Abstract
Poor water solubility, emulsifying, and foaming properties of gluten protein have limited its applications. Gluten is structured by covalent (disulfide bonds) and noncovalent bonds (hydrogen bonds, ionic bonds, hydrophobic bonds) which prone to alteration by various treatments. Enzyme modification has the ability to alter certain properties of gluten and compensate the deficiencies in gluten network. By hydrolyzing mechanisms and softening effects, hydrolytic enzymes affect gluten directly and indirectly and improve dough quality. The present review investigates the effects of some hydrolytic enzymes (protease and peptidase, alcalase, xylanase, pentosanase, and cellulase) on the rheological, functional, conformational, and nutritional features of gluten and dough. Overall, protease, peptidase, and alcalase directly affect peptide bonds in gluten. In contrast, arabinoxylan, pentosan, and cellulose are affected, respectively, by xylanase, pentosanase, and cellulase which indirectly affect gluten proteins. The changes in gluten structure by enzyme treatment allow gluten for being used in variety of purposes in the food and nonfood industry.
Keywords: Enzymatic modification, Gluten, Physicochemical properties, Rheological properties
The present review investigates the effects of some hydrolytic enzymes (protease and peptidase, alcalase, xylanase, pentosanase, and cellulase) on the rheological, functional, conformational, and nutritional features of gluten and dough. Overall, protease, peptidase, and alcalase directly affect peptide bonds in gluten. In contrast, arabinoxylan, pentosan, and cellulose are affected, respectively, by xylanase, pentosanase, and cellulase which indirectly affect gluten proteins. The changes in gluten structure by enzyme treatment allow gluten for being used in variety of purposes in the food and nonfood industry.
Abbreviations
- CD
celiac disease
- DH
degree of hydrolysis
- DSC
differential scanning calorimetry
- FDA
food and drug administration
- FTIR
Fourier transforms infrared spectroscopy
- GRAS
generally recognized as safe
- HMW‐GS
high molecular weight‐glutenin subunits
- LAB
lactic acid bacteria
- LMW proteins
low molecular weight protein
- MW
molecular weight
- PEP
Prolyl endopeptidases
- Rmax
maximum resistance
- SDS‐PAGE
sodium dodecyl sulfate‐poly acryl amide gel electrophoresis
- SH
thiol group
- SS
disulfide bonds
- Tg
glass transition
1. INTRODUCTION
Storage proteins existing in wheat gluten structure cause the exclusive viscoelastic characteristics of wheat dough when gluten is hydrated (Delcour et al., 2012). As vital components in wheat endosperm, gluten proteins are well‐known for their use in bread formulation, determining its quality. According to Osborne fractionation procedures, gluten is classified into storage proteins that confer a viscoelastic behavior in bakery products. Composed of gliadins (soluble in 70% ethanol) and glutenins (insoluble in 70% ethanol), gluten proteins are almost insoluble in water.
Glutenin subunits with size range from about 500,000 to more than 10 million (g/mol) are comprised of aggregated proteins formed by SS bonds and are among the largest proteins in nature. The glutenin subunit is categorized into two types, namely LMW‐GS (30,000 to 45,000) and HMW‐GS (70,000 to 90,000) (Abedi & Pourmohammadi, 2020a, 2020b,2020a, 2020b). Gliadins (prolamins), with a monomeric structure, are categorized into four classes, namely α, β, γ, and ω gliadins (Abedi & Pourmohammadi, 2021a). The relative molecular weights of α, β gliadins are about 30,000–40,000 g/mol, while, due to the existence of notable amount of sulfur amino acids, γ gliadins have higher molecular weight than α, β gliadins. In contrast, the ω‐gliadins with size range from 44,000 to 80,000 (g/mol) contain considerable amounts of glutamine/glutamic acid, proline, and phenylalanine and are completely deficient in sulfur amino acids (Majzoobi, et al., 2012). Therefore, the low gluten solubility is due to the small number of ionizable amino acids and high amounts of glutamine, proline, and glycine in gluten construction.
Despite the myriad interesting functionalities of wheat glutens, this protein has poor water solubility, emulsifying, and foaming properties which have limited its applications. There are various methods for improving the functionality of glutenin and gliadin by modifying their structure. As a biotechnological treatment, enzyme modification is one of the methods which extends gluten applications.
There are numerous limitations in using flours with strong gluten networks; therefore, the hydrolyzing effects of enzymes allow to use these flours in various purposes. Improvements in bread properties obtained by the addition of hydrolytic enzymes have been associated with their impact on the physical properties of the dough during processing. One of the major functions of hydrolytic enzyme addition is to soften dough to improve machining properties and thus enhance bread quality (Figure 1; Harada et al., 2000; Pourmohammadi & Abedi, 2021; Yong et al., 2006).
FIGURE 1.
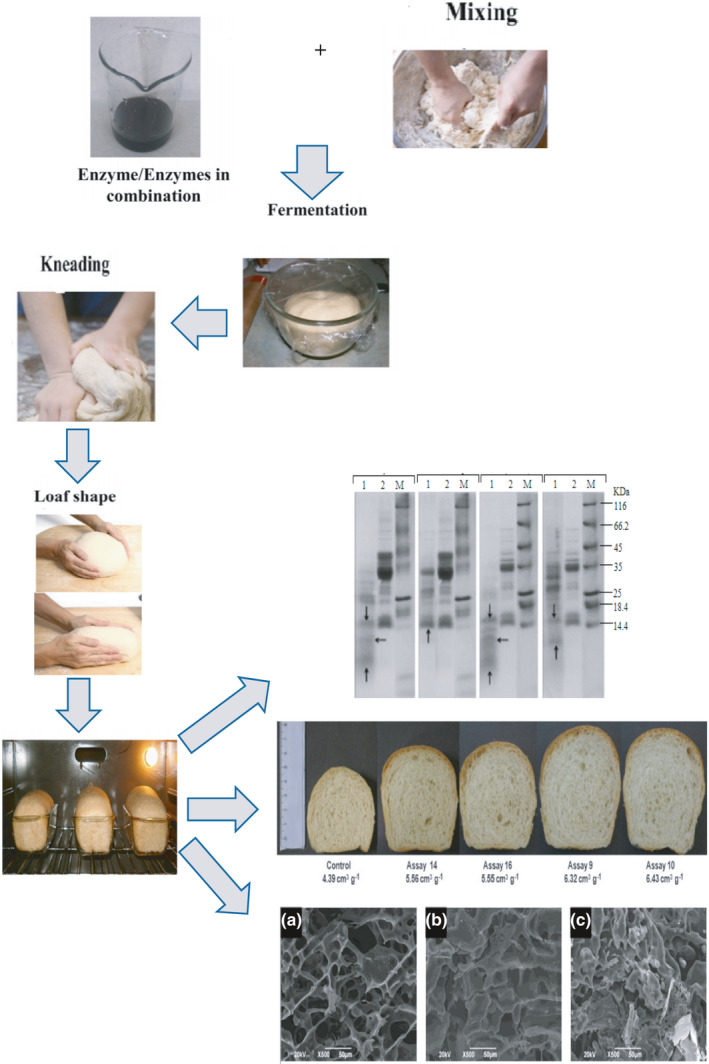
Application of hydrolytic enzymes on dough structure
The present review investigates the modes of action of some hydrolyzing enzymes and their effects on the functional, rheological, conformational, and nutritional characteristics of gluten and dough.
1.1. Protease and peptidase
Wheat gluten hydrolysis to smaller peptides and free amino acids with more hydrophilic polypeptide (Wang et al., 2016; Zhou et al., 2017; Hwang et al., 2016) normally is carried out via peptidase and protease (reaction 1). Hydrolyzing treatment: 1) enhance functional features (solubility, foaming, and emulsifying capacity) (Wang et al., 2016; Wouters et al., 2016; Wouters, Fierens, et al., 2017; Kammoun et al., 2003); 2) improve the safety and nutritional values of gluten protein by reducing the allergenic potential of wheat gluten which cause celiac disease (Elmalimadi et al., 2017; Henggeler et al., 2017; Merz et al., 2015; Merz, Appel, et al., 2016); 3) improve dough handling through managing the viscoelasticity of gluten network and modifying dough rheology; 4) improve the antioxidant activity of hydrolyzed gluten (Abedi & Pourmohammadi, 2020a; Pourmohammadi & Abedi, 2021). The antioxidant properties are the ability to hinder linoleic acid peroxidation inhibition or put out the DPPH (2,2‐diphenyl‐1‐picrylhydrazyl), ABTS (2,2‐azino‐bis (3‐ethylbenzothiazoline‐6‐sulfonic acid)), or other radicals (Elmalimadi et al., 2017; Elmalimadi, 2018; Jin et al., 2016; Wang et al., 2016). In addition, protein hydrolysates are extensively utilized as functional ingredients in food and chemical industries. Some studies, on the other hand, showed that proteolytic enzymes had undesirable impacts on the volume of bread because of the disruption in gluten matrix, particularly glutenin subunits (Kolpakova et al., 2014). Moreover, the combination of hydrolyzing enzymes with different treatments and additives has considerable effects on gluten properties (Table 1, 2).
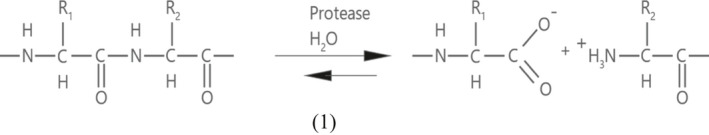
TABLE 1.
Effects of different treatments in combination with hydrolyzing enzymes on gluten properties
| Treatment | Effect | Mechanism | Ref. |
|---|---|---|---|
| Heat treatment +papain | Reduces the free SH in wheat gluten proteins | Makes the structure of wheat gluten more compact | (Wang et al., 2009) |
| Heat treatment +alcalase | Increases the rate of hydrolysis | Improves gluten susceptibility to alcalase owing to the rearrangements of the inter‐ and intramolecular binding | (Mohamed Bashir Elmalimadi, 2018; Saha et al., 2013) |
| Heat treatment +alcalase | Enhances the emulsifying properties of gluten | Exposes the hydrophobic protein interior, improving the adsorption at the interface, forming a cohesive interfacial film with the hydrophobic residues | (Mohamed Bashir Elmalimadi, 2018; Phillips & Beuchat, 1981) s |
| Heat treatment +alcalase | Improves the foaming characteristics | Increases polypeptide chains arising from partial proteolysis, incorporating more air | (Mohamed Bashir Elmalimadi, 2018; Kong et al., 2007a; Wouters et al., 2016) |
| Heat treatment +alcalase | Increases solubility and water‐holding capacity | Augments cleavable peptide bonds and increases the number of exposed ionizable amino and carboxyl groups | (Mohamed Bashir Elmalimadi, 2018; Hardt et al., 2013) |
| Heat treatment +alcalase | Reduces the binding affinity to fat | Hydrolytic degradation of the protein structure | (Mohamed Bashir Elmalimadi, 2018) |
| Heat treatment +alcalase | Increases the DPPH radical scavenging activity (antioxidant ability) | Opens and exposes active amino acid residues, which could react with oxidants or reactive oxygen | (Mohamed Bashir Elmalimadi, 2018; Koo et al., 2014) |
| Agitation +alcalase | Ameliorates the efficiency of gluten hydrolysis | Reduces the particle size and increases the surface area | (Mohamed Bashir Elmalimadi, 2018) |
| Temperature (50 0C) + pH 9 + gluten +alcalase | Improves gluten solubility | ||
| Enhances foaming stability of gluten | Reduces the molecular weight and hydrophobicity of wheat protein and increases the content of polar and ionizing groups | (Jakovetić et al., 2015) | |
| Pancreatin hydrolysis/ Extrusion | Enhances the enzymatic hydrolysis efficiency of wheat gluten | The conformational changes and structural rearrangements of wheat gluten treated with extrusion might modify the catalytic sites of proteases | (Cui, Gong, et al., 2013) |
TABLE 2.
Effects of different additives in combination with hydrolyzing enzymes on gluten properties
| Compounds | Effect | Mechanism | Ref. |
|---|---|---|---|
| Starch +flavourzyme + protamex | |||
| starch granules impede gluten aggregation, which facilitates the hydrolysis | Hinders the gluten aggregation | (Hardt et al., 2015) | |
| Enzymatically hydrolyzed gluten +sucrose | Improves the stability and foaming capacity of hydrolyzed gluten | Increase the affinity of hydrolyzed gluten and adsorption at the water–air interface | (Wouters, Fierens, et al., 2017) |
| Ethanol +trypsin or pepsin | Increases the foaming capacity and reduces the foam stability of gluten | Alters the air–water interfacial behavior of gluten | (Wouters, Fierens, et al., 2017) |
| Cysteine +alcalase | Enhances gluten hydrolysis | Alters gluten viscoelastic behavior (varying from more solid‐like to more fluid‐like) and increases its solubility | (Zhang et al., 2012) |
1.2. Sources of proteases and peptidases for gluten hydrolyzation
Enzymes able to degrade gluten have been detected in various sources including plants (wheat, rye, barley), fungal (A. niger and A. flavus var. oryzae), bacteria (Flavobacterium meningosepticum, Sphingomonas capsulate, Pseudomonas aeruginosa, Myxococcus xanthus, Bacillus sp., Bifidobacterium sp., Lactobacillus sp., and Rothia mucilaginosa), and insects (Rhizopertha dominica) (Table 3).
TABLE 3.
Different sources of proteases and peptidases for gluten hydrolyzation
| Type of enzyme | Object | Approach | Ref. |
|---|---|---|---|
| Plant peptidases | |||
| Cysteine endopeptidase | Wheat gluten | Attack N and C‐terminal sites | (Savvateeva et al., 2015) |
| Cysteine peptidase | Wheat prolamin | Hydrolyzing wheat prolamin down to 5% | (Gänzle et al., 2008) |
| Cysteine peptidase | Rye prolamins | Degradation of 99.5% of rye prolamins | (Gänzle et al., 2008) |
| Triticain‐α | α‐, γ‐, ω‐gliadins and glutenins | Triticain‐α (EC 3.4.22) as a cysteine endopeptidase hydrolyze gluten peptides | (Savvateeva et al., 2015) |
| Endoprotease B, isoform 2 (EP‐B2) | Barley gluten | Degrade peptide bonds following glutamine, with proline often positioned at the P2 | (Savvateeva et al., 2015) |
| Caricain (EC 3.4.22.30), cysteine endopeptidases papain (EC 3.4.22.2), glutaminyl‐peptide cyclotransferase (EC 2.3.2.5), chymopapain (EC 3.4.22.6) | Wheat gliadin | Caricain is a gluten‐degrading enzyme of the most activity | (Buddrick et al., 2015) |
| Ginger protease | Wheat gluten | Production of a new type of wheat gluten hydrolysate | (Taga et al., 2017) |
| Fungal peptidases (Aspergillus flavus var. Oryzae) | |||
| DPP IV (EC 3.4.14.5) | Wheat gluten | Hydrolyze polypeptides and release N‐terminal dipeptide | (Merz et al., 2015) |
| Flavourzyme | Wheat gluten | The addition of flavourzyme to wheat gluten (25 g/L) lead to 9.5 mg residual gliadin/kg hydrolysate | (Eugster et al., 2015; Merz, Kettner, et al., 2016) |
| Fungal peptidases (Aspergillus niger) | |||
| Prolyl endopeptidase | α‐gliadins, γ‐gliadins, HMW‐GS, and LMW‐GS | Degrade CD‐active peptides along with intact α‐gliadins, γ‐gliadins, HMW‐GS, and LMW‐GS | (König et al., 2017; Stepniak et al., 2006) |
| Aspergillopepsin | Wheat gluten | gluten‐hydrolyzing reactions | (Ehren et al., 2009) |
| Fusarium graminearum proteases | Wheat gluten | These proteases were observed to be essentially serine proteases like trypsin cutting the proteins at the lysine or arginine residues | (Koga et al., 2019) |
| Fusarium graminearum proteases | Wheat gluten | Fusarium graminearum proteases reduce glutenin amount and increase gliadin | (Eggert et al., 2011) |
| Fusarium. poae | Wheat gliadin | Gliadin degradation | (Brzozowski et al., 2008) |
| Fungal peptidases (Fusarium graminearum) | |||
| Aspergillus usamii protease | Wheat gluten | Increasing the protein hydrolysates solubility resulting from its secondary structure destruction | (Deng et al., 2016) |
| Aspergillus usamii protease | Wheat gluten | The cleavage of peptides by enzyme and unfolding the globular structure of gluten were able to promote the cross‐linking between peptides—lipid, and contribute to anchoring the peptide molecules at the oil–water interface, improving the emulsifying properties and decreasing the interfacial tension | (Deng et al., 2017) |
| Aspergillus usamii protease | Wheat gluten | Increase the water‐holding capacity of wheat gluten/ increase in β‐sheet ratio | (Saberi et al., 2008) |
| Aspergillus usamii protease | Wheat gluten | Increase the oil holding capacity of wheat gluten/ hydrophobic regions, are more exposed to the aqueous phase | (Saberi et al., 2008) |
| Bacterial peptidases | |||
| Peptidase from B. subtilis and B. licheniformis | Wheat gluten | Hydrolyzing wheat gluten to a degree of 35%–38% | (Stressler et al., 2015) |
| Thermolysin (EC 3.4.24.27) from B. thermoproteolyticus, subtilisin (EC 3.4.21.62) from B. licheniformis | Wheat gliadin | Degradation of wheat gliadin to small residues with low molecular weights (<15,000) | (Socha et al., 2019) |
| Prolyl endopeptidase from Lb. brevis, Lb. alimentarius, Lb. hilgardii, Lb. sanfranciscensis | Wheat gliadin | Hydrolyzing gliadin fractions/the CD patients tolerated the resultant bread | (Saberi et al., 2008) |
| Dipeptidases (EC 3.4.13), including PepD and dipeptidyl‐ and tripeptidylpeptidases (EC 3.4.14) such as proline‐specific Xaa‐Pro dipeptidyl peptidase (PepX), metalloendopeptidases (EC 3.4.24) like PepO, PepF, and aminopeptidases (EC 3.4.11) such as PepN and PepC | Wheat gluten | Gluten degradation | (Taga et al., 2017) |
| Prolyl endopeptidase and peptidases from L. sanfranciscensis DSM20663, L. acidophilus 5e2 | ω‐gliadins and HMW‐glutenins | Degradation of ω‐gliadins and HMW‐glutenins | (Gänzle et al., 2008; Nionelli & Rizzello, 2016) |
| Insect peptidases | |||
| Serine‐type carboxypeptidase (EC 3.4.16)/ serine endopeptidases (EC 3.4.21) from Oryzaephilus surinamensis, Rhizopertha dominica, Tenebrio molitor, Alphitobius diapernius, T. confusum, and T. castaneum | Wheat gluten | Gluten degradation with postproline cleaving patterns | (Mika et al., 2015) |
| Endopeptidases from Pentadomidae and Lygaidae, Nysius huttoni | HMW‐GS | The cleavage of peptides by enzyme, unfolding the globular structure of gluten, promoting the cross‐linking between peptides—lipid, contributing to anchoring the peptide molecules at the oil–water interface, lead to improve emulsifying properties and the interfacial tension decrease | (Koksel, 2001) |
| Endopeptidases from Eurygaster Aelia, E. Maura, E. integriceps | Gliadin | Increase the water‐holding capacity of wheat gluten/ increase in β‐sheet ratio | (Sivri et al., 1998) |
| Endopeptidases from Eurygaster spp, Aelia spp, E. integriceps | Glutenin | Increase the oil holding capacity/ enzymatic hydrolysis cause hydrophobic regions, to be more exposed to the aqueous phase | (Yakovenko et al., 1973) |
| Bug proteolytic enzymes | Glutenin | Reduction in the certain bonds intensity and creation of two new bands in the electrophoretic patterns according to SDS‐PAGE | (Sivri et al., 1998; Yakovenko et al., 1973) |
1.2.1. Microbial peptidases
Microbial prolyl endopeptidases (PEPs) are endoproteolytic enzymes which are capable of degrading gluten proteins according to SDS‐PAGE analysis (Figure 2 A and B; Knorr et al., 2016). This could be obtained via fungal (A. niger, A. oryzae, A. usamii, F. graminearum) or bacterial enzymes (Flavobacterium meningosepticum (FM), Sphingomonas capsulata (SC), Pseudomonas aeruginosa (PA), Myxococcus xanthus (MX), Bacillus sp., Bifidobacterium sp., Lactobacillus sp., and Rothia mucilaginosa (RM)). Peptidases (EC 3.4) generally fall under the hydrolases (EC 3) category, which hydrolyze peptide bonds.
FIGURE 2.
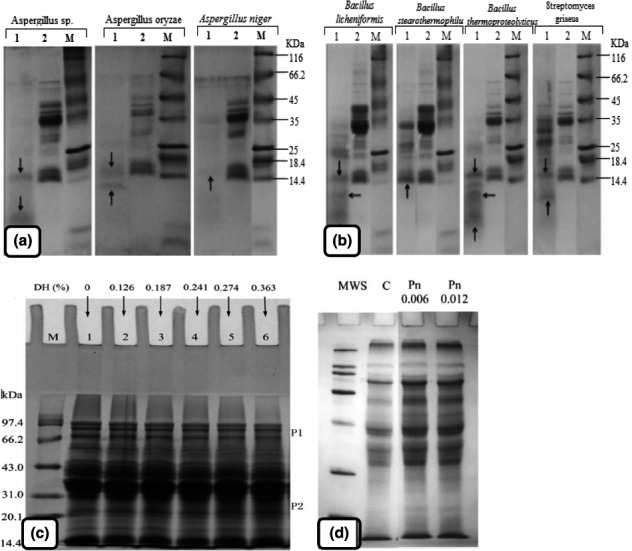
SDS‐PAGE after 60 min of proteolysis of gliadin using specific fungal proteases isolated from Aspergillus oryzae and Aspergillus niger. Lane 1: wheat gliadins treated using fungal proteases; lane 2: untreated wheat gliadins; M: molecular marker; vertical arrows indicate peptides after hydrolysis (A); SDS‐PAGE for gliadin treated with bacterial proteases isolated from Bacillus licheniformis, Bacillus stearothermophilus, Bacillus thermoproteolyticus, and Streptomyces griseus. Lane 1: wheat gliadins treated using bacterial proteases; lane 2: untreated wheat gliadins; M: molecular marker; vertical arrows indicate peptides after hydrolysis (B) (Socha et al., 2020); SDS–PAGE patterns of wheat gluten treated with alcalase. M = protein molecular weight marker; 1 = raw wheat gluten; 2–6 = 0.02%, 0.04%, 0.06%, 0.08%, and 0.10% Alcalase‐treated wheat gluten, respectively (C). Electrophoretic patterns of soluble dough treated with 0.006% and 0.012% of pentosanase (D) (Steffolani et al., 2010)
Fungal peptidases
A. niger, A. oryzae, A. usamii, and F. graminearum are extensively employed in food and feed processing, regarded GRAS by the U.S. FDA. Belonging to A. flavus var. oryzae, food‐grade dipeptidyl peptidase IV (DPPIV) (EC 3.4.14.5) is an exopeptidase releasing an N‐terminal dipeptide from polypeptides. Nevertheless, DPP IV alone is not able to effectively degrade gluten. Capable of hydrolyzing various food proteins, flavourzyme is an industrial prepared from A. flavus var. oryzae. This peptidase is composed of two leucyl aminopeptidases (EC 3.4.11), namely DPP IV and V (EC 3.4.14), three endopeptidases, including neutral peptidase I (EC 3.4.24), neutral peptidase II (EC 3.4.24.39), and alkaline peptidase I (EC 3.4.21.63), and α‐amylase (EC 3.2.1.1) (Merz et al., 2015). Wheat gluten (25 g/L) treated with flavourzyme led to 9.5 mg/kg residual gliadin concentration in the dried hydrolysate, which, through filtration, could be further decreased to around 2 mg/kg (Eugster et al., 2015; Merz, Kettner, et al., 2016). Increase in the degree of hydrolysis (DH) was reported to augment the solubility of hydrolysates obtained from A. oryzae with a fungal protease; however, foaming features are seemingly impaired beyond a certain DH (14%) (Drago & González, 2000). Another study reported that to obtain desirable emulsifying and foaming properties, the DH should be even lower, 5% to be exact (Brzozowski, 2016; Kong et al., 2007b).
Prolyl endopeptidase from A. niger, which called Aspergillus niger prolyl endopeptidase (AN‐PEP) (EC 3.4.21.26), has postproline cleaving activity. It is resistant to digestion by pepsin and active in a pH range of 2–8 with optimum activity at pH 4–5; moreover, AN‐PEP is highly able to effectively degrade CD‐active peptides along with intact α‐gliadins, γ‐gliadins, HMW‐GS, and LMW‐GS (König et al., 2017; Stepniak et al., 2006; Kara et al., 2005; Ahmed et al., 2015). It is also expected that protease therapy with AN enhances the symptoms of nonceliac gluten sensitivity because indigestible gluten‐related proteins have been observed to trigger nonceliac gluten sensitivity (Ido et al., 2018). The enzyme is specifically appropriate for food applications owing to its food‐grade status. AN‐PEP was employed to degrade gluten to levels below 20 mg/kg in wheat starch (Walter et al., 2014), wheat bran (Walter et al., 2014), rye flour and sourdough (Walter et al., 2015), and beer (Knorr et al., 2016). Aspergillopepsin (EC 3.4.23.18) from A. niger further showed gluten‐degrading activity; compared with AN‐PEP, however, it was not nearly as substrate‐specific and efficient, hence the fact that it might only be utilized complementary to Endoprotease B, isoform 2 (EP‐B2), or AN‐PEP, for instance (Ehren et al., 2009).
F. graminearum proteases are not only able to degrade gluten proteins in the grain itself, but also capable of weakening gluten during dough preparation and resting (Koga et al., 2019). These proteases were observed to be essentially trypsin‐like serine proteases cutting the proteins at the lysine or arginine amino acid (Pekkarinen et al., 2007). The primary destruction of HMW‐GS compared to LMW‐GS is possibly explained by their comparatively higher amount of lysine or arginine. Other studies showed reduced glutenin amount in comparison with increased gliadin following F. graminearum infection in wheat grains (Eggert et al., 2011).
A. usamii protease affected gluten through increasing the solubility of protein hydrolysates resulting from its secondary structure destruction and the enzymatic release of smaller polypeptide units from the protein (Deng et al., 2016). The cleavage of peptides by enzyme and unfolding of wheat gluten's globular structure were able to promote the interaction between peptides and lipid and contribute to anchoring the peptide molecules at the oil–water interface. This increased the emulsifying activity and decreased the interfacial tension (Deng et al., 2017). The ratio of turns decreased, and the globular structure of gluten was unfolded due to the enzymatic cleavage of peptide chains; therefore, longer β‐sheet chains were generated and the β‐sheet ratio increased (Barth & Zscherp, 2002). A. usamii protease slightly increased the water‐holding capacity of wheat gluten from 1.47 to 1.75 g/g; however, after hydrolysis, the holding capacity of oil was significantly increased from 0.92 to 2.91 g/g. This is possibly associated with the enzymatic hydrolysis exposing more hydrophobic regions (originally buried within the wheat gluten) to the aqueous phase (Saberi et al., 2008).
Bacterial peptidases
Lactic acid bacteria have a highly convoluted peptidase system (Kunji et al., 1996; M’hir et al., 2012); however, it is not a unique strain possibly possessing the whole pattern of peptidases required to hydrolyze all the potential peptides in which the protein is involved. B. subtilis and B. licheniformis hydrolyzed wheat gluten to a degree of 35%–38%, revealing extracellular peptidase activities comparable to the commercially available endopeptidase preparation alcalase (Stressler et al., 2015). As a nonspecific bacterial protease, alcalase is primarily achieved from Bacillus subtilis. Alcalases are classified to serine protease group that start a nucleophilic assail on the peptide bond via a serine residue at the active site (Apar & Özbek, 2010). Hydrolyzed protein obtained from wheat gluten treated with alcalase possess the maximum degree of hydrolysis (15.8%) values and is more effective in gluten hydrolysis compared with pepsin, pancreatin, neutrase, and protamex (Kong et al., 2007a). Furthermore, thermolysin (EC 3.4.24.27) from B. thermoproteolyticus and subtilisin (EC 3.4.21.62) from B. licheniformis were also able to effectively degrade wheat gliadin to products with molecular weights <15,000 (Socha et al., 2019, 2020). Subtilisin‐modified samples showed the highest extensive change in the immunoreactivity level of gliadin proteins (Leszczyńska et al., 2012). Di Cagno et al., (2002) reported that sourdough lactic acid bacteria positively affected gliadin peptides. The mixed starter containing Lb. brevis, Lb. alimentarius, Lactobacillus hilgardii, and Lb. sanfranciscensis was reported to almost thoroughly hydrolyze gliadin fractions; as shown by intestinal permeability challenge, the CD patients tolerated the resultant bread (Di Cagno et al., 2004). PEP (Prolyl endopeptidases) from Myxococcus xanthus and Sphingomonas capsulate (Gass et al., 2007), and Lactobacillus helveticus (Chen et al., 2003), resulted in similar properties (gluten detoxification).
1.2.2. Plant peptidases
During germination, gluten proteins are degraded to supply the developing embryo with amino acids and nitrogen. Cysteine endopeptidases (endopeptidases function in the middle of polypeptide chains) attack primary cleavage sites in N and C‐terminal domains. This causes the proteins to unfold the central repetitive domain, in turn cleaved at secondary cleavage sites. Cysteine endopeptidases constitute up to 90% of the total degrading activity, followed by metalloendopeptidases (7%), and serine and aspartic endopeptidases. The resultant peptides are further broken down to amino acids by exopeptidases (exopeptidases function close to the polypeptide chains termination) like serine carboxypeptidases and proline‐specific exopeptidases such as DPP II and IV (EC 3.4.14.2), lysosomal Xaa‐Pro carboxypeptidase (EC 3.4.16.2), and Xaa‐Pro aminopeptidase (EC 3.4.11.9) (Simpson, 2001). Peptidases obtained from germinated rye, wheat, and barley grains and bran effectively degraded epitopes into fragments of <9 amino acids (Geßendorfer et al., 2011). Their activities were dependent on cultivar, germination temperature, cereal species, and pH value during application (Kerpes et al., 2016; Schwalb et al., 2012). Wheat grains germinated with high peptidase activity (approximately 70% of the total activity caused by cysteine peptidases) were utilized as raw materials to ferment sourdough with Lactobacillus brevis L62. The combination of sourdough fermentation and germination significantly hydrolyzed wheat prolamin down to <5% (Loponen et al., 2009). Similarly, an approach combined sourdough fermentation and germinated rye, showing more than 99.5% of rye prolamins were degraded to contents of 280 – 430 mg/kg (dry matter) (Gänzle et al., 2008; Loponen et al., 2009). Cereal peptidases have the following upsides: (a) if applied properly, they are stable and highly active, (b) their cleavage specificity is naturally optimized to hydrolyze gluten proteins, (c) they are food‐grade, (d) they are obtainable through such established procedures as malting, (e) they can be integrated into production processes in a relatively facile manner, and (f) they are well accepted by consumers. As far as drawbacks are concerned, the gluten‐degrading activity of cereal extracts was much lower than purified enzymes (Walter et al., 2014) and their activity was inhibited by ethanol (≥ 2%) (Knorr et al., 2016). Taken together, the upsides clearly outweigh the downsides; applying gluten‐degrading cereal peptidases is a promising method for generating high‐quality gluten‐free products derived from gluten‐containing cereals, including gluten‐free and barley‐based beer (Knorr et al., 2016).
1.2.3. Insect peptidases
Because of feeding on cereals, insects, particularly grain pests, probably have endogenous gluten‐degrading enzymes. Among the seven screened beetles, the highest belonged to the proteolytic activity of an aqueous extract from Oryzaephilus surinamensis, Rhizopertha dominica, Tenebrio molitor, Alphitobius diapernius, T. confusum, and T. castaneum against wheat gluten, showing postproline cleaving patterns. Also identified were one serine‐type carboxypeptidase (EC 3.4.16) and two serine endopeptidases (EC 3.4.21), possibly appropriate for extensively degrading gluten (Mika et al., 2015). Wheat gluten was further reported to be damaged by Pentadomidae and Lygaidae (Sivri et al., 1998), Nysius huttoni (Every et al., 1998) (impacting HMW‐GS), Eurygaster and Aelia (Paulian, 1980), E. Maura (Sivri et al., 1998), E. integriceps (Koz’mina & Tvorogova, 1973) (affecting total gluten and gliadin), Eurygaster spp. and Aelia spp. (Every et al., 1998; Sivri et al., 1998), and E. integriceps (Yakovenko et al., 1973) (influencing glutenin). To solubilize the nutrients, these insects attack developing wheat kernels, injecting their salivary secretions into the grain. These secretions have strong proteolytic enzymes persisting in the flour after milling and in the kernel following harvest. During the dough stage of the bread making process, the proteolytic enzymes break down the gluten structure. The doughs prepared from bug‐damaged wheat flour are sticky, generating loaves of poor volume and crumb texture (Every et al., 1998). Koz’mina and Tvorogova (1973) observed a reduction (caused by proteolytic action) in the relative intensities of certain bands in the electrophoretic patterns of total unreduced gluten and gliadin; also, two new bands appeared at the low mobility region in the gliadin patterns of wheat damaged by E.integriceps. Researchers also showed that compared with glutenins, the gliadins had more resistance to bug enzymes (Sivri et al., 1998; Yakovenko et al., 1973).
1.3. Effect on functional properties
By using hydrolyzing enzymes, the emulsifying capacity of gluten augments which is ascribe to changes in the secondary structure of wheat gluten (Sun et al., 2019) depending on the degree of hydrolysis and protease activity (Deng et al., 2016). Increasing the emulsifying capacity of gluten in hydrolyzed samples can be explained by the unfolding of wheat gluten's globular structure and peptide bond's disruption by enzymatic modifications. Disruption of peptide bonds could facilitate the interaction among peptides and lipids and increase the availability of peptide residues at the oil–water interface leading to reduced interfacial tension and increased emulsifying activity. Nonetheless, moving further in the proteolytic activity, a reduction occurs in the emulsifying capacity of the hydrolysates due to extensive gluten degradation (Wang et al., 2016). The foaming capacity of hydrolyzed gluten was significantly improved by elevating the surface activity and reducing the surface strain at the water–air interface. This is possibly attributed to the large amount of polypeptide chains having broad molecular weight distributions generated from partial proteolysis, hence incorporating more air in wheat gluten (Deng et al., 2016) and enhancing the flexibility induced by the reactions of SS/SH interchange (Wouters et al., 2016). On the other hand, foam stability was reduced in more extensive hydrolysis due to the increased polypeptide chains unable to make foam air cells stable (Wouters, Fierens, et al., 2017; Wouters et al., 2016; Wouters, Rombouts, et al., 2017). Gluten treated with alcalase was further reported to increase solubility, emulsifying capacity, foaming stability, and foaming capacity owing to produce lower molecular size hydrolysates (Elmalimadi et al., 2017; He et al., 2019; Kong et al., 2007b).
1.4. Effect on rheological properties
Deng et al., (2016) revealed that enzymatic hydrolysis is capable of breaking some SS bonds to unfold the globular structure of gluten and new SS bonds are generated between both newly generated SH and original free SH groups to stabilize the structure of smaller peptides. According to SDS‐PAGE profile, the molecular weight of all the wheat gluten hydrolysates is drastically decreased (Figure 2) in the hydrolyzed gluten proteins (Wang et al., 2016). Researchers have revealed that the hydrophobicity of hydrolyzed protein is able to augmented or reduced based on enzyme specificity, hydrolysis situations, protein characteristics, and degree of hydrolysis (Wang et al., 2016). At low enzyme concentrations, the hydrophobicity of hydrolyzed gluten, augmented, which could be due to the hydrophobic amino acid exposure through protein unfolding (Wang et al., 2016). In excessive hydrolyzing treatment, hydrophobicity would decrease following two possible reasons: (1) degradation of some exposed hydrophobic regions; (2) burying the hydrophobic amino groups due to hydrolysis reactions (Zhang et al., 2014). As a regard to FTIR results, adding alcalase to wheat gluten reduces α‐helix and β‐turn conformation (Cui, Gong, et al., 2013). The reduction is due to the intermolecular disulfide bonds breakage and the elevation of β‐sheet and random coil caused by the alcalase hydrolyzation of the β‐turns into random coils (Cui, Gong, et al., 2013). Also, Wang et al. (2016) proposed that an acceptable amount of alcalase‐based hydrolysis unfolded the rigid structure of wheat gluten and increased the β‐sheet content.
Based on researches, alcalase‐based partial hydrolysis broke SS bonds, thereby unfolding the protein conformation of wheat gluten and increase the SH content. However, excess hydrolysis exposed many hydrophobic amino acids and formed aggregates belonging to wheat gluten hydrolysate. Besides, free SH groups might have participation in forming such aggregates (Zhao et al., 2013). The antioxidant activity of gluten hydrolyzed by alcalase was also studied, where the antioxidant activity of wheat hydrolysates has positive correlation with the content of hydrophobic amino acids. In other words, by excess hydrolysis, due to the increase in hydrophobic amino acids, the antioxidant activity of gluten would increase (Zhao et al., 2013).
The impact of peptidase hydrolysis on rheological properties is reported in different researches (Ahmed & Ikram, 2015; Koga et al., 2019). Peptidase hydrolysis reduced storage modulus (G´) (Ahmed & Ikram, 2015) and gluten consistency (Koga et al., 2019) due to gluten degradation and digesting effect of protease. In the gluten samples treated with protease, the glutenin polymer size is notably decreased which leads to reduction in maximum resistance to extension (Rmax). Rheological results reveal that glutenin polymer's size reduction by proteolytic hydrolysis in treated flours destroyed gluten structure (Koga et al., 2019).
The interactions among flour components (proteins, starch, fibers, etc) play key roles on the rheological properties of dough. According to researches, proteases diminish the storage (G΄) and complex (G˝) modulus. The weakening effect of proteases on wheat dough relates to the decrease in resistance to extension observed by Indrani et al., (2003). Proteinase activity affects specially to glutenins, which would alter the elasticity of the gluten complex (Caballero et al., 2007). Dough prepared with high levels of protease felt sticky and weak, which probably accounts for its poor performance. This weakness can be attributed to the hydrolysis of gluten proteins which are known to be the major determinant of dough strength (Harada et al., 2000). The hydrolyzing mechanism of protease enzymes results in degrading proteins as enzyme substrate. Hydrolyzed proteins lead to water binding capacity reduction, consequently excess released water, which cause significant reduction in dough viscosity and production of softer dough with better machining properties (Harada et al., 2000). However, it has also been recognized that over enzyme addition can cause overly soft or sticky dough, resulting in machining problems at the sheeter and rounder that lead to a deterioration in bread quality (Harada et al., 2000). Protease and peptidase can be used in bakery industry via their hydrolyzing mechanism (Table 4).
TABLE 4.
Applications of hydrolytic enzymes in gluten‐based products and their mode of actions
| Enzyme | Substrate | Mode of action | Product | Effect | Ref. |
|---|---|---|---|---|---|
| Protease | Gluten | Degrading proteins, water binding capacity reduction, excess released water, reduction in dough viscosity, and production of softer dough | Wheat dough | Diminish the storage (G΄) and complex (G*) modulus, weakening effect, decrease in resistance to extension | (Caballero et al., 2007; Harada et al., 2000; Indrani et al., 2003) |
| Bacterial peptidase/ fungal peptidase/ prolyl endopeptidases (PEPs) | Gliadin | Hydrolyze gliadin into harmless peptides | Wheat gluten | Decrease the gluten concentration and produce safe gluten‐base products for celiac disease | (Heredia‐Sandoval et al., 2016; Scherf et al., 2018; Socha et al., 2019; Wei et al., 2018; G. Wei et al., 2020). |
| Prolyl endopeptidases (PEPs) | Gliadin | Hydrolyze gliadin into harmless peptides | Beer | Produce safe beer for celiac disease | (Guerdrum & Bamforth, 2012) |
| Xylanase | Arabinoxylans | Break glycosidic linkages in arabinoxylans, viscosity reduction, Viscosity reduction, polymer chains get next to each other easier, and the gluten aggregation occurs | Bread | Softening effect on dough, release of excess water, viscosity reduction of dough, better machining properties | (Amiri et al., 2016) |
| Pentosanase | Pentosans | Conversion of water‐unextractable arabinoxylans to water‐extractable forms | Wheat dough | increasing amount of soluble pentosans and released amount of free water and the inhibition of gluten network formation according to glutenin–pentosan interactions, production of weak doughs | (Primo‐Martin et al., 2003) |
| Cellulase | Cellulose | hydrolyze cellulose into cellobiose, glucose, and oligosaccharides | Bread | Decrease in bread hardness and staling, ameliorate the bread sensory evaluations | (Altınel & Ünal, 2017; Nigam, 2013; Park et al., 2019; Wang, Chen, et al., 2018; Yurdugul et al., 2012 |
| Cellulase | Cellulose | Hydrolyze cellulose into cellobiose, glucose, and oligosaccharides | Cracker | Softening effect on dough, shorter baking time | (Carson, 2017) |
| Cellulase | Cellulose | Hydrolyze cellulose into cellobiose, glucose, and oligosaccharides | Steamed bread | Elevate bread sensory evaluation | (Lu et al., 2015) |
Furthermore, SEM analysis (Figure 4a) showed the damaged gluten structure, resulting in increased tan δ. tan δ is an indicator of protein quality (the higher the tan δ, the weaker the gluten network structure) (Kong et al., 2007).
1.5. Effect on thermal properties
Hydrolysis by proteases influenced wheat gluten thermal stability, determined by DSC. The glass transition temperature (Tg) is normally related to the protein thermal stability (more Tg values associated with enhanced wheat gluten thermal stability). Several studies were reported that alcalase significantly increases Tg values due to high quantities of exposed hydrophobic groups (Wang et al., 2017). Furthermore, Tg found to be lower, in excessive hydrolysis, due to splitting hydrophobic groups in to ionized groups. The change in enthalpy (ΔH) shows the extent of arrangements in the protein structure and straightly have relationship with its denaturation. Partially hydrolyzed gluten with alcalase significantly decreases ΔH parameter due to the alteration of the tertiary structure of gluten and less heat energy requirement (Wang, Qin, et al., 2017).
1.6. Effect on sensory characterization
Peptides with more hydrophobic amino acids are more likely to have lower bitter taste thresholds. When wheat gluten was hydrolyzed for 300 min by Proteax (a proteolytic enzyme obtained by Aspergillus oryzae), the hydrolyzed gluten protein showed minimum bitterness but maximum content of small peptides varying from 180 to 500 Da (He et al., 2019; Riu &Riu, 2016).
1.7. Effect on celiac disease
Uncontrolled immune response to wheat gluten causes a chronic enteropathy called celiac disease (CD) which refers to the pathology of the intestine. There are more than 60 immunogenic peptides in gluten derived from Triticum species. 33‐mer peptide with 13 proline residues and 10 glutamine residues is the most important immunogenic peptides, which is resistant to enzymatic proteolysis. Gliadin fractions and other wheat proteins can act as allergens; thus, celiac disease patients are not capable of tolerating these proteins (Heredia‐Sandoval et al., 2016; Bethune et al., 2006). Celiac disease patients carry HLA‐DQ2 and/or ‐DQ8 serotype which has the affinity to connect to antigens like gliadins and increase T‐cell‐mediated autoimmune reaction (Figure 3).
FIGURE 3.
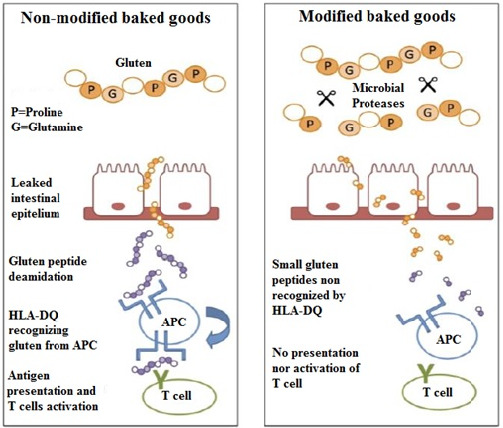
Adaptive immune response to nonmodified baked goods and nonactivation of T cells to modified baked goods by microbial proteases (Heredia‐Sandoval et al., 2016)
Studies have shown that various peptidases of fungal, plant, animal, or bacterial origin are able to hydrolyze gluten into harmless peptides. According to SDS‐PAGE pattern, proteolytic enzymes hydrolyze gliadins (Heredia‐Sandoval et al., 2016; Scherf et al., 2018; Socha et al., 2019; Wei et al., 2018, 2020). Bacterial peptidase (Krishnareddy & Green, 2017), fungal peptidase (Koning et al., 2005), and prolyl endopeptidases (PEPs) (Amador et al., 2019; Janssen et al., 2015; Kerpes et al., 2016; Mamo & Assefa, 2018) thoroughly degrade gliadin fractions to decrease gluten concentration and influence celiac disease. Aspergillus niger derived PEP (AN‐PEP) were assessed in clinical cases for their impact on modifying immune responses to gluten in celiac patients (Lähdeaho et al., 2014). Guerdrum and Bamforth (2012) reported that PEP addition in brewing technology decreased the prolamin and all of the identified immunopathogenic gluten epitopes in beer production (Akeroyd et al., 2016).
On the contrary, many of the recent investigations which employed enzyme‐linked immunosorbent assay (ELISA), mass spectrometry, and Western blot analysis reported that PEP did not thoroughly destroy the whole gluten proteins (Allred et al., 2017; Colgrave et al., 2017; Fiedler et al., 2018; Panda et al., 2015), which indicates that beers treated with PEP are not safe for CD patients.
1.8. Xylanase
Xylanases (EC 3.2.1.8) are able to break glycosidic linkages in arabinoxylans, producing smaller fragments. Xylanase from various sources has different mechanisms: 1) Xylanase obtained from A. niger degrades water‐extractable arabinoxylans exist in flour and reduces the molecular mass and dough viscosity of water‐extractable arabinoxylans; therefore, enhances the gluten agglomeration behavior and the larger gluten aggregates formation; 2) Xylanase generated from B. subtilis solubilizes water‐unextractable arabinoxylans augments the dough viscosity and negatively impacts gluten agglomeration (Romanowska et al., 2006). By removing arabinoxylans from gluten, xylanase alters the water distribution between gluten proteins and arabinoxylans, hence indirectly affect bread quality. Improving bread quality in the presence of xylanase also might be due to pentosan destruction and viscosity reduction effect of this enzyme. Viscosity reduction causes polymer chains to get next to each other easier, and the gluten aggregation would occur (Amiri et al., 2016). According to the competition between gluten and pentosan for water absorption, the degradation of pentosans by xylanase positively affects the gluten water binding characteristics (Figure 5; Amiri et al., 2016). On the contrary, some researchers found no evidence of xylanase removing arabinoxylans from gluten (Steffolani et al., 2010). These researchers believe that the evidence do not demonstrate the cleavage of covalent bonds between arabinoxylans and gluten by xylanase (Amiri, et al., 2016).
1.9. Effects on rheological properties
Steffolani et al., (2010) showed that endo‐xylanases increased SDS‐unextractable proteins, which can be explained by the importance of arabinoxylans in changing the extractability of the glutenin polymers (Figure 4b and Figure 5). Arabinoxylan breakdown with xylanase resulted in a less viscous dough, thereby augmenting the protein fragments mobility and facilitates their hydrophobic connections. These interactions would cause a more rapid protein aggregation which is due to the removing of steric interruption of arabinoxylans. Xylanases also redistributed water from arabinoxylans to the gluten and starch phase, make water more available to plasticize protein, thereby helping the gluten development. This made the dough and bread crumb softer and positively influenced bread making (Altınel & Ünal, 2017; Harada et al., 2000; Nevsky et al., 2018). In certain studies, the dough hardness was clearly reduced by adding high xylanase dosage to flour. The reduced dough hardening indicated the impact of xylanase on the interactions between glutenin and water soluble pentosans which leads to decrease disulfide cross‐linking and increase in SH content (Amiri, et al., 2016). Based on rheological results, xylanase tended to decrease storage modulus (G´) and augment tan δ which both inhibits less elastic behavior of dough in comparison with the control gluten samples. This phenomenon might be attributed to the degradation of pentosans and changing the gluten structure in samples treated with xylanase (Amiri et al., 2016; Steffolani et al., 2010). Moreover, the softening effect of xylanase is attributed to the release of water that occurs when arabinoxylans are hydrolyzed and the water binding capacity is reduced. The released water can reduce dough viscosity resulting in softer dough with better machining properties (Harada et al., 2000).
FIGURE 4.
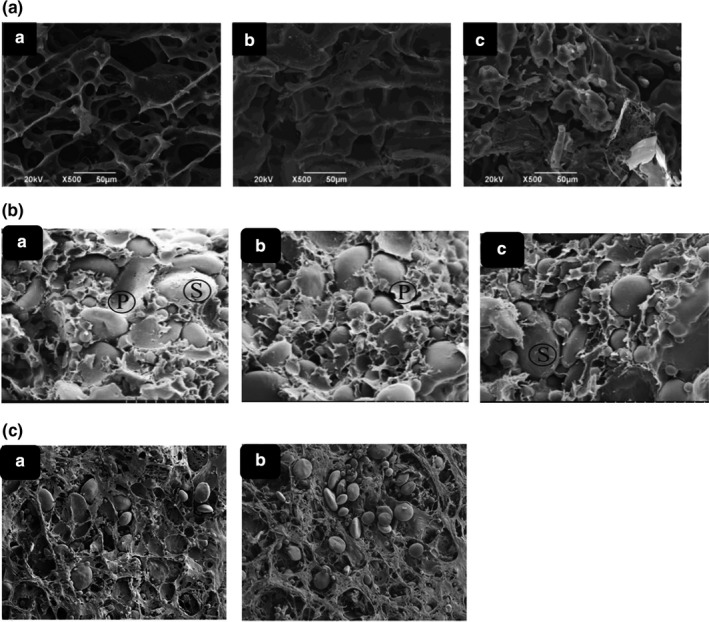
Scanning electron microscopy (SEM) of wheat gluten samples (A) WG (a), WG treated with desired quantity of Alcalase (0.04%, HWG‐4; b) and (0.10%, w/w, HWG‐10; c) (Wang et al., 2016). SEM of gluten (B) without enzymes (a), xylanase (b), and cellulase (c) (Wang et al., 2018). SEM images of dough treated with pentosanase (C), dough without pentosanase (a), and dough treated with pentosanase (b) (Steffolani et al., 2010) (Sun et al., 2019)
FIGURE 5.
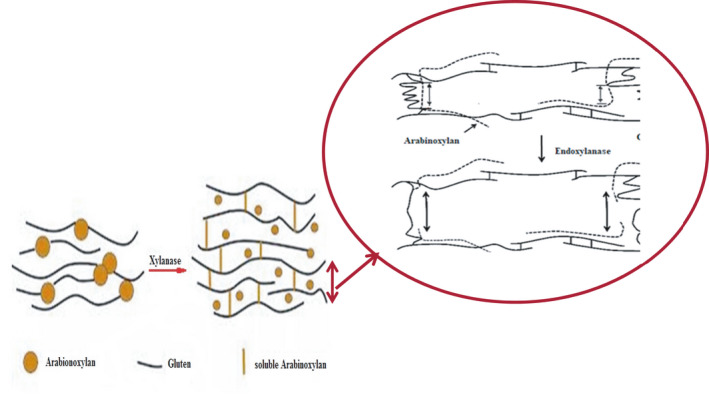
Xylanase reaction with gluten (Steffolani et al., 2010)
1.10. Pentosanase
Pentosanase is responsible for the conversion of water‐unextractable arabinoxylans to water‐extractable forms. Sun et al., (2019) revealed that water‐extractable arabinoxylans positively affect bread volume and textural properties, while water‐unextractable arabinoxylans cause undesirable product quality due to the competition for water and hinders gluten formation during the development of dough (Figures 2D and 4C). This conversion affects gluten network by five possible reactions: 1) Water‐unextractable arabinoxylans have high water‐holding capacity, which might cause lower water availability for gluten development, due to competition for water. Therefore, this conversion is known to improve gluten formation in baked products (Yang et al., 2017); 2) The pentosan–protein network will be weakened during the conversion of water‐unextractable arabinoxylans to their water‐extractable form. The cleavage of pentosan–protein bonds would release water, which is necessary for gluten development (Liu et al., 2018; Verjans et al., 2010); 3) Pentosanase would produce pentosans with smaller size which cause a redistribution of free water and reduce the steric hindrance of insoluble pentosans, thereby elevating the interaction between proteins (Steffolani et al., 2010); 4) Interaction among glutenin and water soluble pentosans lead to increase in SH content and dough softening according to SEM analysis (Figure 4C; Steffolani et al., 2010); 5) the enzyme enhanced gluten coagulation through reducing the steric impediment of the pentosans related to gluten and counteracting the gluten chemical aggregation (Steffolani et al., 2010).
1.11. Effects on rheological properties
Pentosanase produces a dough of greater extensibility and lower resistance to extension by interfering in protein–pentosan interactions (Primo‐Martin et al., 2003). Decrease in resistance values of doughs treated with pentosanase possibly is due to the increasing amount of soluble pentosans and released amount of free water and the inhibition of gluten network formation according to glutenin–pentosan interactions (Primo‐Martin et al., 2003). Revealed a decrease in development time and dough stability in pentosanase treated flours, which leads to production of weak doughs.
1.12. Cellulase
Cellulase (EC 3.2.1.4) is composed of enzymes which hydrolyze cellulose into cellobiose, glucose, and oligosaccharides (Figure 6) (Altınel & Ünal, 2017; Nigam, 2013; Park et al., 2019; Wang, Chen, et al., 2018; Vetrano et al., 2005). According to researches, cellulase used in bread dough resulted in a continuous gluten network (Wang, Chen, et al., 2018; Grigoras, 2017), and subsequently decrease in bread hardness and ameliorate the bread sensory evaluations (Yurdugul et al., 2012). The softening effects of cellulase on dough rheology also allow for a shorter baking time in certain baked goods like crackers (Carson, 2017).
FIGURE 6.
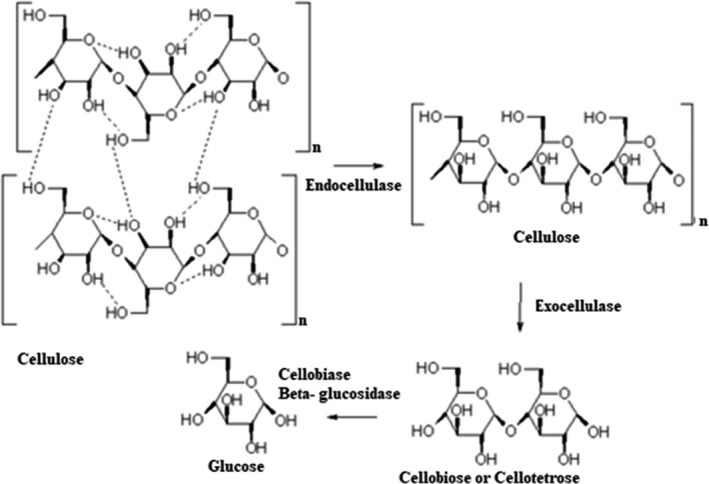
Cellulase mode of action
Extensibility is an important factor reflecting the dough strength. Extensibility of wheat dough is closely related to the content of dietary fiber. According to Lu et al., (2015), cellulase could degrade dietary fiber and reduce the extensibility of wheat dough. Moreover, the softening mechanism of cellulase might be attributed to the release of water that occurs when cellulose, as cellulase substrate is hydrolyzed to reduce its water binding capacity. The released water can reduce dough viscosity resulting in softer dough with better machining properties (Figure 4b; Harada et al., 2000; Zhang et al., 2019). In contrast, according to Liu et al., (2017), the cellulase addition significantly (p <.05) increased the development time, stability, departure time, mixing tolerance index, extensibility, and stickiness of regular dough, and decreased both softening and resistance to extension.
The antistaling effect of cellulase on bread is described by Yurdugul et al., (2012). It can be noted that the cross‐linking between starch–protein is in charge of bread staling. The antistaling effect of cellulase could be according to the enzyme cell wall degradation, and monosaccharides and oligosaccharides resulting from the enzyme action which cause an alteration in water distribution between starch–protein matrix (Yurdugul et al., 2012; Decamps et al., 2016; Joye et al., 2009).
2. CONCLUSIONS
Adding enzymes to wheat flour has recently become a common practice to overcome the gluten deficiencies according to their impact on the properties of gluten protein and network construction through affecting its cross‐linking and bonds. Beside the alteration of gluten functionality, enzyme modification is recognized as a safer and healthier method compared with chemical agents because they are inactivated following the heating process in wheat‐based foods. Enzymatic hydrolysis strongly ameliorates the emulsification, solubility, foaming, and nutritive properties of gluten proteins. The final quality of bakery products significantly owes to flour composition (proteins, starch, arabinoxylan, pentosan, cellulose, etc) and their interactions. Having softening effects, hydrolytic enzymes affect flour ingredients and directly and indirectly affect gluten properties. Taken together, protease, peptidase, and alkalase directly affect gluten, while xylanase (affect arabinoxylan), pentosanase (affect pentosan), and cellulase (affect cellulose) indirectly affect gluten protein. It can be concluded that through enzyme modification, gluten characteristics can be favorably altered in wheat‐based products.
3. ETHICS STATEMENT
Human and animal testing is unnecessary in this study.
CONFLICT OF INTEREST
The authors declare that they do not have any conflict of interest.
AUTHOR CONTRIBUTION
Kiana Pourmohammadi: Conceptualization (equal); Formal analysis (equal); Investigation (equal); Project administration (equal); Visualization (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Elahe Abedi: Conceptualization (equal); Investigation (equal); Methodology (equal); Project administration (equal); Writing‐review & editing (equal).
Pourmohammadi K, Abedi E. Hydrolytic enzymes and their directly and indirectly effects on gluten and dough properties: An extensive review. Food Sci Nutr. 2021;9:3988–4006. 10.1002/fsn3.2344
REFERENCES
- Abedi, E. , & Pourmohammadi, K. (2020a). Chemical modifications and their effects on gluten protein: An extensive review. Food Chemistry, 128398. 10.1016/j.foodchem.2020.128398 [DOI] [PubMed] [Google Scholar]
- Abedi, E. , & Pourmohammadi, K. (2020b). The effect of redox agents on conformation and structure characterization of gluten protein: An extensive review. Food Science & Nutrition, 8(12), 6301–6319. 10.1002/fsn3.1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abedi, E. , & Pourmohammadi, K. (2021a). Physical modifications of wheat gluten protein: An extensive review. Journal of Food Process Engineering, 44(3), e13619. 10.1111/jfpe.13619 [DOI] [Google Scholar]
- Ahmed, R. , Ali, R. , Khan, M. S. , Sayeed, S. A. , Saeed, J. , & Yousufi, F. (2015). Effect of proteases and carbohydarse on dough Rheology and End quality of cookie. American Journal of Food Science and Nutrition Research, 2(2), 62–66. [Google Scholar]
- Ahmed, S. , & Ikram, S. (2015). Chitosan & its derivatives: A review in recent innovations. International Journal of Pharmaceutical Sciences and Research, 6(1), 14. [Google Scholar]
- Akeroyd, M. , van Zandycke, S. , den Hartog, J. , Mutsaers, J. , Edens, L. , van den Berg, M. , & Christis, C. (2016). AN‐PEP, proline‐specific endopeptidase, degrades all known immunostimulatory gluten peptides in beer made from barley malt. Journal of the American Society of Brewing Chemists, 74(2), 91–99. 10.1094/ASBCJ-2016-2300-01 [DOI] [Google Scholar]
- Allred, L. K. , Lesko, K. , McKiernan, D. , Kupper, C. , & Guandalini, S. (2017). The celiac patient antibody response to conventional and gluten‐removed beer. Journal of AOAC International, 100(2), 485–491. 10.5740/jaoacint.16-0184 [DOI] [PubMed] [Google Scholar]
- Altınel, B. , & Ünal, S. S. (2017). The effects of amyloglucosidase, glucose oxidase and hemicellulase utilization on the rheological behaviour of dough and quality characteristics of bread. International Journal of Food Engineering, 13(2). 10.1515/ijfe-2016-0066 [DOI] [Google Scholar]
- Amador, M. D. L. M. , Arevalo‐Rodriguez, M. , Durán, E. M. , Reyes, J. C. M. , & Martin, C. S. (2019). A new microbial gluten‐degrading prolyl endopeptidase: Potential application in celiac disease to reduce gluten immunogenic peptides. PLoS One, 14(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri, A. , Shahedi, M. , & Kadivar, M. (2016). Evaluation of physicochemical properties of gluten modified by Glucose oxidase and Xylanase. Journal of Cereal Science, 71, 37–42. 10.1016/j.jcs.2016.07.013 [DOI] [Google Scholar]
- Apar, D. K. , & Özbek, B. (2010). Corn gluten hydrolysis by alcalase: Kinetics of hydrolysis. Chemical Engineering Communications, 197(7), 963–973. 10.1080/00986440903359368 [DOI] [Google Scholar]
- Barth, A. , & Zscherp, C. (2002). What vibrations tell about proteins. Quarterly Reviews of Biophysics, 35(4), 369. 10.1017/S0033583502003815 [DOI] [PubMed] [Google Scholar]
- Bethune, M. T. , Strop, P. , Tang, Y. , Sollid, L. M. , & Khosla, C. (2006). Heterologous expression, purification, refolding, and structural‐functional characterization of EP‐B2, a self‐activating barley cysteine endoprotease. Chemistry & Biology, 13(6), 637–647. 10.1016/j.chembiol.2006.04.008 [DOI] [PubMed] [Google Scholar]
- Brzozowski, B. (2016). Immunoreactivity of wheat proteins modified by hydrolysis and polymerisation. European Food Research and Technology, 242(7), 1025–1040. [Google Scholar]
- Brzozowski, B. , Stasiewicz, K. , Ostolski, M. , & Adamczak, M. (2020). Reducing Immunoreactivity of Gliadins and Coeliac Toxic Peptides Using Peptidases from L. acidophilus 5e2 and A. niger. Catalysts, 10(8), 923. [Google Scholar]
- Buddrick, O. , Cornell, H. J. , & Small, D. M. (2015). Reduction of toxic gliadin content of wholegrain bread by the enzyme caricain. Food Chemistry, 170, 343–347. 10.1016/j.foodchem.2014.08.030 [DOI] [PubMed] [Google Scholar]
- Caballero, P. A. , Gómez, M. , & Rosell, C. M. (2007). Improvement of dough rheology, bread quality and bread shelf‐life by enzymes combination. Journal of Food Engineering, 81(1), 42–53. 10.1016/j.jfoodeng.2006.10.007 [DOI] [Google Scholar]
- Carson, L. (2017). Dough conditioneres. Bakerpedia.com.
- Chen, Y. S. , Christensen, J. E. , Broadbent, J. R. , & Steele, J. L. (2003). Identification and characterization of Lactobacillus helveticus PepO2, an endopeptidase with post‐proline specificity. Applied and Environmental Microbiology, 69(2), 1276–1282. 10.1128/AEM.69.2.1276-1282.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgrave, M. L. , Byrne, K. , & Howitt, C. A. (2017). Food for thought: Selecting the right enzyme for the digestion of gluten. Food Chemistry, 234, 389–397. 10.1016/j.foodchem.2017.05.008 [DOI] [PubMed] [Google Scholar]
- Cui, C. , Hu, Q. , Ren, J. , Zhao, H. , You, L. , & Zhao, M. (2013). Effect of the structural features of hydrochloric acid‐deamidated wheat gluten on its susceptibility to enzymatic hydrolysis. Journal of Agricultural and Food Chemistry, 61(24), 5706–5714. 10.1021/jf400281v [DOI] [PubMed] [Google Scholar]
- Cui, L. , Gong, J. , Fan, X. , Wang, P. , Wang, Q. , & Qiu, Y. (2013). Transglutaminase‐modified wool keratin film and its potential application in tissue engineering. Engineering in Life Sciences, 13(2), 149–155. 10.1002/elsc.201100206 [DOI] [Google Scholar]
- Decamps, K. , Joye, I. J. , De Vos, D. E. , Courtin, C. M. , & Delcour, J. A. (2016). Molecular oxygen and reactive oxygen species in bread‐making processes: Scarce, but nevertheless important. Critical Reviews in Food Science and Nutrition, 56(5), 722–736. 10.1080/10408398.2013.795929 [DOI] [PubMed] [Google Scholar]
- Delcour, J. A. , Joye, I. J. , Pareyt, B. , Wilderjans, E. , Brijs, K. , & Lagrain, B. (2012). Wheat gluten functionality as a quality determinant in cereal‐based food products. Annual Review of Food Science and Technology, 3, 469–492. 10.1146/annurev-food-022811-101303 [DOI] [PubMed] [Google Scholar]
- Deng, L. , Wang, Z. , Yang, S. , Song, J. , Que, F. , Zhang, H. , & Feng, F. (2016). Improvement of functional properties of wheat gluten using acid protease from Aspergillus usamii. PLoS One, 11(7). 10.1371/journal.pone.0160101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, S. , Zhang, L. , Ji, Y. , Verweij, P. E. , Tsui, K. M. , Hagen, F. , Houbraken, J. , Meis, J. F. , Abliz, P. , & Wang, X. (2017). Triazole phenotypes and genotypic characterization of clinical Aspergillus fumigatus isolates in China. Emerging Microbes & Infections, 6(1), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cagno, R. , De Angelis, M. , Auricchio, S. , Greco, L. , Clarke, C. , De Vincenzi, M. , Giovannini, C. , D'Archivio, M. , Landolfo, F. , Parrilli, G. , Minervini, F. , Arendt, E. , & Gobbetti, M. (2004). Sourdough bread made from wheat and nontoxic flours and started with selected lactobacilli is tolerated in celiac sprue patients. Applied and Environmental Microbiology, 70(2), 1088–1096. 10.1128/AEM.70.2.1088-1096.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cagno, R. , De Angelis, M. , Lavermicocca, P. , De Vincenzi, M. , Giovannini, C. , Faccia, M. , & Gobbetti, M. (2002). Proteolysis by sourdough lactic acid bacteria: Effects on wheat flour protein fractions and gliadin peptides involved in human cereal intolerance. Applied and Environmental Microbiology, 68(2), 623–633. 10.1128/AEM.68.2.623-633.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago, S. R. , & Gonzalez, R. J. (2000). Foaming properties of enzymatically hydrolysed wheat gluten. Innovative Food Science & Emerging Technologies, 1(4), 269–273. 10.1016/S1466-8564(00)00034-5 [DOI] [Google Scholar]
- Eggert, K. , Rawel, H. M. , & Pawelzik, E. (2011). In vitro degradation of wheat gluten fractions by Fusarium graminearum proteases. European Food Research and Technology, 233(4), 697. 10.1007/s00217-011-1566-x [DOI] [Google Scholar]
- Ehren, J. , Morón, B. , Martin, E. , Bethune, M. T. , Gray, G. M. , & Khosla, C. (2009). A food‐grade enzyme preparation with modest gluten detoxification properties. PLoS One, 4(7), e6313. 10.1371/journal.pone.0006313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmalimadi, M. B. (2018). Functional and biological properties of enzymatically modified wheat gluten. Univerzitet u Beogradu‐Tehnološko‐metalurški fakultet. [Google Scholar]
- Elmalimadi, M. B. , Stefanović, A. B. , Šekuljica, N. Ž. , Žuža, M. G. , Luković, N. D. , Jovanović, J. R. , & Knežević‐Jugović, Z. D. (2017). The synergistic effect of heat treatment on alcalase‐assisted hydrolysis of wheat gluten proteins: Functional and antioxidant properties. Journal of Food Processing and Preservation, 41(5), e13207. 10.1111/jfpp.13207 [DOI] [Google Scholar]
- Eugster, P. J. , Salamin, K. , Grouzmann, E. , & Monod, M. (2015). Production and characterization of two major Aspergillus oryzae secreted prolyl endopeptidases able to efficiently digest proline‐rich peptides of gliadin. Microbiology, 161(12), 2277–2288. 10.1099/mic.0.000198 [DOI] [PubMed] [Google Scholar]
- Every, D. , Farrell, J. A. , Stufkens, M. W. , & Wallace, A. R. (1998). Wheat cultivar susceptibility to grain damage by the New Zealand wheat bug, Nysius huttoni, and cultivar susceptibility to the effects of bug proteinase on baking quality. Journal of Cereal Science, 27(1), 37–46. 10.1006/jcrs.1997.0142 [DOI] [Google Scholar]
- Fiedler, J. D. , Fishman, M. R. , Brown, S. D. , Lau, J. , & Finn, M. G. (2018). Multifunctional Enzyme Packaging and Catalysis in the Qβ Protein Nanoparticle. Biomacromolecules, 19(10), 3945–3957. 10.1021/acs.biomac.8b00885 [DOI] [PubMed] [Google Scholar]
- Gänzle, M. G. , Loponen, J. , & Gobbetti, M. (2008). Proteolysis in sourdough fermentations: Mechanisms and potential for improved bread quality. Trends in Food Science & Technology, 19(10), 513–521. 10.1016/j.tifs.2008.04.002 [DOI] [Google Scholar]
- Gass, J. , Bethune, M. T. , Siegel, M. , Spencer, A. , & Khosla, C. (2007). Combination enzyme therapy for gastric digestion of dietary gluten in patients with celiac sprue. Gastroenterology, 133(2), 472–480. 10.1053/j.gastro.2007.05.028 [DOI] [PubMed] [Google Scholar]
- Geßendorfer, B. , Hartmann, G. , Wieser, H. , & Koehler, P. (2011). Determination of celiac disease‐specific peptidase activity of germinated cereals. European Food Research and Technology, 232(2), 205–209. 10.1007/s00217-010-1375-7 [DOI] [Google Scholar]
- Grigoras, A. G. (2017). Catalase immobilization—A review. Biochemical Engineering Journal, 117, 1–20. 10.1016/j.bej.2016.10.021 [DOI] [Google Scholar]
- Guerdrum, L. J. , & Bamforth, C. W. (2012). Prolamin levels through brewing and the impact of prolyl endoproteinase. Journal of the American Society of Brewing Chemists, 70(1), 35–38. 10.1094/ASBCJ-2012-0130-01 [DOI] [Google Scholar]
- Harada, O. , Lysenko, E. D. , & Preston, K. R. (2000). Effects of commercial hydrolytic enzyme additives on Canadian short process bread properties and processing characteristics. Cereal Chemistry, 77(1), 70–76. 10.1094/CCHEM.2000.77.1.70 [DOI] [Google Scholar]
- Hardt, N. A. , Boom, R. M. , & van der Goot, A. J. (2015). Starch facilitates enzymatic wheat gluten hydrolysis. LWT‐Food Science and Technology, 61(2), 557–563. 10.1016/j.lwt.2014.12.010 [DOI] [Google Scholar]
- Hardt, N. A. , Van der Goot, A. J. , & Boom, R. M. (2013). Influence of high solid concentrations on enzymatic wheat gluten hydrolysis and resulting functional properties. Journal of Cereal Science, 57(3), 531–536. 10.1016/j.jcs.2013.03.006 [DOI] [Google Scholar]
- He, W. , Yang, R. , & Zhao, W. (2019). Effect of acid deamidation‐alcalase hydrolysis induced modification on functional and bitter‐masking properties of wheat gluten hydrolysates. Food Chemistry, 277 (November 2018), 655–663. 10.1016/j.foodchem.2018.11.004 [DOI] [PubMed] [Google Scholar]
- Henggeler, J. C. , Veríssimo, M. , & Ramos, F. (2017). Non‐coeliac gluten sensitivity: A review of the literature. Trends in Food Science & Technology, 66, 84–92. 10.1016/j.tifs.2017.05.018 [DOI] [Google Scholar]
- Heredia‐Sandoval, N. G. , Valencia‐Tapia, M. Y. , Calderón de la Barca, A. M. , & Islas‐Rubio, A. R. (2016). Microbial proteases in baked goods: Modification of gluten and effects on immunogenicity and product quality. Foods, 5(3), 59. 10.3390/foods5030059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, C. , Chen, Y. , Luo, C. , & Chiang, W. (2016). Antioxidant and antibacterial activities of peptide fractions from flaxseed protein hydrolysed by protease from Bacillus altitudinis HK02. International Journal of Food Science & Technology, 51(3), 681–689. [Google Scholar]
- Ido, H. , Matsubara, H. , Kuroda, M. , Takahashi, A. , Kojima, Y. , Koikeda, S. , & Sasaki, M. (2018). Combination of gluten‐digesting enzymes improved symptoms of non‐celiac gluten sensitivity: A randomized single‐blind, placebo‐controlled crossover study. Clinical and Translational Gastroenterology, 9(9). 10.1038/s41424-018-0052-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indrani, D. , Prabhasankar, P. , Rajiv, J. , & Rao, G. V. (2003). Scanning electron microscopy, rheological characteristics, and bread‐baking performance of wheat‐flour dough as affected by enzymes. Journal of Food Science, 68(9), 2804–2809. 10.1111/j.1365-2621.2003.tb05809.x [DOI] [Google Scholar]
- Jakovetić, S. , Luković, N. , Jugović, B. , Gvozdenović, M. , Grbavčić, S. , Jovanović, J. , & Knežević‐Jugović, Z. (2015). Production of antioxidant egg white hydrolysates in a continuous stirred tank enzyme reactor coupled with membrane separation unit. Food and Bioprocess Technology, 8(2), 287–300. 10.1007/s11947-014-1402-y [DOI] [Google Scholar]
- Janssen, G. , Christis, C. , Kooy‐Winkelaar, Y. , Edens, L. , Smith, D. , van Veelen, P. , & Koning, F. (2015). Ineffective degradation of immunogenic gluten epitopes by currently available digestive enzyme supplements. PLoS One, 10(6). 10.1371/journal.pone.0128065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, D. , Liu, X. , Zheng, X. , Wang, X. , & He, J. (2016). Preparation of antioxidative corn protein hydrolysates, purification and evaluation of three novel corn antioxidant peptides. Food Chemistry, 204, 427–436. 10.1016/j.foodchem.2016.02.119 [DOI] [PubMed] [Google Scholar]
- Joye, I. J. , Lagrain, B. , & Delcour, J. A. (2009). Use of chemical redox agents and exogenous enzymes to modify the protein network during breadmaking–A review. Journal of Cereal Science, 50(1), 11–21. 10.1016/j.jcs.2009.04.001 [DOI] [Google Scholar]
- Kammoun, R. , Bejar, S. , & Ellouz, R. (2003). Protein size distribution and inhibitory effect of wheat hydrolysates on Neutrase®. Bioresource Technology, 90(3), 249–254. 10.1016/S0960-8524(03)00130-5 [DOI] [PubMed] [Google Scholar]
- Kara, M. , Sivri, D. , & Köksel, H. (2005). Effects of high protease‐activity flours and commercial proteases on cookie quality. Food Research International, 38(5), 479–486. 10.1016/j.foodres.2004.09.012 [DOI] [Google Scholar]
- Kerpes, R. , Knorr, V. , Procopio, S. , Koehler, P. , & Becker, T. (2016). Gluten‐specific peptidase activity of barley as affected by germination and its impact on gluten degradation. Journal of Cereal Science, 68, 93–99. 10.1016/j.jcs.2016.01.004 [DOI] [Google Scholar]
- Knorr, V. , Kerpes, R. , Wieser, H. , Zarnkow, M. , Becker, T. , & Koehler, P. (2016). Production and application of barley malt extract with high peptidase activity for the degradation of gluten in wort. European Food Research and Technology, 242(4), 585–597. 10.1007/s00217-015-2568-x [DOI] [Google Scholar]
- Koga, S. , Rieder, A. , Ballance, S. , Uhlen, A. K. , & Veiseth‐Kent, E. (2019). Gluten‐Degrading Proteases in Wheat Infected by Fusarium graminearum—Protease Identification and Effects on Gluten and Dough Properties. Journal of Agricultural and Food Chemistry, 67(40), 11025–11034. [DOI] [PubMed] [Google Scholar]
- Köksel, H. , Sivri, D. , Ng, P. K. W. , & Steffe, J. F. (2001). Effects of transglutaminase enzyme on fundamental rheological properties of sound and bug‐damaged wheat flour doughs. Cereal Chemistry, 78(1), 26–30. 10.1094/CCHEM.2001.78.1.26 [DOI] [Google Scholar]
- Kolpakova, V. V. , Chumikina, L. V. , Vasil'ev, A. V. , Arabova, L. I. , & Topunov, A. F. (2014). Wheat gluten proteolysis by enzyme preparations of directional action. International Journal of Agronomy and Agricultural Research, 5(2), 72–86. [Google Scholar]
- Kong, X. , Zhou, H. , & Qian, H. (2007a). Enzymatic hydrolysis of wheat gluten by proteases and properties of the resulting hydrolysates. Food Chemistry, 102(3), 759–763. 10.1016/j.foodchem.2006.06.062 [DOI] [Google Scholar]
- Kong, X. , Zhou, H. , & Qian, H. (2007b). Enzymatic preparation and functional properties of wheat gluten hydrolysates. Food Chemistry, 101(2), 615–620. 10.1016/j.foodchem.2006.01.057 [DOI] [Google Scholar]
- König, J. , Holster, S. , Bruins, M. J. , & Brummer, R. J. (2017). Randomized clinical trial: Effective gluten degradation by Aspergillus niger‐derived enzyme in a complex meal setting. Scientific Reports, 7(1), 1–7. 10.1038/s41598-017-13587-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning, F. , Gilissen, L. , & Wijmenga, C. (2005). Gluten: A two‐edged sword. Immunopathogenesis of celiac disease. Springer Seminars in Immunopathology, 27(2), 217–232. 10.1007/s00281-005-0203-9 [DOI] [PubMed] [Google Scholar]
- Koo, S. H. , Bae, I. Y. , Lee, S. , Lee, D.‐H. , Hur, B.‐S. , & Lee, H. G. (2014). Evaluation of wheat gluten hydrolysates as taste‐active compounds with antioxidant activity. Journal of Food Science and Technology, 51(3), 535–542. 10.1007/s13197-011-0515-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koz’mina, N. P. , & Tvorogova, N. N. , (1973). Characteristics of the protein fractions of wheat gluten. Prikladnaia Biokhimiia i Mikrobiologiia, 9(2), 318. [PubMed] [Google Scholar]
- Krishnareddy, S. , & Green, P. H. R. (2017). Celiac Disease, the Microbiome, and Probiotics. In The Microbiota in Gastrointestinal Pathophysiology (pp. 365–371). Elsevier. [Google Scholar]
- Kunji, E. R. , Mierau, I. , Hagting, A. , Poolman, B. , & Konings, W. N. (1996). The proteotytic systems of lactic acid bacteria. Antonie Van Leeuwenhoek, 70(2), 187–221. 10.1007/BF00395933 [DOI] [PubMed] [Google Scholar]
- Lähdeaho, M.‐L. , Kaukinen, K. , Laurila, K. , Vuotikka, P. , Koivurova, O.‐P. , Kärjä‐Lahdensuu, T. , Marcantonio, A. , Adelman, D. C. , & Mäki, M. (2014). Glutenase ALV003 attenuates gluten‐induced mucosal injury in patients with celiac disease. Gastroenterology, 146(7), 1649–1658. 10.1053/j.gastro.2014.02.031 [DOI] [PubMed] [Google Scholar]
- Leszczyńska, J. , Diowksz, A. , LĄcka, A. , Wolska, K. , & Bartos, A. (2012). Evaluation of immunore activity of wheat bread made from fermented wheat flour. Czech Journal of Food Sciences, 30(4), 336–342. 10.17221/137/2011-CJFS [DOI] [Google Scholar]
- Liu, L. , Yang, W. , Cui, S. W. , Jiang, Z. , Chen, Q. , Qian, H. , Wang, L. , & Zhou, S. (2018). Effects of pentosanase and glucose oxidase on the composition, rheology and microstructure of whole wheat dough. Food Hydrocolloids, 84, 545–551. 10.1016/j.foodhyd.2018.06.034 [DOI] [Google Scholar]
- Liu, W. , Brennan, M. A. , Serventi, L. , & Brennan, C. S. (2017). Effect of cellulase, xylanase and α‐amylase combinations on the rheological properties of Chinese steamed bread dough enriched in wheat bran. Food Chemistry, 234, 93–102. 10.1016/j.foodchem.2017.04.160 [DOI] [PubMed] [Google Scholar]
- Loponen, J. , Kanerva, P. , Zhang, C. , Sontag‐Strohm, T. , Salovaara, H. , & Gänzle, M. G. (2009). Prolamin hydrolysis and pentosan solubilization in germinated‐rye sourdoughs determined by chromatographic and immunological methods. Journal of agricultural and food chemistry, 57(2), 746–753. [DOI] [PubMed] [Google Scholar]
- Lu, Q. , He, Y. , & Liu, X. (2015). Property assessment of steamed bread added with cellulase by using fuzzy mathematical model. Journal of Texture Studies, 46(6), 420–428. 10.1111/jtxs.12141 [DOI] [Google Scholar]
- M’hir, S. , Ziadi, M. , Chammem, N. , & Hamdi, M. , (2012). Gluten proteolysis as alternative therapy for celiac patients: A mini‐review. African Journal of Biotechnology, 11(29). [Google Scholar]
- Majzoobi, M. , Abedi, E. , Farahnaky, A. , & Aminlari, M. (2012). Functional properties of acetylated glutenin and gliadin at varying pH values. Food Chemistry, 133(4), 1402–1407. 10.1016/j.foodchem.2012.01.117 [DOI] [Google Scholar]
- Mamo, J. , & Assefa, F. (2018). The role of microbial aspartic protease enzyme in food and beverage industries. Journal of Food Quality, 2018. [Google Scholar]
- Merz, M. , Appel, D. , Berends, P. , Rabe, S. , Blank, I. , Stressler, T. , & Fischer, L. (2016). Batch‐to‐batch variation and storage stability of the commercial peptidase preparation Flavourzyme in respect of key enzyme activities and its influence on process reproducibility. European Food Research and Technology, 242(7), 1005–1012. 10.1007/s00217-015-2606-8 [DOI] [Google Scholar]
- Merz, M. , Eisele, T. , Berends, P. , Appel, D. , Rabe, S. , Blank, I. , Stressler, T. , & Fischer, L. (2015). Flavourzyme, an enzyme preparation with industrial relevance: Automated nine‐step purification and partial characterization of eight enzymes. Journal of Agricultural and Food Chemistry, 63(23), 5682–5693. 10.1021/acs.jafc.5b01665 [DOI] [PubMed] [Google Scholar]
- Merz, M. , Kettner, L. , Langolf, E. , Appel, D. , Blank, I. , Stressler, T. , & Fischer, L. (2016). Production of wheat gluten hydrolysates with reduced antigenicity employing enzymatic hydrolysis combined with downstream unit operations. Journal of the Science of Food and Agriculture, 96(10), 3358–3364. 10.1002/jsfa.7515 [DOI] [PubMed] [Google Scholar]
- Mika, N. , Zorn, H. , & Rühl, M. (2015). Prolyl‐specific peptidases for applications in food protein hydrolysis. Applied Microbiology and Biotechnology, 99(19), 7837–7846. 10.1007/s00253-015-6838-0 [DOI] [PubMed] [Google Scholar]
- Nevsky, A. A. , Tsurikova, N. V. , Dremucheva, G. F. , Nosova, M. V. , Velikoretskaya, I. A. , & Borodulin, D. M. (2018). Effect of enzyme preparations with Endo‐Xylanase and Exo‐Peptidase activities on the bread quality, dough properties and fractional composition of wheat gluten proteins. International Scientific and Practical Conference" Agro‐SMART‐Smart Solutions for Agriculture"(Agro‐SMART 2018). [Google Scholar]
- Nigam, P. S. (2013). Microbial enzymes with special characteristics for biotechnological applications. Biomolecules, 3(3), 597–611. 10.3390/biom3030597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nionelli, L. , & Rizzello, C. G. (2016). Sourdough‐based biotechnologies for the production of gluten‐free foods. Foods, 5(3), 65. 10.3390/foods5030065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda, R. , Zoerb, H. F. , Cho, C. Y. , Jackson, L. S. , & Garber, E. A. E. (2015). Detection and quantification of gluten during the brewing and fermentation of beer using antibody‐based technologies. Journal of Food Protection, 78(6), 1167–1177. 10.4315/0362-028X.JFP-14-546 [DOI] [PubMed] [Google Scholar]
- Park, E. Y. , Fuerst, E. P. , & Baik, B. (2019). Effect of bran hydration with enzymes on functional properties of flour–bran blends. Cereal Chemistry, 96(2), 273–282. [Google Scholar]
- Paulian, F. (1980). Sunn pest or cereal bug. Wheat. [Google Scholar]
- Pekkarinen, A. I. , Longstaff, C. , & Jones, B. L. (2007). Kinetics of the inhibition of Fusarium serine proteinases by barley (Hordeum vulgare L.) inhibitors. Journal of Agricultural and Food Chemistry, 55(7), 2736–2742. [DOI] [PubMed] [Google Scholar]
- Phillips, R. D. , & Beuchat, L. R. (1981). Enzyme modification of proteins. ACS Publications. [Google Scholar]
- Pourmohammadi, K. , & Abedi, E. (2021). Enzymatic modifications of gluten protein: Oxidative enzymes. Food Chemistry, 129679. 10.1016/j.foodchem.2021.129679 [DOI] [PubMed] [Google Scholar]
- Primo‐Martin, C. , Valera, R. , & Martinez‐Anaya, M. A. (2003). Effect of pentosanase and oxidases on the characteristics of doughs and the glutenin macropolymer (GMP). Journal of Agricultural and Food Chemistry, 51(16), 4673–4679. 10.1021/jf0257695 [DOI] [PubMed] [Google Scholar]
- Riu, C. D. , & Riu, J. J. D. (2016). Topical compositions comprising diaminooxidase for the treatment or prevention of diseases associated with high histamine levels which involve an increase in pain. Google Patents. [Google Scholar]
- Romanowska, I. , Polak, J. , & Bielecki, S. (2006). Isolation and properties of Aspergillus niger IBT‐90 xylanase for bakery. Applied Microbiology and Biotechnology, 69(6), 665–671. 10.1007/s00253-005-0011-0 [DOI] [PubMed] [Google Scholar]
- Saberi, A. H. , Kadivar, M. , & Keramat, J. (2008). Improvement of functional properties of glutens extracted from two Iranian wheat varieties (Sardari and Mahdavi ). Employing Chemical and Enzymatic Modifications. 10, 243–252. [Google Scholar]
- Saha, B. C. , Yoshida, T. , Cotta, M. A. , & Sonomoto, K. (2013). Hydrothermal pretreatment and enzymatic saccharification of corn stover for efficient ethanol production. Industrial Crops and Products, 44, 367–372. 10.1016/j.indcrop.2012.11.025 [DOI] [Google Scholar]
- Savvateeva, L. V. , Gorokhovets, N. V. , Makarov, V. A. , Serebryakova, M. V. , Solovyev, A. G. , Morozov, S. Y. , Reddy, V. P. , Zernii, E. Y. , Zamyatnin, A. A. Jr , & Aliev, G. (2015). Glutenase and collagenase activities of wheat cysteine protease Triticain‐α: Feasibility for enzymatic therapy assays. The International Journal of Biochemistry & Cell Biology, 62, 115–124. 10.1016/j.biocel.2015.03.001 [DOI] [PubMed] [Google Scholar]
- Scherf, K. A. , Wieser, H. , & Koehler, P. (2018). Novel approaches for enzymatic gluten degradation to create high‐quality gluten‐free products. Food Research International, 110, 62–72. 10.1016/j.foodres.2016.11.021 [DOI] [PubMed] [Google Scholar]
- Schwalb, T. , Wieser, H. , & Koehler, P. (2012). Studies on the gluten‐specific peptidase activity of germinated grains from different cereal species and cultivars. European Food Research and Technology, 235(6), 1161–1170. [Google Scholar]
- Simpson, D. J. (2001). Proteolytic degradation of cereal prolamins—the problem with proline. Plant Science, 161(5), 825–838. 10.1016/S0168-9452(01)00482-4 [DOI] [Google Scholar]
- Sivri, D. , Köksel, H. , & Bushuk, W. (1998). Effects of wheat bug (Eurygaster maura) proteolytic enzymes on electrophoretic properties of gluten proteins. New Zealand Journal of Crop and Horticultural Science, 26(2), 117–125. [Google Scholar]
- Socha, P. , Mickowska, B. , Urminská, D. , & Kačmárová, K. (2019). The use of different proteases to hydrolyze gliadins. Journal of Microbiology, Biotechnology and Food Sciences, 2019, 101–104. [Google Scholar]
- Socha, P. , Mickowska, B. , Urminská, D. , & Kačmárová, K. (2020). The use of different proteases to hydrolyze gliadins. Journal of Microbiology, Biotechnology and Food Sciences, 9(5), 101–104. [Google Scholar]
- Steffolani, M. E. , Ribotta, P. D. , Pérez, G. T. , & León, A. E. (2010). Effect of glucose oxidase, transglutaminase, and pentosanase on wheat proteins: Relationship with dough properties and bread‐making quality. Journal of Cereal Science, 51(3), 366–373. 10.1016/j.jcs.2010.01.010 [DOI] [Google Scholar]
- Stepniak, D. , Spaenij‐Dekking, L. , Mitea, C. , Moester, M. , De Ru, A. , Baak‐Pablo, R. , Van Veelen, P. , Edens, L. , & Koning, F. (2006). Highly efficient gluten degradation with a newly identified prolyl endoprotease: Implications for celiac disease. American Journal of Physiology ‐ Gastrointestinal and Liver Physiology, 291(4), 621–629. 10.1152/ajpgi.00034.2006 [DOI] [PubMed] [Google Scholar]
- Stressler, T. , Eisele, T. , Baur, C. , Wangler, J. , Kuhn, A. , & Fischer, L. (2015). Extracellular peptidases from insect‐and compost‐associated microorganisms: Screening and usage for wheat gluten hydrolysis. European Food Research and Technology, 241(2), 263–274. 10.1007/s00217-015-2452-8 [DOI] [Google Scholar]
- Sun, Q. , Ma, Z. F. , Zhang, H. , Ma, S. , & Kong, L. (2019). Structural characteristics and functional properties of walnut glutelin as hydrolyzed: Effect of enzymatic modification. International Journal of Food Properties, 22(1), 265–279. 10.1080/10942912.2019.1579738 [DOI] [Google Scholar]
- Taga, Y. , Hayashida, O. , Kusubata, M. , Ogawa‐Goto, K. , & Hattori, S. (2017). Production of a novel wheat gluten hydrolysate containing dipeptidyl peptidase‐IV inhibitory tripeptides using ginger protease. Bioscience, Biotechnology, and Biochemistry, 81(9), 1823–1828. 10.1080/09168451.2017.1345615 [DOI] [PubMed] [Google Scholar]
- Verjans, P. , Dornez, E. , Delcour, J. A. , & Courtin, C. M. (2010). Selectivity for water‐unextractable arabinoxylan and inhibition sensitivity govern the strong bread improving potential of an acidophilic GH11 Aureobasidium pullulans xylanase. Food Chemistry, 123(2), 331–337. 10.1016/j.foodchem.2010.04.039 [DOI] [Google Scholar]
- Vetrano, A. M. , Heck, D. E. , Mariano, T. M. , Mishin, V. , Laskin, D. L. , & Laskin, J. D. (2005). Characterization of the oxidase activity in mammalian catalase. Journal of Biological Chemistry, 280(42), 35372–35381. 10.1074/jbc.M503991200 [DOI] [PubMed] [Google Scholar]
- Walter, T. , Wieser, H. , & Koehler, P. (2014). Degradation of gluten in wheat bran and bread drink by means of a proline‐specific peptidase. Nutrition & Food Sciences. [Google Scholar]
- Walter, T. , Wieser, H. , & Koehler, P. (2015). Degradation of gluten in rye sourdough products by means of a proline‐specific peptidase. European Food Research and Technology, 240(3), 517–524. 10.1007/s00217-014-2350-5 [DOI] [Google Scholar]
- Wang, J. S. , Wei, Z. Y. , Li, L. , Bian, K. , & Zhao, M. M. (2009). Characteristics of enzymatic hydrolysis of thermal‐treated wheat gluten. Journal of Cereal Science, 50(2), 205–209. 10.1016/j.jcs.2009.05.004 [DOI] [Google Scholar]
- Wang, K. , Sun, D.‐W. , Pu, H. , & Wei, Q. (2017). Principles and applications of spectroscopic techniques for evaluating food protein conformational changes: A review. Trends in Food Science & Technology, 67, 207–219. 10.1016/j.tifs.2017.06.015 [DOI] [Google Scholar]
- Wang, L. , Ding, L. , Yu, Z. , Zhang, T. , Ma, S. , & Liu, J. (2016). Intracellular ROS scavenging and antioxidant enzyme regulating capacities of corn gluten meal‐derived antioxidant peptides in HepG2 cells. Food Research International, 90, 33–41. 10.1016/j.foodres.2016.10.023 [DOI] [PubMed] [Google Scholar]
- Wang, P. , Zou, M. , Tian, M. , Gu, Z. , & Yang, R. (2018). The impact of heating on the unfolding and polymerization process of frozen‐stored gluten. Food Hydrocolloids, 85, 195–203. 10.1016/j.foodhyd.2018.07.019 [DOI] [Google Scholar]
- Wang, X. , Qin, X. , Li, D. , Yang, B. , & Wang, Y. (2017). One‐step synthesis of high‐yield biodiesel from waste cooking oils by a novel and highly methanol‐tolerant immobilized lipase. Bioresource Technology, 235, 18–24. 10.1016/j.biortech.2017.03.086 [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Chen, D. , Wang, G. , Zhao, C. , Ma, Y. , & Yang, W. (2018). Immobilization of cellulase on styrene/maleic anhydride copolymer nanoparticles with improved stability against pH changes. Chemical Engineering Journal, 336, 152–159. 10.1016/j.cej.2017.11.030 [DOI] [Google Scholar]
- Wei, C.‐K. , Thakur, K. , Liu, D.‐H. , Zhang, J.‐G. , & Wei, Z.‐J. (2018). Enzymatic hydrolysis of flaxseed (Linum usitatissimum L.) protein and sensory characterization of Maillard reaction products. Food Chemistry, 263, 186–193. 10.1016/j.foodchem.2018.04.120 [DOI] [PubMed] [Google Scholar]
- Wei, G. , Helmerhorst, E. J. , Darwish, G. , Blumenkranz, G. , & Schuppan, D. (2020). Gluten Degrading Enzymes for Treatment of Celiac Disease. Nutrients, 12(7), 2095. 10.3390/nu12072095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters, A. G. B. , Fierens, E. , Rombouts, I. , Brijs, K. , Blecker, C. , & Delcour, J. A. (2017). Air‐water interfacial properties of enzymatically hydrolyzed wheat gluten in the presence of sucrose. Food Hydrocolloids, 73, 284–294. 10.1016/j.foodhyd.2017.07.014 [DOI] [Google Scholar]
- Wouters, A. G. B. , Rombouts, I. , Fierens, E. , Brijs, K. , & Delcour, J. A. (2016). Relevance of the functional properties of enzymatic plant protein hydrolysates in food systems. Comprehensive Reviews in Food Science and Food Safety, 15(4), 786–800. 10.1111/1541-4337.12209 [DOI] [PubMed] [Google Scholar]
- Wouters, A. G. B. , Rombouts, I. , Schoebrechts, N. , Fierens, E. , Brijs, K. , Blecker, C. , & Delcour, J. A. (2017). Foam fractionation as a tool to study the air‐water interface structure‐function relationship of wheat gluten hydrolysates. Colloids and Surfaces B: Biointerfaces, 151, 295–303. 10.1016/j.colsurfb.2016.12.031 [DOI] [PubMed] [Google Scholar]
- Yakovenko, V. A. , Litvinov, A. M. , & Gavrilyuk, I. P. (1973). Electrophoretic characterisation of proteins of chinch‐bug affected wheat. Izv. Vyssh. Uchebn. Zaved . Pishchevaya Tekhnol, 3, 17–19. [Google Scholar]
- Yang, W. , Jiang, Z. , Liu, L. , Lin, Y. , Wang, L. , & Zhou, S. (2017). The effect of pentosanase on the solubilisation and degradation of arabinoxylan extracted from whole and refined wheat flour. Journal of the Science of Food and Agriculture, 97(3), 1034–1041. 10.1002/jsfa.7833 [DOI] [PubMed] [Google Scholar]
- Yong, Y. H. , Yamaguchi, S. , & Matsumura, Y. (2006). Effects of enzymatic deamidation by protein‐glutaminase on structure and functional properties of wheat gluten. Journal of Agricultural and Food Chemistry, 54(16), 6034–6040. 10.1021/jf060344u [DOI] [PubMed] [Google Scholar]
- Yurdugul, S. , Pancevska, N.‐A. , Yildiz, G. G. , & Bozoglu, F. (2012). The influence of a cellulase bearing enzyme complex from anaerobic fungi on bread staling. Rom Agric Res, 29, 2067–5720. [Google Scholar]
- Zhang, H.‐J. , Zhang, H. , Wang, L. , & Guo, X.‐N. (2012). Preparation and functional properties of rice bran proteins from heat‐stabilized defatted rice bran. Food Research International, 47(2), 359–363. 10.1016/j.foodres.2011.08.014 [DOI] [Google Scholar]
- Zhang, W. , Ciclitira, P. , & Messing, J. (2014). PacBio sequencing of gene families—A case study with wheat gluten genes. Gene, 533(2), 541–546. 10.1016/j.gene.2013.10.009 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Chen, M. , Chen, Y. , Hou, Y. , & Hu, S.‐Q. (2019). Characterization and exploration of recombinant wheat catalase for improvement of wheat‐flour‐processing quality. Journal of Agricultural and Food Chemistry, 67(9), 2660–2669. 10.1021/acs.jafc.8b06646 [DOI] [PubMed] [Google Scholar]
- Zhao, L. , Li, L. , Liu, G. , Chen, L. , Liu, X. , Zhu, J. , & Li, B. (2013). Effect of freeze–thaw cycles on the molecular weight and size distribution of gluten. Food Research International, 53(1), 409–416. 10.1016/j.foodres.2013.04.013 [DOI] [Google Scholar]
- Zhou, C. , Hu, J. , Yu, X. , Yagoub, A. E. G. A. , Zhang, Y. , Ma, H. , Gao, X. , & Otu, P. N. Y. (2017). Heat and/or ultrasound pretreatments motivated enzymolysis of corn gluten meal: Hydrolysis kinetics and protein structure. LWT ‐ Food Science and Technology, 77, 488–496. 10.1016/j.lwt.2016.06.048 [DOI] [Google Scholar]


