Abstract
BACKGROUND:
The role of autologous stem cell transplantation (ASCT) in the first complete remission (CR1) of peripheral T-cell lymphomas (PTCLs) is not well defined. This study analyzed the impact of ASCT on the clinical outcomes of patients with newly diagnosed PTCL in CR1.
METHODS:
Patients with newly diagnosed, histologically confirmed, aggressive PTCL were prospectively enrolled into the Comprehensive Oncology Measures for Peripheral T-Cell Lymphoma Treatment (COMPLETE) study, and those in CR1 were included in this analysis.
RESULTS:
Two hundred thirteen patients with PTCL achieved CR1, and 119 patients with nodal PTCL, defined as anaplastic lymphoma kinase–negative anaplastic large cell lymphoma, angioimmunoblastic T-cell lymphoma (AITL), or PTCL not otherwise specified, were identified. Eighty-three patients did not undergo ASCT, whereas 36 underwent consolidative ASCT in CR1. At the median follow-up of 2.8 years, the median overall survival was not reached for the entire cohort of patients who underwent ASCT, whereas it was 57.6 months for those not receiving ASCT (P = .06). ASCT was associated with superior survival for patients with advanced-stage disease or intermediate-to-high International Prognostic Index scores. ASCT significantly improved overall and progression-free survival for patients with AITL but not for patients with other PTCL subtypes. In a multivariable analysis, ASCT was independently associated with improved survival (hazard ratio, 0.37; 95% confidence interval, 0.15–0.89).
CONCLUSIONS:
This is the first large prospective cohort study directly comparing the survival outcomes of patients with nodal PTCL in CR1 with or without consolidative ASCT. ASCT may provide a benefit in specific clinical scenarios, but the broader applicability of this strategy should be determined in prospective, randomized trials. These results provide a platform for designing future studies of previously untreated PTCL.
Keywords: anaplastic lymphoma kinase (ALK)–negative anaplastic large cell lymphoma, angioimmunoblastic T-cell lymphoma (AITL), autologous stem cell transplant, first complete remission, nodal peripheral T-cell lymphoma, peripheral T-cell lymphoma (PTCL) not otherwise specified (NOS)
INTRODUCTION
Peripheral T-cell lymphomas (PTCLs) are a rare and heterogeneous group of diseases principally characterized by aggressive clinical behavior. In the United States, fewer than 14,000 new cases of aggressive PTCLs were diagnosed between 2000 and 2012, and this underscores the rarity of these malignancies.1 Among the more than 20 histologic subtypes, 3 major entities, including PTCL not otherwise specified (NOS), angioimmunoblastic T-cell lymphoma (AITL), and anaplastic large cell lymphoma (ALCL), which are called nodal PTCL, account for approximately 60% of all cases.2 Although some subtypes of PTCL, such as anaplastic lymphoma kinase (ALK)–positive ALCL and subcutaneous panniculitis T-cell lymphoma, are associated with more favorable outcomes, most PTCL cases have a poor prognosis, with the median survival ranging from 22 to 49 months and the 5-year survival rate being less than 30%.1–4
No consensus currently exists on the optimal management of PTCL in the frontline setting. One of the foremost efforts to improve the survival of patients with PTCL has been directed at the use of an aggressive treatment strategy incorporating high-dose chemotherapy followed by autologous stem cell transplantation (ASCT) in first complete remission (CR1). Several groups have reported that the attainment of complete remission (CR) before ASCT is a significant independent predictor of improved survival in patients with PTCL receiving upfront ASCT.5–7 However, there have been no randomized studies specifically designed to evaluate upfront ASCT in comparison with observation in CR1 for the management of PTCL, although some previous randomized trials examining aggressive B- and T-cell non-Hodgkin lymphomas have included a small number of patients with PTCL.8–10 In addition, several retrospective and prospective, single-arm phase 2 trials have reported encouraging results with this approach,5,11–15 but comparing the results of these studies with historical controls is complicated by diverse eligibility criteria, suboptimal rates of transplantation among the intent-to-treat study populations, and differing rates of CR before ASCT.2,16 Furthermore, many phase 2 studies exclude patients with chemoresistant disease (ie, less than CR) before ASCT by default, and they thereby inherently introduce a bias toward a select group of patients with more chemosensitive disease. Clearly, data from large phase 3 randomized trials are needed to further elucidate the role of upfront ASCT in PTCL, but randomizing patients to an intense therapy such as ASCT versus observation is a formidable task, especially with a rare disease such as PTCL. Despite these limitations, the current National Comprehensive Cancer Network guidelines recommend (category 2A) consideration of ASCT in CR1 for most PTCL subtypes with the notable exceptions of low-risk ALK-positive ALCL and extranodal natural killer/T-cell lymphoma (nasal type).17
The current study is designed to better our understanding of whether upfront ASCT confers superior survival outcomes to patients with nodal PTCL who achieve CR1. We analyzed a series of prospectively enrolled patients in the Comprehensive Oncology Measures for Peripheral T-Cell Lymphoma Treatment (COMPLETE) study, a large national cohort study of patients with newly diagnosed PTCL in the United States. Here we report the outcomes for patients who underwent ASCT in CR1 on the basis of multiple variables, including the staging, International Prognostic Index (IPI) score, and histological subtype.
MATERIALS AND METHODS
Study Eligibility
The protocol was approved by the institutional review board (IRB) of each participating institution. Patients were eligible for enrollment in COMPLETE if they provided written informed consent and had a new diagnosis of histologically confirmed PTCL. All subtypes of PTCL were included except for those with a more indolent course, including precursor T/natural killer neoplasms, T-cell large granular lymphocytic leukemia, mycosis fungoides other than transformed mycosis fungoides, Sézary syndrome, and primary cutaneous CD30-positive disorders. Patients were required to be enrolled within 30 days of their initial management and were followed for at least 3 years for disease progression, subsequent treatment, and survival. Investigators were encouraged to approach all eligible patients and to consecutively enroll them after consent was obtained.
Response Assessment and Cohort Selection
Data were recorded prospectively through electronic case report forms. The response assessment was undertaken by the treating investigator according to the Revised Response Criteria for Malignant Lymphoma.18 The best response was recorded at the end of the initial treatment, and any changes in disease status were captured during yearly follow-up intervals. Patients were included in this analysis if their best response to the frontline chemotherapy was CR. Records for these patients were required to be digitally signed and locked by the treating investigator to be included in the analysis. Baseline, treatment, or follow-up records that were incomplete or had outstanding queries at the time of the analysis were excluded. Among the patients who underwent hematopoietic stem cell transplantation (HSCT), those who underwent allogeneic stem cell transplantation were excluded from the final analysis to make the study more consistent with current clinical practice.
Statistical Analysis
Descriptive statistics were used to summarize the baseline characteristics of the study cohort. For categorical and ordinal variables, frequencies and percentages were calculated. For continuous variables, descriptive statistics (numbers of patients, means, medians, standard deviations, and ranges) were provided. Null hypothesis testing used chi-square tests, t tests, and other nonparametric tests as required, with a 2-tailed P value ≤ .05 used to reject the null hypothesis. Survival-based analyses were performed with the Kaplan-Meier methodology with censoring as appropriate and were evaluated with a logrank test, with a 2-tailed P value ≤ .05 used to reject the null hypothesis. A multivariable Cox proportional hazards regression model was used to assess the relation between prespecified variables and overall survival (OS). Hazard ratios (HRs) and associated 95% confidence intervals (CIs) were also calculated. Nonsignificant variables (eg, P > .05) were not removed from the final models. The assumption of proportional hazards for the multivariable Cox regression models was assessed with a test based on weighted Schoenfeld residuals versus the time. All analyses were performed with R version 3.1.0 or higher (R Foundation for Statistical Computing [https://www.r-project.org/]).
RESULTS
Patient Characteristics
A total of 499 patients with PTCL were enrolled in COMPLETE over a period of 4 years between February 2010 and February 2014 from 56 academic and community centers. The characteristics of the patients enrolled in COMPLETE have been reported previously and are comparable to those of patients from the Surveillance, Epidemiology, and End Results program.19 The histology reports were reviewed by 5 independent hematopathologists.20 Before the start of the initial treatment, 18 patients were removed from the study, most commonly because they were lost to follow-up or did not meet the eligibility criteria. Of the remaining patients, 213 achieved CR after frontline therapy and had the required locked records to be included in the analysis. Sixty-four patients (30%) underwent HSCT, whereas 149 patients (70%) were treated without transplantation in CR1. The current analysis focused on 119 patients with nodal PTCL, which was defined as ALK-negative ALCL, AITL, or PTCL NOS, in CR1. Thirty-six patients underwent consolidative ASCT (the ASCT group) whereas 83 were treated without HSCT (the non-ASCT group). The baseline characteristics for the patients in CR1 are shown in Table 1, and those for the patients who did not achieve CR1 are shown in Supporting Table 1. There were no significant differences in demographics (ie, age, race, and sex) between the ASCT and non-ASCT groups, although higher proportions of the ASCT patients were male (P = .21) and younger than 65 years (P = .09) in comparison with non-ASCT patients. PTCL NOS accounted for approximately half of the diagnoses in both groups, but notably, significant differences were seen in the distribution of other PTCL subtypes between the ASCT and non-ASCT groups. The proportion of patients with AITL was higher in the ASCT group than the non-ASCT group (47% vs 22%; P = .01). Conversely, ALCL, which is associated with more favorable outcomes, was a more frequent diagnosis in the non-ASCT group than the ASCT group (31% vs 11%; P = .02). Furthermore, more patients undergoing ASCT in CR1 presented with advanced-stage (III/IV) disease (92% vs 64%; P < .01). The number of patients with advanced PTCL was evenly distributed between the ASCT and non-ASCT groups for ALK-negative ALCL (50% vs 53.8%; P = .89) and AITL (100% vs 83.3%; P = .08), but the largest difference was observed in the PTCL NOS group, in which a significantly higher proportion of patients in the ASCT group had advanced-stage disease in comparison with the non-ASCT group (93% vs 61.5%; P = .02).
TABLE 1.
Characteristics of Patients With PTCL in First Complete Remission
| Characteristic | Non-ASCT (n = 83) | ASCT (n = 36) | P |
|---|---|---|---|
| Age | |||
| <65 y, No. (%) | 39 (47) | 23 (64) | .09 |
| ≥65 y, No. (%) | 44 (53) | 13 (36) | |
| Mean ± SD, y | 64.5 ± 13.9 | 57.5 ± 12.6 | |
| Range, y | 24–89 | 23–75 | |
| Sex, No. (%) | |||
| Female | 28 (34) | 8 (22) | .21 |
| Male | 55 (66) | 28 (78) | |
| Race, No. (%) | |||
| White | 65 (78) | 30 (83) | .32 |
| Black | 11 (13) | 4 (11) | |
| Asian | 3 (4) | 0 | |
| Other/unavailable | 4 (5) | 2 (6) | |
| ECOG performance status, No. (%) | |||
| 0–1 | 79 (95) | 35 (97) | .61 |
| 2 | 4 (5) | 1 (3) | |
| 3 | 0 | 0 | |
| Histologic subtype, No. (%) | |||
| AITL | 18 (22) | 17 (47) | .01 |
| ALK-negative ALCL | 26 (31) | 4 (11) | .02 |
| PTCL NOS | 39 (47) | 15 (42) | .59 |
| Ann Arbor stage, No. (%) | |||
| I/II | 30 (36) | 3 (8) | .01 |
| III/IV | 53 (64) | 33 (92) | |
| Bone marrow involvement, n/N (%) | 11/40 (28) | 8/13 (62) | .03 |
| IPI score, No. (%) | |||
| 0–2 | 59 (71) | 23 (64) | .44 |
| 3–4 | 24 (29) | 13 (36) | |
| PIT score, No. (%) | |||
| 1 | 18 (22) | 9 (25) | .23 |
| 2 | 38 (46) | 15 (42) | |
| 3 | 22 (27) | 6 (17) | |
| 4 | 5 (6) | 6 (17) | |
| First-line chemotherapy | |||
| Anthracycline-containing, No. (%) | 54 (71) | 25 (71) | .97 |
| No. of cycles, mean ± SD | 4.8 ± 2.3 | 4.9 ± 1.9 | .71 |
Abbreviations: AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; ALK, anaplastic lymphoma kinase; ASCT, autologous stem cell transplantation; ECOG, Eastern Cooperative Oncology Group; IPI, International Prognostic Index; NOS, not otherwise specified; PIT, Prognostic Index for PTCL; PTCL, peripheral T-cell lymphoma; SD, standard deviation.
Sums may exceed 100% because of rounding.
Treatment of Patients With PTCL in CR1
Of the 119 patients with nodal PTCL in CR1, 83 received chemotherapy without ASCT consolidation, and 36 underwent ASCT. Transplantation was not considered for the following reasons (as indicated by the treating physician): physician choice (55%), PTCL subtype (21%), patient age/comorbidities (14%), cost/insurance (3%), and others (18%). The majority of patients received an anthracycline-containing regimen as the first-line treatment.19 Various conditioning regimens were used for ASCT according to the institutional standards, although the combination of carmustine, etoposide, cytarabine, and melphalan (BEAM) or different variations of BEAM were most commonly used.
Outcomes of Patients With PTCL in CR1
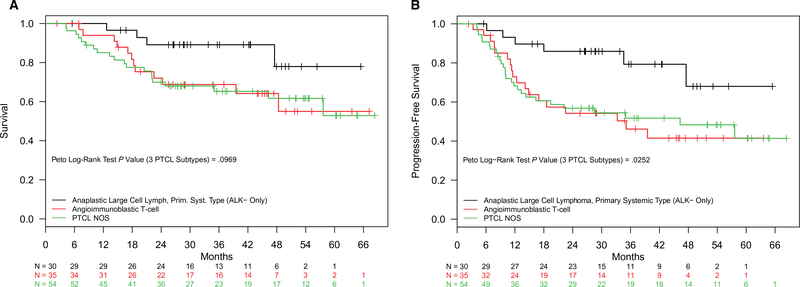
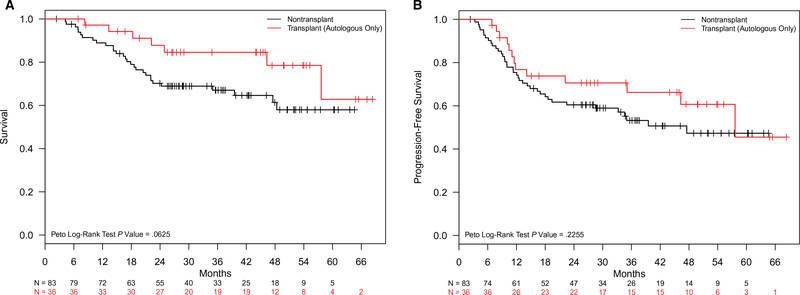
The median follow-up for patients with nodal PTCL in CR1 was 2.8 years (interquartile range, 1.9–4.0 years). The median OS for 119 patients with nodal PTCL in CR1 was not reached, whereas 71 patients with nodal PTCL who did not achieve CR after the frontline chemotherapy had a median OS of 15.9 months (P < .01). The estimated 2-year OS and progression-free survival (PFS) rates for the CR1 patients were 75.3% (95% CI, 67.8%-83.7%) and 63.4% (95% CI, 55.2%-72.9%), respectively, whereas they were 41.9% (95% CI, 31.6%-55.6%) and 19.3% (95% CI, 11.8%-31.8%), respectively, for the patients who did not achieve CR after the initial chemotherapy. Notably, there were no significant differences in survival outcomes by the type of chemotherapy regimen delivered (P = .90) or the treating institution (academic centers vs community centers; P = .15). Estimates of median OS and PFS among patients with PTCL in CR1, stratified by PTCL subtype, are shown in Figure 1. Regardless of their ASCT status, patients with ALK-negative ALCL had significantly longer OS than patients with AITL or PTCL NOS (medians not reached in any group; P = .10). The median PFS was similarly longer for patients with ALK-negative ALCL versus other subtypes. The median PFS was again not reached for ALK-negative ALCL, whereas the median PFS times were 35.1 and 46.1 months for AITL and PTCL NOS, respectively (P = .01). The median OS was not reached in the ASCT group but was 57.6 months for the non-ASCT group; however, this did not meet statistical significance (P = .06; Fig. 2A). The estimated 2-year OS rate was 87.8% (95% CI, 77.3%-99.8%) for the ASCT group and 70.2% (95% CI, 60.9%-80.9%) for the non-ASCT group. The median PFS was 57.6 months in the ASCT group, whereas the non-ASCT group had a median PFS of 47.5 months; again, however, the difference was not statistically significant (P = .23; Fig. 2B).
Figure 1.
(A) Overall survival and (B) progression-free survival for first complete remission patients with nodal PTCL (non-ASCT and ASCT patients combined) by subtypes: ALK-negative anaplastic large cell lymphoma, angioimmunoblastic T-cell lymphoma, and PTCL NOS. ALK indicates anaplastic lymphoma kinase; ASCT, autologous stem cell transplantation; NOS, not otherwise specified; PTCL, peripheral T-cell lymphoma.
Figure 2.
(A) Overall survival and (B) progression-free survival for first complete remission patients with nodal peripheral T-cell lymphoma: ASCT versus non-ASCT. ASCT indicates autologous stem cell transplantation.
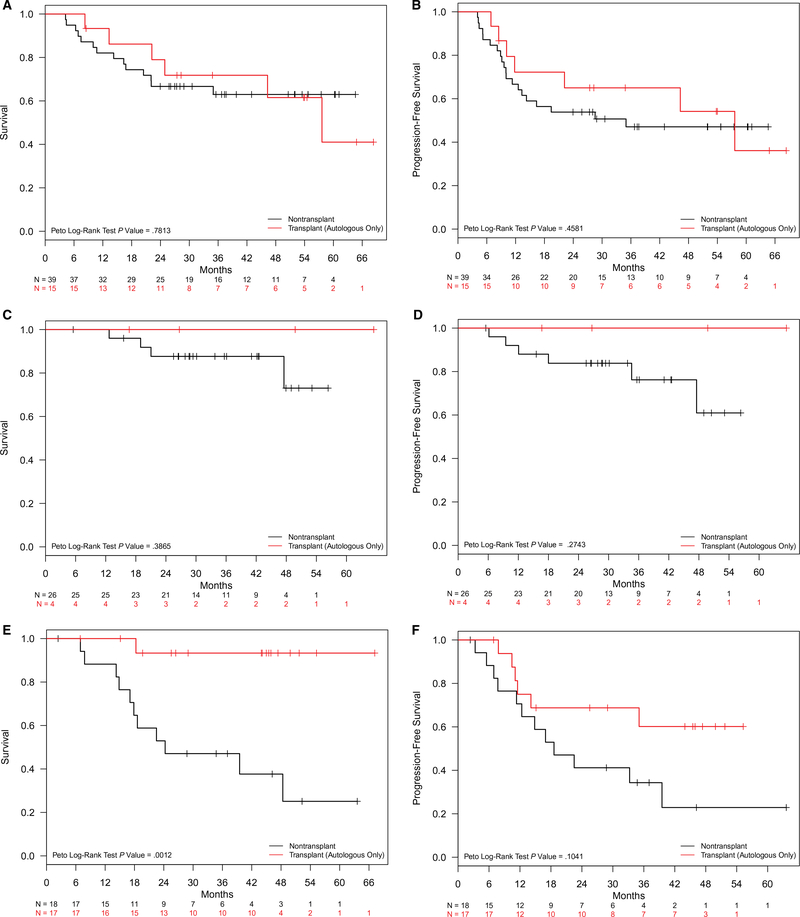
Survival outcomes were compared between the ASCT and non-ASCT groups stratified by PTCL subtypes. There were no significant differences in median OS or PFS between the ASCT and non-ASCT groups for ALK-negative ALCL, with the median OS and PFS not reached in either group (P = .39 and P = .27, respectively; Fig. 3E,F). The estimated 2-year PFS rate for 4 patients with ALK-negative ALCL who underwent ASCT in CR1 was 100%, whereas the estimated 2-year PFS for 26 patients with ALK-negative ALCL who did not undergo ASCT in CR1 was 83.8% (95% CI, 70.5%-99.7%). The survival of patients with AITL appeared more favorable for the ASCT group versus the non-ASCT group (Fig. 3A,B). For the patients with AITL in CR1, the median OS and PFS in the ASCT group were not reached, whereas those in the non-ASCT group had median OS and PFS times of 24.3 and 18.6 months, respectively (P < .01 and P = .10). The estimated 2-year OS and PFS rates for patients with AITL in CR1 were 93.3% (95% CI, 81.5%-100%) and 68.8% (95% CI, 48.4%-95.7%), respectively, in the ASCT group and 52.9% (95% CI, 33.8%-82.9%) and 41.2% (95% CI, 23.3%-72.7%), respectively, in the non-ASCT group. The median OS and PFS were not significantly different between the ASCT and non-ASCT groups for patients with PTCL NOS (P = .78 and P = .46).
Figure 3.
OS and PFS for first complete remission patients with nodal PTCL by subtypes (ASCT versus non-ASCT): (A,B) OS and PFS with angioimmunoblastic T-cell lymphoma, respectively; (C,D) OS and PFS with PTCL not otherwise specified, respectively; and (E,F) OS and PFS with ALK-negative anaplastic large cell lymphoma, respectively. ALK indicates anaplastic lymphoma kinase; ASCT, autologous stem cell transplantation; OS, overall survival; PFS, progression-free survival; PTCL, peripheral T-cell lymphoma.
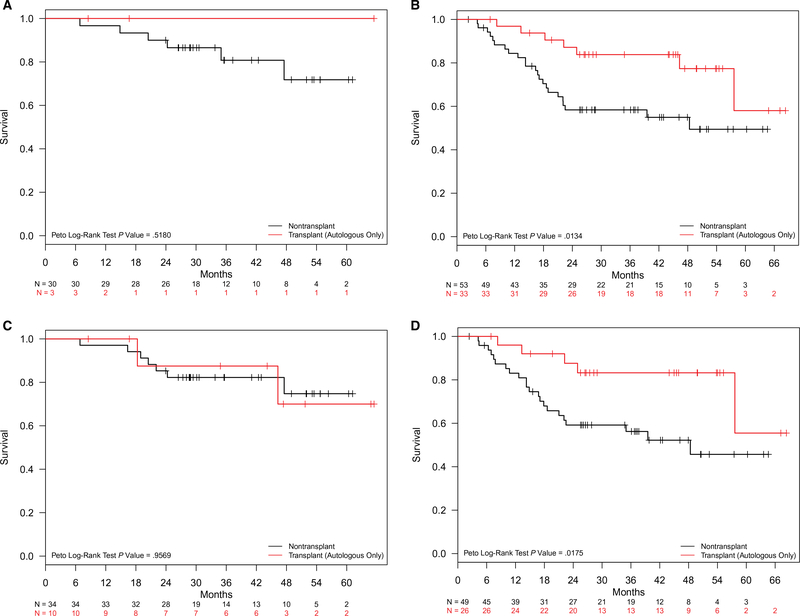
Additional univariate analyses were undertaken to explore the impact of ASCT on survival outcomes among various subgroups of patients with nodal PTCL in CR1. Attention was primarily focused on the characteristics historically associated with prognostic implications, such as the stage, IPI score, and histologic subtype. Among patients with advanced-stage disease (Fig. 4A,B), OS was significantly longer for ASCT patients, for whom the median OS was not reached, in comparison with non-ASCT patients, for whom the median OS was 48.4 months (P = .01). The estimated 2-year OS rate for patients with advanced-stage nodal PTCL was 87.2% (95% CI, 76.2%-99.8%) in the ASCT group and 58.4% (95% CI, 46.2%-73.7%) in the non-ASCT group. An analysis of IPI scores revealed superior OS associated with ASCT among patients with intermediate-to-high IPI scores (2–4; Fig. 4C,D), with the median OS not reached in the ASCT group but 48.4 months in the non-ASCT group (P = .02). The estimated 2-year OS rates for patients with intermediate-to-high IPI scores were 87.6% (95% CI, 75.4%-100%) in the ASCT group and 59.2% (95% CI, 46.6%-75.2%) in the non-ASCT group. In a multivariable analysis of patients with nodal PTCL in CR1, ASCT was associated with superior OS with an HR of 0.37 (95% CI, 0.15–0.89; P = .03) independently of the stage, IPI score, or age (Table 2). An advanced stage (III/IV) was associated with an HR of 2.65 (95% CI, 1.08–6.55; P = .03), whereas ALK-negative ALCL and age had HRs of 0.35 (95% CI, 0.12–1.02; P = .05) and 1.02 (95% CI, 1–1.05; P = .09), respectively.
Figure 4.
Overall survival in first complete remission patients with nodal peripheral T-cell lymphoma by risk factor: (A,B) limited versus advanced stage and (C,D) low IPI scores (0–1) versus intermediate/high (2–4) IPI scores. IPI indicates International Prognostic Index.
TABLE 2.
Cox Regression Model: Overall Survival
| Hazard Ratio | 95% CI | Inverse Hazard Ratio | P | |
|---|---|---|---|---|
| ASCT | 0.37 | 0.15–0.89 | 2.70 | .03 |
| Stage III/IV | 2.65 | 1.08–6.55 | 0.38 | .03 |
| ALK-negative ALCL | 0.35 | 0.12–1.02 | 2.83 | .05 |
| Age (y) | 1.02 | 1.00–1.05 | 0.98 | .09 |
Abbreviations: ALCL, anaplastic large cell lymphoma; ALK, anaplastic lymphoma kinase; ASCT, autologous stem cell transplantation; CI, confidence interval.
DISCUSSION
The current study is the first published report on the role of upfront ASCT for prospectively enrolled patients with PTCL, and it directly compares the outcomes of ASCT and non-ASCT patients in CR1. The majority of reports describing survival outcomes with previously untreated PTCL are based on analyses of retrospective series. The COMPLETE registry represents the largest multi-institutional, prospectively collected PTCL database to date in the United States. In this national cohort study, the most clinically pertinent data, such as performance scores, histological subtypes, and laboratory values, were obtained prospectively to provide a higher level of evidence in the analysis. In addition, the current study secured a large sample size, which allowed the robust selection of PTCL subgroups to closely reflect the real-world data. This is especially important because PTCL is a highly heterogeneous disease. This study, however, is still subject to unintentional bias and confounding variables as an observational study. For example, the decision not to proceed with ASCT in CR1 was made on the basis of the physician’s choice for a relatively large number of patients, and this could have served as a potential source of bias. This serves as an important lesson for similar future prospective analyses: the factors for not pursuing ASCT in these patient populations need to be clearly delineated to eliminate any potential biases. Specifically, the bias is evident in the significant variance between the ASCT and non-ASCT groups with respect to some of the baseline characteristics in the current study. Although major demographic characteristics, including age, sex, and performance status, were similar in the 2 groups, significant differences in the proportions of histologic subtypes and stages were observed between ASCT and non-ASCT patients. Notably, the majority of patients with limited-stage PTCL did not undergo ASCT, whereas a significantly higher percentage of patients with poor-risk histologic subtypes, positive bone marrow involvement, and/or advanced-stage disease underwent ASCT in CR1. The observed variation highlights that baseline risk factors played a significant role in the determination of whether to proceed with ASCT or not in CR1. Nevertheless, it is notable that the ASCT group was associated with superior survival outcomes, even though patients with high-risk features (ie, aggressive PTCL subtypes, advanced stage, and/or bone marrow involvement) were assigned to this group in comparison with the non-ASCT group. This indicates that the survival difference would have been even greater between the ASCT and non-ASCT groups if these potential biases had been removed.
In the absence of randomized clinical trial data, the role of ASCT for PTCL in the frontline setting remains controversial. The current recommendation to consider ASCT in CR1 for most PTCL subtypes is largely based on the results from a small number of phase 2, single-arm studies. A study by the Nordic Lymphoma Group is the largest prospective study of upfront ASCT, and it enrolled 160 patients with PTCL; all subtypes were allowed with the exception of ALK-positive ALCL.11 One hundred thirty patients achieved CR (63%) or a partial response (37%), of whom 115 (88.5%) went on to ASCT. The 5-year OS and PFS rates for the intent-to-treat population were 51% and 44%, respectively. Outcomes by subtype revealed superior OS and PFS for patients with ALK-negative ALCL in comparison with patients with other PTCL subtypes. The second largest prospective study was conducted by Reimer et al12 and enrolled 83 patients with PTCL. Primary cutaneous lymphomas and ALK-positive ALCL were excluded. A total of 59 patients (71%) completed stem cell mobilizing therapy after CR (66%) or a partial response (34%) to induction therapy, with 55 undergoing ASCT. The 3-year OS rate was 48% for the intent-to-treat population. However, these studies all lacked direct comparisons of outcomes between ASCT and non-ASCT patients in CR1.
As expected, patients with nodal PTCL who had CR as their best response to the initial chemotherapy had significantly higher survival rates than those who did not achieve CR in the current study (median OS, not reached vs 15.9 months; P < .01). These data may serve as benchmarks for future studies. Notably, Abramson et al21 also reported that the response to initial therapy was one of the strongest predictors of improved survival in another US cohort study. Furthermore, our data demonstrated that patients with ALK-negative ALCL had better survival outcomes than patients with AITL or PTCL NOS, independently of their transplant status, and these data were concordant with the International T-Cell Project and Nordic Lymphoma Group data.11,22
In this study, there was no significant difference in survival between ASCT and non-ASCT patients when all patients with nodal PTCL were analyzed as a whole, although there was a trend toward improved OS associated with ASCT in CR1 (P = .06). When PTCL subgroups were analyzed, patients with AITL undergoing ASCT had significantly better survival rates than those who did not undergo ASCT in CR1, although definitive conclusions could not be made because of the smaller numbers when PTCL subgroups were analyzed individually. These data further support the potential benefit of upfront ASCT, especially in patients with AITL, shown by the data from the European Group for Blood and Marrow Transplantation group.6 In this retrospective analysis of 146 patients with AITL, the estimated 2-year OS and PFS rates for patients with AITL undergoing ASCT in CR1 were 81% and 70%, respectively, which were similar to our findings. Interestingly, ASCT had no significant effect on survival in patients with PTCL NOS in our study. We speculate that this could be due to the biology of the disease; however, the disease stage might have been a confounder because a disproportional number of patients with PTCL NOS in the ASCT group had advanced-stage disease (93%) in comparison with the non-ASCT group (61.5%). This hypothesis was further supported when survival outcomes were analyzed by disease stage. Upfront ASCT was associated with significantly better survival for patients with advanced-stage disease, regardless of the PTCL subtype. Similarly, ASCT had a significant impact on patient survival for those with intermediate-to-high IPI scores in CR1, regardless of the PTCL subtype. The estimated 2-year OS for these patients (87.6%) appeared higher than what was reported from a phase 2 study by Reimer et al,12 which showed relatively poor survival (estimated 2-year OS ~ 50%) in patients with high IPI scores despite ASCT. This difference can be partly explained by the nature of an intent-to-treat analysis in the phase 2 study, where all intent-to-treat patients were included in the analysis, regardless of their response to the frontline therapy, whereas the current analysis selected only those who achieved CR before ASCT. In contrast to those with high-risk features, the benefit of upfront ASCT appeared less evident in patients with PTCL with limited-stage disease and/or low IPI scores. However, these data should be interpreted with caution because of the limited sample sizes. In our multivariable analysis, ASCT emerged as one of the strongest variables associated with favorable OS (HR, 0.37; 95% CI, 0.15–0.89), whereas an advanced stage was associated with poor OS (HR, 2.65; 95% CI, 1.08–6.55) in patients with nodal PTCL who achieved CR after frontline chemotherapy. This study is limited by the relatively short duration of follow-up (median follow-up ~ 34 months); however, a recent international cohort study by Maurer et al23 demonstrated that event-free survival at 24 months could be a clinically significant endpoint in PTCL, and this supports the high clinical relevance of the current study. Lastly, recent studies have shown the potential role of tumor biology, including chromosomal rearrangements of DUSP22 and TP63, in defining a group of patients with PTCL who may benefit more from ASCT. In a pooled analysis of 170 patients from 3 independent patient cohorts, ASCT was associated with superior survival for patients with PTCL NOS or ALK-negative ALCL with no evidence of chromosomal rearrangement of DUSP22.24 Although the current study lacks any direct tumor analysis, we believe that our clinical findings will provide valuable information for designing future trials, especially when they are combined with the tumor biology data.
In summary, this subset analysis of COMPLETE prospective data represents the largest direct comparison of survival outcomes for patients with nodal PTCL who undergo ASCT and those who do not undergo ASCT in CR1. Our findings demonstrate no statistical difference in survival between the ASCT and non-ASCT groups en masse. However, a relatively large sample size in the current study allowed us to explore various PTCL subgroups with superior statistical power in comparison with previous studies. Our univariate and multivariable analyses suggest that subgroups of patients with nodal PTCL, especially those with AITL and/or high-risk features (ie, advanced-stage disease or intermediate-to-high IPI scores), might benefit from consolidative ASCT in CR1. These results are of high clinical interest because they provide further guidance for the use of ASCT in patients with nodal PTCL. There is currently no such data-driven guidance available in the literature. The full extent of the benefit from upfront ASCT in nodal PTCL should be evaluated in prospective randomized trials, and the current study will serve as a bridge to future trials by better defining the target patient populations that are likely to receive the greatest benefit from ASCT.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
This work, and the COMPLETE study were supported by Spectrum Pharmaceuticals, Inc.
CONFLICT OF INTEREST DISCLOSURES
Steven I. Park reports honoraria from Seattle Genetics; consulting or advisory roles with Teva, Bristol-Myers Squibb, G1 Therapeutics, and Rafael Pharmaceuticals; participation in a speakers’ bureau for Gilead Sciences and Seattle Genetics; and research funding from Teva, Takada, Seattle Genetics, and Bristol-Myers Squibb. Steve M. Horwitz reports consulting or advisory roles with Innate Pharma, Celgene, Millennium/Takeda, Kyowa Hakko Kirin, Seattle Genetics, Forty-Seven, Mundipharma, and Verastem; research funding from Celgene, Millennium/Takeda, Kyowa Hakko Kirin, Seattle Genetics, Forty-Seven, Infinity/Verastem, Spectrum Pharmaceuticals, ADC Therapeutics, Trillium, and Aileron Therapeutics; and personal fees from ADC Therapeutics, Aileron Therapeutics, Portola, and Corvus. Francine M. Foss reports consulting or advisory roles with Celgene, Seattle Genetics, Spectrum Pharmaceuticals, Eisai, Millennium, and Verastem; honoraria from Spectrum, Seattle Genetics, and Miragen; participation in a speakers’ bureau for Seattle Genetics and Celgene; and research funding from Celgene. Lauren C. Pinter-Brown reports consulting or advisory roles with Spectrum, Seattle Genetics, Portola, Miragen, and Kiowa. Kenneth R. Carson reports employment by Flatiron Health, stock or other ownership in Flatiron Health, research funding from Kyowa Hakko Kirin and Celgene, and travel/accommodations/expenses from Flatiron Health. Steven T. Rosen reports honoraria from Celgene, Pharmacyclics, and Valeant Pharmaceuticals International; consulting or advisory roles with Celgene, Genentech, Seattle Genetics, and Aileron Therapeutics; participation in a speakers’ bureau for the Scienomics Group, Xcenda, and AmerisourceBergen; and research funding from Celgene. Barbara Pro reports research grants and personal fees outside the submitted work from Seattle Genetics, Takeda, Kiowa, and Portola. Eric D. Hsi reports consulting or advisory roles with Seattle Genetics and Celgene and research funding from Eli Lilly and AbbVie. Massimo Federico reports consulting or advisory roles with MedNet Solutions, Takeda, and Spectrum and research grants from Spectrum and Takeda. Marc Schwartz is the founder/chief executive officer of MS Biostatistics, LLC, a biostatistics consulting firm; he was formerly an employee of MedNet Solutions, Inc, which was contracted by Spectrum Pharmaceuticals to provide various Electronic Data Capture (EDC) technology and support services to the Comprehensive Oncology Measures for Peripheral T-Cell Lymphoma Treatment (COMPLETE) study; and he is now under subcontract to MedNet Solutions to continue to provide biostatistics services for the COMPLETE study. Lisa A. Bellm reports personal fees from MedNet Solutions during the conduct of the study and stock ownership in Bristol-Myers Squibb, Merck, and Johnson & Johnson. Mark Acosta reports employment by Spectrum Pharmaceuticals, stock or other ownership in Spectrum Pharmaceuticals, and travel/accommodations/expenses from Spectrum Pharmaceuticals. Ranjana H. Advani reports consulting or advisory roles with Sanofi, Sutro, Takeda, Kite, Kyowa, Juno, Genentech/Roche, Bristol-Myers Squibb, Spectrum Pharmaceuticals, Sutter Medical Group, NanoString Technologies, Pharmacyclics, Gilead Sciences, Bayer, Cell Medica, Seattle Genetics, Autolus, and AstraZeneca and research funding from Bristol-Myers Squibb, Millennium, Seattle Genetics, Genentech/Roche, Pharmacyclics, Janssen, Celgene, Agensys, Merck, Kura, Regeneron, Infinity Pharmaceuticals, and Forty Seven. Mary Jo Lechowicz reports other from Spectrum and Soligenix outside the submitted work. Sonali M. Smith reports institutional funding for data collection from Allos during the conduct of the study; consulting or advisory roles with Bayer, Bristol-Myers Squibb, Seattle Genetics, Portola, NanoString Technologies, Genmab, Pharmacyclics/Janssen, Forty Seven, Roche, Juno Therapeutics, Kite Pharma, AbbVie/Genentech, Gilead Sciences, Celgene, TG Therapeutics, Portola Pharmaceuticals, Amgen, and AstraZeneca; and institutional fees for trials from Portola, Pharmacyclics/Janssen, and Celgene. Frederick Lansigan reports consulting or advisory roles with Spectrum Pharmaceuticals and Celgene and research funding from Spectrum Pharmaceuticals. Anil Tulpule reports honoraria, a consulting or advisory role with Seattle Genetics, and travel/accommodations/expenses from Seattle Genetics. Michael D. Craig reports research funding from Celgene, Novartis, and Sanofi and grants from West Virginia University. Brad S. Kahl reports consulting or advisory roles with Seattle Genetics, Millennium, Cell Therapeutics, Celgene, Juno Therapeutics, AbbVie, Genentech/Roche, Pharmacyclics, Acerta Pharma, and ADC Therapeutics; research funding from Genentech, AbbVie, Acerta Pharma, and ADC Therapeutics; travel/accommodations/expenses from Celgene, Juno Therapeutics, Genentech/Roche, AbbVie, Millennium, and Seattle Genetics; and personal fees from Gilead and Bayer. Carla Casulo reports honoraria from Gilead Sciences, research funding from Celgene, and travel/accommodations/expenses from Gilead Sciences and Roche. Andrei R. Shustov reports honoraria from Verastem, nonfinancial support and research funding from Spectrum Pharmaceuticals, and travel/accommodations/expenses from Spectrum Pharmaceuticals.
Footnotes
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1.Adams SV, Newcomb PA, Shustov AR. Racial patterns of peripheral T-cell lymphoma incidence and survival in the United States. J Clin Oncol. 2016;34:963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vose JM. Peripheral T-cell non-Hodgkin’s lymphoma. Hematol Oncol Clin North Am. 2008;22:997–1005. [DOI] [PubMed] [Google Scholar]
- 3.Hapgood G, Savage KJ. The biology and management of systemic anaplastic large cell lymphoma. Blood. 2015;126:17–25. [DOI] [PubMed] [Google Scholar]
- 4.Savage KJ. Peripheral T-cell lymphomas. Blood Rev. 2007;21:201–216. [DOI] [PubMed] [Google Scholar]
- 5.Corradini P, Tarella C, Zallio F, et al. Long-term follow-up of patients with peripheral T-cell lymphomas treated up-front with high-dose chemotherapy followed by autologous stem cell transplantation. Leukemia. 2006;20:1533–1538. [DOI] [PubMed] [Google Scholar]
- 6.Kyriakou C, Canals C, Goldstone A, et al. High-dose therapy and autologous stem-cell transplantation in angioimmunoblastic lymphoma: complete remission at transplantation is the major determinant of outcome—Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26:218–224. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez J, Conde E, Gutierrez A, et al. The adjusted International Prognostic Index and beta-2-microglobulin predict the outcome after autologous stem cell transplantation in relapsing/refractory peripheral T-cell lymphoma. Haematologica. 2007;92:1067–1074. [DOI] [PubMed] [Google Scholar]
- 8.Stiff PJ, Unger JM, Cook JR, et al. Autologous transplantation as consolidation for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 2013;369:1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mounier N, Gisselbrecht C, Briere J, et al. Prognostic factors in patients with aggressive non-Hodgkin’s lymphoma treated by frontline autotransplantation after complete remission: a cohort study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2004;22:2826–2834. [DOI] [PubMed] [Google Scholar]
- 10.Mounier N, Gisselbrecht C, Briere J, et al. All aggressive lymphoma subtypes do not share similar outcome after front-line autotransplantation: a matched-control analysis by the Groupe d’Etude des Lymphomes de l’Adulte (GELA). Ann Oncol. 2004;15:1790–1797. [DOI] [PubMed] [Google Scholar]
- 11.d’Amore F, Relander T, Lauritzsen GF, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30:3093–3099. [DOI] [PubMed] [Google Scholar]
- 12.Reimer P, Rudiger T, Geissinger E, et al. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol. 2009;27:106–113. [DOI] [PubMed] [Google Scholar]
- 13.Feyler S, Prince HM, Pearce R, et al. The role of high-dose therapy and stem cell rescue in the management of T-cell malignant lymphomas: a BSBMT and ABMTRR study. Bone Marrow Transplant. 2007;40:443–450. [DOI] [PubMed] [Google Scholar]
- 14.Mercadal S, Briones J, Xicoy B, et al. Intensive chemotherapy (high-dose CHOP/ESHAP regimen) followed by autologous stem-cell transplantation in previously untreated patients with peripheral T-cell lymphoma. Ann Oncol. 2008;19:958–963. [DOI] [PubMed] [Google Scholar]
- 15.Smith SM, Burns LJ, vanBesien K, et al. Hematopoietic cell transplantation for systemic mature T-cell non-Hodgkin lymphoma. J Clin Oncol. 2013;31:3100–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savage KJ, Chhanabhai M, Gascoyne RD, et al. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol. 2004;15:1467–1475. [DOI] [PubMed] [Google Scholar]
- 17.Horwitz SM, Zelenetz AD, Gordon LI, NCCN, et al. Guidelines Insights: non-Hodgkin’s lymphomas, version 3.2016. J Natl Compr Canc Netw. 2016;14:1067–1079. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. [DOI] [PubMed] [Google Scholar]
- 19.Carson KR, Horwitz SM, Pinter-Brown LC, et al. A prospective cohort study of patients with peripheral T-cell lymphoma in the United States. Cancer. 2017;123:1174–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsi ED, Horwitz SM, Carson KR, et al. Analysis of peripheral T-cell lymphoma diagnostic workup in the United States. Clin Lymphoma Myeloma Leuk. 2017;17:193–200. [DOI] [PubMed] [Google Scholar]
- 21.Abramson JS, Feldman T, Kroll-Desrosiers AR, et al. Peripheral T-cell lymphomas in a large US multicenter cohort: prognostication in the modern era including impact of frontline therapy. Ann Oncol. 2014;25:2211–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savage KJ, Harris NL, Vose JM, et al. ALK– anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111:5496–5504. [DOI] [PubMed] [Google Scholar]
- 23.Maurer MJ, Ellin F, Srour L, et al. International assessment of event-free survival at 24 months and subsequent survival in peripheral T-cell lymphoma. J Clin Oncol. 2017;35:4019–4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedersen MB, Relander T, Luaritzsen GF, et al. The impact of upfront autologous transplant on the survival of adult patients with ALCL and PTCL NOS according to their ALK, DUSP22, and TP63 gene rearrangement status—a joined Nordic Lymphoma Group and Mayo Clinic analysis. Blood. 2017;130:822. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.