Abstract
Age-related declines in fine motor control may impact tool-use and thereby limit functional independence. Most previous research has, however, focused on the effect of aging on gross motor tasks. Few studies have investigated the effects of aging on the strategy or quality of fine motor skills, especially in tool-use, which may better reflect how age impacts complex movement capability. Twenty-two young (ages 19-35) and 18 older adults (ages 58-87) performed a timed upper-extremity task using a tool to acquire and transport objects to different locations. Overall task performance was divided into two phases based on 3-D position of the tool: a gross motor phase (object transport) and a fine motor phase (object acquisition). Overall, older adults took longer to complete the task. A linear model indicated that this was due to the duration of the fine motor phase more so than the gross motor phase. To identify age-related differences in the quality of the fine motor phase, we fit 3-dimensional ellipsoids to individual data and the calculated the ellipsoid volume. Results demonstrated a significant volume-by-age interaction, whereby increased ellipsoid volume (space the tool occupied) related to increased mean dwell time for the older adult group only; younger adults did not demonstrate this relationship. Additionally, older adults with longer movement times during the fine motor phase also had lower cognitive scores. No age-related differences were observed for the gross motor phase, suggesting that age-related declines in tool-use may be due to changes in fine motor control and cognitive status.
Keywords: Functional Movement, Motor Performance, Fine Motor Skill, Older Adult, Cognition
INTRODUCTION
It is well established that aging affects the speed of voluntary upper limb movements (Voelcker-Rehage 2008). Specifically, studies using sequence learning (Shea et al. 2006), joystick aiming (Seidler 2006), and rapid aimed limb movements (Pratt et al. 1994) have shown an overall slowing of movement (e.g., bradykinesia), which has been attributed to a number of sensorimotor factors such as proprioceptive loss (Stelmach and Sirica 1986), sensory loss (Newell et al. 2006), cortical thinning (Ward and Frackowiak 2003), central and peripheral demyelination (Seidler et al. 2010), and slower reaction times and processing speeds (Ratcliff et al. 2001; Ebaid et al. 2017). However, few studies have analyzed what specific aspects or qualities of the underlying motor behavior contribute to the observed age-related differences in upper limb motor tasks.
Although many studies have focused on age-dependent differences in movement of reaching and finger tapping tasks, very few experiments have focused on possible age-dependent movement differences in tool-use tasks, which represent more real-world movement. Understanding motor behavior in more functional tasks that capture activities of daily living is important, as such tasks appear to be more sensitive to functional and cognitive decline in older adults (Schupf et al. 2005; Schaefer et al. 2020). Thus, identifying measures that are sensitive to age-related differences in tool-use may allow us to better understand the mechanisms underlying cognitive and functional changes with advancing age.
One possible explanation for age-dependent differences in tool-use may be explained by embodied cognition, i.e., the interaction between cognition, perception, and action with an implement (Wilson 2002; Glenberg et al. 2013; Vallet 2015; Kuehn et al. 2018). Although research in both humans and animals has demonstrated that tool-use has similar neural representation as hand use (Iriki et al. 1996; Maravita et al. 2002; Umiltà et al. 2008), there are also significant differences in brain regions correlated with tool-use versus non-tool-use motor tasks (Gallivan et al. 2013); however, the effect of aging on this representation (and thus tool-use task performance) is not well known. For example, Costello and colleagues demonstrated that the perceived distance of a target while reaching with a tool was dependent on age, while reaching without a tool was not (Costello et al. 2015). A shift in perception while using an implement is called a “tool-use effect” and, based on this previous work, it is plausible that the strength of this effect may change with age. Thus, age-dependent differences in reaching tasks may not generalize to age dependent changes in tool-use tasks, thereby warranting an investigation of age-related declines in tool-use specifically.
Recent work in young adults demonstrated a temporal dissociation between reaching and object manipulation with a tool (gross and fine motor phases, respectively), suggesting that improvements in tool-use task performance were due to improved movement quality during object manipulation and not simply faster movements (Itaguchi 2020). Furthermore, movement quality may contribute to age-related differences in movement time, although this has been explored only in reaching tasks (Yan et al. 1998; Morrison and Newell 2012). As noted above, identifying changes in movement quality within real-world goal-directed actions with a tool (not just constrained experimental tasks) may be particularly important in older adults as an indicator of functional decline (i.e., declines in activities of daily living) (Dunlop et al. 2005; Zisberg et al. 2011; Colón-Emeric et al. 2013). It also suggests that studying complex tasks involving tool-use may be more sensitive to age than planar point-to-point reaching (Hinder et al. 2013), force modulation during grasping (Voelcker-Rehage and Alberts 2005; Parikh and Cole 2012), or reach-to-grasp (Cole 1991; Roy et al. 1999; Holt et al. 2013), which tend to show only modest changes in speed, accuracy, and movement time between age groups. This may be due to the very constrained nature of these other motor tasks that do not afford the ability to investigate strategic or qualitative differences in movement patterns with advancing age. Furthermore, age-dependent relationships observed from more constrained motor task paradigms (e.g., those that occur in one- or two-dimensions) may not generalize to ecologically-valid, real-world movements that are embedded in activities of daily living (Wulf and Shea 2002). Thus, it is plausible that a fine motor skill in a tool-use task may be a sensitive assay that may reflect cognitive (Hooyman et al. 2020) and functional (Fauth et al. 2017; Schaefer et al. 2020) decline, which are relevant for dementia related diseases (Schupf et al. 2005; Schaefer et al. 2020).
To our knowledge, there have been very few studies that have compared functional tool-use between younger and older adults. Previous research looking at hand prehension during different functional tasks has noted an age effect after 75 years old (Shiffman 1992), yet only through visual inspection of participant video recordings; no quantifiable outcome explaining these age differences was provided. Thus, the purpose of this study was to dissociate age-related changes in movement quality from general age-related slowing of movement during a functional tool-use task (Schaefer and Hengge 2016). To this end, groups of younger and older adults were tested on a timed task involving object manipulation, with task performance being dichotomized into a gross motor phase (i.e., reaching with tool) and a fine motor phase (i.e., object acquisition with tool). Based on previous research (Dixon et al. 1993; Slavin et al. 1996), we hypothesized that the older adult group would have slower trial times than the younger adult group, but that these differences in task performance would be due to age-related differences in movement quality of the fine motor phase rather than general slowing.
METHODS
Participant Characteristics
This study was approved by the Arizona State University Institutional Review Board (Study 00004214 and 00009764), and was therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All individuals provided informed consent prior to participation. Forty adults were recruited into this study (older adult group: n = 18, 6 female, mean±SD age: 70.11±7.57 years, range: 58-87 young adult group: n = 22, 6 female, mean±SD age: 24.32±3.41 years, range: 19-35). All participants were non-demented (Montreal Cognitive Assessment score > 21, mean±SD: 26.87±1.94, range: 23 – 30) with no self-reported history of depression or psychiatric disorders. No participant reported any previous injury or musculoskeletal disability related to either hand. No participants demonstrated any sensory loss/impairment, as measured by the Semmes-Weinstein monofilament test (all were within normal range). Sensorimotor function was also measured in the older adult group with the Grooved Pegboard Test. All participants reported full independence (score = 0) on the Index of Independence in Activities of Daily Living Scale (Katz et al. 1970), thereby indicating a minimal likelihood of apraxia (Hanna-Pladdy et al. 2003).
Functional Tool-use Task
Movement was assessed via a tool manipulation task (Fig. 1), which has been published previously (Schaefer et al. 2015; Schaefer and Duff 2015; Schaefer and Hengge 2016) and viewable on Open Science Framework (https://osf.io/phs57/wiki/Functional_reaching_task/). Importantly, this task has been related to objective and subjective measures of daily functioning in older adults, demonstrating its ecological validity (Schaefer et al. 2020) and is more related to cognition than gross motor assessments like grip strength (Hooyman et al. 2020). Hand dominance was determined with the Edinburgh handedness questionnaire (Oldfield 1971); overall, 39 of the 40 participants in this study were right-handed. Participants completed the task with their nondominant hand to allow for more variance within and between groups, and were instructed to move as quickly yet as accurately as possible. Task performance involved 15 repetitions of acquiring and transporting two objects (hard, kidney-shaped, ~0.5cm3) at a time with a tool (spoon) from a central ‘home’ container (9.5 cm in diameter and 5.8 cm in height) to one of three distal ‘target’ containers that were the same size as the home container. Thirty objects were placed by the experimenter at the start. The target containers were secured radially around the home container at −40°, 0°, and 40° at distance of 16 cm. Participants started by moving to the target cup ipsilateral of the hand used, then returned to the central cup to acquire two more objects at a time to transport to the middle target cup, then the contralateral target cup, and then repeated this 3-cup sequence five times for a total of 15 out-and-back movements. Thus, this task involved both object manipulation (fine motor) and point-to-point reaching (gross motor), similar to Itaguchi (2020). Overall task performance was quantified as the amount of time taken to complete all 15 repetitions (i.e., trial time). Kinematic data were collected throughout the 15 repetitions to analyze both the fine and gross motor stages. Errors such as transporting the wrong number of beans, dropping beans, or reaching in the wrong direction were recorded; however, less than 3% of all repetitions had any errors. Additionally, there was no difference in error rate between age groups (p=.25) nor any relationship between errors and trial time (p=.89). For more exploratory analyses, kinematic data for the dominant hand was also used (see supplementary material).
Figure 1.

Visual depiction of the tool-use task. Participants use the non-dominant hand to scoop and then transport 2 kidney beans at a time from the home container, directly in front of the participant, into one of the three adjacent containers from left to right. Instruction to each participant is to transport the total number of kidney beans quickly and accurately from the home container to the adjacent containers.
Kinematic Analysis
Kinematic data were collected at sampling frequency of 100 Hz with a small 6 DOF electromagnetic sensor (Ascension Model 130, measured at 0.7 cm long and 0.15 cm diameter; Flock of Birds, via MotionMonitor integrated software) placed on the underside of (plastic) spoon handle at its base. Data were low-pass filtered offline at 8Hz with a 4th order Butterworth filter. To parse each repetition into a fine and gross motor stage, we identified three event markers for 1) start of object manipulation, 2) start of reach, and 3) end of reach. The event for start of object manipulation was registered when the spoon was within the container diameter and the vertical velocity (inferior-superior, movement toward superior – out of the container – is positive) changed direction from negative to positive for the first time. The event for start of reach was registered at the latest occurrence when resultant velocity exceeds 0.05 m/s while the spoon is within the container. The end of reach was registered when the spoon reaches its largest y-position (anterior-posterior, movement towards anterior is positive).
The fine motor stage occurred during object acquisition, and was defined from the start of object manipulation (event marker 1) to the start of reach (event marker 2) (Fig. 2A). Kinematic variables of interest for the fine motor stage included: the 3-dimensional position of the spoon, time spent in the fine motor stage (dwell time) and cumulative distance traveled by the spoon. It is noted that this phase is of particular interest in studies of aging, as it is more susceptible to age-related changes in brain structure and function (Floel et al. 2008; Sullivan et al. 2010). The gross motor stage occurred during the reaching movement towards the target, and was defined from the start of reach (event marker 2) to the end of reach (event marker 3). Kinematic variables of interest for the gross motor stage included: the 3-dimensional position of the spoon, transport time, average velocity, average jerk, and average distance (Fig. 2B).
Figure 2.
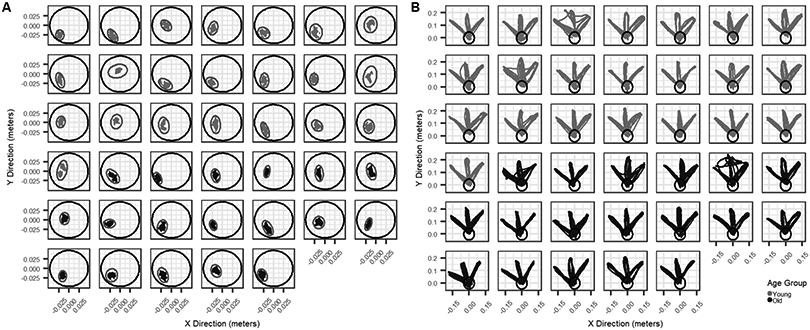
Individual participant tool position during fine and gross motor phase. Black circle is visualization of home container for reference. A) Two-dimensional density plots of tool position in the horizontal plane during the fine motor phase for each participant, colored by group (Young = Grey; Old = Black). X-direction = Anterior/Posterior; Y-direction = Medial/Lateral, Z-direction = Inferior/Superior. B) Tool position data in the horizontal plane during the gross motor phase for each participant, colored by group (Young = Grey; Old = Black). X-direction = Anterior/Posterior; Y-direction = Medial/Lateral. Light grey ellipse represents the calculated ellipse for each participant in the x and y dimension.
Measuring Fine and Gross Motor Phase Quality
The 3-dimensional space the tool occupied during the fine motor phase (see Fig. 2A) was calculated for each participant by computing the ellipsoid hull (Pison et al. 1999) using Titterington’s algorithm (Titterington 1975), i.e., the minimum volume given all points fit just inside the boundary, across all 15 repetitions of the trial. All repetitions were considered since previous research suggests that the last repetitions have different movement patterns than the first repetitions (Schaefer and Hengge 2016). Ellipsoid volume was derived from the 3 semi-major axes from the x (anterior-posterior), y (medial-lateral) and z (superior-inferior) direction using the following equation:
where V is the ellipsoid volume and x, y and z are the semi-major axis derived from the Titterington’s algorithm from the x, y and z axes, respectively. The calculated volume of each individual ellipsoid reflects the overall space in the home container that the tool moved during the fine motor phase. This essentially quantifies how much or how little space within the container the participant used to accomplish the task in the x, y, or z direction. We also analyzed 3D movement variance during the fine motor phase as an adjunct to ellipsoid volume to further assess how movement quality varies as a function of age. Any differences between age groups in the space used (i.e., variance) during the fine motor phase would demonstrate a potential shift in performance strategy or movement quality with aging, as suggested previously (Morrison and Newell 2012; Sternad 2018). Ellipsoid calculation was not used on the gross motor phase since other variables, such as average velocity and cumulative distance, are more commonly used to quantify reaching (see variables above).
Statistical Analysis
All statistical analyses were done in R, Version 4.0.0 (R Core Team 2019). To first test whether there were in fact differences between age groups in overall performance, total time was analyzed using a Welch’s two sample t-test (in the case of unequal variances). Then, to determine if each motor phase (fine vs. gross) had a differential effect on overall performance based on age group, the general linear model was used to test for interaction effects between motor phase and age group on total trial time. Results from this analysis would inform us whether the contributions of the fine and gross motor phases to the overall trial time were different for older vs. young groups. Thus, main effects of group and dwell time were included as predictors of total trial time, as was an interaction term between age group and dwell time. A similar model with interaction terms was used to assess the contribution of the gross motor phase to task performance. Separate linear models were used instead of a singular model to avoid collinearity between predictors, rather than a single model including both phases. Significant interactions within either model between age group and motor phase would suggest strategic differences between older and younger adults. Alternatively, only a main effect of age group, and no presence of interaction, would indicate an overall effect of a slowing of movement due to age and not a change in movement quality or strategy.
Finally, to determine if there was a significant difference in interaction effect magnitude between models, we implemented bootstrapping with resampling to replicate the previous analyses. Both motor phase times were scaled to allow for comparison. We generated 10,000 bootstrapped models to generate separate interaction term distributions and then compared those distributions via an independent t-test to determine mean distribution differences. The application of bootstrapping also allowed us to determine the robustness of the interaction effect independent of possible outliers or skewness within a distribution. An outcome where the dwell time interaction term is significantly larger in mean magnitude than the transport time interaction term would confirm that the dwell time interaction term is not only statistically more precise for predicting reach time between age groups, but is also larger in magnitude than that of the transport time interaction term.
As noted above, the ellipsoid hull (Maechler et al. 2020) of the fine motor phase was computed to yield a single variable that captured how much 3-dimensional movement each participant had in the home cup. Once we confirmed that there were in fact differences in overall performance between age groups, and identified which phase of the task was more sensitive to these differences, we then used robust linear model (Venables and Ripley 2002) to examine how 3-dimensional volume, age group, and age group-by-volume interaction predicted average dwell time, i.e. the fine motor phase. We utilized robust linear model to account for any influential points within the data. A Wald test and false discovery rate correction was applied to account for multiple comparisons (Maechler 2020). This phase was shown to be more sensitive to age-differences in overall performance (see Results), thereby guiding this analysis step. As such, this robust model allowed us to examine if any age group-by-volume interactions could explain differences in the fine motor phase. To avoid structural collinearity (i.e., correlation between group effects and interaction effects with group) within the model, we scaled volume by subtracting the mean and then divided by the standard deviation. A significant interaction between age group and volume represents that the tool was being manipulated differently by older and younger adult groups and this difference resulted in slower average dwell time as a function of age group. We also analyzed how average movement velocity during the fine motor phase may explain differences in ellipsoid volume between age groups. The same analysis was performed for 3-dimensional variance of the kinematic data during the fine motor phase. Supplementary analyses further explored whether the relationship between ellipsoid volume and average dwell time was dependent on which hand was used (non-dominant vs. dominant) (Judge and Stirling 2003).
Finally, although our analyses revealed that the gross motor (i.e., reaching) phase was less sensitive to the age-related differences in overall performance, model analyses were still conducted for each gross motor kinematic variable: average distance, average velocity, and average jerk, as well as each of their possible interactions with age to predict mean transport time. This allowed us to determine if differences in mean transport time (the measure of the gross motor phase) were related to reach velocity, distance traveled, and movement smoothness (jerk) for each age group. Since we have observed the effects of cognition on fine motor performance in other tasks (Fauth et al. 2017), we also tested whether average dwell time and average transport time was related to performance on the Montreal Cognitive Assessment (MoCA).
RESULTS
Age group differences in overall task performance
Welch’s two-sample t-tests determined that total trial time was significantly different between older and younger adults (mean group difference = 10.38 seconds, 95% CI = [2.68, 18.06], p = .01), with older adults taking ~10 seconds longer than younger adults to complete the task (total 15 repetitions).
Comparison of fine vs. gross motor phase relationship to overall task performance
The general linear models for the effects of age group and age interactions with both the gross and fine motor phases on task performance were statistically significant (fine: p = 1.16e-11, R2 = .78; gross: p = 3.68e-8, R2 = .61), indicating that although both phases were significant predictors of total trial time, the fine motor phase explained more variance overall. Significant age group-by-phase interactions were only observed during the fine motor phase (βdwelltime x group = −5.36, 95% CI = [−9.5, −1.19], p = .01), and not the gross motor phase (βtransporttime x group = −3.51, 95% CI = [−9.23, 2.21], p = .22). Comparison of interaction effects between each model using bootstrapping with resampling demonstrated that the dwell time interaction effect was significantly larger than the transport time interaction effect (mean of dwell interaction = −5.32, s.d. = 2.02; mean of transport interaction = −3.36, s.d. = 2.9, p = 2.2e-16). This indicates that age differentially affected how the fine motor phase influenced overall task performance compared to the gross motor phase (Fig. 3A & B), thereby warranting additional investigation (see below). This suggests that the fine motor phase is likely more sensitive to age, consistent with previous studies (Floel et al. 2008; Itaguchi 2020).
Figure 3.
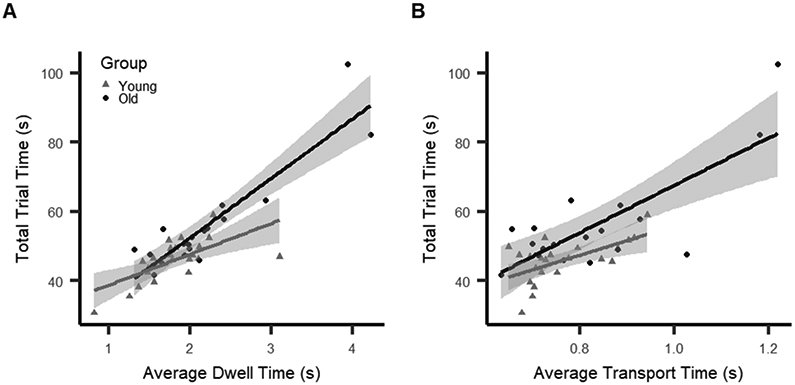
Interactions between age group and motor phase (fine motor phase = dwell time; gross motor phase = transport time) as predictors of overall trial time (task performance). Color of points indicates age group and color of lines models the interaction effect on average time spent in each phase based on age group. Shaded area represents standard error. A) The relationship between average dwell time and total trial time is different for young and older age groups. B). The relationship between average transport time and total trial time is comparable for young and older age groups.
Relationship between ellipsoid volume and the fine motor phase
To determine how volume was sensitive to differences in average dwell time by age group, robust general linear model was used with volume, age group and a volume by age group interaction as independent variables and average dwell time as the dependent variable. Results from our robust model demonstrated associations between each independent variable to average dwell time, application of the Wald test confirmed statistical significance, intercept (βintercept = 2.41, F = 282.48, p = 2.2e-16), age group (βage group = −.65, F = 12.82, p = .001), volume (βvolume = 1.01, F= 11.79, p = .002), and age group-by-volume interaction (βvolume x group = −.98, F = 10.09, p = .003). These data indicate that dwell time increased as volume increased in the older group but not the younger group (Fig. 4).
Figure 4.
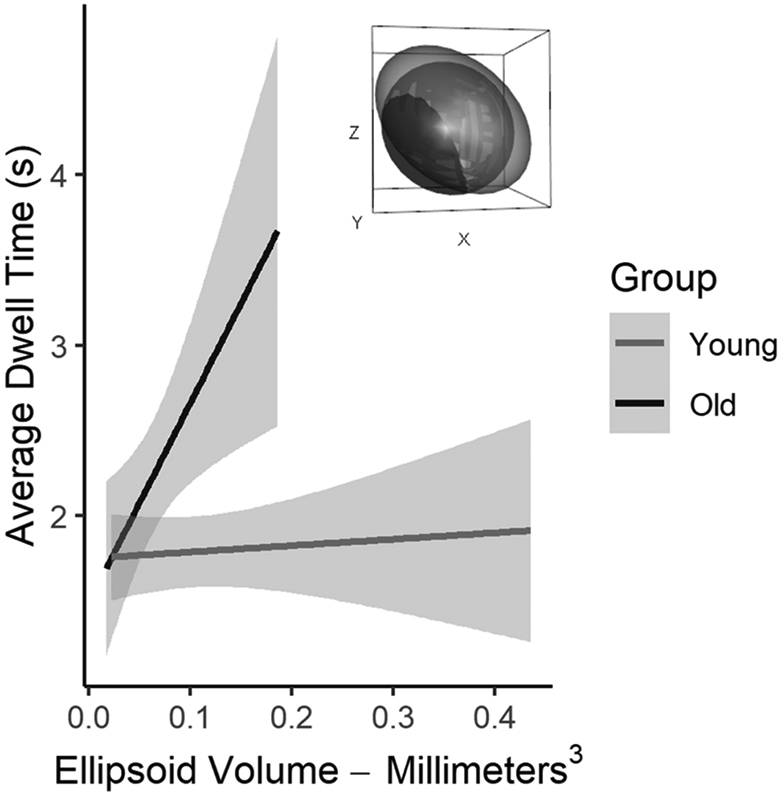
Relationship between fine motor phase ellipsoid volume with average dwell time by age group. Older adults compared to younger adults demonstrate greater increases in average dwell time with increases in variance explained in the y direction. Shaded area represents standard error. Average ellipsoid hull of each age group is visualized within the figure inset with the older adult ellipsoid in black and the young adult ellipsoid in grey.
Our previous research has suggested that the amount of “rooting around” a participant does in the home cup with the tool may be an important factor in overall task performance (Schaefer and Hengge 2016). Computed ellipsoids of the fine motor phase kinematic data now allow us to quantify this ‘rooting’ behavior. The overall linear model using ellipsoid volume as well as age group to predict average dwell time of the fine motor phase was statistically significant. This demonstrates that use of ellipsoid calculations used on this type of unstructured kinematic data can explain differences in tool-use that correspond to individual differences in the time spent in the fine motor phase. Furthermore, there was a significant group-by-volume (i.e., space used) interaction in predicting dwell time (p < .05) which further supports that the extent to which changes in motor quality (as evidenced by different movement patterns) are related to dwell time is age-dependent.
To further explore whether these effects actually reflected differences in movement quality between age groups, average movement velocity during the fine motor phase for each individual was regressed against ellipsoid volume. As shown in Supplementary Material (Fig. 1S), the interaction with age group was consistent with Figure 3, indicating that higher ellipsoid volume was associated with faster velocity within the home cup, but only for the younger age group. This effect was not observed in the older group, such that ellipsoid volume was independent of how quickly the hand/tool was moving during the fine motor phase. In addition, variation in relative shape of individual ellipsoid volume along each semi-major axis, x, y and z, of the ellipsoid was analyzed. We used a general linear model analysis with a Tukey HSD for post-hoc analysis to test for interactions between age group and semi-major axis. There was a significant interaction effect along the x-dimension (Group-by-x dimension difference = .011, p-adjusted = .01). Collectively, these results further support differences in movement quality between the age groups, rather than the older adults simply being more cautious and slower during this phase. Similar results were obtained using the 3-dimensional variance of the kinematic data during the fine motor phase, rather than ellipsoid volume (see Supplementary Material, Fig. 2S). Supplementary data are provided for the dominant hand (see Supplementary Material, Fig. 3S).
Lack of relationship between kinematics and gross motor phase
A single linear model that included all gross motor kinematic variables (average distance, average reach velocity and average reach jerk) and age group as predictors of transport time (i.e., the reach from the home cup to the target cup) was not statistically significant (p = .09). Average velocity was, however, the only significant variable (βvelocity, −1.29, 95% CI [−2.49 to −0.09], p = .04), suggesting that the only kinematic variable related to transport time was (unsurprisingly) average velocity irrespective of age. This supports that the reaching portion of this task is not necessarily sensitive to any differences in movement quality between the age groups.
Associations with sensory and cognitive function in older adults
As noted above, none of the older adults in this study demonstrated any loss in tactile sensation as measured by monofilament testing, suggesting that the interactions between the fine motor phase and age are not attributed to sensory loss. There was also no relationship between performance on the Grooved Pegboard Test and overall task performance (total time), dwell time, or transport time (all p-values > .05), suggesting that manual dexterity is also not a factor in the effects of age group observed in this study. There was, however, a significant negative relationship between global cognition (as measured by the MoCA) and dwell time of the fine motor phase (R2 = .17, βMoCA, −.18, 95% CI [−.33 to −0.03] ,p = .04), such that longer (worse) dwell times were associated with lower (worse) cognitive scores. No significant relationship was observed between cognitive score and overall task performance (p = .06) or the gross motor phase (p = .79).
DISCUSSION
The purpose of this study was to dissociate age-related changes in movement quality from general age-related slowing of movement during a functional tool-use task that had a gross motor phase (i.e., reaching with tool) and a fine motor phase (i.e., object acquisition with tool). Based on previous research (Dixon et al. 1993; Slavin et al. 1996) , we hypothesized that the older group would have worse performance on the task overall (longer trial times) than the young group, but that these differences in task performance would be due to age-related differences in movement quality rather than general slowing. Evidence from this experiment suggests that the fine motor phase (i.e., object manipulation), but not the gross motor phase (i.e., reaching), was related to the differences in overall task performance between age groups. This suggests that age group differences were not necessarily due to an age-dependent slowing of movement but instead due to changes in movement quality specific to the fine motor phase. This is the first experiment to our knowledge that quantifies how these age-related changes in movement quality can result in slower functional task performance, irrespective of movement speed. Recent research has alluded to age-related differences in movement quality in eye-hand coordination during a time-constrained reaching task, but were not able to fully describe the qualitative differences that generated the age effect (O’Rielly and Ma-Wyatt 2018). Our ability to observe behavioral patterns that are significantly related to slower task performance is contrary to other research examining age differences in lifting an object (arguably a more gross motor task) in which older adults had faster, rather than slower, movement times than younger adults (Hoellinger et al. 2017). That further supports that reaching and object manipulation with the hand is different than with a tool. It may very well be that changes in fine motor skill as a result of age may be a stronger proxy for functional decline (Contreras-Vidal et al. 1998; Hoogendam et al. 2014; Schaefer et al. 2020).
Additionally, differences in the gross motor phase (i.e., reaching) were primarily distinguished as an overall slowing of movement that was not dependent on age. Thus, performance on these types of movements may be more indicative of peripheral factors (e.g., skeletal muscle quality or α-motoneuron integrity) than are fine motor changes (Moore et al. 2014). This experiment demonstrates that fine motor performance may rely upon neural mechanisms that are particularly relevant (and sensitive) to the aging process, more so than targeted reaching. This is further illustrated by the differential relationship that these two motor phases had with cognitive function. The observed association between the fine motor phase and cognitive score (MoCA) (and lack thereof for the gross motor phase) may be related to the complexity of movement involved in each phase. Previous research has shown that task complexity increases movement variability among older adults (Morrison and Newell 2012), which, in this study was measured as ellipsoid volume and 3D variance.
Limitations
We acknowledge that the current study did not have a ‘control’ condition of just point-to-point reaching to observe if any non-tool-use gross motor behavior related to changes in tool-use movement time. This theoretically would allow for dissociating simple upper limb movement features from tool-use features that predict age-related differences in overall task performance (Ketcham and Stelmach 2004). Additionally, no measures of object perception were collected to determine where there were group differences in perceived shape. We also acknowledge that participants were not directly screened for apraxia or poor visual acuity, although all participants reported full independence on their activities of daily living, thereby suggesting that deficits were not present in this sample. Furthermore, the error rates for the task were very low and comparable between the age groups. Lastly, we acknowledge that this study focused on the nondominant hand performance, and it is plausible that dominant hand performance may show less age-related differences in movement quality (see Supplementary Material). This may reflect experience-dependent differences, as the dominant hand is more commonly used for unimanual tool-use. This study is therefore crucial for identifying aspects of functional, real-world movement that are sensitive to age and experience. Future studies will now investigate how repetitive practice affects the fine motor phase specifically in both younger and older adults.
Future Directions
It is important to identify possible mechanisms of these changes in movement quality as a function of age. Previous research has demonstrated that executive function may be driving age-related differences in reaching movements (Rodríguez-Aranda et al. 2016). However, impaired spatial memory may reduce the complex maneuvering of the motor system in tool-use tasks, which may require use of visual information to accomplish the task goal , as evidenced by 2-dimensional reaching movements where visual information was the greatest contributor of early reach corrections in older adults (Sarlegna and Sainburg 2009). As noted above, future work will analyze how ellipsoid volume or movement variance during the fine motor phase changes within and across trials as a function of practice, and how those changes are dependent on specific cognitive domains (rather than a global cognitive measure). Additionally, our future research aims to identify possible differences in brain structure and function in relationship to movement time and quality of each movement phase. Differential substrates related to slowing of movement versus movement quality may highlight specific neurodegenerative processes related to aging and motor function (Sprague et al. 2019).
CONCLUSION
Overall, results from this experiment demonstrate that age-related differences in fine movement quality are more sensitive to differences in timed motor performance with a tool than gross motor measures. Furthermore, tool-use tasks with a fine motor component may have a higher cognitive demand than reaching from point-to-point. Future studies can focus whether and why fine motor skill changes with age, above and beyond any sensory loss, and what cognitive domains it is related to in older adults.
Supplementary Material
Funding:
This work was supported by the National Institutes of Health [grant number K01AG047926 to SYS] and the Marriner S. Eccles Foundation. These sponsors did not have any role in the following: the study design; the collection, analysis, or interpretation of data; the writing of the paper; nor the decision to submit the paper for publication.
Footnotes
Conflict of Interest: The authors have no disclosures to report.
Ethics approval: This study was approved by the Arizona State University Institutional Review Board (Study 00004214 and 00009764), and was therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Consent to participate: All individuals provided informed consent prior to participation.
Availability of data and material: Metadata that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability (software application or custom code): Code used to calculate ellipsoid hull can be found at the following github: https://github.com/hooymana/Ellipsoid
REFERENCES
- Cole KJ (1991) Grasp Force Control in Older Adults. J Mot Behav 23:251–258. 10.1080/00222895.1991.9942036 [DOI] [PubMed] [Google Scholar]
- Colón-Emeric CS, Whitson HE, Pavon J, Hoenig H (2013) Functional decline in older adults. Am Fam Physician 88:388–94 [PMC free article] [PubMed] [Google Scholar]
- Contreras-Vidal JL, Teulings H, Stelmach G (1998) Elderly subjects are impaired in spatial coordination in fine motor control. Acta Psychol (Amst) 100:25–35. 10.1016/S0001-6918(98)00023-7 [DOI] [PubMed] [Google Scholar]
- Costello MC, Bloesch EK, Davoli CC, et al. (2015) Spatial representations in older adults are not modified by action: Evidence from tool use. Psychol Aging 30:656–668. 10.1037/pag0000029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, Kurzman D, Friesen IC (1993) Handwriting performance in younger and older adults: Age, familiarity, and practice effects. Psychol Aging 8:360–370. 10.1037/0882-7974.8.3.360 [DOI] [PubMed] [Google Scholar]
- Dunlop DD, Semanik P, Song J, et al. (2005) Risk factors for functional decline in older adults with arthritis. Arthritis Rheum 52:1274–1282. 10.1002/art.20968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebaid D, Crewther SG, MacCalman K, et al. (2017) Cognitive Processing Speed across the Lifespan: Beyond the Influence of Motor Speed. Front Aging Neurosci 9:62. 10.3389/fnagi.2017.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauth EB, Schaefer SY, Zarit SH, et al. (2017) Associations Between Fine Motor Performance in Activities of Daily Living and Cognitive Ability in a Nondemented Sample of Older Adults: Implications for Geriatric Physical Rehabilitation. J Aging Health 29:1144–1159. 10.1177/0898264316654674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floel A, Vomhof P, Lorenzen A, et al. (2008) Levodopa improves skilled hand functions in the elderly. Eur J Neurosci 27:1301–1307. 10.1111/j.1460-9568.2008.06079.x [DOI] [PubMed] [Google Scholar]
- Gallivan JP, Adam McLean D, Valyear KF, Culham JC (2013) Decoding the neural mechanisms of human tool use. Elife 2013:. 10.7554/eLife.00425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenberg AM, Witt JK, Metcalfe J (2013) From the Revolution to Embodiment. Perspect Psychol Sci 8:573–585. 10.1177/1745691613498098 [DOI] [PubMed] [Google Scholar]
- Hanna-Pladdy B, Heilman KM, Foundas AL (2003) Ecological implications of ideomotor apraxia: Evidence from physical activities of daily living. Neurology 60:487–490. 10.1212/WNL.60.3.487 [DOI] [PubMed] [Google Scholar]
- Hinder MR, Carroll TJ, Summers JJ (2013) Inter-limb transfer of ballistic motor skill following non-dominant limb training in young and older adults. Exp Brain Res 227:19–29. 10.1007/s00221-013-3481-9 [DOI] [PubMed] [Google Scholar]
- Hoellinger T, McIntyre J, Jami L, et al. (2017) A strategy of faster movements used by elderly humans to lift objects of increasing weight in ecological context. Neuroscience 357:384–399. 10.1016/J.NEUROSCIENCE.2017.04.010 [DOI] [PubMed] [Google Scholar]
- Holt RJ, Lefevre AS, Flatters IJ, et al. (2013) Grasping the Changes Seen in Older Adults When Reaching for Objects of Varied Texture. PLoS One 8:e69040. 10.1371/journal.pone.0069040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendam YY, van der Lijn F, Vernooij MW, et al. (2014) Older Age Relates to Worsening of Fine Motor Skills: A Population-Based Study of Middle-Aged and Elderly Persons. Front Aging Neurosci 6:259. 10.3389/fnagi.2014.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooyman A, Malek-Ahmadi M, Fauth EB, Schaefer SY (2020) Challenging the relationship of grip strength with cognitive status in older adults. Int J Geriatr Psychiatry gps.5441. 10.1002/gps.5441 [DOI] [PubMed] [Google Scholar]
- Iriki A, Tanaka M, Iwamura Y (1996) Coding of modified body schema during tool use by macaque postcentral neurones. Neuroreport 7:2325–30. 10.1097/00001756-199610020-00010 [DOI] [PubMed] [Google Scholar]
- Itaguchi Y (2020) Toward natural grasping with a tool: effects of practice and required accuracy on the kinematics of tool-use grasping. J Neurophysiol 123:2024–2036. 10.1152/jn.00384.2019 [DOI] [PubMed] [Google Scholar]
- Judge J, Stirling J (2003) Fine motor skill performance in left- and right-handers: Evidence of an advantage for left-handers. Laterality 8:297–306. 10.1080/13576500342000022 [DOI] [PubMed] [Google Scholar]
- Katz S, Downs TD, Cash HR, Grotz RC (1970) Progress in development of the index of ADL. Gerontologist 10:20–30. 10.1093/geront/10.1_part_1.20 [DOI] [PubMed] [Google Scholar]
- Ketcham CJ, Stelmach GE (2004) Movement Control in the Older Adult [Google Scholar]
- Kuehn E, Perez-Lopez MB, Diersch N, et al. (2018) Embodiment in the aging mind. Neurosci Biobehav Rev 86:207–225. 10.1016/J.NEUBIOREV.2017.11.016 [DOI] [PubMed] [Google Scholar]
- Maechler M (2020) sfsmisc: Utilities from “Seminar fuer Statistik” ETH Zurich [Google Scholar]
- Maechler M, Rousseeuw PJ, Struyf A, et al. (2020) cluster: Cluster Analysis Basics and Extensions [Google Scholar]
- Maravita A, Spence C, Kennett S, Driver J (2002) Tool-use changes multimodal spatial interactions between vision and touch in normal humans. Cognition 83:B25–B34. 10.1016/S0010-0277(02)00003-3 [DOI] [PubMed] [Google Scholar]
- Moore AZ, Caturegli G, Metter EJ, et al. (2014) Difference in Muscle Quality over the Adult Life Span and Biological Correlates in the Baltimore Longitudinal Study of Aging. J Am Geriatr Soc 62:230–236. 10.1111/jgs.12653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S, Newell KM (2012) Aging, neuromuscular decline, and the change in physiological and behavioral complexity of upper-limb movement dynamics. J. Aging Res 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell KM, Vaillancourt DE, Sosnoff JJ (2006) Aging, Complexity, and Motor Performance. Handb Psychol Aging 163–182. 10.1016/B978-012101264-9/50011-2 [DOI] [Google Scholar]
- O’Rielly JL, Ma-Wyatt A (2018) Changes to online control and eye-hand coordination with healthy ageing. Hum Mov Sci 59:244–257. 10.1016/J.HUMOV.2018.04.013 [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971) The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- Parikh PJ, Cole KJ (2012) Handling objects in old age: forces and moments acting on the object. J Appl Physiol 112:1095–1104. 10.1152/japplphysiol.01385.2011 [DOI] [PubMed] [Google Scholar]
- Pison G, Struyf A, Rousseeuw PJ (1999) Displaying a clustering with CLUSPLOT. Comput Stat Data Anal 30:381–392. 10.1016/S0167-9473(98)00102-9 [DOI] [Google Scholar]
- Pratt J, Chasteen AL, Abrams RA (1994) Rapid aimed limb movements: age differences and practice effects in component submovements. Psychol Aging 9:325–34. 10.1037//0882-7974.9.2.325 [DOI] [PubMed] [Google Scholar]
- R Core Team (2019) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing [Google Scholar]
- Ratcliff R, Thapar A, McKoon G (2001) The effects of aging on reaction time in a signal detection task. Psychol Aging 16:323–341. 10.1037/0882-7974.16.2.323 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Aranda C, Mittner M, Vasylenko O (2016) Association Between Executive Functions, Working Memory, and Manual Dexterity in Young and Healthy Older Adults. Percept Mot Skills 122:165–192. 10.1177/0031512516628370 [DOI] [PubMed] [Google Scholar]
- Roy EA, Weir PL, Desjardins-Denault S, Winchester T (1999) Pointing Versus Grasping in Young and Older Adults. Dev Neuropsychol 16:19–27. 10.1207/S15326942DN160102 [DOI] [Google Scholar]
- Sarlegna FR, Sainburg RL (2009) The roles of vision and proprioception in the planning of reaching movements. Adv Exp Med Biol 629:317–35. 10.1007/978-0-387-77064-2_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Dibble LE, Duff K (2015) Efficacy and Feasibility of Functional Upper Extremity Task-Specific Training for Older Adults With and Without Cognitive Impairment. Neurorehabil Neural Repair 29:636–644. 10.1177/1545968314558604 [DOI] [PubMed] [Google Scholar]
- Schaefer SY, Duff K (2015) Rapid Responsiveness to Practice Predicts Longer-Term Retention of Upper Extremity Motor Skill in Non-Demented Older Adults. Front Aging Neurosci 7:214. 10.3389/fnagi.2015.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Hengge CR (2016) Testing the concurrent validity of a naturalistic upper extremity reaching task. Exp Brain Res 234:229–240. 10.1007/s00221-015-4454-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Hooyman A, Duff K (2020) Using a Timed Motor Task to Predict One-Year Functional Decline in Amnestic Mild Cognitive Impairment. J Alzheimer’s Dis 1–6. 10.3233/JAD-200518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupf N, Tang M-X, Albert SM, et al. (2005) Decline in cognitive and functional skills increases mortality risk in nondemented elderly. Neurology 65:1218–26. 10.1212/01.wnl.0000180970.07386.cb [DOI] [PubMed] [Google Scholar]
- Seidler RD (2006) Differential effects of age on sequence learning and sensorimotor adaptation. Brain Res Bull 70:337–46. 10.1016/j.brainresbull.2006.06.008 [DOI] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, et al. (2010) Motor control and aging: Links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 34:721–733. 10.1016/J.NEUBIOREV.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea CH, Park J-H, Braden HW (2006) Age-related effects in sequential motor learning. Phys Ther 86:478–88 [PubMed] [Google Scholar]
- Shiffman LM (1992) Effects of Aging on Adult Hand Function. Am J Occup Ther 46:785–792. 10.5014/ajot.46.9.785 [DOI] [PubMed] [Google Scholar]
- Slavin MJ, Phillips JG, Bradshaw JL (1996) Visual cues and the handwriting of older adults: A kinematic analysis. Psychol Aging 11:521–526. 10.1037/0882-7974.11.3.521 [DOI] [PubMed] [Google Scholar]
- Sprague BN, Phillips CB, Ross LA (2019) Age-Varying Relationships Between Physical Function and Cognition in Older Adulthood. Journals Gerontol Ser B 74:772–784. 10.1093/geronb/gbx126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelmach GE, Sirica A (1986) Aging and proprioception. Age (Omaha) 9:99–103. 10.1007/BF02432281 [DOI] [Google Scholar]
- Sternad D (2018) It’s not (only) the mean that matters: variability, noise and exploration in skill learning. Curr. Opin. Behav. Sci 20:183–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan E V, Rohlfing T, Pfefferbaum A (2010) Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: Relations to timed performance. Neurobiol Aging 31:464–481. 10.1016/J.NEUROBIOLAGING.2008.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titterington DM (1975) Optimal Design: Some Geometrical Aspects of D-Optimality. Biometrika 62:313. 10.2307/2335366 [DOI] [Google Scholar]
- Umiltà MA, Escola L, Intskirveli I, et al. (2008) When pliers become fingers in the monkey motor system. Proc Natl Acad Sci U S A 105:2209–13. 10.1073/pnas.0705985105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet GT (2015) Embodied cognition of aging. Front Psychol 6:. 10.3389/fpsyg.2015.00463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables WN, Ripley BD (2002) Modern Applied Statistics with S-Plus, Fourth. Springer [Google Scholar]
- Voelcker-Rehage C (2008) Motor-skill learning in older adults—a review of studies on age-related differences. Eur Rev Aging Phys Act 5:5–16. 10.1007/s11556-008-0030-9 [DOI] [Google Scholar]
- Voelcker-Rehage C, Alberts JL (2005) Age-related changes in grasping force modulation. Exp brain Res 166:61–70. 10.1007/s00221-005-2342-6 [DOI] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RSJ (2003) Age-related changes in the neural correlates of motor performance. Brain 126:873–888. 10.1093/brain/awg071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M (2002) Six views of embodied cognition. Psychon Bull Rev 9:625–636. 10.3758/BF03196322 [DOI] [PubMed] [Google Scholar]
- Wulf G, Shea CH (2002) Principles derived from the study of simple skills do not generalize to complex skill learning. Psychon Bull Rev 9:185–211. 10.3758/BF03196276 [DOI] [PubMed] [Google Scholar]
- Yan JH, Thomas JR, Stelmach GE (1998) Aging and rapid aiming arm movement control. Exp Aging Res 24:155–168. 10.1080/036107398244292 [DOI] [PubMed] [Google Scholar]
- Zisberg A, Shadmi E, Sinoff G, et al. (2011) Low Mobility During Hospitalization and Functional Decline in Older Adults. J Am Geriatr Soc 59:266–273. 10.1111/j.1532-5415.2010.03276.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


