Abstract
Simple Summary
Gastrointestinal stromal tumors (GIST) are potentially malignant tumors and require evidence-based surgical and/or medical treatment. Laparoscopy has similar safety and prognostic outcomes to those of laparotomy and is currently a standard procedure for localized GISTs. However, surgery for gastric GISTs less than 2 cm may be re-evaluated due to the indolent nature of the GIST and other competing risks among GIST patients. A work-up with endoscopy and endoscopic ultrasonography as well as endoscopic or percutaneous biopsy is important for the preoperative diagnosis of GISTs. Medical treatment with tyrosine kinase inhibitors is the mainstay for recurrent/metastatic GISTs. The activity of an individual drug is well correlated with gene alterations, and, in the era of precision medicine, cancer genome profiling should be considered before medical treatment.
Abstract
Gastrointestinal stromal tumors (GISTs) are the most frequent malignant mesenchymal tumors in the gastrointestinal tract. The clinical incidence of GISTs is estimated 10/million/year; however, the true incidence is complicated by frequent findings of tiny GISTs, of which the natural history is unknown. The initial work-up with endoscopy and endoscopic ultrasonography plays important roles in the differential diagnosis of GISTs. Surgery is the only modality for the permanent cure of localized GISTs. In terms of safety and prognostic outcomes, laparoscopy is similar to laparotomy for GIST treatment, including tumors larger than 5 cm. GIST progression is driven by mutations in KIT or PDGFRA or by other rare gene alterations, all of which are mutually exclusive. Tyrosine kinase inhibitors (TKIs) are the standard therapy for metastatic/recurrent GISTs. Molecular alterations are the most reliable biomarkers for TKIs and for other drugs, such as NTRK inhibitors. The pathological and genetic diagnosis prior to treatment has been challenging; however, a newly developed endoscopic device may be useful for diagnosis. In the era of precision medicine, cancer genome profiling by targeted gene panel analysis may enable potential targeted therapy even for GISTs without KIT or PDGFRA mutations.
Keywords: gastrointestinal stromal tumor, submucosal tumor, subepithelial tumor, gene panel analysis, precision medicine
1. Introduction
The gastrointestinal stromal tumor (GIST) is a potentially malignant mesenchymal tumor (sarcoma) that usually expresses KIT or DOG1 proteins by immunohistochemistry (IHC). GISTs are considered to be a lineage of immature mesenchymal cells capable of differentiating into the interstitial cells of Cajal (ICC), which serve as pacemaker cells of the gastrointestinal (GI) tract [1,2,3,4]. Hence, GISTs are exclusively found in various parts of the GI tract, including the stomach (approximately 60–65%, with most found in the upper stomach), the small intestine (20–25%, mainly in the proximal small intestine, the duodenum, and proximal jejunum), and the colon, as well as in the rectum (comprising a low % of GISTs, mostly in the distal rectum), the esophagus (1%), and, rarely, in parts of the extra-GI tract, such as the peritoneum and major omentum. The majority of GISTs have a gain-of-function mutation in either KIT (70%) or PDGFRA (10–15%), and some (nearly 15%) may have other mutations in BRAF, RAS family genes, and NF1, and alterations in the SDH (succinate dehydrogenase; complex III in the mitochondrial electron transport system) complex or in NRTK translocation (Table 1) [4,5,6,7,8,9,10,11]. These mutations and alterations are mutually exclusive in primary GISTs.
Table 1.
Features and mutations of GISTs.
| Alterations | Estimated Frequency | Main Location | Characteristics | Sensitivity to Drugs & Potential Drugs | |||
|---|---|---|---|---|---|---|---|
| KIT mutations in the autoinhibited form | KIT mutation # | exon 9 (or exon 8), typically duplicated insertion of A502-Y503 codons | 5–10% | Small intestine | Spindle cell type Aggressive features |
Less imatinib sensitive, sensitive to sunitinib, regorafenib, ripretinib, avapritinib | |
| exon 11 (deletions, missense, insertions etc.) | ~60% | All sites | Aggressive features with del 557-558, which is very sensitive to imatinib | Sensitive to imatinib, sunitinib, regorafenib, ripretinib, avapritinib | |||
| exon 13 (K642E) | <1% | Sensitive to imatinib, sunitinib, regorafenib, ripretinib, avapritinib | |||||
| exon 17 (D820Y, N822K, Y823D) | 1% | Sensitive to imatinib, regorafenib, ripretinib, avapritinib, and less sensitive to sunitinib | |||||
| PDGFRA mutations in the autoinhibited form | PDGFRA mutation # | exon 12 (V561D etc.) | <1% | Stomach>>small intestine | Epithelioid cell type Indolent clinical course in main |
Probably sensitive to imatinib, sunitinib, regorafenib, ripretinib, avapritinib | |
| exon 14 (N659K) | <1% | Probably sensitive to imatinib, sunitinib, regorafenib, ripretinib, avapritinib | |||||
| exon 18 (del, Y849H etc., other than D842V) | 1–2% | Sensitive to imatinib, sunitinib, regorafenib, ripretinib, avapritinib | |||||
| KIT or PDGFRA mutations in the activated form | PDGFRA exon 18 D842V, rarely KIT exon 17 D816V | ~10% | Stomach>>small intestine | Epithelioid cell type | D842V is resistant to imatinib, sunitinib, regorafenib. D842V is sensitive to avapritinib & ripretinib |
||
| No mutation in KIT and PDGFRA | SDHB-competent | NF1 mutation $ | 1–2% | Small intestine | Spindle cell type Generally indolent clinical course associated with Neurofibromatosis type I |
not sensitive to available drugs | possibly sensitive to MEK inhibitors, such as selumetinib |
| BRAF mutation | <1% | Small intestine/stomach | Spindle cell type VE1-positive |
possibly sensitive to BRAF inhibitors (e.g., vemurafenib, dabrafenib) | |||
| HRAS, NRAS or KRAS mutation | very rare | no data | no data | MEK inhibitors (e.g., trametinib) may possibly have some activities | |||
| Others including PIK3CA, CBL, ETV6–NTRK3 et al. | very rare | no data | no data | NTR-fusion is sensitive to entrectinib and larotrectinib | |||
| SDHB-deficient | SDHA, SDHB, SDHC or SDHD mutation (including Carney-Stratakis syndrome #) | ~3% | Stomach>>small intestine | Epithelioid cell type SDHB-negative Children/adolescent and young adult Frequent lymph node metastasis Indolent clinical course |
not sensitive to available drugs VEGFR inhibitors may have temporary stabilizing effects |
||
| Loss of SDHB expression (including Carney Triad $) | <1% | Stomach | |||||
#: there are some GISTs with germline mutations; familial GIST; $: syndromic GIST.
The incidence of clinical GISTs, symptomatic GISTs or GISTs requiring treatment, is assumed to be 6–22 cases per million per year [1,2,3,4]. However, the true incidence of GISTs is more complicated and unknown because of the presence of mini-GISTs (asymptomatic GISTs less than 2 cm incidentally found by endoscopy) and of micro-GISTs (GISTs that are usually less than 1 cm and incidentally found by pathological examinations of resected specimens) [2,4,12]. Pathological examination of the stomachs and rectums of middle-aged adults reveals micro-GISTs in 10–35% and in 0.1–0.2% of cases, respectively [12,13,14,15]. Small submucosal tumors (SMTs) that are less than 2 cm are relatively frequent endoscopic findings in the stomach. It has been reported that endoscopy may reveal small neoplastic SMTs in 0.15% of middle-aged adults and that half of them are considered to be GISTs [12,16,17]. The natural history of mini-GISTs and micro-GISTs is unknown, and its clinical relevance needs to be elucidated.
2. Diagnosis
There are no symptoms or signs specific to GISTs. The most frequent symptoms include gastrointestinal bleeding and subsequent anemia, followed by abdominal pain, weight loss, and a palpable abdominal mass [4,9]. GISTs are unusually associated with bowel obstruction or perforation, except in cases of large tumors. It should be noted that a significant number of GISTs are asymptomatically found as SMTs by cancer-screening endoscopy or may be incidentally found in explorations of other diseases. GISTs are diagnosed from childhood to late adulthood, and the reported median age is in the 60 s [1,2,3,4]. There is no sex difference in terms of the incidence or clinical and genetic features of GISTs, except GISTs with SDH alterations which appear to be relatively predominant in females. Multiplicity is rarely seen except among patients with familial predispositions for germline mutations in KIT, PDGFRA, or SDH [18,19,20] or for multiple small intestinal GISTs in neurofibromatosis type I patients [21,22] When patients have germline mutations in KIT, PDGFRA, or NF1, they may have early onset of GISTs, ICC hyperplasia in the normally appearing GI tract, and characteristic clinical features, such as skin pigmentation, dysphagia, and other tumors, in addition to multiple GISTs. When there are multiple GISTs in a patient without a hereditary background, we may consider that these tumors are multiple sporadic GISTs if each GIST has different KIT or PDGFRA mutations [23,24]. If they have the same mutation type, they may be considered a metastatic disease. There are no reported environmental risk factors for GISTs.
2.1. Pathological Diagnosis of GIST
The diagnosis of GISTs is based on pathological examinations, but not clinical examinations. Morphologically, GISTs can be divided into three types: the spindle cell type with eosinophilic fibrillary cytoplasm (70%), epithelioid type (20%) with clear eosinophilic cytoplasm, and mixed type with spindle and epithelioid cells (10%) [25,26,27]. Spindle cell-type GISTs should be differentiated from both benign and malignant diseases, including smooth muscle tumors (leiomyoma or leiomyosarcoma), schwannoma, hemangioma, plexiform fibromyxoma, desmoid, inflammatory myofibroblastic tumor (IMT), and solitary fibrous tumor (SFT), and epithelioid-type GISTs from melanoma, perivascular epithelioid cell tumor (PEComa), neuroendocrine tumors, clear cell sarcoma, and epithelioid variants of leiomyosarcoma [4,25,26]. Some characteristic pathological findings of each tumor are shown in Table 2. There are some correlations between clinicopathological features and the genotype of the GIST, as described later [28]. Epithelioid transformation or mixed type may also be found in aggressive GISTs in the small intestine.
Table 2.
Endoscopic and EUS features of gastric submucosal tumor.
| Disease | Endoscopic Findings | EUS Findings | Pathological Features | |||
|---|---|---|---|---|---|---|
| Surface, Form, etc. | Major Location | Main Layer | Echo Findings | Morphology | IHC; Genetic Changes | |
| GIST | hemi-spherical, occasionally with delle or ulcer | body | proper muscle, rarely submucosa | hypoechoic, heterogenous with increased malinancy | spindle cell > epithelioid cell | KIT, DOG1; mutation in KIT or PDGFRA |
| Myogenic tumor & Leiomyoma | hemi-spherical, intact mucosa |
near cardia | proper muscle, sometimes submucosa | round, hypoechoic, homogenous | spindle cell (eosinophilic cell) | Desmin, α-SMA |
| Schwanomma & neurogenic tumor | hemi-spherical, intact mucosa |
body, lesser curvature | proper muscle, sometimes submucosa~deep mucosa | hypoechoic, homogenous~slightly heterogeneous | spindle cell, palisading, Verocay body, lymphoid cuff in Schwannoma | S-100, SOX10, NSE in neurogenic tumor |
| Heterotopic Pancreas | hill-shaped, intact mucosa, maybe dimple or aperture | antrum | submucosa | sometimes lobulated, ductal structure, heterogeneous internal echo, thickend proper muscle | Heimlich classification & | |
| Neuroendocrine tumor | hemi-spherical, mucosal color~yellowish~red, occasionally dimple | body | initially deep mucosa or submucosa | homogenous, heterogeneous with increased malinancy | epithelioid cell, organoid pattern | CD56, synaptophysin, chromogranin A, NSE |
| MALT lymphoma | various surface, multiple lesions | anywhere | deep mucosa~submucosa | beltlike~multiple round, hypoechoic, homogenous | Centrocyte-like, lymphoepithelial lesion, plasma cell differentiation | κ or λ chain; t(11;18)/API2-MALT1 |
| Malignant lymphoma | various surface, multiple lesions | anywhere | initially deep mucosa~submucosa | beltlike~advanced carcinoma-like, hypoechoic, homogenous | CD20+, CD79a+; t(3;14)/BCL6-IGH | |
| Lipoma & lipogenic tumor | hill-shaped to pedunculated, intact mucosa (~yellowish), cushion sign | antrum | submucosa | round~oval, hyperechoic | Lipoblast (spider-web cell) | MDM2, CDK4 in well differenciated liposarcoma |
| Granular cell tumor | hemi-spherical, molar-like appearance, intact~ivoly | body | submucosa | round, heterogenously hypoechoic | eosinophilic granules | S-100, SOX10, CD68 |
| inflammatory fibroid polyp (IFP) | pedunculated or penis-like, may with erosion/ulcer | antrum | deep mucosa~submucosa | hypoechoic, relatively homogeneous | perivascular fibrosis (onion skin pattern), eosinophil infiltration | CD34, α-SMA; mutations in PDGFRA |
| inflammatory myofibroblastic tumor (IMT) | hill-shaped, mucosal color |
fornix~body | hypoechoic (not definite) | spindle cell & inflammatory cell infiltration | ALK, α-SMA, ALK-fusion, CD34, | |
| Solitary fibrous tumor (SFT) | n.d. | n.d. | n.d. | spindle cell, patternless pattern | CD34, nuclear STAT6, bcl2, CD99; NAB2-STAT6 fusion | |
| Glomus tumor | hemi-spherical, same color as mucosa | antrum | proper muscle | relatively hyperechoic~heterogenous | eosinophilic cell with oval nucleus | α-SMA |
| lymphangioma or cavenous hemangioma | flat-elavated, intact mucosa (whitish or dark-reddish, respectively), cushion sign | n.d. | deep mucosa~submucosa | aechoic~hyperechoic, multicystic | endothelial cells | CD31, CD34, Factor VIII in vascular tumor |
| PEComa | hemi-spherical~polypoid, intact mucosa | n.d. | submucosa | hypoechoic, homogenous | epithelioid cell with clear cytoplasm | α-SMA, HMB45, Melan A; LOH of TSC2 |
| Melanoma | pedunculated or lobular protrusion (may with melanosis), occasionally with erosion | n.d. | initially deep mucosa~submucosa | iso-hypoechoic, maybe regional lymph node metastasis | S-100, HMB45, Melan A, SOX10; mutations in BRAF ot KIT | |
| Desmoid | n.d. | n.d. | n.d. | spindle cell | nuclear β-catenin; alterations in CTNNB1 | |
| Metastatic tumor | bull’s eye~various, multiple lesions | n.d. | deep mucosa~submucosa | round~oval, hyperechoic & heterogenous | ||
n.d.: no definite data; &: Heimlich classification; Type 1: normal pancreatic tissue, Type 2: acinar cells & ducts, Type 3: ducts & hyperplasia of smooth muscle fibers.
Differentiation of GISTs from other tumors in the GI tract described above usually requires IHC in addition to hematoxylin and eosin staining, and, occasionally, genotyping (Table 2) [4,25,26,27]. In IHC, KIT (CD117) is expressed in ~95% of GISTs, and DOG1, a calcium-dependent, receptor-activated chloride channel protein, is expressed in ~95% of GISTs [25,26,27,28,29]. These two biomarkers usually show diffuse expression in tumor cells. KIT expression is regulated by ETV1, a transcription factor required for the proliferation of GISTs and ICCs, and the expression of which is, conversely, regulated by the MEK-MAPK pathway downstream of the KIT and PDGFRA tyrosine kinases [30]. It should be noted that melanoma, angiosarcoma, Ewing’s sarcoma, childhood neuroblastoma, seminoma, and small cell lung carcinoma may also show expression of the KIT protein by IHC [4,25,26,29]. In contrast, KIT expression is sometimes weak and faint in PDGFRA-mutated GISTs, in which DOG1 may be expressed [1,2,4,31]. CD34 may be expressed in GISTs but is less specific and less frequent (~70% of GISTs) [4,25]. S-100 is an immunohistochemical marker of neurogenic tumors, and alpha-smooth muscle actin and desmin are markers of myogenic tumors. As most GISTs with loss-of-function mutations in SDH subunits or with loss of expression due to methylation substantially do not express SDH subunit B, they are generally negative for SDHB in IHC [31]. A few GISTs may face diagnostic difficulty even with these IHCs and may require mutation research of the KIT and PDGFRA genes for their diagnosis.
2.2. Molecular Aspects of GIST
Molecularly, GISTs consist of heterogeneous subgroups, including GISTs with mutations in the KIT, PDGFRA, SDH genes, RAS genes, BRAF, NF1, or other rarely mutated genes as well as alterations [1,4,7,29,32,33]. KIT and PDGFRA have similar structures and similar downstream signaling pathways and are a type III receptor tyrosine kinase (RTK), a family including PDGFRB, CSF1R (macrophage colony-stimulating-factor receptor), and FLT3 (FMS-like tyrosine kinase 3) [34]. Small GISTs, including micro-GISTs and mini-GISTs, have KIT or PDGFRA mutations similar to those of clinical GISTs [16,35], and familial GISTs with germline mutations in KIT or PDGFRA accompanied by diffuse hyperplasia of ICC cells and multiple micro-GISTs and mini-GISTs with benign features [18,19,36]. These data and results from knocked-in mice indicate that mutations in the KIT or PDGFRA gene are an early neoplastic event and are considered to be causative of the GIST but are not always involved in malignant transformation [37].
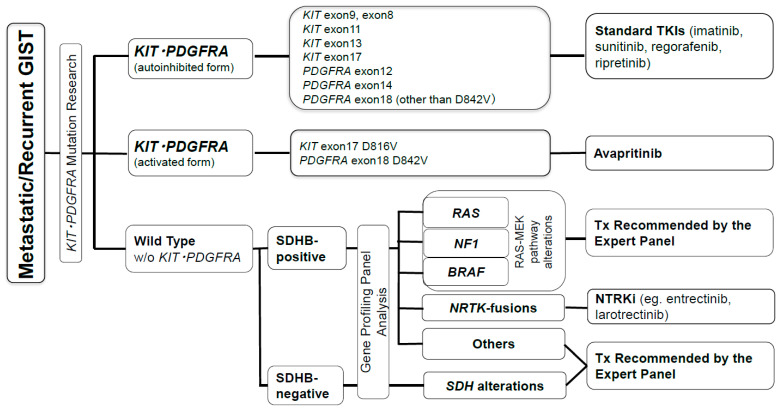
The frequent driver mutations found in GISTs include mutations in KIT (~70%) or PDGFRA (10~15%), followed by mutations in SDH family genes, in NF1, in BRAF, in RAS family genes [1,4,7,29,32,33], or, rarely, in other gene alterations including fusion genes involving the TRK family [38,39]. The molecular subtypes may somewhat correlate with the primary location as well as clinicopathological features (Table 1). For example, PDGFRA-mutated GISTs, found mainly in the stomach, may frequently show epithelioid cell features, and SDH-GISTs are located mainly in the stomach and show epithelioid features separated by fibrous bands, whereas NF1-mutated GISTs usually appear as spindle cell tumors in the small intestine [19,20,21,22,25,40]. More importantly, mutations are considered the most reliable biomarker of medical therapy. The molecular correlation with the clinicopathological features of GISTs and drug sensitivities are briefly summarized in Table 1 and Figure 1. GISTs without KIT or PDGFRA mutations, so-called “Wild-type GISTs”, may be divided into SDHB-competent (SDHB-positive by IHC) and SDHB-deficient (SDHB-negative by IHC) GISTs [4,31]. The latter may have mutations in a subunit of the SDH complex, including SDHA, SDHB, SDHC, or SDHD, or may have downregulated expression of the SDH complex through site-specific hypermethylation of the promoter regions [9,19,20]. The SDH-GIST is resistant to all available tyrosine kinase inhibitors (TKIs) in most cases and may partly show transient stabilization or decrease in size under VEGFR inhibitor treatment because its progression is thought to be driven by the expression of insulin growth factor-1 receptor (IGF1R) and vascular endothelial growth factor receptor (VEGFR) induced by hypoxia-inducible factor-1α (HIF-1α) [4,40]. The former includes GISTs with mutations in NF1, BRAF, or RAS, which are usually accompanied by activation of the MEK-MAPK pathway, implying that GISTs with these mutations may be potentially sensitive to MEK inhibitors and/or BRAF inhibitors (Table 1) [41,42].
Figure 1.
Mutational types and medical treatment for metastatic/recurrent GISTs. Abbreviations: TKI: tyrosine kinase inhibitor, Tx: therapy, NTRKi: NRTK inhibitor.
2.3. Clinical Diagnosis of GIST
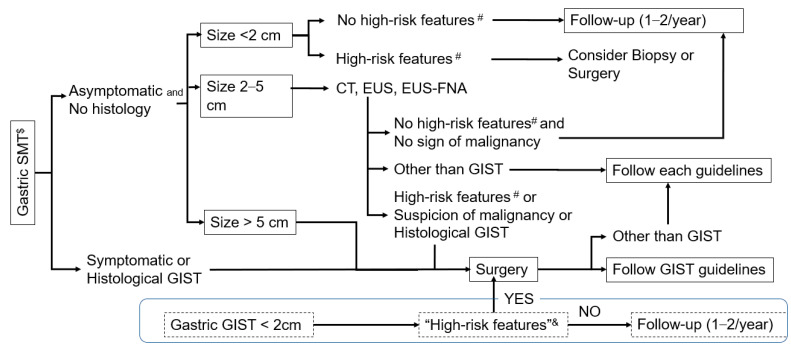
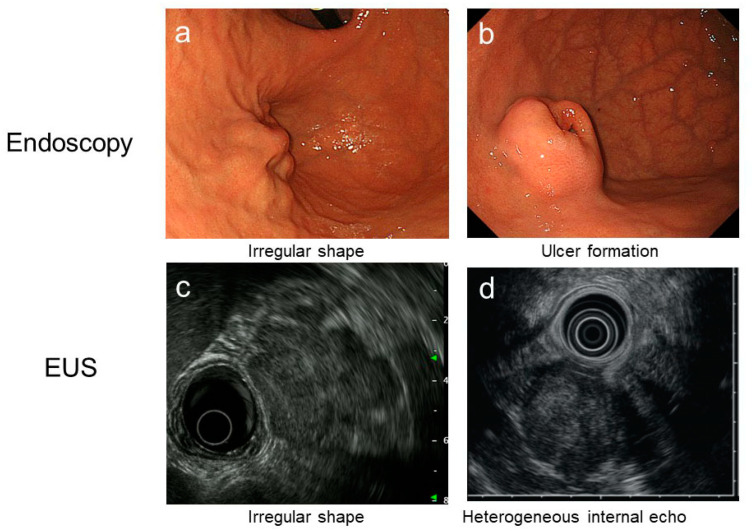
GISTs are initially found as SMTs and/or abdominal masses during exploration of the GI tract due to the abovementioned symptoms and signs or are incidentally found during cancer screening as mentioned. Less frequently, a work-up for emergent admission with GI bleeding or perforation may reveal a GIST [4]. Clinical diagnosis is performed by endoscopy, endoscopic ultrasonography (EUS), ultrasonography and/or CT scan, and a definitive diagnosis can be made only by pathological examinations after surgery or biopsy sampling. Figure 2 shows the diagnostic flow of gastric SMT proposed in the Japanese GIST guidelines [43]. Although CT scans may have advantages in terms of diagnosis, especially for tumors showing extramural growth and for the evaluation of disease spread [44], CT scans have accompanying radiation exposure, and EUS is still a major diagnostic tool for GISTs in the stomach and rectum. Gastric GIST is frequently found by endoscopy and/or fluoroscopy; thus, the initial work-up with endoscopy and EUS is important in the differential diagnosis of GISTs from other neoplastic SMTs. Here, we quickly summarize the characteristic endoscopic and EUS features of several neoplasms found in the stomach (Table 2). The other important role of endoscopy and EUS may be to identify SMTs that require treatment, such as GISTs. An irregular shape (Figure 3a), ulcer formation (Figure 3b), and/or rapid growth between endoscopy intervals together with an irregular shape (Figure 3c), heterogeneous internal echo (Figure 3d), and/or regional lymph node swelling by EUS may indicate malignant tumors including a GIST, and, thus, these findings are considered as high-risk features for SMTs [2,12,45]. If patients with small SMTs have high-risk features, we recommend surgical resection or tissue acquisition for pathological diagnosis depending on tumor size, location, and conditions (Figure 2).
Figure 2.
Diagnostic and treatment flow of gastric submucosal tumors and GISTs. Based on the Japanese GIST guidelines, the diagnostic and treatment flow of gastric submucosal tumors, including GISTs, is shown in the upper panel. In the lower panel, the approach to gastric GISTs < 2 cm is indicated according to the NCCN guidelines. $: SMT: submucosal tumor, #: high-risk features: irregular shape, ulcer formation, and/or rapid growth between intervals by endoscopy, and heterogeneous internal echo, irregular shape, and/or regional lymph node swelling by EUS (Ref. [43]), &: “high-risk features” including irregular border, cystic spaces, ulceration, echogenic foci, and heterogeneity (Ref. [2]). The former high-risk features (#) used in the Japanese GIST guidelines are endoscopic and EUS features of submucosal tumors that require treatment, whereas the latter “high-risk features” (&) in the NCCN guidelines include EUS features suggesting small gastric GISTs with potential disease progression.
Figure 3.
High-risk features by endoscopy and EUS. Representatives of high-risk features by endoscopy and EUS are shown.(a): endoscopic features of GIST with irregular shape; (b): endoscopic features of GIST with ulcer; (c): EUS features of GIST with irregular shape; (d): hypoechoic EUS features of GIST with heterogeneous internal echo.
In practice, the pathological diagnosis of GISTs is infrequent before surgery [4], and the clinical diagnosis is not always consistent with the pathological diagnosis. The pathological diagnosis can be obtained by pre-treatment biopsy. Sampling biopsy is usually performed either endoscopically or percutaneously when neoadjuvant therapy is considered for locally advanced GIST or medical therapy for metastatic, recurrent, and/or unresectable GIST (hereafter “metastatic/recurrent GIST”). The biopsy method may be dependent on tumor location, disease spread, and accessibility.
2.4. Tissue Acquisition for Pathological Diagnosis
The diagnostic yield of conventional endoscopic forceps biopsy is low in GISTs and is accompanied by a relative risk of bleeding [46]. EUS-guided fine needle aspiration (EUS-FNA) is safe and useful for pathological diagnosis, although the rate of tissue acquisition of EUS-FNA for GISTs and SMTs varies dependent on the tumor, location, and skill of the specialist, and is lower than that for pancreatic lesions [46,47,48]. The diagnostic accuracy of EUS-FNA may be improved by the introduction of rapid on-site evaluation (ROSE) [49,50]. Recently, EUS-guided biopsy sampling (EUS-FNB), equipped with the side-fenestrated reverse bevel design needle, was shown to be more reliable in obtaining sufficient tissue (91%) from pancreatic cancer than EUS-FNA (67%) [51]. EUS-FNB may be useful for IHC and genomic sequencing and, thus, may be a promising theranostic of GISTs. Alternative endoscopic approaches may include mucosal incision-assisted biopsy (MIAB) using the technique of endoscopic submucosal dissection (ESD) or endoscopic mucosal resection (EMR). MIAB has been shown to have similar diagnostic accuracy and safety to EUS-FNA with ROSE [50].
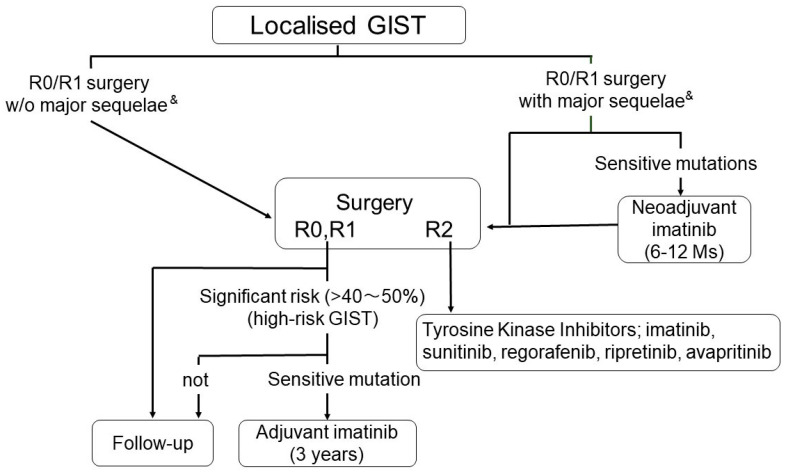
Although EUS-guided biopsy is preferred to percutaneous biopsy in terms of the risk of tumor cell dissemination, a percutaneous biopsy may be required for small intestinal lesions and metastatic diseases, depending on the situation. Several retrospective studies have indicated that percutaneous biopsy does not increase the risk of recurrence among patients with localized high-risk GISTs in the settings of postoperative adjuvant therapy [52,53]. In clinical practice, when a patient presents with an abdominal tumor that is highly likely to be a GIST, and that is resectable without extended surgery, one may consider surgery (Figure 2 and Figure 4). In fact, when surgery is scheduled ahead, EUS-FNA is unlikely to be recommended in the clinical guidelines of the European Society of Gastrointestinal Endoscopy (ESEG) [54]. For metastatic/recurrent diseases, a percutaneous image-guided biopsy is feasible and appropriate. However, when a percutaneous biopsy is not applicable and/or accessible and when medical treatment is urgently required, imatinib may be started without biopsy after clinical diagnosis of GISTs. In these situations, we recommend early response evaluation by enhanced CT scan or PET-CT approximately 1 month after treatment.
Figure 4.
Management of localized GISTs. &: “R0/R1 surgery with major sequela” indicates that surgery could reasonably be considered to be accompanied with risk for postoperative major morbidities and/or functional deficit.
3. Surgery
3.1. Surgical Therapy of Primary GISTs
Surgery is a mainstay and the only modality providing a permanent cure for primary localized GIST [2,4,43,55]. GISTs usually show expansive growth and rarely metastasize to lymph nodes except SHD-GIST. There is no known efficacy of prophylactic dissection of regional lymph nodes, and cherry-picking dissection of potentially metastatic lymph nodes is considered sufficient for GISTs, even for SDH-GISTs, which show frequent metastasis to lymph nodes [2,4]. GISTs are fragile and highly vascularized tumors; thus, they are gently manipulated and carefully de-vascularized during operations to avoid tumor rupture [2]. The principles of surgery in GIST cases include macroscopic complete resection (R0) and functional preservation of resected organs, ideally, by wedge resection [2,43]. R1 surgery may not always require re-excision in the imatinib era, and GISTs may be followed by watchful waiting with low relapsing risk, or may be treated with adjuvant therapy when the GIST is high risk in the risk stratifications and has imatinib-sensitive mutations [56,57,58].
For GISTs with imatinib-sensitive mutations, preoperative imatinib (neoadjuvant therapy) is recommended when the GIST is large, namely, when it is more than 10 cm, and/or is considered marginally resectable on technical grounds and location, or when the GIST is likely to have significant morbidity or functional deficit after surgery (Figure 4) [2,4,55,59,60]. The preoperative treatment period may be between approximately 6 and 12 months and should not exceed 1 year. Early evaluation of imatinib activities approximately 1 month after treatment is important, and imatinib could be continued when there is no disease progression by enhanced CT scan. When imatinib treatment is active, GISTs are decreased not only in size but also in vascularity, which may increase the safety of surgery and may prevent intraoperative rupture [61,62]. In a few cases, however, massive necrosis of GIST tumor cells may cause inflammatory responses and fibrous adhesions to surrounding tissues, and GIST may become brittle. Imatinib neoadjuvant therapy shows significant safety and feasibility [61,63]. The efficacy in prognostic improvement, preservation of organ function, such as in duodenal GISTs and rectal GISTs, and increase in R0 resection, as well as resectability, has yet to be determined, although several retrospective and prospective studies have indicated these possibilities [61,62,63,64]. At present, most GISTs undergoing neoadjuvant therapy are considered to be high-risk GISTs, at least before the treatment, and may be recommended to receive adjuvant therapy even after R0 surgery.
3.2. Surgical Therapy of Small GISTs
The incidence of clinical GISTs is estimated to be 10/million/year, whereas that of mini-GISTs is reportedly 1/1000, as described above. Several retrospective cohort studies have shown that there are a small but significant number of recurrences (less than 10%) after R0 surgery of GISTs less than 2 cm (<2 cm GIST) after 10 years of follow-up [65,66,67]. A subanalysis of the large epidemiologic study of <2 cm GISTs, however, did not show a significant decrease in disease-specific mortality of patients by surgical resection compared with observation (10.9% vs. 27.9%, p = 0.13), probably due to low statistical power [67]. Gastric GISTs have different immunohistochemical and genetic features from small intestinal GISTs [68,69]. The former is thought to be clinically indolent compared with the latter [2,4,11,24,65]. Most gastric mini-GISTs do not show malignant features or behaviors [2,12,13,16]; thus, they may not become clinical GISTs. There is inconsistency in the recommendations for surgical resection of gastric GISTs <2 cm. Surgical resection is recommended for gastric GISTs <2 cm in the Japanese and Asian GIST guidelines (Figure 2; upper panel) [43,55], whereas the NCCN guidelines recommend surgical resection for gastric GISTs <2 cm when they have “high-risk features” based on empirical evidence; otherwise, they could be followed by periodical EUS after shared decision-making (Figure 2; lower panel) [2]. All guidelines, including the NCCN and ESMO guidelines [2,43,55,59], recommend complete resection for GISTs in the rectum that are <2 cm because of their different clinical features and prognostic outcomes.
There have been many reports describing endoscopic resection of small GISTs and SMTs using ESD techniques, endoscopic full-thickness resection (EFTR), or others [70,71,72,73]. Endoscopic resection of small GISTs has been shown to be safe and feasible, and it has shown good prognostic outcomes. However, we need to be careful in interpreting these prognostic outcomes. Most gastric mini-GISTs may have an indolent clinical course and do not progress even without resection [12,16,74,75]. Furthermore, GIST patients have significant competing risks, including secondary cancer and cardiovascular diseases [57,76]. Hence, we need to identify features of small gastric GISTs indicating disease progression, resulting in a poor prognosis without treatment. Thus, it is necessary to narrow down small gastric GISTs requiring surgical resection. In this regard, the clinical features of GISTs indicating highly malignant potential are indicated to include an irregular shape, mucosal ulceration, and tumor size > 2 cm [77]. High-risk features of small gastric GISTs should be re-evaluated by prospective studies, and indications of surgery for gastric GISTs <2 cm should be reconsidered.
3.3. Laparoscopic Surgery for GISTs
Laparotomy has been standard in GIST surgery; now, laparoscopic surgery is also considered the standard procedure for surgery in cases of small GISTs less than 5 cm [4,12]. Laparoscopic surgery for GISTs has been shown to be less invasive, less painful, and have a faster postoperative recovery and better cosmetic outcomes than open surgery, with similar oncologic prognoses [12,78]. In laparoscopy, concomitant use of endoscopy may facilitate securing oncological margins and adequate patency of the remnant gastrointestinal lumen. One of the typical surgeries includes laparoscopic endoscopic cooperative surgery (LECS) and related procedures, which are feasible and safe in short-term outcomes and have similar oncological outcomes to those of open surgery after long-term follow-up [79,80]. These procedures benefit organ function preservation by minimizing resection of normal organs in addition to being less invasive. They may work for surgery of GISTs near the esophagogastric junction (EGJ) or pylorus, although surgery itself is technically demanding in these locations.
Initially, laparoscopic surgery is predominantly performed for GISTs that are smaller than 5 cm, but currently, it is applied to GISTs larger than 5 cm. Several retrospective studies and their meta-analyses suggest that laparoscopic surgery for large GISTs shows similar operation times, and less blood loss, postoperative morbidity, and shorter in-hospital days than open surgery [81,82,83]. Long-term oncologic outcomes in terms of disease-free survival (DFS) and overall survival (OS) are similar between the two. However, evidence of laparoscopy is very limited when the tumor is over 8 cm, and the application of laparoscopy may vary depending on the tumor location and conditions.
3.4. Risk Evaluation in GIST
Risk assessment in localized GISTs aims to identify GISTs likely to recur after surgery, hence, to identify GISTs requiring multidisciplinary treatment and/or intense follow-up. Tumor size, location, and mitotic count of tumor cells under a microscope are well-established independent prognostic factors [2,27,84]. They are included in several risk stratifications and nomograms, such as the National Institutes of Health (NIH) consensus criteria, the Armed Forces Institute of Pathology (AFIP) criteria, the modified NIH classification, and the Gold’s nomogram [84,85,86,87]. In the stratification systems, size was categorized as <2 cm, 2~5 cm, 5~10 cm, and >10 cm, and mitosis was categorized as <5/5 mm2, 5~10/5 mm2, > 10/5 mm2. However, these factors are continuous variables showing a non-linear relationship with recurrence risk [65]. Afterward, the prognostic contour map and the Gold’s nomogram were introduced [84,87]. Genotype, the presence of clinical symptoms, and histological necrosis, among other factors, have been reported to be possible prognostic factors; however, no factor is superior to size, location, or mitosis as an independent prognostic factor [4,88]. For example, GISTs with deletion mutations of codons 557–558 have been shown to have aggressive clinicopathological features and poorer prognosis, and most PDGFRA-mutated GISTs and SDH-GISTs show indolent features and better prognoses; however, they are not always shown to be independent for prognostic evaluation.
The modified NIH classification includes tumor rupture as a prognostic factor [65,84]. Rupture is a clinical factor that was not universally defined. Some retrospective studies have reported rupture as an independent prognostic factor, whereas others have not, probably due to different criteria for tumor rupture [58,89,90,91]. Recently, the universal definition of tumor rupture was proposed [58,89]. The composite definition of the rupture includes tumor fracture or spillage, blood-stained ascites, gastrointestinal perforation at the tumor site, microscopic infiltration of an adjacent organ, piecemeal resection, or incisional biopsy. In contrast, R1 surgery, intraluminal penetration of the tumor, needle biopsy, and peritoneal penetration of tumor cells in pathological examinations were not considered tumor rupture. Even with this definition, 10 to 20% of GISTs with tumor rupture do not recur during follow-up; in particular, GISTs with low mitotic counts show low recurrence rates even in the presence of tumor rupture [92]. The prognostic significance of tumor rupture should be prospectively re-evaluated according to the definition.
When patients have a significant risk of recurrence, imatinib adjuvant therapy is indicated after R0/R1 surgery. Details of the indication and duration of adjuvant therapy are discussed elsewhere [2,4,11,59,84], and, here, we briefly provide an overview of adjuvant therapy. Values indicating “significant risk” may vary depending on individuals. Clinical studies show that patients with high-risk GISTs in the risk stratifications may benefit from adjuvant therapy. Recurrence rates of high-risk GISTs may be estimated to be more than 40~50% after 10 years of follow-up [65,84]. The other important factor to be considered is imatinib sensitivity [4]. The guidelines do not recommend adjuvant therapy for PDGFRA D842V-mutated GISTs [2,4,59], nor is the therapy indicated for GISTs without KIT or PDGFRA mutations (“wild-type GIST”) because of the relatively indolent nature as well as their lack of imatinib responsiveness [59]. Clinical evidence suggests that adjuvant therapy for 3 years improves recurrence-free survival (RFS) as well as OS among patients with high-risk GISTs compared with 1-year adjuvant therapy [92,93]. The duration of adjuvant therapy may depend on the estimated recurrence risk and patient preference as well as conditions [4,59] and has not yet been established. Five years of adjuvant therapy shows that recurrences are rare during therapy and are frequently observed within a couple of years after stopping imatinib [94]. Recurrence after discontinuation of adjuvant therapy is very similar among 1-year, 2-year, 3-year [95], and 5-year adjuvant therapies, suggesting that imatinib activities are cytostatic. Thus, we may consider longer adjuvant therapy for very high-risk GISTs, such as ruptured GISTs. In fact, the ESMO guidelines indicate life-long adjuvant therapy for ruptured GISTs [59], if tolerable and if GISTs have imatinib-sensitive mutations.
4. Medical Therapy
4.1. Medical Therapy for Metastatic/Recurrent GISTs
TKIs are the primary choice for metastatic/recurrent GISTs [2,3,4,11,59]. Currently, five TKIs, namely, imatinib, sunitinib, regorafenib, ripretinib, and avapritinib, have significant clinical evidence for GIST treatment, but insurance reimbursement for recent two TKIs, ripretinib and avapritinib, may depend on the country. The details of the initial three TKIs, namely, imatinib, sunitinib, and regorafenib, are not addressed in this manuscript and are referred elsewhere for this information [2,3,4,11]. This paper focuses on emerging therapy and newly developing drugs for GISTs. Briefly, metastatic/recurrent GISTs with conventional KIT or PDGFRA mutations stabilized in the autoinhibited form (Table 1 and Figure 1) are initially treated with imatinib, and when resistance or intolerance to imatinib develops, sunitinib serves as the second-line treatment. When the GIST becomes refractory to sunitinib, regorafenib is used as the third-line, followed by ripretinib as the fourth-line treatment (Figure 1 and Table 1) [4,96]. When PDGFRA D842V mutations stabilized in the activated form are found, GISTs may be treated with avapritinib [4,97]. When there is no mutation in either the KIT or PDGFRA gene, the GIST should be subjected to targeted gene panel analysis or whole-exome sequencing; then, the patient can be advised to receive potential therapeutic agents based on the results (Figure 1 and Table 1). When no mutational information is available, the conventional first-line imatinib, second-line sunitinib, and third-line regorafenib are recommended. Regarding the correlation between mutation types and TKI activity, sunitinib has significant activity against KIT exon 9-mutant GISTs after imatinib therapy [98]. The majority of GISTs, which are initially responsive to imatinib, become refractory to the drug due to secondary mutations in either the ATP-binding domain (exons 13 and 14) or the activation loop domain (exons 16, 17, and 18) [99]. Sunitinib is active for ATP-binding domain mutations, but not for mutations in the activation loop [100]. Regorafenib, in contrast, has significant activity against GISTs with mutations in the activation loop [101,102].
4.2. Newly Emerging Therapy: New TKIs and Drugs for the NTRK Fusion
Ripretinib, a switch-control TKI inhibiting both KIT and PDGFRA kinases by securing the kinases in an inactive conformation, inhibits most primary and secondary mutations of KIT and PDGFRA in vitro [103]. In a pivotal phase III study, ripretinib was compared with placebo in GIST patients previously treated with imatinib, sunitinib, and regorafenib [96]. Progression-free survival (PFS; median PFS: 6.3 months for ripretinib and 1.0 months for placebo; hazard ratio (HR) = 0.15, p < 0.0001) and OS (median OS: 15.1 months for ripretinib and 6.6 months for placebo; HR = 0.36; p = 0.0004) were better with ripretinib. Toxicity was mild, and the drug was well tolerated. The most common adverse events observed in the ripretinib arm were alopecia, nausea, diarrhea, myalgia, fatigue, and palmar-plantar erythrodysesthesia syndrome. The FDA approved ripretinib for patients with metastatic/recurrent GIST who have received prior treatment with three or more kinase inhibitors. A phase III study comparing ripretinib with sunitinib in the second-line therapy for metastatic/recurrent GISTs is currently in progress.
Avapritinib is another oral TKI designed to selectively target the active conformation of KIT and PDGFRA via a type 1 inhibition mechanism and inhibits various KIT and PDGFRA mutations, including those resistant to the three approved TKIs. In the phase I study, avapritinib showed substantial clinical activity against PDGFRA-mutant GISTs [97]. Among patients with PDGFRA D842V-mutant GISTs, the response rate was 88% (49 of 56 patients), with five (9%) complete responses and 44 (79%) partial responses. There were no dose-limiting toxicities at doses of 30–400 mg per day. The FDA has approved avapritinib for PDGFRA exon 18 mutation-positive unresectable or metastatic GISTs. Avapritinib was evaluated in the third- or fourth-line settings by comparison with regorafenib, and failed to demonstrate superiority to regorafenib in terms of PFS (median PFS 4.2 months for avapritinib and 5.6 months for regorafenib; HR = 1.25; p = 0.055) [104].
GISTs without KIT and PDGFRA mutations may be called “wild-type GISTs”. A small proportion of gastrointestinal mesenchymal tumors and “wild-type GISTs” may have NTRK fusions, which are candidates for TRK inhibitors [105], although there is some discussion on GISTs with NTRK fusions. It is reported that NTRK rearrangement-containing mesenchymal tumors in the GI are clinically and morphologically heterogeneous, and that few may be related to GISTs [39]. Even so, larotrectinib, a selective oral TRK inhibitor, the first drug approved for solid tumors with an NTRK gene fusion, has shown a response rate (RR) of 79% in the pooled analysis of a phase 1 study in adults, a phase 1/2 study in children, and a phase 2 basket study [106]. Common adverse events include fatigue, dizziness, nausea, vomiting, increased AST, and cough. Entrectinib, which inhibits ROS1, ALK, three TRKs, and TRK-fusion tyrosine kinases, has shown an RR of 57% (31 of 54 patients) [107], and is approved by the FDA for NTRK gene fusion-positive solid tumors and ROS1-positive non-small cell lung cancer.
4.3. Developing Therapy
There are many developing drugs for GISTs, and here, we quickly discuss a few emerging drugs other than TKIs. Immune checkpoint inhibitors have been approved for a wide range of tumors, including lung cancer, melanoma, head and neck cancer, esophageal cancer, gastric cancer, urothelial cancer, and breast cancer, among others. Anti-programmed death-1 (PD-1) and anti-programmed death-ligand 1 (PD-L1) antibodies have also been evaluated in GISTs. A randomized phase II trial including 40 metastatic/recurrent GIST patients explored nivolumab, a monoclonal antibody for PD-1, combined with or without ipilimumab, a monoclonal antibody for the human T-cell receptor cytotoxic T-lymphocyte-associated antigen 4 (CTLA4), and the results showed only one response among 12 patients in the combination arm [108]. Pembrolizumab, an anti-PD-L1 antibody, was evaluated with epacadostat, a selective indoleamine 2,3-dioxygenase (IDO1) inhibitor; however, the study was terminated earlier due to insufficient clinical efficacy [109].
HSP90 is a molecular chaperone that stabilizes client proteins, including KIT, BRAF, and SDHs. HSP90 inhibitors have been evaluated in TKI-resistant GISTs. However, the development of a first-generation of HSP90 inhibitors, such as retaspimycin (IPI-504) and luminespib (AUY922), has failed to develop due to drug toxicities [110,111]. Additionally, the second-generation of pimitespib (TAS-116), an oral competitive inhibitor of cytosolic Hsp90α and β, showed a meaningful disease control rate of 85.0% and a median PFS of 4.4 months in the phase II study (n = 40). The drug was compared with placebo in a phase III clinical study. The results showed significantly improved PFS (median PFS = 2.8 months for pimitespib and 1.4 months for placebo; HR = 0.51; p = 0.006), and the median OS times were 13.8 months for pimitespib and 9.6 months (HR = 0.63; p = 0.081) for placebo, with tolerable safety profiles [112,113].
Selinexor, an oral selective inhibiter of nuclear export that functions by blocking exportin 1 (XPO1), is currently being explored in combination with imatinib for GISTs [114]. DS-6157a is an antibody-drug conjugate targeting G protein-coupled receptor 20 (GPR20), which is selectively expressed in GISTs [115], and currently, a phase I study is underway in the US and Japan. XmAb18087, a bispecific antibody against somatostatin receptor 2 (SSTR2) and CD3, is now being explored in a phase I study of patients with metastatic/recurrent GISTs [116].
5. Conclusions
GISTs are potentially malignant tumors in the GI tract, and clinical GISTs require treatment; however, the treatment impact for gastric GISTs that are less than 2 cm may be reconsidered because of their indolent nature and competing risks among these patients. Preoperative diagnosis of GISTs is challenging, and a work-up by means of endoscopy and EUS as well as endoscopic or percutaneous biopsy may be useful for the differential diagnosis and subsequent therapy of GISTs. Laparoscopy has similar safety and prognostic outcomes to those of laparotomy in terms of surgery of GISTs, including for tumors larger than 5 cm. Medical treatment with TKIs is the mainstay for recurrent/metastatic GISTs. The activity of each drug is well correlated with gene mutations and alterations; thus, in the era of precision medicine, cancer genome profiling should be considered when treatments are used. Targeted gene panel analysis and whole-exome sequencing may provide potential targeted therapy for “wild-type GISTs” and GISTs that are refractory to conventional TKIs.
Authors Contribution
Conceptualization, T.N.; Writing, T.N., S.Y., T.T. and Y.N.; Visualization, T.N., S.Y. and T.T.; Funding acquisition, T.N. All authors have read and agreed to the final version of the manuscript.
Acknowledgments
The authors acknowledge Yasuyuki Yoshida and Katsumi Yamamoto, Department of Pathology and Department of Medicine, Community Health-Care Organization Osaka Hospital, for their generous courtesy of pathological review and endoscopic pictures, respectively.
Abbreviations
| AFIP | Armed Forces Institute of Pathology |
| CSF1R | macrophage colony-stimulating-factor 1 receptor |
| CTLA4 | cytotoxic T-lymphocyte-associated antigen 4 |
| DFS | disease-free survival |
| EGJ | esophagogastric junction |
| EMR | endoscopic mucosal resection |
| ESD | endoscopic submucosal dissection |
| ESEG | European Society of Gastrointestinal Endoscopy |
| ESMO | European Society for Medical Oncology |
| EUS | endoscopic ultrasonography |
| EUS-FNA | EUS-guided fine needle aspiration |
| FDA | Food and Drug Administration |
| FLT3 | FMS-like tyrosine kinase 3 |
| GI tract | gastrointestinal tract |
| GIST | gastrointestinal stromal tumor |
| GPR20 | G protein-coupled receptor 20 |
| HIF-1α | hypoxia-inducible factor-1α |
| HR | hazard ratio |
| IGF1R | insulin growth factor-1 receptor |
| IHC | immunohistochemistry |
| IMT | inflammatory myofibroblastic tumor |
| LECS | laparoscopic endoscopic cooperative surgery |
| MIAB | mucosal incision-assisted biopsy |
| NCCN | National Comprehensive Cancer Network |
| NIH | National Institutes of Health |
| OS | overall survival |
| PD-1 | programmed death-1 |
| PD-L1 | programmed death-ligand 1 |
| PEComa | perivascular epithelioid cell tumor |
| PFS | progression-free survival |
| RFS | recurrence-free survival |
| ROSE | rapid on-site evaluation |
| RR | response rate |
| RTK | receptor tyrosine kinase |
| SDH | succinate dehydrogenase |
| SFT | solitary fibrous tumor |
| SMT | submucosal tumor |
| TKI | tyrosine kinase inhibitors |
| Tx | therapy |
| VEGFR | vascular endothelial growth factor receptor |
| SSTR2 | somatostatin receptor 2 |
Funding
The research was partly funded by Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (grant numbers: 20K21639 and 19H03722).
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to the review article and the study not involving humans and animals.
Informed Consent Statement
Patient consent was waived due to the review article and the study not involving humans.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
T.N.: honoraria from Pfizer, EA Pharma, Otsuka, and Shiseido outside the submitted work; Y.Y.: honoraria from Pfizer, Taiho, Nippon Kayaku, Eli Lilly, AstraZeneca, Merck Serono, Bayer, Meiji Seika, Roche Diagnostics, Novartis, Eisai, Chugai Pharmaceutical, Fuji Film Toyama Chemistry, and research funding from Roche Diagnostics. T.T.: honoraria from Pfizer, Taiho, Bayer, Tsumura, Novartis, SBI Pharma, and research funds from Taiho and SBI Pharma. S.Y.: None.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Corless C.L., Barnett C.M., Heinrich M.C. Gastrointestinal stromal tumours: Origin and molecular oncology. Nat. Rev. Cancer. 2011;11:865–878. doi: 10.1038/nrc3143. [DOI] [PubMed] [Google Scholar]
- 2.Demetri G.D., von Mehren M., Antonescu C.R., DeMatteo R.P., Ganjoo K.N., Maki R.G., Pisters P.W., Raut C.P., Riedel R.F., Schuetze S., et al. NCCN Task Force report: Update on the management of patients with gastrointestinal stromal tumors. J. Natl. Compr. Canc. Netw. 2010;8(Suppl. S2):S1–S41. doi: 10.6004/jnccn.2010.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Von Mehren M., Joensuu H. Gastrointestinal Stromal Tumors. J. Clin. Oncol. 2018;36:136–143. doi: 10.1200/JCO.2017.74.9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blay J.Y., Kang Y.K., Nishida T., von Mehren M. Gastrointestinal stromal tumours. Nat. Rev. Dis. Primers. 2021;7:22. doi: 10.1038/s41572-021-00254-5. [DOI] [PubMed] [Google Scholar]
- 5.Hirota S., Isozaki K., Moriyama Y., Hashimoto K., Nishida T., Ishiguro S., Kawano K., Hanada M., Kurata A., Takeda M., et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 6.Nishida T., Doi T., Naito Y. Tyrosine kinase inhibitors in the treatment of unresectable or metastatic gastrointestinal stromal tumors. Expert Opin Pharmacother. 2014;15:1979–1989. doi: 10.1517/14656566.2014.937707. [DOI] [PubMed] [Google Scholar]
- 7.Hemming M.L., Heinrich M.C., Bauer S., George S. Translational insights into gastrointestinal stromal tumor and current clinical advances. Ann. Oncol. 2018;29:2037–2045. doi: 10.1093/annonc/mdy309. [DOI] [PubMed] [Google Scholar]
- 8.Vanden Bempt I., Vander Borght S., Sciot R., Spans L., Claerhout S., Brems H., Lehnert S., Dehaspe L., Fransis S., Neuville B., et al. Comprehensive targeted next-generation sequencing approach in the molecular diagnosis of gastrointestinal stromal tumor. Genes Chromosomes Cancer. 2021;60:239–249. doi: 10.1002/gcc.22923. [DOI] [PubMed] [Google Scholar]
- 9.Janeway K.A., Kim S.Y., Lodish M., Nosé V., Rustin P., Gaal J., Dahia P.L., Liegl B., Ball E.R., Raygada M., et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc. Natl. Acad. Sci. USA. 2011;108:314–318. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huss S., Pasternack H., Ihle M.A., Merkelbach-Bruse S., Heitkötter B., Hartmann W., Trautmann M., Gevensleben H., Büttner R., Schildhaus H.U., et al. Clinicopathological and molecular features of a large cohort of gastrointestinal stromal tumors (GISTs) and review of the literature: BRAF mutations in KIT/PDGFRA wild-type GISTs are rare events. Hum. Pathol. 2017;62:206–214. doi: 10.1016/j.humpath.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Joensuu H., Hohenberger P., Corless C.L. Gastrointestinal stromal tumour. Lancet. 2013;382:973–983. doi: 10.1016/S0140-6736(13)60106-3. [DOI] [PubMed] [Google Scholar]
- 12.Nishida T., Goto O., Raut C.P., Yahagi N. Diagnostic and treatment strategy for small gastrointestinal stromal tumors. Cancer. 2016;122:3110–3118. doi: 10.1002/cncr.30239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawanowa K., Sakuma Y., Sakurai S., Hishima T., Iwasaki Y., Saito K., Hosoya Y., Nakajima T., Funata N. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum. Pathol. 2006;37:1527–1535. doi: 10.1016/j.humpath.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Abraham S.C., Krasinskas A.M., Hofstetter W.L., Swisher S.G., Wu T.T. “Seedling” mesenchymal tumors (gastrointestinal stromal tumors and leiomyomas) are common incidental tumors of the esophagogastric junction. Am. J. Surg. Pathol. 2007;31:1629–1635. doi: 10.1097/PAS.0b013e31806ab2c3. [DOI] [PubMed] [Google Scholar]
- 15.Agaimy A., Wünsch P.H., Dirnhofer S., Bihl M.P., Terracciano L.M., Tornillo L. Microscopic gastrointestinal stromal tumors in esophageal and intestinal surgical resection specimens: A clinicopathologic, immunohistochemical, and molecular study of 19 lesions. Am. J. Surg. Pathol. 2008;32:867–873. doi: 10.1097/PAS.0b013e31815c0417. [DOI] [PubMed] [Google Scholar]
- 16.Rossi S., Gasparotto D., Toffolatti L., Pastrello C., Gallina G., Marzotto A., Sartor C., Barbareschi M., Cantaloni C., Messerini L., et al. Molecular and clinicopathologic characterization of gastrointestinal stromal tumors (GISTs) of small size. Am. J. Surg. Pathol. 2010;34:1480–1491. doi: 10.1097/PAS.0b013e3181ef7431. [DOI] [PubMed] [Google Scholar]
- 17.Hedenbro J.L., Ekelund M., Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg. Endosc. 1991;5:20–23. doi: 10.1007/BF00591381. [DOI] [PubMed] [Google Scholar]
- 18.Nishida T., Hirota S., Taniguchi M., Hashimoto K., Isozaki K., Nakamura H., Kanakura Y., Tanaka T., Takabayashi A., Matsuda H., et al. Familial gastrointestinal stromal tumours with germline mutation of the KIT gene. Nat. Genet. 1998;19:323–324. doi: 10.1038/1209. [DOI] [PubMed] [Google Scholar]
- 19.Chompret A., Kannengiesser C., Barrois M., Terrier P., Dahan P., Tursz T., Lenoir G.M., Bressac-De Paillerets B. PDGFRA germline mutation in a family with multiplecases of gastrointestinal stromal tumor. Gastroenterology. 2004;126:318–321. doi: 10.1053/j.gastro.2003.10.079. [DOI] [PubMed] [Google Scholar]
- 20.Pasini B., McWhinney S.R., Bei T., Matyakhina L., Stergiopoulos S., Muchow M., Boikos S.A., Ferrando B., Pacak K., Assie G., et al. Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur. J. Hum. Genet. 2008;16:79–88. doi: 10.1038/sj.ejhg.5201904. [DOI] [PubMed] [Google Scholar]
- 21.Yantiss R.K., Rosenberg A.E., Sarran L., Besmer P., Antonescu C.R. Multiple gastrointestinal stromal tumors in type I neurofibromatosis: A pathologic and molecular study. Mod. Pathol. 2005;18:475–484. doi: 10.1038/modpathol.3800334. [DOI] [PubMed] [Google Scholar]
- 22.Nishida T., Tsujimoto M., Takahashi T., Hirota S., Blay J.Y., Wataya-Kaneda M. Gastrointestinal stromal tumors in Japanese patients with neurofibromatosis type I. J. Gastroenterol. 2016;51:571–578. doi: 10.1007/s00535-015-1132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haller F., Schulten H.J., Armbrust T., Langer C., Gunawan B., Füzesi L. Multicentric sporadic gastrointestinal stromal tumors (GISTs) of the stomach with distinct clonal origin: Differential diagnosis to familial and syndromal GIST variants and peritoneal metastasis. Am. J. Surg. Pathol. 2007;31:933–937. doi: 10.1097/01.pas.0000213440.78407.27. [DOI] [PubMed] [Google Scholar]
- 24.Shen Y.Y., Ma X.L., Yang L.X., Zhao W.Y., Tu L., Zhuang C., Ni B., Liu Q., Wang M., Cao H. Clinicopathologic characteristics, diagnostic clues, and prognoses of patients with multiple sporadic gastrointestinal stromal tumors: A case series and review of the literature. Diagn. Pathol. 2020;15:56. doi: 10.1186/s13000-020-00939-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miettinen M., Lasota J. Gastrointestinal stromal tumors: Review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch. Pathol. Lab. Med. 2006;130:1466–1478. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 26.Hirota S. Differential diagnosis of gastrointestinal stromal tumor by histopathology and immunohistochemistry. Transl. Gastroenterol. Hepatol. 2018;3:27. doi: 10.21037/tgh.2018.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dei Tos A.P., Hornick J.L., Miettinen M., Wanless I.R., Wardelmann E. Gastrointestinal Stromal Tumour. In: The WHO Classification of Tumours Editorial Board, editor. WHO Classification of Tumours Soft Tissue and Bone Tumours. 5th ed. IARC Press; Lyon, France: 2020. pp. 216–221. [Google Scholar]
- 28.Charville G.W., Longacre T.A. Surgical Pathology of Gastrointestinal Stromal Tumors: Practical Implications of Morphologic and Molecular Heterogeneity for Precision Medicine. Adv. Anat. Pathol. 2017;24:336–353. doi: 10.1097/PAP.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 29.Corless C.L. Gastrointestinal stromal tumors: What do we know now? Mod. Pathol. 2014;27(Suppl. S1):S1–S16. doi: 10.1038/modpathol.2013.173. [DOI] [PubMed] [Google Scholar]
- 30.Chi P., Chen Y., Zhang L., Guo X., Wongvipat J., Shamu T., Fletcher J.A., Dewell S., Maki R.G., Zheng D., et al. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumours. Nature. 2010;467:849–853. doi: 10.1038/nature09409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubin B.P., Heinrich M.C. Genotyping and immunohistochemistry of gastrointestinal stromal tumors: An update. Semin. Diagn. Pathol. 2015;32:392–399. doi: 10.1053/j.semdp.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Heinrich M.C., Corless C.L., Duensing A., McGreevey L., Chen C.J., Joseph N., Singer S., Griffith D.J., Haley A., Town A., et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 33.Agaram N.P., Wong G.C., Guo T., Maki R.G., Singer S., Dematteo R.P., Besmer P., Antonescu C.R. Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2008;47:853–859. doi: 10.1002/gcc.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roskoski R., Jr. Structure and regulation of Kit protein-tyrosine kinase—The stem cell factor receptor. Biochem. Biophys. Res. Commun. 2005;338:1307–1315. doi: 10.1016/j.bbrc.2005.09.150. [DOI] [PubMed] [Google Scholar]
- 35.Corless C.L., McGreevey L., Haley A., Town A., Heinrich M.C. KIT mutations are common in incidental gastrointestinal stromal tumors one centimeter or less in size. Am. J. Pathol. 2002;160:1567–1572. doi: 10.1016/S0002-9440(10)61103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manley P.N., Abu-Abed S., Kirsch R., Hawrysh A., Perrier N., Feilotter H., Pollett A., Riddell R.H., Hookey L., Walia J.S. Familial PDGFRA-mutation syndrome: Somatic and gastrointestinal phenotype. Hum. Pathol. 2018;76:52–57. doi: 10.1016/j.humpath.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 37.Bosbach B., Rossi F., Yozgat Y., Loo J., Zhang J.Q., Berrozpe G., Warpinski K., Ehlers I., Veach D., Kwok A., et al. Direct engagement of the PI3K pathway by mutant KIT dominates oncogenic signaling in gastrointestinal stromal tumor. Proc. Natl. Acad. Sci. USA. 2017;114:E8448–E8457. doi: 10.1073/pnas.1711449114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi E., Chmielecki J., Tang C.M., Wang K., Heinrich M.C., Kang G., Corless C.L., Hong D., Fero K.E., Murphy J.D., et al. FGFR1 and NTRK3 actionable alterations in “Wild-Type” gastrointestinal stromal tumors. J. Transl. Med. 2016;14:339. doi: 10.1186/s12967-016-1075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atiq M.A., Davis J.L., Hornick J.L., Dickson B.C., Fletcher C.D.M., Fletcher J.A., Folpe A.L., Mariño-Enríquez A. Mesenchymal tumors of the gastrointestinal tract with NTRK rearrangements: A clinicopathological, immunophenotypic, and molecular study of eight cases, emphasizing their distinction from gastrointestinal stromal tumor (GIST) Mod. Pathol. 2021;34:95–103. doi: 10.1038/s41379-020-0623-z. [DOI] [PubMed] [Google Scholar]
- 40.Maki R.G., Blay J.Y., Demetri G.D., Fletcher J.A., Joensuu H., Martín-Broto J., Nishida T., Reichardt P., Schöffski P., Trent J.C. Key Issues in the Clinical Management of Gastrointestinal Stromal Tumors: An Expert Discussion. Oncologist. 2015;20:823. doi: 10.1634/theoncologist.2014-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falchook G.S., Trent J.C., Heinrich M.C., Beadling C., Patterson J., Bastida C.C., Blackman S.C., Kurzrock R. BRAF mutant gastrointestinal stromal tumor: First report of regression with BRAF inhibitor dabrafenib (GSK2118436) and whole exomic sequencing for analysis of acquired resistance. Oncotarget. 2013;4:310–315. doi: 10.18632/oncotarget.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergqvist C., Wolkenstein P. MEK inhibitors in RASopathies. Curr. Opin. Oncol. 2021;33:110–119. doi: 10.1097/CCO.0000000000000711. [DOI] [PubMed] [Google Scholar]
- 43.Nishida T., Hirota S., Yanagisawa A., Sugino Y., Minami M., Yamamura Y., Otani Y., Shimada Y., Takahashi F., Kubota T., et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int. J. Clin. Oncol. 2008;13:416–430. doi: 10.1007/s10147-008-0798-7. [DOI] [PubMed] [Google Scholar]
- 44.Huh C.W., Jung D.H., Kim J.S., Shin Y.R., Choi S.H., Kim B.W. CT Versus Endoscopic Ultrasound for Differentiating Small (2–5 cm) Gastrointestinal Stromal Tumors From Leiomyomas. AJR Am. J. Roentgenol. 2019;213:586–591. doi: 10.2214/AJR.18.20877. [DOI] [PubMed] [Google Scholar]
- 45.Von Mehren M., Randall R.L., Benjamin R.S., Boles S., Bui M.M., Casper E.S., Conrad E.U., 3rd, DeLaney T.F., Ganjoo K.N., George S., et al. Gastrointestinal stromal tumors, version 2.2014. J. Natl. Compr. Canc. Netw. 2014;12:853–862. doi: 10.6004/jnccn.2014.0080. [DOI] [PubMed] [Google Scholar]
- 46.Akahoshi K., Oya M., Koga T., Shiratsuchi Y. Current clinical management of gastrointestinal stromal tumor. World. J. Gastroenterol. 2018;24:2806–2817. doi: 10.3748/wjg.v24.i26.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X.C., Li Q.L., Yu Y.F., Yao L.Q., Xu M.D., Zhang Y.Q., Zhong Y.S., Chen W.F., Zhou P.H. Diagnostic efficacy of endoscopic ultrasound-guided needle sampling for upper gastrointestinal subepithelial lesions: A meta-analysis. Surg. Endosc. 2016;30:2431–2441. doi: 10.1007/s00464-015-4494-1. [DOI] [PubMed] [Google Scholar]
- 48.Cazacu I.M., Singh B.S., Chavez A.A.L., Koduru P., Ejaz S., Weston B.R., Ross W.A., Lee J.H., Roy-Chowdhuri S., Bhutani M.S. EUS and EUS-guided FNA/core biopsies in the evaluation of subepithelial lesions of the lower gastrointestinal tract: 10-year experience. Endosc. Ultrasound. 2020;9:329–336. doi: 10.4103/eus.eus_51_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamura T., Yamashita Y., Ueda K., Kawaji Y., Itonaga M., Murata S.I., Yamamoto K., Yoshida T., Maeda H., Maekita T., et al. Rapid On-Site Evaluation by Endosonographers during Endoscopic Ultrasonography-Guided Fine-Needle Aspiration for Diagnosis of Gastrointestinal Stromal Tumors. Clin. Endosc. 2017;50:372–378. doi: 10.5946/ce.2016.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osoegawa T., Minoda Y., Ihara E., Komori K., Aso A., Goto A., Itaba S., Ogino H., Nakamura K., Harada N., et al. Mucosal incision-assisted biopsy versus endoscopic ultrasound-guided fine-needle aspiration with a rapid on-site evaluation for gastric subepithelial lesions: A randomized cross-over study. Digest. Endosc. 2019;31:413–421. doi: 10.1111/den.13367. [DOI] [PubMed] [Google Scholar]
- 51.Hedenström P., Marschall H.U., Nilsson B., Demir A., Lindkvist B., Nilsson O., Sadik R. High clinical impact and diagnostic accuracy of EUS-guided biopsy sampling of subepithelial lesions: A prospective, comparative study. Surg. Endosc. 2018;32:1304–1313. doi: 10.1007/s00464-017-5808-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yeh C.H., Pan K.T., Chu S.Y., Chen C.M., Hsu M.Y., Hung C.F., Tseng J.H. Safety and efficacy of image-guided percutaneous biopsies in the diagnosis of gastrointestinal stromal tumors. Clin. Imaging. 2012;36:19–23. doi: 10.1016/j.clinimag.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Eriksson M., Reichardt P., Sundby Hall K., Schütte J., Cameron S., Hohenberger P., Bauer S., Leinonen M., Reichardt A., Rejmyr Davis M., et al. Needle biopsy through the abdominal wall for the diagnosis of gastrointestinal stromal tumour—Does it increase the risk for tumour cell seeding and recurrence? Eur. J. Cancer. 2016;59:128–133. doi: 10.1016/j.ejca.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 54.Dumonceau J.M., Polkowski M., Larghi A., Vilmann P., Giovannini M., Frossard J.L., Heresbach D., Pujol B., Fernández-Esparrach G., Vazquez-Sequeiros E., et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2011;43:897–912. doi: 10.1055/s-0030-1256754. [DOI] [PubMed] [Google Scholar]
- 55.Koo D.H., Ryu M.H., Kim K.M., Yang H.K., Sawaki A., Hirota S., Zheng J., Zhang B., Tzen C.Y., Yeh C.N., et al. Asian Consensus Guidelines for the Diagnosis and Management of Gastrointestinal Stromal Tumor. Cancer Res. Treat. 2016;48:1155–1166. doi: 10.4143/crt.2016.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCarter M.D., Antonescu C.R., Ballman K.V., Maki R.G., Pisters P.W., Demetri G.D., Blanke C.D., von Mehren M., Brennan M.F., McCall L., et al. Microscopically positive margins for primary gastrointestinal stromal tumors: Analysis of risk factors and tumor recurrence. J. Am. Coll. Surg. 2012;215:53–59. doi: 10.1016/j.jamcollsurg.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cavnar M.J., Seier K., Curtin C., Balachandran V.P., Coit D.G., Yoon S.S., Crago A.M., Strong V.E., Tap W.D., Gönen M., et al. Outcome of 1000 Patients with Gastrointestinal Stromal Tumor (GIST) Treated by Surgery in the Pre- and Post-imatinib Eras. Ann. Surg. 2021;273:128–138. doi: 10.1097/SLA.0000000000003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishida T., Hølmebakk T., Raut C.P., Rutkowski P. Defining Tumor Rupture in Gastrointestinal Stromal Tumor. Ann. Surg Oncol. 2019;26:1669–1675. doi: 10.1245/s10434-019-07297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Casali P.G., Abecassis N., Aro H.T., Bauer S., Biagini R., Bielack S., Bonvalot S., Boukovinas I., Bovee J.V.M.G., Brodowicz T., et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018;29(Suppl. S4):iv68–iv78. doi: 10.1093/annonc/mdy095. [DOI] [PubMed] [Google Scholar]
- 60.Wang S.Y., Wu C.E., Lai C.C., Chen J.S., Tsai C.Y., Cheng C.T., Yeh T.S., Yeh C.N. Prospective Evaluation of Neoadjuvant Imatinib Use in Locally Advanced Gastrointestinal Stromal Tumors: Emphasis on the Optimal Duration of Neoadjuvant Imatinib Use, Safety, and Oncological Outcome. Cancers. 2019;11:424. doi: 10.3390/cancers11030424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kurokawa Y., Yang H.K., Cho H., Ryu M.H., Masuzawa T., Park S.R., Matsumoto S., Lee H.J., Honda H., Kwon O.K., et al. Phase II study of neoadjuvant imatinib in large gastrointestinal stromal tumours of the stomach. Br. J. Cancer. 2017;117:25–32. doi: 10.1038/bjc.2017.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cavnar M.J., Wang L., Balachandran V.P., Antonescu C.R., Tap W.D., Keohan M., Singer S., Temple L., Nash G.M., Weiser M.R., et al. Rectal Gastrointestinal Stromal Tumor (GIST) in the Era of Imatinib: Organ Preservation and Improved Oncologic Outcome. Ann. Surg. Oncol. 2017;24:3972–3980. doi: 10.1245/s10434-017-6087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang D., Zhang Q., Blanke C.D., Demetri G.D., Heinrich M.C., Watson J.C., Hoffman J.P., Okuno S., Kane J.M., von Mehren M., et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: Long-term follow-up results of Radiation Therapy Oncology Group 0132. Ann. Surg. Oncol. 2012;19:1074–1080. doi: 10.1245/s10434-011-2190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vassos N., Jakob J., Kähler G., Reichardt P., Marx A., Dimitrakopoulou-Strauss A., Rathmann N., Wardelmann E., Hohenberger P. Preservation of Organ Function in Locally Advanced Non-Metastatic Gastrointestinal Stromal Tumors (GIST) of the Stomach by Neoadjuvant Imatinib Therapy. Cancers. 2021;13:586. doi: 10.3390/cancers13040586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joensuu H., Vehtari A., Riihimäki J., Nishida T., Steigen S.E., Brabec P., Plank L., Nilsson B., Cirilli C., Braconi C., et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: An analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265–274. doi: 10.1016/S1470-2045(11)70299-6. [DOI] [PubMed] [Google Scholar]
- 66.Yanagimoto Y., Takahashi T., Muguruma K., Toyokawa T., Kusanagi H., Omori T., Masuzawa T., Tanaka K., Hirota S., Nishida T. Re-appraisal of risk classifications for primary gastrointestinal stromal tumors (GISTs) after complete resection: Indications for adjuvant therapy. Gastric Cancer. 2015;18:426–433. doi: 10.1007/s10120-014-0386-7. [DOI] [PubMed] [Google Scholar]
- 67.Coe T.M., Fero K.E., Fanta P.T., Mallory R.J., Tang C.M., Murphy J.D., Sicklick J.K. Population-Based Epidemiology and Mortality of Small Malignant Gastrointestinal Stromal Tumors in the USA. J. Gastrointest. Surg. 2016;20:1132–1140. doi: 10.1007/s11605-016-3134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nishitani A., Hirota S., Nishida T., Isozaki K., Hashimoto K., Nakagomi N., Matsuda H. Differential expression of connexin 43 in gastrointestinal stromal tumours of gastric and small intestinal origin. J. Pathol. 2005;206:377–382. doi: 10.1002/path.1799. [DOI] [PubMed] [Google Scholar]
- 69.Haller F., Happel N., Schulten H.J., von Heydebreck A., Schwager S., Armbrust T., Langer C., Gunawan B., Doenecke D., Füzesi L. Site-dependent differential KIT and PDGFRA expression in gastric and intestinal gastrointestinal stromal tumors. Mod. Pathol. 2007;20:1103–1111. doi: 10.1038/modpathol.3800947. [DOI] [PubMed] [Google Scholar]
- 70.Meng Y., Li W., Han L., Zhang Q., Gong W., Cai J., Li A., Yan Q., Lai Q., Yu J., et al. Long-term outcomes of endoscopic submucosal dissection versus laparoscopic resection for gastric stromal tumors less than 2 cm. J. Gastroenterol. Hepatol. 2017;32:1693–1697. doi: 10.1111/jgh.13768. [DOI] [PubMed] [Google Scholar]
- 71.Ko E.J., Bang B.W., Kwon K.S., Shin Y.W., Kim H.K. Endoscopic Enucleation Is Effective and Relatively Safe in Small Gastric Subepithelial Tumors Originating from Muscularis Propria. Dig. Dis. Sci. 2019;64:524–531. doi: 10.1007/s10620-018-5348-1. [DOI] [PubMed] [Google Scholar]
- 72.Li B., Chen T., Qi Z.P., Yao L.Q., Xu M.D., Shi Q., Cai S.L., Sun D., Zhou P.H., Zhong Y.S. Efficacy and safety of endoscopic resection for small submucosal tumors originating from the muscularis propria layer in the gastric fundus. Surg. Endosc. 2019;33:2553–2561. doi: 10.1007/s00464-018-6549-6. [DOI] [PubMed] [Google Scholar]
- 73.Zhai Y.Q., Chai N.L., Li H.K., Lu Z.S., Feng X.X., Zhang W.G., Liu S.Z., Linghu E.Q. Endoscopic submucosal excavation and endoscopic full-thickness resection for gastric schwannoma: Five-year experience from a large tertiary center in China. Surg. Endosc. 2020;34:4943–4949. doi: 10.1007/s00464-019-07285-w. [DOI] [PubMed] [Google Scholar]
- 74.Ye L.S., Li Y., Liu W., Yao M.H., Khan N., Hu B. Clinical course of suspected small gastrointestinal stromal tumors in the stomach. World J. Gastrointest. Surg. 2020;12:171–177. doi: 10.4240/wjgs.v12.i4.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Song J.H., Kim S.G., Chung S.J., Kang H.Y., Yang S.Y., Kim Y.S. Risk of progression for incidental small subepithelial tumors in the upper gastrointestinal tract. Endoscopy. 2015;47:675–679. doi: 10.1055/s-0034-1391967. [DOI] [PubMed] [Google Scholar]
- 76.Shen C., Wang C., He T., Cai Z., Yin X., Yin Y., Lu D., Zhang B., Zhou Z. Long-term survival among patients with gastrointestinal stromal tumors diagnosed after another malignancy: A SEER population-based study. World J. Surg. Oncol. 2020;18:88. doi: 10.1186/s12957-020-01868-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang Z., Gao Y., Fan X., Zhao X., Zhu S., Guo M., Liu Z., Yang X., Han Y. A multivariate prediction model for high malignancy potential gastric GI stromal tumors before endoscopic resection. Gastrointest. Endosc. 2020;91:813–822. doi: 10.1016/j.gie.2019.09.032. [DOI] [PubMed] [Google Scholar]
- 78.Xiong H., Wang J., Jia Y., Ye C., Lu Y., Chen C., Shen J., Chen Y., Zhao W., Wang L., et al. Laparoscopic surgery versus open resection in patients with gastrointestinal stromal tumors: An updated systematic review and meta-analysis. Am. J. Surg. 2017;214:538–546. doi: 10.1016/j.amjsurg.2017.03.042. [DOI] [PubMed] [Google Scholar]
- 79.Hiki N., Nunobe S., Matsuda T., Hirasawa T., Yamamoto Y., Yamaguchi T. Laparoscopic endoscopic cooperative surgery. Dig. Endosc. 2015;27:197–204. doi: 10.1111/den.12404. [DOI] [PubMed] [Google Scholar]
- 80.Hiki N., Nunobe S. Laparoscopic endoscopic cooperative surgery (LECS) for the gastrointestinal tract: Updated indications. Ann. Gastroenterol. Surg. 2019;3:239–246. doi: 10.1002/ags3.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ye L., Wu X., Wu T., Wu Q., Liu Z., Liu C., Li S., Chen T. Meta-analysis of laparoscopic vs. open resection of gastric gastrointestinal stromal tumors. PLoS ONE. 2017;12:e0177193. doi: 10.1371/journal.pone.0177193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lian X., Feng F., Guo M., Cai L., Liu Z., Liu S., Xiao S., Zheng G., Xu G., Zhang H. Meta-analysis comparing laparoscopic versus open resection for gastric gastrointestinal stromal tumors larger than 5 cm. BMC Cancer. 2017;17:760. doi: 10.1186/s12885-017-3741-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cui J.X., Gao Y.H., Xi H.Q., Cai A.Z., Zhang K.C., Li J.Y., Wei B., Chen L. Comparison between laparoscopic and open surgery for large gastrointestinal stromal tumors: A meta-analysis. World J. Gastrointest. Oncol. 2018;10:48–55. doi: 10.4251/wjgo.v10.i1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Joensuu H. Gastrointestinal stromal tumors: Risk assessment and adjuvant therapy. Hematol. Oncol. Clin. N. Am. 2013;27:889–904. doi: 10.1016/j.hoc.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 85.Fletcher C.D., Berman J.J., Corless C., Gorstein F., Lasota J., Longley B.J., Miettinen M., O’Leary T.J., Remotti H., Rubin B.P., et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum. Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 86.Miettinen M., Lasota J. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin. Diagn. Pathol. 2006;23:70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 87.Gold J.S., Gönen M., Gutiérrez A., Broto J.M., García-del-Muro X., Smyrk T.C., Maki R.G., Singer S., Brennan M.F., Antonescu C.R., et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: A retrospective analysis. Lancet Oncol. 2009;10:1045–1052. doi: 10.1016/S1470-2045(09)70242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Joensuu H., Rutkowski P., Nishida T., Steigen S.E., Brabec P., Plank L., Nilsson B., Braconi C., Bordoni A., Magnusson M.K., et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J. Clin. Oncol. 2015;33:634–642. doi: 10.1200/JCO.2014.57.4970. [DOI] [PubMed] [Google Scholar]
- 89.Hølmebakk T., Bjerkehagen B., Boye K., Bruland Ø., Stoldt S., Sundby Hall K. Definition and clinical significance of tumour rupture in gastrointestinal stromal tumours of the small intestine. Br. J. Surg. 2016;103:684–691. doi: 10.1002/bjs.10104. [DOI] [PubMed] [Google Scholar]
- 90.Nishida T., Cho H., Hirota S., Masuzawa T., Chiguchi G., Tsujinaka T., Kinki GIST Study Group Clinicopathological Features and Prognosis of Primary GISTs with Tumor Rupture in the Real World. Ann. Surg. Oncol. 2018;25:1961–1969. doi: 10.1245/s10434-018-6505-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hølmebakk T., Hompland I., Bjerkehagen B., Stoldt S., Bruland Ø.S., Hall K.S., Boye K. Recurrence-Free Survival After Resection of Gastric Gastrointestinal Stromal Tumors Classified According to a Strict Definition of Tumor Rupture: A Population-Based Study. Ann. Surg. Oncol. 2018;25:1133–1139. doi: 10.1245/s10434-018-6353-5. [DOI] [PubMed] [Google Scholar]
- 92.Casali P.G., Le Cesne A., Poveda Velasco A., Kotasek D., Rutkowski P., Hohenberger P., Fumagalli E., Judson I.R., Italiano A., Gelderblom H., et al. Time to Definitive Failure to the First Tyrosine Kinase Inhibitor in Localized GI Stromal Tumors Treated with Imatinib As an Adjuvant: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup Randomized Trial in Collaboration With the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J. Clin. Oncol. 2015;33:4276–4283. doi: 10.1200/JCO.2015.62.4304. [DOI] [PubMed] [Google Scholar]
- 93.Joensuu H., Eriksson M., Sundby Hall K., Reichardt A., Hermes B., Schütte J., Cameron S., Hohenberger P., Jost P.J., Al-Batran S.E., et al. Survival Outcomes Associated With 3 Years vs 1 Year of Adjuvant Imatinib for Patients with High-Risk Gastrointestinal Stromal Tumors: An Analysis of a Randomized Clinical Trial After 10-Year Follow-up. JAMA Oncol. 2020;6:1241–1246. doi: 10.1001/jamaoncol.2020.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Raut C.P., Espat N.J., Maki R.G., Araujo D.M., Trent J., Williams T.F., Purkayastha D.D., DeMatteo R.P. Efficacy and Tolerability of 5-Year Adjuvant Imatinib Treatment for Patients with Resected Intermediate- or High-Risk Primary Gastrointestinal Stromal Tumor: The PERSIST-5 Clinical Trial. JAMA Oncol. 2018;4:e184060. doi: 10.1001/jamaoncol.2018.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Benjamin R.S., Casali P.G. Adjuvant Imatinib for GI Stromal Tumors: When and For How Long? J. Clin. Oncol. 2016;34:215–218. doi: 10.1200/JCO.2015.64.0102. [DOI] [PubMed] [Google Scholar]
- 96.Blay J.Y., Serrano C., Heinrich M.C., Zalcberg J., Bauer S., Gelderblom H., Schöffski P., Jones R.L., Attia S., D’Amato G., et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:923–934. doi: 10.1016/S1470-2045(20)30168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heinrich M.C., Jones R.L., von Mehren M., Schöffski P., Serrano C., Kang Y.K., Cassier P.A., Mir O., Eskens F., Tap W.D., et al. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): A multicentre, open-label, phase 1 trial. Lancet Oncol. 2020;21:935–946. doi: 10.1016/S1470-2045(20)30269-2. [DOI] [PubMed] [Google Scholar]
- 98.Reichardt P., Demetri G.D., Gelderblom H., Rutkowski P., Im S.A., Gupta S., Kang Y.K., Schöffski P., Schuette J., Soulières D., et al. Correlation of KIT and PDGFRA mutational status with clinical benefit in patients with gastrointestinal stromal tumor treated with sunitinib in a worldwide treatment-use trial. BMC Cancer. 2016;16:22. doi: 10.1186/s12885-016-2051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Serrano C., Mariño-Enríquez A., Tao D.L., Ketzer J., Eilers G., Zhu M., Yu C., Mannan A.M., Rubin B.P., Demetri G.D., et al. Complementary activity of tyrosine kinase inhibitors against secondary kit mutations in imatinib-resistant gastrointestinal stromal tumours. Br. J. Cancer. 2019;120:612–620. doi: 10.1038/s41416-019-0389-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heinrich M.C., Maki R.G., Corless C.L., Antonescu C.R., Harlow A., Griffith D., Town A., McKinley A., Ou W.B., Fletcher J.A., et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J. Clin. Oncol. 2008;26:5352–5359. doi: 10.1200/JCO.2007.15.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.George S., Wang Q., Heinrich M.C., Corless C.L., Zhu M., Butrynski J.E., Morgan J.A., Wagner A.J., Choy E., Tap W.D., et al. Efficacy and safety of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of imatinib and sunitinib: A multicenter phase II trial. J. Clin. Oncol. 2012;30:2401–2407. doi: 10.1200/JCO.2011.39.9394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yeh C.N., Chen M.H., Chen Y.Y., Yang C.Y., Yen C.C., Tzen C.Y., Chen L.T., Chen J.S. A phase II trial of regorafenib in patients with metastatic and/or a unresectable gastrointestinal stromal tumor harboring secondary mutations of exon 17. Oncotarget. 2017;8:44121–44130. doi: 10.18632/oncotarget.17310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Smith B.D., Kaufman M.D., Lu W.P., Gupta A., Leary C.B., Wise S.C., Rutkoski T.J., Ahn Y.M., Al-Ani G., Bulfer S.L., et al. Ripretinib (DCC-2618) is a switch control kinase inhibitor of a broad spectrum of oncogenic and drug-resistant KIT and PDGFRA variants. Cancer Cell. 2019;35:738–751. doi: 10.1016/j.ccell.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 104.Kang Y.K., Suzanne G., Jones R.L., Rutkowski P., Shen L., Mir O., Patel S., Zhou Y., von Mehren M., Hohenberger P., et al. Avapritinib vs Regorafenib in Patients with Locally Advanced Unresectable or Metastatic Gastrointestinal Stromal Tumor (GIST): Efficacy and Safety Data from Phase 3 VOYAGER Study; Proceedings of the CTOS 2020; Boca Raton, FL, USA. 18‒21 November 2020; Poster 114. [Google Scholar]
- 105.Demetri G.D., Antonescu C.R., Bjerkehagen B., Bovée J.V.M.G., Boye K., Chacón M., Dei Tos A.P., Desai J., Fletcher J.A., Gelderblom H., et al. Diagnosis and management of tropomyosin receptor kinase (TRK) fusion sarcomas: Expert recommendations from the World Sarcoma Network. Ann. Oncol. 2020;31:1506–1517. doi: 10.1016/j.annonc.2020.08.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hong D.S., DuBois S.G., Kummar S., Farago A.F., Albert C.M., Rohrberg K.S., van Tilburg C.M., Nagasubramanian R., Berlin J.D., Federman N., et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21:531–540. doi: 10.1016/S1470-2045(19)30856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Doebele R.C., Drilon A., Paz-Ares L., Siena S., Shaw A.T., Farago A.F., Blakely C.M., Seto T., Cho B.C., Tosi D., et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21:271–282. doi: 10.1016/S1470-2045(19)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Singh A.S., Chmielowski B., Hecht J.R., Rosen L.S., Chow W.A., Wang X., Brackert S., Adame C., Bovill J., Schink E., et al. A randomized phase II study of nivolumab monotherapy versus nivolumab combined with ipilimumab in advanced gastrointestinal stromal tumor (GIST) J. Clin. Oncol. 2019;37:11017. doi: 10.1200/JCO.2019.37.15_suppl.11017. [DOI] [Google Scholar]
- 109.Epacadostat Pembrolizumab in Patients with, G.I.S.T. [(accessed on 8 May 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03291054.
- 110.Wagner A.J., Chugh R., Rosen L.S., Morgan J.A., George S., Gordon M., Dunbar J., Normant E., Grayzel D., Demetri G.D. A phase I study of the HSP90 inhibitor retaspimycin hydrochloride (IPI-504) in patients with gastrointestinal stromal tumors or soft-tissue sarcomas. Clin. Cancer Res. 2013;19:6020–6029. doi: 10.1158/1078-0432.CCR-13-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bendell J.C., Bauer T.M., Lamar R., Joseph M., Penley W., Thompson D.S., Spigel D.R., Owera R., Lane C.M., Earwood C., et al. A Phase 2 Study of the Hsp90 Inhibitor AUY922 as Treatment for Patients with Refractory Gastrointestinal Stromal Tumors. Cancer Investig. 2016;34:265–270. doi: 10.1080/07357907.2016.1193746. [DOI] [PubMed] [Google Scholar]
- 112.Doi T., Kurokawa Y., Sawaki A., Komatsu Y., Ozaka M., Takahashi T., Naito Y., Ohkubo S., Nishida T. Efficacy and safety of TAS-116, an oral inhibitor of heat shock protein 90, in patients with metastatic or unresectable gastrointestinal stromal tumour refractory to imatinib, sunitinib and regorafenib: A phase II, single-arm trial. Eur. J. Cancer. 2019;121:29–39. doi: 10.1016/j.ejca.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 113.Honma Y., Kurokawa Y., Sawaki A., Naito Y., Iwagami S., Baba H., Komatsu Y., Nishida T., Doi T. Randomized, double-blind, placebo-controlled, phase III trial of pimitespib (TAS-116), an oral inhibitor of heat shock protein 90 (HSP90), in patients with advanced gastrointestinal stromal tumor refractory to imatinib, sunitinib and regorafenib. [(accessed on 7 June 2021)];J. Clin. Oncol. 2021 39(Suppl. S15):11524. doi: 10.1200/JCO.2021.39.15_suppl.11524. Available online: https://meetinglibrary.asco.org/record/195669/abstract. [DOI] [Google Scholar]
- 114.Selinexor as a Single Agent and in Combination with Imatinib in Patients with Metastatic and/or Unresectable Gastrointestinal Stromal Tumors. (SeliGIST) [(accessed on 8 May 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04138381.
- 115.Iida K., Abdelhamid Ahmed A.H., Nagatsuma A.K., Shibutani T., Yasuda S., Kitamura M., Hattori C., Abe M., Hasegawa J., Iguchi T., et al. Identification and Therapeutic Targeting of GPR20, Selectively Expressed in Gastrointestinal Stromal Tumors, with DS-6157a, a First-in-Class Antibody-Drug Conjugate. [(accessed on 8 May 2021)];Cancer Discov. 2021 11:1508–1523. doi: 10.1158/2159-8290.CD-20-1434. Epub ahead of Print. & Clinical Trial of DS-6157a in Participants With Advanced Gastrointestinal Stromal Tumor (GIST) Available online: https://clinicaltrials.gov/ct2/show/NCT04276415. [DOI] [PubMed] [Google Scholar]
- 116.A Study of XmAb®18087 in Subjects With NET and GIST. [(accessed on 8 May 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03411.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in this article.