detects somatic mutation and copy number alteration in urothelial carcinoma of bladder. Sci Rep. 2018;8:14707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dusoswa SA, Horrevorts SK, Ambrosini M, et al. Glycan modification of glioblastoma-derived extracellular vesicles enhances receptor-mediated targeting of dendritic cells. J Extracell Vesicles. 2019;8: 1648995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scott E, Munkley J. Glycans as biomarkers in prostate cancer. Int J Mol Sci. 2019;20:1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Skotland T, Ekroos K, Kauhanen D, et al. Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur J Cancer. 2017;70:122–132. [DOI] [PubMed] [Google Scholar]
- 119.Myung JH, Hong S. Microfluidic devices to enrich and isolate circulating tumor cells. Lab Chip. 2015;15:4500–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Contreras-Naranjo JC, Wu H, Ugaz VM. Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine. Lab Chip. 2017;17:3558–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gregson AL, Hoji A, Injean P, et al. Altered exosomal RNA profiles in bronchoalveolar lavage from lung transplants with acute rejection. Am J Respir Crit Care Med. 2015;192:1490–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sigdel TK, Ng YW, Lee S, et al. Perturbations in the urinary exosome in transplant rejection. Front Med (Lausanne). 2014;1:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Das S, Halushka MK. Extracellular vesicle microRNA transfer in cardiovascular disease. Cardio Path. 2015;24:199–206. [DOI] [PubMed] [Google Scholar]
- 124.Stern RA, Tripodis Y, Baugh CM, et al. Preliminary study of plasma exosomal tau as a potential biomarker for chronic traumatic encephalopathy. J Alzheimers Dis. 2016;51:1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vella LJ, Hill AF, Cheng L. Focus on extracellular vesicles: exosomes and their role in protein trafficking and biomarker potential in Alzheimer’s and Parkinson’s disease. Int J Mol Sci. 2016;17:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Graham EM, Burd I, Everett AD, Northington FJ. Blood biomarkers for evaluation of perinatal encephalopathy. Front Pharmacol. 2016;7: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Coras R, Narasimhan R, Guma M. Liquid biopsies to guide therapeutic decisions in rheumatoid arthritis. Transl Res. 2018;201:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huang Y, Li R, Ye S, et al. Recent advances in the use of exosomes in Sjögren’s Syndrome. Front Immunol. 2020;11:1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Matsumoto J, Stewart T, Banks WA, et al. The transport mechanism of extracellular vesicles at the blood-brain barrier. Curr Pharm Des. 2017;23:6206–6214. [DOI] [PubMed] [Google Scholar]
- 130.Enderle D, Spiel A, Coticchia CM, et al. Characterization of RNA from exosomes and other extracellular vesicles isolated by a novel spin column-based method. PLoS One. 2015;10:e0136133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Best MG, Sol N, Kooi I, et al. RNA-seq of tumor-educated platelets enables blood-based pan-cancer, multiclass, and molecular pathway cancer diagnostics. Cancer Cell. 2015;28:666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]


