ABSTRACT
The genus Acinetobacter comprises species with ecological significance and opportunistic pathogens and has a complicated taxonomy. Precise species identification is a foundation for understanding bacteria. In this study, we found and characterized two novel Acinetobacter species, namely, Acinetobacter tianfuensis sp. nov. and Acinetobacter rongchengensis sp. nov., based on phenotype examinations and genome analyses of the two strains WCHAc060012T and WCHAc060115T. The two strains had ≤89.69% (mean, 79.28% or 79.72%) average nucleotide identity (ANI) and ≤36.4% (mean, 20.89% or 22.19%) in silico DNA-DNA hybridization (isDDH) values compared with each other and all known Acinetobacter species. Both species can be differentiated from all hitherto known Acinetobacter species by a combination of phenotypic characteristics. We found that Acinetobacter pullorum B301T and Acinetobacter portensis AC 877T are actually the same species with 98.59% ANI and 90.4% isDDH values. We then applied the updated taxonomy to curate 3,956 Acinetobacter genomes in GenBank and found that 6% of Acinetobacter genomes (n = 234) are required to be corrected or updated. We identified 56 novel tentative Acinetobacter species, extending the number of Acinetobacter species to 144, including 68 with species names and 76 unnamed taxa. We also found that ANI and the average amino acid identity (AAI) values among type or reference strains of all Acinetobacter species and taxa are ≥76.97% and ≥66.5%, respectively, which are higher than the proposed cutoffs to define the genus boundary. This study highlights the complex taxonomy of Acinetobacter as a single genus and the paramount importance of precise species identification. The newly identified unnamed taxa warrant further studies.
IMPORTANCE Acinetobacter species are widely distributed in nature and are of important ecological significance and clinical relevance. In this study, first, we significantly update the taxonomy of Acinetobacter by reporting two novel Acinetobacter species, namely, Acinetobacter tianfuensis and Acinetobacter rongchengensis, and by identifying Acinetobacter portensis as a synonym of Acinetobacter pullorum. Second, we curated Acinetobacter genome sequences deposited in GenBank (n = 3,956) using the updated taxonomy by correcting species assignations for 6% (n = 234) genomes and by assigning 94 (2.4%) to 56 previously unknown tentative species (taxa). Therefore, after curation, we further update the genus Acinetobacter to comprise 144 species, including 68 with species names and 76 unnamed taxa. Third, we addressed the question of whether such a large number of species should be divided in different genera and found that Acinetobacter is indeed a single genus. Our study significantly advanced the taxonomy of Acinetobacter, an important genus with science and health implications.
KEYWORDS: Acinetobacter, genome analysis, phylogenetic analysis, quasispecies, species
INTRODUCTION
The genus Acinetobacter, first proposed by Brisou and Prévot (1), is a highly diverse group. Members of the genus Acinetobacter are distributed widely in soil and water (2) and possess versatile metabolic capabilities for the degradation of various compounds, such as long-chain dicarboxylic acids and aromatics, and actively participate in the nutrient cycle in the ecosystem (3, 4). Some Acinetobacter species are also well-known opportunistic pathogens causing a variety of human infections (5–8). Precise species assignation lays a foundation for understanding the habitat, epidemiology, pathogenesis, and microbiological features of bacteria and has important implications for health, industry, and science, while updated and curated taxonomic assignment is the premise of precise species identification (9, 10). Before the present study, the genus Acinetobacter included 67 species with validly published names (11) and 20 additional Acinetobacter species with tentative species designations (www.szu.cz/anemec/Classification.pdf). Validly published names refer to those published in the International Journal of Systematic and Evolutionary Microbiology, the official journal of the International Committee on Systematics of Prokaryotes, including its validation lists (12). New Acinetobacter species are continuingly being reported, and the number of Acinetobacter species increases every year, with 6 novel species in 2017, 3 in 2018, 4 in 2019. and 9 in 2020 (11, 13–18). However, the taxonomy of Acinetobacter is complicated by the presence of synonyms (19–22). In addition, it is not uncommon that bacterial genomes deposited in GenBank are mislabeled for species assignations (10, 23, 24) (https://help.ezbiocloud.net/type-strain-and-reference-strain/). Therefore, there is a need to update the taxonomy of Acinetobacter and to curate the species assignations of Acinetobacter genome sequences deposited in GenBank.
Here, we report two novel Acinetobacter species, namely, Acinetobacter tianfuensis sp. nov. and Acinetobacter rongchengensis sp. nov., based on phenotypic characterization and genomic analysis. We updated the Acinetobacter taxonomy and found a pair of synonyms, Acinetobacter pullorum and Acinetobacter portensis, which has not been identified before. We then used the updated taxonomy to curate 5,997 Acinetobacter genomes available in GenBank (accessed by 1 August 2020), and we identified 56 previously unknown tentative species designations.
RESULTS
Identification of two novel Acinetobacter species, namely, Acinetobacter tianfuensis and Acinetobacter rongchengensis.
Two Acinetobacter strains, namely, WCHAc060012T and WCHAc060115T, were recovered from hospital sewage using an Acinetobacter chromogenic agar plate in 2018. We obtained the nearly complete 16S rRNA gene sequences (1,352 bp) of the two strains using PCR with the universal primers 27F and 1492R (25) and Sanger sequencing as described previously (26) for preliminary species identification. Comparison of the 16S rRNA gene sequences in the EzBioCloud database (27) and the 16S rRNA gene sequence-based phylogenetic tree (see Fig. S1 in the supplemental material) revealed that the two strains indeed belonged to the genus Acinetobacter. Strains WCHAc060012T and WCHAc060115T had the highest identity of 16S rRNA gene sequences with Acinetobacter chengduensis WCHAc060005T (98.96%; accession no. MK796535) and Acinetobacter chinensis WCHAc010005T (98.05%; accession no. NR_165666), respectively. However, it is well known that analysis based on the 16S rRNA sequence is insufficient for accurate taxonomic assignment (28). We then compared partial rpoB sequences (861 bp) of the two strains with those of Acinetobacter type strains. The two strains were also distinct from all known Acinetobacter species and formed two evolutionary clades in the phylogenetic tree based on partial rpoB gene sequences (see Fig. S2 in the supplemental material). Strain WCHAc060012T had the highest identity of the partial rpoB sequence with Acinetobacter wanghuae dk386T (89.08%), while WCHAc060115T had the highest identity with Acinetobacter piscicola KCTC 62134T (95.23%).
Maximum likelihood phylogenetic tree based on 16S rRNA gene sequences (1,352 bp) of WCHAc060012T, WCHAc060115T, and type strains of Acinetobacter species with validly published names. The sequence of Moraxella lacunata ATCC 17967T (GenBank accession no. AF005160) was used as the outgroup. Bootstrap values (≥50%) after 1,000 resamplings are indicated at branch nodes. Shown in parentheses are the DDBJ/ENA/GenBank accession no. for 16S rRNA gene sequences or whole-genome sequences. Bar, 0.2 substitutions per nucleotide position. Download FIG S1, PDF file, 2.6 MB (2.7MB, pdf) .
Copyright © 2021 Qin et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Maximum likelihood phylogenetic tree based on partial rpoB (861 bp) gene sequences of WCHAc060012T, WCHAc060115T, and type strains of Acinetobacter species with validly published names. Evolutionary distances were computed using Kimura’s two-parameter model. Moraxella lacunata ATCC 17967T was used as the outgroup. Bootstrap values ≥ 50% based on 1,000 resamplings are shown. Shown in parentheses are the DDBJ/ENA/GenBank accession no. for rpoB gene sequences or whole-genome sequences. Bar, 0.2 substitutions per nucleotide position. Download FIG S2, PDF file, 2.3 MB (2.3MB, pdf) .
Copyright © 2021 Qin et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
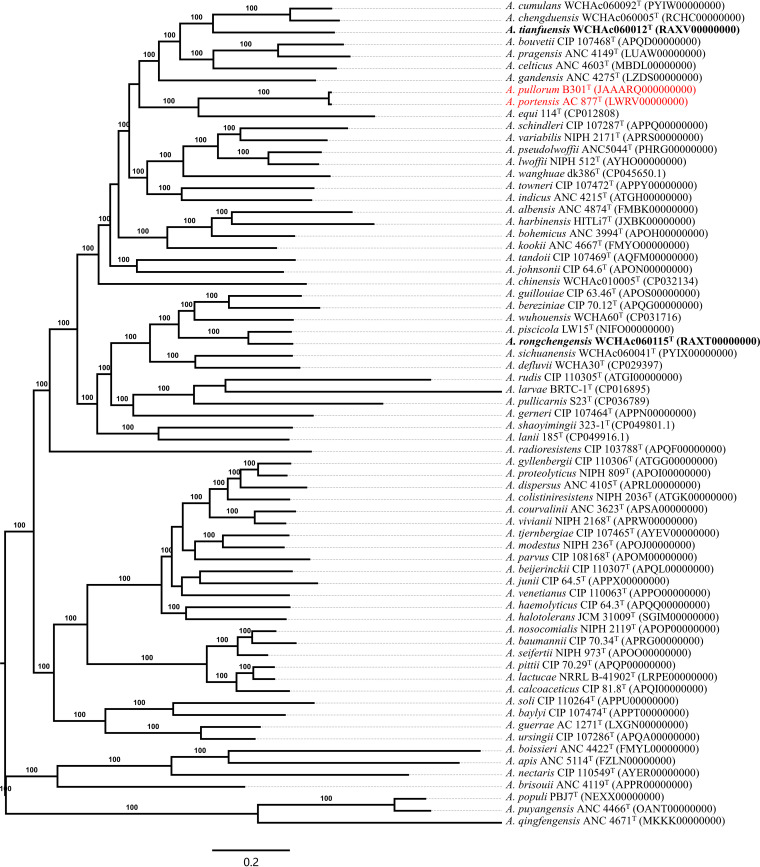
To further explore their precise species assignations, the two strains were subjected to whole-genome sequencing using Illumina HiSeq X10 platform. For strain WCHAc060012T, 6,401,206 reads and 1.92 giga-bases (Gb) were generated with an actual 549.9× coverage, which were assembled into a 3.5-Mb draft genome sequence containing 116 contigs (N50, 77,978 bp) with a G+C content of 42.3% . For strain WCHAc060115T, 5,479,547 reads and 1.64 Gb were generated with an actual 396.8 × coverage, which were assembled into a 4.1-Mb draft genome sequence containing 248 contigs (N50, 68,539 bp) with a G+C content of 37.7%. We determined the average nucleotide identity (ANI) values between WCHAc060012T and WCHAc060115T and between the two strains and type strains of all Acinetobacter species. Compared with type strains of all Acinetobacter species, ANI values of WCHAc060012T ranged from 77.09% (Acinetobacter puyangensis ANC 4466T) to 82.70% (Acinetobacter cumulans WCHAc060092T), while those of WCHAc060115T ranged from 77.71% (A. puyangensis ANC 4466T) to 89.69% (Acinetobacter piscicola LW15T) (Table 1). The ANI value between WCHAc060012T and WCHAc060115T was 79.46% (Table 1). These ANI values are well below the 95% to 96% threshold used to define bacterial species (29). We then performed in silico DNA-DNA hybridization (isDDH) analyses for WCHAc060012T, WCHAc060115T, and type strains of all Acinetobacter species. The isDDH values of WCHAc060012T and type strains of all Acinetobacter species were 19.2% to 23.4%, while those of WCHAc060115T and type strains of all Acinetobacter species were 20.0% to 36.4% (Table 1), which are far below the 70% cutoff used to define a species (30, 31). The isDDH value between WCHAc060012T and WCHAc060115T was 21.7% (Table 1). Both ANI and isDDH analyses clearly indicate that the two strains represent two novel Acinetobacter species. In the phylogenomic tree based on core genes (Fig. 1), WCHAc060012T and WCHAc060115T are most closely related to A. cumulans WCHAc060092T and A. piscicola LW15T, respectively.
TABLE 1.
Average nucleotide identity based on BLAST and in silico DNA-DNA hybridization values
| Acinetobacter species and strain | Accession no. | ANI (%)/isDDH (%)a of: |
GC content (%) | |
|---|---|---|---|---|
| WCHAc060012T | WCHAc060115T | |||
| A. albensis ANC 4874T | FMBK00000000.1 | 79.15/20.2 | 79.48/20.9 | 38.4 |
| A. apis ANC 5114T | FZLN00000000.1 | 77.71/20.3 | 77.99/20.0 | 38.3 |
| A. baumannii ATCC 19606T | APRG00000000.1 | 78.75/19.9 | 79.37/21.1 | 39.1 |
| A. baylyi CIP 107474T | APPT00000000.1 | 78.47/19.7 | 78.91/20.7 | 40.4 |
| A. beijerinckii CIP 110307T | APQL00000000.1 | 78.46/20.8 | 79.42/21.1 | 38.3 |
| A. bereziniae CIP 70.12T | APQG00000000.1 | 79.26/21.1 | 82.98/27.1 | 38.2 |
| A. bohemicus ANC 3994T | APOH00000000.1 | 79.70/21.1 | 80.04/21.8 | 39.6 |
| A. boissieri ANC 4422T | FMYL00000000.1 | 77.81/19.5 | 77.79/20.1 | 38.0 |
| A. bouvetii CIP 107468T | APQD00000000.1 | 81.52/22.4 | 79.36/20.7 | 45.0 |
| A. brisouii CIP 110357T | AYEU00000000.1 | 78.70/21.7 | 79.13/22.3 | 41.7 |
| A. calcoaceticus DSM 30006T | APQI00000000.1 | 78.82/20.2 | 78.99/21.4 | 38.6 |
| A. celticus ANC 4603T | MBDL00000000.1 | 80.30/20.8 | 79.77/21.0 | 39.3 |
| A. chengduensis WCHAc060005T | RCHC00000000.1 | 81.99/22.2 | 79.52/21.8 | 39.9 |
| A. chinensis WCHAc010005T | CP032134.1 | 79.78/21.0 | 80.09/22.9 | 42.4 |
| A. colistiniresistens NIPH 2036T | ATGK00000000.1 | 78.72/20.5 | 81.37/28.6 | 41.0 |
| A. courvalinii ANC 3623T | APSA00000000.1 | 78.74/20.6 | 79.00/21.0 | 42.0 |
| A. cumulans WCHAc060092T | PYIW00000000.1 | 82.70/23.4 | 79.56/21.9 | 40.2 |
| A. defluvii WCHA30T | MAUF00000000.1 | 80.03/21.7 | 83.08/27.6 | 38.0 |
| A. dispersus ANC 4105T | APRL00000000.1 | 78.86/20.2 | 79.19/21.3 | 40.4 |
| A. equi 114T | CP012808.1 | 79.94/21.4 | 79.86/21.9 | 34.9 |
| A. gandensis ANC 4275T | LZDS00000000.1 | 81.09/21.4 | 79.94/21.4 | 39.7 |
| A. gerneri CIP 107464T | APPN00000000.1 | 79.48/22.5 | 80.13/22.9 | 37.7 |
| A. guerrae AC 1271T | LXGN00000000.1 | 78.65/19.2 | 78.89/20.1 | 39.2 |
| A. guillouiae CIP 63.46T | APOS00000000.1 | 79.10/21.3 | 82.02/24.6 | 38.2 |
| A. gyllenbergii CIP 110306T | ATGG00000000.1 | 78.52/20.1 | 79.35/22.5 | 40.8 |
| A. haemolyticus CIP 64.3T | APQQ00000000.1 | 78.95/21.5 | 79.22/22.3 | 39.7 |
| A. halotolerans JCM 31009T | SGIM00000000.1 | 78.58/19.8 | 79.02/20.5 | 40.0 |
| A. harbinensis HITLi7T | JXBK00000000.1 | 79.01/19.9 | 79.18/21.1 | 40.9 |
| A. indicus CIP 110367T | AYET00000000.1 | 79.99/21.3 | 79.69/21.5 | 45.4 |
| A. johnsonii CIP 64.6T | APON00000000.1 | 80.58/21.6 | 80.03/22.6 | 41.5 |
| A. junii CIP 107470T | APPS01000079.1 | 79.07/21.1 | 79.11/21.6 | 38.8 |
| A. kookii ANC 4667T | FMYO00000000.1 | 80.35/21.2 | 79.78/20.9 | 43.0 |
| A. lactucae NRRL B-41902T | LRPE00000000.1 | 78.85/19.8 | 78.98/21.3 | 38.6 |
| A. lanii 185T | CP049916.1 | 79.66/22.2 | 79.76/21.8 | 41.3 |
| A. larvae BRTC-1T | CP016895.1 | 78.06/20.6 | 78.21/21.8 | 41.6 |
| A. lwoffii NIPH 512T | AYHO00000000.1 | 80.01/21.3 | 79.12/22.0 | 43.0 |
| A. modestus NIPH 236T | APOJ00000000.1 | 78.72/20.3 | 79.53/21.9 | 38.4 |
| A. nectaris CIP 110549T | AYER00000000.1 | 77.98/20.1 | 78.08/20.5 | 36.7 |
| A. nosocomialis NIPH 2119T | APOP00000000.1 | 78.72/20.0 | 79.22/21.4 | 38.7 |
| A. parvus CIP 108168T | APOM00000000.1 | 79.12/21.8 | 79.18/22.3 | 41.7 |
| A. piscicola KCTC 62134T | NIFO00000000.1 | 79.33/21.0 | 89.69/36.4 | 37.2 |
| A. pittii ATCC 19004T | APQP01000014.1 | 78.73/20.2 | 78.98/21.2 | 38.8 |
| A. populi PBJ7T | NEXX00000000.1 | 77.54/20.6 | 78.02/21.1 | 40.2 |
| A. portensis AC 877T | LWRV00000000.1 | 80.10/21.2 | 80.14/21.8 | 36.6 |
| A. pragensis ANC 4149T | LUAW00000000.1 | 81.32/22.1 | 79.09/20.5 | 44.0 |
| A. proteolyticus NIPH 809T | APOI00000000.1 | 78.57/19.9 | 79.80/22.2 | 41.1 |
| A. pseudolwoffii ANC 5044T | PHRG00000000.1 | 80.14/21.0 | 79.42/21.3 | 43.3 |
| A. pullicarnis S23T | VCMZ00000000.1 | 78.10/21.7 | 79.92/24.9 | 41.5 |
| A. pullorum B301T | JAAARQ000000000.1 | 80.21/21.5 | 80.14/22.3 | 37.0 |
| A. puyangensis ANC 4466T | OANT00000000.1 | 77.09/20.1 | 77.71/20.5 | 40.2 |
| A. qingfengensis ANC 4671T | MKKK00000000.1 | 77.52/19.9 | 77.88/21.0 | 38.1 |
| A. radioresistens DSM 6976T | APQF00000000.1 | 78.59/19.8 | 78.78/20.8 | 41.7 |
| A. rongchengensis WCHAc060115T | RAXT00000000.1 | 79.46/21.7 | 37.7 | |
| A. rudis CIP 110305T | ATGI00000000.1 | 78.27/20.8 | 78.90/21.0 | 39.0 |
| A. schindleri CIP 107287T | APPQ00000000.1 | 80.38/21.4 | 79.65/21.7 | 42.2 |
| A. seifertii NIPH 973T | APOO00000000.1 | 78.94/20.7 | 79.35/22.6 | 38.6 |
| A. shaoyimingii 323-1T | CP049801.1 | 79.61/22.3 | 79.92/21.7 | 38.3 |
| A. sichuanensis WCHAc060041T | PYIX00000000.1 | 79.86/21.9 | 83.12/27.2 | 37.2 |
| A. soli CIP 110264T | APPU00000000.1 | 78.28/19.7 | 78.64/20.2 | 43.2 |
| A. tandoii CIP 107469T | AQFM00000000.1 | 79.89/20.5 | 80.58/23.2 | 40.0 |
| A. tianfuensis WCHAc060012T | RAXV00000000.1 | 79.46/21.7 | 42.3 | |
| A. tjernbergiae CIP 107465T | AYEV00000000.1 | 78.79/20.1 | 79.63/22.0 | 38.5 |
| A. towneri CIP 107472T | APPY00000000.1 | 80.03/21.7 | 79.97/21.9 | 41.2 |
| A. ursingii CIP 107286T | APQA00000000.1 | 78.73/19.9 | 79.25/22.1 | 40.1 |
| A. variabilis NIPH 2171T | APRS00000000.1 | 79.95/20.9 | 79.76/22.4 | 42.0 |
| A. venetianus CIP 110063T | APPO00000000.1 | 78.77/20.4 | 79.11/20.8 | 39.1 |
| A. vivianii NIPH 2168T | APRW00000000.1 | 78.90/20.6 | 79.20/21.3 | 41.4 |
| A. wanghuae dk386T | CP045650.1 | 79.88/21.0 | 79.65/21.0 | 40.6 |
| A. wuhouensis WCHA60T | MBPR00000000.1 | 79.95/22.1 | 82.04/24.0 | 38.1 |
FIG 1.
Phylogenomic tree of WCHAc060012T, WCHAc060115T, and type strains of Acinetobacter species with validly published names. The phylogenomic tree was inferred based on the alignment of 1,397 core genes. WCHAc060012T and WCHAc060115T are highlighted in bold. A. pullorum and A. portensis, a pair of synonyms, are highlighted in red. DDBJ/ENA/GenBank accession no. are shown in parentheses and 100% bootstraps are indicated. Bar, 0.2 changes per nucleotide position.
After phenotypic characterizations (see below), we propose strain WCHAc060012T with the name Acinetobacter tianfuensis sp. nov. (tian.fu.en’sis. N.L. masc. adj. tianfuensis, referring to Chengdu City, Sichuan Province, China) and WCHAc060115T with the name Acinetobacter rongchengensis sp. nov. (rong.cheng.en’sis. N.L. masc. adj. rongchengensis, another name referring to Chengdu City, Sichuan Province, China). The type strain of Acinetobacter tianfuensis and Acinetobacter rongchengensis is WCHAc060012T (=GDMCC 1.1623T =JCM 33510T) and WCHAc060115T (=GDMCC 1.1625T =JCM 33512T), respectively.
The two novel Acinetobacter species may be able to be differentiated from other Acinetobacter species by a combination of phenotypic characteristics.
The phenotypic characteristics tested using the genus-targeted set of physiological and metabolic tests are presented in the standard way used in previous nomenclatural proposals (32, 33). The phenotypes for the two novel Acinetobacter species, together with those for all known Acinetobacter species with validly published names, are summarized in Data Set S1 in the supplemental material. For both strains, growth occurs at various pHs from 7 to 8 and the temperatures range 20 to 35°C. Strain WCHAc060012T grows at 30°C in the presence of 0% to 3% (wt/vol) NaCl in tryptic soy broth (TSB), while WCHAc060115T grows in 0% to 4% (wt/vol) NaCl. Both strains were positive for the catalase test but negative for the oxidase activity. Cells of the two strains are Gram-negative coccobacilli; strictly aerobic; nonsporogenous; incapable of swimming motility; and capable of growing on media such as tryptic soy agar (TSA), Luria-Bertani (LB) agar, BHI agar, and Müller-Hinton agar (all from Hopebio). Colonies are light yellow, circular, opaque, smooth, convex, with entire margins, and approximately 1.0 to 2.0 mm in diameter after 24 h of incubation at 30°C on BHI agar plates.
Phenotypic properties of A. tianfuensis sp. nov., A. rongchengensis sp. nov. and the Acinetobacter species with validly published names. Download Data Set S1, XLSX file, 0.03 MB (30.3KB, xlsx) .
Copyright © 2021 Qin et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Phenotypic differences between the two novel Acinetobacter species and each of the known species with validly published names are indicated in Data Set S1. When considering only clearly positive or clearly negative results, the most useful combinations of characteristics for differentiating WCHAc060012T from all known Acinetobacter species include growth on l-glutamate, d-malate, malonate, and phenylacetate but no growth on l-arabinose, l-arginine, azelate, and glutarate (Data Set S1). Strain WCHAc060115T could be differentiated from all known Acinetobacter species by the combination of assimilation trans-aconitate, citrate (Simmons’), and l-tartrate but not β-alanine and 4-aminobutyrate (Data Set S1).
We also identified antimicrobial resistance genes from genome sequences of the two strains (see Table S1 in the supplemental material). Both strains had genes mediating resistance to aminoglycosides, sulfonamides, and macrolides, while WCHAc060115T also harbored two carbapenemase genes, namely, blaNDM-1 and blaOXA-58, and WCHAc060012T carried a tetracycline-resistant gene tet(39).
Antimicrobial resistance genes of A. tianfuensis WCHAc060012T and A. rongchengensis WCHAc060115T. Download Table S1, PDF file, 0.1 MB (71.7KB, pdf) .
Copyright © 2021 Qin et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Acinetobacter pullorum Elnar et al. 2020 and Acinetobacter portensis Ana et al. 2020 are the same species.
During the process of studying WCHAc060012T and WCHAc060115T, we also found a pair of synonyms, namely, A. pullorum and A. portensis. A. pullorum B301T was isolated from raw chicken meat at a local market in Korea (14). It has been shown that A. pullorum B301T is closely related to Acinetobacter celticus ANC 4603T (14). Four A. portensis strains were isolated from raw meat samples in supermarkets in Porto, Portugal. A. portensis is also closely related to A. celticus ANC 4603T (15). A comparison of the 16S rRNA gene sequences for the two type strains showed a 99.70% similarity. The draft genome sequence of A. pullorum B301T (GenBank accession no. JAAARQ000000000) and that of A. portensis AC 877T (GenBank accession no. LWRV00000000) have a 90.4% isDDH value and a 98.59% ANI value. Both ANI and isDDH analyses clearly indicate that the two species are actually the same species. In the phylogenomic tree, A. pullorum B301T and A. portensis AC 877T indeed cluster together (Fig. 1, highlighted in red).
A comparison of the physiological and biochemical features of the two type strains shows phenotype coherence, which is summarized in Table S2 in the supplemental material. According to previous reports (14), A. pullorum and A. portensis are different in the acidification of d-glucose and utilization of β-alanine and d-glucose, which is likely due to intraspecies variability or assay conditions. Based on principles by the International Code of Nomenclature of Bacteria (12), A. pullorum has the priority of species name over A. portensis. We therefore propose that A. portensis (15) is a later heterotypic synonym of A. pullorum (14).
The characteristics of strains of A. pullorum and A. portensis. Download Table S2, PDF file, 0.1 MB (143.5KB, pdf) .
Copyright © 2021 Qin et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Curation of Acinetobacter genomes with the updated taxonomy.
Based on the above findings, the valid species names of Acinetobacter should be updated to comprise 68 species at present (Table 2). In addition, there are 20 tentative species designations of Acinetobacter (www.szu.cz/anemec/Classification.pdf) (Table 2). We then applied the updated Acinetobacter taxonomy to curate the 5,997 Acinetobacter strains with genome sequences deposited in GenBank (accessed by 1 August 2020). Before curation, we performed a quality-control check for all of the genomes. Among the 5,997 genomes, 2,041 were discarded due to low quality defined by >300 contigs for individual genomes (n = 444), a <50-kb N50 value (n = 458), <90% genome completeness (n = 20), genome contamination (n = 206), or genome heterogeneity (n = 913). We then used the remaining 3,956 genomes for precise species identification by both ANI and isDDH. Among the 3,956 Acinetobacter genomes, 3,777 were labeled with a known Acinetobacter species name (Data Set S2). The remaining 179 strains were labeled only with Acinetobacter sp. (n = 175), Acinetobacter genomosp. (n = 2), Acinetobacter calcoaceticus/Acinetobacter baumannii complex (n = 1), or uncultured Acinetobacter (n = 1) (Data Set S2), which were updated by our curation. Species were misidentified for 55 Acinetobacter genomes (Data Set S2 and summarized in Table S3 in the supplemental material). The 55 misidentified genomes include 13 labeled with A. baumannii but actually belonging to other Acinetobacter species and four of non-A. baumannii Acinetobacter species actually belonging to other closely related species (Table S3), while the remaining 38 genomes should be assigned to novel taxa (see below for details). Therefore, there were 234 genomes whose species identification needs to be corrected (n = 55) or updated (n = 179) according to the findings in this study (Data Set S2).
TABLE 2.
Updated classification and nomenclature of the genus Acinetobacter before species curation for genomes in GenBank
| Species name | Type strain or reference strain | Accession no. |
|---|---|---|
| Valid (n = 68) | ||
| Acinetobacter albensis | ANC 4874T | FMBK00000000 |
| Acinetobacter apis | ANC 5114T | FZLN00000000 |
| Acinetobacter baumannii | CIP 70.34T | APRG00000000 |
| Acinetobacter baylyi | CIP 107474T | APPT00000000 |
| Acinetobacter beijerinckii | CIP 110307T | APQL00000000 |
| Acinetobacter bereziniae | CIP 70.12T | APQG00000000 |
| Acinetobacter bohemicusa | ANC 3994T | APOH00000000 |
| Acinetobacter boissieri | ANC 4422T | FMYL00000000 |
| Acinetobacter bouvetii | CIP 107468T | APQD00000000 |
| Acinetobacter brisouii | ANC 4119T | APPR00000000 |
| Acinetobacter calcoaceticus | CIP 81.8T | APQI00000000 |
| Acinetobacter celticus | ANC 4603T | MBDL00000000 |
| Acinetobacter chengduensis | WCHAc060005T | RCHC00000000 |
| Acinetobacter chinensis | WCHAc010005T | CP032134 |
| Acinetobacter colistiniresistens | NIPH 2036T | ATGK00000000 |
| Acinetobacter courvalinii | ANC 3623T | APSA00000000 |
| Acinetobacter cumulans | WCHAc060092T | PYIW00000000 |
| Acinetobacter defluvii | WCHA30T | CP029397 |
| Acinetobacter dispersus | ANC 4105T | APRL00000000 |
| Acinetobacter equi | 114T | CP012808 |
| Acinetobacter gandensis | ANC 4275T | LZDS00000000 |
| Acinetobacter gerneri | CIP 107464T | APPN00000000 |
| Acinetobacter guerrae | AC 1271T | LXGN00000000 |
| Acinetobacter guillouiae | CIP 63.46T | APOS00000000 |
| Acinetobacter gyllenbergii | CIP 110306T | ATGG00000000 |
| Acinetobacter haemolyticus | CIP 64.3T | APQQ00000000 |
| Acinetobacter halotolerans | JCM 31009T | SGIM00000000 |
| Acinetobacter harbinensis | HITLi7T | JXBK00000000 |
| Acinetobacter indicusb | ANC 4215T | ATGH00000000 |
| Acinetobacter johnsonii | CIP 64.6T | APON00000000 |
| Acinetobacter juniic | CIP 64.5T | APPX00000000 |
| Acinetobacter kookii | ANC 4667T | FMYO00000000 |
| Acinetobacter lactucaed | NRRL B-41902T | LRPE00000000 |
| Acinetobacter lanii | 185T | CP049916 |
| Acinetobacter larvae | BRTC-1T | CP016895 |
| Acinetobacter lwoffiie | NIPH 512T | AYHO00000000 |
| Acinetobacter modestus | NIPH 236T | APOJ00000000 |
| Acinetobacter nectaris | CIP 110549T | AYER00000000 |
| Acinetobacter nosocomialis | NIPH 2119T | APOP00000000 |
| Acinetobacter parvus | CIP 108168T | APOM00000000 |
| Acinetobacter piscicola | LW15T | NIFO00000000 |
| Acinetobacter pittii | CIP 70.29T | APQP00000000 |
| Acinetobacter populi | PBJ7T | NEXX00000000 |
| Acinetobacter pragensis | ANC 4149T | LUAW00000000 |
| Acinetobacter proteolyticus | NIPH 809T | APOI00000000 |
| Acinetobacter pseudolwoffii | ANC 5044T | PHRG00000000 |
| Acinetobacter pullicarnis | S23T | CP036789 |
| Acinetobacter pullorumf | B301T | JAAARQ000000000 |
| Acinetobacter puyangensis | ANC 4466T | OANT00000000 |
| Acinetobacter qingfengensis | ANC 4671T | MKKK00000000 |
| Acinetobacter radioresistens | CIP 103788T | APQF00000000 |
| Acinetobacter rongchengensis | WCHAc060115T | RAXT00000000 |
| Acinetobacter rudis | CIP 110305T | ATGI00000000 |
| Acinetobacter schindleri | CIP 107287T | APPQ00000000 |
| Acinetobacter seifertii | NIPH 973T | APOO00000000 |
| Acinetobacter shaoyimingii | 323-1T | CP049801 |
| Acinetobacter sichuanensis | WCHAc060041T | PYIX00000000 |
| Acinetobacter soli | CIP 110264T | APPU00000000 |
| Acinetobacter tandoii | CIP 107469T | AQFM00000000 |
| Acinetobacter tianfuensis | WCHAc060012T | RAXV00000000 |
| Acinetobacter tjernbergiae | CIP 107465T | AYEV00000000 |
| Acinetobacter towneri | CIP 107472T | APPY00000000 |
| Acinetobacter ursingii | CIP 107286T | APQA00000000 |
| Acinetobacter variabilis | NIPH 2171T | APRS00000000 |
| Acinetobacter venetianus | CIP 110063T | APPO00000000 |
| Acinetobacter vivianii | NIPH 2168T | APRW00000000 |
| Acinetobacter wanghuae | dk386T | CP045650 |
| Acinetobacter wuhouensis | WCHA60T | CP031716 |
| Tentative designations (n = 20) | ||
| Acinetobacter kyonggiensis | ANC 5109 | FNPK00000000 |
| Acinetobacter marinus | ANC 3699 | FMYK00000000 |
| Acinetobacter oleivorans | DR1 | CP002080 |
| Genomic sp. 6 | CIP a165 | APOK00000000 |
| Genomic sp. 15BJ | CIP 110321 | AQFL00000000 |
| Genomic sp. 16 | CIP 70.18 | APRN00000000 |
| Taxon 21 | ANC 3929 | APRH00000000 |
| Taxon 22 | NIPH 2100 | APSB00000000 |
| Taxon 24A | ANC 4655 | NEGF00000000 |
| Taxon 24B | ANC 4471 | SJNZ00000000 |
| Taxon 25A | ANC 3789 | APOY00000000 |
| Taxon 25B | ANC 4633 | SJNX00000000 |
| Taxon 27 | ANC 4169 | NEGE00000000 |
| Taxon 32 | ANC 4218 | NEGD00000000 |
| Taxon 34 | ANC 4470 | NEGC00000000 |
| Taxon 35 | ANC 4999 | NEGB00000000 |
| Taxon 36 | ANC 4945 | MVKX00000000 |
| Taxon 37 | WCHAc010034 | CP032279 |
| Taxon 38 | ANC 3903 | NEGA00000000 |
| Taxon 39 | ANC 4204 | NEFZ00000000 |
Acinetobacter pakistanensis is a later synonym of Acinetobacter bohemicus (57).
Acinetobacter guangdongensis is a later synonym of Acinetobacter indicus (20).
Acinetobacter grimontii is a later synonym of Acinetobacter junii (21).
Acinetobacter dijkshoorniae is a later synonym of Acinetobacter lactucae (22).
Acinetobacter mesopotamicus is a later synonym of Acinetobacter lwoffii (19).
Acinetobacter portensis is a later synonym of Acinetobacter pullorum (this study).
The 55 Acinetobacter genome sequences with misidentified species in NCBI. Download Table S3, PDF file, 0.1 MB (92.7KB, pdf) .
Copyright © 2021 Qin et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The 3,956 Acinetobacter genome sequences available in GenBank (accessed by 1 August 2020). Download Data Set S2, XLSX file, 0.4 MB (427.5KB, xlsx) .
Copyright © 2021 Qin et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
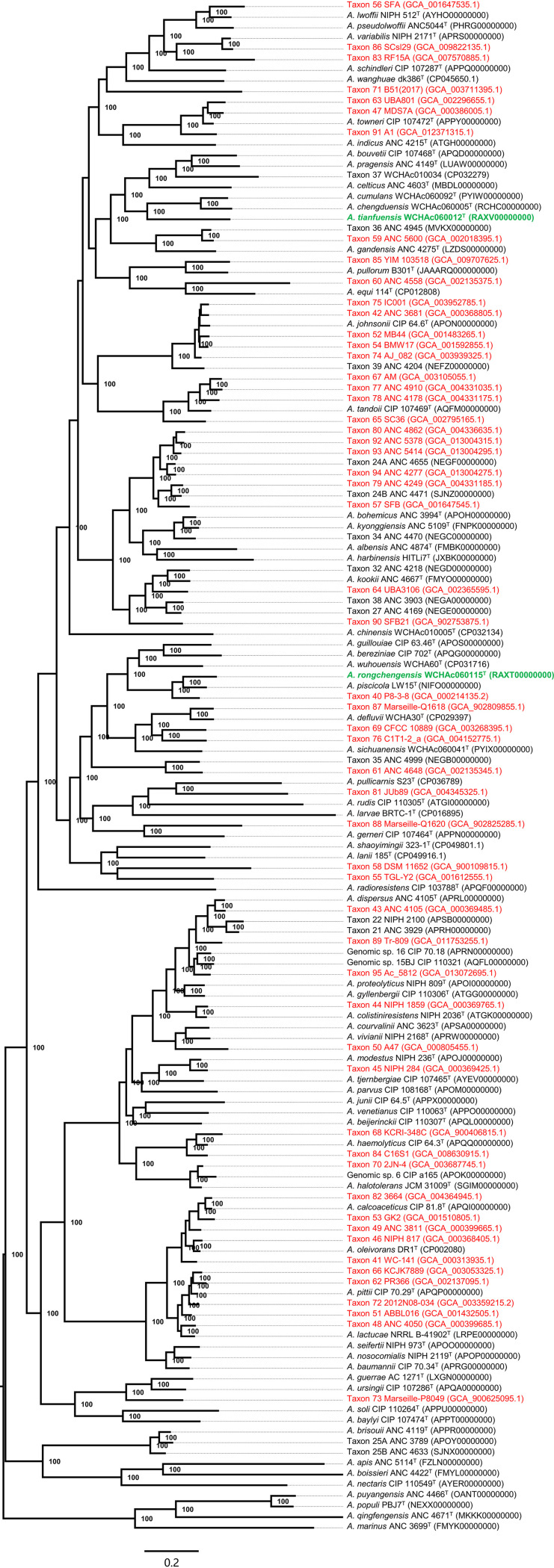
After precise species identification, among the 3,956 Acinetobacter strains with genome sequences available, most (n = 3,124, 79.0%) belonged to A. baumannii, followed by Acinetobacter pittii (n = 174, 4.4%), Acinetobacter nosocomialis (n = 103, 2.6%), and Acinetobacter indicus (n = 68, 1.7%; Table 3). However, 94 (2.4%) strains could not be assigned to any known Acinetobacter species nor to any known tentative species designations (Data Set S3). Instead, the 94 strains could be assigned to 56 potentially novel Acinetobacter species, which are named taxon 40 to 95 here (Table 4 and Fig. 2), as Acinetobacter taxon 39 has been used before. Characterization of taxon 40 to 95 by phenotype methods is warranted to further establish their species status with proper species names under current International Code of Nomenclature of Prokaryotes (12).
TABLE 3.
Species distribution of 3,956 Acinetobacter strains with genome sequences available in GenBank
| Species | No. of genomes | Taxon without a species namea | No. of genomes |
|---|---|---|---|
| Acinetobacter albensis | 2 | Genomic sp. 6 | 2 |
| Acinetobacter apis | 1 | Genomic sp. 15BJ | 1 |
| Acinetobacter baumannii | 3,124 | Genomic sp. 16 | 6 |
| Acinetobacter baylyi | 11 | Taxon 21 | 1 |
| Acinetobacter beijerinckii | 3 | Taxon 22 | 1 |
| Acinetobacter bereziniae | 1 | Taxon 24A | 1 |
| Acinetobacter bohemicus | 1 | Taxon 24B | 3 |
| Acinetobacter boissieri | 1 | Taxon 25A | 2 |
| Acinetobacter bouvetii | 3 | Taxon 25B | 2 |
| Acinetobacter brisouii | 4 | Taxon 27 | 1 |
| Acinetobacter calcoaceticus | 15 | Taxon 32 | 1 |
| Acinetobacter celticus | 1 | Taxon 34 | 1 |
| Acinetobacter chengduensis | 2 | Taxon 35 | 1 |
| Acinetobacter chinensis | 2 | Taxon 36 | 1 |
| Acinetobacter colistiniresistens | 4 | Taxon 37 | 1 |
| Acinetobacter courvalinii | 5 | Taxon 38 | 1 |
| Acinetobacter cumulans | 8 | Taxon 39 | 2 |
| Acinetobacter defluvii | 2 | Taxon 40 | 1 |
| Acinetobacter dispersus | 1 | Taxon 41 | 1 |
| Acinetobacter equi | 1 | Taxon 42 | 3 |
| Acinetobacter gandensis | 1 | Taxon 43 | 2 |
| Acinetobacter gerneri | 2 | Taxon 44 | 2 |
| Acinetobacter guerrae | 3 | Taxon 45 | 5 |
| Acinetobacter guillouiae | 1 | Taxon 46 | 4 |
| Acinetobacter gyllenbergii | 4 | Taxon 47 | 2 |
| Acinetobacter haemolyticus | 20 | Taxon 48 | 1 |
| Acinetobacter halotolerans | 1 | Taxon 49 | 1 |
| Acinetobacter harbinensis | 1 | Taxon 50 | 1 |
| Acinetobacter indicus | 68 | Taxon 51 | 1 |
| Acinetobacter johnsonii | 8 | Taxon 52 | 7 |
| Acinetobacter junii | 27 | Taxon 53 | 2 |
| Acinetobacter kookii | 2 | Taxon 54 | 5 |
| Acinetobacter kyonggiensis | 1 | Taxon 55 | 1 |
| Acinetobacter lactucae | 7 | Taxon 56 | 2 |
| Acinetobacter lanii | 2 | Taxon 57 | 1 |
| Acinetobacter larvae | 1 | Taxon 58 | 1 |
| Acinetobacter lwoffii | 17 | Taxon 59 | 2 |
| Acinetobacter marinus | 1 | Taxon 60 | 1 |
| Acinetobacter modestus | 2 | Taxon 61 | 1 |
| Acinetobacter nectaris | 1 | Taxon 62 | 3 |
| Acinetobacter nosocomialis | 103 | Taxon 63 | 1 |
| Acinetobacter oleivorans | 7 | Taxon 64 | 1 |
| Acinetobacter parvus | 8 | Taxon 65 | 3 |
| Acinetobacter piscicola | 1 | Taxon 66 | 4 |
| Acinetobacter pittii | 174 | Taxon 67 | 1 |
| Acinetobacter populi | 1 | Taxon 68 | 1 |
| Acinetobacter pragensis | 1 | Taxon 69 | 1 |
| Acinetobacter proteolyticus | 5 | Taxon 70 | 1 |
| Acinetobacter pseudolwoffii | 3 | Taxon 71 | 3 |
| Acinetobacter pullicarnis | 1 | Taxon 72 | 1 |
| Acinetobacter pullorum | 2 | Taxon 73 | 1 |
| Acinetobacter puyangensis | 1 | Taxon 74 | 1 |
| Acinetobacter qingfengensis | 2 | Taxon 75 | 1 |
| Acinetobacter radioresistens | 32 | Taxon 76 | 2 |
| Acinetobacter rongchengensis | 1 | Taxon 77 | 1 |
| Acinetobacter rudis | 1 | Taxon 78 | 1 |
| Acinetobacter schindleri | 10 | Taxon 79 | 1 |
| Acinetobacter seifertii | 19 | Taxon 80 | 1 |
| Acinetobacter shaoyimingii | 2 | Taxon 81 | 1 |
| Acinetobacter sichuanensis | 1 | Taxon 82 | 1 |
| Acinetobacter soli | 22 | Taxon 83 | 3 |
| Acinetobacter tandoii | 2 | Taxon 84 | 1 |
| Acinetobacter tianfuensis | 1 | Taxon 85 | 1 |
| Acinetobacter tjernbergiae | 3 | Taxon 86 | 2 |
| Acinetobacter towneri | 11 | Taxon 87 | 1 |
| Acinetobacter ursingii | 29 | Taxon 88 | 1 |
| Acinetobacter variabilis | 6 | Taxon 89 | 1 |
| Acinetobacter venetianus | 10 | Taxon 90 | 1 |
| Acinetobacter vivianii | 5 | Taxon 91 | 1 |
| Acinetobacter wanghuae | 2 | Taxon 92 | 1 |
| Acinetobacter wuhouensis | 6 | Taxon 93 | 1 |
| Taxon 94 | 1 | ||
| Taxon 95 | 1 |
Taxa identified in this study are highlighted in bold.
TABLE 4.
Tentative taxon assignations for novel, unnamed Acinetobacter species identified in this study
| Taxon | Accession no. | Reference straina | Closest species or taxon | ANI (%) | isDDH (%) |
|---|---|---|---|---|---|
| 40 | GCA_000214135.2 | P8-3-8 | A. piscicola | 88.77 | 34.4 |
| 41 | GCA_000313935.1 | WC-141 | A. oleivorans | 93.08 | 49.3 |
| 42 | GCA_000368805.1 | ANC 3681 | A. johnsonii | 95.83 | 66.6 |
| 43 | GCA_000369485.1 | ANC 4105 | A. dispersus | 95.61 | 62.4 |
| 44 | GCA_000369765.1 | NIPH 1859 | A. colistiniresistens | 95.29 | 60.3 |
| 45 | GCA_000369425.1 | NIPH 284 | A. modestus | 94.51 | 54.8 |
| 46 | GCA_000368405.1 | NIPH 817 | A. oleivorans | 95.2 | 61.3 |
| 47 | GCA_000386005.1 | MDS7A | A. towneri | 93.8 | 52.9 |
| 48 | GCA_000399685.1 | ANC 4050 | A. lactucae | 94.01 | 53.5 |
| 49 | GCA_000399665.1 | ANC 3811 | A. oleivorans | 94.28 | 55.8 |
| 50 | GCA_000805455.1 | A47 | A. courvalinii | 88 | 31.9 |
| 51 | GCA_001432505.1 | ABBL016 | A. pittii | 94.84 | 58 |
| 52 | GCA_001483265.1 | MB44 | A. johnsonii | 95.84 | 65.6 |
| 53 | GCA_001510805.1 | GK2 | A. calcoaceticus | 93.65 | 51.5 |
| 54 | GCA_001592855.1 | BMW17 | A. johnsonii | 95.6 | 64.9 |
| 55 | GCA_001612555.1 | TGL-Y2 | A. bohemicus | 80.2 | 22.1 |
| 56 | GCA_001647535.1 | SFA | A. lwoffii | 90.48 | 38.1 |
| 57 | GCA_001647545.1 | SFB | Taxon 24B | 89.6 | 36.8 |
| 58 | GCA_900109815.1 | DSM 11652 | A. cumulans | 80.48 | 21.5 |
| 59 | GCA_002018395.1 | ANC 5600 | Taxon 36 | 95.48 | 62.6 |
| 60 | GCA_002135375.1 | ANC 4558 | A. equi | 81.73 | 23 |
| 61 | GCA_002135345.1 | ANC 4648 | Taxon 35 | 87.82 | 33 |
| 62 | GCA_002137095.1 | PR366 | A. pittii | 95.08 | 59.4 |
| 63 | GCA_002296655.1 | UBA801 | A. towneri | 93.42 | 50.5 |
| 64 | GCA_002365595.1 | UBA3106 | A. kookii | 88.29 | 32.6 |
| 65 | GCA_002795165.1 | SC36 | A. tandoii | 87.13 | 29.9 |
| 66 | GCA_003053325.1 | KCJK7889 | A. pittii | 95.5 | 62.5 |
| 67 | GCA_003105055.1 | AM | A. tandoii | 92 | 43.2 |
| 68 | GCA_900406815.1 | KCRI-348C | A. haemolyticus | 92.5 | 46.7 |
| 69 | GCA_003268395.1 | CFCC 10889 | A. wuhouensis | 85.29 | 29.1 |
| 70 | GCA_003687745.1 | 2JN-4 | A. halotolerans | 95.44 | 59.7 |
| 71 | GCA_003711395.1 | B51(2017) | A. gandensis | 80.94 | 21.9 |
| 72 | GCA_003359215.2 | 2012N08-034 | A. pittii | 95.95 | 65.4 |
| 73 | GCA_900625095.1 | Marseille-P8049 | A. ursingii | 84.88 | 26.8 |
| 74 | GCA_003939325.1 | AJ_082 | A. johnsonii | 95.75 | 65.9 |
| 75 | GCA_003952785.1 | IC001 | A. johnsonii | 95.86 | 66.6 |
| 76 | GCA_004152775.1 | C1T1-2_a | A. sichuanensis | 86.05 | 28.5 |
| 77 | GCA_004331035.1 | ANC 4910 | A. tandoii | 91 | 40 |
| 78 | GCA_004331175.1 | ANC 4178 | A. tandoii | 91.25 | 40.6 |
| 79 | GCA_004331185.1 | ANC 4249 | Taxon 24B | 95.48 | 61 |
| 80 | GCA_004336635.1 | ANC 4862 | Taxon 24A | 92.69 | 47.2 |
| 81 | GCA_004345325.1 | JUb89 | A. pullicarnis | 79.81 | 21.2 |
| 82 | GCA_004364945.1 | 3664 | A. calcoaceticus | 95.97 | 65.8 |
| 83 | GCA_007570885.1 | RF15A | A. variabilis | 83.02 | 24.6 |
| 84 | GCA_008630915.1 | C16S1 | A. haemolyticus | 93.7 | 50.1 |
| 85 | GCA_009707625.1 | YIM 103518 | A. pullorum | 87.32 | 30.6 |
| 86 | GCA_009822135.1 | SCsl29 | A. variabilis | 95.33 | 62.9 |
| 87 | GCA_902809855.1 | Marseille-Q1618 | A. defluvii | 91.33 | 42.3 |
| 88 | GCA_902825285.1 | Marseille-Q1620 | A. gerneri | 81.13 | 22.2 |
| 89 | GCA_011753255.1 | Tr-809 | A. dispersus | 91.34 | 41.3 |
| 90 | GCA_902753875.1 | SFB21 | Taxon 32 | 85.48 | 27.8 |
| 91 | GCA_012371315.1 | A1 | A. towneri | 88.81 | 32.8 |
| 92 | GCA_013004315.1 | ANC 5378 | Taxon 24A | 92.8 | 47.2 |
| 93 | GCA_013004295.1 | ANC 5414 | Taxon 24A | 92.74 | 47 |
| 94 | GCA_013004275.1 | ANC 4277 | Taxon 24A | 95.98 | 64.1 |
| 95 | GCA_013072695.1 | Ac_5812 | Genomic sp. 16 | 92.7 | 47.2 |
The strain with genome sequence deposited in GenBank at the earliest date was selected as the reference strain for the newly identified taxa.
FIG 2.
Phylogenomic tree of Acinetobacter species with validly published names and tentative taxa. The phylogenomic tree was inferred based on the alignment of 1,397 core genes. Strains and their nucleotide accession no. are listed alongside the names of species, and 100% bootstrap are shown. Bar, value indicates the nucleotide substitutions per site. The two novel Acinetobacter species are depicted in green, while novel Acinetobacter taxa identified in this study, namely, taxon 40 to 95, are in red.
The potential tentative species designation. Download Data Set S3, XLSX file, 0.02 MB (16.1KB, xlsx) .
Copyright © 2021 Qin et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Acinetobacter is indeed a single genus comprising 144 species at present.
The identification of the 56 taxa also extends the number of Acinetobacter species to 144, including 68 with species names and 76 unnamed taxa. The large number of species raises the question whether Acinetobacter is indeed a single genus or actually should be divided into different genera. ANI values among type strains of all species and reference strains of all taxa of the genus Acinetobacter are ≥76.97% (76.97% to 95.98%) (see Data Set S4 in the supplemental material). The ANI values are higher than 72.50% to 73.70%, which has been proposed as the 95% confidence interval of the boundary to define a bacterial genus (34). To further verify the genus Acinetobacter, the average amino acid identity (AAI) values among type strains of all species and reference strains of all taxa of the genus Acinetobacter were also calculated, which are >66% (66.5% to 97.4%) (see Data Set S5 in the supplemental material). This is higher than the proposed cutoff of 65% AAI used to define a bacterial genus (34, 35). Both ANI and AAI analyses suggest that all Acinetobacter species and unnamed taxa identified so far indeed belong to a single genus.
Average nucleotide identity between type strains of Acinetobacter species and reference strains of Acinetobacter taxa without species names. Download Data Set S4, XLSX file, 0.1 MB (124.3KB, xlsx) .
Copyright © 2021 Qin et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Average amino acid identity between type strains of Acinetobacter species and reference strains of Acinetobacter taxa without species names. Download Data Set S5, XLSX file, 0.1 MB (129.7KB, xlsx) .
Copyright © 2021 Qin et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DISCUSSION
In this study, we first found and characterized two novel Acinetobacter species. We also found that A. pullorum and A. portensis are synonyms and then updated the taxonomy of the genus Acinetobacter. We applied the updated taxonomic assignments to curate genome sequences deposited in GenBank with the label of Acinetobacter and found that 6% (n = 234) of the 3,956 genomes with good quality need to be corrected or updated for species identification. We also identified 56 previously unknown tentative species designations, which further update the genus Acinetobacter to comprise 144 species, including 68 with species names and 76 taxa without species names. Such a large number of species raises the question whether Acinetobacter should be divided into multiple genera. Although the boundary of bacterial genera based on genome sequences is less established than that of species and requires more studies (9), our ANI and AAI analyses suggest that all Acinetobacter species indeed belong to a single genus. The mechanisms and factors driving the divergence of Acinetobacter to form the evolutionary trajectory and generate the remarkable species diversity and form have not been understood (36, 37).
Along with many Acinetobacter species identified recently (11, 13–18), the above findings highlight that Acinetobacter is a highly diverse and complex group (38). The species status of two novel Acinetobacter species, namely, A. tianfuensis and A. rongchengensis, was established by both genome- and phenotype-based methods. In addition to known species, there were 76 tentative novel Acinetobacter taxa, including 56 identified in this study. The identification of new taxa invites more studies on these tentative species by both genome- and phenotype-based methods to establish their species status and to propose appropriate species names under the current code for prokaryotes (12). Alternatively, it has also been proposed to create a new code that would use DNA sequences as the type material to rule the nomenclature of prokaryotes (39) or to establish placeholder species names using genome-based taxonomy (10). Indeed, there is an urgent need to find a solution to deal with the exploration of new taxonomic findings generated by genome sequencing (40).
In conclusion, we characterized and reported two novel Acinetobacter species, namely, A. tianfuensis and A. rongchengensis. A. tianfuensis may be distinguished from all other Acinetobacter species by its ability to grow on l-glutamate, d-malate, malonate, and phenylacetate but not grow on l-arabinose, l-arginine, azelate, and glutarate. A. rongchengensis may be differentiated from all other Acinetobacter species by the combination of assimilation trans-aconitate, citrate (Simmons’), and l-tartrate but not β-alanine and 4-aminobutyrate. We also found that A. portensis is a later heterotypic synonym of A. pullorum. We demonstrated that some Acinetobacter genome sequences deposited in GenBank are required to be corrected and identified 56 novel tentative Acinetobacter taxa, which warrant further phenotype-based characterizations.
MATERIALS AND METHODS
Strains and preliminary species identification.
Hospital sewage (1 ml) was collected from the influx mainstream of the wastewater treatment plant at West China Hospital in June 2018, which was added in 10 ml nutrient broth (Oxoid, Basingstoke, UK) and was incubated overnight at 30°C with shaking. The culture suspension was diluted to 0.5 McFarland standard and was then further diluted to 1:100 with saline. A 100-μl aliquot was then streaked onto an Acinetobacter chromogenic agar plate (CHROMagar, Paris, France). The plate was then incubated at 30°C overnight. All isolates recovered from the plate were subjected to preliminary species identification by partial sequencing of the RNA polymerase β subunit-encoding rpoB gene using PCR and Sanger sequencing as described previously (5). Isolates with ≤98% identity of the 861-bp partial rpoB sequence (corresponding to nucleotide positions 2915 to 3775 of A. baumannii CIP 70.34T; accession no. DQ207471) to type strains of all known Acinetobacter species may belong to novel species and were characterized as described below. Two Acinetobacter isolates, namely, WCHAc060012T and WCHAc060115T, were recovered from the plate and had ≤98% identity of the 861-bp partial rpoB sequence to type strains of all known Acinetobacter species.
Analysis based on 16S rRNA and rpoB genes.
Boiled lysates were used as the PCR template, and PCR amplicons were sequenced using the Sanger method (26). The nearly complete 16S rRNA gene sequences of WCHAc060012T and WCHAc060115T were obtained using PCR with universal primers 27F and 1492R (25). The 16S rRNA gene sequences of type strains of each Acinetobacter species were retrieved from their depositions in GenBank or from their whole-genome sequences. The longest common fragments of the 16S rRNA gene sequences (1,352 bp) were aligned using MAFFT v7.471 (41), and a maximum likelihood phylogenetic tree (42) based on the 1,352-bp sequences was inferred using RAxML v8.2.12 (43) with the general time reversible (GTR) model.
To further investigate the taxonomic position of WCHAc060012T and WCHAc060115T, 861-bp partial rpoB sequences of type strains of each Acinetobacter species were retrieved from their depositions in GenBank or from their whole-genome sequences. Sequence alignment and the construction of a maximum-likelihood phylogenetic tree were performed as described above.
Whole-genome sequencing of the two strains.
Genomic DNA from an overnight culture of each of the two strains was prepared using the QIAamp DNA minikit (Qiagen, Hilden, Germany) and was then subjected to whole-genome sequencing using the HiSeq X10 sequencing platform (Illumina, San Diego, CA, USA) with an approximate 250× coverage. Reads were de novo assembled into contigs using the program SPAdes v3.15.1 (44). Potential contaminations of WCHAc060012T and WCHAc060115T genomes were checked using CheckM v1.1.3 (45). Antimicrobial resistance genes were identified from genome sequences using the ABRicate program (https://github.com/tseemann/abricate) to query the ResFinder database 4.1 (https://cge.cbs.dtu.dk/services/ResFinder/).
Precise species identification and phylogenomic analysis of the two strains.
Whole-genome sequences of type strains of all Acinetobacter species (Data Set S2) were retrieved from the NCBI database. Genome sequences of WCHAc060012T and WCHAc060115T were compared with those of type strains of Acinetobacter species using the average nucleotide identity based on BLAST (ANI) and in silico DNA-DNA hybridization (isDDH). ANI and isDDH values were calculated using the fastANI v1.32 (46) and genome-to-genome distance calculator (formula 2) (47) with the recommended parameters and/or default settings, respectively. A ≥96% ANI (31) or ≥70.0% isDDH (31, 47) was used as the cutoff to define a bacterial species.
A core genome phylogenetic tree based on concatenated sequences of core genes was constructed as described previously (48). Prokka v1.14.5 (49) and Prodigal v2.6.3 (50) were used to annotate these genome sequences, and protein-encoding sequences for each genome were retrieved for gene alignment and clustering using PIRATE v1.0.4 (51). The gene sequences were aligned and concatenated using MAFFT v7.471 (41) and AMAS v0.98 (52), which were then used to infer a phylogenomic tree using RAxML v8.2.12 (43) with GTR model plus gamma distribution and a 1,000-bootstrap test. The phylogenetic tree was visualized with FigTree (https://github.com/rambaut/figtree).
Phenotypic characterization for strains of two novel species.
The metabolic and physiological properties were assessed using the standardized genus-targeted set of metabolic/physiological tests as described previously (5, 32, 53). The two strains were grown on brain heart infusion (BHI) agar (Oxoid) plates at 30°C overnight, and the colony morphology was observed by naked eyes. Cell morphology was visualized by light microscopy (CX21 microscope; Olympus, Japan). The Gram staining was carried out with a Gram staining kit (bioMérieux, Marcy l'Etoile, France). The cultivation temperature was 30°C unless indicated otherwise. Cell motility was tested in LB medium with 0.4% agar. Growth at various temperatures (20, 25, 32, 35, 37, 41, and 44°C) was tested in 5-ml aliquots of BHI broth dispensed into tubes (16-mm inner diameter) as described previously (5). Salt tolerance tests at different NaCl concentrations (0% to 10%, wt/vol, in increments of 1.0%) were performed in tryptic soy broth (TSB; Hopebio, Qingdao, China) after incubation for 2 days. Growth at pH 4.0 to 11.0 (at intervals of 1 pH unit, adjusted by adding HCl or NaOH) was examined in TSB for 2 days. The anaerobic growth was examined on a BHI agar plate, which was placed in an anaerobic bag (bioMérieux) at 30°C for 7 day (26). Aerobic acid production from glucose and gelatin hydrolysis was performed using the API 20NE system (bioMérieux), and the results were observed after 48 h. Hemolysis of sheep blood and utilization of citrate (Simmons’) were examined according to methods described previously (5). The characteristics for the assimilation of the other carbon sources were determined using the basal mineral medium (54) supplemented with 0.1% (wt/vol) carbon source as described previously (5).
Curation of all available Acinetobacter genomes for precise species identification.
All genome sequences labeled as Acinetobacter species in GenBank (n = 5,997, accessed by 1 August 2020) were retrieved. The assemblies, completeness, contamination, and heterogeneity of the genomes were evaluated using QUAST v5.0.2 (55) and CheckM v1.1.3 (45). Genome assemblies were discarded due to low quality defined by >300 contigs, a <50-kb N50 value, <90% genome completeness, genome contamination indicated by ≥2 in CheckM, or none-zero genome heterogeneity value for individual genomes. ANI and isDDH values between each of the genomes and type strains of Acinetobacter genomes were calculated, using the fastANI v1.32 (46) and genome-to-genome distance calculator (formula 2) (47), respectively. A ≥96% ANI (31) or ≥70.0% isDDH (31, 47) was used as the cutoff to define a bacterial species. AAI was calculated between each pair of genome sequences using CompareM v0.1.2 (56) with the recommended parameters.
Data availability.
The nearly complete 16S rRNA gene sequences, partial rpoB sequences, and the whole-genome shotgun projects of strains A. tianfuensis WCHAc060012T and A. rongchengensis WCHAc060115T have been deposited at DDBJ/ENA/GenBank under accession no. MK796537, MK796539, MK805088, MK805090, RAXV00000000, and RAXT00000000 (Table 2).
ACKNOWLEDGMENTS
We are grateful for Alexandr Nemec and his group at the National Institute of Public Health, Czech Republic for standardizing phenotypic characterizations and the helpful discussion.
The work was supported by grants from the National Natural Science Foundation of China (project no. 81861138055) and West China Hospital of Sichuan University (1.3.5 project for disciplines of excellence, project no. ZYYC08006).
We declare that we have no conflict of interest.
Contributor Information
Zhiyong Zong, Email: zongzhiy@scu.edu.cn.
Rup Lal, University of Delhi.
REFERENCES
- 1.Brisou J, Prevot AR. 1954. Studies on bacterial taxonomy. X. The revision of species under Acromobacter group. Ann Inst Pasteur 86:722–728. [PubMed] [Google Scholar]
- 2.Baumann P. 1968. Isolation of Acinetobacter from soil and water. J Bacteriol 96:39–42. doi: 10.1128/JB.96.1.39-42.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung J, Park W. 2015. Acinetobacter species as model microorganisms in environmental microbiology: current state and perspectives. Appl Microbiol Biotechnol 99:2533–2548. doi: 10.1007/s00253-015-6439-y. [DOI] [PubMed] [Google Scholar]
- 4.de Berardinis V, Durot M, Weissenbach J, Salanoubat M. 2009. Acinetobacter baylyi ADP1 as a model for metabolic system biology. Curr Opin Microbiol 12:568–576. doi: 10.1016/j.mib.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Nemec A, Musílek M, Maixnerová M, De Baere T, van der Reijden TJ, Vaneechoutte M, Dijkshoorn L. 2009. Acinetobacter beijerinckii sp. nov. and Acinetobacter gyllenbergii sp. nov., haemolytic organisms isolated from humans. Int J Syst Evol Microbiol 59:118–124. doi: 10.1099/ijs.0.001230-0. [DOI] [PubMed] [Google Scholar]
- 6.Nemec A, Dijkshoorn L, Cleenwerck I, De Baere T, Janssens D, Van Der Reijden TJ, Jezek P, Vaneechoutte M. 2003. Acinetobacter parvus sp. nov., a small-colony-forming species isolated from human clinical specimens. Int J Syst Evol Microbiol 53:1563–1567. doi: 10.1099/ijs.0.02631-0. [DOI] [PubMed] [Google Scholar]
- 7.Bernards AT, de Beaufort AJ, Dijkshoorn L, Van Boven CPA. 1997. Outbreak of septicaemia in neonates caused by Acinetobacter junii investigated by amplified ribosomal DNA restriction analysis (ARDRA) and four typing methods. J Hosp Infect 35:129–140. doi: 10.1016/S0195-6701(97)90101-8. [DOI] [PubMed] [Google Scholar]
- 8.Nemec A, Dijkshoorn L, JežEk P. 2000. Recognition of two novel phenons of the genus Acinetobacter among non-glucose-acidifying isolates from human specimens. J Clin Microbiol 38:3937–3941. doi: 10.1128/JCM.38.11.3937-3941.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zong Z. 2020. Genome-based taxonomy for bacteria: a recent advance. Trends Microbiol 28:871–874. doi: 10.1016/j.tim.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Parks DH, Chuvochina M, Chaumeil PA, Rinke C, Mussig AJ, Hugenholtz P. 2020. A complete domain-to-species taxonomy for Bacteria and Archaea. Nat Biotechnol 38:1079–1086. doi: 10.1038/s41587-020-0501-8. [DOI] [PubMed] [Google Scholar]
- 11.Parte AC. 2018. LPSN—list of prokaryotic names with standing in nomenclature (bacterio.net), 20 years on. Int J Syst Evol Microbiol 68:1825–1829. doi: 10.1099/ijsem.0.002786. [DOI] [PubMed] [Google Scholar]
- 12.Parker CT, Tindall TJ, Garrity GM. 2019. International Code of Nomenclature of Prokaryotes. Prokaryotic code (2008 revision). Int J Syst Evol Microbiol 69:S1–S111. doi: 10.1099/ijsem.0.000778. [DOI] [PubMed] [Google Scholar]
- 13.Han R-H, Lee J-E, Yoon S-H, Kim G-B. 2020. Acinetobacter pullicarnis sp. nov. isolated from chicken meat. Arch Microbiol 202:727–732. doi: 10.1007/s00203-019-01785-y. [DOI] [PubMed] [Google Scholar]
- 14.Elnar A, Kim M, Lee J, Han R, Yoon S, Lee G, Yang S, Kim G. 2020. Acinetobacter pullorum sp. nov., isolated from chicken meat. J Microbiol Biotechnol 30:526–532. doi: 10.4014/jmb.2002.02033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvalheira A, Gonzales-Siles L, Salvà-Serra F, Lindgren Å, Svensson-Stadler L, Thorell K, Piñeiro-Iglesias B, Karlsson R, Silva J, Teixeira P, Moore E. 2020. Acinetobacter portensis sp. nov. and Acinetobacter guerrae sp. nov., isolated from raw meat. Int J Syst Evol Microbiol 70:4544–4554. doi: 10.1099/ijsem.0.004311. [DOI] [PubMed] [Google Scholar]
- 16.Qin J, Feng Y, Lu X, Zong Z. 2020. Characterization of Acinetobacter chengduensis sp. nov., isolated from hospital sewage and capable of acquisition of carbapenem resistance genes. Syst Appl Microbiol 43:126092. doi: 10.1016/j.syapm.2020.126092. [DOI] [PubMed] [Google Scholar]
- 17.Zhu W, Dong K, Yang J, Lu S, Lai XH, Pu J, Jin D, Huang Y, Zhang S, Zhou J, Huang Y, Xu J. 2020. Acinetobacter lanii sp. nov., Acinetobacter shaoyimingii sp. nov. and Acinetobacter wanghuae sp. nov., isolated from faeces of Equus kiang. Int J Syst Evol Microbiol 71. doi: 10.1099/ijsem.0.004567. [DOI] [PubMed] [Google Scholar]
- 18.Acer Ö, Güven K, Poli A, Di Donato P, Leone L, Buono L, Güven RG, Nicolaus B, Finore I. 2020. Acinetobacter mesopotamicus sp. nov., petroleum-degrading bacterium, isolated from petroleum-contaminated soil in Diyarbakir, in the southeast of Turkey. Curr Microbiol 77:3192–3200. doi: 10.1007/s00284-020-02134-9. [DOI] [PubMed] [Google Scholar]
- 19.Nemec A. 2021. Strain “Acinetobacter mesopotamicus” GC2 does not represent a novel species, but belongs to the species Acinetobacter lwoffii as revealed by whole-genome sequence-based analysis. Curr Microbiol 78:369–370. doi: 10.1007/s00284-020-02275-x. [DOI] [PubMed] [Google Scholar]
- 20.Nemec A, Radolfova-Krizova L. 2017. Acinetobacter guangdongensis Feng et al. 2014 is a junior heterotypic synonym of Acinetobacter indicus Malhotra et al. 2012. Int J Syst Evol Microbiol 67:4080–4082. doi: 10.1099/ijsem.0.002251. [DOI] [PubMed] [Google Scholar]
- 21.Vaneechoutte M, De Baere T, Nemec A, Musilek M, van der Reijden TJ, Dijkshoorn L. 2008. Reclassification of Acinetobacter grimontii Carr et al. 2003 as a later synonym of Acinetobacter junii Bouvet and Grimont 1986. Int J Syst Evol Microbiol 58:937–940. doi: 10.1099/ijs.0.65129-0. [DOI] [PubMed] [Google Scholar]
- 22.Dunlap CA, Rooney AP. 2018. Acinetobacter dijkshoorniae is a later heterotypic synonym of Acinetobacter lactucae. Int J Syst Evol Microbiol 68:131–132. doi: 10.1099/ijsem.0.002470. [DOI] [PubMed] [Google Scholar]
- 23.Wu W, Feng Y, Zong Z. 2020. Precise species identification for Enterobacter: a genome sequence-based study with reporting of two novel species, Enterobacter quasiroggenkampii sp. nov. and Enterobacter quasimori sp. nov. mSystems 5:e00527-20. doi: 10.1128/mSystems.00527-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mateo-Estrada V, Graña-Miraglia L, López-Leal G, Castillo-Ramírez S. 2019. Phylogenomics reveals clear cases of misclassification and genus-wide phylogenetic markers for Acinetobacter. Genome Biol Evol 11:2531–2541. doi: 10.1093/gbe/evz178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lane D. 1991. 16S/23S rRNA sequencing, p 115–175. In Stackebrandt E, Goodfellow M (eds), Nucleic acid techniques in bacterial systematics. John Wiley and Sons, Chichester, UK. [Google Scholar]
- 26.Hu Y, Feng Y, Zhang X, Zong Z. 2017. Acinetobacter defluvii sp. nov., recovered from hospital sewage. Int J Syst Evol Microbiol 67:1709–1713. doi: 10.1099/ijsem.0.001847. [DOI] [PubMed] [Google Scholar]
- 27.Lee I, Chalita M, Ha S-M, Na SI, Yoon S-H, Chun J. 2017. ContEst16S: an algorithm that identifies contaminated prokaryotic genomes using 16S RNA gene sequences. Int J Syst Evol Microbiol 67:2053–2057. doi: 10.1099/ijsem.0.001872. [DOI] [PubMed] [Google Scholar]
- 28.Chun J, Oren A, Ventosa A, Christensen H, Arahal DR, da Costa MS, Rooney AP, Yi H, Xu X-W, De Meyer S, Trujillo ME. 2018. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int J Syst Evol Microbiol 68:461–466. doi: 10.1099/ijsem.0.002516. [DOI] [PubMed] [Google Scholar]
- 29.Konstantinidis KT, Tiedje JM. 2005. Genomic insights that advance the species definition for prokaryotes. Proc Natl Acad Sci U S A 102:2567–2572. doi: 10.1073/pnas.0409727102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stackebrandt E, Goebel BM. 1994. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Evol Microbiol 44:846–849. doi: 10.1099/00207713-44-4-846. [DOI] [Google Scholar]
- 31.Richter M, Rosselló-Móra R. 2009. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci U S A 106:19126–19131. doi: 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krizova L, Maixnerova M, Šedo O, Nemec A. 2015. Acinetobacter albensis sp. nov., isolated from natural soil and water ecosystems. Int J Syst Evol Microbiol 65:3905–3912. doi: 10.1099/ijsem.0.000511. [DOI] [PubMed] [Google Scholar]
- 33.Nemec A, Radolfova-Krizova L, Maixnerova M, Sedo O. 2017. Acinetobacter colistiniresistens sp. nov. (formerly genomic species 13 sensu Bouvet and Jeanjean and genomic species 14 sensu Tjernberg and Ursing), isolated from human infections and characterized by intrinsic resistance to polymyxins. Int J Syst Evol Microbiol 67:2134–2141. doi: 10.1099/ijsem.0.001903. [DOI] [PubMed] [Google Scholar]
- 34.Barco RA, Garrity GM, Scott JJ, Amend JP, Nealson KH, Emerson D. 2020. A genus definition for bacteria and archaea based on a standard genome relatedness index. mBio 11:e02475-19. doi: 10.1128/mBio.02475-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konstantinidis KT, Tiedje JM. 2007. Prokaryotic taxonomy and phylogeny in the genomic era: advancements and challenges ahead. Curr Opin Microbiol 10:504–509. doi: 10.1016/j.mib.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Carvalheira A, Silva J, Teixeira P. 2021. Acinetobacter spp. in food and drinking water—a review. Food Microbiol 95:103675. doi: 10.1016/j.fm.2020.103675. [DOI] [PubMed] [Google Scholar]
- 37.Al Atrouni A, Joly-Guillou ML, Hamze M, Kempf M. 2016. Reservoirs of non-baumannii Acinetobacter species. Front Microbiol 7:49. doi: 10.3389/fmicb.2016.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. 2017. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 30:409–447. doi: 10.1128/CMR.00058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray AE, Freudenstein J, Gribaldo S, Hatzenpichler R, Hugenholtz P, Kämpfer P, Konstantinidis KT, Lane CE, Papke RT, Parks DH, Rossello-Mora R, Stott MB, Sutcliffe IC, Thrash JC, Venter SN, Whitman WB, Acinas SG, Amann RI, Anantharaman K, Armengaud J, Baker BJ, Barco RA, Bode HB, Boyd ES, Brady CL, Carini P, Chain PSG, Colman DR, DeAngelis KM, de los Rios MA, Estrada-de los Santos P, Dunlap CA, Eisen JA, Emerson D, Ettema TJG, Eveillard D, Girguis PR, Hentschel U, Hollibaugh JT, Hug LA, Inskeep WP, Ivanova EP, Klenk H-P, Li W-J, Lloyd KG, Löffler FE, Makhalanyane TP, Moser DP, Nunoura T, Palmer M, et al. 2020. Roadmap for naming uncultivated Archaea and Bacteria. Nat Microbiol 5:987–994. doi: 10.1038/s41564-020-0733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanford RA, Lloyd KG, Konstantinidis KT, Löffler FE. 2021. Microbial taxonomy run amok. Trends Microbiol 29:394–404. doi: 10.1016/j.tim.2020.12.010. [DOI] [PubMed] [Google Scholar]
- 41.Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Felsenstein J. 1981. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol 17:368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- 43.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parks D, Imelfort M, Skennerton C, Hugenholtz P, Tyson G. 2015. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res 25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jain C, Rodriguez RL, Phillippy AM, Konstantinidis KT, Aluru S. 2018. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat Commun 9:5114. doi: 10.1038/s41467-018-07641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meier-Kolthoff JP, Auch AF, Klenk HP, Goker M. 2013. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics 14:60. doi: 10.1186/1471-2105-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ankenbrand MJ, Keller A. 2016. bcgTree: automatized phylogenetic tree building from bacterial core genomes. Genome 59:783–791. doi: 10.1139/gen-2015-0175. [DOI] [PubMed] [Google Scholar]
- 49.Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 50.Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ. 2010. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bayliss SC, Thorpe HA, Coyle NM, Sheppard SK, Feil EJ. 2019. PIRATE: a fast and scalable pangenomics toolbox for clustering diverged orthologues in bacteria. Gigascience 8:giz119. doi: 10.1093/gigascience/giz119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borowiec ML. 2016. AMAS: a fast tool for alignment manipulation and computing of summary statistics. PeerJ 4:e1660. doi: 10.7717/peerj.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Radolfova-Krizova L, Maixnerova M, Nemec A. 2016. Acinetobacter pragensis sp. nov., found in soil and water ecosystems. Int J Syst Evol Microbiol 66:3897–3903. doi: 10.1099/ijsem.0.001285. [DOI] [PubMed] [Google Scholar]
- 54.Cruze JA, Singer JT, Finnerty WR. 1979. Conditions for quantitative transformation in Acinetobacter calcoaceticus. Curr Microbiol 3:129–132. doi: 10.1007/BF02601853. [DOI] [Google Scholar]
- 55.Mikheenko A, Prjibelski A, Saveliev V, Antipov D, Gurevich A. 2018. Versatile genome assembly evaluation with QUAST-LG. Bioinformatics 34:i142–i150. doi: 10.1093/bioinformatics/bty266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benjamin B, Chao X, Daniel HH. 2015. Fast and sensitive protein alignment using DIAMOND. Nat Methods 12:59–60. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- 57.Nemec A, Radolfova-Krizova L. 2016. Acinetobacter pakistanensis Abbas et al. 2014 is a later heterotypic synonym of Acinetobacter bohemicus Krizova et al. 2014. Int J Syst Evol Microbiol 66:5614–5617. doi: 10.1099/ijsem.0.001530. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Maximum likelihood phylogenetic tree based on 16S rRNA gene sequences (1,352 bp) of WCHAc060012T, WCHAc060115T, and type strains of Acinetobacter species with validly published names. The sequence of Moraxella lacunata ATCC 17967T (GenBank accession no. AF005160) was used as the outgroup. Bootstrap values (≥50%) after 1,000 resamplings are indicated at branch nodes. Shown in parentheses are the DDBJ/ENA/GenBank accession no. for 16S rRNA gene sequences or whole-genome sequences. Bar, 0.2 substitutions per nucleotide position. Download FIG S1, PDF file, 2.6 MB (2.7MB, pdf) .
Copyright © 2021 Qin et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Maximum likelihood phylogenetic tree based on partial rpoB (861 bp) gene sequences of WCHAc060012T, WCHAc060115T, and type strains of Acinetobacter species with validly published names. Evolutionary distances were computed using Kimura’s two-parameter model. Moraxella lacunata ATCC 17967T was used as the outgroup. Bootstrap values ≥ 50% based on 1,000 resamplings are shown. Shown in parentheses are the DDBJ/ENA/GenBank accession no. for rpoB gene sequences or whole-genome sequences. Bar, 0.2 substitutions per nucleotide position. Download FIG S2, PDF file, 2.3 MB (2.3MB, pdf) .
Copyright © 2021 Qin et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Phenotypic properties of A. tianfuensis sp. nov., A. rongchengensis sp. nov. and the Acinetobacter species with validly published names. Download Data Set S1, XLSX file, 0.03 MB (30.3KB, xlsx) .
Copyright © 2021 Qin et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Antimicrobial resistance genes of A. tianfuensis WCHAc060012T and A. rongchengensis WCHAc060115T. Download Table S1, PDF file, 0.1 MB (71.7KB, pdf) .
Copyright © 2021 Qin et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The characteristics of strains of A. pullorum and A. portensis. Download Table S2, PDF file, 0.1 MB (143.5KB, pdf) .
Copyright © 2021 Qin et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The 55 Acinetobacter genome sequences with misidentified species in NCBI. Download Table S3, PDF file, 0.1 MB (92.7KB, pdf) .
Copyright © 2021 Qin et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The 3,956 Acinetobacter genome sequences available in GenBank (accessed by 1 August 2020). Download Data Set S2, XLSX file, 0.4 MB (427.5KB, xlsx) .
Copyright © 2021 Qin et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The potential tentative species designation. Download Data Set S3, XLSX file, 0.02 MB (16.1KB, xlsx) .
Copyright © 2021 Qin et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Average nucleotide identity between type strains of Acinetobacter species and reference strains of Acinetobacter taxa without species names. Download Data Set S4, XLSX file, 0.1 MB (124.3KB, xlsx) .
Copyright © 2021 Qin et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Average amino acid identity between type strains of Acinetobacter species and reference strains of Acinetobacter taxa without species names. Download Data Set S5, XLSX file, 0.1 MB (129.7KB, xlsx) .
Copyright © 2021 Qin et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
The nearly complete 16S rRNA gene sequences, partial rpoB sequences, and the whole-genome shotgun projects of strains A. tianfuensis WCHAc060012T and A. rongchengensis WCHAc060115T have been deposited at DDBJ/ENA/GenBank under accession no. MK796537, MK796539, MK805088, MK805090, RAXV00000000, and RAXT00000000 (Table 2).