Abstract
Methcathinone analogs are appearing on the clandestine market at a rate nearly out-pacing the ability of investigators to examine them on an individual basis. In order to formulate structure-activity relationship (SAR) generalities, we examined releasing ability of several simple methcathinone analogs at the three monoamine transporters (i.e., the dopamine, norepinephrine, and serotonin transporters, DAT, NET, and SERT, respectively) using in vitro assay methods. The analogs included methcathinone, and 14 other compounds mono-substituted at either the 2-, 3-, or 4-position. In general, a) the 2-substituted analogs were less potent than either the 3- or 4-substituted analogs, b) the 3- and 4-substituted analogs were relatively similar in potency, c) methcathinone was the most selective as a DAT releasing agent, and d) the 3- and 4-CF3 analogs were the least DAT-selective. For the 15 compounds, there was a significant correlation (r > 0.9) between DAT and NET potency suggesting relatively similar structure-activity relationships (at least for the compounds examined here). Several of the compounds have appeared on the clandestine market since our studies were initiated and the present results provide new information on how they might act.
INTRODUCTION
Over the last several years, >150 methcathinone (1) analogs, or “synthetic cathinones” have appeared on the clandestine market as abused substances.1,2 They vary with respect to their terminal amine substituents, length and nature of their α-alkyl group, and aryl substitution pattern. In theory, many more synthetic cathinone analogs are possible. Some synthetic cathinones behave, primarily, as central stimulants, and a few have been controlled as U.S. Schedule I substances.2 Yet, many more remain to be pharmacologically and mechanistically characterized. Their pharmacological actions can vary from agent to agent, and synthetic cathinones cannot be considered as being an homogeneous group of similar-acting agents.1 Those synthetic cathinones bearing a bulky terminal amine substituent and/or an extended α-alkyl side chain are fairly selective reuptake inhibitors (i.e., blockers) at membrane dopamine and norepinephrine transporters (DAT and NET, respectively).Reviewed: 3 Agents with an N-methyl terminal amine and an α-methyl substituent, termed here as “simple methcathinone analogs”, typically behave as DAT and NET releasing agents (i.e., substrates), but some also act as releasing agents at the serotonin transporter (SERT) depending upon the nature of their 4-position aryl substituent.1,3,4
The appearance on the illicit market of so many synthetic cathinones is out-pacing the ability of investigators (and law enforcement officials) to examine them on an individual basis. An approach we have previously used to circumvent this problem is to systematically examine small sets of methcathinone analogs that vary with regard to specific substituents at a specific location to formulate structure-activity relationship (SAR) generalities that might be useful to better understand larger groups of agents – including agents that have yet to appear on the “street”. In the present investigation, we further define the SAR of simple N-methyl cathinone or methcathinone analogs (i.e., cathinone analogs that possess an N-methyl and α-methyl group).
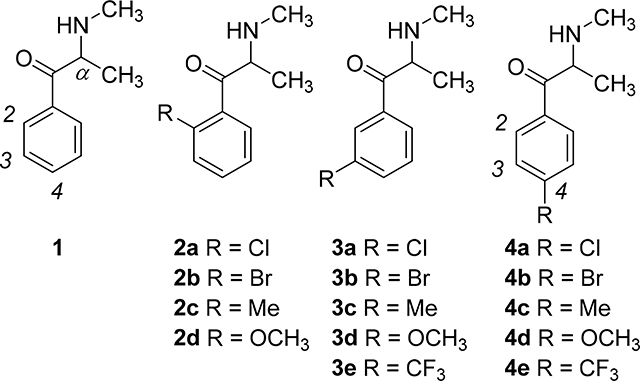
Methcathinone (1) itself, as well as a number of other simple methcathinone analogs, act as releasing agents (i.e., as substrates) at DAT and NET.1–3 However, variation of the aryl 4-position substituent not only influences methcathinone potency, but can shift action from a relatively selective DAT vs SERT releaser, to a much less DAT versus SERT-selective agent, and even to agents with some selectivity for SERT.4 For example, methcathinone (1) displays greater >300-fold selectivity for DAT vs SERT as a releasing agent (see Table 1 for reference), whereas the 4-trifluoromethyl analog of methcathinone (i.e., 4e) displays 14-fold SERT selectivity.4 In an attempt to better understand the role of 4-position substituents on potency and selectivity, we conducted a quantitative structure-activity relationship (QSAR) study and found that SERT better accommodates bulky 4-position substituents than does DAT.5 That is, methcathinone analogs with small 4-position substituents are selective releasing agents at DAT, but as the size of the 4-position substituent is increased, selectivity shifts in favor of SERT.5
Table 1.
Potencies of aryl-substituted methcathinone analogs on transporter-mediated release in rat brain synaptosomes.a
 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC50 nM (SD) | ||||||||||||
| 2-Substituted: | 3-Substituted: | 4-Substituted:b | ||||||||||
| DAT | NET | SERT | DAT vs SERT Selectivityc | DAT | NET | SERT | DAT vs SERT Selectivityc | DAT | NET | SERT | DAT vs SERT Selectivityc | |
| H (1) | 25 (3) | 23 (2) | 2592 (1000) | 103 | 21 (3) | 22 (3) | 5853 (1250) | 279 | 12.5 | 22 (4) | 3860 | 309 |
| Cl | 179 (22) | 93 (6) | 2815 (620) | 16 | 26 (4) | 19 (3) | 211 (33) | 8 | 42.2 | 44 (9) | 144 | 3.4 |
| Br | 650 (80) | 156 (12) | 2837 (260) | 4 | 21 (3) | 25 (4) | 136 (24) | 6 | 59.4 | 100 (16) | 60 | 1.0 |
| Me | 81 (8) | 53 (4) | 490 (15) | 6 | 28 (6) | 27 (4) | 268 (25) | 10 | 49.1 | 63 (16) | 118 | 2.4 |
| OMe | 920 (30) | 339 (12) | 7220 (900) | 8 | 109 (18) | 111 (12) | 683 (92) | 6 | 506 | 111 (24) | 120 | 0.24 |
| CF3 | - | - | - | 714 (73) | 370 (49) | 281 (57) | 0.4 | 2700 | 900 (300) | 190 | 0.07 | |
All data are expressed as mean ± SD for n = 3 experiments performed in triplicate.
DAT and SERT data previously published.4
SERT EC50 ÷ DAT EC50
Here, we investigate a series of 2- and 3-substituted methcathinone analogs (i.e., 2a-d and 3a-e, respectively) to determine their potency and selectivity as releasing agents at DAT and SERT with the intent of conducting a QSAR study much in the same way we examined the 4-substituted analogs.5 Furthermore, we examined each of the compounds for their actions at NET; we have not previously reported NET release data for 4a-4e.
SYNTHESIS
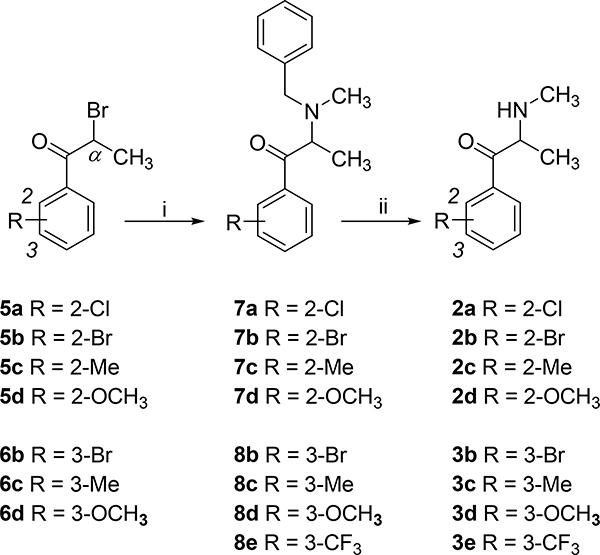
Compounds 1, 3a, and 4a-4e as their water-soluble HCl salts were on-hand from earlier studies4,6 Some of the products and/or intermediates also have been reported by others, but none of the synthetic targets was prepared as shown in Scheme 1.
Scheme 1.
Synthesis of 2- and 3-substituted methcathinone analogs.a
aReagents and conditions: (i) N-benzyl-N-methylamine, THF, reflux, 18–23 h; (ii) (a) 1-chloroethyl chloroformate, CH2Cl2, reflux, 2 h; (b) CH3OH, reflux, 1 h; (c) Et2O, room temperature, 18 h.
Reaction of bromopropiophenones 5a-d and 6b-d with N-benzyl-N-methylamine provided N-benzyl analogs 7a-d and 8b-d, respectively. Deprotection by treatment with 1-chloroethyl chloroformate afforded targets 2a-d and 3b-d. Compound 3e was prepared in the same manner from the known 8e. The synthesis of 3b is described in the Methods section; all other targets were obtained using comparable reaction conditions.
RESULTS
The potency of methcathinone (1) as a releasing agent at DAT and NET was found to be similar (EC50 = 21–25 nM; Table 1), and comparable, to its previously reported potency as a DAT releasing agent.4 Its potency as a releasing agent at SERT was substantially lower (i.e., by 100- to >300-fold; see Table 1). Representative plots for methcathinone (1) and several 3-substituted methcathinone analogs are shown in Supporting Information (Figure S1).
4-Substituted Analogs.
DAT and SERT release potencies have been previously reported by us for 1 and 4a-4e4 and are reproduced in Table 1 for comparison with the new NET release data. NET potency for these compounds appeared to co-vary with their DAT potency (correlation coefficient r > 0.9; n = 6).
2-Substituted Analogs.
In general, where comparisons could be made, the 2-substituted analogs displayed approximately 2- to 10-fold reduced potency at DAT than their corresponding 4-substituted counterparts (Table 1). Potencies at NET closely mirrored potencies at DAT (r > 0.9; n = 5). 2-Substituted methcathinone analogs showed reduced potencies at SERT as compared to DAT release, and were from 4- to 47-fold lower than their corresponding 4-substituted analogs.
3-Substituted Analogs.
We previously reported the potency of 3a as a DAT, NET, and SERT releasing agent (EC50 = 29, 40, and 212 nM, respectively).6 The current study provided comparable values (EC50 = 26, 19, and 211 nM) (Table 1). As with data for the 4-substituted and 2-substituted series, potencies of the 3-substituted analogs at DAT mirrored their potency at NET (r > 0.9). In general, the potencies of the 3-substituted compounds as DAT releasing agents were 2- to 5-fold higher than those of their corresponding 4-substituted counterparts whereas, in contrast, potencies at SERT were consistency lower by about half, and only spanned a 5-fold range from the most potent (i.e., 3b) to the least potent (3d).
As with the 4-substituted methcathinone derivatives (i.e., analogs 4), the high DAT potency of the 3-substituted analogs was associated with an unsubstituted ring (i.e., 1). However, whereas the 4-Cl, 4-Br, and 4-Me analogs 4a-4c, respectively, showed several-fold reduced potency relative to methcathinone (1), their 3-substituted counterparts were very similar in potency to the parent. Nevertheless, as seen in the 4-substituted series, the 3-OMe and 3-CF3 analogs 3d and 3e, respectively, were the least potent.
DISCUSSION
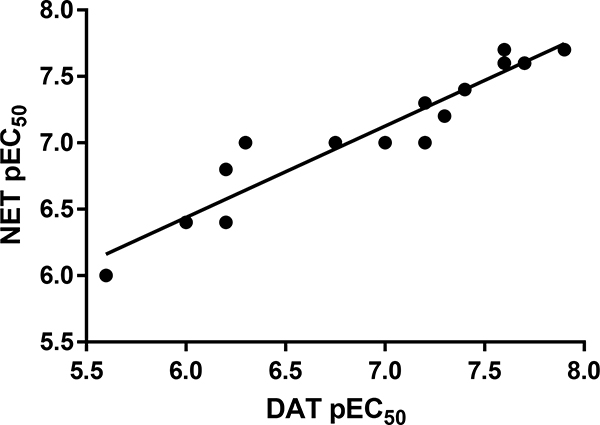
We have now examined a number of 2-, 3-, and 4-substituted simple methcathinone analogs in a fairly systematic manner at the three monoamine transporters (present investigation and reference 4). Each compound examined was a releasing agent at DAT, NET, and SERT. In general, the agents (n = 15) displayed similar potencies at DAT and NET (Figure 1). However, such a relationship was not apparent when DAT pEC50 values were plotted against SERT EC50 values (r = 0.209, n = 15; data not shown). Hence, aryl substitution had a decided influence on DAT/NET versus SERT potency and selectivity.
Figure 1.
A comparison of DAT and NET release potencies for compounds shown in Table 1 (n = 15, r = 0.950); DAT and NET data for methcathinone were only considered once, IC50 = 21 and 22 nM, respectively).
As releasing agents, methcathinone analogs with substituents at the 2-position (i.e. 2 analogs) were not as potent as their 3- or 4-positional isomers (i.e., 3 and 4 analogs, respectively). This is particularly true at SERT where, with one exception, 2c, EC50 values are in the μM range. Given this trend, we did not examine the 2-CF3 analog. In fact, a prior investigation had already demonstrated that the 2-CF3 analog of methcathinone to be >10-fold less potent than 3e as a releasing agent at DAT and SERT.7 In contrast, with the exception of the OMe and CF3 analogs 3d and 3e, substituents at the 3-position had little effect on the potency of substituted methcathinone analogs at DAT and NET, but decreased potency at SERT, relative to their 4-substituted counterparts.
On the basis of functional QSAR studies, we found that there are limitations to the amount of steric bulk associated with the 4-position of simple methcathinone analogs tolerated by DAT as releasing agents.5 In the present investigation, this same trend was evident with the 3-substituted analogs; however, due the narrow potency range, a QSAR study was not feasible here for these compounds. That is, four of the analogs (i.e., 3a-3d) showed only a 4-fold range in potency, and the entire range in potency was only 34-fold (when 3e is considered). Nevertheless, the two lowest potency compounds as DAT releasing agents are the 3-OMe and 3-CF3 analogs 3d and 3e, which are the two largest analogs (or analogs with the largest 3-position substituents) of those examined. Regardless of the steric metric employed (see Supporting Information for a comparison of modified steric E’s values, Charton’s steric parameter ν, and the Kier third-order steric shape index 3κα for the described substituents, and the calculated volume of the molecules as a whole), the 3-OMe and 3-CF3 groups appear to be the most bulky.
DAT does not readily accommodate large substituents at the aryl 4-position of the simple methcathinones as releasing agents;5 this seems to be echoed here by the findings with 3-position substituents (although no QSAR studies were conducted due to the constricted findings). Nevertheless, it would seem that DAT does not appreciate steric bulk on or around the 3- or 4-position of the simple methcathinone analogs as DAT releasing agents; that is, as bulk around the 3- or 4-position increases, DAT release potency decreases. This is not unreasonable considering that these are adjacent positions.
The abuse character of 4-substituted methcathinone analogs has been described;1,4 but, much less is known about the 2- and 3-substituted compounds shown in Table 1. 3-Chloromethcathinone (3a) has been found on the clandestine market,8 but its mechanism of action has not been investigated. Likewise, there have been seizures of 3-bromomethcathinone (3b).9 3-Bromomethcathinone (3b) acts as a reuptake inhibitor at NET and SERT (IC50 = 158 and 2,000 nM, respectively)10 at concentrations higher than those reported here for neurotransmitter release. Because releasing agents can behave as reuptake inhibitors at high concentrations,6 the present results suggest that 3b might produce its effects primarily as a releasing agent. 2-Methylmethcathinone (2c) and, especially, its positional isomer, 3-methylmethcathinone (3c), are fast becoming two of the most popular synthetic cathinones in Europe.11–15 Compound 3c has been reported to be a reuptake inhibitor at DAT, NET, and SERT (IC50 = 2,600, 270, and 9,500 nM, respectively)14 at concentrations well above those shown here to serve as a releasing agent at all three transporters.
In conclusion, 2-substituted methcathinone analogs are not well tolerated by DAT, NET, and SERT as determined by their reduced potency as releasing agents; however, the 3-substituted analogs are nearly equivalent in potency to their 4-substituted counterparts as DAT and NET releasing agents, but somewhat less potent at SERT. As a consequence, the 3-substituted analogs are slightly more DAT selective than the corresponding 4-substituted compounds. Nevertheless, none is as selective for DAT/NET versus SERT as is methcathinone (1) itself. Also, there is no discernable difference in DAT and NET release potencies suggesting a similar SAR for the simple methcathinone analogs at these two transporters. A final, yet unintended, consequence of the present investigation was to shed light on the transporter actions of some newer agents currently found on the clandestine market.
METHODS
Synthesis.
A Thomas-Hoover melting point apparatus was used for the determination of melting points and the values are uncorrected. A Bruker ARX 400 MHz spectrometer was used for recording 1H NMR spectra with tetramethylsilane (TMS) as an internal standard and IR spectra were obtained using a Thermo Nicolet iS10 FT-IR. Elemental analysis was performed by Atlantic Microlab Inc. (Norcross, GA) and observed values were within 0.4 % of theory. A CombiFlash Companion/TS (Telodyne Isco Inc., Lincoln, NE) was used for automated flash chromatography. Silica Gel (230–400 mesh) was used as adsorbent, and RediSep Rf Normal-phase Silica Flash Columns (Teledyne Isco Inc., Lincoln, NE) were used as the stationary phase. Thin-layer chromatography on silica gel GHLF plates (250 μ, 2.5 × 10 cm; Analtech Inc., Newark, DE) was used for monitoring reactions. Note: Although all compounds described here are methcathinone analogs, this section identifies them by their correct chemical names.
1-(3-Bromophenyl)-2-(methylamino)propan-1-one Hydrochloride (3b).
N-Benzyl-N-methylamine (0.6 mL, 5 mmol) was added at room temperature to a stirred solution of 6b16 (0.67 g, 2 mmol) in anhydrous THF (10 mL). The stirred reaction mixture was heated at reflux for 18 h, allowed to cool to room temperature, and solvent was removed under reduced pressure. The residue was dissolved in EtOAc (20 mL), washed with saturated NaHCO3 solution (5 mL), H2O (5 mL), brine (5 mL), dried (Na2SO4), and evaporated to dryness under reduced pressure to yield a crude product that was purified by column chromatography (silica gel; hexane/EtOAc; 95:5) to afford 0.28 g (36%) of 8b as a yellow oil. 1H NMR (CDCl3) δ 1.22 (d, 3H, CH3), 2.11 (s, 3H, CH3), 3.56 (s, 2H, CH2), 4.13 (q, 1H, CH), 7.16−7.26 (m, 6H, ArH), 7.59 (d, 1H, ArH), 7.82 (d, 1H, ArH), 8.09 (t, 1H, ArH).
1-Chloroethyl chloroformate (0.2 mL, 2.0 mmol) was added at room temperature to a stirred solution of 8b (0.28 g, 0.8 mmol) in anhydrous CH2Cl2 (5 mL). The stirred reaction mixture was heated at reflux for 2 h, cooled to room temperature, and concentrated under reduced pressure. The residue was dissolved in CH3OH (5 mL), heated at reflux for 1 h, cooled to room temperature, and evaporated to dryness under reduced pressure. The residue was stirred in Et2O for 18 h. The solid material was collected by filtration to yield a brown solid that was recrystallized from i-PrOH/Et2O to yield 0.09 g (39%) of 3b as a pale brown solid: mp 185 °C (lit.17 mp 178−181 °C, i-PrOH/Et2O). 1H NMR (DMSO-d6) δ 1.42 (d, 3H, CH3), 2.57 (s, 3H, CH3), 5.18 (d, 1H, CH), 7.57 (t, 1H, ArH), 7.95 (d, 1H, ArH), 8.02 (d, 1H, ArH), 8.19 (t, 1H, ArH), 9.16 (br s, 1H, NH), 9.62 (br s, 1H, NH+).
The following targets were prepared in the same manner as 3b.
1-(2-Chlorophenyl)-2-(methylamino)propan-1-one Hydrochloride (2a).
The product was obtained from bromopropiophenone 5a18 as a white solid in 35% yield after recrystallization from MeOH/EtOAc: mp 172−174 °C (lit.19 mp 159−161 °C). 1H NMR (DMSO-d6) δ 1.37 (d, 3H, CH3), 2.66 (s, 3H, CH3), 4.99 (q, 1H, CH), 7.55−7.59 (m, 1H, ArH), 7.66 (d, 2H, ArH), 7.90 (d, 1H, ArH), 9.36 (br s, 2H, NH2+); Due to the discrepancy with the literature melting point, 2a was submitted for elemental analysis. Anal. Calcd for (C10H12ClNO·HCl) C, 51.30; H, 5.60; N, 5.98. Found: C, 51.08; H, 5.51; N, 5.94.
1-(2-Bromophenyl)-2-(methylamino)propan-1-one Hydrochloride (2b).
The product was obtained from 5b20 as a white solid in 29% yield after recrystallization from MeOH/EtOAc: mp 172−174 °C; 1H NMR (DMSO-d6) δ 1.36 (d, 3H, CH3), 2.66 (s, 3H, CH3), 4.98 (q, 1H, CH), 7.54−7.62 (m, 2H, ArH), 7.82−7.84 (m, 1H, ArH), 7.88 (d, 1H, ArH), 9.44 (br s, 2H, NH2+); Anal. Calcd for (C10H12BrNO·HCl) C, 43.12; H, 4.70; N, 5.03. Found: C, 43.21; H, 4.84; N, 4.91.
2-(Methylamino)-1-(2-methylphenyl)propan-1-one Hydrochloride (2c).
The product was obtained from 5c18 as a white solid in 53% yield after recrystallization from MeOH/EtOAc: mp 160 °C (lit.21 mp 163−165 °C). 1H NMR (DMSO-d6) δ 1.36 (d, 3H, CH3), 2.44 (s, 3H, CH3), 2.62 (s, 3H, CH3), 5.06 (q, 1H, CH), 7.39−7.42 (m, 2H, ArH), 7.53−7.57 (m, 1H, ArH), 7.89−7.92 (m, 1H, ArH), 9.24 (br s, 1H, NH), 9.61 (br s, 1H, NH+).
1-(2-Methoxyphenyl)-2-(methylamino)propan-1-one Hydrochloride (2d).
The product was obtained from 5d22 as a white solid in 70% yield after recrystallization from MeOH/EtOAc: mp 184 °C (lit.22 mp 175−177 °C). 1H NMR (DMSO-d6) δ 1.40 (d, 3H, CH3), 2.60 (s, 3H, CH3), 3.96 (s, 3H, CH3), 4.83 (q, 1H, CH), 7.13 (t, 1H, ArH), 7.28 (d, 1H, ArH), 7.70 (t, 1H, ArH), 7.80 (d, 1H, ArH), 9.22 (br s, 2H, NH2+).
2-(Methylamino)-1-(3-methylphenyl)propan-1-one Hydrochloride (3c).
The product was obtained from 6c23 as a grey solid in 70% yield after recrystallization from MeOH/EtOAc: mp 193−195 °C (lit.21 mp 188−190 °C). 1H NMR (DMSO-d6) δ 1.42 (d, 3H, CH3), 2.39 (s, 3H, CH3), 2.57 (s, 3H, CH3), 5.13 (q, 1H, CH), 7.45−7.57 (m, 2H, ArH), 7.81−7.85 (m, 2H, ArH).
2-(Methylamino)-1-(3-methoxyphenyl)propan-1-one Hydrochloride (3d).
The product was obtained from 6d24 as a white solid in 70% yield after recrystallization from EtOH: mp 174−175 °C. 1H NMR (DMSO-d6) δ 1.42 (d, 3H, CH3), 2.57 (s, 3H, CH3), 3.83 (s, 3H, CH3), 5.17 (q, 1H, CH), 7.29–7.33 (m, 1H, ArH), 7.49–7.54 (m, 2H, ArH), 7.60–7.63 (m, 1H, ArH), 9.34 (br s, 2H, NH2+); Although the compound was previously reported,25 it was not characterized. Anal. Calcd for (C11H15NO2·HCl·0.7 H2O) C, 54.52; H, 7.24; N, 5.78. Found: C, 54.63; H, 7.20; N, 5.85.
2-(Methylamino)-1-(3-(trifluoromethyl)phenyl)propan-1-one Hydrochloride (3e).
The product was prepared from amine 8e21 and obtained as a yellow-white solid in 71% yield after recrystallization from MeOH/EtOAc: mp 171 °C (lit.7 mp 163.5−165 °C). 1H NMR (DMSO-d6) δ 1.54 (d, 3H, CH3), 2.70 (s, 3H, CH3), 5.38 (d, 1H, CH), 7.96 (t, 1H, ArH), 8.22 (d, 1H, ArH), 8.42−8.43 (m, 2H, ArH), 9.28 (br s, 1H, NH), 9.76 (br s, 1H, NH+).
In vitro release assay.
Assays were performed as previously described.6 Male Sprague-Dawley rats (Harlan Laboratories, Frederick, MD, USA) weighing 250–350 g were housed three per cage with free access to food and water and maintained on a 12- h light/dark cycle with lights on from 7:00 a.m. to 7:00 p.m. Animal facilities were accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, and procedures were carried out in accordance with the Institutional Animal Care and Use Committee and the National Institutes of Health guidelines on care and use of animal subjects in research (National Research Council.
Rats were euthanized by CO2 narcosis, and brains were processed to yield synaptosomes. Synaptosomes were prepared from striatum for DAT assays, whereas synaptosomes were prepared from whole brain minus striatum and cerebellum for the NET and SERT assays. [3H]1-Methyl-4-phenylpyridinium ([3H]MPP+) (9 nM) was used as the radiolabeled substrate for DAT and NET, whereas [3H]5-HT (5 nM) was used as a substrate for SERT. All buffers used in the release assays contained 1 μM reserpine to block vesicular uptake of substrates. The selectivity of assays was optimized for a single transporter by including unlabeled compounds to prevent the uptake of [3H]MPP+ or [3H]5-HT by competing transporters. Synaptosomes were preloaded with radiolabeled substrate in Krebs-phosphate buffer for 1 h (steady state). Assays were initiated by adding 850 μL of preloaded synaptosomes to 150 μL of test drug. Assays were terminated by vacuum filtration, and retained radioactivity was quantified by liquid scintillation counting. Effects of test drug concentrations were expressed as % Maximum Release, with maximum release (i.e., 100% Emax) defined as the release produced by 10 nM tyramine for DAT and NET assay conditions, and 100 nM tyramine for SERT assay conditions. These concentrations of tyramine evoke the efflux of all “releasable” tritium from synaptosomes as determined previously.26 EC50 values were determined using nonlinear least-squares curve fitting (GraphPad Prism, v. 6.0; GraphPad Scientific, San Diego, CA, USA).
Correlation analysis.
The correlation shown as Figure 1 was obtained using the linear regression analysis feature of GraphPad Prism, v. 7.01 (GraphPad Scientific, San Diego, CA, USA). EC50 values in Table 1 were converted to their pEC50 values prior to analysis.
Supplementary Material
Funding
This work was supported in part by DA033930 and by the National Institute on Drug Abuse Intramural Research Program Grant DA000523.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.Glennon RA Bath salts, mephedrone, and methylenedioxypyrovalerone as emerging illicit drugs that will need targeted therapeutic intervention. (2014) Adv. Pharmacol 69, 581–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumann MH, Glennon RA, and Wiley JL (Eds.), (2017) Neuropharmacology of New Psychoactive Substances (NPS); The Science Behind the Headlines. Springer International. Cham, Switzerland. [Google Scholar]
- 3.Glennon RA, and Dukat M (2017) Structure-activity relationships of synthetic cathinones. Curr. Top. Behav. Neurosci 32, 19–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonano JS, Banks ML, Kolanos R, Sakloth F, Barnier ML, Glennon RA, Cozzi NV, Partilla JS, Baumann MH, and Negus SS (2015) Quantitative structure-activity relationship analysis of the pharmacology of para-substituted methcathinone analogues. Br. J. Pharmacol 172, 2433–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakloth F, Kolanos R, Mosier PD, Bonano JS, Banks ML, Partilla JS, Baumann MH, Negus SS, and Glennon RA (2015) Steric parameters, molecular modeling and hydropathic interaction analysis of the pharmacology of para-substituted methcathinone analogues. Br. J. Pharmacol 172, 2210–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shalabi AR, Walther D, Baumann MH, and Glennon RA (2017) Deconstructed analogues of bupropion reveal structural requirements for transporter inhibition versus substrate-induced neurotransmitter release. ACS Chem. Neurosci 8, 1397–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt SD; Daley PF; Cozzi NV (2012). Analytical characterization of three trifluoromethyl-substituted methcathinone isomers. Drug Test. Anal 4, 525–529. [DOI] [PubMed] [Google Scholar]
- 8.Odoardi S, Romolo FS, and Strano-Rossi S (2016) A snapshot on NPS in Italy: Distribution of drugs in seized materials analysed in an Italian forensic laboratory in the period 2013–2015. Forensic Sci. Int 265, 116–120. [DOI] [PubMed] [Google Scholar]
- 9.Meyer MR, Vollmar C, Schwaninger AE, Wolf E, and Maurer HH (2012) New cathinone-derived designer drugs 3-bromomethcathinone and 3-fluoromethcathinone: studies on their metabolism in rat urine and human liver microsomes using GC-MS and LC-high-resolution MS and their detectability in urine. J. Mass. Spectrom 47, 253–362. [DOI] [PubMed] [Google Scholar]
- 10.Foley KF, and Cozzi NV (2003) Novel aminopropiophenones as potential antidepressants. Drug Dev. Res 60, 252–260. [Google Scholar]
- 11.Adamowicz P, Gieroń J, Gil D, Lechowicz W, Skulska., and Tokarczyk B (2016) The prevalence of new psychoactive substances in biological material – a three-year review of casework in Poland. Drug Test. Anal 8, 63–70. [DOI] [PubMed] [Google Scholar]
- 12.Romanek K, Stenzel J, Schmoll S, Schrettl V, Geith S, Eyer F, and Rabe C (2017) Synthetic cathinones in Southern Germany - characteristics of users, substance-patterns, co-ingestions, and complications. Clin. Toxicol. (Phila) 55, 573–578. [DOI] [PubMed] [Google Scholar]
- 13.European Drug Report (2017) European Monitoring Centre for Drugs and Drug Addiction. Publications Office of the European Union, Luxembourg. [Google Scholar]
- 14.Luethi D, Kolaczynska KE, Docci L, Krähenbühl S, Hoener MC, and Liechti ME, (2018) Pharmacological profile of mephedrone analogs and related new psychoactive substances. Neuropharmacology 134 (Part A), 4–12. [DOI] [PubMed] [Google Scholar]
- 15.Backburg M, Lindeman E, Beck O, and Helander A (2014) Characteristics of analytically confirmed 3-MMC-related intoxications from the Swedish STRIDA project. Clin. Toxicol 53, 46–53. [DOI] [PubMed] [Google Scholar]
- 16.Carroll FI, Blough BE, and Abraham P Preparation of Arylaminoketones as Monoamine Reuptake Inhibitors. WO 2010121022, October 21, 2010. [Google Scholar]
- 17.Foley KF and Cozzi NV (2003) Novel aminopropiophenones as potential antidepressants. Drug Dev. Res 60, 252–260. [Google Scholar]
- 18.Kalendra DM and Sickles BR (2003) Diminished reactivity of ortho-substituted phenacyl bromides toward nucleophilic displacement. J. Org. Chem 68, 1594–1596. [DOI] [PubMed] [Google Scholar]
- 19.Ose S, Takamatsu H, and Saheki T Alkanolamines. JP 36013215, November 30, 1960. [Google Scholar]
- 20.Elson LA, Gibson CS, and Johnson JDA (1930) o- and m-Derivatives of simple alkyl phenyl ketones. J. Chem. Soc 1128–1136. [Google Scholar]
- 21.Power JD, McGlynn P, Clarke K, McDermott SD, Kavanagh P, and O’Brien J (2011) The analysis of substituted cathinones. Part 1: chemical analysis of 2-, 3- and 4-methylmethcathinones. Forensic Sci. Int 212, 6–12. [DOI] [PubMed] [Google Scholar]
- 22.Heinzelmann RV (1953) Physiologically active secondary amines. β-(o-Methoxyphenyl)isopropyl-N-methylamine and related compounds. J. Am. Chem. Soc 75, 921–925. [Google Scholar]
- 23.Boss C, Brotschi C, Heidmann B, Sifferlen T, and Williams JT 3,8-Diazabicyclo[4.2.0]octane derivatives as orexin receptor antagonists and their preparation and use for the treatment of diseases. WO 2012085857, June 28, 2012. [Google Scholar]
- 24.Carroll FI, Blough BE, Mascarella SW, Navaroo HA, Eaton JB, Lukas RJ, and Damaj MI (2010) Synthesis and biological evaluation of bupropion analogues as potential pharmacotherapies for smoking cessation. J. Med. Chem 53, 2204–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Awad T, Clark CR, and DeRuiter J (2006) Chromatographic and mass spectral studies on methoxymethcathinones related to 3,4-methylenedioxymeth- amphetamine. J. Chromatogr. Sci 44, 155–161. [DOI] [PubMed] [Google Scholar]
- 26.Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, and Schindler CW (2013) Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology 38, 552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.