Abstract
Recent studies from high-risk countries such as the US, Denmark, and Ireland have shown rising incidence rates of hormone receptor (HR)-positive and falling rates of HR-negative breast cancers (BC). However, it remains unclear whether a similar pattern occurs in low-risk countries. Detailed clinical and risk factor data were collected from 2,977 female invasive BC patients (≥20 years) in Sarawak General Hospital, Malaysia, representing 93% of the population. The population-at-risk was obtained from Department of Statistics Malaysia. Secular trends in age-standardized incidence rates were assessed using estimated average annual percent changes. Associations between established BC risk factors and tumor subtypes defined by HR or joint HR/HER2 (human epidermal growth factor receptor 2) status were examined by case-case comparisons using logistic regression. From 2006-2015, incidence rates increased for HR-positive cancers by 4.46%/year (95%CI=2.19 to 6.78) and decreased for HR-negative cancers by 2.29%/year (95%CI=−4.31 to −0.24). When further stratified by HER2, the most contrasting difference in linear trends was observed between HR+/HER2− and HR−/HER2− subtypes. After controlling for potential confounders, cases with excess body weight (ORoverweight vs. normal=0.82; 95%CI=0.69-0.98; ORobese vs. normal=0.62; 95%CI=0.48-0.80), later age at first birth (OR≥26 years vs. <23 years=0.82; 95%CI=0.66-1.02), nulliparity (ORnulliparous vs. <23 years=0.74; 95%CI=0.59-0.94), and never-breastfeeding (ORnever vs. ever =0.73; 95%CI=0.55-0.97) were less frequent among HR-negative cases than among HR-positive cases. Diverging incidence trends by HR expression were similar in Sarawak and Western countries, possibly reflecting changes in the prevalence of risk factors with opposing effects by tumor subtypes in low- and high-risk populations.
Keywords: Breast cancer, Incidence trend, Hormone receptor positive cancer, Hormone receptor negative cancer, Malaysia
Introduction
Breast cancer subtypes defined by hormone receptor (HR) status are thought to be associated with different etiologic pathways.1, 2 Therefore, temporal changes in the prevalence of risk factors are likely to have different impact on incidence rates of breast cancer by tumor subtypes. However, subtype-specific incidence trends of breast cancer are largely unknown, except for those reported in a few high-income Western countries. Studies conducted in the United States (US),3, 4 Denmark,5 and Ireland6 demonstrated that rates for HR-positive cancers have been increasing, whereas rates for HR-negative cancers have been decreasing during 1980-2004., likely reflecting changes in mammography screening as well as risk factors that have heterogenous effects on HR-positive and HR-negative cancers.
Compared to high-income Western countries, incidence rates of breast cancer are still substantially lower in most transitioning countries. In 2018, incidence rate in Malaysia was 47.5 per 100,000 women compared to 84.9 in the US and 90.3 in Ireland.7 Nevertheless, breast cancer incidence rates have been rapidly increasing in most traditionally low-risk countries, presumptively due to the adoption of a Westernized lifestyle, and breast cancer is now the most frequently diagnosed female cancer in Malaysia (33%) and in South-East Asia (26.4%).7 Sarawak is the largest state in Malaysia. Based on the 2015 census data, the population was 2,636,000, approximately 60% of which residing in urban areas and consisting predominantly of Malays and Chinese, and a small population of urban natives of Ibans and Bidayuhs.8 We previously reported that women in Sarawak were at lower risks for all breast cancer subtypes defined by HR or joint HR/human epidermal growth factor receptor 2 (HER2) status compared with their counterparts in the US.9 However, secular trends in subtype-specific rates have not been reported in Sarawak or in any low-risk populations. In this study, we further examined trends in subtype-specific incidence rates of breast cancer in Sarawak. To identify risk factors that potentially account for the observed trends, we also extended our previous analysis that compared distributions of well-established breast cancer risk factors by tumor subtypes in this larger study including more patients with extended study period.10, 11
Material and Methods
Study population
Breast cancer cases were diagnosed and treated in the Department of Radiotherapy and Oncology of the Sarawak General Hospital, where about 93% of all newly diagnosed breast cancer cases in Sarawak, Malaysia, are treated.10 We included 2,977 cases (≥20 years) who were diagnosed with invasive breast cancer between 2003 and 2015. Demographic factors and breast cancer risk factors were collected using a standardized questionnaire at the time of breast cancer diagnosis, including age at diagnosis, height and weight for the calculation of body mass index (BMI), family history of breast cancer among first-degree relatives, age at menarche, age at first birth, parity (yes or no), number of children, and breastfeeding (yes or no; cumulative duration in month). Clinical characteristics of cancers were extracted from pathology reports and medical records, which included TNM stage (0, I, II, III, IV), grade (well differentiated, moderately differentiated, poorly differentiated), and expression status of three tumor makers including estrogen receptor (ER), progesterone receptor (PR), and HER2. ER and PR expression were assessed with immunohistochemical (IHC) staining of formalin-fixed, paraffin-embedded tissue sections, while HER2 expression was measured by IHC and fluorescence in situ hybridization (FISH, for HER2 IHC 2+ or 3+).10 Details on the reliability of IHC staining were described previously.9 We defined HR-positive (HR+) cancers as ER-positive or PR-positive; HR-negative (HR−) as ER-negative and PR-negative; and remaining as HR-unknown. Subtypes by joint HR/HER2 status were defined as: HR+/HER2−, HR+/HER2+, HR−/HER2+, and HR−/HER2−.12-14 The study protocol was approved by the National Ethics Committee, Ministry of Health of Malaysia and exempted from review by the National Institutes of Health Office of Human Subject Research (OHSRP#: 5410).
Statistical Analysis
We restricted the trend analysis to cases diagnosed in 2006-2015 because of the incomplete marker data collection in earlier years (Supplementary Figure 1). To estimate population-based subtype-specific incidence rates of breast cancer, we counted observed number of cases for each subtype by age at diagnosis (20-24, 25-29, …, 80-84, 85+) and calendar year of diagnosis (2006, 2007, … 2015), and adjusted the observed number of cases to the expected total population by dividing the observed number of cases by population coverage factor, 0.93.10 We obtained data on population at-risk by age (5-year age groups) in Sarawak from Department of Statistics Malaysia (Supplementary Table 1).15 We estimated subtype-specific age-standardized incidence rates (ASR) of breast cancer overall and by age groups (20-49, 50+ years) using the World Health Organization's new World Standard Population (WHO 2000-2025) as a reference population. The linear trend in ASRs was summarized as average annual percent change in the ASR, which was calculated by weighted log-linear regression under the assumption of a Poisson distribution.16
We used case-case analyses to test heterogeneity in associations of established breast cancer risk factors (family history of breast cancer, obesity, age at menarche, age at first birth, parity, breastfeeding) by tumor subtypes.17 We conducted unconditional logistic regression analyses to calculate odds ratios (ORs) for risk factor differences in HR-positive and HR-negative patients using HR-positive as the reference. Polytomous logistic regression was used to calculate ORs for HR+/HER+, HR−/HER2+, HR−/HER2− subtypes using HR+/HER2−, the most common subtype, as the reference. We tested family history of breast cancer (yes or no), BMI (<25, 25-29.9, 30+ kg/m2), age at menarche (<13, 13, ≥13 years), age at first birth (<23, 23-25, 26+ years, nulliparous) in mutually adjusted models in all cases or in cases stratified by age groups (20-49, 50+ years). Number of children (1-2, 3-4, or ≥5 children) and breastfeeding (ever, never) were additionally tested in parous women only. All ORs were adjusted for age (5-year interval) and year at diagnosis (2003-2006, 2007-2010, 2011-2013, 2014-2015) and ethnicity (Chinese, Malay, others). Statistical analyses were performed using SAS (Version 9.4, The SAS institute, Cary, NC) and MATLAB® R2017b (The MathWorks Inc., MA). All p-values were two-sided and considered statistically significant at p < 0.05.
Ethics approval and consent to participate
All study participants provided written informed consent and the project was approved by the Ethics Committee of the National Institutes of Health, Malaysia. The study was also exempted from review by the Office of Human Subject Research Protections at the National Institutes of Health (NIH) since NIH investigators do not have the access to the personal identifying information.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Characteristics of study participants
There were 2,977 invasive cases diagnosed between 2003 and 2015 in Sarawak, Malaysia, with the majority being Chinese (48.6%) and 40-69 years old (67.4%), and having Stage II (42.1%), moderately differentiated (55.8%), and HR-positive (65%) cancers. HR-positive cases tended to have earlier stage and lower grade cancers than HR-negative cases (PMantel-Haenszel Chi-Square<0.0001, Table 1). While the frequencies of family history of breast cancer, age at menarche, and menopausal status were similar between HR-positive and HR-negative patients, Chinese ethnicity, higher BMI, delayed first birth, and shorter duration of breastfeeding were more common among HR-positive patients than HR-negative patients (Table 1).
Table1.
Characteristics of female invasive breast cancer cases diagnosed between 2003 and 2015 in Sarawak, Malaysia, by hormone receptor status
| Total (n=2977) | HR-positive (n=1925) |
HR-negative (n=981) |
HR-unknown (n=71) |
Pb | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| ASR per 100,000 (SD)a | 40.0 | (0.78) | 28.1 | (0.68) | 12.9 | (0.44) | 0.5 | (0.09) | |
| Race | |||||||||
| Chinese | 1448 | 48.6 | 977 | 50.8 | 444 | 45.3 | 27 | 38.0 | 0.02 |
| Malay | 722 | 24.3 | 448 | 23.3 | 258 | 26.3 | 16 | 22.5 | |
| Others | 807 | 27.1 | 500 | 26.0 | 279 | 28.4 | 28 | 39.4 | |
| Age at diagnosis | |||||||||
| Mean (SD) | 51.6 | (11.1) | 51.6 | (10.9) | 51.4 | (11.5) | 51.8 | (11.0) | 0.62 |
| 20-40 | 367 | 12.3% | 216 | 11.2 | 144 | 14.7 | 7.0 | 9.9 | 0.77 |
| 40-49 | 1006 | 33.8% | 686 | 35.6 | 299 | 30.5 | 21 | 29.6 | |
| 50-59 | 911 | 30.6% | 578 | 30.0 | 305 | 31.1 | 28 | 39.4 | |
| 60-89 | 693 | 23.3% | 445 | 23.1 | 233 | 23.8 | 15 | 21.1 | |
| Year of diagnosis | |||||||||
| 2003-2005 | 481 | 16.2 | 273 | 14.2 | 169 | 17.2 | 39 | 54.9 | <.0001 |
| 2006-2009 | 841 | 28.3 | 521 | 27.1 | 313 | 31.9 | 7 | 9.9 | |
| 2010-2012 | 779 | 26.2 | 508 | 26.4 | 256 | 26.1 | 15 | 21.1 | |
| 2013-2015 | 876 | 29.4 | 623 | 32.4 | 243 | 24.8 | 10 | 14.1 | |
| Stage | |||||||||
| I | 414 | 14.1 | 315 | 16.5 | 97 | 10.0 | 2 | 2.9 | <.0001 |
| II | 1238 | 42.1 | 828 | 43.5 | 395 | 40.8 | 15 | 21.7 | |
| III | 931 | 31.7 | 566 | 29.7 | 343 | 35.4 | 22 | 31.9 | |
| IV | 359 | 12.2 | 196 | 10.3 | 133 | 13.7 | 30 | 43.5 | |
| Unknown | 35 | 20 | 13 | 2 | |||||
| Grade | |||||||||
| Well differentiated | 310 | 10.7 | 267 | 14.1 | 40 | 4.2 | 3 | 5.5 | <.0001 |
| Moderately differentiated | 1622 | 55.8 | 1171 | 61.8 | 420 | 43.8 | 31 | 56.4 | |
| Poorly differentiated | 977 | 33.6 | 456 | 24.1 | 500 | 52.1 | 21 | 38.2 | |
| Unknown | 68 | 31 | 21 | 16 | |||||
| Molecular subtype | |||||||||
| HR+/HER2− | 1411 | 52.2 | 1411 | 78.8 | - | - | - | - | |
| HR+/HER2+ | 379 | 14.0 | 379 | 21.2 | - | - | - | - | |
| HR−/HER2+ | 343 | 12.7 | - | - | 343 | 37.5 | - | - | |
| HR−/HER2− | 571 | 21.1 | - | - | 571 | 62.5 | - | - | |
| Unknown | 273 | 135 | 67 | 71 | |||||
| Family history | |||||||||
| No | 2496 | 85.3 | 1613 | 85.2 | 824 | 85.3 | 59 | 86.8 | 0.95 |
| Yes | 431 | 14.7 | 280 | 14.8 | 142 | 14.7 | 9 | 13.2 | |
| Unknown | 50 | 32 | 15 | 3 | |||||
| BMI (kg/m2) | |||||||||
| Mean (SD) | 24.8 | (4.7) | 25.0 | (4.7) | 24.5 | (4.6) | 24.6 | (4.2) | 0.01 |
| <25 | 1612 | 56.0 | 1008 | 54.1 | 565 | 59.7 | 39 | 57.4 | 0.001 |
| 25-29.9 | 876 | 30.4 | 579 | 31.1 | 276 | 29.2 | 21 | 30.9 | |
| 30+ | 390 | 13.6 | 277 | 14.9 | 105 | 11.1 | 8 | 11.8 | |
| Unknown | 99 | 61 | 35 | 3 | |||||
| Age at menarche | |||||||||
| <13 | 909 | 30.8 | 606 | 31.7 | 277 | 28.5 | 26 | 37.7 | 0.18 |
| 13 | 1003 | 34.0 | 634 | 33.2 | 345 | 35.5 | 24 | 34.8 | |
| 13+ | 1040 | 35.2 | 670 | 35.1 | 351 | 36.1 | 19 | 27.5 | |
| Unknown | 25 | 15 | 8 | 2 | |||||
| Menopausal status | |||||||||
| Pre | 1396 | 46.9 | 906 | 47.1 | 458 | 46.7 | 32 | 45.1 | 0.85 |
| Post | 1581 | 53.1 | 1019 | 52.9 | 523 | 53.3 | 39 | 54.9 | |
| Parity/Age at first birth | |||||||||
| <23 | 891 | 29.9 | 554 | 28.8 | 319 | 32.6 | 18 | 25.4 | 0.01 |
| 23-25 | 619 | 20.8 | 387 | 20.1 | 214 | 21.8 | 18 | 25.4 | |
| 26+ | 807 | 27.1 | 539 | 28.0 | 250 | 25.5 | 18 | 25.4 | |
| Nulliparous | 659 | 22.1 | 445 | 23.1 | 197 | 20.1 | 17 | 23.9 | |
| Unknown | 1 | 1 | |||||||
| Number of childrenc | |||||||||
| 1-2 | 742 | 32.1 | 482 | 32.7 | 251 | 32.1 | 9 | 16.7 | 0.09 |
| 3-2 | 1032 | 44.7 | 677 | 45.9 | 323 | 41.4 | 32 | 59.3 | |
| 5+ | 537 | 23.2 | 317 | 21.5 | 207 | 26.5 | 13 | 24.1 | |
| Unknown | 7 | 4 | 3 | ||||||
| Breastfeedingc | |||||||||
| Never | 341 | 14.7 | 233 | 15.7 | 96 | 12.2 | 12 | 22.2 | 0.01 |
| 1-5 Months | 802 | 34.6 | 522 | 35.3 | 267 | 34.1 | 13 | 24.1 | |
| 6-12 Months | 704 | 30.4 | 446 | 30.1 | 241 | 30.7 | 17 | 31.5 | |
| 13+ Months | 471 | 20.3 | 279 | 18.9 | 180 | 23.0 | 12 | 22.2 | |
ASR, Age-standardised rate; BMI, body mass index; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; SD, standard deviation
ASR per 100,000 woman-years for breast cancer diagnosed from 2006-2015.
P-value for HR-positive versus HR-negative comparison based on Student's t-test, Chi-Square test, or Mantel-Haenszel Chi-Square test whichever appropriate
Among parous women
Time trend in age-standardized breast cancer incidence rates by tumor subtypes
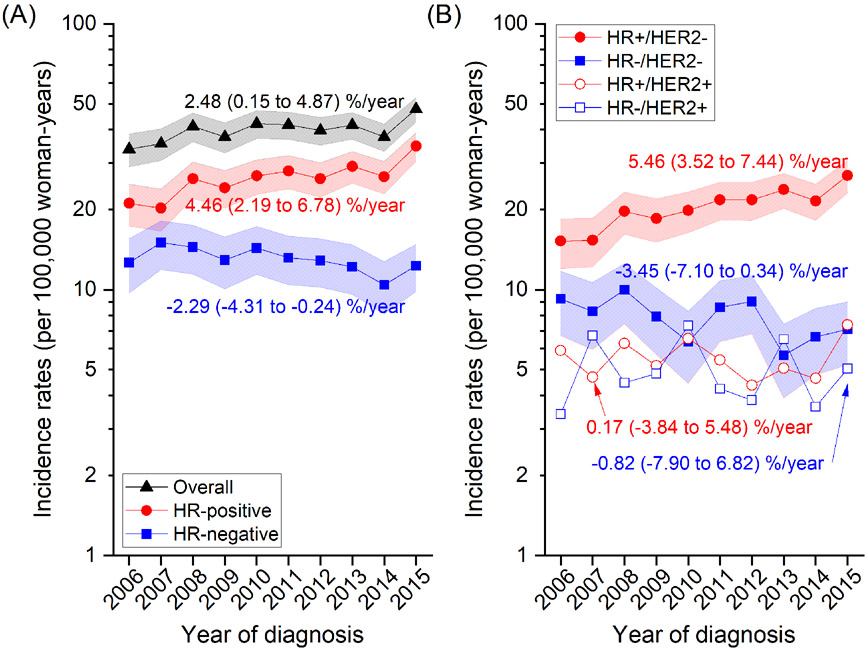
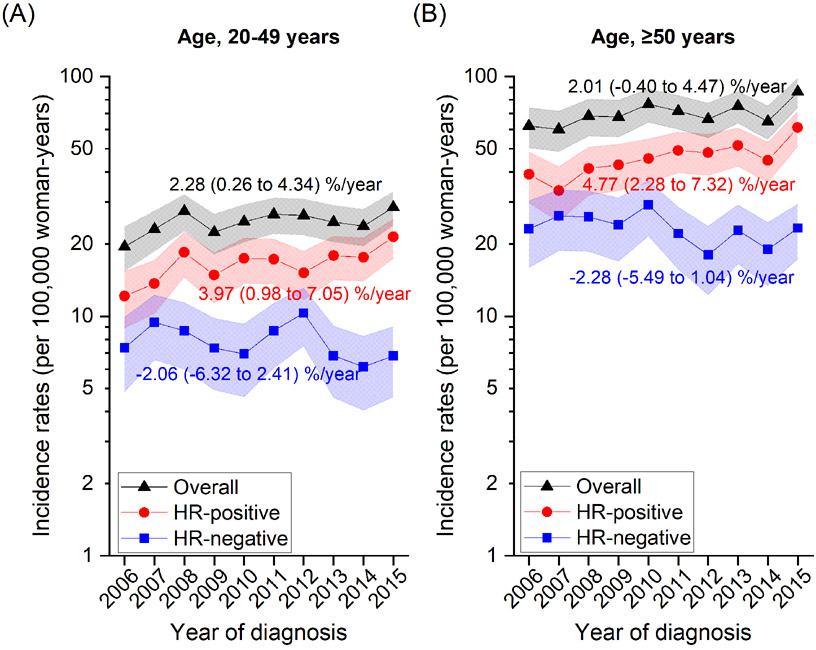
Figure 1 shows overall and subtype-specific incidence rates between 2006 and 2015. Rates for overall breast cancer increased from 33.8 (95% CI=29.0-38.5) in 2006 to 47.7 (95% CI=42.8-5.27) in 2015 at a rate of 2.48% on average per year (95% CI=0.15-4.87). During the same period, the rate for HR-positive cancers increased from 21.1 (95% CI=17.3-24.9) to 34.7 (95% CI=30.5-39.0) with an average increase of 4.46% per year (95% CI=2.19-6.78), but decreased for HR-negative cancers from 12.6 (95% CI=9.70-15.6) in 2006 and 15.0 (95% CI=11.9-18.1) in 2007 to 12.3 (95% CI=9.80-14.8) in 2015 with an average decrease of 2.29% per year (95% CI= −4.31 to −0.24) (Figure 1A; see Supplementary Table 2 for numbers). When further stratified by HER2 status (Figure 1B), the increase of HR-positive cancers was accentuated in HR+/HER2− subtype (5.46%/yr; 95% CI=3.52-7.44) but was not prominent in HR+/HER2+ cancers. For HR-negative cancers, the decrease appeared to be more pronounced for HR−/HER2− cancers but the trend was not statistically significant (−3.45%/yr; 95% CI= −7.10 to 0.34). Analyses by HR (Figure 2; Supplementary Table 3) and HR/HER2 (Supplementary Table 3) subtypes according to age groups (20-49 years, ≥50 years) showed similar trends in younger and older women with a slightly greater annual percent change estimated in older than younger women, although none of the trends in HR-negative tumors were statistically significant.
Figure 1. Trends in breast cancer incidence rates and average annual percent changes (% per year) by (A) HR and (B) HR/HER2 status between 2006 and 2015 in Sarawak, Malaysia.
Symbols represent annual rates adjusted to the World Health Organization's new World Standard Population (WHO 2000-2025) and 95% confidence intervals (CIs) are indicated by shaded bands. Annual rates (95% CI) for HR+/HER2+ and HR−/HER− subtypes are presented in Supplementary Table 1.
Figure 2. Trends in breast cancer incidence rates and average annual percent changes (% per year) by HR status for women aged (A) 20-49 years and (B) 50-89 years between 2006 and 2015 in Sarawak, Malaysia.
Symbols represent annual rates adjusted to the World Health Organization's new World Standard Population (WHO 2000-2025) and 95% confidence intervals (CIs) are indicated by shaded bands. Annual rates (95% CI) by HR/HER2 status according to age groups are presented in Supplementary Table 2.
Association of breast cancer risk factors with tumor subtypes
Table 2 shows associations of BMI and selected reproductive factors with HR-status that remained statistically significant in multivariable models. We found that nulliparity (ORnulliparous vs. <23 years=0.74; 95% CI=0.59-0.94) and later age at first full term birth (OR≥26 vs. <23 years=0.82; 95% CI=0.66-1.02) were less frequent among HR-negative cases than among HR-positive cases after controlling for potential confounders. Among parous women, never-breastfeeding was also less common among HR-negative cases (ORnever vs. ever=0.73; 95% CI=0.55-0.97). In addition, excess body weight was less frequent among HR-negative cases than among HR-positive cases with OR of 0.82 (95% CI=0.69-0.98) for overweight and 0.62 (95% CI=0.48-0.80) for obesity. These associations were similar in older and younger women, but the magnitude of the associations was generally stronger among older women. When HR-positive cases were further stratified by HER2 status (Supplementary Table 4), breast cancer risk factors did not vary between HR+/HER2− and HR+/HER+. Excess body weight and delayed first birth were less frequently observed among cases with HR−/HER2+ and HR−/HER2− cancers than among those with HR+/HER2− cases.
Table 2.
Associations between breast cancer risk factors and hormone receptor status by age group in Sarawak, Malaysia
| HR-positive (n=1925) | HR-negative (n=981) | HR-negative vs. HR-positive | P | |||
|---|---|---|---|---|---|---|
| n | % | n | % | OR (95% CI) | ||
| BMI (kg/m2)a | ||||||
| Overall | ||||||
| <25 | 1008 | 54.1 | 565 | 59.7 | Ref. | |
| 25-29.9 | 579 | 31.1 | 276 | 29.2 | 0.82 (0.69- 0.98) | 0.03 |
| 30+ | 277 | 14.9 | 105 | 11.1 | 0.62 (0.48- 0.80) | 2.5E-04 |
| Ptrend | 1.2E-04 | |||||
| 20-49 years | ||||||
| <25 | 492 | 55.9 | 246 | 57.5 | Ref. | |
| 25-29.9 | 264 | 30.0 | 124 | 29.0 | 0.92 (0.70- 1.21) | 0.55 |
| 30+ | 124 | 14.1 | 58 | 13.6 | 0.84 (0.58- 1.21) | 0.35 |
| Ptrend | 0.33 | |||||
| ≥50 years | ||||||
| <25 | 516 | 52.4 | 319 | 61.6 | Ref. | |
| 25-29.9 | 315 | 32.0 | 152 | 29.3 | 0.76 (0.60- 0.97) | 0.03 |
| 30+ | 153 | 15.6 | 47 | 9.1 | 0.44 (0.30- 0.64) | 1.6E-05 |
| Ptrend | 9.7E-06 | |||||
| Parity/Age at first birtha | ||||||
| Overall | ||||||
| <23 | 554 | 28.8 | 319 | 32.6 | Ref. | |
| 23-25 | 387 | 20.1 | 214 | 21.8 | 0.95 (0.76- 1.19) | 0.66 |
| 26+ | 539 | 28.0 | 250 | 25.5 | 0.82 (0.66- 1.02) | 0.07 |
| Nulliparous | 445 | 23.1 | 197 | 20.1 | 0.74 (0.59- 0.94) | 0.01 |
| Ptrend | 5.4E-03 | |||||
| 20-49 years | ||||||
| <23 | 275 | 30.5 | 145 | 32.8 | Ref. | |
| 23-25 | 164 | 18.2 | 93 | 21.0 | 1.18 (0.84- 1.66) | 0.34 |
| 26+ | 232 | 25.7 | 103 | 23.3 | 0.95 (0.68- 1.32) | 0.75 |
| Nulliparous | 231 | 25.6 | 101 | 22.9 | 0.81 (0.58- 1.13) | 0.21 |
| Ptrend | 0.16 | |||||
| ≥50 years | ||||||
| <23 | 279 | 27.3 | 174 | 32.3 | Ref. | |
| 23-25 | 223 | 21.8 | 121 | 22.5 | 0.80 (0.59- 1.09) | 0.16 |
| 26+ | 307 | 30.0 | 147 | 27.3 | 0.70 (0.52- 0.94) | 0.02 |
| Nulliparous | 214 | 20.9 | 96 | 17.8 | 0.64 (0.46- 0.88) | 0.01 |
| Ptrend | 3.1E-03 | |||||
| Breastfeedingb | ||||||
| Overall | ||||||
| Ever | 1247 | 84.3 | 688 | 87.8 | Ref. | |
| Never | 233 | 15.7 | 96 | 12.2 | 0.73 (0.55- 0.97) | 0.03 |
| 20-49 years | ||||||
| Ever | 578 | 86.1 | 300 | 87.7 | Ref. | |
| Never | 93 | 13.9 | 42 | 12.3 | 0.87 (0.56- 1.37) | 0.56 |
| ≥50 years | ||||||
| Ever | 669 | 82.7 | 388 | 87.8 | Ref. | |
| Never | 140 | 17.3 | 54 | 12.2 | 0.65 (0.45- 0.95) | 0.03 |
BMI, body mass index; CI, confidence interval; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; OR, odds ratio
Model included year of diagnosis, race (Chinese, Malay, or others), age (5-year), BMI (<25, 25-29.9, or 30+), family history of breast cancer (yes or no), age at menarche (<13, 13, or ≥13 years), and parity (parous or nulliparous) as explanatory variables
Among parous women, model included year of diagnosis, race (Chinese, Malay, or others), age (5-year), BMI (<25, 25-29.9, or 30+), family history of breast cancer (yes or no), age at menarche (<13, 13, or ≥13 years), age at first birth (<23, 23-25, or 26+), number of children (1-2, 3-4, or ≥5 children), and breastfeeding (ever or never) as explanatory variables.
Discussion
In Sarawak, Malaysia, breast cancer incidence rates increased for HR-positive cancers but decreased for HR-negative cancers from 2006-2015. Further stratification by HER2 status suggested that the increasing HR-positive trend and the decreasing HR-negative trend was driven by HR+/HER2− and HR−/HER2− cancers, respectively. Frequencies of excess body weight and reproductive factors, particularly age at first birth and breastfeeding, varied by subtypes, which may partly account for rising rates for HR-positive cancers and falling rates for HR-negative cancers in Sarawak. The pattern of divergent trends by HR status in Sarawak is similar to what was previously reported in the US, Denmark, and Ireland.3, 5, 18 This suggests similar changes in the prevalence of common risk factors that may have opposing effects on HR-positive and HR-negative breast cancers, despite the substantial variation in overall incidence rates across populations.19
Although results in different studies were not directly comparable due to differences in study periods, design, and approaches, the annual increase estimated for HR-positive cancers was greater in Sarawak (4.5%/yr from 2005-2014) compared to those in the US (1.2%/yr from 1992-2008), Denmark (3.0%/yr from 1993-2010), and Ireland (2.2%/yr from 2004-2013), whereas there was little variation in annual declines for HR-negative cancers across countries (range, 2.1-3.4%/yr).4-6 While the recent stabilizing trend of overall breast cancer incidence rates in some high-risk countries may reflect the superimposition of the two opposing trends (HR-positive and HR-negative cancers) with similar magnitudes, the greater increase in HR-positive cancers shown in Sarawak, Malaysia, implies that sustained rapid increases of overall breast cancer incidences in historically low-risk regions are driven by increases in HR-positive cancers rather than by HR-negative cancers. In our previous study, the overall ASR for breast cancer was 22.0 per 100,000 women for 1998-2009 in Sarawak10 as compared to 40 per 100,000 women for 2006-2015 estimated in the current study, showing a rapid increase of breast cancer incidence in recent years. Despite the potential impact of improved case ascertainment on cancer incidence rates, especially in transitioning countries, the decline of HR-negative cancers as opposed to the incline of HR-positive cancers implies that the increase of HR-positive cancers may not be solely explained by changes in the cancer registry practice.
Studies examining subtype-specific trends of breast cancer are lacking in other low-risk countries and/or regions. Notwithstanding the existence of high-quality long-term cancer registry data in several Asian countries such as Japan, Korea, Singapore, and Taiwan, incidence trends by HR status have not been reported in Asia due to the lack of tumor marker information in cancer registries. A report from Korea based on hospital-based cancer registry showed that the percentage of ER-positive cancers increased from 58% in 2002 to 74% in 2015,20 which is consistent with the rising trend of HR-positive cancers observed in our study. Following sociocultural changes towards a more Westernized lifestyle, Asian women have experienced rapid changes in their lifestyle and reproductive factors, characterized by earlier onset of menarche, delayed childbirth and fewer children, less breastfeeding, increasing body weight, and less physical activities.21, 22 It is notable that some of these factors are known to have differential associations with HR-positive cancers and HR-negative cancers. For example, while earlier age at first birth and multiparity are protective for HR-positive cancers, they were associated with increased risk of HR-negative cancers, especially triple-negative cancers.1, 23-29 Consistent with these findings, our case-case comparisons showed cases with HR-positive cancers were more likely to be nulliparous or to have delayed their first births compared to cases with HR-negative cancers. In Malaysia, the mean age at first marriage, which could be considered as a proxy for age at first birth, increased from 21.6 years old in 1970 to 25.1 years in 2000.30 The total fertility rate (births per woman) also decreased from 6.5 children per woman in 1960 to 2.0 in 2017.31 Data from a case-control study conducted in Selangor, Malaysia, also showed that women of each ethnic group experienced significant reduction in parity and increase in age at first full term pregnancy across birth cohorts from before 1950 to after 1970.32 The increasing prevalence of delayed childbirth and fewer number of children, which may have opposing effects on breast cancer risk by subtypes, may at least in part explain the observed diverging trend of breast cancer defined by HR status.
Given the declining frequency and duration of breastfeeding during recent decades in Malaysia,33 the observed lower frequency of breastfeeding in HR-positive cases compared to HR-negative cases is also consistent with the rising HR-positive cancers in contrast to the falling HR-negative cancers. However, the association of breastfeeding with cancer subtypes in our study is contradictory with results based on a systematic review summarizing 27 studies that reported a stronger protective effect of breastfeeding for HR-negative cancers (especially for triple-negative cancer) compared to HR-positive cancers.34 Conflicting results exist particularly for non-white women. For example, a previous study using a similar case-only design as our study among women of Mexican descent reported lower prevalence of breastfeeding in luminal A cases vs. triple-negative cases;35 and a multiethnic study showed a significantly shorter duration of breastfeeding among triple-negative breast cancer cases in White, Hispanic and African American but not among Asian women.36 The discrepancies are possibly due to differences in study design, the adjustment of multiparity, definition of HR-positive tumors (based on ER or joint ER/PR), as well as race/ethnicity.37 Large studies, especially those with population-based design and optimally controlling for other parity-related variables, are needed to further investigate the relationship between breastfeeding and breast cancer subtypes in diverse populations/ethnic groups.
Excess body weight has been consistently associated with increased risk of HR-positive breast cancers, particularly among postmenopausal women.38-41 Increasing trends of HR-positive cancers may be partially due to a rapid increase of the obesity prevalence in Malaysia in recent decades. Age-standardized prevalence of obesity (BMI≥30 kg/m2) among women (≥20 years) increased more than 8-fold from 2.3% in 1975 to 18.5% in 2016 in Malaysia, making Malaysia the most obese country in East and South East Asia.42 Although excess body weight has been associated with reduced risk of premenopausal breast cancer and increased risk of postmenopausal women in Western countries,38-41 studies conducted in Asian countries or immigrant Asian women showed that excess body weight was associated with increased breast cancer risk regardless of menopausal status in Asian women.39, 43 We found a slightly faster increase in HR-positive cancers among older than younger women (4.8%/yr versus 4.0%/yr), which is consistent with the greater effect size of the obesity association among postmenopausal women.39, 43
Increasing access to mammography screening may have also contributed to the rise of HR-positive cancers as screening mammography preferentially detects ER-positive cancers with higher sensitivity than ER-negative cancers.44, 45 In Malaysia, a subsidized screening program has been carried out since 2007 by the National Population and Family Development Board Malaysia mammogram screening program.46 However, very few women are eligible to attend this screening, and screening uptake in the general population is low. According to studies conducted in urban and sub-urban localities of Terengganu, Selangor and Kuala Lumpur, screening uptake was between 11% and 32% in the general population of those areas.46 Therefore, mammographic screening may not have major impact on breast cancer incidence changes in Malaysia as it does in high-income Western countries.
Data source from Sarawak offered a unique opportunity, for the first time to our knowledge, to examine subtype-specific incidence trends of breast cancer in a historically low-risk population. The data covered 93% of the target population and subtype information was nearly complete (missing [%], 0 to 2.3% by year for HR status; 0 to 5% by year for HR/HER2 status; Supplementary Figure 1). Further to enhance our understanding of the observed trend, we examined the associations between well-established breast cancer risk factors and HR subtypes, which demonstrated that breast cancer risk factors might act differentially on different subtypes. However, our study has several important limitations. First, breast cancer subtype information was available only for the recent 10 years, which limited our ability to examine a long-term incidence trend by subtype. As additional data, especially from other low-risk populations, become available over time, future analyses are needed to confirm and expand our findings. Second, although our findings on divergent trends of breast cancer accompanied by risk factor analysis may provide important insights on the presence of risk factors with opposing effects by tumor subtypes, our results do not inform a causal relationship of the risk factors on the observed incidence trends. In addition, our study did not assess the impact of changing prevalence of risk factors on the diverging incidence trends. Addressing these limitations in future studies, which require longitudinal collection of risk factor data, would further improve our understanding of the observed trend. Third, our findings were based on a single hospital in the state of Sarawak, which may not be generalizable to the rest of the population in Malaysia considering the heterogeneous ethnic composition across states. In addition, although tumor markers have been measured consistently in a single institution using the same threshold over the 10 years of the study period, it is unknown whether the improved sensitivity in assay techniques to measure tumor markers over time may have affected the observed subtype-specific trend. Lastly, Sarawak is a multiethnic state comprising three major ethnic groups namely Malays, Chinese, and Natives, with varied breast cancer incidence rates and distinct lifestyles in each ethnic group.10 However, our study was not powered to investigate the incidence trends by HR status in each ethnic group separately.
In conclusion, breast cancer incidence rates in Sarawak, Malaysia, have shown diverging trends by HR status. We also showed that risk factors, including nulliparity, late age at first birth, never-breastfeeding, and excess body weight, were more prevalent among HR-positive than HR-negative cancers. The burden of HR-positive breast cancers relative to HR-negative cancers is expected to continue to increase with time, given the rising prevalence of risk factors that are more relevant to the risk of HR-positive cancers. Future studies are warranted to investigate subtype-specific incidence trends in other low-risk populations and further identify factors contributing the trends.
Supplementary Material
Novelty and Impact.
Using data from Sarawak, Malaysia, we first report incidence trend of breast cancer by tumor subtype in a low-risk region. Between 2007 and 2015, incidence rates for hormone-receptor (HR)-positive cancer increased (4.46%/year), while rates for HR-negative cancer decreased (2.29%/year), similar to what previously reported in a few high-income Western countries. Reasons for the divergent trend remain unclear, however, may reflect changes in the prevalence of risk factors with opposing effects on HR-positive vs. HR-negative cancers.
Funding
The work was supported by the Intramural Research Program of American Cancer Society and the National Cancer Institute.
Abbreviations
- ASR
age-standardized incidence rates
- BC
breast cancers
- BMI
body mass index
- CI
confidence interval
- ER
estrogen receptor
- HER2
human epidermal growth factor receptor 2
- HR
hormone-receptor
- IHC
immunohistochemical
- OR
odds ratio
- PR
progesterone receptor
Footnotes
Conflict of interests
Dr Sung is employed by the American Cancer Society, which received a grant from Merck Inc for intramural research outside the submitted work; however, her salary is solely funded through the American Cancer Society. No other disclosures were reported. The other authors declare that they have no competing interests.
References
- 1.Gaudet MM, Gierach GL, Carter BD, Luo J, Milne RL, Weiderpass E, Giles GG, Tamimi RM, Eliassen AH, Rosner B, Wolk A, Adami HO, et al. Pooled Analysis of Nine Cohorts Reveals Breast Cancer Risk Factors by Tumor Molecular Subtype. Cancer Res 2018;78: 6011–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson WF, Pfeiffer RM, Wohlfahrt J, Ejlertsen B, Jensen MB, Kroman N. Associations of parity-related reproductive histories with ER+/− and HER2+/− receptor-specific breast cancer aetiology. International journal of epidemiology 2017;46: 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glass AG, Lacey JV, Carreon D, Hoover RN. Breast cancer incidence, 1980-2006: Combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. Journal of the National Cancer Institute 2007;99: 1152–61. [DOI] [PubMed] [Google Scholar]
- 4.Anderson WF, Katki HA, Rosenberg PS. Incidence of breast cancer in the United States: current and future trends. Journal of the National Cancer Institute 2011;103: 1397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson WF, Rosenberg PS, Petito L, Katki HA, Ejlertsen B, Ewertz M, Rasmussen BB, Jensen MB, Kroman N. Divergent estrogen receptor-positive and -negative breast cancer trends and etiologic heterogeneity in Denmark. International Journal of Cancer 2013;133: 2201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullooly M, Murphy J, Gierach GL, Walsh PM, Deady S, Barron TI, Sherman ME, Rosenberg PS, Anderson WF. Divergent oestrogen receptor-specific breast cancer trends in Ireland (2004-2013): Amassing data from independent Western populations provide etiologic clues. Eur J Cancer 2017;86: 326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68: 394–424. [DOI] [PubMed] [Google Scholar]
- 8.Sarawak: https://en.wikipedia.org/wiki/Sarawak Accessed on Sep 23, 2019. [Google Scholar]
- 9.Horne HN, Devi CRB, Sung H, Tang TS, Rosenberg PS, Hewitt SM, Sherman ME, Anderson WF, Yang XHR. Greater absolute risk for all subtypes of breast cancer in the US than Malaysia. Breast cancer research and treatment 2015;149: 285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devi, Tang TS, Corbex M. Incidence and risk factors for breast cancer subtypes in three distinct South-East Asian ethnic groups: Chinese, Malay and natives of Sarawak, Malaysia. Int J Cancer 2012;131: 2869–77. [DOI] [PubMed] [Google Scholar]
- 11.Abubakar M, Sung H, Bcr D, Guida J, Tang TS, Pfeiffer RM, Yang XR. Breast cancer risk factors, survival and recurrence, and tumor molecular subtype: analysis of 3012 women from an indigenous Asian population. Breast Cancer Res 2018;20: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke CA, Keegan TH, Yang J, Press DJ, Kurian AW, Patel AH, Lacey JV Jr., Age-specific incidence of breast cancer subtypes: understanding the black-white crossover. J Natl Cancer Inst 2012;104: 1094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bastien RR, Rodriguez-Lescure A, Ebbert MT, Prat A, Munarriz B, Rowe L, Miller P, Ruiz-Borrego M, Anderson D, Lyons B, Alvarez I, Dowell T, et al. PAM50 breast cancer subtyping by RT-qPCR and concordance with standard clinical molecular markers. BMC Med Genomics 2012;5: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauer K, Parise C, Caggiano V. Use of ER/PR/HER2 subtypes in conjunction with the 2007 St Gallen Consensus Statement for early breast cancer. Bmc Cancer 2010;10: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Department of Statistics Malaysia, Official Portal. https://www.dosm.gov.my Access on Mar 1, 2019, 2013.
- 16.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19: 335–51. [DOI] [PubMed] [Google Scholar]
- 17.Martinez ME, Cruz GI, Brewster AM, Bondy ML, Thompson PA. What can we learn about disease etiology from case-case analyses? Lessons from breast cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2010;19: 2710–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson WF, Katki HA, Rosenberg PS. Incidence of Breast Cancer in the United States: Current and Future Trends. J Natl Cancer I 2011;103: 1397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2016;25: 16–27. [DOI] [PubMed] [Google Scholar]
- 20.Kang SY, Kim YS, Kim Z, Kim HY, Lee SK, Jung KW, Youn HJ, Korean Breast Cancer S. Basic Findings Regarding Breast Cancer in Korea in 2015: Data from a Breast Cancer Registry. J Breast Cancer 2018;21: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan L, Zheng Y, Yu KD, Liu GY, Wu J, Lu JS, Shen KW, Shen ZZ, Shao ZM. Breast cancer in a transitional society over 18 years: trends and present status in Shanghai, China. Breast cancer research and treatment 2009;117: 409–16. [DOI] [PubMed] [Google Scholar]
- 22.Porter P "Westernizing" women's risks? Breast cancer in lower-income countries. The New England journal of medicine 2008;358: 213–6. [DOI] [PubMed] [Google Scholar]
- 23.Palmer JR, Boggs DA, Wise LA, Ambrosone CB, Adams-Campbell LL, Rosenberg L. Parity and lactation in relation to estrogen receptor negative breast cancer in African American women. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2011;20: 1883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson WF, Pfeiffer RM, Wohlfahrt J, Ejlertsen B, Jensen MB, Kroman N. Associations of parity-related reproductive histories with ER +/− and HER2 +/− receptor-specific breast cancer aetiology (vol 46, pg 86, 2016). International journal of epidemiology 2017;46: 373-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnard ME, Boeke CE, Tamimi RM. Established breast cancer risk factors and risk of intrinsic tumor subtypes. Bba-Rev Cancer 2015;1856: 73–85. [DOI] [PubMed] [Google Scholar]
- 26.Tamimi RM, Colditz GA, Hazra A, Baer HJ, Hankinson SE, Rosner B, Marotti J, Connolly JL, Schnitt SJ, Collins LC. Traditional breast cancer risk factors in relation to molecular subtypes of breast cancer. Breast cancer research and treatment 2012;131: 159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warner ET, Colditz GA, Palmer JR, Partridge AH, Rosner BA, Tamimi RM. Reproductive factors and risk of premenopausal breast cancer by age at diagnosis: Are there differences before and after age 40? Breast cancer research and treatment 2013;142: 165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phipps AI, Chlebowski RT, Prentice R, McTiernan A, Wactawski-Wende J, Kuller LH, Adams-Campbell LL, Lane D, Stefanick ML, Vitolins M, Kabat GC, Rohan TE, et al. Reproductive History and Oral Contraceptive Use in Relation to Risk of Triple-Negative Breast Cancer. Journal of the National Cancer Institute 2011;103: 470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang XR, Chang-Claude J, Goode EL, Couch FJ, Nevanlinna H, Milne RL, Gaudet M, Schmidt MK, Broeks A, Cox A, Fasching PA, Hein R, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. Journal of the National Cancer Institute 2011;103: 250–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Population and Housing Census in Malaysia, 1970-2000. EDIT. [Google Scholar]
- 31.The World Back Database Archives (beta) Total Fertility Rates (Births Per Woman) https://data.worldbank.org/indicator/SP.DYN.TFRT.IN?locations=MY Accessed on July 18, 2019.
- 32.Tan MM, Ho WK, Yoon SY, Mariapun S, Hasan SN, Lee DS, Hassan T, Lee SY, Phuah SY, Sivanandan K, Ng PP, Rajaram N, et al. A case-control study of breast cancer risk factors in 7,663 women in Malaysia. Plos One 2018;13: e0203469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sulaiman Z, Liamputtong P, Amir LH. Timing of return to work and women's breastfeeding practices in urban Malaysia: A qualitative study. Health Soc Care Community 2018;26: 48–55. [DOI] [PubMed] [Google Scholar]
- 34.Islami F, Liu Y, Jemal A, Zhou J, Weiderpass E, Colditz G, Boffetta P, Weiss M. Breastfeeding and breast cancer risk by receptor status-a systematic review and meta-analysis. Annals of Oncology 2015;26: 2398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez ME, Wertheim BC, Natarajan L, Schwab R, Bondy M, Daneri-Navarro A, Meza-Montenegro MM, Gutierrez-Millan LE, Brewster A, Komenaka IK, Thompson PA. Reproductive factors, heterogeneity, and breast tumor subtypes in women of mexican descent. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2013;22: 1853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinde SS, Forman MR, Kuerer HM, Yan K, Peintinger F, Hunt KK, Hortobagyi GN, Pusztai L, Symmans WF. Higher parity and shorter breastfeeding duration: association with triple-negative phenotype of breast cancer. Cancer 2010;116: 4933–43. [DOI] [PubMed] [Google Scholar]
- 37.Lee H, Li JY, Fan JH, Li J, Huang R, Zhang BN, Zhang B, Yang HJ, Xie XM, Tang ZH, Li H, He JJ, et al. Risk factors for breast cancer among Chinese women: a 10-year nationwide multicenter cross-sectional study. J Epidemiol 2014;24: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. Brit Med J 2007;335: 1134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371: 569–78. [DOI] [PubMed] [Google Scholar]
- 40.Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body Mass Index and Breast Cancer Risk According to Postmenopausal Estrogen-Progestin Use and Hormone Receptor Status. Epidemiologic Reviews 2014;36: 114–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki R, Orsini N, Saji S, Key TJ, Wolk A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status-A meta-analysis. Int J Cancer 2009;124: 698–712. [DOI] [PubMed] [Google Scholar]
- 42.Collaboration NCDRF. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017;390: 2627–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wada K, Nagata C, Tamakoshi A, Matsuo K, Oze I, Wakai K, Tsuji I, Sugawara Y, Mizoue T, Tanaka K, Iwasaki M, Inoue M, et al. Body mass index and breast cancer risk in Japan: a pooled analysis of eight population-based cohort studies. Annals of Oncology 2014;25: 519–24. [DOI] [PubMed] [Google Scholar]
- 44.Gilliland FD, Joste N, Stauber PM, Hunt WC, Rosenberg R, Redlich G, Key CR. Biologic characteristics of interval and screen-detected breast cancers. Journal of the National Cancer Institute 2000;92: 743–9. [DOI] [PubMed] [Google Scholar]
- 45.Porter PL, El-Bastawissi AY, Mandelson MT, Lin MG, Khalid N, Watney EA, Cousens L, White D, Taplin S, White E. Breast tumor characteristics as predictors of mammographic detection: comparison of interval- and screen-detected cancers. Journal of the National Cancer Institute 1999;91: 2020–8. [DOI] [PubMed] [Google Scholar]
- 46.Mahmud A, Aljunid SM. Availability and accessibility of subsidized mammogram screening program in peninsular Malaysia: A preliminary study using travel impedance approach. Plos One 2018;13: e0191764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.