Abstract
With the Pfizer-BioNTech, Moderna, and now Johnson and Johnson COVID-19 vaccines readily available to the general population, the appearance of vaccine-induced axillary adenopathy on imaging has become more prevalent. We are presenting follow up to the first reported four cases of vaccine induced unilateral axillary adenopathy on imaging to our knowledge, which demonstrate expected self-resolving adenopathy. Our hope is that by providing this follow-up and reviewing current management guidelines, clinicians as well as patients will appreciate that this is an expected, benign, and self-resolving finding. In addition, we hope to quell any vaccine hesitancy brought about by recent mainstream media attention to this topic and ultimately empower patients to receive both the COVID-19 vaccine and undergo routine screening mammography, as both are vital to their health.
Keywords: COVID-19, Vaccine, Unilateral axillary Adenopathy, Breast imaging, Screening
1. Introduction
We previously reported four cases of patients who were found to have unilateral axillary adenopathy on breast imaging after receiving either the first or second dose of the Pfizer-BioNTech or Moderna COVID-19 vaccine in the ipsilateral upper extremity.1 Of the four cases, three patients (Case 1, Case 2, and Case 4) have presented for follow-up imaging demonstrating resolution of the previously noted unilateral axillary adenopathy. One patient (Case 3) was noted to have resolution of the unilateral axillary adenopathy on physical exam.
2. Case series
2.1. Case 1
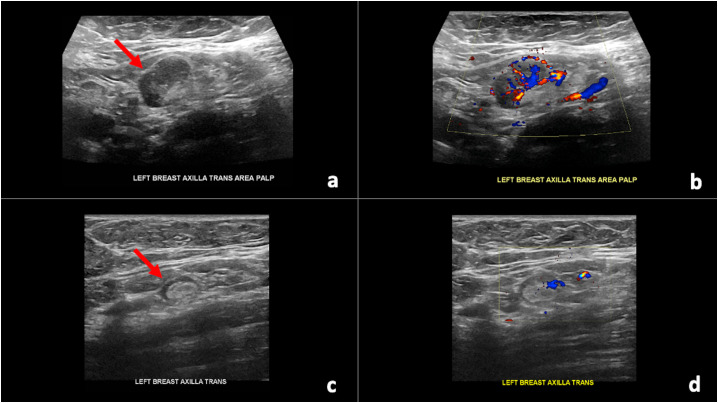
59-year-old female initially presented for evaluation of a palpable lump in her left axilla with targeted ultrasound demonstrating a left axillary lymph node measuring 2.6 × 1.5 × 1.6 cm with uniform cortical thickening of 0.7 cm corresponding to the patient's palpable area of concern. She had received the first dose of the Pfizer-BioNTech COVID-19 vaccine in the left upper extremity nine days prior to imaging. At that time, the abnormal axillary lymph node was thought to most likely be reactive, attributed to recent ipsilateral upper extremity vaccination, and assessed as BI-RADS category 3. Short-term follow-up targeted ultrasound of the left axilla in 4–12 weeks was recommended to ensure resolution. The patient presented for follow-up targeted ultrasound of the left axilla seven weeks later, which was five weeks after receiving the second dose of the Pfizer-BioNTech COVID-19 vaccine and was noted to have resolution of the previously noted unilateral left axillary adenopathy (Fig. 1 ).
Fig. 1.
59-year-old female with unilateral left axillary adenopathy noted approximately 9 days after receiving the first dose of the Pfizer-BioNTech COVID-19 vaccine in her left upper extremity which resolved on follow-up targeted ultrasound performed 7 weeks after initial presentation, which was 5 weeks after receiving the second dose. (a) Gray-scale and (b) color Doppler images of an enlarged left axillary lymph node measuring 2.6 × 1.5 × 1.6 cm with uniform cortical thickening up to 0.7 cm (arrow). (c) Gray-scale and (b) color Doppler images from targeted ultrasound performed 7 weeks after initial presentation demonstrated resolution of the previously noted unilateral left axillary adenopathy (arrow).
2.2. Case 2
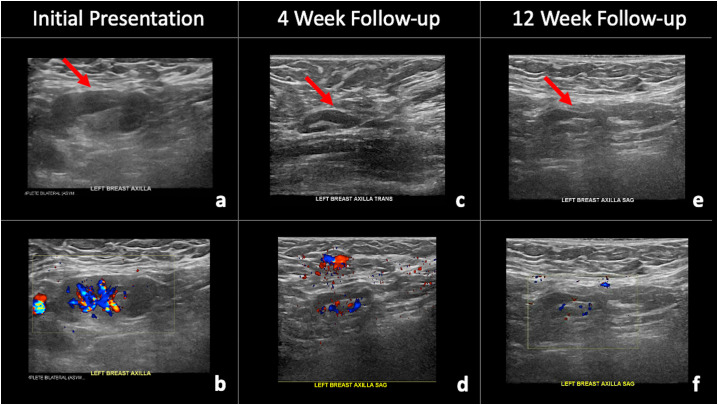
42-year-old female presented for routine screening mammogram with screening ultrasound, and on screening ultrasound was noted to have multiple left axillary lymph nodes with uniformly thickened cortices, the largest of which measured 2.7 × 1.2 × 1.0 cm (Fig. 2a, b). She had received the second dose of the Pfizer-BioNTech COVID-19 vaccine in her left upper extremity five days prior to imaging. At that time, the abnormal axillary lymph nodes were thought to most likely be reactive, attributed to recent ipsilateral upper extremity vaccination, and assessed as BI-RADS category 3. Short-term follow-up targeted ultrasound of the left axilla in 4–12 weeks was recommended to ensure resolution. The patient presented for follow-up targeted ultrasound of the left axilla 4 weeks after initial presentation (Fig. 2c, d) and was noted to have decreased, but persistent, cortical thickening of left axillary lymph nodes. Given that the abnormal left axillary lymph nodes persisted, these were assessed as BI-RADS category 3 and the patient was advised to return for short-term follow-up targeted ultrasound in eight weeks, which would be 12 weeks after her initial presentation, to ensure complete resolution. The patient returned eight weeks later (12 weeks after initial presentation), and as expected there had been complete resolution of the previously noted unilateral left axillary adenopathy (Fig. 2e, f).
Fig. 2.
42-year-old female with unilateral left axillary adenopathy noted 5 days after receiving the second dose of the Pfizer-BioNTech COVID-19 vaccine in her left upper extremity, which improved on follow-up targeted ultrasound performed 4 weeks after initial presentation and ultimately resolved on follow-up targeted ultrasound performed 12 weeks after initial presentation. (a) Gray-scale and (b) color Doppler images demonstrate an enlarged left axillary lymph node with cortical thickening (arrow). (c) Gray-scale and (d) color Doppler images from a targeted ultrasound performed 4 weeks after initial presentation demonstrated interval decreased cortical thickening of the left axillary lymph node (arrow). (e) Gray-scale and (f) color Doppler images from a targeted ultrasound performed 12 weeks after initial presentation demonstrated interval resolution of the previously noted left axillary adenopathy (arrow).
2.2.1. Case 3
42-year-old female presented for sonographic follow-up of probably benign bilateral breast masses and was found to have diffuse cortical thickening of a left axillary lymph node. She had received the first dose of the Moderna COVID-19 vaccine in her left upper extremity 13 days prior to imaging. The lymph node was thought to be reactive and attributed to recent ipsilateral vaccination. As the patient was seen in early December of 2020, prior to institutional policy on management of these cases and prior to the published recommendations set forth by the Society of Breast Imaging,2 the lymph node was deemed to be reactive and benign and assessed as a BI-RADS category 2 with the recommendation to resume routine screening in 1 year. Although the patient has not received interval imaging follow-up, she was noted to have resolution of the previously noted unilateral left axillary adenopathy on physical exam two months subsequent to presentation for imaging.
2.3. Case 4
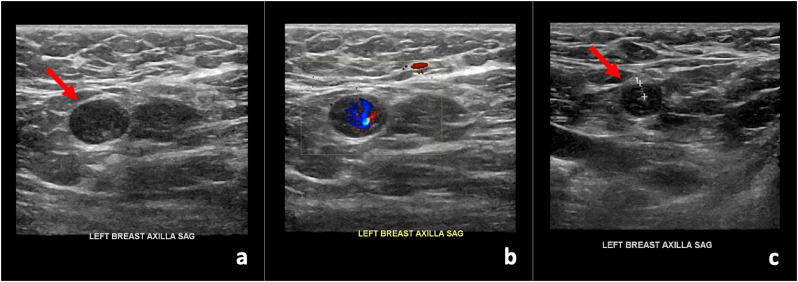
57-year-old female presented for routine screening mammogram with screening ultrasound, and on screening ultrasound was noted to have a single prominent left axillary lymph node measuring 1.0 cm in short axis with diffuse cortical thickening and near complete obliteration of fatty hilum. She had received the first dose of the Pfizer-BioNTech COVID-19 vaccine in her left upper extremity eight days prior to imaging. At that time, the abnormal axillary lymph node was thought to most likely be reactive, attributed to recent ipsilateral upper extremity vaccination, and assessed as BI-RADS category 3. Short-term follow-up targeted ultrasound of the left axilla in 4–12 weeks was recommended to ensure resolution. The patient presented for follow-up targeted ultrasound of the left axilla 17 weeks later, which was 13 weeks after receiving the second dose of the Pfizer-BioNTech COVID-19 vaccine and was noted to have significant decrease in cortical thickness to normal size of 0.3 cm of the previously noted unilateral left axillary adenopathy, compatible with benign reactive adenopathy (Fig. 3 ).
Fig. 3.
57-year-old female with unilateral left axillary adenopathy noted 8 days after receiving the first dose of the Pfizer-BioNTech COVID-19 vaccine in her left upper extremity, noted to have significant decrease in cortical thickness to normal size of 0.3 cm on follow-up targeted ultrasound performed 17 weeks after initial presentation, which was 13 weeks after receiving the second dose, compatible with benign reactive adenopathy. (a) Gray-scale and (b) color Doppler images of a single enlarged left axillary lymph node with diffuse cortical thickening (arrow). (c) Gray-scale image from targeted ultrasound performed 17 weeks after initial presentation, demonstrated significant decrease in cortical thickness to normal size of 0.3 cm (arrow), compatible with benign reactive adenopathy.
3. Discussion
With increased rollout of the Pfizer-BioNTech and Moderna COVID-19 vaccines since December of 2020 to the general population, the appearance of vaccine-induced axillary adenopathy on imaging has been prevalent. Given that this is a benign and expected finding following vaccination, it is important for radiologists to be familiar with the imaging presentation of COVID-19 vaccine induced hyperplastic unilateral axillary adenopathy and to consider this as a potential differential diagnosis for unilateral axillary adenopathy. Therefore, we previously reported four cases of patients who were found to have unilateral axillary adenopathy on breast imaging after receiving either the first or second dose of the Pfizer-BioNTech or Moderna COVID-19 vaccine in the ipsilateral upper extremity.1
On January 22, 2021, the Society of Breast Imaging (SBI) published recommendations for the management of axillary adenopathy in patients with recent COVID-19 vaccination. Specifically, the SBI recommended that if unilateral axillary adenopathy is detected at screening, a BI-RADS category 0 assessment is warranted, and following appropriate diagnostic work-up, short-term follow-up exam in 4–12 weeks (BI-RADS category 3) following the second vaccine dose should be recommended. Should the axillary adenopathy persist on short-term follow-up imaging, lymph node sampling should be considered to exclude breast and non-breast malignancy. Furthermore, radiologists should consider obtaining COVID-19 vaccination history on intake forms, with specific attention to the timing and side of vaccination.2 These recommendations are in line with what was recommendation for our Cases 1, 2, and 4.
Per the SBI guidelines, the recommendations for biopsy on follow-up imaging is in the setting of persistent adenopathy. In the setting of improved, but not fully resolved unilateral axillary adenopathy, the decision to follow-up is at the discretion of the radiologist. One can argue, however, that malignant adenopathy should not spontaneously improve, thus any improvement in cortical lymph node thickening, decrease in overall lymph node size, or increased prominence of the benign hilum and morphology can be used as proof of benignity with no additional follow-up needed. This is congruent with recommendations to down-grade a probably benign mass (BI-RADS category 3) to a benign mass (BI-RADS category 2) on follow-up if the mass is decreasing in size.3 Alternatively, some may question if the differences may be technical in nature and thus recommend additional follow-up to resolution. Furthermore, although the four cases we have presented returned and demonstrated resolution of unilateral axillary adenopathy in the recommended time frame, it has been our experience that resolution/improved adenopathy may persist for longer than 12 weeks on imaging, in particular sonography, given the higher sensitivity compared to physical exam, as previously demonstrated on Positron Emission Tomography – Computed Tomography (PET/CT), in which inflammatory activity may be detected in non-enlarged lymph nodes.4
In February 2021, shortly after the SBI guidelines were published, radiologists from Massachusetts General Hospital (MGH) published their approach for managing unilateral axillary adenopathy detected on breast imaging after receiving the COVID-19 vaccine, which is based on the patient's clinical presentation as either asymptomatic for screening, symptomatic breast and/or axilla for diagnosis, or recent breast cancer diagnosis.5 Specifically, for asymptomatic patients presenting for screening with no imaging findings beyond the unilateral axillary adenopathy ipsilateral to recent (prior six weeks) vaccination, a BI-RADS category 2 assessment is appropriate with no further imaging recommended if no lymph nodes are palpable six weeks after the patient receives the last dose of the COVID-19 vaccine. For patients who present for breast imaging with palpable axillary adenopathy, with history of recently receiving the vaccine in the ipsilateral upper extremity, clinical follow-up of the axilla should be recommended, with axillary ultrasound to be recommended if clinical concern persists six weeks after the patient receives the last dose of the COVID-19 vaccine. Lastly, for patients with a recent diagnosis of breast cancer in the pre- or peri-treatment setting, prompt imaging is recommended. Although these recommendations were published after our four cases had initially presented for imaging, they are in line with what was recommended for our Case 3, prior to the development of our institutional policy on the management of such cases.
Of note, our case series only included patients who had received the Pfizer-BioNTech and Moderna COVID-19 vaccines, as the Johnson & Johnson COVID-19 vaccine had not yet been approved by the U.S. Food and Drug Administration (FDA). We expect similar, benign and self-resolving findings of unilateral axillary adenopathy to be seen in those patients who receive the Johnson & Johnson COVID-19 vaccine, and therefore they should be managed similarly.
With the COVID-19 vaccines now available to the general population, the self-resolving and benign finding of unilateral axillary adenopathy has become more prevalent and has garnered the attention of the mainstream media, with published coverage in numerous news outlets around the world, including the U.S. News & World Report and Forbes magazine.6., 7., 8., 9., 10., 11., 12. Unfortunately, one consequence of this mainstream media attention is the incorrect notion that unilateral axillary adenopathy found on breast imaging, following the COVID-19 vaccine, could be mistaken for malignancy. Patients should be reminded that unilateral axillary adenopathy after receiving an ipsilateral vaccine is not only an expected, benign, self-resolving finding, but a good indication that their immune system is responding appropriately to the vaccine.13 , 14 To quell patient anxiety and potential screening hesitancy, the SBI recommends that women should schedule routine screening mammography either before receiving the first dose of the COVID-19 vaccine or 4–6 weeks after receiving the second dose to avoid a false positive finding.2 Given that this may not always be feasible, women should be empowered to share their recent vaccine history with their mammography technologist or breast radiologist to provide an accurate context within which the results of their imaging will be interpreted.15 Ultimately, it is imperative that patients do not delay undergoing routine screening mammography in lieu of receiving the COVID-19 vaccine, or vice versa, as both are vital to their health.
With the rollout of the COVID-19 vaccine and increasing prevalence of vaccine related unilateral adenopathy seen in the general population, the approach to screening mammography in the setting of recent COVID-19 vaccination and follow-up of vaccine-induced ipsilateral axillary adenopathy is evolving. The original recommendations issued by the SBI were published early in the vaccine rollout period and were by design conservative. As the prevalence of vaccine-induced adenopathy increases, the flexibility of both breast imaging practices as well as patients for scheduling screening appointments and the need for follow-up imaging is diminishing. As such, and in the context of appropriate history of recent ipsilateral upper extremity vaccination, and no signs of breast cancer in the breast on mammography and/or ultrasound, alternative approaches such as suggested by Lehman et al. from MGH5 for a less conservative approach to management can be considered as acknowledged in the SBI recommendations.2 To decrease confusion and patient anxiety, selecting and providing a consistent approach by all practice members is prudent.
References
- 1.Mehta N., Sales R.M., Babagbemi K., et al. Unilateral axillary Adenopathy in the setting of COVID-19 vaccine. Clin Imaging. 2021;75:12–15. doi: 10.1016/j.clinimag.2021.01.016. Epub 2021/01/19. PubMed PMID: 33486146; PubMed Central PMCID: PMC7817408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grimm L.D., Stamatia, Dogan Basak, et al. In: SBI Recommendations for the Management of Axillary Adenopathy in Patients with Recent COVID-19 Vaccination. Committee SoBIPCaD, editor. 2021. p. 3. [Google Scholar]
- 3.Lee K.A., Talati N., Oudsema R., Steinberger S., Margolies L.R. BI-RADS 3: current and future use of probably benign. Curr Radiol Rep. 2018;6(2):5. doi: 10.1007/s40134-018-0266-8. Epub 2018/01/27. PubMed PMID: 29399419; PubMed Central PMCID: PMC5787219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee K.A., Talati N., Oudsema R., Steinberger S., Margolies LR. BI-RADS 3: current and future use of probably benign. Curr Radiol Rep. 2018;6(2):5. doi: 10.1007/s40134-018-0266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehman C.D., Lamb L.R., D’Alessandro H.A. Mitigating the impact of coronavirus disease (COVID-19) vaccinations on patients undergoing breast imaging examinations: a pragmatic approach. AJR Am J Roentgenol. 2021 doi: 10.2214/AJR.21.25688. Epub 2021/02/22. PubMed PMID: 33617288. [DOI] [PubMed] [Google Scholar]
- 6.Thompson D. COVID vaccine reaction can mimic breast cancer symptoms, but doctors say 'don't panic': U.S. news & world report. 2021. https://www.usnews.com/news/health-news/articles/2021-02-11/covid-vaccine-reaction-can-mimic-breast-cancer-symptoms-but-doctors-say-dont-panic [updated February 11, 2021.]. Available from:
- 7.Shapiro N. Local reactions after Covid-19 vaccine can mimic signs of breast cancer: Forbes. 2021. https://www.forbes.com/sites/ninashapiro/2021/02/18/local-reactions-after-covid-19-vaccine-can-mimic-signs-of-breast-cancer/?sh=61cb6ed03451 [updated February 18, 2021]. Available from:
- 8.Lee BY. Covid-19 coronavirus vaccine side effect can make your lymph nodes swell: Forbes.; 2021. [updated March 3, 2021.]. Available from: https://www.forbes.com/sites/brucelee/2021/03/03/can-this-covid-19-coronavirus-vaccine-side-effect-be-mistaken-for-cancer/?sh=10ed829e76e9.
- 9.Means SP. Women who get COVID-19 vaccine should delay their regular mammogram, Utah doctor says: the salt Lake tribune; [updated February 9, 2021.]. Available from: https://www.sltrib.com/news/2021/02/09/women-who-get-covid/)/.
- 10.Kern J. La vaccination provoque des gonflements visibles sur les mammographies: Yahoo! France; [updated March 6, 2021.]. Available from: https://fr.news.yahoo.com/vaccination-provoque-gonflements-visibles-mammographies-090000926.html?guccounter=1&guce_referrer=aHR0cHM6Ly93d3cuYWx0bWV0cmljLmNvbS9kZXRhaWxzLzk3OTYzNTYxL25ld3M&guce_referrer_sig=AQAAAEzA5RScFG6-PuruG6Qa0AzxrNB14m_C7rkoUd79BHUPOoEfxXzhJcmfythAvrtaRqWVd8BSRVOgIUPLI1b5y-BR-7q_coBPC-fHqa1hjHN3Q-C4CM9Pc4mPrBEEdAbLuGQIH9nKUn0fo7t410WYZQZf1wtPSeAwQSL5V0jXwODj.
- 11.Pablos T. Ultrasound shows lymph node changes after COVID-19 vaccine: AuntMinnie.com; 2021. [updated January 22, 2021]. Available from: https://www.auntminnie.com/index.aspx?sec=sup⊂=ult&pag=dis&ItemID=131373.
- 12.Palmer W.J. COVID-19 vaccine-linked adenopathies could mimic breast malignancies: diagnostic imaging. 2021. https://www.diagnosticimaging.com/view/covid-19-vaccine-linked-adenopathies-could-mimic-breast-malignancies [updated January 20, 2021]. Available from:
- 13.Watson S. A warning to women about mammograms after COVID-19 vaccination: Survivornet; 2021 [updated February 22, 2021]. Available from: https://www.survivornet.com/articles/a-warning-to-women-about-mammograms-after-covid-19-vaccination/.
- 14.Scherer L. Mammograms should be scheduled before COVID-19 vaccine or 4 to 6 weeks after, experts say: everyday health; 2021. [updated February 26, 2021.]. Available from: https://www.everydayhealth.com/cancer/breast-cancer/mammograms-should-be-scheduled-before-or-4-to-6-weeks-after-covid-19-vaccine-experts-say/.
- 15.Nelson R. Armpit swelling after COVID-19 vaccine may mimic breast Cancer: Medscape. 2021. https://www.medscape.com/viewarticle/946448#vp_1 [updated February 25, 2021.]. Available from: