Abstract
Diabetes is a metabolic disease that involves the death or dysfunction of the insulin-secreting β cells in the pancreas. Consequently, most diabetes research is aimed at understanding the molecular and cellular bases of pancreatic development, islet formation, β-cell survival, and insulin secretion. Complex interactions of signaling pathways and transcription factor networks regulate the specification, growth, and differentiation of cell types in the developing pancreas. Many of the same regulators continue to modulate gene expression and cell fate of the adult pancreas. The transcription factor NEUROD1 is essential for the maturation of β cells and the expansion of the pancreatic islet cell mass. Mutations of the Neurod1 gene cause diabetes in humans and mice. However, the different aspects of the requirement of NEUROD1 for pancreas development are not fully understood. In this study, we investigated the role of NEUROD1 during the primary and secondary transitions of mouse pancreas development. We determined that the elimination of Neurod1 impairs the expression of key transcription factors for α- and β-cell differentiation, β-cell proliferation, insulin production, and islets of Langerhans formation. These findings demonstrate that the Neurod1 deletion altered the properties of α and β endocrine cells, resulting in severe neonatal diabetes, and thus, NEUROD1 is required for proper activation of the transcriptional network and differentiation of functional α and β cells.
Keywords: transcriptional network, pancreatic development, mouse model, genetic mutation, NEUROD1
1. Introduction
All forms of diabetes mellitus are characterized by the dysfunction and reduction of insulin-producing β-cells. The most critical step for understanding the pathophysiology of diabetes and for restoring lost and dysfunctional endocrine β cells is the identification of molecular cues that can be used for direct transformation in situ. Besides β cells, the pancreatic endocrine islets contain four additional hormone-secreting cell types; glucagon-secreting α cells, somatostatin-releasing ∂ cells, pancreatic polypeptide (PPY)-secreting PP cells, and a minority of ghrelin-producing ε cells [1,2]. In the mouse, all pancreatic endocrine cells differentiate from PDX1+ multipotent progenitors that transiently expressed Neurogenin 3 (Neurog3) [3]. The first endocrine cells appearing in the dorsal pancreatic bud at embryonic day (E) 9.5 are cells expressing glucagon and ghrelin [4,5,6]. The insulin expressing cells are detected around E11.5, followed by somatostatin+ and PPY+ cells [4,5]. Endocrine cell formation coincides with the two morphogenesis stages, the primary (E9.0–E12.5) and secondary (E13.5–E15.5) epithelial-to-mesenchymal transitions, during mouse pancreas development [7]. The formation of the endocrine islets begins with the secondary transition, and immature differentiated α and β cells exponentially proliferate to produce the islet cell mass [7,8,9]. These functionally immature cells undergo maturation to obtain a hormone-producing glucose-responsive phenotype during the late fetal and postnatal period [9].
Complex interactions of transcription factor networks regulate the specification, differentiation, expansion, and maturation of endocrine cells in the developing pancreas (as reviewed in [2,10]. NEUROD1, a basic helix–loop–helix (bHLH) transcription factor, is crucial for pancreatic development, as mice with deletions of Neurod1 die perinatally due to severe diabetes [11,12]. Mutations in the NEUROD1 gene in humans are linked to maturity-onset diabetes of the young (MODY), specifically MODY6 [13,14], and to susceptibility to the acute-onset of type I diabetes mellitus [15]. Inactivation of Neurod1 in human embryonic stem cells results in failure to activate a β cell transcriptional network and differentiate into functional β cells [12]. NEUROD1 together with PDX1, ISL1 and MAFA, are key transcription factors regulating insulin synthesis in pancreatic β cells in response to blood glucose [16]. Consistently with these observations, conditional deletion of Neurod1 in the insulin-producing cell population at the onset of their formation during pancreas development results in severe glucose intolerance and immature β cell characteristics, although these mice formed islets comparable in size to controls and survived to adulthood [17].
During mouse pancreatic development, Neurod1 mRNA is first expressed in the pancreatic primordium at E9.5 along with the first glucagon+ cells and continues to be expressed in the endocrine precursors of α and β cells during the first and second transition wave in the mouse [11]. Although glucagon-producing α and insulin-producing β cells are found during the secondary transition in the Neurod1-null pancreas, Neurod1 elimination results in the failure to increase β-cell mass due to reduced proliferation at E17.5 [12] or apoptosis [11], as it is in dispute. This fact suggests a functional role of NEUROD1 during the expansion phase of β cells. However, the role of NEUROD1 in early pancreas development and the differentiation of α and β cells is still unclear.
Given the importance of NEUROD1 in diabetes research, we examined different aspects of NEUROD1 requirements for pancreas development in this study. We eliminated Neurod1 during early pancreas development and performed detailed molecular analyses of primary and secondary transitions, and subsequent α and β cell differentiation, and organization of pancreatic islets. For the first time, we demonstrated the effects of NEUROD1 deficiency during the primary transition for the formation of the endocrine precursor population. Additionally, we showed that the elimination of Neurod1 impairs the expression of key transcription factors regulating pancreatic endocrine cell differentiation during the secondary transition of pancreas development, and thus, negatively, affecting the formation of α and β cells. Comparative molecular analyses revealed that the deletion of Neurod1 affected the transcriptional networks important for α- and β-cell differentiation and the proliferation potential of β cells, resulting in a significant reduction of functional islet cell mass, and disorganization of islet architecture in the developing pancreas.
2. Results
2.1. Conditional Deletion of Neurod1 Results in Neonatal Diabetes
To assess the role of Neurod1 in early pancreatic development, we introduced a somatic Neurod1 mutation using a floxed allele (Neurod1loxP/loxP) [18] and an Isl1Cre [19], generating Neurod1CKO conditional mutants with a Neurod1loxP/loxP;Isl1Cre/+ genotype (breeding scheme in Figure 1A). We compared Neurod1CKO mutants to control animals of Neurod1loxP/loxP or Neurod1loxP/+ genotypes. Neurod1CKO embryos were recovered at expected Mendelian ratios in the examined embryonic days (Figure 1B). However, Neurod1CKO did not survive postnatally due to severe neonatal diabetes (Figure 1C), corresponding to the phenotype of the global deletion of Neurod1 [11].
Figure 1.
Neurod1CKO mutants demonstrate a neonatal diabetic phenotype. (A) Breeding scheme shows genotypes for heterozygous (HET), homozygous mutant Neurod1CKO and control mice. The Isl1Cre transgene is transmitted paternally to eliminate any potential maternal influence on the developing embryos. (B) The survival of Neurod1CKO embryos is not affected, as shown by chi-squared test. (C) The blood glucose levels of control and Neurod1CKO pups fed ad libitum at P0 and P2-P3. Data are presented as mean ± SD, One-Way ANOVA (**** p < 0.0001).
2.2. Altered Formation of Endocrine Cells in Neurod1CKO during the Primary Transition of Pancreas Development Corresponds to NEUROD1 Elimination
Pancreas development begins with PDX1 expression and evagination of a dorsal pancreatic bud from the foregut endoderm around E8.5, followed by the formation of the ventral pancreatic bud [2]. PDX1 specifies multipotent pancreatic progenitors and is required for the formation of NEUROG3+ endocrine precursors [20]. In differentiating endocrine cells, PDX1 is restricted to β cells, and is downregulated in cells directed to the α fate, glucagon-expressing cells [21]. A loss of NEUROD1 expression and the formation of cell clusters co-expressing NEUROD1 and Isl1-Cre in Neurod1CKO was already detected in the PDX1+ pancreatic domain as early as E9.5 (Figure 2A). There were diminished delaminating NEUROD1+ clusters with a significant loss (61%) of NEUROD1 in the Neurod1CKO dorsal pancreas compared to the littermate controls at E10.5 (Figure 2A,B). The first sign of endocrine cell differentiation is the formation of glucagon-expressing cells in the dorsal pancreatic bud during the primary transition [5]; therefore, we first evaluated glucagon expression (Figure 3A,B). There was an increased number of cells co-expressing PDX1 and glucagon in the Neurod1CKO pancreas, indicating abnormalities in the differentiation of endocrine precursors (Figure 3A′,B′). The formation of glucagon+ cell clusters was unaffected in Neurod1CKO during the primary transition of the developing pancreas (Figure 3C–E). In contrast to littermate controls, insulin expressing cells were not detected in the Neurod1CKO pancreas at E12.5, indicating a delay in the differentiation of insulin+ endocrine cells (Figure 3F,G). Interestingly, there was a moderate reduction of cells expressing NEUROG3, a marker of early endocrine progenitors, in the dorsal pancreas of Neurod1CKO compared to littermate controls at E11.5 (Figure 3H–J), suggesting a possible regulatory feedback loop between Neurod1 and Neurog3 during the primary transition of pancreatic development.
Figure 2.
NEUROD1 is efficiently reduced during the primary transition of early pancreas development in Neurod1CKO. (A) Representative whole-mount immunolabeling of dorsal pancreas shows reduction of NEUROD1+ clusters in the PDX1+ pancreatic domain (green) of Neurod1CKO compare to the control pancreas at E9.5 and E10.5. Isl1Cre expression is indicated by anti-Cre antibody (red). Large NEUROD1+ and Isl1-Cre+ cell clusters are detected in the control dorsal pancreas (arrowheads) but not in Neurod1CKO. The inverted single-channel image shows NEUROD1 expression. Asterisks indicate autofluorescent red blood cells. Scale bar: 50 μm. (B) Quantification of the NEUROD1+ area as a percentage of the total PDX1+ area in the dorsal pancreas at E10.5 using ImageJ program. Data are presented as mean ± SD, n = 4 embryos/genotype, Unpaired t-test (*** p = 0.0005).
Figure 3.
The formation of glucagon+ clusters is unaffected but glucagon+ cells co-express PDX1 in Neurod1CKO during the primary transition. (A,B) Representative immunolabeling of the first endocrine cells expressing glucagon (GCG) is shown in the dorsal pancreatic epithelium delineated by the expression of PDX1 (whole-mounts). (A′,B′) Higher-magnification images show increased co-expression of PDX1 and GCG in Neurod1CKO compared to control pancreatic epithelium. (C–E) Representative whole-mount immunolabeling shows dorsal and ventral pancreatic buds with GCG+ cells at E12.5. GCG+ area in the dorsal pancreas was quantified using ImageJ program and depicted as a percentage of GCG+ of the PDX1+ area in control and Neurod1CKO littermates (E). (F,G) Insulin (INS) expression in endocrine clusters is immunodetected in the control pancreas (arrowheads) but not in Neurod1CKO at E12.5 (vibratome sections). (H,I) NEUROG3 expressing endocrine precursors are shown in the whole-mount of control and Neurod1CKO PDX1+ dorsal pancreas. (J) NEUROG3+ cells were counted in the PDX1+ dorsal pancreas at E11.5. Data are presented as mean ± SD, Unpaired t-test (* p = 0.0354). Scale bars: 50 μm.
2.3. Differentiation of α and β Cell Lineages Is Affected in the Neurod1CKO Pancreas during the Early Phase of the Secondary Transition
The period of the secondary transition is characterized by a major wave of endocrine cell differentiation, the formation of endocrine protoislets consisting of Neurog3+ endocrine progenitors delaminating from the bipotent trunk epithelium, and the presence of all five hormone-expressing endocrine lineages [2]. To define changes in endocrine differentiation in the Neurod1CKO pancreas during the secondary transition, we quantified mRNA levels of genes encoding transcription factors necessary for the differentiation of α and β cell lineages (Figure 4A), and pancreatic endocrine hormones (Figure 4B). These data indicate that the elimination of Neurod1 affected the formation of pancreatic endocrine α and β populations. We found a significant reduction in the mRNA expression of Arx, Pou3f4, Pax6, and MafB transcription factors that affect aspects of α-cell fate and differentiation [22,23,24,25]. Pax6 and MafB transcription factors are also crucial for other pancreas endocrine cell differentiation, particularly β cells [22,26]. Significantly decreased expression was also detected for MafA, Pax4, Insm1, Foxa2, Nkx2.2, and Pdx1 transcription factors that have been shown to regulate β cell differentiation [22,27,28,29,30]. Except for MafB, Neurog3, and Pou3f4, all these genes have been identified as direct targets of NEUROD1 in ChIP-Seq analysis of regulatory regions in murine islets [31]. Although all pancreatic endocrine cells differentiate from NEUROG3+ progenitors in mice, there are sequential competence states of these precursors to generate a subset of endocrine cell types [5,32]. Our qPCR analyses showed changes only in the expression of genes encoding insulin and glucagon endocrine hormones, but not PPY, ghrelin, and somatostatin (Figure 4B). Notably, the expression of Neurog3 was reduced in Neurod1CKO. This trend corresponds to a decrease in the number of cells expressing NEUROG3 at E11.5 (Figure 3J), indicating a negative effect of Neurod1 elimination on the formation of the endocrine NEUROG3+ progenitors. To determine whether decreased Neurog3 levels in the Neurod1CKO pancreas were due to decreased proliferation of NEUROG3+ progenitors, we evaluated proliferation of NEUROG3+ cells in the developing pancreas at E15.5, at which there is a peak in Neurog3 expression [5,32]. The numbers of proliferating NEUROG3+ cells were increased in the Neurod1CKO pancreas compared to controls (Figure 5).
Figure 4.
Reduced expression of genes important for the differentiation and function α and β cells in the Neurod1CKO pancreas at E14.5. Quantitative RT-PCR analyses of mRNA levels of transcription factors (A) and endocrine hormones (B) in E14.5 total pancreas of Neurod1CKO and control embryos. Genes highlighted in bold print indicate NEUROD1 target genes, as determined by ChIP-Seq analysis of NEUROD1 binding at mouse islet enhancers [31]. Data are presented as mean ± SEM (n = 8), Unpaired t-test (**** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05). Ins1, insulin 1; Ins2, insulin 2; Gcg, glucagon; Sst, somatostatin; Ppy, pancreatic polypeptide; Ghrl, ghrelin.
Figure 5.
Percentage of proliferating NEUROG3+ endocrine precursors is higher in the Neurod1CKO pancreas compared to the littermate controls. (A,B) Immunostaining for proliferating cell nuclear antigen Ki67 (red) in vibratome sections of the control and Neurod1CKO pancreas at E15.5 (arrows indicate NEUROG3+ proliferating cells). Nuclei are stained with Hoechst. (C) Quantification of proliferating NEUROG3+ cells per NEUROG3+ population (n = 3 embryos per genotype and 3–4 fields per section). Data are presented as mean ± SD, Unpaired t-test (* p = 0.03). Scale bar: 50 μm.
2.4. Deletion of Neurod1 Is Associated with Reduced Insulin Production, Disorganized Architecture of Islets of Langerhans, and Reduced Proliferation of β Cells
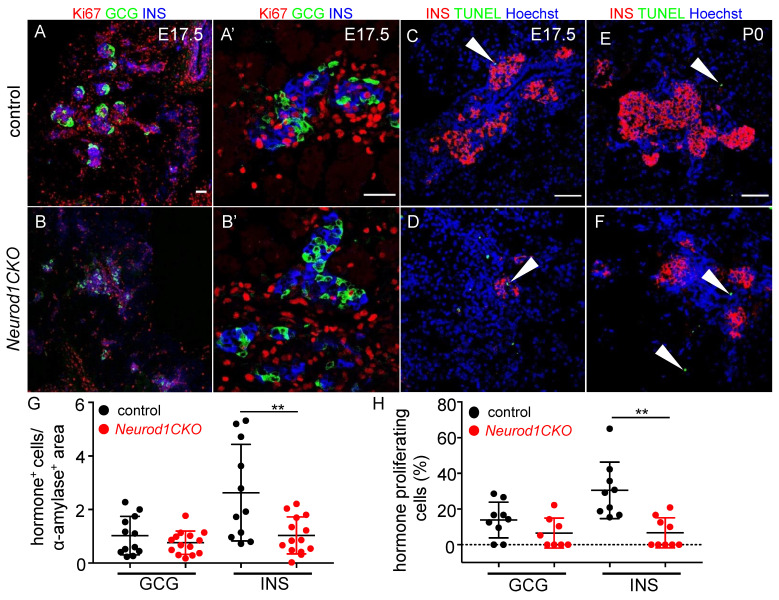
Next, we evaluated the formation of islets of Langerhans and the differentiation of α and β cells. The secondary transition wave from E13.5 to E15.5 is characterized by a massive differentiation of pancreatic lineages from delaminating epithelial cells [1,2,9]. Starting at E16, endocrine α and β cells proliferate to increase islet cell mass [9]. Consistent with the previous study [11], we found glucagon-producing α and insulin-producing β cells in the Neurod1CKO pancreas (Figure 6). While β cells co-expressing insulin and PDX1 (a differentiation marker for β cells) were present at E15.5, insulin production appeared reduced in Neurod1CKO (Figure 6A–B′). The reduced insulin production corresponds to the quantification of mRNA levels of insulin genes, Ins1 and Ins2, in the E14.5 Neurod1CKO pancreas. Compared to the control islets with β cells found in the core and α cells at the periphery, the Neurod1CKO architecture of islets of Langerhans was disorganized, with both cell types intermingled within the islets, as shown at E17.5 (Figure 6C–D′).
Figure 6.
The formation of pancreatic islets is altered in the Neurod1CKO pancreas. Representative images of immunolabeling showing glucagon (GCG) producing α cells and β cells expressing PDX1, a marker of differentiated β cells, and insulin (INS) in the control and Neurod1CKO pancreas at E15.5 (A,B), and E17.5 (C,D). Higher-magnification images show a detail of the architecture of the islets of Langerhans at E15.5 (A′,B′), and E17.5 (C′,D′). Note decreased INS production, GCG+ cells co-expressing PDX1 (arrowhead), and the disrupted β-cell core/α-cell mantle organization of the islets in Neurod1CKO. Scale bars: 50 μm.
Then, we questioned whether the expansion of β-cells is affected in the Neurod1CKO pancreas at E17.5, during a period characterized by a major expansion of the β cell population and islet cell mass production. Previous studies using Neurod1-null mice [11] and mice with a conditional Neurod1 deletion in the NEUROG3+ endocrine cells by Neurog3Cre [12] open to dispute the main reason for the diabetic phenotype and perinatal lethality of these Neurod1 mutants. The studies concluded that β cells differentiate during the second transition but fail to form islets due to reduced proliferation at E17.5 [12] or apoptosis [11]. To resolve this contradiction, we compared proliferation and apoptosis in β cells in our Neurod1CKO mutant at E17.5 (Figure 7). Consistent with previous reports, the morphometric quantification indicated that the insulin-producing cell mass was significantly decreased at E17.5 (Figure 7G). At this time point, we found a significant reduction of proliferating β cells (Figure 7A,B,A′,B′,H) without noticeable increase in apoptotic cells in Neurod1CKO, as evaluated by TUNEL (Figure 7C,D, the Supplementary Material, Table S1). We also analyzed apoptosis in the pancreas of newborn pups at P0, as at this stage, substantial apoptosis was also reported for Neurod1-null mice [11]. We did not detect measurably increased apoptosis in the Neurod1CKO pancreas (Figure 7E,F). This fact argues that the proliferative potential of β cells in the Neurod1CKO islets is significantly reduced, ruling out any significant contribution of apoptosis to the loss of β-cell mass.
Figure 7.
Proliferation potential of β cells is reduced in the Neurod1CKO pancreas. (A,B) Representative sections from the control and Neurod1CKO pancreas immunostained for proliferating cell nuclear antigen Ki67 (red) in endocrine α (glucagon, GCG) and β cells (insulin, INS) at E17.5. Note a disrupted formation of the islets of Langerhans in Neurod1CKO pancreas (A′,B′) Higher-magnification images show a detail of proliferating GCG+ and INS+ cells. (C–F) Expression analysis of insulin (INS) and TUNEL staining performed on sections of E17.5 and P0 pancreata. Arrowheads indicate TUNEL+ cells. Nuclei are stained with Hoechst. (G) Relative quantification of GCG+ and INS+ cells per α-amylase+ area (marker of exocrine tissue) and (H) the percentage of GCG+ and INS+ cells expressing Ki67 per total number of GCG+ and INS+ cells in 80-μm vibratome sections of the pancreas at E17.5. Data are presented as mean ± SD; n = 3/genotype/3–5 fields of view, Unpaired t-test (** p = 0.0056 (G); ** p = 0.0011 (H)). Scale bars: 50 μm.
2.5. Abnormalities in the Expression of Pancreatic Endocrine Markers Are Associated with a Severe Diabetic Phenotype of Neurod1CKO after Birth
Because of the abnormalities in the formation of islets of Langerhans at E17.5 with the altered distribution of α and β cells, and severe neonatal diabetes in Neurod1CKO, we further investigated the formation of islets and insulin production at P0. We found clusters of cells expressing PDX1, a marker of differentiated β cells, without any detectable insulin expression in Neurod1CKO (arrows in Figure 8B). Compared to α cells in the control pancreas (Figure 8A), we also found cells co-expressing glucagon and PDX1, indicating abnormalities in the differentiation of α cells (Figure 8B, arrowheads). Since insulin production was noticeably reduced in Neurod1CKO, we evaluated the protein level of proinsulin 1 and proinsulin 2, encoded by the Ins1 and Ins2 genes, respectively. Rodents express two nonallelic insulin genes, Ins1 and Ins2, and double deficiency for Ins1 and Ins2 results in acute diabetes and neonatal death [33]. Interestingly, the single Ins1−/− or Ins2−/− deletion mutants survive by β-cell mass increase to compensate for low insulin production [34]. We used anti-C-peptide 1 or anti-C-peptide 2 antibodies equimolar to insulin 1 and insulin 2, respectively, to compare the expression levels in pancreatic sections between Neurod1CKO and controls. A dramatic decrease in immunostaining for C-peptide 1 compared to C-peptide 2 was detected in the remaining β cells in the P0 Neurod1CKO pancreas (Figure 8C–F). Diminished levels of C-peptide 1 were also found in the Neurod1CKO pancreas at E17.5 (Figure 8G,H), indicating a reduced activation of the Ins1 gene during embryonic development. These data are consistent with the previous study showing that deletion of Neurod1 results in a loss of Ins1 expression, whereas Ins2 expression is relatively unaffected [17]. We used RT-qPCR to quantify the expression of hormones and selected β cell markers (Figure 8I). Significantly decreased insulin and glucagon mRNA levels corresponded to the reduction of α and β cells noted in the immunohistochemical morphometric analyses of the Neurod1CKO pancreas. We also detected a significant decrease in the mRNA Pdx1 [28] and MafA [35], essential markers of differentiated β cells, and reduced Pax6 levels indicating decreased endocrine cells in the Neurod1CKO pancreas [36]. Thus, abnormalities in the cytoarchitecture of islets of Langerhans, a failure to generate β-cell mass, and deficiency in Ins1 expression correlated with severe neonatal diabetes and postnatal lethal phenotype of Neurod1CKO mice.
Figure 8.
Insulin production is reduced in β cells and the differentiation of α cells is altered in the Neurod1CKO pancreas. (A,B) Representative vibratome sections from the control and Neurod1CKO pancreas show the expression of insulin (INS), glucagon (GCG) and PDX1, a marker of differentiated β cells. Note perturbations in the Neurod1CKO endocrine α cells co-expressing GCG and PDX1 (arrowheads), and β cells with PDX1 expressing cells without the expression of INS (arrows). (C–F) Immunostaining for C-peptide 1 and C-peptide 2 shows a significant loss of C-peptide 1 levels in the Neurod1CKO pancreas. Nuclei are stained with Hoechst. (G,H) Reduced expression of C-peptide 1 is noticeable in the Neurod1CKO pancreas compared to littermate control at E17.5. Arrowheads indicate INS expressing cells without C-peptide 1 expression. (I) Quantitative RT-PCR analyses of mRNA levels of transcription factors and endocrine hormones in P1 total pancreas of Neurod1CKO and controls, indicating a major loss of β and α cells in the Neurod1CKO mutant. Data are presented as mean ± SEM (n = 8), Unpaired t-test (**** p < 0.0001, *** p < 0.001, ** p < 0.01). Ins1, insulin 1; Ins2, insulin 2; Gcg, glucagon. Scale bars: 50 μm.
3. Discussion
The different aspects of NEUROD1 requirements for pancreas development are not fully understood. Our study revisited the major events of pancreas endocrine development to address the different regulatory roles of NEUROD1. We eliminated Neurod1 at the onset of pancreas endocrine cell formation and performed molecular and phenotypic analyses of different stages of pancreas development. We show for the first time that the elimination of Neurod1 significantly affected the transcription factor networks of α and β endocrine lineages during the early phase of the secondary transition of pancreas development, including reduced expression of Arx, Pdx1, Nkx2.2, MafA, MafB, Insm1, Pax6, Pax4, and Pou3f4. Although the generation of glucagon-producing cells during the first transition was unaffected in the Neurod1CKO pancreas compared to littermate controls, most of these cells co-expressed PDX1, indicating abnormalities in the differentiation of α endocrine cells. These results reveal a function of NEUROD1 in early α and β specification and differentiation processes of pancreatic progenitors prior to the endocrine cell expansion stage. Additionally, our study confirms a previous report that the proliferation potential of β cells in the Neurod1 deletion mutant is reduced, resulting in a diminished β-cell mass at birth [12]. However, we found significant abnormalities in the architecture of islets of Langerhans that cannot be explained only by the defects in the proliferation of β cells. Therefore, we propose that the leading cause of disorganized pancreatic islets and deficiency in β-cell proliferation in the Neurod1CKO pancreas is an altered transcriptional network, and thus, that NEUROD1 is required for the early stages of the developmental programs of α and β endocrine lineages, as well as for later stages of endocrine differentiation and maturation.
The role of NEUROD1 as a key transcription factor in the differentiation of β cells was shown in the differentiation of human embryonic stem cells (HESC) from pancreatic progenitors into insulin-producing cells [12]. Similar to our results, the elimination of Neurod1 in HESCs resulted in a significant reduction of the crucial β-cell transcription factors, including MafA, Pax6, Nkx2.2, Insm1, and Pdx1 [12]. Combinatorial interactions of Neurod1 and Nkx2.2 [37], and Neurod1 with Insm1 and Foxa2 are important for β-cell development and function. Furthermore, we showed that the levels of Arx, MafB, and Pou3f4 transcription factors essential for α cell differentiation were reduced together with aberrant co-expression of PDX1 and glucagon in the Neurod1CKO developing pancreas, indicating abnormalities in α-cell differentiation. This conclusion is consistent with the role of NEUROD1 in alpha cell lineage specification [37,38]. Surprisingly, we also found a significant reduction of NEUROG3+ endocrine progenitors and decreased Neurog3 expression in the Neurod1CKO pancreas during the primary and secondary transitions, at E11.5 and E14.5, respectively. These results suggest a putative interaction between NEUROD1 and NEUROG3 for the coordinated regulation of endocrine cell formation during early pancreas development. Our conclusions are in line with the finding of a study showing that the overlapping and integrative networks of the transcription factors NEUROD1 and NEUROG3 are essential for the differentiation of pancreatic endocrine cells, as the absence or presence of Neurod1 in the PDX1+ pancreatic progenitors affected the lineage potential and altered cell fate decisions [37]. Thus, our results demonstrate an important regulatory function of NEUROD1 for the differentiation of α and β pancreatic endocrine cells prior to the proliferation of these cells. These effects of Neurod1 deletion on early pancreas development may have previously been overlooked for two reasons: first, because of limited markers [11], and second, due to limited molecular analyses focused only on the later stage of pancreas development, the expansion phase of β cells at E17 [12] and on the formation of β cells [17]. Previously published conditional Neurod1 deletion mutants used either the BAC transgenic Neurog3-Cre driver [12] with reported Cre activity at E13.5 at the earliest [29,39] or Cre driver under the control of the rat insulin II promoter [40] limiting the deletion of Neurod1 to developing insulin-producing β cells [17]. Compared to these Neurod1 mutants, our model of Neurod1 deficiency in the developing pancreas during the primary transition demonstrates previously unappreciated roles of NEUROD1 in the formation of endocrine precursors and activity of transcriptional networks in developing α and β cells.
A recent study showing that endocrine differentiation and islet morphology are directly related [8] is in line with our conclusion that the abnormalities in the cytoarchitecture of pancreatic islets of Neurod1CKO are tied to the changes in the differentiation programs of endocrine α and β cells rather than only as a result of proliferation deficiency. This fact is further supported by the specific deletion of Neurod1 in developing β cells that did not affect β-cell numbers but disrupted pancreatic islet architecture [17]. Although the mechanisms regulating the formation of the islets of Langerhans during development remain unresolved, a loss of the core–mantle-like cytoarchitecture of islets has been associated with defects in β-cell differentiation [35,41,42,43].
Additionally, we showed that the remaining β cells lacking Neurod1 have reduced insulin expression resulting from diminished Ins1 gene transcription. A similar deficiency in Ins1 transcription was demonstrated in mice with Neurod1 deletion in developing insulin-producing β cells [17]. Although these mice survive postnatal development and form islets similar in size to those of controls, they are severely glucose intolerant. In contrast to this model, our Neurod1CKO mice form disorganized and reduced islets of Langerhans and die of severe diabetes soon after birth. Given the substantial reduction in the proliferation of β cells in the Neurod1CKO mutant, we hypothesized that the deficiency in Ins1 transcription and thus, reduced insulin production, cannot be compensated by increasing the β-cell mass, as reported for the single Ins1−/− deletion mutant [34], and therefore, contributing to the severe diabetes phenotype of our Neurod1CKO.
In summary, our study revisits the central events during pancreas development and provides novel insight into the role of NEUROD1. Our data indicate differential temporal and spatial transcriptional regulatory activities of NEUROD1 during pancreas development. In the course of primary and secondary transitions, Neurod1 elimination affected the expression of essential transcription factors for the differentiation of α and β lineages, indicating that NEUROD1 is required for the early α and β differentiation similar to human β-cell development. Furthermore, we postulate that changes in the differentiation programs of α and β cells result in a severe diabetic phenotype combining deficiency in insulin expression, diminished proliferation potential of β cells, and disorganized cytoarchitecture of pancreatic islets.
4. Materials and Methods
4.1. Mouse Model
All experiments using animals were performed according to protocols approved by the Animal Care and Use Ethics Committee of the Institute of Molecular Genetics, Czech Academy of Sciences (protocol code 98/2018 and date of approval 15 January 2019). The experimental mice were housed in a controlled environment (12-h light-12-h dark cycles) with free access to food and water. All experiments were performed with littermates (males and females) cross-bred from two transgenic mouse lines: floxed Neurod1 (Neurod1loxP/loxP) [18] and Isl1Cre (Isl1-Cre; Isl1tm1(cre)Sev/J) from The Jackson Laboratory. The expression of Isl1Cre during the formation of dorsal pancreatic bud at E9.5 corresponds to the expression of Isl1 gene [44], and it is an appropriate Cre driver for Neurod1loxP/LoxP elimination during the initial stage of endocrine pancreas development. Phenotypes were analyzed on a mixed C57Bl/6 × 129 genetic background and mutants were always compared with littermates. Breeding pairs contain a mouse with two floxed Neurod1 alleles (Neurod1loxP/loxP) and a mouse with one floxed Neurod1 allele together with one Isl1Cre allele (Isl1Cre/+;Neurod1loxP/+). The Isl1Cre transgene was transmitted only paternally in order to eliminate any potential influence of maternal ISL1 haploinsufficiency on the developing embryos. Genotyping was performed by PCR on tail DNA. The specific primers used were the following: Isl1-Cre F 5′-GCC TGC ATT ACC GGT CGA TGC AAC GA-3′ and Isl1-Cre R 5′-GTG GCA GAT GGC GCG GCA ACA CCA TT-3′ with a 700 bp product; Neurod1 F 5′-ACC ATG CAC TCT GTA CGC ATT-3′ and Neurod1 R 5′-GAG AAC TGA GAC ACT CAT CTG-3′ with a 400 bp product for the WT allele or 600 bp for the floxed allele. Heterozygous mice Isl1Cre/+;Neurod1loxP/+ (HET) were comparable to the control mice (Isl1+/+;Neurod1loxP/loxP or Isl1+/+;Neurod1loxP/+) without any detectable morphological and functional differences. Neurod1CKO offspring were recovered at expected Mendelian ratios from E9.5 to birth; chi-square p = 0.6131 (117 litters collected and genotyped, 212 Neurod1CKO: 241 HET: 462 control offspring). Blood glucose levels were measured in animals by glucometer (COUNTOUR TS, Bayer); blood glucose levels maintained above 13.9 mmol/L are classified as diabetic.
4.2. Immunohistochemistry
Embryos were dissected in cold PBS, and the dissected pancreas was fixed in 4% paraformaldehyde (PFA) in PBS. For vibratome sections, samples were embedded in 4% agarose and sectioned at 80 μm on a Leica VT1000S vibratome. Samples were then incubated with primary antibodies at 4 °C for 72 h. The primary antibodies used: anti-glucagon (1:400, ab10988, Abcam (Waltham, MA, USA) or 1:500, 4660-1140, BIO-RAD (Hercules, CA, USA)), anti-PDX1 (1:2000, ab47267, Abcam), anti-insulin (1:400, C27C9, Cell Signalling (Danvers, MA, USA) or 1:50, ab7842, Abcam), anti-Ngn3 (1:3, the F25A1B3 monoclonal antibody was obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242, USA), anti-alpha-amylase (1:2500, A8273, Sigma-Aldrich (Saint Louis, MO, USA)), anti-Ki67 (1:400, 9129, Cell Signalling), anti-Neurod1 (1:100, sc-1084, Santa Cruz Biotechnology (Dallas, TX, USA)), anti-Cre (1:500, 908001, BioLegend (San Diego, CA, USA)), and anti-peptide C1 and anti-peptide C2 (1:1000 and 1:3000, Beta Cell Biology Consortium). The secondary antibodies used were Alexa Fluor® 488 AffiniPure Goat Anti-Mouse IgG (1:500, #115-545-146, Jackson ImmunoResearch (West Grove, PA, USA)), Alexa Fluor® 594 AffiniPure Goat Anti-Rabbit (1:500, #111-585-144, Jackson ImmunoResearch), and DyLight488-conjugated AffiniPure Mouse Anti-Goat IgG (1:500, #205-485-108, Jackson ImmunoResearch). The nuclei were counterstained with Hoechst 33342. Image acquisition was completed using the Zeiss LSM 880 NLO scanning confocal, with ZEN lite program. The number of insulin+, NEUROG3+, and Ki67+ cells was counted in vibratome sections with the largest pancreatic footprint per photographic field using the Cell Counter plugin of ImageJ program (NIH), as described [45]. We analyzed 6 sections of the pancreas from 3 embryos for each genotype. Tissue paraffin sections (8 μm) of dissected E17.5 and P0 pancreases were treated with 20 μg/mL proteinase K for 20 min at room temperature. The sections were incubated with the TUNEL labeling kit (Roche, Basel, Switzerland) for 1 h at 37 °C, Hoechst 33,342 was used as a nuclear counterstain, and immunolabeled by anti-insulin antibody. The sections were analyzed under a Nikon Eclipse E400 fluorescent microscope.
4.3. Reverse Transcription-Quantitative Real-Time Polymerase Chain Reaction
RT-qPCR was performed as described previously [46]. Briefly, total RNA was isolated from the whole pancreas at embryonic day E14.5 or postnatal day P1 (n = 8 samples/group) by Trizol RNA extraction. Following RT, quantitative real-time PCR (qPCR) was performed with the initial AmpliTaq activation at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 30 s, as described. The Hprt1 gene was selected as the best reference gene for our analyses from a panel of 12 control genes (TATAA Biocenter AB, Sweden). The relative expression of a target gene was calculated based on qPCR efficiencies and the quantification cycle (Cq) difference (Δ) of an experimental sample (mutant) versus control (the 2−ΔΔCt method). Primers were designed using Primer Blast tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/ (accessed on 13 July 2018 and 15 January 2018)). Primers were selected according to the following parameters: length between 18 and 24 bases, melting temperature (Tm) between 58° C and 60 °C, G + C content between 40 and 60% (optimal 50%) and efficiency above 80%. Primer sequences are presented in the Supplementary Material, Table S2.
4.4. Quantification and Statistical Analysis
Data in the figures were represented as mean ± SD (standard error deviation) or ±SEM (standard error of mean). Chi-square test, Student’s t-test and One-Way ANOVA were used for statistical comparison between groups that are normally distributed using the built-in function of GraphPad Prism 7 program (GraphPad Software, San Diego, CA, USA). p-values of less than 0.05 were considered significant. Significance was determined as p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), or p < 0.0001 (****). Sample sizes and individual statistical results for all analyses are provided in the figure legends.
Acknowledgments
A. Pavlinek (King’s College London) for editing the MS. We acknowledge Imaging Methods Core Facility at BIOCEV supported by the MEYS CR (Large RI Project LM2018129 Czech-BioImaging) and ERDF (project No. CZ.02.1.01/0.0/0.0/18_046/0016045) for its support with obtaining imaging data presented in this paper.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms22136713/s1, Table S1: Quantification of apoptotic cells in the E17.5 pancreas, Table S2: Primer sequences for qPCR.
Author Contributions
Conceptualization, F.S., and G.P.; Methodology, R.B., O.S., Z.N., and Z.B.; Experimental work and Analyses, R.B., O.S., J.M., and Z.B.; Writing—Original Draft Preparation, G.P.; Writing—Review & Editing, R.B., O.S., G.P., and F.S.; Funding Acquisition, G.P. and O.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Czech Science Foundation (No. 19-07378S); from Charles University Grant Agency (No. 176120); and by the institutional support of the Czech Academy of Sciences RVO: 86652036.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Animal Care and Use Ethics Committee of the Institute of Molecular Genetics, Czech Academy of Sciences (protocol code 98/2018 and date of approval 15 January 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pan F.C., Wright C. Pancreas organogenesis: From bud to plexus to gland. Dev. Dyn. 2011;240:530–565. doi: 10.1002/dvdy.22584. [DOI] [PubMed] [Google Scholar]
- 2.Bastidas-Ponce A., Scheibner K., Lickert H., Bakhti M. Cellular and molecular mechanisms coordinating pancreas development. Development. 2017;144:2873–2888. doi: 10.1242/dev.140756. [DOI] [PubMed] [Google Scholar]
- 3.Gu G., Dubauskaite J., Melton D.A. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 4.Herrera P.L., Huarte J., Sanvito F., Meda P., Orci L., Vassalli J.D. Embryogenesis of the murine endocrine pancreas; early expression of pancreatic polypeptide gene. Development. 1991;113:1257–1265. doi: 10.1242/dev.113.4.1257. [DOI] [PubMed] [Google Scholar]
- 5.Johansson K.A., Dursun U., Jordan N., Gu G., Beermann F., Gradwohl G., Grapin-Botton A. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev. Cell. 2007;12:457–465. doi: 10.1016/j.devcel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Heller R.S., Jenny M., Collombat P., Mansouri A., Tomasetto C., Madsen O.D., Mellitzer G., Gradwohl G., Serup P. Genetic determinants of pancreatic epsilon-cell development. Dev. Biol. 2005;286:217–224. doi: 10.1016/j.ydbio.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 7.Bastidas-Ponce A., Tritschler S., Dony L., Scheibner K., Tarquis-Medina M., Salinno C., Schirge S., Burtscher I., Bottcher A., Theis F.J., et al. Comprehensive single cell mRNA profiling reveals a detailed roadmap for pancreatic endocrinogenesis. Development. 2019;146 doi: 10.1242/dev.173849. [DOI] [PubMed] [Google Scholar]
- 8.Sharon N., Chawla R., Mueller J., Vanderhooft J., Whitehorn L.J., Rosenthal B., Gurtler M., Estanboulieh R.R., Shvartsman D., Gifford D.K., et al. A Peninsular Structure Coordinates Asynchronous Differentiation with Morphogenesis to Generate Pancreatic Islets. Cell. 2019;176:790–804.e13. doi: 10.1016/j.cell.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu W.L., Zhang Y.W., Feng Y., Li L.C., Yang L., Xu C.R. Deciphering Pancreatic Islet beta Cell and alpha Cell Maturation Pathways and Characteristic Features at the Single-Cell Level. Cell Metab. 2017;25:1194–1205.e4. doi: 10.1016/j.cmet.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Jennings R.E., Scharfmann R., Staels W. Transcription factors that shape the mammalian pancreas. Diabetologia. 2020;63:1974–1980. doi: 10.1007/s00125-020-05161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naya F.J., Huang H.P., Qiu Y., Mutoh H., DeMayo F.J., Leiter A.B., Tsai M.J. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev. 1997;11:2323–2334. doi: 10.1101/gad.11.18.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romer A.I., Singer R.A., Sui L., Egli D., Sussel L. Murine Perinatal beta-Cell Proliferation and the Differentiation of Human Stem Cell-Derived Insulin-Expressing Cells Require NEUROD1. Diabetes. 2019;68:2259–2271. doi: 10.2337/db19-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malecki M.T., Jhala U.S., Antonellis A., Fields L., Doria A., Orban T., Saad M., Warram J.H., Montminy M., Krolewski A.S. Mutations in NEUROD1 are associated with the development of type 2 diabetes mellitus. Nat. Genet. 1999;23:323–328. doi: 10.1038/15500. [DOI] [PubMed] [Google Scholar]
- 14.Rubio-Cabezas O., Minton J.A., Kantor I., Williams D., Ellard S., Hattersley A.T. Homozygous mutations in NEUROD1 are responsible for a novel syndrome of permanent neonatal diabetes and neurological abnormalities. Diabetes. 2010;59:2326–2331. doi: 10.2337/db10-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamada S., Motohashi Y., Yanagawa T., Maruyama T., Kasuga A., Hirose H., Matsubara K., Shimada A., Saruta T. NeuroD/beta2 gene G-->A polymorphism may affect onset pattern of type 1 diabetes in Japanese. Diabetes Care. 2001;24:1438–1441. doi: 10.2337/diacare.24.8.1438. [DOI] [PubMed] [Google Scholar]
- 16.Qiu Y., Guo M., Huang S., Stein R. Insulin gene transcription is mediated by interactions between the p300 coactivator and PDX-1, BETA2, and E47. Mol. Cell Biol. 2002;22:412–420. doi: 10.1128/MCB.22.2.412-420.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu C., Stein G.H., Pan N., Goebbels S., Hornberg H., Nave K.A., Herrera P., White P., Kaestner K.H., Sussel L., et al. Pancreatic beta cells require NeuroD to achieve and maintain functional maturity. Cell Metab. 2010;11:298–310. doi: 10.1016/j.cmet.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goebbels S., Bode U., Pieper A., Funfschilling U., Schwab M.H., Nave K.A. Cre/loxP-mediated inactivation of the bHLH transcription factor gene NeuroD/BETA2. Genesis. 2005;42:247–252. doi: 10.1002/gene.20138. [DOI] [PubMed] [Google Scholar]
- 19.Yang L., Cai C.L., Lin L., Qyang Y., Chung C., Monteiro R.M., Mummery C.L., Fishman G.I., Cogen A., Evans S. Isl1Cre reveals a common Bmp pathway in heart and limb development. Development. 2006;133:1575–1585. doi: 10.1242/dev.02322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burlison J.S., Long Q., Fujitani Y., Wright C.V., Magnuson M.A. Pdx-1 and Ptf1a concurrently determine fate specification of pancreatic multipotent progenitor cells. Dev. Biol. 2008;316:74–86. doi: 10.1016/j.ydbio.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y.P., Thorel F., Boyer D.F., Herrera P.L., Wright C.V. Context-specific alpha- to-beta-cell reprogramming by forced Pdx1 expression. Genes Dev. 2011;25:1680–1685. doi: 10.1101/gad.16875711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Artner I., Le Lay J., Hang Y., Elghazi L., Schisler J.C., Henderson E., Sosa-Pineda B., Stein R. MafB: An activator of the glucagon gene expressed in developing islet alpha- and beta-cells. Diabetes. 2006;55:297–304. doi: 10.2337/diabetes.55.02.06.db05-0946. [DOI] [PubMed] [Google Scholar]
- 23.Gosmain Y., Cheyssac C., Heddad Masson M., Dibner C., Philippe J. Glucagon gene expression in the endocrine pancreas: The role of the transcription factor Pax6 in alpha-cell differentiation, glucagon biosynthesis and secretion. Diabetes Obes. Metab. 2011;13(Suppl. 1):31–38. doi: 10.1111/j.1463-1326.2011.01445.x. [DOI] [PubMed] [Google Scholar]
- 24.Collombat P., Mansouri A., Hecksher-Sorensen J., Serup P., Krull J., Gradwohl G., Gruss P. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev. 2003;17:2591–2603. doi: 10.1101/gad.269003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heller R.S., Stoffers D.A., Liu A., Schedl A., Crenshaw E.B., 3rd, Madsen O.D., Serup P. The role of Brn4/Pou3f4 and Pax6 in forming the pancreatic glucagon cell identity. Dev. Biol. 2004;268:123–134. doi: 10.1016/j.ydbio.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Gosmain Y., Katz L.S., Masson M.H., Cheyssac C., Poisson C., Philippe J. Pax6 is crucial for beta-cell function, insulin biosynthesis, and glucose-induced insulin secretion. Mol. Endocrinol. 2012;26:696–709. doi: 10.1210/me.2011-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia S., Ivanov A., Blasevic D., Muller T., Purfurst B., Sun W., Chen W., Poy M.N., Rajewsky N., Birchmeier C. Insm1 cooperates with Neurod1 and Foxa2 to maintain mature pancreatic beta-cell function. EMBO J. 2015;34:1417–1433. doi: 10.15252/embj.201490819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao T., McKenna B., Li C., Reichert M., Nguyen J., Singh T., Yang C., Pannikar A., Doliba N., Zhang T., et al. Pdx1 maintains beta cell identity and function by repressing an alpha cell program. Cell Metab. 2014;19:259–271. doi: 10.1016/j.cmet.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mastracci T.L., Wilcox C.L., Arnes L., Panea C., Golden J.A., May C.L., Sussel L. Nkx2.2 and Arx genetically interact to regulate pancreatic endocrine cell development and endocrine hormone expression. Dev. Biol. 2011;359:1–11. doi: 10.1016/j.ydbio.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osipovich A.B., Long Q., Manduchi E., Gangula R., Hipkens S.B., Schneider J., Okubo T., Stoeckert C.J., Jr., Takada S., Magnuson M.A. Insm1 promotes endocrine cell differentiation by modulating the expression of a network of genes that includes Neurog3 and Ripply3. Development. 2014;141:2939–2949. doi: 10.1242/dev.104810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tennant B.R., Robertson A.G., Kramer M., Li L., Zhang X., Beach M., Thiessen N., Chiu R., Mungall K., Whiting C.J., et al. Identification and analysis of murine pancreatic islet enhancers. Diabetologia. 2013;56:542–552. doi: 10.1007/s00125-012-2797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J., Banerjee A., Herring C.A., Attalla J., Hu R., Xu Y., Shao Q., Simmons A.J., Dadi P.K., Wang S., et al. Neurog3-Independent Methylation Is the Earliest Detectable Mark Distinguishing Pancreatic Progenitor Identity. Dev. Cell. 2019;48:49–63.e7. doi: 10.1016/j.devcel.2018.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duvillie B., Cordonnier N., Deltour L., Dandoy-Dron F., Itier J.M., Monthioux E., Jami J., Joshi R.L., Bucchini D. Phenotypic alterations in insulin-deficient mutant mice. Proc. Natl. Acad. Sci. USA. 1997;94:5137–5140. doi: 10.1073/pnas.94.10.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leroux L., Desbois P., Lamotte L., Duvillie B., Cordonnier N., Jackerott M., Jami J., Bucchini D., Joshi R.L. Compensatory responses in mice carrying a null mutation for Ins1 or Ins2. Diabetes. 2001;50(Suppl. 1):S150. doi: 10.2337/diabetes.50.2007.S150. [DOI] [PubMed] [Google Scholar]
- 35.Artner I., Hang Y., Mazur M., Yamamoto T., Guo M., Lindner J., Magnuson M.A., Stein R. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes. 2010;59:2530–2539. doi: 10.2337/db10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krentz N.A.J., Lee M.Y.Y., Xu E.E., Sproul S.L.J., Maslova A., Sasaki S., Lynn F.C. Single-Cell Transcriptome Profiling of Mouse and hESC-Derived Pancreatic Progenitors. Stem Cell Rep. 2018;11:1551–1564. doi: 10.1016/j.stemcr.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mastracci T.L., Anderson K.R., Papizan J.B., Sussel L. Regulation of Neurod1 contributes to the lineage potential of Neurogenin3+ endocrine precursor cells in the pancreas. PLoS Genet. 2013;9:e1003278. doi: 10.1371/journal.pgen.1003278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chao C.S., Loomis Z.L., Lee J.E., Sussel L. Genetic identification of a novel NeuroD1 function in the early differentiation of islet alpha, PP and epsilon cells. Dev. Biol. 2007;312:523–532. doi: 10.1016/j.ydbio.2007.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schonhoff S.E., Giel-Moloney M., Leiter A.B. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev. Biol. 2004;270:443–454. doi: 10.1016/j.ydbio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 40.Herrera P.L. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- 41.Gannon M., Ray M.K., Van Zee K., Rausa F., Costa R.H., Wright C.V. Persistent expression of HNF6 in islet endocrine cells causes disrupted islet architecture and loss of beta cell function. Development. 2000;127:2883–2895. doi: 10.1242/dev.127.13.2883. [DOI] [PubMed] [Google Scholar]
- 42.Doyle M.J., Sussel L. Nkx2.2 regulates beta-cell function in the mature islet. Diabetes. 2007;56:1999–2007. doi: 10.2337/db06-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Du A., Hunter C.S., Murray J., Noble D., Cai C.L., Evans S.M., Stein R., May C.L. Islet-1 is required for the maturation, proliferation, and survival of the endocrine pancreas. Diabetes. 2009;58:2059–2069. doi: 10.2337/db08-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahlgren U., Pfaff S.L., Jessell T.M., Edlund T., Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385:257–260. doi: 10.1038/385257a0. [DOI] [PubMed] [Google Scholar]
- 45.Bohuslavova R., Cerychova R., Papousek F., Olejnickova V., Bartos M., Gorlach A., Kolar F., Sedmera D., Semenza G.L., Pavlinkova G. HIF-1alpha is required for development of the sympathetic nervous system. Proc. Natl. Acad. Sci. USA. 2019;116:13414–13423. doi: 10.1073/pnas.1903510116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cerychova R., Bohuslavova R., Papousek F., Sedmera D., Abaffy P., Benes V., Kolar F., Pavlinkova G. Adverse effects of Hif1a mutation and maternal diabetes on the offspring heart. Cardiovasc. Diabetol. 2018;17:68. doi: 10.1186/s12933-018-0713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article.