Abstract
Although much has been learned about circadian clocks and rhythms over the past few decades, translation of this foundational science underlying the temporal regulation of physiology and behavior to clinical applications has been slow. Indeed, acceptance of the modern study of circadian rhythms has been blunted because the phenomenology of cyclic changes had to counteract the 20th century dogma of homeostasis in the biological sciences and medicine. We are providing this review of clinical data to highlight the emerging awareness of circadian variation in efficacy of medications for physicians, clinicians, and pharmacists. We are suggesting that gold-standard double-blind clinical studies should be conducted to determine the best time of day for optimal effectiveness of medications; also, we suggest that time of day should be tracked and reported as an important biological variable in ongoing clinical studies hereafter. Furthermore, we emphasize that time of day is, and should be considered, a key biological variable in research design similar to sex. In common with biomedical research data that have been historically strongly skewed towards males, most pharmaceutical data have been skewed towards morning dosing without strong evidence that this is the optimal time of efficacy.
Keywords: chronopharmacology, chronotherapy, circadian rhythms, diel cycles, biological rhythms, chronomodulation, time of day
Introduction
Virtually all animal processes display daily rhythms, termed circadian rhythms, that persist in the absence of external factors. Accordingly, virtually all physiological processes including respiration, metabolism, endocrinological, immunological, cardiovascular, and neuronal, display circadian rhythms in their function. In the presence of environmental light-dark cycles, these self-sustaining endogenous rhythms display 24-hour periods. In the absence of environmental light-dark cycles, these rhythms ‘free-run’ at periods of about 24 hours. Circadian rhythms display both ‘bottom-up’ and ‘top-down’ organization (1).
Molecular networks of gene transcription and translation form the basis of circadian rhythms in cells. The molecular clock in the suprachiasmatic nuclei (SCN) of the hypothalamus comprises a set of transcriptional–translational feedback loops that drive rhythmic 24-hour expression of the core clock components (2). In the primary feedback loop, Circadian Locomotor Output Cycles Kaput (CLOCK) and Brain and Muscle ARNT-Like Protein (BMAL1) proteins form heterodimers that activate gene expression of Period (Per) and Cryptochrome (Cry). PER and CRY proteins then heterodimerize and translocate into the nucleus, where they suppress their own transcription by acting on the CLOCK/BMAL1 complexes. In an interacting feedback loop, CLOCK/BMAL1 complexes promote expression of retinoic acid-related orphan nuclear receptors, Rev-erbα and RORα. Their protein products feedback to regulate Bmal1 by competitively binding retinoic acid-related orphan receptor response elements in the Bmal1 promoter. Reverse viral erythroblastosis oncogene products repress the transcription of Bmal1, whereas RORs activate it. These two loops form the primary basis of the molecular clock, but a complex network of interacting genes and post-translational modifications ensure that the process takes ~24 h to complete (2, 3). Again, this transcriptional–translational feedback loop is the basis of intrinsic daily circadian rhythms.
The top-down organization of circadian rhythms is coordinated in mammals by the SCN (4, 5). Environmental light is detected by specialized photoreceptors, termed intrinsically photosensitive retinal ganglion cells (ipRGCs). Unlike rods and cones, ipRGCs perform non-image-forming functions, sending direct projections to the SCN to synchronize circadian cellular rhythms of clock gene transcription and translation, which in turn transduces this circadian information to a network of peripheral clocks to coordinate bodily functions (Figure 1) (1, 6, 7).
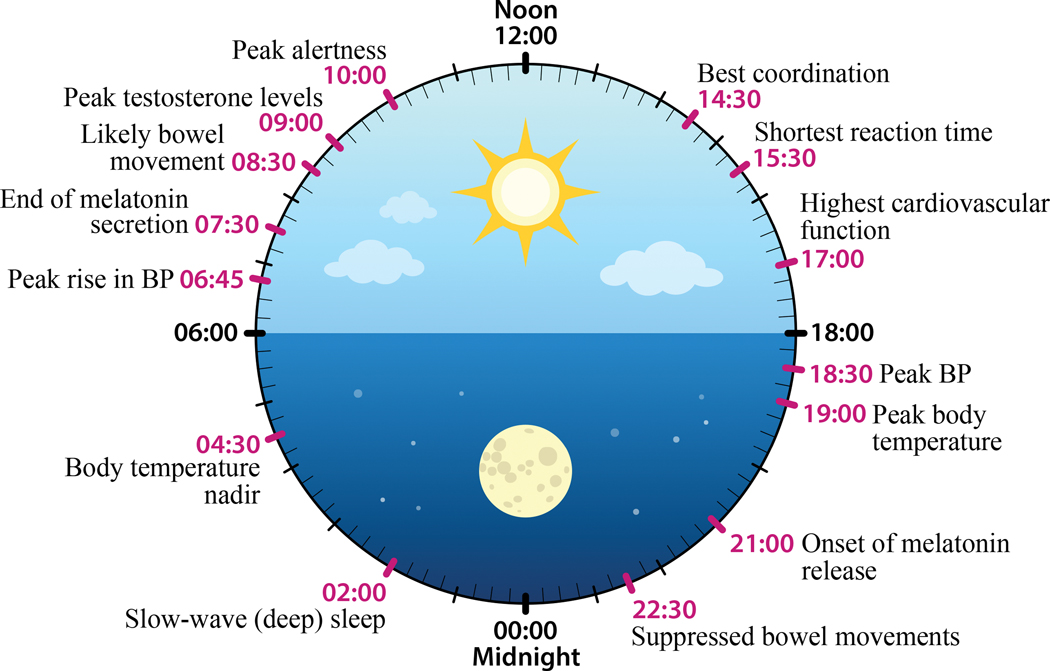
Figure 1. Circadian rhythms in physiology.
In humans, environmental light information is detected by specialized retinal ganglion cells in the eyes that project to the master circadian clock, the suprachiasmatic nuclei in the hypothalamus. In a top-down fashion, the master clock then transduces photic information to drive neural and hormonal signals, such as melatonin and cortisol, which in turn synchronize circadian cellular rhythms in the network of peripheral clocks which control and coordinate physiology and body function. Peak and nadir circadian timing of some of these and other physiological and bodily functions are depicted here (redrawn from [7]).
Although much has been learned about circadian clocks and rhythms over the past few decades, translation of this foundational science underlying the temporal regulation of physiology and behavior to clinical applications has been slow. Indeed, the modern study of circadian rhythms as a scientific discipline was initially blunted because the phenomenology of cyclic changes had to counteract the 20th century dogma of homeostasis in the biological sciences and medicine (8–10). Large fluctuations in physiological processes were considered pathological, and many physicians resisted the idea of the programmed changes in physiology and behavior that we now understand to underlie homeostatic processes. Additional barriers to incorporating circadian rhythmicity into the study and treatment of diseases are based on practical considerations, such as patient compliance with dosing times, and additional cost incurred by including multiple dosing times to clinical trials. Despite recent recognition of the field of circadian biology with the awarding of a Nobel Prize to prominent circadian biologists in 2017, translation to clinical practice remains virtually nonexistent.
Although there have been scattered reports of beneficial effects of differential timing of chemotherapy, anesthesia, and effectiveness of specific disease-associated medications, to our knowledge, there have been few recent systematic reviews of circadian variation in efficacy of pharmaceuticals (e.g., (11)). The goal of this review is twofold: 1) to provide a comprehensive review of medications emphasizing the time of day when benefits are highest and contraindications are at a nadir. Our approach was to use biomedical database search engines (PubMed, Web of Science, EBSCO, etc.) along with key words indicative of assessment of circadian or diel efficacy (circadian, time of day, morning, evening, chrono*) in reported trials and studies for specific conditions or drugs; most of the cited studies are small human clinical studies. There are some publications on dosing time dependent clinical trials on other diseases or types of drugs which may not have been included in this review for brevity. We are are not recommending changing dosing regimens based on the cited studies, but we are suggesting that gold-standard double-blind clinical studies should be conducted to determine the best time of day for optimal effectiveness of various medications. 2) The second goal of this review is to emphasize that time of day should be considered a key biological variable similar to sex. In common with biomedical research data that have been historically strongly skewed towards males (12), most pharmaceutical data have been skewed towards morning dosing without strong evidence that this is the optimal time of efficacy. As Colin Pittendrigh noted, “A rose is not necessarily and unqualifiedly a rose...it is a very different biochemical system at noon and at midnight” (13). The same can be said of humans. Accounting for time-of-day as a biological variable begins with the design of research studies, and should include data collection and analysis of results, as well as reporting of findings. Furthermore, to provide a better mechanistic understanding of chronopharmacology in future clinical trials, it is important to consider time of day in both the pharmacokinetics (14) and pharmacodynamics (15) of the drug and the disease being treated; both factors can interact, or act independently to influence both drug effectiveness and outcomes. Consideration of time-of-day of dosing may be critical to the interpretation, validation, and replication of research results.
Hypertension.
The current clinical practice guidelines for management of hypertension published by the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines (16) neither address nor provide guidance on timing of medications across the day. However, clinical data have been reported suggesting that dosing at specific time-of-day provides benefits in efficacy for various medications used to treat various factors contributing to hypertension; studies reporting these data are reviewed below and listed in Table 1.
Table 1.
Clinical trials and studies considering time of day in treatment for hypertension.
| DRUG CLASS | DRUG(S) | STUDY DESIGN; POPULATION | STUDY CONCLUSIONS | SUGGESTED TIME OF DAY | CITATION |
|---|---|---|---|---|---|
| Anti-hypertensives | |||||
| Calcium Channel Blockers | Amlodipine | Single center, randomized crossover study; Hypertensive / normotensive subjects. | Highest effect on reducing BP & HR when taken in the morning (0800 h) compared to evening (2000 h). | Morning | Khodadoustan, Nasri Ashrafi et al. 2017 |
| Nifedipine GITS | Multicenter, double-blind, randomized clinical trial; 180 Hypertensive patients (86 males, 94 females) | Bedtime dose was more effective and was associated with decreased negative side effects. | Bedtime | Hermida, Ayala et al. 2008 | |
| Verapamil - COER | 8-wk prospective, multicenter, randomized, double-blind clinical trial; 193 dipper and 64 non-dipper hypertensives | A dose at 2200 h reduced 24h BP on dippers and non-dippers, but had a greater reduction in nocturnal BP on non-dippers. | Night (2200 h) | White, Mehrotra et al. 1997 | |
| Open-label, multiple-dose, four-period, crossover study; 29 healthy men | There were no differences between an 0800 h and a 2200 h dose on BP, however time of administration did affect the rate of absorption (morning dose was slower). | Morning | Gupta, Yih et al. 1995 | ||
| CODAS - verapamil | 8-week, double-blind, placebo-controlled trial; 257 patients:193 dippers & 64 nondippers | Taken between 2100–2300 h produced best effects in the morning (between 0600 – 12000 h). | Night (2100 – 2300 h) | Smith, Neutel et al. 2001; Prisant 2003 | |
| Open-label, multicenter dose-titration study; Elderly (≥65 y/o) 628 patients. | 200 mg/d at bedtime, dosing was titrated to a maximum of 400 mg/d | Night | Weber, Prisant et al. 2004 | ||
| Nitrendipine | 6 hospitalized and drug-free patients with essential hypertension. | More effective at awakening (0600 h) or supper compared to breakfast (0830 h) and supper administration brought a deeper asleep BP decline. | Awakening or Supper | Umeda, Naomi et al. 1994, | |
| Isradipine Sustained Release | Double-blind, randomized, cross-over design; Non-dipping chronic renal failure patients | Both 0800 h and 2000 h effectively reduced 24-h BP, but 2000 h administration showed a more pronounced effect during the night. | Night (2000h) | Portaluppi, Vergnani et al. 1995 | |
| Diltiazem Retard | Open, non-randomized study; 13 dipper, essential hypertension patients | Morning (0800 h, n = 7) dose had the most marked antihypertensive effects during nighttime BP, while evening dose (1900 h, n = 6) dose exerted greatest effects during daytime activity with inhibition of the morning BP rise. | Evening | Kohno, Iwasaki et al. 1997 | |
| Open, non-randomized study; 5 dipper, essential hypertension patients | Dosing 3x a day had the best effect during daytime activity. | 3x daily | |||
| Diltiazem | Open, non-randomized study; 8 non-dipper, essential hypertension patients | Most pronounced antihypertensive effects during nightly rest. Evening dose seems to be more efficacious than the other dosage schedules. | Evening | ||
| Diltiazem Extended Release (ER) | Open-label, randomized, two-way crossover study; 48 healthy volunteers | Administration in the evening (2200 h) exhibited 17% and 22% greater bioavailability compared to morning (0700 or 0800 h) administration under single-dose and steady-state conditions, respectively. The two times of drug administration were bioinequivalent in both studies. Evening schedule provided more than twofold higher plasma diltiazem levels in the critical morning hours. | Evening | Sista, Lai et al. 2003 | |
| NSAIDS | Aspirin | Prospective trial; 328 untreated patients with grade 1 hypertension | 100mg at bedtime reduced ambulatory BP, while a dose upon awakening increased the 24h mean BP | Evening | Hermida et al., 2005 |
| Pregnant women | Bedtime (but not morning) aspirin dosing was best for preventing pregnancy-induced hypertension and preeclampsia. | Bedtime | Smolensky & Haus, 2001 | ||
| Randomized control trial | Low dose aspirin administered in the evening is more effective at reducing morning platelet activity than morning dosing. | Evening | Bonten, Snoep et al. 2015, van Diemen, Fuijkschot et al. 2016, Racca, van Diemen et al. 2019 | ||
| ACE inhibitors | Benazepril | Most effective at controlling nocturnal and early morning BP and consequently, normalizing the circadian BP profile, when dosed at night | Evening | Palatini, Mos et al. 1993 | |
| Enalapril | Randomized, crossover study; 8 hypertensive patients | Witte, Weisser et al. 1993 | |||
| Perindopril | 20 hypertensive patients | Early morning BP rise is reduced more with night (2100 h) administration. However, it does not reduce BP over 24 h as is achieved with the morning (0900 h) dose. | Night (2100 h) | Morgan, Anderson et al. 1997 | |
| Quinapril | Double-blind clinical trial; 18 hypertensive patients | 24-hour BP profiles showed a more sustained antihypertensive action with the evening (2200 h) administration compared with the morning (0800 h) administration. | Evening | Palatini 1992, Palatini, Racioppa et al. 1992 | |
| Ramipril | Open, randomized, crossover trial; 33 patients with mild-to-moderate essential hypertension | A significant decrease from baseline BP was observed with a once-daily dose either at morning (0800 – 1100 h) or evening (2000 – 2300 h), and provided equal or better, BP control when taken in the morning. | Morning (0800 – 1100 h) | Myburgh, Verho et al. 1995 | |
| Prospective, randomized, open-label, parallel-group, blinded end point multicenter clinical trial; 115 untreated hypertensive patients | Diurnal BP reduction was similar with awakening or bedtime treatment. However, Bedtime administration was significantly more efficient at reducing asleep BP. | Bedtime | Hermida and Ayala 2009 | ||
| Spirapril | 165 previously untreated subjects | The BP reduction during diurnal activity was similar for both treatment times. However, bedtime administration was more efficient than morning administration in reducing asleep BP. The awake/asleep BP ratio was decreased with the upon-awakening schedule but significantly increased toward a more dipping pattern with the bedtime treatment schedule. | Bedtime | Hermida, Ayala et al. 2010 | |
| Lisinopril | 40 subjects with primary mild to moderate hypertension | Greater reduction of systolic BP and diastolic BP from 0600 h to 1100 h after 2100 PM dosing. | Night (2100 h) | Macchiarulo, Pieri et al. 1999 | |
| Zofenopril | 33 untreated patients with grade 1 or 2 uncomplicated essential hypertension | Nocturnal BP regulation is better achieved at bedtime administration as compared to at awakening, without any loss in efficacy during diurnal active hours. | Bedtime | Balan, Popescu et al. 2011 | |
| Trandolapril | 37 hypertensive patients | The 24-h systolic BP was reduced with both morning and bedtime regimes, but bedtime dosing had a greater decrease in pre-waking and morning systolic BP. | Bedtime | Kuroda, Kario et al. 2004 | |
| Captopril + Hydrochlorothiazide | 20 hypertensive patients | Morning dose resulted in reduced daytime BP, and an evening dose reduced evening BP. | Morning intake controls morning BP; Evening intake controls evening BP | Middeke et al., 1991 | |
| α-Adrenoceptor Antagonists | Doxazosin | Clinical trial; 111 patients with mild hypertension | Slightly reduced the 24-h systolic BP and diastolic BP ratio, having no significant effect in asleep BP; evening, dose of these drugs had a significant 24-h SBP and DBP-lowering effect throughout the entire day, with the greatest effects on early morning BP. | Evening | Pickering, Levenstein et al. 1994 |
| Doxazosin GITS | 91 subjects: 49 men and 42 women with grade 1–2 essential hypertension | 24 h mean BP reduction was larger and statistically significant (6.9 and 5.9 mm for systolic and diastolic BP, respectively, in monotherapy; 5.3 and 4.5 mm Hg in polytherapy) when doxazosin GITS was scheduled at bedtime. This BP-lowering effect was similar during both the day and nighttime hours. Doxazosin GITS ingested daily on awakening failed to provide full 24h therapeutic coverage | Evening | Hermida, Calvo et al. 2004 | |
| β-adrenoceptor antagonists | Propranolol | 4 subjects | No circadian variation in the maximum decrease in HR, but the time to peak effect dependended on time intake. Circadian variation in sympathetic tone and vascular reactivity is mainly responsible for the circadian changes in the effects of propranolol. | No difference | Langner and Lemmer 1988 |
| Nebivolol | Hypertensive patients with a non-dipper BP profile | Either morning or evening administration, had significant BP-lowering effects throughout the day, with more marked effects on the awake BP mean. However, morning administration had a greater attenuation of the nocturnal BP decline, which effectively increased the number of non-dipper patients. | Morning | Hermida, Calvo et al. 2006 | |
| 19–76 y/o with mild to moderate hypertension | Morning and evening were equally effective but evening administration had greater anti-hypertensive effects on prewaking BP. | Equaly effective; evening had added benefits | Acelejado et al., 2012 | ||
| Angiotensin II Receptor Blockers | Valsartan | Patients with essential hypertension | 1600mg at night was more effective at reducing nocturnal BP than when taken during the morning. | Night | Hermida, Calvo et al. 2003, Hermida, Calvo et al. 2005, Hermida, Calvo et al. 2005 |
| Non-sleepy (at nighttime) hypertensives w/obstructive sleep apnea | Evening dose better controls BP; there are differential BP patterns between am and pm | Evening | Kasiakogias, Tsioufis et al. 2015; Reviewed in Bowles, Thosar et al. 2018 | ||
| Olmesartan | 40 Patients: 23 Females 17 Males | Night-time to create dipper phenotype | Evening | Hermida, Ayala et al. 2009, Tofe Povedano and Garcia De La Villa 2009 | |
| Candesartan | Evening doses were more effective at reducing the risk of microalbuminuria compared to morning dosing. | Evening | Kario, Hoshide et al. 2010 | ||
| Diuretics | Torsemide | Randomized control study; 113 grade 1 and 2 hypertensive patients, 51.7+/−10.6 yrs of age | A 5 mg dose at bedtime, but not upon awakening, was effective at reducing the 24h-SBP:DBP, and providing complete 24-h BP coverage. | Bedtime | (Hermida et al., 2008) |
| Combination Therapies | Hydrochlothiazide + Valsartan | Greatest ambulatory BP control when taken in the evening, compared to the morning. | Evening | Hermida, Ayala et al. 2011 | |
| Hydrochlothiazide + Amlodipine | Greater control of BP across the day when taken in the evening compared to the morning. | Evening | Hermida, Ayala et al. 2010, Zeng, Jia et al. 2011 | ||
| Hypertensives with chronic kidney disease | Evening anti-hypertensive dosing was more effective than morning dosing for controlling nocturnal BP and lowering the percentage of non-dipper BP patterns. | Evening | Crespo, Pineiro et al. 2013, Wang, Ye et al. 2017 | ||
| Hypertensive African Americans | No beneficial effects of evening dosing over morning dosing were reported. | No difference | Rahman, Greene et al. 2013 | ||
Calcium Channel Blockers.
Essentially, calcium channel blockers vary by type for when they are most effective (Table 1). These include amlodipine, nifedipine gastrointestinal therapeutic system (GITS), verapamil, diltiazem, and isradipine (17). For instance, nifedipine GITS administered at bedtime was more effective than morning administration for treating hypertension; nighttime administration was associated with decreased negative side effects (18). Similarly, evening administration of amlodipine has the highest effects on reducing blood pressure (BP) and heart rate (HR) when compared to morning administration (19). In one study verapamil (controlled-onset, extended-release version [COER]) administered at 2200 h reduced BP values throughout the day in both dippers and non-dippers, with a more pronounced effect on the nocturnal BP decline of non-dippers (20). Another study comparing a 0800 h dose to a 2200 h dose of verapamil-COER found no differences in effects on BP, however, the rate of absorption was slowed with a morning administration (21). Similarly, chronotherapeutic oral drug absorption system (CODAS)-verapamil taken during the evening (2100–2300 h) produced the best anti-hypertensive benefits during the morning (0600–1200 h) (22, 23). Blood pressure in mild to moderate hypertensive patients displayed best 24-hour control of BP when taking CODAS-verapamil at bedtime (22, 23). CODAS-verapamil also best regulated BP throughout the day in patients >65 years of age when taken at night (24). The dihydropyridine derivative nitrendipine was more effective at reducing morning BP surge when taken upon awakening (0600 h) or after dinner (1800 h) than at breakfast (0830 h); 1800 h dosing resulted in the greatest decline in nocturnal BP (25). Compared to morning (0800 h) dosing, non-dipping chronic renal failure patients had better nocturnal BP control when dosed at night (2000 h) with the dihydropyridine derivative isradipine; night dosing also displayed a resetting effect of the misaligned 24-h BP and HR profiles often observed in these patients as a result of their renal failure condition (26). Although graded-release long-acting diltiazem has been approved for once-daily use either at morning or nighttime, evening administration exhibits a pharmacokinetic profile better aligned with the ideal therapeutic time of day (between 1000 – 1200 h) for patients with essential hypertension (27, 28).
NSAIDS (aspirin).
Circadian variation in effectiveness of anti-hypertensive medications has been reported with aspirin. For example, a 100 mg dose of aspirin at bedtime reduced ambulatory BP in mild hypertensive patients, whereas a morning dose increased the 24-h BP mean (29). Furthermore, for patients who were non-dippers at baseline, bedtime administration of aspirin doubled the reduction in nocturnal BP. Bedtime (but not morning) aspirin dosing was best for preventing pregnancy-induced hypertension and preeclampsia (30). Other studies have reported similar results of reduced BP after evening administration of aspirin (reviewed in (31)). Additionally, low dose aspirin administered in the evening is more effective at reducing morning platelet activity than morning dosing (32–34).
Angiotensin-Converting Enzyme (ACE) Inhibitors.
Considered together, the ACE-inhibitors work when administered in the morning or evening, however, they best adjust patients’ BP towards the physiological circadian BP rhythm when taken at night. Their effect on decreasing nocturnal BP drives patients towards a more dipper status, effectively reducing the asleep to awake BP ratio (35). The ACE-inhibitors benazepril (36) enalapril (37), perindopril (38), quinapril (39, 40), ramipril (41, 42), spirapril (43, 44), lisinopril (45), zofenopril (46), and trandolapril (47) were most effective at controlling nocturnal and early morning BP and consequently, normalizing the circadian BP profile, when dosed at night. Intake of either ramipril (41), spirapril (43, 44), or perindopril (38) exerted maximum diurnal BP control when administered during the morning, while optimal nighttime BP control, with these drugs, was best achieved when dosed during the evening. Combined dosing of captopril and hydrochlorothiazide (a diuretic) resulted in reduced daytime BP when administered in the morning and reduced evening BP when taken during the evening (48). Other ACE-inhibitors have been assessed, but possibly due to a small sample size, have not demonstrated a time-of-day-associated difference in effectiveness (reviewed in (35)). Details regarding the studies referenced can be found in Table 1.
α-Adrenoceptor Antagonists.
The effects of α-Adrenoceptor antagonists appear to be circadian-stage dependent, as they have the highest BP-reducing effects in the morning, the time-of-day when BP-spikes typically occur (49, 50). A morning dose of either doxazosin or doxazosin GITS (controlled rate release) only slightly reduced the 24-h systolic BP (SBP) and diastolic BP (DBP) ratio, having no significant effect in asleep BP. Moreover, an evening dose of these drugs had a significant 24-h SBP and DBP-lowering effect throughout the entire day, with the greatest effects on early morning BP (51). Taken together these findings suggest that independent of the drug concentration (pharmacokinetics), the physiological response to it (pharmacodynamics) follows a circadian pattern, which should be taken into consideration when determining optimal timing of dosing.
β-adrenoceptor Antagonists.
Beta blockers generally have greater impact on diurnal BP, yet display little or no effect on circadian BP patterns, or they lean towards a pattern of a non-dipper BP profile (17). This is the case for propranolol (52). Nebivolol, administered during either the morning or evening, had significant BP-lowering effects throughout the day, with more marked effects on the awake BP mean (53). However, morning administration had a greater attenuation of the nocturnal BP decline, which effectively increased the number of non-dipper patients. Hence, according to these data, evening dose might be more beneficial in controlling 24-h BP, as well as the tendency towards the nocturnal BP dip. A more recent study similarly reported that both morning and evening administration of nebivolol control overall 24-h BP, however evening, but not morning, administration significantly reduced prewaking SBP (54). Taken together, the tendency towards a more dipping pattern at night and the increased reduction in prewaking BP suggest that evening administration might have an additional benefit over morning dosing.
Angiotensin II Receptor Blockers.
Angiotensin receptor blockers have been reported to have significant, 24h-reduction in BP when taken either in the morning or night (55), but have a significant increase in the day-night BP ratio, reducing the number of non-dipper patients by 73%, when administered at night. For example, a dose of 160 mg of valsartan taken at night was more effective at reducing nocturnal BP than when taken during the morning (55–57). Furthermore, this increase towards a dipper profile is also seen in non-sleepy (at nighttime) hypertensive patients with obstructive sleep apnea (58) not only supporting the data indicating that hypertensive medications more effectively control BP when administered during the evening, but also suggesting differential BP patterns between day and night. Similarly, olmesartan had an overall greater reduction in nocturnal BP and improved the awake-asleep BP ratio without affecting the overall daily effectiveness (59, 60). Generally, time of administration can be determined according to the dipping status of patients to maximize antihypertensive effects of the drugs, but there may also be other time of day specific benefits to consider. For example, independent of time of day differences in dosing on controlling BP, evening doses of candesartan were more effective at reducing the risk of microalbuminuria compared to morning dosing (61).
Diuretics.
The BP-lowering effects of diuretics in relation to treatment time have not been well studied. The first trial of this kind studied the loop diuretic torsemide. A 5 mg dose of torsemide was most effective at reducing the 24h-SBP to DBP ratio, as well as providing complete 24-h BP coverage when administered at bedtime, but not upon awakening (62). When taken in combination therapy along with the angiotensin receptor blocker valsartan (see previous paragraph), the diuretic hydrochlorothiazide provided the greatest ambulatory BP control when taken in the evening compared to morning dosing (63). Additionally, hydrochlorothiazide in combination therapy with the calcium channel blocker amlodipine similarly provided greater control of BP across the day when taken in the evening compared to the morning (64, 65).
Combination Therapies.
Most of the previously discussed studies focused on monotherapy for treatment of hypertension, however, effective treatment frequently includes combination therapy with multiple classes of antihypertensives along with comorbidities such as diabetes, obstructive sleep apnea, and chronic kidney disease. Meta analyses of extant clinical studies have provided some insight. Analyses of 175 clinical trials concluded that across anti-hypertensive medications, evening dosing evoked the lowest adverse cardiovascular events and BP when compared to morning dosing (66). Meta-analyses of studies in hypertensive patients with chronic kidney disease similarly concluded that evening anti-hypertensive dosing was more effective than morning dosing for controlling nocturnal BP and lowering the percentage of non-dipper BP patterns (67, 68); however, it is important to note no beneficial effects of evening dosing over morning dosing were reported in African Americans (69). A prospective randomized study of 2,012 hypertensive patients (MAPEC study) reported that, compared to morning administration, evening administration of all hypertensive medications provided the greatest reduction in BP, with the added benefit of significantly reducing the risk of developing new-onset type 2 diabetes (T2DM) with evening dosing (70). For treatment of hypertension in T2DM patients, evening administration of antihypertensive agents was similarly reported to be most effective at blood pressure control compared to morning dosing (60, 71, 72). Very few studies have focused on the circadian differences in the effectiveness of antihypertensives in patients with obstructive sleep apnea and/or diabetes (reviewed in (73). However, it is apparent that additional clinical trials are needed to directly test the conclusions of these meta-analyses and to compare circadian patterns of effectiveness of anti-hypertensive medications, alone and in combination, in essential hypertension and across hypertension with comorbidities.
Endocrine Disorders
Adrenal Insufficiency (AI) and Congenital Adrenal Hyperplasia (CAH).
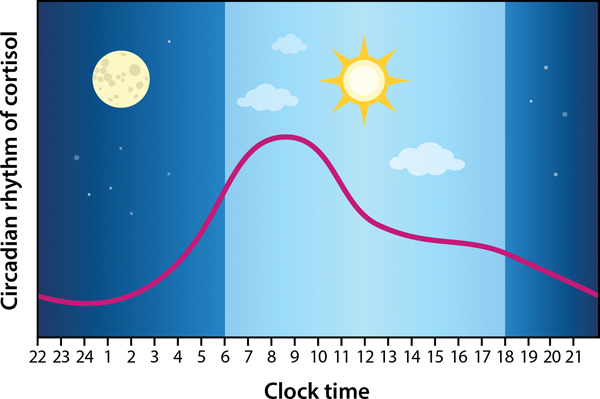
AI and CAH are conditions characterized by deficiencies in adrenal corticosteroid (and androgen) production. Optimal treatment approaches for CAH and AI must mimic the normal endogenous circadian rhythm of glucocorticoids (74, 75), which is comprised of a sharp rise beginning 2–3 hours before waking that peaks shortly after waking, and then declines gradually across the day reaching nadir in the middle of the night (Figure 2). Daily dosing regimens can mimic the daytime levels of cortisol, but they fail to recapitulate the late nocturnal rise. To recapitulate the late nocturnal rise with current formulations, one must either be on a continuous infusion paradigm or interrupt sleep to dose in the middle of the night, neither of which are practical or lead to compliance. Current clinical practice guidelines for both AI (76) and CAH (77) acknowledge the difficult circadian aspects of replacement therapy, yet current pharmacological approaches still fall far short of this goal. Thus, both guidelines call for more research and robust clinical trials into optimal dosing regimens for replacement therapy. Additionally, physiological glucocorticoid replacement must also fall within a narrow range for each individual; dosing above or below this range can lead to severe adverse effects and poor clinical outcomes (78).
Figure 2. Endogenous circadian rhythm of cortisol.
Cortisol, the primary glucocorticoid in humans, is produced by the adrenal glands and is regulated by one of the major neuroendocrine systems, namely, the hypothalamic-pituitary-adrenal (HPA) axis. Typical physiological cortisol concentrations function to regulate energy mobilization in the body and thus follows a circadian rhythm in which changes in circulating cortisol generally precede daily activity patterns; cortisol values rise sharply just prior to the onset of activity (prewaking) and then gradually decline across the day to reach nadir at the end of the active period (onset of sleep) (based on [74]). The HPA axis also functions to integrate physiological systems (e.g., immune, cardiovascular, reproductive, metabolism, central nervous system) to rapidly adapt to changes in the environment. Strong bidirectional feedback between the HPA axis and these systems place it as the fulcrum of typical physiology; thus, HPA axis dysregulation is implicated in many medical conditions.
The most common treatment approach for AI and CAH is administration of the glucocorticosteroid hydrocortisone (HC), but there is no consensus on the timing and regimen of dosing, which can range from once daily to over four times daily (76, 77, 79). One underpowered study of two AI and two CAH patients suggests that a 24-hour infusion regimen of HC that mimics circadian rhythms of cortisol levels can restore circulating cortisol rhythms, restore levels of adrenocorticotropic hormone (ACTH), and reduce levels of plasma 17-hydroxyprogesterone (17-OHP) (80). Other small studies supporting these results demonstrated the effectiveness of continuous subcutaneous HC administration on restoring cortisol and ACTH rhythms as well as increasing nocturnal growth hormone and insulin growth factor levels in AI patients (81, 82) (Table 2). In an attempt to more closely mimic normal endogenous circadian rhythms in cortisol, several formulations of modified-release glucocorticoids are currently undergoing clinical trials for approval (reviewed in (83)). Continued research with careful attention paid to circadian parameters will be needed to elucidate optimal treatment approaches for patients with AI and CAH.
Table 2.
Clinical trials and studies considering time of day in the treatment of endocrine disorders.
| DRUG CLASS | DRUG(S) | POPULATION | STUDY CONCLUSIONS | SUGGESTED TIME OF DAY | CITATION |
|---|---|---|---|---|---|
| Adrenal Insufficiency (AI) and Congenital Adrenal Hyperplasia (CAH) | |||||
| Glucocorticosteroid | Hydrocortisone | 2 AIH + 2 CAG patients | 24-hour infusion regimen of HC that mimics circadian rhythms of cortisol levels can restore circulating cortisol rhythms, restore levels of ACTH, and reduce levels of plasma 17-OHP. | 24-h | Merza, Rostami-Hodjegan et al. 2006 |
| AI patients | 24-h sub-cutaneous HC administration on restoring cortisol, ACTH, and 17-OH rhythms as well as increasing nocturnal growth hormone and insulin growth factor levels in AI patients | 24-h | Lovas and Husebye 2007; Bjornsdottir, Oksnes et al. 2015 | ||
| Type I Diabetes Mellitus | |||||
| Hormone - Insulin Analogue | Insulin | Open, randomized, cross-over design; 14 patients who experience evening hypoglycemia | Nighttime subcuntaneous continuous injections seem to be more effective at hypoglycemic control. | Continous nighttime | Kanc, Janssen et al. 1998 |
| Insulin Ultratard | 9 Patients | No significant difference in blood glucose levels at any point. | No difference | Edsberg, Dejgaard et al. 1987 | |
| Pediatric patients | Continuous subcutaneous injections of insulin glargine reduced HbA1C levels and controlled pre-meal glucose levels better than multiple daily injections | Continuous | Doyle, Weinzimer et al. 2004 | ||
| Insulin Glargine | 292 Patients | Similar improvements were seen in morning, evening, or split dose groups. Split dosing results in weight gain. | No difference | Garg, Gottlieb et al. 2004 | |
| Patients whose HbA1C and glycemic levels were not controlled by single injections | Split dosing was effective. | Split dosing | Albright, Desmond et al. 2004 | ||
| 18 Patients with poorly managed T1DM | Transitioning from evening to morning administration, independent of dose, resulted in more favorable glucose control and lipid profile without affecting body weight. | Morning | Gradiser, Bilic-Curcic et al. 2015 | ||
| Insulin Glargine + Lispro | HbA1C levels and 24-hour glycemic control did not differ among groups administring insulin glargine in the morning, evening, or bedtime in conjunction with prandial insulin lispro; morning administration resulted in fewer nocturnal hypoglcemic episodes | No differences; morning had added benefits | Hamann, Matthaei et al. 2003 | ||
| Lispro | Randomized, cross-over study. 23 patients | Administration of insulin glargine at lunch, dinner, or bedtime resulted in hypoglycemia at distinct timepoints after each injection; the night-time hyperglycemia after bedtime glargine injections was avoided with lunch or dinner injection schedules | Ashwell, Gebbie et al. 2006 | ||
| 13 Patients | More effective at evening and nocturnal glycemic control when the bedtime dose is greater than mealtime doses; Lower mealtime and higher bedtime doses might be most effective at evening glycemic control. | Low mealtime, higher bedtime | Ahmed, Mallias et al. 1998 | ||
| Detemir + Aspart | Combination therapy at mealtime provided equally effective glycemic control when administered as a morning/dinner or a morning/bedtime dose; however both regimens provided better glycemic control with no weight gain when compared to NPH morning/evening insulin regimen. | Pieber, Draeger et al. 2005 | |||
| Octapeptides | Octreotide | 8 T1DM patients who experience evening hypoglycemia: 4 Females 4 Males | Continuous subcuntaneous night injection is more effective at reducing hyperglycemia and growth hormone levels than single injections across the night. | Continous nighttime | Lunetta, Di Mauro et al. 1998 |
| Type 2 Diabetes Mellitus | |||||
| Hormone | Insulin | 100 Patients | Higher morning:evening ratio seems to have greater safety and efficacy. | Higher morning:evening | Jung, Park et al. 2014 |
| 143 Patients | For twice-daily doses of insulin, a higher morning:evening ratio might be more effective at managing glycemic levels. | Higher morning:evening | Lee, Lee et al. 2012, | ||
| Insulin Glargine + Glimepiride | 624 patients | Single daily dose was equally effective at glycemic control when given in the morning or evening. | No difference | Standl, Maxeiner et al. 2006 | |
| Insulin Glargine | 10 Patients | Total insulin activity is similar between morning/evening doses. However, evening administration controls nocturnal EGP, lipolysis, and glucagon concentration more consistently, whereas morning administration has greater protection against nocturnal hypoglycemia. | No difference | Porcellati, Lucidi et al. 2015 | |
| Incretin Mimetics | Lixisenatide | 680 T2DM patients with inadequate control of glucose levels by metformin | Morning and evening injections similarly improve glucose control. | No difference | Ahren, Leguizamo Dimas et al. 2013 |
| Meglitinide - Antidiabetic | Repaglinide | 19 T2DM patients | Mealtime dosing is more effective than morning/evening split dose. | Mealtime | Schmitz, Lund et al. 2002 |
| Dipeptidyl Peptidase-4 Inhibitor | Vildagliptin | 48 Patients | Morning and evening dosing were equally effective at post-prandial and 24-h glucose control; however an evening dose was effective at reducing fasting plasma glucose. | No difference, but evening has additional benefits | He, Valencia et al. 2010 |
| Gestational Diabetes Mellitus | |||||
| Hormone | Insulin | 274 Females w/ Gestational Diabetes 118 Females with Pregestational Diabetes | Insulin administered four times daily is more effective at glycemic control than twice daily. 30 mins before each meal and before bedtime. | Four times/day | Nachum, Ben-Shlomo et al. 1999 |
| 480 Females, >30 weeks pregnant. | Four times daily. 30 mins before each meal, and before bed-time. | Four times/day | Saleem, Godman et al. 2016 | ||
| Hypothyroidism | |||||
| Thyroid hormone | Levothyroxine | 50 Patients | Morning dose is more effective, but if evening dose is necessary for compliance, evening dose is acceptable. | Morning, before mealtime | Ala, Akha et al. 2015 |
| 12 Females | Bedtime administration seems to improve thyroid hormone levels and reduced TSH levels. | Bedtime | Bolk, Visser et al. 2007, Banerjee, Hossain et al. 2018 | ||
| 105 Patients | Bedtime administration improved thyroid hormone levels. | Bedtime | Bolk, Visser et al. 2010 | ||
| 152 Patients | Morning and evening doses are equally effective | No difference | Rajput, Chatterjee et al. 2011 | ||
| 163 Children: 125 Females 38 Males | No difference between bedtime and morning treatments. | No difference | Akin 2018 | ||
| Older adults | Clinical trial currently underway | TBD | Giassi, Piccoli et al. 2019 | ||
| Fat-soluble vitamin | Vitamin D3 | 13 Patients: 5 Females, 8 Males with secondary hyperparathyroidism in end-stage renal failure. | Evening dose is more effective at managing hyperparathyroidism in patients with renal osteodystrophy. | Evening | Tsuruoka, Wakaumi et al. 2003 |
| Osteoporosis | |||||
| Mineral | Calcium | 14 patients | Calcium-supplemented meals did not affect the levels of bone resorption or the circadian patterns of resorption in comparison to evening-only supplements | No difference | Aerssens, Declerck et al. 1999 |
| 26 early-menopausal females | Split morning:evening dose of 500:1000 mg (Tot. 1500mg) | Higher evening:morning | Scopacasa, Need et al. 2002 | ||
| 19 post-menopausal females | Single evening 1000 mg dose only suppressed bone resorption during the night. | Split morning/evening | Scopacasa, Horowitz et al. 1998 | ||
| 19 Females | Split dosing improved daytime bone resorption but not nighttime resorption. | Split morning/evening | Scopacasa F, Need AG, Horowitz M, Wishart JM, Morris HA and Nordin BE (2000) Inhibition of bone resorption by divided-dose calcium supplementation in early postmenopausal women. Calcif Tissue Int 67:440–442. | ||
| 30 Females 21–34 y | Split, morning, or 4 x daily doses showed no difference on bone resorption across the day, However parathyroid hormones were differently affected based on the size and timing of calcium dose, Need for longitudinal studies. | No difference | Kärkkäinen MU, Lamberg-Allardt CJ, Ahonen S and Välimäki M (2001) Does it make a difference how and when you take your calcium? The acute effects of calcium on calcium and bone metabolism. Am J Clin Nutr 74:335–342. | ||
| Estrogen receptor modulator | Raloxifene | 39 Post-menopausal females | The only difference between morning/evening dose was the increase of plasminogen activator inhibitor (PAI)-1 with morning administration. Authors recommend evening administration. | Evening | Ando, Otoda et al. 2013 |
| Parathyroid Hormone | Teriparatide | 50 Females, post-menopausal | Morning administration resulted in increase in lumbar spine BMD. | Morning | Michalska, Luchavova et al. 2012 |
| Etidronate | retrospective longitudinal study | Dosing was similarly effective when taken as single doses across the day if the patient adhered to a 2 h fast before and after dosing | No difference | Cook, Blake et al. 2000 | |
| Cathepsin K Inhibitor | ONO-5334 | 14 Females; single-blind crossover study | Morning dose is more effective at reducing bone resorption than evening dose. | Morning | Eastell, Dijk et al. 2016 |
| Hormone | Salmon Calcitonin | 9 Females, Post-Menopausal | Both 0800 h and 2100 h administration are effective with no obvious advantage to either. 0800 h versus 2100 h treatment transiently reduced bone resorption but did not effectively alter the circadian pattern of bone resorption. | No difference | Schlemmer, Ravn et al. 1997 |
| 81 Females between 40–70 y/o | Pre-dinner (1700 h) administration resulted in the greatest reduction in bone resorption, when compared to 0800 h or 2200 h administration. | Evening | Karsdal, Byrjalsen et al. 2008 | ||
| Growth Hormone Deficiency | |||||
| Hormones | Growth Hormone | Evening administration of GH was more effective at restoration of normal hormone and metabolite circadian patterns. | Evening | Jorgensen, Moller et al. 1990 | |
| 8 adult patients | Compared to one dose at 1900 h, split dosing at 1900 h (2/3 dose) and 0800 h (1/3 dose), better matched normal physiological GH profile, increased serum IGF-1, and decreased serum IGFBP-1 while lowering non-esterified fatty acids. | Split dose at 0800 h, 1900 h | Laursen, Jorgensen et al. 1994 | ||
| 34 children | No differences between morning, afternoon or evening administration, in growth, IGF-1, or GH-BP after 6 or 12 months of GH treatment | No difference | Zadik, Lieberman et al. 1993 | ||
| Glucocorticoid | Prednisolone | 8 Patients: 4 Females 4 Males | Morning administration attenuates nocturnal growth hormone suppression, therefore potentially attenuating stunted growth. | Morning | Wolthers, Ramshanker et al. 2017 |
| Turner Syndrome | |||||
| Hormone | Estradiol | 9 girls with Turner Syndrome receiving GH injections | Estradiol was more effective at managing insulin, glucagon, IGF-1 levels when administered in the evening compared to morning, but further studies are needed. | Evening | Naeraa, Gravholt et al. 2001 |
| Other Endocrine Treatments | |||||
| Hormone Therapy | Cyclo-Progynova Therapy | 62 patients | No obvious difference in efficacy of morning/evening treatment. | No difference | Pongsatha, Chainual et al. 2005 |
| Artifical Hormones | Hydrocortisone | 6 females | Morning and evening administration is equally effective. | No difference | Kiriwat and Fotherby 1983 |
Diabetes.
Therapeutic regimens for diabetic patients need to consider both insulin requirements after food ingestion and the diurnal variations in insulin requirements unrelated to meals (84). Chronotherapy for diabetes management has been investigated, but optimal treatment across the day can vary among individuals and should ultimately be based on individual diagnosis, lifestyle, and blood glucose patterns (85, 86) (Table 2). The ideal timing of daily insulin treatments for patients with type 1 diabetes mellitus (T1DM) is variable and may depend on a range of factors. Multiple studies have reported that long-acting insulins, such as glargine or ultratard insulin, are similarly effective at glycemic control when administered at different times throughout the day, albeit with some differences between timing (87–91). For example, split dosing rather than single morning or evening dosing resulted in significant weight gain (1.4 ± 0.5 kg vs. 1.1 ± 0.6 kg and 0.6 ± 0.5 kg) and decreased quality of life (88). However, one study reported that split dosing was effective for patients whose HbA1C and glycemic levels were not controlled by single injections (92). Another study similarly reported that HbA1C levels and 24-hour glycemic control did not differ among groups administering insulin glargine in the morning, evening, or bedtime in conjunction with prandial insulin lispro; however, morning administration resulted in fewer nocturnal hypoglycemic episodes (90). A randomized crossover study in 23 patients using prandial insulin lispro reported that administration of insulin glargine at lunch, dinner, or bedtime resulted in hypoglycemia at distinct timepoints after each injection; the night-time hyperglycemia after bedtime glargine injections was avoided with lunch or dinner injection schedules (93). Another study in 18 patients with poorly managed T1DM, reported that transitioning from evening to morning administration of insulin glargine, independent of dose, resulted in more favorable glucose control and lipid profile without affecting body weight (94). A study of 13 T1DM patients reported that insulin lispro is more effective at evening and nocturnal glycemic control when the bedtime dose is greater than mealtime doses (95). Insulin detemir, in combination with mealtime insulin aspart, provided equally effective glycemic control when administered as a morning/dinner or a morning/bedtime dose; however both regimens provided better glycemic control with no weight gain when compared to morning/evening NPH insulin regimen (96). For T1DM patients who experience evening hypoglycemia, continuous nocturnal subcutaneous infusions of either insulin or octreotide, compared to multiple injection regimens, better reduced nocturnal and morning hyperglycemia and lowered growth hormone levels (97, 98). Similarly, a pediatric study also demonstrated that continuous subcutaneous injections of insulin glargine reduced HbA1C levels and controlled pre-meal glucose levels better than multiple daily injections (99).
Optimal timing of treatment for type 2 diabetes mellitus (T2DM) is also variable and can depend on drug and treatment schedule based on individual diagnosis, lifestyle, and blood glucose patterns (86) (Table 2). When treating T2DM with morning and evening doses of insulin or insulin analogues, a high morning dose (in ratios between 53:47 and 75:25 morning:evening) was reported by two studies to be more effective for glycemic control (100, 101). When administered as a single daily dose, insulin glargine in conjunction with morning glimepiride was equally effective at glycemic control when given in the morning or evening in a randomized study of 624 patients (102). An additional euglycemic clamp study in 10 patients similarly reported that the timing of single insulin glargine administration did not affect total 24-h insulin activity, although nocturnal administration improved 24-h suppression of both plasma glucagon and markers of lipolysis (103). In T2DM patients who experience inadequate control of glucose levels by metformin, a double-blind placebo-controlled trial in 680 patients reported that morning and evening lixisenatide injections similarly improve glucose control (104). Repaglinide, a fast-acting meglitinide, was also reported to be more effective when administered at mealtimes rather than as split morning/evening doses in a double-blind randomized parallel-group study of 19 T2DM patients (105). In a randomized double-blind crossover study in 48 T2DM patients morning or evening dosing of vildagliptin, a dipeptidyl peptidase-4 inhibitor, was reported to be equally effective at post-prandial and 24-h glucose control; however only evening dosing was effective at reducing fasting plasma glucose (106). Finally, for treatment of gestational diabetes mellitus, dosing regimens of regular and intermediate insulin that follow a four-times daily administration schedule were reported to provide better glycemic control and maternal/fetal outcomes when compared to twice daily regimens (107, 108).
Hypothyroidism and Hyperparathyroidism.
The primary drug used for treatment of hypothyroidism is levothyroxine, a synthetic thyroid hormone. Despite multiple attempts to elucidate effective circadian timing of administration for disorder control, there is contradicting evidence suggesting when the drug should be administered (Table 2). Some reports suggest that the levothyroxine should be taken in the morning before mealtimes (109). Conversely, other groups report improved thyroid hormone control after administering the drug in the evening compared to morning (110–112), or no variation in efficacy between morning and evening treatment in adults (113) or children (114). A clinical trial is currently underway to compare efficacy of levothyroxine morning dosing to evening dosing in older adults (115). A brief review on the circadian variations of drug efficacy suggests that administration of levothyroxine should be coordinated primarily with timing of meals until further research demonstrates a consistently optimal time of day for drug administration (116). Finally, a randomized cross-over study of 13 patients with hyperparathyroidism providing morning (0800 h) or evening (2000 h) doses of vitamin D3 reported that evening doses were more effective at condition management (117). Due to conflicting reports, no conclusions can be drawn on optimal timing of treatment for thyroid disorders, however it is obvious that further clinical research with a clear consideration of time of day as a biological variable is necessary to optimize efficacy of treatment.
Osteoporosis.
A circadian pattern of markers of bone metabolism in serum, saliva, and urine suggests that chronotherapy should be considered for the treatment of osteoporosis (118–122). Clinical practice guidelines published by various professional organizations do not form a consensus on best approaches (reviewed in (123)), yet one commonality among them is the lack of consideration of circadian rhythms in bone metabolism, likely due to the paucity of clinical studies. Treatments for osteoporosis include nonpharmacological interventions (diet, calcium/vitamin D supplements) and pharmacological interventions (antiresorptive or anabolic medications).
Chronotherapeutic studies of non-pharmacological treatments of osteoporosis have primarily examined the timing of supplemental calcium administration (Table 2). One study of 14 patients with osteoporosis reported that calcium-supplemented meals did not affect the levels of bone resorption or the circadian patterns of resorption in comparison to evening-only supplements (124). Analysis of urinary bone resorption markers in healthy postmenopausal women revealed that a split morning evening dose of 500:1000 mg of calcium suppressed bone resorption across the 24 h period (125), whereas a single evening 1000 mg dose only suppressed bone resorption during the night (126). A randomized trial in 30 healthy adult females reported that altering acute oral calcium load by split, morning, or 4 x daily doses of calcium did not differentially affect bone resorption across the day, however parathyroid hormones were differently affected based on the size and timing of calcium dose, thus the authors concluded that longitudinal studies were needed to determine most effective timing for chronic calcium supplementation on bone health (127).
Chronotherapeutic approaches have also been examined for pharmacological approaches to treating osteoporosis (Table 2). A study in 39 post-menopausal women with osteoporosis treated with raloxifene (an estrogen receptor modulator) reported that morning (0730–0900 h) or evening (1800–2030 h) dosing similarly reduced levels of markers of bone metabolism; however the authors recommended evening doses due to morning dose-induced upregulation of plasminogen activator inhibitor-1, which is associated with increased venous thromboembolism risk (128). A longitudinal study in osteoporotic patients administered teriparatide, an anabolic drug, reported greater lumbar bone mass density increase with evening administration compared to morning dosing (129). A retrospective longitudinal study examining the timing of etidronate administration determined that dosing was similarly effective when taken as single doses across the day if the patient adhered to a 2 h fast before and after dosing (130). Finally, in a single-blind crossover study examining bone resorption, morning administration of ONO-5334 (a cathepsin K inhibitor) was more effective than evening administration at suppressing C-terminal telopeptide of type I collagen (a marker of bone turnover) across the day (131).
Optimal timing for administration of calcitonin, an antiresorptive hormonal treatment for osteoporosis and Paget’s disease, depends on route of administration. For example, 0800 h or 2100 h nasal salmon calcitonin treatment transiently reduced bone resorption but did not effectively alter the circadian pattern of bone resorption (132). However, a randomized, double-blind study in 81 postmenopausal women reported that oral salmon calcitonin (0.8 mg) administered before dinner (1700 h) resulted in the largest 24 h suppression of bone resorption relative to placebo, when compared to morning (0800 h) or evening (2200) dosing (133). Thus, evening dosing of oral salmon calcitonin appears to most effectively blunt the circadian peak in bone resorption (134), however extended calcitonin use has been associated with increased liver cancer risk and has been pulled from several markets (135). To our knowledge, there have been no recent clinical trials on optimal timing of calcitonin injection. Taken together, these studies highlight the need for including time of day as a biological variable in future studies of efficacy of treatments for osteoporosis.
Growth Hormone.
When treating growth hormone (GH) deficiency, early studies indicated that evening administration of GH was more effective at restoration of normal hormone and metabolite circadian patterns (136) (Table 2). A crossover study in 8 adult patients dosed either once at 1900 h or twice-daily at 1900 h (2/3 dose) and 0800 h (1/3 dose) reported that twice daily dosing better matched normal physiological GH profile, increased serum IGF-1, and decreased serum IGFBP-1 while lowering non-esterified fatty acids (137). A study in 34 children undergoing GH therapy either in the morning, afternoon, or evening, reported no differences among groups in growth, IGF-1, or GH-BP after 6 or 12 months of GH treatment (138). Additional circadian considerations for GH treatment were highlighted in a small study examining prednisone administration to 10 healthy children, which reported that evening administration suppressed GH secretion whereas morning administration did not (139). It is also important to note that a common limitation of biomedical studies is a male bias and, as is the case with most physiological processes, there is a significant sex difference in human GH patterns (140). Thus, future studies must consider both time of day and sex as biological variables to optimize GH treatment. One case where sex differences are not an issue is with the treatment of Turner syndrome. A double-blind placebo controlled crossover study in nine girls with Turner syndrome receiving evening GH injections reported that estradiol was more effective at managing insulin, glucagon, IGF-1 levels when administered in the evening compared to morning (141).
Other Endocrine Treatments.
A randomized crossover study on hormone therapy for the treatment of climacteric symptoms in perimenopausal women reported no difference between morning or evening administration of cycloprogynova (142). In regard to the time of day effects of the estrogen receptor modulator raloxifene on osteoporosis, please see the study in that section by Ando (128). Finally, regulation of ovulatory cycles via once-daily oral contraceptives were reported to be equally effective when administered as a morning or evening dose (143).
Immune System
Glucocorticoids.
The circadian rhythm of glucocorticoids is discussed in detail in the AI/CAH section above. Glucocorticoids are steroidal hormones that function to reduce inflammation, in addition to regulating glucose and metabolism. Glucocorticoids act by binding to glucocorticoid receptors, resulting in dimerization and translocation to the nucleus, repressing inflammatory transcription factors (144). These anti-inflammatory properties make this class of steroids a common treatment for immune disorders and diseases such as asthma, rheumatoid arthritis, and multiple sclerosis. Physiological production and secretion of glucocorticoids occurs in the adrenal glands in a distinct circadian pattern (Figure 2) (145, 146), suggesting that the timing of treatment with glucocorticoids could play an important role in immunotherapeutics, especially for chronic inflammatory diseases (147).
Rheumatoid Arthritis.
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by joint and tissue damage caused by chronic inflammation of the synovial membrane, resulting in joint pain, stiffness, and swelling of the joint. Symptoms of RA show circadian rhythmicity with a peak occurring in the morning; due in part to blunted levels of rhythmic cortisol secretion that lead to an abnormal elevated morning peak of circulating proinflammatory factors such as tumor necrosis factor (TNF-α) and interleukin-6 (IL-6) (reviewed in (148, 149)).
To determine circadian variations in anti-inflammatory capacity of corticosteroids, 41 RA patients in a crossover study were administered 5.8 mg of prednisolone either in the morning (0600 – 0700 h) or at bedtime (between 2200 – 2300 h); bedtime ingestion of the corticosteroid significantly reduced morning stiffness as compared to patients who took their dose in the morning (0700 h) (150). Another study evaluating timing administration of prednisolone in 85 women reported that mean pain score, based from the disease activity score 28 (DAS28), duration of morning stiffness, and erythrocyte sedimentation rate were decreased when administered at (2200 h) compared to (0800 h) (151); however, this study was neither a crossover study, nor randomized, so it is unclear if the effects were due to timing of the dose or duration of treatment. A randomized, double-blind multicenter trial in 288 patients comparing prednisone delivered in a modified release formulation in the evening, so as to have its peak effects coincide with nocturnal IL-6 upregulation, to prednisone delivered in a rapid release formulation in the morning, reported that morning pain intensity and duration, DAS28, and plasma IL-6 levels were significantly reduced with the evening modified release dose (152, 153).
In addition to short-term use of corticosteroids, NSAIDS and disease-modifying antirheumatic drugs (DMARDs) are also used to treat RA. Switching methotrexate dosing from morning to bedtime led to significant improvements in both DAS28 and modified health questionnaire (MHAQ) scores in a cohort of RA patients (154). Time-of-day specific effects for other DMARDs used to treat RA, such as baricitinib and tofacitinib, have not been clinically tested to our knowledge. A double-blind crossover study with the NSAID flurbiprofen reported that twice-daily dosing regimens that included an evening dose were more effective in reducing RA symptoms and increasing grip strength (155). Evening administration (2000 h) of the NSAID indomethacin reduced morning pain symptoms in a double-blind crossover study in 66 patients with osteoarthritis; this study further reported that evening dosing resulted in the fewest undesirable effects and that worsening afternoon or evening pain was best relieved by administration in morning (0800 h) or afternoon (1200 h) (156). In an double-blind randomized trial in 117 osteoarthritis patients, evening dosing (2000 h) of ketoprofen was reported to cause longer duration of analgesia with fewer adverse effects when compared to morning dosing (0800 h) (157).
In spite of the clinical evidence (Table 3), the current clinical practice guidelines for RA (158) do not address the circadian aspects of RA nor discuss chronobiological approaches in treatment. However, chronotherapeutic approaches for optimal timing of treatments for RA have been proposed based on this clinical evidence (Figure 3) (159). Future studies should incorporate time of day as a biological variable to optimize efficacy of treatments for RA.
Table 3.
Clinical trials and studies considering time of day in the treatment of disorders involving the immune system.
| DRUG CLASS | DRUG(S) | STUDY DESIGN; POPULATION | STUDY CONCLUSIONS | SUGGESTED TIME OF DAY | CITATION |
|---|---|---|---|---|---|
| Rheumatoid Arthritis | |||||
| Glucocorticoids | Prednisolone | 41 patients | Bedtime dose (between 2200 −2300 h) significantly reduced morning stiffness as compared to morning (0700 h) dose. | Bedtime (between 2200 −2300 h) | De Silva, Binder et al. 1984 |
| 85 women | Mean pain score based from the disease activity score 28, duration of morning stiffness, and erythrocyte sedimentation rate were decreased when administered at (2200 h) compared to (0800 h) | Night (2200 h) | Gul H 2017 | ||
| Prednisone Modified Release (PMR) v. Prednisone Rapid Release (PRR) | 288 patients | PMR significantly reduced morning pain intensity and duration, DAS28, and plasma IL-6 levels, compared to PRR in the morning. | Evening | Buttgereit, Doering et al. 2008, Buttgereit, Doering et al. 2010 | |
| DMARDS | Methotrexate | Prospective, single-arm study; 17 patients | Methotrexate dosing from morning to bedtime led to significant improvements in both DAS28 and modified health questionnaire (MHAQ) scores in a cohort of RA patients | Bedtime, 3x/wk | To, Yoshimatsu et al. 2011 |
| Osteoarthritis | |||||
| NSAIDs (Aspirin) | Flurbiprofen | Double-blind crossover study; 17 patients | Twice-daily dosing regimens that included an evening dose were more effective in reducing RA symptoms and increasing grip strength. | 2x/daily with an evening dose | Kowanko, Pownall et al. 1981 |
| Indomethacin | Double-blind crossover study in 66 patients with osteoarthritis | Evening administration (2000 h) reduced morning pain and reported the fewest undesirable effects and that worsening afternoon or evening pain was best relieved by administration in morning (0800 h) or afternoon (1200 h). | Evening | Levi, Le Louarn et al. 1985 | |
| Ketoprofen | double-blind randomized trial in 117 osteoarthritis patients | Evening dosing (2000 h) caused longer duration of analgesia with fewer adverse effects when compared to morning dosing (0800 h). | Evening | Perpoint, Mismetti et al. 1994 | |
| Multiple Sclerosis | |||||
| Cytokines | IFN-β1 | 16 patients with relapsing/remitting MS | On day 1 of treatment, morning injection resulted in higher plasma IL-10; evening injection caused an earlier and more robust peak in cortisol, increased soluble tumor necrosis factor receptor 1 & 2 (sTNF-R), and increased plasma IL-1, which was associated with more intense negative side effects; after 6 months of IFN-β therapy, elevated sTNF-R1 in the morning group was the only difference reported. | Inconclusive | Kumpfel, Schwan et al. 2007 |
| IFN-β | 105 patients | Switching from evening to morning injections of IFN-β qualitatively improved flu-like symptoms (58%) and sleep quality (48%), common side effects from INF-β delivery | Morning | Nadjar, Coutelas et al. 2011 | |
| IFN-β1a | Randomized controlled parallel-group trial in 200 patients with relapsing MS | Morning administration reported more intense flu-like symptoms at weeks 4 & 8; by week 12 there were no differences in symptoms between groups. No reported effects of time-of-day on dosing, sleep quality, fatigue severity, or circulating leptin, resistin, and adiponectin after 12 weeks of therapy. | No differences | Patti, Zimatore et al. 2020 | |
| Glucocorticoid | Methylprednisolone | 17 patients | Night (2200 – 0200 h) administration vs. day (1000 – 1400 h) was reported to reduce serum MMP-9 and adverse events, including symptoms such as insomnia, depression, headaches, restlessness, gastrointestinal symptoms, palpitations | Night (2200 – 0200 h) | Glass-Marmor, Paperna et al. 2007 |
| Asthma treatments | |||||
| Glucocorticoids | Triamcinolone | 30 patients | Equally effective when administered as a single 800 μg dose at 1500 h when compared to 200 μg 4x/day. Authors suggest 1x/d dose should increase compliance of steroid use, | 1x/d at 1730 h or 4x/d | Pincus, Szefler et al. 1995 |
| 59 subjects | Equally effective administered as a single dose at 1730 h or 4x/d, but a single dose at 0800 h was less beneficial in comparison to the other dosing regimens. | Afternoon (1500 −1730 h) | Pincus, Humeston et al. 1997 | ||
| Mometasone Furoate | Open-label, randomized, parallel-group study; 1537 subjects with mild to moderate asthma | No difference between morning or evening administration on subjective symptoms. | No difference | Zetterstrom, Dahl et al. 2008 | |
| Fluticasone Fluroate | Randomized double-blind clinical trial; 28 patients | No difference between morning or evening administration on subjective symptoms. | No difference | Kempsford, Bal et al. 2016 | |
| Glucocorticoids + β-agonist | Fluticasone Furoate + β-agonist Vilanterol | Randomized, double-blind crossover clinical trial; 26 subjects | No difference between morning or evening administration on subjective symptoms. | No difference | Kempsford, Oliver et al. 2013 |
| Bambuterol | Double-blind, randomized, placebo-controlled, crossover study; 29 patients | Reduced symptoms at either 0700 h or 2200 h; evening administration produced the most improvement in morning forced expiratory volume. | Evening | D’Alonzo, Smolensky et al. 1995 | |
| Nonselective phosphodisterase enzyme inhibitor | Sustained release theophylline | 25 adult patients | Once (2000 h) or twice (0800 & 2000 h) daily doses had similar improvement in airflow. Single evening dose significantly improved peak expiratory flow rate and forced expiratory volume between 0200 h and 0600 h. | Similar improvements; evening dose had additional benefits. | D’Alonzo, Smolensky et al. 1990 |
| Extended release theophilline | Double-blind crossover study; 8 pediatric patients | Treatment irrespective of dosing time resulted in comparable enhancement of the group24-hr mean, minimum and maximum values of airways patency with reference to placebo baselines. However, dosing at 1500 or 2100 h, resulted in the best effect on the airways as assessed by the 24-hr mean forced expiratory volume. | Evening (1500 h) or Night (2100 h) | Smolensky, Scott et al. 1987 | |
| Allergic Rhinitis | |||||
| H1 Histamine Antagonist | Mequitazine | Multicenter | Dinner-time dosing was more effective at controlling morning peak and 24-h symptoms, as compared to breakfast dosing. | Dinner-time | Reinberg, Gervais et al. 1985 |
| Desloratadine | randomized study in 663 adult AR patients | No difference in morning vs evening administration. | No difference | Haye, Hoye et al. 2005 | |
| Cetirizine | two multicenter, randomized, double-blind, parallel-group studies | Morning and evening administration was equally effective at symptom relief in seasonal AR. | No difference | Urdaneta, Patel et al. 2018 | |
| Nasal Decongestants | Pseudoephedrine | Randomized, double-blind, crossover study in 9 male athletes | Morning (0700 h) but not afternoon (1700 h) supra-therapeutic dose boosted muscle contraction velocity in squat exercises. | Morning (0700 h) | Pallares, Lopez-Samanes et al. 2015 |
| Cystic Fibrosis | |||||
| Antibiotic | Tobramycin | Randomized trial in 18 children | Morning (0800 h) compared to evening (2000 h) administration showed no differences in pharmokinetics due to time of day, however urinary KIM-1 (kidney injury molecule) was higher in the 2000 h group, indicating greater potential for kidney damage with evening dosing | Morning due to evening side effects | Prayle, Jain et al. 2016 |
| 25 adult CF patients | No differences in pharmacokinetics due to time of day, but the evening group (2200 h) had increased serum blood urea nitrogen compared to the morning (0800 h) group | Morning due to evening side effects | van Maarseveen, van der Meer et al. 2020 | ||
| Eczema / Psoriasis | |||||
| Corticosteroids | Betamethasone | Maximal therapeutic effects achieved on healthy skin with a late afternoon application. | Late afternoon | Pershing, Corlett et al. 1994 | |
| Evening application was more effective than morning application; however, its effects were attenuated after 5 nights of application. | Evening | Nguyen, Lacour et al. 2017 | |||
| Vaccines | |||||
| Vaccines | Influenza and Hepatitis A | Morning vaccinations produce enhanced antibody responses compared to those given in the afternoon. | Morning | Phillips, Gallagher et al. 2008, Kirby 2016, Long, Drayson et al. 2016 | |
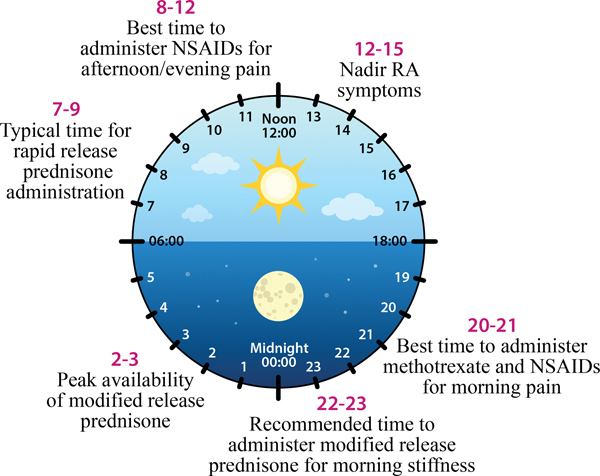
Figure 3. Chronotherapy for rheumatoid arthritis (RA).
Suggested optimal timing of various treatments for circadian RA symptoms (redrawn from [159]).
Multiple Sclerosis.
Multiple Sclerosis (MS) is an autoimmune inflammatory disorder characterized by demyelination and neurodegeneration of the central nervous system. This results in varying symptoms depending on the severity and location of nerve damage, but often includes pain, numbness, tremors, unsteady gait, and loss of coordination as the disease progresses. Recent evidence strongly implicates environmental and genetic influences on the circadian system in the development and progression of MS. For example, disruption of circadian rhythms by shift work at an early age is associated with increased risk of developing MS (160). Polymorphisms in the genes for ARNTL and CLOCK, core components of the molecular circadian clock, are associated with MS risk (161). Furthermore, seasonal relapses in MS are associated with changes in melatonin, which is a circadian rhythm-dependent hormone (162). Finally, a study comparing 34 healthy adults with 34 MS patients reported that serum inflammatory markers, and diurnal rhythms in some markers, differ between healthy adults and MS patients, and between MS patients with active and inactive lesions (163).
Pharmacological treatment approaches are trifold: first to modify the disease course, second to treat exacerbations, and third to manage the multitude of symptoms. Interferon-beta (IFN-β) therapy is one of the commonly prescribed disease modifying approaches. A small study in 16 patients with relapsing/remitting MS comparing IFN-β1a injected at 0800 h to 1800 h, reported that on the first day of treatment, morning injection resulted in higher plasma IL-10 whereas evening injection caused an earlier and more robust peak in cortisol, increased soluble tumor necrosis factor receptor 1 & 2 (sTNF-R), and increased plasma IL-1, which was associated with more intense negative side effects; after 6 months of IFN-β therapy, elevated sTNF-R1 in the morning group was the only difference reported (164). Another clinical study in 105 MS patients reported that switching from evening to morning injections of IFN-β qualitatively improved flu-like symptoms (58%) and sleep quality (48%), common side effects from INF-β delivery (165). Conversely, a recent randomized controlled parallel-group trial in 200 patients with relapsing MS comparing morning and evening dosing of IFN-β1a across 12 weeks of treatment reported that the morning administration group reported more intense flu-like symptoms at weeks 4 and 8, however by week 12 there we no differences in symptoms between groups. Furthermore, there were no reported effects of time of day of dosing on sleep quality, fatigue severity, or circulating leptin, resistin, and adiponectin after 12 weeks of IFN-β1a therapy. (166). Limited and conflicting data make it impossible to determine maximal effectiveness of IFN-β1a therapy based on time of day of dosing (Table 3). To our knowledge, no other disease modifying treatments (such as glatiramer acetate, dimethyl fumarate, teriflunomide, siponimod, mitoxantrone, natalizumab, fingolimod, etc.) have been publicly evaluated in the context of administration time in relation to therapeutic effectiveness.
For managing relapses in MS, immunosuppressive doses of corticosteroids, or adrenocorticotropic hormone (ACTH) to stimulate endogenous corticosteroid release, are generally given during the flare up. Corticosteroid rhythms and time-of-day specific effects of corticosteroid treatments are reviewed above in the context of congenital adrenal hyperplasia and rheumatoid arthritis, however examination of time of day effects of treatment in the context of treating MS are even more limited (Table 3). Methylprednisolone, when administered intravenously at night (2200–0200 h) compared to daytime (1000–1400 h) in 17 MS patients, was reported to reduce serum MMP-9 and adverse events, including symptoms such as insomnia, depression, headaches, restlessness, gastrointestinal symptoms, palpitations (167). Pharmacological approaches to managing the multitude of MS symptoms are greatly varied and are symptom specific. To our knowledge, scant few (if any) studies have directly investigated time-of-day specific effects of symptom modifying drugs in the context of MS. Regardless, taken together it is apparent that treatment of MS should use a chronobiological approach, and the few clinical studies that have investigated time-of-day effects are very limited and grossly underpowered. Finally, the preclinical and clinical studies of remyelination by oligodendrocytes for disease mitigation should take into account circadian rhythms of those cells (168).
Asthma.
Asthma is a chronic inflammatory disease characterized by episodes of shortness of breath, wheezing, and chest tightness due to narrowing or swelling of the airways. Both symptoms and treatments for asthma appear to be affected by circadian rhythms (169, 170), and reported optimal treatment times vary based on drug. The inhaled glucocorticoid triamcinolone was reported to be equally effective when administered as a single 800 μg dose at 1500 h when compared to 200 μg 4 times daily in a study with 30 participants (171). A follow up study in 59 subjects using the same total daily dose similarly reported triamcinolone was equally effective administered as a single dose at 1730 h or four times daily, however a single dose administered at 0800 h was reported less beneficial in comparison to the other dosing regimens (172). Studies evaluating other steroid inhalants, mometasone furoate (randomized parallel trial in 1537 subjects; (173) and fluticasone furoate (randomized double-blind trial in 28 subjects; (174), reported no difference between morning or evening administration on subjective symptoms. Similarly, no difference in effectiveness based on the time of day of administration was reported when fluticasone furoate was administered in combination with the beta-agonist vilanterol in a randomized, double-blind crossover trial in 26 subjects (175).
Bambuterol, a long-acting β-adrenoreceptor agonist, effectively reduced symptoms of asthma when administered once daily at either 0700 h or 2200 h in a double-blind, randomized, placebo-controlled, crossover study in 29 patients; however, evening administration produced the most improvement in morning forced expiratory volume, suggesting an optimal time of dosing for patients with nocturnal asthma (176). Similarly, a study in 25 adult patients comparing a once-daily administration of sustained release theophylline at 2000 h to twice-daily (0800 and 2000 h) reported that both regimens had similar overall improvements in airflow, however the single evening dose significantly improved peak expiratory flow rate and forced expiratory volume between 0200 h and 0600 h (177). However, a double-blind crossover study in 8 asthmatic children (178) reported that extended release theophylline formulated for once per day administration in the morning, if taken in the afternoon or evening (1500 h or 2100 h), resulted in serum theophylline concentrations greater than 20 μg/mL, which are toxic levels (179). Thus, in common with cortisol replacement therapy described in the adrenal insufficiency section above, theophylline provides another cautionary example of the critical need to account for chronopharmacology and circadian rhythms in clinical research and outcomes.
A third approach for treating asthma has been with leukotriene receptor antagonists such as montelukast (Singulair). Evening dosing is recommended for montelukast; however, there does not appear to be any clinical support for this recommendation. Indeed the both the US FDA and the manufacturer state in the product insert that “There have been no clinical trials in patients with asthma to evaluate the relative efficacy of morning versus evening dosing” (180), thus the evening dosing recommendation may likely represent the time of day for best patient compliance. For exercise induced asthma in children, one study reported no differences in protection from bronchial constriction when comparing in morning to evening dosing of montelukast (181). Similarly, when used for the treatment allergic rhinitis (see below), montelukast is reported to be equally effective when taken once a day in the morning or evening (182).
Allergic rhinitis.
Allergic rhinitis (AR) is an upper airway inflammatory disease characterized by sneezing, congestion of the nasal region, and rhinorrhea in response to allergens present in the environment, including particles such as pollen, dust, and mold. Symptoms of this condition display circadian rhythmicity, with a peak occurring in the early morning (183, 184), in common with what is observed in lower airway inflammation in asthma (185). Current clinical practice guidelines for AR do not discuss or provide guidance on the circadian aspects of this disease, although the guidelines do suggest clinicians should consider sleep-disordered breathing and sleep disturbance in their assessments (186).
Studies on chronotherapy for AR have generally focused on the most common therapeutic approach, histamine H1 receptor antagonists, however results from these limited studies have been equivocal (Table 3). When compared to breakfast dosing, dinner-time dosing of the H1 antagonist mequitazine was reported to be more effective at controlling both morning peak and 24-h symptoms of AR in a multicenter study (187). The effectiveness of the antihistamine desloratadine (5 mg/day for two weeks) was reported to not differ between morning or evening administration in a randomized study in 663 adult AR patients (188). Similarly, cetirizine was reported to be equally effective at symptom relief in seasonal AR independent of time of day of dosing in two multicenter, randomized, double-blind, parallel-group studies (189). A randomized, double-blind, crossover study in 9 male athletes comparing morning (0700 h) to early evening (1700 h) supra-therapeutic dosing (180 mg) of pseudoephedrine, a common over the counter nasal decongestant used to treat AR, reported that only a morning dose boosted muscle contraction velocity in squat exercises (190). The data are insufficient to draw any recommendations on chronotherapy for AR, however it is apparent that future studies into the effectiveness of treatments should record time of day as a biological variable.
Cystic fibrosis.
Tobramycin is a water-soluble aminoglycoside antibiotic commonly used to treat Cystic Fibrosis (CF) patients. A randomized trial in 18 children comparing dosing at 0800 h to 2000 h reported no differences in pharmacokinetics due to time of day, however urinary KIM-1 (kidney injury molecule) was higher in the 2000 h group, indicating greater potential for kidney damage with evening dosing (191). Consistent with the results in children, a study in 25 adult CF patients reported the same pattern; there were no differences in pharmacokinetics due to time of day, but the evening group (2200 h) had increased serum blood urea nitrogen compared to the morning (0800 h) group (192). Taken together, these studies (Table 3) suggest a chronotherapeutic approach may avoid contraindications caused by time of day of treatment for CF, and as such future studies should consider time of day as a biological variable.
Psoriasis and Eczema.
In common with other organs and physiological processes, the skin also displays circadian rhythms in normal function and disease, such as night time flares of atopic dermatitis (193). Additionally, disruption of circadian rhythms by shift work increases the risk of developing psoriasis (194), suggesting chronobiological approaches should be considered for treatment and prevention of skin disorders. As discussed previously, corticosteroids to treat inflammatory conditions generally have their greatest effect when applied at the nadir of the endogenous circadian cortisol rhythm. This appears to be the case for topical administration of betamethasone, which has its maximal effects on healthy skin with a late afternoon application (195). For acute treatment of psoriasis, evening application of betamethasone was reported to be more effective than morning application (196), however, its effects were attenuated after five nights of application, possibly due to skin saturation with corticosteroids.
Vaccines.
Circadian regulation of the adaptive immune system predicts time of day specific differences in response to antigens in vaccines (197), and thus potential beneficial effects of time of day specific vaccination. Morning vaccinations for influenza and hepatitis A produce enhanced antibody responses compared to those given in the afternoon (198–200), however some of these enhanced responses can vary by strain (201). Temporally-specific effects of vaccination are still debated as antibody titer is influenced by time of day of sample collection among the elderly (202). However, the aforementioned studies (Table 3) indicate that morning vaccination may enhance efficacy, especially when followed by a quality night of sleep after vaccination (203). Taken together, these few studies indicate that future vaccine trials should incorporate time of day as a biological variable in their design.
Metabolic Disorders
Statins.
Current clinical guidelines for the treatment and management of blood cholesterol (204) neither mention nor provide guidance on circadian aspects of cholesterol biosynthesis and management. However, this has been an active area of clinical investigation for over 30 years, with a clear recognition that time of day must be considered in both dietary and pharmacological approaches to management of blood cholesterol and treatment of atherosclerosis (205, 206). Statins can reduce serum low-density lipoprotein (LDL)-cholesterol ~30% by inhibiting the rate-limiting enzyme in cholesterol biosynthesis, HMG-CoA reductase (207).
Evening administration of an immediate release (IR) formulation of simvastatin (dose of either 2.5 or 5 mg) had a significantly higher percent reduction of plasma LDL-C concentrations when compared to morning administration (208). Similarly, switching adults who were receiving either 10 or 20 mg dose of IR simvastatin in the evening to a morning dose resulted in a 10% increase in LDL-C levels, suggesting that taking IR simvastatin during the evening is most effective (209). Nevertheless, administration of a controlled release (CR) formulation of simvastatin in the morning compared to evening reported no differential effects on reduction of LDL-C (210). When comparing efficacy and safety of morning intake of CR simvastatin to evening intake of IR simvastatin, there were no differences reported between treatment groups (211). In common with CR simvastatin, extended-release fluvastatin is equally effective in reducing LDL when administered in the morning or evening (212). Other clinical statin studies have evaluated lovastatin (213), pravastatin (214), atorvastatin (215, 216), and rosuvastatin (217). These studies had limitations either from confounding factors, failure to reach concrete conclusions, failure to reach statistical significance, or lacking in diversity among the populations studied, which ultimately limits their ability to be extrapolated (reviewed in (218, 219)).
Taken together (Table 4), these results highlight the importance of choosing the optimal time of day for statin administration, dependent upon the circadian pharmacokinetics and pharmacodynamics of each drug and formulation, and in the cases where no differences are observed, time of administration may be chosen that best supports patient compliance. Generally, the effects of statin treatment are improved when drug availability coincides with the time of day of maximum biosynthesis, thus it is likely more effective with bioavailability at night compared to morning. The limitations of previous studies provide insight that time of fday should be considered as a key biological variable in future research design into the effectiveness of statins on hyperlipidemia and atherosclerosis.
Table 4.
Clinical trials and studies considering time of day in the treatment of metabolic disorders.
| DRUG CLASS | DRUG(S) | STUDY DESIGN; POPULATION | STUDY CONCLUSIONS | SUGGESTED TIME OF DAY | CITATION |
|---|---|---|---|---|---|
| Statins: Management of Blood Cholesterol | |||||
| Statins | Simvastatin Immediate Release (IR) | Double-blind, placebo-controlled study; 172 patients | Evening administration had a significantly higher reduction of plasma [LDL-C] compared to morning administration. | Evening | Saito, Yoshida et al. 1991 |
| Adults receiving either 10 or 20 mg of IR Simvastatin | Switching adults from an evening to a morning dose resulted in a 10% increase in LDL-C levels, suggesting that taking IR simvastatin during the evening is most effective. | Evening | Wallace, Chinn et al. 2003 | ||
| Simvastatin Continuous Release (CR) | Prospective, randomized, double-blind, multicenter, placebo-controlled Phase III study; 132 patients with hypercholesterolemia | Morning compared to evening reported no differential effects on reduction of LDL-C. | No difference | Kim, Kim et al. 2013 | |
| Prospective, multicenter, double-blind, Phase IV trial with an active comparator. 122 patients with chronic kidney disease and dyslipidemia | There were no differences reported between morning versus evening administration. | No difference | Yi, Kim et al. 2014 | ||
| Fluvastatin Extended Release (ER) | Equally effective in reducing LDL when administered in the morning or evening. Authors suggest that morning administration is expected to increase patient compliance. | No difference | Scharnagl, Vogel et al. 2006 | ||
| Lovastatin | These studies had limitations either from confounding factors, failure to reach concrete conclusions, failure to reach statistical significance, or lacking in diversity among the populations studied, which ultimately limits their ability to be extrapolated. (Reviewed in (Plakogiannis and Cohen 2007, Awad and Banach 2018) | Illingworth et al., 1986 | |||
| Pravastatin | Hunninghake, Mellies et al. 1990 | ||||
| Atorvastatin | Cilla, Gibson et al. 1996, Plakogiannis, Cohen et al. 2005 | ||||
| Rosuvastatin | Martin, Mitchell et al. 2002 | ||||
| Glaucoma | |||||
| Prostaglandin Analogs | Travapost | Randomized crossover study; 30 patients; age > 18 | No difference between morning and evening administration. | No difference | Ford, Gooi et al. 2013 |
| Prostaglandin Analogs + Beta Blocker | Fixed combination of travoprost and timolol | prospective randomized crossover comparison study in 32 patients | Evening dosing provided better 24-h pressure control and lower peak IOP when compared to morning dosing. | Evening | Konstas, Tsironi et al. 2009 |
| Prostaglandin Analogs + Beta Blocker | Preservative-free tafluprost/timolol | placebo-controlled crossover trial in 42 patients | Evening dosing provided greater reductions in IOP and 24-h IOP fluctuation when compared to morning dosing. | Evening | Konstas, Katsanos et al. 2018 |
Glaucoma.
Production and flow of aqueous humor in the eyes follows a circadian rhythm, with peak during the day and nadir occurring at night (220). Intraocular pressure (IOP) also displays a circadian rhythm, with the peak generally occurring at the end of the nocturnal period and nadir at the end of the day (221, 222); however, these circadian patterns may be shifted in some individuals with glaucoma (223). Thus, although glaucoma treatments may likely benefit from a chronotherapeutic approach, optimal dosing times may need to be tailored to patients’ circadian rhythm of IOP and humor flow. Regardless, few studies have evaluated the differential effects of time-of-day specific administration of medications in glaucoma (Table 4). In a randomized crossover study in 30 patients, treatment with travoprost alone either in the morning or evening was equally effective at lowering IOP (224). However, in a prospective randomized crossover comparison study in 32 patients given a fixed combination of travoprost and timolol, evening dosing provided better 24-h pressure control and lower peak IOP when compared to morning dosing (225). Similarly, a recent placebo-controlled crossover trial in 42 patients given preservative-free tafluprost/timolol also reported that evening dosing provided greater reductions in IOP and 24-h IOP fluctuation when compared to morning dosing (226).
Cancer Treatments
Chemotherapy.
Chemotherapy is a mainstay in cancer treatment; the CDC estimates that as many as 600,000 patients will receive chemotherapy treatment in an outpatient setting in this year alone. Chrono-chemotherapy aims to optimally time chemotherapy administration to reduce adverse side effects and increase anti-tumor efficacy. Treatment regimens implementing chrono-chemotherapy time chemotherapy administration at the point in the circadian cycle during which adverse side effects will be the lowest, allowing for an increase in the maximal tolerated dose of chemotherapy to increase anti-tumor efficacy (227). Despite chrono-chemotherapy first being demonstrated to be beneficial more than 40 years ago, (228–230), it remains understudied and underappreciated. Studies examining the beneficial effects of chrono-chemotherapy in humans have primarily focused on treatments for ovarian, renal cell, and colorectal cancers, with few studies examining chronomodulated chemotherapy treatment for breast, lung, and pancreatic cancers (Table 5) (231–233).
Table 5.
Clinical trials and studies considering time of day in the treatment of cancer.
| DRUG(S) | CANCER TYPE | STUDY DESIGN; POPULATION | STUDY CONCLUSIONS | SUGGESTED TIME OF DAY | CITATION |
|---|---|---|---|---|---|
| Chemotherapeutics | |||||
| Doxorubicin (DOX) + Cisplatin (CDDP) | Ovarian Cancer | 31 patients with advanced ovarian cancer | DOX treatment at O600 h followed by CDDP treatment 1800 h, compared to DOX treatment at 1600 h followed by CDDP treatment at 0600 h, had fewer dose reductions, treatment delays, and treatment complications. | DOX at 0600 h + CDDP at 1800 h | Hrushesky 1985 |
| Patients with advanced ovarian cancer | 0600 h DOX treatment with 1600 h CDDP treatment is advantageous in reducing adverse side effects; this study demonstrated that this treatment schedule increased the 5 year survival rate from 11% to 44%. | DOX at 0600 h + CDDP at 1800 h | Hrushesky and Bjarnason 1993 | ||
| 4’−0-tetrahydropyranyl doxorubicin (THP) + Cisplatin (CDDP) | Randomized phase II trial; 31 patients with advanced ovarian cancer | Patients receiving a THP intravenous bolus at 0600 h followed by a 4-hour infusion of CDDP at 1600 h, had less neutropenia, thrombocytopenia, anemia, and renal toxicity relative to patients beginning THP treatment at 1800 h, followed by CDDP at 0400 h. | THP at 0600 h + CDDP at 1600 h | Levi, Benavides et al. 1990 | |
| Cisplatin (CDDP) | Advanced non-small cell lung cancer | Patients with advanced non-small cell lung cancer | Evening treatment caused less adverse effects such as leucopenia, neutropenia, and gastrointestinal toxicity relative to a morning treatment group. | Evening | Li, Chen et al. 2015 |
| Etoposide + Cisplatin | Advanced lung cancer, mixture of solid tumors, or metastatic cancer | advanced lung cancer, mixture of solid tumors, or metastatic cancer | Morning (0600 h or 0700 h) etoposide and evening CDDP (1800 h) treatment demonstrated less adverse hematological toxicities relative to patients receiving etoposide at 1800 h and CDDP at 0600 h. | Etopside at 0600 or 0700 h + CDDP at 1800 h | Krakowski, Levi et al. 1988, Krakowski, Levi et al. 1988, Focan 1995 |
| Floxuridine (FUDR) | Advanced Metastatic Cancer | 54 patients with advanced metastatic cancer | Patients receiving chronomodulated variable rate infusions had less frequent and less severe diarrhea, nausea, and vomiting. Additionally, patients receiving variable rate infusions increased their maximally tolerated dose by 45%. | Chronomodulated variable rate infusions | von Roemeling and Hrushesky 1989 |
| Oxaliplatin (1-OHP) + 5-Fluorouracil (5-FU) and Folinic Acid | Metastatic Colorectal Cancer | 92 patients with metastatic colorectal cancer | Chronomodulated variable rate infusions of chemotherapeutics demonstrated five times less severe stomatitis, the dose limiting adverse effect of 5-FU, an increased in the maximal tolerated dose of 5-FU, and an increase in median survival (19 months vs 14.9) relative to constant rate infusions. | Chronomodulated variable rate infusions | Levi, Zidani et al. 1994 |
| 5-FU and 1-OHP | Randomised multicentre trial; 186 patients with untreated metastases from colorectal cancer | Patients receiving chronomodulated variable rate infusions had five time less severe mucosal toxicity and half as much peripheral neuropathy. However, median survival and three year survival were unchanged relative to constant rate infusion. | Chronomodulated variable rate infusions | Levi, Zidani et al. 1997 | |
| 5-FU and cisplatin (CDDP) | Less adverse effects with chronomodulated variable rate infusions. | Chronomodulated variable rate infusions | Levi, Tubiana-Mathieu et al. 2004 | ||
| 5-FU | Failed to demonstrate beneficial effects of chronomodulated variable rate infusions. | No differences | Price, Ross et al. 2004, Garufi, Vanni et al. 2006, Ramanathan, Bjarnason et al. 2008, Huang, Yu et al. 2017 | ||
| 5-FU | Chronomodulation only demonstrated favorable effects on males. | Giacchetti, Bjarnason et al. 2006, Levi, Innominato et al. 2009, Giacchetti, Dugue et al. 2012 | |||
| Docetaxel (DOC) | Advanced Nasopharyngeal Carcinoma | Chronomodulated variable rate infusions of chemotherapeutics resulted in less adverse effects. | Chronomodulated variable rate infusions | Bi, Jin et al. 2015, Mao 2015, Liao 2016, Gou, Jin et al. 2018, Wan 2018, Zhang, Jin et al. 2018 | |
| Cisplatin (CDDP) | |||||
| 5-FU + radiotherapy | Improved cellular immune numbers in virtually all clinical trials. | ||||
| 5-FU without radiotherapy | |||||
| 6-Mercaptopurine and Methotrexate | Childhood Acute Lymphoblastic Leukemia | Evening administration of 6-mercaptopurine and methotrexate increased disease-free survival and reduced the risk of relapse by 2.5 times. | Evening | Rivard, Infante-Rivard et al. 1993 | |
| Phase II clinical trial; | Similar effects of increased event free survival from evening consumption. | Evening | Schmiegelow, Glomstein et al. 1997 | ||
| Capecitabine | Advanced Colorectal cancer | Chronomodulated asymmetrical dose did not significantly reduce toxicity or improve efficacy. | Qvortrup, Jensen et al. 2010 | ||
| Radiotherapeutics | |||||
| Radiotherapy | Breast Cancer | Retrospective study of 878 patients | Examining adverse effects following radiotherapy, the authors conclude that patients receiving radiotherapy in the afternoon (i.e. after 1200) had less adverse reactions. | Afternoon (after 1200 h) | Johnson, Chang-Claude et al. 2019 |
| Radiotherapy | Afternoon (after 1500 h) timed radiotherapy was associated with higher incidences of adverse skin reactions relative to morning-timed. | Morning | Noh, Choi et al. 2014) | ||
| Radiotherapy | Cervical cancer | Inconsistent results in adverse effects. | Inconclusive | Shukla, Gupta et al. 2010, Chang, Li et al. 2016 | |
| Radiotherapy | Head and Neck Carcinoma or Squamous Cell Carcinoma of Oral Cavity/Pharynx/Larynx | No significant differences in adverse effects. | No differences | Bjarnason, Mackenzie et al. 2009, Goyal, Shukla et al. 2009 | |
| Radiotherapy | Advanced Rectal Cancer | Afternoon-timed radiotherapy was associated with a higher incidence of complete or moderate pathological response and improved nodal downstaging in advanced rectal cancer. | Afternoon | Squire, Buchanan et al. 2017 | |
| Radiotherapy | Brain Metastases | Increased overall survival in patients receiving radiotherapy in the morning (before 1200 h). | Morning (before 1200 h) | Rahn, Ray et al. 2011, Badiyan, Ferraro et al. 2013 | |
| Radiotherapy | No difference between morning and afternoon groups. | No differences | Kabolizadeh, Wegner et al. 2011, Chan, Rowbottom et al. 2016 | ||
| Sunitinib | No differences in adverse events or overall survival. | No differences | Escudier, Roigas et al. 2009, George, Blay et al. 2009 | ||
One of the first studies examining the effects of chrono-chemotherapy for the treatment of advanced ovarian cancer in 31 patients, administered doxorubicin at either 0600 h or 1800 h followed by cisplatin (CDDP) twelve hours after the completion of doxorubicin treatment, reported that patients with doxorubicin treatment in the morning and cisplatin treatment in the evening had fewer dose reductions, treatment delays, and treatment complications (230). Morning doxorubicin treatment followed with evening cisplatin treatment was also reported to be advantageous in reducing adverse side effects in two other phase III clinical trials for the treatment of ovarian and bladder cancers, with an increase in 5 year survival rate from 11% to 44% in patients with advanced ovarian cancer (231). Similar beneficial effects of morning-timed doxorubicin treatment followed by evening-timed cisplatin treatments have been demonstrated using doxorubicin derivative 4’−0-tetrahydropyranyl doxorubicin (THP) (234). Indeed, in a phase II clinical trial evaluating circadian timing of chemotherapy treatment for advanced ovarian cancer in 31 patients reported that patients receiving a THP intravenous bolus at 0600 h followed 10 hours later by a 4-hour infusion of cisplatin, had less neutropenia, thrombocytopenia, anemia, and renal toxicity relative to patients beginning THP treatment at 1800 h (234). Furthermore, the favorable effects of evening-timed cisplatin treatments are not limited to ovarian cancer. A study in 41 patients with advanced non-small cell lung cancer reported that patients receiving cisplatin treatment in the evening (1800 h) developed less adverse effects such as leucopenia, neutropenia, and gastrointestinal toxicity relative to the 0600 h treatment group (235). Additionally, studies examining morning-timed (0600 h or 0700 h) etoposide followed with evening-timed cisplatin (1800 h) treatment for advanced lung cancer, mixture of solid tumors, or metastatic cancer demonstrated less adverse hematological toxicities relative to patients receiving etoposide at 1800 h followed by cisplatin at 0600 h (236–238).
Unlike differentially timed chemotherapy boluses described above, most clinical trials evaluating chronomodulated chemotherapy treatment regimens have compared chronomodulated to continuous chemotherapy infusions, primarily in treatment of colorectal and renal cancers. One of the first such studies compared chronomodulated to flat continuous chemotherapy infusions of floxuridine (FUDR) for 14 days in 54 patients with advanced metastatic cancer (239). To achieve the variable rate infusion, the daily dose was broken into 4 segments (6hrs each) of 68%,15%, 2%, and 15% of the total daily dose with the peak dose infusion time from 1500 h to 2100 h (239). Patients receiving chronomodulated variable rate infusions had less frequent and less severe diarrhea, nausea, and vomiting, in addition to a 45% increase in maximally tolerated dose (239). Similar favorable effects of chronomodulated variable rate infusions of FUDR have been reported in multiple clinical trials for patients with renal cancer and colorectal cancers (232).
The antimetabolite, 5-Fluorouracil (5-FU), is likely the most studied chemotherapeutic in the context of chronomodulated chemotherapy treatment regimens (233). To date more than 10 clinical trials have assessed the effects of chronomodulated 5-FU treatments with more than half of the trials evaluating treatment of metastatic colorectal cancer (233). One of the first studies to demonstrate the favorable outcomes of chronomodulated variable rate 5-FU treatment compared to continuous 5-FU infusions involved 92 patients with metastatic colorectal cancer across seven different European cancer centers (240). Patients were randomly assigned to chronomodulated or constant rate treatment regimens and chemotherapeutics were administered consistently for 5 days and repeated every 21 days. Patients in the chronomodulated treatment regimen received oxaliplatin (1-OHP) for 11.5 hours from 1015 h to 2145 h, with a sinusoidally delivery rate and peak delivery at 1600 h; 5-FU and folinic acid were infused from 2215 h to 0945 h, with peak delivery at 0400 h. Patients receiving chronomodulated variable rate infusions of chemotherapeutics demonstrated five times less severe stomatitis, the dose limiting adverse effect of 5-FU, had increased maximal tolerated dose of 5-FU, and increased median survival (19 months vs 14.9) relative to constant rate infusions (240). A follow up randomized multicenter trial involving 93 patients with metastatic colorectal cancer reported that, compared to constant rate infusion, patients receiving chronomodulated variable rate infusions of 5-FU and 1-OHP had a five-fold reduction in mucosal toxicity and half of the peripheral neuropathy, however median survival and three year survival were unchanged by dosing regimen (241). A subsequent multicenter randomized trial utilizing chronomodulated variable rate infusions of 5-FU and cisplatin for the treatment of metastatic pancreatic cancer also reported reduced adverse effects when compared to constant rate infusions (242). More recent studies however, have failed to demonstrate similar beneficial effects of chronomodulated variable rate infusions of 5-FU for the treatment of metastatic colorectal cancer (243–246), or have only demonstrated favorable effects on males (247–249).
Recent studies in the field of cancer chronotherapy have examined chronomodulated variable rate infusions of docetaxel, cisplatin, or 5-FU with or without radiotherapy for the treatment of advanced nasopharyngeal carcinoma (250–255). Chronomodulated variable rate infusions of chemotherapeutics resulted in less adverse effects (250–253, 255) and improved cellular immune numbers in virtually all clinical trials (250–252, 254, 255).
Few studies have examined the effects of chronomodulated oral chemotherapy treatment (256–258). One such retrospective study compared the effects of morning to evening dosing of 6-mercaptopurine and methotrexate for the treatment of childhood acute lymphoblastic leukemia in 118 children and reported that evening dosing increased disease free survival and reduced the risk of relapse by 2.5 times (256). Similarly, when compared to morning dosing, evening dosing of 6-mercaptopurine and methotrexate increased event free survival in a study in 294 children with acute lymphoblastic leukemia (257). However, a phase II clinical trial in patients with advanced colorectal cancer compared the effects of equidose to chronomodulated asymmetrical dose (i.e., 80% of the daily dose consumed at night) of the oral chemotherapeutic capecitabine; the authors concluded that the chronomodulated asymmetrical dose did not significantly reduce toxicity or improve efficacy (258).
Radiotherapy and Other Cancer Treatments.
As evident above, chrono-chemotherapy has beneficial effects relative to conventional chemotherapy in most clinical trials. In contrast, studies examining chrono-radiotherapy are scarce and even fewer studies have evaluated cancer treatments outside of chemotherapy and radiotherapy (Table 5). To date, approximately 13 studies have evaluated chrono-radiotherapy as an improved treatment option compared to conventional radiotherapy, and although somewhat promising, the results lack consistency across studies (259). For example, a retrospective study of 878 breast cancer patients examining adverse effects following radiotherapy concluded that patients receiving radiotherapy in the afternoon (i.e. after 1200) had less adverse reactions (260). However, a prior study had concluded that afternoon (i.e. after 1500 h) timed radiotherapy was associated with higher incidences of adverse skin reactions relative to morning-timed (i.e., before 1000 h) radiotherapy (261). Similarly inconsistent results in adverse effects have been reported in two studies that compared morning to evening timed radiotherapy in the treatment of cervical cancer (262, 263). Additionally, no differences in adverse effects were reported due to time of day of radiotherapy treatment for head and neck carcinoma or squamous cell carcinoma of oral cavity/pharynx/larynx (264, 265). However, afternoon-timed radiotherapy was associated with a higher incidence of complete or moderate pathological response and improved nodal downstaging in advanced rectal cancer (266). Furthermore, studies evaluating chrono-radiotherapy for the treatment of brain metastases have reported either increased overall survival in patients receiving radiotherapy in the morning (i.e., before 1200 h) (267, 268) or reported no difference between morning and afternoon groups (269, 270). Overall, the data examining chrono-radiotherapy as an improved treatment option is limited and equivocal. Further investigations, primarily randomized clinical trials, are necessary to determine the efficacy of chrono-radiotherapy.
Data on chronomodulated cancer treatments outside of chemotherapy and radiotherapy are extremely scarce. Only a few studies have compared morning to evening consumption of the oral tyrosine kinase inhibitor, Sunitinib, and have reported no differences in adverse events or overall survival (271, 272). However, given the demonstrated circadian timing for tolerability, pharmacokinetics, and antitumor efficacy of antiangiogenic drugs, cell cycle inhibitors, and other cancer treatments in laboratory rodents (273), this is an area desperate for clinical investigation and represents an emerging area of research within the field of chronotherapeutics.
Perspectives and future directions.
In this review we have emphasized what is known and not known about the time of day when benefits are highest and contraindications are at a nadir for medications used in the treatment of multiple conditions. We have also highlighted the lack of consideration of time of day as a biological variable in both clinical research and in current clinical practice guidelines. Taken together, however, it is apparent that time of day is critical in consideration of pharmacokinetics, pharmacodynamics, toxicity, and efficacy of medications. It is also apparent that this factor is woefully understudied and we join our colleagues in highlighting that circadian rhythms must be considered as a key biological variable in both clinical studies and practice (274, 275). As noted, we are are not recommending changing dosing regimens based on the cited studies, but suggest that clinical trials attend to and report on time of day as a key variable for effectiveness.
Acknowledgments
Funding: National Institute of Health R01NS092388; National Institute of General Medical Sciences of the National Institutes of Health 5U54GM104942-03
Footnotes
Publisher's Disclaimer: Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
COI: All authors declared no competing interests for this work.
References
- (1).Roenneberg T. & Merrow M. The circadian clock and human health. Current biology : CB 26, R432–43 (2016). [DOI] [PubMed] [Google Scholar]
- (2).Ko CH & Takahashi JS Molecular components of the mammalian circadian clock. Hum Mol Genet 15 Spec No 2, R271–7 (2006). [DOI] [PubMed] [Google Scholar]
- (3).Partch CL, Green CB & Takahashi JS Molecular architecture of the mammalian circadian clock. Trends Cell Biol 24, 90–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Moore RY & Eichler VB Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res 42, 201–6 (1972). [DOI] [PubMed] [Google Scholar]
- (5).Stephan FK & Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A 69, 1583–6 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Bedrosian TA & Nelson RJ Timing of light exposure affects mood and brain circuits. Transl Psychiatry 7, e1017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Smolensky MH & Lamberg L. The body clock guide to better health. 1st edn. (H. Holt: New York, 2000). [Google Scholar]
- (8).Smolensky MH & D’Alonzo GE Biologic rhythms and medicine. Am J Med 85, 34–46 (1988). [DOI] [PubMed] [Google Scholar]
- (9).Halberg F. et al. Transdisciplinary unifying implications of circadian findings in the 1950s. J Circadian Rhythms 1, 2 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Reinberg A. & Smolensky MH Biological rhythms and medicine : cellular, metabolic, physiopathologic, and pharmacologic aspects (Springer-Verlag: New York, 1983). [Google Scholar]
- (11).Ohdo S, Koyanagi S. & Matsunaga N. Chronopharmacological strategies focused on chrono-drug discovery. Pharmacology & therapeutics 202, 72–90 (2019). [DOI] [PubMed] [Google Scholar]
- (12).Beery AK & Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev 35, 565–72 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Pittendrigh C. Biological clocks: The functions, ancient and modern, of circadian oscillations. Science and the Sixties, 96–111 (1965). [Google Scholar]
- (14).Bruguerolle B Chronopharmacokinetics. Current status. Clin Pharmacokinet 35, 83–94 (1998). [DOI] [PubMed] [Google Scholar]
- (15).Erkekoglu P. & Baydar T. Chronopharmacodynamics of drugs in toxicological aspects: A short review for clinical pharmacists and pharmacy practitioners. J Res Pharm Pract 1, 41–7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Whelton PK et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol 71, e127–e248 (2018). [DOI] [PubMed] [Google Scholar]
- (17).Hermida RC, Ayala DE, Calvo C, Portaluppi F. & Smolensky MH Chronotherapy of hypertension: Administration-time-dependent effects of treatment on the circadian pattern of blood pressure. Adv Drug Deliv Rev 59, 923–39 (2007). [DOI] [PubMed] [Google Scholar]
- (18).Hermida RC, Ayala DE, Mojon A. & Fernandez JR Chronotherapy with nifedipine GITS in hypertensive patients: Improved efficacy and safety with bedtime dosing. Am J Hypertens 21, 948–54 (2008). [DOI] [PubMed] [Google Scholar]
- (19).Khodadoustan S, Nasri Ashrafi I, Vanaja Satheesh K, Kumar C, Hs S. & C S. Evaluation of the effect of time dependent dosing on pharmacokinetic and pharmacodynamics of amlodipine in normotensive and hypertensive human subjects. Clin Exp Hypertens 39, 520–6 (2017). [DOI] [PubMed] [Google Scholar]
- (20).White WB, Mehrotra DV, Black HR & Fakouhi TD Effects of controlled-onset extended-release verapamil on nocturnal blood pressure (dippers versus nondippers). COER-Verapamil Study Group. Am J Cardiol 80, 469–74 (1997). [DOI] [PubMed] [Google Scholar]
- (21).Gupta SK, Yih BM, Atkinson L. & Longstreth J. The effect of food, time of dosing, and body position on the pharmacokinetics and pharmacodynamics of verapamil and norverapamil. J Clin Pharmacol 35, 1083–93 (1995). [DOI] [PubMed] [Google Scholar]
- (22).Smith DH, Neutel JM & Weber MA A new chronotherapeutic oral drug absorption system for verapamil optimizes blood pressure control in the morning. Am J Hypertens 14, 14–9 (2001). [DOI] [PubMed] [Google Scholar]
- (23).Prisant LM, Weber M, Black HR Role of circadian rhythm in cardiovascular function — efficacy of a chronotherapeutic approach to controlling hypertension with Verelan PM (verapamil HCL). Today’s Therapeutic Trends 21, 201–13 (2003). [Google Scholar]
- (24).Weber MA, Prisant LM, Black HR & Messerli FH Treatment of elderly hypertensive patients with a delayed-release verapamil formulation in a community-based trial. Am J Geriatr Cardiol 13, 131–6 (2004). [DOI] [PubMed] [Google Scholar]
- (25).Umeda T. et al. Timing for administration of an antihypertensive drug in the treatment of essential hypertension. Hypertension 23, I211–4 (1994). [DOI] [PubMed] [Google Scholar]
- (26).Portaluppi F, Vergnani L, Manfredini R, degli Uberti EC & Fersini C. Time-dependent effect of isradipine on the nocturnal hypertension in chronic renal failure. Am J Hypertens 8, 719–26 (1995). [DOI] [PubMed] [Google Scholar]
- (27).Sista S, Lai JC, Eradiri O. & Albert KS Pharmacokinetics of a novel diltiazem HCl extended-release tablet formulation for evening administration. J Clin Pharmacol 43, 1149–57 (2003). [DOI] [PubMed] [Google Scholar]
- (28).Kohno I. et al. Administration-time-dependent effects of diltiazem on the 24-hour blood pressure profile of essential hypertension patients. Chronobiol Int 14, 71–84 (1997). [DOI] [PubMed] [Google Scholar]
- (29).Hermida RC, Ayala DE, Calvo C. & Lopez JE Aspirin administered at bedtime, but not on awakening, has an effect on ambulatory blood pressure in hypertensive patients. J Am Coll Cardiol 46, 975–83 (2005). [DOI] [PubMed] [Google Scholar]
- (30).Smolensky MH & Haus E. Circadian rhythms and clinical medicine with applications to hypertension. Am J Hypertens 14, 280S–90S (2001). [DOI] [PubMed] [Google Scholar]
- (31).Ruan Y. Effect of administration of low-dose aspirin before bedtime on blood pressure in hypertensive patients: A meta-analysis. Int J Cardiol 152, S85–S6 (2011). [Google Scholar]
- (32).Bonten TN et al. Time-dependent effects of aspirin on blood pressure and morning platelet reactivity: A randomized cross-over trial. Hypertension 65, 743–50 (2015). [DOI] [PubMed] [Google Scholar]
- (33).Racca C. et al. Aspirin intake in the morning is associated with suboptimal platelet inhibition, as measured by serum Thromboxane B2, during infarct-prone early-morning hours. Platelets 30, 871–7 (2019). [DOI] [PubMed] [Google Scholar]
- (34).van Diemen JJ, Fuijkschot WW, Wessels TJ, Veen G, Smulders YM & Thijs A. Evening intake of aspirin is associated with a more stable 24-h platelet inhibition compared to morning intake: A study in chronic aspirin users. Platelets 27, 351–6 (2016). [DOI] [PubMed] [Google Scholar]
- (35).Hermida RC et al. Administration-time differences in effects of hypertension medications on ambulatory blood pressure regulation. Chronobiol Int 30, 280–314 (2013). [DOI] [PubMed] [Google Scholar]
- (36).Palatini P. et al. Effect of evening versus morning benazepril on 24-hour blood pressure: a comparative study with continuous intraarterial monitoring. Int J Clin Pharmacol Ther Toxicol 31, 295–300 (1993). [PubMed] [Google Scholar]
- (37).Witte K. et al. Cardiovascular effects, pharmacokinetics, and converting enzyme inhibition of enalapril after morning versus evening administration. Clin Pharmacol Ther 54, 177–86 (1993). [DOI] [PubMed] [Google Scholar]
- (38).Morgan T, Anderson A. & Jones E. The effect on 24 h blood pressure control of an angiotensin converting enzyme inhibitor (perindopril) administered in the morning or at night. J Hypertens 15, 205–11 (1997). [DOI] [PubMed] [Google Scholar]
- (39).Palatini P. Can an angiotensin-converting enzyme inhibitor with a short half-life effectively lower blood pressure for 24 hours? Am Heart J 123, 1421–5 (1992). [DOI] [PubMed] [Google Scholar]
- (40).Palatini P, Racioppa A, Raule G, Zaninotto M, Penzo M. & Pessina AC Effect of timing of administration on the plasma ACE inhibitory activity and the antihypertensive effect of quinapril. Clin Pharmacol Ther 52, 378–83 (1992). [DOI] [PubMed] [Google Scholar]
- (41).Myburgh DP, Verho M, Botes JH, Erasmus TP & Luus HG 24-hour blood pressure control with ramipril: Comparison of once-daily morning and evening administration. Curr Ther Res 56, 1298–306 (1995). [Google Scholar]
- (42).Hermida RC & Ayala DE Chronotherapy with the angiotensin-converting enzyme inhibitor ramipril in essential hypertension: improved blood pressure control with bedtime dosing. Hypertension 54, 40–6 (2009). [DOI] [PubMed] [Google Scholar]
- (43).Hermida RC, Ayala DE, Fontao MJ, Mojon A, Alonso I. & Fernandez JR Administration-time-dependent effects of spirapril on ambulatory blood pressure in uncomplicated essential hypertension. Chronobiol Int 27, 560–74 (2010). [DOI] [PubMed] [Google Scholar]
- (44).Hermida RC, Calvo C, Ayala DE, Chayan L, Rodriguez M. & Lopez JE Chronotherapy with spirapril in hypertensive patients: Changes in the diurnal/nocturnal blood pressure ratio as a function of the circadian time of administration. J Hypertens 24, S88-S (2006). [Google Scholar]
- (45).Macchiarulo C, Pieri R, Mitolo DC & Pirrelli A. Management of antihypertensive treatment with Lisinopril: A chronotherapeutic approach. Eur Rev Med Pharmacol Sci 3, 269–75 (1999). [PubMed] [Google Scholar]
- (46).Balan H, Popescu E. & Angelescu G. Comparing different treatment schedules of Zomen (zofenopril). Rom J Intern Med 49, 75–84 (2011). [PubMed] [Google Scholar]
- (47).Kuroda T. et al. Effects of bedtime vs. morning administration of the long-acting lipophilic angiotensin-converting enzyme inhibitor trandolapril on morning blood pressure in hypertensive patients. Hypertens Res 27, 15–20 (2004). [DOI] [PubMed] [Google Scholar]
- (48).Middeke M, Kluglich M. & Holzgreve H. Chronopharmacology of captopril plus hydrochlorothiazide in hypertension: Morning versus evening dosing. Chronobiol Int 8, 506–10 (1991). [DOI] [PubMed] [Google Scholar]
- (49).Panza JA, Epstein SE & Quyyumi AA Circadian variation in vascular tone and its relation to alpha-sympathetic vasoconstrictor activity. N Engl J Med 325, 986–90 (1991). [DOI] [PubMed] [Google Scholar]
- (50).Pickering TG, Levenstein M. & Walmsley P. Nighttime dosing of doxazosin has peak effect on morning ambulatory blood pressure. Results of the HALT Study. Hypertension and Lipid Trial Study Group. Am J Hypertens 7, 844–7 (1994). [DOI] [PubMed] [Google Scholar]
- (51).Hermida RC et al. Administration-time-dependent effects of doxazosin GITS on ambulatory blood pressure of hypertensive subjects. Chronobiol Int 21, 277–96 (2004). [DOI] [PubMed] [Google Scholar]
- (52).Langner B. & Lemmer B. Circadian changes in the pharmacokinetics and cardiovascular effects of oral propranolol in healthy subjects. Eur J Clin Pharmacol 33, 619–24 (1988). [DOI] [PubMed] [Google Scholar]
- (53).Hermida RC, Calvo C, Ayala DE, Rodriguez M, Chayan L. & Lopez JE Administration time-dependent effects of nebivolol on the diurnal/nocturnal blood pressure ratio in hypertensive patients. J Hypertens 24, S89-S (2006). [Google Scholar]
- (54).Acelajado MC, Pisoni R, Dudenbostel T, Oparil S, Calhoun DA & Glasser SP Both morning and evening dosing of nebivolol reduces trough mean blood pressure surge in hypertensive patients. J Am Soc Hypertens 6, 66–72 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Hermida RC et al. Administration time-dependent effects of valsartan on ambulatory blood pressure in hypertensive subjects. Hypertension 42, 283–90 (2003). [DOI] [PubMed] [Google Scholar]
- (56).Hermida RC et al. Administration time-dependent effects of valsartan on ambulatory blood pressure in elderly hypertensive subjects. Chronobiol Int 22, 755–76 (2005). [DOI] [PubMed] [Google Scholar]
- (57).Hermida RC et al. Treatment of non-dipper hypertension with bedtime administration of valsartan. J Hypertens 23, 1913–22 (2005). [DOI] [PubMed] [Google Scholar]
- (58).Kasiakogias A. et al. Evening versus morning dosing of antihypertensive drugs in hypertensive patients with sleep apnoea: a cross-over study. J Hypertens 33, 393–400 (2015). [DOI] [PubMed] [Google Scholar]
- (59).Hermida RC, Ayala DE, Chayan L, Mojon A. & Fernandez JR Administration-time-dependent effects of olmesartan on the ambulatory blood pressure of essential hypertension patients. Chronobiol Int 26, 61–79 (2009). [DOI] [PubMed] [Google Scholar]
- (60).Tofe Povedano S. & Garcia De La Villa B. 24-hour and nighttime blood pressures in type 2 diabetic hypertensive patients following morning or evening administration of olmesartan. J Clin Hypertens (Greenwich) 11, 426–31 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Kario K. et al. Effect of dosing time of angiotensin II receptor blockade titrated by self-measured blood pressure recordings on cardiorenal protection in hypertensives: the Japan Morning Surge-Target Organ Protection (J-TOP) study. J Hypertens 28, 1574–83 (2010). [DOI] [PubMed] [Google Scholar]
- (62).Hermida RC et al. Comparison of the effects on ambulatory blood pressure of awakening versus bedtime administration of torasemide in essential hypertension. Chronobiol Int 25, 950–70 (2008). [DOI] [PubMed] [Google Scholar]
- (63).Hermida RC, Ayala DE, Mojon A, Fontao MJ & Fernandez JR Chronotherapy with valsartan/hydrochlorothiazide combination in essential hypertension: improved sleep-time blood pressure control with bedtime dosing. Chronobiol Int 28, 601–10 (2011). [DOI] [PubMed] [Google Scholar]
- (64).Zeng J. et al. Fixed-combination of amlodipine and diuretic chronotherapy in the treatment of essential hypertension: Improved blood pressure control with bedtime dosing-a multicenter, open-label randomized study. Hypertens Res 34, 767–72 (2011). [DOI] [PubMed] [Google Scholar]
- (65).Hermida RC, Ayala DE, Fontao MJ, Mojon A. & Fernandez JR Chronotherapy with valsartan/amlodipine fixed combination: Improved blood pressure control of essential hypertension with bedtime dosing. Chronobiol Int 27, 1287–303 (2010). [DOI] [PubMed] [Google Scholar]
- (66).Roush GC, Fapohunda J. & Kostis JB Evening dosing of antihypertensive therapy to reduce cardiovascular events: A third type of evidence based on a systematic review and meta-analysis of randomized trials. J Clin Hypertens (Greenwich) 16, 561–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Wang C. et al. Evening versus morning dosing regimen drug therapy for chronic kidney disease patients with hypertension in blood pressure patterns: a systematic review and meta-analysis. Intern Med J 47, 900–6 (2017). [DOI] [PubMed] [Google Scholar]
- (68).Crespo JJ et al. Administration-time-dependent effects of hypertension treatment on ambulatory blood pressure in patients with chronic kidney disease. Chronobiol Int 30, 159–75 (2013). [DOI] [PubMed] [Google Scholar]
- (69).Rahman M. et al. A trial of 2 strategies to reduce nocturnal blood pressure in blacks with chronic kidney disease. Hypertension 61, 82–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Hermida RC, Ayala DE, Mojon A. & Fernandez JR Bedtime ingestion of hypertension medications reduces the risk of new-onset type 2 diabetes: A randomised controlled trial. Diabetologia 59, 255–65 (2016). [DOI] [PubMed] [Google Scholar]
- (71).Moya A. et al. Effects of time-of-day of hypertension treatment on ambulatory blood pressure and clinical characteristics of patients with type 2 diabetes. Chronobiol Int 30, 116–31 (2013). [DOI] [PubMed] [Google Scholar]
- (72).Hermida RC, Ayala DE, Mojon A. & Fernandez JR Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care 34, 1270–6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Bowles NP, Thosar SS, Herzig MX & Shea SA Chronotherapy for hypertension. Curr Hypertens Rep 20, 97 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Chan S. & Debono M. Replication of cortisol circadian rhythm: New advances in hydrocortisone replacement therapy. Ther Adv Endocrinol Metab 1, 129–38 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Oprea A, Bonnet NCG, Polle O. & Lysy PA Novel insights into glucocorticoid replacement therapy for pediatric and adult adrenal insufficiency. Ther Adv Endocrinol Metab 10, 2042018818821294 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Bornstein SR et al. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 101, 364–89 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Speiser PW et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 103, 4043–88 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Rahvar AH, Haas CS, Danneberg S. & Harbeck B. Increased cardiovascular risk in patients with adrenal insufficiency: A short review. Biomed Res Int 2017, 3691913 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Murray RD et al. Management of glucocorticoid replacement in adrenal insufficiency shows notable heterogeneity - data from the EU-AIR. Clin Endocrinol (Oxf) 86, 340–6 (2017). [DOI] [PubMed] [Google Scholar]
- (80).Merza Z. et al. Circadian hydrocortisone infusions in patients with adrenal insufficiency and congenital adrenal hyperplasia. Clin Endocrinol (Oxf) 65, 45–50 (2006). [DOI] [PubMed] [Google Scholar]
- (81).Lovas K. & Husebye ES Continuous subcutaneous hydrocortisone infusion in Addison’s disease. Eur J Endocrinol 157, 109–12 (2007). [DOI] [PubMed] [Google Scholar]
- (82).Bjornsdottir S. et al. Circadian hormone profiles and insulin sensitivity in patients with Addison’s disease: A comparison of continuous subcutaneous hydrocortisone infusion with conventional glucocorticoid replacement therapy. Clin Endocrinol (Oxf) 83, 28–35 (2015). [DOI] [PubMed] [Google Scholar]
- (83).Stewart PM Modified-release hydrocortisone: Is it time to change clinical practice? J Endocr Soc 3, 1150–3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Geffner ME, Frank HJ, Kaplan SA, Lippe BM & Levin SR Early-morning hyperglycemia in diabetic individuals treated with continuous subcutaneous insulin infusion. Diabetes Care 6, 135–9 (1983). [DOI] [PubMed] [Google Scholar]
- (85).American Diabetes, A. Standards of medical care in diabetes-2020 abridged for primary care providers. Clin Diabetes 38, 10–38 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Garber AJ et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm - 2020 executive summary. Endocr Pract 26, 107–39 (2020). [DOI] [PubMed] [Google Scholar]
- (87).Edsberg B, Dejgaard A. & Kuhl C. Comparison of glycaemic control in diabetic patients treated with morning or evening human ultratard insulin. Diabet Med 4, 53–5 (1987). [DOI] [PubMed] [Google Scholar]
- (88).Garg SK et al. Improved glycemic control without an increase in severe hypoglycemic episodes in intensively treated patients with type 1 diabetes receiving morning, evening, or split dose insulin glargine. Diabetes Res Clin Pract 66, 49–56 (2004). [DOI] [PubMed] [Google Scholar]
- (89).Bergenstal RM et al. Comparison of insulin glargine 300 units/mL and 100 units/mL in adults with type 1 diabetes: Continuous glucose monitoring profiles and variability using morning or evening injections. Diabetes Care 40, 554–60 (2017). [DOI] [PubMed] [Google Scholar]
- (90).Hamann A, Matthaei S, Rosak C, Silvestre L. & Group HOES A randomized clinical trial comparing breakfast, dinner, or bedtime administration of insulin glargine in patients with type 1 diabetes. Diabetes Care 26, 1738–44 (2003). [DOI] [PubMed] [Google Scholar]
- (91).Home PD et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 1 diabetes: A randomized, phase 3a, open-label clinical trial (EDITION 4). Diabetes Care 38, 2217–25 (2015). [DOI] [PubMed] [Google Scholar]
- (92).Albright ES, Desmond R. & Bell DS Efficacy of conversion from bedtime NPH insulin injection to once- or twice-daily injections of insulin glargine in type 1 diabetic patients using basal/bolus therapy. Diabetes Care 27, 632–3 (2004). [DOI] [PubMed] [Google Scholar]
- (93).Ashwell SG, Gebbie J. & Home PD Optimal timing of injection of once-daily insulin glargine in people with type 1 diabetes using insulin lispro at meal-times. Diabet Med 23, 46–52 (2006). [DOI] [PubMed] [Google Scholar]
- (94).Gradiser M, Bilic-Curcic I, Djindjic B. & Berkovic MC The effects of transition from bedtime to morning glargine administration in patients with poorly regulated type 1 diabetes mellitus: Croatian pilot study. Diabetes Ther 6, 643–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Ahmed AB, Mallias J. & Home PD Optimization of evening insulin dose in patients using the short-acting insulin analog lispro. Diabetes Care 21, 1162–6 (1998). [DOI] [PubMed] [Google Scholar]
- (96).Pieber TR, Draeger E, Kristensen A. & Grill V. Comparison of three multiple injection regimens for type 1 diabetes: Morning plus dinner or bedtime administration of insulin detemir vs. morning plus bedtime NPH insulin. Diabet Med 22, 850–7 (2005). [DOI] [PubMed] [Google Scholar]
- (97).Lunetta M, Di Mauro M. & Le Moli R. Different effects of octreotide by continuous night infusion at increasing rate or by evening injections at different times on morning hyperglycemia and growth hormone levels in insulin-dependent diabetic patients. J Endocrinol Invest 21, 454–8 (1998). [DOI] [PubMed] [Google Scholar]
- (98).Kanc K. et al. Substitution of night-time continuous subcutaneous insulin infusion therapy for bedtime NPH insulin in a multiple injection regimen improves counterregulatory hormonal responses and warning symptoms of hypoglycaemia in IDDM. Diabetologia 41, 322–9 (1998). [DOI] [PubMed] [Google Scholar]
- (99).Doyle EA, Weinzimer SA, Steffen AT, Ahern JA, Vincent M. & Tamborlane WV A randomized, prospective trial comparing the efficacy of continuous subcutaneous insulin infusion with multiple daily injections using insulin glargine. Diabetes Care 27, 1554–8 (2004). [DOI] [PubMed] [Google Scholar]
- (100).Lee YH, Lee BW, Kwon HJ, Kang ES, Cha BS & Lee HC Higher morning to evening ratio in total dose of twice-daily biphasic insulin analog might be effective in achieving glucose control in patients with poorly controlled type 2 diabetes. Diabetes Technol Ther 14, 508–14 (2012). [DOI] [PubMed] [Google Scholar]
- (101).Jung CH et al. The optimal morning:evening ratio in total dose of twice-daily biphasic insulin analogue in poorly controlled type 2 diabetes: a 24-week multicentre prospective, randomized controlled, open-labelled clinical study. Diabet Med 31, 68–75 (2014). [DOI] [PubMed] [Google Scholar]
- (102).Standl E, Maxeiner S, Raptis S. & Group HOES Once-daily insulin glargine administration in the morning compared to bedtime in combination with morning glimepiride in patients with type 2 diabetes: an assessment of treatment flexibility. Horm Metab Res 38, 172–7 (2006). [DOI] [PubMed] [Google Scholar]
- (103).Porcellati F. et al. Pharmacokinetics and pharmacodynamics of insulin glargine given in the evening as compared with in the morning in type 2 diabetes. Diabetes Care 38, 503–12 (2015). [DOI] [PubMed] [Google Scholar]
- (104).Ahren B, Leguizamo Dimas A, Miossec P, Saubadu S. & Aronson R. Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M). Diabetes Care 36, 2543–50 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Schmitz O, Lund S, Andersen PH, Jonler M. & Porksen N. Optimizing insulin secretagogue therapy in patients with type 2 diabetes: A randomized double-blind study with repaglinide. Diabetes Care 25, 342–6 (2002). [DOI] [PubMed] [Google Scholar]
- (106).He YL et al. Hormonal and metabolic effects of morning or evening dosing of the dipeptidyl peptidase IV inhibitor vildagliptin in patients with type 2 diabetes. Br J Clin Pharmacol 70, 34–42 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Nachum Z, Ben-Shlomo I, Weiner E. & Shalev E. Twice daily versus four times daily insulin dose regimens for diabetes in pregnancy: Randomised controlled trial. BMJ 319, 1223–7 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Saleem N, Godman B. & Hussain S. Comparing twice- versus four-times daily insulin in mothers with gestational diabetes in Pakistan and its implications. J Comp Eff Res 5, 453–9 (2016). [DOI] [PubMed] [Google Scholar]
- (109).Ala S, Akha O, Kashi Z, Asgari H, Bahar A. & Sasanpour N. Dose administration time from before breakfast to before dinner affect thyroid hormone levels? Caspian J Intern Med 6, 134–40 (2015). [PMC free article] [PubMed] [Google Scholar]
- (110).Bolk N, Visser TJ, Kalsbeek A, van Domburg RT & Berghout A. Effects of evening vs morning thyroxine ingestion on serum thyroid hormone profiles in hypothyroid patients. Clin Endocrinol (Oxf) 66, 43–8 (2007). [DOI] [PubMed] [Google Scholar]
- (111).Bolk N, Visser TJ, Nijman J, Jongste IJ, Tijssen JG & Berghout A. Effects of evening vs morning levothyroxine intake: a randomized double-blind crossover trial. Arch Intern Med 170, 1996–2003 (2010). [DOI] [PubMed] [Google Scholar]
- (112).Banerjee M, Hossain S, Mondal S. & Maiti A. A comparative study on effect of evening versus morning intake of levothyroxine in patients of hypothyroidism. Thyroid Res Pract 15, 89–93 (2018). [Google Scholar]
- (113).Rajput R, Chatterjee S. & Rajput M. Can levothyroxine be taken as evening dose? Comparative evaluation of morning versus evening dose of levothyroxine in treatment of hypothyroidism. J Thyroid Res 2011, 505239 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Akin O. Morning vs. bedtime levothyroxine administration: What is the ideal choice for children? J Pediatr Endocrinol Metab 31, 1249–55 (2018). [DOI] [PubMed] [Google Scholar]
- (115).Giassi K, Piccoli V, da Costa Rodrigues T. & Gorga Bandeira de Mello R. Evaluation of evening versus morning levothyroxine intake in elderly (MONIALE). Trials 20, 742 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Garufi LC, Jabbour K, Hansen SE, Stello B. & Petre KA Morning vs. evening administration of levothyroxine. Am Fam Physician 98, 532–4 (2018). [Google Scholar]
- (117).Tsuruoka S, Wakaumi M, Sugimoto K, Saito T. & Fujimura A. Chronotherapy of high-dose active vitamin D3 in haemodialysis patients with secondary hyperparathyroidsm: A repeated dosing study. Br J Clin Pharmacol 55, 531–7 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Greenspan SL, Dresner-Pollak R, Parker RA, London D. & Ferguson L. Diurnal variation of bone mineral turnover in elderly men and women. Calcif Tissue Int 60, 419–23 (1997). [DOI] [PubMed] [Google Scholar]
- (119).Schlemmer A, Hassager C, Jensen SB & Christiansen C. Marked diurnal variation in urinary excretion of pyridinium cross-links in premenopausal women. J Clin Endocrinol Metab 74, 476–80 (1992). [DOI] [PubMed] [Google Scholar]
- (120).Song C. et al. Insights into the role of circadian rhythms in bone metabolism: A promising intervention target? Biomed Res Int 2018, 9156478 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Swanson C. et al. 24-hour profile of serum sclerostin and its association with bone biomarkers in men. Osteoporos Int 28, 3205–13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Pellegrini GG et al. Salivary bone turnover markers in healthy pre- and postmenopausal women: Daily and seasonal rhythm. Clin Oral Investig 16, 651–7 (2012). [DOI] [PubMed] [Google Scholar]
- (123).Tu KN et al. Osteoporosis: A review of treatment options. P T 43, 92–104 (2018). [PMC free article] [PubMed] [Google Scholar]
- (124).Aerssens J, Declerck K, Maeyaert B, Boonen S. & Dequeker J. The effect of modifying dietary calcium intake pattern on the circadian rhythm of bone resorption. Calcif Tissue Int 65, 34–40 (1999). [DOI] [PubMed] [Google Scholar]
- (125).Scopacasa F, Need AG, Horowitz M, Wishart JM, Morris HA & Nordin BE Effects of dose and timing of calcium supplementation on bone resorption in early menopausal women. Horm Metab Res 34, 44–7 (2002). [DOI] [PubMed] [Google Scholar]
- (126).Scopacasa F. et al. Calcium supplementation suppresses bone resorption in early postmenopausal women. Calcif Tissue Int 62, 8–12 (1998). [DOI] [PubMed] [Google Scholar]
- (127).Karkkainen MU, Lamberg-Allardt CJ, Ahonen S. & Valimaki M. Does it make a difference how and when you take your calcium? The acute effects of calcium on calcium and bone metabolism. Am J Clin Nutr 74, 335–42 (2001). [DOI] [PubMed] [Google Scholar]
- (128).Ando H. et al. Dosing time-dependent effect of raloxifene on plasma plasminogen activator inhibitor-1 concentrations in post-menopausal women with osteoporosis. Clin Exp Pharmacol Physiol 40, 227–32 (2013). [DOI] [PubMed] [Google Scholar]
- (129).Michalska D, Luchavova M, Zikan V, Raska I Jr., Kubena AA & Stepan JJ Effects of morning vs. evening teriparatide injection on bone mineral density and bone turnover markers in postmenopausal osteoporosis. Osteoporos Int 23, 2885–91 (2012). [DOI] [PubMed] [Google Scholar]
- (130).Cook GJ, Blake GM & Fogelman I. The time of day that etidronate is ingested does not influence its therapeutic effect in osteoporosis. Scand J Rheumatol 29, 62–4 (2000). [DOI] [PubMed] [Google Scholar]
- (131).Eastell R. et al. Morning vs evening dosing of the cathepsin K inhibitor ONO-5334: Effects on bone resorption in postmenopausal women in a randomized, phase 1 trial. Osteoporos Int 27, 309–18 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Schlemmer A, Ravn P, Hassager C. & Christiansen C. Morning or evening administration of nasal calcitonin? Effects on biochemical markers of bone turnover. Bone 20, 63–7 (1997). [DOI] [PubMed] [Google Scholar]
- (133).Karsdal MA, Byrjalsen I, Riis BJ & Christiansen C. Investigation of the diurnal variation in bone resorption for optimal drug delivery and efficacy in osteoporosis with oral calcitonin. BMC Clin Pharmacol 8, 12 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Karsdal MA et al. Lessons learned from the development of oral calcitonin: the first tablet formulation of a protein in phase III clinical trials. J Clin Pharmacol 51, 460–71 (2011). [DOI] [PubMed] [Google Scholar]
- (135).Sun LM, Lin MC, Muo CH, Liang JA & Kao CH Calcitonin nasal spray and increased cancer risk: A population-based nested case-control study. J Clin Endocrinol Metab 99, 4259–64 (2014). [DOI] [PubMed] [Google Scholar]
- (136).Jorgensen JO, Moller N, Lauritzen T, Alberti KG, Orskov H. & Christiansen JS Evening versus morning injections of growth hormone (GH) in GH-deficient patients: Effects on 24-hour patterns of circulating hormones and metabolites. J Clin Endocrinol Metab 70, 207–14 (1990). [DOI] [PubMed] [Google Scholar]
- (137).Laursen T, Jorgensen JO & Christiansen JS Metabolic effects of growth hormone administered subcutaneously once or twice daily to growth hormone deficient adults. Clin Endocrinol (Oxf) 41, 337–43 (1994). [DOI] [PubMed] [Google Scholar]
- (138).Zadik Z, Lieberman E, Altman Y, Chen M, Limoni Y. & Landau H. Effect of timing of growth hormone administration on plasma growth-hormone-binding activity, insulin-like growth factor-I and growth in children with a subnormal spontaneous secretion of growth hormone. Horm Res 39, 188–91 (1993). [DOI] [PubMed] [Google Scholar]
- (139).Wolthers OD, Ramshanker N, Heuck C. & Frystyk J. The timing of administration of exogenous glucocorticoid affects 24hour growth hormone secretion in children. Growth Horm IGF Res 35, 40–4 (2017). [DOI] [PubMed] [Google Scholar]
- (140).Jessup SK, Dimaraki EV, Symons KV & Barkan AL Sexual dimorphism of growth hormone (GH) regulation in humans: endogenous GH-releasing hormone maintains basal GH in women but not in men. J Clin Endocrinol Metab 88, 4776–80 (2003). [DOI] [PubMed] [Google Scholar]
- (141).Naeraa RW, Gravholt CH, Kastrup KW, Svenstrup B. & Christiansen JS Morning versus evening administration of estradiol to girls with turner syndrome receiving growth hormone: impact on growth hormone and metabolism. A randomized placebo-controlled crossover study. Acta Paediatr 90, 526–31 (2001). [PubMed] [Google Scholar]
- (142).Pongsatha S, Chainual A. & Morakote N. Morning and evening administration of hormone therapy in perimenopausal women. Int J Gynaecol Obstet 91, 77–8 (2005). [DOI] [PubMed] [Google Scholar]
- (143).Kiriwat O. & Fotherby K. Pharmacokinetics of oral contraceptive steroids after morning or evening administration. Contraception 27, 153–60 (1983). [DOI] [PubMed] [Google Scholar]
- (144).Barnes PJ Anti-inflammatory actions of glucocorticoids: Molecular mechanisms. Clin Sci (Lond) 94, 557–72 (1998). [DOI] [PubMed] [Google Scholar]
- (145).Krieger DT, Allen W, Rizzo F. & Krieger HP Characterization of the normal temporal pattern of plasma corticosteroid levels. J Clin Endocrinol Metab 32, 266–84 (1971). [DOI] [PubMed] [Google Scholar]
- (146).Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF & Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab 33, 14–22 (1971). [DOI] [PubMed] [Google Scholar]
- (147).Scherholz ML, Schlesinger N. & Androulakis IP Chronopharmacology of glucocorticoids. Adv Drug Deliv Rev 151–152, 245–61 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Straub RH & Cutolo M. Circadian rhythms in rheumatoid arthritis: Implications for pathophysiology and therapeutic management. Arthritis Rheum 56, 399–408 (2007). [DOI] [PubMed] [Google Scholar]
- (149).Cutolo M. Circadian rhythms and rheumatoid arthritis. Joint Bone Spine 86, 327–33 (2019). [DOI] [PubMed] [Google Scholar]
- (150).De Silva M, Binder A. & Hazleman BL The timing of prednisolone dosage and its effect on morning stiffness in rheumatoid arthritis. Ann Rheum Dis 43, 790–3 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (151).Gul H NA, Salim B . Effects of timing on prednisolone on the duration of early morning stiffness, pain and disease activity score (DAS-28) in patients with rheumatoid arthritis. Pak Armed Forces Med J 67, 832–37 (2017). [Google Scholar]
- (152).Buttgereit F. et al. Efficacy of modified-release versus standard prednisone to reduce duration of morning stiffness of the joints in rheumatoid arthritis (CAPRA-1): a double-blind, randomised controlled trial. Lancet 371, 205–14 (2008). [DOI] [PubMed] [Google Scholar]
- (153).Buttgereit F. et al. Targeting pathophysiological rhythms: Prednisone chronotherapy shows sustained efficacy in rheumatoid arthritis. Ann Rheum Dis 69, 1275–80 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).To H. et al. Methotrexate chronotherapy is effective against rheumatoid arthritis. Chronobiol Int 28, 267–74 (2011). [DOI] [PubMed] [Google Scholar]
- (155).Kowanko IC, Pownall R, Knapp MS, Swannell AJ & Mahoney PG Circadian variations in the signs and symptoms of rheumatoid arthritis and in the therapeutic effectiveness of flurbiprofen at different times of day. Br J Clin Pharmacol 11, 477–84 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Levi F, Le Louarn C. & Reinberg A. Timing optimizes sustained-release indomethacin treatment of osteoarthritis. Clin Pharmacol Ther 37, 77–84 (1985). [DOI] [PubMed] [Google Scholar]
- (157).Perpoint B. et al. Dosing time optimizes sustained-release ketoprofen treatment of osteoarthritis. Chronobiol Int 11, 119–25 (1994). [DOI] [PubMed] [Google Scholar]
- (158).Singh JA et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 68, 1–25 (2016). [DOI] [PubMed] [Google Scholar]
- (159).Cutolo M. Glucocorticoids and chronotherapy in rheumatoid arthritis. RMD Open 2, e000203 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Hedstrom AK, Akerstedt T, Hillert J, Olsson T. & Alfredsson L. Shift work at young age is associated with increased risk for multiple sclerosis. Annals of neurology 70, 733–41 (2011). [DOI] [PubMed] [Google Scholar]
- (161).Lavtar P. et al. Association of circadian rhythm genes ARNTL/BMAL1 and CLOCK with multiple sclerosis. PLoS One 13, e0190601 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (162).Farez MF et al. Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell 162, 1338–52 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (163).Wipfler P. et al. Circadian rhythmicity of inflammatory serum parameters: A neglected issue in the search of biomarkers in multiple sclerosis. J Neurol 260, 221–7 (2013). [DOI] [PubMed] [Google Scholar]
- (164).Kumpfel T. et al. Time of interferon-beta 1a injection and duration of treatment affect clinical side effects and acute changes of plasma hormone and cytokine levels in multiple sclerosis patients. Mult Scler 13, 1138–45 (2007). [DOI] [PubMed] [Google Scholar]
- (165).Nadjar Y. et al. Injection of interferon-beta in the morning decreases flu-like syndrome in many patients with multiple sclerosis. Clin Neurol Neurosurg 113, 316–22 (2011). [DOI] [PubMed] [Google Scholar]
- (166).Patti F. et al. Administration of subcutaneous interferon beta 1a in the evening: Data from RELIEF study. J Neurol, (2020). [DOI] [PubMed] [Google Scholar]
- (167).Glass-Marmor L, Paperna T, Ben-Yosef Y. & Miller A. Chronotherapy using corticosteroids for multiple sclerosis relapses. J Neurol Neurosurg Psychiatry 78, 886–8 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (168).Colwell CS & Ghiani CA Potential circadian rhythms in oligodendrocytes? Working together through time. Neurochemical research 45, 591–605 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (169).Muers MF Diurnal variation in asthma. Arch Dis Child 59, 898–901 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (170).Smolensky MH, McGovern JP, Scott PH & Reinberg A. Chronobiology and asthma. II. Body-time-dependent differences in the kinetics and effects of bronchodilator medications. J Asthma 24, 91–134 (1987). [DOI] [PubMed] [Google Scholar]
- (171).Pincus DJ, Szefler SJ, Ackerson LM & Martin RJ Chronotherapy of asthma with inhaled steroids: The effect of dosage timing on drug efficacy. J Allergy Clin Immunol 95, 1172–8 (1995). [DOI] [PubMed] [Google Scholar]
- (172).Pincus DJ, Humeston TR & Martin RJ Further studies on the chronotherapy of asthma with inhaled steroids: the effect of dosage timing on drug efficacy. J Allergy Clin Immunol 100, 771–4 (1997). [DOI] [PubMed] [Google Scholar]
- (173).Zetterstrom O, Dahl R, Lindqvist A. & Olsson P. Comparable morning versus evening administration of once-daily mometasone furoate dry powder inhaler. Respir Med 102, 1406–11 (2008). [DOI] [PubMed] [Google Scholar]
- (174).Kempsford RD, Bal J, Baines A, Renaux J, Ravindranath R. & Thomas PS The efficacy of fluticasone furoate administered in the morning or evening is comparable in patients with persistent asthma. Respir Med 112, 18–24 (2016). [DOI] [PubMed] [Google Scholar]
- (175).Kempsford RD, Oliver A, Bal J, Tombs L. & Quinn D. The efficacy of once-daily fluticasone furoate/vilanterol in asthma is comparable with morning or evening dosing. Respir Med 107, 1873–80 (2013). [DOI] [PubMed] [Google Scholar]
- (176).D’Alonzo GE, Smolensky MH, Feldman S, Gnosspelius Y. & Karlsson K. Bambuterol in the treatment of asthma. A placebo-controlled comparison of once-daily morning vs evening administration. Chest 107, 406–12 (1995). [DOI] [PubMed] [Google Scholar]
- (177).D’Alonzo GE et al. Twenty-four hour lung function in adult patients with asthma. Chronoptimized theophylline therapy once-daily dosing in the evening versus conventional twice-daily dosing. Am Rev Respir Dis 142, 84–90 (1990). [DOI] [PubMed] [Google Scholar]
- (178).Smolensky MH et al. Administration-time-dependency of the pharmacokinetic behavior and therapeutic effect of a once-a-day theophylline in asthmatic children. Chronobiol Int 4, 435–47 (1987). [DOI] [PubMed] [Google Scholar]
- (179).Journey JD & Bentley TP Theophylline toxicity. In: StatPearls (Treasure Island (FL), 2020). [PubMed] [Google Scholar]
- (180).USFDA & Merck & Co., I. SINGULAIR Prescribing Information. <https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021409s036lbl.pdf https://www.merck.com/product/usa/pi_circulars/s/singulair/singulair_pi.pdf> (Accessed 08 September, 2020).
- (181).Pajaron-Fernandez M, Garcia-Rubia S, Sanchez-Solis M. & Garcia-Marcos L. Montelukast administered in the morning or evening to prevent exercise-induced bronchoconstriction in children. Pediatr Pulmonol 41, 222–7 (2006). [DOI] [PubMed] [Google Scholar]
- (182).Adelsberg J, Philip G, Menten J, Malice, m.p. & Reiss, T. Flexible dosing of montelukast for treatment of seasonal allergic rhinitis: Morning or evening. Journal of Allergy and Clinical Immunology 111, S146 (2003). [Google Scholar]
- (183).Reinberg A, Gervais P, Levi F, Smolensky M, Del Cerro L. & Ugolini C. Circadian and circannual rhythms of allergic rhinitis: An epidemiologic study involving chronobiologic methods. J Allergy Clin Immunol 81, 51–62 (1988). [DOI] [PubMed] [Google Scholar]
- (184).Nakao A, Nakamura Y. & Shibata S. The circadian clock functions as a potent regulator of allergic reaction. Allergy 70, 467–73 (2015). [DOI] [PubMed] [Google Scholar]
- (185).Smolensky MH, Lemmer B. & Reinberg AE Chronobiology and chronotherapy of allergic rhinitis and bronchial asthma. Adv Drug Deliv Rev 59, 852–82 (2007). [DOI] [PubMed] [Google Scholar]
- (186).Seidman MD et al. Clinical practice guideline: Allergic rhinitis. Otolaryngol Head Neck Surg 152, S1–43 (2015). [DOI] [PubMed] [Google Scholar]
- (187).Reinberg A, Gervais P, Ugolini C, Del Cerro L, Ricakova-Rocher A. & Nicolai A. A multicentric chronotherapeutic study of mequitazine in allergic rhinitis. Annu Rev Chronopharmacol 3, 441–4 (1985). [Google Scholar]
- (188).Haye R, Hoye K, Berg O, Frones S. & Odegard T. Morning versus evening dosing of desloratadine in seasonal allergic rhinitis: A randomized controlled study [ISRCTN23032971]. Clin Mol Allergy 3, 3 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (189).Urdaneta ER, Patel MK, Franklin KB, Tian X. & Wu MM Assessment of different cetirizine dosing strategies on seasonal allergic rhinitis symptoms: Findings of two randomized trials. Allergy Rhinol (Providence) 9, 2152656718783630 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (190).Pallares JG et al. Pseudoephedrine and circadian rhythm interaction on neuromuscular performance. Scand J Med Sci Sports 25, e603–12 (2015). [DOI] [PubMed] [Google Scholar]
- (191).Prayle AP et al. The pharmacokinetics and toxicity of morning vs. evening tobramycin dosing for pulmonary exacerbations of cystic fibrosis: A randomised comparison. J Cyst Fibros 15, 510–7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (192).van Maarseveen EM, van der Meer R, Neef C, Heijerman HGM & Touw DJ Does circadian rhythm affect the pharmacokinetics of once-daily tobramycin in adults with cystic fibrosis? Ther Drug Monit, (2020). [DOI] [PubMed] [Google Scholar]
- (193).Fishbein AB et al. Nocturnal eczema: Review of sleep and circadian rhythms in children with atopic dermatitis and future research directions. J Allergy Clin Immunol 136, 1170–7 (2015). [DOI] [PubMed] [Google Scholar]
- (194).Li WQ, Qureshi AA, Schernhammer ES & Han J. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol 133, 565–7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (195).Pershing LK, Corlett JL, Lambert LD & Poncelet CE Circadian activity of topical 0.05% betamethasone dipropionate in human skin in vivo. J Invest Dermatol 102, 734–9 (1994). [DOI] [PubMed] [Google Scholar]
- (196).Nguyen S, Lacour JP & Passeron T. Topical corticosteroids application in the evening is more effective than in the morning in psoriasis: in reply. J Eur Acad Dermatol Venereol 31, e406–e7 (2017). [DOI] [PubMed] [Google Scholar]
- (197).Downton P, Early JO & Gibbs JE Circadian rhythms in adaptive immunity. Immunology, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (198).Phillips AC, Gallagher S, Carroll D. & Drayson M. Preliminary evidence that morning vaccination is associated with an enhanced antibody response in men. Psychophysiology 45, 663–6 (2008). [DOI] [PubMed] [Google Scholar]
- (199).Long JE, Drayson MT, Taylor AE, Toellner KM, Lord JM & Phillips AC Morning vaccination enhances antibody response over afternoon vaccination: A cluster-randomised trial. Vaccine 34, 2679–85 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (200).Kirby T. Influenza vaccination in the morning improves response. Lancet Respir Med 4, 435 (2016). [DOI] [PubMed] [Google Scholar]
- (201).Long JE, Drayson MT, Taylor AE, Toellner KM, Lord JM & Phillips AC Corrigendum to ‘Morning vaccination enhances antibody response over afternoon vaccination: A cluster-randomised trial’ [Vaccine 34 (2016) 2679–2685]. Vaccine 34, 4842 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (202).Kurupati RK et al. The effect of timing of influenza vaccination and sample collection on antibody titers and responses in the aged. Vaccine 35, 3700–8 (2017). [DOI] [PubMed] [Google Scholar]
- (203).Lange T, Dimitrov S, Bollinger T, Diekelmann S. & Born J. Sleep after vaccination boosts immunological memory. J Immunol 187, 283–90 (2011). [DOI] [PubMed] [Google Scholar]
- (204).Grundy SM et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol 73, e285–e350 (2019). [DOI] [PubMed] [Google Scholar]
- (205).Jones PJ & Schoeller DA Evidence for diurnal periodicity in human cholesterol synthesis. J Lipid Res 31, 667–73 (1990). [PubMed] [Google Scholar]
- (206).Winter C. & Soehnlein O. The potential of chronopharmacology for treatment of atherosclerosis. Curr Opin Lipidol 29, 368–74 (2018). [DOI] [PubMed] [Google Scholar]
- (207).Lennernas H. & Fager G. Pharmacodynamics and pharmacokinetics of the HMG-CoA reductase inhibitors. Similarities and differences. Clin Pharmacokinet 32, 403–25 (1997). [DOI] [PubMed] [Google Scholar]
- (208).Saito Y, Yoshida S, Nakaya N, Hata Y. & Goto Y. Comparison between morning and evening doses of simvastatin in hyperlipidemic subjects. A double-blind comparative study. Arterioscler Thromb 11, 816–26 (1991). [DOI] [PubMed] [Google Scholar]
- (209).Wallace A, Chinn D. & Rubin G. Taking simvastatin in the morning compared with in the evening: Randomised controlled trial. BMJ 327, 788 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (210).Kim SH et al. Efficacy and safety of morning versus evening dose of controlled-release simvastatin tablets in patients with hyperlipidemia: a randomized, double-blind, multicenter phase III trial. Clin Ther 35, 1350–60 e1 (2013). [DOI] [PubMed] [Google Scholar]
- (211).Yi YJ et al. Comparison of the efficacy and safety profile of morning administration of controlled-release simvastatin versus evening administration of immediate-release simvastatin in chronic kidney disease patients with dyslipidemia. Clin Ther 36, 1182–90 (2014). [DOI] [PubMed] [Google Scholar]
- (212).Scharnagl H, Vogel M, Abletshauser C, Freisinger F, Stojakovic T. & Marz W. Efficacy and safety of fluvastatin-extended release in hypercholesterolemic patients: Morning administration is equivalent to evening administration. Cardiology 106, 241–8 (2006). [DOI] [PubMed] [Google Scholar]
- (213).Illingworth DR Comparative efficacy of once versus twice daily mevinolin in the therapy of familial hypercholesterolemia. Clin Pharmacol Ther 40, 338–43 (1986). [DOI] [PubMed] [Google Scholar]
- (214).Hunninghake DB et al. Efficacy and safety of pravastatin in patients with primary hypercholesterolemia. II. Once-daily versus twice-daily dosing. Atherosclerosis 85, 219–27 (1990). [DOI] [PubMed] [Google Scholar]
- (215).Plakogiannis R, Cohen H. & Taft D. Effects of morning versus evening administration of atorvastatin in patients with hyperlipidemia. Am J Health Syst Pharm 62, 2491–4 (2005). [DOI] [PubMed] [Google Scholar]
- (216).Cilla DD Jr., Gibson DM, Whitfield LR & Sedman AJ Pharmacodynamic effects and pharmacokinetics of atorvastatin after administration to normocholesterolemic subjects in the morning and evening. J Clin Pharmacol 36, 604–9 (1996). [DOI] [PubMed] [Google Scholar]
- (217).Martin PD, Mitchell PD & Schneck DW Pharmacodynamic effects and pharmacokinetics of a new HMG-CoA reductase inhibitor, rosuvastatin, after morning or evening administration in healthy volunteers. Br J Clin Pharmacol 54, 472–7 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (218).Plakogiannis R. & Cohen H. Optimal low-density lipoprotein cholesterol lowering--morning versus evening statin administration. Ann Pharmacother 41, 106–10 (2007). [DOI] [PubMed] [Google Scholar]
- (219).Awad K. & Banach M. The optimal time of day for statin administration: A review of current evidence. Curr Opin Lipidol 29, 340–5 (2018). [DOI] [PubMed] [Google Scholar]
- (220).Reiss GR, Lee DA, Topper JE & Brubaker RF Aqueous humor flow during sleep. Invest Ophthalmol Vis Sci 25, 776–8 (1984). [PubMed] [Google Scholar]
- (221).Liu JH, Bouligny RP, Kripke DF & Weinreb RN Nocturnal elevation of intraocular pressure is detectable in the sitting position. Invest Ophthalmol Vis Sci 44, 4439–42 (2003). [DOI] [PubMed] [Google Scholar]
- (222).Mosaed S, Liu JH & Weinreb RN Correlation between office and peak nocturnal intraocular pressures in healthy subjects and glaucoma patients. Am J Ophthalmol 139, 320–4 (2005). [DOI] [PubMed] [Google Scholar]
- (223).Huang J, Katalinic P, Kalloniatis M, Hennessy MP & Zangerl B. Diurnal intraocular pressure fluctuations with self-tonometry in glaucoma patients and suspects: A clinical trial. Optom Vis Sci 95, 88–95 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (224).Ford BA, Gooi M, Carlsson A. & Crichton AC Morning dosing of once-daily glaucoma medication is more convenient and may lead to greater adherence than evening dosing. J Glaucoma 22, 1–4 (2013). [DOI] [PubMed] [Google Scholar]
- (225).Konstas AG et al. Intraocular pressure control over 24 hours using travoprost and timolol fixed combination administered in the morning or evening in primary open-angle and exfoliative glaucoma. Acta Ophthalmol 87, 71–6 (2009). [DOI] [PubMed] [Google Scholar]
- (226).Konstas AG et al. Preservative-free tafluprost/timolol fixed combination: Comparative 24-h efficacy administered morning or evening in open-angle glaucoma patients. Expert Opin Pharmacother 19, 1981–8 (2018). [DOI] [PubMed] [Google Scholar]
- (227).Levi F. Circadian chronotherapy for human cancers. Lancet Oncol 2, 307–15 (2001). [DOI] [PubMed] [Google Scholar]
- (228).Focan C. Sequential chemotherapy and circadian rhythm in human solid tumours. A randomised trial. Cancer Chemother Pharmacol 3, 197–202 (1979). [DOI] [PubMed] [Google Scholar]
- (229).Levi FA et al. Reduction of cis-diamminedichloroplatinum nephrotoxicity in rats by optimal circadian drug timing. Cancer Res 42, 950–5 (1982). [PubMed] [Google Scholar]
- (230).Hrushesky WJ Circadian timing of cancer chemotherapy. Science 228, 73–5 (1985). [DOI] [PubMed] [Google Scholar]
- (231).Hrushesky WJ & Bjarnason GA Circadian cancer therapy. J Clin Oncol 11, 1403–17 (1993). [DOI] [PubMed] [Google Scholar]
- (232).Bjarnason GA Chronobiology. Implications for cancer chemotherapy. Acta Oncol 34, 615–24 (1995). [DOI] [PubMed] [Google Scholar]
- (233).Innominato PF, Levi FA & Bjarnason GA Chronotherapy and the molecular clock: Clinical implications in oncology. Adv Drug Deliv Rev 62, 979–1001 (2010). [DOI] [PubMed] [Google Scholar]
- (234).Levi F. et al. Chemotherapy of advanced ovarian cancer with 4’-O-tetrahydropyranyl doxorubicin and cisplatin: a randomized phase II trial with an evaluation of circadian timing and dose-intensity. J Clin Oncol 8, 705–14 (1990). [DOI] [PubMed] [Google Scholar]
- (235).Li J, Chen R, Ji M, Zou SL & Zhu LN Cisplatin-based chronotherapy for advanced non-small cell lung cancer patients: a randomized controlled study and its pharmacokinetics analysis. Cancer Chemother Pharmacol 76, 651–5 (2015). [DOI] [PubMed] [Google Scholar]
- (236).Focan C. Circadian rhythms and cancer chemotherapy. Pharmacology & therapeutics 67, 1–52 (1995). [DOI] [PubMed] [Google Scholar]
- (237).Krakowski I. et al. Dose intensity of etoposide (Vp16) - cisplatin (CDDP) depends upon dosing time. P Am Assoc Canc Res 29, 195– (1988). [Google Scholar]
- (238).Krakowski I. et al. Etoposide and cisplatin in advanced solid tumors: Results of a study of chronotolerance. Annu Rev Chronopharmacol 5, 373–76 (1988). [Google Scholar]
- (239).von Roemeling R. & Hrushesky WJ Circadian patterning of continuous floxuridine infusion reduces toxicity and allows higher dose intensity in patients with widespread cancer. J Clin Oncol 7, 1710–9 (1989). [DOI] [PubMed] [Google Scholar]
- (240).Levi FA et al. Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (leucovorin) in patients with colorectal cancer metastases: a randomized multi-institutional trial. J Natl Cancer Inst 86, 1608–17 (1994). [DOI] [PubMed] [Google Scholar]
- (241).Levi F, Zidani R. & Misset JL Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet 350, 681–6 (1997). [DOI] [PubMed] [Google Scholar]
- (242).Levi FA et al. Chronomodulated (Chrono) vs constant (Cst) rate infusional 5-fluorouracil (FU) with or without cisplatin (CDOP) in patients with advanced or metastatic pancreatic cancer. A multicenter randomized trial of the Chronotherapy Group of the European Organisation for Research and Treatment of Cancer (EORTC 05962). J Clin Oncol 22, 342s-s (2004). [Google Scholar]
- (243).Price TJ et al. Phase III study of mitomycin-C with protracted venous infusion or circadian-timed infusion of 5-fluorouracil in advanced colorectal carcinoma. Clin Colorectal Cancer 3, 235–42 (2004). [DOI] [PubMed] [Google Scholar]
- (244).Garufi C. et al. Randomised phase II study of standard versus chronomodulated CPT-11 plus chronomodulated 5-fluorouracil and folinic acid in advanced colorectal cancer patients. Eur J Cancer 42, 608–16 (2006). [DOI] [PubMed] [Google Scholar]
- (245).Ramanathan RK et al. A four-arm, randomized, multicenter phase II trial of oxaliplatin combined with varying schedules of 5-fluorouracil as first-line therapy in previously untreated advanced colorectal cancer. Clin Colorectal Cancer 7, 134–9 (2008). [DOI] [PubMed] [Google Scholar]
- (246).Huang Y. et al. Efficacy and safety of chronomodulated chemotherapy for patients with metastatic colorectal cancer: A systematic review and meta-analysis. Asia Pac J Clin Oncol 13, e171–e8 (2017). [DOI] [PubMed] [Google Scholar]
- (247).Giacchetti S. et al. Phase III trial comparing 4-day chronomodulated therapy versus 2-day conventional delivery of fluorouracil, leucovorin, and oxaliplatin as first-line chemotherapy of metastatic colorectal cancer: the European Organisation for Research and Treatment of Cancer Chronotherapy Group. J Clin Oncol 24, 3562–9 (2006). [DOI] [PubMed] [Google Scholar]
- (248).Giacchetti S. et al. Sex moderates circadian chemotherapy effects on survival of patients with metastatic colorectal cancer: A meta-analysis. Ann Oncol 23, 3110–6 (2012). [DOI] [PubMed] [Google Scholar]
- (249).Levi F. et al. Meta-analysis of gender effect for first-line chronomodulated 5-fluorouracil-leucovorin-oxaliplatin (ChronoFLO) compared with FOLFOX or constant infusion (conventional delivery, CONV) against metastatic colorectal cancer (MCC) in three international controlled phase III randomized trials (RT). J Clin Oncol 27, (2009). [Google Scholar]
- (250).Mao Z, Jin F, Weili WU, Yuanyuan LI, Long J, Gong X, Bo QU . Clinical study of chrono-chemotherapy in treating nasopharyngeal carcinoma patients with distant metastasis at preliminary diagnosis. . Chinese J Clin Oncol 14, 709–15 (2015). [Google Scholar]
- (251).Bi T. et al. Phase II clinical trial of two different modes of administration of the induction chemotherapy for locally advanced nasopharyngeal carcinoma. Zhonghua Zhong Liu Za Zhi 37, 676–81 (2015). [PubMed] [Google Scholar]
- (252).Liao J, Huang L, Wang X, Guo Z, Jianming YE, & Fuping TU . Clinical study of docetaxel and cisplatin chrono-chemotherapy in locally advanced nasopharyngeal carcinoma. Chinese J Biochem Pharmaceut 36, 51–4 (2016). [Google Scholar]
- (253).Gou XX et al. Induction chronomodulated chemotherapy plus radiotherapy for nasopharyngeal carcinoma: A Phase II prospective randomized study. J Cancer Res Ther 14, 1613–9 (2018). [DOI] [PubMed] [Google Scholar]
- (254).Wan S, Jin F, Weili WU, Yuanyuan LI, Long J, Chen G, Fang YU An analysis on the combination of chrono-chemotherapy with different speed rate and concomitant intensity-modulated radiotherapy in the treatment of locally advanced nasopharyngeal carcinoma. Chinese J Radiol Med Protect 38, 278–84 (2018). [Google Scholar]
- (255).Zhang PX et al. A randomized phase II trial of induction chemotherapy followed by cisplatin chronotherapy versus constant rate delivery combined with radiotherapy. Chronobiol Int 35, 240–8 (2018). [DOI] [PubMed] [Google Scholar]
- (256).Rivard GE, Infante-Rivard C, Dresse MF, Leclerc JM & Champagne J. Circadian time-dependent response of childhood lymphoblastic leukemia to chemotherapy: A long-term follow-up study of survival. Chronobiol Int 10, 201–4 (1993). [DOI] [PubMed] [Google Scholar]
- (257).Schmiegelow K, Glomstein A, Kristinsson J, Salmi T, Schroder H. & Bjork O. Impact of morning versus evening schedule for oral methotrexate and 6-mercaptopurine on relapse risk for children with acute lymphoblastic leukemia. Nordic Society for Pediatric Hematology and Oncology (NOPHO). J Pediatr Hematol Oncol 19, 102–9 (1997). [DOI] [PubMed] [Google Scholar]
- (258).Qvortrup C. et al. A randomized study comparing short-time infusion of oxaliplatin in combination with capecitabine XELOX(30) and chronomodulated XELOX(30) as first-line therapy in patients with advanced colorectal cancer. Ann Oncol 21, 87–91 (2010). [DOI] [PubMed] [Google Scholar]
- (259).Harper E. & Talbot CJ Is it time to change radiotherapy: The dawning of chronoradiotherapy? Clin Oncol (R Coll Radiol) 31, 326–35 (2019). [DOI] [PubMed] [Google Scholar]
- (260).Johnson K. et al. Genetic variants predict optimal timing of radiotherapy to reduce side-effects in breast cancer patients. Clin Oncol (R Coll Radiol) 31, 9–16 (2019). [DOI] [PubMed] [Google Scholar]
- (261).Noh JM et al. Comparison of acute skin reaction following morning versus late afternoon radiotherapy in patients with breast cancer who have undergone curative surgical resection. J Radiat Res 55, 553–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (262).Shukla P. et al. Circadian variation in radiation-induced intestinal mucositis in patients with cervical carcinoma. Cancer 116, 2031–5 (2010). [DOI] [PubMed] [Google Scholar]
- (263).Chang L. et al. Research on radiotherapy at different times of the day for inoperable cervical cancer. Int J Clin Pharmacol Ther 54, 856–64 (2016). [DOI] [PubMed] [Google Scholar]
- (264).Bjarnason GA et al. Comparison of toxicity associated with early morning versus late afternoon radiotherapy in patients with head-and-neck cancer: a prospective randomized trial of the National Cancer Institute of Canada Clinical Trials Group (HN3). Int J Radiat Oncol Biol Phys 73, 166–72 (2009). [DOI] [PubMed] [Google Scholar]
- (265).Goyal M. et al. Oral mucositis in morning vs. evening irradiated patients: A randomised prospective study. Int J Radiat Biol 85, 504–9 (2009). [DOI] [PubMed] [Google Scholar]
- (266).Squire T. et al. Does chronomodulated radiotherapy improve pathological response in locally advanced rectal cancer? Chronobiol Int 34, 492–503 (2017). [DOI] [PubMed] [Google Scholar]
- (267).Rahn DA 3rd et al. Gamma knife radiosurgery for brain metastasis of nonsmall cell lung cancer: Is there a difference in outcome between morning and afternoon treatment? Cancer 117, 414–20 (2011). [DOI] [PubMed] [Google Scholar]
- (268).Badiyan SN et al. Impact of time of day on outcomes after stereotactic radiosurgery for non-small cell lung cancer brain metastases. Cancer 119, 3563–9 (2013). [DOI] [PubMed] [Google Scholar]
- (269).Kabolizadeh P, Wegner R, Bernard M, Heron D, Mintz A. & Burton S. The effect of treatment time on outcome in non-small cell lung cancer brain metastases treated with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 81, S301-S (2011). [Google Scholar]
- (270).Chan S. et al. Could time of whole brain radiotherapy delivery impact overall survival in patients with multiple brain metastases? Ann Palliat Med 5, 267–79 (2016). [DOI] [PubMed] [Google Scholar]
- (271).Escudier B. et al. Phase II study of sunitinib administered in a continuous once-daily dosing regimen in patients with cytokine-refractory metastatic renal cell carcinoma. J Clin Oncol 27, 4068–75 (2009). [DOI] [PubMed] [Google Scholar]
- (272).George S. et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer 45, 1959–68 (2009). [DOI] [PubMed] [Google Scholar]
- (273).Levi F, Okyar A, Dulong S, Innominato PF & Clairambault J. Circadian timing in cancer treatments. Annu Rev Pharmacol Toxicol 50, 377–421 (2010). [DOI] [PubMed] [Google Scholar]
- (274).Ruben MD, Smith DF, FitzGerald GA & Hogenesch JB Dosing time matters. Science 365, 547–9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (275).Cederroth CR et al. Medicine in the fourth dimension. Cell Metab 30, 238–50 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]