Abstract
As of today, little data is available on COVID-19 in African countries, where the case management relied mainly on a treatment by association between hydroxychloroquine (HCQ) and azithromycin (AZM). This study aimed to understand the main clinical outcomes of COVID-19 hospitalized patients in Senegal from March to October 20202. We described the clinical characteristics of patients and analysed clinical status (alive and discharged versus hospitalized or died) at 15 days after Isolation and Treatment Centres (ITC) admission among adult patients who received HCQ plus AZM and those who did not receive this combination. A total of 926 patients were included in this analysis. Six hundred seventy-four (674) (72.8%) patients received a combination of HCQ and AZM. Results showed that the proportion of patient discharge at D15 was significantly higher for patients receiving HCQ plus AZM (OR: 1.63, IC 95% (1.09–2.43)). Factors associated with a lower proportion of patients discharged alive were: age ≥ 60 years (OR: 0.55, IC 95% (0.36–0.83)), having of at least one pre-existing disorder (OR: 0.61, IC 95% (0.42–0.90)), and a high clinical risk at admission following NEWS score (OR: 0.49, IC 95% (0.28–0.83)). Few side effects were reported including 2 cases of cardiac rhythmic disorders in the HCQ and AZM group versus 13 in without HCQ + AZM. An improvement of clinical status at 15 days was found for patients exposed to HCQ plus AZM combination.
Keywords: SARS-CoV-2, hydroxychloroquine, azithromycin, treatment, Senegal
1. Introduction
On 31 December 2019, the WHO China Country Office was informed of cases of pneumonia of unknown aetiology detected in Wuhan City, Hubei Province of China [1]. The COVID-19 was declared as a pandemic by WHO [2]. From the middle of February 2020, the outbreak started in Africa. Senegal was the second sub-Saharan country to report confirmed cases of COVID-19 with an imported case detected on 2 March 2020. From March to October 2020 in Senegal, a total of 15,605 cases has been identified and 323 deaths reported [3].
However few data on COVID-19 in sub-Saharan African populations is available. In Senegal as in most countries, several debates occurred regarding care management and the use of specific treatments for COVID-19 patients. The treatment using hydroxychloroquine (HCQ) alone or a combination of HCQ and azithromycin (AZM) has been controversial. After initial publications of the potential efficacy of this regimen [4,5,6,7], a large use of this treatment was observed in many countries, including China, France, Italy, Netherlands, and Korea [8]. Some in vitro studies have shown that CQ and HCQ possess antiviral activities against many viruses, including influenza viruses and arboviruses [9]. Some authors have reported that QC/HCQ significantly inhibits virus entry and at least five steps in the replication of SARS-CoV-2 [10]. Significant effect was observed on duration of fever, duration of cough, clinical recovery, death and/or transfer to intensive care [11]. Although AZM activity against several viruses has been studied in vitro and in clinical trials [12]. Recently, the combination of HCQ and AZM has been shown to have a synergistic effect in vitro on SARS-CoV-2 and, when administered over a period of at least three days, to improve the clinical condition of patients [13]. However, other studies including clinical trials showed no effect for this treatment on hospitalized patients with COVID-19 in terms of overall mortality, initiation of ventilation, or duration of hospital stay [14,15,16,17,18]. Moreover, concerns regarding safety and especially occurrence of cardiac rhythmic disorders have been raised after administration of HCQ [19,20] and Word Health Organization (WHO) edited a recommendation against the use of HCQ in patients with COVID-19 regardless of disease severity [21]. However, the use of HCQ for COVID-19 treatment has been maintained in many countries [22].
In Senegal, case management for COVID-19 was based on symptomatic treatment at the discretion of the treating clinician. At the beginning of the epidemic, with the debate about the use of HCQ, the first patients were treated only symptomatically. In mid-March, HCQ was used by several practitioners and in early April HCQ was combined with AZM.
Here, we described the clinical outcome of patients hospitalized in five Isolation and Treatment Centres (ITC) in Senegal between 2 March 2020 and 31 October 2020. We analysed outcomes associated with the clinical status of patients within 15 days after ITC admission.
2. Methods
2.1. Study Design
We put in place a retrospective data analysis of medical records of patients, with a SARS-CoV-2 confirmed infection who were admitted in five ITCs. A care protocol was adapted from the International Severe Acute Respiratory and emerging Infections Consortium (ISARIC)/WHO Clinical Characterisation Protocol (CCP) v3.1 study [23,24].
2.2. Setting
In Dakar, the study was implemented in the ITCs of Fann Hospital, Dalal Jamm Hospital, and Hospital Principal of Dakar. All these three ITCs included an Intensive Care Unit. Two other ITCs were involved: one in the Diamniadio Children Hospital and the other corresponding to the Darou Marnane ITC, a community health care in the city of Touba, Diourbel region. All centres used the same protocol but the only difference between them was the availability of Intensive Care unit (ICU).
Data were collected on patients hospitalized from 2 March 2020 to 31 October 2020. According to the national strategy implement by the Senegalese Ministry of health for COVID-19 confirmed cases, after the 1st positive detection of the virus by RT-PCR, SARS-CoV-2 molecular detection was performed through nasopharyngeal and oropharyngeal swabs every 48–72 h until negativity. Hospital discharge was allowed in case of two consecutive negative RT-PCRs. The commercial LightMix Wuhan CoV E-gene and LightMix Modular Wuhan CoV RdRP-gene kits from TIB MOLBIOL (Berlin, Germany) were used for the SARS-CoV-2 qRT-PCR diagnostic tests [25]. A sample is positive if the cycle threshold (Ct) is lower or equal to 36.
2.3. Participants
Patients with a COVID-19 infection confirmed by RT-PCR were included in the cohort survey. Given the high number of patients exposed to the combination of HCQ-AZM, we excluded from analysis patients less than 18 years-old to avoid bias regarding indications of treatment. Due to a short-term use of treatment with HCQ without AZM, we also excluded from analysis patients receiving HCQ only.
Patients who did not receive a combination of HCQ and AZM for at least 3 days corresponding to AZM treatment duration were excluded. Finally, to avoid the impact of late treatment initiation on Clinical status at D15, we excluded patients who received a combination of HCQ and AZM before ITC admission. After admission in ITC, patients who stayed more than 3 days before being treated with HCQ and AZM were also excluded.
Patients were not treated with HCQ and AZM if they were children or had a known allergy to any of the products or had any other known contra indication for treatment, including retinopathy, QT prolongation, cardiac rhythm disorder and if they were pregnant or breastfeeding. At the beginning of the epidemic, patients were not treated with HCQ, only symptomatic treatment was recommended by the local authorities and then HCQ was introduced for a few weeks. Then they decided to treat all patients with HCQ + AZM.
2.4. Data Sources
Clinical data were collected from patients’ medical records. Data collected included patients’ sociodemographic details, medical history in terms of comorbidities and clinical symptoms and medication administration. Additionally, intensive care unit (ICU) status and ventilator use were reported.
A dedicated paper Case Report Form (CRF) adapted from ISARIC nCoV CRF v1.2 (Oxford, UK) [26] was filled at site level. Electronic anonymized data entry was secondarily performed using an instance of the Research Electronic Data Capture system (REDCap, Nashville, TN, USA) [27].
2.5. Variables Assessed
Given the high number of COVID-19 patient exposed to HCQ-AZM combination during the survey, we distinguished patients exposed or not to this treatment regimen. The HCQ + AZM group was defined as patients who have received HCQ and AZM concomitantly for at least 72 h. AZM was administered once a day at a dose of 500 mg per administration, during 3 days. HCQ was administered three times a day at a dose of 200 mg per administration, during 10 days. The group that neither received HCQ alone nor HCQ-AZM combination was defined as the control group (patients who have not received HCQ concomitantly or not with AZM). For the safety analysis, side effects potentially linked to the HCQ plus AZM regimen were self-declared by the clinician in charge of the patient.
2.6. Illness Severity
The illness severity of COVID-19 was defined by the National Early Warning Score (NEWS Score) version 2 [28].
2.7. End Point
Clinical status at Day 15 defined by discharge alive within 15 days after ITC admission versus dead or still hospitalized was considered.
2.8. Statistical Analysis
Continuous and categorical variables were presented as median, interquartile range (IQR) and as frequencies and percentages, respectively. We compared categorical variables using Fisher’s exact test and continuous variables using ANOVA. To explore the risk factors associated with clinical status at Days 15, unadjusted and covariate adjusted multivariate logistic models were performed. All covariates included in the multivariate models were chosen for their clinical relevance. Collinearity analysis has been performed. We conducted all analyses with R software, version 4.0.1 (Vienna, Austria) [29].
2.9. Ethical Aspects
This study was approved by the Senegalese National Ethics Committee for Research in Health (reference number 00000068/MSAS/CNERS/Sec, accessed on 10 April 2020).
3. Results
3.1. Characteristics of Patients
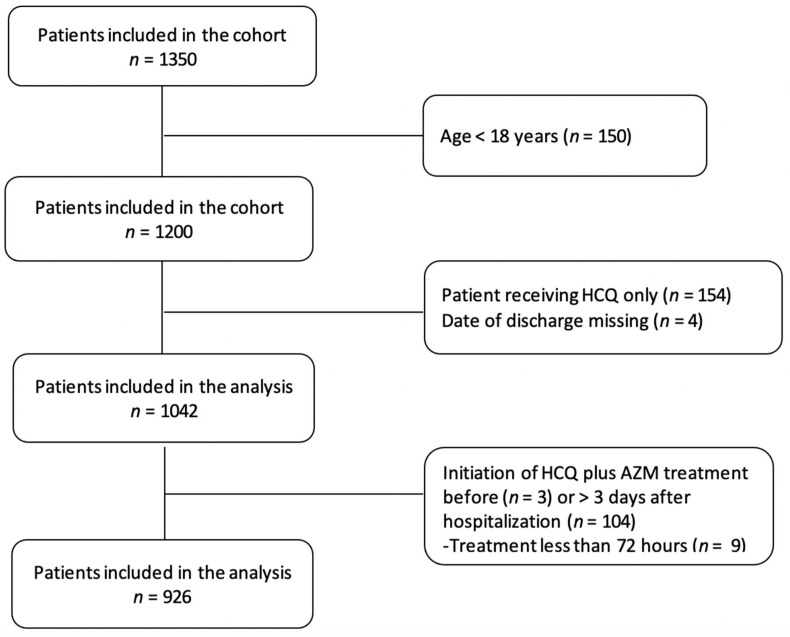
A total of 1350 patients with a confirmed COVID-19 infection and admitted between 2 March and 31 October 2020 have been included. Patients less than 18 years of age, those receiving HCQ only, those with a missing date of discharge, and those who received a combination of HCQ and AZM before admission to the hospital were excluded. Patients stayed more than three days in hospital before being treated were also excluded from the analysis. Overall, 424 patients were excluded from this study. A total of 926 patients were included in the analysis (Figure 1).
Figure 1.
Flowchart of participants included in the Cohort study. HCQ: hydroxychloroquine; AZM: azithromycin.
3.2. Characteristics of Patient at ITC Admission Time
Patients’ median (IQR) age was 45 years (31–60) (Table 1). A total of 412 (44.4%) were female. The main coexisting illnesses reported were hypertension, diabetes and chronic lung disease in 155 (17.1%), 132 (14.5%) and 73 (8%) patients respectively. At least one pre-existing disorder was recorded at admission time in 33.6% of patients.
Table 1.
Patients’ characteristics at the time of admission in the Isolation and Treatment Centre.
| Patient’s Characteristics | Without HCQ + AZM (n = 252) | HCQ + AZM (n = 674) | Total (n = 926) | p-Value |
|---|---|---|---|---|
| Age | <0.001 1 | |||
| Median | 57 | 42 | 45 | |
| Q1, Q3 | 36, 67 | 30, 58 | 31, 60 | |
| Age categories | <0.001 2 | |||
| 18–59 | 140 (55.6%) | 521 (77.3%) | 661 (71.4%) | |
| ≥60 | 112 (44.4%) | 153 (22.7%) | 265 (28.6%) | |
| Sex | 0.824 2 | |||
| Female | 114 (45.2%) | 298 (44.2%) | 412 (44.5%) | |
| Number of preexisting disorders | <0.001 2 | |||
| 0 | 118 (46.8%) | 464 (68.8%) | 582 (62.9%) | |
| 1 | 68 (27.0%) | 124 (18.4%) | 192 (20.7%) | |
| ≥2 | 66 (26.2%) | 86 (12.8%) | 152 (16.4%) | |
| Preexisting disorder | ||||
| Hypertension | 72 (30.1%) | 83 (12.5%) | 155 (17.1%) | <0.001 2 |
| Diabetes | 58 (23.8%) | 74 (11.1%) | 132 (14.5%) | <0.001 2 |
| Chronic lung disease * | 4 (1.6%) | 15 (2.2%) | 19 (2.1%) | 0.794 2 |
| Obesity | 17 (7.1%) | 21 (3.2%) | 38 (4.2%) | 0.014 2 |
| Chronic Heart dysfunction | 13 (5.2%) | 15 (2.2%) | 28 (3.0%) | 0.028 2 |
| Chronic hematologic dysfunction | 3 (1.2%) | 7 (1.0%) | 10 (1.1%) | 0.736 2 |
| Chronic renal disease | 4 (1.6%) | 1 (0.1%) | 5 (0.5%) | 0.020 2 |
| Others | 34 (13.7%) | 66 (9.9%) | 100 (10.9%) | 0.121 2 |
| Symptoms | ||||
| No COVID symptoms | 37 (14.7%) | 213 (32.2%) | 250 (27.4%) | <0.001 2 |
| Fever | 116 (49.2%) | 261 (39.5%) | 377 (42.1%) | 0.011 2 |
| Cough | 115 (48.9%) | 207 (31.6%) | 322 (36.1%) | <0.001 2 |
| Runny nose | 24 (10.3%) | 72 (11.0%) | 96 (10.8%) | 0.902 2 |
| Taste or smell lost | 33 (14.1%) | 107 (16.3%) | 140 (15.7%) | 0.465 2 |
| Myalgia | 67 (28.4%) | 144 (22.0%) | 211 (23.7%) | 0.050 2 |
| Asthenia | 47 (24.9%) | 80 (13.5%) | 127 (16.2%) | <0.001 2 |
| Diarrhea and/or Nausea | 19 (8.7%) | 44 (7.0%) | 63 (7.4%) | 0.373 2 |
| Median no. of days since symptom onse—median (IQR) | 0.359 1 | |||
| Median | 6 | 6 | 6 | |
| Q1, Q3 | 3, 8 | 3, 9 | 3, 8 | |
| Delay between first positive PCR and ITC admission, days—median (IQR) | 0.333 1 | |||
| Median | 3 | 3 | 3 | |
| Q1, Q3 | 2, 4 | 2, 4 | 2, 4 | |
| Systolic Blood Pressure (mmHg) | <0.001 1 | |||
| Median | 135 | 130 | 130 | |
| Q1, Q3 | 120, 150 | 120, 140 | 120, 145 | |
| Diastolic Blood Pressure (mmHg) | 0.697 1 | |||
| Median | 80 | 80 | 80 | |
| Q1, Q3 | 72, 90 | 77, 90 | 76, 90 | |
| Heart Rate (beats/min) | <0.001 1 | |||
| Median | 96 | 87 | 89 | |
| Q1, Q3 | 84, 107 | 76, 98 | 78, 100 | |
| Respiratory rate (breaths/min) | <0.001 1 | |||
| Median | 24 | 22 | 22 | |
| Q1, Q3 | 20, 30 | 20, 24 | 20, 26 | |
| NEWS score at admission time | <0.001 2 | |||
| Low | 135 (53.6%) | 519 (77.0%) | 654 (70.6%) | |
| Low medium | 16 (6.3%) | 60 (8.9%) | 76 (8.2%) | |
| Medium | 44 (17.5%) | 48 (7.1%) | 92 (9.9%) | |
| High | 57 (22.6%) | 47 (7.0%) | 104 (11.2%) | |
| Admitted to intensive care at admission (ICU) | 30 (11.9%) | 10 (1.5%) | 40 (4.3%) | <0.001 2 |
| Virological results at admission † | ||||
| Negative | - | - | 45 (4.86%) | |
| Positive | - | - | 881 (95.14%) | |
| Ct values—median (IQR) | - | - | 30 (23.7,33) |
HCQ: Hydroxychloroquine; AZM: Azithromycin; † first PCR results following ICT admission; * Chronic lung disease was defined as chronic obstructive pulmonary disease, asthma, or chronic bronchitis. 1 Anova test; 2 Exact Fisher test.
On admission, 250 (27%) of patients presented no signs of COVID-19 infection. Among the 676 patients who presented COVID-19 symptoms, fever was the most observed symptoms for 377 (55.8%), followed by cough for 322 (47.6%), myalgia for 211 (31.2%) and loss of taste or smell affected 140 (20.7%) patients. The degree of severity of COVID-19 was categorized as low in 656 (70.2%) patients. The combination of HCQ and AZM was initiated within 72 h after admission for 674 (72.8%) patients. On admission, 881 (95.1%) patients presented a positive SARS-CoV-2 PCR result. Patients without a positive PCR at baseline had a positive PCR later on were mainly suspect cases.
3.3. Patients’ Characteristics during Hospitalization
Median duration of hospitalization was 11 days (IQR: 8–16) (Table S1). Only 326 (35.2%) patients were categorized as low clinical risk following the NEWS score. Oxygen supplementation was required for 269 (29%) patients and 125 (13.4%) needed intensive care. Among the studied population, 91 (9.8%) deaths occurred.
Exposition to at least one antibiotic was observed for 807 (87.2%) patients. Macrolides were the most widely administered antibiotics (80.6%) followed by 3rd generation of cephalosporin (16.7%) of patients exposed.
3.4. Clinical Status at Day 15
Globally, 565 (61%) patients were discharged alive from the ITC within the 15 days after their admission.
In univariate analysis, treatment with combination of HCQ and AZM and absence of pre-existing disorder were significantly associated with a higher proportion of patients discharged alive within 15 days after their admission (both p < 0.001) (Table S2).
Patients older than 60 years (p < 0.001) were significantly associated with a lower proportion of discharge before 15 days after admission. Regarding symptoms at ICT admission time, absence of COVID symptoms (p = 0.012) was significantly associated with a higher proportion of patients discharged alive whereas cough (p = 0.012) and a NEWS gravity score higher than “low level” class were significantly associated with a higher proportion of patients still hospitalized or dead 15 days after their admission.
In the multivariate analysis, the treatment regimen with HCQ-AZM combination (OR: 1.63, IC 95% (1.10–2.42), p = 0.016) and the delay between hospitalization and symptoms onset (OR: 1.05, IC 95% (1.00–1.10), p = 0.042) were both significantly associated with a higher proportion of patients discharged within 15 days after their admission to ITC.
Older age (OR: 0.55 IC 95% (0.36–0.83), p = 0.004), existence of at least one comorbidity (OR: 0.59, IC 95% (0.40–0.86), p = 0.006) and a high NEWS severity score at admission (OR: 0.50, IC 95% (0.29–0.85), p = 0.011) were significantly associated with a higher proportion of patients dead or still hospitalized 15 days after their admission to ITC (Figure 2).
Figure 2.
Multivariate analysis of clinical status at D15.
3.5. Reported Side Effects
A total of 395 side effects were reported. We observed that 91 (23%) deaths, 82 (20.7%) headache, and muscular skeletal disorders 72 (18.2%) were the most common side effects. Overall, 15 cardiac rhythm disorders were observed, 2 (13.3%) in the HCQ and AZM group and 13 (87.7%) in the without HCQ + AZM group (Table 2). Cardiac rhythm disorders observed in the HCQ + AZM group were: QT prolongation and bradycardia requiring stopping the treatment with no sequelae.
Table 2.
Reported side effects in the two groups of treatment.
| Without HCQ + AZM | HCQ + AZI | Total | |
|---|---|---|---|
| Abdominal discomfort | 14 (4.32%) | 6 (8.45%) | 20 (5.06%) |
| Cardiac rhythm disorder | 13 (4.01%) | 2 (2.82%) | 15 (3.80 %) |
| Death | 67 (20.68%) | 24 (33.80%) | 91 (23.04%) |
| Diarrhea | 18 (5.56%) | 19 (26.76%) | 37 (9.37%) |
| Headache | 78 (24.07%) | 4 (5.63%) | 82 (20.76%) |
| Hematologic disorders | 6 (1.85%) | 1 (1.41%) | 7 (1.77%) |
| Musculoskeletal disorders | 70 (21.60%) | 2 (2.82%) | 72 (18.23%) |
| Upset stomach | 43 (13.27%) | 5 (7.04%) | 48 (12.15%) |
| Vomit | 15 (4.63%) | 8 (11.27%) | 23 (5.82%) |
| Total side effect reported | 324 | 71 | 395 |
| Absence of side effect reported | 88 (9.5%) | 618 (66.74%) | 706 (76.24%) |
4. Discussion
As of today, few data are available regarding clinical and virological outcomes of COVID-19 patients living in sub-Saharan Africa. We exposed results of this first descriptive cohort in Senegal.
The population hospitalized, from March to October for COVID-19 infection described in this cohort, was younger and with less comorbidities compared to the population previously reported in Europe or the United States [30,31]. Among patients with pre-existing disorders, hypertension and diabetes were the most common. Regarding clinical presentation, the majority of patients presented a low level of severity at admission and the number of patients without any COVID-19 symptoms was high. When symptoms were present, fever, cough and myalgia were the most frequent as commonly described [32]. The political measures taken by the Senegalese government immediately after the first cases of COVID-19, and which were based on a systematic screening and testing of contact cases as well as a systematic hospitalization of all positive cases regardless of symptom presentation, could explain the higher proportion of low severity clinical presentation in hospitalized patients at the time of admission.
Patients hospitalized at an early stage of COVID-19 developed secondary symptoms, explaining the higher proportion of patients categorized as medium or high clinical risk. Complications related to the hospitalization and isolation could also explain part of this increase.
Exposure to antibiotics was extremely high due to the large prescription of macrolides. This broad prescription may have contributed to the prevention of the occurrence of infectious complications [33].
In our cohort, 61% of patients were discharged alive within 15 days after their admission. This proportion was lower than described in the multicentre, randomized, open-label, conducted in Brazil [15]. This study showed no difference in terms of improvement of clinical status at 15 days between standard care and standard care plus combination of HCQ and AZM. Hospital duration depends on criteria for admission and discharge [34]. In Senegal, following national guidelines, hospital discharge was allowed only after obtaining two consecutive negative PCRs. Moreover, recruitment of our cohort was conducted in five sites including three specialized treatment centres which included intensive care units. This specificity contributes to a higher level of severity of hospitalized patients and a higher rate of death.
A majority of the described patients (72.4%) received a combination of HCQ and AZM within 72 h following their admission to the ITC. This cohort’s results showed, in multivariate analysis, a higher proportion of patients discharged alive for patients taking combination of HCQ and AZM regimen early after their admission. However, another study in Africa confirms this finding with the combination of chloroquine and azithromycin (CQ/AZM). It shows that its patients who received CQ/AZ had significantly lower mortality than those on other treatments [35].
These results, while consistent with some studies [5,6,35,36,37], are in contradiction with clinical trials and other large-scale studies on efficacy of HCQ with or without AZM which were conducted in high income countries [15,17,18,38]. Treatment initiation at an early stage of the disease, age of the population, local hospital discharge indications may contribute to this result.
Few side effects were documented in our study including only two cases of cardiac rhythm disorder in the HCQ and AZM group. A study in Africa where the majority of patients (n = 630) were treated with CQ/AZ reported that eighteen out of 545 patients on CQ/AZ reported at least one side effect, which was mostly pruritus, rash, gastrointestinal disturbances, palpitations or bradycardia [35]. Chloroquine is the most effective and widely used drug in many parts of the world, and particularly in Africa, as a reliable treatment for uncomplicated forms of malaria [39]. It was a frequently kept medicine at home and used for self-treatment in most African countries. Between 1995 and 2003, chloroquine was the only drug recommended by the Senegalese health authorities [40]. Moreover, a prospective longitudinal study carried out in a Senegalese village between 1990 and 2012 reported few serious side effects linked to chloroquine [41]. Furthermore, the spread of chloroquine resistance in Plasmodium falciparum has had a considerable impact on the high level of malaria mortality in most epidemiological settings in Africa [42]. Hydroxychloroquine (HCQ) is a less toxic metabolite of chloroquine [43]. Most toxic effects occur at high concentrations, after prolonged administration of the drug or in combination with other drugs [44].
Delay between first symptoms onset and treatment was also correlated to a higher proportion of patients discharged at D15. This result may be related to the national recommendations for hospital discharge which was conditioned by a PCR negativity. Thus, the negativity of the nasopharyngeal swab could be obtained earlier for patients who developed their symptoms far from admission.
We showed a significantly higher proportion of patients dead or still hospitalized after 15 days post-admission among older patients, patients with at least one reported pre-existing disorder and patients categorized as high clinical risk at admission time following NEWS score. These results were in line with previously reported data [32,45,46]. The number of hospitalised cases depends more on the availability of resources and diagnostic capacity. In many African countries, due to resource limitations, the number of hospitalised cases appears to be insignificant, in contrast to the hospitalised numbers in Europe during this period. It also depends on different case detection and management policy established by the health authorities.
Limitations of the Study
Our study has some limitations. First, some cases had incomplete documentation of the disease history before hospitalization, daily clinical symptoms and laboratory testing. Data generation was clinically driven and not systematic. Second, recruitment was performed in five ITC including three specialized treatment centres which included intensive care units. This aspect may have an overrepresentation of severe cases.
Combination of HCQ-AZM was positively correlated with an increase in the proportion of patients discharged alive at 15 days. However, further prospective trials are needed to examine this impact. Future analyses will allow more detailed description of the biological, virological and immunological profiles of the patients.
5. Conclusions
Our results also need to be confirmed by randomised controlled trials that strictly evaluate the efficacy of HCQ + AMZ treatment for COVID-19 in hospitalised patients in Africa. Our findings also suggest that HCQ + AMZ treatment may play an important role in reducing the length of hospital stay.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10132954/s1, Table S1: Patients’ clinical and virological characteristics during hospitalization period. Table S2: Clinical status of patients at day 15, risk factors associated with hospital discharge.
Author Contributions
Conceptualization, M.S., F.T., C.L. and A.A.S.; methodology, M.S., F.T., P.D.; software, M.D. (Mamadou Diop), M.C.; validation, N.D., O.F., C.T.D., A.S., F.D.S. and I.V.-W.; formal analysis, A.G., C.T., C.L.; investigation, B.T., F.D.S., M.D. (Moustapha Diop), K.D.M., N.A.L., D.T., A.M.N., P.S.B., M.D. (Margarite Diatta), N.M.F., M.A.B., A.S.B.; resources, N.A.L.; writing—original draft preparation, F.T.; writing—review and editing, C.T., F.T.; visualization, M.D. (Mamadou Diop), M.C.; supervision, M.S., A.A.S., L.F., V.M.-P.C.D., D.K.; funding acquisition, A.A.S. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Institut Pasteur de Dakar and Welcome Trust Grant 221012/Z/20/Z. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Senegalese National Ethics Committee for Research in Health (reference number 00000068/MSAS/CNERS/Sec, accessed on 10 April 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Novel Coronavirus–China 12 January 2020. [(accessed on 1 June 2020)]; Available online: http://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/
- 2.WHO WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19-11 March 2020. [(accessed on 1 June 2020)]; Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- 3.SMoHaS, Coronavirus (COVID-19) Situation Dashboard. [(accessed on 30 October 2020)]; Available online: https://cartosantesen.maps.arcgis.com/apps/opsdashboard/index.html#/260c7842a77a48c191bf51c8b0a1d3f6.
- 4.Gautret P., Hoang V.T., Lagier J.-C., Raoult D. Effect of hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial, an update with an intention-to-treat analysis and clinical outcomes. Int. J. Antimicrob. Agents. 2021;57 doi: 10.1016/j.ijantimicag.2020.106239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Lagier J.-C., Million M., Gautret P., Colson P., Cortaredona S., Giraud-Gatineau A., Honoré S., Gaubert J.-Y., Fournier P.-E., Tissot-Dupont H., et al. Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis. Travel Med. Infect. Dis. 2020;36 doi: 10.1016/j.tmaid.2020.101791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagens A., Sigfrid L., Cai E., Lipworth S., Cheung V., Harris E., Bannister P., Rigby I., Horby P. Scope, quality, and inclusivity of clinical guidelines produced early in the covid-19 pandemic: Rapid review. BMJ. 2020;369:m1936. doi: 10.1136/bmj.m1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devaux C.A., Rolain J.-M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID-19? Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6 doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Million M., Gautret P., Colson P., Roussel Y., Dubourg G., Chabriere E., Honore S., Rolain J.-M., Fenollar F., Fournier P.-E., et al. Clinical efficacy of chloroquine derivatives in COVID-19 infection: Comparative meta-analysis between the big data and the real world. New Microbes New Infect. 2020;38 doi: 10.1016/j.nmni.2020.100709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damle B., Vourvahis M., Wang E., Leaney J., Corrigan B. Clinical Pharmacology Perspectives on the Antiviral Activity of Azithromycin and Use in COVID-19. Clin. Pharmacol. Ther. 2020;108:201–211. doi: 10.1002/cpt.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andreani J., Le Bideau M., Duflot I., Jardot P., Rolland C., Boxberger M., Wurtz N., Rolain J.-M., Colson P., La Scola B., et al. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb. Pathog. 2020;145 doi: 10.1016/j.micpath.2020.104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO Solidarity Trial Consortium. Pan H., Peto R., Henao-Restrepo A.-M., Preziosi M.-P., Sathiyamoorthy V., Abdool Karim Q., Alejandria M.M., Hernández García C., Kieny M.-P., et al. Repurposed Antiviral Drugs for Covid-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021;384:497–511. doi: 10.1056/nejmoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C., Veiga V.C., Avezum A., Damiani L.P., Marcadenti A., Kawano-Dourado L., Lisboa T., et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N. Engl. J. Med. 2020;383:2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G., Labella A., Manson D.K., Kubin C., Barr R.G., et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitjà O., Corbacho-Monné M., Ubals M., Tebé C., Peñafiel J., Tobias A., Ballana E., Alemany A., Riera-Martí N., Pérez A.C., et al. Hydroxychloroquine for Early Treatment of Adults with Mild Coronavirus Disease 2019: A Randomized, Controlled Trial. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The RECOVERY Collaborative Group. Horby P., Mafham M., Linsell L., Bell J.L., Staplin N., Emberson J.R., Wiselka M., Us-tianowski A., Elmahi E., et al. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020;383:2030–2040. doi: 10.1056/nejmoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bessière F., Roccia H., Delinière A., Charrière R., Chevalier P., Argaud L., Cour M. Assessment of QT Intervals in a Case Series of Patients with Coronavirus Disease 2019 (COVID-19) Infection Treated with Hydroxychloroquine Alone or in Combination with Azithromycin in an Intensive Care Unit. JAMA Cardiol. 2020;5:1067–1069. doi: 10.1001/jamacardio.2020.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg E.S., Dufort E.M., Udo T., Wilberschied L.A., Kumar J., Tesoriero J., Weinberg P., Kirkwood J., Muse A., DeHovitz J., et al. Association of Treatment with Hydroxychloroquine or Azithromycin with In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA. 2020;323:2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization . COVID-19 Clinical Management: Living Guidance 25 January 2021. World Health Organization; Geneva, Switzerland: 2021. [Google Scholar]
- 22.Belayneh A. Off-Label Use of Chloroquine and Hydroxychloroquine for COVID-19 Treatment in Africa Against WHO Recommendation. Res. Rep. Trop. Med. 2020;11:61–72. doi: 10.2147/rrtm.s269936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simpson C.R., Beever D., Challen K., De Angelis D., Fragaszy E., Goodacre S., Hayward A., Lim W.S., Rubin G.J., Semple M., et al. The UK’s pandemic influenza research portfolio: A model for future research on emerging infections. Lancet Infect. Dis. 2019;19:e295–e300. doi: 10.1016/S1473-3099(18)30786-2. [DOI] [PubMed] [Google Scholar]
- 24.WHO Clinical Characterisation Protocol (CCP) ISARIC. [(accessed on 1 June 2020)]; Available online: https://isaric.org/research/covid-19-clinical-research-resources/clinical-characterisation-protocol-ccp/
- 25.Hoehl S., Rabenau H., Berger A., Kortenbusch M., Cinatl J., Bojkova D., Behrens P., Böddinghaus B., Götsch U., Naujoks F., et al. Evidence of SARS-CoV-2 Infection in Returning Travelers from Wuhan, China. N. Engl. J. Med. 2020;382:1278–1280. doi: 10.1056/NEJMc2001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ISARIC . Novel Coronavirus (NCoV) Acute Respiratory Infection Clinical Characterisation Data Tool. Version 1.2 28 JAN 2020. World Health Organization; Geneva, Switzerland: 2020. [Google Scholar]
- 27.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O’Neal L., McLeod L., Delacqua G., Delacqua F., Kirby J., et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Royal College of Physicians . National Early Warning Score (NEWS) 2: Standardising the Assessment of Acute-Ilness Severity in the NHS. Updated Report of a Working Party. RCP; London, UK: 2017. [Google Scholar]
- 29.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2020. [Google Scholar]
- 30.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., Holden K.A., Read J.M., Dondelinger F., Carson G., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., the Northwell COVID-19 Research Consortium Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bacharier L.B., Guilbert T.W., Mauger D.T., Boehmer S.J., Beigelman A., Fitzpatrick A.M., Jackson D.J., Baxi S.N., Benson M., Burnham C.-A.D., et al. Early Administration of Azithromycin and Prevention of Severe Lower Respiratory Tract Illnesses in Preschool Children with a History of Such Illnesses: A Randomized Clinical Trial. JAMA. 2015;314:2034–2044. doi: 10.1001/jama.2015.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rees E.M., Nightingale E.S., Jafari Y., Waterlow N.R., Clifford S., Pearson C.A.B., CMMID Working Group. Jombart T., Procter S., Knight G.M. COVID-19 length of hospital stay: A systematic review and data synthesis. BMC Med. 2020;18:1–22. doi: 10.1186/s12916-020-01726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nachega J.B., Ishoso D.K., Otokoye J.O., Hermans M.P., Machekano R.N., Sam-Agudu N.A., Nswe C.B.-P., Mbala-Kingebeni P., Madinga J.N., Mukendi S., et al. Clinical Characteristics and Outcomes of Patients Hospitalized for COVID-19 in Africa: Early Insights from the Democratic Republic of the Congo. Am. J. Trop. Med. Hyg. 2020;103:2419–2428. doi: 10.4269/ajtmh.20-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Million M., Lagier J.-C., Gautret P., Colson P., Fournier P.-E., Amrane S., Hocquart M., Mailhe M., Esteves-Vieira V., Doudier B., et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille, France. Travel Med. Infect. Dis. 2020;35 doi: 10.1016/j.tmaid.2020.101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arshad S., Kilgore P., Chaudhry Z.S., Jacobsen G., Wang D.D., Huitsing K., Brar I., Alangaden G.J., Ramesh M.S., McKinnon J.E., et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int. J. Infect. Dis. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenthal N., Cao Z., Gundrum J., Sianis J., Safo S. Risk Factors Associated with In-Hospital Mortality in a US National Sample of Patients With COVID-19. JAMA Netw. Open. 2020;3:e2029058. doi: 10.1001/jamanetworkopen.2020.29058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jensen M., Mehlhorn H. Seventy-five years of Resochin® in the fight against malaria. Parasitol. Res. 2009;105:609–627. doi: 10.1007/s00436-009-1524-8. [DOI] [PubMed] [Google Scholar]
- 40.Trape J.F. The public health impact of chloroquine resistance in Africa. Am. J. Trop. Med. Hyg. 2001;64:12–17. doi: 10.4269/ajtmh.2001.64.12. [DOI] [PubMed] [Google Scholar]
- 41.Trape J.-F., Tall A., Sokhna C., Ly A.B., Diagne N., Ndiath O., Mazenot C., Richard V., Badiane A., Dieye-Ba F., et al. The rise and fall of malaria in a west African rural community, Dielmo, Senegal, from 1990 to 2012: A 22 year longitudinal study. Lancet Infect. Dis. 2014;14:476–488. doi: 10.1016/S1473-3099(14)70712-1. [DOI] [PubMed] [Google Scholar]
- 42.Trape J.-F., Pison G., Preziosi M.-P., Enel C., du Loû A.D., Delaunay V., Samb B., Lagarde E., Molez J.-F., Simondon F. Impact of chloroquine resistance on malaria mortality. Comptes Rendus Acad. Sci. Ser. III Sci. Vie. 1998;321:689–697. doi: 10.1016/S0764-4469(98)80009-7. [DOI] [PubMed] [Google Scholar]
- 43.Stokkermans T.J., Goyal A., Bansal P., Trichonas G. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2021. Chloroquine and Hydroxychloroquine Toxicity. [PubMed] [Google Scholar]
- 44.Cardiac Effects and Toxicity of Chloroquine: A Short Update—ScienceDirect. [(accessed on 5 June 2021)]; Available online: https://www.sciencedirect.com/science/article/pii/S0924857920302272.
- 45.Huang C., Soleimani J., Herasevich S., Pinevich Y., Pennington K.M., Dong Y., Pickering B.W., Barwise A.K. Clinical Characteristics, Treatment, and Outcomes of Critically Ill Patients With COVID-19: A Scoping Review. Mayo Clin. Proc. 2021;96:183–202. doi: 10.1016/j.mayocp.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu Z., McGoogan J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.