Abstract
Introduction: Surveillance for hepatocellular carcinoma (HCC) is recommended by national and international guidelines. However, there are no trial data on whether surveillance improves clinical outcomes in a UK cirrhosis population of mixed aetiology. Our aim was to determine the impact of, and adherence to, surveillance on overall survival. Methods: We prospectively collected data on consecutive patients diagnosed with HCC between January 2009 and December 2015 at two large UK centres. We assessed outcomes depending on whether they had been entered into an HCC surveillance programme, and if they had adhered to that. Results: Out of 985 patients diagnosed with HCC in this study, 40.0% had been enrolled in a surveillance programme. Of these, 76.6% were adherent with surveillance and 24.4% were not. Adherence to surveillance was significantly associated with improved overall survival, even when accounting for lead-time bias using different approaches (HR for 270 days lead-time adjustment 0.64, 0.53 to 0.76, p < 0.001). Conclusions: When adjusted for lead-time bias, HCC surveillance is associated with improved overall survival; however, the beneficial effect of surveillance on survival was lower than reported in studies that did not account fully for lead-time bias.
Keywords: HCC, hepatocellular carcinoma, cirrhosis, surveillance, cancer screening, lead-time
1. Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and remains a major public health burden worldwide [1,2]. Major increases have been reported in HCC incidence in the last 25 years, particularly in Europe and North America [3]. In the UK, there has been a 63% increase in incidence and 55% increase in mortality from HCC over the past decade [4,5], which is highest in Scotland [6]. Incidence rates for HCC are projected to rise by 38% (43% for males and 21% for females) in the UK by 2035, accompanied by a 58% rise in mortality [4].
Cirrhosis is the main risk factor for HCC, with up to a third of patients developing HCC. Patients with cirrhosis have a 1 to 5.8% risk per year of developing HCC [7], but regular surveillance may allow early detection and increase access to potentially curative therapies. Five-year survival rates for early stage HCC is more than 70%, compared with less than 5% when diagnosed at an advanced stage [8,9,10]. Most guidelines recommend surveillance is undertaken by 6-monthly ultrasound (US) scan, which has a sensitivity of 58–89% and specificity greater than 90%, and some centres also incorporate alpha-fetoprotein (AFP) measurement [11,12,13,14,15].
Lead-time bias greatly affects studies of interventions which aim to detect cancer early. When this bias arises, survival time is inflated, making an intervention appear to have a greater effect than it actually has [16]. Many non-randomised, observational studies have suggested a survival benefit from HCC surveillance [17,18], but did not investigate whether adherence to surveillance is associated with survival benefit. The aims of this study were to estimate the survival of patients diagnosed with HCC who had been entered into a surveillance programme, and to assess the effect of adherence to the programme on outcomes.
2. Materials and Methods
2.1. Study Design and Population
Consecutive patients who were diagnosed with HCC in a 7-year period from January 2009 to December 2015 were entered prospectively into this study. These patients were identified through regional multidisciplinary team meetings (MDTs) in Glasgow and Edinburgh, the two largest centres in Scotland. There are higher levels of socioeconomic deprivation in Glasgow than Edinburgh and we took this into account as described in our modelling process. Results are reported according to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) and Statistical Analyses and Methods in the Published Literature (SAMPL) guidelines [19,20]. To establish the denominator of patients who may have been eligible for inclusion in our study, we extracted the total number of patients with the ICD code ‘C22.0′ for Hepatocellular Carcinoma from the Scottish Cancer Registry.
2.2. Inclusion Criteria
The decision to enter a patient with known cirrhosis into a surveillance programme was made by the responsible Gastroenterologist or regional Hepatologist. The aetiology of underlying liver disease was determined by the patient’s history (including alcohol intake), clinical evaluation, laboratory parameters including viral and autoimmune serology, genetic testing where appropriate, imaging, and histology when available. The Barcelona Clinic Liver Cancer (BCLC) staging system was used to evaluate the HCC stage at diagnosis [21].
2.3. Outcomes
The primary outcome was overall survival, measured in years from diagnosis of HCC. Surviving patients were followed up until 1 August 2019.
2.4. Surveillance Adherence
A pragmatic approach for adherence with surveillance was adopted, with adherence defined as a US scan performed within a surveillance programme up to 9 months prior to HCC diagnosis. Based upon enrolment in the surveillance programme and adherence, patients were considered as never entered into surveillance (no surveillance group) or having been entered into surveillance. Within the group who were enrolled into surveillance, they were then considered as having been (1) adherent with surveillance or (2) not-adherent with surveillance.
2.5. Lead-Time Bias Estimation
Surveillance aims to detect lesions at a pre-symptomatic stage. Patients who have lesions detected by surveillance, therefore, have the additional survival time from when lesions are detected to when they become symptomatic and thus, will appear to have better survival (known as lead-time bias). Three separate approaches were used to account for the effects of lead-time bias on overall survival using:
2.5.1. Rate of Transition to Symptomatic Disease
This approach estimates the additional follow-up time observed purely as a result of lead-time in patients with a surveillance-detected cancer. Described by Duffy et al. [22], the rate of transition is from when a cancer is asymptomatic, but surveillance detectable to a cancer with symptoms is λ. Thus, the expected additional time, E(s), due to lead-time bias is given by:
| (1) |
where t is equal to the survival time from HCC diagnosis observed in a patient with a surveillance-detected cancer. We used data from studies that estimated the time taken for screen-detectable HCC to transition to symptomatic disease to be between 70 to 140 days [23,24]. We also tested this for the time specified as being non-adherent to surveillance (9 months) and for the most extreme values, we could find them in the literature for cirrhotic disease (1.57 years) and non-cirrhotic disease as the upper bound to provide a conservative estimate (2.66 years) [25].
2.5.2. Describing Lead-Time Using Tumour Size
Assuming surveillance detects tumours when they are smaller and asymptomatic, we used the maximum tumour diameter at HCC diagnosis, measured on cross-sectional imaging (either CT or MRI), to generate a scaled and centred variable describing lead-time. We then used a gamma distribution model to predict the scaled lead-time estimation for patients who were not in surveillance, based on the maximum size of their tumours and their BCLC stage (to account for disease spread in addition to just size alone). The gamma distribution was used as lead-times are always positive and have skewed distributions. This variable was then included in models as an interaction term with surveillance group, as the causal effect of surveillance is dependent on lead-time (i.e., patients with early asymptomatic lesions have much longer survival times, as a combination of long lead-times and being in surveillance). We performed this analysis in the Edinburgh cohort, as tumour size was only collected in this cohort.
2.5.3. Counterfactual Estimation
Given there are situations in the management of HCC where there is no anticancer therapy provided and no effective therapy for cirrhosis, we hypothesised that if we observed improved survival in the supportive care only group, the difference would be due to lead-time, rather than due to a survival benefit gained by being in surveillance. We compared the groups who received supportive care only and using a flexible parametric survival model, applied the differences to our study population. We term here the additional survival time for patients with a surveillance-detected cancer E(scf). Using these three approaches, we estimated plausible values for the effect of HCC surveillance on overall survival from the point of HCC diagnosis and the effect of surveillance adherence within these patient populations.
2.6. Data Handling and Statistical Analysis
Data were summarised using percentages and counts for categorical data, mean average where continuous data were normally distributed (presented with standard deviation—SD) and median average where continuous data did not fit the normal distribution (presented with 25th and 75th centiles). To test for baseline differences across summarised groups, we used the chi-square test (or Fisher’s exact where appropriate) for categorical data, Welch’s t-test for continuous normally distributed data, Wilcoxon signed rank test for non-normal continuous data, or the Kruskal–Wallis test for continuous data where there were more than two groups. To see whether we could identify predictors of entering and adhering to surveillance, multilevel regression modelling was used to account for variation observed across the centres and patient-level characteristics. Patient-level characteristics were modelled as fixed effects, with centre specified as a random effect. For these models, variables were selected on the basis they were clinically relevant and were unlikely to be confounded. Continuous variables were centred or separated into clinically relevant categories prior to inclusion in models. Final model selection was guided by the minimisation of the Akaike information criterion (AIC). Effects estimates are represented as odds ratios (OR) with the corresponding 95% confidence interval (95% CI).
Where missing data for predictor variables were present, multiple imputation by chained equations was planned to impute these data. Missing data rates were very low, and imputation was not required.
For survival analyses, as we were modifying the survival times based on our lead-time bias estimates, we used a flexible parametric model to estimate hazards at a given time. We specified centre as a random effect variable within this model to account for centre-level variation. Survival effect estimates are represented as Hazard Ratios (HR) with the corresponding 95% confidence interval. First order interactions were examined for each variable entered into every multivariable model and significant interactions retained.
Statistical significance was set at the level of p < 0.05. All statistical analysis was performed using R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) using the Tidyverse, flexsurv and finalfit packages.
3. Results
We included 985 patients diagnosed with HCC in the two centres between 1 January 2009 and 31 December 2015 inclusive. From the Scottish Cancer Registry, for the population covered by the two centres, there were a total number of 1117 incident cases over the same study time period, giving a case ascertainment rate of 88.18%. Of these 985 patients, 638 (64.8%) were from Centre 1 (Glasgow) and 347 (35.2%) were from Centre 2 (Edinburgh). Data are shown by centre in Supplementary Table S1).
Missing data in all fields were ≤1%; therefore, no imputation was required. During the study period between 2009 and 2015, the number of patients diagnosed annually with HCC rose from 96 to 182.
Table 1 shows the characteristics of the included patients; the median age of the patients was 69 years (25th centile—61; and 75th centile—77) and 789 (80.1%) patients were male.
Table 1.
Patient characteristics by surveillance adherence group.
| Total N | Missing N | Levels | No Surveillance | Not Adherent to Surveillance | Adherent to Surveillance | p | |
|---|---|---|---|---|---|---|---|
| Total N (%) | 591 (60.0) | 92 (9.3) | 302 (30.7) | ||||
| Age at diagnosis | 985 | 0 | Median (25th, 75th centile) | 73.0 (64.5 to 79) | 67.0 (60.8 to 73) | 64.0 (58 to 71) | <0.001 † |
| Sex | 985 | 0 | Female | 106 (17.9) | 22 (23.9) | 68 (22.5) | 0.161 |
| Male | 485 (82.1) | 70 (76.1) | 234 (77.5) | ||||
| Year of diagnosis | 985 | 0 | Median (25th, 75th centile) | 2012.0 (2011 to 2014) | 2012.0 (2011 to 2014) | 2013.0 (2011 to 2014) | 0.007 † |
| Child–Pugh Stage at diagnosis | 985 | 0 | No Cirrhosis | 142 (24.0) | 5 (5.4) | 10 (3.3) | <0.001 |
| A | 169 (28.6) | 32 (34.8) | 150 (49.7) | 0.023 | |||
| B | 209 (35.4) | 46 (50.0) | 114 (37.7) | ||||
| C | 71 (12.0) | 9 (9.8) | 28 (9.3) | ||||
| Alcoholic Liver Disease | 985 | 0 | No | 331 (56.0) | 40 (43.5) | 142 (47.0) | 0.009 |
| Yes | 260 (44.0) | 52 (56.5) | 160 (53.0) | ||||
| Viral hepatitis | 985 | 0 | No | 513 (86.8) | 68 (73.9) | 194 (64.2) | <0.001 |
| Yes | 78 (13.2) | 24 (26.1) | 108 (35.8) | ||||
| Non-alcoholic fatty liver disease | 985 | 0 | No | 447 (75.6) | 69 (75.0) | 236 (78.1) | 0.670 |
| Yes | 144 (24.4) | 23 (25.0) | 66 (21.9) | ||||
| Other | 985 | 0 | No | 387 (65.5) | 69 (75.0) | 250 (82.8) | <0.001 |
| Yes | 204 (34.5) | 23 (25.0) | 52 (17.2) |
All tests are chi-square, except when denoted by † where Kruskal–Wallis tests used. SD—Standard Deviation.
The most common aetiology for underlying liver disease was Alcoholic Liver Disease (47.9%), followed by Non-alcoholic Fatty Liver Disease (23.7%) and viral hepatitis (21.5%; see Table 1). There were no changes in the distribution of patient characteristics over time. Characteristics were similar across centres, with the exception of disease aetiology, where a greater proportion of patients in Centre 1 had ALD (53.0%, 338/638 in Centre 1 versus 38.6%, 134/347 in Centre 2); and for BCLC stage at HCC diagnosis, where Centre 1 had a lower proportion of patients with BCLC Stage 0/A disease (17.1%, 109/638 in Centre 1 versus 41.2%, 143/347 in Centre 2; Supplementary Table S2).
3.1. Enrolment and Adherence to Surveillance
Out of the 985 included patients, 591 (60.0%) had not been enrolled in HCC surveillance, 302 (30.7%) had been enrolled and were adherent with surveillance, and 92 (9.3%) had been enrolled but were not adherent with surveillance. The overall adherence for surveillance was 76.6% (302/394). Patients in the surveillance groups were younger than those not in surveillance, were more likely to have ALD, viral hepatitis or ‘other aetiology’ and had lower Child–Pugh stage at diagnosis of HCC (Table 1). Using multilevel modelling, female sex, younger age, the presence of alcoholic liver disease, viral hepatitis and NAFLD were associated with entry into surveillance (Table 2). We found no clinical characteristics predicted adherence to surveillance (Supplementary Table S3).
Table 2.
Model for surveillance group membership (entry to surveillance).
| Dependent: Entry to Surveillance | No Surveillance | Entered into Surveillance | OR (Univariable) | OR (Multilevel) | |
|---|---|---|---|---|---|
| Age at diagnosis | Mean (SD) | 70.9 (11.4) | 64.8 (9.7) | 0.95 (0.94–0.96, p < 0.001) | 0.97 (0.95–0.98, p < 0.001) |
| Sex | Female | 106 (54.1) | 90 (45.9) | - | - |
| Male | 485 (61.5) | 304 (38.5) | 0.74 (0.54–1.01, p = 0.059) | 0.60 (0.42–0.85, p = 0.004) | |
| Alcoholic Liver Disease | No | 331 (64.5) | 182 (35.5) | - | - |
| Yes | 260 (55.1) | 212 (44.9) | 1.48 (1.15–1.92, p = 0.003) | 1.75 (1.20–2.55, p = 0.004) | |
| Viral hepatitis | No | 513 (66.2) | 262 (33.8) | - | - |
| Yes | 78 (37.1) | 132 (62.9) | 3.31 (2.42–4.56, p < 0.001) | 2.35 (1.49–3.69, p < 0.001) | |
| Non-alcoholic fatty liver disease | No | 447 (59.4) | 305 (40.6) | - | - |
| Yes | 144 (61.8) | 89 (38.2) | 0.91 (0.67–1.22, p = 0.520) | 1.29 (0.85–1.94, p = 0.231) | |
| Other | No | 387 (54.8) | 319 (45.2) | - | - |
| Yes | 204 (73.1) | 75 (26.9) | 0.45 (0.33–0.60, p < 0.001) | 0.99 (0.62–1.57, p = 0.963) |
SD—Standard Deviation, OR—Odds Ratio.
3.2. Stage at Diagnosis
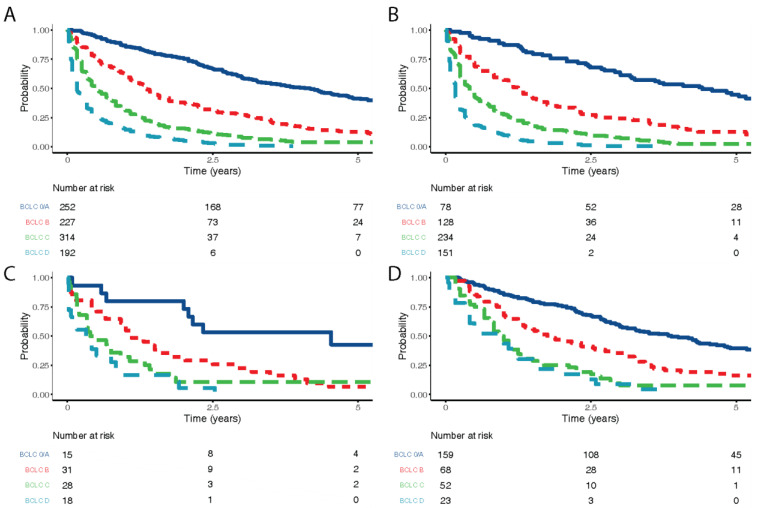
Over half the patients in the adherent with surveillance group (52.6%, 159/302) were diagnosed with BCLC stage 0/A disease, compared to 16.3% (15/92) in the not adherent group and 13.5% (78/591) in the no surveillance group (p < 0.001; Table 3). In both the non-adherent to surveillance and not under surveillance groups, disease at presentation was more advanced. Patients with higher BCLC stages had poorer overall survival, which was consistent across all surveillance groups (Figure 1).
Table 3.
Stage of disease and subsequent therapy by surveillance adherence group.
| Label | Total N | Missing N | Levels | No Surveillance | Not Adherent to Surveillance | Adherent to Surveillance | p |
|---|---|---|---|---|---|---|---|
| Total N (%) | 591 (60.0) | 92 (9.3) | 302 (30.7) | ||||
| BCLC Stage at diagnosis | 985 | 0 | 0/A | 78 (13.2) | 15 (16.3) | 159 (52.6) | <0.001 |
| B | 128 (21.7) | 31 (33.7) | 68 (22.5) | ||||
| C | 234 (39.6) | 28 (30.4) | 52 (17.2) | ||||
| D | 151 (25.5) | 18 (19.6) | 23 (7.6) | ||||
| AFP level | 941 | 44 | <100 | 299 (50.6) | 61 (66.3) | 241 (79.8) | <0.001 |
| >1000 | 165 (27.9) | 16 (17.4) | 16 (5.3) | ||||
| 100–1000 | 87 (14.7) | 10 (10.9) | 41 (13.6) | ||||
| Not known | 5 (0.8) | 0 (0.0) | 0 (0.0) | ||||
| (Missing) | 35 (5.9) | 5 (5.4) | 4 (1.3) | ||||
| Treatment | 983 | 2 | Liver resection | 36 (6.1) | 4 (4.3) | 24 (7.9) | <0.001 |
| Liver transplant | 8 (1.4) | 3 (3.3) | 45 (14.9) | ||||
| Ablative therapies | 20 (3.4) | 9 (9.8) | 43 (14.2) | ||||
| Sorafenib | 22 (3.7) | 2 (2.2) | 7 (2.3) | ||||
| Supportive care only | 382 (64.6) | 47 (51.1) | 85 (28.1) | ||||
| TACE | 122 (20.6) | 27 (29.3) | 97 (32.1) | ||||
| (Missing) | 1 (0.2) | 0 (0.0) | 1 (0.3) | ||||
| Treatment type | 983 | 2 | Curative therapy | 64 (10.8) | 16 (17.4) | 112 (37.1) | <0.001 |
| Palliative therapy | 144 (24.4) | 29 (31.5) | 104 (34.4) | ||||
| Supportive care only | 382 (64.6) | 47 (51.1) | 85 (28.1) | ||||
| (Missing) | 1 (0.2) | 0 (0.0) | 1 (0.3) | ||||
| Treatment intent | 983 | 2 | Curative | 64 (10.8) | 16 (17.4) | 112 (37.1) | <0.001 |
| Palliative | 526 (89.0) | 76 (82.6) | 189 (62.6) | ||||
| (Missing) | 1 (0.2) | 0 (0.0) | 1 (0.3) |
AFP—Alpha-fetoprotein, BCLC—Barcelona Clinic Liver Cancer, TACE—Transarterial Chemoembolisation. All tests are chi-square.
Figure 1.
Overall survival by BCLC stage. (A) For all patients included in the study, (B) for patients never entered into surveillance, (C) overall survival by BCLC stage for those not adherent to surveillance, (D) overall survival for those adherent with surveillance.
3.3. Treatment Intent
Patients adherent to surveillance were most likely to receive potentially curative treatments (37%), compared with those not adherent (17.4%) and those not in surveillance (10.8%) p < 0.001 (Table 3). When adjusted for potential confounders using a multilevel logistic regression model (Table 4), surveillance remained significantly associated with receipt of curative therapy. When BCLC stage was included as an interaction term due to its impact on receipt of curative therapy, adherence to surveillance remained significantly associated with the use of curative therapies, regardless of BCLC stage (Table 4).
Table 4.
Model for receiving potentially curative therapy.
| Dependent: Treatment Intent | Palliative | Curative | OR (Univariable) | OR (Multilevel) | |
|---|---|---|---|---|---|
| Surveillance group | No surveillance/not compliant | 602 (88.3) | 80 (11.7) | - | - |
| Adherent to surveillance | 189 (62.8) | 112 (37.2) | 0.73 (0.43–1.24, p = 0.249) | 0.67 (0.38–1.20, p = 0.182) | |
| BCLC Stage at diagnosis | 0/A | 98 (39.0) | 153 (61.0) | - | - |
| B | 210 (92.9) | 16 (7.1) | 0.02 (0.01–0.05, p < 0.001) | 0.02 (0.01–0.06, p < 0.001) | |
| C | 296 (94.3) | 18 (5.7) | 0.02 (0.01–0.05, p < 0.001) | 0.02 (0.01–0.05, p < 0.001) | |
| D | 187 (97.4) | 5 (2.6) | 0.00 (0.00–0.01, p < 0.001) | 0.00 (0.00–0.02, p < 0.001) | |
| Age at diagnosis | Median (25th to 75th centile) | 71 (63 to 78) | 71 (63 to 78) | 0.95 (0.93–0.96, p < 0.001) | 0.95 (0.92–0.97, p < 0.001) |
| Sex | Female | 153 (78.1) | 43 (21.9) | - | - |
| Male | 638 (81.1) | 149 (18.9) | 0.83 (0.57–1.23, p = 0.343) | 1.06 (0.62–1.80, p = 0.829) | |
| Alcoholic Liver Disease | No | 406 (79.3) | 106 (20.7) | - | - |
| Yes | 385 (81.7) | 86 (18.3) | 0.86 (0.62–1.17, p = 0.334) | 0.69 (0.40–1.18, p = 0.173) | |
| Child–Pugh Stage | No Cirrhosis | 130 (83.3) | 26 (16.7) | - | - |
| A | 243 (69.4) | 107 (30.6) | 2.20 (1.38–3.61, p = 0.001) | 0.66 (0.31–1.37, p = 0.264) | |
| B | 319 (86.4) | 50 (13.6) | 0.78 (0.47–1.33, p = 0.354) | 0.42 (0.19–0.92, p = 0.029) | |
| C | 99 (91.7) | 9 (8.3) | 0.45 (0.19–0.98, p = 0.054) | 0.78 (0.19–3.31, p = 0.739) | |
| Viral hepatitis | No | 648 (83.8) | 125 (16.2) | - | - |
| Yes | 143 (68.1) | 67 (31.9) | 2.43 (1.71–3.43, p < 0.001) | 0.96 (0.50–1.88, p = 0.913) | |
| Non-alcoholic fatty liver disease | No | 608 (81.1) | 142 (18.9) | - | - |
| Yes | 183 (78.5) | 50 (21.5) | 1.17 (0.81–1.67, p = 0.396) | 1.41 (0.75–2.64, p = 0.288) | |
| Other | No | 556 (78.9) | 149 (21.1) | - | - |
| Yes | 235 (84.5) | 43 (15.5) | 0.68 (0.47–0.98, p = 0.044) | 1.31 (0.64–2.67, p = 0.459) | |
| Adherent to surveillance: BCLC B | Interaction | - | - | 4.50 (1.41–14.81, p = 0.011) | 4.63 (1.41–15.23, p = 0.012) |
| Adherent to surveillance: BCLC C | Interaction | - | - | 4.85 (1.51–14.90, p = 0.006) | 4.46 (1.35–14.72, p = 0.014) |
| Adherent to surveillance: BCLC D | Interaction | - | - | 48.37 (6.26–1006.51, p = 0.001) | 47.55 (4.40–513.48, p = 0.001) |
BCLC—Barcelona Clinic Liver Cancer, SD—Standard Deviation, OR —Odds Ratio. Curative therapies are Surgery and Transplant.
3.4. Survival
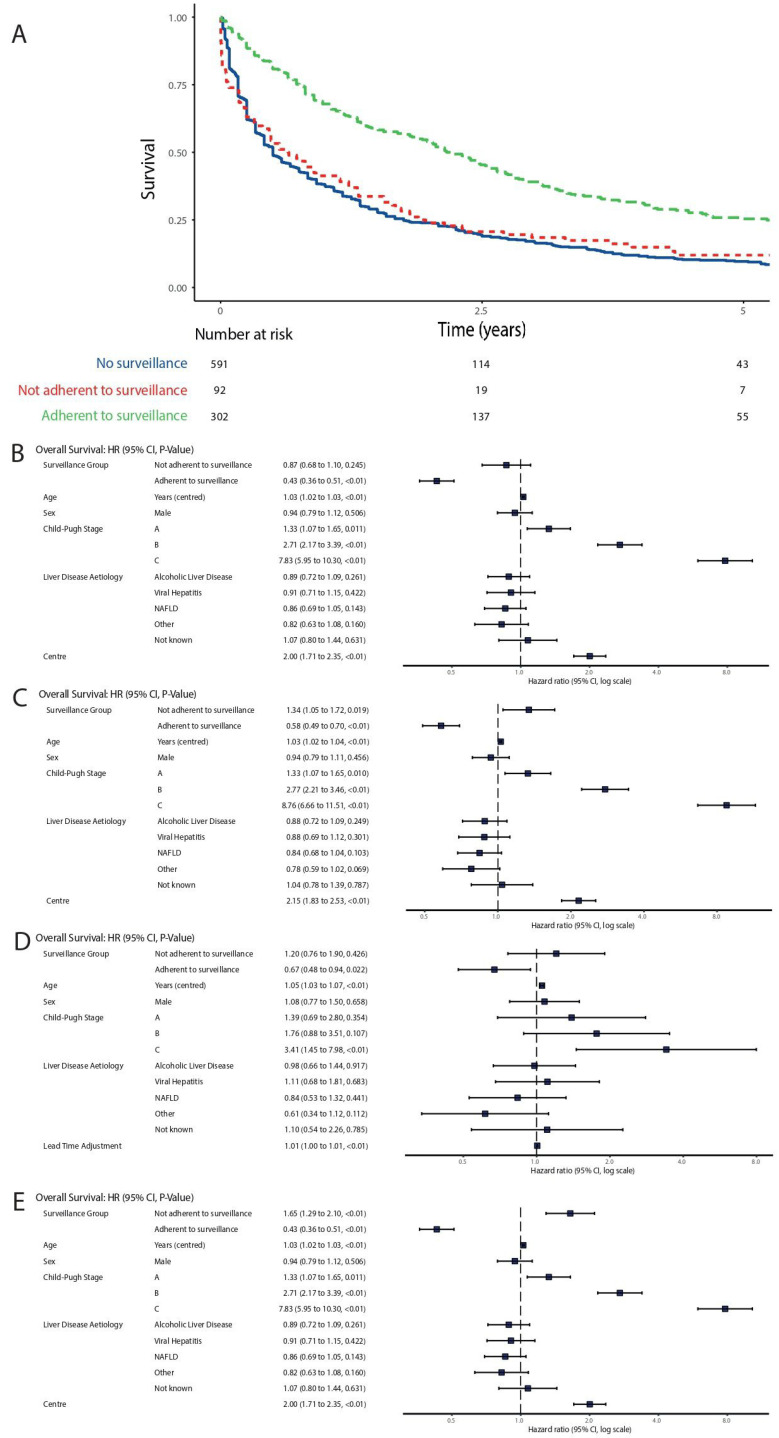
Median overall survival was 6.0 months in the no surveillance group (25th centile 2.0 months, 75th centile 21.0 months), 10.1 months in the not adherent with surveillance group (25th centile 2.4 months, 75th centile 25.2 months) and 28.7 months in the adherent with surveillance group (25th centile 11.4 months, 75th centile 52.0 months). At the time of data collection, a total of 1881 years of follow-up had been accrued by all patients in the study. When lead-time bias was not accounted for, adherence with surveillance was associated with longer survival (Figure 2A). When adjusted for explanatory variables, this association persisted (Figure 2B).
Figure 2.
Corrections for lead-time bias. (A) Overall survival by surveillance adherence, without adjustment for lead-time, (B) flexible parametric survival model for overall survival with no adjustment for lead-time bias, (C) flexible parametric survival model for overall survival, adjusted for lead-time bias using rate of transition to symptomatic disease method (270 days), (D) flexible parametric survival model for overall survival, adjusted for lead-time bias using tumour size method, (E) flexible parametric survival model for overall survival, adjusted for lead-time bias using counterfactual method.
Child–Pugh grade, increasing age and centre were the only other variables associated with survival, with cirrhosis of any grade associated with worse survival (Figure 2B and Table 4).
When using the transition to symptomatic disease method to account for lead-time bias, adherence to surveillance remained associated with a survival benefit (Figure 2C). As a sensitivity analysis, we entered further permutations of lead-time (Supplementary Figure S1, Kaplan–Meier for symptom transition method. A—70 days, B—140 days, C—270 days, D—1.57 years, E—2.66 years). For all permutations of transition time except 2.66 years (conservative estimate in methodology as above), a significant association between survival and adherence to surveillance remained. Patients who were not adherent to surveillance continued to have a similar survival to those who were not entered into surveillance, up to and including 140 days adjustment. When adjusted lead-time exceeded 140 days, we observed worse survival in the not adherent group, supporting the hypothesis that both lead-time and surveillance group contribute to the total observed effect of surveillance for HCC.
When we described lead-time as a variable in our model based on tumour size at presentation in the Edinburgh cohort, we found the beneficial effect of surveillance adherence on overall survival persisted independent of lead-time (Figure 2D). Patients with shorter lead-times/larger tumours at HCC diagnosis had shorter survival.
Finally, using the counterfactual approach, adherence to surveillance was not significantly associated with improved survival, but did not reverse the effect of surveillance (Figure 2E, Supplementary Figure S2).
3.5. Sensitivity Analysis
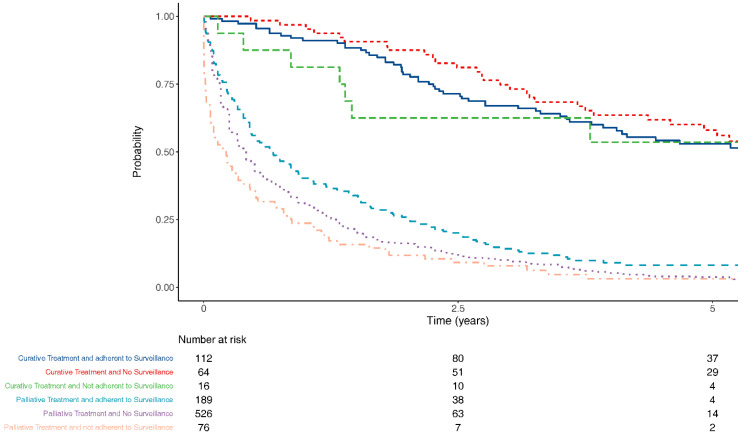
Patients in surveillance were significantly more likely to receive potentially curative therapies, even when lead-time bias was accounted for (Figure 3).
Figure 3.
Overall survival by surveillance group and treatment intent, 270 days lead-time bias accounted for.
4. Discussion
This study found that in a large patient population undergoing real world surveillance in the two largest Scottish regions, HCC surveillance was associated with earlier disease stage at presentation, increased use of therapy with curative intent, and improved overall survival from diagnosis. However, patients with poor adherence to surveillance had outcomes similar to those never entered into surveillance. The beneficial effect of surveillance on overall survival was lower than that reported in many other studies that did not fully consider lead-time bias.
There is a lack of high-quality, randomised evidence to clarify the best approach to HCC surveillance based either on outcomes or cost efficiency. Most of the evidence regarding the effectiveness of HCC surveillance is from observational studies and only two RCTs have been published, both from China in HBV infected populations [26,27]. One RCT used both USS and AFP for surveillance and reported a 37% reduction in mortality despite suboptimal adherence, whereas the other used AFP measurement only and reported no difference in survival. It is unclear whether the results can be extrapolated to a Western population with different aetiology of liver disease.
Adherence is a critical factor in determining the effectiveness of a given intervention. We found that patients with poor surveillance adherence had similar survival to patients with no surveillance. Indeed, our sensitivity analyses and adjustments for lead-time bias suggested this group may even have poorer survival. This could be an artefactual finding arising from our modelling techniques, or it could be that patients who are poorly adherent may have different behavioural or healthcare-seeking characteristics from the other groups [28]. The importance of adherence to surveillance should be a key component of the initial discussion with a patient who is being considered for entry into an HCC surveillance programme.
Our study has several strengths. Firstly, the study population was highly annotated consecutive patients diagnosed with HCC, with cirrhosis of various aetiologies, and included referrals from smaller local hospitals. Therefore, our study is likely to represent a real-world scenario. Much of the current evidence is from areas in the world where HBV and/or HCV are endemic. It is well described that there are differences in the clinical characteristics and genetic drivers of HCC arising on a background of viral liver disease compared with other aetiologies; therefore, our study helps address this area of uncertainty [29].
We also used a variety of statistical approaches to account for lead-time bias. Most studies of HCC surveillance rely on a single method using assumptions such as tumour volume doubling time, which may be unreliable given the heterogeneous nature of HCC tumours [30]. In addition, by analysis of data from the Scottish Cancer Registry, we confirmed that we managed to capture 88.18% of all HCC cases from our centres over the study period.
Our study has several limitations. The data originate from a prospectively collected observational database. We chose a pragmatic definition of adherence to surveillance defined as a US scan performed within 9 months prior to HCC diagnosis. However, this time period has been used by other studies in this field and takes into account potential organisational delays in coordinating US scans [31,32]. Furthermore, we did not collect detailed data on number of tumours, size of tumour at diagnosis or details on the presence portal hypertension. This was to make the study feasible by minimizing the number of variables to be collected. This meant that we grouped BCLC stage 0 and A together. Although this could be considered an important distinction, patients in both stages are eligible for curative therapy and are likely to have the best prognosis. Therefore, we believe this grouping is unlikely to have had a major effect on our findings.
Given our findings, controversy about the clinical and cost-effectiveness of HCC surveillance is likely to continue. Estimating the true effect of HCC surveillance on overall survival would ideally be carried out in a large RCT. However, this would be extremely challenging due to issues of patient acceptability, controversy around the clinical equipoise of surveillance, current guidelines recommending surveillance, and potential new developments in surveillance technologies. These difficulties were underlined when investigators tested the feasibility of conducting an RCT in 205 patients with liver cirrhosis and 99.5% of patients declined randomisation, with 88% choosing non-randomised surveillance [33]. The fact that only 37.1% patients adhering to surveillance in our study underwent curative therapy underlines the limitations of the surveillance investigations and methods that are currently recommended by most guidelines. This emphasises the importance of finding new and better surveillance tests that may include biomarkers and abbreviated MRI.
In conclusion, current evidence for HCC surveillance in a population with mixed causes of cirrhosis is confounded by lead-time bias. We have demonstrated that the effect size of HCC surveillance on overall survival is smaller than previously described. Despite this, adherence to surveillance is associated with earlier stage at HCC diagnosis, increased access to curative therapies, and increased survival. However, patients with poor adherence to surveillance have outcomes similar to patients never enrolled in surveillance, which is an important educational point to discuss with patients being entered into a surveillance programme.
Acknowledgments
Thomas G Bird was supported by a Wellcome Trust Intermediate Fellowship (WT/107492Z) for the course of this work. R Khasturi, D Kay, Ballantyne and K Slaven, Dept Radiology and Interventional Radiology, Gartnavel General Hospital, and Queen Elizabeth University Hospital Glasgow. H Ireland, Dept Radiology and Interventional Radiology, Royal Infirmary of Edinburgh. Anya Adair, E Harrison, James Powell and S Wigmore, Dept Surgery & Liver Unit, Royal Infirmary of Edinburgh.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10132770/s1, Figure S1: Kaplan–Meier for symptom transition method. A—70 days, B—140 days, C—270 days, D—1.57 years, E—2.66 years, Figure S2: Kaplan–Meier plot by surveillance adherence, with adjustment for counterfactual method, Table S1: Characteristics by Centre, Table S2: Stage of disease by centre and surveillance adherence, Table S3: Predictors of adherence to surveillance (including centre).
Author Contributions
Data curation, M.I.H., T.M.D., T.L.G. and A.A.; Formal analysis, A.J.S.; Investigation, S.B. (Stuart Ballantyne); Methodology, A.J.S.; Software, T.M.D. and T.L.G.; Supervision, A.J.S.; Visualization, A.J.S.; Writing—original draft, M.I.H. and T.M.D.; Writing—review and editing, M.I.H., T.M.D., E.F., S.B. (Stephen Barclay), R.G., M.P., J.E., J.G., D.C.M., P.C.H., T.G.B. and A.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by HUNTER. “T.G.B was funded by the Welcome Trust (WT107492Z) and a CRUK/AIRC Accelerator Award (A26813)”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Pineros M., Znaor A., Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer. 2018;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:194–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Liu Z., Jiang Y., Yuan H., Fang Q., Cai N., Suo C., Jin L., Zhang T., Chen X. The trends in incidence of primary liver cancer caused by specific etiologies: Results from the Global Burden of Disease Study 2016 and implications for liver cancer prevention. J. Hepatol. 2019;70:674–683. doi: 10.1016/j.jhep.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Research UK. [(accessed on 20 October 2018)]; Available online: www.cancerresearchuk.org/liver-cancer.
- 5.Information Services Division (ISD Scotland) [(accessed on 20 October 2018)]; Available online: www.isdscotland.org/Health-Topics/Cancer/Cancer-Statistics/Liver.
- 6.Leon D.A., McCambridge J. Liver cirrhosis mortality rates in Britain from 1950 to 2002: An analysis of routine data. Lancet. 2006;367:52–56. doi: 10.1016/S0140-6736(06)67924-5. [DOI] [PubMed] [Google Scholar]
- 7.Ioannou G.N., Splan M.F., Weiss N.S., McDonald G.B., Beretta L., Lee S.P. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2007;5:938–945. doi: 10.1016/j.cgh.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 8.El-Serag H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 9.Bruix J., Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forner A., Llovet J.M., Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 11.Ryder S. Guidelines for the diagnosis and treatment of hepatocellular carcinoma. Gut. 2003;52(Suppl. S3):iii1–iii8. doi: 10.1136/gut.52.suppl_3.iii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institute for Health and Care Excellence Hepatitis B (Chronic): Diagnosis and management. (NICE Guideline 165) [(accessed on 26 May 2021)];2013 Available online: https://www.nice.org.uk/guidance/cg165.
- 13.National Institute for Health and Care Excellence Cirrhosis in over 16s: Assessment and Management. (NICE Guideline 50) [(accessed on 26 May 2021)];2016 Available online: https://www.nice.org.uk/guidance/ng50. [PubMed]
- 14.European Association for the Study of the Liver. European Organisation for Research and Treatment of Cancer EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Heimbach J.K., Kulik L.M., Finn R., Sirlin C.B., Abecassis M., Roberts L.R., Zhu A.X., Murad M.H., Marrero J.A. AASLD guidelines for the treatment of HCC. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 16.Cucchetti A., Trevisani F., Pecorelli A., Erroi V., Farinati F., Ciccarese F., Rapaccini G.L., di Marco M., Caturelli E., Giannini E.G., et al. Estimation of lead-time bias and its impact on the outcome of surveillance for the early diagnosis of hepatocellular carcinoma. J. Hepatol. 2014;61:333–341. doi: 10.1016/j.jhep.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 17.Singal A.G., Pillai A., Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: A meta-analysis. PLoS Med. 2014;11:e1001624. doi: 10.1371/journal.pmed.1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelizzaro F., Vitale A., Sartori A., Vieno A., Penzo B., Russo F.P., Frigo A.C., Giannini E.G., Piccinnu M., Rapaccini G.L., et al. Surveillance as determinant of long-term survival in non-transplanted hepatocellular carcinoma patients. Cancers. 2021;13:897. doi: 10.3390/cancers13040897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P., Initiative F.T.S. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007;4:e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang T.A., Altman D.G. Basic statistical reporting for articles published in biomedical journals: The “Statistical Analyses and Methods in the Published Literature” or the SAMPL Guidelines. Int. J. Nurs. Stud. 2015;52:5–9. doi: 10.1016/j.ijnurstu.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Forner A., Reig M., Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 22.Duffy S.W., Agbaje O., Tabar L., Vitak B., Bjurstam N., Björneld L., Myles J.P., Warwick J. Overdiagnosis and overtreatment of breast cancer: Estimates of overdiagnosis from two trials of mammographic screening for breast cancer. Breast Cancer Res. 2005;7:258–265. doi: 10.1186/bcr1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartolomeo N., Trerotoli P., Serio G. Progression of liver cirrhosis to HCC: An application of hidden Markov model. BMC Med. Res. Methodol. 2011;11:38. doi: 10.1186/1471-2288-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thein H.-H., Campitelli M.A., Yeung L.T., Zaheen A., Yoshida E.M., Earle C.C. Improved survival in patients with viral hepatitis-induced hepatocellular carcinoma undergoing recommended abdominal ultrasound surveillance in ontario: A population-based retrospective cohort study. PLoS ONE. 2015;10:e0138907. doi: 10.1371/journal.pone.0138907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen T.H.H., Chen C.J., Yen M.F., Lu S.-N., Sun C.-A., Huang G.-T., Yang P.-M., Lee H.-S., Duffy S.W. Ultrasound screening and risk factors for death from hepatocellular carcinoma in a high risk group in Taiwan. Int. J. Cancer. 2002;98:257–261. doi: 10.1002/ijc.10122. [DOI] [PubMed] [Google Scholar]
- 26.Zhang B.H., Yang B.H., Tang Z.Y. Randomized controlled trial of screening for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2004;130:417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PubMed] [Google Scholar]
- 27.Chen J.G., Parkin D.M., Chen Q.G., Lu J.-H., Shen Q.-J., Zhang B.-C., Zhu Y.-R. Screening for liver cancer: Results of a randomised controlled trial in Qidong, China. J. Med. Screen. 2003;10:204–209. doi: 10.1258/096914103771773320. [DOI] [PubMed] [Google Scholar]
- 28.Von Wagner C., Verstraete W., Stoffel S. Oxford Research Encyclopedias. Oxford University Press; Oxford, UK: 2019. [(accessed on 26 May 2021)]. Psychological aspects of cancer screening. Available online: https://oxfordre.com/psychology/view/ [Google Scholar]
- 29.Hashimoto E., Taniai M., Kaneda H., Tokushige K., Hasegawa K., Okuda H., Shiratori K., Takasaki K. Comparison of hepatocellular carcinoma patients with alcoholic liver disease and non-alcoholic steatohepatitis. Alcohol. Clin. Exp. Res. 2004;28:164S–168S. doi: 10.1111/j.1530-0277.2004.tb03237.x. [DOI] [PubMed] [Google Scholar]
- 30.Kansagara D., Papak J., Pasha A.S., O’Neil M., Freeman M., Relevo R., Quiñones A., Motu’Apuaka M., Jou J.H. Screening for hepatocellular carcinoma in chronic liver disease: A systematic review. Ann. Intern. Med. 2014;161:261–269. doi: 10.7326/M14-0558. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed M.H.A., Yang J.D., Giama N.H., Choi J., Ali H.M., Mara K.C., Harmsen W.S., Wiesner R.H., Leise M.D., Therneau T.M., et al. Factors influencing surveillance for hepatocellular carcinoma in patients with liver cirrhosis. Liver Cancer. 2017;6:126–136. doi: 10.1159/000450833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Meer S., de Man R.A., Coenraad M.J., Sprengers D., van Nieuwkerk K.M.J., Klümpen H.J., Jansen P.L.M., Jzermans j.M.I., van Oijen M.G.H., Siersema P.D., et al. Surveillance for hepatocellular carcinoma is associated with increased survival: Results from a large cohort in the Netherlands. J. Hepatol. 2015;63:1156–1163. doi: 10.1016/j.jhep.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Poustchi H., Farrell G.C., Strasser S.I., Lee A.U., McCaughan G.W., George J. Feasibility of conducting a randomized control trial for liver cancer screening: Is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology. 2011;54:1998–2004. doi: 10.1002/hep.24581. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable.