Abstract
Testosterone exerts an important regulation of cardiovascular function through genomic and nongenomic pathways. It produces several changes in cardiomyocytes, the main actor of cardiomyopathies, which are characterized by pathological remodeling, eventually leading to heart failure. Testosterone is involved in contractility, in the energy metabolism of myocardial cells, apoptosis, and the remodeling process. In myocarditis, testosterone directly promotes the type of inflammation that leads to fibrosis, and influences viremia with virus localization. At the same time, testosterone exerts cardioprotective effects that have been observed in different studies. There is increasing evidence that low endogenous levels of testosterone have a negative impact in some cardiomyopathies and a protective impact in others. This review focuses on the interrelationships between testosterone and cardiomyopathies and heart failure.
Keywords: androgens, cardiomyocytes, heart disease, myocardial, prognosis
INTRODUCTION
Testosterone (T) is the principal male sex hormone and is secreted primarily by the testes.1 Only 1%–2% of testosterone circulates in the blood as free testosterone, and the remaining is bound to proteins including albumin and sex steroid hormone-binding globulin (SHBG).1 Plasma levels of T vary with circadian rhythm and fluctuate throughout life.2 Aging is associated with a gradual decline in testosterone levels in men and an increase in circulating SHBG levels.3 In addition to its anabolic and androgenic effects, testosterone has important effects on the cardiovascular system. The cardiac effects of testosterone are influenced by plasma levels, cellular metabolism, modulation of intracellular pathways, and androgen receptor expression.4 Androgens exert their physiological effects through a genomic mechanism that is mediated by the androgen receptor,5 but several possible nongenomic pathways have also been identified (Figure 1). These nongenomic pathways seem to be more rapid than the genomic mechanisms. The nongenomic effects of androgen characteristically involve the rapid induction of conventional second messenger signal transduction cascades, including increases in cytosolic calcium levels and activation of protein kinase A, protein kinase C, and mitogen-activated protein kinase (MAPK).6
Figure 1.
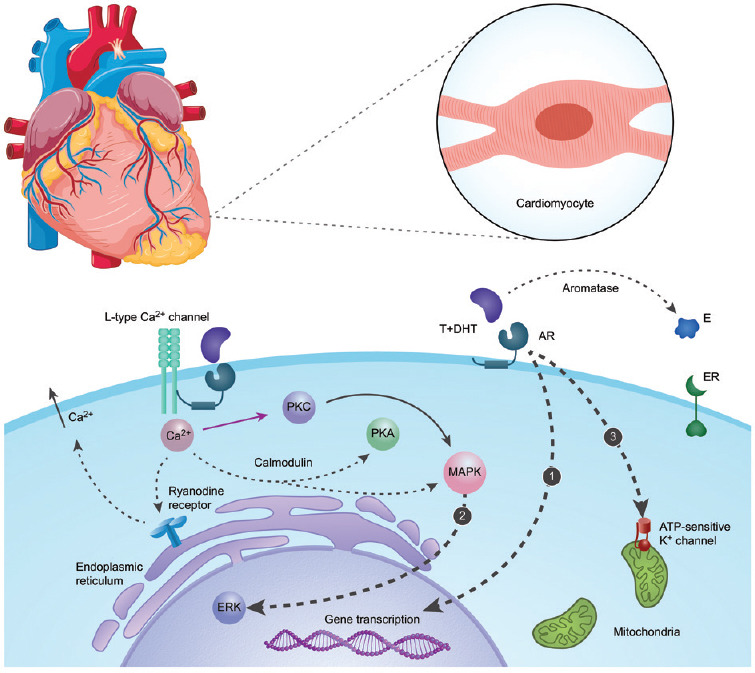
The main actions of androgen in cardiomyocytes. (1) Genomic pathway: androgen freely passes through the membrane and binds cytoplasmic AR. Bound AR translocates to the nucleus, binds to a DNA response element on the promoter of an androgen-responsive gene, and stimulates transcription. (2) Nongenomic pathways: androgen interacts with a membrane-associated androgen receptor, leading to the activation of L-type calcium channels. This increase in intracellular calcium can lead to the activation of PKC and, via calmodulin, lead to the activation of the PKA and MAPK pathways. The initial influx of Ca2+ further stimulates the efflux of Ca2+ from the sarcoplasmic reticulum via ryanodine receptor type-2 channels. Ca2+ from these sources converges on the MAPK/ERK pathway, through which ERK subsequently translocates to the nucleus and interacts with transcriptional factors. (3) Testosterone induces cytoprotection by activating ATP-sensitive K+ channels in the cardiac mitochondrial inner membrane. T: testosterone; DHT: dihydrotestosterone; E: estradiol; AR: androgen receptor; ER: estrogen receptor; PKA: protein kinase A; PKC: protein kinase C; MAPK: mitogen-activated protein kinase; ERK: extracellular signal-regulated kinase.
Several studies have shown that low serum T concentrations are associated with increased cardiovascular risk and mortality.7,8 It is not clear whether there is a causative relationship or an indirect association between T concentration and cardiovascular function.
Testosterone replacement therapy (TRT) has been shown to alleviate myocardial ischemia in men with coronary artery disease;9,10,11 increase exercise capacity in men with heart failure;12,13,14 and improve serum glucose levels, hemoglobin A1c (HbA1c) levels, and insulin resistance in men with diabetes and prediabetes.15,16 On the other hand, TRT has been linked to prostate cancer, polycythemia, and obstructive sleep apnea, and its long-term effects are unknown.17
This review focuses on the interrelationships between testosterone and cardiomyopathies and between testosterone and heart failure.
TESTOSTERONE AND CARDIAC CONTRACTILE FUNCTION
Testosterone influences the cardiovascular system by acting directly on cardiac cells. Cardiomyocytes express receptors for all major sex steroid hormones including testosterone and are thus exposed to their modulatory effects on myocardial cell physiology. Testosterone has an important role in intracellular Ca2+ homeostasis. Mediated by the androgen receptor, testosterone activates L-type calcium channels, which increase the intracellular levels of calcium and thus regulate myocardial contractility.18
Gonadectomy in adult male rats reduces the contractility of isolated cardiac myocytes,19 and short-term androgen exposure stimulates the contractility of isolated rat ventricular myocytes.20 Additionally, in castrated animals, androgen therapy improves coronary blood flow and increases both fractional shortening and peak myocardial oxygen consumption, thereby improving cardiac function.21
In an ancillary study of a randomized controlled trial involving men and women with advanced head-and-neck or cervix cancers,22 the patients were randomly allocated to receive weekly injections of either 100 mg of testosterone enanthate (6 patients, 1 male) or placebo (10 patients) in addition to receiving standard cancer treatment. After 7 weeks of treatment, testosterone improved left ventricular ejection fraction (LVEF: +6.2% ± 4.3%, vs placebo: −1.8% ± 4.3%; P < 0.05) as well as ventricular–vascular coupling.
Longitudinal observational studies of prostate cancer patients have shown that androgen ablation, a treatment option for locally advanced and metastatic prostate cancer, is associated with an increased incidence of cardiovascular diseases (CVD).23,24,25,26
In addition to increasing the risk of myocardial infarction (MI) and coronary heart disease (CHD), androgen deprivation therapy is also associated with a decrease in left ventricular longitudinal, circumferential, and radial strain measures, as assessed by speckle-tracking echocardiography.27
CARDIOPROTECTIVE MECHANISMS OF TESTOSTERONE
Testosterone has been shown to elicit cardioprotective effects, and several intracellular mechanisms have been identified. Through genomic and nongenomic mechanisms, testosterone influences apoptosis,28 regulates leukocyte migration and reactive oxygen species (ROS) generation,29 and the nitric oxide (NO) - guanosine 3',5'-cyclic monophosphate (cGMP) pathway.30
Cardioprotection allows myocardial cells to survive severe stress induced by ischemia–reperfusion or other types of metabolic factors by activating specific intracellular signaling factors, including protein kinases, enzymes, and transcription factors. There is a long list of factors and procedures that promote cardioprotection.31 The best-known protective mechanism of the heart under conditions of hypoxia is ischemic preconditioning, which was first described by Murry et al.32 in 1986. In a model of ischemia-reperfusion injury, testosterone was shown to preserve cardiomyocytes by activating ATP-sensitive K channels and upregulating cardiac alpha(1)-adrenoceptors.33 Ischemic preconditioning is well known to reduce the infarct size in young male rats, but both in the aged heart and the female heart, this protective effect is less evident.34 Additionally, T reduces the expression of caspase-3, a protein critical for apoptosis.35
Testosterone induces ROS generation in vascular smooth muscle cells isolated from normotensive and hypertensive rats, and this effect is followed by an increase in nicotinamide adenine dinucleotide phosphate oxidase.36 Testosterone protects against angiotensin II-induced vascular remodeling and controls superoxide production through the inhibition of the production of nicotinamide adenine dinucleotide phosphate oxidase.37
AMP-activated protein kinase (AMPK) is a serine/threonine kinase that has been implicated in cardiomyocyte metabolism38 and has cardioprotective properties, such as anti-apoptotic and anti-hypertrophic effects.39,40,41 In vitro studies have shown that short-term stimulation of cardiomyocytes with testosterone increases AMPK phosphorylation through calcium/calmodulin-dependent protein kinase type II (CaMKII).42
Altieri et al.43 compared the effects of pretreatment with sex hormones (testosterone and 17β-estradiol) on doxorubicin-induced toxicity in the rat embryonic cardiac cell line H9c2 and neonatal mouse cardiomyocytes. They showed that testosterone exerted protective effects against senescence caused by doxorubicin by modulating telomere-binding factor 2 via the pathway involving the androgen receptor, phosphatidylinositol 3 kinase, protein kinase B (Akt), and nitric oxide synthase 3. In another study on female embryonic heart H9c2 cells, which express a low number of androgen receptors, testosterone was shown to exert protective effects against 2,4-dinitrophenol, an inhibitor of oxidative phosphorylation. The postulated mechanism is the conversion of 2,4-dinitrophenol to metabolites that activate estrogen receptors and upregulate mitochondrial sulfonylurea receptor 2B intraexonic splice variant (IES SUR2B) expression.44
The heart demands a continuous supply of energy to maintain muscle excitation–contraction coupling, and in heart failure, energy is wasted at the cellular level.45 In a rat model of doxorubicin-induced cardiomyopathy, decreased effectiveness of all energy-producing systems is observed. Combined administration of testosterone and myocardial extract to rats with doxorubicin-induced cardiomyopathy activates energy supply mechanisms.46
Normal cardiomyocytes produce ATP mainly via fatty acid oxidation,47 but energy substrate utilization may change under pathological conditions.48 Testosterone improves insulin sensitivity and glucose uptake in the heart, which could lead to an increase in ATP production when cardiac output is increased.49,50 Testosterone improves mitochondrial function in cardiac cells by regulating the expression of mitochondrial genes.51 Androgen levels decrease with age, so cardiac cells exhibit fewer mitochondria, resulting in lower energy production efficiency.52
TESTOSTERONE IN HEART FAILURE PATIENTS
Low testosterone levels are frequently seen in men with chronic heart failure (HF), and some studies have associated low testosterone levels with increased mortality; however, the current data are conflicting.
The testosterone concentration is lower in men with HF of any etiology than that in healthy men and is correlated with the severity of the disease. There are many possible causes of low T levels in heart failure. Testosterone levels could be low because of chronic inflammation. Pro-inflammatory cytokines (tumor necrosis factor-α [TNF-α] and interleukin-1β [IL-1β] and IL-6) are known to regulate the hypothalamic–pituitary axis, resulting in reduced testicular production of testosterone.53 Another mechanism could be an increase in SHBG levels in the presence of inflammation. This increase in SHBG levels leads to increased estrogen production and decreased T activity.54 The low level of testosterone in heart failure could also be caused by drugs. Spironolactone, which is both an androgen receptor blocker and an aldosterone receptor blocker, decreases 5α-reductase activity via increased clearance of testosterone secondary to augmented liver hydroxylase activity.55 Studies suggest that the beneficial effects of spironolactone on cardiomyocytes may be reduced by blocking the anti-apoptotic effect of testosterone and that eplerenone should be used instead.56 Finally, a hemodynamic factor could be involved in the decrease in testosterone levels, as suggested by the fact that mechanical circulatory support in end-stage cardiac failure has been shown to improve the levels of testosterone in circulation.4
There are significant differences in the clinical profile, etiology, demographics, comorbidities, and management of heart failure depending on left ventricular ejection fraction. Most studies addressing the issue of testosterone in heart failure have examined patients with systolic dysfunction (Table 1).
Table 1.
Studies concerning testosterone relation to prognosis in chronic heart failure
| Study | Patients | Findings |
|---|---|---|
| Jankowska et al.57 | 208 men (LVEF <45%) compared to 366 healthy men | A low T level in all NYHA classes is a marker of poor prognosis |
| Güder et al.62 | 191 men; 96 with LVEF ≤40% and 95 with LVEF >40% | FT, but not TT, levels are inversely associated with NYHA class; lower FT and DHEAS levels and higher SHBG levels predict all-cause mortality risk, but this relationship is confounded by indicators of a poor health state |
| Wu et al.58 | 175 older men with LVEF ≤45% | TT and eFT levels are decreased and related to disease severity but are not independent predictors of mortality |
| Santos et al.59 | 110 hospitalized men with LVEF < 45% and NYHA class IV | A low T level is an independent risk factor for hospital readmission within 90 days and increased mortality |
| Han et al.60 | 167 men | A low T level is associated with increased readmission rate and mortality |
| Yoshihisa et al.61 | 618 men discharged with decompensated HF | A low T level is associated with myocardial damage and lower exercise capacity; the TT level is an independent predictor of all-cause mortality |
DHEAS: dehydroepiandrosterone sulfate; eFT: estimated free testosterone; FT: free testosterone; HF: heart failure; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; SHBG: sex hormone-binding globulin; T: testosterone; TT: total testosterone
Jankowska et al.57 studied the prevalence and prognosis of deficiencies in total and estimated free testosterone, dehydroepiandrosterone sulfate, and insulin-like growth factor-1 levels in 208 men with chronic heart failure (LVEF <45%) compared to 366 healthy men. In the heart failure group, the median age was 63 years, 81% of the men had ischemic etiology, the median LVEF was 33%, and 28% of the men had diabetes. Deficiency in the circulating testosterone level was most common in patients aged ≤45 years and those aged ≥66 years when assessed based on both total T (TT) levels (39% and 27%, respectively) and estimated free testosterone (eFT) levels (62% and 36%, respectively). Deficiencies in dehydroepiandrosterone sulfate (DHEAS) and insulin-like growth factor 1 (IGF-1) levels were observed in all age groups among men with chronic heart failure (CHF). There was a statistically significant decrease in TT, eFT, and DHEAS concentrations in subjects with more severe CHF, as assessed by New York Heart Association (NYHA) class, but there was no relationship between decreases in these levels and LVEF or plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) levels. Regarding prognosis, deficiencies in the levels of multiple anabolic hormones correlated with increased mortality (χ2 = 26.12, P < 0.0001). Normal circulating levels of all anabolic hormones were associated with an 83% 3-year survival rate (95% confidence interval [CI]: 67%–98%). In patients with deficiencies in three anabolic axes, survival was 27% (95% CI: 5%–49%).
The prognostic value of androgen hormone levels (total testosterone, sex hormone-binding globulin, and estimated free testosterone) in chronic heart failure with reduced left ventricular ejection fraction (LVEF ≤45% measured by echocardiography) was assessed by Wu et al.58 in 175 men aged ≥60 years. The mean age was 68.5 ± 5 years, 54.9% of the men had ischemic etiology of CHF, and the median follow-up was 3.46 years. The median level of TT was 20.3 nmol l−1 (25th–75th percentile: 17.0–27.2 nmol l−1), and that of eFT was 0.28 nmol l−1 (25th–75th percentile: 0.21–0.35 nmol l−1). The researchers found a relatively high prevalence of reduced serum concentrations of androgens: the prevalence of TT deficiency was 21.7%, and the prevalence of eFT deficiency was 27.4%. Total testosterone and estimated free testosterone levels were inversely associated with left ventricular ejection fraction and NT-proBNP but not with NYHA class; also, they were not significantly associated with survival after adjustment for clinical variables.
Testosterone was associated not only with mortality but also with the length of hospitalization and readmission rate in 110 men with a left ventricular ejection fraction <45% and NYHA class IV heart failure symptomatology.59
The prognostic value of testosterone was demonstrated in 167 Chinese men with CHF who were followed up for at least 3 years.60 Compared with those with normal testosterone levels, patients with low testosterone levels had more severe cardiac dysfunction, a higher prevalence of ischemic etiology, and more comorbidities. Readmission rates were also higher for patients with low testosterone levels than for patients in the normal testosterone group. Predictors of mortality in multivariable models of Cox proportional hazard analyses were LVEF (hazard ratio [HR]: 0.885, 95% CI: 0.829–0.945, P = 0.0003), free testosterone levels (HR: 6.301, 95% CI: 3.187–12.459, P < 0.0001), and glomerular filtration rate (GFR; HR: 0.967, 95% CI: 0.937–0.998, P = 0.0348).
A prospective observational study of 618 men discharged with decompensated HF showed that all-cause mortality was inversely correlated with the quartile of the total testosterone level.61 On multivariate analysis, TT was independently associated with mortality (HR: 0.929, P = 0.042). Left ventricular ejection fraction and B-type natriuretic peptide levels were not different among quartiles, but the lowest quartile (TT ≤300 ng dl−1) had higher levels of troponin, which is a marker of myocardial injury.
Finally, the study by Güder et al.62 is noteworthy because it also included patients with heart failure and preserved ejection fraction. Free serum testosterone, DHEAS, and SHBG levels were measured in 191 consecutive men with heart failure (mean age: 64 years), 96 of whom had reduced LVEF (≤ 40%) and 95 of whom had preserved LVEF (>40%). The median observation period was 859 days. Free serum testosterone levels were inversely related to NYHA class, C-reactive protein levels, and NT-proBNP levels. There was no relationship between free serum testosterone levels and ejection fraction. Lower free testosterone and DHEAS levels and higher SHBG levels predicted all-cause mortality risk (HR: 0.89, 95% CI: 0.82–0.96 per 1 ng dl−1 free testosterone, P = 0.004; adjusted for age and NYHA class), but this was not confirmed after further adjustments. This study suggests that there is no relationship between testosterone levels and the type of heart failure (systolic or nonsystolic).
Data from epidemiologic studies and clinical trials have suggested that testosterone replacement therapy has beneficial systemic effects in testosterone-deficient men with heart failure.12,13,14,63 Testosterone replacement therapy in patients with heart failure has promising results in improving functional capacity and quality of life. Two meta-analyses, one including four randomized controlled trials (n = 198; 84% men; mean age: 67 years) and the other including eight trials (n = 393; 96% men; mean age: 65 years), showed that TRT could significantly improve exercise capacity, muscle strength, and electrocardiogram indicators without inducing significant changes in ejection fraction, systolic blood pressure, diastolic blood pressure, NT-proBNP levels, TNF-α levels, high-sensitivity C-reactive protein levels, or IL-6 levels.64,65 Another meta-analysis of eight randomized controlled trials including 170 patients in the testosterone supplementation group and 162 in the control group showed that testosterone supplementation within a physiological range was not associated with significantly improved exercise capacity, cardiac function, quality of life, or clinical outcome.66
TESTOSTERONE AND MYOCARDITIS
Sex hormones play opposing roles in the immune system, with estrogen having stimulatory effects and androgens being immunosuppressive.67 Androgens decrease B-cell maturation, reduce B-cell synthesis of antibodies (Abs), and suppress auto-Ab production in humans.68 It is known that autoimmune disorders predominantly affect women and that affected men have lower serum concentrations of androgens.69 Androgens have immunomodulatory properties, suppressing macrophage production of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) and increasing the production of anti-inflammatory cytokines (IL-10).70,71 In male rats with heart failure, testosterone treatment suppresses ventricular remodeling and improves cardiac function by diminishing the imbalance between IL-10 and TNF-α.72 In contrast, T promotes the pro-inflammatory Th1 and/or Th17-type immune response73 that is characteristic of acute myocarditis.
Myocarditis is a form of inflammatory cardiomyopathy that typically develops secondary to viral infection, often in young, healthy men. It is one of the causes of dilated cardiomyopathy (DCM) and heart failure.74
Several experimental studies have suggested that testosterone promotes a type of inflammation that may be involved in fibrosis in the setting of myocarditis.75,76,77,78
Human data and clinical studies in this context are lacking. In an in vivo study, male and female BALB/c mice were inoculated with various concentrations of coxsackievirus. Lower viral doses induced severe myocarditis in male mice but caused little injury in females. Sex steroid hormones influence viremia and virus localization; in females given exogenous testosterone and progesterone, the amount of virus in the heart is ten times higher than that in animals given estradiol. The hormones may act by increasing viral receptor expression on endothelial cells and myocytes.79 Testosterone increases Toll-like receptor (TLR4) and IL-1β expression on CD11b+GR1+F4/80+ macrophages and CD11b+CD117+ mast cells in male BALB/c mice with myocarditis.80 Gonadectomy reduces CD11b+ inflammation and causes a reversal of the Th1 response in males to a Th2 response.81 Studies suggest that IL-1β produced by TLR4 is critical for the induction of fibrosis, which leads to DCM.82
Serum-soluble interleukin-1 receptor-like 1 (ST2) is a marker of heart failure. Elevated levels predict acute and chronic heart failure mortality regardless of cause. Soluble ST2 (sST2) levels are elevated in young men with myocarditis and correlate with NYHA class III–IV heart failure. Additionally, in several studies, sST2 levels were found to be significantly elevated in healthy men compared with healthy women. In mice with myocarditis, testosterone, but not estradiol, increases sST2 levels.83 The soluble form of ST2 can promote myocardial damage by binding to IL-33 and blocking the cardioprotective effects generated by the interaction between IL-33 and the transmembrane ST2 ligand.83
Testosterone increases the expression of genes associated with cardiac remodeling during acute coxsackievirus B3-induced myocarditis, including tissue inhibitor of metalloproteinases-1 (TIMP-1), serpin A 3n, IL-1β, and neutrophil collagenase (MMP-8). The regulation of IL-1β and serpin A 3n by testosterone in the heart plays a major role in the progression from myocarditis to DCM in males. Serpin A 3n is elevated not only in myocarditis but also in the heart of patients with DCM.84
TESTOSTERONE IN DILATED CARDIOMYOPATHY
Several studies have shown that the incidence rate of DCM is higher in men than that in women. Women with DCM have better survival than men, which may partly be due to less severe left ventricular dysfunction and a smaller scar burden.84 The exact reason for this gender-specific discrepancy has yet to be elucidated.
In heart failure studies, female gender has been associated with better cardiac function and survival.85 In the aging female heart, hypertrophy, apoptosis, and fibrosis are less pronounced than in the aging male heart.86 Higher testosterone levels in women with polycystic ovary syndrome are associated with cardiovascular and metabolic diseases.87 Additionally, women with cardiovascular disease have higher levels of free androgen than controls.88 In postmenopausal women, the ovaries produce significant amounts of androgens, which can increase the incidence of cardiac events in women in the absence of estrogen.89
Experimental studies indicate additive cardioprotection when treatment with a combination of estrogen and testosterone treatment is administered.90,91 This additive effect could be caused by the synergistic and codependency of these hormones.92 Additionally, the aromatization of testosterone to estradiol is a critical mechanism involved in the beneficial effect of testosterone on the cardiovascular system.93 This is supported by the fact that conversion of androgens to estrogens takes place in the heart and by the nongonadal expression of aromatase, which is higher in males than females.94
Xiong et al.95 demonstrated lower T levels (540.8 ± 186.0 pg ml−1 vs 656.3 ± 112.9 pg ml−1, P < 0.001) but higher SHBG and serum bisphenol-A (BPA) levels in DCM patients than those in controls. Serum BPA levels are statistically significantly associated with increased SHBG levels, but no significant difference in estradiol levels has been noted.95 Pascual-Figal et al.96 demonstrated that SHBG levels are associated with the severity of heart failure and a higher risk of cardiac death. Another study that evaluated the association between the BPA concentration and sex hormone levels in 290 men with or without BPA exposure in the workplace showed that exposure to a high amount of BPA impacts the levels of sex hormone levels in men. An increased serum BPA concentration is statistically significantly associated with decreased androstenedione levels, decreased free testosterone levels, decreased free androgen index, and increased SHBG levels.97 This environmental endocrine-disrupting compound may be associated with the gender disparity in the incidence of DCM.
Kontoleon et al.98 compared 23 men with NYHA class II–IV heart failure due to idiopathic dilated cardiomyopathy and LVEF ≤35% to 20 healthy men and found significantly lower FT levels in men with heart failure (48.6 ± 23.8 pmol l−1 vs 105.0 ± 17 pmol l−1, P < 0.0.01). Naderi et al.99 assessed the levels of several hormones and markers in 33 men with idiopathic dilated cardiomyopathy and NYHA class II-III heart failure. The free testosterone level was within the normal range, and there was no association between free testosterone levels and LVEF, NT-proBNP levels, or high-sensitivity C-reactive protein (hsCRP) levels. There was a weak correlation between free testosterone levels and 6-min walking test (6MWT) distance.
In a study reported only in abstract form, which included men with dilated cardiomyopathy and heart failure, a combination of intramuscular testosterone injections and standard HF therapy significantly improved patients' clinical status.100
HYPERTROPHIC CARDIOMYOPATHY
Hypertrophic cardiomyopathy (HCM) is a genetic disorder inherited as a Mendelian autosomal dominant trait. Although the prevalence of HCM in the general population should be equal between the two genders, studies have shown a higher predominance in male patients than in female patients. Differences in phenotypic expression could be caused by social and endocrine factors. Some familial hypertrophic cardiomyopathies, such as Danon disease, are more severe in males than in females,101 but in other forms, women are more symptomatic than men, often due to outflow tract obstruction, and show a higher risk of progression to advanced heart failure or death.102 In patients without obstruction, on magnetic resonance imaging, females have a lower left ventricle (LV) remodeling index (LV mass/LV end-diastolic volume) than males, suggesting a difference in LV remodeling in HCM.103 There are differences in remodeling between males and females.104
In animal models, testosterone has been found to enhance cardiac remodeling, whereas estrogen is protective.105 In a mouse model of myocardial infarction, there is an obvious gender difference in cardiac remodeling and function. Male mice have a higher incidence of cardiac rupture and a lower LVEF than females. Additionally, in males, castration significantly reduces both mortality and rupture. Testosterone replacement adversely affects myocardial healing and early remodeling during the acute phase of myocardial infarction (MI) in both male and female mice. Moreover, this effect is more pronounced in castrated females.106
In another study, testosterone deficiency induced by orchidectomy in rats was shown to alter the pattern of late remodeling postinfarction, with a lower degree of pathological cardiac hypertrophy and an improvement in contractile parameters.107
The main effect of testosterone on post-MI remodeling may occur in myocytes, mainly through the induction of hypertrophy via androgen receptors108 and increased apoptosis.107 In humans with heart failure post-MI, the rates of myocyte necrosis and apoptosis are higher in men than in women, and these differences are associated with the earlier onset of the disease in males than in females.109
The effects of gender and reproductive hormones on cardiac mass have led to the hypothesis that testosterone influences human left ventricular hypertrophy. Androgen activity is regulated through both AR-dependent and AR-independent mechanisms. Testosterone induces hypertrophy via a direct AR-mediated pathway110 and through cytosolic and nuclear signaling pathways. Testosterone activates the nuclear factor of activated T cells (NFAT) in cardiac myocytes through calcineurin activation and glycogen synthase kinase3 (GSK-3β) inhibition.111 The transcriptional activity of NFAT is regulated tightly by intracellular Ca2+ through calcineurin.
In vitro treatment with testosterone leads to GSK-3β inhibition and increases in intracellular levels of calcium and activation of calcineurin and NFAT, resulting in cardiomyocyte hypertrophy.112
Deletion of GSK-3β in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast hyperproliferation.113 Therefore, GSK-3β has antihypertrophic and antiapoptotic roles114 and could be a viable drug target.
Another hypertrophic mechanism regulated by androgens involves activation of the mTORC1/S6K1 axis through IP3/Ca2+ and MAPK kinase (MEK)/extracellular-regulated kinase (ERK) 1/2.115
Hypertrophied cardiomyocytes increase glycolysis-dependent ATP production, and glucose uptake is stimulated by testosterone, which involves the participation of the CaMKII and AMPK signaling pathways.42
Poorer survival in males than in females has been reported in a variety of rat models of cardiac hypertrophy and heart failure involving pressure (aortic banding) or volume (fistula) overload, with androgens being the important drivers of pathological cardiac hypertrophy.116 Compared to normal testosterone levels, testosterone deficiency in rats with severe left ventricle volume overload results in less cardiac hypertrophy, less LV dilation, better function, and a strong tendency for better survival of the animals.117 Castration in male guanylyl cyclase-A-deficient mice alleviates not only cardiac hypertrophy but also cardiac fibrosis.118
Testosterone can be transformed by the enzyme 5α reductase to 5α-dihydrotestosterone (DHT), a more potent androgen with five times more affinity for the androgen receptor and a ten-fold more potent effect on signaling.1 It has been speculated that the conversion of testosterone to DHT is required for regulation of some of the effects of androgen on the cardiovascular system. DHT induces cardiac hypertrophy In vitro and in rats.119 Antiandrogenic therapy with finasteride, which inhibits the transformation of testosterone to DHT, attenuates cardiac hypertrophy and left ventricular dysfunction in mice of both sexes.120
Androgen receptor (AR) plays an important role in cardiac development and function. This is highlighted by the phenotype of AR knockout animals. Compared with wild-type animals, AR knockout mice exhibit a significant reduction in cardiac hypertrophy and cardiac fibrosis induced by angiotensin (Ang) II.121 Flutamide, an AR antagonist, remarkably alleviates cardiac hypertrophy.122
The effects of physiological androgen levels on cardiac remodeling and hypertrophy remain controversial, and further research is necessary to understand their roles.
RESTRICTIVE CARDIOMYOPATHY
Restrictive cardiomyopathy (RCM), the least common cardiomyopathy, is characterized by the presence of restrictive ventricular physiology with a normal or reduced ventricular volume and normal wall thickness.123
There have been few studies on sex differences in RCM, but these studies have shown a higher occurrence but better survival in females than in males.103 However, RCM represents a heterogeneous group of cardiac diseases with different pathophysiological processes, clinical presentations, treatments, and prognoses.123 Endomyocardial fibrosis is a form of endemic restrictive cardiomyopathy primarily observed in Africa, Latin America, and Asia. The exact epidemiology, etiology, and pathogenesis of endomyocardial fibrosis remain unknown, and it is characterized by endocardial fibrosis of the apex and inflow tracts of the right ventricle, left ventricle, or both.124 It is more common in children and young females, and affected males have clinical feminization. In a study of 19 male patients with endomyocardial fibrosis, testosterone serum levels were significantly lower in patients than those in controls.125 Fibrosis also occurs in skeletal muscle, and some patients exhibit skeletal muscle atrophy.124 Transforming growth factor (TGF)-β and Ang II are pivotal in the progression of cardiac fibrosis. Androgens can regulate the effects of TGF-β and Ang II.126
Cardiac amyloidosis is the most common form of restrictive cardiomyopathy in high-income countries. It is characterized by deposition of amyloid protein in the cardiac muscle and surrounding tissues.123 Hereditary transthyretin-related amyloidosis ATTR-CM is a condition more commonly reported in men than in women. Some biological characteristics associated with female gender protect against myocardial involvement in familial ATTR. Females have a less aggressive disease phenotype than males at the time of diagnosis, are significantly older at diagnosis, and have a higher left ventricle ejection fraction.127 Wild-type transthyretin cardiac amyloidosis is a common aging phenomenon in the elderly population. It predominantly affects elderly men, and affected men have a greater increase in LV thickness than affected women.128
Fabry disease, a form of restrictive cardiomyopathy, is an X-linked lysosomal storage disease most often associated with renal dysfunction and death due to renal failure. The disease affects both men and women of all ethnic groups, but men are usually more severely affected.123 In α-galactosidase A knockout mice, a model of Fabry disease, AR activity is increased in the heart and kidneys. Castration and consequent hypogonadism or AR antagonism therapy alleviates heart and kidney phenotypes in Fabry disease model mice.129
NONCOMPACTION CARDIOMYOPATHY
Noncompaction cardiomyopathy is a rare structural myocardial disorder of acquired or congenital origin characterized by prominent trabeculations and recesses in the LV walls.130 Stöllberger et al.131 reported that the prevalence of noncompaction cardiomyopathy is higher in males than in females, females exhibit more extensive disease, and that mortality is the same between males and females. There are no data regarding the possible involvement of testosterone in this disease.
ARRHYTHMOGENIC RIGHT VENTRICULAR CARDIOMYOPATHY (ARVC)
ARVC is a genetic form of primary cardiomyopathy in which the right ventricle myocardium is typically replaced by fibrous tissue and fat, with scattered residual myocardial cells, resulting in structural changes such as myocardial thinning, localized or generalized dilatation of the right ventricle, and an increased risk of ventricular arrhythmias and sudden death.132 Because ARVC is an autosomal-dominant disease, an equal number of men and women are genetically affected. However, recent large studies have revealed a male predominance, with 75% of the patients being male and 25% of the patients being female, and have shown that men have a higher all-life risk of ventricular arrhythmias. Although total cardiac mortality is similar between sexes, women with ARVC have a significant risk for HF.133 Differences in ARVC phenotypic expression may be affected by sex hormones. A gender difference in the ventricular repolarization rate due to a potassium channel (Kv1.5) was reported to be androgen dependent in mice.134
Akdis et al.135 measured the levels of testosterone and other hormones and biomarkers in 54 ARVC patients (72% male) who were followed up for a median of 1.1 years. The researchers found that total and free testosterone levels are significantly increased in males with major arrhythmic events (defined as sudden cardiac death, survival after sudden cardiac death, ventricular fibrillation, sustained ventricular tachycardia, or arrhythmic syncope). Total testosterone levels >13.5 nmol l−1 predicted unfavorable outcomes with a sensitivity of 84% and a specificity of 75%. For free testosterone levels, a cutoff of >264 pmol l−1 had a sensitivity of 74% and a specificity of 75%, and for bioavailable testosterone, a cutoff level of >6.2 nmol l−1 had 79% sensitivity and 75% specificity. Moreover, testosterone plasma levels in male ARVC patients are higher than those in healthy males.136 In an induced pluripotent stem cell-derived ARVC cardiomyocyte model, testosterone worsens and estradiol alleviates cardiomyocyte apoptosis and lipogenesis, which are hallmarks of ARVC.135
TAKOTSUBO CARDIOMYOPATHY
The mechanism of Takotsubo cardiomyopathy (TTS) is still elusive, but there are several etiological theories, such as catecholamine-induced acute myocardial injury, vasospasm (epicardial coronary and/or microvascular), impairment of the coronary microcirculation, and myocardial stunning.123 Due to its predominance in postmenopausal females, it has been suggested that sex hormones may play a role in the disease, but the limited available data are contradictory.137
Cardiomyocyte contractility studies have demonstrated that estrogen and testosterone exert contrasting inotropic actions and modulate Ca2+ handling differently.138 Myocytes from female patients with elevated Ang II levels appear to be more susceptible to contractility deficits, which may explain the female predominance among TTS patients.139
Brenner et al.140 analyzed the levels of sex hormones in postmenopausal women with TTS and age-matched females with and without myocardial infarction and found significantly higher estrogen levels at hospital admission in TTS patients than in age-matched females. Male sex is considered a mortality predictor in TTS. Male patients have a higher frequency of both chronic comorbidities and acute illnesses141 associated with low serum testosterone levels, and this could be an interesting topic for future study.
CONCLUSIONS
Cardiovascular disease remains a major cause of death, particularly in men and postmenopausal women. The cardiovascular protection observed in females has been attributed to the beneficial effects of estrogen, but little is known about the effects of testosterone. Evidence from basic science and clinical studies has shown that testosterone influences the cardiovascular system, but these effects are still poorly understood and contradictory. Additionally, the effects of testosterone may be different under normal physiological conditions and in pathological states. Testosterone could simultaneously benefit and harm the cardiovascular system through different pathways. Testosterone deficiency is common in men with dilated cardiomyopathy and heart failure, and studies have shown an association between low testosterone levels and poor cardiovascular outcomes.
AUTHOR CONTRIBUTIONS
RD, ID, and OM contributed to the conception of the work, conducted the study, revised the draft, and agreed with all aspects of the work. TAB contributed to the conception of the work, revised it critically for important intellectual content, and agreed with all aspects of the work. All authors have read and approved the final version of the manuscript and agree with the order of presentation of the authors.
COMPETING INTERESTS
All authors declare no competing interests.
REFERENCES
- 1.Matsumoto A, Bremner W. Testicular disorders. In: Melmed S, Polonsky K, Larsen P, Kronenberg H, editors. Williams Textbook of Endocrinology. New York: Elsevier Health Sciences; 2015. pp. 688–777. [Google Scholar]
- 2.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–98. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 3.Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23:490–4. doi: 10.2337/diacare.23.4.490. [DOI] [PubMed] [Google Scholar]
- 4.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24:313–40. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 5.Magee JA, Chang LW, Stormo GD, Milbrandt J. Direct, androgen receptor-mediated regulation of the FKBP5 gene via a distal enhancer element. Endocrinology. 2006;147:590–8. doi: 10.1210/en.2005-1001. [DOI] [PubMed] [Google Scholar]
- 6.Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002;16:2181–7. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- 7.Militaru C, Donoiu I, Dracea O, Ionescu DD. Serum testosterone and short-term mortality in men with acute myocardial infarction. Cardiol J. 2010;17:249–53. [PubMed] [Google Scholar]
- 8.Kirby M, Hackett G, Ramachandran S. Testosterone and the heart. Eur Cardiol Rev. 2019;14:103–10. doi: 10.15420/ecr.2019.13.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris PD, Channer KS. Testosterone and cardiovascular disease in men. Asian J Androl. 2012;14:428–35. doi: 10.1038/aja.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones TH, Kelly DM. Randomized controlled trials - mechanistic studies of testosterone and the cardiovascular system. Asian J Androl. 2018;20:120–30. doi: 10.4103/aja.aja_6_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malkin CJ, Pugh PJ, Morris PD, Kerry KE, Jones RD, et al. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart. 2004;90:871–6. doi: 10.1136/hrt.2003.021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caminiti G, Volterrani M, Iellamo F, Marazzi G, Massaro R, et al. Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure a double-blind, placebo-controlled, randomized study. J Am Coll Cardiol. 2009;54:919–27. doi: 10.1016/j.jacc.2009.04.078. [DOI] [PubMed] [Google Scholar]
- 13.Malkin CJ, Pugh PJ, West JN, van Beek EJ, Jones TH, et al. Testosterone therapy in men with moderate severity heart failure: a double-blind randomized placebo controlled trial. Eur Heart J. 2006;27:57–64. doi: 10.1093/eurheartj/ehi443. [DOI] [PubMed] [Google Scholar]
- 14.Pugh PJ, Jones RD, West JN, Jones TH, Channer KS. Testosterone treatment for men with chronic heart failure. Heart. 2004;90:446–7. doi: 10.1136/hrt.2003.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154:899–906. doi: 10.1530/eje.1.02166. [DOI] [PubMed] [Google Scholar]
- 16.Jones TH. Effects of testosterone on type 2 diabetes and components of the metabolic syndrome. J Diabetes. 2010;2:146–56. doi: 10.1111/j.1753-0407.2010.00085.x. [DOI] [PubMed] [Google Scholar]
- 17.Rhoden EL, Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med. 2004;350:482–92. doi: 10.1056/NEJMra022251. [DOI] [PubMed] [Google Scholar]
- 18.Curl CL, Delbridge LM, Canny BJ, Wendt IR. Testosterone modulates cardiomyocyte Ca(2+) handling and contractile function. Physiol Res. 2009;58:293–7. doi: 10.33549/physiolres.931460. [DOI] [PubMed] [Google Scholar]
- 19.Golden KL, Marsh JD, Jiang Y, Brown T, Moulden J. Gonadectomy of adult male rats reduces contractility of isolated cardiac myocytes. Am J Physiol Endocrinol Metab. 2003;285:E449–53. doi: 10.1152/ajpendo.00054.2003. [DOI] [PubMed] [Google Scholar]
- 20.Ayaz O, Banga S, Heinze-Milne S, Rose RA, Pyle WG, et al. Long-term testosterone deficiency modifies myofilament and calcium-handling proteins and promotes diastolic dysfunction in the aging mouse heart. Am J Physiol Heart Circ Physiol. 2019;316:H768–80. doi: 10.1152/ajpheart.00471.2018. [DOI] [PubMed] [Google Scholar]
- 21.Pugh PJ, English KM, Jones TH, Channer KS. Testosterone: a natural tonic for the failing heart? QJM. 2000;93:689–94. doi: 10.1093/qjmed/93.10.689. [DOI] [PubMed] [Google Scholar]
- 22.Scott JM, Dillon EL, Kinsky M, Chamberlain A, McCammon S, et al. Effects of adjunct testosterone on cardiac morphology and function in advanced cancers: an ancillary analysis of a randomized controlled trial. BMC Cancer. 2019;19:778. doi: 10.1186/s12885-019-6006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–56. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 24.Saigal CS, Gore JL, Krupski TL, Hanley J, Schonlau M, et al. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110:1493–500. doi: 10.1002/cncr.22933. [DOI] [PubMed] [Google Scholar]
- 25.Tsai HK, D'Amico AV, Sadetsky N, Chen MH, Carroll PR. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99:1516–24. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- 26.D'Amico AV, Denham JW, Crook J, Chen MH, Goldhaber SZ, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007;25:2420–5. doi: 10.1200/JCO.2006.09.3369. [DOI] [PubMed] [Google Scholar]
- 27.Kanar BG, Ozben B, Sunbul M, Şener E, Ozkan O, et al. Androgen-deprivation therapy impairs left ventricle functions in prostate cancer patients. Int Urol Nephrol. 2019;51:1107–12. doi: 10.1007/s11255-019-02184-4. [DOI] [PubMed] [Google Scholar]
- 28.Lopes RA, Neves KB, Pestana CR, Queiroz AL, Zanotto CZ, et al. Testosterone induces apoptosis in vascular smooth muscle cells via extrinsic apoptotic pathway with mitochondria-generated reactive oxygen species involvement. Am J Physiol Heart Circ Physiol. 2014;306:H1485–94. doi: 10.1152/ajpheart.00809.2013. [DOI] [PubMed] [Google Scholar]
- 29.Chignalia AZ, Oliveira MA, Debbas V, Dull RO, Laurindo FR, et al. Testosterone induces leucocyte migration by NADPH oxidase-driven ROS- and COX2-dependent mechanisms. Clin Sci (Lond) 2015;129:39–48. doi: 10.1042/CS20140548. [DOI] [PubMed] [Google Scholar]
- 30.Comeglio P, Cellai I, Filippi S, Corno C, Corcetto F, et al. Differential effects of testosterone and estradiol on clitoral function: an experimental study in rats. J Sex Med. 2016;13:1858–71. doi: 10.1016/j.jsxm.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Jovanović A. Cardioprotective signalling: past, present and future. Eur J Pharmacol. 2018;833:314–9. doi: 10.1016/j.ejphar.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 32.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 33.Tsang S, Wu S, Liu J, Wong TM. Testosterone protects rat hearts against ischaemic insults by enhancing the effects of alpha(1)-adrenoceptor stimulation. Br J Pharmacol. 2008;153:693–709. doi: 10.1038/sj.bjp.0707624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev. 2014;66:1142–74. doi: 10.1124/pr.113.008300. [DOI] [PubMed] [Google Scholar]
- 35.Wang XF, Qu XQ, Zhang TT, Zhang JF. Testosterone suppresses ventricular remodeling and improves left ventricular function in rats following myocardial infarction. Exp Ther Med. 2015;9:1283–91. doi: 10.3892/etm.2015.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montezano AC, Tsiropoulou S, Dulak-Lis M, Harvey A, Camargo Lde L, et al. Redox signaling, Nox5 and vascular remodeling in hypertension. Curr Opin Nephrol Hypertens. 2015;24:425–33. doi: 10.1097/MNH.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ikeda Y, Aihara K, Yoshida S, Sato T, Yagi S, et al. Androgen-androgen receptor system protects against angiotensin II-induced vascular remodeling. Endocrinology. 2009;150:2857–64. doi: 10.1210/en.2008-1254. [DOI] [PubMed] [Google Scholar]
- 38.Arad M, Seidman CE, Seidman JG. AMP-activated protein kinase in the heart: role during health and disease. Circ Res. 2007;100:474–88. doi: 10.1161/01.RES.0000258446.23525.37. [DOI] [PubMed] [Google Scholar]
- 39.Pang T, Rajapurohitam V, Cook MA, Karmazyn M. Differential AMPK phosphorylation sites associated with phenylephrine vs. antihypertrophic effects of adenosine agonists in neonatal rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2010;298:H1382–90. doi: 10.1152/ajpheart.00424.2009. [DOI] [PubMed] [Google Scholar]
- 40.Meng R, Pei Z, Zhang A, Zhou Y, Cai X, et al. AMPK activation enhances PPARα activity to inhibit cardiac hypertrophy via ERK1/2 MAPK signaling pathway. Arch Biochem Biophys. 2011;511:1–7. doi: 10.1016/j.abb.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Chen C, Yao F, Su Q, Liu D, et al. AMPK inhibits cardiac hypertrophy by promoting autophagy via mTORC1. Arch Biochem Biophys. 2014;558:79–86. doi: 10.1016/j.abb.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 42.Wilson C, Contreras-Ferrat A, Venegas N, Osorio-Fuentealba C, Pávez M, et al. Testosterone increases GLUT4-dependent glucose uptake in cardiomyocytes. J Cell Physiol. 2013;228:2399–407. doi: 10.1002/jcp.24413. [DOI] [PubMed] [Google Scholar]
- 43.Altieri P, Barisione C, Lazzarini E, Garuti A, Bezante GP, et al. Testosterone antagonizes doxorubicin-induced senescence of cardiomyocytes. J Am Heart Assoc. 2016;5:e002383. doi: 10.1161/JAHA.115.002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ballantyne T, Du Q, Jovanović S, Neemo A, Holmes R, et al. Testosterone protects female embryonic heart H9c2 cells against severe metabolic stress by activating estrogen receptors and up-regulating IES SUR2B. Int J Biochem Cell Biol. 2013;45:283–91. doi: 10.1016/j.biocel.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anker SD, Sharma R. The syndrome of cardiac cachexia. Int J Cardiol. 2002;85:51–66. doi: 10.1016/s0167-5273(02)00233-4. [DOI] [PubMed] [Google Scholar]
- 46.Oleynikov D, Vasilieva S, Jashin A. Testosterone and myocardial extract as a correction scheme in case of experimental doxorubicin cardiomyopathy. Can Sci J. 2015;1:4–10. [Google Scholar]
- 47.Scolletta S, Biagioli B. Energetic myocardial metabolism and oxidative stress: let's make them our friends in the fight against heart failure. Biomed Pharmacother. 2010;64:203–7. doi: 10.1016/j.biopha.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 49.Kolwicz SC, Jr, Tian R. Glucose metabolism and cardiac hypertrophy. Cardiovasc Res. 2011;90:194–201. doi: 10.1093/cvr/cvr071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nascimben L, Ingwall JS, Lorell BH, Pinz I, Schultz V, et al. Mechanisms for increased glycolysis in the hypertrophied rat heart. Hypertension. 2004;44:662–7. doi: 10.1161/01.HYP.0000144292.69599.0c. [DOI] [PubMed] [Google Scholar]
- 51.Juang HH, Hsieh ML, Tsui KH. Testosterone modulates mitochondrial aconitase in the full-length human androgen receptor-transfected PC-3 prostatic carcinoma cells. J Mol Endocrinol. 2004;33:121–32. doi: 10.1677/jme.0.0330121. [DOI] [PubMed] [Google Scholar]
- 52.Petersson SJ, Christensen LL, Kristensen JM, Kruse R, Andersen M, et al. Effect of testosterone on markers of mitochondrial oxidative phosphorylation and lipid metabolism in muscle of aging men with subnormal bioavailable testosterone. Eur J Endocrinol. 2014;171:77–88. doi: 10.1530/EJE-14-0006. [DOI] [PubMed] [Google Scholar]
- 53.Goodale T, Sadhu A, Petak S, Robbins R. Testosterone and the heart. Methodist Debakey Cardiovasc J. 2017;13:68–72. doi: 10.14797/mdcj-13-2-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamazaki H, Kushiyama A, Sakoda H, Fujishiro M, Yamamotoya T, et al. Protective effect of sex hormone-binding globulin against metabolic syndrome: In vitro evidence showing anti-inflammatory and lipolytic effects on adipocytes and macrophages. Mediators Inflamm. 2018;2018:3062319. doi: 10.1155/2018/3062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corvol P, Michaud A, Menard J, Freifeld M, Mahoudeau J. Antiandrogenic effect of spirolactones: mechanism of action. Endocrinology. 1975;97:52–8. doi: 10.1210/endo-97-1-52. [DOI] [PubMed] [Google Scholar]
- 56.Anker SD, Caprio M, Vitale C. Possible interactive effect of testosterone and aldosterone receptor antagonists on cardiac apoptosis. Rev Esp Cardiol. 2010;63:760–2. doi: 10.1016/s1885-5857(10)70159-6. [DOI] [PubMed] [Google Scholar]
- 57.Jankowska EA, Biel B, Majda J, Szklarska A, Lopuszanska M, et al. Anabolic deficiency in men with chronic heart failure: prevalence and detrimental impact on survival. Circulation. 2006;114:1829–37. doi: 10.1161/CIRCULATIONAHA.106.649426. [DOI] [PubMed] [Google Scholar]
- 58.Wu HY, Wang XF, Wang JH, Li JY. Testosterone level and mortality in elderly men with systolic chronic heart failure. Asian J Androl. 2011;13:759–63. doi: 10.1038/aja.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santos MR, Sayegh AL, Groehs RV, Fonseca G, Trombetta IC, et al. Testosterone deficiency increases hospital readmission and mortality rates in male patients with heart failure. Arq Bras Cardiol. 2015;105:256–64. doi: 10.5935/abc.20150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han Y, Sun W, Sun G, Hou X, Gong Z, et al. A 3-year observation of testosterone deficiency in Chinese patients with chronic heart failure. Oncotarget. 2017;8:79835–42. doi: 10.18632/oncotarget.19816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshihisa A, Suzuki S, Sato Y, Kanno Y, Abe S, et al. Relation of testosterone levels to mortality in men with heart failure. Am J Cardiol. 2018;121:1321–7. doi: 10.1016/j.amjcard.2018.01.052. [DOI] [PubMed] [Google Scholar]
- 62.Güder G, Frantz S, Bauersachs J, Allolio B, Ertl G, et al. Low circulating androgens and mortality risk in heart failure. Heart. 2010;96:504–9. doi: 10.1136/hrt.2009.181065. [DOI] [PubMed] [Google Scholar]
- 63.Mirdamadi A, Garakyaraghi M, Pourmoghaddas A, Bahmani A, Mahmoudi H, et al. Beneficial effects of testosterone therapy on functional capacity, cardiovascular parameters, and quality of life in patients with congestive heart failure. Biomed Res Int. 2014;2014:392432. doi: 10.1155/2014/392432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toma M, McAlister FA, Coglianese EE, Vidi V, Vasaiwala S, et al. Testosterone supplementation in heart failure: a meta-analysis. Circ Heart Fail. 2012;5:315–21. doi: 10.1161/CIRCHEARTFAILURE.111.965632. [DOI] [PubMed] [Google Scholar]
- 65.Wang W, Jiang T, Li C, Chen J, Cao K, et al. Will testosterone replacement therapy become a new treatment of chronic heart failure? A review based on 8 clinical trials. J Thorac Dis. 2016;8:E269–77. doi: 10.21037/jtd.2016.03.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tao J, Liu X, Bai W. Testosterone supplementation in patients with chronic heart failure: a meta-analysis of randomized controlled trials. Front Endocrinol (Lausanne) 2020;11:110. doi: 10.3389/fendo.2020.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cutolo M. Hormones and cytokines: gender-specific effects. In: Legato MJ, editor. Principles of Gender-Specific Medicine. San Diego: Elsevier; 2010. pp. 592–6. [Google Scholar]
- 68.Taneja V. Sex hormones determine immune response. Front Immunol. 2018;9:1931. doi: 10.3389/fimmu.2018.01931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bupp MR, Jorgensen TN. Androgen-induced immunosuppression. Front Immunol. 2018;9:794. doi: 10.3389/fimmu.2018.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fairweather D, Petri MA, Coronado MJ, Cooper LT. Autoimmune heart disease: role of sex hormones and autoantibodies in disease pathogenesis. Expert Rev Clin Immunol. 2012;8:269–84. doi: 10.1586/eci.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, et al. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–8. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 72.Zhang YZ, Xing XW, He B, Wang LX. Effects of testosterone on cytokines and left ventricular remodeling following heart failure. Cell Physiol Biochem. 2007;20:847–52. doi: 10.1159/000110444. [DOI] [PubMed] [Google Scholar]
- 73.Onyimba JA, Coronado MJ, Garton AE, Kim JB, Bucek A, et al. The innate immune response to coxsackievirus B3 predicts progression to cardiovascular disease and heart failure in male mice. Biol Sex Differ. 2011;2:2. doi: 10.1186/2042-6410-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–48. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 75.Huber SA. Autoimmunity in coxsackievirus B3 induced myocarditis. Autoimmunity. 2006;39:55–61. doi: 10.1080/08916930500484906. [DOI] [PubMed] [Google Scholar]
- 76.Fairweather D, Rose NR. Coxsackievirus-induced myocarditis in mice: a model of autoimmune disease for studying immunotoxicity. Methods. 2007;41:118–22. doi: 10.1016/j.ymeth.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ellis NM, Kurahara DK, Vohra H, Mascaro-Blanco A, Erdem G, et al. Priming the immune system for heart disease: a perspective on group A Streptococci. J Infect Dis. 2010;202:1059–67. doi: 10.1086/656214. [DOI] [PubMed] [Google Scholar]
- 78.Huber SA, Job LP. Cellular immune mechanisms in Coxsackievirus group B, type 3 induced myocarditis in Balb/C mice. Adv Exp Med Biol. 1983;161:491–508. doi: 10.1007/978-1-4684-4472-8_29. [DOI] [PubMed] [Google Scholar]
- 79.Lyden DC, Olszewski J, Feran M, Job LP, Huber SA. Coxsackievirus B-3-induced myocarditis. Effect of sex steroids on viremia and infectivity of cardiocytes. Am J Pathol. 1987;126:432–8. [PMC free article] [PubMed] [Google Scholar]
- 80.Frisancho-Kiss S, Davis SE, Nyland JF, Frisancho JA, Cihakova D, et al. Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J Immunol. 2007;178:6710–4. doi: 10.4049/jimmunol.178.11.6710. [DOI] [PubMed] [Google Scholar]
- 81.Frisancho-Kiss S, Coronado MJ, Frisancho JA, Lau VM, Rose NR, et al. Gonadectomy of male BALB/C mice increases Tim-3(+) alternatively activated M2 macrophages, Tim-3(+) T cells, Th2 cells and Treg in the heart during acute coxsackievirus-induced myocarditis. Brain Behav Immun. 2009;23:649–57. doi: 10.1016/j.bbi.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coronado MJ, Bruno KA, Blauwet LA, Tschöpe C, Cunningham MW, et al. Elevated sera sST2 is associated with heart failure in men ≤50 years old with myocarditis. J Am Heart Assoc. 2019;8:e008968. doi: 10.1161/JAHA.118.008968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bayés-Genis A, González A, Lupón J. ST2 in heart failure. Circ Heart Fail. 2018;11:e005582. doi: 10.1161/CIRCHEARTFAILURE.118.005582. [DOI] [PubMed] [Google Scholar]
- 84.Halliday BP, Gulati A, Ali A, Newsome S, Lota A, et al. Sex- and age-based differences in the natural history and outcome of dilated cardiomyopathy. Eur J Heart Fail. 2018;20:1392–400. doi: 10.1002/ejhf.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Regitz-Zagrosek V, Oertelt-Prigione S, Seeland U, Hetzer R. Sex and gender differences in myocardial hypertrophy and heart failure. Circ J. 2010;74:1265–73. doi: 10.1253/circj.cj-10-0196. [DOI] [PubMed] [Google Scholar]
- 86.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–7. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 87.Studen KB, Pfeifer M. Cardiometabolic risk in polycystic ovary syndrome. Endocr Connect. 2018;7:R238–51. doi: 10.1530/EC-18-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rexrode KM, Manson JE, Lee IM, Ridker PM, Sluss PM, et al. Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation. 2003;108:1688–93. doi: 10.1161/01.CIR.0000091114.36254.F3. [DOI] [PubMed] [Google Scholar]
- 89.Sluijmer AV, Heineman MJ, De Jong FH, Evers JL. Endocrine activity of the postmenopausal ovary: the effects of pituitary down-regulation and oophorectomy. J Clin Endocrinol Metab. 1995;80:2163–7. doi: 10.1210/jcem.80.7.7608272. [DOI] [PubMed] [Google Scholar]
- 90.Herring MJ, Oskui PM, Hale SL, Kloner RA. Testosterone and the cardiovascular system: a comprehensive review of the basic science literature. J Am Heart Assoc. 2013;2:e000271. doi: 10.1161/JAHA.113.000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Koeverden ID, de Bakker M, Haitjema S, van der Laan SW, de Vries JP, et al. Testosterone to oestradiol ratio reflects systemic and plaque inflammation and predicts future cardiovascular events in men with severe atherosclerosis. Cardiovasc Res. 2019;115:453–62. doi: 10.1093/cvr/cvy188. [DOI] [PubMed] [Google Scholar]
- 92.Perrino C, Ferdinandy P, Bøtker HE, Brundel B, Collins P, et al. Improving translational research in sex-specific effects of comorbidities and risk factors in ischemic heart disease and cardioprotection: position paper and recommendations of the ESC working group on cellular biology of the heart. Cardiovasc Res. 2020 doi: 10.1093/cvr/cvaa155. Doi: 10.1093/cvr/cvaa155. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vitale C, Mendelsohn ME, Rosano GM. Gender differences in the cardiovascular effect of sex hormones. Nat Rev Cardiol. 2009;6:532–42. doi: 10.1038/nrcardio.2009.105. [DOI] [PubMed] [Google Scholar]
- 94.Jazbutyte V, Stumpner J, Redel A, Lorenzen JM, Roewer N, et al. Aromatase inhibition attenuates desflurane-induced preconditioning against acute myocardial infarction in male mouse heart in vivo. PLoS One. 2012;7:e42032. doi: 10.1371/journal.pone.0042032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xiong Q, Liu X, Shen Y, Yu P, Chen S, et al. Elevated serum bisphenol A level in patients with dilated cardiomyopathy. Int J Environ Res Public Health. 2015;12:5329–37. doi: 10.3390/ijerph120505329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pascual-Figal DA, Tornel PL, Nicolás F, Sánchez-Más J, Martínez MD, et al. Sex hormone-binding globulin: a new marker of disease severity and prognosis in men with chronic heart failure. Rev Esp Cardiol. 2009;62:1381–7. doi: 10.1016/s1885-5857(09)73532-7. [DOI] [PubMed] [Google Scholar]
- 97.Zhou Q, Miao M, Ran M, Ding L, Bai L, et al. Serum bisphenol-A concentration and sex hormone levels in men. Fertil Steril. 2013;100:478–82. doi: 10.1016/j.fertnstert.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 98.Kontoleon PE, Anastasiou-Nana MI, Papapetrou PD, Alexopoulos G, Ktenas V, et al. Hormonal profile in patients with congestive heart failure. Int J Cardiol. 2003;87:179–83. doi: 10.1016/s0167-5273(02)00212-7. [DOI] [PubMed] [Google Scholar]
- 99.Naderi N, Heidarali M, Barzegari F, Ghadrdoost B, Amin A, et al. Hormonal profile in patients with dilated cardiomyopathy. Res Cardiovasc Med. 2015;4:e27631. doi: 10.5812/cardiovascmed.27631v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abdullaev T, Mardanov B, Kurbanov N. The value of testosterone on cardiac function in patients with dilated cardiomyopathy. Int J Cardiol. 2008;125:S51. [Google Scholar]
- 101.D'Souza RS, Levandowski C, Slavov D, Graw SL, Allen LA, et al. Danon disease: clinical features, evaluation, and management. Circ Heart Fail. 2014;7:843–9. doi: 10.1161/CIRCHEARTFAILURE.114.001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Olivotto I, Maron MS, Adabag AS, Casey SA, Vargiu D, et al. Gender-related differences in the clinical presentation and outcome of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:480–7. doi: 10.1016/j.jacc.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 103.Meyer S, van der Meer P, van Tintelen JP, van den Berg MP. Sex differences in cardiomyopathies. Eur J Heart Fail. 2014;16:238–47. doi: 10.1002/ejhf.15. [DOI] [PubMed] [Google Scholar]
- 104.Kessler EL, Rivaud MR, Vos MA, van Veen TA. Sex-specific influence on cardiac structural remodeling and therapy in cardiovascular disease. Biol Sex Differ. 2019;10:7. doi: 10.1186/s13293-019-0223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ikeda Y, Aihara K, Yoshida S, Akaike M, Matsumoto T. Effects of androgens on cardiovascular remodeling. J Endocrinol. 2012;214:1–10. doi: 10.1530/JOE-12-0126. [DOI] [PubMed] [Google Scholar]
- 106.Cavasin MA, Sankey SS, Yu AL, Menon S, Yang XP. Estrogen and testosterone have opposing effects on chronic cardiac remodeling and function in mice with myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;284:H1560–9. doi: 10.1152/ajpheart.01087.2002. [DOI] [PubMed] [Google Scholar]
- 107.Corrêa RA, Júnior RF, Mendes SB, Dos Santos PM, da Silva MV, et al. Testosterone deficiency reduces the effects of late cardiac remodeling after acute myocardial infarction in rats. PLoS One. 2019;14:e0213351. doi: 10.1371/journal.pone.0213351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marsh JD, Lehmann MH, Ritchie RH, Gwathmey JK, Green GE, et al. Androgen receptors mediate hypertrophy in cardiac myocytes. Circulation. 1998;98:256–61. doi: 10.1161/01.cir.98.3.256. [DOI] [PubMed] [Google Scholar]
- 109.Guerra S, Leri A, Wang X, Finato N, Di Loreto C, et al. Myocyte death in the failing human heart is gender dependent. Circ Res. 1999;85:856–66. doi: 10.1161/01.res.85.9.856. [DOI] [PubMed] [Google Scholar]
- 110.Davey RA, Grossmann M. Androgen receptor structure, function and biology: from bench to bedside. Clin Biochem Rev. 2016;37:3–15. [PMC free article] [PubMed] [Google Scholar]
- 111.Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–4. doi: 10.1126/science.275.5308.1930. [DOI] [PubMed] [Google Scholar]
- 112.Duran J, Oyarce C, Pavez M, Valladares D, Basualto-Alarcon C, et al. GSK-3β/NFAT signaling is involved in testosterone-induced cardiac myocyte hypertrophy. PLoS One. 2016;11:e0168255. doi: 10.1371/journal.pone.0168255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kerkela R, Kockeritz L, Macaulay K, Zhou J, Doble BW, et al. Deletion of GSK-3beta in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast hyperproliferation. J Clin Invest. 2008;118:3609–18. doi: 10.1172/JCI36245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hardt SE, Sadoshima J. Negative regulators of cardiac hypertrophy. Cardiovasc Res. 2004;63:500–9. doi: 10.1016/j.cardiores.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 115.Altamirano F, Oyarce C, Silva P, Toyos M, Wilson C, et al. Testosterone induces cardiomyocyte hypertrophy through mammalian target of rapamycin complex 1 pathway. J Endocrinol. 2009;202:299–307. doi: 10.1677/JOE-09-0044. [DOI] [PubMed] [Google Scholar]
- 116.Gardner JD, Brower GL, Janicki JS. Gender differences in cardiac remodeling secondary to chronic volume overload. J Card Fail. 2002;8:101–7. doi: 10.1054/jcaf.2002.32195. [DOI] [PubMed] [Google Scholar]
- 117.Beaumont C, Walsh-Wilkinson É, Drolet MC, Roussel É, Melançon N, et al. Testosterone deficiency reduces cardiac hypertrophy in a rat model of severe volume overload. Physiol Rep. 2019;7:e14088. doi: 10.14814/phy2.14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li Y, Kishimoto I, Saito Y, Harada M, Kuwahara K, et al. Androgen contributes to gender-related cardiac hypertrophy and fibrosis in mice lacking the gene encoding guanylyl cyclase-A. Endocrinology. 2004;145:951–8. doi: 10.1210/en.2003-0816. [DOI] [PubMed] [Google Scholar]
- 119.Hou M, Gu HC, Wang HH, Liu XM, Zhou CL, et al. Prenatal exposure to testosterone induces cardiac hypertrophy in adult female rats through enhanced Pkcδ expression in cardiac myocytes. J Mol Cell Cardiol. 2019;128:1–10. doi: 10.1016/j.yjmcc.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 120.Zwadlo C, Schmidtmann E, Szaroszyk M, Kattih B, Froese N, et al. Antiandrogenic therapy with finasteride attenuates cardiac hypertrophy and left ventricular dysfunction. Circulation. 2015;131:1071–81. doi: 10.1161/CIRCULATIONAHA.114.012066. [DOI] [PubMed] [Google Scholar]
- 121.Ikeda Y, Aihara K, Sato T, Akaike M, Yoshizumi M, et al. Androgen receptor gene knockout male mice exhibit impaired cardiac growth and exacerbation of angiotensin II-induced cardiac fibrosis. J Biol Chem. 2005;280:29661–6. doi: 10.1074/jbc.M411694200. [DOI] [PubMed] [Google Scholar]
- 122.Baltatu O, Cayla C, Iliescu R, Andreev D, Jordan C, et al. Abolition of hypertension-induced end-organ damage by androgen receptor blockade in transgenic rats harboring the mouse ren-2 gene. J Am Soc Nephrol. 2002;13:2681–7. doi: 10.1097/01.asn.0000033327.65390.ca. [DOI] [PubMed] [Google Scholar]
- 123.Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, et al. Classification of the cardiomyopathies: a position statement from the European society of cardiology working group on myocardial and pericardial diseases. Eur Heart J. 2008;29:270–6. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 124.Duraes AR, Bitar YS, Roever L, Neto MG. Endomyocardial fibrosis: past, present, and future. Heart Fail Rev. 2020;25:725–30. doi: 10.1007/s10741-019-09848-4. [DOI] [PubMed] [Google Scholar]
- 125.Bolarin DM, Andy JJ. Clinical feminization and serum testosterone levels in male patients with chronic African endomyocardial fibrosis. Trop Geogr Med. 1982;34:309–12. [PubMed] [Google Scholar]
- 126.Chung CC, Hsu RC, Kao YH, Liou JP, Lu YY, et al. Androgen attenuates cardiac fibroblasts activations through modulations of transforming growth factor-β and angiotensin II signaling. Int J Cardiol. 2014;176:386–93. doi: 10.1016/j.ijcard.2014.07.077. [DOI] [PubMed] [Google Scholar]
- 127.Batra J, Rosenblum H, Defilippis EM, Griffin JM, Saith SE, et al. Sex differences in the phenotype of transthyretin cardiac amyloidosis due to Val122Ile mutation: insights from non-invasive pressure-volume analysis. J Card Fail. 2020 doi: 10.1016/j.cardfail.2020.08.007. Doi: 10.1016/j.cardfail.2020.08.007. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bukhari S, Barakat A, Mulukutla S, Thoma F, Eisele YS, et al. Faster progression of left ventricular thickness in men compared to women in wild-type transthyretin cardiac amyloidosis. J Am Coll Cardiol. 2020;75:812. [Google Scholar]
- 129.Shen JS, Meng XL, Wight-Carter M, Day TS, Goetsch SC, et al. Blocking hyperactive androgen receptor signaling ameliorates cardiac and renal hypertrophy in Fabry mice. Hum Mol Genet. 2015;24:3181–91. doi: 10.1093/hmg/ddv070. [DOI] [PubMed] [Google Scholar]
- 130.Mirea O, Berceanu M, Constantin A, Mănescu M, Târtea G, et al. Non-compaction cardiomyopathy – brief review. J Mind Med Sci. 2017;4:115–24. [Google Scholar]
- 131.Stöllberger C, Blazek G, Winkler-Dworak M, Finsterer J. Sex differences in left ventricular noncompaction in patients with and without neuromuscular disorders. Rev Esp Cardiol. 2008;61:130–6. [PubMed] [Google Scholar]
- 132.Corrado D, Link MS, Calkins H. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2017;376:61–72. doi: 10.1056/NEJMra1509267. [DOI] [PubMed] [Google Scholar]
- 133.Bauce B, Frigo G, Marcus FI, Basso C, Rampazzo A, et al. Comparison of clinical features of arrhythmogenic right ventricular cardiomyopathy in men versus women. Am J Cardiol. 2008;102:1252–7. doi: 10.1016/j.amjcard.2008.06.054. [DOI] [PubMed] [Google Scholar]
- 134.Brouillette J, Trépanier-Boulay V, Fiset C. Effect of androgen deficiency on mouse ventricular repolarization. J Physiol. 2003;546:403–13. doi: 10.1113/jphysiol.2002.030460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Akdis D, Saguner AM, Shah K, Wei C, Medeiros-Domingo A, et al. Sex hormones affect outcome in arrhythmogenic right ventricular cardiomyopathy/dysplasia: from a stem cell derived cardiomyocyte-based model to clinical biomarkers of disease outcome. Eur Heart J. 2017;38:1498–508. doi: 10.1093/eurheartj/ehx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ren J, Chen L, Zhang N, Chen X, Zhao Q, et al. Plasma testosterone and arrhythmic events in male patients with arrhythmogenic right ventricular cardiomyopathy. ESC Heart Fail. 2020;7:1547–59. doi: 10.1002/ehf2.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang Y, Xia L, Shen X, Han G, Feng D, et al. A new insight into sudden cardiac death in young people: a systematic review of cases of takotsubo cardiomyopathy. Medicine (Baltimore) 2015;94:e1174. doi: 10.1097/MD.0000000000001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bell JR, Bernasochi GB, Varma U, Raaijmakers AJ, Delbridge LM. Sex and sex hormones in cardiac stress--mechanistic insights. J Steroid Biochem Mol Biol. 2013;137:124–35. doi: 10.1016/j.jsbmb.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 139.Mellor KM, Curl CL, Chandramouli C, Pedrazzini T, Wendt IR, et al. Ageing-related cardiomyocyte functional decline is sex and angiotensin II dependent. Age (Omaha) 2014;36:1155–67. doi: 10.1007/s11357-014-9630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brenner R, Weilenmann D, Maeder MT, Jörg L, Bluzaite I, et al. Clinical characteristics, sex hormones, and long-term follow-up in Swiss postmenopausal women presenting with takotsubo cardiomyopathy. Clin Cardiol. 2012;35:340–7. doi: 10.1002/clc.21986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pérez-Castellanos A, Martínez-Sellés M, Mejía-Rentería H, Andrés M, Sionis A, et al. Takotsubo syndrome in men: rare, but with poor prognosis. Rev Esp Cardiol (Engl Ed) 2018;71:703–8. doi: 10.1016/j.rec.2017.07.021. [DOI] [PubMed] [Google Scholar]


