Abstract
Regenerating gene (REG) family proteins serve as multifunctional secretory molecules with trophic, antiapoptotic, anti-inflammatory, antimicrobial and probably immuno-regulatory effects. Since their discovery, accumulating evidence has clarified the potential roles of the REG family in the occurrence, progression and development of a wide range of inflammatory and inflammation-associated diseases of the gastrointestinal (GI) tract. However, significant gaps still exist due to the undefined nature of certain receptors, regulatory signaling pathways and possible interactions among distinct Reg members. In this narrative review, we first describe the structural features, distribution pattern and purported regulatory mechanisms of REG family proteins. Furthermore, we summarize the established and proposed roles of REG proteins in the pathogenesis of various inflammation-associated pathologies of the GI tract and the body as a whole, focusing particularly on carcinogenesis in the ulcerative colitis—colitic cancer sequence and gastric cancer. Finally, the clinical relevance of REG products in the context of diagnosis, treatment and prognostication are also discussed in detail. The current evidence suggests a need to better understanding the versatile roles of Reg family proteins in the pathogenesis of inflammatory-associated diseases, and their broadened future usage as therapeutic targets and prognostic biomarkers is anticipated.
Keywords: REG family proteins, inflammation, inflammatory bowel disease, gastric cancer, colorectal cancer, mitogenesis
1. Introduction
Regenerating gene (Reg) family proteins are divided into four subgroups (Reg I, Reg II, Reg III and Reg IV) on the basis of their primary structure. The expression patterns and pathophysiological roles of these proteins have been extensively investigated in rodents and humans. In rodents, Reg family proteins comprise Reg I, Reg II, Reg IIIα, Reg IIIβ, Reg IIIγ, Reg IIIδ and Reg IV, while those in human are REG Iα, REG Iβ, REG IIIα, REG IIIγ and REG IV [1]. REG proteins contain a C-type-like lectin domain, which facilitates selective binding to a variety of carbohydrates and ligands [2]. The binding interaction is mediated by the expression of specific anionic residues as well as in a Ca2+-independent manner. For instance, REG IV, pertaining to human beings, represents binding affinity to mannan or heparin via two Ca2+-independent sites, while REG III recognizes peptidoglycan carbohydrate moiety through a tripeptide motif [3,4]. Another structural feature of REG proteins is characterized by a cleavage site for trypsin in the vicinity of the N-terminus [5]. A soluble short peptide and the remaining insoluble fragment consisting of approximate 130 residues are generated in response to trypsin digestion [6]. The latter is activated upon cleavage of a peptide sequence without definite physiological role. Given the fact that the various REG genes show approximately 50–70% homology, they appear to function similarly in terms of mitogenic and antiapoptotic activity and also differentiation induction in a wide range of diseases and physiological conditions. We and others have shown that REG proteins are involved in the pathogenesis of several conditions driven by inflammation in the gastrointestinal (GI) tract and body as a whole, including inflammatory bowel disease (IBD), indomethacin-induced GI injury and rheumatoid arthritis (RA) [7,8,9]. Additionally, REG family proteins can be used for prognostication of digestive tract malignancies, thus serving as potential diagnostic markers or therapeutic targets [10].
In the present review, we provide an overview of REG family proteins, including their regulatory signaling pathways and possible functions, and discuss their potential roles in the occurrence and development of inflammatory diseases (predominantly those of the GI tract), inflammation-associated carcinogenesis and their clinical application as biomarkers of inflammatory/neoplastic pathologies.
2. Background of the Reg Family
The first Reg gene was discovered by Terazono et al. in 1988 and was shown to contribute to β-cell regeneration in vitro and in vivo [11,12]. REG genes each have six exons and five introns, with the exception of REG IV, which has seven exons. In humans, REG IV gene is located on chromosome 1p11-3, whereas the others are on chromosome 2p12 [13]. REG family proteins have a molecular mass of 16-17 kDa. Reg I is present in pancreatic acinar cells, which are capable of transdifferentiation to islets in pathological states but not under healthy conditions [14,15]. Recently, it has been shown that Reg I is expressed in gastric enterochromaffin-like (ECL) cells and that its production is stimulated by gastrin, acting as a mitogenic factor to enhance the proliferation of gastric epithelial cells [16,17]. Reg II is detected only in mice and has no human ortholog, and Reg II mRNA is known to be expressed predominantly in normal pancreatic acini and hyperplastic islets [18]. The third subgroup of Reg proteins is expressed mainly in the pancreas, acting to ameliorate stress and prevent pancreatic inflammation. In this context, Reg IIIβ may be expressed in response to nuclear factor kappa B (NF-κB) and tumor necrosis factor-α (TNF-α), subsequently exerting antiapoptotic and anti-inflammatory actions during oxidative stress [19,20,21]. On the other hand, IL-22 signaling can induce intestinal expression of REG IIIα and REG IIIγ to enhance the survival of intestinal stem cells and Paneth cells [22,23]. The most recently discovered member, REG IV, was originally isolated by Hartupee et al. from an ulcerative colitis (UC) cDNA library [24]. REG IV is expressed in various human organs and tissues, such as the colon, small intestine, stomach, pancreas and appendix [25,26].
3. Regulation of Reg Family Genes
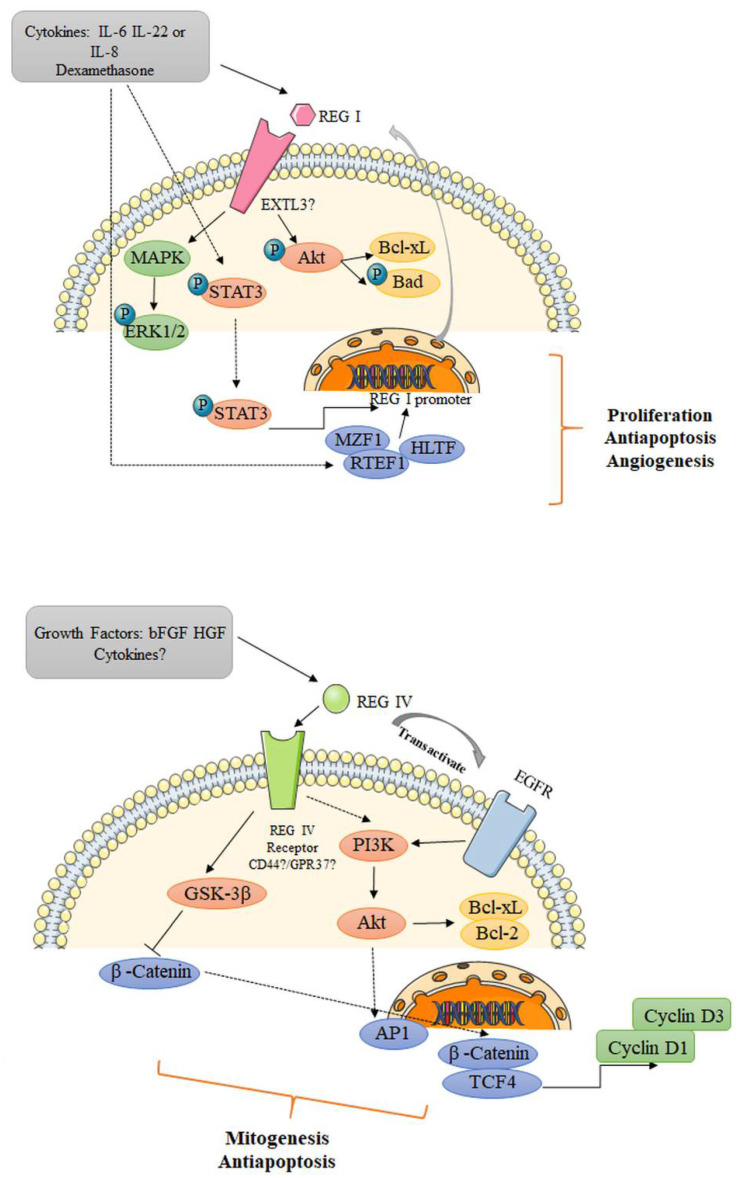
The specific receptors of Reg remain elusive, and this has hindered classification of the molecular pathways in which these proteins function. Kobayashi et al. identified a cDNA for the rat Reg I protein receptor based on an islet cDNA expression library using a 125I-labeled protein probe [27]. This receptor corresponded to human multiple exostoses-like gene 3 (EXTL3), which contributes to the biosynthesis of heparan sulfate (Figure 1). The EXTL3 mRNA is broadly present in GI tract epithelia, as well as the pancreas, liver, spleen, kidney, neurons and various glands. Further studies have revealed that overexpression of EXTL3 in β-cells triggers the expression of activating transcription factor-2 (ATF-2) [28], and that REG Iα regulates neurite outgrowth via its receptor EXTL3 in neuronal cell lines and primary hippocampal neurons [29]. These findings suggest the possible involvement of the REG I–REG receptor signaling system in a wide range of cell types. On the other hand, it has been speculated that CD44 may be a REG IV receptor, as the two show interaction in colorectal cancer (CRC) proliferation [30]. Intriguingly, Wang et al. have shown that G protein-coupled receptor 37 (GPR37) is part of the same complex as REG IV, which mediates its signal transduction and promotes peritoneal metastasis of gastric cancer cells [31].
Figure 1.
Potential mechanism of REG I and REG IV signaling. Upon stimulation from exogenous growth factors or cytokines, REG I/REG IV bind to their putative receptors and subsequently activate or transactivate several signaling cascades, serving as mitogenic, antiapoptotic and/or angiogenic factors in the pathogenesis of various pathological conditions. EXTL3, exostoses-like gene 3; MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase; STAT3, signal transducer and activator of transcription 3; MZF1, myeloid zinc finger 1; RTEF1, related transcriptional enhancer factor-1; HLTF, helicase-like transcription factor; bFGF, basic fibroblast growth factor; HGF, hepatocyte growth factor; AP1, activator protein-1; EGFR, epidermal growth factor receptor; GSK-3β, glycogen synthase kinase 3β; TCF4, T-cell factor 4.
There is now mounting evidence that exogenous growth factors and several cytokines induce REG gene expression (Figure 1). Akiyama et al. have reported that a combination of IL-6 and dexamethasone enhanced the expression of Reg I mRNA in the rat insulinoma cell line RINm5F. This effect was attributable to the presence of a cis-element of the Reg gene promoter (T−81GCCCCTCCCAT−70) and poly(ADP-ribose) synthetase/polymerase inhibitors promoting Reg production and secretion at the transcriptional level [32]. Dusetti et al. have also shown that IL-6 and dexamethasone together strongly upregulate Reg I in acinar AR-42J cells, whereas this positive effect is partly eliminated by addition of IL-1 [33]. It has been reported in ECL cells, a cytokine-induced neutrophil chemoattractant, dose-dependently increased the expression of Reg I mRNA and protein via CXC receptor 2 during the healing of gastric mucosal damage [34]. In H. pylori-infected human gastric mucosa, IL-8 might stimulate REG protein production [35]. In addition, both IL-22 and IL-10, which share IL-10 receptor β, can upregulate Reg II by activating the signal transducer and activator of transcription 3 (STAT3) in acinar cells [36,37]. Sekikawa et al. have shown that addition of IL-6 promotes the expression of REG Iα protein via the STAT3 pathway [38]. The REG Iα promoter region is located in the sequence from −142 to −134 as an IL-6 responsive element, and enhanced REG Iα activates Akt, p-Bad and Bcl-xL as an antiapoptotic factor in gastric cancer cells. A REG IIIα-JAK2/STAT3 positive loop has been suggested to promote tumor formation in human pancreatic cancer, which is mediated by EGFR [39]. Furthermore, Naito et al., using a reporter gene assay, have revealed that transcriptional caudal type homeobox 2 (CDX2) DNA-binding elements are located in the 5’-flanking region of the REG IV gene [40]. The ability of CDX2 to bind directly to REG IV has been further verified by chromatin immunoprecipitation assays.
4. Reg and Inflammatory Diseases (GI Tract and Systemic Inflammation)
IBD is an inflammatory GI tract disease affecting millions of patients worldwide. The putative etiology includes excessive exposure of the intestine to microbiota, aberrant activation of the immune system, impaired epithelial barrier function and subsequent injury of intestinal tissues. Two distinct IBD disorders have been identified: Crohn’s disease (CD) and UC. UC and CD each have a discrete cytokine profile. In UC, T-helper type 2 (Th2) cytokine IL-5/IL-13 are the main players [41], whereas in CD, the Th1 cytokine interferon-γ and the Th17 cytokines IL-17/IL-22 are actively involved. The possible mechanisms, pathogenetic involvement and clinical relevance of REG proteins in IBD have recently been outlined by others [1], and we will mention them only briefly here. In a dextran sulfate sodium (DSS)-induced mouse model of colitis, Ogawa et al. found that epithelial Reg IIIβ and Reg IIIγ were overexpressed, possibly indicating mucosal inflammation driven by commensal bacteria [42]. In the same disease model, Xu et al. demonstrated that Reg IIIβ and Reg IIIγ were alternatively increased in the colonic epithelia, their expression levels being correlated with those of STAT3-associated cytokines such as IL-6, IL-17 and IL-22 [43]. In LoVo and SW403 colon cancer cells, IL-22 stimulation has been suggested to enhance the expression of REG Iα by activating its gene promoter through the STAT3 pathway [7]. Another study has revealed that REG IIIα dose dependently repressed the production of proinflammatory cytokines and adhesive molecules in intestinal mucosa collected from subjects with active CD [42]. Intriguingly, intrarectal administration of Reg IIIα or transgenic overexpression of Reg IIIα both mitigate colonic inflammation and damage, probably through reduction of oxidative stress by preserving the gut microbiota [44]. It has also been shown that DSS-induced colitis is aggravated in Reg IIIβ−/− mice relative to wild-type mice, suggesting that attenuation of colitis and ileitis is dependent on the function of Reg IIIβ [45]. Bishnupuri et al. showed that REG IV protein activates the epidermal growth factor/Akt/ activator protein-1 signaling pathway in vitro [30]. The same research group also identified REG IV as a potent regulator of mitotic division of CRC cells in humans [46]. These findings suggest that the REG IV-mediated increase in nuclear β-catenin might elicit TCF-4 transcriptional activities to promote the expression of cell cycle-regulatory genes (cyclin D1 and D3) and associated cyclin-dependent kinases. Another study has suggested that stimulation of SW430 cells with basic fibroblast growth factor and hepatocyte growth factor enhances the expression of REG IV mRNA via the mitogen-activated protein kinase (MAPK)-dependent pathway [47]. An increased number of studies have analyzed the expression of REG family proteins in IBD mucosa separately or in combination [1]. More recently, Takasawa and colleagues have comprehensively examined the expression of all five REG family mRNAs in biopsy samples harvested from IBD patients using quantitative RT-PCR [48,49]. Their results suggested that REG Iα, REG Iβ and REG IV mRNAs are overexpressed in the CD colon, whereas the REG IV gene is overexpressed in UC samples. The IL-22/IL-6-induced upregulation of REG Iα and REG Iβ is attributed to several transcription factors including myeloid zinc finger 1, related transcriptional enhancer factor-1/ TEA domain transcription factor 4, STAT3 and helicase-like transcription factor/forkhead box protein N2 in the LS-174T and HT-29 cell lines [49]. On the other hand, GATA DNA-binding protein 6 (GATA6) is pivotal for REG IV expression in colon epithelial cells under TNF-α suppression.
Helicobacter pylori has been identified as a critical pathogen in the development of chronic gastritis, gastric ulcer and gastric cancer. Fukui et al. have reported that the Reg gene is associated with hypergastrinemia and fundic mucosal inflammation and might participate in H. pylori-induced gastritis [50]. In a study of indomethacin-induced lesions, Sun et al. suggested that Reg I might play a pivotal role in maintenance of GI mucosal integrity by enhancing cell proliferation and conferring resistance to the impact of apoptosis [8]. Intriguingly, in that study, the expression of Reg IIIβ and Reg IIIγ was also significantly increased in the upper GI tract during mucosal damage, indicating that Reg family proteins might exert trophic, antiapoptotic and antimicrobial effects. In vitro data suggest that REG Iα protein might contribute to the maintenance of mucosal barrier function by inducing tight junction proteins such as claudin 3 and 4 [51]. Amebic colitis due to Entamoeba histolytica infection is characterized by intestinal mucosal ulcers. Peterson et al. showed that the expression of REG Iα and REG Iβ is elevated during amebiasis and thus may function to prevent parasite-induced apoptosis [52].
Reg III proteins appear to have multiple functions in the intestine. Ogawa et al. have found that epithelial expression of Reg III is upregulated under conditions of mucosal inflammation initiated by exposure to commensal bacteria [42]. Another study has shown that Reg IIIβ binds to bacterial pathogens and might interfere with their mode of action [53]. This preventive activity against intestinal translocation is observed with respect to the gram-negative bacterium S. enteritidis but not the gram-positive bacterium L. monocytogenes. Zheng et al. have reported that exogenous mouse or human Reg IIIγ markedly improves the survival of IL-22-knockout mice after infection with C. rodentium [54]. In contrast to Reg III, Reg IV is produced predominantly by deep crypt secretory cells and intestinal enteroendocrine cells in the colon [55]. Gut Reg IV- and complement factor D-mediated membrane attack complexes may help to maintain gut homeostasis by elimination of inflammatory E. coli [56].
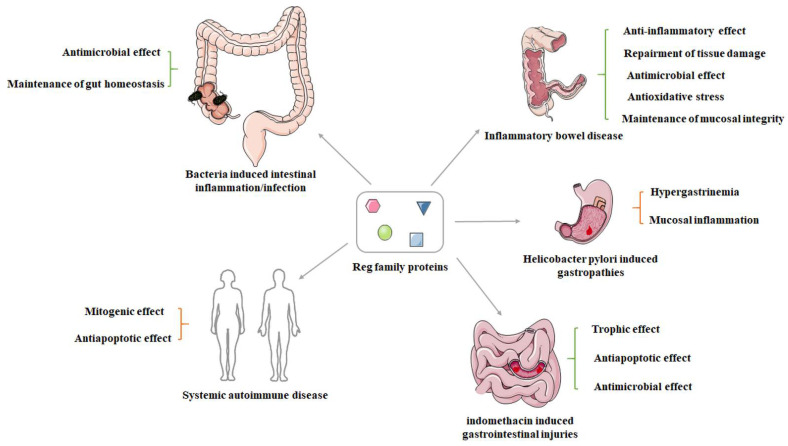
In addition to inflammatory response in the GI tract, REG family proteins are also involved in the pathogenesis of several systemic autoimmune diseases. Fujishiro et al. showed that REG Iα plays a significant role in the pathogenesis of RA through abnormal activation of synovial fibroblasts, subsequently giving rise to pannus formation [57]. Sjögren’s syndrome is a chronic autoimmune disease without a clear etiology, characterized by lymphocytic infiltration of salivary and lacrimal glands. Fukui et al. have suggested that REG Iα might play a role in the regeneration of ductal epithelial cells in the minor salivary glands [58]. Another study by Yoshimoto et al. has confirmed the expression of REG Iα mRNA in the salivary glands and REG Iα in the ductal epithelial cells of minor salivary glands from patients with primary Sjögren’s syndrome [59]. One detailed study has clarified that IL-6 stimulation triggers REG Iα transcription by activating STAT3 and its binding to the Reg Iα promoter in salivary ductal cells, which might be responsible for the progression of Sjögren’s syndrome [60]. A summary of these contents is illustrated in Figure 2.
Figure 2.
The potential role of Reg family proteins in the onset, development and progression of versatile inflammatory diseases. Mounting evidence has suggested that the Reg family proteins may serve as both protective and detrimental pathophysiological factors in the context of distinct conditions.
5. Involvement of Reg Family Proteins in Inflammation-Associated Carcinogenesis
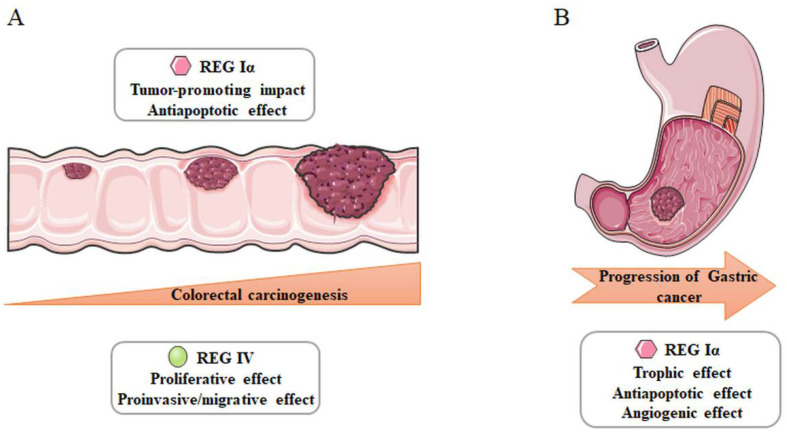
It has been widely accepted that inflammation contributes to the onset and progression of many of malignancies. In fact, patients with longstanding and extensive IBD are at increased risk of CRC [61,62]. In UC, this malignant transformation is believed to result from a chronic inflammation–dysplasia–carcinoma sequence [63]. Current practice is to perform surveillance examinations every 1-3 years for detection and removal of lesions. In the development of UC-associated neoplasia, Tanaka et al. have shown that the pattern of REG Iα immunostaining is significantly correlated with p53 overexpression [64]. Sekikawa et al. have demonstrated that REG Iα is expressed not only in the epithelia of UC mucosa but also in precancerous dysplastic epithelial cells and seven colon cancer cell lines [65]. The growth-promoting and antiapoptotic properties of REG Iα might be responsible for its role in the UC–colitic cancer sequence. REG IV, as a multifunctional secreted protein, acts on intestinal cell proliferation, migration and invasion in an autocrine and paracrine manner in HT-29 cell lines [66]. Zhang et al. have demonstrated that REG IV expression is positively correlated with severity of dysplasia in adenoma, suggesting that overexpression of REG IV might be an early event in colorectal carcinogenesis [67].
As discussed above, REG protein is overexpressed in mucosal cells from H. pylori-infected subjects with concomitant chronic gastritis, which is a risk factor for the development and progression of gastric cancer [68]. Kadowaki et al. have reported that REG appears to be highly expressed in a large number of gastric cancers in vivo [69]. Reg could dose-dependently stimulate thymidine incorporation in MKN45 and AGS gastric cancer cells, partly through activation of the extracellular signal-regulated kinase (ERK) 1/2 and MAPK pathways. Moreover, Fukui et al. have demonstrated the expression of both REG Iα and REG receptor mRNA in seven human gastric cancer cell lines [70]. REG Iα-positive early gastric cancer also exhibits a markedly higher proliferating cell nuclear antigen labeling index and more severe inflammatory cell infiltration in the vicinity of gastric mucosa [71]. Another study has suggested that the expression of REG Iα is positively associated with microvessel density in gastric cancer [72]. Administration of REG Iα enhanced the proliferation and survival of endothelial cells via the ERK and Akt signaling pathways. Collectively, the evidence suggests that REG Iα protein serves as a trophic, antiapoptotic and angiogenetic factor for gastric cancer. A summary of these contents is demonstrated in Figure 3.
Figure 3.
Involvement of REG family proteins in inflammation-associated carcinogenesis. REG Iα and REG IV are responsible for the tumorigenesis of colorectal (A) and gastric cancer (B).
6. Reg Family Proteins as Biological Markers of Inflammatory/Neoplastic Diseases
A proteomic analysis using mass spectrometry has suggested that Reg Iα might be a strong biomarker in patients with infliximab-treated RA [73] (Table 1). In patients with celiac disease, Reg Iα expression is increased in the targeted tissue and also in serum during damage/inflammation, whereas it is decreased after a gluten-free diet, suggesting its potential applicability for diagnosis and monitoring of the disease [74]. In posttraumatic sepsis, serum Reg Iα is related to the severity of inflammation and activates neutrophil granulocytes [75]. An investigation by Que et al. has found that plasma Reg Iα was significantly higher in patients with septic shock than in those with severe sepsis and that the plasma Reg Iα concentration was the only biomarker associated with in-hospital mortality [76]. On the other hand, it has been shown that the plasma Reg Iα level does not differ between children with systemic inflammatory response syndrome and those with septic conditions until signs of organ dysfunction are present [77]. Ferrara et al. have reported that Reg IIIα can be used as a plasma biomarker of GI graft-versus-host disease in combination with clinical stage and histologic grade to improve risk stratification of patients [22]. The biomarker potential of Reg Iα/Reg IIIα has also been validated for identifying septic complication/infection in patients following a variety of surgical procedures [78,79].
Yonemura et al. have indicated that REG Iα gene expression is closely linked to the infiltrative potential of gastric carcinoma and might function as a prognostic indicator for differentiated adenocarcinoma of the stomach [80]. REG Iα expression is also an independent predictor of unfavorable outcomes in gastric cancers, irrespective of intestinal mucin phenotype [81]. In patients with stage IV gastric cancer, REG Iα is a potent predictive marker of resistance to chemotherapeutic drugs [82], and furthermore, serum REG IV protein is a novel predictive marker of resistance to 5-FU-based chemotherapy in patients with gastric cancer [83]. Tao et al. have found that, in TNM stage I and II patients with gastric cancer, the proportion of serum samples positive for REG IV is significantly higher than that of samples positive for carcinoembryonic antigen or carbohydrate antigen 19-9 [84].
Zheng et al. have reported that both REG Iβ and REG IIIα show lower expression in CRCs with venous invasion, lymph node metastasis and deeper invasion, suggesting their possible use as prognostic indicators [85]. In patients with early CRCs without metastasis undergoing potentially curative surgery, the expression of REG I, both alone and in combination with REG IIIα, predicts an adverse survival outcome [86]. Advanced CRC with metastatic hepatic recurrence is reported to show REG IV staining more frequently than that without such metastasis [87]. Increased preoperative serum REG IV levels have been found solely in stage IV CRC patients with liver metastasis. In addition, it has been proven that REG IV acts as a crucial modulator of CRC cell sensitivity and susceptibility to radiotherapy [88,89].
Table 1.
Reg family members as biomarkers for inflammatory/neoplastic diseases.
| Reg Family | Diseases | Findings | References |
|---|---|---|---|
| REG Iα | RA | The efficacy of infliximab treatment | [73] |
| REG Iα | Celiac disease | For diagnosis and monitor | [74] |
| REG Iα | Posttraumatic sepsis | Related to the severity of inflammation | [75] |
| REG Iα | Septic shock | Increased and associated with in-hospital mortality | [76] |
| REG Iα | SIRS | Increased at the onset of organ dysfunction | [77] |
| REG Iα | GC | Relevant to infiltrating pattern and function as prognostic factor | [80] |
| REG Iα | GC | Independent predictor of poor outcomes | [81] |
| REG Iα | Stage IV GC | Potent indicator for resistance to chemotherapy | [82] |
| REG I/REG IIIα | Early CRC | Indicator of adverse effect on survival | [86] |
| REG Iα/REG IIIα | Abdominal/cardiac surgery | Identification of septic complications | [78,79] |
| REG Iβ/ REG IIIα | CRC | Predictor of favorable prognosis | [85] |
| REG IIIα | Gastrointestinal GVHD | Prediction of response to therapy and 1-year survival following HCT | [22] |
| REG IIIα | Mucosal enteropathies | Useful marker to distinguish mucosal enteropathies from IBS | [90] |
| REG IIIα | CD | Increased in active conditions | [91] |
| REG IV | GC | Predictor of resistance to 5-FU-based chemotherapy | [83] |
| REG IV | GC | Prognostic indicator and early diagnosis of GC | [84] |
| REG IV | Advanced CRC | Prognosticator of poor survival with liver metastasis | [87] |
| REG IV | CRC | Crucial modulator of sensitivity and specificity to radiotherapy | [88,89] |
| REG IV | UC | Upregulation in remitting mucosa | [92] |
RA, rheumatoid arthritis; SIRS, systemic inflammatory response syndrome; GVHD, graft-versus-host disease; HCT, hematopoietic cell transplantation; GC, gastric cancer; CRC, colorectal cancer; CD, Crohn’s disease; UC, ulcerative colitis; IBS, irritable bowel syndrome.
Marafini et al. have reported that serum REG IIIα assay is useful for distinguishing between mucosal enteropathies and functional intestinal conditions [90]. Moreover, serum REG IIIα levels decrease significantly following successful treatment with infliximab, supporting the feasibility of REG IIIα as an indicator of therapeutic efficacy. However, Nunes et al. have concluded that serum REG IIIα is upregulated in patients with active CD relative to those in remission, although its predictive capacity is relatively low, with an AUC of 0.69 [91]. Another study has shown that the expression of both REG IV mRNA and protein is markedly upregulated in the colonic mucosa of UC patients in remission, suggesting that further investigation of its possible role as a predictor of progression to CRC might be warranted [92].
7. Conclusions
Reg family proteins have versatile roles as trophic, antiapoptotic, angiogenetic, anti-inflammatory and bactericidal secretory molecules. The data available so far indicate that members of the Reg protein family are intimately involved in the occurrence, progression and development of many inflammation-associated GI tract conditions. It is anticipated that further research will lead to the adoption of these proteins and genes as therapeutic targets and prognostic biomarkers.
Abbreviations
| REG | regenerating gene in human |
| Reg | regenerating gene in mouse/rat |
| GI | gastrointestinal |
| IBD | inflammatory bowel disease |
| RA | rheumatoid arthritis |
| ECL | enterochromaffin-like |
| NF-κB | nuclear factor kappa B |
| TNF-α | tumor necrosis factor-α |
| UC | ulcerative colitis |
| EXTL3 | exostoses-like gene 3 |
| ATF-2 | activating transcription factor-2 |
| CRC | colorectal cancer |
| GPR37 | G protein-coupled receptor 37 |
| STAT3 | signal transducer and activator of transcription 3 |
| CDX2 | caudal type homeobox 2 |
| CD | Crohn’s disease |
| Th2 | T-helper type 2 |
| DSS | dextran sulfate sodium |
| GATA6 | GATA DNA-binding protein 6 |
| ERK | extracellular signal-regulated kinase |
| MAPK | mitogen-activated protein kinase |
| MZF1 | myeloid zinc finger 1 |
| RTEF1 | related transcriptional enhancer factor-1 |
| HLTF | helicase-like transcription factor |
| bFGF | basic fibroblast growth factor |
| HGF | hepatocyte growth factor |
| AP1 | activator protein-1 |
| EGFR | epidermal growth factor receptor |
| GSK-3β | glycogen synthase kinase 3β |
| TCF4 | T-cell factor 4 |
| SIRS | systemic inflammatory response syndrome |
| GVHD | graft-versus-host disease |
| HCT | hematopoietic cell transplantation |
| GC | gastric cancer |
| IBS | irritable bowel syndrome |
Author Contributions
X.W., Y.H. and C.S. prepared the original draft. H.F., C.S., B.W. and H.M. reviewed and made critical revisions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by Grants-in-aid for Scientific Research 21K08016 from the Ministry of Education, Culture, Sports, Science and Technology, Japan (2 April 2021).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Edwards J.A., Tan N., Toussaint N., Ou P., Mueller C., Stanek A., Zinsou V., Roudnitsky S., Sagal M., Dresner L., et al. Role of regenerating islet-derived proteins in inflammatory bowel disease. World J. Gastroenterol. 2020;26:2702–2714. doi: 10.3748/wjg.v26.i21.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dieckgraefe B.K., Crimmins D.L., Landt V., Houchen C., Anant S., Porche-Sorbet R., Ladenson J.H. Expression of the regenerating gene family in inflammatory bowel disease mucosa: Reg Iα upregulation, processing, and antiapoptotic activity. J. Investig. Med. 2002;50:421–434. doi: 10.1136/jim-50-06-02. [DOI] [PubMed] [Google Scholar]
- 3.Ho M.R., Lou Y.C., Wei S.Y., Luo S.C., Lin W.C., Lyu P.C., Chen C. Human RegIV protein adopts a typical C-type lectin fold but binds mannan with two calcium-independent sites. J. Mol. Biol. 2010;402:682–695. doi: 10.1016/j.jmb.2010.07.061. [DOI] [PubMed] [Google Scholar]
- 4.Lehotzky R.E., Partch C.L., Mukherjee S., Cash H.L., Goldman W.E., Gardner K.H., Hooper L.V. Molecular basis for peptidoglycan recognition by a bactericidal lectin. Proc. Natl. Acad. Sci. USA. 2010;107:7722–7727. doi: 10.1073/pnas.0909449107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Reggi M., Gharib B. Protein-X, pancreatic stone-, pancreatic thread-, reg-protein, P19, lithostathine, and now what? Characterization, structural analysis and putative function(s) of the major non-enzymatic protein of pancreatic secretions. Curr. Protein Pept. Sci. 2001;2:19–42. doi: 10.2174/1389203013381233. [DOI] [PubMed] [Google Scholar]
- 6.Graf R., Schiesser M., Scheele G.A., Marquardt K., Frick T.W., Ammann R.W., Bimmler D. A family of 16-kDa pancreatic secretory stress proteins form highly organized fibrillar structures upon tryptic activation. J. Biol. Chem. 2001;276:21028–21038. doi: 10.1074/jbc.M010717200. [DOI] [PubMed] [Google Scholar]
- 7.Sekikawa A., Fukui H., Suzuki K., Karibe T., Fujii S., Ichikawa K., Tomita S., Imura J., Shiratori K., Chiba T., et al. Involvement of the IL-22/REG Iα axis in ulcerative colitis. Lab. Invest. 2010;90:496–505. doi: 10.1038/labinvest.2009.147. [DOI] [PubMed] [Google Scholar]
- 8.Sun C., Fukui H., Hara K., Kitayama Y., Eda H., Yang M., Yamagishi H., Tomita T., Oshima T., Watari J., et al. Expression of Reg family genes in the gastrointestinal tract of mice treated with indomethacin. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;308:G736–G744. doi: 10.1152/ajpgi.00362.2014. [DOI] [PubMed] [Google Scholar]
- 9.Hakata Y., Fukui H., Sekikawa A., Yamagishi H., Ichikawa K., Tomita S., Imura J., Kawamata H., Imai Y., Fujimori T. Expression of β-catenin and REG Iα in relation to cell proliferative ability in salivary gland tumors. Exp. Ther. Med. 2010;1:437–443. doi: 10.3892/etm_00000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takasawa S. Regenerating gene (REG) product and its potential clinical usage. Expert Opin. Ther. Targets. 2016;20:541–550. doi: 10.1517/14728222.2016.1123691. [DOI] [PubMed] [Google Scholar]
- 11.Terazono K., Yamamoto H., Takasawa S., Shiga K., Yonemura Y., Tochino Y., Okamoto H. A novel gene activated in regenerating islets. J. Biol. Chem. 1988;263:2111–2114. doi: 10.1016/S0021-9258(18)69176-8. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe T., Yonemura Y., Yonekura H., Suzuki Y., Miyashita H., Sugiyama K., Moriizumi S., Unno M., Tanaka O., Kondo H., et al. Pancreatic beta-cell replication and amelioration of surgical diabetes by Reg protein. Proc. Natl. Acad. Sci. USA. 1994;91:3589–3592. doi: 10.1073/pnas.91.9.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parikh A., Stephan A.F., Tzanakakis E.S. Regenerating proteins and their expression, regulation and signaling. Biomol. Concepts. 2012;3:57–70. doi: 10.1515/bmc.2011.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tezel E., Nagasaka T., Tezel G., Kaneko T., Takasawa S., Okamoto H., Nakao A. REG I as a marker for human pancreatic acinoductular cells. Hepato-Gastroenterol. 2004;51:91–96. [PubMed] [Google Scholar]
- 15.Zenilman M.E., Magnuson T.H., Swinson K., Egan J., Perfetti R., Shuldiner A.R. Pancreatic thread protein is mitogenic to pancreatic-derived cells in culture. Gastroenterology. 1996;110:1208–1214. doi: 10.1053/gast.1996.v110.pm8613011. [DOI] [PubMed] [Google Scholar]
- 16.Asahara M., Mushiake S., Shimada S., Fukui H., Kinoshita Y., Kawanami C., Watanabe T., Tanaka S., Ichikawa A., Uchiyama Y., et al. Reg gene expression is increased in rat gastric enterochromaffin-like cells following water immersion stress. Gastroenterology. 1996;111:45–55. doi: 10.1053/gast.1996.v111.pm8698224. [DOI] [PubMed] [Google Scholar]
- 17.Fukui H., Kinoshita Y., Maekawa T., Okada A., Waki S., Hassan S., Okamoto H., Chiba T. Regenerating gene protein may mediate gastric mucosal proliferation induced by hypergastrinemia in rats. Gastroenterology. 1998;115:1483–1493. doi: 10.1016/S0016-5085(98)70027-7. [DOI] [PubMed] [Google Scholar]
- 18.Unno M., Yonekura H., Nakagawara K., Watanabe T., Miyashita H., Moriizumi S., Okamoto H., Itoh T., Teraoka H. Structure, chromosomal localization, and expression of mouse reg genes, reg I and reg II. A novel type of reg gene, reg II, exists in the mouse genome. J. Biol. Chem. 1993;268:15974–15982. doi: 10.1016/S0021-9258(18)82347-X. [DOI] [PubMed] [Google Scholar]
- 19.Ortiz E.M., Dusetti N.J., Vasseur S., Malka D., Bödeker H., Dagorn J.C., Iovanna J.L. The pancreatitis-associated protein is induced by free radicals in AR4-2J cells and confers cell resistance to apoptosis. Gastroenterology. 1998;114:808–816. doi: 10.1016/S0016-5085(98)70595-5. [DOI] [PubMed] [Google Scholar]
- 20.Malka D., Vasseur S., Bödeker H., Ortiz E.M., Dusetti N.J., Verrando P., Dagorn J.C., Iovanna J.L. Tumor necrosis factor alpha triggers antiapoptotic mechanisms in rat pancreatic cells through pancreatitis-associated protein I activation. Gastroenterology. 2000;119:816–828. doi: 10.1053/gast.2000.16491. [DOI] [PubMed] [Google Scholar]
- 21.Vasseur S., Folch-Puy E., Hlouschek V., Garcia S., Fiedler F., Lerch M.M., Dagorn J.C., Closa D., Iovanna J.L. p8 improves pancreatic response to acute pancreatitis by enhancing the expression of the anti-inflammatory protein pancreatitis-associated protein I. J. Biol. Chem. 2004;279:7199–7207. doi: 10.1074/jbc.M309152200. [DOI] [PubMed] [Google Scholar]
- 22.Ferrara J.L., Harris A.C., Greenson J.K., Braun T.M., Holler E., Teshima T., Levine J.E., Choi S.W., Huber E., Landfried K., et al. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood. 2011;118:6702–6708. doi: 10.1182/blood-2011-08-375006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao D., Kim Y.H., Jeong S., Greenson J.K., Chaudhry M.S., Hoepting M., Anderson E.R., van den Brink M.R.M., Peled J.U., Gomes A.L.C., et al. Survival signal REG3α prevents crypt apoptosis to control acute gastrointestinal graft-versus-host disease. J. Clin. Invest. 2018;128:4970–4979. doi: 10.1172/JCI99261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartupee J.C., Zhang H., Bonaldo M.F., Soares M.B., Dieckgraefe B.K. Isolation and characterization of a cDNA encoding a novel member of the human regenerating protein family: Reg IV. Biochim. Biophys. Acta. 2001;1518:287–293. doi: 10.1016/S0167-4781(00)00284-0. [DOI] [PubMed] [Google Scholar]
- 25.Li F.Y., Ren X.B., Xu E.P., Huang Q., Sheng H.Q., Lv B.J., Lai M.D. RegIV expression showing specificity to gastrointestinal tract and its potential role in diagnosing digestive tract neuroendocrine tumor. J. Zhejiang Univ. Sci. B. 2010;11:258–266. doi: 10.1631/jzus.B0900383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Numata M., Oshima T. Significance of regenerating islet-derived type IV gene expression in gastroenterological cancers. World J. Gastroenterol. 2012;18:3502–3510. doi: 10.3748/wjg.v18.i27.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi S., Akiyama T., Nata K., Abe M., Tajima M., Shervani N.J., Unno M., Matsuno S., Sasaki H., Takasawa S., et al. Identification of a receptor for reg (regenerating gene) protein, a pancreatic beta-cell regeneration factor. J. Biol. Chem. 2000;275:10723–10726. doi: 10.1074/jbc.275.15.10723. [DOI] [PubMed] [Google Scholar]
- 28.Takasawa S., Ikeda T., Akiyama T., Nata K., Nakagawa K., Shervani N.J., Noguchi N., Murakami-Kawaguchi S., Yamauchi A., Takahashi I., et al. Cyclin D1 activation through ATF-2 in Reg-induced pancreatic beta-cell regeneration. FEBS. Lett. 2006;580:585–591. doi: 10.1016/j.febslet.2005.12.070. [DOI] [PubMed] [Google Scholar]
- 29.Acquatella-Tran Van Ba I., Marchal S., Francois F., Silhol M., Lleres C., Michel B., Benyamin Y., Verdie J.M., Trousse F., Marcilhac A. Regenerating islet-derived 1α (Reg-1α) protein is new neuronal secreted factor that stimulates neurite outgrowth via exostosin Tumor-like 3 (EXTL3) receptor. J. Biol. Chem. 2012;287:4726–4739. doi: 10.1074/jbc.M111.260349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bishnupuri K.S., Luo Q., Murmu N., Houchen C.W., Anant S., Dieckgraefe B.K. Reg IV activates the epidermal growth factor receptor/Akt/AP-1 signaling pathway in colon adenocarcinomas. Gastroenterology. 2006;130:137–149. doi: 10.1053/j.gastro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Wang H., Hu L., Zang M., Zhang B., Duan Y., Fan Z., Li J., Su L., Yan M., Zhu Z., et al. REG4 promotes peritoneal metastasis of gastric cancer through GPR37. Oncotarget. 2016;7:27874–27888. doi: 10.18632/oncotarget.8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akiyama T., Takasawa S., Nata K., Kobayashi S., Abe M., Shervani N.J., Ikeda T., Nakagawa K., Unno M., Matsuno S., et al. Activation of Reg gene, a gene for insulin-producing beta -cell regeneration: Poly(ADP-ribose) polymerase binds Reg promoter and regulates the transcription by autopoly(ADP-ribosyl)ation. Proc. Natl. Acad. Sci. USA. 2001;98:48–53. doi: 10.1073/pnas.240458597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dusetti N.J., Mallo G.V., Ortiz E.M., Keim V., Dagorn J.C., Iovanna J.L. Induction of lithostathine/reg mRNA expression by serum from rats with acute pancreatitis and cytokines in pancreatic acinar AR-42J cells. Arch. Biochem. Biophys. 1996;330:129–132. doi: 10.1006/abbi.1996.0234. [DOI] [PubMed] [Google Scholar]
- 34.Kazumori H., Ishihara S., Hoshino E., Kawashima K., Moriyama N., Suetsugu H., Sato H., Adachi K., Fukuda R., Watanabe S., et al. Neutrophil chemoattractant 2 beta regulates expression of the Reg gene in injured gastric mucosa in rats. Gastroenterology. 2000;119:1610–1622. doi: 10.1053/gast.2000.20262. [DOI] [PubMed] [Google Scholar]
- 35.Yoshino N., Ishihara S., Rumi M.A., Ortega-Cava C.F., Yuki T., Kazumori H., Takazawa S., Okamoto H., Kadowaki Y., Kinoshita Y. Interleukin-8 regulates expression of Reg protein in Helicobacter pylori-infected gastric mucosa. Am. J. Gastroenterol. 2005;100:2157–2166. doi: 10.1111/j.1572-0241.2005.41915.x. [DOI] [PubMed] [Google Scholar]
- 36.Aggarwal S., Xie M.H., Maruoka M., Foster J., Gurney A.L. Acinar cells of the pancreas are a target of interleukin-22. J. Interferon Cytokine Res. 2001;21:1047–1053. doi: 10.1089/107999001317205178. [DOI] [PubMed] [Google Scholar]
- 37.Folch-Puy E., Granell S., Dagorn J.C., Iovanna J.L., Closa D. Pancreatitis-associated protein I suppresses NF-kappa B activation through a JAK/STAT-mediated mechanism in epithelial cells. J. Immunol. 2006;176:3774–3779. doi: 10.4049/jimmunol.176.6.3774. [DOI] [PubMed] [Google Scholar]
- 38.Sekikawa A., Fukui H., Fujii S., Ichikawa K., Tomita S., Imura J., Chiba T., Fujimori T. REG Iα protein mediates an anti-apoptotic effect of STAT3 signaling in gastric cancer cells. Carcinogenesis. 2008;29:76–83. doi: 10.1093/carcin/bgm250. [DOI] [PubMed] [Google Scholar]
- 39.Liu X., Wang J., Wang H., Yin G., Liu Y., Lei X., Xiang M. REG3A accelerates pancreatic cancer cell growth under IL-6-associated inflammatory condition: Involvement of a REG3A-JAK2/STAT3 positive feedback loop. Cancer Lett. 2015;362:45–60. doi: 10.1016/j.canlet.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Naito Y., Oue N., Hinoi T., Sakamoto N., Sentani K., Ohdan H., Yanagihara K., Sasaki H., Yasui W. Reg IV is a direct target of intestinal transcriptional factor CDX2 in gastric cancer. PLoS ONE. 2012;7:e47545. doi: 10.1371/journal.pone.0047545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strober W., Fuss I.J. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–1767. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogawa H., Fukushima K., Naito H., Funayama Y., Unno M., Takahashi K., Kitayama T., Matsuno S., Ohtani H., Takasawa S., et al. Increased expression of HIP/PAP and regenerating gene III in human inflammatory bowel disease and a murine bacterial reconstitution model. Inflamm. Bowel Dis. 2003;9:162–170. doi: 10.1097/00054725-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Xu X., Fukui H., Ran Y., Wang X., Inoue Y., Ebisudani N., Nishimura H., Tomita T., Oshima T., Watari J., et al. The link between type III Reg and STAT3-associated cytokines in inflamed colonic tissues. Mediat. Inflamm. 2019;2019:7859460. doi: 10.1155/2019/7859460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darnaud M., Dos Santos A., Gonzalez P., Augui S., Lacoste C., Desterke C., De Hertogh G., Valentino E., Braun E., Zheng J., et al. Enteric delivery of regenerating family member 3 alpha alters the intestinal microbiota and controls inflammation in mice with colitis. Gastroenterology. 2018;154:1009–1023. doi: 10.1053/j.gastro.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Shindo R., Katagiri T., Komazawa-Sakon S., Ohmuraya M., Takeda W., Nakagawa Y., Nakagata N., Sakuma T., Yamamoto T., Nishiyama C., et al. Regenerating islet-derived protein (Reg)3β plays a crucial role in attenuation of ileitis and colitis in mice. Biochem. Biophys. Rep. 2020;21:100738. doi: 10.1016/j.bbrep.2020.100738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bishnupuri K.S., Sainathan S.K., Bishnupuri K., Leahy D.R., Luo Q., Anant S., Houchen C.W., Dieckgraefe B.K. Reg4-induced mitogenesis involves Akt-GSK3β-β-Catenin-TCF-4 signaling in human colorectal cancer. Mol. Carcinog. 2014;53(Suppl. 1):E169–E180. doi: 10.1002/mc.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nanakin A., Fukui H., Fujii S., Sekikawa A., Kanda N., Hisatsune H., Seno H., Konda Y., Fujimori T., Chiba T. Expression of the REG IV gene in ulcerative colitis. Lab. Invest. 2007;87:304–314. doi: 10.1038/labinvest.3700507. [DOI] [PubMed] [Google Scholar]
- 48.Tsuchida C., Sakuramoto-Tsuchida S., Takeda M., Itaya-Hironaka A., Yamauchi A., Misu M., Shobatake R., Uchiyama T., Makino M., Pujol-Autonell I., et al. Expression of REG family genes in human inflammatory bowel diseases and its regulation. Biochem. Biophys. Rep. 2017;12:198–205. doi: 10.1016/j.bbrep.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takasawa S., Tsuchida C., Sakuramoto-Tsuchida S., Takeda M., Itaya-Hironaka A., Yamauchi A., Misu M., Shobatake R., Uchiyama T., Makino M., et al. Expression of human REG family genes in inflammatory bowel disease and their molecular mechanism. Immunol. Res. 2018;66:800–805. doi: 10.1007/s12026-019-9067-2. [DOI] [PubMed] [Google Scholar]
- 50.Fukui H., Franceschi F., Penland R.L., Sakai T., Sepulveda A.R., Fujimori T., Terano A., Chiba T., Genta R.M. Effects of Helicobacter pylori infection on the link between regenerating gene expression and serum gastrin levels in Mongolian gerbils. Lab. Invest. 2003;83:1777–1786. doi: 10.1097/01.LAB.0000106501.56339.CE. [DOI] [PubMed] [Google Scholar]
- 51.Kitayama Y., Fukui H., Hara K., Eda H., Kodani M., Yang M., Sun C., Yamagishi H., Tomita T., Oshima T., et al. Role of regenerating gene I in claudin expression and barrier function in the small intestine. Transl. Res. 2016;173:92–100. doi: 10.1016/j.trsl.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Peterson K.M., Guo X., Elkahloun A.G., Mondal D., Bardhan P.K., Sugawara A., Duggal P., Haque R., Petri W.A. The expression of REG 1A and REG 1B is increased during acute amebic colitis. Parasitol. Int. 2011;60:296–300. doi: 10.1016/j.parint.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Ampting M.T., Loonen L.M., Schonewille A.J., Konings I., Vink C., Iovanna J., Chamaillard M., Dekker J., van der Meer R., Wells J.M., et al. Intestinally secreted C-type lectin Reg3b attenuates salmonellosis but not listeriosis in mice. Infect. Immun. 2012;80:1115–1120. doi: 10.1128/IAI.06165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng Y., Valdez P.A., Danilenko D.M., Hu Y., Sa S.M., Gong Q., Abbas A.R., Modrusan Z., Ghilardi N., de Sauvage F.J., et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 55.Sasaki N., Sachs N., Wiebrands K., Ellenbroek S.I., Fumagalli A., Lyubimova A., Begthel H., van den Born M., van Es J.H., Karthaus W.R., et al. Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proc. Natl. Acad. Sci. USA. 2016;113:E5399–E5407. doi: 10.1073/pnas.1607327113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qi H., Wei J., Gao Y., Yang Y., Li Y., Zhu H., Su L., Su X., Zhang Y., Yang R. Reg4 and complement factor D prevent the overgrowth of E. coli in the mouse gut. Commun. Biol. 2020;3:483. doi: 10.1038/s42003-020-01219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujishiro M., Nozawa K., Kawasaki M., Yamaguchi A., Iwabuchi K., Yanagida M., Suzuki F., Miyazawa K., Fukui H., Kaneko K., et al. Regenerating gene (REG) 1 alpha promotes pannus progression in patients with rheumatoid arthritis. Mod. Rheumatol. 2012;22:228–237. doi: 10.3109/s10165-011-0564-y. [DOI] [PubMed] [Google Scholar]
- 58.Kimura T., Fukui H., Sekikawa A., Yamagishi H., Ichikawa K., Tomita S., Fujii S., Imura J., Kawamata H., Chiba T., et al. Involvement of REG Iα protein in the regeneration of ductal epithelial cells in the minor salivary glands of patients with Sjögren’s syndrome. Clin. Exp. Immunol. 2009;155:16–20. doi: 10.1111/j.1365-2249.2008.03806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshimoto K., Fujimoto T., Itaya-Hironaka A., Miyaoka T., Sakuramoto-Tsuchida S., Yamauchi A., Takeda M., Kasai T., Nakagawara K., Nonomura A., et al. Involvement of autoimmunity to REG, a regeneration factor, in patients with primary Sjögren’s syndrome. Clin. Exp. Immunol. 2013;174:1–9. doi: 10.1111/cei.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fujimura T., Fujimoto T., Itaya-Hironaka A., Miyaoka T., Yoshimoto K., Sakuramoto-Tsuchida S., Yamauchi A., Takeda M., Tsujinaka H., Tanaka Y., et al. Significance of interleukin-6/STAT pathway for the gene expression of REG Iα, a new autoantigen in Sjögren’s syndrome patients, in salivary duct epithelial cells. Clin. Rev. Allergy Immunol. 2017;52:351–363. doi: 10.1007/s12016-016-8570-7. [DOI] [PubMed] [Google Scholar]
- 61.Wijnands A.M., de Jong M.E., Lutgens M., Hoentjen F., Elias S.G., Oldenburg B. Dutch Initiative on Crohn and Colitis (ICC). Prognostic factors for advanced colorectal neoplasia in inflammatory bowel disease: Systematic review and meta-analysis. Gastroenterology. 2021;160:1584–1598. doi: 10.1053/j.gastro.2020.12.036. [DOI] [PubMed] [Google Scholar]
- 62.Nebbia M., Yassin N.A., Spinelli A. Colorectal cancer in inflammatory bowel disease. Clin. Colon Rectal Surg. 2020;33:305–317. doi: 10.1055/s-0040-1713748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rabbenou W., Ullman T.A. Risk of colon cancer and recommended surveillance strategies in patients with ulcerative colitis. Gastroenterol. Clin. North. Am. 2020;49:791–807. doi: 10.1016/j.gtc.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 64.Tanaka H., Fukui H., Fujii S., Sekikawa A., Yamagishi H., Ichikawa K., Tomita S., Imura J., Yasuda Y., Chiba T., et al. Immunohistochemical analysis of REG Iα expression in ulcerative colitis-associated neoplastic lesions. Digestion. 2011;83:204–209. doi: 10.1159/000321808. [DOI] [PubMed] [Google Scholar]
- 65.Sekikawa A., Fukui H., Fujii S., Nanakin A., Kanda N., Uenoyama Y., Sawabu T., Hisatsune H., Kusaka T., Ueno S., et al. Possible role of REG Iα protein in ulcerative colitis and colitic cancer. Gut. 2005;54:1437–1444. doi: 10.1136/gut.2004.053587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rafa L., Dessein A.F., Devisme L., Buob D., Truant S., Porchet N., Huet G., Buisine M., Lesuffleur T. REG4 acts as a mitogenic, motility and pro-invasive factor for colon cancer cells. Int. J. Oncol. 2010;36:689–698. doi: 10.3892/ijo_00000544. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y., Lai M., Lv B., Gu X., Wang H., Zhu Y., Zhu Y., Shao L., Wang G. Overexpression of Reg IV in colorectal adenoma. Cancer Lett. 2003;200:69–76. doi: 10.1016/S0304-3835(03)00460-9. [DOI] [PubMed] [Google Scholar]
- 68.Kinoshita Y., Ishihara S., Kadowaki Y., Fukui H., Chiba T. Reg protein is a unique growth factor of gastric mucosal cells. J. Gastroenterol. 2004;39:507–513. doi: 10.1007/s00535-004-1354-5. [DOI] [PubMed] [Google Scholar]
- 69.Kadowaki Y., Ishihara S., Miyaoka Y., Rumi M.A., Sato H., Kazumori H., Adachi K., Takasawa S., Okamoto H., Chiba T., et al. Reg protein is overexpressed in gastric cancer cells, where it activates a signal transduction pathway that converges on ERK1/2 to stimulate growth. FEBS. Lett. 2002;530:59–64. doi: 10.1016/S0014-5793(02)03398-7. [DOI] [PubMed] [Google Scholar]
- 70.Fukui H., Fujii S., Takeda J., Kayahara T., Sekikawa A., Nanakin A., Suzuki K., Hisatsune H., Seno H., Sawada M., et al. Expression of REG Iα protein in human gastric cancers. Digestion. 2004;69:177–184. doi: 10.1159/000078762. [DOI] [PubMed] [Google Scholar]
- 71.Sekikawa A., Fukui H., Fujii S., Takeda J., Nanakin A., Hisatsune H., Seno H., Takasawa S., Okamoto H., Fujimori T., et al. REG Iα protein may function as a trophic and/or anti-apoptotic factor in the development of gastric cancer. Gastroenterology. 2005;128:642–653. doi: 10.1053/j.gastro.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 72.Hara K., Fukui H., Sun C., Kitayama Y., Eda H., Yamasaki T., Kondo T., Tomita T., Oshima T., Watari J., et al. Effect of REG Iα protein on angiogenesis in gastric cancer tissues. Oncol. Rep. 2015;33:2183–2189. doi: 10.3892/or.2015.3878. [DOI] [PubMed] [Google Scholar]
- 73.Sekigawa I., Yanagida M., Iwabuchi K., Kaneda K., Kaneko H., Takasaki Y., Jung G., Sone S., Tanaka Y., Ogawa H., et al. Protein biomarker analysis by mass spectrometry in patients with rheumatoid arthritis receiving anti-tumor necrosis factor-alpha antibody therapy. Clin. Exp. Rheumatol. 2008;26:261–267. [PubMed] [Google Scholar]
- 74.Vives-Pi M., Takasawa S., Pujol-Autonell I., Planas R., Cabre E., Ojanguren I., Montraveta M., Santos A.L., Ruiz-Ortiz E. Biomarkers for diagnosis and monitoring of celiac disease. J. Clin. Gastroenterol. 2013;47:308–313. doi: 10.1097/MCG.0b013e31827874e3. [DOI] [PubMed] [Google Scholar]
- 75.Keel M., Harter L., Reding T., Sun L.K., Hersberger M., Seifert B., Bimmler D., Graf R. Pancreatic stone protein is highly increased during posttraumatic sepsis and activates neutrophil granulocytes. Crit. Care Med. 2009;37:1642–1648. doi: 10.1097/CCM.0b013e31819da7d6. [DOI] [PubMed] [Google Scholar]
- 76.Que Y.A., Delodder F., Guessous I., Graf R., Bain M., Calandra T., Liaudet L., Eggimann P. Pancreatic stone protein as an early biomarker predicting mortality in a prospective cohort of patients with sepsis requiring ICU management. Crit. Care. 2012;16:R114. doi: 10.1186/cc11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiri Z., Kyr M., Vavrina M., Fedora M. Pancreatic stone protein—a possible biomarker of multiorgan failure and mortality in children sepsis. Cytokine. 2014;66:106–111. doi: 10.1016/j.cyto.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 78.Fisher O.M., Oberkofler C.E., Raptis D.A., Soll C., Béchir M., Schiesser M., Graf R. Pancreatic stone protein (PSP) and pancreatitis-associated protein (PAP): A protocol of a cohort study on the diagnostic efficacy and prognostic value of PSP and PAP as postoperative markers of septic complications in patients undergoing abdominal surgery (PSP study) BMJ Open. 2014;4:e004914. doi: 10.1136/bmjopen-2014-004914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klein H.J., Csordas A., Falk V., Slankamenac K., Rudiger A., Schönrath F., Rodriguez Cetina Biefer H., Starck C.T., Graf R. Pancreatic stone protein predicts postoperative infection in cardiac surgery patients irrespective of cardiopulmonary bypass or surgical technique. PLoS ONE. 2015;10:e0120276. doi: 10.1371/journal.pone.0120276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yonemura Y., Sakurai S., Yamamoto H., Endou Y., Kawamura T., Bandou E., Elnemr A., Sugiyama K., Sasaki T., Akiyama T., et al. REG gene expression is associated with the infiltrating growth of gastric carcinoma. Cancer. 2003;98:1394–1400. doi: 10.1002/cncr.11658. [DOI] [PubMed] [Google Scholar]
- 81.Yamagishi H., Fukui H., Sekikawa A., Kono T., Fujii S., Ichikawa K., Tomita S., Imura J., Hiraishi H., Chiba T., et al. Expression profile of REG family proteins REG Iα and REG IV in advanced gastric cancer: Comparison with mucin phenotype and prognostic markers. Mod. Pathol. 2009;22:906–913. doi: 10.1038/modpathol.2009.41. [DOI] [PubMed] [Google Scholar]
- 82.Sekikawa A., Fukui H., Zhang X., Maruo T., Tsumura T., Okabe Y., Wakasa T., Osaki Y., Chiba T., Tomita T., et al. REG Iα is a biomarker for predicting response to chemotherapy with S-1 plus cisplatin in patients with unresectable stage IV gastric cancer. Br. J. Cancer. 2013;108:395–401. doi: 10.1038/bjc.2012.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mitani Y., Oue N., Matsumura S., Yoshida K., Noguchi T., Ito M., Tanaka S., Kuniyasu H., Kamata N., Yasui W. Reg IV is a serum biomarker for gastric cancer patients and predicts response to 5-fluorouracil-based chemotherapy. Oncogene. 2007;26:4383–4393. doi: 10.1038/sj.onc.1210215. [DOI] [PubMed] [Google Scholar]
- 84.Tao H.Q., He X.J., Ma Y.Y., Wang H.J., Xia Y.J., Ye Z.Y., Zhao Z.S. Evaluation of REG4 for early diagnosis and prognosis of gastric cancer. Hum. Pathol. 2011;42:1401–1409. doi: 10.1016/j.humpath.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 85.Zheng H.C., Sugawara A., Okamoto H., Takasawa S., Takahashi H., Masuda S., Takano Y. Expression profile of the REG gene family in colorectal carcinoma. J. Histochem. Cytochem. 2011;59:106–115. doi: 10.1369/jhc.2010.956961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Macadam R.C., Sarela A.I., Farmery S.M., Robinson P.A., Markham A.F., Guillou P.J. Death from early colorectal cancer is predicted by the presence of transcripts of the REG gene family. Br. J. Cancer. 2000;83:188–195. doi: 10.1054/bjoc.2000.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oue N., Kuniyasu H., Noguchi T., Sentani K., Ito M., Tanaka S., Setoyama T., Sakakura C., Natsugoe S., Yasui W. Serum concentration of Reg IV in patients with colorectal cancer: Overexpression and high serum levels of Reg IV are associated with liver metastasis. Oncology. 2007;72:371–380. doi: 10.1159/000113147. [DOI] [PubMed] [Google Scholar]
- 88.Kobunai T., Watanabe T., Fukusato T. REG4, NEIL2, and BIRC5 gene expression correlates with gamma-radiation sensitivity in patients with rectal cancer receiving radiotherapy. Anticancer Res. 2011;31:4147–4153. [PubMed] [Google Scholar]
- 89.Bishnupuri K.S., Luo Q., Sainathan S.K., Kikuchi K., Sureban S.M., Sabarinathan M., Gross J.H., Aden K., May R., Houchen C.W., et al. Reg IV regulates normal intestinal and colorectal cancer cell susceptibility to radiation-induced apoptosis. Gastroenterology. 2010;138:616–626, 626.e1–2. doi: 10.1053/j.gastro.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marafini I., Di Sabatino A., Zorzi F., Monteleone I., Sedda S., Cupi M.L., Antenucci C., Biancheri P., Giuffrida P., Di Stefano M., et al. Serum regenerating islet-derived 3-alpha is a biomarker of mucosal enteropathies. Aliment. Pharmacol. Ther. 2014;40:974–981. doi: 10.1111/apt.12920. [DOI] [PubMed] [Google Scholar]
- 91.Nunes T., Etchevers M.J., Sandi M.J., Pino Donnay S., Grandjean T., Pellisé M., Panés J., Ricart E., Iovanna J.L., Dagorn J.C., et al. Pancreatitis-associated protein does not predict disease relapse in inflammatory bowel disease patients. PLoS ONE. 2014;9:e84957. doi: 10.1371/journal.pone.0084957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Planell N., Lozano J.J., Mora-Buch R., Masamunt M.C., Jimeno M., Ordás I., Esteller M., Ricart E., Piqué J.M., Panés J., et al. Transcriptional analysis of the intestinal mucosa of patients with ulcerative colitis in remission reveals lasting epithelial cell alterations. Gut. 2013;62:967–976. doi: 10.1136/gutjnl-2012-303333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.