Abstract
Erythropoietin (EPO) acts on multiple tissues through its receptor EPOR, a member of a cytokine class I receptor superfamily with pleiotropic effects. The interaction of EPO and EPOR triggers the activation of several signaling pathways that induce erythropoiesis, including JAK2/STAT5, PI3K/AKT, and MAPK. The canonical EPOR/JAK2/STAT5 pathway is a known regulator of differentiation, proliferation, and cell survival of erythroid progenitors. In addition, its role in the protection of other cells, including cancer cells, is under intense investigation. The involvement of EPOR/JAK2/STAT5 in other processes such as mRNA splicing, cytoskeleton reorganization, and cell metabolism has been recently described. The transcriptomics, proteomics, and epigenetic studies reviewed in this article provide a detailed understanding of EPO signalization. Advances in this area of research may be useful for improving the efficacy of EPO therapy in hematologic disorders, as well as in cancer treatment.
Keywords: STAT5, erythropoietin, erythropoietin receptor
1. STAT5 Signaling and Erythropoiesis
In classical erythropoiesis, erythropoietin (EPO) binds to an EPO receptor (EPOR) homo-dimer, activating EPO/EPOR signal transduction. EPO has two non-identical binding sites on EPORs: a high-affinity G151 (nano-molar) on the first and a low-affinity R103 (micro-molar) on the second receptor. The main signal cascades activated by EPO are JAK2/STAT5 cascade, phosphatidylinositol 3-kinase (PI3K) cascade, RAS/MAP kinase cascade, and protein kinase C (PKC) cascade [1].
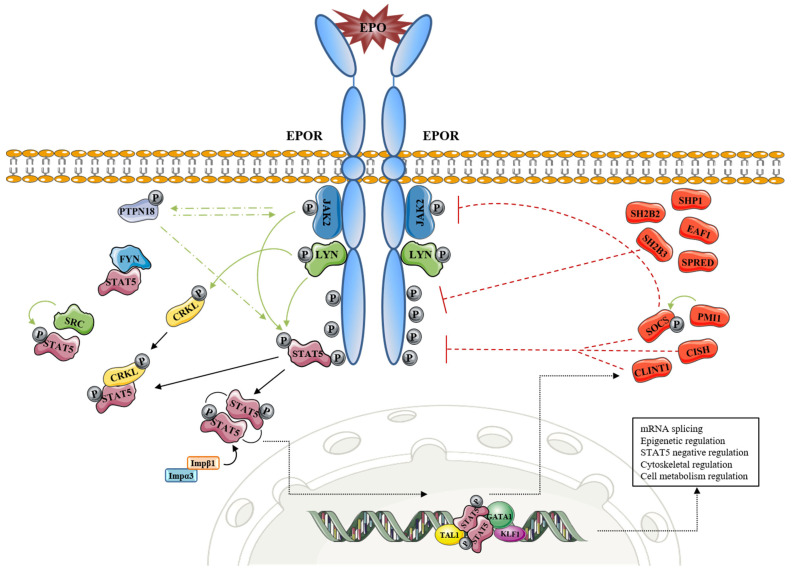
Janus kinase 2 (JAK2) is a non-receptor tyrosine kinase that represents a pivotal role in signal transduction via its association with EPOR. There are diverse models of EPO/EPOR signaling. One supposes that EPO binding stimulates dimerization of the EPOR, which is important for signalization [2]. Another suggests the existence of a pre-assembled EPOR homodimer and JAK2 attached via the box1-2 motif to each monomer of EPOR. EPO induces a conformational twist of the two EPOR monomers, resulting in cross-inhibition of JAK2 kinase domains and JAK2 phosphorylation [3]. Investigations by McGraw et al. [4] have shown that EPO also induces EPOR translocation to membrane rafts, accompanied by the incorporation of JAK2, STAT5, and tyrosine-protein kinase LYN.
Signal transducer and activator of transcription 5 (STAT5) refer to two proteins that share 94% structural homology and are transcribed from separate genes, STAT5A and STAT5B. However, they are both essential in erythroid differentiation [5]. STAT5 protein is recruited to phosphorylated tyrosine residue 344 (pY343) of the EPOR via its SH2 domain [6] and is phosphorylated by JAK2, although non-tyrosine-containing sequences in the cytoplasmic region of EPOR can also stimulate STAT5 [7]. Subsequently, STAT5 undergoes a conformational change and forms parallel dimers via the SH2 domain. Such conformational reorganization is possible due to the flexible linkers that connect the core fragment of STAT5 with its N- and C-terminus [8]. Established STAT5 dimers are recognized by importins—importin α3 and β1, respectively—which allow them to translocate into the nucleus [9]. STAT5 binds as a dimer or tetramer to palindromic gamma-activated sequences called GAS0 motifs (TTCYXRGAA) in promoters and enhancers of target genes, resulting in their transcription [10]. The proto-oncogene tyrosine-protein kinase Src (SRC) can directly phosphorylate tyrosine residue 694 (pY694) of STAT5 and so contribute to EPO-induced signal transduction via STAT5 [11]. FYN, an Src-family tyrosine kinase, also participates in the EPO signaling pathway, since Fyn−/− mice exhibit reduced tyrosine phosphorylation of EPOR and decreased STAT5 activity. The importance of FYN in erythropoiesis is also supported by the blunted responsiveness of Fyn−/− mice to stress erythropoiesis [12]. Another member of the Src-family tyrosine kinases, LYN, is involved in tyrosine phosphorylation and activation of STAT5, mainly on tyrosine residue 694 (pY694) [13]. Besides, CRK-like adapter protein (CRKL) seemed to have a role in STAT5 activation since direct binding of CRKL to STAT5 was observed after EPO stimulation in an (EPO)-dependent cell line UT-7/EPO [14].
Recently, 121 new signals and/or EPO/EPOR/JAK2 target proteins, together with their EPO-modulated domains and phosphosites (e.g., pT349, pS358 and others), were identified. These included actin assemblage modifiers with adaptors DLG-1, DLG-3, WAS, WASL, and CD2AP, and erythroid cytoskeletal targets such as spectrin A, spectrin B, adducin 2, and glycophorin C. Metabolic regulators such as aldolase A, pyruvate dehydrogenase α1, and thioredoxin-interacting protein were also found as an EPO target set [15]. Furthermore, the same authors identified several novel EPO-modified kinases and phosphatases, including membrane palmitoylated protein 1, guanylate kinase 1, pseudopodium enriched atypical kinase 1, AP2 associated kinase 1, protein tyrosine phosphatase receptor type A, phosphohistidine phosphatase 1, tensin 2, ubiquitin-associated and SH3 domain-containing B (UBASH3B), and protein tyrosine phosphatase non-receptor type 18 (PTPN18). Due to its high expression in hematopoietic progenitors, PTPN18 has been further investigated, and it was revealed that PTPN18 promotes ERK1/2, AKT, STAT5, and JAK2 signals for EPO-dependent hematopoietic cell growth (Figure 1, Table 1) [15].
Figure 1.
EPOR-STAT5 signaling and its involvement in processes other than inhibition of apoptosis, cell cycle progression, and cell cycle arrest. Green line: activation effect (phosphorylation); red line: inhibition effect; black line: transition.
Table 1.
Main players of JAK2/STAT5 signaling cascade.
| Gene | Gene ID | Protein Name | Mechanism | Reference |
|---|---|---|---|---|
| JAK2 | 3717 | Tyrosine-protein kinase JAK2 | EPORactivation | [16] |
| STAT5A | 20850 | Signal transducer and activator of transcription 5A | Signal transduction and activation of transcription of genes of erythroid differentiation | [5] |
| STAT5B | 6777 | Signal transducer and activator of transcription 5B | ||
| LYN | 4067 | Tyrosine-protein kinase Lyn | Phosphorylation/activation of STAT5 Phosphorylation/activation of CRKL |
[13] |
| SRC | 6714 | Proto-oncogene tyrosine-protein kinase Src | Phosphorylation/activation of STAT5 | [11] |
| FYN | 2534 | Tyrosine-protein kinase Fyn | Phosphorylation/activation of STAT5 | [17] |
| CRKL | 1399 | CRK-Like protein | Phosphorylation/activation of STAT5 | [14,18] |
| PTPN18 | 26469 | Tyrosine-protein phosphatase non-receptor type 18 | Activation of STAT5 | [15] |
| KPNA4 | 3840 | Importin subunit alpha-3 | Nuclear import/export | [9,19] |
| KPNB1 | 3837 | Importin subunit beta-1 | ||
| XPO1 | 7514 | Exportin-1 (CRM1) | ||
| GATA1 | 2623 | Erythroid transcription factor | Regulation of STAT5 target gene transcription Transcription factor |
[20] |
| KLF1 | 10661 | Krueppel-like factor 1 | Regulation of STAT5 target gene transcription Transcription factor |
[20] |
| TAL1 (SCL) | 6886 | T-cell acute lymphocytic leukemia protein1 | Regulation of STAT5 target gene transcription Transcription factor STAT5 target gene |
[21] |
| BCL2L1 | 598 | Bcl-2-like protein 1 (apoptosis regulator Bcl-X) | STAT5 target gene | [20] |
| CLINT1 | 9685 | Clathrin interactor 1 (EPSINR) | STAT5 target gene EPOR internalization & negative feedback for EPO-induced signaling |
[20] |
| CISH | 1154 | Cytokine-inducible SH2-containing protein | STAT5 target gene Inhibition of STAT5 signaling Degradation of EPOR |
[20,22,23] |
| SOCS1 | 8651 | Suppressor of cytokine signaling 1 | STAT5 target gene JAK2 degradation Inhibitor of EPO signaling/Inhibition of STAT5 signaling |
[22] |
| SOCS2 | 8835 | Suppressor of cytokine signaling 2 | STAT5 target gene Inhibition of STAT5 signaling |
[22] |
2. The Interplay of EPO Signaling, Transcription Patterns, and Epigenetics
EPO-dependent STAT5 target genes involved in erythropoiesis are well-known. These are the following: Bcl-XL, Bcl2l1, Pim1, Cish, Socs3, Podocalyxin, Maltr3, Chac1, Ccrn4l, Socs2, Tnfr-sf13c, Lrp8, Cmtm6, Gdf3, Oncostatin-m, Rpl12, Lyl1, Gas5, Pim3, Bim, Trb3, Serpina-3G, Irs2, Suv420h2, Gypc Cdc25a, Btg3, p27-kip1, Trb2, Klf3, Cyclin-G2, Cyclin-D2, CyclinB1-IP-1, Spi2A, and others [20,24,25]. Some of these STAT5 target genes are negative regulators of STAT5 signaling. For example, proto-oncogene serine/threonine-protein kinase Pim-1 (PIM1) suppresses cytokine signaling 1 (SOCS1) and SOCS3. PIM1, however, does not bind directly to STAT5. It interacts with SOCS1 and SOCS3 and potentiates their inhibitory effects on STAT5, most likely via phosphorylation-mediated stabilization of the SOCS proteins [24]. STAT5 activity is also regulated via the inhibitory mechanism of tyrosine-protein phosphatase non-receptor type 6 (SHP-1, encoded by PTPN^6 gene) which associates with the tyrosine-phosphorylated cytoplasmic domain of the EPOR. Moreover, SH2B adapter protein 3 (Lnk—signal transduction protein) involved in the signaling of cytokine receptors inhibits erythropoiesis and EPO-dependent JAK2 activation together via downstream signaling pathways [26]. The authors demonstrated that Lnk blocks STAT5 pathways induced by EPO in primary erythroblasts. In addition, SH2B adapter protein 2 suppresses EPO-induced STAT5 activation through the binding and a masking effect on STAT5 docking sites of EPOR. Negative feedback on EPO-induced signaling also provides CISH protein (the CISH gene is a direct target of STAT5), which binds activated pY401 of EPOR. Similar to other SOCS family members, CISH forms a complex with elongin B, elongin C, cullin5, and Rbx2. Such complex functions as an E3 ubiquitin ligase for ubiquitination and subsequent proteasomal degradation of EPOR [20]. SOCS3, however, provides a slightly different feedback dynamic compared to that of CISH. It also contains an N-terminal KIR peptide that directly affects STAT5 binding via JAK2. Gillinder et al. [20] suggested another STAT5-dependent negative feedback loop of EPOR downregulation. They reported that Clint1 (EpsinR) might enhance the internalization and/or endocytosis of EPOR through the association with clathrin-coated vesicles (Figure 1, Table 1). Other than Socs1 and Socs3, Socs2, Spred1, Spred2, and Eaf1 were also found as EPO/EPOR target genes and negative-feedback components of EPO-induced erythropoiesis during anemia [25].
Many new targets have emerged in EPOR/JAK2/STAT5 signaling in erythroid cells. Singh et al. [25] found 160 EPO/EPOR target transcripts that were significantly modulated (2—21.8-fold) in primary bone marrow-derived CFUe-like progenitors. In another study, over 300 genomic locations, predominantly in promoter distal enhancer regions, were occupied by STAT5 within 30 min of EPO stimulation in murine J2E cells. Several newly discovered EPO-responsive target genes were involved in driving erythroid cell differentiation, including those involved in mRNA splicing (Rbm25), epigenetic regulation (Suv420h2), and EPOR turnover (Clint1/EpsinR) [20]. Interestingly, STAT5-binding locations linked to erythroid-related genes were co-occupied by GATA1, KLF1, and/or TAL1 transcription factor [20]. However, housekeeping genes were bound by STAT5 without GATA1 and KLF1 [21]. While STAT5 was not enriched at the promoters of the master regulator genes in J2E cells, it was enriched at the GATA1 and TAL1 promoters of human erythroleukemic K562 cells [27].
The occupation of STAT-binding sites seems to be cell-type- and developmental-stage-specific. That makes the signaling pathways, epigenetic, and transcriptional changes downstream of EPOR unique in different kinds of cells. For example, in the well-designed study, novel factors such as REST, an epigenetic modifier central to neural differentiation and plasticity, and NFR1, a key regulator of antioxidant response and mitochondrial biogenesis, were found in fetal neural progenitor cells. EPO treatment of these cells also altered the epigenetic landscape, identifying 1150 differentially bound histone H3 lysine 4 dimethylated regions, which are known as active and poised enhancers and transcription start sites [28]. The most recent studies revealed that the EPOR signaling axis is comprised of an exceedingly complex system of molecular signaling, epigenetic modifications, and transcriptional dynamics. The data of Schulz et al. [29] demonstrated that chromatin states vary by individual erythroid stages, with significant transition between some stages. This suggests that stage-specific transcription of genes is gradual and hierarchical process with many cis-regulatory elements included [30]. Recently, Perreault et al. [31] discovered that rapid EPO-induced transcription in murine model of erythropoiesis occurs within invariant pre-established chromatin structure. HiChIP analysis revealed chromatin contacts between H3K27ac (epigenetic modification to the DNA packaging protein Histone H3) and ubiquitous transcription factor Yin Yang 1 which was distributed to surrounding areas of EPO-regulated genes.
In fact, evidence is emerging that specific chromatin remodeling is required for STAT binding to a subset of loci, and epigenetic regulation is a crucial and dynamic part of their gene regulation activity. Multiple studies have shown that STAT5 can also function as a transcriptional repressor by recruiting demethylating or deacetylating epigenetic modifiers to specific gene loci [32]. An experiment with EPO starvation to monitor the gene expression profile of pro-erythroblasts derived from primary fetal liver cells was carried out. In this regard, EPO induced the expression of hundreds of genes involved in the cell signaling related to the survival and identity of cells [21]. Cell identity is established and propagated primarily through epigenetic mechanisms, including the cell type-specific repertoire of enhancers [33]. The function of EPO signaling and its impact on epigenome became a high point of interest. Recently, the locations of the landscape of erythroid-specific enhancers have been determined in murine and human cell systems [34]. However, the detailed epigenetic and transcriptional patterns by which EPO signaling controls erythroid gene expression remain incompletely understood. Although transcription factors frequently bind to promoters, their predominant genome-wide distributions reside within distal enhancer regions. Enhancers are distinguished from promoters by histone modifications. Promoters are marked by histone 3 lysine 4 trimethylation (H3K4me3), while enhancer elements are demarcated by histone 3 lysine 4 monomethylation (H3K4me1) and histone 3 lysine 27 acetylation (H3K27ac) [35].
To uncover how EPO modulates the erythroid epigenome, an epigenetic profiling study was performed using an ex vivo murine cell system that underwent synchronous erythroid maturation in response to EPO stimulation [36]. Authors revealed a cis-regulatory network of thousands of EPO-responsive enhancers, of which EPO stimulation altered the histone mark signatures, highlighting EPO’s important role in reprogramming the epigenome. However, to what extent are these results comparable to humans remains unclear. Moreover, it was found that many enhancers modulated by EPO stimulation were linked to genes involved in cell-signaling pathways, such as JAK/STAT (n = 24), PI3K (n = 45), and FOXO (n = 25). The epigenetic landscape near genes involved in regulating the actin cytoskeleton (n = 31), glycosylation (n = 153), and transport (n = 221) was also altered within the first hour of exposure, suggesting that cytoskeletal reorganization may be regulated in part at the epigenetic level.
3. EPO/EPOR/STAT5 Signaling in Cancer Cells
3.1. In Vitro Studies
Mutational analysis of the eight EPOR tyrosine residues indicated that three of them, Y343, Y460, and Y464, are required for the JAK2 V617F mutant to exhibit its oncogenic activity. Moreover, phosphorylation at these three residues was necessary for full activation of STAT5, which is a critical downstream mediator of JAK2 V617F-induced oncogenic signaling [37]. Jeong et al. [38] showed that both the prostate cancer cell lines, as well as the primary prostate tissue, express functional EPOR, which was demonstrated via the dose-dependent proliferative response to EPO and EPO-induced STAT5B phosphorylation in cells. The activation of STAT5 was also confirmed during EPO-directed suppression of apoptosis in erythroleukemic cell lines, so STAT5 was functionally implicated in the EPO-dependent survival of cells [39]. The antiapoptotic effects of EPO in both neuronal differentiated neuroblastoma SH-SY5Y and pheochromocytoma PC-12 cells require “classical” homodimeric EPOR and the combinatorial activation of multiple signaling pathways, including STAT5, AKT, and potentially MAPK, which is comparable to that observed in hematopoietic cells (Table 2) [40].
Table 2.
The list of cell lines and tumors with impact of EPOR/STAT5 signaling on the cell processes.
| Cell Line/Tumor Type | Effect of EPOR/STAT5 | References |
|---|---|---|
| Myeloproliferative neoplasms, secondary acute myeloid leukemia | Cell survival, proliferation and migration | [51] |
| Prostate cancer cell lines, primary prostate tissue | Proliferation | [38] |
| Erythroleukemic cell lines | Cell survival | [39] |
| Neuronal differentiated neuroblastoma SH-SY5Y, Pheochromocytoma PC-12 | Cell survival | [40] |
| Breast cancer cells | Cell survival | [41] |
| Non-small-cell lung carcinoma (NSCLC) cell line H838 | Cell survival | [45] |
| NSCLC cell lines A427, A549, and NCI-H358 cell lines | No effect | [46] |
| Head and neck squamous cell carcinoma cells | Tumor invasion | [48] |
| Multiple myeloma endothelial cells (MMEC) | Angiogenesis | [52] |
| Benign non-invasive rat mammary cell lines stably transfected with human EPOR | Malignant cell behavior | [42] |
Although EPO stimulation of cultured EPOR-expressing breast cancer cells did not result in increased proliferation, over activation of EPOR (receptor phosphorylation) or a consistent activation of canonical EPOR signaling pathway mediators such as JAK2, STAT3, STAT5, or AKT were observed [41]. EPO-induced activation of the JAK2/STAT5, PI3K/AKT, and RAS/ERK pathways has been shown to promote malignant cell behavior in benign, non-invasive rat mammary cell lines stably transfected with human EPOR [42]. However, EPO stimulated the activation of the extracellular signal-regulated kinase p38 and c-Jun-NH(2)-kinase in MCF-7 cells but did not activate AKT or STAT5 [43]. Similarly, EPO had no effect on the JAK2/STAT5 signal transduction in MDA-MB-231 or in MDA-MB-435 cells [44].
Non-small cell lung carcinoma (NSCLC) cell line H838 expresses functional EPORs and their treatment with EPO-reduced cisplatin-induced apoptosis [45]. Another three NSCLC cell lines, A427, A549, and NCI-H358, were analyzed for the expression of EPOR and its downstream signaling pathways JAK2, STAT5, PI3K, and ERK. All cell lines expressed both mRNA and proteins of EPOR, which were found constitutively phosphorylated with downstream STAT5, AKT, and ERK1/2 independently activated of EPO treatment [46]. Additionally, pharmacological concentrations of EPO activated JAK2/STAT5, RAS/ERK, and PI3K/AKT pathways in NSCLC cells without growth advantage for these cells [47]. On the contrary, EPO mediated the invasion of head and neck squamous cell carcinoma cells through the JAK2/STAT signaling pathway (Table 2) [48].
Similarly, the co-existence of JAK2 and phosphorylated-JAK2, and STAT5 and phosphorylated STAT5, which are all involved in the mitogenic signaling of EPO, has been found frequently in the cells of malignant tumors of female reproductive organs, stomach, choriocarcinoma, melanoma, and capillary endothelial cells. Most of the phosphorylated JAK2 or phosphorylated STAT5-positive cells, however, had disappeared after the injection of monoclonal antibodies against EPO or a soluble form of EPOR [49]. In HUVEC cells, the conditioned media of hypoxic and EPO-treated A2780 cells induced significant phosphorylation of STAT5 [50].
3.2. In Vivo Studies and Patients
To date, epoetin alpha, beta, and darbepoetin alpha have been approved for anemia treatment in cancer patients. Although epoetins act on the same EPOR, differences in pharmacodynamics and pharmacokinetics through variations in glycolylation degree are expected. However, though both epoetins alpha and beta demonstrated comparable efficacy and safety profiles, epoetin beta achieved targeted hematocrit levels with lower doses [53] and kept the occurrence of hemoglobin levels above 13 g/dL in EPO-treated end-stage renal disease hemodialysis patients [54].
Under physiological conditions, STAT5 expression is regulated by prolactin, growth hormone, insulin growth factor, estrogen, and progesterone signaling pathways, and plays a significant role in a normal mammary cell differentiation, proliferation, and survival [55]. Watowich et al. [56] have shown a lack of specificity of STAT signaling during red blood cell development. However, the importance of STAT5A/B proteins in the erythroid differentiation activated by EPO/EPOR/JAK2 signaling has been well demonstrated [20,21]. Although deletion of erythroid STAT5A/B resulted in anemia [57], Villarino et al. [58] found no difference in the hematocrit of single-allele expressing mice. STAT5A/B are upregulated in myeloproliferative neoplasms, acute lymphoblastic leukemia (ALL), chronic myelogenous leukemia (CML), and B-cell and peripheral T-cell leukemia/lymphoma [51]. Increased STAT5A/B activation is achieved by gains in copy number, by upregulation of protein expression, or gain-of-function (GOF) mutations, which can lead to a higher phosphorylated form of STAT5A/B levels (pYSTAT5A/B) followed by tumor cell survival and disease progression [5]. The research in mature NK/T-cell neoplasms is currently focused on the recurrent point mutations localized mainly in the SH2 domain of STAT5B. The most frequent mutation, STAT5BN642H, was observed in many forms of peripheral T-cell leukemia/lymphomas [59,60,61] and has also been detected in myeloid neoplasia with eosinophilia [62] and neutrophilic leukemia [63]. Moreover, a transgenic mouse model with STAT5BN642H, under the hematopoietic vav-promoter, developed an aggressive CD8+ T-cell leukemia with organ infiltrations by CD8+, CD4+, and γδ T-cells [64,65]. The oncogenic role for STAT5BN642H was confirmed by a transplantation model derived from the transgenic mouse in NKT [66] and γδ T-cells [65] mentioned above.
Bone marrow-derived endothelial cells from monoclonal gammopathy (MGEC) of undetermined significance and multiple myeloma endothelial cells (MMEC) were assessed for the possibility that EPO might play a role in MGECs- and MMECs-mediated angiogenesis. Lamanuzzi et al. [52] showed that EPOR is expressed in both MGECs and MMECs, and at higher levels in the former (Table 2). Both EC types respond to recombinant human EPO (rHuEPO) in terms of cell proliferation and activation of JAK2/STAT5 and PI3K/AKT pathways. On the other hand, bone marrow-derived dendritic cells, which are direct targets of EPO, have demonstrated STAT3 rather than STAT5 activation [67]. Interestingly, the overexpression of HIF-1a, with stimulated EPO expression, diminished ROS levels and weakened cell damage caused by the iron overload in myelodysplastic syndrome patients [68]. Similarly, iron chelation therapy reduced the expression of SOCS1, recovered EPOR/STAT5 signalization, and reduced the apoptosis of immature erythrocytes in the bone marrow of low-risk myelodysplastic syndrome patients [69].
Similar signal transduction, including the STAT5 pathway, is mediated via EPOR-βcR, although EPOR splice variants [70] and EPOR-βcR heterodimers [71] have received much less consideration. The only difference in EPOR-βcR signaling compared with EPOR homodimer is the high concentration of EPO required. Ephrin-type B-receptor 4 (EPHB4) was also identified as an alternative EPOR that triggers downstream signaling via STAT3 and promotes recombinant human EPO-induced tumor growth and progression [72].
4. EPO/EPOR/STAT5 Induced Protection of Normal Cells
Protection mediated by EPO/EPOR/JAK2 results in pancreatic β cell proliferation and survival, increased angiogenesis, and reduced inflammation in the islets [73], as well as impaired pathogen clearance due to reduced production of anti-microbial TNF-α and NO molecules [74]. Recently, Li et al. [75] demonstrated that kidney-secreted cytokine EPO controls blood lipids in kidney disease. EPO has been shown to prevent necrotic ischemic injury and cisplatin-induced nephrotoxicity in primary mouse renal tubular epithelial cells [76] and human renal proximal tubular epithelial cells [77], possibly via JAK2/Y343/STAT5 signaling and Pim3 as a target gene of STAT5 [76]. EPO induced JAK2/STAT5 phosphorylation and nuclear translocation of STAT5, without the activation of MAPK38α and ERK1/2, followed by pronounced release of miRNA-210 from peripheral blood mononuclear cells in vitro. Hemodialyzed end-stage renal disease patients also demonstrated EPO-stimulated miRNA-210 as a result of JAK2/STAT5 signal transduction in peripheral blood mononuclear cells. Whereas some miRNAs can be used as a marker of urological [78] or prostate [79] cancer diagnosis, plasmatic miRNA-210 negatively correlates with the EPO resistance index and is considered to be a prognostic marker of EPO responsiveness and an index for anemia management in hemodialyzed end-stage renal disease patients [80].
STAT5 is also required for lipid degradation and its deficiency supports adiposity and impairs lipid mobilization in the adipocytes of mice [75,81,82]. In this regard, EPO stimulated lipid catabolism through the activation of JAK2/STAT5 signaling in peripheral adipose tissue and ameliorated non-alcoholic fatty liver disease via EPO/EPOR-induced STAT3 and STAT5 activation [83]. EPO and darbepoetin alpha improved glucose tolerance and insulin resistance, inhibited lipid accumulation in the liver and white adipose tissue, and reduced body weight. Their administration led to downregulation of proteins involved in the synthesis of lipids in the liver, such as fatty acid synthase, acetyl-CoA carboxylase, and sterol regulatory element-binding protein 1. They additionally led to upregulation of proteins involved in the lipolysis of visceral white adipose tissue, such as anti-adipose triglyceride lipase and hormone sensitive lipase [83,84]. In the brown fat tissue of young male mice, EPO therapy regulated differentiation of brown adipocyte, STAT3 activation, secretion of FGF21, and improved insulin sensitivity and glucose tolerance via upregulation of master transcriptional coregulator PRDM16 [84]. Recently, Suresh et al. [85] demonstrated that endogenous EPO signalization modulates differentiation of bone marrow stromal cells, and yet the aberrant EPO reduces osteogenesis and favors adipogenesis. It therefore seems that EPO plays a significant role in osteoblastosis and is important for both normal bone formation and bone loss during EPO-induced erythropoiesis [86,87,88]. Bone loss could be partly mediated by EPO targeted B-cells via both increased expression of osteoclastogenic molecules, as well as by transdifferentiation of B-cells into osteoblasts [89].
The upregulation of STAT5 and its downstream gene products, Bcl-xL and XIAP, demonstrated significant protective effects on astrocytes (during 24–48 h anoxia) and A9 dopaminergic neurons of the substantia nigra [90]. Both effects were attenuated by inhibition of JAK2/STAT5 signaling [91]. In cultured cerebrocortical neurons, EPO/EPOR-triggered phosphorylation of STAT5 has been shown to activate nuclear factor κB (NF-κB) and subsequently induce expression of the antiapoptotic proteins X-linked inhibitor of apoptosis (XIAP) and c-inhibitor of apoptosis-2 (cIAP2), which in turn suppress caspase 3, 8, and 9 [92]. Interestingly, EPO-mediated neuroprotection involves cross-talk between JAK2 and NF-κB signal cascades [93]. Although not required for neuroprotection of EPO in hippocampal neurons, STAT5 is essential for EPO-neurotrophic activity [94]. In the detached retina, EPO activates both PI3K/AKT and MAPK/ERK-1/2 signal transduction pathways. Intravitreal injection of EPO has been demonstrated to protect both retinal vascular and neuronal cells in early diabetes via the activation of ERK but not the STAT5 pathway [95]. Conversely, EPO reduces insulin resistance and increases glucose uptake in adipocytes through the activation of AKT and STAT5 [96].
In rats with experimental epilepsy, EPO protected myocardial cells from apoptosis via the JAK2/STAT5 pathway, and the inhibition of JAK2/STAT5 signalization by AG490 reduced the antiapoptotic effects of EPO [97]. On the other hand, carbamyl-EPO variants and pharmacological doses of EPO (250 IU/mL) exerted antiapoptotic activity in myocardial cells [98] and vascular smooth muscle cells (VSMCs) [99], respectively, via a pathway independent of JAK2/STAT5 signaling. In addition, modulating activities of EPO on adaptive and innate cells of immune systems are reviewed in Cantarelli et al. [100].
5. Conclusions
Recently, several new target genes of the EPO/EPOR/STAT5 signaling cascade have been discovered, especially in erythroid cells. These genes and their protein products are mainly involved in the processes of epigenetic regulation, mRNA splicing, molecular signaling, EPOR turnover, and negative regulation of STAT5 signaling. However, they can also be implicated in the regulation of cell metabolism and cytoskeletal reorganization. Transcriptomic analyses and mapping of EPO/EPOR target genes are also necessary for tumor and normal cells, on which only signaling and the effect of EPO/EPOR have been described so far, e.g., protective, antiapoptotic, trophic, and other. New EPO/EPOR target genes in cancer cells could serve as markers for various means of monitoring both disease activity and cell responses to various therapies, including the administration of rHuEPO to tumor patients.
6. Source of Data
We reviewed most recent scientific papers and the data from the biomedical literature found in PubMed. Studies were obtained using the following keywords: “STAT5” or “erythropoietin” or “erythropoietin receptor” and “erythropoiesis” or “cancer cell” or “normal cell” or “signaling”.
Acknowledgments
This study was supported by the Scientific Grant Agency of the Ministry of Education of the Slovak Republic by grant No. VEGA1/0536/19.
Author Contributions
Z.T., J.T. and P.S. were responsible for the conception, collection of data and design of figure and tables. Manuscript was drafted by Z.T. and P.S. and critically revised by J.T. and N.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Debeljak N., Sytkowski A.J. Erythropoietin: New approaches to improved molecular designs and therapeutic alternatives. Curr. Pharm. Des. 2008;14:1302–1310. doi: 10.2174/138161208799316393. [DOI] [PubMed] [Google Scholar]
- 2.Kim A.R., Ulirsch J.C., Wilmes S., Unal E., Moraga I., Karakukcu M., Yuan D., Kazerounian S., Abdulhay N.J., King D.S., et al. Functional Selectivity in Cytokine Signaling Revealed Through a Pathogenic EPO Mutation. Cell. 2017;168:1053–1064.e1015. doi: 10.1016/j.cell.2017.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moraga I., Wernig G., Wilmes S., Gryshkova V., Richter C.P., Hong W.J., Sinha R., Guo F., Fabionar H., Wehrman T.S., et al. Tuning cytokine receptor signaling by re-orienting dimer geometry with surrogate ligands. Cell. 2015;160:1196–1208. doi: 10.1016/j.cell.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGraw K.L., Fuhler G.M., Johnson J.O., Clark J.A., Caceres G.C., Sokol L., List A.F. Erythropoietin receptor signaling is membrane raft dependent. PLoS ONE. 2012;7:e34477. doi: 10.1371/journal.pone.0034477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maurer B., Kollmann S., Pickem J., Hoelbl-Kovacic A., Sexl V. STAT5A and STAT5B-Twins with Different Personalities in Hematopoiesis and Leukemia. Cancers. 2019;11:1726. doi: 10.3390/cancers11111726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menon M.P., Karur V., Bogacheva O., Bogachev O., Cuetara B., Wojchowski D.M. Signals for stress erythropoiesis are integrated via an erythropoietin receptor-phosphotyrosine-343-Stat5 axis. J. Clin. Investig. 2006;116:683–694. doi: 10.1172/JCI25227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon D., Watowich S.S. Hematopoietic cell survival signals are elicited through non-tyrosine-containing sequences in the membrane-proximal region of the erythropoietin receptor (EPOR) by a Stat5-dependent pathway. Exp. Hematol. 2003;31:1310–1316. doi: 10.1016/j.exphem.2003.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cumaraswamy A.A., Lewis A.M., Geletu M., Todic A., Diaz D.B., Cheng X.R., Brown C.E., Laister R.C., Muench D., Kerman K., et al. Nanomolar-Potency Small Molecule Inhibitor of STAT5 Protein. ACS Med. Chem. Lett. 2014;5:1202–1206. doi: 10.1021/ml500165r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ernst S., Muller-Newen G. Nucleocytoplasmic Shuttling of STATs. A Target for Intervention? Cancers. 2019;11:1815. doi: 10.3390/cancers11111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sathyanarayana B.K., Li P., Lin J.X., Leonard W.J., Lee B. Molecular Models of STAT5A Tetramers Complexed to DNA Predict Relative Genome-Wide Frequencies of the Spacing between the Two Dimer Binding Motifs of the Tetramer Binding Sites. PLoS ONE. 2016;11:e0160339. doi: 10.1371/journal.pone.0160339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okutani Y., Kitanaka A., Tanaka T., Kamano H., Ohnishi H., Kubota Y., Ishida T., Takahara J. Src directly tyrosine-phosphorylates STAT5 on its activation site and is involved in erythropoietin-induced signaling pathway. Oncogene. 2001;20:6643–6650. doi: 10.1038/sj.onc.1204807. [DOI] [PubMed] [Google Scholar]
- 12.Beneduce E., Matte A., De Falco L., Mbiandjeu S., Chiabrando D., Tolosano E., Federti E., Petrillo S., Mohandas N., Siciliano A., et al. Fyn kinase is a novel modulator of erythropoietin signaling and stress erythropoiesis. Am. J. Hematol. 2019;94:10–20. doi: 10.1002/ajh.25295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chin H., Arai A., Wakao H., Kamiyama R., Miyasaka N., Miura O. Lyn physically associates with the erythropoietin receptor and may play a role in activation of the Stat5 pathway. Blood. 1998;91:3734–3745. doi: 10.1182/blood.V91.10.3734. [DOI] [PubMed] [Google Scholar]
- 14.Ota J., Kimura F., Sato K., Wakimoto N., Nakamura Y., Nagata N., Suzu S., Yamada M., Shimamura S., Motoyoshi K. Association of CrkL with STAT5 in hematopoietic cells stimulated by granulocyte-macrophage colony-stimulating factor or erythropoietin. Biochem. Biophys. Res. Commun. 1998;252:779–786. doi: 10.1006/bbrc.1998.9445. [DOI] [PubMed] [Google Scholar]
- 15.Held M.A., Greenfest-Allen E., Jachimowicz E., Stoeckert C.J., Stokes M.P., Wood A.W., Wojchowski D.M. Phospho-proteomic discovery of novel signal transducers including thioredoxin-interacting protein as mediators of erythropoietin-dependent human erythropoiesis. Exp. Hematol. 2020;84:29–44. doi: 10.1016/j.exphem.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghoreschi K., Laurence A., O’Shea J.J. Janus kinases in immune cell signaling. Immunol. Rev. 2009;228:273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Able A.A., Burrell J.A., Stephens J.M. STAT5-Interacting Proteins: A Synopsis of Proteins that Regulate STAT5 Activity. Biology. 2017;6:20. doi: 10.3390/biology6010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arai A., Kanda E., Nosaka Y., Miyasaka N., Miura O. CrkL is recruited through its SH2 domain to the erythropoietin receptor and plays a role in Lyn-mediated receptor signaling. J. Biol. Chem. 2001;276:33282–33290. doi: 10.1074/jbc.M102924200. [DOI] [PubMed] [Google Scholar]
- 19.Reich N.C. STATs get their move on. JAKSTAT. 2013;2:e27080. doi: 10.4161/jkst.27080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillinder K.R., Tuckey H., Bell C.C., Magor G.W., Huang S., Ilsley M.D., Perkins A.C. Direct targets of pSTAT5 signalling in erythropoiesis. PLoS ONE. 2017;12:e0180922. doi: 10.1371/journal.pone.0180922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perreault A.A., Venters B.J. Integrative view on how erythropoietin signaling controls transcription patterns in erythroid cells. Curr. Opin. Hematol. 2018;25:189–195. doi: 10.1097/MOH.0000000000000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jegalian A.G., Wu H. Differential roles of SOCS family members in EpoR signal transduction. J. Interferon Cytokine Res. 2002;22:853–860. doi: 10.1089/107999002760274863. [DOI] [PubMed] [Google Scholar]
- 23.Bachmann J., Raue A., Schilling M., Bohm M.E., Kreutz C., Kaschek D., Busch H., Gretz N., Lehmann W.D., Timmer J., et al. Division of labor by dual feedback regulators controls JAK2/STAT5 signaling over broad ligand range. Mol. Syst. Biol. 2011;7:516. doi: 10.1038/msb.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peltola K.J., Paukku K., Aho T.L., Ruuska M., Silvennoinen O., Koskinen P.J. Pim-1 kinase inhibits STAT5-dependent transcription via its interactions with SOCS1 and SOCS3. Blood. 2004;103:3744–3750. doi: 10.1182/blood-2003-09-3126. [DOI] [PubMed] [Google Scholar]
- 25.Singh S., Dev A., Verma R., Pradeep A., Sathyanarayana P., Green J.M., Narayanan A., Wojchowski D.M. Defining an EPOR- regulated transcriptome for primary progenitors, including Tnfr-sf13c as a novel mediator of EPO- dependent erythroblast formation. PLoS ONE. 2012;7:e38530. doi: 10.1371/journal.pone.0038530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong W., Zhang J., Lodish H.F. Lnk inhibits erythropoiesis and Epo-dependent JAK2 activation and downstream signaling pathways. Blood. 2005;105:4604–4612. doi: 10.1182/blood-2004-10-4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J., Friedman G., Doyon Y., Wang N.S., Li C.J., Miller J.C., Hua K.L., Yan J.J., Babiarz J.E., Gregory P.D., et al. Targeted gene addition to a predetermined site in the human genome using a ZFN-based nicking enzyme. Genome Res. 2012;22:1316–1326. doi: 10.1101/gr.122879.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sollinger C., Lillis J., Malik J., Getman M., Proschel C., Steiner L. Erythropoietin Signaling Regulates Key Epigenetic and Transcription Networks in Fetal Neural Progenitor Cells. Sci Rep. 2017;7:14381. doi: 10.1038/s41598-017-14366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schulz V.P., Yan H., Lezon-Geyda K., An X., Hale J., Hillyer C.D., Mohandas N., Gallagher P.G. A Unique Epigenomic Landscape Defines Human Erythropoiesis. Cell Rep. 2019;28:2996–3009.e2997. doi: 10.1016/j.celrep.2019.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonifer C., Cockerill P.N. Chromatin priming of genes in development: Concepts, mechanisms and consequences. Exp. Hematol. 2017;49:1–8. doi: 10.1016/j.exphem.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Perreault A.A., Brown J.D., Venters B.J. Erythropoietin Regulates Transcription and YY1 Dynamics in a Pre-established Chromatin Architecture. iScience. 2020;23:101583. doi: 10.1016/j.isci.2020.101583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X.P., Ghoreschi K., Steward-Tharp S.M., Rodriguez-Canales J., Zhu J., Grainger J.R., Hirahara K., Sun H.W., Wei L., Vahedi G., et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat. Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shlyueva D., Stampfel G., Stark A. Transcriptional enhancers: From properties to genome-wide predictions. Nat. Rev. Genet. 2014;15:272–286. doi: 10.1038/nrg3682. [DOI] [PubMed] [Google Scholar]
- 34.Huang J., Liu X., Li D., Shao Z., Cao H., Zhang Y., Trompouki E., Bowman T.V., Zon L.I., Yuan G.C., et al. Dynamic Control of Enhancer Repertoires Drives Lineage and Stage-Specific Transcription during Hematopoiesis. Dev. Cell. 2016;36:9–23. doi: 10.1016/j.devcel.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calo E., Wysocka J. Modification of enhancer chromatin: What, how, and why? Mol. Cell. 2013;49:825–837. doi: 10.1016/j.molcel.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perreault A.A., Benton M.L., Koury M.J., Brandt S.J., Venters B.J. Epo reprograms the epigenome of erythroid cells. Exp. Hematol. 2017;51:47–62. doi: 10.1016/j.exphem.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ueda F., Tago K., Tamura H., Funakoshi-Tago M. Three Tyrosine Residues in the Erythropoietin Receptor Are Essential for Janus Kinase 2 V617F Mutant-induced Tumorigenesis. J. Biol Chem. 2017;292:1826–1846. doi: 10.1074/jbc.M116.749465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeong J.Y., Hoxhaj G., Socha A.L., Sytkowski A.J., Feldman L. An erythropoietin autocrine/paracrine axis modulates the growth and survival of human prostate cancer cells. Mol. Cancer Res. 2009;7:1150–1157. doi: 10.1158/1541-7786.MCR-08-0243. [DOI] [PubMed] [Google Scholar]
- 39.Bittorf T., Seiler J., Ludtke B., Buchse T., Jaster R., Brock J. Activation of STAT5 during EPO-directed suppression of apoptosis. Cell Signal. 2000;12:23–30. doi: 10.1016/S0898-6568(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 40.Pregi N., Wenker S., Vittori D., Leiros C.P., Nesse A. TNF-alpha-induced apoptosis is prevented by erythropoietin treatment on SH-SY5Y cells. Exp. Cell Res. 2009;315:419–431. doi: 10.1016/j.yexcr.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Reinbothe S., Larsson A.M., Vaapil M., Wigerup C., Sun J., Jogi A., Neumann D., Ronnstrand L., Pahlman S. EPO-independent functional EPO receptor in breast cancer enhances estrogen receptor activity and promotes cell proliferation. Biochem. Biophys. Res. Commun. 2014;445:163–169. doi: 10.1016/j.bbrc.2014.01.165. [DOI] [PubMed] [Google Scholar]
- 42.Shi Z., Hodges V.M., Dunlop E.A., Percy M.J., Maxwell A.P., El-Tanani M., Lappin T.R. Erythropoietin-induced activation of the JAK2/STAT5, PI3K/Akt, and Ras/ERK pathways promotes malignant cell behavior in a modified breast cancer cell line. Mol. Cancer Res. 2010;8:615–626. doi: 10.1158/1541-7786.MCR-09-0264. [DOI] [PubMed] [Google Scholar]
- 43.Gewirtz D.A., Di X., Walker T.D., Sawyer S.T. Erythropoietin fails to interfere with the antiproliferative and cytotoxic effects of antitumor drugs. Clin. Cancer Res. 2006;12:2232–2238. doi: 10.1158/1078-0432.CCR-05-2287. [DOI] [PubMed] [Google Scholar]
- 44.Chan K.K., Matchett K.B., Coulter J.A., Yuen H.F., McCrudden C.M., Zhang S.D., Irwin G.W., Davidson M.A., Rulicke T., Schober S., et al. Erythropoietin drives breast cancer progression by activation of its receptor EPOR. Oncotarget. 2017;8:38251–38263. doi: 10.18632/oncotarget.16368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merkle R., Steiert B., Salopiata F., Depner S., Raue A., Iwamoto N., Schelker M., Hass H., Wasch M., Bohm M.E., et al. Identification of Cell Type-Specific Differences in Erythropoietin Receptor Signaling in Primary Erythroid and Lung Cancer Cells. PLoS Comput. Biol. 2016;12:e1005049. doi: 10.1371/journal.pcbi.1005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frille A., Leithner K., Olschewski A., Olschewski H., Wohlkonig C., Hrzenjak A. No erythropoietin-induced growth is observed in non-small cell lung cancer cells. Int. J. Oncol. 2018;52:518–526. doi: 10.3892/ijo.2017.4225. [DOI] [PubMed] [Google Scholar]
- 47.Dunlop E.A., Percy M.J., Boland M.P., Maxwell A.P., Lappin T.R. Induction of signalling in non-erythroid cells by pharmacological levels of erythropoietin. Neurodegener Dis. 2006;3:94–100. doi: 10.1159/000092099. [DOI] [PubMed] [Google Scholar]
- 48.Lai S.Y., Childs E.E., Xi S., Coppelli F.M., Gooding W.E., Wells A., Ferris R.L., Grandis J.R. Erythropoietin-mediated activation of JAK-STAT signaling contributes to cellular invasion in head and neck squamous cell carcinoma. Oncogene. 2005;24:4442–4449. doi: 10.1038/sj.onc.1208635. [DOI] [PubMed] [Google Scholar]
- 49.Yasuda Y., Fujita Y., Masuda S., Musha T., Ueda K., Tanaka H., Fujita H., Matsuo T., Nagao M., Sasaki R., et al. Erythropoietin is involved in growth and angiogenesis in malignant tumours of female reproductive organs. Carcinogenesis. 2002;23:1797–1805. doi: 10.1093/carcin/23.11.1797. [DOI] [PubMed] [Google Scholar]
- 50.Kriska J., Solar P., Varinska L., Solarova Z., Kimakova P., Mojzis J., Fedorocko P., Sytkowski A.J. Human erythropoietin increases the pro-angiogenic potential of A2780 ovarian adenocarcinoma cells under hypoxic conditions. Oncol. Rep. 2013;30:1455–1462. doi: 10.3892/or.2013.2566. [DOI] [PubMed] [Google Scholar]
- 51.Orlova A., Wingelhofer B., Neubauer H.A., Maurer B., Berger-Becvar A., Keseru G.M., Gunning P.T., Valent P., Moriggl R. Emerging therapeutic targets in myeloproliferative neoplasms and peripheral T-cell leukemia and lymphomas. Expert Opin. Ther. Targets. 2018;22:45–57. doi: 10.1080/14728222.2018.1406924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lamanuzzi A., Saltarella I., Ferrucci A., Ria R., Ruggieri S., Racanelli V., Rao L., Annese T., Nico B., Vacca A., et al. Role of erythropoietin in the angiogenic activity of bone marrow endothelial cells of MGUS and multiple myeloma patients. Oncotarget. 2016;7:14510–14521. doi: 10.18632/oncotarget.7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loughnan A., Ali G.R., Abeygunasekara S.C. Comparison of the therapeutic efficacy of epoetin beta and epoetin alfa in maintenance phase hemodialysis patients. Ren Fail. 2011;33:373–375. doi: 10.3109/0886022X.2011.559675. [DOI] [PubMed] [Google Scholar]
- 54.Azmandian J., Abbasi M.R., Pourfarziani V., Nasiri A.A., Ossareh S., Ezzatzadegan Jahromi S., Sanadgol H., Amini S., Shahvaroughi Farahani A. Comparing Therapeutic Efficacy and Safety of Epoetin Beta and Epoetin Alfa in the Treatment of Anemia in End-Stage Renal Disease Hemodialysis Patients. Am. J. Nephrol. 2018;48:251–259. doi: 10.1159/000493097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Furth P.A., Nakles R.E., Millman S., Diaz-Cruz E.S., Cabrera M.C. Signal transducer and activator of transcription 5 as a key signaling pathway in normal mammary gland developmental biology and breast cancer. Breast Cancer Res. 2011;13:220. doi: 10.1186/bcr2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watowich S.S., Mikami A., Busche R.A., Xie X., Pharr P.N., Longmore G.D. Erythropoietin receptors that signal through Stat5 or Stat3 support fetal liver and adult erythropoiesis: Lack of specificity of stat signals during red blood cell development. J. Interferon Cytokine Res. 2000;20:1065–1070. doi: 10.1089/107999000750053726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Z., Medrzycki M., Bunting S.T., Bunting K.D. Stat5-deficient hematopoiesis is permissive for Myc-induced B-cell leukemogenesis. Oncotarget. 2015;6:28961–28972. doi: 10.18632/oncotarget.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Villarino A., Laurence A., Robinson G.W., Bonelli M., Dema B., Afzali B., Shih H.Y., Sun H.W., Brooks S.R., Hennighausen L., et al. Signal transducer and activator of transcription 5 (STAT5) paralog dose governs T-cell effector and regulatory functions. Elife. 2016;5 doi: 10.7554/eLife.08384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schrader A., Crispatzu G., Oberbeck S., Mayer P., Putzer S., von Jan J., Vasyutina E., Warner K., Weit N., Pflug N., et al. Actionable perturbations of damage responses by TCL1/ATM and epigenetic lesions form the basis of T-PLL. Nat. Commun. 2018;9:697. doi: 10.1038/s41467-017-02688-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dufva O., Kankainen M., Kelkka T., Sekiguchi N., Awad S.A., Eldfors S., Yadav B., Kuusanmaki H., Malani D., Andersson E.I., et al. Aggressive natural killer-cell leukemia mutational landscape and drug profiling highlight JAK-STAT signaling as therapeutic target. Nat. Commun. 2018;9:1567. doi: 10.1038/s41467-018-03987-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pham H.T.T., Hengstschlager M., Moriggl R. A haunted beast: Targeting STAT5BN642H in T-Cell Neoplasia. Mol. Cell. Oncol. 2018;5:e1435181. doi: 10.1080/23723556.2018.1435181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cross N.C.P., Hoade Y., Tapper W.J., Carreno-Tarragona G., Fanelli T., Jawhar M., Naumann N., Pieniak I., Lubke J., Ali S., et al. Recurrent activating STAT5B N642H mutation in myeloid neoplasms with eosinophilia. Leukemia. 2018;33:415–425. doi: 10.1038/s41375-018-0342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luo Q., Shen J., Yang Y., Tang H., Shi M., Liu J., Liu Z., Shi X., Yi Y. CSF3R T618I, ASXL1 G942 fs and STAT5B N642H trimutation co-contribute to a rare chronic neutrophilic leukaemia manifested by rapidly progressive leucocytosis, severe infections, persistent fever and deep venous thrombosis. Br. J. Haematol. 2018;180:892–894. doi: 10.1111/bjh.14456. [DOI] [PubMed] [Google Scholar]
- 64.Pham H.T.T., Maurer B., Prchal-Murphy M., Grausenburger R., Grundschober E., Javaheri T., Nivarthi H., Boersma A., Kolbe T., Elabd M., et al. STAT5BN642H is a driver mutation for T-cell neoplasia. J. Clin. Investig. 2018;128:387–401. doi: 10.1172/JCI94509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Araujo E.D., Erdogan F., Neubauer H.A., Meneksedag-Erol D., Manaswiyoungkul P., Eram M.S., Seo H.S., Qadree A.K., Israelian J., Orlova A., et al. Structural and functional consequences of the STAT5B(N642H) driver mutation. Nat. Commun. 2019;10:2517. doi: 10.1038/s41467-019-10422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klein K., Witalisz-Siepracka A., Maurer B., Prinz D., Heller G., Leidenfrost N., Prchal-Murphy M., Suske T., Moriggl R., Sexl V. STAT5B(N642H) drives transformation of NKT cells: A novel mouse model for CD56(+) T-LGL leukemia. Leukemia. 2019;33:2336–2340. doi: 10.1038/s41375-019-0471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lifshitz L., Prutchi-Sagiv S., Avneon M., Gassmann M., Mittelman M., Neumann D. Non-erythroid activities of erythropoietin: Functional effects on murine dendritic cells. Mol. Immunol. 2009;46:713–721. doi: 10.1016/j.molimm.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 68.Zheng Q.Q., Zhao Y.S., Guo J., Zhao S.D., Song L.X., Fei C.M., Zhang Z., Li X., Chang C.K. Iron overload promotes erythroid apoptosis through regulating HIF-1a/ROS signaling pathway in patients with myelodysplastic syndrome. Leuk Res. 2017;58:55–62. doi: 10.1016/j.leukres.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y. Effect of iron chelation therapy on EPO-STAT5 signalling pathway and EPO resistance in iron-overloaded low-risk myelodysplastic syndrome patients. Hematology. 2020;25:10. doi: 10.1080/16078454.2019.1700330. [DOI] [PubMed] [Google Scholar]
- 70.Arcasoy M.O., Jiang X., Haroon Z.A. Expression of erythropoietin receptor splice variants in human cancer. Biochem Biophys Res. Commun. 2003;307:999–1007. doi: 10.1016/S0006-291X(03)01303-2. [DOI] [PubMed] [Google Scholar]
- 71.Broxmeyer H.E. Erythropoietin: Multiple targets, actions, and modifying influences for biological and clinical consideration. J. Exp. Med. 2013;210:205–208. doi: 10.1084/jem.20122760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pradeep S., Huang J., Mora E.M., Nick A.M., Cho M.S., Wu S.Y., Noh K., Pecot C.V., Rupaimoole R., Stein M.A., et al. Erythropoietin Stimulates Tumor Growth via EphB4. Cancer Cell. 2015;28:610–622. doi: 10.1016/j.ccell.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choi D., Schroer S.A., Lu S.Y., Wang L., Wu X., Liu Y., Zhang Y., Gaisano H.Y., Wagner K.U., Wu H., et al. Erythropoietin protects against diabetes through direct effects on pancreatic beta cells. J. Exp. Med. 2010;207:2831–2842. doi: 10.1084/jem.20100665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nairz M., Sonnweber T., Schroll A., Theurl I., Weiss G. The pleiotropic effects of erythropoietin in infection and inflammation. Microbes Infect. 2011;14:238–246. doi: 10.1016/j.micinf.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li J., Yang M., Yu Z., Tian J., Du S., Ding H. Kidney-secreted erythropoietin lowers lipidemia via activating JAK2-STAT5 signaling in adipose tissue. EBioMedicine. 2019;50:317–328. doi: 10.1016/j.ebiom.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Breggia A.C., Wojchowski D.M., Himmelfarb J. JAK2/Y343/STAT5 signaling axis is required for erythropoietin-mediated protection against ischemic injury in primary renal tubular epithelial cells. Am. J. Physiol. Renal Physiol. 2008;295:F1689–F1695. doi: 10.1152/ajprenal.90333.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salahudeen A.K., Haider N., Jenkins J., Joshi M., Patel H., Huang H., Yang M., Zhe H. Antiapoptotic properties of erythropoiesis-stimulating proteins in models of cisplatin-induced acute kidney injury. Am. J. Physiol. Renal. Physiol. 2008;294:F1354–F1365. doi: 10.1152/ajprenal.00131.2008. [DOI] [PubMed] [Google Scholar]
- 78.Chen Z., Zhan Y., Chi J., Guo S., Zhong X., He A., Zheng J., Gong Y., Li X., Zhou L. Using microRNAs as Novel Predictors of Urologic Cancer Survival: An Integrated Analysis. EBioMedicine. 2018;34:94–107. doi: 10.1016/j.ebiom.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alhasan A.H., Scott A.W., Wu J.J., Feng G., Meeks J.J., Thaxton C.S., Mirkin C.A. Circulating microRNA signature for the diagnosis of very high-risk prostate cancer. Proc. Natl. Acad. Sci. USA. 2016;113:10655–10660. doi: 10.1073/pnas.1611596113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Felaco P., Felaco M., Franceschelli S., Ferrone A., Gatta D.M.P., Speranza L., Patruno A., De Lutiis M.A., Ballerini P., Sirolli V., et al. Erythropoietin induces miRNA-210 by JAK2/STAT5 signaling in PBMCs of End-stage Renal Disease patients. FEBS J. 2020;287:5167–5182. doi: 10.1111/febs.15302. [DOI] [PubMed] [Google Scholar]
- 81.Kaltenecker D., Mueller K.M., Benedikt P., Feiler U., Themanns M., Schlederer M., Kenner L., Schweiger M., Haemmerle G., Moriggl R. Adipocyte STAT5 deficiency promotes adiposity and impairs lipid mobilisation in mice. Diabetologia. 2017;60:296–305. doi: 10.1007/s00125-016-4152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaltenecker D., Spirk K., Ruge F., Grebien F., Herling M., Rupprecht A., Kenner L., Pohl E.E., Mueller K.M., Moriggl R. STAT5 is required for lipid breakdown and beta-adrenergic responsiveness of brown adipose tissue. Mol. Metab. 2020;40:101026. doi: 10.1016/j.molmet.2020.101026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsuma Y., Mori J., Ota T., Kawabe Y., Morimoto H., Fukuhara S., Kodo K., Umemura A., Nakajima H., Hosoi H. Erythropoietin and long-acting erythropoiesis stimulating agent ameliorate non-alcoholic fatty liver disease by increasing lipolysis and decreasing lipogenesis via EPOR/STAT pathway. Biochem. Biophys. Res. Commun. 2019;509:306–313. doi: 10.1016/j.bbrc.2018.12.131. [DOI] [PubMed] [Google Scholar]
- 84.Noguchi C.T. Erythropoietin regulates metabolic response in mice via receptor expression in adipose tissue, brain, and bone. Exp. Hematol. 2020;92:32–42. doi: 10.1016/j.exphem.2020.09.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suresh S., Rajvanshi P.K., Noguchi C.T. The Many Facets of Erythropoietin Physiologic and Metabolic Response. Front. Physiol. 2019;10:1534. doi: 10.3389/fphys.2019.01534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Suresh S., Alvarez J.C., Dey S., Noguchi C.T. Erythropoietin-Induced Changes in Bone and Bone Marrow in Mouse Models of Diet-Induced Obesity. Int. J. Mol. Sci. 2020;21:1657. doi: 10.3390/ijms21051657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Suresh S., Lee J., Noguchi C.T. Effects of Erythropoietin in White Adipose Tissue and Bone Microenvironment. Front. Cell Dev. Biol. 2020;8:584696. doi: 10.3389/fcell.2020.584696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Suresh S., Lee J., Noguchi C.T. Erythropoietin signaling in osteoblasts is required for normal bone formation and for bone loss during erythropoietin-stimulated erythropoiesis. FASEB J. 2020;34:11685–11697. doi: 10.1096/fj.202000888R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deshet-Unger N., Kolomansky A., Ben-Califa N., Hiram-Bab S., Gilboa D., Liron T., Ibrahim M., Awida Z., Gorodov A., Oster H.S., et al. Erythropoietin receptor in B-cells plays a role in bone remodeling in mice. Theranostics. 2020;10:8744–8756. doi: 10.7150/thno.45845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marcuzzi F., Zucchelli S., Bertuzzi M., Santoro C., Tell G., Carninci P., Gustincich S. Isoforms of the Erythropoietin receptor in dopaminergic neurons of the Substantia Nigra. J. Neurochem. 2016;139:596–609. doi: 10.1111/jnc.13757. [DOI] [PubMed] [Google Scholar]
- 91.Al-Sarraf H., Malatiali S., Al-Awadi M., Redzic Z. Effects of erythropoietin on astrocytes and brain endothelial cells in primary culture during anoxia depend on simultaneous signaling by other cytokines and on duration of anoxia. Neurochem. Int. 2018;113:34–45. doi: 10.1016/j.neuint.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 92.Digicaylioglu M., Lipton S.A. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- 93.Pajonk F., Weil A., Sommer A., Suwinski R., Henke M. The erythropoietin-receptor pathway modulates survival of cancer cells. Oncogene. 2004;23:8987–8991. doi: 10.1038/sj.onc.1208140. [DOI] [PubMed] [Google Scholar]
- 94.Byts N., Samoylenko A., Fasshauer T., Ivanisevic M., Hennighausen L., Ehrenreich H., Siren A.L. Essential role for Stat5 in the neurotrophic but not in the neuroprotective effect of erythropoietin. Cell Death Differ. 2008;15:783–792. doi: 10.1038/cdd.2008.1. [DOI] [PubMed] [Google Scholar]
- 95.Zhang J., Wu Y., Jin Y., Ji F., Sinclair S.H., Luo Y., Xu G., Lu L., Dai W., Yanoff M., et al. Intravitreal injection of erythropoietin protects both retinal vascular and neuronal cells in early diabetes. Investig. Ophthalmol. Vis. Sci. 2008;49:732–742. doi: 10.1167/iovs.07-0721. [DOI] [PubMed] [Google Scholar]
- 96.Pan Y., Shu J.L., Gu H.F., Zhou D.C., Liu X.L., Qiao Q.Y., Fu S.K., Gao F.H., Jin H.M. Erythropoietin improves insulin resistance via the regulation of its receptor-mediated signaling pathways in 3T3L1 adipocytes. Mol. Cell. Endocrinol. 2013;367:116–123. doi: 10.1016/j.mce.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 97.Chai H.T., Yip H.K., Sun C.K., Hsu S.Y., Leu S. AG490 suppresses EPO-mediated activation of JAK2-STAT but enhances blood flow recovery in rats with critical limb ischemia. J. Inflamm. 2016;13:18. doi: 10.1186/s12950-016-0126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ma B.X., Li J., Li H., Wu S.S. Recombinant Human Erythropoietin Protects Myocardial Cells from Apoptosis via the Janus-Activated Kinase 2/Signal Transducer and Activator of Transcription 5 Pathway in Rats with Epilepsy. Curr. Ther. Res. Clin. Exp. 2015;77:90–98. doi: 10.1016/j.curtheres.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Janmaat M.L., Heerkens J.L., de Bruin A.M., Klous A., de Waard V., de Vries C.J. Erythropoietin accelerates smooth muscle cell-rich vascular lesion formation in mice through endothelial cell activation involving enhanced PDGF-BB release. Blood. 2010;115:1453–1460. doi: 10.1182/blood-2009-07-230870. [DOI] [PubMed] [Google Scholar]
- 100.Cantarelli C., Angeletti A., Cravedi P. Erythropoietin, a multifaceted protein with innate and adaptive immune modulatory activity. Am. J. Transplant. 2019;19:2407–2414. doi: 10.1111/ajt.15369. [DOI] [PMC free article] [PubMed] [Google Scholar]