Abstract
Background
Emerging evidence indicates that propolis as a novel potential antioxidant has unique benefits. This study aimed to evaluate the effect of propolis on oxidative stress, inflammation, body composition, and athletic performance in healthy active subjects.
Methods
This clinical trial was conducted on 54 male military cadets. Eligible subjects were randomly allocated to receive a single dose of 450 mg propolis twice daily for four weeks or a matching placebo containing microcrystalline cellulose. Cooper 12‐min run test and running‐based anaerobic sprint test were performed to measure aerobic and anaerobic performance. Blood samples were obtained immediately after Cooper's test to evaluate oxidative stress and inflammation status. Fat mass and fat‐free mass were analyzed using bioelectrical impedance.
Results
Mean changes in fat mass, fat‐free mass, anaerobic powers, fatigue index, and VO2 max did not differ significantly between the two groups after the adjustment for baseline values (P‐value>0.05). A significant change was observed in plasma levels of IL‐6 (−1.43 ± 0.11pg/mL), total oxidant status (−3.9 ± 0.2µmol/L), total antioxidant capacity (164 ± 12 µmol/L), malondialdehyde (−0.52 ± 0.03µmol/L), oxidative stress index (−0.45 ± 0.04), and glutathione (48.72±2µmol/L) in the propolis group compared with the placebo group after the adjustment for baseline values and weight changes (P‐value<0.05). Although IL‐10 concentrations had no significant changes in both groups, the ratio of IL‐6/IL‐10 significantly reduced in the propolis group compared with the placebo group (−0.174 ± 0.015 versus. 0.051 ± 0.014; P‐value: 0.041).
Conclusions
Our results indicated that propolis might have beneficial effects on oxidative stress and inflammation following intense activities in healthy male subjects.
Keywords: athletic performance, body composition, inflammation, oxidative stress, propolis, VO2 max
As far as we know, this is the first trial to evaluate the effect of propolis supplementation on exercise performance. This clinical trial study shows that propolis supplementation can significantly reduce plasma levels of total oxidants, IL‐6, malondialdehyde, oxidative stress index, and IL‐6/IL‐10 ratio and can significantly improve plasma levels of total antioxidant capacity and glutathione following intense physical activity. However, our trial indicates that propolis intake had no significant effect on anaerobic and aerobic performance and body composition.

1. INTRODUCTION
Intense physical activities can increase oxygen consumption in the active muscles 10–15 times more than in the resting state (Joyner & Case y, 2015). The rise in oxygen delivery to active skeletal muscles is required to produce adenosine triphosphate (ATP) through the electron transport chain (ETC) to continue the activities (Miyazaki et al., 2001). The ETC is located in the inner mitochondrial membrane, which is not only the principal site of ATP production in skeletal muscles but also a potential source of reactive oxygen species (ROS) production (Leeuwenburgh & Heinecke, 2001). In addition to ETC, xanthine oxidase, lipoxygenase, phospholipase A2, myostatin, and catecholamines also contribute to ROS formation in contracting muscles (Powers & Jackson, 2008; Steinbacher & Eckl, 2015).
Some researchers have suggested that low ROS concentrations in skeletal muscles can increase the muscle's ability to produce force (Reid, 2001). However, what is certain is that ROS in high concentrations results in muscle force decline and fatigue (Powers et al., 2016). In addition, overproduction of ROS following intense activates can cause irreversible damage to intracellular organelles, inflammation, and eventually muscle breakdown (Suzuki et al., 2020). The susceptibility of skeletal muscle to oxidative stress depends on the antioxidant defense system capability. This system comprises nonenzymatic antioxidants, such as glutathione (GSH), and enzymatic antioxidants such as superoxide dismutase (SOD) and catalase (CAT). Nutritional status influences the capacity and ability of the body's antioxidant defense to neutralize ROS (Chow, 1979). In addition, dietary antioxidants can modulate the immune response and inflammatory pathways which can help the enhancement of physical performance and recovery from exercise in athletes (Mason et al., 2020).
Propolis is a sticky substance produced by honey bees (Apis mellifera L.) from buds and various plants' exudates. Numerous polyphenol compounds have been identified in propolis from different geographical regions of the world (Huang et al., 2014). Propolis has long been used as a popular therapeutic agent to promote the body's health and treat wounds and infections (Sforcin, 2016). Recent studies have shown the antioxidant, anti‐inflammatory, and immunomodulatory activities of propolis from different parts of the world (Franchin et al., 2018; Soleimani, Miryan, et al., 2021; Wang et al., 2018). Animal studies support that propolis and its derivatives can ameliorate exercise‐induced damage through enhancing the antioxidant defense system and suppressing nuclear factor‐kappa B (NF‐κB) (Kwon et al., 2014; Shen et al., 2013). Quercetin, the predominant flavonoid compound identified in propolis (Zheng et al., 2017), has been shown to promote exercise performance (Davis et al., 2010) and muscle mitochondrial biogenesis (Islam et al., 2020). This evidence indicates that propolis might help athletes protect their muscles against exercise‐induced oxidative and inflammatory damage and improve exercise performance. The current trial aimed to assess the effect of propolis supplementation on pro/anti‐inflammatory cytokines, antioxidant/oxidant status, and anaerobic/aerobic endurance among active subjects.
2. METHODS AND MATERIALS
2.1. Study design and participants
This randomized, triple‐blind, placebo‐controlled clinical trial was designed to evaluate the effect of propolis supplementation on pro/anti‐inflammatory cytokines, antioxidant/oxidant status, and exercise performance. Participants were recruited from new male cadets entering AJA University of Medical Sciences in Tehran, Iran. A total of 107 young males were enrolled in the trial, of whom 54 eligible participants underwent randomization to assign either the propolis group or the placebo group (Figure 1). The protocol, available at the Iranian Registry of Clinical Trials (IRCT20180824040857N2), was reviewed and approved by the ethics committee at the AJA University of Medical Sciences (IR.AJAUMS.REC.1399.107). Each participant was provided verbally with information on the objectives of the trial and its benefits and possible health risks at the time of enrollment. Each participant provided written informed consent. This trial was conducted in accordance with the principles of the Helsinki Declaration.
FIGURE 1.
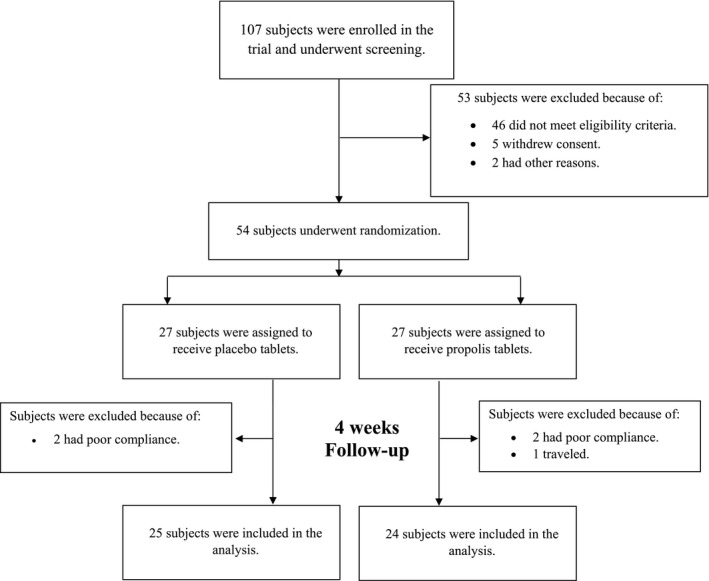
Screening, Randomization, Treatment, and Follow‐up
2.2. Eligibility criteria
Cadets, who agreed to participate in this trial, were screened based on eligibility criteria. Inclusion criteria were male gender, age of 20–30 years, body mass index (BMI) of 18.5–25 kg/m2, a minimum of 6 hr/wk of sports activities in the last year, and written informed consent. Those who had a history of adverse reaction to bee products, musculoskeletal dysfunction, previous musculoskeletal injuries during training or exercise, heart diseases, diabetes mellitus, metabolic diseases, regular use of antioxidant supplements (e.g., vitamin E, vitamin C, β‐carotene, selenium, and propolis) or anti‐inflammatory drugs in the last three months, or specific dietary regimen (e.g., vegetarian diets) and energy‐restricted diets, in the last six months were not eligible to participate in the trial.
2.3. Trial randomization and blinding
Cadets who met the eligibility criteria underwent randomization after the screening visit. Randomization sequences were generated using a random‐number table and opaque, sealed, numbered envelopes. Randomization was stratified according to age (20–25 years versus. 26–30 years). Eligible participants were randomly assigned in a 1:1 ratio with a blinded manner to the intervention or control group. Participants and investigators, except the trial pharmacist, were concealed from the study‐group assignment until the end of the trial and data analyses.
2.4. Intervention
Participants in the intervention group were assigned to take ethanolic‐extracted poplar propolis at a single dose of 450 mg twice daily, before lunch and dinner, for 4 weeks. In contrast, those in the control group received a matching placebo containing microcrystalline cellulose. The dose of propolis was selected according to the previous phase ΙΙ trial (Zhao et al., 2016). Propolis extract was standardized based on total polyphenols and flavonoids content, according to Bankova's recommendation (Bankova, 2005). Each propolis tablet contained 180 mg polyphenols and 134 mg flavonoids. The Reyhan Naghsh Jahan Pharmaceutical Company manufactured both the propolis and matching placebo tablets under good manufacturing practice (GMP) conditions. Adherence was estimated by counting unused tablets. During the trial, all cadets were in the same place, had the same physical activity, ate the same meals, and slept the same number of hours. Participants were also followed by face‐to‐face visits every week. During the intervention period, participants who were unwilling to continue the trial were sensitive or unable to take the assigned intervention (<80%), or used other dietary supplements and drugs were excluded from the trial. Among 54 eligible participants, 49 completed the 4‐week intervention period. Two subjects in the propolis group and two subjects in the placebo group were withdrawn from the trial because of poor adherence to the trial‐group assignment. Also, one in the propolis group discontinued the trial for a reason unrelated to the study (Figure 1).
2.5. Anthropometric assessment
Before and after the intervention period, body weight, fat mass (FM), and fat‐free mass (FFM) were measured using the bioelectrical impedance analysis (BIA) technique (Tania BC‐ 418 machine, Tania Corp., Tokyo, Japan). All participants were asked to be well hydrated and to abstain from exercising and caffeine‐containing products in the 6 hr and 24 hr before the BIA measure, respectively (Kyle et al., 2004). Height was measured using a portable stadiometer (Seca 213, Hamburg, Germany) without shoes in a standing position to the nearest 1 cm. Then, BMI was computed by dividing weight in kilograms by height in meters squared.
2.6. Exercise performance assessment
Aerobic endurance was measured using the Cooper 12‐min run test. This test was designed by Dr. Ken Cooper to evaluate the aerobic capacity of the US military (Cooper, 1968). Cooper's test is a valid and reliable method of estimating the maximum oxygen uptake (VO2 max) (Bandyopadhyay, 2015). Before and after the intervention period, participants conducted the 12 min of continuous running in the 400‐m running track, and then, covered distance was used to calculate VO2 max according to Cooper's equation as follows:
VO2 max (ml/kg/min) ₌ (Distance ‐ 504.9) ÷ 44.73.
We also used the running‐based anaerobic sprint test (RAST) to measure anaerobic endurance before and after the intervention period. This test provides a valid and reliable method for estimating anaerobic power and fatigue index (Zagatto et al., 2009). The RAST test comprises six consecutive 35‐m sprints with a rest time of 10 s between sprints. Participants conducted the RAST test, and time spent in each attempt was automatically recorded using an electronic timing system. Then, we computed anaerobic power output along with fatigue index (FI) as the indicator of the drop in power by following equations (Adamczyk, 2011):
Power output (watts) ₌ (Weight ×Distance 2) / Time 3
FI (watts/s) = (Maximum Power – Minimum Power) / Time spent in six sprints
In the familiarization session, all participants were informed about doing sports tests and were motivated to do them with maximal effort. They also were abstained from intense physical activities in the 24 hr before sports tests. All participants had a five‐minute warm‐up period of light aerobic activities prior to performing exercise tests and were verbally encouraged throughout performing exercise tests.
2.7. Biochemical assessment
Heparinized blood samples were taken from the cubital vein immediately after Cooper's test. Then, samples were centrifuged at 3,000 rpm for 10 min at 4°C, and supernatants were used to assess inflammation, antioxidants, and oxidative stress. Plasma levels of the interleukin (IL) 6 and IL‐10 were measured based on the biotin double antibody sandwich method using commercial enzyme‐linked immunosorbent assay (ELISA) kits (Diaclone, Besancon, France). Then, the ratio of the IL‐6 and IL‐10 was used to indicate the degree of inflammation status. Plasma levels of total antioxidants capacity (TAC), GSH, and total oxidant status (TOS) were measured based on the enzymatic colorimetric method using commercial kits (ZellBio GmbH, Ulm, Germany). The ratio of the TAC to TOS was used to compute the oxidative stress index (OSI) as follows (Buico et al., 2009): OSI =100 × (TOS/TAC). In addition, malondialdehyde (MDA) concentrations as an indicator of the degree of oxidative damage were assessed by the colorimetric reagent kit (ZellBio GmbH, Ulm, Germany). All tests were read using an ELISA reader (Stat Fax 2,100, Awareness Technology, Inc., USA).
2.8. Statistical analysis
Data were analyzed using SPSS software, version 16 (SPSS Inc., Chicago, IL, USA). We estimated that a sample of 27 subjects in each study arm would provide a power of 80% to detect an effect size of 1.4 gr/L in GSH concentrations response to propolis intake (Zhao et al., 2016) and a withdrawal rate of 10% at a significance level of 5% (two‐tailed). Kolmogorov–Smirnov test was used to examine the normal distribution of quantitative data. Within‐group comparisons were done using paired Student's t test for normally distributed data and Wilcoxon rank‐sum test for ordinal or non‐normally distributed data. Independent Student's t test for normally distributed data, Mann–Whitney U test for ordinal or non‐normally distributed data, and the chi‐square test or Fisher's exact test for nominal data were also used to ascertain between‐group comparisons. We estimated the adjusted effects of propolis using analysis of Covariance (ANCOVA) test with baseline values as covariates. P‐values of less than 0.05 were considered to indicate statistically significant differences.
3. RESULTS
The participants had a mean age of 24.21 ± 2.09 years and a BMI of 23.52 ± 1.31 kg/m2. The mean age and BMI of participants were 24.21 ± 1.98 years and 23.82 ± 1.06 kg/m2 in the propolis group and 24.20 ± 2.24 years and 23.22 ± 1.47 kg/m2 in the placebo group. There were no significant differences in age and BMI between the two groups (P‐value >0.05). The mean compliance rate was 96.9% for propolis and 97.1% for placebo. Participants throughout the trial reported no adverse effects.
The adjusted mean changes in the anthropometric characteristics are shown in Table 1. The baseline values of the anthropometric variables were similar in both groups (P‐value >0.05). The mean weight and FM reduced significantly in the placebo group, while no significant changes were observed in the propolis group throughout the trial. However, mean changes in weight and FM did not differ significantly in the placebo group than the propolis group after the adjustment for the baseline values (P‐value >0.05).
TABLE 1.
Adjusted changes in the anthropometric variables from baseline to the end of the trial
| Variables | Group | Before | After | P‐value a | Changes b | P‐value b | |
|---|---|---|---|---|---|---|---|
| Weight; kg | Propolis (N:24) | 74.42 ± 2.86 | 74.19 ± 3.24 | 0.323 | −0.244 ± 0.049 | 0.562 | |
| Placebo (N:25) | 72.73 ± 3.35 | 72.29 ± 3.42 | 0.039 | −0.430 ± 0.044 | |||
| FM; kg | Propolis (N:24) | 18.47 ± 4.83 | 18.44 ± 4.70 | 0.887 | −0.058 ± 0.036 | 0.294 | |
| Placebo (N:25) | 20.33 ± 4.97 | 19.98 ± 4.96 | 0.011 | −0.324 ± 0.034 | |||
| FFM; kg | Propolis (N:24) | 55.85 ± 5.55 | 55.75 ± 5.94 | 0.393 | −0.251 ± 0.043 | 0.483 | |
| Placebo (N:25) | 52.50 ± 6.38 | 52.31 ± 6.52 | 0.613 | −0.040 ± 0.042 | |||
Data are presented as mean ±standard deviation.
Abbreviations: FFM, fat‐free mass; FM, fat mass.
Values were obtained from paired‐sample t test;
Values were obtained from ANCOVA test with baseline values as a covariate.
The adjusted mean changes in athletic performances (aerobic & anaerobic) are shown in Table 2. There were no significant differences in the mean aerobic and anaerobic parameters between the two groups at the baseline examination (P‐value >0.05). At the end of the trial, only mean VO2 MAX significantly changed in the propolis group at the end of the trial. However, this change was not significant as compared with the placebo group after the adjustment for baseline values and changes in FFM (P‐value: 0.772). Also, the mean changes in anaerobic Powers and Fatigue Index did not differ significantly from baseline to the end of the trial in both groups (P‐value>0.05).
TABLE 2.
Adjusted mean changes in the Cooper and RAST tests from baseline to the end of the trial
| Variables | Group | Before | After | P‐value a | Changes b | P‐value b |
|---|---|---|---|---|---|---|
| VO2 MAX; ml/kg/min | Propolis (N:24) | 48.04 ± 2.19 | 49.54 ± 2.38 | 0.026 | 1.25 ± 0.109 | 0.772 |
| Placebo (N:25) | 49.12 ± 2.63 | 49.84 ± 3.05 | 0.212 | 0.99 ± 0.107 | ||
| Power MIN; watts | Propolis (N:24) | 346.48 ± 75.65 | 335.01 ± 54.78 | 0.534 | −1.46 ± 3.09 | 0.118 |
| Placebo (N:25) | 323.59 ± 76.79 | 365.75 ± 86.94 | 0.055 | 32.54 ± 2.97 | ||
| Power MEAN; watts | Propolis (N:24) | 459.08 ± 48.96 | 457.68 ± 53.61 | 0.902 | 2.41 ± 3.03 | 0.207 |
| Placebo (N:25) | 439.78 ± 50.93 | 473.03 ± 89.25 | 0.079 | 29.59 ± 2.95 | ||
| Power MAX; watts | Propolis (N:24) | 654.74 ± 146.43 | 680.66 ± 138.29 | 0.482 | 42.77 ± 6.17 | 0.734 |
| Placebo (N:25) | 619.75 ± 116.01 | 664.19 ± 152.61 | 0.265 | 28.25 ± 5.92 | ||
| Fatigue Index; watts/s | Propolis (N:24) | 8.71 ± 4.13 | 9.92 ± 3.94 | 0.297 | 1.389 ± 0.169 | 0.292 |
| Placebo (N:25) | 8.39 ± 3.34 | 8.74 ± 4.01 | 0.753 | 0.155 ± 0.162 |
Abbreviations: FFM, fat‐free mass; FM, fat mass; RAST, Running‐based Anaerobic Sprint Test.
Note: Data are presented as mean ±standard deviation.
Values were obtained from paired‐sample t test;
Values were obtained from ANCOVA test with baseline values and changes in fat‐free mass as covariates.
The adjusted mean changes in the biochemical parameters measured immediately after Cooper's test are shown in Table 3. The baseline values did not differ significantly between the two groups (P‐value>0.05). The mean IL‐6, IL‐6/IL‐10, MDA, and TOS significantly reduced, while GSH and TAC significantly increased in the propolis group at the end of the trial. But, these variables did not have significant changes in the placebo group. The adjusted mean changes in GSH (Effect Size±Std. Error: 50.39 ± 13.46 µmol/L; P‐value: 0.001), TAC (Effect Size±Std. Error: 259.83 ± 82.7 µmol/L; P‐value: 0.003), IL‐6 (Effect Size±Std. Error: −2.01 ± 0.731 pg/ml; P‐value: 0.011), IL‐6/IL‐10(Effect Size±Std. Error: −0.229 ± 0.101; P‐value: 0.041), MDA (Effect Size±Std. Error: −0.53 ± 0.18 µmol/L; P‐value: 0.011), and TOS (Effect Size±Std. Error: −4.92 ± 1.46 µmol/L; P‐value: 0.001) were significant in the propolis group compared with the control group. Also, significant reductions in OSI (Effect Size: 0.64 ± 0.28; P‐value: 0.032) were observed in the propolis group compared with the control group after the adjustment for baseline values and weight changes.
TABLE 3.
Adjusted changes in the biochemical assessments from baseline to the end of the trial
| Variables | Group | Before | After | P‐value a | Changes b | P‐value b |
|---|---|---|---|---|---|---|
| IL−10; pg/ml | Propolis (N:24) | 9.95 ± 1.65 | 10.49 ± 2.01 | 0.245 | 0.489 ± 0.085 | 0.848 |
| Placebo (N:25) | 10.15 ± 4.21 | 10.21 ± 4.19 | 0.893 | 0.067 ± 0.082 | ||
| IL−6; pg/ml | Propolis (N:24) | 10.30 ± 3.38 | 8.85 ± 2.96 | 0.011 | −1.43 ± 0.108 | 0.011 |
| Placebo (N:25) | 9.93 ± 2.92 | 10.65 ± 3.67 | 0.247 | 0.578 ± 0.106 | ||
| GSH; µmol/L | Propolis (N:24) | 229.18 ± 32.06 | 277.85 ± 62.48 | 0.001 | 48.72 ± 2 | 0.001 |
| Placebo (N:25) | 244.87 ± 53.39 | 243.24 ± 52.55 | 0.811 | −1.70 ± 1.87 | ||
| TAC; µmol/L | Propolis (N:24) | 1,105 ± 357 | 1,259 ± 447 | 0.026 | 164 ± 12 | 0.003 |
| Placebo (N:25) | 1,083 ± 496 | 1,017 ± 392 | 0.280 | −96 ± 11 | ||
| TOS; µmol/L | Propolis (N:24) | 13.72 ± 4.23 | 9.13 ± 3.56 | 0.001 | −3.94 ± 0.199 | 0.001 |
| Placebo (N:25) | 11.81 ± 4.85 | 13.40 ± 6.44 | 0.200 | 0.969 ± 0.191 | ||
| MDA; µmol/L | Propolis (N:24) | 3.25 ± 1.22 | 2.68 ± 0.92 | 0.005 | −0.523 ± 0.029 | 0.011 |
| Placebo (N:25) | 3.07 ± 1.16 | 3.13 ± 0.87 | 0.761 | 0.008 ± 0.028 | ||
| IL−6/IL−10 | Propolis (N:24) | 1.05 ± 0.39 | 0.88 ± 0.41 | 0.033 | −0.174 ± 0.015 | 0.041 |
| Placebo (N:25) | 1.09 ± 0.46 | 1.14 ± 0.45 | 0.515 | 0.051 ± 0.014 | ||
| OSI | Propolis (N:24) | 1.42 ± 0.74 | 1.00 ± 1.02 | 0.069 | −0.453 ± 0.042 | 0.032 |
| Placebo (N:25) | 1.49 ± 1.27 | 1.65 ± 1.17 | 0.494 | 0.199 ± 0.041 |
Abbreviations: GSH, glutathione; IL, interleukin; MDA, malondialdehyde; OSI, oxidative stress index; TAC, total antioxidant capacity; TOS, total oxidant status.
Note: OSI was calculated as follows: 100 × (TOS/TAC). Data are presented as mean ±standard deviation.
Values were obtained from paired‐sample t test;
Values were obtained from ANCOVA test with baseline values and changes in weight as covariates.
4. DISCUSSION
This trial's main finding was that the administration of propolis at a daily dose of 900 mg for four weeks enhanced the antioxidant status and reduced oxidative stress and inflammation following intense exercise. Nevertheless, propolis had no significant effects on body composition and anaerobic and aerobic endurance (Figure 2). To our knowledge, this is the first trial to evaluate the efficacy of propolis supplementation on exercise performance.
FIGURE 2.
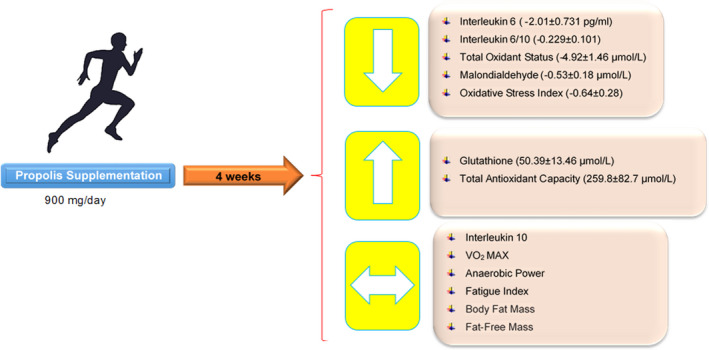
The Effect of Propolis Supplementation on Athletic Performance, Body Composition, Inflammation, and Oxidative Stress following intense exercise. [Down Arrow (Decrease); Up Arrow (Increase); Double‐sided Arrow (Without Change)]
Our trial shows that propolis administration has no effects on weight, FFM, and FM in subjects within the normal weight range. In a 4‐month randomized clinical trial, Soleimani et al. found that supplementation with 500 mg/day of propolis had no significant effect on weight, FFM, and FM among patients with nonalcoholic fatty liver disease (Soleimani, Rezaie, et al., 2021). Likewise, Mujica et al. reported that the administration of 30 drops/day of propolis solution (Beepolis®) for three months had no effects on weight and waist circumference among patients with cardiometabolic risk factors (Mujica et al., 2017). Also, Zakerkish et al. showed that the daily intake of 1,000 mg/day of propolis for three months had no effects on weight in diabetic subjects (Zakerkish et al., 2019). Conversely, Samadi et al. reported that the administration of 900 mg/day of propolis in diabetic subjects over a period of 3 months reduced weight and BMI while did not affect waist circumference (Samadi et al., 2017). This controversy can be related to confounders' effect, such as changes in energy intake and physical activity throughout the study of Samadi et al.
Our trial shows that propolis supplementation improves TAC and GSH and reduces TOS, MDA, and OSI following intense exercise. These findings on the improvement of the antioxidant defense system's capacity are in line with the observations of previous clinical trials. In an 18‐week randomized clinical trial, Zhao et al. reported that among diabetic subjects, propolis supplementation at a daily dose of 900 mg/day elevated serum levels of GSH and total polyphenols (Zhao et al., 2016). In the study of Mujica et al., the administration of propolis significantly increased the levels of GSH in red blood cells (RBCs) and decreased the plasma MDA concentrations after 3 months (Mujica et al., 2017). Likewise, Hesami et al. showed that 1,500 mg/day of propolis administration significantly increased catalase activity and reduced oxidized low‐density lipoprotein (LDL) concentrations in diabetic subjects (Hesami et al., 2019). Propolis contains high amounts of phenolic compounds which directly react with and quench free radicals. In addition, propolis and its derivatives have been shown to activate the nuclear factor erythroid‐2‐related factor 2 (Nrf2) pathway, which mainly regulates the expression of a large battery of genes related to endogenous antioxidants (Jin et al., 2015). In this line, propolis has been shown in vivo to increase the levels of heme oxygenase‐1 (HO‐1), glutamate‐cysteine ligase (GCL: the rate‐limiting enzyme for the biosynthesis of GSH), and thioredoxin reductase 1 (TrxR1) (Zhang et al., 2015). The activation of the Nrf2 pathway not only up‐regulates the GCL mRNA expression but also facilitates the cellular uptake of cystine as the rate‐limiting precursor for the synthesis of GSH via enhancing the cystine/glutamate amino acid antiporter (Xc‐system) (Correa et al., 2011).
Our trial shows that propolis supplementation reduces the IL‐6 concentrations and IL‐6/IL‐10 ratio in plasma following intense exercise. Intense physical activities stimulate inflammatory cytokines, which attenuate muscle force production, immune response, and recovery times (Nemet et al., 2002). There are some inconsistencies regarding the effect of propolis on inflammatory cytokines in previous clinical trials. Zakerkish et al.'s study showed that propolis supplementation reduced TNF‐α while had no effect on IL‐6 and IL‐1β among diabetic subjects (Zakerkish et al., 2019). The study of Zhao et al. also reported that propolis reduced TNF‐α concentrations, whereas increased IL‐1β and IL‐6 levels among diabetic subjects (Zhao et al., 2016). Fukuda et al. found that propolis supplementation (226.8 mg/day for 8 weeks) had no effect on TNF‐α and IL‐6 concentrations among diabetic subjects (Fukuda et al., 2015). Khayyal et al. found that propolis supplementation (260 mg/day for 8 weeks) reduced TNF‐α, intercellular adhesion molecule‐1 (ICAM‐1), IL‐6, and IL‐8 and increased IL‐10 in asthmatic patients (Khayyal et al., 2003). Afsharpour et al. reported that propolis supplementation (1,500 mg/day for 8 weeks) reduced TNF‐α among diabetic subjects (Afsharpour et al., 2017). Taken together, a recent meta‐analysis revealed a significant reduction in IL‐6, TNF‐α, and high‐sensitivity C‐reactive protein (hs‐CRP) concentrations by 17.96 pg/ml, 34.08 pg/ml, and 1.16 pg/ml, respectively, following propolis supplementation. Nonetheless, no significant reduction was reported in IL‐1β concentrations with propolis consumption (Shang et al., 2020). Recent scientific evidence (in vitro and in vivo) shows that propolis and its bioactive compounds attenuate inflammatory cytokines synthesis and leukocyte recruitment into the inflammatory site through various mechanisms of action such as blocking the activation of NF‐κB, extracellular signal‐regulated kinase (ERK), and Jun N‐terminal Kinase (JNK) signaling pathways, inhibiting the release of CXCL1/KC and CXCL2/MIP‐2 chemokines, and suppressing the expression of ICAM‐1, vascular cell adhesion molecule 1 (VCAM‐1), and E‐selectin (Franchin et al., 2018). Nuclear factor‐kappa B (NF‐κB) is a critical transcription factor for the activation of inflammatory gene expression in response to various stimuli such as ROS/RNS. Caffeic acid phenyl ester which is identified in propolis has been shown to reduce exercise‐induced skeletal muscle injury by inhibiting the activation of NF‐κB as well as the generation of ROS (Shen et al., 2013). Collectively, propolis may be a promising candidate to treat acute and chronic inflammatory diseases (Franchin et al., 2018; Soleimani, Miryan, et al., 2021).
Our results failed to show a significant improvement in aerobic and anaerobic endurance. Scientific evidence on the effect of antioxidants on exercise performance is limited and conflicting. In a 1‐week randomized trial, Davis et al. found that the daily administration of 1,000 mg of quercetin, which is identified in propolis, increased VO2 max (3.9%) and bike‐ride times to fatigue (13.2%) among untrained subjects (Davis et al., 2010). In a 3‐week randomized trial, Jourkesh et al. reported that a daily intake of 400 mg of vitamin E along with 1,000 mg of vitamin C improved VO2 max. However, it had no significant effect on anaerobic power among male students (Jourkesh et al., 2007). Conversely, the administration of 1,000 mg/day of vitamin C for 4 weeks had no significant effect on VO2 max among recreationally active men (Roberts et al., 2011). Likewise, in another clinical trial, Paulsen et al. reported that the daily intake of 1,000 mg of vitamin C and 235 mg of vitamin E for 11 weeks did not affect VO2 max on healthy subjects (Paulsen et al., 2014). Taken together, it seems that long‐term antioxidant supplementation may attenuate its favorable effects on exercise performance. In this line, a body of evidence suggests that high levels of antioxidants in skeletal muscles can attenuate exercise adaptations and mitochondrial biogenesis by suppressing redox signaling pathways related to exercise‐induced ROS production (Cobley et al., 2015; Strobel et al., 2011). Consistent with this hypothesis, it has been shown that ROS can modulate the force production in unfatigued skeletal muscles (Powers & Jackson, 2008).
The lack of access to an ergo‐spirometer for direct evaluation of VO2 max via a breath‐by‐breath gas analyzing system was a limitation of this study. However, we used the Cooper test to evaluate the aerobic capacity of the military (Cooper, 1968). This test is a valid and reliable method for the estimation of VO2 max in the field (Bandyopadhyay, 2015). Another limitation of this study is that it was conducted only among men and the results may not be generalizable to women. This trial's strengths were the triple‐blind design, high compliance rates, matched control, the similarity in terms of living place, physical activities, meals, and sleep duration among all participants, minimal drop‐out rates, and adjustment for potential confounding factors.
In conclusion, our findings demonstrate that supplementation with propolis might have beneficial effects on oxidative stress and inflammation status following intense physical activities while not affecting athletic performance in healthy active subjects. Further studies are needed to ascertain the effect of propolis on exercise performance in trained and nontrained subjects.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
ETHICS APPROVAL
The trial protocol was approved by the research ethics committee by the ethics committee at the AJA University of Medical Sciences (IR.AJAUMS.REC.1399.107). All patients provided written consent for participation in this study.
ACKNOWLEDGMENTS
The authors would like to thank the AJA University of Medical Sciences for the financial support of this research. We also sincerely appreciate all those who participated in this study.
Soleimani D, Miryan M, Hadi V, et al. Effect of propolis supplementation on athletic performance, body composition, inflammation, and oxidative stress following intense exercise: A triple‐blind randomized clinical trial. Food Sci Nutr. 2021;9:3631–3640. 10.1002/fsn3.2319
Funding information
This work was supported by the Vice‐Chancellor for Research at the AJA University of Medical Sciences, Tehran, Iran (ID: 97001031).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Adamczyk, J. (2011). The estimation of the RAST test usefulness in monitoring the anaerobic capacity of sprinters in athletics. Polish Journal of Sport and Tourism, 18(3), 214–218. 10.2478/v10197-011-0017-3 [DOI] [Google Scholar]
- Afsharpour, F. , Hashemipour, S. , Khadem‐Haghighian, H. , & Koushan, Y. (2017). Effects of Iranian propolis on glucose metabolic changes, inflammatory factors, liver enzymes levels in type 2 diabetic patients: A randomized, double‐blind, placebo‐controlled, clinical trial. Journal of Nutrition Science Dietetics, 3(2), 10. [Google Scholar]
- Bandyopadhyay, A. (2015). Validity of Cooper's 12‐minute run test for estimation of maximum oxygen uptake in male university students. Biology of Sport, 32(1), 59–63. 10.5604/20831862.1127283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankova, V. (2005). Chemical diversity of propolis and the problem of standardization. Journal of Ethnopharmacology, 100(1–2), 114–117. 10.1016/j.jep.2005.05.004 [DOI] [PubMed] [Google Scholar]
- Buico, A. , Cassino, C. , Ravera, M. , Betta, P. G. , & Osella, D. (2009). Oxidative stress and total antioxidant capacity in human plasma. Redox Report, 14(3), 125–131. 10.1179/135100009x392557 [DOI] [PubMed] [Google Scholar]
- Chow, C. K. (1979). Nutritional influence on cellular antioxidant defense systems. American Journal of Clinical Nutrition, 32(5), 1066–1081. 10.1093/ajcn/32.5.1066 [DOI] [PubMed] [Google Scholar]
- Cobley, J. N. , McHardy, H. , Morton, J. P. , Nikolaidis, M. G. , & Close, G. L. (2015). Influence of vitamin C and vitamin E on redox signaling: Implications for exercise adaptations. Free Radical Biology and Medicine, 84, 65–76. 10.1016/j.freeradbiomed.2015.03.018 [DOI] [PubMed] [Google Scholar]
- Cooper, K. H. (1968). A means of assessing maximal oxygen intake. Correlation between field and treadmill testing. JAMA, 203(3), 201–204. 10.1001/jama.1968.03140030033008 [DOI] [PubMed] [Google Scholar]
- Correa, F. , Ljunggren, E. , Mallard, C. , Nilsson, M. , Weber, S. G. , & Sandberg, M. (2011). The Nrf2‐inducible antioxidant defense in astrocytes can be both up‐ and down‐regulated by activated microglia: Involvement of p38 MAPK. Glia, 59(5), 785–799. 10.1002/glia.21151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, J. M. , Carlstedt, C. J. , Chen, S. , Carmichael, M. D. , & Murphy, E. A. (2010). The dietary flavonoid quercetin increases VO(2max) and endurance capacity. International Journal of Sport Nutrition and Exercise Metabolism, 20(1), 56–62. 10.1123/ijsnem.20.1.56 [DOI] [PubMed] [Google Scholar]
- Franchin, M. , Freires, I. A. , Lazarini, J. G. , Nani, B. D. , da Cunha, M. G. , Colón, D. F. , de Alencar, S. M. , & Rosalen, P. L. (2018). The use of Brazilian propolis for discovery and development of novel anti‐inflammatory drugs. European Journal of Medicinal Chemistry, 153, 49–55. 10.1016/j.ejmech.2017.06.050 [DOI] [PubMed] [Google Scholar]
- Fukuda, T. , Fukui, M. , Tanaka, M. , Senmaru, T. , Iwase, H. , Yamazaki, M. , Aoi, W. , Inui, T. , Nakamura, N. , & Marunaka, Y. (2015). Effect of Brazilian green propolis in patients with type 2 diabetes: A double‐blind randomized placebo‐controlled study. Biomed Rep, 3(3), 355–360. 10.3892/br.2015.436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesami, S. , Hashemipour, S. , Shiri‐Shahsavar, M. R. , Koushan, Y. , & Khadem Haghighian, H. (2019). Administration of Iranian Propolis attenuates oxidative stress and blood glucose in type II diabetic patients: A randomized, double‐blind, placebo‐controlled, clinical trial. Caspian Journal of Internal Medicine, 10(1), 48–54. 10.22088/cjim.10.1.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S. , Zhang, C. P. , Wang, K. , Li, G. Q. , & Hu, F. L. (2014). Recent advances in the chemical composition of propolis. Molecules, 19(12), 19610–19632. 10.3390/molecules191219610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, H. , Hood, D. A. , & Gurd, B. J. (2020). Looking beyond PGC‐1α: Emerging regulators of exercise‐induced skeletal muscle mitochondrial biogenesis and their activation by dietary compounds. Applied Physiology, Nutrition and Metabolism, 45(1), 11–23. 10.1139/apnm-2019-0069 [DOI] [PubMed] [Google Scholar]
- Jin, X. , Liu, Q. , Jia, L. , Li, M. , & Wang, X. (2015). Pinocembrin attenuates 6‐OHDA‐induced neuronal cell death through Nrf2/ARE pathway in SH‐SY5Y cells. Cellular and Molecular Neurobiology, 35(3), 323–333. 10.1007/s10571-014-0128-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourkesh, M. , Ostojic, S. M. , & Azarbayjani, M. A. (2007). The effects of vitamin E and vitamin C supplementation on bioenergetics index. Res Sports Med, 15(4), 249–256. 10.1080/15438620701693249 [DOI] [PubMed] [Google Scholar]
- Joyner, M. J. , & Casey, D. P. (2015). Regulation of increased blood flow (hyperemia) to muscles during exercise: A hierarchy of competing physiological needs. Physiological Reviews, 95(2), 549–601. 10.1152/physrev.00035.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayyal, M. T. , El‐Ghazaly, M. A. , El‐Khatib, A. S. , Hatem, A. M. , de Vries, P. J. F. , El‐Shafei, S. , & Khattab, M. M. (2003). A clinical pharmacological study of the potential beneficial effects of a propolis food product as an adjuvant in asthmatic patients. Fundamental & Clinical Pharmacology, 17(1), 93–102. 10.1046/j.1472-8206.2003.00117.x [DOI] [PubMed] [Google Scholar]
- Kwon, T. D. , Lee, M. W. , & Kim, K. H. (2014). The effect of exercise training and water extract from propolis intake on the antioxidant enzymes activity of skeletal muscle and liver in rat. J Exerc Nutrition Biochem, 18(1), 9–17. 10.5717/jenb.2014.18.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle, U. G. , Bosaeus, I. , De Lorenzo, A. D. , Deurenberg, P. , Elia, M. , Gómez, J. M. , & Pichard, C. (2004). Bioelectrical impedance analysis–part I: Review of principles and methods. Clinical Nutrition, 23(5), 1226–1243. 10.1016/j.clnu.2004.06.004 [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh, C. , & Heinecke, J. W. (2001). Oxidative stress and antioxidants in exercise. Current Medicinal Chemistry, 8(7), 829–838. 10.2174/0929867013372896 [DOI] [PubMed] [Google Scholar]
- Mason, S. A. , Trewin, A. J. , Parker, L. , & Wadley, G. D. (2020). Antioxidant supplements and endurance exercise: Current evidence and mechanistic insights. Redox Biology, 35, 101471. 10.1016/j.redox.2020.101471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki, H. , Oh‐ishi, S. , Ookawara, T. , Kizaki, T. , Toshinai, K. , Ha, S. , Haga, S. , Ji, L. L. , & Ohno, H. (2001). Strenuous endurance training in humans reduces oxidative stress following exhausting exercise. European Journal of Applied Physiology, 84(1–2), 1–6. 10.1007/s004210000342 [DOI] [PubMed] [Google Scholar]
- Mujica, V. , Orrego, R. , Pérez, J. , Romero, P. , Ovalle, P. , Zúñiga‐Hernández, J. , Arredondo, M. , & Leiva, E. (2017). The Role of Propolis in Oxidative Stress and Lipid Metabolism: A Randomized Controlled Trial. Evidence‐Based Complementary and Alternative Medicine: Ecam, 2017, 4272940. 10.1155/2017/4272940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemet, D. , Oh, Y. , Kim, H. S. , Hill, M. , & Cooper, D. M. (2002). Effect of intense exercise on inflammatory cytokines and growth mediators in adolescent boys. Pediatrics, 110(4), 681–689. 10.1542/peds.110.4.681 [DOI] [PubMed] [Google Scholar]
- Paulsen, G. , Cumming, K. T. , Holden, G. , Hallén, J. , Rønnestad, B. R. , Sveen, O. , Skaug, A. , Paur, I. , Bastani, N. E. , Østgaard, H. N. , Buer, C. , Midttun, M. , Freuchen, F. , Wiig, H. , Ulseth, E. T. , Garthe, I. , Blomhoff, R. , Benestad, H. B. , & Raastad, T. (2014). Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: A double‐blind, randomised, controlled trial. Journal of Physiology, 592(8), 1887–1901. 10.1113/jphysiol.2013.267419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers, S. K. , & Jackson, M. J. (2008). Exercise‐induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiological Reviews, 88(4), 1243–1276. 10.1152/physrev.00031.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers, S. K. , Radak, Z. , & Ji, L. L. (2016). Exercise‐induced oxidative stress: Past, present and future. Journal of Physiology, 594(18), 5081–5092. 10.1113/jp270646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, M. B. (2001). Nitric oxide, reactive oxygen species, and skeletal muscle contraction. Medicine and Science in Sports and Exercise, 33(3), 371–376. 10.1097/00005768-200103000-00006 [DOI] [PubMed] [Google Scholar]
- Roberts, L. A. , Beattie, K. , Close, G. L. , & Morton, J. P. (2011). Vitamin C consumption does not impair training‐induced improvements in exercise performance. International Journal of Sports Physiology and Performance, 6(1), 58–69. 10.1123/ijspp.6.1.58 [DOI] [PubMed] [Google Scholar]
- Samadi, N. , Mozaffari‐Khosravi, H. , Rahmanian, M. , & Askarishahi, M. (2017). Effects of bee propolis supplementation on glycemic control, lipid profile and insulin resistance indices in patients with type 2 diabetes: A randomized, double‐blind clinical trial. Journal of Integrative Medicine, 15(2), 124–134. 10.1016/s2095-4964(17)60315-7 [DOI] [PubMed] [Google Scholar]
- Sforcin, J. M. (2016). Biological properties and therapeutic applications of propolis. Phytotherapy Research, 30(6), 894–905. 10.1002/ptr.5605 [DOI] [PubMed] [Google Scholar]
- Shang, H. , Srikanth Bhagavathula, A. , Ali Aldhaleei, W. , Rahmani, J. , Karam, G. , Rinaldi, G. , Clark, C. , Salehisahlabadi, A. , & Yuan, Q. (2020). Effect of propolis supplementation on C‐reactive protein levels and other inflammatory factors: A systematic review and meta‐analysis of randomized controlled trials. Journal of King Saud University‐Science, 32(2), 1694–1701. 10.1016/j.jksus.2020.01.003 [DOI] [Google Scholar]
- Shen, Y. C. , Yen, J. C. , & Liou, K. T. (2013). Ameliorative effects of caffeic acid phenethyl ester on an eccentric exercise‐induced skeletal muscle injury by down‐regulating NF‐κb mediated inflammation. Pharmacology, 91(3–4), 219–228. 10.1159/000348412 [DOI] [PubMed] [Google Scholar]
- Soleimani, D. , Miryan, M. , Tutunchi, H. , Navashenaq, J. G. , Sadeghi, E. , Ghayour‐Mobarhan, M. , Ferns, G. A. , & Ostadrahimi, A. (2021). A systematic review of preclinical studies on the efficacy of propolis for the treatment of inflammatory bowel disease. Phytotherapy Research, 35(2), 701–710. 10.1002/ptr.6856 [DOI] [PubMed] [Google Scholar]
- Soleimani, D. , Rezaie, M. , Rajabzadeh, F. , Gholizadeh Navashenaq, J. , Abbaspour, M. , Miryan, M. , & Nematy, M. (2021). Protective effects of propolis on hepatic steatosis and fibrosis among patients with nonalcoholic fatty liver disease (NAFLD) evaluated by real‐time two‐dimensional shear wave elastography: A randomized clinical trial. Phytotherapy Research, 35(3), 1669–1679. 10.1002/ptr.6937 [DOI] [PubMed] [Google Scholar]
- Steinbacher, P. , & Eckl, P. (2015). Impact of oxidative stress on exercising skeletal muscle. Biomolecules, 5(2), 356–377. 10.3390/biom5020356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel, N. A. , Peake, J. M. , Matsumoto, A. , Marsh, S. A. , Coombes, J. S. , & Wadley, G. D. (2011). Antioxidant supplementation reduces skeletal muscle mitochondrial biogenesis. Medicine and Science in Sports and Exercise, 43(6), 1017–1024. 10.1249/MSS.0b013e318203afa3 [DOI] [PubMed] [Google Scholar]
- Suzuki, K. , Tominaga, T. , Ruhee, R. T. , & Ma, S. (2020). Characterization and modulation of systemic inflammatory response to exhaustive exercise in relation to oxidative stress. Antioxidants (Basel), 9(5), 401. 10.3390/antiox9050401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, K. , Jin, X. , Li, Q. , Sawaya, A. , Le Leu, R. K. , Conlon, M. A. , & Hu, F. (2018). Propolis from Different Geographic Origins Decreases Intestinal Inflammation and Bacteroides spp. Populations in a Model of DSS‐Induced Colitis. Molecular Nutrition & Food Research, 62(17), e1800080. 10.1002/mnfr.201800080 [DOI] [PubMed] [Google Scholar]
- Zagatto, A. M. , Beck, W. R. , & Gobatto, C. A. (2009). Validity of the running anaerobic sprint test for assessing anaerobic power and predicting short‐distance performances. Journal of Strength and Conditioning Research, 23(6), 1820–1827. 10.1519/JSC.0b013e3181b3df32 [DOI] [PubMed] [Google Scholar]
- Zakerkish, M. , Jenabi, M. , Zaeemzadeh, N. , Hemmati, A. A. , & Neisi, N. (2019). The effect of Iranian propolis on glucose metabolism, lipid profile, insulin resistance, renal function and inflammatory biomarkers in patients with type 2 diabetes mellitus: a randomized double‐blind clinical trial. Scientific Reports, 9(1), 7289. 10.1038/s41598-019-43838-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Cao, X. , Ping, S. , Wang, K. , Shi, J. , Zhang, C. , Zheng, H. , & Hu, F. (2015). Comparisons of ethanol extracts of chinese propolis (poplar type) and poplar gums based on the antioxidant activities and molecular mechanism. Evidence‐Based Complementary and Alternative Medicine: Ecam, 2015, 307594. 10.1155/2015/307594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, L. , Pu, L. , Wei, J. , Li, J. , Wu, J. , Xin, Z. , Gao, W. , & Guo, C. (2016). Brazilian Green Propolis Improves Antioxidant Function in Patients with Type 2 Diabetes Mellitus. International Journal of Environmental Research and Public Health, 13(5), 498– 10.3390/ijerph13050498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Y. Z. , Deng, G. , Liang, Q. , Chen, D. F. , Guo, R. , & Lai, R. C. (2017). Antioxidant Activity of Quercetin and Its Glucosides from Propolis: A Theoretical Study. Scientific Reports, 7(1), 7543. 10.1038/s41598-017-08024-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


