Abstract
Simple Summary
Fertility sparing treatment can be considered for young women with clinical stage 1A endometrial cancer (EC) without myometrial invasion (MI). Surgical diagnostic procedures (SDP) were needed to make diagnosis of EC, but different extents of SDP including diagnostic hysteroscopic biopsy (DHB, group 1), operative hysteroscopic partial resection (OHPR, group 2), operative hysteroscopic complete resection (OHCR, group 3), and cervical dilatation and fractional curettage (D&C, group 4) may affect the accuracy of MI assessment by magnetic resonance imaging (MRI) after SDP. Here, we retrospectively review those initially diagnosed with stage 1A EC and compare MI status on MRI reports and final histopathology of hysterectomy. We found that the MRI accuracy of MI was better in patients with EC diagnosed with D&C. Three diagnostic procedures using hysteroscopy might interfere with the diagnostic power of MI on MRI. Thus, D&C for diagnosis of EC and further hysteroscopic complete resection with hormone as a fertility sparing treatment for those confirmed as stage 1A without MI from MRI may be a choice in the future.
Abstract
Young women with endometrial cancer (EC) can choose fertility-sparing treatment for stage 1A disease without myometrial invasion (MI). The surgical diagnostic procedure (SDP) may affect the accuracy of magnetic resonance imaging (MRI) to assess MI. Here, we evaluated different SDP and compared the MI on MRI results with further pathologic results after hysterectomy. We retrospectively collected data on 263 patients with clinical stage IA EC diagnosed between January 2013 and December 2015. Patients were classified into four groups based on SDP, including diagnostic hysteroscopic biopsy (DHB, group 1), operative hysteroscopic partial resection (OHPR, group 2), operative hysteroscopic complete resection (OHCR, group 3), and cervical dilatation and fractional curettage (D&C, group 4). The sensitivity, specificity, diagnostic accuracy, positive predictive value, and negative predictive value of MRI to assess MI were 73.1%, 46.7%, 63.9%, 71.8%, and 48.3%, respectively. Three hysteroscopic procedures (groups 1 to 3) had a trend with a higher odds ratio of discrepancy between MRI and histopathology (p = 0.068), especially in group 2 (odds ratio 2.268, p = 0.032). Here, we found MRI accuracy of MI was better in patients with EC diagnosed with D&C. Three diagnostic procedures using hysteroscopy might interfere with the diagnostic power of MI on MRI.
Keywords: endometrial cancer, myometrial invasion, fertility-sparing, magnetic resonance imaging, hysteroscopy, dilation and curettage of endometrium
1. Introduction
Endometrial cancer (EC) is one of the most prevalent and emerging gynecologic malignancies, with over 382,000 newly diagnosed cases annually worldwide [1]. A trend of diagnosis in younger patients was found in the United States [2], which disclosed a need for fertility-sparing treatment (FST). In Taiwan, 10.3% of the cases were diagnosed at an age of less than 40 years in 2015, and the percentage was 8.4% before 2005 [3].
The treatment of EC is comprehensive staging surgery, including hysterectomy, bilateral salpingo-oophorectomy, and lymphadenectomy [4]. The FST uses progestin-containing hormonal therapy, either oral medication or levonorgestrel-releasing intrauterine system. Among the criteria for selecting suitable patients for FST, the presence of progesterone receptor (PR), no metastasis, and lack of myometrial invasion (MI) are the most important [3].
Currently, to evaluate the depth of myometrial invasion (MI), contrast-enhanced magnetic resonance imaging (MRI) is substantially better than ultrasonography and computed tomography (CT) [5]. The surgical diagnostic procedures (SDP) of EC included diagnostic hysteroscopic biopsy (DHB), operative hysteroscopic partial resection (OHPR), operative hysteroscopic complete resection, OHCR, and cervical dilatation and fractional curettage. Most of the MRI scans were performed after histologic proof of EC. The current retrospective study aimed to evaluate the accuracy of MRI in the assessment of MI for early-stage EC and its correlation with SDP.
2. Materials and Methods
2.1. Patients and Study Design
This retrospective study collected data on 365 patients diagnosed and treated for clinical stage IA EC from the electronic medical records from January 2013 to December 2015 at Chang Gung Memorial Hospital of Linkou branch, a tertiary medical center in northern Taiwan. The study was approved by the local ethics committee (IRB No. 201701008B0C501). After excluding 102 patients without preoperative MRI or total hysterectomy, 263 patients were finally included in the study.
The accuracy of MRI reports was evaluated based on a comparison of myometrial invasion between the MRI reports and the subsequent histopathological results of the hysterectomy specimen, which was considered as the gold standard. The MRI images were reviewed by a radiologist, Huang, who was a member of our multi-disciplinary gynecologic oncology team for more than 10 years. Additionally, the hysteroscopic biopsy in office was only grasp biopsy, and the operative hysteroscopic surgery was done by Mazzon’s technique. To evaluate the accuracy of MRI reports on MI, the study also evaluated different factors including age, SDP, tumor markers such as CA125, or the presence of estrogen receptor (ER) and PR. We classified the SDP into DHP (<5% tumor excision) as group 1, OHPR (5–70% tumor excision) as group 2, OHCR (>70% tumor excision) as group 3, and D&C as group 4. The degree of hysteroscopic tumor excision was based on surgical reports and operative images. The ER and PR were examined by immunochemistry after hysterectomy. The CA125 was checked after diagnosis of endometrial cancer and before the hysterectomy.
2.2. MRI Protocol
A 3T-MRI system (Tim Trio, Siemens, Erlangen, Germany) was used for the preoperative MRI assessment. The lower nine elements of the integrated spine coil and the lower six elements of the body-phased array coil were used to cover the entire pelvis [6]. Axial T1WI (repetition time msec/echo time msec: 626/11; average = 2; 256 × 320 matrix; 20 cm field of view (FOV)) and axial and sagittal T2WI (5630/87; average = 3; 256 × 320 matrix; 20 cm FOV) with a 4 mm section thickness/1 mm gap were applied.
Both axial and sagittal diffusion-weighted images (DWI) were obtained. DWI was performed using a single-shot echo-planar technique with fat suppression (3300 ms/79; average = 4; 4 mm section thickness; 1 mm gap; 128 × 128 matrix; 30 cm field of view). Apparent diffusion coefficient (ADC) maps were generated from isotropic DWI, with b-values of 0 and 1000 s/mm2, by calculating the slope of the logarithmic decay curve for signal intensity against b-value (Syngo, Siemens, Erlangen, Germany).
Axial and sagittal contrast-enhanced TIWI with fat saturation (567/10; average = 2; 4 mm section thickness; 1 mm gap; 256 × 320 matrix; 20 cm FOV) was acquired at approximately 120–180 s equilibrium phases after intravenous injection (0.1 mmol/kg bodyweight of contrast medium (Gadopentetate dimeglumine, Magnevist, Schering, Berlin, Germany), followed by a 20 mL saline flush at a rate of 2–3 mL/s). The study was performed during free breathing. No premedication or antiperistalsis agent was administered.
2.3. Histopathologic Analysis
Permanent paraffin sections were prepared to determine the final diagnosis of myometrium invasion. After the hysterectomy, the uterus was resected and cut into 5 mm thick sagittal sections to evaluate the gross extent of myometrial invasion. The pathologist assessed the deepest site of invasion and stained it with hematoxylin and eosin for microscopy. The endometrium was distinguished from the myometrium by the internal components of stroma cells and smooth muscle cells by microscopy. We further evaluated the endometrial–myometrial junctional line, which was smooth and intact in a healthy uterus but was disrupted by tumor cells invading the myometrium in patients with endometrial cancer. Moreover, the myometrial invasion depth could be assessed using a full-thickness cut section of the endometrial tumors at the deepest myometrial invasion point.
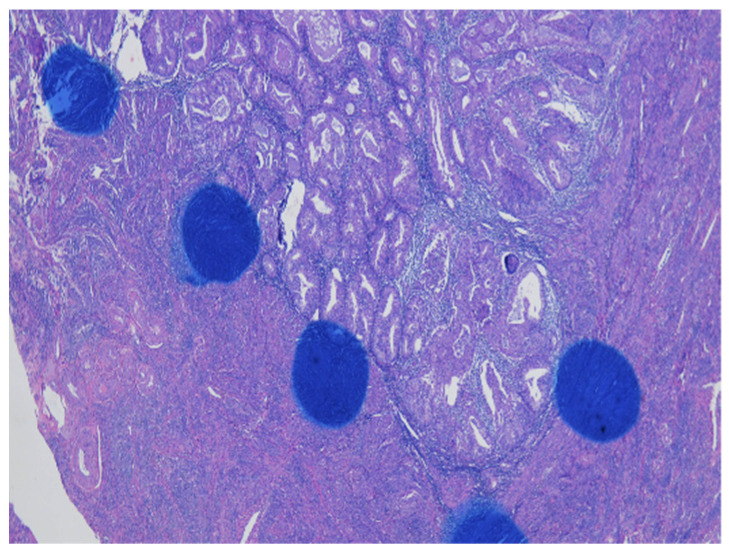
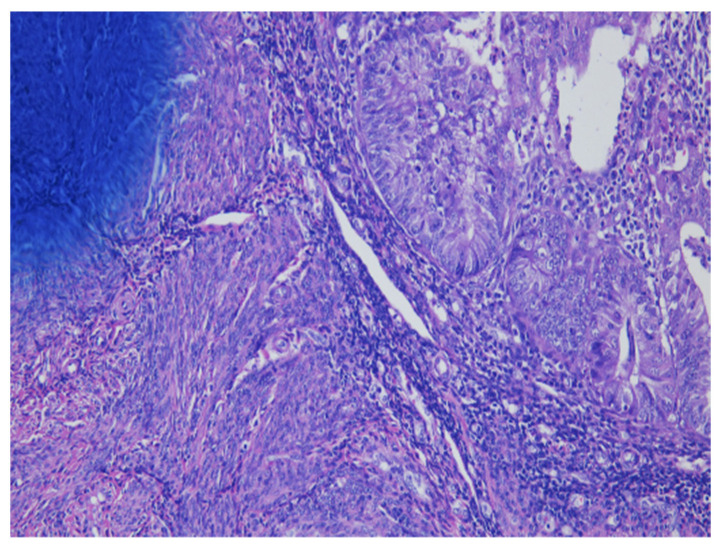
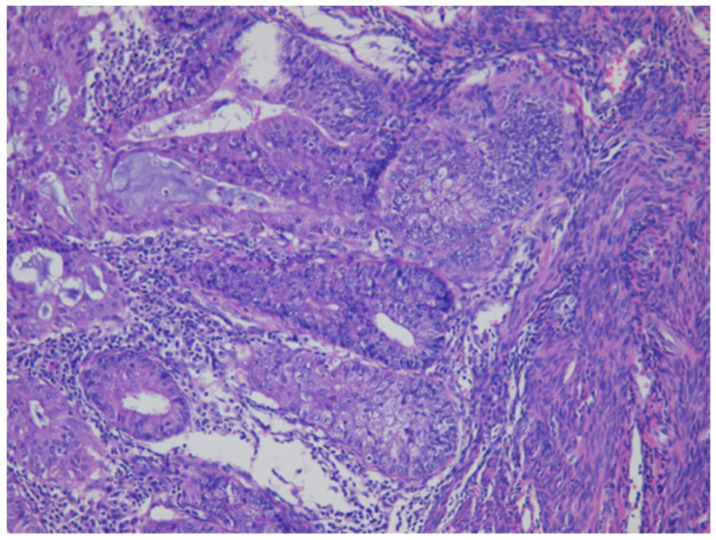
As shown in Figure 1 of EC at the uterine cornus, negative MI was found with an intact thin rim of stroma between the tumor–myometrium junction on the left side and with positive MI featuring an absence of stroma between the junction on the right side. A further higher power scope of the left side and right side of Figure 1 is shown in Figure 2 and Figure 3, respectively. Figure 2 demonstrated a thin rim of endometrial stroma between the tumor-myometrium junction (dark blue area: dark blue nuclei of stromal cells), and Figure 3 revealed the superficial early MI. MI determined by histopathology was the gold standard for comparison.
Figure 1.
Endometrial cancer at the cornus. Intact tumor–myometrial junction without myometrial invasion at the left side. Myometrial invasion without stroma presence between the junction at the right side (100× magnification).
Figure 2.
Thin rim of endometrial stroma between tumor–myometrium junction means no myometrial invasion (200× magnification).
Figure 3.
The absence of stromal cells in between tumor and myometrium hints the superficial myometrial invasion (200× magnification).
2.4. Statistical Analysis
The differences in MI determined by MRI and histopathology were compared using the chi-square test using SPSS (version 22.0, IBM). Logistic regression analysis was used to evaluate the different variables related to the accuracy of MRI reports for MI. The analyses were considered significant when the p-value was less than 0.05.
3. Results
A total of 263 patients were included, and their characteristics are shown in Table 1. The mean age of our cohort was 53.5 years. The endometrioid type comprised 94.7% of the histology, and other types included clear cell carcinoma, serous carcinoma, undifferentiated carcinoma, adenosquamous carcinoma, and well-differentiated carcinoma. The grade of differentiation was 1 in 62.7%, 2 in 26.6%, and 3 in 10.6% of patients. Table 2 shows the different MI degrees determined by histopathology or preoperative MRI. All MI depth assessed by MRI was less than 50% after radiologist review. From the MRI assessment in our cases, 33.8% had no MI observed and 66.2% had <50% of MI depth, respectively. The MI depth determined by histopathology was negative, less than 50%, and over 50% in 35.0%, 55.1%, and 9.9% of all patients, respectively.
Table 1.
Patients’ Characteristics.
| Number of Cases | 263 |
|---|---|
| Age, years (mean, +/− SD) | 53.5 +/− 10.3 |
| Histological type, N (%) | |
| Endometrioid | 249 (94.7) |
| Clear cell | 5 (1.9) |
| Serous | 5 (1.9) |
| Undifferentiated | 2 (0.8) |
| Adenosquamous | 1 (0.4) |
| Well-differentiated | 1 (0.4) |
| Tumor grade, N (%) | |
| 1 | 165 (62.7) |
| 2 | 70 (26.6) |
| 3 | 28 (10.6) |
Table 2.
The myometrial invasion assessment by MRI and histopathology.
| Myometrial Invasion | By Histology, N (%) | By MRI, N (%) |
|---|---|---|
| Negative | 92 (35.0) | 89 (33.8) |
| Positive, <50% thickness | 145 (55.1) | 174 (66.2) |
| Positive, ≥50% thickness | 26 (9.9) | 0 |
Table 3 shows a comparison of MI between MRI and histopathology. In the 92 patients without MI, MRI also showed negative results in 43 of them. In the 171 patients with MI, MRI was positive in 125 patients. The accuracy of MRI was 63.9%, and further evaluations with positive prediction value (PPV), negative prediction value (NPV), sensitivity, and specificity were 71.8%, 48.3%, 73.1%, and 46.7%, respectively. The kappa value was 0.2. In addition, the accuracies were 57.6%, 50.0%, 65.2%, and 69.4% in groups 1, 2, 3, and 4, respectively.
Table 3.
The analysis of sensitivity, specificity, and accuracy for assessment of myometrial invasion in MRI.
| Total Cohorts | Pathology | |||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| MRI | Positive | 125 (47.5%) | 49 (18.6%) | 174 (66.2%) |
| Negative | 46 (17.4%) | 43 (16.3%) | 89 (33.8%) | |
| Total | 171 (65.0%) | 92 (35.0%) | 263 (100%) | |
| Group 1 | Pathology | |||
| Positive | Negative | Total | ||
| MRI | Positive | 15 (45.5%) | 6 (18.2%) | 21 (63.6%) |
| Negative | 8 (24.2%) | 4 (12.1%) | 12 (36.3%) | |
| Total | 23 (69.7%) | 10 (30.3%) | 33 (100%) | |
| Group 2 | Pathology | |||
| Positive | Negative | Total | ||
| MRI | Positive | 15 (41.7%) | 9 (25.0%) | 24 (66.7%) |
| Negative | 9 (25.0%) | 3 (8.3%) | 12 (33.3%) | |
| Total | 24 (66.7%) | 12 (33.3%) | 36 (100%) | |
| Group 3 | Pathology | |||
| Positive | Negative | Total | ||
| MRI | Positive | 19 (41.3%) | 12 (26.1%) | 31 (67.4%) |
| Negative | 4 (8.7%) | 11 (23.9%) | 15 (32.6%) | |
| Total | 23 (50.0%) | 23 (50.0%) | 46 (100%) | |
| Group 4 | Pathology | |||
| Positive | Negative | Total | ||
| MRI | Positive | 69 (51.5%) | 19 (14.2%) | 88 (65.7%) |
| Negative | 22 (16.4%) | 24 (17.9%) | 46 (34.3%) | |
| Total | 91 (67.9%) | 43 (32.1%) | 134 (100%) | |
Table 4 shows the univariate analysis by logic regression of different variables regarding the accuracy of preoperative MRI for MI. To compare the consistency of results between MRI and histopathology results, a higher odds ratio detected from logistic regression analysis indicated less accuracy of MRI prediction power. No significant differences were detected in age, ER, PR, preoperative CA125, histology type, family history of EC, personal diabetes history, previous radiation history, or body matrix index. Although not significant, MRI was less accurate for patients with menopause onset after age 55 compared with those with menopause onset before age 55 (OR 1.853, CI 0.901–3.809, p = 0.093). The three diagnostic methods using hysteroscopy, including DHB (group 1), OHPR (group 2), and OHCR (group 3), showed a trend of less accuracy than D&C (group 4). However, the difference was not statistically significant. Among the three groups of hysteroscopic procedures, MRI in OHPR was the least accurate, which was statistically significant compared with MRI in D&C (OR 2.268, CI 1.072–4.80, p = 0.032). All the above details were listed in the Supplementary Table S1.
Table 4.
Univariate logistic regression analysis of myometrial invasion in MRI.
| Variables | N | OR (95% CI) | p-Value |
|---|---|---|---|
| Age | 263 | 0.978 (0.953–1.002) | 0.074 |
| ER | |||
| Negative | 17 | Reference | |
| Positive | 103 | 0.826 (0.281–2.430) | 0.729 |
| PR | |||
| Negative | 18 | Reference | |
| Positive | 102 | 1.243 (0.409–3.780) | 0.701 |
| CA125 (U/mL) | |||
| <35 | 196 | Reference | |
| ≥35 | 57 | 0.686 (0.363–1.296) | 0.245 |
| Menopause age (year) | |||
| <55 | 120 | Reference | |
| ≥55 | 42 | 1.853 (0.901–3.809) | 0.093 |
| Family History of EC | |||
| No | 258 | Reference | |
| Yes | 5 | 1.183 (0.194–7.207) | 0.856 |
| Diabetes history | |||
| No | 215 | Reference | |
| Yes | 48 | 0.861 (0.444–1.667) | 0.657 |
| BMI | |||
| <30 | 205 | Reference | |
| ≥30 | 58 | 1.461 (0.806–2.648) | 0.211 |
| Radiation history | |||
| No | 261 | Reference | |
| Yes | 2 | 1.777 (0.110–28.732) | 0.686 |
| Pre-debulking surgery | |||
| 1 | 33 | 0.737 (0.285–1.906) | 0.529 |
| 2 | 36 | Reference | |
| 3 | 46 | 0.533 (0.219–1.301) | 0.167 |
| Pre-debulking surgery | |||
| 1, 2 | 69 | 1.902 (1.078–3.571) | 0.027 |
| 3 | 46 | 1.21 (0.595–2.459) | 0.599 |
| 4 | 134 | Reference | |
| Pre-debulking surgery | |||
| 1, 2, 3 | 115 | 1.625 (0.964–2.739) | 0.068 |
| 4 | 134 | Reference | |
| Pre-debulking surgery | |||
| 1 | 33 | 1.671 (0.765–3.653) | |
| 2 | 36 | 2.268 (1.072–4.80) | 0.198 |
| 3 | 46 | 1.21 (0.595–2.459) | 0.032 |
| 4 | 134 | Reference | 0.599 |
4. Discussion
The incidence of EC has increased in recent decades, and the age at diagnosis tends to decrease [2]. FST is a common issue in current practice, and most of them are achieved by progestin-containing hormonal therapy. High-dose progesterone (megestrol acetate, 160 mg/day) after hysteroscopic resection of endometrial tumor obtained a complete response in 81.1% of patients and a subsequent pregnancy rate of 13.3% [7]. Approximately 50% of the complete responders experienced recurrence and even late recurrence at 156 months after treatment [8]. For obese patients or those who experience side effects after the administration of oral progesterone [9], the levonorgestrel intrauterine device (LNG-IUD) was reported to achieve a complete response rate of 80% after 10.2 months of the observation period [10,11]. Metformin had anticancer effects including mTOR pathway blockade leading to downregulation of neovascularization [12], tumor cell apoptosis induction at the mitochondrial level [13], and inhibition of epithelial-to-mesenchymal transition [14]. Metformin combined with cyproterone/ethinyl estradiol resulted in 100% regression of endometrial cancer without MI [15].
The selection criteria for FST include younger age, nulliparity or not, cell type, tumor grading, presence of PR, serum tumor marker level, and negative or superficial MI [3]. Among these, MI is not only a prognostic factor of survival or lymph node metastasis [16] but also a predictor of the feasibility of fertility-sparing management in EC [3,17,18,19]. Contrast-enhanced MRI detects MI more accurately than ultrasound, CT, or non-contrast MRI, as shown in a meta-analysis [5]. Dynamic contrast-enhanced MRI detects the disruption of sub endometrial enhancement and irregular peritumoral enhancement to achieve an accuracy rate of 85–91%, which is higher than the 68–82% of non-contrast MRI [20,21,22], especially in those with unclear junctional zones such as postmenopausal patients [23]. Previous studies have suggested that diffusion-weighted image (DWI) MRI has a better ability for deep MI detection than dynamic contrast-enhanced MRI [24]. However, one meta-analysis showed only slightly higher specificity toward DWI MRI images without significance [25].
The guidelines of the European Society of Urogenital Radiology (ESUR) suggested the use of MRI to assess MI in patients of childbearing age for FST [26]. Table 5 lists several suggestions for MI assessment that are quoted in the published clinical guideline, and MRI was still the preferred tool, if available, when compared with computed tomography (CT) and positron emission tomography (PET). The detection accuracy was reported as only 12% for lesion size of 4 mm or less by PET-CT, which was unsuitable for making an evaluation of intrauterine disease, including MI or endocervix [27]. PET-CT had limited detection of pelvic lymph node metastasis in early stage EC, so the discrimination of low or intermediate risk was also insufficient by PET-CT alone [28], although PET-MRI maybe an alternate selection [29]. Adenomyosis was another challenge to differentiate from EC since both involve interruption of the endometrial–myometrial interface, and MRI with diffusion-weighted imaging can be considered as a diagnostic tool [30]. Clear cell EC may have myometrial infiltration without obvious endometrial thickening, which is difficult to detect by hysteroscopy due to disease being confined to the myometrium and because it may be mistaken for adenomyosis [31]. Doppler ultrasound to evaluate different subendometrial vascular patterns can be helpful [31,32]
Table 5.
Current clinical guidelines related to MRI assessment of MI in EC.
| The Guidelines | Quote the Content Related with MI Assessment |
|---|---|
| British Gynecologic Cancer Society (BCGS) [33] |
|
| European Society for Medical Oncology (ESMO), European Society for Radiotherapy and Oncology (ESTRO), and European Society of Gynaecological Oncology (ESGO) consensus conference [34] |
|
| European Society of Urogenital Radiology (ESUR) [26] |
|
| GEICO (Spanish Group for Investigation in Ovarian Cancer) and SEOM (Spanish Society of Medical Oncology) [35] |
|
| Japan Society of Gynecologic Oncology (JSGO) [36] |
|
| National Comprehensive Cancer Network (NCCN) guideline [37] |
|
| Society of Gynecologic Oncology (SGO) [38] |
|
From Table 4, patients at late menopause, later than 55-years-old, may have a more obscured junctional zone in the uterus due to age [23], and the accuracy for MRI assessment of MI was limited when compared with those at menopause earlier than 55-years-old. Additionally, such patients should undergo hysterectomy as standard protocol instead of FST. For premenopausal women, dynamic contrast-enhanced MRI was superior to DWI in the assessment of MI [39]. In our study, the accuracy rate was 63%. MRI underestimated 51.7% of patients with MI and overestimated another 35% of patients without MI.
Our study demonstrated a lower accuracy rate of MI in patients after hysteroscopic diagnostic procedures than in conventional D&C. This might be due to the thermal effect of tissue injury by cauterization. To the best of our knowledge, the present study is the first to compare the influence of different diagnostic procedures on the detection of MI. Since hysteroscopy has the advantage of seeing a small lesion that might be missed by conventional D&C, it was therefore favorable in these patients whose ultrasound showed a small focal lesion. Conventional D&C is suitable for patients with obvious lesions on ultrasound for histopathology proof and for decreasing the tumor burden to enhance the FST.
Although the image results and histopathology were reviewed, the limitation of our retrospective study still showed possible inconsistencies in diagnostic procedures, chart records, and sequence of clinical management. Future research is needed to identify other potential biomarkers, including polymerase epsilon (POLE) mutation, microsatellite instability (MSI), TP53 mutation, CTNNB1 mutations (encoding β-catenin), L1CAM (L1 cell adhesion molecule), or PTEN mutation for a more precise selection of patients for fertility-sparing management.
5. Conclusions
Our study showed that conventional D&C had the least interference with MI depth assessment from MRI and that OHPR had the most disturbance. To make a better selection for fertility-sparing treatment of early EC, DHB, OHCR, and conventional D&C should be performed in different situations.
Acknowledgments
This work was supported by the Chang-Gung Memorial Hospital integrated research project (CORPG3G0401). The results of the current study were presented at the bi-annual meeting of the Asian Society of Gynecologic Oncology in 2017. The authors acknowledge the statistical assistance provided by the Clinical Trial Center, Chang Gung Memorial Hospital, Linkou, Taiwan, which was founded by the Ministry of Health and Welfare of Taiwan; MOHW108-TDU-B-212-133005.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13133275/s1, Table S1: patient list.
Author Contributions
Conceptualization, W.-C.C., H.-H.C. and T.-C.C.; methodology, W.-C.C., L.-T.H., Y.-T.H., S.-H.U. and T.-C.C.; software, L.-T.H. and Y.-B.P.; validation, W.-C.C., L.-T.H. and T.-C.C.; formal analysis, W.-C.C., L.-T.H. and T.-C.C.; investigation, W.-C.C., L.-T.H., Y.-T.H. and S.-H.U.; resources, T.-C.C.; data curation, W.-C.C., L.-T.H. and Y.-B.P.; writing—original draft preparation, W.-C.C.; writing—review and editing, W.-C.C., H.-H.C. and T.-C.C.; visualization, T.-C.C.; supervision, H.-H.C. and T.-C.C.; project administration, T.-C.C.; funding acquisition, T.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Chang-Gung Memorial Hospital integrated research project, grant number “CORPG3G0401”.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of Chang Gung Memorial Hospital (IRB No. 201701008B0C501, on 13 April 2018).
Informed Consent Statement
Patient consent was waived since this was a retrospective study using existing data in our electronic chart system, and there was no further use of existing specimens for further staining. Therefore, the patients’ informed consent was waived after approval by the Institutional Review Board of Chang Gung Memorial Hospital.
Data Availability Statement
The data presented in this study are available in Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the current study.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang S., Gong T.T., Liu F.H., Jiang Y.T., Sun H., Ma X.X., Zhao Y.H., Wu Q.J. Global, Regional, and National Burden of Endometrial Cancer, 1990–2017: Results from the Global Burden of Disease Study, 2017. Front. Oncol. 2019;9:1440. doi: 10.3389/fonc.2019.01440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2012. CA Cancer J. Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Lai C.-H., Wang C.-J., Chao A. The Clinical Management of Endometrial Cancer in Young Women. Curr. Obstet. Gynecol. Rep. 2013;2:26–31. doi: 10.1007/s13669-012-0032-5. [DOI] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists ACOG practice bulletin, clinical management guidelines for obstetrician-gynecologists, number 65, August 2005: Management of endometrial cancer. Obs. Gynecol. 2005;106:413–425. doi: 10.1097/00006250-200508000-00050. [DOI] [PubMed] [Google Scholar]
- 5.Kinkel K., Kaji Y., Yu K.K., Segal M.R., Lu Y., Powell C.B., Hricak H. Radiologic staging in patients with endometrial cancer: A meta-analysis. Radiology. 1999;212:711–718. doi: 10.1148/radiology.212.3.r99au29711. [DOI] [PubMed] [Google Scholar]
- 6.Lin G., Ng K.K., Chang C.J., Wang J.J., Ho K.C., Yen T.C., Wu T.I., Wang C.C., Chen Y.R., Huang Y.T., et al. Myometrial invasion in endometrial cancer: Diagnostic accuracy of diffusion-weighted 3.0-T MR imaging-initial experience. Radiology. 2009;250:784–792. doi: 10.1148/radiol.2503080874. [DOI] [PubMed] [Google Scholar]
- 7.Chao A.S., Chao A., Wang C.J., Lai C.H., Wang H.S. Obstetric outcomes of pregnancy after conservative treatment of endometrial cancer: Case series and literature review. Taiwan J. Obstet. Gynecol. 2011;50:62–66. doi: 10.1016/j.tjog.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Wang C.J., Chao A., Yang L.Y., Hsueh S., Huang Y.T., Chou H.H., Chang T.C., Lai C.H. Fertility-preserving treatment in young women with endometrial adenocarcinoma: A long-term cohort study. Int. J. Gynecol. Cancer. 2014;24:718–728. doi: 10.1097/IGC.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 9.Shan W., Wang C., Zhang Z., Gu C., Ning C., Luo X., Zhou Q., Chen X. Conservative therapy with metformin plus megestrol acetate for endometrial atypical hyperplasia. J. Gynecol. Oncol. 2014;25:214–220. doi: 10.3802/jgo.2014.25.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown A.J., Westin S.N., Broaddus R.R., Schmeler K. Progestin intrauterine device in an adolescent with grade 2 endometrial cancer. Obs. Gynecol. 2012;119:423–426. doi: 10.1097/AOG.0b013e318234d97c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim M.L., Seong S.J. Clinical applications of levonorgestrel-releasing intrauterine system to gynecologic diseases. Obs. Gynecol. Sci. 2013;56:67–75. doi: 10.5468/OGS.2013.56.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boussios S., Mikropoulos C., Samartzis E., Karihtala P., Moschetta M., Sheriff M., Karathanasi A., Sadauskaite A., Rassy E., Pavlidis N. Wise Management of Ovarian Cancer: On the Cutting Edge. J. Pers. Med. 2020;10:41. doi: 10.3390/jpm10020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J.S., Turbov J., Rosales R., Thaete L.G., Rodriguez G.C. Combination simvastatin and metformin synergistically inhibits endometrial cancer cell growth. Gynecol. Oncol. 2019;154:432–440. doi: 10.1016/j.ygyno.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 14.Lee T.Y., Martinez-Outschoorn U.E., Schilder R.J., Kim C.H., Richard S.D., Rosenblum N.G., Johnson J.M. Metformin as a Therapeutic Target in Endometrial Cancers. Front. Oncol. 2018;8:341. doi: 10.3389/fonc.2018.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X., Guo Y.R., Lin J.F., Feng Y., Billig H., Shao R. Combination of Diane-35 and Metformin to Treat Early Endometrial Carcinoma in PCOS Women with Insulin Resistance. J. Cancer. 2014;5:173–181. doi: 10.7150/jca.8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boronow R.C., Morrow C.P., Creasman W.T., Disaia P.J., Silverberg S.G., Miller A., Blessing J.A. Surgical staging in endometrial cancer: Clinical-pathologic findings of a prospective study. Obs. Gynecol. 1984;63:825–832. [PubMed] [Google Scholar]
- 17.Zivanovic O., Carter J., Kauff N.D., Barakat R.R. A review of the challenges faced in the conservative treatment of young women with endometrial carcinoma and risk of ovarian cancer. Gynecol. Oncol. 2009;115:504–509. doi: 10.1016/j.ygyno.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Yang J.X. Fertility-preserving treatment in women with early endometrial cancer: The Chinese experience. Cancer Manag. Res. 2018;10:6803–6813. doi: 10.2147/CMAR.S188087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitsuhashi A., Habu Y., Kobayashi T., Kawarai Y., Ishikawa H., Usui H., Shozu M. Long-term outcomes of progestin plus metformin as a fertility-sparing treatment for atypical endometrial hyperplasia and endometrial cancer patients. J. Gynecol. Oncol. 2019;30:e90. doi: 10.3802/jgo.2019.30.e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujii S., Kido A., Baba T., Fujimoto K., Daido S., Matsumura N., Konishi I., Togashi K. Subendometrial enhancement and peritumoral enhancement for assessing endometrial cancer on dynamic contrast enhanced MR imaging. Eur. J. Radiol. 2015;84:581–589. doi: 10.1016/j.ejrad.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Ito K., Matsumoto T., Nakada T., Nakanishi T., Fujita N., Yamashita H. Assessing myometrial invasion by endometrial carcinoma with dynamic MRI. J. Comput. Assist. Tomogr. 1994;18:77–86. doi: 10.1097/00004728-199401000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Sironi S., Colombo E., Villa G., Taccagni G., Belloni C., Garancini P., DelMaschio A. Myometrial invasion by endometrial carcinoma: Assessment with plain and gadolinium-enhanced MR imaging. Radiology. 1992;185:207–212. doi: 10.1148/radiology.185.1.1523309. [DOI] [PubMed] [Google Scholar]
- 23.Cunha T.M., Félix A., Cabral I. Preoperative assessment of deep myometrial and cervical invasion in endometrial carcinoma: Comparison of magnetic resonance imaging and gross visual inspection. Int. J. Gynecol. Cancer. 2001;11:130–136. doi: 10.1046/j.1525-1438.2001.011002130.x. [DOI] [PubMed] [Google Scholar]
- 24.Beddy P., Moyle P., Kataoka M., Yamamoto A.K., Joubert I., Lomas D., Crawford R., Sala E. Evaluation of depth of myometrial invasion and overall staging in endometrial cancer: Comparison of diffusion-weighted and dynamic contrast-enhanced MR imaging. Radiology. 2012;262:530–537. doi: 10.1148/radiol.11110984. [DOI] [PubMed] [Google Scholar]
- 25.Andreano A., Rechichi G., Rebora P., Sironi S., Valsecchi M.G., Galimberti S. MR diffusion imaging for preoperative staging of myometrial invasion in patients with endometrial cancer: A systematic review and meta-analysis. Eur. Radiol. 2014;24:1327–1338. doi: 10.1007/s00330-014-3139-4. [DOI] [PubMed] [Google Scholar]
- 26.Nougaret S., Horta M., Sala E., Lakhman Y., Thomassin-Naggara I., Kido A., Masselli G., Bharwani N., Sadowski E., Ertmer A., et al. Endometrial Cancer MRI staging: Updated Guidelines of the European Society of Urogenital Radiology. Eur. Radiol. 2019;29:792–805. doi: 10.1007/s00330-018-5515-y. [DOI] [PubMed] [Google Scholar]
- 27.Kitajima K., Murakami K., Yamasaki E., Kaji Y., Sugimura K. Accuracy of integrated FDG-PET/contrast-enhanced CT in detecting pelvic and paraaortic lymph node metastasis in patients with uterine cancer. Eur. Radiol. 2009;19:1529–1536. doi: 10.1007/s00330-008-1271-8. [DOI] [PubMed] [Google Scholar]
- 28.Franchi M., Garzon S., Zorzato P.C., Laganà A.S., Casarin J., Locantore L., Raffaelli R., Ghezzi F. PET-CT scan in the preoperative workup of early stage intermediate- and high-risk endometrial cancer. Minim. Invasive Ther. Allied Technol. 2020;29:232–239. doi: 10.1080/13645706.2019.1624576. [DOI] [PubMed] [Google Scholar]
- 29.Tsuyoshi H., Tsujikawa T., Yamada S., Okazawa H., Yoshida Y. Diagnostic value of 18F-FDG PET/MRI for staging in patients with endometrial cancer. Cancer Imaging. 2020;20:75. doi: 10.1186/s40644-020-00357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otero-García M.M., Mesa-Álvarez A., Nikolic O., Blanco-Lobato P., Basta-Nikolic M., de Llano-Ortega R.M., Paredes-Velázquez L., Nikolic N., Szewczyk-Bieda M. Role of MRI in staging and follow-up of endometrial and cervical cancer: Pitfalls and mimickers. Insights Imaging. 2019;10:19. doi: 10.1186/s13244-019-0696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scioscia M., Noventa M., Laganà A.S. Abnormal uterine bleeding and the risk of endometrial cancer: Can subendometrial vascular ultrasound be of help to discriminate cancer from adenomyosis? Am. J. Obs. Gynecol. 2020;223:605–606. doi: 10.1016/j.ajog.2020.05.049. [DOI] [PubMed] [Google Scholar]
- 32.Cunningham R.K., Horrow M.M., Smith R.J., Springer J. Adenomyosis: A Sonographic Diagnosis. Radiographics. 2018;38:1576–1589. doi: 10.1148/rg.2018180080. [DOI] [PubMed] [Google Scholar]
- 33.Sundar S., Balega J., Crosbie E., Drake A., Edmondson R., Fotopoulou C., Gallos I., Ganesan R., Gupta J., Johnson N., et al. BGCS uterine cancer guidelines: Recommendations for practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017;213:71–97. doi: 10.1016/j.ejogrb.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Colombo N., Creutzberg C., Amant F., Bosse T., González-Martín A., Ledermann J., Marth C., Nout R., Querleu D., Mirza M.R., et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-up. Int. J. Gynecol. Cancer. 2016;26:2–30. doi: 10.1097/IGC.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santaballa A., Matías-Guiu X., Redondo A., Carballo N., Gil M., Gómez C., Gorostidi M., Gutierrez M., Gónzalez-Martín A. SEOM clinical guidelines for endometrial cancer (2017) Clin. Transl. Oncol. 2018;20:29–37. doi: 10.1007/s12094-017-1809-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamagami W., Mikami M., Nagase S., Tabata T., Kobayashi Y., Kaneuchi M., Kobayashi H., Yamada H., Hasegawa K., Fujiwara H., et al. Japan Society of Gynecologic Oncology 2018 guidelines for treatment of uterine body neoplasms. J. Gynecol. Oncol. 2020;31:e18. doi: 10.3802/jgo.2020.31.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Network N.C.C. Uterine Neoplasm (Version 3.2021) [(accessed on 3 June 2021)]; Available online: https://www.nccn.org/professionals/physician_gls/pdf/uterine_blocks.pdf.
- 38.Hamilton C.A., Pothuri B., Arend R.C., Backes F.J., Gehrig P.A., Soliman P.T., Thompson J.S., Urban R.R., Burke W.M. Endometrial cancer: A society of gynecologic oncology evidence-based review and recommendations, part II. Gynecol. Oncol. 2021;160:827–834. doi: 10.1016/j.ygyno.2020.12.024. [DOI] [PubMed] [Google Scholar]
- 39.Lin G., Huang Y.T., Chao A., Ng K.K., Yang L.Y., Ng S.H., Lai C.H. Influence of menopausal status on diagnostic accuracy of myometrial invasion in endometrial cancer: Diffusion-weighted and dynamic contrast-enhanced MRI at 3 T. Clin. Radiol. 2015;70:1260–1268. doi: 10.1016/j.crad.2015.06.097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in Supplementary Materials.