Abstract
Purpose:
To evaluate the effectiveness of propranolol at mitigating FDG uptake in brown adipose tissue (BAT) of pediatric patients with known or suspected malignancies.
Methods:
PET/CT scans of 3 cohorts of patients treated from 2005–2017 were scored for presence of FDG uptake by BAT at 7 sites: right or left neck/supraclavicular area, right or left axilla, anterior mediastinum, posterior thorax, and abdomen/pelvis. Uptake was scored as follows: 0-none, 1-mild uptake < liver, 2-moderate uptake = liver, 3-intense uptake > liver. Group 1 consisted of 323 patients (630 scans) who had no specific preparation to mitigate FDG uptake by BAT. Group 2 consisted of 345 patients (705 scans) who underwent only warming in an uptake room with a fixed temperature at 24°C. Group 3 consisted of 622 patients (1457 scans) who underwent warming. In Group 3, patients 8 years and older, 471 patients (1114 scans), were also pre-medicated with oral propranolol 60 minutes before injection of FDG. Generalized estimation equation, using the logit link method, was used to model the relationship between the incidence of BAT score > 0, in any site, as a function of age, sex, seasonal effect, and body surface area (BSA).
Results:
In patients aged 8 years or older, the incidence of BAT uptake was 35 – 44% and declined to 15% with propranolol. BAT was most frequent in the neck (26%), axilla (18%), posterior thorax (18%), mediastinum (14%), and abdomen/pelvis (8%); BAT was less common in warm months (p=0.001). No substantial benefit was shown with pre-injection warming alone. No significant effect was found for age, sex, or BSA separately. When BAT uptake was present, it was usually intense.
Conclusion:
Propranolol preparation minimizes FDG uptake by BAT and should be considered routine for pediatric FDG PET/CT cancer-related protocols in children, adolescents, and young adults.
Keywords: PET/CT, propranolol, brown adipose tissue, pediatric, FDG
Introduction
Visualization of 18F-fluorodeoxyglucose (FDG) uptake by brown adipose tissue (BAT) is a well-recognized physiologic variant and benign finding on pediatric PET/CT. Two principal types of adipocytes, or fat cells, exist in the body, although an intermediate type is now recognized. White fat cells are composed of a single, large lipid droplet with flattened eccentric nuclei and scanty cytoplasm. By contrast, brown adipocytes are polygonal with rounded central nuclei and have multiple lipid droplets of varying sizes tucked between numerous mitochondria [1–3]. BAT contains the genetic signature of a muscle cell [2], is highly vascularized, and highly innervated [4], and due to its abundance of mitochondria, BAT is well suited for its role in heat generation [1, 2]; the iron from the mitochondria renders the adipocytes brown [5]. Fatty acids in brown adipocytes are oxidized in mitochondria by mitochondrial uncoupling protein (UCP), generating heat via non-shivering thermogenesis [1, 2, 4]. UCP leads to proton leakage across the inner mitochondrial membrane and decreased coupling of respiration to adenosine diphosphate phosphorylation [6]. Thus, uncoupling of the mitochondrial respiratory chain occurs in BAT, leading to heat production rather than adenosine triphosphate energy storage [2, 4, 6, 7].
BAT is more common in children (1 in 3) than in adults (1 in 20) and is a rapid source of energy in infants and adolescents, constituting 5% of their body weight and then declining with age. BAT uptake is estimated to occur in one-third of pediatric patients undergoing PET/CT for cancer staging [8, 9]. The distribution of FDG uptake by BAT has been described in the pediatric population, affecting posterior cervical-supraclavicular regions, and less so in the axilla, posterior thoracic and abdominal paraspinal regions [10]. BAT uptake of FDG can confound FDG PET/CT interpretation by mimicking or obscuring nearby sites of tumor uptake [8, 9, 11–13].
With the advent of PET/CT hybrid scanners, physicians have become familiar with the patterns and typical uptake locations of FDG into BAT. However, despite the improved ability to identify BAT independent of general FDG uptake patterns in patients, the presence of FDG uptake in BAT still compromises image interpretation, particularly when uptake by both lymph node tissue and BAT in the neck and/or chest are present. Several studies have suggested using high-fat, very-low-carbohydrate protocols [14], ambient temperature [15], or pharmacological methods [16–20] to control uptake by BAT. Preparing children and adults for FDG PET/CT imaging can include the proposed pharmacological interventions diazepam, fentanyl, and propranolol to decrease the incidence and intensity of uptake in BAT [21]. Propranolol is postulated to decrease visualization by inhibiting sympathetic stimulation of BAT. Due to its documented effect on reducing BAT uptake of FDG, its widespread availability, and known safety profile, we chose to incorporate propranolol into routine preparation in our patients 8 years and older undergoing FDG PET/CT scans.
The purpose of this study was (1) to examine the frequency and distribution of BAT uptake in a large cohort of children, teenagers, and young adults with known or suspected neoplasms undergoing an FDG PET CT examination and (2) to evaluate the potential utility of environmental modification (temperature control) and pre-injection propranolol to reduce BAT uptake of FDG.
Materials and methods
Study design and patients
The study has been approved by the institutional review board, and the need for informed consent was waived. Diagnosis, treatment, and demographic information were obtained from a central patient database including information on age, sex, disease type, month of scan, and time elapsed between scan and diagnosis (Table 1, Supplemental Table 1). FDG PET/CT whole body scans in patients with known or suspected neoplasms treated from 2005 to 2017 were acquired over 3 separate time periods with different PET/CT preparation protocols.
Table 1.
Group demographics
| Group 1 No specific anti-BAT preparation | Group 2 Patients were in uptake room 24C for one hour prior to FDG | Group 3 Same preparation as Group 2 plus oral propranolol if applicable one hour prior to FDG | |
|---|---|---|---|
| Sex | |||
| Female | 144 (45%) | 159 (46%) | 280 (45%) |
| Male | 179 (55%) | 186 (54%) | 342 (55%) |
| Diagnosis | |||
| LL | 155 (48%) | 136 (39%) | 247 (40%) |
| Solid Tumor | 92 (28%) | 117 (34%) | 229 (37%) |
| Other | 76 (24%) | 92 (27%) | 146 (23%) |
| Age (y) at Time of Scan | |||
| 0 – 3.9 | 52 (8%) | 46 (7%) | 106 (7%) |
| 4 – 7.9 | 79 (13%) | 86 (12%) | 107 (7%) |
| 8 – 11.9 | 73 (12%) | 110 (16%) | 241 (17%) |
| 12 – 15.9 | 137 (22%) | 160 (23%) | 400 (27%) |
| 16 – 19.9 | 168 (27%) | 177 (25%) | 417 (29%) |
| ≥ 20 | 121 (19%) | 126 (18%) | 186 (13%) |
LL — Leukemia, lymphoma & related such as Langerhans cell histiocytosis (LCH)
Solid Tumor – Wilms tumor, neuroblastoma, sarcomas
Other – Thyroid, CNS, nasopharyngeal carcinomas, miscellaneous
Group 1 was the earliest cohort and had no specific preparation to reduce FDG uptake by BAT. This historical comparison group was of patients imaged when only a single uptake room was available, and reserving the room for use prior to patient injection was not feasible; no warming or other interventions were performed in this group. Group 1 consisted of patients (630 scans in 323 patients; F144:M179, age 13.8 y ± 6.4) imaged from January 3rd, 2005, to December 30th, 2005. Group 1a consisted only of those scans performed during this time period in patients 8 years and older (499 scans in 257 patients).
Group 2 consisted of patients (705 scans in 345 patients; F159:M186, age 13.6 y ± 5.8) imaged from July 1st, 2013, to June 30th, 2014, when the room temperature was fixed at 24°C (75°F). Group 2a consisted only of those scans performed during this time period in patients 8 years and older (572 scans in 273 patients). All Group 2 patients were instructed to lie quietly in an uptake room with dimmed lights and an elevated ambient room temperature for one hour prior to FDG injection and during the approximately one-hour uptake time before PET/CT examination.
Group 3 consisted of patients (622 patients; 1457 scans, F280: M342, age 13.7y ± 5.6) imaged from April 15th, 2015, to October 30th, 2017. Patients were prepared identically to Group 2, but patients aged 8 years or older were pre-medicated with propranolol (0.5 mg/kg orally 60–75 minutes prior to FDG injection, maximum 40 mg). Group 3a consisted only of those scans from patients aged 8 years or older that were performed with propranolol preparation (471 patients; 1114 scans, F206: M265, age 15.6y ± 3.9) The mean administered propranolol dose was 27.7 mg ± 10.0.
FDG PET/CT Scans
For Group 1, FDG PET/CT scans were performed after a 4-h or overnight fast (6 hours for patients undergoing sedation/anesthesia). First, the patients were given intravenous injections of 5.6 MBq (0.15 mCi)/kg 18F- FDG (maximum 444 MBq [12 mCi]). Approximately 1 h later, after the patient voided, image acquisition began with a non-contrast CT scan for attenuation correction and lesion localization followed by emission imaging using a GE Discovery LS PET CT scanner. Then, images from the top of the head through the feet were acquired at 5 minutes per bed position using 2D acquisition.
For Groups 2 and 3, a GE Discovery 690 PET/CT system was used, with images acquired at 3 minutes per bed position in 3D mode. Patients were maintained in a warm 24°C temperature-controlled environment for one hour prior to injection. Group 3 patients aged 8 years or older received oral propranolol one hour prior to injection (Group 3a). Promptly after receiving propranolol, the patients were escorted to an uptake room, where they remained at rest for an hour during the warming period, and for an additional hour following 18F-FDG administration.
Evaluation of FDG uptake by BAT
Reconstructed patient images were scored for the presence of FDG uptake by BAT as follows: (0) no BAT uptake visible, (1) intensity less than that of liver, (2) intensity equal to that of liver, and (3) intensity greater than that of liver. FDG uptake by BAT uptake was recorded for 7 locations: neck (left and right), axilla (left and right), mediastinum, posterior thorax, and abdomen/pelvis. Assessments of BAT uptake patterns were based on literature reporting anatomical sites negatively impacted by BAT: cervical, supraclavicular fossa, axillary, mediastinal, thoracic paravertebral, and abdominal locations [2, 6]. FDG uptake by BAT was considered present in any patient with at least one anatomical region with a score of ⩾ 1. Three experienced nuclear medicine physicians (KKW, MD, BLS) without knowledge of the patients’ treatment or diagnoses used a HERMES workstation (Gold Ver 4.7B, Stockholm, Sweden) or an Intelerad viewer (InteleViewer, Montreal CA) to evaluate the 7 selected locations for the presence or absence of FDG uptake by BAT.
Statistical analyses
The patient population was categorized by age group (0–3, 4–7, 8–11, 12–15, 16–19, and ⩾ 20 years), demographic variables, and disease diagnosis. Descriptive statistics were used to describe the patients of each group. Because a patient could have received multiple scans, generalized estimating equation (GEE) using the logit link method for repeated measurements was used to model the potential factors of incidence of BAT (BAT with score > 0 in any area) and to compare incidence among the 3 groups. Comparisons were made among Groups 1a, 2a, and 3a to assess the effect of warming and that of warming with propranolol in patients of similar ages. Data from the entire Group 1 and Group 2 were used to compare incidence of BAT uptake of FDG with and without warming. Each potential factor, including age, sex, body surface area (BSA), and season (dichotomized as warm months or cool months), was analyzed in the GEE model separately to estimate its individual effect. GEE with logit link was also used to determine group effect that affected the BAT after controlling for other potential confounding factors listed above. Warm months were defined as June through September. The remaining months were defined as cool months. Data were analyzed using the SAS software (SAS Institute, Inc., Cary, NC). Two-sided significance levels were calculated; p < 0.05 was considered to indicate statistical significance.
Results
Figures 1–4 are examples of BAT uptake in our patients studied.
Figure 1:
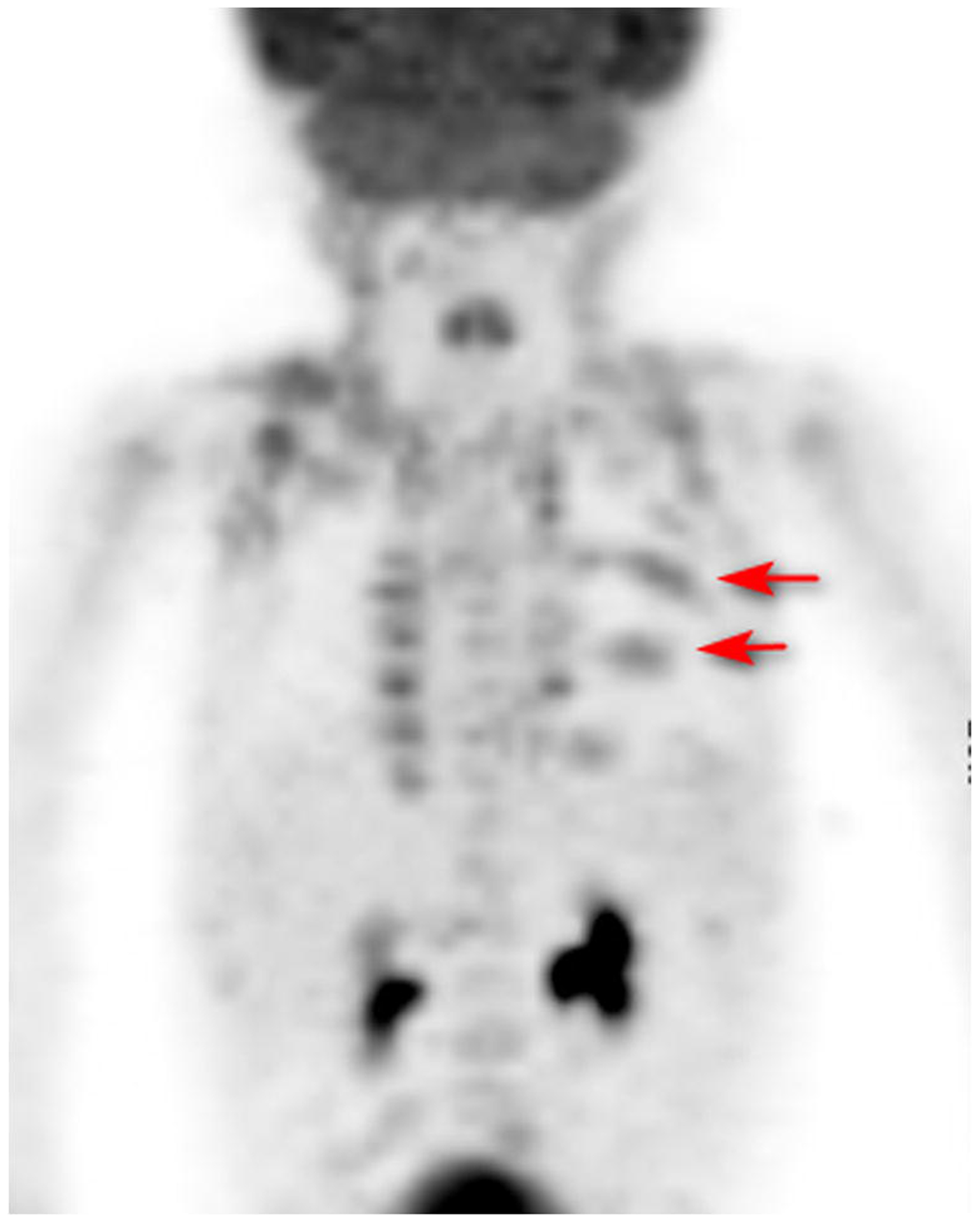
FDG PET/CT anterior MIP images of an 18 month old boy with Langerhans cell histiocytosis for therapy response evaluation. Patient did not receive propranolol. SUVmax BAT right supraclavicular area was 4.0, left supraclavicular 3.2. Arrows point to regions of histiocytic involvement posterior ribs.
Figure 4.
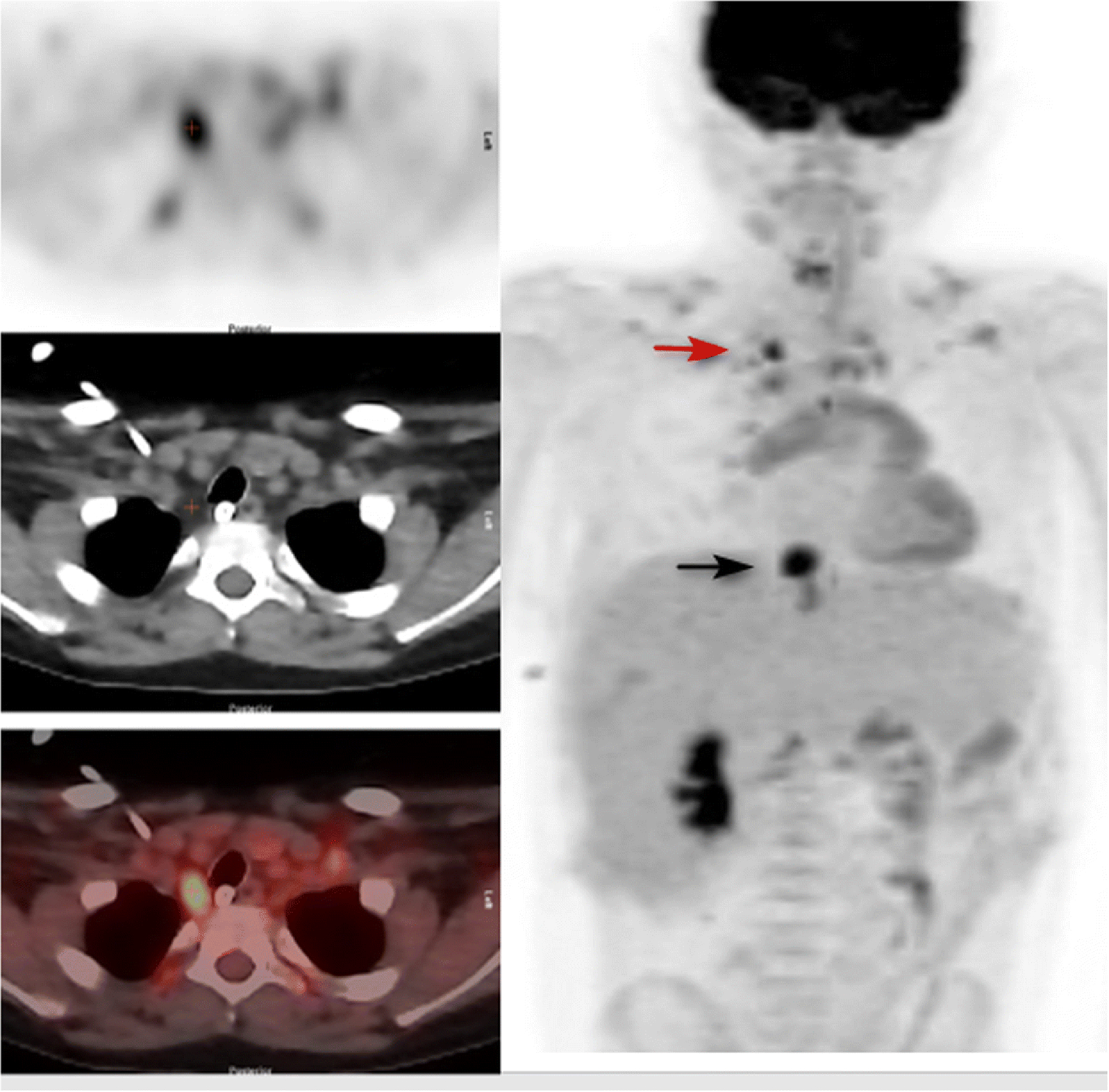
15 year old male with resected hepatoblastoma studied for rising alfa fetoprotein levels. Left panel top: PET image through the upper chest. Left panel middle: corresponding attenuation correction CT image. Left panel bottom: fusion image. Right panel: Anterior MIP of neck, chest, abdomen and upper pelvis. There is intense uptake in BAT in the lower right side of the neck paratracheal region, (identified by + on the cross-sectional images and red arrow on the MIP), and less intense uptake in supraclavicular BAT bilaterally. SUVmax of this area of BAT was 8.0. Black arrow points to a paraesophageal site of recurrent tumor. The patient did not receive propranolol as he was treated with metoprolol for diastolic dysfunction.
Demographic features of each group
Table 1 describes the demographic characteristics of each group. Diseases studied were categorized as LL (leukemia, lymphoma, and related disorders), Solid Tumor (sarcomas, neuroblastoma, Wilms tumor), or Other (thyroid, nasopharyngeal carcinomas, miscellaneous).
Location and intensity of uptake (Figure 1)
The reviewers’ scores are shown in Tables 2 and 3. Table 2 is a comparison of all patients in Group 1 and Group 2 only, enabling comparison of no preparation (Group 1) and warming (Group 2). The most common site of uptake was the neck, followed by the axilla and mediastinum. This pattern of uptake was common to all age groups throughout both the cool and warm seasons.
Table 2.
Incidence of regional FDG uptake by BAT by reviewer score
| Group 1 | No Preparation (n = 630; BAT incident rate = 32%) Number of scans in parentheses | ||||
|---|---|---|---|---|---|
| Score | Necka | Axillab | Mediastinum | Posterior Thoracic | Abdominopelvic |
| 0 | 69% (434) |
77% (488) |
82% (514) |
75% (472) |
90% (567) |
| 1 | 5% (31) |
5% (30) |
4% (27) |
3% (20) |
1% (6) |
| 2 | 5% (34) |
4% (27) |
5% (33) |
9% (55) |
2% (11) |
| 3 | 21% (131) |
13% (85) |
9% (56) |
13% (83) |
7% (46) |
| Group 2 | Warming Preparation (n = 705; BAT incident rate = 41%) | ||||
| Score | Necka | Axillab | Mediastinum | Posterior Thoracic | Abdominopelvic |
| 0 | 60% (423) |
74% (519) |
78% (549) |
71% (503) |
86% (604) |
| 1 | 4% (30) |
2% (16) |
3% (18) |
3% (22) |
0.3% (2) |
| 2 | 9% (62) |
3% (18) |
1% (8) |
6% (42) |
0.4% (3) |
| 3 | 27% (190) |
22% (152) |
18% (130) |
20% (138) |
14% (96) |
Left or right neck
Left or right
Table 3.
Incidence of regional FDG uptake by BAT in patients aged 8 years or older by reviewer score
| Group1a | No Preparation (n = 499; BAT incident rate = 35%) | ||||
|---|---|---|---|---|---|
| Score | Necka | Axillab | Mediastinum | Posterior Thoracic | Abdominopelvic |
| 0 | 66% (331) |
75% (375) |
79% (395) |
72% (358) |
89% (443) |
| 1 | 5% (23) |
5% (24) |
4% (22) |
3% (17) |
1% (5) |
| 2 | 5% (27) |
5% (23) |
6% (31) |
9% (47) |
2% (10) |
| 3 | 24% (118) |
15% (77) |
10% (51) |
15% (77) |
8% (41) |
| Group2a | Warming Preparation (n = 572; BAT incident rate = 44%) | ||||
| Score | Necka | Axillab | Mediastinum | Posterior Thoracic | Abdominopelvic |
| 0 | 56% (323) |
70% (403) |
75% (429) |
67% (384) |
83% (477) |
| 1 | 4% (21) |
3% (15) |
3% (15) |
4% (21) |
0.4% (2) |
| 2 | 10% (55) |
3% (16) |
1% (6) |
6% (37) |
0.4% (2) |
| 3 | 30% (173) |
24% (138) |
21% (122) |
23% (130) |
16% (91) |
| Group 3a | Warming + Propranolol Preparation (n = 1114; BAT incident rate = 15%) | ||||
| Score | Necka | Axillab | Mediastinum | Posterior Thoracic | Abdominopelvic |
| 0 | 85% (950) |
90% (998) |
94% (1044) |
93% (1038) |
97% (1079) |
| 1 | 3% (28) |
2% (25) |
1% (9) |
1% (12) |
0.1% (1) |
| 2 | 4% (47) |
3% (30) |
2% (20) |
2% (19) |
0.2% (2) |
| 3 | 8% (89) |
5% (61) |
4% (41) |
4% (45) |
3% (32) |
Left or right neck
Left or right axilla
For Group 1, the incidence of FDG uptake by BAT (score 1–3) was 32.2% (203 of 630) scans and of moderate-intense uptake (score 2–3) 26.7% (168 of 630) scans. Relative population occurrence of FDG uptake by BAT (calculated as the number of scans with BAT in each age group divided by the total number of scans with BAT) was 5% (0–3 years), 9% (4–7 years), 11% (8–11 years), 33% (12–15 years), 29% (16–19 years), and 13% (⩾ 20 years).
For Group 2, the incidence of FDG uptake by BAT visualization (score 1–3) was 40.6% (286 of 705) scans and of moderate-intense BAT (score 2–3) 36% (253 of 705) scans. Of the 705 patient scans from the Group 2 warming cohort [where the room temperature was fixed at 24°C (75°F)], the relative population occurrence of FDG uptake by BAT was 2% (0–3 years), 9% (4–7 years), 22% (8–11 years), 27% (12–15 years), 23% (16–19 years), and 17% (⩾ 20 years).
Table 3 is a comparison of all 3 groups, restricted to patients aged 8 years or older, to allow comparison of aged match patients for Group 3a, the patients treated with propranolol. Tables 2 and 3 show that for any region, when FDG uptake by BAT was present, the uptake was most commonly rated as intense (i.e., a score of 3). The most commonly involved areas, as expected, were the neck and axilla. Table 4 depicts the regional incidence of FDG uptake by BAT by age groups for patients 8 years and older. Effect of Propranolol
Table 4.
Incidence of FDG uptake by BAT in patients aged 8 years or older
| Necka | |||
|---|---|---|---|
| Age (y) | Group 1a | Group 2a | Group 3a |
| 8 – 11.9 | 27% | 56% | 12% |
| 12 – 15.9 | 47% | 47% | 20% |
| 16 – 19.9 | 35% | 37% | 16% |
| ⩾20 | 21% | 37% | 4% |
| Axillab | |||
| Age (y) | Group 1a | Group 2a | Group 3a |
| 8 – 11.9 | 23% | 35% | 10% |
| 12 – 15.9 | 36% | 36% | 13% |
| 16 – 19.9 | 26% | 23% | 11% |
| ⩾20 | 12% | 26% | 2% |
| Mediastinum | |||
| Age (y) | Group 1a | Group 2a | Group 3a |
| 8 – 11.9 | 21% | 25% | 6% |
| 12 – 15.9 | 27% | 30% | 9% |
| 16 – 19.9 | 22% | 24% | 7% |
| ⩾20 | 12% | 20% | 0% |
| Posterior Thoracic | |||
| Age (y) | Group 1a | Group 2a | Group 3a |
| 8 – 11.9 | 27% | 36% | 7% |
| 12 – 15.9 | 40% | 38% | 9% |
| 16 – 19.9 | 27% | 28% | 7% |
| ⩾20 | 17% | 30% | 1% |
| Abdominopelvic | |||
| Age (y) | Group 1a | Group 2a | Group 3a |
| 8 – 11.9 | 8% | 20% | 4% |
| 12 – 15.9 | 15% | 19% | 4% |
| 16 – 19.9 | 13% | 16% | 3% |
| ⩾20 | 6% | 11% | 1% |
left or right neck
left or right axilla
Table 3 indicates that for the propranolol-treated group, Group 3a, the incidence of FDG uptake by BAT visualization (score 1–3) was 15% (171 of 1114) and of moderate-intense BAT 13% (141 of 1114). Of the 1114 patient scans from the Group 3a propranolol-treated cohort, 85% (943 of 1114) did not have FDG uptake by BAT in any location. Relative occurrence of FDG uptake by BAT by age in the propranolol cohort was 15% (8–11 years), 44% (12–15 years), 36% (16–19 years), and 5% (⩾ 20 years). Of patients in the propranolol treated group who had BAT uptake - 15% were 8–11 years of age, 44% were 12–15 years, 36% were16–19 years, and 5% were 20 years and older). When BAT was present in the propranolol-treated cohort, the distribution was similar to that found in patients who did not receive propranolol.
FDG uptake by BAT was significantly more common in Group 1a than in Group 3a (estimate = 1.160, OR = 3.19, p<0.0001); FDG uptake by BAT in Group 2a was significantly more than that in Group 3a (estimate = 1.424, OR = 4.15, p<0.0001). Compared to patients of the same age, sex, BSA, and seasonal group in Group 3a, Group 1a had a 219% increase in odds of FDG uptake by BAT, and Group 2a had a 315% increase. To compare Group 1 and Group 2, all the patients are included in the model. The FDG uptake by BAT was significantly different (estimate = 0.304, OR = 1.36, p = 0.03). Compared to patients of the same age, sex, BSA, and seasonal group in Group 1, Group 2 had a 36% increase in odds of FDG uptake.
Seasonal variations
Figure 5 depicts the percentage of monthly variation in BAT uptake on scans of patients older than 8 years for all years in this retrospective study. The average uptake was lower in the warm months, with the maximum in December and the minimum in August. The odds of FDG uptake by BAT in colder weather increased 33% over those in warm months (estimate = 0.284, OR = 1.33, p=0.001). No significant effect was found for age, sex, or BSA with regard to seasonal variation.
Figure 5:
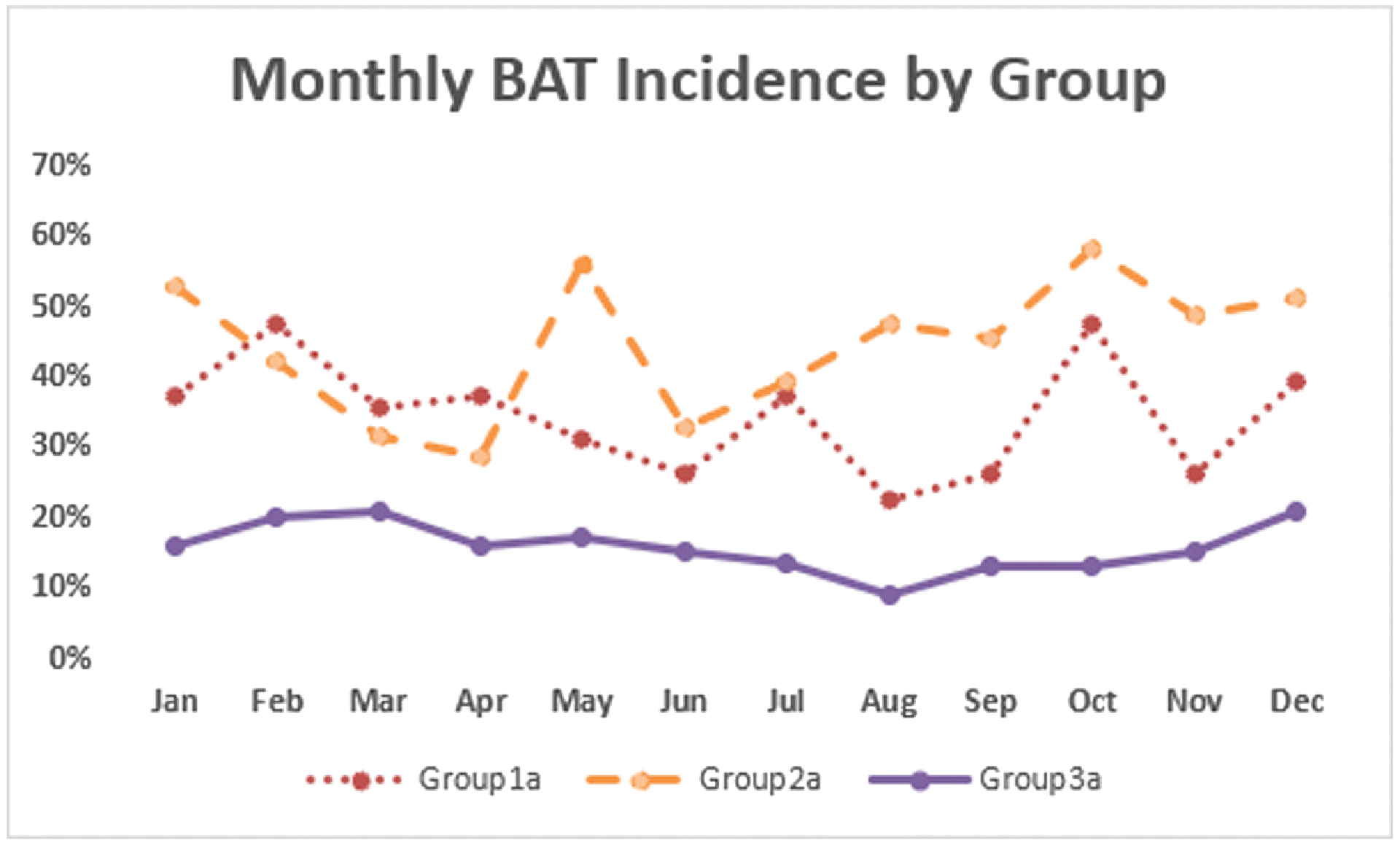
Seasonal FDG uptake by BAT. Warm months were defined as June through September; all other months were considered cool.
Discussion
This large retrospective analysis of children and young adults (1,290 patients; 2,792 scans), with suspected or known neoplasms who underwent FDG PET/CT imaging indicates that FDG uptake by BAT is common in these age groups. These study data agree with observations that BAT increases during puberty and is proportional to muscle mass [22–24]. Pubertal patients are reported to have 10 times more FDG uptake by BAT than those < 10 years old; only 20% of PET scans in pre-pubertal children had FDG uptake by BAT, whereas >75% of those in pubertal and adolescent cohorts had FDG uptake by BAT [17, 22, 24, 25]. Metabolism in white fat, brown fat, and muscle are intimately related to BAT activation associated with less weight gain and adiposity in adulthood [4]. Children with activated BAT had less subcutaneous white fat deposits and weight gain [26].
In this study of pediatric patients and young adults, FDG uptake by BAT most commonly occurred in the neck and supraclavicular regions, similar to the distributions described previously in pediatric patients [9, 17] and in adults [27]. The incidence of FDG uptake by BAT is much higher in this study than found in most studies of adults [27] but less than the 47% found by Gelfand and colleagues in 118 scans of pediatric patients [17]. Although mediastinal FDG uptake by BAT was uncommon in their patient population (2 of 118 scans), it was much more frequent in the patients in our study: ~25% in patients aged 12–19 years. The analysis of this study demonstrated peak occurrence of FDG uptake by BAT in patients aged 8–20 years, with declines thereafter (Table 4).
BAT is more common in cold climates and is dependent on the photopeak and day length, as shown by Gilsanz et al. [4]. In addition to white fat and brown fat, a category termed beige, or brite, fat has been reported [6]: this intermediate type of adipose tissue has been postulated to be influenced by cold weather [6]. As in other studies [15, 23, 28], our data, collected over 3.5 years, did show evidence for a small, warm-cool variation in FDG uptake by BAT. Seasonally influenced variation, however, may be impacted by extensive use of air conditioning in the warm months and limited outdoor time when ambient temperatures are highest, resulting in greater BAT uptake in warm months than might otherwise be expected.
FDG uptake by BAT in children and young adults with neoplastic disease has been studied at baseline and after therapy for different evaluation times to investigate the incidence of increasing or decreasing BAT uptake after therapy. A study by Gilsanz et al. that demonstrated a greater uptake by BAT in pediatric patients after successful therapy of lymphoma, when the patients were disease free [23]. Other studies have not described patients studied serially, except those whose studies were repeated because of extensive interference from FDG uptake by BAT [5, 29], and these studies did not include patients with neoplastic disease at different time points after therapy [5, 29]. Analysis of patients studied serially in relation to their clinical course was beyond the scope of this project but may be worthwhile for future data analysis.
Strategies to reduce brown fat [21] include warming techniques [8], and a high-fat diet for 12 hours prior may also reduce the incidence [14, 30], although the association with diet is being debated [4]. PET preparation in pediatric cases includes recommendations for control of ambient temperature [21]. Zukotynski et al. suggested that a constant ambient temperature of 24°C can reduce FDG uptake by BAT in children undergoing scans in the winter [15]. Our study fixed ambient temperature for Groups 2 and 3 at 24°C, as suggested, but did not find a statistical reduction in FDG uptake in BAT when comparing Groups 1 and 2, suggesting that environmental control was not effective for our population of pediatric and young adult patients. This was a surprising finding as we anticipated a substantial benefit to warming, which did not occur. Although no single cause explanation is convincing, it is possible that, for those undergoing warming, the additional hour of waiting in the imaging suite prior to injection evoked additional anxiety which in part negated potentially beneficial effects of warming. It is also possible that the advance in image quality provided by the scanner used in Group 2 facilitated greater confidence in identifying areas of BAT that might not have been identified in Group 1. Furthermore, it is possible that noncompliance blunted potential effects of warming on BAT uptake. A limitation for environmental control was that temperatures at or above 24°C were occasionally uncomfortable, resulting in patient or parent nonadherence. Nonadherence manifested as manipulation of the air temperature controller. Once access to the thermostats was removed, patients or parents might attempt to lower the room temperature by leaving the door ajar.
Brown fat is activated by norepinephrine binding of beta3-adrenergic receptors, via a mechanism of cold-induced sympathetic stimulation [1]. Pharmacologic interventions have been used to reduce brown fat accumulation of FDG. Gelfand and colleagues found that intravenous fentanyl (0.75–1.0 microgram/kg), up to 50 micrograms 10 minutes prior to FDG injection, reduced FDG uptake by BAT [9, 17]. There is conflicting evidence about the effect of diazepam on BAT. Rakhega et al. reported that intravenous diazepam significantly reduces FDG uptake by BAT[31]. However, other authors have found that diazepam does not reduce FDG uptake by BAT [9, 17, 32]. Use of a non-selective beta adrenergic antagonist, such as propranolol, is another strategy to reduce FDG uptake by BAT [1, 3, 6, 9, 18, 20, 33]. Although the dose predicted to be required to reduce heat production by BAT via beta3-adrenergic receptors blockage is 100-fold the tolerated dose, it appears that 20 to 80 mg of propranolol is adequate [1]. Furthermore, propranolol does not affect tumor uptake of FDG [1, 2]. Agrawal et al. investigated the utility of an oral 40-mg dose of propranolol one hour prior to FDG administration [16]. In 40 patients undergoing repeat studies, 36 (90%) showed complete resolution of FDG uptake by BAT. Soderlund et al. reported results of 11 patients with strong FDG uptake by BAT who received a single dose of 80 mg of propranolol 2 hours before injection of FDG [20]. There was complete or near-complete resolution in each patient and no effect on tumor uptake; similar results were reported by Parysow et al. [18].
Brown fat is present throughout life [34]. In adults, uptake of FDG can be induced by cold activation [35] and by beta 3 receptor agonists [36]. We believe that the frequent uptake of FDG in adolescents and young adults, compared to infants, children, and older adults is due to activation rather than being related to actual quantity. While uncommon, we have noted intense uptake within BAT in children as young as 18 months (Figure 1), and adults within our cohort up to age 27 (Figure 2)
Figure 2.
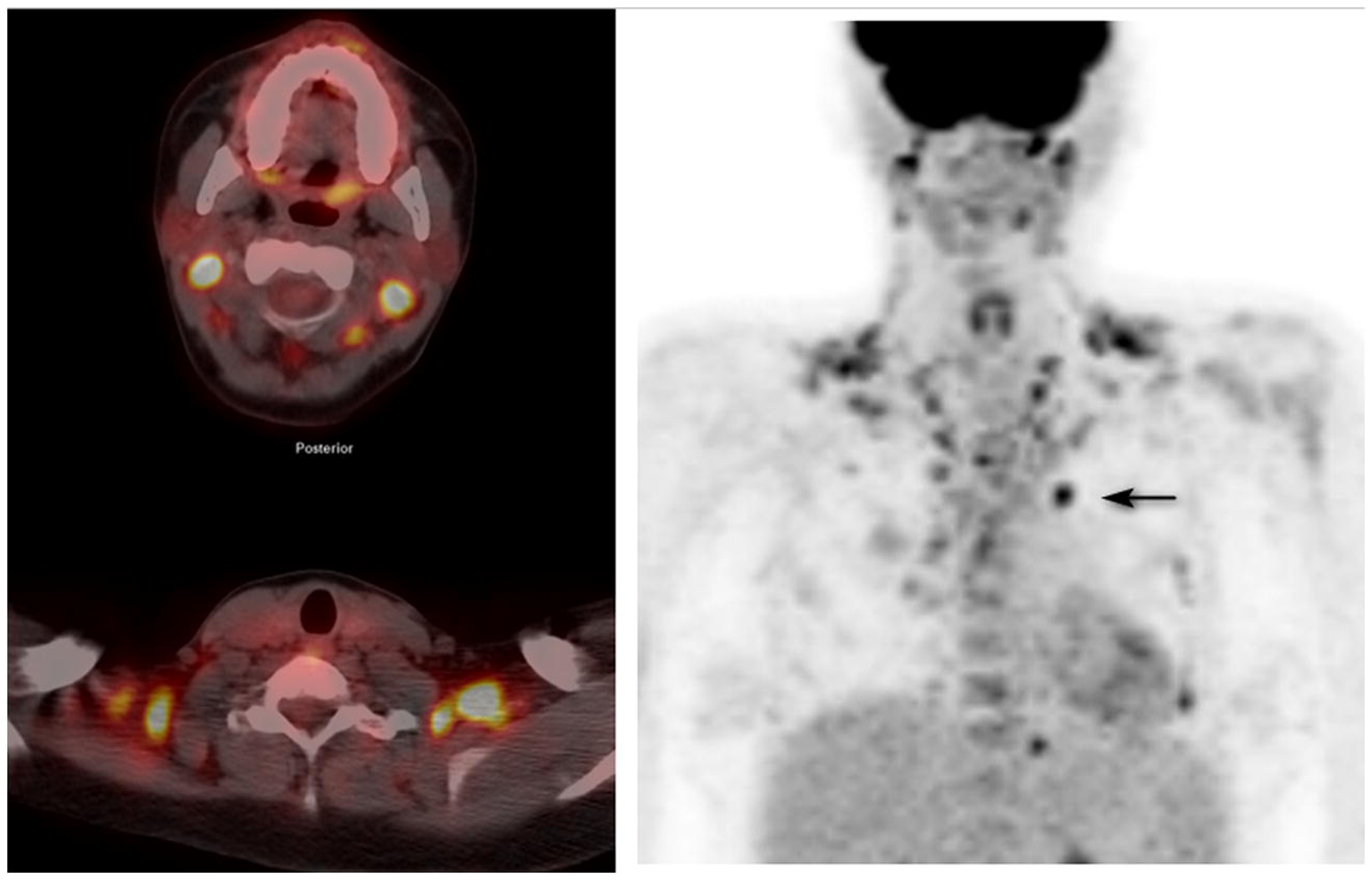
A 27-year-old woman with relapsed non Hodgkin lymphoma who has received an autologous hematopoetic stem cell transplant and allogeneic hematopoetic stem cell transplant for restaging prior to second allograft. Left panel top; fusion image through the upper neck. Left panel bottom: fusion image through the supraclaviclar regions. Right panel: anterior MIP. Extensive BAT uptake in the neck and chest renders the image quality suboptimal and distinction of BAT from pathologic activity difficult, even with close reference to the attenuation correction CT. A black arrow identifies an area of abnormal uptake in the pre-vascular region. SUVmax of BAT in the prevascular region 7.1, right upper cervical 7.6, left upper cervical 7.4, right supraclavicular 5.7, SUVmax left supraclavicular 7.8. This patient was part of Group 1 and had no specific BAT activity prevention. The study illustrates the need for BAT activity suppression.
Our study reports the results of brown fat uptake in nearly 3000 FDG PET/CT scans in pediatric, adolescent, and young adult patients studied for malignant diseases. Over 1,100 scans were performed with propranolol preparation, with successful reduction in BAT in each age group that received it. Per consensus agreement of institutional specialists, vital signs in patients receiving propranolol were not routinely monitored. Although we cannot entirely exclude the presence of uncommon side effects, such as transient orthostasis, bradycardia, hypotension, or wheezing, no adverse events were specifically identified. Following oral administration of propranolol, patients were escorted to an uptake room where they were instructed to lie recumbent for an hour prior to FDG administration, then an additional hour during the uptake period. Side effects, had they occurred, were likely militated by the long period of recumbency. Patients with potential adverse reactions to propranolol were identified as part of the ordering process for FDG PET/CT via a pop-up box querying potential risks. The safety profile for propranolol in pediatric patients has been described and is quite favorable [35]. With long term use of propranolol in patients with infantile hemangiomas, the principal adverse effects encountered were sleep disorders, diarrhea, peripheral coldness, and agitation. The dose of propranolol used is 2 fold that we have used. Thus, administering a single, low dose of propranolol to prevent BAT uptake appears to be both safe and effective under the conditions used. For unclear reasons, propranolol was not uniformly successful. Among the possibilities are that the dosage for those patients was insufficient, that the time from propranolol administration to FDG injection was inadequate, and that those patients with uptake of FDG by BAT require multiple doses of propranolol, supplementation with diazepam, and/or other additional pharmacologic and non pharmacologic interventions. The main limitation of this study was that it was a single institutional work with a nearly exclusive pediatric oncologic population and may not extrapolate to other institutions in different climates or with different patient populations. Cross-institutional prospective studies in other patient groups may be useful to verify these results.
Conclusion
In conclusion, propranolol administered one hour before FDG administration in pediatric, adolescent, and young adult patients markedly reduces the frequency of FDG uptake by BAT compared to patients without specific preparation and patients undergoing warming alone. In our population, maintaining uptake room temperatures at 24°C for one hour prior to injection did not reduce uptake of FDG by BAT. Propranolol is safe and effective for preventing FDG uptake in BAT in pediatric, adolescent, and young adult patients undergoing FDG PET/CT, resulting in higher-quality scans and facilitating interpretation.
Supplementary Material
Figure 3:
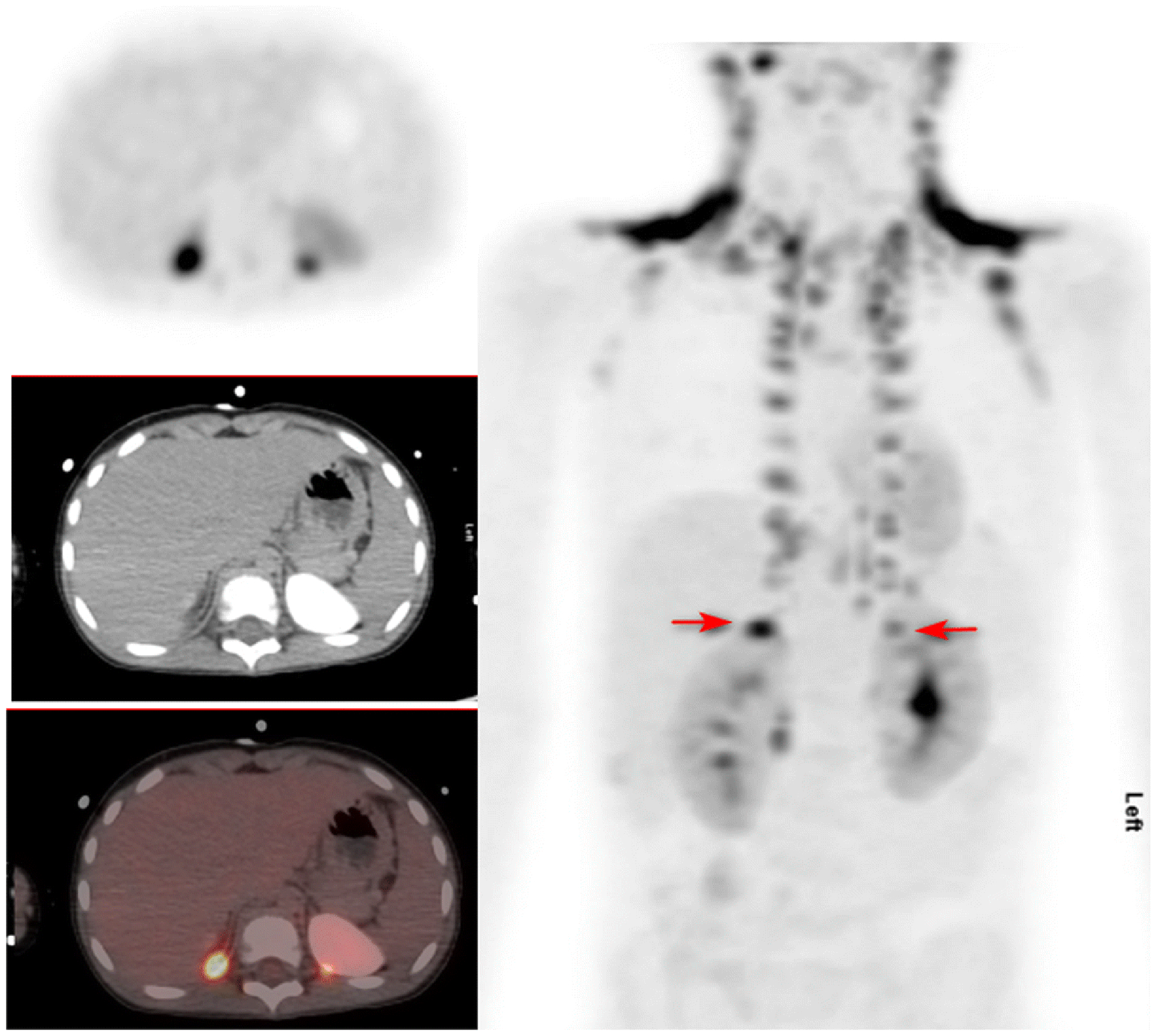
FDG PET/CT images of a 5 year old boy with Burkitt lymphoma for therapy response evaluation. Patient did not receive propranolol. Left panel top: Transverse PET image through the upper abdomen. Left panel middle: corresponding attenuation correction CT image. Left panel bottom: fusion image. Right panel: Anterior MIP of neck, chest, and abdomen. There is intense uptake in BAT in the neck, particularly supraclavicular regions bilaterally, thoracic costovertebral junctions, with less intense uptake in the axillae and mediastinum. Arrows point to subdiaphragmatic uptake bilaterally. The patient had had a CT with intravenous contrast prior to the PET/CT. SUVmax of BAT in the right subdiaphragmatic uptake was 9.3, left 5.1, right supraclavicular 17.8, left supraclavicular 15.4.
Acknowledgements:
The authors thank the nuclear medicine technologists Leigh Ann Davis (Supervisor), William Davis, Tiffany Holman, and Shannon James for their help with this study; Vella Laws-Bell for assistance in preparing the manuscript; and Cherise Guess, PhD, ELS, for scientific editing. This study was supported, in part, by the American Lebanese Syrian Associated Charities (ALSAC) and National Cancer Institute (NCI) Award Number 5R25CA023944 (CBC salary support).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest (COI):
The authors have no disclosures or conflicts of interest to report.
Ethics approval. The local Institutional Review Board for the Conduct of Studies in Human Subjects approved the study protocol and waived the requirement for individual consent in this retrospective analysis.
References
- 1.Basu S Functional imaging of brown adipose tissue with PET: can this provide new insights into the pathophysiology of obesity and thereby direct antiobesity strategies? Nucl Med Commun. 2008;29:931–3. doi: 10.1097/MNM.0b013e328310af46. [DOI] [PubMed] [Google Scholar]
- 2.Haas B, Schlinkert P, Mayer P, Eckstein N. Targeting adipose tissue. Diabetology & metabolic syndrome. 2012;4:43. doi: 10.1186/1758-5996-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virtanen KA. The rediscovery of BAT in adult humans using imaging. Best practice & research Clinical endocrinology & metabolism. 2016;30:471–7. doi: 10.1016/j.beem.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Gilsanz V, Hu HH, Kajimura S. Relevance of brown adipose tissue in infancy and adolescence. Pediatr Res. 2013;73:3–9. doi: 10.1038/pr.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enerback S The origins of brown adipose tissue. The New England journal of medicine. 2009;360:2021–3. doi: 10.1056/NEJMcibr0809610. [DOI] [PubMed] [Google Scholar]
- 6.Bauwens M, Wierts R, van Royen B, Bucerius J, Backes W, Mottaghy F, et al. Molecular imaging of brown adipose tissue in health and disease. European journal of nuclear medicine and molecular imaging. 2014;41:776–91. doi: 10.1007/s00259-013-2611-8. [DOI] [PubMed] [Google Scholar]
- 7.Lecoultre V, Ravussin E. Brown adipose tissue and aging. Curr Opin Clin Nutr Metab Care. 2011;14:1–6. doi: 10.1097/MCO.0b013e328341221e. [DOI] [PubMed] [Google Scholar]
- 8.Grant FD. Normal variations and benign findings in pediatric 18F-FDG-PET/CT. PET Clin. 2014;9:195–208. doi: 10.1016/j.cpet.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Hong TS, Shammas A, Charron M, Zukotynski KA, Drubach LA, Lim R. Brown adipose tissue 18F-FDG uptake in pediatric PET/CT imaging. Pediatric radiology. 2011;41:759–68. doi: 10.1007/s00247-010-1925-y. [DOI] [PubMed] [Google Scholar]
- 10.Yeung HW, Grewal RK, Gonen M, Schoder H, Larson SM. Patterns of (18)F-FDG uptake in adipose tissue and muscle: a potential source of false-positives for PET. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2003;44:1789–96. [PubMed] [Google Scholar]
- 11.Iyer RB, Guo CC, Perrier N. Adrenal pheochromocytoma with surrounding brown fat stimulation. AJR American journal of roentgenology. 2009;192:300–1. doi: 10.2214/AJR.08.1166. [DOI] [PubMed] [Google Scholar]
- 12.Shammas A, Lim R, Charron M. Pediatric FDG PET/CT: physiologic uptake, normal variants, and benign conditions. Radiographics. 2009;29:1467–86. doi: 10.1148/rg.295085247. [DOI] [PubMed] [Google Scholar]
- 13.Truong MT, Erasmus JJ, Munden RF, Marom EM, Sabloff BS, Gladish GW, et al. Focal FDG uptake in mediastinal brown fat mimicking malignancy: a potential pitfall resolved on PET/CT. AJR American journal of roentgenology. 2004;183:1127–32. doi: 10.2214/ajr.183.4.1831127. [DOI] [PubMed] [Google Scholar]
- 14.Williams G, Kolodny GM. Method for decreasing uptake of 18F-FDG by hypermetabolic brown adipose tissue on PET. AJR American journal of roentgenology. 2008;190:1406–9. doi: 10.2214/AJR.07.3205. [DOI] [PubMed] [Google Scholar]
- 15.Zukotynski KA, Fahey FH, Laffin S, Davis R, Treves ST, Grant FD, et al. Constant ambient temperature of 24 degrees C significantly reduces FDG uptake by brown adipose tissue in children scanned during the winter. European journal of nuclear medicine and molecular imaging. 2009;36:602–6. doi: 10.1007/s00259-008-0983-y. [DOI] [PubMed] [Google Scholar]
- 16.Agrawal A, Nair N, Baghel NS. A novel approach for reduction of brown fat uptake on FDG PET. The British journal of radiology. 2009;82:626–31. doi: 10.1259/bjr/24661539. [DOI] [PubMed] [Google Scholar]
- 17.Gelfand MJ, O’Hara SM, Curtwright LA, Maclean JR. Pre-medication to block [(18)F]FDG uptake in the brown adipose tissue of pediatric and adolescent patients. Pediatric radiology. 2005;35:984–90. doi: 10.1007/s00247-005-1505-8. [DOI] [PubMed] [Google Scholar]
- 18.Parysow O, Mollerach AM, Jager V, Racioppi S, San Roman J, Gerbaudo VH. Low-dose oral propranolol could reduce brown adipose tissue F-18 FDG uptake in patients undergoing PET scans. Clinical nuclear medicine. 2007;32:351–7. doi: 10.1097/01.rlu.0000259570.69163.04. [DOI] [PubMed] [Google Scholar]
- 19.Rakhega R, Ciarallo A, Alabed YZ, Hickeson M. Intravenous administration of diazepam significantly reduces brown fat activity on 18F-FDG PET/CT. Am J Nucl Med Mol Imaging. 2011;1:29–35. [PMC free article] [PubMed] [Google Scholar]
- 20.Soderlund V, Larsson SA, Jacobsson H. Reduction of FDG uptake in brown adipose tissue in clinical patients by a single dose of propranolol. European journal of nuclear medicine and molecular imaging. 2007;34:1018–22. doi: 10.1007/s00259-006-0318-9. [DOI] [PubMed] [Google Scholar]
- 21.Gelfand MJ, Sharp SE. Patient Preparation and Performance of PET/CT Scans in Pediatric Patients. PET Clin. 2008;3:473–85. doi: 10.1016/j.cpet.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Gilsanz V, Chung SA, Jackson H, Dorey FJ, Hu HH. Functional brown adipose tissue is related to muscle volume in children and adolescents. J Pediatr. 2011;158:722–6. doi: 10.1016/j.jpeds.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilsanz V, Hu HH, Smith ML, Goodarzian F, Carcich SL, Warburton NM, et al. The depiction of brown adipose tissue is related to disease status in pediatric patients with lymphoma. AJR American journal of roentgenology. 2012;198:909–13. doi: 10.2214/AJR.11.7488. [DOI] [PubMed] [Google Scholar]
- 24.Hu HH, Gilsanz V. Developments in the imaging of brown adipose tissue and its associations with muscle, puberty, and health in children. Front Endocrinol (Lausanne). 2011;2:33. doi: 10.3389/fendo.2011.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilsanz V, Smith ML, Goodarzian F, Kim M, Wren TA, Hu HH. Changes in brown adipose tissue in boys and girls during childhood and puberty. J Pediatr. 2012;160:604–9 e1. doi: 10.1016/j.jpeds.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chalfant JS, Smith ML, Hu HH, Dorey FJ, Goodarzian F, Fu CH, et al. Inverse association between brown adipose tissue activation and white adipose tissue accumulation in successfully treated pediatric malignancy. Am J Clin Nutr. 2012;95:1144–9. doi: 10.3945/ajcn.111.030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2003;44:170–6. [PubMed] [Google Scholar]
- 28.Cohade C, Mourtzikos KA, Wahl RL. “USA-Fat”: prevalence is related to ambient outdoor temperature-evaluation with 18F-FDG PET/CT. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2003;44:1267–70. [PubMed] [Google Scholar]
- 29.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–56. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Shao D, Tian XW, Gao Q, Liang CH, Wang SX. Preparation methods prior to PET/CT scanning that decrease uptake of 18F-FDG by myocardium, brown adipose tissue, and skeletal muscle. Acta radiologica (Stockholm, Sweden: : 1987). 2016. doi: 10.1177/0284185116633917. [DOI] [PubMed] [Google Scholar]
- 31.Rakheja R, Ciarallo A, Alabed YZ, Hickeson M. Intravenous administration of diazepam significantly reduces brown fat activity on 18F-FDG PET/CT. Am J Nucl Med Mol Imaging. 2011;1:29–35. [PMC free article] [PubMed] [Google Scholar]
- 32.Sturkenboom MG, Hoekstra OS, Postema EJ, Zijlstra JM, Berkhof J, Franssen EJ. A randomised controlled trial assessing the effect of oral diazepam on 18F-FDG uptake in the neck and upper chest region. Mol Imaging Biol. 2009;11:364–8. doi: 10.1007/s11307-009-0207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindholm H, Brolin F, Jonsson C, Jacobsson H. Effects on the FDG distribution by a high uptake of brown adipose tissue at PET examination. EJNMMI Res. 2014;4:72. doi: 10.1186/s13550-014-0072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heaton J The distribution of brown adipose tissue in the human. J Anat. 1972;112:35–9. [PMC free article] [PubMed] [Google Scholar]
- 35.Leaute-Labreze C, Boccara O, Degrugillier-Chopinet C, Mazereeuw-Hautier J, Prey S, Lebbe G, et al. Safety of Oral Propranolol for the Treatment of Infantile Hemangioma: A Systematic Review. Pediatrics. 2016;138. doi: 10.1542/peds.2016-0353. [DOI] [PubMed] [Google Scholar]
- 36.Cypress AM, Weiner LS, Roberts-Toler Carla, Elia EF, Kessler SH, Kahn PA, et al. Activation of Human Brown Adipose Tissue by a β3-Adrenergic Receptor Agonist. Cell Metabolism. 2015. 21:33–8. doi: 10.1016/j.cmet.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


