Abstract
Background
Accumulating evidence have shown that the intestinal microbiota plays an important role in prevention of host obesity and metabolism disorders. Recent studies also demonstrate that early life is the key time for the colonization of intestinal microbes in host. However, there are few studies focusing on possible association between intestinal microbiota in the early life and metabolism in adulthood. Therefore the present study was conducted to examine whether the short term antibiotic and/or probiotic exposure in early life could affect intestinal microbes and their possible long term effects on host metabolism.
Results
A high-fat diet resulted in glucose and lipid metabolism disorders with higher levels of visceral fat rate, insulin-resistance indices, and leptin. Exposure to ceftriaxone in early life aggravated the negative influences of a high-fat diet on mouse physiology. Orally fed TMC3115 protected mice, especially those who had received treatment throughout the whole study, from damage due to a high-fat diet, such as increases in levels of fasting blood glucose and serum levels of insulin, leptin, and IR indices. Exposure to ceftriaxone during the first 2 weeks of life was linked to dysbiosis of the fecal microbiota with a significant decrease in the species richness and diversity. However, the influence of orally fed ceftriaxone on the fecal microbiota was limited to 12 weeks after the termination of treatment. Of note, at week 12 there were still some differences in the composition of intestinal microbiota between mice provided with high fat diet and antibiotic exposure and those only fed a high fat diet.
Conclusions
These results indicated that exposure to antibiotics, such as ceftriaxone, in early life may aggravate the negative influences of a high-fat diet on the physiology of the host animal. These results also suggest that the crosstalk between the host and their intestinal microbiota in early life may be more important than that in adulthood, even though the same intestinal microbes are present in adulthood.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12866-021-02263-6.
Keywords: Early life, Intestinal microbiota, High-fat diet, Metabolism, Visceral obesity
Background
Obesity is regarded as a worldwide public health issue, and the Global Burden of Disease study shows that the prevalence of obesity has more than doubled since 1980 [1]. Widely published studies have identified obesity as a major risk factor for various noncommunicable diseases (NCD) [2, 3], including diabetes, hypertension, dyslipidemia, insulin resistance, and cardiovascular diseases [4, 5]. Importantly, early life, especially the first 1000 days, is regarded as the key period for infant growth and has long-term effects even on adulthood health and diseases [6]. Mounting evidence has identified that the intestinal microbiota in early life has strong correlations with NCD during adulthood, including obesity, but the underlying mechanisms and possible preventative treatments remain unclear [7, 8].
The intestinal microbiota has been widely proven to contribute to human health [9, 10]. Therefore, the colonization of the intestinal microbiota during early development is important for host health. Previous studies have shown that there were significant differences between the intestinal microbiota of infants and adults and that infants were more sensitive to environmental factors than adults, suggesting that early life may be of key importance to the establishment of the intestinal microbiota [11]. Our previous study also indicated that early life is the key time for the formation of intestinal microbiota in infants [12]. Therefore, research on the association between the formation of the intestinal microbiota in infancy and long-term effects on adulthood health and diseases may contribute to the prevention of obesity in adulthood.
Antibiotics have been used to protect humans from severe infections and can be beneficial to patient health for extended periods. However, recent studies have indicated that antibiotics can alter the intestinal microbiota of patients [13]. Neonatal mice who received antibiotic treatment had a higher ratio of Firmicutes species to Bacteroides species–a key bacterial indicator for obesity–and lower alpha diversity, which is considered harmful to host health [14, 15]. Evidence also suggests that antibiotic use can alter the composition of the microbial community, and patients who had been treated with broad-spectrum antibiotics were found to be more consistently associated with overweight or obesity symptoms [16, 17]. Therefore, the dysbiosis of the intestinal microbiota may be an underlying mechanism whereby antibiotic use leads to obesity and metabolic disorders.
Probiotics are defined as “live microorganisms” that “when administered in adequate amounts, confer a health benefit to the host” and are regarded as functional foods with protective effects against obesity and metabolic diseases [18]. Recent studies have demonstrated that supplementation with several probiotics may have beneficial effects on host metabolism, regardless of whether these are humans or animals [19, 20]. An analysis of the whole genome of Bifidobacterium bifidum TMC3115 (TMC3115) isolated from healthy infants revealed encoded loci for the utilization of human milk oligosaccharides. These studies have indicated that the abnormal intestinal microbiota induced by antibiotic treatment in early life could impair the epithelium and affect immunity through to adulthood and that TMC3115 may alleviate the side effects caused by ceftriaxone [21]. However, it remains unclear whether TMC3115 could mitigate the metabolic disorders induced by the dysbiosis of the intestinal microbiota.
Our previous study identified that short-term use of ceftriaxone can damage the intestinal microbiota in young mice [22]. Therefore, this study was conducted to determine whether the short-term antibiotic and/or probiotic exposure in early life could affect the construction of the intestinal microbiota and the possible long-term effects of these treatments on host metabolism.
Results
Body weight and visceral fat rate
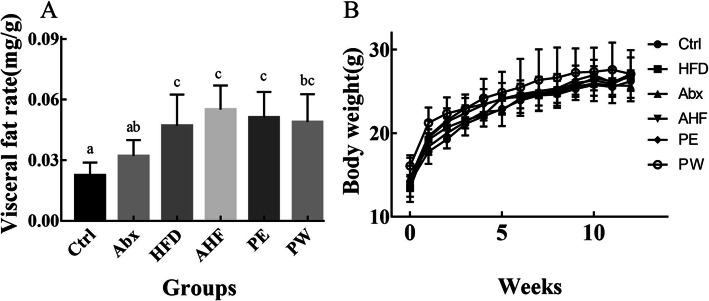
Although there was no significant difference between mouse body weights (Fig. 1), the visceral fat rate (total visceral fat/body weight) was significantly different among the six groups (p < 0.05). Providing mice with a high-fat diet resulted in a higher visceral fat rate relative to the normal diet group (p < 0.05). However, probiotic treatment (both PE and PW groups) did not reduce the visceral fat rate of mice compared with that of the AHF group (p < 0.05) (Fig. 1).
Fig. 1.
The result of body weight and visceral fat rate (n = 12/group). Values are expressed as mean ± SEM (a) The visceral fat rate of mice in different groups. (b) The body weight of mice in different groups. There were no significant differences among groups with a common letter P < 0.05
Impairment of FBG and OGTT
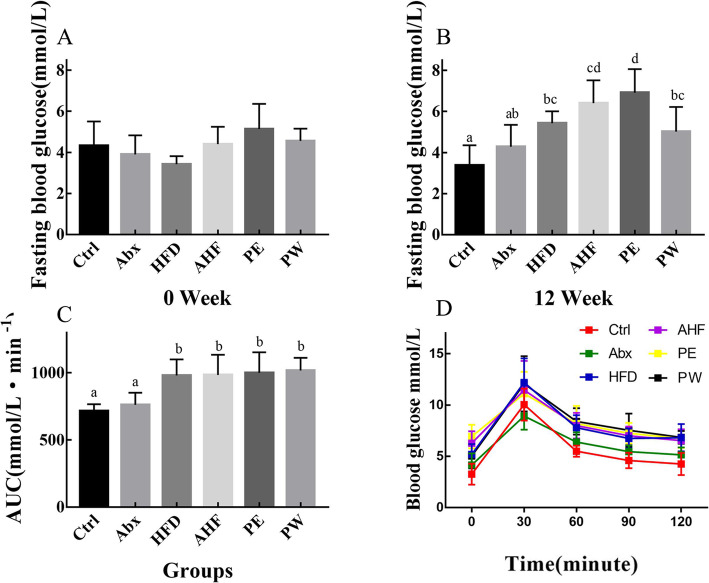
At the end of the antibiotic treatment (week 0), the level of FBG in mice in the test groups was not significantly different from that in mice in the Ctrl group (Fig. 2). Mice fed with a high-fat diet had significantly higher FBG levels and higher AUC values compared with those of the Ctrl group mice (p < 0.05). Additionally, at week 12, there was an increasing trend in the FBG levels of mice in the AHF group compared with the HFD group, whereas the PW group displayed a decreasing trend of FBG levels compared with the AHF group (Fig. 2). However, the AUC values did not show any differences among mice fed with a high-fat diet.
Fig. 2.
The result of fasting blood glucose and oral glucose tolerance test (n = 12/group). Values are expressed as mean ± SEM (a) The fasting blood glucose of mice at week 0. (b) The fasting blood glucose of mice at week 12. (c) The area under the curve values of mice in different groups. (d) The oral glucose tolerance test of mice in different groups. There were no significant differences among groups with a common letter P < 0.05
Level of serum and liver lipid metabolism
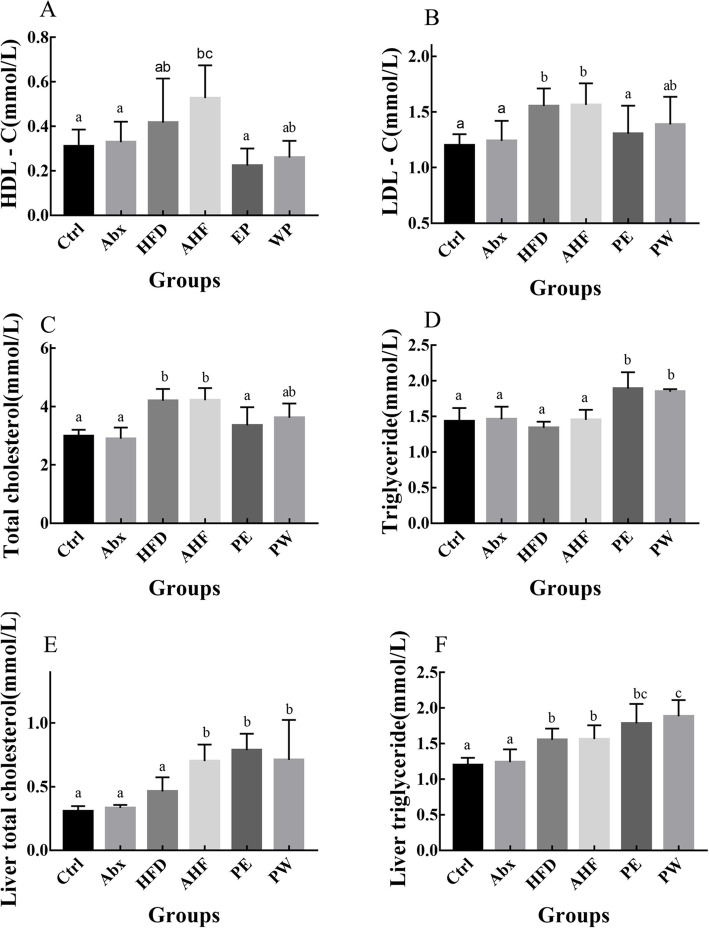
No significant differences were observed in the serum levels of HDL-c among the six groups. However, when compared with the Ctrl group, mice in the HFD and AHF groups had increased levels of LDL-c and serum total cholesterol (p < 0.05), although this was alleviated by TMC3115 treatment (Fig. 3). In addition, a high-fat diet lead to higher levels of liver triglyceride and liver total cholesterol (p < 0.05), and these were not affected by probiotic treatment (Fig. 3).
Fig. 3.
The lipid metabolism-related index in mice (n = 12/group). Values are expressed as mean ± SEM (a) Plasma levels of high-density lipoprotein cholesterol. (b) Plasma levels of low-density lipoprotein cholesterol. (c) Plasma levels of total cholesterol. (d) Plasma levels of triglyceride (e) Liver levels of total cholesterol. (f) Liver levels of triglyceride. There were no significant differences among groups with a common letter P < 0.05
Serum level of insulin, adiponectin, and leptin
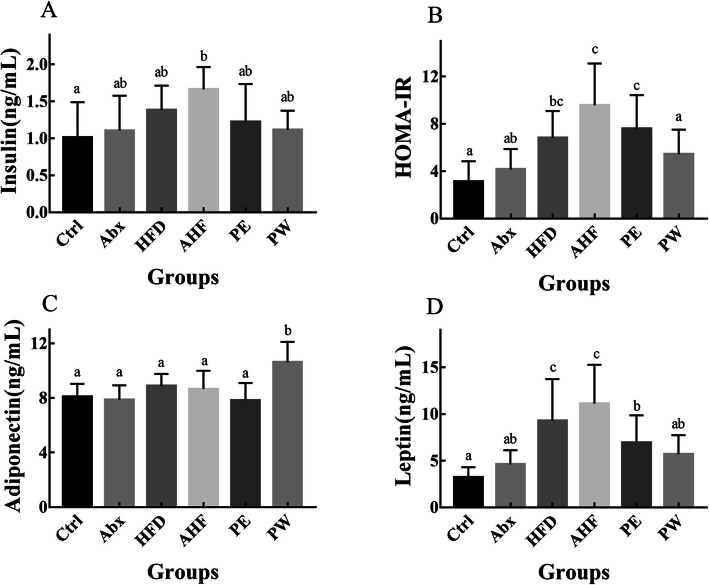
Although mice in the HFD group did not have higher serum insulin levels compared with those in the Ctrl group, mice in the AHF group did have significantly higher levels of serum insulin compared with mice in the Ctrl group (p < 0.05) (Fig. 4). Meanwhile, there was a decreasing trend of serum insulin levels in the PW group compared with the HFD and AHF groups. Mice fed a high-fat diet had higher levels of leptin and IR compared to mice provided with normal diet (p < 0.05), and antibiotic exposure resulted in an increasing trend of leptin and IR levels, both in mice in the normal diet group and in the high-fat diet group. Importantly, significantly lower levels of leptin and IR (p < 0.05) and a higher level of adiponectin were observed in the PW group compared with the AHF group (Fig. 4).
Fig. 4.
The result of metabolism-related hormones and insulin resistance (n = 12/group). Values are expressed as mean ± SEM (a) The serum level of insulin (b) The insulin-resistance indices of mice in different groups. (c) The serum level of adiponectin. (d) The serum level of leptin. There were no significant differences among groups with a common letter P < 0.05
Fecal microbiota analysis
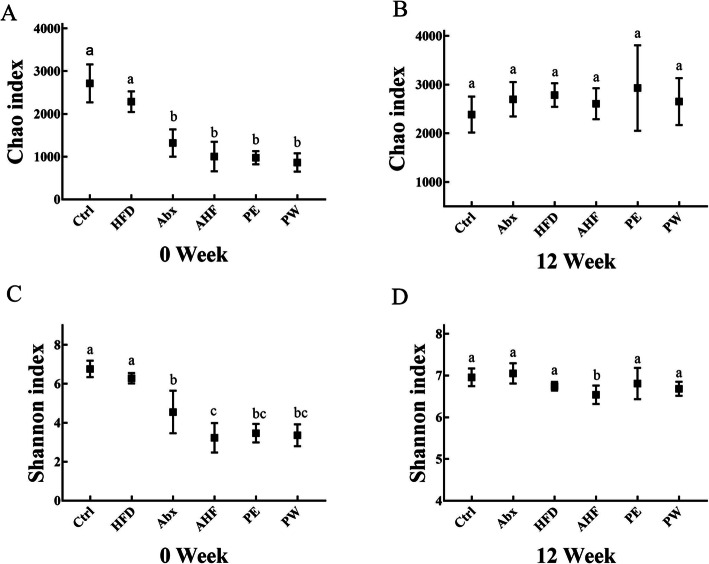
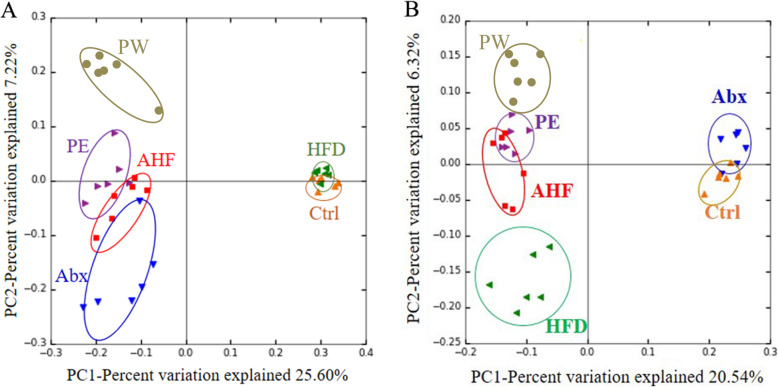
After gavage, the Chao and Shannon indices both significantly decrease in mice treated with an antibiotic (p < 0.05) (Fig. 5). After 12 weeks, only the Shannon indices of the AHF group exhibited a significant decrease compared with that of the Ctrl and Abx groups (Fig. 5). PCoA analysis based on unweighted UniFrac distance was used to reflect the construction of the intestinal microbiota and demonstrated that the intestinal microbiota composition of each group formed a significant cluster(Fig. 6). At week 0, principal component (PC)1 and PC2 explained 25.60 and 7.22% of variability, respectively, and after 12 weeks, PC1 and PC2 explained 20.54 and 6.32% of variability, respectively.
Fig. 5.
The α-diversity of intestinal microbiota. (a) The Chao indices of intestinal microbiota at week 0 (b) The Chao indices of intestinal microbiota at week 12. (c) The Shannon indices of intestinal microbiota at week 0. (d) The Shannon indices of intestinal microbiota at week 12
Fig. 6.
The β-diversity of of intestinal microbiota. (a) Principal coordinate analysis based on the unweighted UniFrac distance of operational taxonomic units at week 0. (b) Principal coordinate analysis based on the unweighted UniFrac distance of operational taxonomic units at week 12
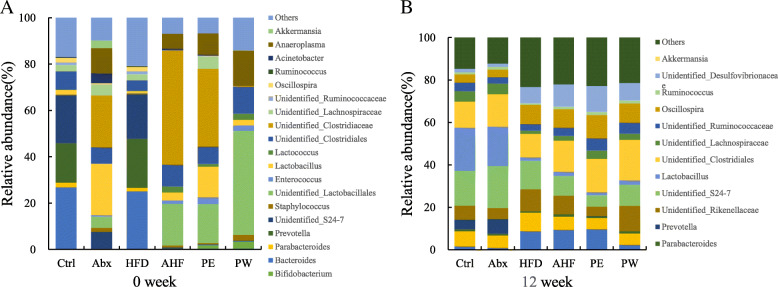
At the phylum level, the relative abundance of Firmicutes increased sharply, whereas that of Bacteroidetes decreased significantly in the Abx/AHF/PE/PW groups because of antibiotic treatment at week 0 (Fig. 7). Notably, the relative abundance of Actinobacteria included Bifidobacterium TMC3115 and was observed to increase significantly in both the PE and PW groups. After 12 weeks, the Abx group exhibited a similar species abundance at the phylum level compared with that of the Ctrl group. In addition, there was a significant increase of Proteobacteria in mice fed with a high-fat diet. Meanwhile, the relative abundance of Proteobacteria increased in the AHF group compared with the HFD group. At the genus level, antibiotic treatment led to a significant decrease of Parabacteroides, Prevotella, Ruminococcus, Oscillospira, and Bacteroides genera after antibiotic treatment (Fig. 7). Additionally, the PE and PW groups had a higher abundance of Bifidobacterium species. After 12 weeks, a high-fat diet resulted in a significant decrease of Prevotella, Ruminococcus, Bifidobacterium, and Ruminococcaceae species. Then, as expected, the abundance of Bifidobacterium species in the groups treated with TMC3115, especially in the PW group, significantly increased.
Fig. 7.
Effects of antibiotic/probiotic treatment and high fat diet on intestinal microbiota. (a) Relative abundance at the phylum level at week 0 (b) Relative abundance at the phylum level at week 12. (c) Relative abundance at the genus level at week 0. (d) Relative abundance at the genus level at week 12
Discussion
A high-fat diet is known to potentially result in obesity, and long-term microbiome dysbiosis induced by antibiotic treatment can lead to further weight gain and higher fat mass in mice fed with HFD [23]. Female BALB/c mice fed with a 12-week high-fat diet developed higher visceral obesity, although there were no significant differences in body weight among the tested mice, which may be explained by the strain, sex, and food intake of mice [24]. Additionally, the mice treated with ceftriaxone had a higher trend of visceral fat rate, and the same trend was observed between mice fed a HFD treated with or without ceftriaxone. These results indicate that antibiotic treatment, even in early life, may promote a higher deposition of visceral fat caused by a high-fat diet. In our previous studies, TMC3115 was proven to help in alleviating the dysbiosis of the intestinal microbiota induced by antibiotics [21]. In this study, although there was no significant difference in the visceral obesity of mice treated with TMC3115 and those without TMC3115, the long-term use of TMC3115 did show a decreased trend in visceral obesity. Thus, our results suggest that exposure to antibiotics in infancy may influence the accumulation of visceral fat in the host during adulthood and that the presence of TMC3115 may mitigate these effects.
The FBG levels and AUC values in OGTT were determined to assess the effects of HFD and antibiotic/probiotic treatment in the glucose metabolism of mice. Previous studies have shown that a high-fat diet could result in higher FBG levels and AUC values [25]. In this study, at week 0, there were no significant differences among the tested mice, whereas at week 12, mice fed a high-fat diet all had higher FBG levels than mice fed a normal diet. Additionally, recent research has shown that probiotic treatment, especially with Bifidobacterium species, may contribute to FBG levels and AUC values [26, 27]. Our results also demonstrated that TMC3115 was associated with a decreased trend in FBG levels in mice but did not improve the AUC values. Mice exposed to antibiotics and fed with a high-fat diet had an increased trend in FBG levels compared with mice exposed to ceftriaxone alone, whereas those treated with TMC3115 only had a decreasing trend in FBG levels. Therefore, these results indicate that the dysbiosis of the intestinal microbiota in early life can aggravate the dysmetabolism of the host in adulthood. Meanwhile, the long-term use of TMC3115 may partly resist the effects induced by antibiotic treatment in early life.
Similarly to other studies, a high-fat diet significantly increased TC and LDL-C in the tested mice without influencing the TG level [28, 29]. A high-fat diet also led to higher levels of liver TG and liver TC than mice fed a normal diet. Antibiotic treatment resulted in a further elevation of liver TC levels in mice fed with a high-fat diet. Although previous studies have reported that a number of Bifidobacterium species can improve dyslipidemia caused by a high-fat diet, this was not the case with TMC3115. However, considering that the long-term use of TMC3115 was associated with a decreasing trend of visceral obesity, these results may be explained by the use of TMC3115 to inhibit the absorption of serum lipids, further affect lipid metabolism, and alleviate the accumulation of visceral fat. Therefore, these results may demonstrate that exposure to antibiotics in early life may promote lipid dysmetabolism in adulthood and that TMC3115 may alter the lipid dysmetabolism induced by a high-fat diet in the host.
The serum levels of insulin, leptin, and adiponectin are known to be closely related to host metabolism [30, 31]. For instance, adiponectin can improve IR by decreasing the muscular lipid contents in mice, and leptin can promote energy expenditure by increasing thyroid hormone signaling [32, 33]. Antibiotic treatment in early life also led to a higher trend of leptin levels compared with those of mice fed on the same diet. The use of TMC3115 in early life significantly decreased the levels of leptin, and the long-term use of TMC3115 significantly increased the levels of adiponectin. Although research has reported that a high-fat diet can increase insulin levels and decrease adiponectin levels, there were no significant differences in adiponectin levels between mice fed with a normal diet and those fed with a high-fat diet [34]. Previous studies have demonstrated that the sensitivity of adiponectin levels to a high-fat diet in female mice was different compared with those in male mice, which may explain the results of serum adiponectin in our studies [35, 36].
A high-fat diet can also result in a higher level of insulin and HOMA-IR [37, 38]. Similarly, the results from the present study also showed that a high-fat diet led to an increased trend in insulin and IR levels. Moreover, antibiotic treatment in early life further impaired the insulin levels and HOMA-IR in mice fed with a high-fat diet. In contrast to the effects on mice treated with antibiotics, even the use of TMC3115 in early life led to a decreased trend in insulin and IR levels. In addition, the long-term use of TMC3115 showed a further decreased trend in serum insulin levels and a significant decrease in IR compared with those in mice in the PE group. These results further indicated that antibiotic treatment in early life might aggravate the dysmetabolism of the host with an unhealthy diet in adulthood, whereas TMC3115 could partly alter this effect.
The intestinal microbiota is a key factor that influences host health, including immunity, metabolism, and even neurobehavioral traits [39, 40]. One of those underlying mechanisms may be the metabolites of intestinal microbiota, such as short-chain fatty acids or bile acids that have been shown to influence host health. The short-chain fatty acids fermented by the intestinal microbiota can regulate energy uptake and secretion of hormones, such as peptide YY (PYY) and glucagon-like peptide 1 (GLP-1), by activating G protein-coupled receptor 43 (GPR 43) and G protein-coupled receptor 41 (GPR 41) [41, 42]. Meanwhile, the intestinal microbiota can also affect bile acids that regulate host metabolic pathways via the farnesoid X receptor (FXR) and G protein-coupled membrane receptor 5 (TGR 5) [43, 44]. Furthermore, previous studies also indicated that the balance of the intestinal microbiota community composition, especially that of Firmicutes and Bacteroidetes, might play an important role in the metabolism of host [15, 45]. In this study, it was clear that antibiotic treatment dramatically altered the intestinal microbiota composition immediately, but the effects induced by a short-term therapeutic dose of ceftriaxone on intestinal microbiota at least at the phylum level did not appear to continue to adulthood. Recently, research has explored the relationship between obesity and antibiotic treatment, especially in infancy or childhood, but the results lacked consistency [46–48]. In our study, antibiotic use induced the dysbiosis of the intestinal microbiota during early life, although this effect did not continue to adulthood. However, antibiotic treatment in early life increased the sensitivity of the host animal to a high-fat diet and enhanced the negative effects of a high-fat diet on host metabolism, although the alpha-diversity and beta-diversity of intestinal microbiota seemed to recover in adulthood after the termination of the antibiotic treatment. Thus, these results indicate that exposure to antibiotics in early life might damage or alter the physiological function and metabolism of the host animal in a complex and incurable manner. Moreover, these results indicate that the crosstalk between the host and their intestinal microbiota in early life might be more important than in adulthood, even with the same intestinal microbes. These results could also provide possible reasons for the differences among recent epidemiological investigations concerning the association between antibiotic treatment and obesity as the timing of the antibiotic treatment may be critical.
Proteobacteria have a relatively low abundance at the phylum level in a healthy host. However, previous studies have shown that the relative abundance of Proteobacteria in patients with obesity was significantly higher [49]. Further research also indicated that abnormal inflammation, obesity, and insulin resistance were related to a lower relative abundance of Proteobacteria, especially that of Enterobacteriaceae and Desulfovibrionaceae genera [50]. In this study, the relative abundance of Proteobacteria in mice fed with a high-fat diet was significantly higher than mice fed a normal diet, and treatment with antibiotics in early life resulted in an increased trend of Proteobacteria abundance. However, probiotic treatment did not alter the relative abundance of Proteobacteria. In addition, Parabacteroides species are known to contribute to alleviation of obesity and obesity-related dysfunctions in mice [51], whereas Prevotella, Ruminococcus, and Bacteroides species were considered to be beneficial to host health [52]. Previous studies have demonstrated that perturbations caused by short-term antibiotic treatment can recovery to baseline spontaneously [53]. In our study, antibiotic exposure in early life did lead to a significant decrease of these beneficial microbes at week 0, but the abundance of these species returned to their normal level after the 12 weeks normal diet. Importantly, the high-fat diet did decrease the abundance of Prevotella, Parabacteroides, and Ruminococcus genera. Although TMC3115 did not decrease the abundance of these genera, the abundance of Bifidobacterium species was increased. These results suggest that the crosstalk between the intestinal microbes and host may be timing dependent and that several microbial genera may have more impact on the host in early life.
Our study used the Chao indices (reflecting species richness) and Shannon indices (reflecting species diversity) to assess the alpha diversity of intestinal microbiota. Previous studies have demonstrated that lower alpha diversity was related to various diseases such as nonalcoholic fatty liver disease and obesity [54, 55]. Here, antibiotic treatment significantly decreased the Chao and Shannon indices in early life, whereas these influences did not last to adulthood. After week 12, no differences were observed in the Chao indices among the tested mice, a high-fat diet did lead to a decreasing trend in the Shannon indices, and antibiotic treatment resulted in a further decrease of the Shannon indices. The results of the UniFrac-based principal coordinate analysis showed that the short-term use of antibiotics in early life can dramatically alter the composition of the intestinal microbiota. After 12 weeks of antibiotic treatment, mice that had been administered solely with antibiotic by gavage had the most similarity in the composition of the intestinal microbiota compared with mice in other groups, whereas those fed with a high-fat diet had a significantly different composition of intestinal microbiota. Furthermore, mice treated with a high-fat diet and antibiotic in early life had a varied composition of intestinal microbiota compared with mice fed with a high-fat diet. These results indicated that the unhealthy composition of the intestinal microbiota induced by ceftriaxone in early life could promote intestinal microbiota disorders in the host later induced by a high-fat diet. By contrast, probiotic treatment with TMC3115, especially long term, could significantly alleviate these effects.
Conclusion
In conclusion, these results demonstrated that exposure to antibiotics in infancy can influence the long-term health of host, for instance, in the accumulation of visceral fat, changes to the glycolipid metabolism, and levels of several related hormones, although the dysbiosis of the intestinal microbiota had mostly recovered in adulthood. Therefore, the crosstalk between the intestinal microbes and host animal may be timing dependent, and the host early life may be the key time for intestinal microbes to affect physical function and metabolism. Furthermore, the results from our study suggest that probiotic treatment might be used as a complementary strategy to protect people from damage caused by the dysbiosis of the intestinal microbiota and unhealthy diet habits.
Methods
Mice
Two-week-old female BALB/c mice from Chengdu Dossy Experimental Animals Co., Ltd. (Chengdu, China), were divided into (n = 12) the control group (Ctrl), antibiotic exposure group (Abx), high-fat diet group (HFD), antibiotic exposure + high-fat diet group (AHF), probiotic used in early life group (PE), and probiotic used throughout whole life group (PW). A specific pathogen-free facility with a 12-h light/dark cycle was used at an ambient temperature of 23 °C ± 3 °C and humidity of 40–70%. This study was approved by the Experimental Animal Management Committee of Sichuan Government (Approval number: SYXK2013–011).
At the end of the study, all mice were anesthetized by intraperitoneal injection of 2,2,2- tribromoethanol (125 mg/kg, CAS:75–80-9 Sigma-Aldrich Chemie GmbH, Steinheim, Germany) to collect blood samples via heart puncture. Then mice were euthanized by injection of an overdose of 2,2,2- tribromoethanol (500 mg/kg) and decapitated.
Experimental schedule
The mouse experimental schedule is shown in Fig. S1. In the first 2 weeks (week − 2 to week 0), the Abx/AHF/PE/PW groups were treated with 0.2 mL (100 mg/kg) of ceftriaxone (Shanghai Aladdin Bio-Chem Technology Co., Ltd., Shanghai, China) once a day, and the Ctrl/HFD group received an equal volume of sterile saline. Meanwhile, the PE/PW groups were treated with 0.2 mL of 5 × 109 CFU/mL TMC3115 (Hebei Inatural Biotech Co., Ltd., Hebei, China) after 2 h of antibiotic treatment, whereas mice in the other groups were administered an equal volume of sterile saline by gavage. For the next 12 weeks (week 0 to week 12), the PW group was treated with 0.2 mL of 5 × 109 CFU/mL TMC3115 once a day, whereas the other groups received an equal volume of sterile saline.
All mice received a normal diet in early life (week − 2 to week 0). Then, the Ctrl/Abx group continued received a normal diet, and the HFD/AHF/PE/PW group received a high-fat diet (Research Diet D12492; Research Diets, New Brunswick, NJ, USA) for 12 weeks (week 0 to week 12).
Fasting blood glucose(FBG) and oral glucose tolerance test (OGTT)
All mice were fasted from 9:00 p.m. to 9:00 a.m., and blood glucose levels were determined using a portable glucometer (ACCU-CHEK) at 0 and 12 weeks. At the end of week 12, after a 12 h fast, all mice were orally dosed with a glucose solution (2 g/kg). Blood glucose levels were determined using the portable glucometer at 0, 30, 60, 90, and 120 min after oral administration, and area under the curve (AUC) values were used to estimate the extent of glucose tolerance impairment.
Serum and liver tissue supernatant analysis
Mouse blood was centrifuged at 2000×g for 20 min. Then, the serum was collected and was centrifuged again at 2000×g for 5 min. The serum level of insulin was determined using an enzyme-linked immunoassay (ELISA) kit (Merck Millipore, Burlington, MA, USA), and the insulin resistance indices(IR) was calculated by (FBG × fasting insulin)/22.5. The serum levels of adiponectin and leptin were measured using their respective ELISA kits (R&D Systems Inc., Minneapolis, MN, USA).
An Automatic Chemistry Analyzer-Chemray240 (Shenzhen Rayto Life and Analytical Sciences Co., Ltd.) was used to determine the serum level of triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL), and low-density lipoprotein (LDL).
The levels of TG and TC in the liver were determined using commercially available kits: tissue total cholesterol assay kit-E1015 and tissue triglyceride assay kit-E1013 (Applygen Technologies Inc., Beijing, China).
Fecal microbiota community determination by 16S rRNA sequencing
According to previous methods, the 16S rRNA sequencing was performed as follow [56]. The Qubit fluorometer (Life Technologies) and the TapeStation (Agilent) were used to concentrate and purify DNA and the 16S library was constructed in strict accordance recommended by Illumina. The V3-V4 region of the 16S rRNA gene was amplified using 341F and 806R fusion primers containing identification indexes. PCR was performed on a TaKaRa Cycler Dice Touch (TaKaRa) with 2 × KAPA HiFi HotStart ReadyMix (Kapa Biosystems Inc., USA) under the following conditions: initial denaturation at 95 °C for 3 min, followed by 25 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, and ended with an extension step at 72 °C for 5 min. Qubit fluorometer and TapeStation were used to analyze the DNA concentration and size. The AMPure XP magnetic beads (Beckman Coulter Inc., USA) were used to purify the above PCR products and these products were diluted and pooled. Next the Nextera XT Index Kit (Illumina, United States) was used to add the barcodes into the above pooled PCR products and the MiSeq Reagent Kit v3 (600-cycle) (Illumina) was used to purify and pool the indexed PCR products. USEARCH (v 6.1.544) software was used to remove the chimeric check and singleton and the low-quality sequences were filtered by default 0.97. The QIIME (v 1.9.1) pipeline was used to identify representative sequences for each operational taxonomic unit (OTU) and the GreenGenes 13.8 database was used to align them. Further processions of OTU tables were completed at genus and phylum levels.
The QIIME script core_diversity_analyses.py was used to perform the calculation of the Alpha and beta diversity of gut microbiota and the principal coordinate analysis plots (PCoA) based on unweighted UniFrac was used to calculate Beta diversity.
Statistical analysis
All statistical analyses, except the results of the 16S rRNA sequencing, were performed using GraphPad Prism 7.0 software (GraphPad Software Inc., San Diego, CA, USA). The Shapiro–Wilk normality test was used to assess the normality. The Holm–Sidak’s multiple comparisons test or Kruskal–Wallis test was used for multiple comparisons. A p-value ≤0.05 was considered statistically significant.
Supplementary Information
Acknowledgments
The 16S rRNA sequencing and bioinformatics analysis were assisted by Dr. Gaku Harata (Technical research laboratory, Takanashi Milk Products Co., Ltd., Yokohama, Japan). We also appreciate the support of Public health and Preventive Medicine Provincial Experiment Teaching Center at Sichuan University and Food Safety Monitoring and Risk Assessment Key Laboratory of Sichuan Province.
Abbreviations
- IR
Insulin resistance indices
- FBG
Fasting blood glucose:
- TMC3115
Bifidobacterium bifidum TMC3115
- Ctrl
Control group
- Abx
Antibiotic exposure group
- HFD
High-fat diet group
- AHF
Antibiotic exposure + high-fat diet group
- PE
Probiotic used in early life group
- PW
Probiotic used throughout whole study group
- PCoA
Principal coordinates analysis
- PYY
Peptide YY
- GLP-1
Glucagon-like peptide 1
- GPR
G protein-coupled receptor
- FXR
Farnesoid X receptor
- TGR
G protein-coupled membrane receptor
Authors’ contributions
FH, RC, ZM, and WZ designed the study. HF, ML and XS obtained funding for the study and formulated laboratory methods. ZM, WZ, HL, and FJ performed the study. ZM, WZ, FJ, and HL analyzed the data. ZM, JL, and HL draft the manuscript. ML, FH, XS, and RC promoted analysis of the samples, contributed to interpret the data and provided important critical review. XS and FH had responsibility for the final manuscript. All authors have read and approved of the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant number 81973042) and the National Natural Science Foundation of China does not participate in the design, data collection, analysis or interpretation of the research or in the writing of the manuscript.
Availability of data and materials
Raw reads of 16S rRNA sequencing data is submitted to Sequence Read Archive (SRA) under the BioProject ID PRJNA636965 in NCBI.
Declarations
Ethics approval and consent to participate
This study was approved by the Experimental Animal Management Committee of Sichuan Government (Approval number: SYXK2013–011).
Consent for publication
Not applicable.
Competing interests
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
X. Shen, Email: Im_nutr@scu.edu.cn
F. He, Email: hf18602880124@163.com
References
- 1.Gregg EW, Shaw JE. Global Health effects of overweight and obesity. N Engl J Med. 2017;377(1):80–81. doi: 10.1056/NEJMe1706095. [DOI] [PubMed] [Google Scholar]
- 2.Bhupathiraju SN, Hu FB. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res. 2016;118(11):1723–1735. doi: 10.1161/CIRCRESAHA.115.306825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carpaij OA, van den Berge M. The asthma-obesity relationship: underlying mechanisms and treatment implications. Curr Opin Pulm Med. 2018;24(1):42–49. doi: 10.1097/MCP.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 4.Hall JE, Do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat Rev Nephrol. 2019;15(6):367–385. doi: 10.1038/s41581-019-0145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93(1):359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 6.Agosti M, Tandoi F, Morlacchi L, Bossi A. Nutritional and metabolic programming during the first thousand days of life. Pediatr Med Chir. 2017;39(2):157. doi: 10.4081/pmc.2017.157. [DOI] [PubMed] [Google Scholar]
- 7.Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8(1):39. doi: 10.1186/s13073-016-0294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leclercq S, Mian FM, Stanisz AM, Bindels LB, Cambier E, Ben-Amram H, Koren O, Forsythe P, Bienenstock J. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat Commun. 2017;8(1):15062. doi: 10.1038/ncomms15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulange CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8(1):42. doi: 10.1186/s13073-016-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paun A, Danska JS. Modulation of type 1 and type 2 diabetes risk by the intestinal microbiome. Pediatr Diabetes. 2016;17(7):469–477. doi: 10.1111/pedi.12424. [DOI] [PubMed] [Google Scholar]
- 11.Derrien M, Alvarez AS, de Vos WM. The gut microbiota in the first decade of life. Trends Microbiol. 2019;27(12):997–1010. doi: 10.1016/j.tim.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Shen X, Wang M, Zhang X, He M, Li M, Cheng G, Wan C, He F. Dynamic construction of gut microbiota may influence allergic diseases of infants in Southwest China. BMC Microbiol. 2019;19(1):123. doi: 10.1186/s12866-019-1489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lange K, Buerger M, Stallmach A, Bruns T. Effects of antibiotics on gut microbiota. Dig Dis. 2016;34(3):260–268. doi: 10.1159/000443360. [DOI] [PubMed] [Google Scholar]
- 14.Cheng RY, Li M, Li SS, He M, Yu XH, Shi L, He F. Vancomycin and ceftriaxone can damage intestinal microbiota and affect the development of the intestinal tract and immune system to different degrees in neonatal mice. Pathog Dis. 2017;75(8):ftx104. [DOI] [PubMed]
- 15.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 16.Haak BW, Lankelma JM, Hugenholtz F, Belzer C, de Vos WM, Wiersinga WJ. Long-term impact of oral vancomycin, ciprofloxacin and metronidazole on the gut microbiota in healthy humans. J Antimicrob Chemother. 2019;74(3):782–786. doi: 10.1093/jac/dky471. [DOI] [PubMed] [Google Scholar]
- 17.Miller SA, Wu RKS, Oremus M. The association between antibiotic use in infancy and childhood overweight or obesity: a systematic review and meta-analysis. Obes Rev. 2018;19(11):1463–1475. doi: 10.1111/obr.12717. [DOI] [PubMed] [Google Scholar]
- 18.Cani PD. Microbiota and metabolites in metabolic diseases. Nat Rev Endocrinol. 2019;15(2):69–70. doi: 10.1038/s41574-018-0143-9. [DOI] [PubMed] [Google Scholar]
- 19.Koutnikova H, Genser B, Monteiro-Sepulveda M, Faurie JM, Rizkalla S, Schrezenmeir J, Clement K. Impact of bacterial probiotics on obesity, diabetes and non-alcoholic fatty liver disease related variables: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2019;9(3):e017995. doi: 10.1136/bmjopen-2017-017995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naudin CR, Maner-Smith K, Owens JA, Wynn GM, Robinson BS, Matthews JD, Reedy AR, Luo L, Wolfarth AA, Darby TM, Ortlund EA, Jones RM. Lactococcus lactis subspecies cremoris elicits protection against metabolic changes induced by a Western-style diet. Gastroenterology. 2020;159(2):639–651.e5. doi: 10.1053/j.gastro.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Cheng R, Guo J, Pu F, Wan C, Shi L, Li H, Yang Y, Huang C, Li M, He F. Loading ceftriaxone, vancomycin, and Bifidobacteria bifidum TMC3115 to neonatal mice could differently and consequently affect intestinal microbiota and immunity in adulthood. Sci Rep. 2019;9(1):3254. doi: 10.1038/s41598-018-35737-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miao Z, Cheng R, Zhang Y, Liang H, Jiang F, Shen X, Chen G, Zhang Q, He F, Li M. Antibiotics can cause weight loss by impairing gut microbiota in mice and the potent benefits of lactobacilli. Biosci Biotechnol Biochem. 2020;84(2):411-20. [DOI] [PubMed]
- 23.Mahana D, Trent CM, Kurtz ZD, Bokulich NA, Battaglia T, Chung J, Muller CL, Li H, Bonneau RA, Blaser MJ. Antibiotic perturbation of the murine gut microbiome enhances the adiposity, insulin resistance, and liver disease associated with high-fat diet. Genome Med. 2016;8(1):48. doi: 10.1186/s13073-016-0297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulecka M, Paziewska A, Zeber-Lubecka N, Ambrozkiewicz F, Kopczynski M, Kuklinska U, Pysniak K, Gajewska M, Mikula M, Ostrowski J. Prolonged transfer of feces from the lean mice modulates gut microbiota in obese mice. Nutr Metab (Lond) 2016;13(1):57. doi: 10.1186/s12986-016-0116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han L, Zhao LH, Zhang ML, Li HT, Gao ZZ, Zheng XJ, Wang XM, Wu HR, Zheng YJ, Jiang XT, Ding QY, Yang HY, Jia WP, Tong XL. A novel antidiabetic monomers combination alleviates insulin resistance through bacteria-Cometabolism-inflammation responses. Front Microbiol. 2020;11:173. doi: 10.3389/fmicb.2020.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Tang H, Zhang C, Zhao Y, Derrien M, Rocher E, van-Hylckama Vlieg JE, Strissel K, Zhao L, Obin M, et al. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015;9(1):1–15. doi: 10.1038/ismej.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao D, Zhu H, Gao F, Qian Z, Mao W, Yin Y, Tan J, Chen D. Antidiabetic effects of selenium-enriched Bifidobacterium longum DD98 in type 2 diabetes model of mice. Food Funct. 2020;11(7):6528–6541. doi: 10.1039/D0FO00180E. [DOI] [PubMed] [Google Scholar]
- 28.He C, Cheng D, Peng C, Li Y, Zhu Y, Lu N. High-fat diet induces Dysbiosis of gastric microbiota prior to gut microbiota in association with metabolic disorders in mice. Front Microbiol. 2018;9:639. doi: 10.3389/fmicb.2018.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Xi Y, Xin X, Tian H, Hu Y. Gypenosides regulate farnesoid X receptor-mediated bile acid and lipid metabolism in a mouse model of non-alcoholic steatohepatitis. Nutr Metab (Lond) 2020;17(1):34. doi: 10.1186/s12986-020-00454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farr OM, Gavrieli A, Mantzoros CS. Leptin applications in 2015: what have we learned about leptin and obesity? Curr Opin Endocrinol Diabetes Obes. 2015;22(5):353–359. doi: 10.1097/MED.0000000000000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yanai H, Yoshida H. Beneficial effects of adiponectin on glucose and lipid metabolism and atherosclerotic progression: mechanisms and perspectives. Int J Mol Sci. 2019;20(5):1190. [DOI] [PMC free article] [PubMed]
- 32.Stern JH, Rutkowski JM, Scherer PE. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016;23(5):770–784. doi: 10.1016/j.cmet.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7(8):941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 34.Ma Q, Zhou X, Sun Y, Hu L, Zhu J, Shao C, Meng Q, Shan A. Threonine, but not lysine and methionine, reduces fat accumulation by regulating lipid metabolism in obese mice. J Agric Food Chem. 2020;68(17):4876–4883. doi: 10.1021/acs.jafc.0c01023. [DOI] [PubMed] [Google Scholar]
- 35.Amengual-Cladera E, Llado I, Gianotti M, Proenza AM. Sex differences in the effect of high-fat diet feeding on rat white adipose tissue mitochondrial function and insulin sensitivity. Metabolism. 2012;61(8):1108–1117. doi: 10.1016/j.metabol.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 36.Litzenburger T, Huber EK, Dinger K, Wilke R, Vohlen C, Selle J, Kadah M, Persigehl T, Heneweer C, Dotsch J, et al. Maternal high-fat diet induces long-term obesity with sex-dependent metabolic programming of adipocyte differentiation, hypertrophy and dysfunction in the offspring. Clin Sci (Lond) 2020;134(7):921–939. doi: 10.1042/CS20191229. [DOI] [PubMed] [Google Scholar]
- 37.Kuo CS, Chen JS, Lin LY, Schmid-Schonbein GW, Chien S, Huang PH, Chen JW, Lin SJ. Inhibition of serine protease activity protects against high fat diet-induced inflammation and insulin resistance. Sci Rep. 2020;10(1):1725. doi: 10.1038/s41598-020-58361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu T, Qi X, Liu Y, Guo J, Zhu R, Chen W, Zheng X, Yu T. Dietary supplementation with purified mulberry (Morus australis Poir) anthocyanins suppresses body weight gain in high-fat diet fed C57BL/6 mice. Food Chem. 2013;141(1):482–487. doi: 10.1016/j.foodchem.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 39.Baothman OA, Zamzami MA, Taher I, Abubaker J, Abu-Farha M. The role of gut microbiota in the development of obesity and diabetes. Lipids Health Dis. 2016;15(1):108. doi: 10.1186/s12944-016-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou L, Sonnenberg GF. Essential immunologic orchestrators of intestinal homeostasis. Sci Immunol. 2018;3(20):eaao1605. [DOI] [PMC free article] [PubMed]
- 41.Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, Takahashi T, Miyauchi S, Shioi G, Inoue H, Tsujimoto G. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4(1):1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moran BM, Flatt PR, McKillop AM. G protein-coupled receptors: signalling and regulation by lipid agonists for improved glucose homoeostasis. Acta Diabetol. 2016;53(2):177–188. doi: 10.1007/s00592-015-0826-9. [DOI] [PubMed] [Google Scholar]
- 43.Wahlstrom A, Sayin SI, Marschall HU, Backhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24(1):41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Wahlstrom A, Kovatcheva-Datchary P, Stahlman M, Khan MT, Backhed F, Marschall HU. Induction of farnesoid X receptor signaling in germ-free mice colonized with a human microbiota. J Lipid Res. 2017;58(2):412–419. doi: 10.1194/jlr.M072819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Komaroff AL. The microbiome and risk for obesity and diabetes. JAMA. 2017;317(4):355–356. doi: 10.1001/jama.2016.20099. [DOI] [PubMed] [Google Scholar]
- 46.Block JP, Bailey LC, Gillman MW, Lunsford D, Daley MF, Eneli I, Finkelstein J, Heerman W, Horgan CE, Hsia DS, et al. Early antibiotic exposure and weight outcomes in young children. Pediatrics. 2018;142(6):e20180290. [DOI] [PMC free article] [PubMed]
- 47.Leong KSW, McLay J, Derraik JGB, Gibb S, Shackleton N, Taylor RW, Glover M, Audas R, Taylor B, Milne BJ, Cutfield WS. Associations of prenatal and childhood antibiotic exposure with obesity at age 4 years. JAMA Netw Open. 2020;3(1):e1919681. doi: 10.1001/jamanetworkopen.2019.19681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edmonson MB, Eickhoff JC. Weight gain and obesity in infants and young children exposed to prolonged antibiotic prophylaxis. JAMA Pediatr. 2017;171(2):150–156. doi: 10.1001/jamapediatrics.2016.3349. [DOI] [PubMed] [Google Scholar]
- 49.Valsecchi C, Carlotta Tagliacarne S, Castellazzi A. Gut Microbiota and Obesity. J Clin Gastroenterol, Suppl 2, Proceedings from the 8th Probiotics, Prebiotics & New Foods for Microbiota and Human Health meeting held in Rome, Italy on September 13-15, 2015(50), S157-S158. [DOI] [PubMed]
- 50.Zhao L. The gut microbiota and obesity: from correlation to causality. Nat Rev Microbiol. 2013;11(9):639–647. doi: 10.1038/nrmicro3089. [DOI] [PubMed] [Google Scholar]
- 51.Wang K, Liao M, Zhou N, Bao L, Ma K, Zheng Z, Wang Y, Liu C, Wang W, Wang J, Liu SJ, Liu H. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 2019;26(1):222–235. doi: 10.1016/j.celrep.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 52.Shen F, Zheng RD, Sun XQ, Ding WJ, Wang XY, Fan JG. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2017;16(4):375–381. doi: 10.1016/S1499-3872(17)60019-5. [DOI] [PubMed] [Google Scholar]
- 53.MacPherson CW, Mathieu O, Tremblay J, Champagne J, Nantel A, Girard SA, Tompkins TA. Gut bacterial microbiota and its Resistome rapidly recover to basal state levels after short-term amoxicillin-clavulanic acid treatment in healthy adults. Sci Rep. 2018;8(1):11192. doi: 10.1038/s41598-018-29229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao R, Zhu C, Li H, Yin M, Pan C, Huang L, Kong C, Wang X, Zhang Y, Qu S, Qin H. Dysbiosis signatures of gut microbiota along the sequence from healthy, young patients to those with overweight and obesity. Obesity (Silver Spring) 2018;26(2):351–361. doi: 10.1002/oby.22088. [DOI] [PubMed] [Google Scholar]
- 55.Wang B, Jiang X, Cao M, Ge J, Bao Q, Tang L, Chen Y, Li L. Altered fecal microbiota correlates with liver biochemistry in nonobese patients with non-alcoholic fatty liver disease. Sci Rep. 2016;6(1):32002. doi: 10.1038/srep32002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fiedorova K, Radvansky M, Nemcova E, Grombirikova H, Bosak J, Cernochova M, Lexa M, Smajs D, Freiberger T. The impact of DNA extraction methods on stool bacterial and fungal microbiota community recovery. Front Microbiol. 2019;10:821. doi: 10.3389/fmicb.2019.00821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw reads of 16S rRNA sequencing data is submitted to Sequence Read Archive (SRA) under the BioProject ID PRJNA636965 in NCBI.