1. Introduction
Immunodeficiency is a hallmark of CLL and the cause of much morbidity and mortality. As modern treatments increasingly provide long-term survival, the question how one might restore immune function is taking center stage. Here we first review disease and treatment related factors that contribute to immunodeficiency and then address strategies that may boost immune function. Encouragingly, novel therapies are often less immunosuppressive than prior approaches using chemotherapy. At the same time, the advent of the coronavirus disease 2019 (COVID-19) pandemic heightens the urgency of developing effective strategies to protect patients with CLL from infection. Hopefully the past 10 years of progress in treating CLL can be the harbinger of forthcoming progress in restoring immunocompetence for patients.
2. Disease-Related Immunodeficiency
2.1. Adaptive Immunity
Hypogammaglobulinemia
Hypogammaglobulinemia is a common contributor to immunosuppression in patients with advanced CLL (Figure 1).1 Immunoglobulin G (IgG) and its subclasses, IgG1, IgG2, IgG3, and IgG4, as well as IgA and IgM may be deficient.2 The severity of hypogammaglobulinemia is correlated with stage, duration of disease, and susceptibility to severe and recurrent infections.1,3 Both malignant and non-malignant immune cells appear to suppress the normal antibody response. Co-culture experiments have shown that Fas/Fas ligand interactions between tumor cells and bone marrow plasma cells inhibit antibody production.4 T and natural killer cells from CLL patients decrease antibody secretion by activated B cells from healthy donors.5–7 CD30+ T cells, which are frequently expanded in CLL, inhibit isotype switching to IgG and IgA in nonclonal B cells.8 In addition, there are fewer newly produced B cells, and consequently a smaller pool of antibody-producing cells, in CLL patients compared to healthy controls.9
Figure 1. Components of immunodeficiency in CLL.
Patients with CLL have abnormal innate and adaptive immunity. Innate immune defects include reduced levels of complement, neutropenia related to treatment or less commonly, bone marrow infiltration of CLL, and increased MDSCs, which inhibit T-cell responses. Adaptive immune defects include hypogammaglobulinemia, Th2 polarization, T-cell expression of inhibitory receptors such as PD-1 and CTLA-4, and loss of immune synapse formation between T cells and target cells.
Cell-Mediated Immunity
The T-cell compartment in patients with CLL is simultaneously immunosuppressive and tumor supportive. CD8+ T cells highly express inhibitory receptors and have diminished proliferative capacity (Figure 1).10 Abnormalities in granzyme packaging, degranulation, and immune synapse formation reduce the cytolytic activity of CD8+ T cells (Figure 1).10,11 CD4+ T cells are polarized toward an immunosuppressive Th2 phenotype.12,13
In the tumor microenvironment, T cells interact directly with CLL cells via CD40L-CD40 and secrete soluble factors, such as interleukin-4 (IL-4) and interferon-gamma (IFN- γ), which promote tumor survival and proliferation.14–16 Autologous CD4+ T cells have also been shown to facilitate engraftment and clonal expansion of CLL cells in patient-derived xenografts.17
2.2. Innate Immunity
Clearance of pathogens, in particular of encapsulated bacteria, requires opsonization by the complement system.18 Complement deficiency is frequently observed in patients with CLL and affects components of the classical, alternative, and terminal pathways (Figure 1).19 Patients deficient in one or more complement components are more susceptible to infection and have shorter overall survival.19,20 In addition, low levels of complement have been shown to limit complement-dependent cytotoxicity of anti-CD20 monoclonal antibodies against primary CLL cells.21
Neutropenia caused by bone marrow infiltration of CLL cells, albeit less common than anemia and thrombocytopenia, can be a complication of active disease and an indication for treatment (Figure 1).22 More often, neutropenia is a treatment related toxicity. Grade ≥3 neutropenia affects approximately one-third of patients treated with chemoimmunotherapy,23 half of patients treated with venetoclax and anti-CD20 mAb, and 10% of patient treated with ibrutinib monotherapy.24 Qualitative defects in neutrophil function have also been reported.25,26
Myeloid derived suppressor cells (MDSCs) expand regulatory T cells, inhibit T-cell activation, and thereby suppress immune surveillance.27–29 These cells are present at increased frequency in CLL patients compared to healthy individuals and associated with more aggressive disease (Figure 1).30 MDSCs differentiate to tumor associated macrophages (TAMs), also referred to as nurse-like cells, in the tumor microenvironment.28 In co-culture experiments, TAMs have been shown to enhance CLL cell survival via direct contact and secretion of immunosuppressive cytokines.31,32
2.3. Clinical Manifestations
Infections
Patients with CLL are at increased risk of infection-related morbidity and mortality.33 In a retrospective study of 125 patients over a 10-year period, severe infections occurred in 26% of patients and accounted for 30% of deaths.34 Among patients with treatment naïve CLL, bacterial pneumonia, involving Streptococcus pneumonia, Staphylococcus aureus, and Hemophilus influenza, is the most common serious infection.35 Recurrent infections may evolve into chronic sinusitis and bronchiectasis.36 Other sites of infection in order of frequency are the upper respiratory tract, genitourinary tract, blood, skin and soft tissue, gastrointestinal tract, and central nervous system.37
The severity of hypogammaglobulinemia correlates with infection risk.38 IgG levels <3 g/L confer a high risk of infections and depressed IgA was associated with inferior survival independent of disease stage.39,40
Defective cell-mediated immunity increases the frequency and severity of herpes family virus infection and reactivation.41 Disseminated and atypical herpes simplex 1 or 2 and herpes zoster, such as that involving the eye or liver, have been described.42,43 Cytomegalovirus (CMV) disease and Epstein-Barr associated lymphoproliferative disorders are rare in untreated patients with CLL.44
In a retrospective cohort study of patients with hematologic malignancies, there were 5 cases of invasive fungal infections (IFIs) among 1,104 newly diagnosed CLL patients over a 5-year period.45 All cases were caused by Aspergillus sp. and resulted in death in 4 of 5 patients.45 Uncontrolled malignancy, severe lymphopenia, CMV disease, prolonged neutropenia, and recent history of IFIs have been identified as treatment-independent risk factors for IFIs.46 Pneumocystis jirovecii pneumonia (PJP) is uncommonly reported in treatment-naïve patients and more frequent in patients treated with purine analogs or steroids.47
Second Primary Malignancies
The incidence of second primary malignancies (SPMs) in patients with CLL is increased. Disease, host, environment, and treatment-related factors influence malignancy type and overall risk. Overall, non-melanoma skin cancers are most common, followed by solid tumors and second lymphoid malignancies, including Richter transformation. Richter transformation is variably considered a SPM and can be a leading second cancer diagnosis in patients treated with chemoimmunotherapy.48,49 Myeloid neoplasms are overall less common but may develop as a complication of genotoxic therapy, accounting for up to 10–14% of all second cancers in patients treated with fludarabine, cyclophosphamide, with or without rituximab.49,50 In recent series, standardized incidence ratios of SPM in CLL patients range from 1.19 to 2.2.51–54
T-cell dysfunction is thought to contribute to increased incidence of SPMs in patients with CLL.51,53 Epidemiological support for a role of immune dysfunction is the increased relative risk for SPMs in patients with CLL that are more common in immunosuppressed patients including melanoma, kidney cancer, Hodgkin Lymphoma and the virally induced Merkel cell carcinoma and Kaposi sarcoma.53 A disease related contribution to cancer risk is also suggested by the observation that patients with CLL have an increased risk of lung cancer and melanoma but patients with DLBCL do not.55 In Australia, a country with high levels of ultraviolet radiation exposure, the standardized incidence of melanoma in patients with CLL is 7.7.54
Whether targeted therapy changes the risk of SPMs is unclear. Patients on BTKi have an increased incidence of SPMs compared to data in the Surveillance, Epidemiology, and End Results (SEER) Program.51 However, the majority of these patients had CLL for many years and were previously treated with alkylating agents or purine analogs; 44% had a smoking history. In the front-line CLL14 trial that compared venetoclax with obinutuzumab versus chlorambucil with obinutuzumab, the reported rate of SPMs was 13.7% versus 10.3% of patients, respectively.56 Additional studies will be needed to estimate the impact of targeted therapy on the risk of SPMs.
3. Treatment-Related Immune Changes
3.1. Anti-CD20 Antibodies
Anti-CD20 antibody therapy depletes normal B cells for several months but does not directly impact plasma cells. Rituximab use has been associated with development or worsening of hypogammaglobulinemia, especially in patients receiving maintenance therapy.57 A confounding factor is the frequent co-administration of chemotherapy. Overall, anti-CD20 antibodies are well tolerated. Of specific clinical importance is the risk of hepatitis B reactivation; all patients should be screened for past or active infection before the initiation of anti-CD20 antibody therapy.58 Retrospective case series uncovered an increased incidence of progressive multifocal leukoencephalopathy (PML), which remains a rare occurrence.59 As a consequence of B cell depletion lasting many months beyond the last infusion, anti-CD20 therapy is thought to interfere with humoral vaccine responses.60–62 Interestingly, cellular vaccine response was not affected by B cell depletion.61
3.2. Chemotherapy and Chemoimmunotherapy
Purine analogs can profoundly impact immune function ranging from an increased risk of opportunistic infections to precipitation of autoimmune cytopenia. Use of purine analogs is associated with neutropenia and lymphopenia and CD4 counts can remain depressed for years after conclusion of treatment.60 Chemoimmunotherapy was the mainstay of CLL therapy for many years and has been associated with increased incidence of neutropenic and opportunistic infections including PJP and herpes virus reactivations.63
3.3. Phosphatidylinositol-3-Kinase Inhibitors
The phosphatidylinositol-3-kinase (PI3K) inhibitors idelalisib and duvelisib are approved in the United States for the treatment of relapsed CLL and follicular lymphoma.64,65 Both drugs target the PI3K-δ isoform in CLL cells that participates in signal transduction downstream of the BCR.66 Duvelisib also inhibits PI3K-γ expressed by non-malignant immune cells in the tumor microenvironment.67,68
Treatment with idelalisib increases the risk of PJP and CMV disease.69,70 Higher mortality from opportunistic and bacterial infections among patients treated with idelalisib-based combination therapies resulted in the early termination of six clinical trials for CLL and indolent non-Hodgkin lymphoma.71,72 Despite consensus recommendations for PJP prophylaxis, a survey of Medicare beneficiaries found that less than 40% received prophylaxis during idelalisib therapy.73
Hepatotoxicity, colitis, pneumonitis, and rash are immune-related toxicities associated with PI3K inhibitors.74 Quantitative and qualitive reductions in regulatory T-cell (Treg) function have been implicated. Murine models of PI3Kδ loss have shown that impaired Treg function leads to a CD8+ T-cell response and fatal autoimmune colitis.75–77 Patients experiencing immune-related toxicities have decreased circulating Treg cells and increased infiltrating CD8+ T cells in affected tissues.78,79
3.4. BTK Inhibitors
Ibrutinib and acalabrutinib are oral irreversible inhibitors of Bruton tyrosine kinase (BTK) and widely used to treat CLL. The risk of infection is highest during the first 6 months, then improves with extended treatment.80–82 This improvement correlates with an increase in IgA, which may be an indicator or surrogate of humoral immune reconstitution (Figure 2).81,82 Compared to chemoimmunotherapy, neutropenia and febrile neutropenia are less common among patients receiving BTK inhibitor therapy.83
Figure 2. Immunologic alterations during treatment with ibrutinib.
Ibrutinib increases the level of serum IgA, restores immune synapses between T cells and CLL cells, and inhibits Th17 differentiation. Ibrutinib also reduces the frequency of MDSCs as well as their secretion of immunosuppressive factors such as NO and TNFα and migration toward chemokines such as CCL3 and CXCL12. Many inflammatory cytokines in the serum decrease on ibrutinib, including IFN-χ, IL-17, IL-4, IL-6 and TNFα.
IFIs, predominantly involving aspergillus, have been reported in patients receiving ibrutinib.84,85 Most patients who develop IFIs have predisposing factors including neutropenia, corticosteroid use, and antecedent chemotherapy.84 Nevertheless, susceptibility to invasive aspergillosis has been demonstrated in BTK knockout mice.85 In addition, patients treated with single-agent ibrutinib can develop PJP, mostly presenting with mild symptoms or radiographic changes only.86
Ibrutinib reverses some aspects of T-cell dysfunction in CLL. Downregulation of inhibitory receptors, restoration of immune synapses, and suppression of Th2 differentiation are among the favorable changes observed (Figure 2).87–89 These changes appear to benefit patients treated with T-cell directed immunotherapy. For example, expansion of autologous anti-CD19 chimeric antigen receptor (CAR) T-cells is enhanced by antecedent treatment with ibrutinib.90 Concurrent ibrutinib reduces the severity of cytokine release syndrome during CAR T-cell therapy.91 Bispecific antibodies engage T cells and CLL cells simultaneously to facilitate cell-mediated lysis of tumor cells. Prior therapy with ibrutinib increases CLL cell killing by autologous T cells in response to a bispecific antibody targeting CD3 and CD19 in vitro and in vivo.92 Interestingly, the benefit of ongoing therapy with ibrutinib was still maintained in patients with early progressive disease and mutations in BTK or PLCG2 that have been associated with ibrutinib resistance.92 Others have observed increased efficacy of a ROR1 targeting BITE in PBMCs from ibrutinib-treated patients.93 While the mechanism for improved T cell cytotoxicity still requires additional studies, improved immune synapse formation between T cells and CLL cells in ibrutinib-treated patients may be partially responsible.93,94 Preclinical testing suggests that coadministration of acalabrutinib could also enhance T cell cytotoxic functions.95
3.5. Venetoclax
Venetoclax is effective in relapsed refractory and frontline settings.22,56 Venetoclax has been combined with anti-CD20 antibodies,22,56 and investigated in combination with ibrutinib.96 Tolerability of these regimens has generally been good. Neutropenia is a common adverse event on venetoclax. The rate of neutropenia in patients treated with venetoclax and obinutuzumab or chlorambucil and obtinutuzumab was comparable, as was the rate of febrile neutropenia at 5.2% versus 3.7%,respectively.56 Little is known about the impact of venetoclax on specific immune responses. Combined treatment with venetoclax and obinutuzumab reduced healthy B cells, T cells and NK cells.97 The CD4/CD8 ratio and differentiation state of T cells did not change during therapy.
3.6. Lenalidomide
Lenalidomide, an analog of thalidomide and commonly used agent in patients with multiple myeloma, has been extensively studied in CLL.98 Although lenalidomide has considerable anti-CLL activity, its adoption has been hampered by concerns about toxicity, predominantly with regard to infections and SPMs.99,100 Paradoxically, lenalidomide exerts multiple and potentially beneficial immunologic effects in patients with CLL.101 Lenalidomide upregulates costimulatory molecules on tumor cells, improves T-cell immune synapse formation, and enhances cytotoxic effector functions.11,102 At treatment initiation, activation of T cells results in tumor flare reactions with release of inflammatory cytokines, which often manifests clinically as painful swelling of lymphoid tissues, fever, and malaise.102,103 The degree of this tumor flare reaction has been correlated with improved treatment response and superior progression-free survival.104,105 In paired lymph node biopsies obtained from patients with CLL pre-treatment and on day 8 of lenalidomide therapy, we observed increased tumor infiltration by T cells and a shift towards Th1 polarization that positively correlated with anti-tumor response.106 Maintenance therapy with lenalidomide after chemoimmunotherapy has been shown to lead to sustained improvements in T-cell responses and to delay disease progression.107–109
Lenalidomide stimulates the production of immunoglobulins by normal B cells. During treatment with lenalidomide, IgG, IgA, and IgM increase, albeit with considerable interpatient variability.104,110 There was no correlation between serum Ig levels and the rate of infections in a small cohort of patients.104 Mechanistically, class switching and increased antibody secretion from normal B cells depends on lenalidomide mediated upregulation of CD154, the ligand of CD40, on CLL cells. Further studies will hopefully reveal how to harness the beneficial effects and avoid concerning toxicities of this class of agents.
4. Strategies to Boost Immunity
4.1. Vaccinations
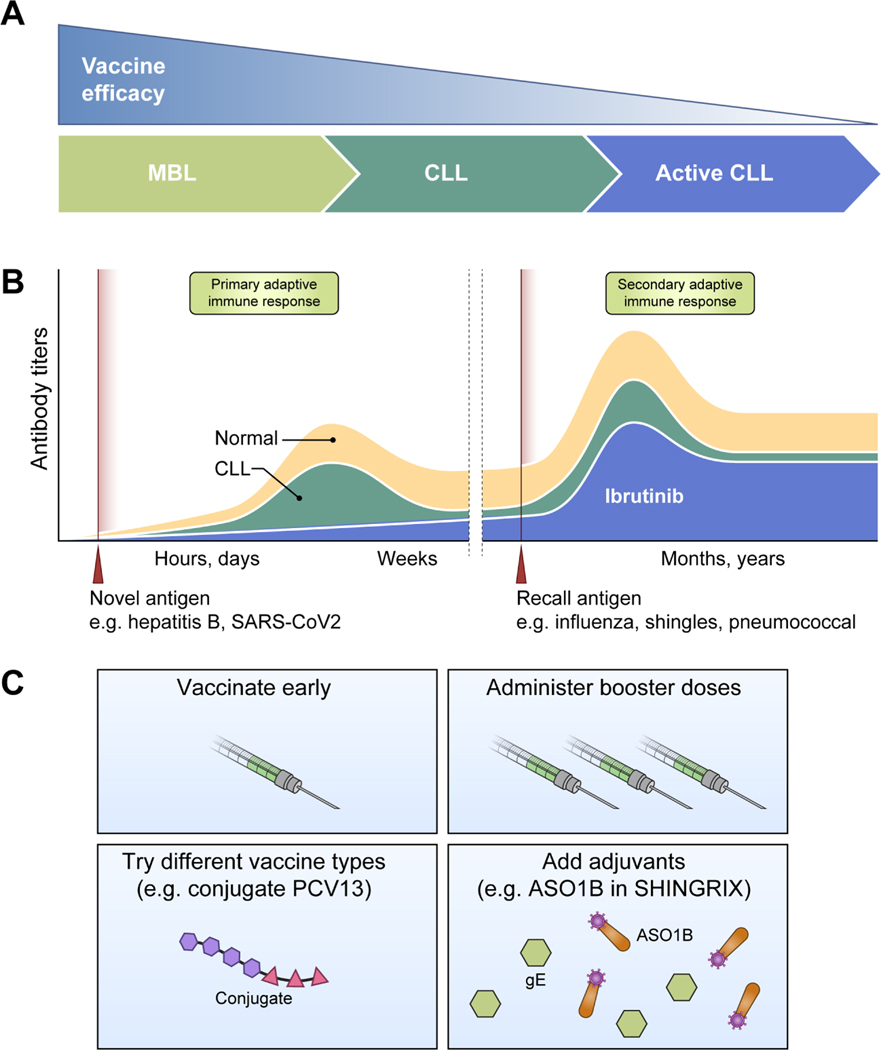
Patients with CLL have consistently demonstrated a weakened and less durable response to immunizations (Figure 3). Patient factors associated with blunted vaccine responses include advanced disease, older age, and hypogammaglobulinemia.111–113 Conversely, patients with monoclonal B lymphocytosis, a precursor state of CLL, mount a better response to influenza vaccination than patients with CLL.114 From a safety perspective, live attenuated vaccines carry a risk of virus replication and disease in immunocompromised individuals. Patients with CLL have died from disseminated varicella zoster virus (VZV) infection after administration of live attenuated zoster vaccine.115,116 Subunit, recombinant, polysaccharide, conjugate, or mRNA vaccines and those comprised of inactivated virus, and toxins do not have this risk. Taken together, patients with CLL should receive non-live vaccinations at diagnosis when immunity is least suppressed by disease and subsequent therapies (Figure 3).
Figure 3. Vaccination overview in CLL.
(A) Vaccine efficacy diminishes as patients progress from MBL to CLL to active disease requiring treatment. (B) The antibody response to a vaccine depends on treatment history and whether the vaccine antigen induces a primary or secondary adaptive immune response. Patients with CLL have an impaired response to immunization compared to the general population. Treatment with a BTK inhibitor further suppresses this response, particularly against novel vaccine antigens such as hepatis B. (C) Potential strategies to improve vaccine response include vaccination early in diagnosis, administration of booster doses, modification of vaccine type, and addition of adjuvants to increase immunogenicity.
Vaccine types have evolved with the aim of eliciting better responses with variable success for patients with CLL (Figure 3). For example, conjugation of polysaccharide to a carrier generates a T-cell dependent humoral response and improves immunological memory compared to polysaccharide only vaccine.117 A randomized study of the 13-valent pneumococcal conjugate vaccine (PCV13) versus the 23-valent pneumococcal polysaccharide vaccine (PPSV23) in 128 patients with treatment naïve CLL found higher antibody titers against 10 of 12 serotypes one month after PCV13.118 Nevertheless, only 58.3% of patients with treatment naïve CLL develop an adequate antibody response to PCV13 compared to 100% in healthy subjects.119
Other potential strategies to enhance vaccine response in patients with CLL are coadministration of drugs and booster doses (Figure 3). Lenalidomide modulates multiple facets of the immune system as described above. While concomitant lenalidomide increased serum immunoglobulins in patients with CLL, it did not improve the response to PCV13.120 Ranitidine has been investigated for its antagonistic effect on histamine, which inhibits antibody responses in mice.121 Ranitidine given concurrently with the Hemophilus influenza type B conjugated vaccine increased post-vaccination titers compared to controls.122,123 These published series are small, the benefit was limited to conjugated vaccines, and the concept was not further tested in larger studies. Vaccine type may determine the efficacy of booster vaccination. A booster dose of PCV13 administered 2 months after the initial vaccine resulted in seroconversion to at least 1 serotype in all participants.120 However, repeated vaccination with inactivated subunit influenza vaccine 3 weeks apart did not increase the rate of seroprotection or hemagglutinin inhibition titers.124
Due to its mechanism of action, BTK inhibitors have the potential to inhibit or abrogate the humoral immune response to vaccines. Early immunization studies in ibrutinib-treated patients tested the antibody response to influenza vaccination.125,126 This response depends on preexisting memory B cells from prior immunization or infection.127,128 Seroconversion was observed in 7% to 26% of patients, with a lower rate among patients who had received prior lines of therapy.125,126 Treatment with ibrutinib significantly reduced the antibody response to influenza vaccination and PCV13 compared to patients with untreated CLL (Figure 3).129
To test the humoral immune response against novel antigens, hepatitis B vaccination was administered to treatment naïve CLL patients and patients on ibrutinib or acalabrutinib.130 All patients had undetectable anti-HBs antibodies prior to vaccination and no history of hepatitis B infection or vaccination.130 While treatment naïve patients had an inferior response of 28.1% compared to >95% in healthy subjects, response was nearly absent (one responder, 3.8%) in patients treated with BTK inhibitor (Figure 3).130 In contrast, 59.1% of treatment-naïve patients and 41.5% of patients on a BTK inhibitor responded to varicella zoster vaccination, which induces a recall immune response. Consistent with relatively preserved recall responses, B-cell repertoire analysis of patients on ibrutinib have shown partial preservation of non-malignant antigen-experienced B cells during treatment.131
Vaccination prevents, and in some instances have eradicated, infections in the general population. Unfortunately, vaccine efficacy is diminished in patients with CLL who are particularly vulnerable to infectious complications. Studies to enhance vaccine response in CLL are urgently needed.
4.2. Immunoglobulin Replacement
Few treatments have been associated with improvements in Ig levels: lenalidomide has been found to increase IgG, IgA, and IgM, and BTK inhibitor therapy increases IgA levels in many patients. Thus the mainstay of improving hypogammaglobulinemia is exogenous administration of purified IgG pooled from donors. Immunoglobulin replacement therapy (IgRT) can reduce frequency of infections, rates of hospital admissions, and antibiotic usage. However, there is no demonstrated impact on (short-term) mortality and some question the cost-effectiveness of (routine) IgRT. However, others make the case that IgRT is the standard of care to prevent infections in patients with primary immunodeficiency disorders who share an increased susceptibility to the same infections and advanced hypogammaglobulinemia as patients with CLL.132
Randomized trials of IgRT in patients with CLL have used different inclusion criteria, dosage and frequency of administration (reviewed in 132). Overall, IgRT should be considered for patients with bacterial infections and IgG levels <4 g/L.132 While the risk of infection may increase at IgG levels <6g/L, one study reported 2/3 of infections in 1/4 of patients having IgG <3g/L.39 Immunoglobulin preparations dosed at 100–800mg/kg given intravenously every 3–5 weeks have been investigated. One study comparing 100mg/kg, 400mg/kg and 800mg/kg dose levels concluded 400mg/kg given every 3 weeks for 4 doses followed by maintenance every 5 weeks was optimal.133 Notably, this dose is lower than what may be required in patients with MM who have a more rapid clearance of immunoglobulins. One study reported that 50% of patients with breakthrough infections became infection-free when the dose was increased from 18 g to 24 g every 3 weeks, lending support to strategies that individualize dosing based on clinical outcomes.39 IgRT can be achieved with intravenous or subcutaneous administration. Subcutaneous administration of 75mg/kg weekly compared to intravenous 300mg/kg every 4 weeks achieved higher IgG trough levels and was associated with lower incidence of infections and less antibiotics usage. Subcutaneous obviates the need for intravenous access and offers the possibility of self-administration at home.
IgRT is typically well tolerated with minor systemic reactions after infusion or local irritation at the injection site with subcutaneous administration. Infrequent anaphylactoid reactions and an increased risk of thromboembolic events have been associated with intravenous administration.134 For a detailed discussion and proposed treatment algorithm for the use of IgRT we refer to the review by Lachance and colleagues.132
A variation of IgRT is convalescent plasma from donors who have recovered from COVID-19. Immunosuppressed patients may not be able to mount an antibody response leading to protracted COVID-19 infection.135,136 In this setting, administration of convalescent plasma containing high-titer neutralizing antibodies to a patient with CLL has been reported to resolve the infection.135 However, the titer of neutralizing antibodies in donor plasma can be highly variable and may be critical for success.135,137 Engineered monoclonal SARS-CoV-2-neutralizing antibodies have also been developed and could contribute to humoral anti-viral defense.138
5. Discussion
While the clinical sequelae of immunodeficiency in CLL are readily apparent, the mechanistic aspects of how CLL cells interfere with normal immune function still need to be better understood. BTK and PI3K kinase inhibitors have shown both beneficial and deterimental effects on humoral and cellular immune responses. There is still much to learn about how to best harness these properties for the treatment of CLL and to reverse or even prevent disease-related immunodeficiency in patients with CLL. At present, the best approaches may be to avoid immunosuppressive therapies, boost specific immune responses by vaccinating early in the diseae course, and to take appropriate supportive measures for patients with recurrent infections and severe hypogammaglobulinemia.
Early intervention trials have failed to show a survival benefit and observation in the absence of active disease remains the recommended approach to patients with CLL. Most endpoints in early intervention trials incorporate traditional response criteria especially PFS, exposing the trials to possible criticism that the outcome of comparing an active treatment regimen versus placebo is a forgone conclusion.139 It is tempting to speculate that early intervention could forestall the immunodeficiency that seems unvavoidable if the disease is left to run its course. On the other hand, early intervention, depending on the regimen chosen, could worsen immunodeficiency or expose patients unnecessarily to the risk of serious adverse events. Ideally, assessment of immune status would be incorporated as an objective for all clinical trials in CLL akin to safety and efficacy endpoints.
Notable among ongoing early interevention trials is the PreVent-ACaLL study that uses a recently developed scoring system to identify patients with untreated CLL who do not meet criteria for treatment initiation but are predicted to have a high risk of infection and/or need of CLL treatment within 2 years of diagnosis.140 Patients are randomized to observation versus 12 weeks of therapy with acalabrutinib and venetoclax. The primary endpoint is the rate of grade ≥3 infection-free, CLL treatment-free survival at 24 weeks.141 A possible extension of the observation period to 2 years from enrollment is considered. An emphasis on systematically testing immunorestorative interventions is required to build upon the significant therapeutic advancements that have already been made toward the outcome of patients with CLL.
6. Clinical Care Points
Patient with CLL are at increased risk for Infections and second primary malignancies due to their underlying immunodeficiency.
Vaccinations should be administered early in the disease course to improve the likelihood of an immune response.
Non-live virus vaccines are generally safe in patients with CLL and, while response rates tend to be lower than in the general population, are an important component of preventing infections.
Patients with IgG levels < 4g/L and history of bacterial infections may benefit from immunoglobulin replacement therapy to reduce the rate of infections and hospitalizations.
Patients should be counseled about the benefit of cancer screening, especially skin exams.
Key Points:
Immunodeficiency in CLL is caused by disease intrinsic and treatment related factors that impair adaptive and innate immunity.
Available treatments have differing impact on immune function in CLL: chemoimmunotherapy is associated with opportunistic infections and increased risk of myeloid malignancies; anti-CD20 antibody therapy and BTK inhibitors increase the risk of hepatitis B reactivation; neutropenia, but not neutropenic fever, is a frequent adverse event on venetoclax; oppoportunistic infections and autoimmune complications are increased on idelalisib.
Hypogamaglobulinemia generally worsens over the course of the disease and low levels of IgG are associated with an increased risk of infections.
BTK inhibitor therapy can improve aspects of T-cell function and promote a moderate increase in IgA that is associated with lower rates of infection.
Synopsis.
Reversing or preventing immunodeficiency in patients with chronic lymphocytic leukemia (CLL) is of the highest priority. The past decade of research has met the challenge of treating CLL for most patients. However, patients continue to struggle with infections and second primary malignancies related to immunodeficiency. Strategies addressing this need are currently limited to vaccinations, with suboptimal efficacy, and immunoglobulin replacement. Correlative studies have provided insights into immunologic alterations on treatment. Understanding vulnerabilities in the immune system may help identify potential interventions to boost immunity. An emphasis on systematically testing such interventions is required to restore immunocompetence in patients with CLL.
Acknowledgments
Disclosure Statement: C.S. received research funding from Genmab. A.W. received research funding from Pharmacyclics, an Abbvie company, Acerta Pharma, B.V., Merck, Nurix, Verastem, and Genmab.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hamblin AD, Hamblin TJ. The immunodeficiency of chronic lymphocytic leukaemia. Br Med Bull. 2008;87:49–62. [DOI] [PubMed] [Google Scholar]
- 2.Freeman JA, Crassini KR, Best OG, et al. Immunoglobulin G subclass deficiency and infection risk in 150 patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2013;54(1):99–104. [DOI] [PubMed] [Google Scholar]
- 3.Griffiths H, Lea J, Bunch C, Lee M, Chapel H. Predictors of infection in chronic lymphocytic leukaemia (CLL). Clin Exp Immunol. 1992;89(3):374–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sampalo A, Navas G, Medina F, Segundo C, Camara C, Brieva JA. Chronic lymphocytic leukemia B cells inhibit spontaneous Ig production by autologous bone marrow cells: role of CD95-CD95L interaction. Blood. 2000;96(9):3168–3174. [PubMed] [Google Scholar]
- 5.Kunicka JE, Platsoucas CD. Defective helper function of purified T4 cells and excessive suppressor activity of purified T8 cells in patients with B-cell chronic lymphocytic leukemia. T4 suppressor effector cells are present in certain patients. Blood. 1988;71(6):1551–1560. [PubMed] [Google Scholar]
- 6.Chiorazzi N, Fu SM, Montazeri G, Kunkel HG, Rai K, Gee T. T cell helper defect in patients with chronic lymphocytic leukemia. J Immunol. 1979;122(3):1087–1090. [PubMed] [Google Scholar]
- 7.Kay NE, Perri RT. Evidence that large granular lymphocytes from B-CLL patients with hypogammaglobulinemia down-regulate B-cell immunoglobulin synthesis. Blood. 1989;73(4):1016–1019. [PubMed] [Google Scholar]
- 8.Cerutti A, Kim EC, Shah S, et al. Dysregulation of CD30+ T cells by leukemia impairs isotype switching in normal B cells. Nat Immunol. 2001;2(2):150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motta M, Chiarini M, Ghidini C, et al. Quantification of newly produced B and T lymphocytes in untreated chronic lymphocytic leukemia patients. J Transl Med. 2010;8:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riches JC, Davies JK, McClanahan F, et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121(9):1612–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramsay AG, Johnson AJ, Lee AM, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest. 2008;118(7):2427–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Podhorecka M, Dmoszynska A, Rolinski J, Wasik E. T type 1/type 2 subsets balance in B-cell chronic lymphocytic leukemia--the three-color flow cytometry analysis. Leuk Res. 2002;26(7):657–660. [DOI] [PubMed] [Google Scholar]
- 13.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8(12):1353–1362. [DOI] [PubMed] [Google Scholar]
- 14.Buschle M, Campana D, Carding SR, Richard C, Hoffbrand AV, Brenner MK. Interferon gamma inhibits apoptotic cell death in B cell chronic lymphocytic leukemia. J Exp Med. 1993;177(1):213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dancescu M, Rubio-Trujillo M, Biron G, Bron D, Delespesse G, Sarfati M. Interleukin 4 protects chronic lymphocytic leukemic B cells from death by apoptosis and upregulates Bcl-2 expression. J Exp Med. 1992;176(5):1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schattner EJ, Mascarenhas J, Reyfman I, et al. Chronic lymphocytic leukemia B cells can express CD40 ligand and demonstrate T-cell type costimulatory capacity. Blood. 1998;91(8):2689–2697. [PubMed] [Google Scholar]
- 17.Bagnara D, Kaufman MS, Calissano C, et al. A novel adoptive transfer model of chronic lymphocytic leukemia suggests a key role for T lymphocytes in the disease. Blood. 2011;117(20):5463–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20(1):34–50. [DOI] [PubMed] [Google Scholar]
- 19.Schlesinger M, Broman I, Lugassy G. The complement system is defective in chronic lymphatic leukemia patients and in their healthy relatives. Leukemia. 1996;10(9):1509–1513. [PubMed] [Google Scholar]
- 20.Varga L, Czink E, Miszlai Z, et al. Low activity of the classical complement pathway predicts short survival of patients with chronic lymphocytic leukaemia. Clin Exp Immunol. 1995;99(1):112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Middleton O, Cosimo E, Dobbin E, et al. Complement deficiencies limit CD20 monoclonal antibody treatment efficacy in CLL. Leukemia. 2015;29(1):107–114. [DOI] [PubMed] [Google Scholar]
- 22.Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–2760. [DOI] [PubMed] [Google Scholar]
- 23.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. [DOI] [PubMed] [Google Scholar]
- 24.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med. 2015;373(25):2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kontoyiannis DP, Georgiadou SP, Wierda WG, et al. Impaired bactericidal but not fungicidal activity of polymorphonuclear neutrophils in patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2013;54(8):1730–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manukyan G, Papajik T, Gajdos P, et al. Neutrophils in chronic lymphocytic leukemia are permanently activated and have functional defects. Oncotarget. 2017;8(49):84889–84901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanna BS, McClanahan F, Yazdanparast H, et al. Depletion of CLL-associated patrolling monocytes and macrophages controls disease development and repairs immune dysfunction in vivo. Leukemia. 2016;30(3):570–579. [DOI] [PubMed] [Google Scholar]
- 28.Nagaraj S, Youn JI, Gabrilovich DI. Reciprocal relationship between myeloid-derived suppressor cells and T cells. J Immunol. 2013;191(1):17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giannoni P, Pietra G, Travaini G, et al. Chronic lymphocytic leukemia nurse-like cells express hepatocyte growth factor receptor (c-MET) and indoleamine 2,3-dioxygenase and display features of immunosuppressive type 2 skewed macrophages. Haematologica. 2014;99(6):1078–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gustafson MP, Abraham RS, Lin Y, et al. Association of an increased frequency of CD14+ HLA-DR lo/neg monocytes with decreased time to progression in chronic lymphocytic leukaemia (CLL). Br J Haematol. 2012;156(5):674–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herman SE, Wiestner A. Preclinical modeling of novel therapeutics in chronic lymphocytic leukemia: the tools of the trade. Semin Oncol. 2016;43(2):222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96(8):2655–2663. [PubMed] [Google Scholar]
- 33.Morrison VA. Infections in patients with leukemia and lymphoma. Cancer treatment and research. 2014;161:319–349. [DOI] [PubMed] [Google Scholar]
- 34.Molica S, Levato D, Levato L. Infections in chronic lymphocytic leukemia. Analysis of incidence as a function of length of follow-up. Haematologica. 1993;78(6):374–377. [PubMed] [Google Scholar]
- 35.Dearden C. Disease-specific complications of chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2008:450–456. [DOI] [PubMed] [Google Scholar]
- 36.Vanura K, Le T, Esterbauer H, et al. Autoimmune conditions and chronic infections in chronic lymphocytic leukemia patients at diagnosis are associated with unmutated IgVH genes. Haematologica. 2008;93(12):1912–1916. [DOI] [PubMed] [Google Scholar]
- 37.Travade P, Dusart JD, Cavaroc M, Beytout J, Rey M. [Severe infections associated with chronic lymphoid leukemia. 159 infectious episodes in 60 patients]. Presse Med. 1986;15(34):1715–1718. [PubMed] [Google Scholar]
- 38.Morrison VA. Infectious complications in patients with chronic lymphocytic leukemia: pathogenesis, spectrum of infection, and approaches to prophylaxis. Clin Lymphoma Myeloma. 2009;9(5):365–370. [DOI] [PubMed] [Google Scholar]
- 39.Boughton BJ, Jackson N, Lim S, Smith N. Randomized trial of intravenous immunoglobulin prophylaxis for patients with chronic lymphocytic leukaemia and secondary hypogammaglobulinaemia. Clin Lab Haematol. 1995;17(1):75–80. [DOI] [PubMed] [Google Scholar]
- 40.Rozman C, Montserrat E, Vinolas N. Serum immunoglobulins in B-chronic lymphocytic leukemia. Natural history and prognostic significance. Cancer. 1988;61(2):279–283. [DOI] [PubMed] [Google Scholar]
- 41.Arvin AM, Pollard RB, Rasmussen LE, Merigan TC. Cellular and humoral immunity in the pathogenesis of recurrent herpes viral infections in patients with lymphoma. J Clin Invest. 1980;65(4):869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82(11):1341–1349. [DOI] [PubMed] [Google Scholar]
- 43.Muller SA, Herrmann EC, Jr., Winkelmann RK. Herpes simplex infections in hematologic malignancies. Am J Med. 1972;52(1):102–114. [DOI] [PubMed] [Google Scholar]
- 44.Melchardt T, Weiss L, Greil R, Egle A. Viral infections and their management in patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2013;54(8):1602–1613. [DOI] [PubMed] [Google Scholar]
- 45.Pagano L, Caira M, Candoni A, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 2006;91(8):1068–1075. [PubMed] [Google Scholar]
- 46.Stanzani M, Vianelli N, Cavo M, Kontoyiannis DP, Lewis RE. Development and internal validation of a model for predicting 60-day risk of invasive mould disease in patients with haematological malignancies. J Infect. 2019;78(6):484–490. [DOI] [PubMed] [Google Scholar]
- 47.Strich JR, Jerussi TD, Wiestner A, Holland SM. Pneumocystis jirovecii Pneumonia in a Treatment-Naive Patient With Chronic Lymphocytic Leukemia. Infect Dis Clin Pract (Baltim Md). 2016;24(6):e86–e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maurer C, Langerbeins P, Bahlo J, et al. Effect of first-line treatment on second primary malignancies and Richter’s transformation in patients with CLL. Leukemia. 2016;30(10):2019–2025. [DOI] [PubMed] [Google Scholar]
- 49.Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127(2):208–215. [DOI] [PubMed] [Google Scholar]
- 50.Thompson PA, Tam CS, O’Brien SM, et al. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood. 2016;127(3):303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bond DA, Huang Y, Fisher JL, et al. Second cancer incidence in CLL patients receiving BTK inhibitors. Leukemia. 2020;34(12):3197–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maurer C, Langerbeins P, Bahlo J, et al. Effect of first-line treatment on second primary malignancies and Richter’s transformation in patients with CLL. Leukemia. 2016. [DOI] [PubMed] [Google Scholar]
- 53.Zheng G, Chattopadhyay S, Sud A, et al. Second primary cancers in patients with acute lymphoblastic, chronic lymphocytic and hairy cell leukaemia. Br J Haematol. 2019;185(2):232–239. [DOI] [PubMed] [Google Scholar]
- 54.Royle JA, Baade PD, Joske D, Girschik J, Fritschi L. Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: a population-based study. Br J Cancer. 2011;105(7):1076–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morton LM, Curtis RE, Linet MS, et al. Second malignancy risks after non-Hodgkin’s lymphoma and chronic lymphocytic leukemia: differences by lymphoma subtype. J Clin Oncol. 2010;28(33):4935–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. N Engl J Med. 2019;380(23):2225–2236. [DOI] [PubMed] [Google Scholar]
- 57.Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk. 2013;13(2):106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gea-Banacloche JC. Rituximab-associated infections. Semin Hematol. 2010;47(2):187–198. [DOI] [PubMed] [Google Scholar]
- 59.Focosi D, Tuccori M, Maggi F. Progressive multifocal leukoencephalopathy and anti-CD20 monoclonal antibodies: What do we know after 20 years of rituximab. Rev Med Virol. 2019;29(6):e2077. [DOI] [PubMed] [Google Scholar]
- 60.Ysebaert L, Gross E, Kuhlein E, et al. Immune recovery after fludarabine-cyclophosphamide-rituximab treatment in B-chronic lymphocytic leukemia: implication for maintenance immunotherapy. Leukemia. 2010;24(7):1310–1316. [DOI] [PubMed] [Google Scholar]
- 61.Neelapu SS, Kwak LW, Kobrin CB, et al. Vaccine-induced tumor-specific immunity despite severe B-cell depletion in mantle cell lymphoma. Nat Med. 2005;11(9):986–991. [DOI] [PubMed] [Google Scholar]
- 62.Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis. 2019;19(9):988–1000. [DOI] [PubMed] [Google Scholar]
- 63.Tam CS, O’Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112(4):975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flinn IW, Hillmen P, Montillo M, et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;132(23):2446–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3’-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118(13):3603–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmid MC, Avraamides CJ, Dippold HC, et al. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3kgamma, a single convergent point promoting tumor inflammation and progression. Cancer Cell. 2011;19(6):715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Konrad S, Ali SR, Wiege K, et al. Phosphoinositide 3-kinases gamma and delta, linkers of coordinate C5a receptor-Fcgamma receptor activation and immune complex-induced inflammation. J Biol Chem. 2008;283(48):33296–33303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zelenetz AD, Barrientos JC, Brown JR, et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: interim results from a phase 3, randomised, double-blind, placebo-controlled trial. The Lancet Oncology. 2017;18(3):297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sehn LH, Hallek M, Jurczak W. A Retrospective Analysis of Pneumocystis Jirovecii Pneumonia Infection in Patients Receiving Idelalisib in Clinical Trials. American Society of Hematology 58th Annual Meeting, San Diego, CA. 2016:abstr 3705. [Google Scholar]
- 71.U.S. Food & Drug Administration. FDA Alerts Healthcare Professionals About Clinical Trials with Zydelig (idelalisib) in Combination with other Cancer Medicines. 2016; https://www.fda.gov/Drugs/DrugSafety/ucm490618.htm. Accessed 04/12/2017.
- 72.Cheah CY, Fowler NH. Idelalisib in the management of lymphoma. Blood. 2016;128(3):331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bird ST, Tian F, Flowers N, et al. Idelalisib for Treatment of Relapsed Follicular Lymphoma and Chronic Lymphocytic Leukemia: A Comparison of Treatment Outcomes in Clinical Trial Participants vs Medicare Beneficiaries. JAMA Oncol. 2020;6(2):248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hanlon A, Brander DM. Managing toxicities of phosphatidylinositol-3-kinase (PI3K) inhibitors. Hematology Am Soc Hematol Educ Program. 2020;2020(1):346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ali K, Soond DR, Pineiro R, et al. Inactivation of PI(3)K p110delta breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510(7505):407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Okkenhaug K, Bilancio A, Farjot G, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297(5583):1031–1034. [DOI] [PubMed] [Google Scholar]
- 77.Uno JK, Rao KN, Matsuoka K, et al. Altered macrophage function contributes to colitis in mice defective in the phosphoinositide-3 kinase subunit p110delta. Gastroenterology. 2010;139(5):1642–1653, 1653 e1641–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lampson BL, Kasar SN, Matos TR, et al. Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood. 2016;128(2):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weidner AS, Panarelli NC, Geyer JT, et al. Idelalisib-associated Colitis: Histologic Findings in 14 Patients. Am J Surg Pathol. 2015;39(12):1661–1667. [DOI] [PubMed] [Google Scholar]
- 80.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun C, Tian X, Lee YS, et al. Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Blood. 2015;126(19):2213–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pleyer C, Wiestner A, Sun C. Immunological changes with kinase inhibitor therapy for chronic lymphocytic leukemia. Leuk Lymphoma. 2018;59(12):2792–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N Engl J Med. 2019;381(5):432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ghez D, Calleja A, Protin C, et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood. 2018;131(17):1955–1959. [DOI] [PubMed] [Google Scholar]
- 85.Lionakis MS, Dunleavy K, Roschewski M, et al. Inhibition of B Cell Receptor Signaling by Ibrutinib in Primary CNS Lymphoma. Cancer Cell. 2017;31(6):833–843 e835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ahn IE, Jerussi T, Farooqui M, Tian X, Wiestner A, Gea-Banacloche J. Atypical Pneumocystis jirovecii pneumonia in previously untreated patients with CLL on single-agent ibrutinib. Blood. 2016;128(15):1940–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Niemann CU, Herman SE, Maric I, et al. Disruption of in vivo Chronic Lymphocytic Leukemia Tumor-Microenvironment Interactions by Ibrutinib--Findings from an Investigator-Initiated Phase II Study. Clin Cancer Res. 2016;22(7):1572–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Long M, Beckwith K, Do P, et al. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest. 2017;127(8):3052–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Papazoglou D, Lesnick CE, Wang V, Kay NE, Shanafelt TD, Ramsay AG. Ibrutinib-Based Therapy Improves Anti-Tumor T Cell Killing Function Allowing Effective Pairing with Anti-PD-L1 Immunotherapy Compared to Traditional FCR Chemoimmunotherapy; Implications for Therapy and Correlative Immune Functional Data from the Phase III E1912 Trial. 2018;132(Suppl 1):236–236. [Google Scholar]
- 90.Fraietta JA, Beckwith KA, Patel PR, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood. 2016;127(9):1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gauthier J, Hirayama AV, Hay KA, et al. Comparison of Efficacy and Toxicity of CD19-Specific Chimeric Antigen Receptor T-Cells Alone or in Combination with Ibrutinib for Relapsed and/or Refractory CLL. 2018;132(Suppl 1):299–299. [Google Scholar]
- 92.Robinson HR, Qi J, Cook EM, et al. A CD19/CD3 bispecific antibody for effective immunotherapy of chronic lymphocytic leukemia in the ibrutinib era. Blood. 2018;132(5):521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gohil SH, Evans R, Harasser M, et al. Ibrutinib enhances the efficacy of ROR1 bispecific T cell engager mediated cytotoxicity in chronic lymphocytic leukaemia. Br J Haematol. 2019;186(2):380–382. [DOI] [PubMed] [Google Scholar]
- 94.Vardi A, Vlachonikola E, Papazoglou D, et al. T-Cell Dynamics in Chronic Lymphocytic Leukemia under Different Treatment Modalities. Clin Cancer Res. 2020;26(18):4958–4969. [DOI] [PubMed] [Google Scholar]
- 95.Qin JS, Johnstone TG, Baturevych A, et al. Antitumor Potency of an Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy, Lisocabtagene Maraleucel in Combination With Ibrutinib or Acalabrutinib. J Immunother. 2020;43(4):107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jain N, Keating M, Thompson P, et al. Ibrutinib and Venetoclax for First-Line Treatment of CLL. N Engl J Med. 2019;380(22):2095–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Weerdt I, Hofland T, de Boer R, et al. Distinct immune composition in lymph node and peripheral blood of CLL patients is reshaped during venetoclax treatment. Blood Adv. 2019;3(17):2642–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Itchaki G, Brown JR. Lenalidomide in the treatment of chronic lymphocytic leukemia. Expert Opin Investig Drugs. 2017;26(5):633–650. [DOI] [PubMed] [Google Scholar]
- 99.Chanan-Khan A, Egyed M, Robak T, et al. Randomized phase 3 study of lenalidomide versus chlorambucil as first-line therapy for older patients with chronic lymphocytic leukemia (the ORIGIN trial). Leukemia. 2017;31(5):1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Furstenau M, Fink AM, Schilhabel A, et al. B-cell Acute Lymphoblastic Leukemia in Patients with Chronic Lymphocytic Leukemia Treated with Lenalidomide. Blood. 2020. [DOI] [PubMed] [Google Scholar]
- 101.Riches JC, Gribben JG. Mechanistic and Clinical Aspects of Lenalidomide Treatment for Chronic Lymphocytic Leukemia. Curr Cancer Drug Targets. 2016;16(8):689–700. [DOI] [PubMed] [Google Scholar]
- 102.Aue G, Njuguna N, Tian X, et al. Lenalidomide-induced upregulation of CD80 on tumor cells correlates with T-cell activation, the rapid onset of a cytokine release syndrome and leukemic cell clearance in chronic lymphocytic leukemia. Haematologica. 2009;94(9):1266–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Andritsos LA, Johnson AJ, Lozanski G, et al. Higher doses of lenalidomide are associated with unacceptable toxicity including life-threatening tumor flare in patients with chronic lymphocytic leukemia. J Clin Oncol. 2008;26(15):2519–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Badoux XC, Keating MJ, Wen S, et al. Lenalidomide as initial therapy of elderly patients with chronic lymphocytic leukemia. Blood. 2011;118(13):3489–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chanan-Khan A, Miller KC, Lawrence D, et al. Tumor flare reaction associated with lenalidomide treatment in patients with chronic lymphocytic leukemia predicts clinical response. Cancer. 2011;117(10):2127–2135. [DOI] [PubMed] [Google Scholar]
- 106.Aue G, Sun C, Liu D, et al. Activation of Th1 Immunity within the Tumor Microenvironment Is Associated with Clinical Response to Lenalidomide in Chronic Lymphocytic Leukemia. J Immunol. 2018;201(7):1967–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shanafelt TD, Ramsay AG, Zent CS, et al. Long-term repair of T-cell synapse activity in a phase II trial of chemoimmunotherapy followed by lenalidomide consolidation in previously untreated chronic lymphocytic leukemia (CLL). Blood. 2013;121(20):4137–4141. [DOI] [PubMed] [Google Scholar]
- 108.Chanan-Khan AA, Zaritskey A, Egyed M, et al. Lenalidomide maintenance therapy in previously treated chronic lymphocytic leukaemia (CONTINUUM): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Haematology. 2017;4(11):e534–e543. [DOI] [PubMed] [Google Scholar]
- 109.Fink AM, Bahlo J, Robrecht S, et al. Lenalidomide maintenance after first-line therapy for high-risk chronic lymphocytic leukaemia (CLLM1): final results from a randomised, double-blind, phase 3 study. The Lancet Haematology. 2017;4(10):e475–e486. [DOI] [PubMed] [Google Scholar]
- 110.Lapalombella R, Andritsos L, Liu Q, et al. Lenalidomide treatment promotes CD154 expression on CLL cells and enhances production of antibodies by normal B cells through a PI3-kinase-dependent pathway. Blood. 2010;115(13):2619–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gribabis DA, Panayiotidis P, Boussiotis VA, Hannoun C, Pangalis GA. Influenza virus vaccine in B-cell chronic lymphocytic leukaemia patients. Acta Haematol. 1994;91(3):115–118. [DOI] [PubMed] [Google Scholar]
- 112.Mauro FR, Giannarelli D, Galluzzo CM, et al. Response to the conjugate pneumococcal vaccine (PCV13) in patients with chronic lymphocytic leukemia (CLL). Leukemia. 2020. [DOI] [PubMed] [Google Scholar]
- 113.Hartkamp A, Mulder AH, Rijkers GT, van Velzen-Blad H, Biesma DH. Antibody responses to pneumococcal and haemophilus vaccinations in patients with B-cell chronic lymphocytic leukaemia. Vaccine. 2001;19(13–14):1671–1677. [DOI] [PubMed] [Google Scholar]
- 114.Whitaker JA, Parikh SA, Shanafelt TD, et al. The humoral immune response to high-dose influenza vaccine in persons with monoclonal B-cell lymphocytosis (MBL) and chronic lymphocytic leukemia (CLL). Vaccine. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Alexander KE, Tong PL, Macartney K, Beresford R, Sheppeard V, Gupta M. Live zoster vaccination in an immunocompromised patient leading to death secondary to disseminated varicella zoster virus infection. Vaccine. 2018;36(27):3890–3893. [DOI] [PubMed] [Google Scholar]
- 116.Costa E, Buxton J, Brown J, Templeton KE, Breuer J, Johannessen I. Fatal disseminated varicella zoster infection following zoster vaccination in an immunocompromised patient. BMJ Case Rep. 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Clutterbuck EA, Lazarus R, Yu LM, et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis. 2012;205(9):1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Svensson T, Kattstrom M, Hammarlund Y, et al. Pneumococcal conjugate vaccine triggers a better immune response than pneumococcal polysaccharide vaccine in patients with chronic lymphocytic leukemia A randomized study by the Swedish CLL group. Vaccine. 2018;36(25):3701–3707. [DOI] [PubMed] [Google Scholar]
- 119.Pasiarski M, Rolinski J, Grywalska E, et al. Antibody and plasmablast response to 13-valent pneumococcal conjugate vaccine in chronic lymphocytic leukemia patients--preliminary report. PLoS One. 2014;9(12):e114966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thangavadivel S, Zhao Q, Epperla N, et al. Early Intervention with Lenalidomide in Patients with High-risk Chronic Lymphocytic Leukemia. Clin Cancer Res. 2020;26(23):6187–6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jutel M, Blaser K, Akdis CA. The role of histamine in regulation of immune responses. Chem Immunol Allergy. 2006;91:174–187. [DOI] [PubMed] [Google Scholar]
- 122.Van der Velden AM, Van Velzen-Blad H, Claessen AM, et al. The effect of ranitidine on antibody responses to polysaccharide vaccines in patients with B-cell chronic lymphocytic leukaemia. Eur J Haematol. 2007;79(1):47–52. [DOI] [PubMed] [Google Scholar]
- 123.Jurlander J, de Nully Brown P, Skov PS, et al. Improved vaccination response during ranitidine treatment, and increased plasma histamine concentrations, in patients with B cell chronic lymphocytic leukemia. Leukemia. 995;9(11):1902–1909. [PubMed] [Google Scholar]
- 124.van der Velden AM, Mulder AH, Hartkamp A, Diepersloot RJ, van Velzen-Blad H, Biesma DH. Influenza virus vaccination and booster in B-cell chronic lymphocytic leukaemia patients. Eur J Intern Med. 2001;12(5):420–424. [DOI] [PubMed] [Google Scholar]
- 125.Douglas AP, Trubiano JA, Barr I, Leung VK, Slavin MA, Tam CS. Ibrutinib may impair serological responses to influenza vaccination. Haematologica. 2017(102):e397–e399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sun C, Gao J, Couzens L, et al. Seasonal Influenza Vaccination in Patients With Chronic Lymphocytic Leukemia Treated With Ibrutinib. JAMA Oncol. 2016;2(12):1656–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ellebedy AH, Krammer F, Li GM, et al. Induction of broadly cross-reactive antibody responses to the influenza HA stem region following H5N1 vaccination in humans. Proc Natl Acad Sci U S A. 2014;111(36):13133–13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Abreu RB, Kirchenbaum GA, Clutter EF, Sautto GA, Ross TM. Preexisting subtype immunodominance shapes memory B cell recall response to influenza vaccination. JCI Insight. 2020;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Andrick B, Alwhaibi A, DeRemer DL, et al. Lack of adequate pneumococcal vaccination response in chronic lymphocytic leukaemia patients receiving ibrutinib. Br J Haematol. 2018;182(5):712–714. [DOI] [PubMed] [Google Scholar]
- 130.Pleyer C, Ali MA, Cohen JI, et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood. 2021;137(2):185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schliffke S, Sivina M, Kim E, et al. Dynamic changes of the normal B lymphocyte repertoire in CLL in response to ibrutinib or FCR chemo-immunotherapy. Oncoimmunology. 2018;7(4):e1417720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lachance S, Christofides AL, Lee JK, et al. A Canadian perspective on the use of immunoglobulin therapy to reduce infectious complications in chronic lymphocytic leukemia. Curr Oncol. 2016;23(1):42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sklenar I, Schiffman G, Jonsson V, et al. Effect of various doses of intravenous polyclonal IgG on in vivo levels of 12 pneumococcal antibodies in patients with chronic lymphocytic leukaemia and multiple myeloma. Oncology. 1993;50(6):466–477. [DOI] [PubMed] [Google Scholar]
- 134.Ammann EM, Jones MP, Link BK, et al. Intravenous immune globulin and thromboembolic adverse events in patients with hematologic malignancy. Blood. 2016;127(2):200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Honjo K, Russell RM, Li R, et al. Convalescent plasma-mediated resolution of COVID-19 in a patient with humoral immunodeficiency. Cell Rep Med. 2021;2(1):100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Roeker LE, Knorr DA, Pessin MS, et al. Anti-SARS-CoV-2 antibody response in patients with chronic lymphocytic leukemia. Leukemia. 2020;34(11):3047–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Boonyaratanakornkit J, Morishima C, Selke S, et al. Clinical, laboratory, and temporal predictors of neutralizing antibodies against SARS-CoV-2 among COVID-19 convalescent plasma donor candidates. J Clin Invest. 2021;131(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen P, Nirula A, Heller B, et al. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N Engl J Med. 2021;384(3):229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Muchtar E, Kay NE, Parikh SA. Early intervention in asymptomatic chronic lymphocytic leukemia. Clin Adv Hematol Oncol. 2021;19(2):92–103. [PubMed] [Google Scholar]
- 140.Agius R, Brieghel C, Andersen MA, et al. Machine learning can identify newly diagnosed patients with CLL at high risk of infection. Nat Commun. 2020;11(1):363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Da Cunha-Bang C, Agius R, Kater AP, et al. PreVent-ACaLL Short-term combined acalabrutinib and venetoclax treatment of newly diagnosed patients with CLL at high risk of infection and/or early treatment, who do not fulfil IWCLL treatment criteria for treatment. A randomized study with extensive immune phenotyping. Blood. 2019;134(Supplement_1):4304–4304. [Google Scholar]