Abstract
Intracytoplasmic sperm injection (ICSI) efficiently addresses male factor infertility. However, the occurrence of abnormal fertilization, mainly characterized by abnormal pronuclei (PN) patterns, merits investigation. To investigate abnormal fertilization patterns following ICSI and identify their respective associations with abnormal parameters in semen analysis (SA), a retrospective observational study including 1855 cycles was performed. Male infertility diagnosis relied on the 2010 WHO criteria. The population was divided into groups based on their SA results. The presence of 2PNs and extrusion of the second polar body (PB) indicated normal fertilization. A Kruskal–Wallis test along with a Wilcoxon post hoc evaluation and Bonferroni correction was employed for comparison among the groups. For the pregnancy rate, logistic regression was employed. No correlation was established between the SA abnormalities and the 1PN or 3PN formation rates. The highest and lowest 0PN rates were reported for the oligoasthenoteratozoospermic and normal groups, respectively. The lowest cleavage formation rates were identified in the oligoasthenozoospermic and oligoasthenoteratozoospermic groups. The aforementioned groups along with the oligoteratozoospermic group similarly presented the lowest blastocyst formation rates. For the clinical pregnancy rate, no statistically significant difference was observed. In conclusion, the incidence of two or more abnormal SA parameters – with the common denominator being oligozoospermia – may jeopardize normal fertilization, cleavage, and blastocyst rates. Once the developmental milestone of achieving blastocyst stage status was achieved, only oligoasthenozoospermia and oligoasthenoteratozoospermia were associated with lower rates. Interestingly, following adjustment for the number of blastocysts, no statistically significant differences were observed.
Keywords: assisted reproduction techniques, fertilization, intracytoplasmic sperm injection, oligoasthenoteratozoospermia, pronuclei, semen analysis
INTRODUCTION
Male factor infertility constitutes a multifaceted and versatile phenomenon.1 According to the World Health Organization (WHO) criteria,2 male factor infertility may be phenotypically expressed as oligozoospermia, referring to a low sperm concentration of <15 × 106 ml−1 and a total spermatozoa number lower than 39 × 106 ml−1. Asthenozoospermia is described as <32% progressively motile spermatozoa. Teratozoospermia refers to a sample including a percentage of normal spermatozoa morphology <4%. Oligoasthenoteratozoospermia combines the above three compromised parameters, whereas azoospermia describes a case where no spermatozoa are detected in the ejaculate. Failure to produce ejaculate is called aspermia, and leukocytospermia is diagnosed when >1 × 106 ml−1 leukocytes are detected in the ejaculate.3 Moreover, a combination of the aforementioned categories is regularly encountered in infertile patients, and appropriate terms are employed to describe the coexistence of impaired parameters, namely, oligoasthenozoospermia, oligoteratozoospermia, and asthenoteratozoospermia.
In assisted reproduction technology (ART), intracytoplasmic sperm injection (ICSI) has emerged to address patients with severely compromised spermatogenesis.4 ICSI constitutes an invasive procedure entailing the use of a micromanipulator inverted microscope featuring a hydraulic system that enables the procedure. During ICSI, a single spermatozoon is selected, immobilized, and injected directly into the cytoplasm of the oocyte. The techniques include penetration of the zona pellucida followed by oolemma breakage prior to deposition of the injected spermatozoon into the ooplasm. ICSI application enables the employment of ejaculated spermatozoa irrespective of any compromised semen analysis (SA) parameters.5,6 However, a positive fertilization outcome may remain uncertain even when ICSI is used. The etiology of abnormal fertilization types, their causality, and how they are managed within the in vitro fertilization (IVF) laboratory remain a heated topic of continuous research.7
Normal fertilization is confirmed by the observation of two pronuclei (PN) and the extrusion of the second polar body (PB). The spermatozoon's penetration into the oocyte's cytoplasm instigates resumption of the second meiotic division, resulting in the formation and extrusion of the second PB. Following penetration of the spermatozoon, the enclosed paternal genetic material forms the paternal pronucleus. At the same time, the maternal pronucleus enclosing the maternal genetic material is also organized. Sixteen to eighteen hours following insemination in the IVF laboratory, fertilization is assessed.8 When no PNs are visible (identified by 0PN), the oocyte has failed to fertilize. Apart from the 0PN confirming the failure of fertilization, the presence of a single PN or 3PNs similarly constitutes a pathological finding.8 The additional pronucleus may be of paternal or maternal origin, while the number of the detected PBs may serve as a way to determine the pronucleus' true identity.9,10 Nonetheless, the management of such cases may vary significantly in clinical decision-making, namely, 1PN embryos may either be discarded or considered for embryo transfer in case of absence of an adequate number of normally fertilized embryos.11 The percentages of 1PN and polypronuclear zygotes following conventional IVF vary from 2.7% to 5.5% and from 4.7% to 12.5%, and for ICSI, from 4.9% to 11.4% and from 5.1% to 7.4%, respectively.12 Nonetheless, chromosomal profiling is strongly suggested prior to initiating such a practice.10
Various patterns have been described to understand cases featuring the abnormal presence of PNs.13,14,15,16 An increased number of PNs is usually attributed to triploidy affecting 1%–3% of pregnancies and characterized by the presence of three instead of two haploid chromosome complements. This may be attributed to various underlying etiological factors, including polyspermy, the fertilization of an oocyte by a diploid spermatozoon leading to diandric triploidy,17 the failure of the second PB extrusion,11 or fertilization of a diploid oocyte leading to digynic triploidy.10 Regarding the incidence of a 1PN zygote, the presence of 1PN in the cytoplasm of an oocyte following fertilization may be attributed to an event of parthenogenetic activation, the premature fusion of maternal PN and paternal PN,13 or to asynchrony in PN formation at the time of assessment.18
ICSI may be related to a reduced likelihood of abnormal fertilization patterns because this technique safely ensures that a singular spermatozoon is selected and injected. Spermatozoon selection relies on the criterion of good morphological features, provided that the respective sample allows this option and includes some spermatozoa with good motility and morphology. Nonetheless, there is an increased number of couples persistently presenting with poor quality or abnormal fertilization despite the implementation of ICSI and reporting high fertility success rates overall.19 The aim of this study is to investigate the incidence of abnormal fertilization originating from ICSI cases and explore the possibility of respective associations with abnormal semen analysis parameters. We sought to test the hypothesis that abnormal fertilization patterns following ICSI may not be random observations but directly associated with specific patterns of abnormal semen analysis parameters – a hypothesis that has not been hitherto investigated in the literature. The goal in conducting this research was to provide further understanding of the associations and define the expectations related to abnormal fertilization. Semen analysis, as the foremost and primary assessment in infertility investigation, may help further guide treatment strategies, developing from simply prescribing ICSI to anticipating certain fertilization patterns and how this information can be employed in optimal management to avoid ART overuse.
MATERIALS AND METHODS
The phenomenon of abnormal fertilization was studied in 1855 ICSI cycles performed between 2014 and 2018 in Genesis Athens Clinic, Athens, Greece. Medical records from couples undergoing a first fresh embryo transfer autologous ICSI cycle employing fresh semen samples between 2014 and 2019 were retrieved and included in the study. The Ethics Board of the Centre of Human Reproduction (Athens, Greece) approved the study protocol in accordance with the Helsinki declaration (136/4-6-2019). All participants signed an informed consent. The inclusion criteria for the present study were being a male aged 18–50 years or a female aged 18–40 years using the standard gonadotropin-releasing hormone (GnRH) long agonist protocol. All couples were subjected to extensive infertility investigations, including sexually transmitted disease testing and hormonal evaluations for both males and their female counterparts, hormonal and ultrasonographical assessments to report ovarian functions, and, subsequently, hysterosalpingography to report the tubal patency and anatomical physiology of the uterus for the female counterpart. The extensive infertility investigation prior to initiating treatment in our clinic included the molecular karyotyping of both partners. Furthermore, patients presenting with an initial compromised semen analysis were advised to visit a urologist for further evaluation.
Semen analysis and sperm preparation
For the diagnosis of male infertility, a semen analysis according to the WHO criteria was performed.2 Semen samples were collected following 2–5 days of sexual abstinence, as suggested. The semen analysis results presented in this study refer to the semen analysis performed on the oocyte retrieval day. Semen concentration and motility were assessed using a Makler counting chamber (Sefi Medical Instruments, Haifa, Israel). Spermatozoa viability was assessed via an eosin–nigrosin assay (Merck, Athens, Greece), and morphology was evaluated under a light microscope (Eclipse TE 2000 U, Nikon, Tokyo, Japan) with ×400 magnification employing the Papanicolaou staining technique. Following the initial semen analysis, sperm preparation was performed employing the density gradient centrifugation method, according to the WHO. Specifically, a maximum of 2.5 ml of the semen sample was gently stratified above the two gradients' layers (80% and 40% sperm gradients, Cook Medical LLC, Bloomington, IN, USA) and centrifuged (Centrifuge 5702 R, Eppendorf, Wien, Austria) at 400g for 20 min. The pellet was then aspirated and resuspended in 3 ml of the sperm medium (Cook Medical LLC) prior to being further washed and centrifuged at 200g for 10 min. Following the second centrifugation, the final pellet was resuspended in the appropriate volume of sperm medium to provide the desired concentration for ICSI performance. Where possible, the concentration of the final prepped sample to be employed for ICSI should be approximately 10 × 106 – 15 × 106 spermatozoa per ml. The same sperm preparation technique was employed for all samples.
Exclusion criteria
Patients presenting with abnormalities in the ovaries, Fallopian tubes, or uterus were excluded from this study. Furthermore, women reporting genetic diseases or chromosomal abnormalities, a cancer diagnosis, reduced ovarian reserves, or endocrine disorders were similarly excluded. Only men reporting with a normal molecular karyotype were recruited in the study, excluding any genetic or chromosomal abnormalities including Y chromosome microdeletions. Couples opting for preimplantation genetic testing were excluded on the basis of possible unreported genetic disorders. Cases of azoospermia, cryptozoospermia, aspermia, severe hypospermia (<1 ml), necrozoospermia, or semen analysis with a lymphocyte or erythrocyte concentration >1 × 106 ml-1 were also excluded. Patients diagnosed with a varicocele or with a prior varicocelectomy were likewise excluded from the current study.
Study groups
Participants were divided into eight major groups according to male infertility diagnosis and the respective combinations of individual abnormal parameters. Men with a normal semen analysis comprised the normal group, which served as the control group. Male infertility was diagnosed as oligozoospermia, referring to a low spermatozoa concentration of <15 × 106 ml−1 or a total spermatozoa number lower than 39 × 106 ml−1; asthenozoospermia, describing a percentage of <32% progressively motile spermatozoa present or a percentage of <40% motile spermatozoa; or teratozoospermia, describing <4% morphologically normal spermatozoa. Men were categorized in each group according to the semen analysis performed on the day of ICSI. The groups created according to male infertility diagnosis were normal (n = 162), oligozoospermia (n = 229), asthenozoospermia (n = 295), teratozoospermia (n = 83), oligoasthenozoospermia (n = 232), oligoteratozoospermia (n = 213), asthenoteratozoospermia (n = 285), and oligoasthenoteratozoospermia (n = 356).
ICSI criteria and assessment of fertilization
ICSI was performed on samples of male factor etiology presenting at least one abnormal semen analysis parameter, referring to oligozoospermic, asthenozoospremic, and teratozoospermic patients, as well as their respective combinations and subgroups. ICSI was performed on the normal semen analysis samples when the infertility investigation indicated unexplained infertility.20 Eighteen hours following the ICSI procedure, an assessment of fertilization was performed. Oocytes that sustained degeneration or lysis following the ICSI procedure or that failed to be fertilized were similarly recorded.
Embryo quality and transfer
Normally fertilized oocytes were placed into an embryo culture dish with a Continuous Single Culture media (Fujifilm Irvine Scientific Inc., Santa Ana, CA, USA) under mineral oil and were cultured under 37°C, 6% CO2, 5% O2, and 89% N2 in a humidified incubator. On day three, the embryos were classified according to the ALPHA/ESHRE 2011 consensus.21 The evaluation of day-five blastocysts was performed according to Gardner's criteria.22 Decision-making on the embryo transfer day relied on the number and quality of embryos on day three. For couples with less than five embryos on day three or those presenting poor-quality embryos, a cleavage-stage embryo transfer was performed. All embryo transfers included two embryos, abiding by Greek Laws.
Outcome measures
To assess the impact of semen parameters on fertilization regardless of the number of metaphase II (MII) oocytes retrieved, we employed the mean rates for each group instead of the absolute means. The 2PN, 3PN, 1PN, 0PN, and lysed oocyte rates are defined as the number of the fertilized or lysed oocytes over the number of MII oocytes. Similarly, to assess embryo development regardless of fertilization, we defined the cleavage formation rate as the number of cleaved embryos/the number of 2PN zygotes, and the blastocyst formation rate as the number of blastocysts/the number of 2PN zygotes. The rates correspond to each cycle; thus, for each group, the mean rate is presented. The total fertilization failure is defined as the percentage of cycles presenting without any normally fertilized (2PN) zygotes.
Subgroup analysis
A subgroup analysis of the severity of oligozoospermia was performed. Oligozoospermic patients were categorized as mild oligozoospermia (O-M) if they presented with a concentration of 5 × 106 ml-1 – 15 × 106 ml−1, as severe oligozoospermia (O-S) if they presented with a concentration of 1 × 106 ml-1 – 5 × 106 ml−1, and as very severe oligozoospermia (O-VS) if they presented with a concentration of less than 1 × 106 ml−1. A subgroup analysis of the severity of asthenozoospermia was also performed. Patients presenting with only nonprogressive and immotile spermatozoa were classified as the nonprogressive asthenozoospermic group (A-NP), whereas those presenting with only immotile spermatozoa were classified in the immotility group (A-IM); and the remaining samples were classified in the mild asthenozoospermic subgroup (A-M). In patients presenting with only immotile spermatozoa, the selection of viable spermatozoa was performed by employing the laser-assisted selection method first described by Aktan et al.23 The new oligozoospermic and asthenozoospermic subgroups were compared with the normal group and the oligoasthenoteratozoospermic group, as these groups were associated with the best and worst prognoses, respectively. The significance level remained at a P = 0.00625 to minimize the possible multiple comparison bias.
Statistical analyses
The statistical analysis was performed using the R programming language for statistical purposes via the RStudio interpreter version 1.2.5 (RStudio, Boston, MA, USA). The normality of the distribution was assessed by a Shapiro–Wilk test. Because a normal distribution was not followed, for continuous outcomes, a Kruskal–Wallis test was employed to assess the differences between groups. A post hoc analysis was performed employing the Wilcoxon post hoc test for pair-wise comparisons. For the assessment of differences between the groups regarding clinical pregnancy, a logistic regression analysis was employed. The variables serving as confounding factors for this study were embryo number, quality, and day of transfer because none of the other parameters (e.g., male or female age and the number of inseminated oocytes) differed statistically significantly. To avoid a multiple comparison bias, Bonferroni correction was employed, setting the level of statistical significance to a P = 0.00625 (eight analyses) in the first analysis. We decided to maintain the significance level at P = 0.00625 (eight analyses) during the subgroup analysis to avoid the aforementioned bias.
RESULTS
A total of 1855 couples undergoing their first ICSI cycle were included in the present study and were grouped according to male infertility diagnosis. Data on patients' general characteristics are presented in Table 1. No statistically significant difference was observed for any of the parameters presented in Table 1 (i.e., male and female age, follicle-stimulating hormone [FSH], estradiol [E2], number of oocytes retrieved, and the number of mature MII oocytes obtained). For all cycles, at least 5 MII oocytes were acquired and inseminated via ICSI.
Table 1.
Descriptive statistics (mean±standard deviation) for the male partner’s age, female partner’s age, reproductive hormone levels, number of oocytes retrieved, and number of mature metaphase II oocytes obtained
| Groups | O (n=229) | A (n=295) | T (n=83) | OA (n=232) | OT (n=213) | AT (n=285) | OAT (n=356) | N (n=162) | P |
|---|---|---|---|---|---|---|---|---|---|
| Male age (year) | 41.01±3.45 | 40.55±3.16 | 41.21±2.25 | 41.12±3.31 | 41.69±3.27 | 41.39±2.88 | 41.17±3.01 | 40.72±3.11 | 0.85 |
| Female age (year) | 35.72±1.87 | 36.03±2.01 | 35.76±2.22 | 35.76±1.95 | 35.70±1.48 | 35.68±1.65 | 35.71±1.78 | 35.72±1.91 | 0.76 |
| FSH (IU l−1; on day 3) | 7.76±2.35 | 7.95±2.43 | 8.09±2.21 | 7.10±2.38 | 7.35±2.19 | 7.86±2.25 | 8.25±2.87 | 7.59±2.91 | 0.78 |
| E2 (pg ml−1; on hCG day) | 3269±652 | 3176±588 | 3007±635 | 2861±699 | 2784±711 | 3104±667 | 3062±658 | 2874±609 | 0.85 |
| Number of oocytes retrieved | 15.94±4.64 | 14.08±4.84 | 14.37±4.44 | 15.01±4.71 | 13.69±4.67 | 13.86±4.49 | 14.14±4.73 | 14.14±4.60 | 0.09 |
| Number of MII oocytes | 14.81±3.99 | 12.53±4.29 | 14.02±4.04 | 13.53±4.53 | 12.44±4.13 | 12.69±4.35 | 12.14±4.04 | 12.31±4.80 | 0.07 |
| Cycles with total fertilization failure | 3 | 6 | 3 | 4 | 5 | 5 | 10 | 3 | 0.97 |
A Kruskal–Wallis test and a Wilcoxon post hoc analysis were employed for the statistical evaluation. FSH: follicle-stimulating hormone recorded on day 3 of menstrual cycle; E2: estradiol recorded on the day of hCG triggering; hCG: human chorionic gonadotropin; O: oligozoospermia; A: asthenozoospermia; T: teratozoospermia; OA: oligoasthenozoospermia; OT: oligoteratozoospermia; AT: asthenoteratozoospermia; OAT: oligoasthenoteratozoospermia; N: normal semen analysis; MII: metaphase II
Normal two pronuclei zygote formation rate
For the formation of normal 2PN zygotes, a statistically significant difference among the groups was observed (P < 0.0001). The 2PN rates for each group are presented in Table 2. The normozoospermic group, with a mean 2PN rate of 0.82 (standard deviation [s.d.]: 0.05), presented a statistically significantly higher 2PN rate compared to that of any other group (P < 0.0001). The oligoasthenoteratozoospermic group, with a mean 2PN rate of 0.601 (s.d.: 0.081), presented a statistically significantly lower 2PN rate compared to that of any other group (P < 0.0001). The oligozoospermic group, with a mean 2PN rate of 0.761 (s.d.: 0.072), presented no statistically significant difference compared to that of the asthenozoospermic group, the teratozoospermic group, and the oligoasthenozoospermic group, but did present a statistically higher 2PN rate compared to that of the oligoteratozoospermic (P < 0.0001) and the asthenoteratozoospermic groups (P = 0.0029). The asthenozoospermic group, with a mean 2PN rate of 0.75 (s.d.: 0.05), presented no statistically significant difference compared to that of the teratozoospermic, asthenoteratozoospermic, and oligoasthenozoospermic groups but did present a statistically significantly higher 2PN rate compared to that of the oligoteratozoospermic group (P < 0.0001). The teratozoospermic group presented a statistically significantly higher 2PN rate compared to that of the oligoteratozoospermic group (P = 0.0025). The oligoasthenozoospermic group presented a statistically significantly higher 2PN rate compared to that of the asthenoteratozoospermic (P = 0.0017) and oligoteratozoospermic groups (P < 0.0001). The asthenoteratozoospermic group presented a statistically significantly higher 2PN rate compared to that of the oligoteratozoospermic group (P < 0.0001). A graphical representation of the mean 2PN rate for each group is presented in Figure 1a.
Table 2.
Descriptive statistics (mean±standard deviation) for the 2PN, 1PN, 3PN, lysed, fertilization failure (0PN), and cleavage formation rates
| Groups | O (n=229) | A (n=295) | T (n=83) | OA (n=232) | OT (n=213) | AT (n=285) | OAT (n=356) | N (n=162) |
|---|---|---|---|---|---|---|---|---|
| 2PN rate | 0.76±0.07N,OT,AT,OAT | 0.75±0.05N,OT,OAT | 0.74±0.09N,OT,OAT | 0.75±0.03N,OT,AT,OAT | 0.71±0.08* | 0.74±0.05* | 0.60±0.08* | 0.82±0.05* |
| 1PN rate | 0.0152±0.0347 | 0.0126±0.0286 | 0.0125±0.0314 | 0.01±0.03 | 0.0076±0.0252 | 0.015±0.034 | 0.0181±0.0391 | 0.0056±0.0199 |
| 3PN rate | 0.0223±0.0398 | 0.0202±0.0396 | 0.0172±0.0361 | 0.0184±0.0350 | 0.0139±0.0339 | 0.0254±0.0433 | 0.0514±0.0543 | 0.0078±0.0211 |
| Lysed rate | 0.0226±0.0393 | 0.0165±0.0357 | 0.0167±0.0352 | 0.013±0.030 | 0.0316±0.0114 | 0.0160±0.0342 | 0.0355±0.0497 | 0.0076±0.0218 |
| 0PN rate | 0.170±0.065N,A,OA,OT,AT,OAT | 0.195±0.068N,O,OT,OAT | 0.190±0.074N,OT,OAT | 0.201±0.057N,O,OT,OAT | 0.216±0.065* | 0.197±0.061N,O,OA,OAT | 0.246±0.075* | 0.155±0.051* |
| Cleavage formation rate | 0.817±0.051OA,OAT | 0.794±0.084OA,OAT | 0.81±0.05OA,OAT | 0.773±0.086N,O,A,T,OT,AT | 0.814±0.508OA,OAT | 0.821±0.098OA,OAT | 0.78±0.10N,O,A,T,OT,AT | 0.801±0.087OA,OAT |
A Kruskal–Wallis test and a Wilcoxon post hoc analysis were employed for the statistical evaluation. Statistical significance is set at P=0.00625. The letters in superscript indicate statistically significant differences for their respective groups. *Statistically significant differences for all other groups. PN: pronuclei; O: oligozoospermia; A: asthenozoospermia; T: teratozoospermia; OA: oligoasthenozoospermia; OT: oligoteratozoospermia; AT: asthenoteratozoospermia; OAT: oligoasthenoteratozoospermia; N: normal semen analysis
Figure 1.
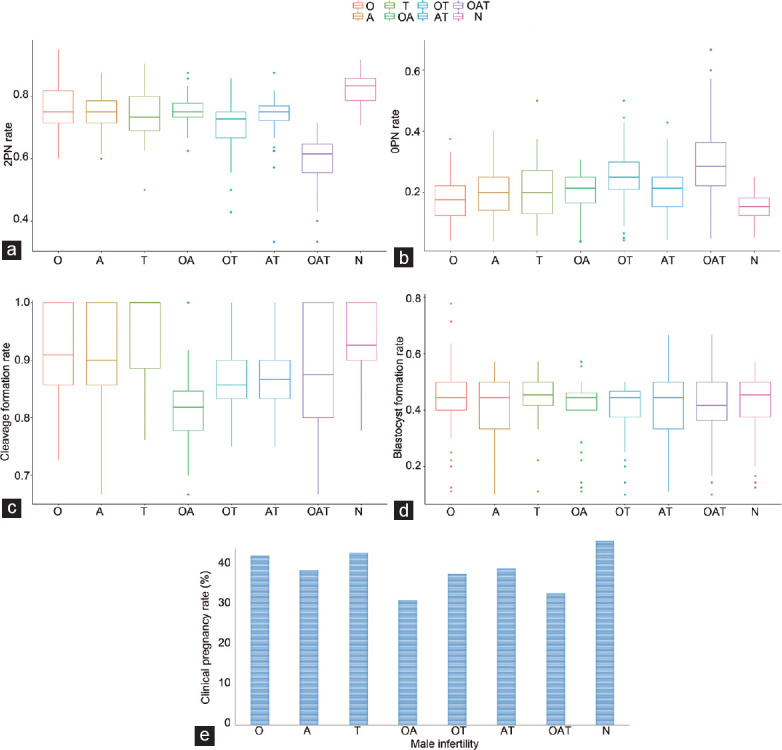
Graphical representation of the statistically significant findings between the groups. (a) The mean 2PN rate for each group. (b) The mean 0PN rate for each group. (c) The mean cleavage formation rate for each group. (d) The mean blastocyst formation rate for each group. (e) The clinical pregnancy rate for each group. Statistical significance is set at P = 0.00625. PN: pronuclei; O: oligozoospermia; A: asthenozoospermia; T: teratozoospermia; OA: oligoasthenozoospermia; OT: oligoteratozoospermia; AT: asthenoteratozoospermia; OAT: oligoasthenoteratozoospermia; N: normal semen analysis.
Abnormal zygote formation rate, 0PN rate, and lysed rate
No statistically significant difference between the groups was presented for the 1PN rate (P = 0.3495), 3PN rate (P = 0.1489), and lysed rate (P = 0.4041). The 0PN rate differed statistically significantly between the groups (P < 0.0001). The 1PN, 3PN, 0PN, and lysed rates for each group are presented in Table 2. The normal group, with a mean 0PN rate of 0.155 (s.d.: 0.051), presented a statistically significantly lower 0PN rate compared to that of any other group (P < 0.0001). The oligoasthenoteratozoospermic group, with a mean 0PN rate of 0.246 (s.d.: 0.075), presented a statistically significantly higher 0PN rate compared to that of any other group (P < 0.0001). The oligozoospermic group presented no statistically significant difference compared to that of the teratozoospermic group (P = 0.0075) but did present a statistically significantly lower 0PN rate compared to that of the asthenozoospermic (P = 0.0007), oligoasthenozoospermic (P < 0.0001), oligoteratozoospermic (P < 0.0001), and asthenoteratozoospermic (P < 0.0001) groups. The asthenozoospermic group, with a mean 0PN rate of 0.195 (s.d.: 0.068), presented no statistically significant difference compared to that of the teratozoospermic, asthenoteratozoospermic, and oligoasthenozoospermic groups, but did present a statistically lower 0PN rate compared to that of the oligoteratozoospermic (P < 0.0001) group. The teratozoospermic group presented no statistically significant difference compared to that of the oligoasthenozoospermic and the asthenoteratozoospermic groups, but did present a statistically lower fertilization failure rate compared to that of the oligoteratozoospermic group (P < 0.0001). No statistically significant difference was observed when comparing the oligoasthenozoospermic and asthenoteratozoospermic groups (mean ± s.d.: 0.201 ± 0.057 vs 0.197 ± 0.061), although both presented a statistically lower fertilization failure rate compared to that of the oligoteratozoospermic group (P < 0.0001). A graphical representation of the mean 0PN rate for each group is presented in Figure 1b.
Cleavage formation rate, blastocyst formation rate, and clinical pregnancy rates
A statistically significant difference was observed between the groups for the cleavage formation rate. The cleavage and blastocyst formation rates for each group are presented in Table 2. Following the post-hoc analysis, only the oligoasthenozoospermic and oligoasthenoteratozoospermic groups presented a statistically significantly lower cleavage formation rate compared to that of other groups (P < 0.001). No statistically significant difference was observed between the two groups. Couples with six or more cleavage-stage embryos proceeded to the blastocyst culture (n = 1361). The blastocyst formation rates differed statistically significantly between the groups (P < 0.0001). The normal group presented no statistically significant difference compared to that of the oligozoospermic, asthenozoospermic, teratozoospermic, and asthenoteratozoospermic groups, but did present a statistically significantly higher blastocyst formation rate compared to that of the oligoasthenozoospermic, oligoteratozoospermic, and oligoasthenoteratozoospermic groups (P < 0.0001). The oligozoospermic, asthenozoospermic, and teratozoospermic groups presented no statistically significant difference in comparison to that of any of the other groups. The oligoasthenozoospermic, oligoteratozoospermic, asthenoteratozoospermic, and oligoasthenoteratozoospermic groups presented no other statistically significant difference. A graphical representation of the mean blastocyst formation rate is presented in Figure 1 for the clinical pregnancy rates following an adjustment for the number and quality of embryos and the day of the embryo transfer. The mean cleavage formation rate, mean blastocyst formation rate, and clinical pregnancy rate for each group are graphically represented in Figure 1c–1e, respectively. The 2PN, 1PN, 3PN, lysed, 0PN, and cleavage formation rates for each group are presented in Table 2.
Total motile sperm count
The total motile sperm count (TMSC) positively correlated with the 2PN rate (rho: 0.285; P < 0.0001) and negatively correlated with the 3PN rate (rho: −0.095; P = 0.0005) and 0PN rate (rho: −0.223; P < 0.0001). No statistically significant difference was observed between the TMSC and the 1PN rate (rho: −0.018; P = 0.44) or lysed rate (rho: −0.028; P = 0.23). The TMSC positively correlated with the cleavage formation rate (rho: 0.193; P < 0.0001). However, no statistically significant correlation was observed between the TMSC and blastocyst formation rate (rho: 0.023; P = 0.34). When evaluating the TMSC according to morphology, if the normal forms were within a normal range, TMSC positively correlated with the 2PN rate (rho: 0.262; P < 0.0001), negatively correlated with the 0PN rate (rho: −0.179; P < 0.0001), and positively correlated with the cleavage formation rate (rho: 0.273; P < 0.0001) and blastocyst formation rate (rho: 0.085; P = 0.01). In couples with an abnormal range of normal forms, the TMSC similarly positively correlated with the 2PN rate (rho: 0.141; P = 0.0002), negatively correlated with the 0PN rate (rho: −0.131; P = 0.0006), and positively correlated with the cleavage formation rate (rho: 0.101; P = 0.002). No statistically significant correlation was observed between the TMSC and blastocyst formation rate (rho: −0.0519; P = 0.06).
Subgroup analysis for the oligozoospermia group
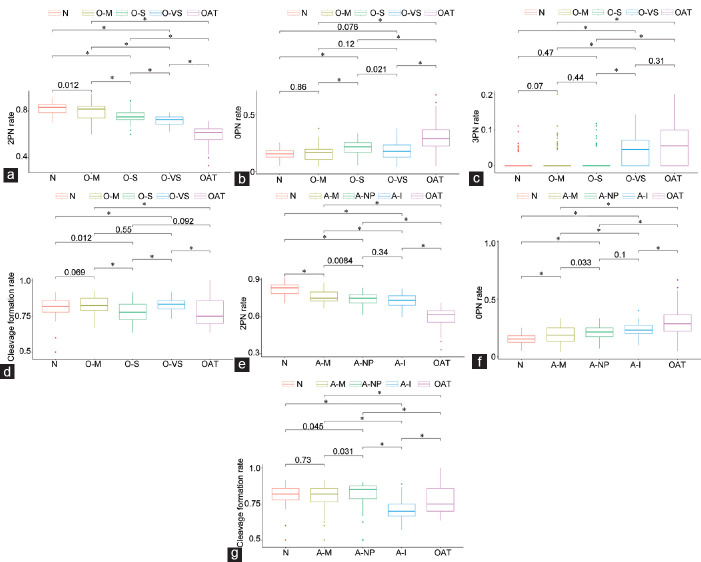
Table 3 provides a presentation of the 2PN, 1PN, 3PN, lysed, fertilization failure, cleavage formation, blastocyst formation, and clinical pregnancy rates for the oligozoospermia subgroup. Figure 2a–2d graphically represents the mean 2PN, 0PN, 3PN, and cleavage formation rate in the oligozoospermic subgroups compared to that of the normal and oligoasthenoteratozoospermic groups.
Table 3.
Descriptive statistics (mean±standard deviation) for the 2PN, 1PN, 3PN, lysed, fertilization failure (0PN), cleavage formation, blastocyst formation, and clinical pregnancy rates in the oligozoospermic subgroup
| Groups | O-M (n=91) | O-S (n=77) | O-VS (n=61) | OAT (n=356) | N (n=162) |
|---|---|---|---|---|---|
| 2PN rate | 0.7950±0.0779O-S,O-VS,0AT | 0.7545±0.0604N,O-M,OAT | 0.7169±0.0451N,O-M,OAT | 0.5977±0.0752* | 0.8242±0.0499O-S,O-VS,OAT |
| 1PN rate | 0.0072±0.0305 | 0.0169±0.0333 | 0.0252±0.0396 | 0.0181±0.0391 | 0.0056±0.0199 |
| 3PN rate | 0.0163±0.0357O-VS,OAT | 0.01±0.03O-VS,OAT | 0.0437±0.0481N,O-M,O-S | 0.0514±0.0543N,O-M,O-S | 0.0078±0.0211O-VS,OAT |
| Lysed rate | 0.0226±0.0412 | 0.0132±0.0306 | 0.0343±0.0434 | 0.0355±0.0497 | 0.0076±0.0218 |
| 0PN rate | 0.1589±0.0635O-S,OAT | 0.2031±0.0681N,O-M,O-VS | 0.7861±0.0668O-S,OAT | 0.246±0.075N,O-M,O-VS | 0.155±0.051O-S,OAT |
| Cleavage formation rate | 0.8293±0.0602O-S | 0.7861±0.0668O-M,O-VS | 0.8359±0.0466O-S | 0.78±0.10N | 0.801±0.087OAT |
| Blastocyst formation rate | 0.4754±0.0676 | 0.4638±0.0806 | 0.479±0.074 | 0.4526±0.0681N | 0.4835±0.0411OAT |
| Clinical pregnancy rate, n/total (%) | 40/91 (43.96) | 31/77 (40.26) | 24/61 (39.34) | 115/356 (32.30) | 73/162 (45.06) |
A Kruskal–Wallis test and a Wilcoxon post hoc analysis were employed for the statistical evaluation. Statistical significance is set at P=0.00625. The letters in superscript indicate statistically significant differences for their respective groups. *Statistically significant differences for all other groups. PN: pronuclei; O-M: oligozoospermia-mild; O-VS: oligozoospermia- severe; O-VS: oligozoospermia-very severe; OAT: oligoasthenoteratozoospermia; N: normal semen analysis
Figure 2.
Graphical representation of statistically significant findings in the asthenozoospermic subgroups compared to those of the normal and oligoasthenoteratozoospermic groups. (a) The 2PN rate in the oligozoospermic subgroups compared to that of the normal and oligoasthenoteratozoospermic groups. (b) The 0PN rate in the oligozoospermic subgroups compared to that of the normal and oligoasthenoteratozoospermic groups. (c) The 3PN rate in the oligozoospermic subgroups compared to that of the normal and oligoasthenoteratozoospermic groups. (d) The cleavage formation rate in the oligozoospermic subgroups compared to that of the normal and oligoasthenoteratozoospermic groups. (e) The 2PN rate in the asthenozoospermic subgroups compared to that of the normal and oligoasthenoteratozoospermic groups. (f) The 0PN rate in the asthenozoospermic subgroups compared to that of the normal and oligoasthenoteratozoospermic groups. (g) The cleavage formation rate in the asthenozoospermic subgroups compared to that of the normal and oligoasthenoteratozoospermic groups. Statistical significance is set at P = 0.00625. PN: pronuclei; N: Normal; A-M: the mild asthenozoospermic subgroup; A-NP: nonprogressive asthenozoospermic group; A-I: the immotility group; O-M: oligozoospermia-mild; O-S: oligozoospermia-severe; O-VS: oligozoospermia-very severe; OAT: oligoasthenozoospermia.
A statistically significant difference was observed in the 2PN formation rate (P < 0.0001) among the subgroups. The O-M group presented a similar 2PN formation rate compared to that of the normal group, and both of them presented superior 2PN formation rates compared to those of both the O-S and O-VS groups. All oligozoospermic subgroups presented a higher 2PN formation rate compared to that of the oligoasthenoteratozoospermia group.
No statistically significant difference was identified regarding the 1PN rate (P = 0.327) or the lysed rate (P = 0.223). The 0PN rate differed statistically significantly among the groups (P < 0.0001). The OAT and the O-S groups presented the highest 0PN rate. The normal, O-M, and O-VS groups presented no statistically significant differences between each other.
The 3PN rate differed statistically significantly between the groups (P < 0.0001). The OAT and O-VS groups presented the highest 3PN formation rate. The normal, O-M, and O-S groups presented no statistically significant differences in the 3PN rate between each other.
The cleavage formation rate differed statistically significantly between the groups (P < 0.0001). The normal group presented no statistically significant difference compared to the O-M, O-VS, and O-S groups. The O-M group presented no statistically significantly different cleavage formation rate compared to that of the O-VS group but did present a statistically higher cleavage formation rate compared to that of O-S group (P = 0.0003). Interestingly, the O-S group presented a statistically significantly lower cleavage formation rate compared to that of the O-VS group (P = 0.0007). A statistically significant difference between the groups was observed in the blastocyst formation rate (P < 0.0001). The only statistically significant difference was observed between the normal and oligoasthenoteratozoospermic groups. No statistically significant difference was observed regarding the clinical pregnancy rates.
Subgroup analysis for the asthenozoospermia group
Table 4 provides a presentation of the 2PN, 1PN, 3PN, lysed, fertilization failure, cleavage formation, blastocyst formation, and clinical pregnancy rates for the asthenozoospermic subgroup. Figure 2e–2g graphically represent the mean 2PN, 0PN, and cleavage formation rate in the asthenozoospermic subgroups compared to that of the normal and oligoasthenoteratozoospermic groups, respectively.
Table 4.
Descriptive statistics (mean±standard deviation) for the 2PN, 1PN, 3PN, lysed, fertilization failure (0PN), cleavage formation, blastocyst formation, and clinical pregnancy rates in the asthenozoospermic subgroup
| Groups | A-NP (n=56) | A-IM (n=57) | A-M (n=182) | OAT (n=356) | N (n=162) |
|---|---|---|---|---|---|
| 2PN rate | 0.7358±0.0559N,A-M,OAT | 0.7260±0.0519N,A-M,OAT | 0.7620±0.0484N,A-NP,A-IM,OAT | 0.5977±0.0752* | 0.8242±0.0499* |
| 1PN rate | 0.0107±0.0307 | 0.0078±0.0237 | 0.0147±0.0292 | 0.018±0.039 | 0.0056±0.0199 |
| 3PN rate | 0.0260±0.0448 | 0.0153±0.0392 | 0.0201±0.038 | 0.0514±0.0543 | 0.0078±0.0211 |
| Lysed rate | 0.0176±0.0411 | 0.0193±0.0411 | 0.0153±0.0321 | 0.0355±0.0497 | 0.0076±0.0218 |
| 0PN rate | 0.2099±0.0644N,OAT | 0.2317±0.0704N,A-M,OAT | 0.1880±0.0687N,A-IM,OAT | 0.2460±0.0749* | 0.1551±0.0511* |
| Cleavage formation rate | 0.8190±0.0835A-IM,OAT | 0.7138±0.0906 | 0.8045±0.0729A-IM,OAT | 0.78±0.10 | 0.801±0.087A-IM,OAT |
| Blastocyst formation rate | 0.4871±0.0585 | 0.4390±0.0642N | 0.4672±0.0496 | 0.4526±0.0681N | 0.4835±0.0411A-IM,OAT |
| Clinical pregnancy rate, n/total (%) | 18/56 (32.14) | 21/57 (36.84) | 73/182 (40.11) | 115/356 (32.30) | 73/162 (45.06) |
A Kruskal–Wallis test and a Wilcoxon post hoc analysis were employed for the statistical evaluation. Statistical significance is set at P=0.00625. The letters in superscript indicate statistically significant differences for their respective groups. *Statistically significant differences for all other groups. PN: pronuclei; A-NP: nonprogressive asthenozoospermic group; A-IM: the immotility group; A-M: the mild asthenozoospermic subgroup; OAT: oligoasthenoteratozoospermia; N: normal semen analysis
A statistically significant difference was observed in the 2PN formation rate (P < 0.0001). The normal group presented a statistically significantly higher 2PN formation rate compared to that of all asthenozoospermic subgroups. Among the subgroups, the A-M groups presented the highest 2PN rate, and no statistically significant difference was observed between the A-NP and A-IM groups. The oligoasthenoteratozoospermic group presented a statistically significantly lower 2PN rate compared to that of the A-M (P < 0.0001), A-NP (P < 0.0001), and A-IM groups (P < 0.0001).
No statistically significant difference was observed in the 1PN, 3PN, and lysed rate. The 0PN rate differed statistically significantly between the groups (P < 0.0001). The normal group presented a statistically significantly lower fertilization failure rate compared to that of all asthenozoospermic subgroups. The A-M group presented a lower 0PN rate compared to that of the A-IM group. The highest 0PN rate was observed in the oligoasthenoteratozoospermic group.
The cleavage formation rate differed statistically significantly among the groups (P < 0.0001). The normal group, the A-M group, and the A-NP group presented no statistically significant difference between each other. The OAT group presented a lower cleavage formation rate than that of the aforementioned groups. The A-IM group presented the lowest cleavage formation rate among the groups.
A statistically significant difference among the groups was observed in the blastocyst formation rate (P < 0.0001). The only statistically significant differences observed were between the normal and oligoasthenoteratozoospermic groups, as previously reported, and a statistically significantly lower blastocyst formation rate was observed in the A-IM group compared to that of the normal group (P = 0.0004). No statistically significant difference was observed in the clinical pregnancy rates.
DISCUSSION
This retrospective observational study set out to investigate the possible associations between abnormal fertilization patterns, focusing on 1PN and 3PN zygotes using poor semen analysis parameters following ICSI for couples diagnosed with male factor infertility. Understandably, information on the oocyte's or the spermatozoon's chromosomal complements prior to proceeding with insemination could not be extracted nor is such information established as part of standard practice. Hence, for assisting the practitioner, seeking associations between the parameters accessible within the ART laboratory may be of added value. To our knowledge, no other study has attempted to determine the associations between abnormal semen analysis parameters – when recruiting via the controlled act of insemination employing ICSI – and subsequent abnormal fertilization patterns, such as 1PN or 3PN zygotes. We ultimately decided to classify couples according to male infertility diagnosis to enable the assessment of the cumulative effects of combinations of abnormal parameters on the IVF outcomes. This classification is essential, as male infertility diagnosis plays an important role during decision-making in a clinical IVF setting.
No statistically significant correlations between 1PN or 3PN events and certain pathologies were detected. Our study indicated that only the number of normal 2PN zygotes may be affected by a compromised semen analysis parameter. The correlation between normal fertilization and semen analysis parameters was previously reported in the literature.24 When the appearance of a third PN is coupled with the observation of a single PB following ICSI implementation, the second PB may be assumed to have been retained by a fertilized zygote, falsely designating the presence of 3PN.17 This phenomenon has been suggested to be associated with damage to the oocyte's cytoskeleton.25 Nonetheless, none of the cases included herein presented a single PB following ICSI. 3PNs' incidents are thought to be primarily linked with the oocyte's integrity and aging, while severe semen abnormalities also constitute a major factor related to abnormal PN patterns following ICSI.17,26,27,28 The total fertilization failure in our study was 2.1% (39/1855), which is similar to the rate reported in the literature.7,29
Based on our results, sperm pathologies seem to exert no impact on the prevalence of a single pronucleus. An abnormal chromosomal status that may not be necessarily associated with the spermatozoa's impairment in morphology or motility (as documented in the literature)30 could explain the occurrence of 1PN. Moreover, as described in 1985, semen parameters constitute only a “rough guide” to evaluate fertility potential.31 Today, this is still the case in modern clinical practice. Moreover, incidents of 1PN could be attributed to either a compromised oocyte or the oocyte's behavior during ICSI performance.32,33 ICSI performance can refer to either resistance during ICSI penetration or oolemma breakage. Penetration difficulty is categorized as either high, normal, or a lack of resistance.34 Oolemma breakage is categorized as sudden, normal, or difficult.33 Different oocyte behavior during ICSI performance may be an indicator of oocyte fragility,35 which may be correlated with abnormal fertilization patterns and fertilization failure.
The lack of association between specific abnormal fertilization parameters and type of male infertility compelled us to perform a subgroup analysis based on the severity of the oligozoospermia and asthenozoospermia. Severe oligozoospermia is the only parameter that appears to be correlated with an increased rate of 3PN formation. One might question why the parameter of motility and morphology has no apparent association. It should be highlighted that during the ICSI procedure, an elective process of identifying spermatozoa with good motility and morphology is performed by the practitioner.6 In the cases of asthenozoospermia, the practitioner aims to select the spermatozoon with good motility, bypassing the abnormalities characterizing the overall sample. The same is true for the cases of teratozoospermia. This fact may serve as an adequate explanation for the lack of association between the diagnosis of asthenozoospermia or teratozoospermia and the prevalence of a third PN. Interestingly, in an oligozoospermic sample, the spermatozoon selection during ICSI could not overcome the fact that the spermatozoon selected still represented a semen sample classified as oligozoospermic. This fact may justify the correlation between the third PN formation and oligozoospermia.
As expected, the normal semen samples presented a lower rate of unfertilized oocytes (0PN). Conversely, oligoasthenoteratozoospermia cases presented the highest 0PN rate, producing the following pattern: when the morphology, concentration, and motility are concurrently compromised, the sample's potential to contribute to fertilization is severely jeopardized. Oligozoospermia and teratozoospermia resulted in similar rates of nonfertilized oocytes, while oligozoospermia resulted in an enhanced fertilization rate compared to that of asthenozoospermia, oligoasthenozoospermia, asthenoteratozoospermia, and oligoteratozoospermia. It may be extrapolated that pathologies entailing two or more compromised semen analysis parameters may have a negative association with fertilization. It is possible that the reason behind the 0PN oocytes may be the different expressions of sperm proteins that initiate the molecular pathway of oocyte activation, such as phospholipase C zeta (PLCζ).36 Recent studies have observed significant correlations between semen parameters – namely, sperm concentration, motility, and mainly sperm morphology – and PLCζ.37 Considering the rate of lysed zygotes, no correlation was observed with any pathologies or the compromised semen parameters. Thus, the assumption that lysed zygotes may originate from a fragile oocyte or poor ICSI procedure performance may be valid.
For the cleavage formation rate, the oligoasthenozoospermic and oligoasthenoteratozoospermic patients presented lower cleavage formation rates. This finding is in accordance with some of the literature38 but contradicts other studies.24,39 A similar observation was reported for the blastocyst formation rate. The aforementioned groups, along with oligoteratozoospermia, presented lower blastocyst formation rates. The decrease in the blastocyst formation rate according to semen quality is in agreement with the literature.38
An interesting observation was made regarding clinical pregnancy rates. Notably, the numerous groups with abnormal parameters in the semen analysis were associated in various ways with the embryo's developmental capacity and potential. However, it was evident that regardless of the underlying pathology affecting the spermatozoon's quality, if blastocyst formation was achieved, the clinical pregnancy rate was similar among the various groups. When reporting on the spermatozoon's role in achieving a pregnancy, it seems that specific abnormal parameters in semen analysis may only affect development up to the time point where the embryo is required to reach the blastocyst stage. Once the developmental milestone of achieving the blastocyst stage is reached, the spermatozoon's pathology does not appear to affect the cycle's outcome. Notably, a lower blastocyst formation rate may lead to a lower number of cryopreserved surplus embryos, thereby producing a lower cumulative clinical pregnancy rate. The study design did not include the outcome of the cumulative pregnancy rate, which could be regarded as a limitation of the present study. The rationale behind this intentional omission was that this outcome could produce excessive bias in the study because the decision to cryopreserve surplus embryos is strictly based on a couple's desire to proceed accordingly.
Interesting observations were made when evaluating the TMSC and the fertilization and IVF outcomes. It should be mentioned that TMSC is considered to be a valuable prognostic marker.40 The TMSC positively correlated with a normal fertilization rate, which agrees with the negative correlation observed with the 0PN rate. It has been reported in the literature that men with high TMSC present higher fertilization rates compared to men with low TMSC.41 TMSC further positively correlated with the cleavage formation rate, an observation contradictory to that of the literature, as the cleavage formation rate has been observed to be independent of TMSC.39 However, TMSC has been associated with cleavage embryo quality. When evaluating the TMSC according to sperm morphology, it was observed that in men presenting normal morphological forms within a normal range, the correlations were stronger. Moreover, a positive correlation between TMSC and the blastocyst formation rate was observed. This observation agrees with the current literature.41 On the other hand, in men presenting normal forms within an abnormal range, no statistically significant correlation was observed between TMSC and the blastocyst formation rate. Interestingly, if this correlation reached the statistical significance threshold, it would be a negative correlation. The lack of an association between TMSC and the blastocyst formation rate could be solely attributed to this difference. The employment of TMSC combined with morphology may lead to the development of a male infertility marker superior to TMSC. However, for this proposal to be validated, large multicentric studies are required.
One may expect to observe solid correlations with extreme cases of teratozoospermia while investigating abnormalities in embryo morphology. However, because practitioners selectively proceed with insemination using spermatozoa with good morphology in an otherwise teratozoospermic sample, the hypothesis that the detrimental impact of a sample with poor morphology may be limited; to what extent this detriment may be cancelled out merits thorough investigation. Based on the assumption that practitioners similarly aim to select a spermatozoon of optimal motility in an otherwise asthenozoospermic sample for insemination, could the same be true for an asthenozoospermic sample? This suggestion requires further examination because we also need to consider the media employed during ICSI and the spermatozoon handling and immobilization process. Polyvinylpyrrolidone (PVP) is commonly selected to assist the embryologist in immobilizing spermatozoa due to its highly viscous nature. In this case, the spermatozoon's motility may not be thoroughly evaluable when employing this step, so the ability of the embryologist to select the spermatozoon with the highest motility may be compromised. However, when a culture media (HEPES-buffered or not) is chosen for ICSI practice,42 the spermatozoon's motility may be assessed. In this case, proper examination may enable the embryologist to perform ICSI while employing a spermatozoon with good motility in an otherwise asthenozoospermic sample. Considering these hypotheses, specific abnormal fertilization patterns may provide an explanation for the lack of association between asthenozoospermic and teratozoospermic samples. Performing ICSI may bypass morphological and motility abnormalities by allowing the embryologist to select a motile spermatozoon with normal morphology in an otherwise compromised sample. The factors that influence oligozoospermia are still not fully explained;1,43 thus, an unknown factor may hinder the success of an ICSI cycle in that context.
Deviations in penetration difficulty and normal oolemma breakage, which are commonly encountered during ICSI, are factors that may exert a negative impact on the zygote. Limitations of this study include the lack of data on the oocytes' behavior during ICSI and the lack of a DNA fragmentation index, which has been proposed as a valuable complementary tool to evaluate sperm quality.44 All the included ICSI cycles were autologous, suggesting that the effect of the female factor on the outcome measures cannot be excluded from the equation. Nonetheless, as couples presenting with female factor infertility were excluded from this study, the outcome measure may be primarily affected by the SA parameters studied herein. The retrospective nature of this study may also correlate with bias. Nonetheless, this bias was largely compensated by the large sample size included in the study.
Male factor infertility is widely known and commonly accepted as a good prognosis in an IVF context. Our initial hypothesis was based on the observation that although male factor infertility is a good prognosis etiology that should be theoretically accompanied by impressive fertilization results, in clinical practice, a wide range of unfortunate events are still regularly encountered. Fertilization failure, abnormal fertilization, and poor embryo quality are only a few of the numerous paradigms demonstrating that the male factor has a crucial impact on various levels of in vitro culture. This empirical perception compelled us to design a study to validate our hypothesis that perhaps certain abnormalities in semen analysis parameters or a combination of those parameters may be indicative of the cycles' outcomes related to fertilization.
The data presented herein may serve as a foundation for future studies to investigate whether the meticulous selection of the most competent spermatozoon would enhance clinical outcomes. In the cases of male factor infertility involving two or more abnormal parameters, should a more thorough observation of spermatozoa or intracytoplasmic morphologically selected sperm injection (IMSI) be performed? To ensure that a more sophisticated technique would improve the ICSI outcomes, further studies are required.
Our results may serve as the impetus to ultimately test whether the incidence of two or more abnormal parameters in semen analysis (with oligozoospermia being the common denominator) could provide direction for decision-making in IVF laboratories.
Our conclusions may also be of benefit in the era of individualized medicine for case management and decision-making within IVF laboratories. Our results may be of particular importance when prior ICSI cycle data or specific semen analysis abnormalities are available and taken into account in treatment plans and strategies.
AUTHOR CONTRIBUTIONS
KP, KS, and MS conceived and designed the study. EM, AR, EK, AG-P, and TV contributed to data acquisition. EM and AR contributed to the interpretation and analysis of the data. EM, AR, EK, and MS drafted the manuscript. KP, KS, MK, and MS critically revised the manuscript. KP and MS supervised the study. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
The authors are very appreciative to all the clinicians, embryologists, and scientists at the Centre for Human Reproduction at the Genesis Hospital and at the Department of Physiology of the National and Kapodistrian University of Athens Medical School.
REFERENCES
- 1.Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: a review of literature. J Hum Reprod Sci. 2015;8:191–6. doi: 10.4103/0974-1208.170370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 3.Krausz C. Male infertility: pathogenesis and clinical diagnosis. Best Pract Res Clin Endocrinol Metab. 2011;25:271–85. doi: 10.1016/j.beem.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–8. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- 5.O'Neill CL, Chow S, Rosenwaks Z, Palermo GD. Development of ICSI. Reproduction. 2018;156:F51–8. doi: 10.1530/REP-18-0011. [DOI] [PubMed] [Google Scholar]
- 6.Simopoulou M, Gkoles L, Bakas P, Giannelou P, Kalampokas T, et al. Improving ICSI: a review from the spermatozoon perspective. Syst Biol Reprod Med. 2016;62:359–71. doi: 10.1080/19396368.2016.1229365. [DOI] [PubMed] [Google Scholar]
- 7.Kahyaoglu I, Demir B, Turkkanı A, Cınar O, Dilbaz S, et al. Total fertilization failure: is it the end of the story? J Assist Reprod Genet. 2014;31:1155–60. doi: 10.1007/s10815-014-0281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kai Y, Iwata K, Iba Y, Mio Y. Diagnosis of abnormal human fertilization status based on pronuclear origin and/or centrosome number. J Assist Reprod Genet. 2015;32:1589–95. doi: 10.1007/s10815-015-0568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garello C, Baker H, Rai J, Montgomery S, Wilson P, et al. Pronuclear orientation, polar body placement, and embryo quality after intracytoplasmic sperm injection and in-vitro fertilization: further evidence for polarity in human oocytes? Hum Reprod. 1999;14:2588–95. doi: 10.1093/humrep/14.10.2588. [DOI] [PubMed] [Google Scholar]
- 10.Rosenbusch BE. Mechanisms giving rise to triploid zygotes during assisted reproduction. Fertil Steril. 2008;90:49–55. doi: 10.1016/j.fertnstert.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 11.Reichman DE, Jackson KV, Racowsky C. Incidence and development of zygotes exhibiting abnormal pronuclear disposition after identification of two pronuclei at the fertilization check. Fertil Steril. 2010;94:965–70. doi: 10.1016/j.fertnstert.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Feenan K, Herbert M. Can 'abnormally' fertilized zygotes give rise to viable embryos? Hum Fertil Camb Engl. 2006;9:157–69. doi: 10.1080/14647270600636269. [DOI] [PubMed] [Google Scholar]
- 13.Balakier H. Tripronuclear human zygotes: the first cell cycle and subsequent development. Hum Reprod. 1993;8:1892–7. doi: 10.1093/oxfordjournals.humrep.a137955. [DOI] [PubMed] [Google Scholar]
- 14.Levron J, Munné S, Willadsen S, Rosenwaks Z, Cohen J. Male and female genomes associated in a single pronucleus in human zygotes. Biol Reprod. 1995;52:653–7. doi: 10.1095/biolreprod52.3.653. [DOI] [PubMed] [Google Scholar]
- 15.Nagy ZP, Liu J, Joris H, Devroey P, Van Steirteghem A. Time-course of oocyte activation, pronucleus formation and cleavage in human oocytes fertilized by intracytoplasmic sperm injection. Hum Reprod. 1994;9:1743–8. doi: 10.1093/oxfordjournals.humrep.a138786. [DOI] [PubMed] [Google Scholar]
- 16.Staessen C, Janssenswillen C, Devroey P, Van Steirteghem AC. Cytogenetic and morphological observations of single pronucleated human oocytes after in-vitro fertilization. Hum Reprod. 1993;8:221–3. doi: 10.1093/oxfordjournals.humrep.a138026. [DOI] [PubMed] [Google Scholar]
- 17.Rosen MP, Shen S, Dobson AT, Fujimoto VY, McCulloch CE, et al. Triploidy formation after intracytoplasmic sperm injection may be a surrogate marker for implantation. Fertil Steril. 2006;85:384–90. doi: 10.1016/j.fertnstert.2005.07.1321. [DOI] [PubMed] [Google Scholar]
- 18.Munné S, Tang YX, Grifo J, Cohen J. Origin of single pronucleated human zygotes. J Assist Reprod Genet. 1993;10:276–9. doi: 10.1007/BF01204942. [DOI] [PubMed] [Google Scholar]
- 19.Neri QV, Lee B, Rosenwaks Z, Machaca K, Palermo GD. Understanding fertilization through intracytoplasmic sperm injection (ICSI) Cell Calcium. 2014;55:24–37. doi: 10.1016/j.ceca.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Check JH, Yuan W, Garberi-Levito MC, Swenson K, McMonagle K. Effect of method of oocyte fertilization on fertilization, pregnancy and implantation rates in women with unexplained infertility. Clin Exp Obstet Gynecol. 2011;38:203–5. [PubMed] [Google Scholar]
- 21.ALPHA Scientists in Reproductive Medicine, ESHRE Special Interest Group Embryology. Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reprod Biomed Online. 2011;22:632–46. doi: 10.1016/j.rbmo.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11:307–11. doi: 10.1097/00001703-199906000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Aktan TM, Montag M, Duman S, Gorkemli H, Rink K, et al. Use of a laser to detect viable but immotile spermatozoa. Andrologia. 2004;36:366–9. doi: 10.1111/j.1439-0272.2004.00636.x. [DOI] [PubMed] [Google Scholar]
- 24.Verza S, Esteves SC. Sperm defect severity rather than sperm Source is associated with lower fertilization rates after intracytoplasmic sperm injection. Int Braz J Urol. 2008;34:49–56. doi: 10.1590/s1677-55382008000100008. [DOI] [PubMed] [Google Scholar]
- 25.Flaherty SP, Dianna P, Swann NJ, Matthews CD. Aetiology of failed and abnormal fertilization after intracytoplasmic sperm injection. Hum Reprod. 1995;10:2623–9. doi: 10.1093/oxfordjournals.humrep.a135757. [DOI] [PubMed] [Google Scholar]
- 26.Macas E, Imthurn B, Keller PJ. Increased incidence of numerical chromosome abnormalities in spermatozoa injected into human oocytes by ICSI. Hum Reprod. 2001;16:115–20. doi: 10.1093/humrep/16.1.115. [DOI] [PubMed] [Google Scholar]
- 27.Egozcue S. Diploid sperm and the origin of triploidy. Hum Reprod. 2002;17:5–7. doi: 10.1093/humrep/17.1.5. [DOI] [PubMed] [Google Scholar]
- 28.Nagy ZP, Staessen C, Liu J, Joris H, Devroey P, Van Steirteghem AC. Prospective, auto-controlled study on reinsemination of failed-fertilized oocytes by intracytoplasmic sperm injection. Fertil Steril. 1995;64:1130–5. [PubMed] [Google Scholar]
- 29.Bhattacharya S, Hamilton MP, Shaaban M, Khalaf Y, Seddler M, et al. Conventional in-vitro fertilisation versus intracytoplasmic sperm injection for the treatment of non-male-factor infertility: a randomised controlled trial. Lancet. 2001;357:2075–9. doi: 10.1016/s0140-6736(00)05179-5. [DOI] [PubMed] [Google Scholar]
- 30.Sun F, Ko E, Martin RH. Is there a relationship between sperm chromosome abnormalities and sperm morphology? Reprod Biol Endocrinol. 2006;4:1. doi: 10.1186/1477-7827-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alper MM, Lee GS, Seibel MM, Smith D, Oskowitz SP, et al. The relationship of semen parameters to fertilization in patients participating in a program of In vitro fertilization. J In vitro Fert Embryo Transf. 1985;2:217–23. doi: 10.1007/BF01201800. [DOI] [PubMed] [Google Scholar]
- 32.Palermo GD, Cohen J, Rosenwaks Z. Intracytoplasmic sperm injection: a powerful tool to overcome fertilization failure. Fertil Steril. 1996;65:899–908. doi: 10.1016/s0015-0282(16)58257-0. [DOI] [PubMed] [Google Scholar]
- 33.Danfour MA, Elmahaishi MS. Human oocyte oolemma characteristic is positively related to embryo developmental competence after ICSI procedure. Middle East Fertil Soc J. 2010;15:269–73. [Google Scholar]
- 34.Pereira N, Neri QV, Lekovich JP, Palermo GD, Rosenwaks Z. The role of in-vivo and in-vitro maturation time on ooplasmic dysmaturity. Reprod Biomed Online. 2016;32:401–6. doi: 10.1016/j.rbmo.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Palermo GD, Alikani M, Bertoli M, Colombero LT, Moy F, et al. Oolemma characteristics in relation to survival and fertilization patterns of oocytes treated by intracytoplasmic sperm injection. Hum Reprod. 1996;11:172–6. doi: 10.1093/oxfordjournals.humrep.a019012. [DOI] [PubMed] [Google Scholar]
- 36.Kashir J, Heindryckx B, Jones C, De Sutter P, Parrington J, et al. Oocyte activation, phospholipase C zeta and human infertility. Hum Reprod Update. 2010;16:690–703. doi: 10.1093/humupd/dmq018. [DOI] [PubMed] [Google Scholar]
- 37.Tavalaee M, Kiani-Esfahani A, Nasr-Esfahani MH. Relationship between phospholipase C-zeta, semen parameters, and chromatin status. Syst Biol Reprod Med. 2017;63:259–68. doi: 10.1080/19396368.2017.1298006. [DOI] [PubMed] [Google Scholar]
- 38.Loutradi KE, Tarlatzis BC, Goulis DG, Zepiridis L, Pagou T, et al. The effects of sperm quality on embryo development after intracytoplasmic sperm injection. J Assist Reprod Genet. 2006;23:69–74. doi: 10.1007/s10815-006-9022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng J, Lu Y, Qu X, Wang P, Zhao L, et al. Decreased sperm motility retarded ICSI fertilization rate in severe oligozoospermia but good-quality embryo transfer had achieved the prospective clinical outcomes. PLoS One. 2016;11:e0163524. doi: 10.1371/journal.pone.0163524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamilton JA, Cissen M, Brandes M, Smeenk JM, de Bruin JP, et al. Total motile sperm count: a better indicator for the severity of male factor infertility than the WHO sperm classification system. Hum Reprod. 2015;30:1110–21. doi: 10.1093/humrep/dev058. [DOI] [PubMed] [Google Scholar]
- 41.Borges E, Jr, Setti AS, Braga DP, Figueira RC, Iaconelli A., Jr Total motile sperm count has a superior predictive value over the WHO 2010 cut-off values for the outcomes of intracytoplasmic sperm injection cycles. Andrology. 2016;4:880–6. doi: 10.1111/andr.12199. [DOI] [PubMed] [Google Scholar]
- 42.Anbari F, Halvaei I, Nabi A, Ghazali S, Khalili MA, et al. The quality of sperm preparation medium affects the motility, viability, and DNA integrity of human spermatozoa. J Hum Reprod Sci. 2016;9:254–8. doi: 10.4103/0974-1208.197691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Punab M, Poolamets O, Paju P, Vihljajev V, Pomm K, et al. Causes of male infertility: a 9-year prospective monocentre study on 1737 patients with reduced total sperm counts. Hum Reprod. 2017;32:18–31. doi: 10.1093/humrep/dew284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boushaba S, Belaaloui G. Sperm DNA fragmentation and standard semen parameters in Algerian infertile male partners. World J Mens Health. 2015;33:1–7. doi: 10.5534/wjmh.2015.33.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]