Abstract
Background
Coagadex is a high‐purity plasma‐derived factor X concentrate (pdFX) developed to treat hereditary factor X deficiency (FXD).
Objective
Evaluate the efficacy and safety of pdFX administered to patients with hereditary FXD.
Methods
This was an open‐label, multicenter, retrospective analysis of patients receiving pdFX for compassionate use. Efficacy end points included treatments administered, the number and treatment of bleeds, and investigator assessments. Adverse drug reactions (ADRs) were monitored.
Results
Fifteen patients were included: seven received routine prophylaxis, seven received on‐demand treatment, and one alternated. Most were aged ≥12 years (n = 13) and had severe hereditary FXD (n = 12). The median follow‐up time was 19.2 months (range, 3.5‐48.8). The number of infusions per patient per month was higher for the routine prophylaxis group (median [range], 5.4 [1.4‐10.1]) than for the on‐demand group (0.8 [0.1‐2.3]), as was the dose per infusion (27.9 [21.9‐53.6] IU/kg vs 20.0 [13.6‐27.7] IU/kg). Patients experienced 88 bleeds (34 minor, 7 major, 47 unclassified). The monthly bleed rate per patient was 0.04 in the routine prophylaxis group (based on 17 bleeds in four patients) and 0.8 in the on‐demand group (based on 71 bleeds in eight patients). pdFX was used to treat 79 bleeds and was rated effective in all instances. In an overall assessment, investigators rated pdFX as excellent for 14 patients (93.3%) and good for 1 patient (6.3%). No ADRs or safety concerns were reported.
Conclusions
This analysis supports the use of pdFX as a safe, effective treatment for hereditary FXD. Routine prophylaxis with pdFX may reduce bleed frequency.
Keywords: blood coagulation disorders and inherited, compassionate use trials, factor X deficiency, retrospective studies, treatment outcome
Essentials.
Plasma‐derived factor X (pdFX) is used to treat hereditary factor X deficiency.
This was an open‐label, retrospective analysis of compassionate use of pdFX in 15 patients.
Overall, investigators rated pdFX as excellent for 14 patients and good for 1 patient.
No adverse drug reactions or safety concerns were reported.
1. INTRODUCTION
Hereditary factor X deficiency (FXD) is a rare autosomal recessive bleeding disorder that affects approximately 1:1 000 000 individuals worldwide. 1 , 2 Bleeding patterns are similar to those seen in hemophilia A and B and may involve menorrhagia for female patients 1 or bleeds in the joints, muscles, or mucous membranes for both male and female patients. 1 , 3 FXD severity can vary and is classified by the endogenous level of factor X (FX) in the plasma; at the time of this analysis, patients with FX activity (FX:C) of 6 to 10, 1 to 5, and <1 U/dL were considered mildly, moderately, and severely affected, respectively, 4 though changes to this rating system have been suggested. 5
Though treatment guidelines now recommend using single‐factor concentrate whenever possible for rare bleeding disorder management, 6 , 7 patients with hereditary FXD experiencing bleeds have historically been treated with fresh frozen plasma (FFP) and prothrombin complex concentrates (PCCs). 8 These treatment options are not specific to FXD, and levels of some factors in PCCs may be inconsistent. 9 The volume of FFP necessary to treat a bleed may lead to blood volume overload and the infusion of unnecessary factors with either treatment may increase the risks of thrombosis and/or anaphylaxis. 1 , 4 , 9
Recently, a high‐purity plasma‐derived FX concentrate (pdFX; Coagadex, Bio Products Laboratory, Elstree, UK), manufactured from plasma obtained from healthy US donors who have passed viral screening tests, was developed and licensed for use in the United States, the United Kingdom, and the European Union. In clinical trials, pdFX demonstrated efficacy when used for short‐term prophylaxis, on‐demand treatment, 10 and surgical coverage 11 among adults with hereditary FXD, as well as when used for prophylactic and on‐demand treatment in children aged <12 years. 12 No adverse events considered related to pdFX treatment and no inhibitor development were observed in these clinical trials. 10 , 11 , 12 Many of the patients included in the previous clinical trials also received pdFX on a compassionate‐use basis. Data from these patients can provide additional information on pdFX use in real‐world settings, including data on efficacy during various treatment regimens and safety during long‐term use (up to 4 years). In this retrospective analysis, the efficacy and safety of pdFX was further evaluated in patients with hereditary FXD. pdFX was administered on a compassionate‐use basis as part of routine prophylaxis, on‐demand treatment, short‐term prevention, or a perisurgical coverage regimen.
2. MATERIALS AND METHODS
2.1. Study design
This was an open‐label, multicenter, international, retrospective analysis of the use of pdFX given on a compassionate‐use basis from March 30, 2011, through December 31, 2015. Data were collected retrospectively from 12 sites across five countries: Germany, Spain, Turkey, the United Kingdom, and the United States.
Patients were eligible if they had hereditary FXD, regardless of age or FXD severity, and had received pdFX on a compassionate‐use basis. The study protocol was approved by the appropriate independent ethics committees, and all adult patients provided written informed consent. For minors, written informed consent was provided by a parent or guardian and, where appropriate, assent was provided by the child.
2.2. Treatments
pdFX is a lyophilized powder that comes in single‐use vials of approximately 250 or 500 IU. After reconstitution in sterile water, the resulting concentration is approximately 100 IU/mL and is administered via intravenous infusion at a rate of 10 mL/min (20 mL/min maximum). The potency of FX activity in pdFX concentrate, measured in IU, is determined using an in vitro chromogenic assay and an FX concentrate reference standard calibrated against the World Health Organization Third International Standard for Blood Coagulation Factors II and X, Concentrate.
Patients received pdFX as routine prophylaxis, on‐demand treatment, short‐term prevention, and/or perisurgical hemostatic cover. Routine prophylaxis was defined as a dose of ≥25 IU/kg at least once weekly for patients aged ≥12 years. Short‐term prevention was defined as less frequent dosing or doses <25 IU/kg per infusion. Across all groups, however, the specific dosing regimen was left to the discretion of the investigator and individually tailored to the patient.
2.3. Assessments
Primary efficacy analyses were completed on a per‐subject basis. For routine prophylaxis, the average number of bleeds per year and per month (including severity, location, and cause) and the total dose per year and per month were evaluated. For patients receiving on‐demand treatment, investigators provided a retrospective assessment of pdFX efficacy (effective, not effective, or unknown) in treating each bleed, as well as the dose of pdFX used. “Effective” was defined as achieving hemostasis with the expected number of pdFX doses for the severity of the bleed. Investigators also provided a retrospective assessment of the overall efficacy (excellent, good, poor, or unassessable) of pdFX. Efficacy was rated as “excellent” (regularly met or exceeded expectations), “good” (less than expected but still adequate), “poor” (did not provide satisfactory hemostasis), or “unassessable” (could not be assessed, eg, no bleeds requiring pdFX occurred during the analysis period or another replacement therapy was given for all bleeds during the analysis period). Additional assessments included the total dose, the number of infusions, the average dose per infusion, and the number of exposure days per subject. When possible, bleed severity was classified as major or minor. Severe gastrointestinal bleeding, intracerebral hemorrhage, severe hemarthrosis, major menorrhagia, and large and/or complicated muscle hematoma were defined as major bleeds. Epistaxis, gum bleed, mild menorrhagia, and superficial hematoma were defined as minor bleeds.
Safety was assessed on the basis of the incidence of adverse drug reactions (ADRs) and serious adverse reactions. When available, FX activity (FX:C) trough levels and tolerability were assessed.
2.4. Statistical analyses
Due to inclusion criteria, the per‐protocol population (all patients who consented) and the safety/intent‐to‐treat population (all patients who received at least one dose of pdFX) were identical. Two primary subpopulations were analyzed: the routine prophylaxis population and the on‐demand treatment population. Two additional subpopulations were analyzed: the short‐term preventative treatment population and the perisurgical coverage population. (All of these patients were also counted in either the routine prophylaxis population or the on‐demand population.)
3. RESULTS
3.1. Patients
Fifteen patients who received pdFX for compassionate use agreed to be included in the analysis. Seven patients received pdFX as routine prophylaxis, seven used pdFX as on‐demand treatment, and one alternated between routine prophylaxis and on‐demand treatment. Three patients on routine prophylaxis also used pdFX for perisurgical hemostatic cover. Six patients used pdFX for short‐term preventative use, three were receiving routine prophylaxis, two were receiving on‐demand treatment, and one alternated between both. Individual patient characteristics are provided in Table 1. Most patients were aged ≥12 years (n = 13) and had severe hereditary FXD (n = 12). All 13 patients aged ≥12 years had already been administered pdFX as part of a previous clinical trial. 10 The two patients aged <12 years were administered pdFX in a subsequent pediatric clinical trial. 12 This report does not include data on exposure or outcomes that occurred during these clinical trials.
TABLE 1.
Patient characteristics
| Patient number | Age at pdFX start, y | Sex | Lowest FX:C result, IU/dL | Bleeds in the 12 months preceding the study period | Time on study, months | Treatment type(s) received in the study |
|---|---|---|---|---|---|---|
| 1 | 23 | Male | <1 | 1–5 | 16.1 | RP, STP |
| 2 | 21 | Male | <1 | >10 | 24.2 | RP |
| 3 | 32 | Female | <1 | >10 | 21.7 | RP, OD, surgery |
| 4 | 17 | Male | <1 | 1–5 | 20.1 | RP, OD, STP, surgery |
| 5 | 22 | Male | <1 | 6–10 | 28.5 | RP, OD, STP |
| 6 | 15 | Female | <5 | >10 | 36.5 | RP, STP |
| 7 | 6 | Female | 4 | 0 | 48.8 | RP |
| 8 | 1 | Male | 2 | 6–10 | 12.9 | RP, surgery |
| 9 | 37 | Male | <1 | 1–5 | 18 | OD |
| 10 | 43 | Female | <1 | 1–5 | 19.2 | OD |
| 11 | 22 | Female | <1 | >10 | 4.1 | OD |
| 12 | 17 | Female | <1 | >10 | 3.5 | OD |
| 13 | 21 | Female | <1 | >10 | 6.4 | OD |
| 14 | 13 | Male | 1 | >10 | 15.7 | OD, STP |
| 15 | 14 | Female | <1 | >10 | 28.3 | OD, STP |
Abbreviations: FX:C, factor X activity; OD, on demand; pdFX, plasma‐derived factor X concentrate; RP, routine prophylaxis; STP, short‐term prevention.
3.2. Treatments administered
The median (range) duration of compassionate use among all 15 patients was 19.2 (3.5‐48.8) months, or approximately 1.6 (0.3‐4.0) years. This amounts to 304.1 subject‐months (or 25.2 subject‐years) of exposure from 1373 infusions. Overall, the median (range) pdFX per patient per month was 55.9 (2.5‐540.2) IU/kg, with a median total amount of pdFX per patient of 1462.7 (46‐26 357) IU/kg, administered over a median of 49 (2‐492) infusions. The monthly pdFX was lower for patients aged ≥12 years (median [range], 37.5 [2.5‐155.7] IU/kg over 2.0 [0.1‐6.9] infusions) than for patients aged <12 years (median [range], 517.0 [493.9‐540.2] IU/kg over 10.1 [10.1‐10.2] infusions).
As expected, patients receiving routine prophylaxis received, on average, larger and more frequent doses of pdFX than patients in the other treatment groups (Table 2). A total of 1239 prophylactic infusions were administered to eight patients: three received infusions every 3 days, four received weekly infusions (including two patients who briefly switched to dosing every 2 days), and one received pdFX every 15 days, per individual investigator’s discretion. The 2 patients aged <12 years received a larger dose per infusion (median [range], 51.1 [48.5‐53.6] IU/kg) than the six patients aged ≥12 years (median [range], 27.2 [21.9‐29.9] IU/kg). Of the three patients who also received pdFX perisurgically, two underwent a dental procedure and required only one presurgical pdFX infusion each (27.1 and 28.5 IU/kg). The third perisurgical patient underwent an implanted port insertion and required six infusions to maintain hemostasis (two infusions on the day of the surgery totaling 72.8 IU/kg and four infusions of 48.5 IU/kg on days 1, 2, 3, and 5). All patients in the on‐demand treatment group (n = 8) experienced at least one bleed. For the six patients who received short‐term preventative treatment, the number of exposure days ranged from 1 to 6, with a median of 3.5 exposure days and a collective total of 21 exposure days. The median (range) dose per infusion was 23.2 (7.3‐57.1) IU/kg.
TABLE 2.
Summary of pdFX usage
| Characteristic |
All uses (N = 15) |
Routine prophylaxis (n = 8) |
On‐demand treatment (n = 8) |
Short‐term preventative treatment (n = 6) |
Perisurgical treatment (n = 3) |
|---|---|---|---|---|---|
| Number of infusions/subject | |||||
| Mean (SD) | 91.5 (130.1) | 154.9 (150.6) | 11.0 (7.6) | 3.5 (1.9) | 2.7 (2.9) |
| Median (range) | 49 (2‐492) | 98.5 (39‐492) | 9.5 (2‐26) | 3.5 (1‐6) | 1 (1‐6) |
| Number of infusions/subject/month | |||||
| Mean (SD) | 3.7 (3.4) | 5.6 (3.2) | 0.9 (0.7) | 0.2 (0.1) | NA |
| Median (range) | 2.1 (0.1‐10.2) | 5.4 (1.4‐10.1) | 0.8 (0.1‐2.3) | 0.2 (0.0‐0.3) | NA |
| Total dose/subject, IU/kg | |||||
| Mean (SD) | 3417.3 (6648.3) | 5985.2 (8425.1) | 237.1 (192.9) | 120.9 (121.9) | 107.5 (138.1) |
| Median (range) | 1462.7 (46‐26, 357) | 2527.2 (1069‐26, 357) | 149.1 (46‐571) | 92.8 (14.5‐342.6) | 28.5 (27.1‐267.0) |
| Dose/infusion/subject, IU/kg | |||||
| Mean (SD) | NR | 32.5 (11.8) | 20.5 (5.1) | 28.9 (17.2) | 33.4 (9.7) |
| Median (range) | NR | 27.9 (21.9‐53.6) | 20.0 (13.6‐27.7) | 23.2 (7.3‐57.1) | 28.5 (27.1‐44.5) |
| Dose/subject/month, IU/kg | |||||
| Mean (SD) | 124.6 (168.3) | 206.2 (190.4) | 18.2 (10.8) | 5.2 (4.6) | NA |
| Median (range) | 55.9 (2.5‐540.2) | 135.5 (37.5‐540.2) | 18.2 (2.5‐37.5) | 4.8 (0.63‐12.1) | NA |
| Dose/subject/year, IU/kg | |||||
| Mean (SD) | 1504.6 (2032.6) | 2490.3 (2299.1) | 220.1 (130.0) | 62.9 (55.7) | NA |
| Median (range) | 674.7 (30.6‐6522.3) | 1635.6 (453.1‐6522.3) | 220.0 (30.6‐452.2) | 58.2 (7.6‐146.2) | NA |
Abbreviations: NA, not applicable; NR, not reported; pdFX, plasma‐derived factor X concentrate; SD, standard deviation.
3.3. Bleeding episodes
Eighty‐eight bleeds, with a median (range) duration of 1 (1‐22) days, were reported by 12 patients, all of whom were ≥12 years old (Figure 1). This amounts to a median of 5.5 bleeds per patient and a monthly per‐patient bleed rate of 0.4. Of the 88 bleeds, 41 had a recorded severity classification, with 34 of 41 classified as minor. Seventy‐nine bleeds were treated with pdFX across 99 exposure days. The median (range) dose per infusion was 22.0 (13.6‐29.7) IU/kg. Investigators rated all treatments as effective. Information regarding treatments for bleeds not treated with pdFX was not recorded.
FIGURE 1.
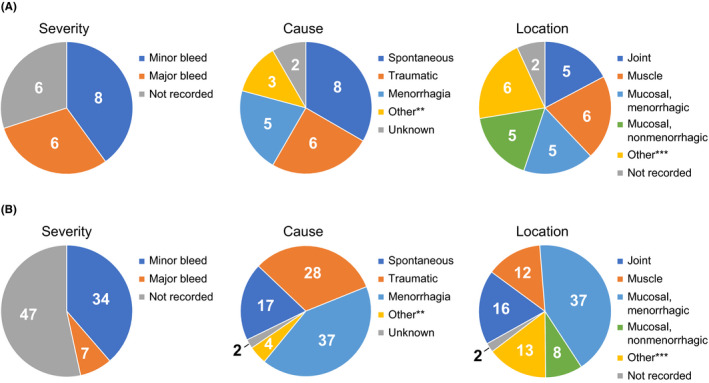
Characteristics of bleeding episodes by (A) number of subjects exhibiting the defined type of bleed (n = 12)* and (B) number of bleeds (n = 88). *Patients may have reported more than 1 type of bleed severity, cause, or location. **Included miscarriage (occurred twice in one subject), cut on right hand (occurred once in one subject), and postpartum bleed (occurred once in one subject). ***Included left leg or right leg (occurred four times in the same subject), renal hemorrhage (occurred once in one subject), soft tissue of left forearm (occurred once in one subject), subcutaneous (occurred three times in one subject), subdural hematoma and external ear (each occurred once in the same subject), and vaginal (occurred twice in one subject)
Among the eight patients in the routine prophylaxis population, 17 bleeds were reported by four patients (all aged ≥12 years) (Table 3). The overall median bleed rate per patient was 1 bleed per patient, equivalent to 0.04 bleeding episodes per patient per month. For the four patients on prophylaxis who reported bleeds, the median bleed rate was 3.5 bleeds per patient, or 0.1 bleeding episodes per patient per month. More specifically, these four patients experienced 2, 3, 4, and 8 bleeds while being treated with pdFX once per week, once every 3 days, once every 15 days, and once per week, respectively. All four patients had severe FXD, and three of the four patients experienced 1 major bleed; in the patient with 4 bleeds, all 4 were rated as minor (Table 3). Ten of the 17 total bleeds were treated with pdFX, with a median (range) dose of 28.4 (21.9‐29.7) IU/kg per infusion. Of the four patients on prophylaxis who did not experience bleeds, two were aged <12 years, one with severe FXD and one with moderate FXD; they received pdFX once every 3 days for an average of 540.1 IU/kg and 473.2 IU/kg per month, respectively. The two patients aged ≥12 years who did not bleed on prophylactic therapy had severe FXD; one was treated once a week, followed by once every 2 days and then once a week, for an overall dose of 148.5 IU/kg per month, and the other was treated once a week for 5 months, followed by a 10‐month period of on‐demand treatment and then once every 2 weeks for 7 months, for an overall dose of 37.5 IU/kg per month.
TABLE 3.
Number of bleeds recorded during compassionate use by treatment group (on‐demand or routine prophylaxis)
| Patient number | No. of bleeds | Severity | Cause | Location | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minor | Major | Not recorded | Spontaneous | Traumatic | Menorrhagia | Other | Unknown | Joint | Muscle | Mucosal/menorrhagic | Mucosal/nonmenorrhagic | Other | Unknown | ||
| Routine prophylaxis | |||||||||||||||
| 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 2 | 4 | 4 | — | — | 4 | — | — | — | — | — | — | — | 1 | 3 a | — |
| 3 | 8 | 7 | 1 | — | 2 | 6 | — | — | — | 6 | 2 | — | — | — | — |
| 4 | 2 | 1 | 1 | — | 2 | — | — | — | — | — | — | — | 1 | 1 b | — |
| 5 c | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 6 | 3 | 1 | 1 | 1 | — | — | 1 | 2 d | — | — | — | 1 | — | 2 | — |
| 7 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 8 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| On demand | |||||||||||||||
| 9 | 2 | 1 | — | 1 | 1 | 1 | — | — | — | 1 | 1 | — | — | — | — |
| 10 | 7 | 6 | 1 | — | 3 | 3 | — | — | 1 | 4 | 2 | — | 1 | — | — |
| 5 c | 6 | 5 | 1 | — | 2 | 4 | — | — | — | 1 | 1 | — | 2 | 2 e | — |
| 11 | 5 | — | — | 5 | — | — | 5 | — | — | — | — | 5 | — | — | — |
| 12 | 5 | — | — | 5 | — | — | 3 | 1 f | 1 | — | 1 | 3 | — | — | 1 |
| 13 | 9 | — | — | 9 | — | 4 | 5 | — | — | — | — | 5 | — | 4 g | — |
| 14 | 11 | 9 | 2 | — | 1 | 10 | — | — | — | 4 | 5 | — | — | 1 h | 1 |
| 15 | 26 | — | — | 26 | 2 | — | 23 | 1 i | — | — | — | 23 | 3 | — | — |
Subcutaneous for all.
Left arm soft tissue.
Patient 5 alternated between on‐demand treatment and routine prophylaxis. All 6 bleeds reported for this patient occurred during on‐demand treatment.
Miscarriages.
Subdural hematoma and external ear.
Cut on right arm.
Left or right leg for all.
Renal hemorrhage.
Postpartum.
The remaining 71 bleeds were reported by the on‐demand population (Table 3), and 69 of these bleeds were treated with pdFX. This includes 6 bleeds that occurred during approximately 10 months of on‐demand treatment for the patient who alternated between routine prophylaxis and on‐demand treatment. This patient did not experience any bleeds during approximately 12 months of routine prophylaxis. The resulting overall bleed rate for the on‐demand population was 8.9 bleeds per patient, equivalent to 0.8 bleeding episodes per patient per month. The minimum number of bleeds in any patient was 2, occurring in 1 patient and separated by 18 months, whereas the maximum number of bleeds was 26, occurring in one patient over 28.3 months. The median (range) pdFX dose per infusion was 20.0 (13.6‐27.7) IU/kg.
There were no bleeds recorded for patients receiving short‐term preventative treatment, and no excessive bleeding was reported during surgery.
3.4. Investigator assessments of efficacy
For 14 patients, investigators assessed the overall efficacy of pdFX during compassionate use as excellent, defined as “efficacy of pdFX regularly [having] met or exceeded expectations.” For the remaining patient, the investigator rated the overall efficacy of pdFX as good, defined as “efficacy of pdFX less than expected, but still adequate.”
3.5. FX activity
Predose FX:C was recorded 18 times from four patients, all of whom were receiving prophylactic treatment. Postdose FX:C was recorded eight times from three patients (Table 4). No patient had both pre‐ and postdose measurements, so incremental recovery could not be calculated. The overall median (range) predose FX:C was 6.0 (2.0‐54.0) IU/dL, with FX:C <5.0 IU/dL for 6 of the 18 predose measurements. In contrast, the median (range) postdose FX:C was 76.5 (46.0‐114.0) IU/dL, and no patient was severely deficient after dosing. Predose FX:C was higher for patients aged ≥12 years (median [range], 29.5 [5.0‐54.0] IU/dL) than for patients aged <12 years (6.0 [2.0‐40.0] IU/dL; Table 4). Likewise, postdose FX:C was higher for patients aged ≥12 years (median [range], 103.0 [92.0‐114.0] IU/dL) than for patients aged <12 years (66.0 [46.0‐84.0] IU/dL).
TABLE 4.
Summary of available FX:C levels pre‐ and post dose
| Characteristic | Predose | Post dose | ||||
|---|---|---|---|---|---|---|
| <12 years (n = 16) | ≥12 years (n = 2) | All (N = 18) | <12 years (n = 6) | ≥12 years (n = 2) | All (n = 8) | |
| FX:C, IU/dL | ||||||
| Mean (SD) | 9.4 (9.2) | 29.5 (34.6) | 11.6 (13.7) | 67.7 (14.5) | 103.0 (15.6) | 76.5 (21.3) |
| Median (range) | 6.0 (2.0‐40.0) | 29.5 (5.0‐54.0) | 6.0 (2.0‐54.0) | 66.0 (46.0‐84.0) | 103.0 (92.0–114.0) | 76.5 (46.0‐114.0) |
| Last weight, kg | ||||||
| Mean (SD) | 13.1 (1.7) | 65.8 (6.7) | 18.9 (17.2) | 11.5 (1.9) | 60.4 (0.9) | 23.7 (22.7) |
| Median (range) | 14.0 (10.3‐14.0) | 65.8 (61.0‐70.5) | 14.0 (10.3–70.5) | 10.3 (10.3‐14.0) | 60.35 (59.7‐61.0) | 12.15 (10.3‐61.0) |
| Last dose, IU/kg | ||||||
| Mean (SD) | 45.4 (7.1) | 26.5 (2.7) | 43.2 (9.2) | 38.6 (11.6) | 24.9 (0.4) | 35.2 (11.7) |
| Median (range) | 43.9 (24.3‐53.6) | 26.5 (24.6‐28.4) | 43.9 (24.3‐53.6) | 42.1 (24.3‐48.5) | 24.9 (24.6‐25.1) | 30.9 (24.3‐48.5) |
| Time since last dose, days | ||||||
| Mean (SD) | 3.2 (3.0) | 3.5 (2.1) | 3.2 (2.8) | 0 | 0 | 0 |
| Median (range) | 3 (1‐14) | 3.5 (2‐5) | 3 (1‐14) | 0 | 0 | 0 |
Abbreviations: FX:C, factor X activity; SD, standard deviation.
3.6. Safety
No ADRs, including inhibitor development, infusion‐site reactions, thromboembolic events, or other safety concerns or tolerability issues, were reported during the study. Likewise, no deaths or serious adverse drug reactions were reported. Two patients reported successful pregnancies and childbirths, with no abnormal bleeding complications and no efficacy or safety concerns. Details regarding these patients have previously been published. 13
4. DISCUSSION
In this retrospective analysis, data were collected on the efficacy and safety of long‐term compassionate use of pdFX in 15 patients with hereditary FXD. The median treatment duration was 1.6 years, with a maximum duration of 4 years. All patients received pdFX as either routine prophylaxis or an on‐demand treatment. At times, some patients used a short‐term preventative or a perisurgical coverage regimen instead. Overall, patients aged ≥12 years received a lower dose and fewer infusions than patients aged <12 years. pdFX was used to treat most of the bleeds that occurred during the study period, with effectiveness in all cases, per investigators. Even with data on up to 4 years of pdFX treatment, no ADRs, tolerability issues, or other safety concerns were reported.
Dosing for patients included in this analysis was at the discretion of the investigator (data were collected retrospectively). As might be expected based on the inter‐ and intrapatient variability of FXD disease course, there were large variations in the number of infusions (2‐492) and the total dose per subject (46‐26, 357 IU/kg). The observed variability in dosing may be explained in part by the age range of the included patients (1‐43 years), as younger patients have been shown to have a lower incremental recovery than older patients and thus require a higher dose. 12 , 14 In a study of patients aged <12 years, incremental recovery was significantly lower for patients aged 0 to 5 years than for those aged 6 to 11 years. 12 Furthermore, in the same study, patients aged <12 years had a lower incremental recovery than those in a separate study of patients aged ≥12 years. 10 , 12 Accordingly, the pdFX product label now recommends higher dosing for patients aged <12 years than for those aged ≥12 years. 14 However, in the current analysis, the effects of age on factor half‐life or incremental recovery could not be assessed. Doses were calculated to the nearest 0.1 mL, and any surplus solution was discarded. Clinicians should consider using a similar method to ensure that doses given to pediatric patients do not exceed the maximum daily dose recommended in the prescribing information (60 IU/kg).
Patients who received pdFX as routine prophylaxis had a lower bleed rate (0.04 bleeds per month) than patients who received only on‐demand treatment (0.8 bleeds per month). The patient who switched between prophylactic and on‐demand treatment received 30.8 IU/kg weekly and then 23.1 IU/kg every 2 weeks. This lower dose was the same as doses used to treat bleeding episodes that occurred during the on‐demand treatment period (with the exception of a major central nervous system bleed for which the patient received 12 doses of treatment in the week following the event). This patient experienced no bleeds on routine prophylaxis and 6 bleeds during on‐demand treatment, suggesting that this prophylactic dose was sufficient to prevent bleeding. This may be due to an increase in FX:C with prophylactic treatment, since greater FX:C is associated with reduced clinical bleeding severity. 15 In a separate prospective study, prophylactic administration of pdFX to children aged <12 years maintained trough FX:C levels >5 IU/dL and resulted in a mean incremental recovery of 1.74 IU/dL. 12 Although the current study was not designed to determine incremental recovery, the lowest postdose FX:C recorded was 46.0. These results suggest that regular pdFX use increases FX:C to a sufficient extent to prevent bleeds.
Investigators rated the overall efficacy of pdFX treatment during compassionate use as excellent for 14 of 15 patients and effective for the treatment of all individual bleeds. Similar results were observed in a prospective study of 16 patients with moderate to severe hereditary FXD receiving pdFX as on‐demand treatment. 10 Patients rated pdFX treatment as successful for 98.4% of bleeds, and investigators rated treatment as successful for 97.6% of bleeds. Overall, investigators rated pdFX treatment as excellent in 80% of patients and good in 20%. Likewise, in a pediatric study of 9 children (aged <12 years) with moderate to severe hereditary FXD receiving 6 months of prophylactic treatment, investigators rated the overall efficacy of pdFX as excellent. No adverse events related to pdFX treatment were observed in either study. 10 , 12
At times, the patients in the current study also received pdFX for short‐term prevention or perisurgical coverage. Although these doses were, on average, lower and less frequent than those administered in the routine prophylaxis group, patients treated for short‐term prevention or perisurgical coverage did not experience any bleeds. In an earlier study, five patients with mild to severe hereditary FXD undergoing surgery received pdFX both pre‐ and postoperatively. 11 Again, investigators rated pdFX as excellent in preventing bleeds and achieving hemostasis; no blood transfusions were required and there were no ADRs.
Limitations of this study include the retrospective nature of the data collection, the small number of patients, the lack of a placebo control, and the variability in patient characteristics and treatments. Furthermore, comparisons of specific treatment regimens (ie, routine prophylaxis vs on‐demand treatment) could not be made. However, these limitations are due to the low prevalence of hereditary FXD and the limited alternative treatment options available.
In this small retrospective study of patients with hereditary FXD, a rare disease, compassionate use of pdFX was safe and effective when used for routine prophylaxis, on‐demand treatment, short‐term prophylaxis, or perisurgical cover for the treatment of hereditary FXD. Patients receiving routine prophylaxis experienced fewer bleeding episodes than those receiving on‐demand treatment. No ADRs were observed, even with up to 4 years of treatment. These findings are consistent with previous prospective studies of pdFX and support the continued use of pdFX to treat patients with hereditary FXD.
AUTHOR CONTRIBUTIONS
CA was responsible for study concept and design. All authors contributed to acquisition, analysis, and interpretation of data, oversaw the drafting of the manuscript, critically revised the manuscript for important intellectual content, and approved the manuscript for publication.
RELATIONSHIP DISCLOSURE
JNH has received research funding from Baxter, Biogen, and Pfizer and has been a study investigator for Bio Products Laboratory. RL has been as a consultant and advisory board member for Baxalta, Bayer, Bio Products Laboratory, Novo Nordisk, Octapharma, Roche, and Sobi; has received honoraria from Baxalta Bayer, Novo Nordisk, Octapharma, Roche, and Sobi; and has been a research funding recipient and speakers’ bureau participant for Baxalta, Bayer, Novo Nordisk, Octapharma, Roche, Sobi, and Sobi/Bioverativ. CA is an employee of Bio Products Laboratory. SKA has been a consultant and advisory board member for Baxalta, Bayer, Bio Products Laboratory, CSL Behring, Novo Nordisk, Pfizer, and Sobi. KK has been a consultant to Baxalta, Bio Products Laboratory, CSL Behring, Novo Nordisk, and Octapharma; has received honoraria from Baxalta, Bayer, Pfizer, and Shire; has been an advisory board member for Baxalta, Bayer, Novo Nordisk, Pfizer, and Shire; and has provided educational and investigational support to Baxalta, Bayer, Bio Products Laboratory, CSL Behring, Novo Nordisk, Octapharma, and Pfizer.
ACKNOWLEDGMENTS
Bio Products Laboratory (Elstree, UK) provided funding for medical writing and editorial assistance in the development of this manuscript. Mollie Marko, PhD (Ashfield MedComms, an Ashfield Health company, Middletown, CT, USA), drafted and revised the manuscript based on input from authors, and Joshua Safran (Ashfield MedComms) copyedited and styled the manuscript per journal requirements.
Huang JN, Liesner R, Austin SK, Kavakli K, Akanezi C. Plasma‐derived factor X concentrate compassionate use for hereditary factor X deficiency: Long‐term safety and efficacy in a retrospective data‐collection study. Res Pract Thromb Haemost. 2021;5:e12550. 10.1002/rth2.12550
Handling Editor: Pantep Angchaisuksiri
Funding information
This study was funded by Bio Products Laboratory Ltd.
REFERENCES
- 1. Brown DL, Kouides PA. Diagnosis and treatment of inherited factor X deficiency. Haemophilia. 2008;14(6):1176‐1182. [DOI] [PubMed] [Google Scholar]
- 2. Herrmann FH, Auerswald G, Ruiz‐Saez A, et al. Factor X deficiency: clinical manifestation of 102 subjects from Europe and Latin America with mutations in the factor 10 gene. Haemophilia. 2006;12(5):479‐489. [DOI] [PubMed] [Google Scholar]
- 3. Peyvandi F, Mannucci PM, Lak M, et al. Congenital factor X deficiency: spectrum of bleeding symptoms in 32 Iranian patients. Br J Haematol. 1998;102(2):626‐628. [DOI] [PubMed] [Google Scholar]
- 4. Bolton‐Maggs PH, Perry DJ, Chalmers EA, et al. The rare coagulation disorders–review with guidelines for management from the United Kingdom Haemophilia Centre Doctors' Organisation. Haemophilia. 2004;10(5):593‐628. [DOI] [PubMed] [Google Scholar]
- 5. Peyvandi F, Di Michele D, Bolton‐Maggs PH, et al. Classification of rare bleeding disorders (RBDs) based on the association between coagulant factor activity and clinical bleeding severity. J Thromb Haemost. 2012;10(9):1938‐1943. [DOI] [PubMed] [Google Scholar]
- 6. Giangrande P, Seitz R, Behr‐Gross ME, et al. Kreuth III: European consensus proposals for treatment of haemophilia with coagulation factor concentrates. Haemophilia. 2014;20(3):322‐325. [DOI] [PubMed] [Google Scholar]
- 7. National Hemophila Foundation . MASAC Recommendations Concerning Products Licensed for the Treatment of Hemophilia and Other Bleeding Disorders; 2020. https://www.hemophilia.org/sites/default/files/document/files/263_treatment.pdf. Updated September 3, 2020. [Google Scholar]
- 8. Mumford AD, Ackroyd S, Alikhan R, et al. Guideline for the diagnosis and management of the rare coagulation disorders: a United Kingdom Haemophilia Centre Doctors' Organization guideline on behalf of the British Committee for Standards in Haematology. Br J Haematol. 2014;167(3):304‐326. [DOI] [PubMed] [Google Scholar]
- 9. Menegatti M, Peyvandi F. Factor X deficiency. Semin Thromb Hemost. 2009;35(4):407‐415. [DOI] [PubMed] [Google Scholar]
- 10. Austin SK, Kavakli K, Norton M, et al. Efficacy, safety and pharmacokinetics of a new high‐purity factor X concentrate in subjects with hereditary factor X deficiency. Haemophilia. 2016;22(3):419‐425. [DOI] [PubMed] [Google Scholar]
- 11. Escobar MA, Auerswald G, Austin S, Huang JN, Norton M, Millar CM. Experience of a new high‐purity factor X concentrate in subjects with hereditary factor X deficiency undergoing surgery. Haemophilia. 2016;22(5):713‐720. [DOI] [PubMed] [Google Scholar]
- 12. Liesner R, Akanezi C, Norton M, Payne J. Prophylactic treatment of bleeding episodes in children <12 years with moderate to severe hereditary factor X deficiency (FXD): efficacy and safety of a high‐purity plasma‐derived factor X (pdFX) concentrate. Haemophilia. 2018;24(6):941‐949. [DOI] [PubMed] [Google Scholar]
- 13. Kulkarni R, James AH, Norton M, Shapiro A. Efficacy, safety and pharmacokinetics of a new high‐purity factor X concentrate in women and girls with hereditary factor X deficiency. J Thromb Haemost. 2018;16(5):849‐857. [DOI] [PubMed] [Google Scholar]
- 14. Coagadex® (Coagulation factor X [human]) [prescribing information]. Elstree, UK: Bio Products Laboratory Ltd; 2018. [Google Scholar]
- 15. Peyvandi F, Palla R, Menegatti M, et al. Coagulation factor activity and clinical bleeding severity in rare bleeding disorders: results from the European Network of Rare Bleeding Disorders. J Thromb Haemost. 2012;10(4):615‐621. [DOI] [PubMed] [Google Scholar]


