Abstract
Background
The triglyceride-glucose (TyG) index is an alternative marker of insulin resistance (IR) and is closely associated with the prevalence and prognosis of atherosclerotic cardiovascular disease (ASCVD). However, the association between the TyG index and in-stent restenosis (ISR) after drug-eluting stent (DES) implantation in patients with acute coronary syndrome (ACS) remains unknown.
Methods
The present study retrospectively recruited patients who were admitted for ACS and underwent coronary angiography at 6 to 24 months after successful DES-based percutaneous coronary intervention (PCI). In addition, we calculated the TyG index with the following formula: Ln(fasting triglyceride [mg/dL] × fasting blood glucose [mg/dL]/2) and divided patients into 3 groups according to the tertile of the TyG index. Most importantly, multivariate logistic regression analysis models were also constructed to assess the association between the TyG index and DES-ISR in patients with ACS.
Results
A total of 1574 patients with ACS (58.4 ± 9.4 years, 77.4% male) were included in this study. At the median follow-up time of 12 (9–14) months, the prevalence of DES-ISR increased stepwise with the increasing tertile of the TyG index (11.6% vs 17.3% vs 19.4%, p = 0.002), and the TyG index was also higher in the ISR group than in the non-ISR group (9.00 ± 0.58 vs 8.84 ± 0.61, p < 0.001). In addition, the positive association between the TyG index and the prevalence of DES-ISR was also determined in the fully adjusted model (TyG, per 1-unit increase: OR 1.424, 95% CI 1.116 to 1.818, p = 0.005; tertile of TyG, the OR (95% CI) values for tertile 2 and tertile 3 were 1.454 (1.013 to 2.087) and 1.634 (1.125 to 2.374), respectively, with tertile 1 as a reference). The association was also reflected in most subgroups. Moreover, adding the TyG index to the predictive model for DES-ISR in patients with ACS could contribute to an increase in C-statistics (0.675 vs 0.659, p = 0.010), categorical net reclassification improvement (0.090, p < 0.001), and integrated discrimination improvement (0.004, p = 0.040).
Conclusion
An elevated TyG index was independently and positively associated with DES-ISR in patients with ACS who underwent PCI. However, the incremental predictive value of the TyG index for DES-ISR was slight. To further confirm our findings, future studies are needed.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-021-01332-4.
Keywords: Triglyceride-glucose index, Insulin resistance, In-stent restenosis, Drug-eluting stents, Acute coronary syndrome, Percutaneous coronary intervention
Background
Despite considerable improvement in the anti-restenotic performance of drug-eluting stents (DESs), technological evolution of percutaneous coronary intervention (PCI), medical therapy and other secondary prevention strategies in recent years, in-stent restenosis (ISR) remains a major challenge after PCI, and its occurrence ranges from 3 to 20% [1–3]. The predisposing factors and underlying mechanisms of DES-ISR are complex and remain unclear [3–5]. Therefore, further identifying and controlling the residual risk factors for DES-ISR may have great clinical importance in developing promising strategies for reducing the incidence of DES-ISR.
Insulin resistance (IR), a well-established hallmark of metabolic disorders and systemic inflammation [6], is not only a substantial risk factor for atherosclerotic cardiovascular disease (ASCVD) but also contributes to a worse prognosis [7–9]. Meanwhile, there is also evidence suggesting that IR may play an important role in the development and progression of ISR [10]. However, IR has not received enough attention at present, and the current methods used to assess IR, including hyperinsulinaemia-euglycaemic clamps and homeostatic model assessment (HOMA), are time-consuming, complex, expensive, and not widely available [11, 12]. Therefore, there has been growing interest in determining a reliable, simple, and accessible index to assess IR quantitively.
Accumulating evidence proposes that the triglyceride-glucose (TyG) index, which is derived from fasting triglyceride (TG) and fasting blood glucose (FBG), could serve as a credible and convenient surrogate marker for evaluating IR in clinical practice [12–14]. Mounting evidence has demonstrated that the TyG index is significantly associated with an increased risk of diabetes mellitus (DM), hypertension, metabolic syndrome, atherosclerosis, and even the progression of atherosclerosis [15–19]. Furthermore, recent data also confirmed that the TyG index is strongly associated with worse long-term prognosis and could be used to optimize early risk stratification in patients with coronary artery disease (CAD) [20, 21].
However, to the best of our knowledge, the association between the TyG index and DES-ISR remains unknown, and no relevant studies have been designed to investigate this topic to date. To address this knowledge gap, we performed the present study to investigate the association between the TyG index and ISR in patients with acute coronary syndrome (ACS) after successful DES-based PCI.
Methods
Study population
Patients who were diagnosed with ACS and underwent follow-up angiography ranging from 6 to 24 months after successful PCI from January 2018 to August 2020 in Beijing Anzhen Hospital, Capital Medical University, were reviewed retrospectively. The main exclusion criteria were as follows: (1) age less than 18 years; (2) history of coronary artery bypass grafting (CABG); (3) culprit lesion treated with bare metal stent (BMS)/only underwent balloon angioplasty without DES implantation; (4) suspected familial hypertriglyceridaemia (triglyceride ≥ 5.65 mmol/L); (5) severe hepatic and renal dysfunction (estimated glomerular filtration rate (eGFR < 30 mL/min/1.73 m2)); and (6) acute/chronic inflammatory disease, malignancy, and body mass index (BMI) ≥ 45 kg/m2. Most importantly, this retrospective study was performed in line with the Declaration of Helsinki and was approved by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University. Additionally, written/oral informed consent was also obtained from the participants.
Intervention and management
Coronary intervention and periprocedural management were performed according to current guidelines in our centre [22]. Before intervention, all enrolled patients were prescribed a loading dose of aspirin (300 mg) and clopidogrel (300 mg) or ticagrelor (180 mg). In addition to antiplatelet therapy, anticoagulation with unfractionated heparin (70–100 IU/kg, with an additional bolus if necessary) was also used to maintain an activated clotting time > 250 s. During the intervention, radial access was regarded as the standard approach, unless there were overriding procedural considerations. The application of intracoronary imaging, type of second-generation DES, and size of stents were all at the discretion of the operator. Following the intervention, secondary prevention strategies recommended by current guidelines were also prescribed to patients.
Data collection and definitions
Patient demographics and clinical characteristics, including age, sex, smoking status, previous medical history, left ventricular ejection fraction (LVEF), angiographic evaluation results, procedure details, and medication used at discharge, were all collected from the electronic medical recording system by trained physicians who were blinded to the aim of the study. In addition, peripheral venous blood samples were obtained after overnight fasting (> 8 h) for analysis. Then, FBG, uric acid, eGFR, high sensitivity-C reactive protein (Hs-CRP) and serum lipid profiles, including TG, total cholesterol (TC), low-density lipoprotein-C (LDL-C), and high-density lipoprotein-C (HDL-C), which were determined in the central laboratory of Beijing Anzhen Hospital, Capital Medical University, were also recorded.
The TyG index was determined with the following formula: Ln (fasting TG [mg/dL] × FBG [mg/dL]/2) [14], and BMI was calculated as weight (kg)/height squared (m2). Smoking status was stratified into 3 levels: never, former (quit smoking > 1 month), and current. Hypercholesteraemia was defined as a fasting serum TC > 6.22 mmol/L, LDL-C > 4.14 mmol/L or treatment with lipid-lowering drugs [23]. The diagnosis of DM was confirmed by a previous history of DM, active treatment with antidiabetic medication, or the typical symptoms of DM with FBG > 7 mmol/L and/or random blood glucose > 11.1 mmol/L [24]. Additionally, based on angiographic evaluation results, multivessel disease was defined as ≥ 2 vessels with significant diameter stenosis (≥ 50%). Chronic total occlusion (CTO) was defined as completed obstruction of a native coronary artery ≥ 3 months.
Follow-up angiography and evaluation of ISR
All the patients included in the analysis underwent follow-up angiography using the standard Judkin technique at 6 to 24 months after successful PCI. According to angiographic follow-up results, patients were divided into the non-ISR group or ISR group, which was defined as the presence of significant diameter stenosis (≥ 50%) at the segment inside the stent or involving its 5-mm edges, which is in line with previous studies [25, 26]. Of note, the follow-up angiography was interpreted by 2 independent and experienced cardiologists who were unaware of the patients’ information. Discrepancies encountered in the processes of identifying ISR were resolved by discussion with a senior researcher.
Statistical analysis
Participants recruited were mainly classified based on the tertile of the TyG index in the present study. To summarize the clinical characteristics of participants, continuous variables were expressed as the mean ± standard deviation or the median with interquartile range depending on the normality of the data distribution, and categorical variables were presented as absolute values (percentages). The differences in continuous variables with a normal distribution across the TyG tertile were compared with one-way analysis of variance. For continuous parameters with skewed distribution, the Kruskal–Wallis H test was performed to detect differences. Differences in categorical variables were analysed by the chi-square test or Fisher’s exact test. In addition, the association between the TyG index and other cardiometabolic risk factors was also assessed by using Pearson’s correlation test or Spearman’s rank test when appropriate.
To identify determinants of ISR in patients after successful PCI with DES, univariate logistic regression analysis was performed. The baseline variables were selected and included in the multivariable logistic regression analysis model if they showed p < 0.1 in univariate analysis or were clinically relevant to DES-ISR. Finally, 3 models were established to control confounding variables and evaluate the association between the TyG index (modulated as continuous or categorical variables) and DES-ISR. Model 1 was adjusted for age, sex, and BMI; model 2 was adjusted for variables included in model 1 plus LVEF, Hs-CRP, hypertension, DM, and previous PCI; and model 3, which is the fully adjusted model, was adjusted for variables in model 2 plus SYNTAX score, target vessel in the left anterior descending artery (LAD), target vessel in the right coronary artery (RCA), intracoronary imaging, DES-sirolimus, total length of stents, and minimal stent diameter.
Furthermore, to evaluate the predictive value of the TyG index for DES-ISR, the area under the curve (AUC) and the optimal cut-off value were assessed through receiver operating characteristic (ROC) curve analysis. Meanwhile, to evaluate whether introducing the TyG index into the model of established risk factors could improve the predictive value, the C-statistic was calculated and compared by De-Long’s test. Additionally, the categorical net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were also calculated to further evaluate the incremental predictive value of the TyG index.
All statistical analyses in the present study were performed with SPSS 20.0 (IBM, Armonk, New York), R Programming Language 4.0.2, and MedCalc 19.1 (MedCalc software, Belgium). Most importantly, statistical significance was regarded as a two-sided p value < 0.05.
Results
Baseline characteristics
A total of 1574 patients who underwent follow-up angiography at a median follow-up time of 12 (9–14) months after successful DES-based PCI were enrolled. As shown in Table 1, the mean age of the study population was 58.40 ± 9.40 years old, and 1218 (77.4%) participants were male. The prevalence of current smoking, hypertension, DM, and previous PCI were 35.3%, 64.5%, 34.6% and 18.4%, respectively. Regarding angiographic findings, multivessel/left main artery (LM) disease (75.9%) was common, and half of the patients had implanted multiple stents (≥ 2).
Table 1.
Baseline characteristics of patients stratified by tertile of TyG index
| Total (n = 1574) | Tertile of TyG index | P-value | |||
|---|---|---|---|---|---|
| I (Lowest) (n = 525) | II (Median) (n = 533) | III (Highest) (n = 516) | |||
| 6.55 < TyG ≦ 8.58 | 8.58 < TyG ≦ 9.11 | 9.11 < TyG ≦ 10.89 | |||
| Age, years | 58.40 ± 9.40 | 59.54 ± 9.23 | 58.94 ± 9.46 | 56.67 ± 9.28 | < 0.001 |
| Male, n (%) | 1218 (77.4) | 423 (80.6) | 406 (76.2) | 389 (75.4) | 0.097 |
| BMI, kg/m2 | 25.96 ± 3.23 | 25.28 ± 3.06 | 26.04 ± 3.18 | 26.57 ± 3.32 | < 0.001 |
| LVEF, % | 62.17 ± 7.01 | 62.55 ± 6.61 | 61.93 ± 7.14 | 62.04 ± 7.26 | 0.312 |
| Diagnosis, n (%) | 0.061 | ||||
| UA | 1334 (84.8) | 459 (87.4) | 452 (84.8) | 423(82.0) | |
| NSTEMI | 116 (7.4) | 33 (6.3) | 44 (8.3) | 39 (7.6) | |
| STEMI | 124 (7.9) | 33 (6.3) | 37 (6.9) | 54 (10.5) | |
| Medical history, n (%) | |||||
| Current smoking | 556 (35.3) | 173 (33.0) | 184 (34.5) | 199 (38.6) | 0.030 |
| Hypertension | 1016 (64.5) | 313 (59.6) | 344 (64.5) | 359 (69.6) | 0.004 |
| Hypercholesteraemia | 610 (38.8) | 192 (36.6) | 198 (37.1) | 220 (42.6) | 0.086 |
| Diabetes mellitus | 544 (34.6) | 111 (21.1) | 173 (32.5) | 260 (50.4) | < 0.001 |
| Previous stroke | 127 (8.1) | 46 (8.8) | 43 (8.1) | 38 (7.4) | 0.710 |
| Previous PCI | 289 (18.4) | 95 (18.1) | 108 (20.3) | 86 (16.7) | 0.317 |
| Laboratory tests | |||||
| Hs-CRP, mg/L | 1.43 (0.56, 3.97) | 0.93 (0.40, 3.11) | 1.44 (0.59, 3.78) | 1.89 (0.82, 4.58) | < 0.001 |
| eGFR, mL/min/1.73 m2 | 96.54 ± 14.72 | 96.71 ± 13.93 | 96.56 ± 14.46 | 96.34 ± 15.78 | 0.921 |
| Uric acid, umol/L | 345.49 ± 84.69 | 332.35 ± 74.62 | 345.42 ± 81.91 | 358.92 ± 94.61 | < 0.001 |
| Homocysteine, umol/L | 12.50 (9.70, 16.10) | 12.80 (9.80, 16.55) | 12.70 (9.85, 16.1) | 12.00 (9.50, 15.68) | 0.095 |
| FBG, mmol/L | 6.57 ± 2.31 | 5.50 ± 0.97 | 6.19 ± 1.54 | 8.04 ± 3.06 | < 0.001 |
| Triglycerides, mmol/L | 1.40 (1.02, 1.98) | 0.90 ± 0.26 | 1.47 ± 0.33 | 2.47 ± 0.88 | < 0.001 |
| TC, mmol/L | 4.11 ± 1.07 | 3.75 ± 0.92 | 4.09 ± 0.97 | 4.50 ± 1.17 | < 0.001 |
| HDL-C, mmol/L | 1.07 ± 0.24 | 1.15 ± 0.26 | 1.06 ± 0.23 | 0.99 ± 0.21 | < 0.001 |
| LDL-C, mmol/L | 2.47 ± 0.89 | 2.24 ± 0.82 | 2.51 ± 0.86 | 2.67 ± 0.93 | < 0.001 |
| Angiography | |||||
| LM disease, n (%) | 139 (8.8) | 53 (10.1) | 50 (9.4) | 36 (7.0) | 0.179 |
| Multivessel/LM disease, n (%) | 1195 (75.9) | 384 (73.1) | 423 (79.4) | 388 (75.2) | 0.055 |
| Chronic total occlusion, n (%) | 367 (23.3) | 104 (19.8) | 130 (24.4) | 133 (25.8) | 0.058 |
| SYNTAX score | 13.93 ± 7.42 | 13.72 ± 7.83 | 14.36 ± 7.43 | 13.70 ± 6.97 | 0.253 |
| Intervention | |||||
| Target vessel, n (%) | |||||
| LM | 78 (5.0) | 30 (5.7) | 30 (5.6) | 18 (3.5) | 0.175 |
| LAD | 889 (56.5) | 304 (57.9) | 296 (55.5) | 289 (56.0) | 0.714 |
| LCX | 434 (27.6) | 125 (23.8) | 151 (28.3) | 158 (30.7) | 0.042 |
| RCA | 640 (40.7) | 214 (40.8) | 217 (40.7) | 209 (40.5) | 0.996 |
| Intracoronary imagine, n (%) | 100 (6.4) | 40 (7.6) | 31 (5.8) | 29 (5.6) | 0.343 |
| DES-sirolimus, n (%) | 849 (53.9) | 288 (54.9) | 281 (52.7) | 280 (54.3) | 0.772 |
| DES-zotarolimus, n (%) | 345 (21.9) | 127 (24.2) | 117 (22.0) | 101 (19.6) | 0.198 |
| DES-everolimus, n (%) | 597 (37.9) | 187 (35.6) | 210 (39.4) | 200 (38.8) | 0.400 |
| Number of stent, /patients | 2.00 (1.00, 2.00) | 1.00 (1.00, 2.00) | 2.00 (1.00, 2.00) | 1.00 (1.00, 2.00) | 0.227 |
| Multiple stents (n ≥ 2) | 789 (50.1) | 256 (48.8) | 280 (52.5) | 253 (49.0) | 0.392 |
| Length of stents, mm/patients | 36 (23, 61) | 36 (21, 58.50) | 38 (23, 62) | 35 (23, 63.75) | 0.223 |
| Minimal stent diameter, mm | 2.84 ± 0.46 | 2.88 ± 0.47 | 2.83 ± 0.46 | 2.82 ± 0.46 | 0.105 |
| Medications at discharge, n (%) | |||||
| Aspirin | 1574 (100.0) | 525 (100.0) | 533 (100.0) | 516 (100.0) | > 0.99 |
| Clopidogrel/Ticagrelor | 1574 (100.0) | 525 (100.0) | 533 (100.0) | 516 (100.0) | > 0.99 |
| Statin | 1570 (99.7) | 523 (99.6) | 532 (99.8) | 515 (99.8) | 0.779 |
| β-block | 1088 (69.2) | 337(64.2) | 378 (71.1) | 373 (72.3) | 0.009 |
| ACEI/ARB | 722 (45.9) | 206 (39.2) | 250 (46.9) | 266 (51.6) | < 0.001 |
| Insulin | 174 (11.1) | 37 (7.0) | 52 (9.8) | 85 (16.5) | < 0.001 |
| Other hypoglycemic agents | 431 (27.4) | 86 (16.4) | 133 (25.0) | 212 (41.1) | < 0.001 |
TyG: triglyceride-glucose index; BMI: body mass index; LVEF: left ventricular ejection fraction; UA: unstable angina; NSTEMI: non ST-segment elevation myocardial infarction; STEMI: ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; Hs-CRP: high sensitivity-C reactive protein; eGFR: estimated glomerular filtration rate; FBG: fasting blood glucose; TC: total cholesterol; HDL-C: high-density lipoprotein-C; LDL-C: low-density lipoprotein-C; LM: left main artery; LAD: left anterior descending artery; LCX: left circumflex artery; RCA: right coronary artery; DES: drug-eluting stent; ACEI/ARB: angiotensin-converting enzyme inhibitor/angiotensin receptor blocker
Based on the tertile of the TyG index, the patients recruited were further divided into 3 groups (Table 1). As demonstrated in Table 1, serum Hs-CRP, uric acid, FBG, TG, TC, and LDL-C; the prevalence of current smoking, hypertension, and DM; and BMI were all significantly increased with increasing TyG index tertile. Meanwhile, the patients in the higher TyG index group also had a trend for a higher proportion of multivessel/LM disease and CTO. Nevertheless, the patients with a higher TyG index were relatively younger and had a lower HDL-C.
Additionally, the differences between the ISR (n = 253) and non-ISR groups were also analysed (Additional file 1: Table S1). As revealed in Additional file 1: Table S1, patients in the ISR group were more likely to have DM and previous PCI, have higher concentrations of Hs-CRP and FBG, and suffer from more severe CAD. For the procedure details, those with ISR were more likely to undergo PCI for LAD and RCA lesions, use DES-sirolimus, and have longer total length of stents. However, PCI under the guidance of intracoronary imaging was more common in those without ISR.
Association between the TyG index and other cardiometabolic risk factors
To further identify the association between the TyG index and other cardiometabolic risk factors, Spearman’s rank or Pearson’s correlation analysis was performed, and the results are described in Table 2. The TyG index correlated positively with BMI, Hs-CRP, uric acid, TC, and LDL-C. In contrast, the TyG index was negatively associated with age (r = − 0.154, p < 0.001) and HDL-C (r = − 0.300, p < 0.001).
Table 2.
Association between TyG index and other cardiometabolic risk factors
| Variables | Correlation coefficient (r) | P-value |
|---|---|---|
| Age | − 0.154 | < 0.001 |
| BMI | 0.174 | < 0.001 |
| Hs-CRP | 0.167 | < 0.001 |
| eGFR | − 0.012 | 0.648 |
| Uric acid | 0.123 | < 0.001 |
| TC | 0.293 | < 0.001 |
| HDL-C | − 0.300 | < 0.001 |
| LDL-C | 0.194 | < 0.001 |
| Homocysteine | − 0.044 | 0.083 |
TyG: triglyceride-glucose index; BMI: body mass index; Hs-CRP: high sensitivity-C reactive protein; eGFR: estimated glomerular filtration rate; FBG: fasting blood glucose; TC: total cholesterol; HDL-C: high-density lipoprotein-C; LDL-C: low-density lipoprotein-C
TyG index and the prevalence of ISR after successful DES-based PCI
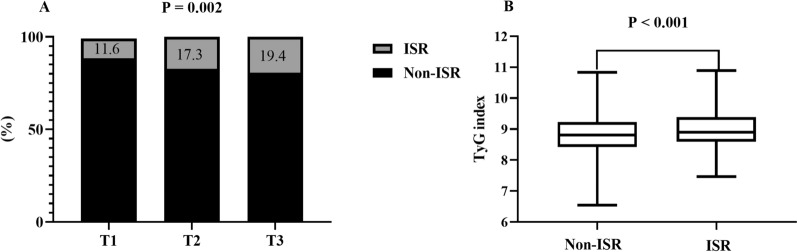
As shown in Fig. 1A, the prevalence of ISR had stepwise increase with the increasing tertile of the TyG index (11.6% vs 17.3% vs 19.4%, p = 0.002). Additionally, it is noteworthy that the ISR group also had a significantly higher TyG index than the non-ISR group (9.00 ± 0.58 vs 8.84 ± 0.61, P < 0.001, Fig. 1B).
Fig. 1.
The impacts of the TyG index on the prevalence of DES-ISR (A) and comparison of the TyG index level between the ISR and non-ISR groups (B) in the overall study population. TyG index: triglyceride-glucose index; DES: drug-eluting stent; ISR: in-stent restenosis
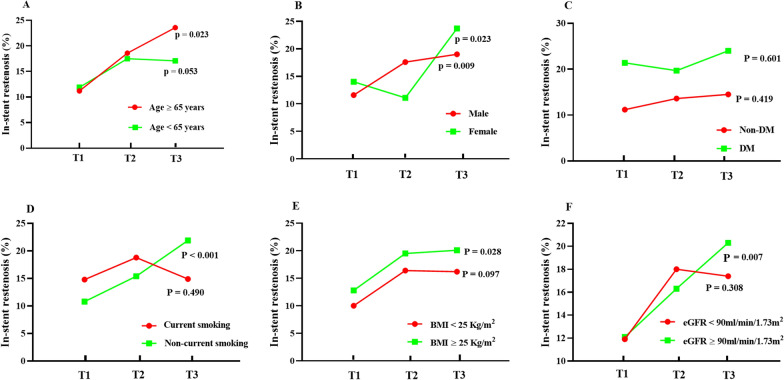
The impacts of the TyG index on the prevalence of ISR were also analysed across the different subgroups. As demonstrated in Fig. 2, the subgroup analysis demonstrated that the prevalence of ISR was increased across the tertiles of the TyG index in both male (11.6% vs 17.6% vs 19.0%, p = 0.009) and female (14.0% vs 11.1% vs 23.7%, p = 0.023) subjects. In addition, a trend towards a higher percentage of ISR across the tertiles of the TyG index was also observed in the subgroups of age ≥ 65 years (11.2% vs 18.6% vs 23.6%, p = 0.023), noncurrent smoking (10.8% vs 15.4% vs 21.9%, p < 0.001), BMI ≥ 25 kg/m2 (12.8% vs 19.5% vs 20.1%, p = 0.028), and eGFR ≥ 90 mL/min/1.73 m2 (12.1% vs 16.3% vs 20.3%, p = 0.007). Meanwhile, comparisons of the TyG index between patients with or without ISR in those subgroups were also analysed and are presented in Additional file 1: Figure S1.
Fig. 2.
The impact of the TyG index on the prevalence of DES-ISR across subgroups of age (A), sex (B), DM status (C), current smoking status (D), dichotomized baseline BMI (E), and dichotomized baseline eGFR (F). TyG index: triglyceride-glucose index; DES: drug-eluting stent; ISR: in-stent restenosis; DM: diabetes mellitus; BMI: body mass index; eGFR: estimated glomerular filtration rate
Association of the TyG index and the risk of DES-ISR in univariate analysis and multivariable analysis
In univariate logistic regression analysis, the TyG index (per 1-unit increase), as a continuous variable, had a positive association with the risk of ISR after successful DES-based PCI (OR = 1.522, 95% CI 1.222 to 1.895, p < 0.001). When the TyG index was modulated as a categorical variable and the tertile 1 group was used as a reference, the incidence of DES-ISR was elevated in the tertile 2 (OR = 1.587, 95% CI 1.119 to 2.249, p = 0.009) and tertile 3 groups (OR = 1.828, 95% CI 1.295 to 2.581). Meanwhile, DES-ISR also correlated significantly with some other variables in the univariate analysis, which is demonstrated in Additional file 1: Table S2.
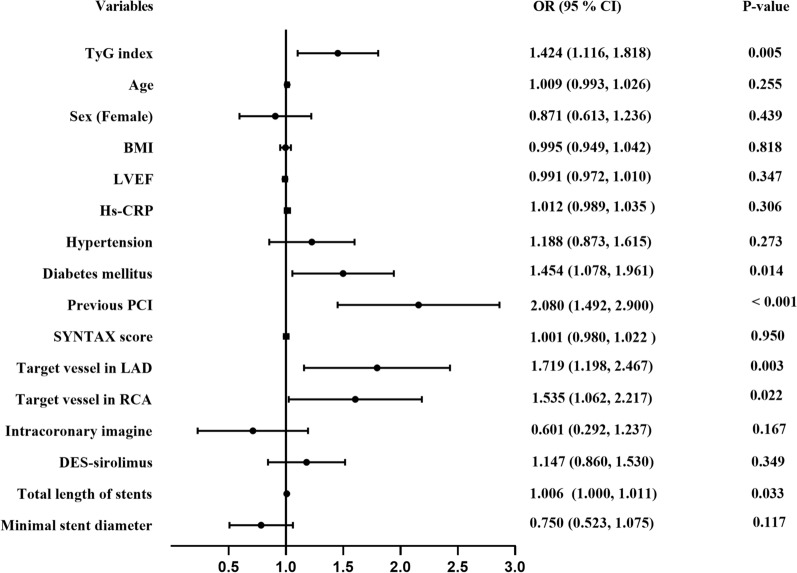
In multivariable logistic regression models, the TyG index was modulated as a continuous variable first. As presented in Table 3 and Fig. 3, a 1-unit increase in the TyG index was independently associated with an increased risk of DES-ISR in model 1 (OR = 1.569, 95% CI 1.253 to 1.965, p < 0.001), model 2 (OR = 1.396, 95% 1.101 to 1.711, p = 0.006), and even in the fully adjusted model (model 3) (OR = 1.424, 95% CI 1.116 to 1.818, p = 0.005). When the TyG index was evaluated as a tertile, the association persisted in the 3 models (Table 3). As shown in Table 3, after fully adjusting for the potential confounding factors in model 3, the adjusted OR (95% CI) values for patients in tertile 2 and tertile 3 were 1.454 (1.013 to 2.087, p = 0.043) and 1.634 (1.125 to 2.374, p = 0.010), respectively, compared to reference tertile 1.
Table 3.
Association of TyG index with DES-ISR in multivariable logistic regression models
| OR | 95% CI | P-value | |
|---|---|---|---|
| Model 1 | |||
| TyG, per 1-unit increase | 1.569 | 1.253 to 1.965 | < 0.001 |
| Tertile 1 | Reference | ||
| Tertile 2 | 1.599 | 1.125 to 2.272 | 0.009 |
| Tertile 3 | 1.890 | 1.328 to 2.688 | < 0.001 |
| Model 2 | |||
| TyG, per 1-unit increase | 1.396 | 1.101 to 1.711 | 0.006 |
| Tertile 1 | Reference | ||
| Tertile 2 | 1.473 | 1.031 to 2.104 | 0.033 |
| Tertile 3 | 1.619 | 1.120 to 2.339 | 0.010 |
| Model 3 | |||
| TyG, per 1-unit increase | 1.424 | 1.116 to 1.818 | 0.005 |
| Tertile 1 | Reference | ||
| Tertile 2 | 1.454 | 1.013 to 2.087 | 0.043 |
| Tertile 3 | 1.634 | 1.125 to 2.374 | 0.010 |
Model 1: adjusted for age, sex, and BMI
Model 2: adjust for age, sex, BMI, LVEF, Hs-CRP: hypertension, diabetes mellitus, and previous PCI
Model 3: adjust for age, sex, BMI, LVEF, Hs-CRP, hypertension, diabetes mellitus, previous PCI, SYNTAX score, target vessel in LAD, target vessel in RCA, the application of intracoronary imagine; DES-sirolimus; total length of stents, and minimal stent diameter
TyG: triglyceride-glucose index; DES: drug-eluting stents; ISR: in-stent restenosis; OR: odds ratio; CI: confidence interval; BMI: body mass index; LVEF: left ventricular ejection fraction; Hs-CRP: high sensitivity-C reactive protein; PCI: percutaneous coronary intervention; LAD: left anterior descending artery; RCA: right coronary artery
Fig. 3.
Forest plot of the multivariable logistic regression analysis model in patients with ACS exploring the association between the TyG index and DES-ISR. ACS: acute coronary syndrome; TyG index: triglyceride-glucose index; DES: drug-eluting stent; ISR: in-stent restenosis; BMI: body mass index; LVEF: left ventricular ejection fraction; Hs-CRP: high sensitivity-C reactive protein; PCI: percutaneous coronary intervention; LAD: left anterior descending artery; RCA: right coronary artery; OR: odds ratio; CI: confidence interval
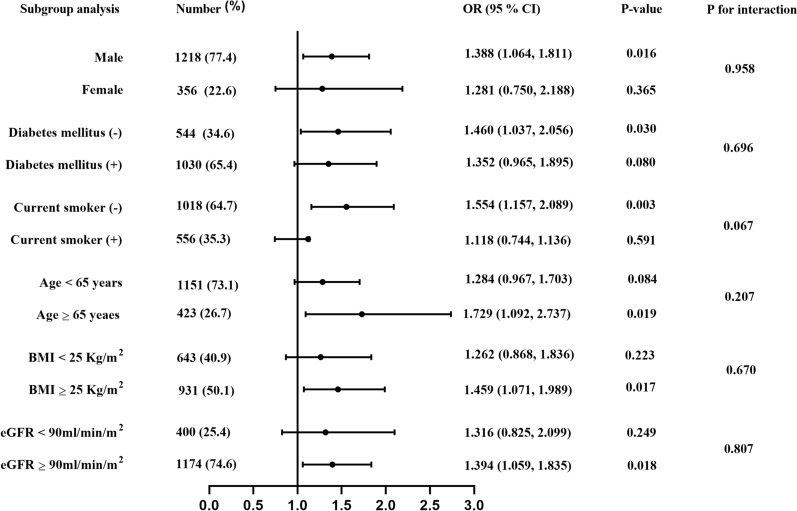
Subsequently, the independent association between the TyG index and DES-ISR was also assessed in various subgroups (Fig. 4). After adjustment for baseline variables with p < 0.1 in the univariate analysis, the positive association between the TyG index and the risk of DES-ISR was mainly reflected in the subgroups of age ≥ 65 years, male sex, eGFR ≥ 90 mL/min/1.73 m2, BMI ≥ 25 kg/m2, without DM, and noncurrent smokers. In addition, the trend was observed in subgroups of age < 65 years (p = 0.084) and those with DM (p = 0.08). Notably, a marginally significant (p = 0.067) interaction existed between the TyG index and smoking status with regard to the risk of DES-ISR.
Fig. 4.
Forest plot investigating the association between the TyG index and the prevalence of DES-ISR in different subgroups. TyG index: triglyceride-glucose index; DES: drug-eluting stent; ISR: in-stent restenosis; BMI: body mass index; eGFR: estimated glomerular filtration rate; OR: odds ratio; CI: confidence interval
Incremental effects of the TyG index on the predictive value of DES-ISR
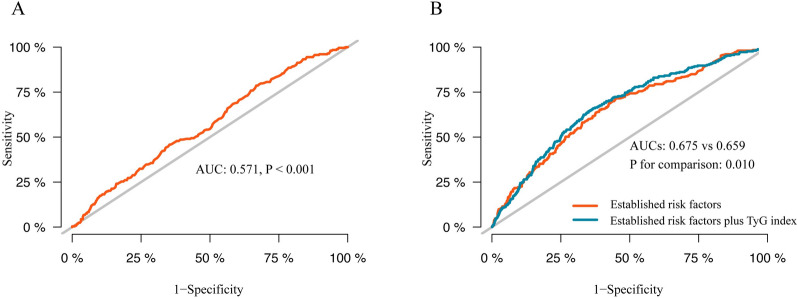
As presented in Fig. 5A, the ROC curve analysis revealed that the TyG index could provide mild predictive value for DES-ISR in patients with ACS who had an AUC of 0.571 (95% CI 0.533 to 0.608, p < 0.001). The optimal cut-off value was 8.55 (sensitivity: 78.7%, specificity: 33.2%). Meanwhile, as presented in Table 4, the C-statistic obtained from the model of established risk factors, which consisted of DM, previous PCI, target vessel in the LAD, target vessel in the RCA, and total length of stents, was 0.659 (95% CI 0.623 to 0.696, p < 0.001). Furthermore, Table 4 and Fig. 5B demonstrate that adding the TyG index to the model of established risk factors could lead to an increase in C-statistics (0.659 (0.623 to 0.696) vs 0.675 (0.639 to 0.711), p = 0.010), categorical NRI (0.090 (0.037 to 0.142), p < 0.001), and IDI (0.004 (0.0002 to 0.008), p = 0.040).
Fig. 5.
Receiver operating characteristic curve analysis of the TyG index to predict DES-ISR (A) and comparison of the C-statistics between the models (B). TyG index: triglyceride-glucose index; DES: drug-eluting stent; ISR: in-stent restenosis; AUC: area under curve
Table 4.
Evaluate the predictive power of models for ISR after successful DES-based PCI
| C-statistic | P-value | Pfor comparison | Categorical NRI | P-value | IDI | P-value | |
|---|---|---|---|---|---|---|---|
| Established risk factors | 0.659 (0.623 to 0.696) | < 0.001 | Ref | Ref | Ref | ||
| Established risk factors plus TyG index | 0.675 (0.639 to 0.711) | < 0.001 | 0.010 | 0.090 (0.037 to 0.142) | < 0.001 | 0.004(0.0002 to 0.008) | 0.040 |
ISR: in-stent restenosis; DES: drug-eluting stent; TyG index: triglyceride-glucose index; PCI: percutaneous coronary intervention; NRI: net reclassification improvement; IDI: integrated discrimination improvement
Discussion
To the best of our knowledge, the present study was the first to investigate the association between IR assessed by the TyG index and DES-ISR in patients with ACS. The main findings of this study were as follows: (1) the TyG index, as a surrogate marker of IR, was significantly associated with cardiometabolic risk factors; (2) patients with a higher TyG index were more likely to have DES-ISR after successful PCI and vice versa; (3) the TyG index, either as a continuous or categorical variable, was independently associated with an increased risk of DES-ISR in the fully adjusted model; and (4) taking the TyG index into consideration may have clinical significance in optimizing the early risk stratification of DES-ISR in patients with ACS.
IR is a pathological condition characterized by defects in the uptake and utilization of glucose, which could lead to chronic hyperglycaemia and unique dyslipidaemia characterized by increased circulating TG and low concentrations of HDL-C [7]. Based on this theoretical background, the TyG index calculated from TG and FBG has been proposed as a surrogate indicator of IR [12, 13]. According to previous studies, the TyG index was significantly associated with IR assessed by the gold standard hyperinsulinaemic-euglycaemic clamp method and may even perform better than the HOMA among subjects with a wide range of body weights and glucose tolerances [14, 27]. In addition to better performance in the estimation of IR, the TyG index also correlated well with various metabolic abnormalities. Recent evidence has suggested that patients with a higher TyG index were more likely to have DM and hypertension, which is also demonstrated in our studies [15, 16]. Furthermore, in line with previous studies [21, 28], our present study also suggested that the TyG index was associated with other cardiometabolic risk factors.
In recent years, mounting clinical trials have also been designed to investigate the association of IR assessed by the TyG index with ASCVD. In the meta-analysis of cohort studies, Ding et al. reported that the TyG index, either as a continuous or categorical variable, was independently associated with the increased prevalence of ASCVD, and the association was not significantly affected by age, sex, or diabetes status in the subgroup analysis [29]. Meanwhile, a series of studies suggested that the TyG index may serve as a simple and effective tool in clinical practice to identify patients at risk of subclinical atherosclerosis, which is evaluated by the coronary artery calcium score, carotid ultrasound (intima-media thickness or plaque), and pulse wave velocity [28, 30–32]. Furthermore, recent evidence also indicated that the TyG index was not only positively associated with the rapid progression of coronary atherosclerosis and calcification but was also an independent risk factor for poor long-term prognosis in patients with stable CAD [19, 20, 33, 34]. Additionally, the positive association between the TyG index and adverse prognosis also persists in patients with ACS [21, 35], which is the leading cause of morbidity and mortality from cardiovascular disease worldwide [36]. Extending the above findings, our present work revealed that the TyG index was an independent predictor of DES-ISR in the entire study population. Based on our findings, novel therapeutic interventions to reduce the TyG index may be of importance for the prevention of DES-ISR in the future.
Subsequently, the stability of the association between the TyG index and DES-ISR was examined through subgroup analysis. This association was stable and persisted in most subgroups. Unexpectedly, this association seems to be more prominent in noncurrent smokers, and a non-significant interaction exist between the TyG index and current smoking status on DES-ISR. Although the mechanisms underlying this phenomenon remain unknown, it is necessary for us to take the current smoking status into consideration when we manage patients after PCI for ISR prevention, according to the TyG index.
Regarding the predictive power of the TyG index for CAD, accumulating evidence has demonstrated that the predictive value of the TyG index for CAD is mild to moderate, and introducing the TyG index into the Framingham model would improve the predictive value for CAD [37, 38]. Furthermore, Zhang et al. and Wang et al. reported that adding the TyG index into a baseline risk model could significantly improve the predictive accuracy for major adverse cardiac events (MACEs) in patients with ACS, although the predictive value of the TyG index for MACEs was mild [39, 40]. Consistent with previous studies, our present study suggested that introducing the TyG index into a model of established risk factors could improve our ability to identify patients at risk for DES-ISR. Although its incremental predictive value for DES-ISR was limited, considering a large and increasing number of patients ACS admitted for PCI every year [35, 36], it still seemed to be clinically important to perform assessments of the risk of DES-ISR combined with established risk factors.
The exact mechanisms underlying the close association of the TyG index with DES-ISR remain unknown. However, we speculated that TyG is a reliable marker of IR, which may mainly be due to the association. First, IR could aggravate the excessive proliferation of vascular smooth muscle cells via various potential signalling pathways, which is a prominent feature in the pathology of ISR [3, 41]. Second, IR could lead to endothelial dysfunction, which is a well-established risk factor for ISR [42, 43], by inducing inflammation, oxidative stress, and metabolic alterations [7, 44]. Third, the TyG index, as a surrogate marker of IR, was also strongly associated with the prevalence and progression of coronary artery calcification [28, 34], which could be another important mechanism. Finally, our present study and previous studies indicated that the TyG index, as a surrogate index of IR, was closely related to various cardiometabolic risk factors [21, 28, 34], which may account for the association.
Meanwhile, some limitations should be acknowledged in our present study. First, this is a single-centre, retrospective, and observational study. Therefore, this study could not determine the causality between the TyG index and DES-ISR. Second, our present study only included patients with ACS who underwent follow-up angiography within 6 to 24 months after PCI, which may cause selection bias and limit the generalizability of our findings to patients with chronic coronary syndrome. And the follow-up time was not long enough. Third, the identification of ISR in the present study mainly relied on visual assessment of angiography by 2 experienced cardiologists rather than more accurate and informative intracoronary imaging. Fourth, the present study fails to compare the role of HOMA-IR and the TyG index in DES-ISR, because fasting insulin is not routinely measured in our centre. Finally, the TyG index was evaluated only once after admission. Information on the change in the TyG index level during follow-up was limited.
Conclusions
The TyG index, as a novel surrogate marker of IR, had an independent and positive association with the risk of ISR in patients with ACS after DES implantation. However, its incremental predictive value for DES-ISR was slight and needs to be further investigated. Additionally, multicentre, prospective, and randomized clinical trials are required in the future to investigate whether measures targeting the TyG index could provide favourable effects for the prevention of DES-ISR.
Supplementary Information
Additional file 1: Table S1. Baseline characteristics of patients with and without ISR. Table S2. Association of DES-ISR and other clinical variables in the univariate analysis. Figure S1. Comparison of the TyG index between patients with or without ISR in the subgroups of sex (A), DM status (B), current smoking status (C), age (D), dichotomized baseline BMI (E), and dichotomized baseline eGFR (F). TyG index, triglyceride-glucose index; ISR, in-stent restenosis; DM, diabetes mellitus; BMI, body mass index; eGFR, estimated glomerular filtration rate.
Acknowledgements
Not applicable.
Abbreviations
- DESs
Drug-eluting stents
- PCI
Percutaneous coronary intervention
- ISR
In-stent restenosis
- IR
Insulin resistance
- ASCVD
Atherosclerotic cardiovascular disease
- HOMA
Homeostatic model assessment
- TyG index
Triglyceride-glucose index
- TG
Triglyceride
- FBG
Fasting blood glucose
- DM
Diabetes mellitus
- CAD
Coronary artery disease
- ACS
Acute coronary syndrome
- CABG
Coronary artery bypass grafting
- BMS
Bare metal stent
- eGFR
Estimated glomerular filtration rate
- BMI
Body mass index
- LVEF
Left ventricular ejection fraction
- Hs-CRP
High sensitivity-C reactive protein
- TC
Total cholesterol
- LDL-C
Low-density lipoprotein-C
- HDL-C
High-density lipoprotein-C
- CTO
Chronic total occlusion
- LAD
Left anterior descending artery
- RCA
Right coronary artery
- AUC
Area under curve
- ROC
Receiver-operating characteristics
- NRI
Net reclassification improvement
- IDI
Integrated discrimination improvement
- MACEs
Major adverse cardiac events
Authors' contributions
YZ and KSL made substantial contributions to data analysis, data interpretation, and manuscript writing; MCL, YL, AG and CPH were responsible for data collection; HGZ and JWZ contributed to interpretation of coronary angiography; HL, HYH, and YXZ performed manuscript revision; YXZ was also responsible for the study design. All authors read and approved the final manuscript.
Funding
This work was supported by the grant from National Key Research and Development Program of China (2017YFC0908800), the “Beijing Municipal Administration of Hospitals” Ascent Plan (DFL20150601) and Mission plan (SML20180601), and Beijing Municipal Health Commission “Project of Science and Technology Innovation Centre” (PXM2019_026272_000006) (PXM2019_026272_000005).
Availability of data and materials
The datasets used/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The present study was performed in line with Declaration of Helsinki and was approved by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University. Additionally, the written/oral informed consent was also obtained by us from the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Grüntzig Lecture ESC 2014. Eur Heart J. 2015;36(47):3320–3331. doi: 10.1093/eurheartj/ehv511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfonso F, Byrne RA, Rivero F, Kastrati A. Current treatment of in-stent restenosis. J Am Coll Cardiol. 2014;63(24):2659–2673. doi: 10.1016/j.jacc.2014.02.545. [DOI] [PubMed] [Google Scholar]
- 3.Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56(23):1897–1907. doi: 10.1016/j.jacc.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Cassese S, Byrne RA, Tada T, Pinieck S, Joner M, Ibrahim T, King LA, Fusaro M, Laugwitz K, Kastrati A. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart. 2013;100(2):153–159. doi: 10.1136/heartjnl-2013-304933. [DOI] [PubMed] [Google Scholar]
- 5.Liu SY, Yang YY, Jiang SY, Tang NN, Tian JW, Ponnusamy M, Tariq MA, Lian ZX, Xin H, Yu T. Understanding the role of non-coding RNA (ncRNA) in stent restenosis. Atherosclerosis. 2018;272:153–161. doi: 10.1016/j.atherosclerosis.2018.03.036. [DOI] [PubMed] [Google Scholar]
- 6.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339(6116):172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10(5):293–302. doi: 10.1038/nrendo.2014.29. [DOI] [PubMed] [Google Scholar]
- 9.Caccamo G, Bonura F, Bonura F, Vitale G, Novo G, Evola S, Evola G, Grisanti MR, Novo S. Insulin resistance and acute coronary syndrome. Atherosclerosis. 2010;211(2):672–675. doi: 10.1016/j.atherosclerosis.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 10.Zhao L, Xu W, Wang L, Li H, Shao C, Gu H, Chan S, Xu H, Yang X. Influence of insulin resistance on in-stent restenosis in patients undergoing coronary drug-eluting stent implantation after long-term angiographic follow-up. Coronary Artery Dis. 2015;26(1):5–10. doi: 10.1097/MCA.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Ding X, Hua B, Liu Q, Gao H, Chen H, Zhao X, Li W, Li H. High triglyceride-glucose index is associated with poor cardiovascular outcomes in nondiabetic patients with ACS with LDL-C below 1.8 mmol/L. J Atheroscler Thromber. 2021 doi: 10.5551/jat.61119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiorentino TV, Marini MA, Succurro E, Andreozzi F, Sesti G. Relationships of surrogate indexes of insulin resistance with insulin sensitivity assessed by euglycemic hyperinsulinemic clamp and subclinical vascular damage. BMJ Open Diabetes Res Care. 2019;7(1):e911. doi: 10.1136/bmjdrc-2019-000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unger G, Benozzi SF, Perruzza F, Pennacchiotti GL. Triglyceride and glucose index: a useful indicator of insulin resistance. Endocrinol Nutr. 2014;61(10):533–540. doi: 10.1016/j.endonu.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, Jacques-Camarena O, Rodríguez-Morán M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–51. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 15.Da Silva A, Caldas APS, Rocha DMUP, Bressan J. Triglyceride-glucose index predicts independently type 2 diabetes mellitus risk: a systematic review and meta-analysis of cohort studies. Prim Care Diabetes. 2020;14(6):584–593. doi: 10.1016/j.pcd.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Yang W, Jiang X. Association between triglyceride-glucose index and hypertension: a meta-analysis. Front Cardiovasc Med. 2021;8:644035. doi: 10.3389/fcvm.2021.644035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angoorani P, Heshmat R, Ejtahed H, Motlagh ME, Ziaodini H, Taheri M, Aminaee T, Goodarzi A, Qorbani M, Kelishadi R. Validity of triglyceride–glucose index as an indicator for metabolic syndrome in children and adolescents: the CASPIAN-V study. Eat Weight Disord. 2018;23(6):877–883. doi: 10.1007/s40519-018-0488-z. [DOI] [PubMed] [Google Scholar]
- 18.Hong S, Han K, Park C. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: a population-based study. BMC Med. 2020;18(1):361. doi: 10.1186/s12916-020-01824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Won K, Lee BK, Park H, Heo R, Lee S, Rizvi A, Lin FY, Kumar A, Hadamitzky M, Kim Y, et al. Quantitative assessment of coronary plaque volume change related to triglyceride glucose index: the progression of AtheRosclerotic PlAque DetermIned by Computed TomoGraphic Angiography IMaging (PARADIGM) registry. Cardiovasc Diabetol. 2020;19(1):113. doi: 10.1186/s12933-020-01081-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin J, Cao Y, Wu L, You X, Guo Y, Wu N, Zhu C, Gao Y, Dong Q, Zhang H, et al. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J Thorac Dis. 2018;10(11):6137–6146. doi: 10.21037/jtd.2018.10.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao Q, Zhou D, Li Y, Wang Y, Xu S, Zhao X. The triglyceride-glucose index predicts coronary artery disease severity and cardiovascular outcomes in patients with Non-ST-segment elevation acute coronary syndrome. Dis Markers. 2019;2019:6891537. doi: 10.1155/2019/6891537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 23.Deng X, Wang X, Yu H, Chen S, Xu X, Huai W, Liu G, Ma Q, Zhang Y, Dart AM, et al. Admission macrophage migration inhibitory factor predicts long-term prognosis in patients with ST-elevation myocardial infarction. Eur Heart J Qual Care Clin Outcomes. 2018;4(3):208–219. doi: 10.1093/ehjqcco/qcy020. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Associations 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2020. Diabetes Care. 2019;43(Supplement 1):S14–S31. doi: 10.2337/dc20-S002. [DOI] [PubMed] [Google Scholar]
- 25.Alfonso F, Pérez-Vizcayno MJ, Cuesta J, García Del Blanco B, García-Touchard A, López-Mínguez JR, Masotti M, Zueco J, Cequier A, Velázquez M, et al. 3-Year clinical follow-up of the RIBS IV Clinical Trial. JACC Cardiovasc Interv. 2018;11(10):981–991. doi: 10.1016/j.jcin.2018.02.037. [DOI] [PubMed] [Google Scholar]
- 26.Xu X, Pandit RU, Han L, Li Y, Guo X. Remnant lipoprotein cholesterol independently associates with in-stent restenosis after drug-eluting stenting for coronary artery disease. Angiology. 2019;70(9):853–859. doi: 10.1177/0003319719854296. [DOI] [PubMed] [Google Scholar]
- 27.Vasques ACJ, Novaes FS, de Oliveira MDS, Matos Souza JR, Yamanaka A, Pareja JC, Tambascia MA, Saad MJA, Geloneze B. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pr. 2011;93(3):e98–e100. doi: 10.1016/j.diabres.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 28.Kim MK, Ahn CW, Kang S, Nam JS, Kim KR, Park JS. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Cardiovasc Diabetol. 2017;16(1):108. doi: 10.1186/s12933-017-0589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride–glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol. 2021;20(1):76. doi: 10.1186/s12933-021-01268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambrinoudaki I, Kazani MV, Armeni E, Georgiopoulos G, Tampakis K, Rizos D, Augoulea A, Kaparos G, Alexandrou A, Stamatelopoulos K. The TyG index as a marker of subclinical atherosclerosis and arterial stiffness in lean and overweight postmenopausal women. Heart Lung Circ. 2018;27(6):716–724. doi: 10.1016/j.hlc.2017.05.142. [DOI] [PubMed] [Google Scholar]
- 31.Irace C, Carallo C, Scavelli FB, De Franceschi MS, Esposito T, Tripolino C, Gnasso A. Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. Int J Clin Pract. 2013;67(7):665–72. doi: 10.1111/ijcp.12124. [DOI] [PubMed] [Google Scholar]
- 32.Won K, Park G, Lee S, Cho I, Kim HC, Lee BK, Chang H. Relationship of insulin resistance estimated by triglyceride glucose index to arterial stiffness. Lipids Health Dis. 2018;17(1):268. doi: 10.1186/s12944-018-0914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin JL, Sun D, Cao YX, Guo YL, Wu NQ, Zhu CG, Gao Y, Dong QT, Zhang HW, Liu G, et al. Triglyceride glucose and haemoglobin glycation index for predicting outcomes in diabetes patients with new-onset, stable coronary artery disease: a nested case-control study. Ann Med. 2018;50(7):576–586. doi: 10.1080/07853890.2018.1523549. [DOI] [PubMed] [Google Scholar]
- 34.Park K, Ahn CW, Lee SB, Kang S, Nam JS, Lee BK, Kim JH, Park JS. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care. 2019;42(8):1569–1573. doi: 10.2337/dc18-1920. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Ding X, Hua B, Liu Q, Gao H, Chen H, Zhao X, Li W, Li H. High triglyceride-glucose index is associated with adverse cardiovascular outcomes in patients with acute myocardial infarction. Nutr Metab Cardiovasc Dis. 2020;30(12):2351–2362. doi: 10.1016/j.numecd.2020.07.041. [DOI] [PubMed] [Google Scholar]
- 36.Eisen A, Giugliano RP, Braunwald E. Updates on acute coronary syndrome: a review. JAMA Cardiol. 2016;1(6):718–730. doi: 10.1001/jamacardio.2016.2049. [DOI] [PubMed] [Google Scholar]
- 37.Park B, Lee Y, Lee HS, Jung D. The triglyceride-glucose index predicts ischemic heart disease risk in Koreans: a prospective study using National Health Insurance Service data. Cardiovasc Diabetol. 2020;19(1):210. doi: 10.1186/s12933-020-01186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46(2):189–197. doi: 10.1111/eci.12583. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Cong H, Zhang J, Hu Y, Wei A, Zhang Y, Yang H, Ren L, Qi W, Li W, et al. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):80. doi: 10.1186/s12933-020-01054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Ding X, Hua B, Liu Q, Gao H, Chen H, Zhao X, Li W, Li H. Predictive effect of triglyceride-glucose index on clinical events in patients with type 2 diabetes mellitus and acute myocardial infarction: results from an observational cohort study in China. Cardiovasc Diabetol. 2021;20(1):43. doi: 10.1186/s12933-021-01236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qi W, Li Q, Liew CW, Rask-Madsen C, Lockhart SM, Rasmussen LM, Xia Y, Wang X, Khamaisi M, Croce K, et al. SHP-1 activation inhibits vascular smooth muscle cell proliferation and intimal hyperplasia in a rodent model of insulin resistance and diabetes. Diabetologia. 2017;60(3):585–596. doi: 10.1007/s00125-016-4159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lafont A, Durand E, Samuel JL, Besse B, Addad F, Lévy BI, Desnos M, Guérot C, Boulanger CM. Endothelial dysfunction and collagen accumulation: two independent factors for restenosis and constrictive remodeling after experimental angioplasty. Circulation. 1999;100(10):1109–1115. doi: 10.1161/01.CIR.100.10.1109. [DOI] [PubMed] [Google Scholar]
- 43.Kitta Y, Nakamura T, Kodama Y, Takano H, Umetani K, Fujioka D, Saito Y, Kawabata K, Obata J, Ichigi Y, et al. Endothelial vasomotor dysfunction in the brachial artery is associated with late in-stent coronary restenosis. J Am Coll Cardiol. 2005;46(4):648–655. doi: 10.1016/j.jacc.2005.04.055. [DOI] [PubMed] [Google Scholar]
- 44.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14(5):575–585. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Baseline characteristics of patients with and without ISR. Table S2. Association of DES-ISR and other clinical variables in the univariate analysis. Figure S1. Comparison of the TyG index between patients with or without ISR in the subgroups of sex (A), DM status (B), current smoking status (C), age (D), dichotomized baseline BMI (E), and dichotomized baseline eGFR (F). TyG index, triglyceride-glucose index; ISR, in-stent restenosis; DM, diabetes mellitus; BMI, body mass index; eGFR, estimated glomerular filtration rate.
Data Availability Statement
The datasets used/or analyzed during the current study are available from the corresponding author on reasonable request.