Abstract
Spermatogenic dysfunction caused by cyclophosphamide (CP) chemotherapy has seriously influenced the life quality of patients. Unfortunately, treatments for CP-induced testicular spermatogenic dysfunction are limited, and the molecular mechanisms are not fully understood. For the first time, here, we explored the effects of bone marrow mesenchymal stem cell-derived exosomes (BMSC-exos) on CP-induced testicular spermatogenic dysfunction in vitro and in vivo. BMSC-exos could be taken up by spermatogonia (GC1-spg cells). CP-injured GC1-spg cells and BMSC-exos were cocultured at various doses, and then, cell proliferation was measured using 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay. In addition, photophosphorylation of extracellular-regulated kinase (ERK), p38 mitogen-activated protein kinase (p38MAPK), and protein kinase B (AKT) proteins was evaluated by western blotting as well as apoptosis in GC1-spg cells measured using flow cytometry. Treatment with BMSC-exos enhanced cell proliferation and reduced apoptosis of CP-injured GCI-spg cells. Phosphorylated levels of ERK, AKT, and p38MAPK proteins were reduced in CP-injured spermatogonia when co-treated with BMSC-exos, indicating that BMSC-exos acted against the reproductive toxicity of CP via the p38MAPK/ERK and AKT signaling pathways. In experiments in vivo, CP-treated rats received BMSC-exos by injection into the tail vein, and testis morphology was compared between treated and control groups. Histology showed that transfusion of BMSC-exos inhibited the pathological changes in CP-injured testes. Thus, BMSC-exos could counteract the reproductive toxicity of CP via the p38MAPK/ERK and AKT signaling pathways. The findings provide a potential treatment for CP-induced male spermatogenic dysfunction using BMSC-exos.
Keywords: bone marrow mesenchymal stem cells, cyclophosphamide, exosomes, reproductive toxicity
INTRODUCTION
Cyclophosphamide (CP) is widely used in chemotherapy for malignant tumors and as an immunosuppressant for the prevention of graft rejection and treatment of chronic autoimmune diseases.1 It is one of the most effective and safe drugs used for such diseases.2 However, its detrimental side effects cannot be ignored. Thus, CP can cause testicular damage, leading to oligospermia or azoospermia and human male infertility.3,4,5 Studies have suggested that free radical-mediated damage is the core mechanism of CP-induced reproductive toxicity. Activities of the necessary free radical scavenger enzymes, 3b-hydroxysteroid dehydrogenase and 17b-hydroxysteroid dehydrogenase, were significantly diminished in CP-treated rat testes, where free radical accumulation and lipid peroxidation resulted in spermatogenic cell damage.6 CP-induced testicular spermatogenic dysfunction seriously diminishes the quality of life in human patients and has attracted considerable attention. However, treatments are limited.
Mesenchymal stem cells (MSCs) are capable of self-renewal and have differentiation potential, when isolated from tissues such as bone marrow, adipose tissue, cord blood, and dental pulp. MSCs have great prospects in the field of human male reproductive health.7 Bone marrow-derived mesenchymal stem cells (BMSCs) were found to alleviate the gonadotoxic effects of lead poisoning, reverse histopathological changes in the testis, improve semen quality, and decrease the amount of fragmented DNA.8 Transplanted BMSCs could improve spermatogenesis in the seminiferous tubules of busulfan-treated azoospermic hamsters.9 Furthermore, BMSCs could repair cisplatin-induced gonadotoxicity through suppression of oxidative stress and inhibition of inflammation and apoptosis.10 Other studies have demonstrated that the functions of MSCs mostly depend on exosomes.11,12
Exosomes are small membrane vesicles of 30–100 nm in diameter, produced by most eukaryotic cells. They contain bioactive constituents such as functional messenger RNA (mRNAs), microRNAs (miRNAs), and long noncoding (lnc) RNAs. They can be transmitted between different cell types and are important for intercellular communication, immunoregulation, metabolism, and damage repair.12 Several studies have demonstrated that BMSC-derived exosomes (BMSC-exos) have therapeutic potential for disorders such as spinal cord injury, liver fibrosis, and degenerative eye diseases.13,14,15 Previously, we found that BMSC-exos protected against testicular ischemia/reperfusion injury in rats through antioxidant, anti-inflammatory, and antiapoptotic mechanisms.16
However, few studies have focused on the effects of BMSC-exos on CP-induced spermatogenic dysfunction. Here, for the first time, we investigated the effects of BMSC-exos on CP-induced testicular spermatogenic dysfunction in vitro and in vivo and the potential molecular mechanisms. We found that BMSC-exos could inhibit the reproductive toxicity of CP, probably via the extracellular-regulated kinase (ERK), p38 mitogen-activated protein kinase (p38MAPK), and protein kinase B (AKT) pathways.
MATERIALS AND METHODS
Identification of BMSC-exos
Exosomes were isolated from culture supernatants of fourth-generation BMSCs, using differential centrifugation and ultracentrifugation (XPN-100, Beckman, Brea, CA, USA). Transmission electron microscopy (TEM; H-9500, Hitachi, Tokyo, Japan) was used to observe cell morphology. Nanoparticle tracking analysis (NTA; LM10, NanoSight, Salisbury, UK) was used to measure exosome size. Surface markers of exosomes, such as CD63 and TSG101, were detected using western blotting.
Exosome uptake assay
To explore whether spermatogonia (GC1-spg cells) could take up exosomes, we cocultured mouse cells with BMSC-exos labeled with PKH67 green fluorescent dye (Sigma, St. Louis, MO, USA). After being cultured for 12 h, nuclear staining with 4',6-diamidino-2-phenylindole (DAPI; Sigma) was performed followed by observations using confocal microscope (FV1000, Olympus, Tokyo, Japan).
The effect of BMSC-exos on CP-injured GC1-spg cells
Mouse spermatogonia, CG1 cells (CRL-2053) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). They were cultured in complete Dulbecco's Modified Eagle's medium (DMEM) with high glucose (Gibco, Carlsbad, CA, USA) and 10% fetal bovine serum (FBS; Gibco) at 37°C under 5% CO2 in humidified air. To test the effect of exosomes on the CP-induced decrease in cell viability, we performed 3-4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay. GC1-spg cells in the logarithmic growth phase were seeded into 96-well plates at 1.5 × 104 cells per ml (100 μl per well). After 3 h, CP or exosomes were added as follows: 0 μg ml−1 CP, 20 μg ml−1 CP, 20 μg ml−1 CP + 0 μg ml−1 exosomes, 20 μg ml−1 CP + 50 μg ml−1 exosomes, 20 μg ml−1 CP + 100 μg ml−1 exosomes, and 20 μg ml−1 CP + 200 μg ml−1 exosomes, respectively. Phosphate-buffered saline (PBS; Gibco) was added to peripheral wells of 96-well plates at 100 μl per well followed by culture as above for 24 h, 48 h, 72 h, and 96 h. A concentration of 5 mg ml−1 MTT was added in a ratio of 1:10; 100 μl of the culture solution was added to 10 μl of the test solution. After incubating at 37°C for 4 h, the supernatant was discarded followed by the addition of 100 μl dimethyl sulfoxide (DMSO; Cytiva, Marlborough, MA, USA). The mixture was then shaken well to dissolve the crystals of MTT. A microplate reader (Synergy HTX, BioTek, Winooski, VT, USA) was used to measure absorbance at 490 nm, while the value-added inhibition rate was calculated as the cell proliferation inhibition rate = (optical density [OD] value of control group − OD value of experimental group)/OD value of control group × 100%.
CP-induced testicular spermatogenic dysfunction in rats
Healthy Sprague–Dawley male rats (weighing 316.7 ± 9.0 g), aged 8 weeks, were purchased from Liaoning Changsheng Biotech Co., Ltd., Liaoning, China. The experiments on animals were conducted within Southern Medical University Experimental Animal Ethics Committee (Guangzhou, China), and institutional animal ethics approval was granted (1107262011). During all experiments, they were feed and given water ad libitum with a 12-h light/12-h dark cycle at 24°C ± 1°C. All rats were injected abdominally with CP (30 mg kg−1) once a day for 5 days to induce testicular toxicity, except for the blank control group. Eighteen rats were divided randomly into three equal groups as follows: blank control group; an exosome-treatment group receiving 1 ml exosomes (100 μg ml−1) by intravenous tail injection every day for 7 days; and a saline control group receiving 1 ml normal saline by intravenous tail injection every day for 7 days. After injection, all rats were housed as normal for 2 weeks. Then, the testes were collected for histological examination.
Histopathology
At the end of the experiment, the rats were euthanized and one testis was fixed and embedded in paraffin wax for sectioning. Hematoxylin and eosin (HE) staining was performed, and testicular tissues were evaluated by microscopy. Histopathological score was evaluated by pathology doctors according to the modified Johnsen scoring system.17 The criteria are as follows: score 10 – full spermatogenesis; score 9 – slightly impaired spermatogenesis with many late spermatids; score 8 – less than five spermatozoa per tubule and a few late spermatids; score 7 – no spermatozoa or late spermatids but many early spermatids; score 6 – no spermatozoa or late spermatids but few early spermatids; score 5 – no spermatozoa or spermatids but many spermatocytes; score 4 – no spermatozoa or spermatids and a few spermatocytes; score 3 – only spermatogonia; score 2 – no germinal epithelial cells, only Sertoli cells; and score 1 – no seminiferous epithelium.
Flow cytometry for cell apoptosis
Mouse GC1-spg cells were prepared by trypsin (Gibco) digestion and centrifuged (L550, Xiangyi, Changsha, China) at 1000g for 5 min. Then, the cells were resuspended in PBS and counted. Aliquots of 100 000 resuspended cells were centrifuged at 1000g for 5 min. The supernatant was discarded and 195 μl Annexin V-FITC (Beyotime, Shanghai, China) was added. Cells were resuspended and treated with 5 μl of Annexin V-FITC, 10 μl of propidium iodide staining solution (Beyotime) was added, and cells were subjected to flow cytometry (Biosciences, Franklin Lakes, NJ, USA).
Western blotting analysis
After extracting cellular proteins (40 μg), the target protein was detected by separation on 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane (BioTrace, Pall, Mexico); 5% skim milk was then used to block the membrane, followed with incubation with a primary antibody (anti-p38MAPK/p-p38MAPK/ERK/p-ERK/AKT/p-AKT/β-actin antibody, Biogot, Nanjing, China) at 4°C overnight. After that, the membrane was washed twice with Tris-buffered saline Tween (TBST) for 7 min and then incubated with the corresponding diluted secondary antibody (horseradish peroxidase-labeled secondary antibody, Forevergen, Guangzhou, China) for 1–2 h at room temperature. Before the characterization of enhanced chemiluminescence (ECL; Forevergen), the membrane was washed three times with TBST every 7 min. ImageJ (https://imagej.net/Welcome) was used to analyze the optical density of the target strip. Differences in the expression levels of the above proteins were compared after different treatments.
Statistical analyses
All experimental data are presented as the mean ± standard deviation and were analyzed statistically using GraphPad Prism software version 7 (GraphPad Software, La Jolla, CA, USA). Group results were analyzed using one-way analysis of variance (ANOVA) followed by Duncan's test. P < 0.05 was considered statistically significant.
RESULTS
Identification and characterization of BMSC-exos
Figure 1a shows a TEM image of extracted exosomes as round vesicles with bilayered membranes. Nanoparticle tracking analysis showed diameters of 20–150 nm (mean: 109 nm; Figure 1b). Western blotting confirmed that the BMSC-exos contained the known exosomal markers CD63 and TSG101, but not calnexin (Figure 1c). These results confirmed that we had successfully extracted exosomes derived from BMSCs.
Figure 1.
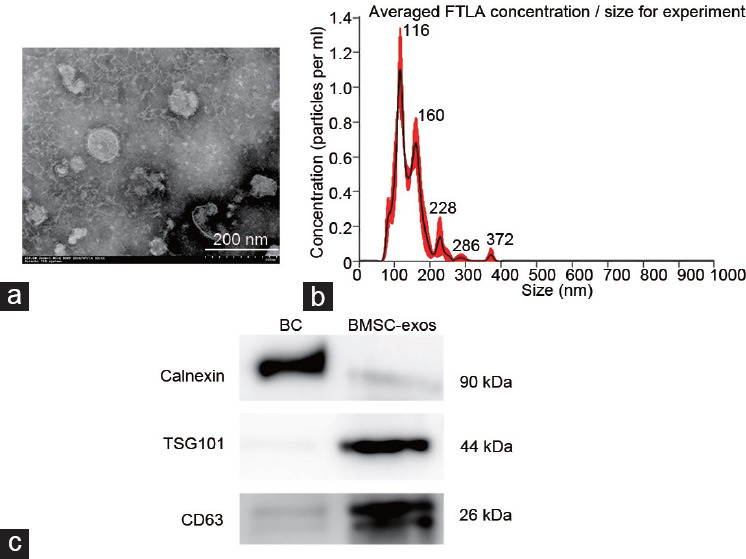
Identification and characterization of BMSC-exos. (a) Morphology of exosomes was observed by transmission electron microscopy. (b) Nanoparticle tracking analysis of the size distribution of BMSC-exos. (c) Western blotting for markers CD63 and TSG101 of the exosomes. BMSC-exos: bone marrow mesenchymal stem cell-derived exosomes; BC: bone marrow mesenchymal stem cells; FTLA: fiber to the last amplifier.
Uptake of BMSC-exos by GC1-spg cells
BMSC-exos were labeled with the green fluorescent dye PKH67 and then cocultured with GC1-spg cells. After 12 h, GC1-spg exhibited uptake of BMSC-exos efficiently, as evidenced by confocal microscopy (Figure 2, green fluorescence inside the cells). This confirmed that GC1-spg cells could ingest exosomes derived from BMSCs.
Figure 2.
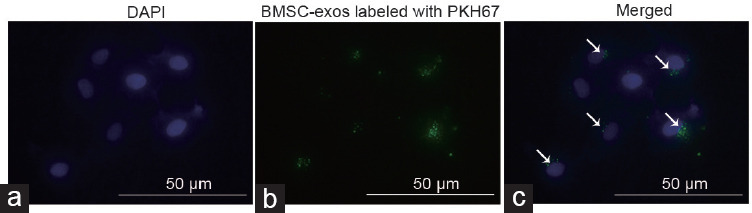
BMSC-exos could be taken up by GC1-spg cells. (a) Nuclei were stained with DAPI. (b) BMSC-exos were marked with the green fluorescence dye PKH67 and then cocultured with GC1-spg cells. (c) Green staining indicates exosomes taken up by these cells as indicated by white arrows. GC1-spg: GC1 spermatogonia; DAPI: 4′,6-diamidino-2-phenylindole; BMSC-exos: bone marrow mesenchymal stem cell-derived exosomes.
BMSC-exos protected against CP-induced reproductive toxicity
Inhibition of cell proliferation in the experimental groups is illustrated in Figure 3. Cell growth in the CP group was significantly inhibited in a time-dependent manner. The treatment group (cocultured with BMSC-exos) showed a protective effect from CP-induced inhibition of cell proliferation, and BMSC-exos at a concentration of 100 g ml−1 gave the best protection. Flow cytometry showed that the apoptosis rate of GC1-spg in the CP-treated groups was significantly higher than that in the control group (19.16% ± 0.80% vs 8.16% ± 0.23%, P < 0.001; Figure 4a and 4b). Similarly, the apoptosis rate of GC1-spg was significantly lower in the CP+BMSC-exos group than that in the CP-treated group (12.01% ± 0.64% vs 19.16% ± 0.80%, P < 0.001; Figure 4b–4d). These results confirmed that BMSC-exos could reduce the apoptosis of GC1-spg cells caused by CP treatment.
Figure 3.
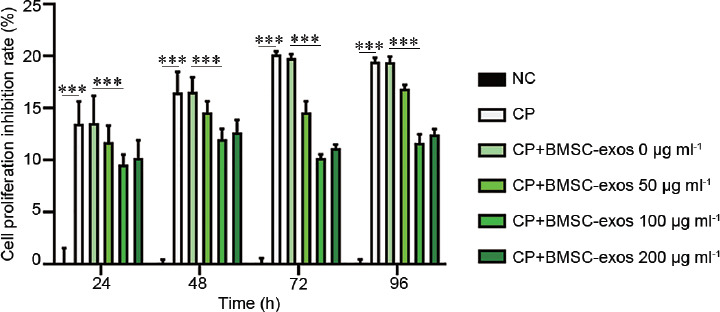
Inhibition rate of GC1-spg proliferation measured by MTT assay. The treatment group (CP + BMSC-exos, cocultured with BMSC-exos) showed a protective effect from CP-induced inhibition of cell proliferation (CP + BMSC-exos 0 μg ml−1 vs CP + BMSC-exos 100 μg ml−1, ***P < 0.001). NC: negative control; CP: cyclophosphamide; BMSC-exos: bone marrow mesenchymal stem cell-derived exosomes; MTT: 3-4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide.
Figure 4.
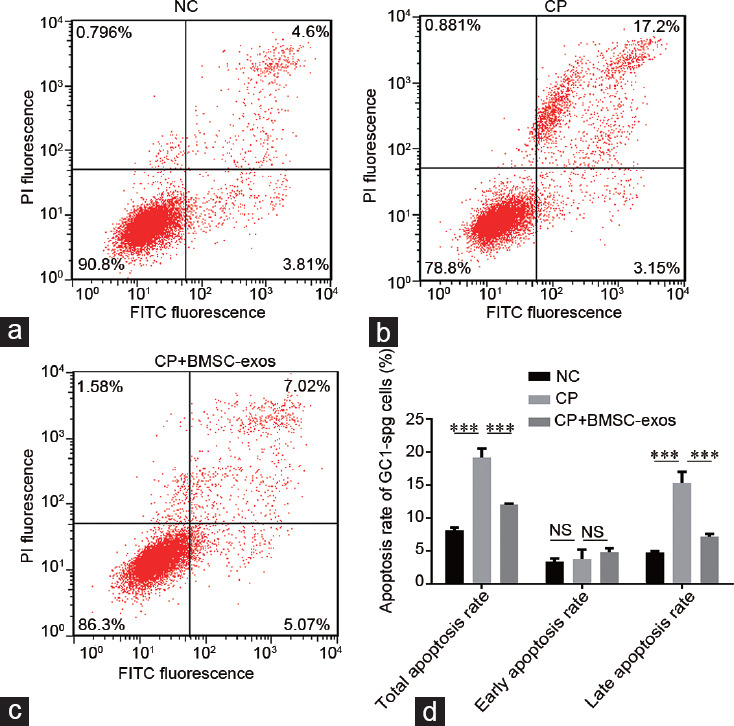
Flow cytometry analysis of cell apoptosis. (a) NC. (b) CP. (c) CP + BMSC-exos. (d) Statistical analysis of the apoptosis rate of GC1-spg cells in the indicated groups. The apoptosis rate of the CP-treated cells was significantly higher than that in the NC cells, while the apoptosis rate of the CP + BMSC-exos cells was significantly lower than those in the CP-treated cells; ***P < 0.001. NC: negative control; CP: cyclophosphamide-treated group; BMSC-exos: bone marrow mesenchymal stem cell-derived exosomes; NS: no significance; PI: Propidium Iodide; GC1-spg: GC1 spermatogonia; FITC: fiber to the last amplifier.
BMSC-exos reduced the reproductive toxicity of CP via the p38MAPK/ERK and AKT signaling pathways
The above results indicated that BMSC-exos had a protective effect against CP-induced reproductive toxicity. To evaluate the possible molecular mechanisms, we examined the phosphorylation levels of several signaling pathway proteins. We found that inhibiting the p38 MAPK/ERK signal pathway could improve CP tolerance by germ cells (Figure 5). The phosphorylation levels of p38MAPK, AKT, and ERK proteins were significantly elevated in the CP-treated group (P < 0.0001). In contrast, the BMSC-exos treatment group showed significant reductions in the phosphorylation of p38, AKT, and ERK proteins (P < 0.01). These results suggested that BMSC-exos could minimize the reproductive toxicity of CP probably via the p38 MAPK/ERK/AKT signaling pathways.
Figure 5.
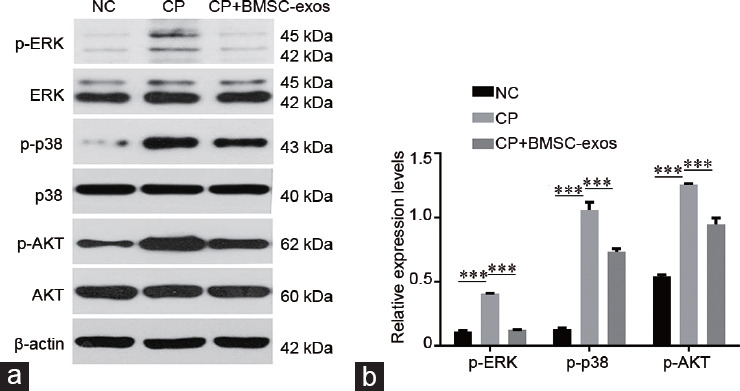
BMSC-exos reduced the reproductive toxicity of CP in GC1-spg cells which is associated with the p38MAPK/ERK and AKT signaling pathways. (a) Western blotting analysis of the indicated proteins in each group. (b) Quantitative analysis of phosphorylation levels of ERK, p38, and AKT in each group; ***P < 0.001. NC: negative control; CP: cyclophosphamide; BMSC-exos: bone marrow mesenchymal stem cell-derived exosomes; ERK: extracellular-regulated kinase; p38: p38 mitogen-activated protein kinase; AKT: protein kinase B; p: phosphorylation; GC1-spg: GC1 spermatogonia.
BMSC-exos diminished the pathological changes in CP-injured testicular tissues
Our rat model of CP-induced testicular spermatogenic dysfunction was successful (Figure 6). Histology of the testes in the CP-treated group showed decreased spermatogenic cells and a disorganized arrangement. In the rats treated with BMSC-exos, the spermatogenic cells in the seminiferous tubules were arranged normally, and the mean Johnsen's score was significantly higher. This indicated that BMSC-exos could counteract the reproductive toxicity of CP in vivo.
Figure 6.
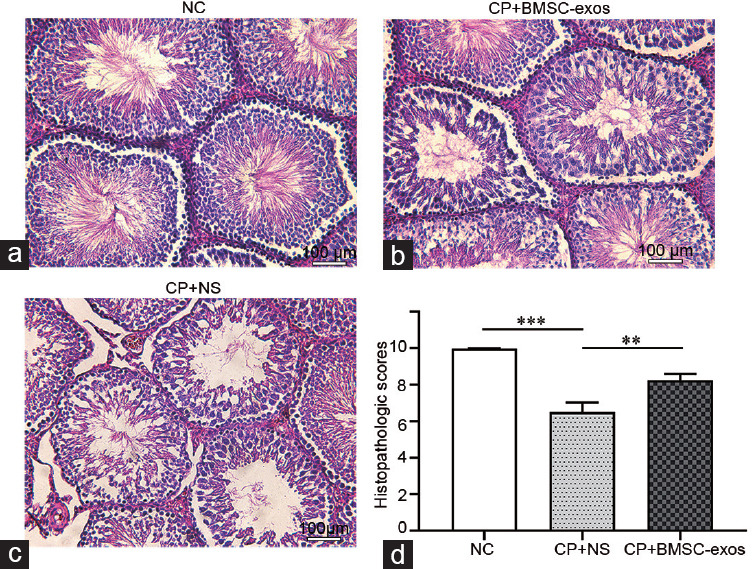
BMSC-exos diminished the pathological changes in CP-injured testicular tissues. (a) NC. (b) CP + BMSC-exos. (c) CP + NS. (d) Histopathologic scores of testicular tissue in the 3 groups (n = 18); ***P < 0.001, **P < 0.01. NC: negative control; CP: cyclophosphamide; NS: normal saline; BMSC-exos: bone marrow mesenchymal stem cell-derived exosomes.
DISCUSSION
Previous studies have demonstrated that rats treated with CP showed decrease in sperm count, viability, and motility and increase in testicular atrophy.5,18 After discontinuing CP treatments, sperm counts could recover slightly, but the testicular atrophy was irreversible.4 With improvements in chemotherapeutic efficacy, most cancers in children and young patients are now treatable. There is a definite improvement in long-term survival and cure rates. Fertility is an important issue for those cancer survivors.19,20 However, most of them suffer from infertility and more than 50% of childhood cancer survivors developed oligospermia or azoospermia in adulthood after chemotherapy with CP.3
Although CP is widely used in the clinical treatment of various diseases, its reproductive toxicity cannot be ignored. The drugs available for limiting spermatogenic dysfunction caused by CP are limited, and the effects are unsatisfactory. Here, we aimed to develop an efficient way to improve CP-induced testicular spermatogenic dysfunction. Several studies have shown that BMSC-exos have therapeutic potential for treating some diseases.13,14,15 To clarify the effect of BMSC-exos on CP-induced testicular spermatogenic dysfunction, we performed in vivo and in vitro experiments. A previous study showed that human umbilical cord mesenchymal stem cell-derived exosomes could be internalized by vaginal epithelial cells.21 Moreover, BMSC-exos can be taken up by adipocytes, myocytes, and hepatocytes.22 In our study, spermatogonia (GC1-spg cells) showed uptake of BMSC-exos efficiently, consistent with previous research. How exosomal vesicles (EVs) interact with cells is not clear: through endocytosis or direct membrane fusion? Elucidating the mechanism of EV uptake by cells is a key to their development as drug carriers. Many studies have demonstrated that absorption by target cells depends on the EV surface membrane proteins interacting with the membrane receptors of target cells.23,24,25,26 For example, the CD9 and CD81 proteins participate in the attachment and uptake of EVs via dendritic cells.24 Further experiments are needed to understand the mechanisms behind these observations.
Here, we investigated whether BMSC-exos could counteract the reproductive toxicity of CP in vivo and in vitro. We found that BMSC-exos had a protective effect against CP-induced spermatogenic dysfunction, including reducing the inhibition of germ cell proliferation, inhibiting apoptosis, and reducing pathological changes. Interestingly, the best protective effect of BMSC-exos was observed at a concentration of 100 μg ml−1 instead of 200 μg ml−1. Possibly, some harmful factors in the exosomes accumulated in the cells, which affected normal metabolism. Further research is needed to evaluate this possibility. We found previously that BMSC-exos protect against testicular ischemia–reperfusion injury in rats.16 Based on the existing evidence, we surmise that BMSC-exos act by passing through the blood–testis barrier and then are taken up by the spermatogenic cells, but this needs further research.
AKT is a critical regulator kinase in multiple cellular processes such as survival and metabolism.27,28 AKT signaling is considered to play an essential role in the regulation of cell apoptosis.29,30 In addition, AKT was also found to participate in CP-induced toxicity in the heart and ovary.31,32 Thus, a human placental extract (HPE)-inhibited p-Rictor reduced the expression of BCL2-associated agonist of cell death (Bad) protein, BCL2-associated X (Bax), and peroxisome proliferator-activated receptor (PPAR) and activated Akt and Forkhead box O3 (Foxo3a). HPE likely protects follicular granulosa cells from undergoing significant apoptosis and reduces atretic follicle formation, thereby alleviating CP-induced ovarian injury.27 We found that phosphorylation of AKT proteins was increased in GC1-spg cells after treatment with CP. The p38MAPK/ERK signal pathway plays a vital role in cell growth, differentiation, and apoptosis.33 The p38MAPK is reported to be activated by various stimuli, such as drugs (e.g., doxorubicin, CP, isoproterenol, and arsenic trioxide), pressure overload, ischemia/reperfusion (I/R), oxidative stress, sepsis, ultraviolet light, and lipopolysaccharides (LPS).28 ERK regulates cell growth and differentiation, while p38 preferentially responds to various stimulating factors.34 Moreover, ERK is activated during disruption of the blood–testis barrier, causing varying degrees of spermatogenic dysfunction by regulating downstream signal molecules such as the tight junction proteins occludin, connexin 43, and N-cadherin.35 Furthermore, inhibition of the p38MAPK/ERK signal pathway restored CP-induced renal oxidative stress, improved CP tolerance by germ cells, and reduced apoptosis induced by CP and its metabolite acrolein.36,37 Therefore, we suspect that the p38MAPK/ERK and AKT signaling pathways might play key roles in blocking CP reproductive toxicity via the use of BMSC-exos. Thus, we examined the expression levels and phosphorylation levels of these proteins. In GC1-spg cells, the phosphorylation levels of ERK, p38, and AKT proteins were increased after treatment with CP, consistent with a previous study.36 However, phosphorylation levels of ERK, p38, and AKT proteins were inhibited in spermatogonia co-treated with CP and BMSC-exos. The crosstalk between p38 and AKT deserves further investigation. Based on the literature and our experimental results, we speculate that BMSC-exos protect against CP-induced spermatogenic dysfunction via inhibiting the AKT and p38MAPK/ERK signal pathways.
Seminiferous tubules contain somatic cells (Sertoli and Leydig cells) and germ cells at different stages of spermatogenesis (spermatogonia, spermatocytes, and spermatids). Our data were obtained using CG1-spg cells from the ATCC. If primary mouse spermatogonia could be used to confirm these effects of exosomes, our results would be more convincing. Isolation of enriched populations of spermatogenic cells as well as Sertoli and Leydig cells from testes is a crucial step needed. Various approaches have been used to isolate testicular cells successfully. Thus, fluorescence-activated cell sorting (FACS) has been used to isolate testicular cells.38,39 Although it may be useful for specific applications, cells isolated using FACS may not be suitable for functional studies because the fluorescent dyes used for cell sorting are toxic. Velocity sedimentation separation using STA-PUT (sedimentation velocity at unit gravity, with 2%–4% BSA gradient in DMEM/F12 medium) chambers is another approach used to isolate spermatogenic cells.40 Although it is a good method for isolating pure germ cell populations, the STA-PUT approach is not feasible for isolating somatic and testicular cells.41 Serial digestion using low concentrations of collagenase has also been used to isolate Leydig cells from other testicular cell types.42 However, this method does not separate the different germ cell populations and Sertoli cells. Chang et al.30 have described a cost-effective and time-saving protocol to isolate multiple somatic and spermatogenic cell populations with high purity from the testis. Further research is needed to confirm the feasibility of this method.
Studies have found that BMSC-exos have tremendous potential value in disease therapy. BMSC-exos can active the MAPK pathway, increase the expression of glucose transporter type 3 (GLUT3) protein, and improve osteoporosis through promoting osteoblast proliferation.43 BMSC-exos stimulated by interferon gamma have therapeutic potential in treating neurodegenerative disorders, which involve anti-inflammatory RNAs and proteins, such as macrophage inhibitory cytokines and galectin-1.44 Exosomes and their differentially expressed miRNAs provide a novel approach to the diagnosis and treatment of diseases as they have unique biological structures and functions.45,46,47 Exosomes are innoxious with high target specificity, which is considered to be essential for next-generation drug delivery systems.48 Our study has broad biological significance as it has identified BMSC-exos as novel therapeutic agents for the treatment of male spermatogenic dysfunction induced by CP. Mounting evidence suggests that exosomes are able to mediate intercellular communication by transferring proteins, lipids, miRNAs, mRNAs, and lncRNAs to recipient cells.49,50,51 We speculate that BMSC-exos might ameliorate the reproductive toxicity of CP by transmitting specific miRNAs or lncRNAs to germ cells. Further research is needed to test this speculation. The potential biological role of exosomes taken up by GC1-spg needs further study. Elucidating how BMSC-exos act may help establish a new approach for clinical research of male infertility.
This study sheds more light on the potential mechanism of testicular spermatogenic dysfunction caused by CP and the protective effect of BMSC-exos. Exploring the crosstalk of these pathways and identifying the core protective molecules deserve to be further investigated.
CONCLUSIONS
Here, we demonstrated that BMSC-exos could ameliorate CP-induced testicular spermatogenic dysfunction. BMSC-exos reduced the reproductive toxicity of CP probably through inhibiting the p38MAPK/ERK and AKT signaling pathways. Elucidating how BMSC-exos act may help establish a new approach for clinical research on male infertility. Clearly, BMSC-exos have great value in reproductive biology and are worth further study.
AUTHOR CONTRIBUTIONS
CDL and QZZ designed the study. XBG and JWZ carried out the cell biology experiments. WBG, HX, JHZ, and CY analyzed the experimental data and drafted the manuscript. JKY, KYX, and MX participated in revising the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (No. 81772257) and the Guangdong Provincial Natural Science Foundation of China (No. 2018A030313697).
REFERENCES
- 1.Ghobadi E, Moloudizargari M, Asghari MH, Abdollahi M. The mechanisms of cyclophosphamide-induced testicular toxicity and the protective agents. Expert Opin Drug Metab Toxicol. 2017;13:525–36. doi: 10.1080/17425255.2017.1277205. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. The selection and use of essential medicines. World Health Organ Tech Rep Ser. 2011;965:i–xiv. 1–249. [PubMed] [Google Scholar]
- 3.Green DM, Liu W, Kutteh WH, Ke RW, Shelton KC, et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort Study. Lancet Oncol. 2014;15:1215–23. doi: 10.1016/S1470-2045(14)70408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delbes G, Vaisheva F, Luu T, Marcon L, Hales BF, et al. Reversibility of the effects of the chemotherapeutic regimen for non-Hodgkin lymphoma, cyclophosphamide, doxorubicin, vincristine, and prednisone, on the male rat reproductive system and progeny outcome. Reprod Toxicol. 2010;29:332–8. doi: 10.1016/j.reprotox.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Salimnejad R, Soleimani Rad J, Mohammad Nejad D, Roshangar L. Effect of ghrelin on total antioxidant capacity, lipid peroxidation, sperm parameters and fertility in mice against oxidative damage caused by cyclophosphamide. Andrologia. 2018;50:e12883. doi: 10.1111/and.12883. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh D, Das UB, Ghosh S, Mallick M, Debnath J. Testicular gametogenic and steroidogenic activities in cyclophosphamide treated rat: a correlative study with testicular oxidative stress. Drug Chem Toxicol. 2002;25:281–92. doi: 10.1081/dct-120005891. [DOI] [PubMed] [Google Scholar]
- 7.Kadam P, Van Saen D, Goossens E. Can mesenchymal stem cells improve spermatogonial stem cell transplantation efficiency? Andrology. 2017;5:2–9. doi: 10.1111/andr.12304. [DOI] [PubMed] [Google Scholar]
- 8.Hassan AI, Alam SS. Evaluation of mesenchymal stem cells in treatment of infertility in male rats. Stem Cell Res Ther. 2014;5:131. doi: 10.1186/scrt521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamadon A, Mehrabani D, Rahmanifar F, Jahromi AR, Panahi M, et al. Induction of spermatogenesis by bone marrow-derived mesenchymal stem cells in busulfan-induced azoospermia in Hamster. Int J Stem Cells. 2015;8:134–45. doi: 10.15283/ijsc.2015.8.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherif IO, Sabry D, Abdel-Aziz A, Sarhan OM. The role of mesenchymal stem cells in chemotherapy-induced gonadotoxicity. Stem Cell Res Ther. 2018;9:196. doi: 10.1186/s13287-018-0946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kishore R, Verma SK, Mackie AR, Vaughan EE, Abramova TV, et al. Bone marrow progenitor cell therapy-mediated paracrine regulation of cardiac miRNA-155 modulates fibrotic response in diabetic hearts. PLoS One. 2013;8:e60161. doi: 10.1371/journal.pone.0060161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100–4. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mead B, Tomarev S. Bone marrow-derived mesenchymal stem cells-derived exosomes promote survival of retinal ganglion cells through miRNA-dependent mechanisms. Stem Cells Transl Med. 2017;6:1273–85. doi: 10.1002/sctm.16-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rong X, Liu J, Yao X, Jiang T, Wang Y, et al. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/beta-catenin pathway. Stem Cell Res Ther. 2019;10:98. doi: 10.1186/s13287-019-1204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan X, Wu Q, Wang P, Jing Y, Yao H, et al. Exosomes derived from pericytes improve microcirculation and protect blood-spinal cord barrier after spinal cord injury in mice. Front Neurosci. 2019;13:319. doi: 10.3389/fnins.2019.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W, Yang C, Guo W, Guo X, Bian J, et al. Protective effect of bone marrow mesenchymal stem cells-derived exosomes against testicular ischemia-reperfusion injury in rats. Nan Fang Yi Ke Da Xue Xue Bao. 2018;38:910–6. doi: 10.3969/j.issn.1673-4254.2018.08.02. Article in Chinese. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gozen A, Demiryurek S, Taskin A, Ciralik H, Bilinc H, et al. Protective activity of ischemic preconditioning on rat testicular ischemia: effects of Y-27632 and 5-hydroxydecanoic acid. J Pediatr Surg. 2013;48:1565–72. doi: 10.1016/j.jpedsurg.2012.10.074. [DOI] [PubMed] [Google Scholar]
- 18.Lu WP, Mei XT, Wang Y, Zheng YP, Xue YF, et al. Zn(II)-curcumin protects against oxidative stress, deleterious changes in sperm parameters and histological alterations in a male mouse model of cyclophosphamide-induced reproductive damage. Environ Toxicol Pharmacol. 2015;39:515–24. doi: 10.1016/j.etap.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Aslam I, Fishel S, Moore H, Dowell K, Thornton S. Fertility preservation of boys undergoing anti-cancer therapy: a review of the existing situation and prospects for the future. Hum Reprod. 2000;15:2154–9. doi: 10.1093/humrep/15.10.2154. [DOI] [PubMed] [Google Scholar]
- 20.Ajala T, Rafi J, Larsen-Disney P, Howell R. Fertility preservation for cancer patients: a review. Obstet Gynecol Int. 2010;2010:160386. doi: 10.1155/2010/160386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Z, Zhang Y, Zhang Y, Zhang H, Liu W, et al. Exosomes derived from human umbilical cord mesenchymal stem cells accelerate growth of VK2 vaginal epithelial cells through MicroRNAs in vitro. Hum Reprod. 2019;34:248–60. doi: 10.1093/humrep/dey344. [DOI] [PubMed] [Google Scholar]
- 22.Su T, Xiao Y, Xiao Y, Guo Q, Li C, et al. Bone marrow mesenchymal stem cells-derived exosomal MiR-29b-3p regulates aging-associated insulin resistance. ACS Nano. 2019;13:2450–62. doi: 10.1021/acsnano.8b09375. [DOI] [PubMed] [Google Scholar]
- 23.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3:24641. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–66. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 25.Glebov OO, Bright NA, Nichols BJ. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol. 2006;8:46–54. doi: 10.1038/ncb1342. [DOI] [PubMed] [Google Scholar]
- 26.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–66. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hers I, Vincent EE, Tavare JM. Akt signalling in health and disease. Cell Signal. 2011;23:1515–27. doi: 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Dan HC, Sun M, Kaneko S, Feldman RI, Nicosia SV, et al. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP) J Biol Chem. 2004;279:5405–12. doi: 10.1074/jbc.M312044200. [DOI] [PubMed] [Google Scholar]
- 30.Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, et al. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 31.Zhang BF, Hu Y, Liu X, Cheng Z, Lei Y, et al. The role of AKT and FOXO3 in preventing ovarian toxicity induced by cyclophosphamide. PLoS One. 2018;13:e0201136. doi: 10.1371/journal.pone.0201136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iqubal A, Iqubal MK, Sharma S, Ansari MA, Najmi AK, et al. Molecular mechanism involved in cyclophosphamide-induced cardiotoxicity: old drug with a new vision. Life Sci. 2019;218:112–31. doi: 10.1016/j.lfs.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 33.Kello M, Kulikova L, Vaskova J, Nagyova A, Mojzis J. Fruit peel polyphenolic extract-induced apoptosis in human breast cancer cells is associated with ROS production and modulation of p38MAPK/Erk1/2 and the Akt signaling pathway. Nutr Cancer. 2017;69:920–31. doi: 10.1080/01635581.2017.1339819. [DOI] [PubMed] [Google Scholar]
- 34.Ng DC, Bogoyevitch MA. The mechanism of heat shock activation of ERK mitogen-activated protein kinases in the interleukin 3-dependent ProB cell line BaF3. J Biol Chem. 2000;275:40856–66. doi: 10.1074/jbc.M004639200. [DOI] [PubMed] [Google Scholar]
- 35.Li MW, Mruk DD, Lee WM, Cheng CY. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol. 2009;41:2302–14. doi: 10.1016/j.biocel.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu YP, Yang XM, Duan ZH, Luo P, Shang JH, et al. Inhibition of chemotherapy-induced apoptosis of testicular cells by squid ink polysaccharide. Exp Ther Med. 2017;14:5889–95. doi: 10.3892/etm.2017.5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salas-Ramirez KY, Bagnall C, Frias L, Abdali SA, Ahles TA, et al. Doxorubicin and cyclophosphamide induce cognitive dysfunction and activate the ERK and AKT signaling pathways. Behav Brain Res. 2015;292:133–41. doi: 10.1016/j.bbr.2015.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mays-Hoopes LL, Bolen J, Riggs AD, Singer-Sam J. Preparation of spermatogonia, spermatocytes, and round spermatids for analysis of gene expression using fluorescence-activated cell sorting. Biol Reprod. 1995;53:1003–11. doi: 10.1095/biolreprod53.5.1003. [DOI] [PubMed] [Google Scholar]
- 39.Kubota H, Avarbock MR, Brinster RL. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci U S A. 2003;100:6487–92. doi: 10.1073/pnas.0631767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han SY, Zhou L, Upadhyaya A, Lee SH, Parker KL, et al. TFIIAalpha/beta-like factor is encoded by a germ cell-specific gene whose expression is up-regulated with other general transcription factors during spermatogenesis in the mouse. Biol Reprod. 2001;64:507–17. doi: 10.1095/biolreprod64.2.507. [DOI] [PubMed] [Google Scholar]
- 41.Bellve AR. Purification, culture, and fractionation of spermatogenic cells. Methods Enzymol. 1993;225:84–113. doi: 10.1016/0076-6879(93)25009-q. [DOI] [PubMed] [Google Scholar]
- 42.Sun J, Zhong L, Zhu Y, Liu G. Research on the isolation of mouse Leydig cells using differential digestion with a low concentration of collagenase. J Reprod Dev. 2011;57:433–6. doi: 10.1262/jrd.10-123n. [DOI] [PubMed] [Google Scholar]
- 43.Zhao P, Xiao L, Peng J, Qian YQ, Huang CC. Exosomes derived from bone marrow mesenchymal stem cells improve osteoporosis through promoting osteoblast proliferation via MAPK pathway. Eur Rev Med Pharmacol Sci. 2018;22:3962–70. doi: 10.26355/eurrev_201806_15280. [DOI] [PubMed] [Google Scholar]
- 44.Riazifar M, Mohammadi MR, Pone EJ, Yeri A, Lasser C, et al. Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano. 2019;13:6670–88. doi: 10.1021/acsnano.9b01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piehl LL, Fischman ML, Hellman U, Cisale H, Miranda PV. Boar seminal plasma exosomes: effect on sperm function and protein identification by sequencing. Theriogenology. 2013;79:1071–82. doi: 10.1016/j.theriogenology.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 46.Abu-Halima M, Ludwig N, Hart M, Leidinger P, Backes C, et al. Altered micro-ribonucleic acid expression profiles of extracellular microvesicles in the seminal plasma of patients with oligoasthenozoospermia. Fertil Steril. 2016;106:1061–9.e3. doi: 10.1016/j.fertnstert.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 47.Du J, Shen J, Wang Y, Pan C, Pang W, et al. Boar seminal plasma exosomes maintain sperm function by infiltrating into the sperm membrane. Oncotarget. 2016;7:58832–47. doi: 10.18632/oncotarget.11315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vakhshiteh F, Atyabi F, Ostad SN. Mesenchymal stem cell exosomes: a two-edged sword in cancer therapy. Int J Nanomedicine. 2019;14:2847–59. doi: 10.2147/IJN.S200036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–8. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Tang Y, Fan C, Guo C, Zhou Y, et al. The role of exosomal non-coding RNAs in cancer metastasis. Oncotarget. 2018;9:12487–502. doi: 10.18632/oncotarget.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang M, Zhou L, Yu F, Zhang Y, Li P, et al. The functional roles of exosomal long non-coding RNAs in cancer. Cell Mol Life Sci. 2019;76:2059–76. doi: 10.1007/s00018-019-03018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


